Abstract
Objective
Although electronic cigarettes (e-cigarettes) are frequently initiated for smoking cessation, results from the first two clinical trials testing this suggest that the perceived benefits of vaping may be influenced by non-nicotine factors, including cognitive outcome expectancies. The current study investigated the separate and combined effects of nicotine delivery and outcome expectancies on cravings for cigarettes and e-cigarettes using a balanced-placebo experiment.
Method
Drug dosage (contains nicotine or not) was crossed with instructional set (told nicotine or non-nicotine) during ad-lib e-cigarette use sessions by 128 current e-cigarette users (52 identifying as current cigarette smokers, or “dual users”). It was hypothesized that reduction in craving for both cigarettes and e-cigarettes following e-cigarette administration would be driven primarily by the instructional set manipulation, reflecting the influence of outcome expectancies.
Results
As hypothesized, among dual users, a main effect of instructional set emerged on reductions in craving to smoke cigarettes, with participants who were told that their e-cigarette contained nicotine reporting greater craving reduction (p = .046). With respect to reduced cravings for e-cigarettes, we found an interaction between drug dose and instructional set (p = .02) such that nicotine e-cigarettes reduced cravings more than non-nicotine e-cigarettes only among participants told to expect nicotine.
Conclusions
Findings suggest that cognitive expectancies contribute to the acute effects of e-cigarettes on craving, which may provide guidance for their potential as smoking cessation aids.
Keywords: e-cigarette, cigarette, smoking, expectancies, balanced-placebo, nicotine
Recently, there has been a shift in the landscape of tobacco use with the introduction of novel products such as electronic cigarettes (e-cigarettes), which are portable, battery-powered devices containing a heating element that aerosolizes a liquid solution. Using e-cigarettes is often referred to as “vaping,” and users may self-identify as “vapers.” It has been estimated that 2.4% of US adults use e-cigarettes regularly, with the vast majority being current or former smokers (Zhu, Zhuang, Wong, Cummins, & Tedeschi, 2017). Vapers report that their primary reason for using e-cigarettes is to quit or reduce smoking of traditional, combustible cigarettes (e.g., Siegel, Tanwar, & Wood, 2011). To date, only two double-blind randomized controlled trials (RCTs) of e-cigarettes for smoking cessation have been published. One study (Bullen et al., 2013) randomly assigned transdermal nicotine patches, nicotine e-cigarettes, and placebo (non-nicotine) e-cigarettes to participants interested in quitting smoking. Another trial (Caponnetto et al., 2013) randomized non-treatment seeking participants to receive one of two nicotine doses or placebo e-cigarettes. Although both trials indicated that e-cigarettes were effective in promoting smoking reduction or cessation, no significant differences were found based on nicotine content.
Cravings to use a drug are often considered the final common pathway in theoretical models of drug use motivation (Baker, Morse, & Sherman, 1986). In the case of tobacco smoking, the FDA-approved medications (NRTs, bupropion, and varenicline) have all been shown to reduce cue- and abstinence-induced cravings to smoke in laboratory paradigms (Ferguson & Shiffman, 2009). In addition, research has found that e-cigarette use reduces cravings to smoke and to vape, with mixed evidence regarding the role of nicotine per se (Dawkins, Turner, Hasna, & Soar, 2012; Perkins, Karelitz, & Michael, 2017). There is also some evidence that the sensorimotor aspects of vaping (i.e., its similarity to smoking behavior) contributes to craving reduction (Van Heel, Van Gucht, Vanbrabant, & Baeyens, 2017).
Drug use and addictive behaviors may also be influenced by outcome expectancies, which are learned, cognitive intervening variables. Drug-related expectancies refer to the degree that individuals expect positive and negative outcomes from drug use. Expectancies have been predictive of initiation, maintenance, cessation, and relapse to alcohol, tobacco, and other substances (Brandon, Juliano, & Copeland, 1999; Goldman, 1999). Research on expectancies for e-cigarette use has been limited to survey data (e.g. Harrell, Marquinez et al., 2015).
Prior studies have indicated that expectancies can sometimes influence immediate drug use behaviors and outcomes to a greater degree than drug dosage itself (Kirsch, 1985)—often referred to as “the placebo effect.” This phenomenon has been studied through simultaneous expectancy and pharmacological manipulation using the balanced-placebo design (BPD), initially to study the effects of alcohol (Hull & Bond, 1986; Marlatt, Demming, & Reid, 1973), and more recently with tobacco and NRT (Dar & Barrett, 2014; Juliano & Brandon, 2002). This paradigm utilizes a 2x2 factorial design in which drug type (active or placebo) is crossed with instructional set (told active or placebo). From this, the effects of both the pharmacologic properties of the drug and expectancies about the drug can be independently evaluated as causal influences. In general, the results from these studies demonstrate that active drug delivery appears to have primary influence over physiological or objective domains, whereas drug expectancies may influence more emotionally salient or subjective domains, such as craving. These effects have been observed in both laboratory studies and more naturalistic field studies (Dar & Barrett, 2014). Thus, the BPD can be used to test the independent and synergistic effects of nicotine delivery and nicotine expectancies upon craving reduction.
The primary goal of the present study was to investigate the effects of nicotine and expectancies on cravings to smoke and cravings to vape. Current e-cigarette users were randomized to use e-cigarettes that contained either nicotine or non-nicotine solutions, and were independently instructed that the e-cigarette contained nicotine or non-nicotine, resulting in four experimental conditions as illustrated in Table 1. It was hypothesized that instructional set should produce differences in craving reduction such that told nicotine would produce greater reduction in craving than told no-nicotine, reflecting the role of nicotine-related expectancies. There were no a priori hypotheses regarding main effects of nicotine content or interactions.
Table 1.
Experimental design
| Drug Content | Instructional Set | |
|---|---|---|
| E-cigarette contains nicotine | E-cigarette does not contain nicotine | |
|
|
||
| Nicotine | True positive | False Negative, “Anti-Placebo” |
|
|
||
| Non-Nicotine | False positive, “Placebo” | True Negative |
|
|
||
Method
Participants
Participants were 130 individuals recruited primarily from flyers at local vape shops (Table 2). Participants were screened by telephone for the following eligibility criteria: 1) ≥ 18 years old; 2) Current e-cigarette users (daily nicotine solution use for ≥ 30 days); 3) History of cigarette smoking (≥ 100 lifetime cigarettes; ≥ 1 cigarette/day for ≥ 30 days); 4) No current e-cigarette cessation attempt; and 5) Not currently pregnant, attempting to get pregnant, or nursing.
Table 2.
Participant demographics (N=128)
| Variable | Description | Mean (SD) or % |
|---|---|---|
| Age | (range 18–76) | 36.40 (13.79) |
| Sex | Female | 38% |
| Race | Black / African American | 12% |
| White / European Origin | 83% | |
| Other | 6% | |
| Ethnicity | Hispanic / Latino | 16% |
| Marital Status | Single | 60% |
| Sexual Orientation | Identify as LGBT+ | 13% |
| Education | High school or less | 25% |
| Some College | 30% | |
| Tech School / Associate’s | 29% | |
| 4-year College Degree or beyond | 16% | |
| Income | Under $10,000 | 22% |
| $10,000 – $29,999 | 28% | |
| $30,000 or more | 49% |
Note: No significant differences between conditions were found for any of the variables.
Experimental Procedure
Eligible participants were asked to abstain from using e-cigarettes and combustible cigarettes for three hours prior to the session. To increase adherence, participants were told that a breath carbon monoxide (CO) reading would be administered. Upon arrival, research staff obtained written informed consent, and then collected the CO sample (M = 12.63, SD = 8.63 ppm among smokers; M = 6.00, SD = 4.21 ppm among former smokers). Participants were then randomized to condition and their e-cigarette solution prepared by a researcher with no participant contact, ensuring a double blind for the drug manipulation. Randomization used a 4-block pattern with stratification based on sex, cigarette smoking status (current [defined by smoking >1 cigarette per week] or former), and flavor preference (tobacco, menthol, or fruit).
Participants completed demographic and baseline measures, followed by the first administration of craving measures. Participants were then provided an e-cigarette with instructions and labeling consistent with the instructional set conditions. They were instructed to take at least 10 puffs over the 10 minute session. Following the ad-lib session, the craving measures were re-administered. (Secondary outcome variables—affect, appetite, reinforcement, and attention—were then collected and will be reported elsewhere.)
Apparatus
Participants were provided with an eGo-style 3.6–4.2 Volt, 1100 mAh battery with a 2.8-Ohm, 510-style clearomizer. This device contained an LCD display showing number of puffs. This e-cigarette style is considered “second generation,” which deliver nicotine more consistently and are preferred among experienced vapers compared to first generation “cig-alike” styles (e.g., Dawkins, Kimber, Puwanesarasa, & Soar, 2015).
The solution used was a 50% vegetable glycerin (VG), 50% propylene glycol (PG) liquid. Target nicotine content was either 0 mg/ml or 12 mg/ml, with the latter having produced similar plasma nicotine concentrations (as measured in venous blood samples) as traditional cigarettes (Ramôa et al., 2015). Participants were given the choice of tobacco, menthol, or fruit flavors. This solution was a custom-made “research blend” (Avail Vapor, LLC). The solutions were retested using mass spectrometry and liquid chromatography, which verified that the non-nicotine solutions contained 0 mg/ml nicotine. Final concentrations of the nicotine solutions were 11.2 mg/ml (tobacco), 10.3 mg/ml (menthol), and 10.0 mg/ml (fruit).
Assessments
Baseline assessments
Participants completed questionnaires capturing basic demographic information, smoking and vaping history, and device preference. Dependence on e-cigarettes was measured using the Penn State Electronic Cigarette Dependence Index (ECDI; Foulds et al., 2014; α = 0.70), whereas cigarette dependence was measured with the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991; α = 0.56 for current smokers).
Craving to smoke and craving to use e-cigarettes
A 3-item adaptation of the Questionnaire of Smoking Urges-Brief (QSU; Cox, Tiffany, & Christen, 2001; Kozlowski, Pillitteri, Sweeney, Whitfield, & Graham, 1996) was used to assess craving to smoke (α = 0.90 – 0.95) and craving to vape (α = 0.91 – 0.94). The items, assessing “desire,” “craving,” and “wanting,” were rated 0–6, yielding scores from 0–24.
Nicotine dosing estimate
As a check on the instructional set manipulation, participants completed a brief questionnaire post ad-lib session asking them to estimate the nicotine dose of the provided e-cigarette (0mg/ml, 6mg/ml, 12 mg/ml, 18 mg/ml, or 24 mg/ml).
Data Analysis Plan
Factorial 2 X 2 analyses of variances (ANOVAs) and χ2 tests were used to test for baseline differences between factorial conditions (drug, instructional set). T-tests were used to test for baseline differences by sex and smoking status. Next, 2 (drug) X 2 (instructional set) X 2 (sex) ANOVAs were used to test the main effects and interactions of the drug and instructional set manipulations on pre-to-post changes in craving. Given previous sex differences found on both general nicotine effects (Perkins, 1996) and e-cigarette outcome expectancies (Copp et al., 2015; Piñeiro et al., 2016), sex was included as a third factor.
RESULTS
Preliminary Analyses
Two participants were removed (instructional non-compliance, incorrect randomization) from final analyses N (= 128). Drug X instructional set ANOVAs failed to reveal significant differences between conditions on any demographic, baseline, pre-test variables, or puff count. No differences were found between sexes on pre-ad-lib outcome variables. Current smokers and former smokers were compared on several descriptive variables, as shown in Table 3.
Table 3.
Smoking and vaping characteristics of the full sample, and broken down by current smokers (dual users) and former smokers.
| Variable | Full sample (N=128) | Current smokers (N=52) | Former smokers (N=76) | p |
|---|---|---|---|---|
| Mean cigarettes per day on days smoked (SD)1 | 13.33 (11.31) | 8.02 (8.57) | 16.96 (11.56) | <.001 |
| Mean days cigarettes smoked per week (SD)1 | 6.01 (1.93) | 5.01 (2.35) | 6.70 (1.15) | <.001 |
| Mean number of daily e-cigarette uses (SD) | 36.50 (53.36) | 26.66 (42.40) | 43.91 (59.60) | n.s |
| % reporting vaping continuously all day (SD) | 47% | 35% | 66% | .001 |
| Personal device used2: | ||||
| Disposable, “cig-a-like” | 11% | 14% | 5% | n.s |
| Refillable tank system | 9% | 14% | 11% | n.s |
| Tank system with modifiable electric components | 66% | 52% | 75% | n.s |
| Mean nicotine content of solution in personal device (mg/ml) | 8.80 (7.51) | 10.57 (8.07) | 7.71 (6.98) | .046 |
| Flavor used most often: | ||||
| Tobacco | 11% | 17% | 7% | n.s |
| Menthol | 21% | 21% | 21% | |
| Fruit | 41% | 40% | 42% | n.s |
| Other (e.g. custard, dessert, beverages) | 23% | 12% | 30% | .013 |
| Reported e-cigarette initiation to quit smoking | 68% | 50% | 80% | <.001 |
| Flavor requested for ad-lib session: | ||||
| Tobacco | 12% | 17% | 8% | n.s |
| Menthol | 25% | 25% | 25% | n.s |
| Fruit | 63% | 58% | 67% | n.s |
| Mean EDCI | 10.05 (4.66) | 8.44 (5.10) | 11.15 (4.00) | .001 |
| Mean FTND1 | 4.50 (2.89) | 3.69 (3.28) | 5.05 (2.45) | .008 |
Notes:
Among former smokers, cigarette quantity, frequency, and dependence reflect reported levels prior to quitting smoking.
Personal device used was coded from participants’ self-reported device brand/model.
Above p values represent significant differences between current and former smokers. n.s = not significant. No significant differences were found between conditions on these variables. ECDI = Penn State Electronic Cigarette Dependence Inventory. FTND = Fagerström Test for Nicotine Dependence.
Craving Reduction
Cravings to smoke
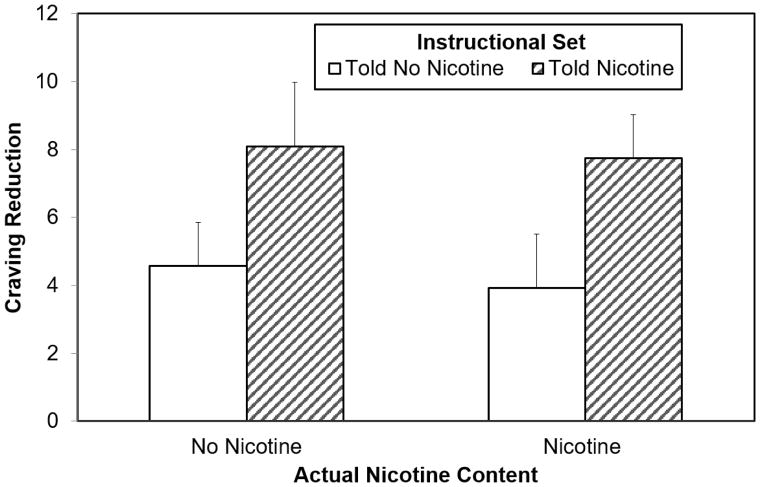
Among current smokers (n = 52), the hypothesized main effect of instructional set was observed on change in craving to smoke as measured by the QSU, F (1, 44) = 4.21, p = .046, η2 = 0.09. Greater craving reduction was found among those told they received nicotine (M = 7.92, SD = 6.59) than those told they did not receive nicotine (M = 4.25, SD = 5.31; see Table 4 and Figure 1). No main effect of drug was found, nor were there interactions between instruction, drug, or sex.
Table 4.
Manipulation Effects on Craving: Nicotine, Instruction, and Interactions
| Variable | Condition means | Marginal means Drug Content | Marginal means Instructional Set | F (Nicotine) | F (Instruction) | F (Nicotine X Instruction) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| True Positive | Placebo | Anti- Placebo | True Negative | Nicotine | No Nicotine | Told Nicotine | Told No Nicotine | ||||
| QSU- Smoke | 7.75 | 8.08 | 3.93 | 4.57 | 5.69 | 6.19 | 7.92a | 4.25a | 0.15 | 4.21* | 0.02 |
| Modified QSU-Vape | 8.00a,b | 3.68a | 3.84b | 4.82 | 5.92 | 4.26 | 5.87 | 4.34 | 1.73 | 1.31 | 5.56* |
Notes: QSU = 3-item version of Questionnaire of Smoking Urges-Brief. Positive difference scores represent reductions in value from pre- to post-tests.
p < .05.
Shared superscripts indicate significant differences in cell means:
p < .05,
= p < .01.
Figure 1. Manipulation Effects on Desire to Smoke Among Current Smokers (n = 52).
Note: Main effect of instructional set significant at p = .046. Error bars are standard error of the mean.
Craving to vape
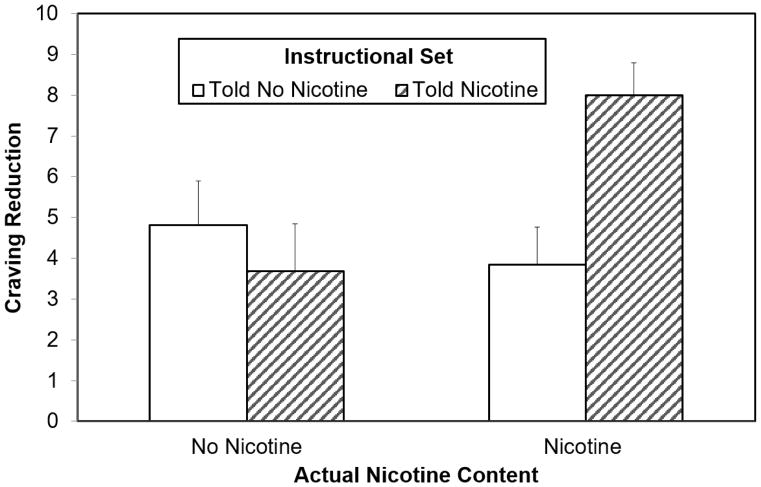
Using the full sample, no main effects of drug, instructional set, or sex emerged, but a significant drug X instructional set interaction was found on craving to vape, F (1, 120) = 5.56, p = .020, η2 = 0.04 (see Figure 2). Post-hoc analyses revealed a significant effect of nicotine dose only when participants were told to expect nicotine, t (62) = −2.75, p = .008, d = .69. Additional paired-comparisons revealed that the True Positive group showed significantly greater reductions in craving to vape than the Placebo group, t (61) = −2.57, p = .013, d = .65, and the Anti-Placebo group, t (62) = −2.75, p = .008, d = .69, and marginally greater than the True Negative group t (63) = −1.96, p = .05, d = .49.
Figure 2. Manipulation Effects on Desire to Vape Among Full Sample (N = 128).
Note: Interaction p = .020. Error bars are standard error of the mean.
Nicotine Dosing Estimate
A 2x2 ANOVA revealed main effects of both instruction (F [1, 124] = 47.17, p < .001) and drug content (F [1, 124] = 15.71, p < .001) on estimated nicotine content. Told nicotine produced higher nicotine estimates (M = 10.98, SD = 6.64 vs M = 4.12, SD = 5.24), as did actual nicotine content (M = 9.50, SD = 7.56 vs M = 5.50, SD = 5.47).
To further explore the relationship between perceived nicotine dose and craving relief, correlations between these variables were examined. Among smokers, higher nicotine dose estimates were associated with greater cigarette craving reduction, r (50) = .37, p = .007. Among the full sample, nicotine dose estimate was not associated with e-cigarette craving reduction, r (126) = .15, ns.
DISCUSSION
This study aimed to address specific motivational factors involved with e-cigarette use, primarily their immediate ability to ameliorate cravings. This study is, to our knowledge, the first fully-crossed BPD conducted to parse the independent and synergistic influences of both nicotine delivery and expectancies on craving-related outcomes of e-cigarette use.
Among dual users, the hypothesized main effect of instructional set was observed upon reduction in cravings to smoke, which suggests that the craving reduction was driven by participants’ expectancies about the effects of nicotine rather than the pharmacological properties of nicotine. The correlation between nicotine dosing estimate and craving reduction provides further support for the role of expectancies. These results are consistent with contemporary models and research indicating that drug delivery alone is insufficient for explaining drug craving and its alleviation (e.g., Baker, Piper, McCarthy, Majeskie, & Fiore, 2004). Findings are also consistent with previous BPDs with cigarettes (Juliano & Brandon, 2002), and with a non-BPD study showing that nicotine instructional set influenced craving reduction following use of nicotine-free e-cigarettes (Copp et al., 2015).
Notably, the main effect of instructional set rather than drug content is consistent with the findings from the two extant clinical trials of e-cigarettes for smoking cessation, which both failed to find statistically superior cessation outcomes from nicotine versus placebo products (Bullen et al., 2013; Caponnetto et al., 2013), and it suggests that any therapeutic benefit of e-cigarettes may derive, at least in part, from users’ cognitive expectancies about them.
Whereas the findings on cravings to smoke address the potential of e-cigarettes as a substitute for smoking, the effects upon cravings to vape address the maintenance of e-cigarette use itself. Here no main effects were found, but an interaction was found between the two experimental factors that indicated the greatest craving reduction when participants were accurately told that they were receiving nicotine (i.e. True Positive). The contrast with the findings on craving to smoke suggest that nicotine delivered via e-cigarettes may reduce cravings for the same product, but it may not transfer to a different nicotine-delivery product, combustible cigarettes.
The results of this study should be considered within the context of some methodological limitations. Both instructional set and, to a lesser degree, actual nicotine dose were found to have influenced participants’ estimates of the dose they had received. The former finding indicates that the instructional set manipulation was successful. The latter is not surprising, yet reflects a common challenge with the BPD (Dar & Barrett, 2014) that must be considered when interpreting BPD results. Although we collected puff count, additional topography or blood nicotine measures would have provided more complete data on nicotine delivery. Several of the measures used in this study were adapted from validated cigarette measures, but have not yet been validated specifically for e-cigarettes. Inclusion of a behavioral outcome might have enhanced the self-report findings. Additionally, it should be noted that the analysis of craving to vape utilized a larger sample, which may have yielded more stable sample statistics with greater power. Finally, the majority of participants used modifiable e-cigarette systems, which may deliver nicotine more effectively than the standard device used in the study.
Emerging evidence suggests that e-cigarettes may be an effective tool for smoking cessation (Zhu et al., 2017). The results of the present study suggest that nicotine delivery may not be necessary for the acute management of cravings to smoke via vaping. Thus, the possibility of further harm-reduction through the elimination or reduction of nicotine content without sacrificing e-cigarettes’ potential efficacy for smoking cessation is promising. Public health campaigns and clinicians could endorse alternative expectancies about the benefits of vaping upon cigarette craving, reducing the emphasis on nicotine per se. Moreover, in other domains, placebo medications have retained their efficacy even after their content has been revealed to patients (i.e., open-label placebo; Kaptchuk et al., 2010). Future studies should extend this line of research beyond abstinence-induced craving to those induced by negative affect and conditioned stimuli (i.e., “smoking triggers”).
Public Health Impact.
This study demonstrated that use of an electronic cigarette may reduce nicotine craving (i.e., desire to smoke or vape) via nonpharmacological routes, including beliefs about nicotine, rather than simply via nicotine delivery itself. This finding has implications for understanding e-cigarette use as well as its potential as a smoking cessation aid.
Acknowledgments
The authors would like to acknowledge Alessandra Santa-Ana, Walid Hazem, Alina Hoehn, Ursula Martinez, John Correa, and Lancia Darville for their assistance in conducting this project, as well as Mark Goldman, Joseph Vandello, and Vani Nath Simmons for their consultation.
This research was supported in part by funding from the University of South Florida, the National Institute on Drug Abuse grant R01 DA037961, and the Proteomics Core at the H. Lee Moffitt Cancer Center & Research Institute, a comprehensive cancer center designated by the National Cancer Institute (P30 CA076292). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Thomas H. Brandon has had past research support from Pfizer, Inc.
References
- Baker TB, Morse E, Sherman JE. The motivation to use drugs: A psychobiological analysis of urges. Nebraska Symposium on Motivation. 1986;34:257–323. [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Juliano LM, Copeland AL. Expectancies for tobacco smoking. In: Kirsh, Irving, editors. How expectancies shape experience. Washington, DC: American Psychological Association; 1999. pp. 263–299. [Google Scholar]
- Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, Walker N. Electronic cigarettes for smoking cessation: A randomised controlled trial. The Lancet. 2013;382:1629–1637. doi: 10.1016/S0140-6736(13)61842-5. [DOI] [PubMed] [Google Scholar]
- Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, Russo C, Polosa R. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: A prospective 12-month randomized control design study. PLoS One. 2013;8:e66317. doi: 10.1371/journal.pone.0066317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Dar R, Barrett SP. The effects of beliefs regarding drug assignment in experimental and field studies of nicotine delivery devices: A review. Journal of Psychopharmacology. 2014;28:1071–1079. doi: 10.1177/0269881114548295. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Kimber C, Puwanesarasa Y, Soar K. First- versus second-generation electronic cigarettes: Predictors of choice and effects on urge to smoke and withdrawal symptoms. Addiction. 2015;110:669–677. doi: 10.1111/add.12807. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Turner J, Hasna S, Soar K. The electronic-cigarette: Effects on desire to smoke, withdrawal symptoms and cognition. Addictive Behaviors. 2012;37:970–973. doi: 10.1016/j.addbeh.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. Journal of Substance Abuse Treatment. 2009;36:235–243. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Foulds J, Veldheer S, Yingst J, Hrabovsky S, Wilson SJ, Nichols TT, Eissenberg T. Development of a questionnaire to assess dependence on electronic cigarettes in a large sample of ex-smoking e-cig users. Nicotine & Tobacco Research. 2014;17:186–192. doi: 10.1093/ntr/ntu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb AM, Killen JD, Marlatt GA, Taylor CB. Psychological and pharmacological influences in cigarette smoking withdrawal: Effects of nicotine gum and expectancy on smoking withdrawal symptoms and relapse. Journal of Consulting and Clinical Psychology. 1987;55:606–608. doi: 10.1037/0022-006X.55.4.606. [DOI] [PubMed] [Google Scholar]
- Harrell PT, Marquinez NS, Correa JB, Meltzer LR, Unrod M, Sutton SK, … Brandon TH. Expectancies for cigarettes, e-cigarettes, and nicotine replacement therapies among e-cigarette users (“Vapers”) Nicotine & Tobacco Research. 2015;17:193–200. doi: 10.1093/ntr/ntu149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hull JG, Bond CF. Social and behavioral consequences of alcohol consumption and expectancy: A meta-analysis. Psychological Bulletin. 1986;99:347–360. [PubMed] [Google Scholar]
- Juliano LM, Brandon TH. Effects of nicotine dose, instructional set, and outcome expectancies on the subjective effects of smoking in the presence of a stressor. Journal of Abnormal Psychology. 2002;111:88–97. doi: 10.1037//0021-843x.111.1.88. [DOI] [PubMed] [Google Scholar]
- Kaptchuk TJ, Friedlander E, Kelley JM, Sanchez MN, Kokkotou E, Singer JP, … Lembo AJ. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PloS one. 2010;5:e15591. doi: 10.1371/journal.pone.0015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch I. Response expectancy as a determinant of experience and behavior. American Psychologist. 1985;40:1189–1202. [Google Scholar]
- Kozlowski LT, Pillitteri JL, Sweeney CT, Whitfield KE, Graham JW. Asking questions about urges or cravings for cigarettes. Psychology of Addictive Behaviors. 1996;10:248. [Google Scholar]
- Marlatt GA, Demming B, Reid JB. Loss of control drinking in alcoholics: An experimental analogue. Journal of Abnormal Psychology. 1973;81:233–241. doi: 10.1037/h0034532. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Sex differences in nicotine versus nonnicotine reinforcement as determinants of tobacco smoking. Experimental and Clinical Psychopharmacology. 1996;4:166. [Google Scholar]
- Perkins KA, Karelitz JL, Michael VC. Effects of nicotine versus placebo e-cigarette use on symptom relief during initial tobacco abstinence. Experimental and Clinical Psychopharmacology. 2017;25:249–254. doi: 10.1037/pha0000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeiro B, Correa JB, Simmons VN, Harrell PT, Menzie NS, Unrod M, … Brandon TH. Gender differences in use and expectancies of e-cigarettes: Online survey results. Addictive Behaviors. 2016;52:91–97. doi: 10.1016/j.addbeh.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramôa CP, Hiler MM, Spindle TR, Lopez AA, Karaoghlanian N, Lipato T, … Eissenberg T. Electronic cigarette nicotine delivery can exceed that of combustible cigarettes: a preliminary report. Tobacco Control. 2015;25:e6–e9. doi: 10.1136/tobaccocontrol-2015-052447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel MB, Tanwar KL, Wood KS. Electronic cigarettes as a smoking-cessation tool: Results from an online survey. American Journal of Preventative Medicine. 2011;40:472–475. doi: 10.1016/j.amepre.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Van Heel M, Van Gucht D, Vanbrabant K, Baeyens F. The importance of conditioned stimuli in cigarette and e-cigarette craving reduction by e-cigarettes. International Journal of Environmental Research and Public Health. 2017;14:193–211. doi: 10.3390/ijerph14020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu SH, Zhuang YL, Wong S, Cummins SE, Tedeschi GJ. E-cigarette use and associated changes in population smoking cessation: Evidence from US current population surveys. BMJ. 2017;358:j3262. doi: 10.1136/bmj.j3262. [DOI] [PMC free article] [PubMed] [Google Scholar]