Abstract
Numerous studies in animals and humans have established that oxytocin (OT) reduces anxiety. In rats, the prelimbic (PL) subregion of the medial prefrontal cortex (mPFC) is among the brain areas implicated in the anxiolytic actions of OT. However, questions remain about the anatomical and receptor specificity of OT and its mechanism of action. Here we assessed whether the regulation of anxiety by mPFC OT is restricted to the PL subregion and evaluated whether oxytocin receptor (OTR) activation is required for OT to have an anxiolytic effect. We also examined whether OT interacts with GABA in the mPFC to reduce anxiety and investigated the extent to which OT in the mPFC affects activation of mPFC GABA neurons as well as neuronal activation in the amygdala, a primary target of the mPFC which is part of the neural network regulating anxiety. We found that OT reduced anxiety-like behavior when delivered to the PL, but not infralimbic or anterior cingulate subregions of the mPFC. The anxiolytic effect of OT in the PL mPFC was blocked by pretreatment with an OTR, but not a vasopressin receptor antagonist as well as with a GABAA receptor antagonist. Lastly, administration of OT to the PL mPFC was accompanied by increased activation of GABA neurons in the PL mPFC and altered neuronal activation of the amygdala following anxiety testing. These results demonstrate that OT in the PL mPFC attenuates anxiety-related behavior and may do so by engaging GABAergic neurons which ultimately modulate downstream brain regions implicated in anxiety.
1. Introduction
In addition to its well-known role in various social behaviors, (Bale et al., 2001; Bosch and Neumann, 2012; Calcagnoli et al., 2015; Caldwell, 2012; Engelmann et al., 1998; Lim and Young, 2006; Meyer-Lindenberg et al., 2011) the neuropeptide oxytocin (OT) has been implicated in the regulation of anxiety (Benarroch, 2013; MacDonald and Feifel, 2014; Neumann and Landgraf, 2012; Veenema and Neumann, 2008). In rats and mice, exogenous OT has repeatedly been shown to attenuate anxiety-like behavior when administered peripherally or centrally (Ayers et al., 2011; Bale et al., 2001; Blume et al., 2008; Mak et al., 2012; McCarthy et al., 1996; Ring et al., 2006; Sabihi et al., 2014b; Slattery and Neumann, 2010; Uvnas-Moberg et al., 1994; Windle et al., 1997). The anxiolytic effect of OT in rodents translates to humans with several studies demonstrating that intranasal administration of OT suppresses anxiety responses in healthy individuals as well as patients with anxiety disorders (de Oliveira et al., 2012; Feifel et al., 2011; Guastella et al., 2009; Heinrichs et al., 2003; MacDonald and Feifel, 2014).
Numerous brain regions have been identified as sites of action for the anxiolytic effect of OT, including the hypothalamic paraventricular nucleus (Blume et al., 2008; Smith et al., 2016), amygdala (Bale et al., 2001; Neumann, 2002), raphe nucleus (Yoshida et al., 2009), and most recently, the prelimbic (PL) region of the medial prefrontal cortex (mPFC) (Sabihi et al., 2014a; Sabihi et al., 2014b). In addition to the PL region, the mPFC of the rodent brain also includes the infralimbic (IL) and anterior cingulate (Cg1) cortices. The various subregions of the mPFC show different patterns of connectivity with subcortical and cortical structures which are known to regulate the expression of anxiety-like behavior (Calhoon and Tye, 2015; Hoover and Vertes, 2007; Likhtik et al., 2005; Myers-Schulz and Koenigs, 2012; Vertes, 2004) and as such have been shown in some studies to differentially contribute to anxiety (Albrechet-Souza et al., 2009; Bi et al., 2013; Gonzalez et al., 2000; Jinks and McGregor, 1997; Maaswinkel et al., 1996; Resstel et al., 2008; Saitoh et al., 2014; Shah et al., 2004; Stern et al., 2010; Suzuki et al., 2016). Thus, it is possible that the effect of exogenous OT within the mPFC on anxiety-like behavior may be subregion specific.
Oxytocin receptors (OTR) are expressed in the mPFC (Gould and Zingg, 2003; Insel and Shapiro, 1992; Liu et al., 2005; Mitre et al., 2016; Smeltzer et al., 2006) and so it is reasonable to assume that OT in the PL mPFC reduces anxiety by activating the OTR. However, receptors for the structurally similar neuropeptide, vasopressin (AVP), are also found in the mPFC (Kozorovitskiy et al., 2006; Smeltzer et al., 2006). Cross-reactivity at the receptor level has been described (Postina et al., 1998) due to OT’s moderate to strong affinity for the V1a subtype of the AVP receptor (Chini et al., 1996; Hicks et al., 2012) and there are studies showing that some behavioral effects of OT involve the V1a receptor (Bowen and McGregor, 2014; Hicks et al., 2012; Ramos et al., 2013; Sala et al., 2011). It remains to be determined whether exogenous OT may be acting as a partial agonist at the AVP V1a receptor to alter anxiety-like behavior.
Besides open questions about anatomical and receptor specificity, OT’s mechanism of action within the PL mPFC remains unclear. Several lines of evidence suggest that OT may be interacting with GABA, the main inhibitory neurotransmitter in the brain, to reduce anxiety (Nuss, 2015; Smith et al., 2016). For example, central OT has been shown to act directly on extrasynaptic GABAA receptors which are involved in the regulation of anxiety (Bowen et al., 2015). Further, within the amygdala (Huber et al., 2005; Knobloch et al., 2012) and PVN (Smith et al., 2016), GABA mediates the anxiolytic action of OT. Recent work has also shown that OTR are located on GABAergic interneurons (Marlin et al., 2015; Nakajima et al., 2014) in the cortex where OT has been found to increase GABA levels (Qi et al., 2012). When combined with the observation that activation of GABAA receptors in the mPFC produces an anxiolytic-like response (Mihalek et al., 1999; Solati et al., 2013), it could be postulated that OT in the PL mPFC attenuates anxiety by enhancing local GABA activity. The resulting increase in inhibition might in turn inhibit glutamatergic projections from the PL mPFC to attenuate activity of downstream limbic areas that generate anxiety-like behavior. Of particular interest are the basolateral (BLA) and central (CEA) amygdala, two components of the neural network regulating anxiety that receive direct and indirect input from the mPFC (Adhikari, 2014; Berretta et al., 2005; Brinley-Reed et al., 1995; Gabbott et al., 2005; Leuner and Shors, 2013; McDonald et al., 1996; Vertes, 2004).
In the present study, we assessed whether the regulation of anxiety by OT within the mPFC is restricted to the PL subregion and evaluated whether OTR activation is required for OT to have an anxiolytic effect. We also examined whether OT interacts with the GABA system in the PL mPFC to reduce anxiety and investigated the extent to which OT in the PL mPFC affects activation of mPFC GABA neurons as well as neuronal activation in the BLA and CEA.
2. Materials and methods
2.1 Animals
Adult (9–12 weeks of age) male (300–350g) Sprague-Dawley rats (Taconic; Germantown, NY) were housed individually in a temperature and humidity controlled room and maintained on a 12h/12h light/dark cycle (lights on at 0600 hr) with access to food and water ad libitum. All procedures were conducted in accordance with The Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and approved by The Ohio State University Institutional Animal Care and Use Committee.
2.2 Surgical procedures
After at least 7 d of acclimation to the colony, rats were anesthetized with a 2–4% isoflurane gas/air mixture and aligned on a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). Body temperature was maintained throughout the surgery with a warming pad. For Experiments 1, 2, 3 and 5, bilateral cannula guides (pedestal mounted 22-gauge stainless steel tubes with 1.5 mm separation and cut either 1.4 mm (Cg1), 3.5 mm (PL), or 4.6 mm (IL) below the pedestal; Plastics One, Roanoke, VA) were secured in a stereotaxic holder and lowered into one of the three mPFC subregions (Cg1: AP: + 2.7 mm, ML: ± 0.5 mm, DV: −1.4 mm; PL: AP: + 3.2 mm, ML: ± 0.5 mm, DV: −3.5 mm; IL: AP: + 3.2 mm, ML: ± 0.5 mm, DV: −4.6 mm) (Paxinos and Watson, 1998). For Experiment 4, bilateral cannula guides were cut 2.7 mm below the pedestal and lowered into the PL mPFC. The cannula were secured by stainless steel screws and dental cement. A bilateral stainless steel obturator (0.35 mm diameter; Plastics One) extending 0.2 mm beyond the tip of the guide cannula was placed into the guide cannula after surgeries. Obturators for animals in Experiment 4 extended 1.0 mm beyond the tip of the guide cannula in order to minimize damage to the PL region. The scalp was closed around the protruding portion of the cannula with sutures. Rats were allowed to recover 7 d before behavioral testing.
2.3 Central infusions
On days 3 and 5 post-surgery, rats were habituated to the handling and infusion procedures. During habituation, rats were removed from their home cage and handled for 3 min while being lightly restrained in a terrycloth towel. The obturators were then removed and a 28-gauge bilateral injection cannula extending 0.2 mm (or 1.0 mm for Experiment 4) beyond the tip of the guide cannula was inserted into the guide. The injection cannula was left in place for 3 min then removed and the obturator replaced. On the day of testing, rats underwent the same procedure as described above except that an injection cannula attached to a 1 µl Hamilton Syringe via PE-10 tubing was inserted into the guide cannula. Infusions were made using a Harvard Apparatus Pico Plus Elite infusion pump (Holliston, MA) which delivered a 0.5 µl volume over 1.5 min. The injector was left in place for an additional 1 min before withdrawal. Testing for anxiety-related behavior was done 15 min after the OT or saline infusion which is consistent with other studies examining the behavioral effects of OT (Bale et al., 2001; Lukas et al., 2011; Sabihi et al., 2014b). Because of the short half-life of brain OT (approximately 30 min), all behavioral testing was completed within 30 min of infusion (Gimpl and Fahrenholz, 2001).
2.4 Anxiety-like behavior
Anxiety-like behavior was evaluated one week post-surgery using the elevated plus maze (EPM) and/or social interaction (SI) test (Lapiz-Bluhm et al., 2008; Rotzinger et al., 2010). When both the EPM and SI test were done in the same animal, the order of the two tests was counterbalanced and were done 5 min apart.
The EPM consisted of a cross-shaped platform (height: 50 cm) with four arms (width: 10 cm, length: 50 cm), two of which were enclosed by walls 50 cm in height. Rats were placed in the center of the platform (10 × 10 cm), facing a junction between an open and closed arm and allowed to explore for 5 min. The number of entries into the open arms and the percentage of time spent in the open arms (time in open arms/time in open and closed arms × 100) were used as measures of anxiety-like behavior (Cruz et al., 1994; Lapiz-Bluhm et al., 2008; Pellow et al., 1985). An increase in the percentage of time spent in the open arms and a greater number of open arm entries are indicative of reduced anxiety. Closed arm entries were used as a measure of locomotion independent of anxiety (Cruz et al., 1994; Lapiz-Bluhm et al., 2008; Pellow et al., 1985).
For the SI test, a 60 × 60 cm Plexiglas arena with walls 40 cm high was used. The experimental rat and an age and weight (+/− 10 g) matched novel male conspecific were placed in opposite corners of the arena for a 5 min test. Conspecifics were used a maximum of two times and were never used twice in the same day. The assignment of a conspecific to an experimental rat was random and not restricted to a particular drug or dose of drug. Active social behaviors initiated by the experimental rat were scored including grooming, sniffing, approaching, following, and climbing on or under the conspecific. Time spent simply in contact (e.g. if the two rats lay down next to each other, but with the attention of the test rat clearly oriented away from the stimulus rat) was not counted. The total time the experimental rat spent socially interacting with the novel conspecific was used as a measure of anxiety-like behavior. Greater social interaction time is indicative of reduced anxiety (File and Seth, 2003).
All behavioral tests were performed under normal fluorescent overhead ambient lighting of ~550 lux which generated high levels of anxiety in the EPM. Although light phase and time of day have not been shown to influence behavior on the EPM, tests were performed within the same time range each day (approximately 0900–1300h), which is sufficiently separated from light-dark transitions to avoid any potential diurnal variations in exploratory behavior (Becker and Grecksch, 1996; Lapiz-Bluhm et al., 2008; Pellow et al., 1985). Tests were digitally recorded and later scored blind by a trained observer using BEST Collection and BEST Analysis software (Education Consulting Inc., Hobe Sound, FL).
2.5 Experimental design
Experiment 1 investigated the extent to which the anxiolytic effect of OT in the mPFC is subregion specific. Separate groups of male rats received infusions of OT (cat# O6379; Sigma, St. Louis, MO) into one of the three regions of the mPFC (Fig.1). OT was dissolved in 0.5 µl saline at a dose of 0.1 µg (Cg1: n = 6; PL: n = 6; IL: n = 9) or 1.0 µg (Cg1: n = 6; PL: n = 6; IL n = 10). Doses were selected based on prior studies of anxiety-like behavior using site specific administration of OT (Ayers et al., 2011; Bale et al., 2001; Lee et al., 2005; Sabihi et al., 2014b). Control rats received a 0.5 µl infusion of saline (Cg1: n = 7; PL: n = 6; IL: n = 8). All rats were tested for anxiety-like behavior on both the EPM and SI test. For this experiment and all others, the number of animals in each experimental group does not include missed cannula placements and thus represent the final number of animals with correct cannula placements that were included in statistical analyses.
Figure 1.
OT in the PL mPFC decreases anxiety-like behavior (Experiment 1). (a) Schematic representation of mPFC regions in which bilateral cannula were implanted (blue = Cg1, red = PL, black = IL; adapted from Paxinos and Watson 1998). In the EPM, (b) the number of open arms entries and (c) the percentage of time spent in the open arms was greatest in the group that received 1.0 µg OT in the PL region of the mPFC. (d) Locomotor activity, as measured by the number of closed arm entries, was not altered by infusion type or region of infusion. (e) In the SI test, the group infused with the 1.0 µg dose of OT in the PL mPFC spent a greater amount of time interacting with a novel conspecific. Bars represent mean + SEM; *p < 0.05 within PL mPFC 1.0 µg OT vs. 0.1 µg OT and saline. Abbreviations: OT = oxytocin, PL = prelimbic, mPFC = medial prefrontal cortex, Cg1 = anterior cingulate, IL = infralimbic, EPM = elevated plus maze, SI = social interaction.
Experiment 2 evaluated whether the anxiolytic effect of OT in the PL mPFC is dependent on OTR activation. Separate groups of male rats received an infusion of saline, OTR antagonist (OTR-A; 0.1 µg) or AVPR antagonist (AVPR-A; 0.1 µg) followed 10 min later (Sala et al., 2011; Yosten and Samson, 2010) by an infusion of saline or OT (1 µg) resulting in the following six groups: saline+saline (n = 7), saline+OT (n = 7), OTR-A+saline (n = 5), OTR-A+OT (n = 7), AVPR-A+saline (n = 5), AVPR-A+OT (n = 7). Testing for anxiety-related behavior was done using only the SI test since both anxiety tests yielded similar results in Experiment 1. All drugs were dissolved in 0.5 µl saline. The OTR-A (desGly-NH2-d(CH2)5[D-Tyr2,Thr4]OVT, courtesy of Dr. Maurice Manning, University of Toledo) is highly specific, being 95 times more selective for the OTR over the V1a receptor (Manning et al., 2008). The AVPR-A (d[Tyr(Me)2, Dab5]AVP, courtesy of Dr. Manning) is highly specific to the V1a receptor and is devoid of any anti-OT activity in vivo, thus allowing for the discrimination between V1a and OT receptors (Manning et al., 2012). Both antagonists have been used in rodent behavioral studies at the dose used here (Lukas et al., 2011; Sabihi et al., 2014a). This dose was lower than that of the peptide (Bales et al., 2004; Cho et al., 1999) since OTR and AVPR antagonists have been shown to be approximately 10–100 times more effective in receptor binding than the natural ligands (Barberis and Tribollet, 1996).
Experiment 3 evaluated whether OT in the PL mPFC exerts an anxiolytic effect by recruiting GABA neurons. Separate groups of male rats received one 0.5 µl infusion of saline followed by an infusion of 1.0 µg OT (S+OT; n = 6), one infusion of bicuculline methiodide (BIC), a specific GABAA receptor antagonist at a dose of 2.5 ng followed by an infusion of 1.0 µg OT (BIC+OT; n = 7), or one infusion of BIC (2.5 ng) followed by an infusion of saline (BIC+S; n=7). OT and BIC were both dissolved in 0.5 µl saline. An additional group of rats received two 0.5 µl infusions of saline (S+S; n = 6). In each case, the second infusion occurred 5 min after the first. Anxiety-like behavior was evaluated in both the EPM and SI test since it could be possible for OT-GABA interactions to affect anxiety differently in non-social versus social contexts.
Experiment 4 examined how OT in the PL mPFC affects activation of GABA neurons in this region following exposure to the EPM. Separate groups of male rats received infusions of OT dissolved in 0.5 µl saline at a dose of 1.0 µg (n = 9) or 0.5 µl saline (n = 8) and were tested for anxiety-like behavior in the EPM. Rats were euthanized and brains removed ~70 min after anxiety testing consistent with previous studies and peak c-Fos expression (Bossert et al., 2011; Knapska and Maren, 2009; Rey et al., 2014).
Experiment 5 examined how OT in the PL mPFC affects neuronal activation in the BLA and CeA following exposure to the EPM. Separate groups of male rats received infusions of OT dissolved in 0.5 µl saline at a dose of 1.0 µg (n = 6) or 0.5 µl saline (n = 5) and were tested for anxiety-like behavior in the EPM. Rats were euthanized and brains removed ~70 min after anxiety testing.
2.6 Tissue Collection and Processing
Rats were deeply anesthetized and transcardially perfused with 4% paraformaldehyde. For experiments 1–3, brains were postfixed for 24 hr at 4°C, transferred to 0.1M PBS, and a Vibratome used to obtain 40 µm thick coronal sections throughout the area of the cannula implant. Sections were stained with 0.2% cresyl violet for verification of correct placement. Those animals with cannula placements outside of the intended region of the mPFC (n = 5 Experiment 1, n = 1 Experiment 2, and n = 1 Experiment 3) were excluded from analyses. These missed cannula placements either hit the ventricles, dorsal peduncular cortex, or were unevenly placed and for Experiment 1 included different drug and dosage groups. As such, statistical analyses could not be completed in order to examine behavioral effects of OT outside of the intended region.
For experiment 4, brains were postfixed overnight at 4 °C and then transferred to 30% sucrose in 0.1M PBS until sectioning. 50 µm coronal sections extending through the mPFC (AP: 4.2 to 2.0 mm) were obtained with a cryostat and stored in a sucrose-based cryoprotectant at −20°C until immunohistochemical processing on free-floating sections. Four sections (1:6 series) were processed for double-labeling for GAD67 and c-Fos. GAD67 was chosen as a marker of inhibitory neurons as it is an isoform of glutamate decarboxyalse (GAD), the enzyme that synthesizes GABA from glutamate (Tillakaratne et al., 1995). Further, since it is found throughout the cytoplasm (Lonstein et al., 2014) it can be easily colocalized with c-Fos. Briefly, sections were washed in 0.1M PBS and then incubated with 0.1% Tween in for 10 min. Next, sections were blocked in 10% normal goat serum (NGS) and 0.3% Triton X in PBS for 60 min followed by incubation in rabbit anti-Fos primary antibody (1:300; Santa Cruz Biotechnology, Santa Cruz, CA) in PBS with 1% Triton X and 3% NGS for 72 hr at 4°C. After a PBS rinse, sections were incubated for 2.5 hr at room temperature in goat anti-rabbit secondary antibody with Alexa 488 (1:500; Vector Laboratories, Burlingame, CA) and rinsed in PBS. Sections were then incubated in mouse anti-GAD67 primary antibody (1:2000; Millipore, Billerica, MA) in PBS with 0.5% tween overnight at 4°C. After a PBS rinse, sections were incubated for 1 hr at room temperature in DyLight 549 horse anti-mouse secondary antibody (1:500; Vector Laboratories, Burlingame, CA). After a final rinse, all sections were mounted on SupraFrost Plus microscope slides, coverslipped with DABCO and kept in the dark at 4°C until imaging. As the 200 micron microinjection needle creates mechanical tissue disturbance in some of the PL mPFC, we preferentially selected sections with minimal or no tissue damage for quantification (Smith et al., 2016).
For experiment 5, brains were kept in 4% paraformaldehyde for 4 hr at 4°C then switched to a solution of 0.1M PBS with 0.01% NaN3 until coronal sections (1:6 series) extending through the amygdala (AP: −1.6 to −2.8 mm) were obtained with a Vibratome. For c-Fos immunohistochemistry, sections were washed in 0.1M PBS, quenched for 30 min with 3% H202 in 50% EtOH, and rinsed with PBS. Sections were then blocked for 60 min in 3% NGS in 0.6% Triton-X followed by incubation in rabbit anti-Fos primary antibody (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA) for 48 hr at 4°C. After a PBS rinse, sections were incubated for 2 hr in biotinylated goat anti-rabbit secondary antibody (1:200; Vector Laboratories, Burlingame, CA), rinsed in PBS, and then incubated for 1 hr in avidin-biotin complex (ABC Vectastain kit, Vector Laboratories). Following another rinse in PBS, sections were reacted in diaminobenzadine (Vector Laboratories) for 7 min. After a final rinse, sections were mounted on SupraFrost Plus microscope slides, dehydrated through a graded alcohol series, cleared with xylene, and coverslipped with Permount.
For Experiments 4 and 5, separate sections throughout the area of the cannula implant were stained with 0.2% cresyl violet for verification of correct placement. There were no animals with cannula placements outside of the intended region of the mPFC, however, the integrity of brain tissue slices from one animal receiving OT in Experiment 4 was compromised during tissue collection and slicing and was consequently removed from the c-Fos analysis.
2.7 c-Fos Analysis
For Experiment 4, a Nikon 90i confocal microscope was used to obtain four unilateral image stacks of the PL mPFC at 20× (~100 steps in 0.3 µm intervals along the z-plane). Identification of this region was conducted with reference to illustrations from a standard stereotaxic rat brain atlas (Paxinos and Watson, 1998) and landmarks such as the location of the corpus callosum. Image stacks were projected using NIS Elements software and on these images, c-Fos+, GAD67+, and GAD67+/c-Fos+ double-labeled cells were quantified in 4 regions of interest (ROIs) by a rater blind to experimental conditions. For each ROI, counts were divided by the area of the ROI (250 mm2) to yield the number of single- or double-labeled cells per 1 mm2. Percentages of GAD67+ cells expressing c-Fos were calculated by dividing the number of cells expressing c-Fos by the number of single-labeled cells expressing GAD67. Densities and percentages were averaged across ROIs for each animal and the group mean determined from these values.
For experiment 5, densities of c-Fos+ cells were quantified in the BLA and CEA. Identification of these regions was conducted with reference to illustrations from a standard stereotaxic rat brain atlas (Paxinos and Watson, 1998) and landmarks such as the location of white matter tracts (e.g., fornix, optic tracts), corpus callosum, caudate putamen, and ventricles. Images of four bilateral sections per rat were taken at 10× with a camera (Zeiss AxioCam and software) affixed to a microscope (Zeiss Axio Imager M2). On these images, c-Fos+ cells (i.e. round or oval-shaped nuclei with brown-black immunostaining darker than background) were counted manually by a rater blind to experimental conditions in 4–6 ROIs using StereoInvestigator (Williston, VT). For each ROI, counts were divided by the area of the ROI (averaging 0.112 mm2 for the BLA and 0.097 mm2 for the CEA) then averaged for each animal and the group mean determined from these values. Data are expressed as the number of c-Fos+ cells per 1 mm2.
2.8 Statistical analysis
All statistical analyses were performed using GraphPad Prism software version 5.01 (La Jolla, CA). For Experiment 1, data from the EPM (percent time in open arms, number of open arm entries, number of closed arm entries) and SI test (time interacting) were analyzed using a two-way Analysis of Variance (ANOVA) with region (Cg1, PL or IL) and infusion type (saline, 0.1 µg OT or 1.0 µg OT) as factors followed by Bonferroni’s post-hoc comparison test. SI test data from Experiment 2, as well as SI and EPM data from Experiment 3, were analyzed using one-way ANOVA followed by Tukey’s HSD post-hoc comparison test. c-Fos and EPM data from Experiments 4 and 5 were analyzed using Student’s one-tailed t-tests. Data are expressed as the mean + SEM. Significance was set at p < 0.05.
3. Results
3.1 Experiment 1: OT in the PL, but not IL or AC, mPFC reduces anxiety-like behavior
In the EPM, OT had a subregion specific effect on anxiety-like behavior (Fig.1). For the number of open arm entries (Fig. 1b) there was a significant main effect of infusion type (F2,55 = 4.48, p = 0.016) and brain region (F2,55 = 6.98, p = 0.002) as well as an infusion type X brain region interaction (F4,55 = 3.51, p = 0.013). Post-hoc analysis revealed that within the PL mPFC, the group receiving 1.0 µg OT had a greater number of open arm entries as compared to groups receiving either the 0.1 µg dose of OT or saline (p’s < 0.05) which did not differ from one another (p > 0.05). For the percentage of time spent in the open arms (Fig. 1c), there were also significant main effects of infusion type (F2,55 = 4.56, p = 0.015) and brain region (F2,55 = 7.55, p = 0.001) and a trend for an infusion type X brain region interaction (F4,55 = 2.24, p = 0.076) likely driven by the high percentage in the group that received 1.0 µg OT in the PL mPFC. Indeed, within the PL mPFC, the group receiving 1.0 µg OT spent a greater percentage of time in the open arms of the EPM compared to those receiving either the 0.1 µg dose of OT or saline (p’s < 0.05), which did not differ from one another (p > 0.05). Locomotor activity as measured by the number of closed arm entries (Fig. 1d) was not altered by infusion type (F2,55 = 2.21, p = 0.120) or region of infusion (F2,55 = 0.08, p = 0.920) and there was no significant infusion type X brain region interaction (F4,55 = 0.36, p = 0.830).
In the SI test (Fig. 1e), there was a significant main effect of infusion type (F2,55 = 3.47, p = 0.038) and brain region (F2,55 = 8.54, p = 0.001) and a significant infusion type X brain region interaction (F4,55 = 4.33, p = 0.004) on the amount of time spent interacting with an unknown stimulus rat. Post-hoc analysis showed that within the PL mPFC, the group receiving 1.0 µg OT spent more time interacting with a novel conspecific as compared to those receiving either the 0.1 µg dose of OT or saline (p’s < 0.05) which did not differ from one another (p > 0.05).
3.2 Experiment 2: The anxiolytic effect of OT in the PL mPFC is dependent on OTR activation
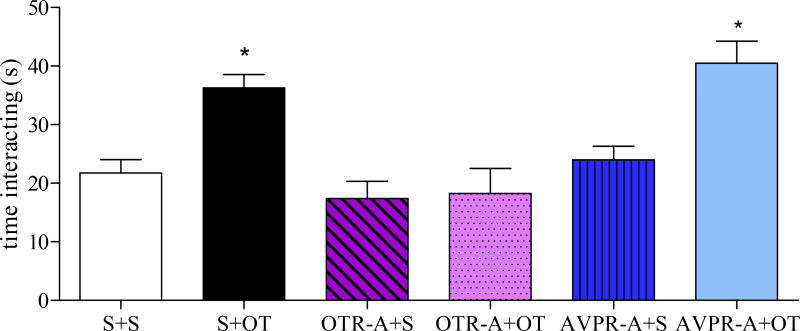
The anxiolytic effect of OT in the PL mPFC was blocked by pretreatment with an OTR-A, but not an AVPR-A (Fig. 2). There was a significant effect of treatment in the SI test (F5,41 = 9.14, p < 0.0001) with post-hoc analysis revealing that the group infused with S+OT and AVPR-A+OT spent more time interacting with an unknown conspecific than the group treated with OTR-A+OT (p’s < 0.05) which did not differ from the S+S group (p > 0.05). Neither antagonist alone affected anxiety-like behavior as social interaction time did not differ among the S+S, OTR-A+S or AVPR-A+S groups (p’s > 0.05).
Figure 2.
OT acting on the OTR in the PL mPFC decreases anxiety-like behavior in the SI test (Experiment 2). The AVPR-A+OT and S+OT groups spent more time interacting with an unknown conspecific than the group treated with OTR-A+OT indicating that the anxiolytic effect of OT in the PL mPFC was blocked by pretreatment with an OTR-A, but not an AVPR-A. The S+S, OTR-A+OT, OTR-A+S and AVPR-A+S groups all showed similar levels of anxiety-like behavior in the SI test. Bars represent mean + SEM; *p < 0.05 S+OT and AVPR-A+OT vs. all other groups. Abbreviations: S = saline, OT = oxytocin, OTR = oxytocin receptor, PL = prelimbic, mPFC = medial prefrontal cortex, SI = social interaction, OTR-A = oxytocin receptor antagonist, AVPR-A = vasopressin receptor antagonist.
3.3 Experiment 3: The anxiolytic effect of OT in the PL mPFC relies on GABAergic neurotransmission
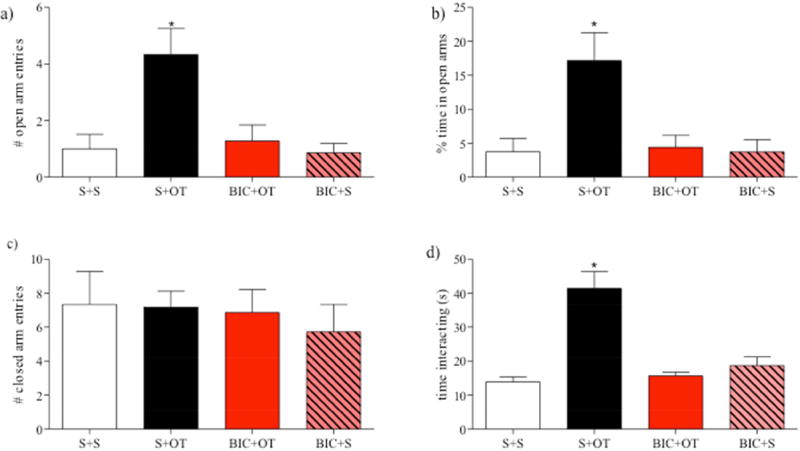
The anxiolytic effect of OT in the PL mPFC was blocked by pretreatment with the GABAA receptor antagonist, BIC (Fig. 3). In the EPM, there was a significant effect of treatment for the number of open arm entries (Fig. 3a; F3,25 = 7.16, p = 0.002) and percentage of time spent in the open arms (Fig. 3b; F3,25 = 6.68, p = 0.002). Post-hoc analysis revealed that the S+OT group made a greater number of entries into the open arms and spent more time in the open arms as compared to all other groups (p’s < 0.05), which did not differ from each other (p’s > 0.05) This anxiolytic effect of OT in the PL mPFC was prevented by pretreatment with BIC as the number of open arm entries and the percentage of time spent in the open arms did not differ between the BIC+OT and S+S groups (p > 0.05). In addition, the BIC+S group did not differ from the S+S group on either measure of anxiety-like behavior indicating the BIC itself was without effect (p’s > 0.05). Locomotor activity, as measured by the number of closed arm entries (Fig. 3c), was not altered by treatment (F3,25 = 0.24, p = 0.868).
Figure 3.
OT interacts with GABA in the PL mPFC to decrease anxiety-like behavior (Experiment 3). In the EPM, the group that received an infusion of OT in the PL mPFC (a) made more open arm entries and (b) spent a greater percentage of time in the open arms unless they also received an mPFC infusion of the GABAA antagonist, BIC which by itself did not affect anxiety-like behavior in the EPM. (c) Locomotor activity (closed arm entries) was not altered by infusion type. (d) In the SI test, OT increased time spent interacting with an unknown stimulus rat, but BIC blocked this anxiolytic effect. Bars represent mean + SEM; *p < 0.05 S+OT vs. all other groups. Abbreviations: S = saline, OT = oxytocin, PL = prelimbic, mPFC = medial prefrontal cortex, EPM = elevated plus maze, BIC = bicuculline methiodide, SI = social interaction.
In the SI test (Fig. 3d), there was also a significant effect of treatment (F3,25 = 19.43, p < 0.0001) on the amount of time spent interacting with an unknown stimulus rat. Post-hoc analysis showed that the S+OT group spent a greater amount of time interacting with a novel conspecific when compared to all other groups (p’s < 0.05) which did not differ from each other (p’s > 0.05). Again, this anxiolytic effect of OT in the PL mPFC was prevented by pretreatment with BIC as indicated by a similar amount of time spent interacting with an unknown conspecific (Fig. 3d) in the BIC+OT and S+S groups (p > 0.05). BIC itself did not affect anxiety-like behavior in the SI test as the BIC+S group did not differ from the S+S group (p > 0.05).
3.4 Experiment 4: OT in the PL mPFC increases activation of GABAergic neurons in this region
Reduced anxiety-like behavior following OT in the PL mPFC was accompanied by greater activation of GABA neurons (Fig. 4). Compared to the saline group, the OT group displayed more open arm entries (Fig. 4a; t(15) = 3.50, p = 0.003) and an increase in the percentage of time spent in the open arms (Fig. 4b; t(15) = 3.99, p < 0.0001) but no change in the number of closed arm entries (Fig. 4c; t(15) = 0.26, p = 0.796). Furthermore, OT in the PL mPFC resulted in more c-Fos+ cells (Fig. 4d; t(15) = 2.58, p = 0.021) without affecting the density of PL mPFC GAD67+ cells (Fig. 4e; t(15) = 0.35, p = 0.731). OT in the PL mPFC also yielded a significant increase in the density (Fig. 4f; t(15) = 2.35, p = 0.033) and percentage (Fig. 4g; t(15) = 2.46, p = 0.027) of GAD67+ cells co-expressing c-Fos in the PL mPFC indicating greater activation of GABA neurons.
Figure 4.
OT in the PL mPFC decreased anxiety-like behavior and increased activation of GABAergic neurons in the PL mPFC (Experiment 4). OT in the PL mPFC increased the (a) number of open arm entries and (b) time spent in the open arms of the EPM but did not alter (c) closed arm entries. OT in the PL mPFC also resulted in (d) more c-Fos+ cells (per 1 mm2) without affecting the density of GAD67+ cells (e). In addition, the density (f) and percentage (g) of GAD67+ neurons expressing c-Fos in the PL mPFC was increased by OT. (h) Confocal images of a cell positive for c-Fos (green, top), GAD67 (red, middle), and GAD67/c-Fos colabeling (bottom). Bars represent mean + SEM; *p < 0.05. Abbreviations: OT = oxytocin, PL = prelimbic, mPFC = medial prefrontal cortex, EPM = elevated plus maze.
3.5 Experiment 5: OT in the PL mPFC alters neuronal activation in the amygdala
OT in the PL mPFC reduced anxiety-like behavior and altered neuronal activation in the amygdala (Fig. 5). Once again, OT increased both the number of open arm entries (Fig. 5a; t9 = 2.51, p = 0.017) and the percentage of time spent in the open arms (Fig. 5b; t9 = 2.08, p = 0.034) without altering the number of closed arm entries (Fig. 5c; t9 = 0.91, p = 0.194). Compared to saline, OT in the PL mPFC was also accompanied by fewer c-Fos+ cells in the BLA (Fig. 5d, e; t8 = 2.38, p = 0.022). In contrast, the density of c-Fos+ cells in the CEA was increased following OT in the PL mPFC (Fig. 5d, f; t8 = 2.94, p = 0.009).
Figure 5.
OT in the PL mPFC reduces anxiety-like behavior and alters neuronal activation in the amygdala (Experiment 5). In the EPM, OT increased both (a) the number of open arm entries and (b) the percentage of time spent in the open arms without altering (c) the number of closed arm entries. (d) Schematic representation of regions throughout the BLA and CEA in which c-Fos counts were taken. Grey shaded regions = CEA; black shaded regions =BLA (adapted from Paxinos and Watson, 1998). (e) In the BLA, fewer c-Fos+ cells (per 1 mm2) were observed following an infusion of OT in the PL mPFC. (f) In contrast, the density of c-Fos+ cells was increased in the CEA following OT in the PL mPFC. Scale bars = 100µm. Bars represent mean + SEM; *p < 0.05. Abbreviations: OT = oxytocin, PL = prelimbic, mPFC = medial prefrontal cortex, EPM = elevated plus maze, BLA = basolateral amygdala, CEA = central amygdala.
4. Discussion
Numerous studies in animals and humans have established that OT reduces anxiety (Ayers et al., 2011; Bale et al., 2001; Blume et al., 2008; de Oliveira et al., 2012; Feifel et al., 2011; Guastella et al., 2009; Heinrichs et al., 2003; MacDonald and Feifel, 2014; Mak et al., 2012; McCarthy et al., 1996; Ring et al., 2006; Sabihi et al., 2014b; Slattery and Neumann, 2010; Uvnas-Moberg et al., 1994; Windle et al., 1997). In rodents, the mPFC is among the brain regions implicated in the anxiolytic actions of OT (Sabihi et al., 2014a; Sabihi et al., 2014b). Here we assessed whether the regulation of anxiety by OT within the mPFC is restricted to the PL subregion and evaluated whether OTR activation is required for OT to have an anxiolytic effect. We also examined whether OT interacts with the GABA system in the PL mPFC to reduce anxiety and investigated the extent to which OT in the mPFC affects activation of mPFC GABA neurons and neuronal activation of the BLA and CeA, two primary targets of the mPFC which are components of the neural network regulating anxiety (Adhikari, 2014; Calhoon and Tye, 2015). We confirm and extend previous work by demonstrating that infusion of OT into the PL region of the mPFC, but not the IL or Cg1 regions, decreased anxiety-like behavior. We also show that the attenuation in anxiety-like behavior following OT administration into the PL mPFC is abolished when the binding of OT to the OTR was impeded by pretreatment with a selective OTR-A or with a GABAA receptor antagonist. Further, the reduction in anxiety-like behavior following OT in the PL mPFC was accompanied by increased c-Fos expression in GAD67+ neurons in the PL mPFC suggesting greater activation of GABA neurons. Lastly, administration of OT to the PL region had differential effects on neuronal activation in the BLA and CEA following EPM exposure. Taken together, these results demonstrate that OT, acting on OTR in the PL mPFC, attenuates anxiety-related behavior and may do so by engaging GABAergic neurons which ultimately modulate downstream brain regions implicated in anxiety.
Here we used two ethologically relevant tests for anxiety-like behavior in rodents – the EPM and the SI test. While the EPM features exploration and relies on the rat's innate fear of open spaces, the SI test places emphasis on social behavioral responses (Lapiz-Bluhm et al., 2008; Rotzinger et al., 2010). The anxiolytic effect of OT in the PL mPFC in both tests suggests that anxiety in non-social and social contexts is sensitive to PL OT although for the SI test, it is difficult to attribute the increased social interaction time to diminished anxiety alone since OT administration is also widely known to promote prosocial behavior (Benarroch, 2013; Lukas et al., 2011; Meyer-Lindenberg et al., 2011; Neumann and Slattery, 2016). Thus, while the SI test has been validated behaviorally, physiologically, and pharmacologically as a test for anxiety rather than a test for social behavior (File and Seth, 2003) it is still possible that the anxiolytic effects observed could be due to OT attenuation of anxiety-like behavior, OT facilitation of social interaction, or both (Bethlehem et al., 2014).
The EPM testing conditions in the current study produced high levels of anxiety in the control groups making it necessary that a large threshold be breached for an anxiolytic effect of OT to be observed. Our results from Experiment 1 show that a 1 µg, but not a 0.1 µg, dose of OT in the PL mPFC was able to overcome this threshold and reduce anxiety. Although 1 µg OT is a relatively high dose, this dose was also shown to be anxiolytic in the amygdala (Bale et al., 2001). However, in the Cg and IL subregions of the mPFC, neither the 1 µg nor the 0.1 µg dose of OT was effective, even though both regions have also been implicated in fear and anxiety regulation (Cg1: Bissiere et al., 2008; Albrechet-Souza et al., 2009; but see Bissiere et al., 2006; IL: Vidal-Gonzalez et al., 2006; Sierra-Mercado et al., 2011; Bi et al., 2013). Importantly, OT’s influence was likely limited to each subregion of the mPFC targeted by the microinjection as a previous study found that when a larger volume of radiolabeled OT was injected into the CEA, which has a slightly smaller area than subregions of the mPFC, there was no spread beyond this region (Lee et al., 2005). As such, it appears that anxiety-like behavior is differentially sensitive to exogenous OT depending on the mPFC subregion although it could be the case that the Cg and IL subregions may require a different OT dose than tested here for an effect on anxiety to be revealed. As nonlinear effects of OT have been reported (Figueira et al., 2008; Leuner et al., 2012), a dose lower than 0.1 µg could possibly yield a different outcome. Consistent with this possibility are findings showing that 0.01 µg OT in the IL mPFC promotes fear extinction (Lahoud and Maroun, 2013), although these results could also suggest that learned fear versus innate anxiety, and the different paradigms used to assess these processes, may not be equally sensitive to mPFC OT. It is also important to consider emerging evidence that OT is not solely involved in reducing fear and anxiety but can also have the opposite action and can increase fear and anxiety (Bartz et al., 2011; Grillon et al., 2013; Guzman et al., 2013; Lahoud and Maroun, 2013; Toth et al., 2012). However, as noted above, anxiety levels in the EPM were already fairly high which could have precluded the ability to detect an anxiogenic effect of OT. In this regard, it is worth noting that baseline anxiety levels in the SI test were not high and yet, like the EPM, OT was anxiolytic when administered in the PL mPFC at a dose of 1 µg. Even so, it is possible that different EPM testing conditions would yield anxiolytic effects of OT at lower doses in the PL mPFC and/or in the other mPFC subregions. It is also possible that different testing conditions could reveal increased anxiety after OT administration.
OTR are located in the mPFC (Duchemin et al., 2017; Gould and Zingg, 2003; Insel and Shapiro, 1992; Liu et al., 2005; Mak et al., 2012; Mitre et al., 2016; Nakajima et al., 2014; Smeltzer et al., 2006) although little is known about subregional differences in OTR density which may be another factor contributing to the observed behavioral variations across mPFC subregions. Nonetheless, it is reasonable to assume that within the PL mPFC, OT reduces anxiety by acting on the OTR. However, receptors for the structurally similar neuropeptide, vasopressin (AVP), are also found in the mPFC (Kozorovitskiy et al., 2006; Smeltzer et al., 2006). Cross-reactivity at the receptor level has been described (Chini et al., 1996; Hicks et al., 2012; Postina et al., 1998) due to OT’s moderate to strong affinity for the V1a subtype of the AVP receptor and there are studies showing that some behavioral effects of OT, particularly social behaviors, involve the V1a receptor (Bowen and McGregor, 2014; Hicks et al., 2012; Mak et al., 2012; Ramos et al., 2013; Sala et al., 2011). Thus, central OT at high concentrations could possibly act as a partial agonist at the AVP V1a receptor to alter anxiety. Although this is unlikely because activation of the V1a receptor has been linked to anxiogenic effects (Neumann and Landgraf, 2012), we addressed this issue directly in Experiment 2 using a co-administration design in which OT was administered along with highly specific antagonists for either the OTR or the V1a receptor prior to the SI test (Manning et al., 2012). Our results demonstrate that the anxiolytic effect of OT in the PL region of the mPFC was blocked by the OTR-A, but not the AVPR-A, and thus confirm the anxiolytic actions of OT through its binding to the OTR. In doing so, these data eliminate the possibility that OT’s anxiety reducing actions occur through effects on the AVPR and indicate that activation of the OTR is required to have behavioral significance, at least for anxiety-like behavior as assessed in the SI test.
In contrast to exogenous OT, endogenous OT in the PL mPFC does not appear to be directly involved in regulating anxiety-like behavior in the SI test since administration of an OTR-A alone was without effect in Experiment 2. This finding is consistent with numerous studies using anxiety tests which lack a social component (i.e. EPM, OF, light-dark box) that have shown an anxiolytic effect of endogenous brain OT only during periods of high OT system activity such as the postpartum period (Lukas et al., 2011; Neumann, 2002; Neumann et al., 2000; Sabihi et al., 2014a; Slattery and Neumann, 2010). That we did not find an anxiolytic effect of endogenous PL OT on social anxiety may be somewhat surprising given the prosocial effects of endogenous brain OT (Dumais and Veenema, 2016) and emerging studies implicating PFC OT in various social behaviors (Marlin et al., 2015; Nakajima et al., 2014; Sabihi et al., 2014a; Sabihi et al., 2014b; Young et al., 2014). Methodological aspects of the SI test versus other tests of social behavior (i.e. social preference, social memory) may explain this discrepancy although the possibility remains that anxiety in a social context is meditated by components of the endogenous brain OT system other than the PL mPFC (Neumann and Slattery, 2016). It should also be noted that like the OTR-A, administration of the AVP V1a receptor antagonist into the PL mPFC had no effect on anxiety-like behavior in the SI test. Thus, although AVP also modulates anxiety-like behavior and some aspects of social behavior (Dumais and Veenema, 2016; Neumann and Landgraf, 2012; Veenema and Neumann, 2008), our data suggest that endogenous action of AVP at the V1a receptor in the PL mPFC is not essential.
While the mPFC is often regarded as having an inhibitory influence on fear and anxiety, numerous studies suggest that it can also stimulate fear and anxiety (Calhoon and Tye, 2015; Quirk and Beer, 2006). This bidirectional regulation of anxiety by the mPFC can be attributable to differences among the subregions with the more dorsally located PL region having an excitatory influence through its glutamatergic projections to downstream limbic regions including the amygdala (Adhikari, 2014; Berretta et al., 2005; Brinley-Reed et al., 1995; Gabbott et al., 2005; Leuner and Shors, 2013; McDonald et al., 1996; Vertes, 2004). Because these glutamatergic projection neurons within the PL mPFC are modulated by local GABA interneurons, which have been shown to express OTR (Marlin et al., 2015; Nakajima et al., 2014) one way OT could attenuate anxiety would be by engaging local GABAergic inhibition. This possibility is supported by evidence that OT increases GABA release in the mPFC (Qi et al., 2012) and balances cortical inhibition (Marlin et al., 2015) as well the the demonstration that activation of mPFC GABAA receptors is anxiolytic (Solati et al., 2013). Our results from Experiments 3 and 4 provide further support for an OT-GABA interaction in the PL mPFC. First, in Experiment 3, we show that the anxiolytic effect of OT in the PL mPFC was inhibited by concomitant treatment with the GABAA receptor antagonist BIC. Notably, higher concentrations of BIC infusions in the mPFC (> 0.25 µg) have been reported to increase anxiety (Solati et al., 2013) while the current study used a lower concentration (2.5 ng) of BIC which did not. Second, in Experiment 4, we found that the decrease in anxiety-like behavior following OT delivery to the PL mPFC was accompanied by increased activation of GAD67 neurons in this region. These findings suggest that OT in the PL mPFC may reduce anxiety-like behavior by acting on receptors located on GABAergic interneurons, thus increasing local inhibition and altering GABAergic output. Interestingly, GABA has also been previously shown to mediate the anxiolytic action of OT within the amygdala (Huber et al., 2005; Knobloch et al., 2012) and PVN (Smith et al., 2016) suggesting a similar mechanism across these brain regions. Although it also possible that OT may directly influence glutamatergic neurotransmission in the mPFC to affect anxiety (Ninan, 2011; Qi et al., 2012; Qi et al., 2009), this seems unlikely as all studies to date have only found OTR on GABAergic interneurons (Nakajima et al., 2014).
Increased activation of GABAergic interneurons in the mPFC could ultimately impact downstream limbic regions, such as the amygdala. Indeed, our results from Experiment 5 show that OT in the PL mPFC was associated with altered amygdala activation such that c-Fos expression was reduced in the BLA but increased in the CEA. The BLA is the main target of glutamatergic projections from the PL mPFC (Gabbott et al., 2005; Vertes, 2004) and increased activation of the BLA is associated with heightened anxiety (Hale et al., 2006; Tye et al., 2011; Wang et al., 2011). Thus, reduced neuronal activation of the BLA after OT administration and the accompanying attenuation of anxiety-like behavior is consistent with diminished excitatory drive to BLA neurons and corroborates other work showing reduced amygdala activation following peripheral OT administration (Sobota et al., 2015). The reason for the opposite pattern of c-Fos expression in the CEA, which receives robust direct projections from the BLA (Tye et al., 2011), isn’t entirely clear but may be explained by the fact that there are also intercalated cells (ITCs) interposed between the BLA and CEA (Cassell et al., 1999; Millhouse, 1986) which generate feedforward inhibition in CEA (Pare et al., 2003; Royer et al., 1999). Thus, the c-Fos responses detected in the CEA might in part be attributable to changes in BLA efferent activity via the ITC. Without examining the phenotype of the activated cells or differentiating among the different subregions within the CEA (medial vs lateral CeA), which can have opposing influences on anxiety (Calhoon and Tye, 2015; Ressler, 2010), it is difficult to make any further conclusions.
In summary, these results demonstrate that OT in the PL mPFC attenuates anxietyrelated behavior via a GABAergic mechanism which may ultimately calm the downstream excitatory circuitry that would otherwise produce an anxious state. In doing so, our results provide novel mechanistic insights into OT’s anxiolytic actions for which little is currently known despite its potential therapeutic uses.
Highlights.
OT infusion into the PL mPFC decreases anxiety-like behavior
Anxiety-like behavior is not altered following OT infusion into the Cg1 or IL subregions of the mPFC
Blockade of OTR, but not AVPR, prevents the anxiolytic effect of OT in the PL mPFC
GABAA receptor antagonism blocks the anxiolytic effect of OT in the PL mPFC
OT in the PL mPFC increases mPFC GABAergic neuronal activity and alters activation in the amygdala
Acknowledgments
The authors wish to thank Dr. Maurice Manning (University of Toledo, Toledo, OH, USA) for kindly providing the OTR-A and AVPR-A as well as Ginamarie Shrader and Joshua Fedder for their assistance.
Funding
This research was supported by NIH grants R00MH084148 and R21HD083791 to BL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None of the authors have financial interests or potential conflicts of interest to report.
References
- Adhikari A. Distributed circuits underlying anxiety. Front Behav Neurosci. 2014;8:112. doi: 10.3389/fnbeh.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrechet-Souza L, Borelli KG, Carvalho MC, Brandao ML. The anterior cingulate cortex is a target structure for the anxiolytic-like effects of benzodiazepines assessed by repeated exposure to the elevated plus maze and Fos immunoreactivity. Neuroscience. 2009;164:387–397. doi: 10.1016/j.neuroscience.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Ayers LW, Missig G, Schulkin J, Rosen JB. Oxytocin Reduces Background Anxiety in a Fear-Potentiated Startle Paradigm: Peripheral vs Central Administration. Neuropsychopharmacology. 2011;36:2488–2497. doi: 10.1038/npp.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci. 2001;21:2546–2552. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Kim AJ, Lewis-Reese AD, Sue Carter C. Both oxytocin and vasopressin may influence alloparental behavior in male prairie voles. Horm Behav. 2004;45:354–361. doi: 10.1016/j.yhbeh.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Barberis C, Tribollet E. Vasopressin and oxytocin receptors in the central nervous system. Crit Rev Neurobiol. 1996;10:119–154. doi: 10.1615/critrevneurobiol.v10.i1.60. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Oxytocin and vasopressin: Social neuropeptides with complex neuromodulatory functions. Neurology. 2013;80:1521–1528. doi: 10.1212/WNL.0b013e31828cfb15. [DOI] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Pare D. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience. 2005;132:943–953. doi: 10.1016/j.neuroscience.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlehem RA, Baron-Cohen S, van Honk J, Auyeung B, Bos PA. The oxytocin paradox. Front Behav Neurosci. 2014;8:48. doi: 10.3389/fnbeh.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi LL, Wang J, Luo ZY, Chen SP, Geng F, Chen YH, Li SJ, Yuan CH, Lin S, Gao TM. Enhanced excitability in the infralimbic cortex produces anxiety-like behaviors. Neuropharmacology. 2013;72C:148–156. doi: 10.1016/j.neuropharm.2013.04.048. [DOI] [PubMed] [Google Scholar]
- Bissiere S, McAllister KH, Olpe HR, Cryan JF. The rostral anterior cingulate cortex modulates depression but not anxiety-related behaviour in the rat. Behav Brain Res. 2006;175:195–199. doi: 10.1016/j.bbr.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Bissiere S, Plachta N, Hoyer D, McAllister KH, Olpe HR, Grace AA, Cryan JF. The rostral anterior cingulate cortex modulates the efficiency of amygdala-dependent fear learning. Biol Psychiatry. 2008;63:821–831. doi: 10.1016/j.biopsych.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume A, Bosch OJ, Miklos S, Torner L, Wales L, Waldherr M, Neumann ID. Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. Eur J Neurosci. 2008;27:1947–1956. doi: 10.1111/j.1460-9568.2008.06184.x. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, Shaham Y. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci. 2011;14:420–422. doi: 10.1038/nn.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen MT, McGregor IS. Oxytocin and vasopressin modulate the social response to threat: a preclinical study. Int J Neuropsychopharmacol. 2014;17:1621–1633. doi: 10.1017/S1461145714000388. [DOI] [PubMed] [Google Scholar]
- Bowen MT, Peters ST, Absalom N, Chebib M, Neumann ID, McGregor IS. Oxytocin prevents ethanol actions at delta subunit-containing GABAA receptors and attenuates ethanol-induced motor impairment in rats. Proc Natl Acad Sci U S A. 2015;112:3104–3109. doi: 10.1073/pnas.1416900112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley-Reed M, Mascagni F, McDonald AJ. Synaptology of prefrontal cortical projections to the basolateral amygdala: an electron microscopic study in the rat. Neurosci Lett. 1995;202:45–48. doi: 10.1016/0304-3940(95)12212-5. [DOI] [PubMed] [Google Scholar]
- Calcagnoli F, Kreutzmann JC, de Boer SF, Althaus M, Koolhaas JM. Acute and repeated intranasal oxytocin administration exerts anti-aggressive and pro-affiliative effects in male rats. Psychoneuroendocrinology. 2015;51:112–121. doi: 10.1016/j.psyneuen.2014.09.019. [DOI] [PubMed] [Google Scholar]
- Caldwell HK. Neurobiology of sociability. Adv Exp Med Biol. 2012;739:187–205. doi: 10.1007/978-1-4614-1704-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoon GG, Tye KM. Resolving the neural circuits of anxiety. Nat Neurosci. 2015;18:1394–1404. doi: 10.1038/nn.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci. 1999;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- Chini B, Mouillac B, Balestre MN, Trumpp-Kallmeyer S, Hoflack J, Hibert M, Andriolo M, Pupier S, Jard S, Barberis C. Two aromatic residues regulate the response of the human oxytocin receptor to the partial agonist arginine vasopressin. FEBS Lett. 1996;397:201–206. doi: 10.1016/s0014-5793(96)01135-0. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- de Oliveira DC, Chagas MH, Garcia LV, Crippa JA, Zuardi AW. Oxytocin interference in the effects induced by inhalation of 7.5% CO(2) in healthy volunteers. Hum Psychopharmacol. 2012;27:378–385. doi: 10.1002/hup.2237. [DOI] [PubMed] [Google Scholar]
- Duchemin A, Seelke AM, Simmons TC, Freeman SM, Bales KL. Localization of oxytocin receptors in the prairie vole (Microtus ochrogaster) neocortex. Neuroscience. 2017 doi: 10.1016/j.neuroscience.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Veenema AH. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front Neuroendocrinol. 2016;40:1–23. doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Ebner K, Wotjak CT, Landgraf R. Endogenous oxytocin is involved in short-term olfactory memory in female rats. Behav Brain Res. 1998;90:89–94. doi: 10.1016/s0166-4328(97)00084-3. [DOI] [PubMed] [Google Scholar]
- Feifel D, MacDonald K, McKinney R, Heisserer N, Serrano V. A randomized, placebo-controlled investigation of intranasal oxytocin in patients with anxiety. Neuropsychopharmacology. 2011;36:S324–S449. [Google Scholar]
- Figueira RJ, Peabody MF, Lonstein JS. Oxytocin receptor activity in the ventrocaudal periaqueductal gray modulates anxiety-related behavior in postpartum rats. Behav Neurosci. 2008;122:618–628. doi: 10.1037/0735-7044.122.3.618. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Gonzalez LE, Rujano M, Tucci S, Paredes D, Silva E, Alba G, Hernandez L. Medial prefrontal transection enhances social interaction. I. behavioral studies. Brain Res. 2000;887:7–15. doi: 10.1016/s0006-8993(00)02931-0. [DOI] [PubMed] [Google Scholar]
- Gould BR, Zingg HH. Mapping oxytocin receptor gene expression in the mouse brain and mammary gland using an oxytocin receptor-LacZ reporter mouse. Neuroscience. 2003;122:155–167. doi: 10.1016/s0306-4522(03)00283-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Krimsky M, Charney DR, Vytal K, Ernst M, Cornwell B. Oxytocin increases anxiety to unpredictable threat. Mol Psychiatry. 2013;18:958–960. doi: 10.1038/mp.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Howard AL, Dadds MR, Mitchell P, Carson DS. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology. 2009;34:917–923. doi: 10.1016/j.psyneuen.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Guzman YF, Tronson NC, Jovasevic V, Sato K, Guedea AL, Mizukami H, Nishimori K, Radulovic J. Fear-enhancing effects of septal oxytocin receptors. Nat Neurosci. 2013;16:1185–1187. doi: 10.1038/nn.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MW, Bouwknecht JA, Spiga F, Shekhar A, Lowry CA. Exposure to high- and low-light conditions in an open-field test of anxiety increases c-Fos expression in specific subdivisions of the rat basolateral amygdaloid complex. Brain Res Bull. 2006;71:174–182. doi: 10.1016/j.brainresbull.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Hicks C, Jorgensen W, Brown C, Fardell J, Koehbach J, Gruber CW, Kassiou M, Hunt GE, McGregor IS. The nonpeptide oxytocin receptor agonist WAY 267,464: receptor-binding profile, prosocial effects and distribution of c-Fos expression in adolescent rats. J Neuroendocrinol. 2012;24:1012–1029. doi: 10.1111/j.1365-2826.2012.02311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks AL, McGregor IS. Modulation of anxiety-related behaviours following lesions of the prelimbic or infralimbic cortex in the rat. Brain Res. 1997;772:181–190. doi: 10.1016/s0006-8993(97)00810-x. [DOI] [PubMed] [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem. 2009;16:486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Hughes M, Lee K, Gould E. Fatherhood affects dendritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nat Neurosci. 2006;9:1094–1095. doi: 10.1038/nn1753. [DOI] [PubMed] [Google Scholar]
- Lahoud N, Maroun M. Oxytocinergic manipulations in corticolimbic circuit differentially affect fear acquisition and extinction. Psychoneuroendocrinology. 2013;38:2184–2195. doi: 10.1016/j.psyneuen.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Lapiz-Bluhm MD, Bondi CO, Doyen J, Rodriguez GA, Bedard-Arana T, Morilak DA. Behavioural assays to model cognitive and affective dimensions of depression and anxiety in rats. J Neuroendocrinol. 2008;20:1115–1137. doi: 10.1111/j.1365-2826.2008.01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Social interaction deficits caused by chronic phencyclidine administration are reversed by oxytocin. Neuropsychopharmacology. 2005;30:1883–1894. doi: 10.1038/sj.npp.1300722. [DOI] [PubMed] [Google Scholar]
- Leuner B, Caponiti JM, Gould E. Oxytocin stimulates adult neurogenesis even under conditions of stress and elevated glucocorticoids. Hippocampus. 2012;22:861–868. doi: 10.1002/hipo.20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Shors TJ. Stress, anxiety, and dendritic spines: What are the connections? Neuroscience. 2013;251:108–119. doi: 10.1016/j.neuroscience.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Pare D. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm & Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Liu W, Pappas GD, Carter CS. Oxytocin receptors in brain cortical regions are reduced in haploinsufficient (+/−) reeler mice. Neurol Res. 2005;27:339–345. doi: 10.1179/016164105X35602. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Maguire J, Meinlschmidt G, Neumann ID. Emotion and mood adaptations in the peripartum female: complementary contributions of gamma-aminobutyric acid and oxytocin. J Neuroendocrinol. 2014;26:649–664. doi: 10.1111/jne.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:2159–2168. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaswinkel H, Gispen WH, Spruijt BM. Effects of an electrolytic lesion of the prelimbic area on anxiety-related and cognitive tasks in the rat. Behav Brain Res. 1996;79:51–59. doi: 10.1016/0166-4328(95)00261-8. [DOI] [PubMed] [Google Scholar]
- MacDonald K, Feifel D. Oxytocins role in anxiety: A critical appraisal. Brain Res. 2014;1580:22–56. doi: 10.1016/j.brainres.2014.01.025. [DOI] [PubMed] [Google Scholar]
- Mak P, Broussard C, Vacy K, Broadbear JH. Modulation of anxiety behavior in the elevated plus maze using peptidic oxytocin and vasopressin receptor ligands in the rat. J Psychopharmacol. 2012;26:532–542. doi: 10.1177/0269881111416687. [DOI] [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J Neuroendocrinol. 2012;24:609–628. doi: 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M, Stoev S, Chini B, Durroux T, Mouillac B, Guillon G. Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents. Prog Brain Res. 2008;170:473–512. doi: 10.1016/S0079-6123(08)00437-8. [DOI] [PubMed] [Google Scholar]
- Marlin BJ, Mitre M, D'Amour JA, Chao MV, Froemke RC. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature. 2015;520:499–504. doi: 10.1038/nature14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, McDonald CH, Brooks PJ, Goldman D. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol Behav. 1996;60:1209–1215. doi: 10.1016/s0031-9384(96)00212-0. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci U S A. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhouse OE. The intercalated cells of the amygdala. J Comp Neurol. 1986;247:246–271. doi: 10.1002/cne.902470209. [DOI] [PubMed] [Google Scholar]
- Mitre M, Marlin BJ, Schiavo JK, Morina E, Norden SE, Hackett TA, Aoki CJ, Chao MV, Froemke RC. A Distributed Network for Social Cognition Enriched for Oxytocin Receptors. J Neurosci. 2016;36:2517–2535. doi: 10.1523/JNEUROSCI.2409-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Mol Psychiatry. 2012;17:132–141. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Gorlich A, Heintz N. Oxytocin Modulates Female Sociosexual Behavior through a Specific Class of Prefrontal Cortical Interneurons. Cell. 2014;159:295–305. doi: 10.1016/j.cell.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID. Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo-pituitary-adrenal axis. Prog Brain Res. 2002;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Slattery DA. Oxytocin in General Anxiety and Social Fear: A Translational Approach. Biol Psychiatry. 2016;79:213–221. doi: 10.1016/j.biopsych.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Torner L, Wigger A. Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience. 2000;95:567–575. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- Ninan I. Oxytocin suppresses basal glutamatergic transmission but facilitates activity-dependent synaptic potentiation in the medial prefrontal cortex. J Neurochem. 2011;119:324–331. doi: 10.1111/j.1471-4159.2011.07430.x. [DOI] [PubMed] [Google Scholar]
- Nuss P. Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr Dis Treat. 2015;11:165–175. doi: 10.2147/NDT.S58841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Royer S, Smith Y, Lang EJ. Contextual inhibitory gating of impulse traffic in the intra-amygdaloid network. Ann N Y Acad Sci. 2003;985:78–91. doi: 10.1111/j.1749-6632.2003.tb07073.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Postina R, Kojro E, Fahrenholz F. Identification of neurohypophysial hormone receptor domains involved in ligand binding and G protein coupling. Adv Exp Med Biol. 1998;449:371–385. doi: 10.1007/978-1-4615-4871-3_48. [DOI] [PubMed] [Google Scholar]
- Qi J, Han WY, Yang JY, Wang LH, Dong YX, Wang F, Song M, Wu CF. Oxytocin regulates changes of extracellular glutamate and GABA levels induced by methamphetamine in the mouse brain. Addict Biol. 2012;17:758–769. doi: 10.1111/j.1369-1600.2012.00439.x. [DOI] [PubMed] [Google Scholar]
- Qi J, Yang JY, Wang F, Zhao YN, Song M, Wu CF. Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neurotransmission in the medial prefrontal cortex of mice in reinstatement. Neuropharmacology. 2009;56:856–865. doi: 10.1016/j.neuropharm.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Ramos L, Hicks C, Kevin R, Caminer A, Narlawar R, Kassiou M, McGregor IS. Acute prosocial effects of oxytocin and vasopressin when given alone or in combination with 3,4-methylenedioxymethamphetamine in rats: involvement of the V1A receptor. Neuropsychopharmacology. 2013;38:2249–2259. doi: 10.1038/npp.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ. Amygdala activity, fear, and anxiety: modulation by stress. Biol Psychiatry. 2010;67:1117–1119. doi: 10.1016/j.biopsych.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LB, Souza RF, Guimaraes FS. Anxiolytic-like effects induced by medial prefrontal cortex inhibition in rats submitted to the Vogel conflict test. Physiol Behav. 2008;93:200–205. doi: 10.1016/j.physbeh.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Rey CD, Lipps J, Shansky RM. Dopamine D1 receptor activation rescues extinction impairments in low-estrogen female rats and induces cortical layer-specific activation changes in prefrontal-amygdala circuits. Neuropsychopharmacology. 2014;39:1282–1289. doi: 10.1038/npp.2013.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology. 2006;185:218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- Rotzinger S, Lovejoy DA, Tan LA. Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides. 2010;31:736–756. doi: 10.1016/j.peptides.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Royer S, Martina M, Pare D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabihi S, Dong SM, Durosko NE, Leuner B. Oxytocin in the medial prefrontal cortex regulates maternal care, maternal aggression and anxiety during the postpartum period. Front Behav Neurosci. 2014a;8:258. doi: 10.3389/fnbeh.2014.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabihi S, Durosko NE, Dong SM, Leuner B. Oxytocin in the prelimbic medial prefrontal cortex reduces anxiety-like behavior in female and male rats. Psychoneuroendocrinology. 2014b;45:31–42. doi: 10.1016/j.psyneuen.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh A, Ohashi M, Suzuki S, Tsukagoshi M, Sugiyama A, Yamada M, Oka J, Inagaki M, Yamada M. Activation of the prelimbic medial prefrontal cortex induces anxiety-like behaviors via N-Methyl-D-aspartate receptor-mediated glutamatergic neurotransmission in mice. J Neurosci Res. 2014;92:1044–1053. doi: 10.1002/jnr.23391. [DOI] [PubMed] [Google Scholar]
- Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, Finardi A, Donzelli A, Pattini L, Rubino T, Parolaro D, Nishimori K, Parenti M, Chini B. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry. 2011;69:875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Shah AA, Sjovold T, Treit D. Inactivation of the medial prefrontal cortex with the GABAA receptor agonist muscimol increases open-arm activity in the elevated plus-maze and attenuates shock-probe burying in rats. Brain Res. 2004;1028:112–115. doi: 10.1016/j.brainres.2004.08.061. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID. Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacol. 2010;58:56–61. doi: 10.1016/j.neuropharm.2009.06.038. [DOI] [PubMed] [Google Scholar]
- Smeltzer MD, Curtis JT, Aragona BJ, Wang Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci Lett. 2006;394:146–151. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Smith AS, Tabbaa M, Lei K, Eastham P, Butler MJ, Linton L, Altshuler R, Liu Y, Wang Z. Local oxytocin tempers anxiety by activating GABAA receptors in the hypothalamic paraventricular nucleus. Psychoneuroendocrinology. 2016;63:50–58. doi: 10.1016/j.psyneuen.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobota R, Mihara T, Forrest A, Featherstone RE, Siegel SJ. Oxytocin reduces amygdala activity, increases social interactions, and reduces anxiety-like behavior irrespective of NMDAR antagonism. Behav Neurosci. 2015;129:389–398. doi: 10.1037/bne0000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solati J, Hajikhani R, Golub Y. Activation of GABAA receptors in the medial prefrontal cortex produces an anxiolytic-like response. Acta Neuropsychiatr. 2013;25:221–226. doi: 10.1111/acn.12016. [DOI] [PubMed] [Google Scholar]
- Stern CA, Do Monte FH, Gazarini L, Carobrez AP, Bertoglio LJ. Activity in prelimbic cortex is required for adjusting the anxiety response level during the elevated plus-maze retest. J Neurosci. 2010;170:214–222. doi: 10.1016/j.neuroscience.2010.06.080. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Saitoh A, Ohashi M, Yamada M, Oka J, Yamada M. The infralimbic and prelimbic medial prefrontal cortices have differential functions in the expression of anxiety-like behaviors in mice. Behav Brain Res. 2016;304:120–124. doi: 10.1016/j.bbr.2016.01.044. [DOI] [PubMed] [Google Scholar]
- Tillakaratne NJ, Medina-Kauwe L, Gibson KM. gamma-Aminobutyric acid (GABA) metabolism in mammalian neural and nonneural tissues. Comp Biochem Physiol A Physiol. 1995;112:247–263. doi: 10.1016/0300-9629(95)00099-2. [DOI] [PubMed] [Google Scholar]
- Toth I, Neumann ID, Slattery DA. Central administration of oxytocin receptor ligands affects cued fear extinction in rats and mice in a timepoint-dependent manner. Psychopharmacology (Berl) 2012;223:149–158. doi: 10.1007/s00213-012-2702-4. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnas-Moberg K, Ahlenius S, Hillegaart V, Alster P. High doses of oxytocin cause sedation and low doses cause an anxiolytic-like effect in male rats. Pharmacol Biochem Behav. 1994;49:101–106. doi: 10.1016/0091-3057(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog Brain Res. 2008;170:261–276. doi: 10.1016/S0079-6123(08)00422-6. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Wang J, Liu ZH, Ming Y, Zhang SW. Increased Posterior Cingulate, Medial Frontal and Decreased Temporal Regional Homogeneity in Depressed Mothers. A Resting-State Functional Magnetic Resonance Study. 2011 International Conference on Environment Science and Biotechnology (Icesb 2011) 2011;8:737–743. [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, Nishimori K. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J Neurosci. 2009;29:2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosten GL, Samson WK. The melanocortins, not oxytocin, mediate the anorexigenic and antidipsogenic effects of neuronostatin. Peptides. 2010;31:1711–1714. doi: 10.1016/j.peptides.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Liu Y, Gobrogge KL, Wang H, Wang Z. Oxytocin reverses amphetamine-induced deficits in social bonding: evidence for an interaction with nucleus accumbens dopamine. J Neurosci. 2014;34:8499–8506. doi: 10.1523/JNEUROSCI.4275-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]