Abstract
Biodegradable magnesium metal filaments placed inside biodegradable nerve conduits might provide the physical guidance support needed to improve the rate and extent of regeneration of peripheral nerves across injury gaps. In this study, we examined basic issues of magnesium metal resorption and biocompatibility by repairing sub-critical size gap injuries (6 mm) in one sciatic nerve of 24 adult male Lewis rats. Separated nerve stumps were connected with poly(caprolactone) nerve conduits, with and without magnesium filaments (0.25 mm diameter, 10 mm length), with two different conduit filler substances (saline and keratin hydrogel). At 6 weeks after implantation, magnesium degradation was examined by micro-computed tomography and histological analyses. Magnesium degradation was significantly greater when the conduits were filled with an acidic keratin hydrogel than with saline (p < 0.05). But magnesium filaments in some animals remained intact for 6 weeks. Using histological and immunocytochemical analyses, good biocompatibility of the magnesium implants was observed at 6 weeks, as shown by good development of regenerating nerve mini-fascicles and only mild inflammation in tissues even after complete degradation of the magnesium. Nerve regeneration was not interrupted by complete magnesium degradation. An initial functional evaluation, determination of size recovery of the gastrocnemius muscle, showed a slight improvement due to magnesium with the saline but not the keratin filler, compared with respective control conduits without magnesium. These results suggest that magnesium filament implants have the potential to improve repair of injured peripheral nerve defects in this rodent model.
Keywords: Peripheral nerve repair, nerve regeneration, magnesium, biodegradable metal, micro-computed tomography, rat, sciatic nerve, magnesium
Introduction
Peripheral nerve (PN) injuries are reported in over 3% of emergency room visits in the United States every year.1,2 Due to the delicate and complex nature of PNs and the neuromuscular junction, full functional recovery is rarely achieved after severe trauma, even with the current “gold standard” of treatment, which is a surgical autograft bridging the separated nerve segments. However, harvesting a donor nerve for an autograft typically requires a second surgical site, resulting in donor site morbidity, and this method often still results in limited functional recovery.3 Thus, novel solutions are greatly needed.
Since 1995, the FDA has approved 11 different bio-degradable nerve conduits,4 but they are only approved and effective for maximum nerve gaps upto 20–25 mm in humans4,5 (physiologically equivalent to a 15 mm critical size gap in rodents). With longer gaps, robust axonal regrowth from proximal to distal nerve stumps has not been demonstrated.6 One of the hypothesized reasons for this failure is that proteinaceous fibrin bridges, which provide physical “contact guidance” support for fibroblast and Schwann cell migration in shorter gaps, cannot form in longer gaps and/or if they form, they are not stabilized, so they may not persist long enough to support migration.7
To overcome this critical gap length limit, researchers have previously explored, with some promising results, the addition of linearly aligned biomaterial substrates6,8 or filamentous biomaterial fillers like matrix proteins, polymers, or hydrogels9 to the lumen of nerve conduits. These linear materials inside the conduits are proposed to substitute for the fibrin bridges and provide physical support for migrating fibroblasts and Schwann cells. We propose that single magnesium (Mg) microfilaments (wires of <1 mm diameter), placed within the lumen of a biomaterial nerve conduit using simple surgical techniques, could provide this contact guidance and act to improve regeneration of PNs. Our proposal is that this solid material, which will cross the nerve gap within the conduit, will provide a physical support comparable to the fibrin bridges that will allow attachment of the fibroblasts and Schwann cells migrating out of the proximal stump and this then will support migration to the distal nerve stump. A significant beneficial feature of Mg microfilaments is that they will degrade rapidly and completely, producing primarily physiologically appropriate Mg2+ and Mg complexes. Mg implants, while not FDA approved, are being tested in Europe in the form of stents, with few safety concerns detected so far.10,11 Also, a Mg screw for toe fixation has recently received a CE mark in Europe (www.Syntellix.de).12 This rapid and safe degradation of Mg metal is potentially advantageous over other biomaterials because some biomaterials can take months or years to degrade6 or can cause inflammation during resorption.13
Another potential benefit of using an implant of Mg metal is that the release of Mg2+ ions during degradation might aid nerve recovery after injury. Mg2+ levels drop in brain tissues after stroke or traumatic brain injury,14 and systemic infusions of Mg2+ solutions have improved functional recovery in animal studies after traumatic brain injury15 or stroke.16,17 Elevated dietary Mg2+ improved functional recovery after a PN crush.18 We have recently shown that in cultures of primary mouse neuronal stem cells, addition of Mg salt solutions can improve cell numbers and neurite production.19 While animal data were promising, clinical studies have, to date, shown efficacy of systemic applications in only a few instances.14 Thus, there is significant support for the concept that application of Mg2+ can be neuroprotective after injury. A further benefit that may occur is that the conductivity of Mg metal might aid nerve regeneration by allowing a path for electrical stimulation to injured nerves and perhaps to the corresponding muscle. The benefit of establishing a conductive path across nerve gaps has been observed with conductive nerve conduits.20,21
While these mechanisms are possible, the primary goal of this study was to determine the basic resorption and biocompatibility characteristics of Mg microfilaments and to begin studies of the effectiveness of using scaffolds of Mg filaments inside polymeric biomaterial nerve conduits to enhance the rate of repair of 6 mm, non-critical size injury gaps in adult rat sciatic nerves, with the eventual goal of extending these studies to longer gaps.
Methods
Mg wire preparation
Mg wires (99.9% pure; Goodfellow, PA) of 10 mm length and 0.250 mm diameter were cleaned (sonication in 100% ethanol for 10 min) and sterilized (UV exposure, 20 min per side). The filaments had a grey oxidation layer that was not removed prior to implantation.
Animals and surgery
All animal treatment protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee and were in accordance with the Guide for the Care and Use of Laboratory Animals, as adopted by the National Institutes of Health. All procedures and housing were in AAALAC approved animal facilities. Twenty-four adult male Lewis rats (Harlan, Indianapolis, IN, 215–235 g weight) were anesthetized (isoflurane gas) and the right sciatic nerve was exposed by gluteal incision as per published methods.22 The nerves were transected 5 mm proximal to the sciatic/peroneal bifurcation and allowed to retract for 30 s. A 6 mm defect was created by inserting the proximal and distal stumps into an 8 mm long poly(caprolactone) (PCL) nerve conduit (internal diameter 0.7 mm, wall thickness 300–400 μm), overlapping the ends by 1 mm, and suturing them in place (8-0 polypropylene, Ethicon Endo Surgery, NJ) (Figure 1a). PCL conduits were prepared as described previously.23 Mg filaments were embedded into the nerve stumps (2 mm deep) with the conduit threaded over all (Figure 1b and c). The conduit lumens were filled via syringe with a keratin hydrogel or sterile saline. Muscles and skin were closed (4-0 Vicryl sutures, Ethicon Endo Surgery, NJ). The four experimental groups (all animals had conduits) were: (a) saline filler only (NoMgSa, n = 4 animals, one sacrificed at 1 week); (b) saline and Mg (MgSa, n = 4, one sacrificed at 1 week); (c) keratin hydrogel only (keratin proteins purified from human hair using a chemical oxidant, generously provided by Dr Mark van Dyke24 and reconstituted with sterile saline) (NoMgKr, n = 8); and (d) keratin and Mg (MgKr, n = 9). Lidocaine HCl gel (Akorn, IL) was applied to the wound after closure and the injured feet and legs were sprayed weekly or more often with Bitter Apple Spray (Petsmart, Cincinnati, OH) to prevent autophagy. No signs of autophagy (toenail or toe chewing) or animal discomfort were observed in any animal, at any time.
Figure 1.

Surgery and micro-CT analyses: (a) a scaffold diagram (arrows = sutures), (b) surgical pictures of the Mg insertion into nerve at surgery and (c) the PCL conduit in place; (d) after sacrifice at 6 weeks, regenerated nerves plus conduit were removed and placed into a holder to image by micro-CT, where a suture marks the proximal end and bar = 5 mm. Micro-CT images of conduits removed after 6 weeks are shown for: (e) MgKr and (f) MgSa animals (bar in f = 1 mm); (g) micro-CT images were lined up with graphs of Mg cavity areas (mm2) versus section position (mm) relative to the conduit midpoint (0 mm, distal is to the left in images and in negative numbers on graph) (bar = 1 mm); (h) Mg cavity measurements were made in sections for all MgSa (MS, n = 3) and MgKr (MK, n = 8) animals (**p < 0.001, data normally distributed; *p < 0.05, data not normally distributed).
Micro-CT: micro-computed tomography; Mg: magnesium; MgSa: saline and Mg; MgKr: keratin and Mg; PCL: poly(caprolactone).
Animal sacrifice and tissue processing
Two animals (2) were sacrificed on post-surgical day 7 to evaluate metal resorption, which, if too rapid, might cause bubbling within the conduit and damage to the animal. The conduits and attached nerves were in good condition, and the metal filaments were intact, as determined by micro-computed tomography (micro-CT) imaging (data not shown), so the remainder of the animals were sacrificed on day 42 (6 weeks, n = 22). At sacrifice, conduits plus ~2–5 mm of both nerve stumps and contralateral nerves were removed, fixed (4% paraformaldehyde in phosphate-buffered saline; 1–2 h for micro-CT imaging, 48 h for tissue processing), and placed in a fluid-filled boat for imaging by micro-CT (Figure 1d). These were then paraffin embedded. For animals sacrificed at 6 weeks, the gastrocnemius muscles from both legs were carefully dissected such that they were clear of all other muscles and fixed, after which slabs were cut from the thickest point of each muscle and photographed to calculate cross-sectional area. The animal weights and sizes of uninjured muscles did not differ between groups.
Micro-CT imaging
To image Mg filament degradation, conduits containing Mg (in saline for imaging) were assessed by micro-CT in a Siemens Inveon Multimodality System (San Diego, CA) at the University of Cincinnati Vontz Imaging Core Facility. Samples were scanned at half-degree increments with 384 steps (step and shoot) for 192°. Images were acquired with high magnification and a pixel matrix binning of 2, resulting in an effective voxel size of 17.27 μm, using 80 kVp voltage and 300 μA current, with the exposure time being 2100 ms with 25 ms settle time. Inveon software was used to create three-dimensional reconstructions and then single frames were selected for figures.
Tissue sectioning and staining
Paraffin-embedded conduits with nerve stumps were sectioned (10 μm thick) and either stained with hematoxylin & eosin (H&E) (every 500 μm through the entire conduit) or immunostained (standard methods, selected sections) for axons (rabbit antibody to the 200 MW neurofilament protein, NF200, 1:500 dilution, Sigma, St Louis, MO), Schwann cells (rabbit antibody to the protein S100, 1:500, Dako, Carpenterio, CA), macrophages (mouse monoclonal ED1 antibody, binds to the cytokine CD68, 1:500, Abcam, Cambridge, MA), and for an intact nerve-blood barrier (mouse monoclonal to the glucose transporter-1 protein, GLUT1, 1:500, Thermo, Fremont, CA). Cell nuclei were labelled with 4′,6-diamidino-2-phenylindole (DAPI) (1:1000) (Sigma-Aldrich). The secondary antibodies were anti-mouse Alexa 488 and anti-rabbit Alexa 594 (1:1000, Invitrogen, Grand Island, NY).
Photography and image analysis
Stained sections were photographed using an upright Zeiss Axioplan Imaging 2e fluorescence microscope with a Zeiss Axiocam digital camera. For immunofluorescence analyses, gray scale images were taken at 100 × magnification in each of the three channels, combined into a composite, and colored using Photoshop. Analyses were done using Photoshop, Matlab, NIH ImageJ, and/or visual counts.
Statistics
Statistical analyses used were t-tests or one-way ANOVAs, with the Student–Neuman–Keuls (SNK) post-hoc test if data were normally distributed and the Wilcoxon Rank Sum test or the Kruskall–Wallis ANOVA on ranks with Dunn’s post-hoc test if not normally distributed. Analyses were done with Matlab or SigmaPlot 12 software. A p value <0.05 was considered significant and error bars show 1 standard deviation.
Results
Mg degradation in vivo
After 6 weeks in vivo, micro-CT imaging of nerve tissues from the Mg-containing groups showed more significant Mg resorption in animals in the MgKr group (from n = 8 animals, Figure 1e) than in the MgSa group (conduits were taken from n = 3 animals, but Figure 1f shows 2 because one micro-CT image was omitted because of technical issues). In all, the gaps or thinning occurred predominantly in the middle of the filaments, with less resorption at either end.
Mg resorption was also examined by measuring the area of the cavities left by the unresorbed Mg metal in H&E-stained paraffin sections (with ImageJ, 10+ sections per animal). In freshly cut sections, metal pieces could be observed, but all cut metal pieces disappeared during staining (presumably either corroding or detaching), leaving round, and empty “cavities” in the tissue sections. The areas of the Mg cavities, plotted against the section position relative to the conduit middle, were matched with the corresponding micro-CT image. Representative animals from the MgSa (MS03, Figure 1g, top) and MgKr (MK01, Figure 1g, bottom) groups are shown. While most of the major gaps were detected with both techniques, obviously the differences in sampling frequency resulted in a much more complete picture of resorption with micro-CT. Quantification of the cavity areas in the sections (Figure 1h) showed that all cavity measures were significantly larger for MgSa versus MgKr animals, confirming that Mg resorption was affected by the conduit filler.
Regenerated tissues
After 6 weeks in vivo, regenerating tissues filled all conduits at the midpoint (Figure 2). All Mg filaments were centrally located and completely surrounded by regenerating tissues (Figures 2b and d and 3b). No necrotic tissue was observed, either adjacent to the Mg cavities or elsewhere. This supports the idea that Mg was compatible with tissue regeneration, and the central position suggests that the metal might have served as a guiding material for cellular growth.
Figure 2.

H&E staining of regenerating tissues: (a–d) mid-conduit sections from each group are shown after 6 weeks in vivo (*cavity left by Mg, b and d). Note the central location of Mg within the regenerated tissues (bar = 300 μm, applies to all).
H&E: hematoxylin & eosin; Mg: magnesium.
Figure 3.

Immunostained regenerating tissues after 6 weeks; (a and b) mid-conduit sections were stained for GLUT1 (green)/S100 (red)/DAPI (blue) in (a) NoMgSa and (b) MgSa animals (*cavity left by Mg, arrows delineate conduit material, bar = 300 μm). (c and d) Regenerating nerve mini-fascicles, indicated by arrows, run close to the Mg cavities, which are marked by * and arrowheads. The same staining was done on the section in (c), while the section in (d) was stained with ED1 (green)/NF200 (red)/DAPI (blue). Note GLUT1+ perineurium in (c) and thin layer of ED1+ macrophages above arrowheads in (d) (bar in c and d = 50 μm). The conduit material (shown in the H&E staining in (e)) contained clusters of overlapping nuclei (arrows) (bar = 50 μm. A graph of ED1+ cells per nerve per group is shown in (f) (cont = undamaged nerve).
H&E: hematoxylin & eosin; NoMgSa: saline filler only; MgSa: saline and Mg; GLUT1: glucose transporter 1; DAPI: 4′,6-diamidino-2-phenylindole.
Regenerating tissues consisted of connective tissue with blood vessels and mini-fascicles of nerve tissue that were recognizable in H&E-stained sections and stained positive for S100 or NF200 (Figure 3a–d). Almost all mini-fascicles were encapsulated by a perineurial sheath that stained strongly for the glucose transporter 1 (GLUT1) protein (Figure 3a–c). GLUT1 staining suggests that the perineurium was functioning as a nerve tissue barrier because this staining is lost when nerve injury disrupts nerve structure.25 Fascicle size and clustering were variable, but with keratin hydrogel groups, there was a trend towards smaller fascicle size, weaker or incomplete GLUT1 staining, greater scatter of fascicles, and less concentration centrally (not quantified). This might be consistent with the hydrogel itself serving as guiding bridges.
Tissue reactions to Mg metal
Inflammation was evaluated in H&E-stained sections throughout the conduit and was classified as mild (representative sections shown in Figures 2 and 4) because there was minimal-to-mild and patchy infiltration of mononuclear leukocytes and a slight widening of blood vessels. Hemosiderin-laden macrophages (suggesting digestion of red blood cells, which may accompany either inflammation or neovascularization) were detected occasionally, with no consistent pattern across groups. Closest to the Mg cavities were one to two layers of ED1-positive (ED1+) macrophages (Figure 3d, at arrowheads), followed externally by a few layers of flattened fibroblasts. No giant cells were observed in this region. Non-cellular material that fluoresced in all channels was seen in or at the edges of the cavities and was assumed to be remnants of Mg degradation products. The greatest degree of inflammation was seen in areas that were undergoing more active Mg resorption, as determined by smaller and more irregular Mg cavities (only seen in MgKr animals). Biocompatibility with regenerating nerve tissue was suggested by the fact that the mini-fascicles were often found close to the Mg cavities, immediately external to the flattened fibroblasts (Figure 3c and d). In contrast, a greater degree of inflammation was observed in the conduit material, with numerous potential giant cells (Figure 3e, arrows).
Figure 4.

Effects of Mg resorption prior to sacrifice: (a) in a MgKr animal, sections were lined up relative to the micro-CT image (bar = 1 mm) and (b–d) comparable regions (*) in the proximal (top row), mid-gap (middle row), and distal (bottom row) sections were stained for H&E (bar = 50 μm) or (e–g) ED1 (green)/NF200 (red)/DAPI (blue) (bar = 50 μm, arrows = NF200+ axons).
H&E: hematoxylin & eosin; Micro-CT: micro-computed tomography; Mg: magnesium; MgKr: keratin and Mg; DAPI: 4′,6-diamidino-2-phenylindole.
As a quantification of one measure of inflammation, ED1+ macrophages were counted (one observer, blinded to conditions) in mid-conduit sections, in regions that contained NF200+ nerve bundles (100 μm square regions, one per NF200/ED1-stained section, two sections per animal, with three contralateral nerves for the controls) (Figure 3f). There were no significant differences (Kruskall–Wallis ANOVA on ranks, p < 0.05) although there appeared to be a trend toward higher macrophages in all injured nerves compared with unoperated nerves. Thus, specifically within regenerating nerve tissue, the presence of Mg did not alter macrophage numbers. Autofluorescence of the conduit material (Figure 3a and b, between arrows) prevented quantification of macrophages throughout the entire conduit.
Tissues in areas where Mg had degraded completely
To examine the effects of complete Mg degradation, paraffin sections were identified where the Mg had resorbed before sacrifice. Such “gap” sections were identified as those that were lacking a central Mg cavity, while both proximal and distal sections contained cavities and, as additional confirmation, this was compared with the corresponding micro-CT image. Representative images from an MgKr animal are shown in Figure 4, with staining for H&E (Figure 4b–d) and NF200/ED1/DAPI (Figure 4–g), and the sections were aligned with the appropriate micro-CT image (Figure 4a). Evidence of inflammation, including enlarged blood vessels, but no necrosis, was observed in the proximal and distal sections (which had reduced Mg cavity areas) (Figure 4b and d). The section without an Mg cavity (Figure 4c) showed less inflammation than these adjacent sections, and the region where the Mg cavity had been expected (Figure 4c, asterisk) was filled with normal-appearing connective tissue with few nerve fascicles. No significant accumulations of ED1+ cells were observed in this central connective tissue (Figure 4e–g). The NF200+ nerve mini-fascicles remained in roughly the same positions in all sections (external to the Mg cavity or where it would have been) and were always present distal to such gaps. This suggests that Mg resorption was not detrimental to the regenerating nerves or tissues.
Muscle measurements
The size of the gastrocnemius muscles at 6 weeks after nerve repair was measured by determining cross-sectional area of muscle slabs taken at the widest area of each muscle to begin to understand possible functional recovery with this repair. Nerve regeneration should trigger recovery from denervation atrophy, and while this is not the most definitive measure and often there is no functional recovery by this this time point,26 it has been reported that this measure is useful in detecting differences between treatments as early as 6 weeks after injury.27 Figure 5 shows the relative muscle sizes. Significant differences were found (one-way ANOVA, p < 0.05, n = 3, 3, 8, 8, SNK post-hoc analysis) between NoMgSa and both the MgSa and NoMgKr groups. The increase with the NoMgKr group was expected because the keratin hydrogel alone has been shown to increase nerve regeneration.2,28 In contrast, muscles in the MgKr group were not significantly different from any other condition, which suggests no additive or synergistic effect of Mg in the presence of keratin. These data provide a positive suggestion that this repair method, with the saline filler solution, might be better than an empty conduit and at least similar to that afforded by the keratin hydrogel.
Figure 5.
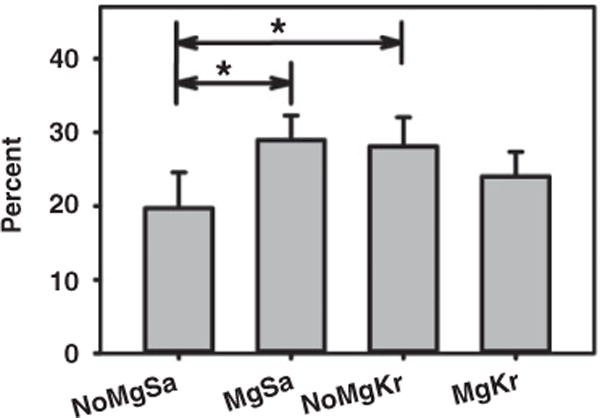
Significant differences between groups were detected for gastrocnemius muscle cross-sectional areas, with injured as a percentage of the non-injured, contralateral muscle for the same animal (*p < 0.05).
Mg: magnesium; NoMgSa: saline filler only; MgSa: saline and Mg; NoMgKr: keratin hydrogel only; MgKr: keratin and Mg.
Discussion
Mg implants have not previously been used to bridge and repair nerve gaps as described in this study, although one earlier study described inserting an Mg filament in a rabbit nerve.29 Research on Mg implants in general is hampered by the fact that resorption of Mg in cell culture systems or in vitro in aqueous solutions occurs much faster than resorption in vivo.30 Similarly, we observed little resorption after 6 weeks in vivo, while nothing remains intact past 1 week when similar wires are placed in cell culture medium (our lab, data not shown). Our findings support the concepts proposed by others, that in vitro systems are not appropriate for studying Mg metal implants.11
One challenge in designing a bioresorbable device is controlling the rate of degradation/resorption. With Mg metal, the resorption in vivo is influenced by many factors within the local aqueous tissue environment, including vascular and lymphatic flow, local pH, and mechanical stress on the implant, all of which can vary by tissue type and implant location.31 There are also reciprocal influences of the degrading Mg on the local environment. In this study, Mg resorption was monitored by micro-CT, which is a sensitive and standard method for visualizing metal implants.32 The information obtained by the micro-CT imaging was superior to that obtained through a more labor-intensive histological analysis. Both techniques have limitations that prevent determination of the exact volumes of the metal, but both methods provided internally consistent results. Both showed that resorption rate was dependent on the fluid used to fill the conduits.
We compared two conduit fillers, saline and a keratin hydrogel, that can enhance regeneration, even across long nerve gaps.2,28,33,34 Initially, we predicted that combining the hydrogel with Mg might have a synergistic effect on regeneration; however, the MgKr group did not demonstrate a significant increase in muscle size over the keratin alone (or MgSa group either), which means that there was no synergistic increase in recovery. The MgKr group showed greater Mg resorption than MgSa, which we speculate may have occurred because the keratin used was prepared by oxidization, which results in abundant acidic sulfhydryl residues on the keratin filaments.24 This could have rendered the local environment more acidic, which would have been more corrosive to Mg than saline. The fact that the gaps were more obvious at the midpoints supports this idea because cells migrating onto the Mg filament from the nerve stumps presumably covered the ends of the filaments quicker and prevented contact between the metal and the filler, thereby slowing metal resorption in the case of the keratin.
There was greater resorption of the Mg filaments in the MgKr group than in the MgSa group, so presumably this degradation released more Mg2+; but the MgKr group did not show improved muscle size over the MgSa or NoMgKr group. Instead, muscle size was increased in the MgSa group over its NoMgSa control, where the MgSa filament degradation was slower. Although further research is needed, this supports the hypothesis of the presence of an intact physical support system and maintenance of an intact Mg filament for at least 6 weeks and that release of ions is probably not a mechanism underlying any enhancement of PN regeneration by these filaments.
This study focused on the biocompatibility of Mg metal with regenerating nerves and not on a return of function because of the early time point of sacrifice. However, muscle size was still quantified as one possible measure of functional recovery. The changes indicate that recovery of muscle size after denervation atrophy was significantly greater in the MgSa group versus the NoMgSa group. While this 6 week time point is early for functional recovery, which explains the very modest changes between groups, other researchers have shown differences in muscle weights between sciatic nerve repairs at 6 weeks.27 This measure is also not robust because of the low number of animals in our groups. But it does provide at least one suggestion that this treatment may be better than an empty conduit. Another measure of relative success would have been to quantify the total amount of neurofilament-positive fibers. However, this was impeded by autofluorescence of the conduit material, which often formed pockets or indentations into the tissues, and autofluorescence of the Mg-degradation products that surrounded the Mg metal pieces (this layer is also autofluorescent in bench or cell culture studies, not shown). While a muscle difference supports the idea that Mg can support nerve regeneration that is re-innervating the muscles, it is also possible that the presence of Mg slowed the rate of muscle atrophy by initially providing an electrically conductive path for transmission of nerve impulses across the gap, thus stimulating the muscle, which then, in turn, might slow atrophy. Thus, in future experiments, the temporal pattern of atrophy over time will be studied, as well as exploring later time periods, longer gaps, and including additional measures of functional recovery.26
If the Mg filaments are providing contact guidance, then they need to remain intact long enough to support the migration of fibroblasts and Schwann cells into the conduit from both nerve stumps. After that, the Schwann cells will support axon outgrowth. Previous studies have shown that for a 10 mm gap repaired with a silicon conduit, non-neuronal cells reached the mid-point (5 mm) at 3–4 weeks (a speed of ~0.18–0.24 mm/day).35 Here, with saline as the filler, the Mg filaments remained intact for 6 weeks, which theoretically might support repair over the critical size nerve gap of 15 mm in rodents. This estimate remains preliminary because of the low number of animals tested to date and the uncertainty that the cell migration rate using our conditions is the same as that in published studies. We project that, for long injury gaps, especially in larger animals or humans, commercial Mg filaments may require modification to slow resorption, i.e. by using a different Mg alloy, surface treatments for Mg, alternate conduit fillers, or perhaps a different conduit substance.
Tissue reaction to a foreign body is manifested with fibrous encapsulation by macrophages, fibroblasts, and, later, giant cells.36 A very thin layer of macrophages was observed around any of the Mg cavities and this was surrounded by a relatively thin layer of fibroblasts. No giant cells were observed in these layers. Macrophage numbers in the regenerating nerve fascicles were not altered by Mg or either conduit filler. In areas that were presumed to be undergoing active resorption of Mg, as determined by the size of the Mg cavity and its relationship to gap regions, inflammation was more obvious than in other regions, although still minimal to mild. No necrosis was observed in these regions. In contrast, the PCL conduit material (an FDA-approved implant material) contained numerous potential giant cells and more significant infiltration with macrophages. Further evidence of Mg biocompatibility is provided by the fact that regenerating nerve mini-fascicles were found very close to the metal and they were not disrupted by Mg resorption.
Conclusions
This study demonstrates that Mg filaments in nerve conduits are biocompatible with PN regeneration. Tissue tolerance of the Mg metal was evident in that inflammation was mild, nerve regeneration was histologically apparent, and necrosis was not observed. Although this study focused primarily on Mg metal resorption rates and biocompatibility, the changes in muscle size recovery, observed with the saline filler, offer some promise for this repair treatment. Furthermore, the Mg filament degradation that occurred in the MgSa group was on a slow enough time scale such that the filaments could provide support of nerve regeneration over these short gaps and perhaps even longer critical size PN gaps.
Acknowledgments
Kathleen LaSance provided micro-CT imaging and guidance on CT image manipulation. Dr Mark van Dyke (Virginia Tech University) provided the keratin hydrogel. Ms Ami Cohen and Ms Erin Armao (UC) provided technical assistance.
Funding
This research was supported by grants from the NSF ERC for Revolutionizing Metallic Biomaterials (EEC-0812348, NCAT 260116C supplement); the American Academy of Otolaryngology, American Academy of Facial Plastic and Reconstructive Surgery (AAFPRS) Leslie Bernstein Resident Research Grant number 277259; the American Association of Hand Surgeons (AAHS) Annual Research Grant; an Institutional Clinical and Translational Science Award, NIH/NCATS Grant number 8UL1TR000077-05; and funding from the University of Cincinnati Department of Otolaryngology—Head & Neck Surgery. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of any of the funding agencies.
Footnotes
Declaration of conflicting interests
None declared.
References
- 1.Robinson LR. Traumatic injury to peripheral nerves. Muscle Nerve. 2000;23(6):863–873. doi: 10.1002/(sici)1097-4598(200006)23:6<863::aid-mus4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Hill PS, Apel PJ, Barnwell J, et al. Repair of peripheral nerve defects in rabbits using keratin hydrogel scaffolds. Tissue Eng Part A. 2011;17(11–12):1499–1505. doi: 10.1089/ten.TEA.2010.0184. [DOI] [PubMed] [Google Scholar]
- 3.Pfister BJ, Gordon T, Loverde JR, et al. Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges. Crit Rev Biomed Eng. 2011;39(2):81–124. doi: 10.1615/critrevbiomedeng.v39.i2.20. [DOI] [PubMed] [Google Scholar]
- 4.Kehoe S, Zhang XF, Boyd D. FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury. 2012;43(5):553–572. doi: 10.1016/j.injury.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 5.Bell JHA, Haycock JW. Next generation nerve guides: materials, fabrication, growth factors, and cell delivery. Tissue Eng Part B Rev. 2012;18(2):116–128. doi: 10.1089/ten.TEB.2011.0498. [DOI] [PubMed] [Google Scholar]
- 6.Deumens R, Bozkurt A, Meek MF, et al. Repairing injured peripheral nerves: bridging the gap. Prog Neurobiol. 2010;92(3):245–276. doi: 10.1016/j.pneurobio.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Yannas IV, Zhang M, Spilker MH. Standardized criterion to analyze and directly compare various materials and models for peripheral nerve regeneration. J Biomater Sci Polymer Ed. 2007;18(8):943–966. doi: 10.1163/156856207781494386. [DOI] [PubMed] [Google Scholar]
- 8.Clements IP, Kim YT, English AW, et al. Thin-film enhanced nerve guidance channels for peripheral nerve repair. Biomaterials. 2009;30(23–24):3834–3846. doi: 10.1016/j.biomaterials.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belkas JS, Shoichet MS, Midha R. Peripheral nerve regeneration through guidance tubes. Neurol Res. 2004;26(2):151–160. doi: 10.1179/016164104225013798. [DOI] [PubMed] [Google Scholar]
- 10.Witte F. The history of biodegradable magnesium implants: a review. Acta Biomater. 2010;6(5):1680–1692. doi: 10.1016/j.actbio.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 11.Kirkland NT. Magnesium biomaterials: past, present and future. Corros Eng Sci Technol. 2012;47(5):322–328. [Google Scholar]
- 12.Windhagen H, Radtke K, Weizbauer A, et al. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: short term results of the first prospective, randomized, controlled clinical pilot study. Biomed Eng Online. 2013;12:62. doi: 10.1186/1475-925X-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haggerty AE, Oudega M. Biomaterials for spinal cord repair. Neurosci Bull. 2013;29(4):445–459. doi: 10.1007/s12264-013-1362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vink R, Cook NL, van den Heuvel C. Magnesium in acute and chronic brain injury: an update. Magnesium Res. 2009;22(3):158–162. [PubMed] [Google Scholar]
- 15.Browne KD, Leoni MJ, Iwata A, et al. Acute treatment with MgSO4 attenuates long-term hippocampal tissue loss after brain trauma in the rat. J Neurosci Res. 2004;77(6):878–883. doi: 10.1002/jnr.20215. [DOI] [PubMed] [Google Scholar]
- 16.Vink R, Bullock MR. Traumatic brain injury: therapeutic challenges and new directions. Neurotherapeutics. 2010;7(1):1–2. doi: 10.1016/j.nurt.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoane MR. Magnesium therapy and recovery of function in experimental models of brain injury and neurodegenerative disease. Clin Calcium. 2004;14(8):65–70. [PubMed] [Google Scholar]
- 18.Pan HC, Sheu ML, Su HL, et al. Magnesium supplement promotes sciatic nerve regeneration and down-regulates inflammatory response. Magnes Res. 2011;24(2):54–70. doi: 10.1684/mrh.2011.0280. [DOI] [PubMed] [Google Scholar]
- 19.Vennemeyer JJ, Hopkins T, Kuhlmann J, et al. Effects of elevated magnesium and substrate on neuronal numbers and neurite outgrowth of neural stem/progenitor cells in vitro. Neurosci Res. 2014;84:72–78. doi: 10.1016/j.neures.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Gordon T, Sulaiman OAR, Ladak A. Chapter 24: Electrical stimulation for improving nerve regeneration: where do we stand? Int Rev Neurobiol. 2009;87:433–444. doi: 10.1016/S0074-7742(09)87024-4. [DOI] [PubMed] [Google Scholar]
- 21.Urbanchek MG, Shim BS, Baghmanli Z, et al. Conduction properties of decellularized nerve biomaterials. IFMBE Proc. 2010;32:430–433. doi: 10.1007/978-3-642-14998-6_109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kokai LE, Bourbeau D, Weber D, et al. Sustained growth factor delivery promotes axonal regeneration in long gap peripheral nerve repair. Tissue Eng Part A. 2011;17(9–10):1263–1275. doi: 10.1089/ten.tea.2010.0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kokai LE, Lin YC, Oyster NM, et al. Diffusion of soluble factors through degradable polymer nerve guides: controlling manufacturing parameters. Acta Biomater. 2009;5(7):2540–2550. doi: 10.1016/j.actbio.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 24.de Guzman RC, Merrill MR, Richter JR, et al. Mechanical and biological properties of keratose biomaterials. Biomaterials. 2011;32(32):8205–8217. doi: 10.1016/j.biomaterials.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 25.Stark B, Carlstedt T, Cullheim S, et al. Developmental and lesion-induced changes in the distribution of the glucose transporter glut-1 in the central and peripheral nervous system. Exp Brain Res. 2000;131(1):74–84. doi: 10.1007/s002219900300. [DOI] [PubMed] [Google Scholar]
- 26.Vleggeert-Lankamp CLAM. The role of evaluation methods in the assessment of peripheral nerve regeneration through synthetic conduits: a systematic review. Laboratory investigation. J Neurosurg. 2007;107(6):1168–1189. doi: 10.3171/JNS-07/12/1168. [DOI] [PubMed] [Google Scholar]
- 27.Siemionow M, Duggan W, Brzezicki G, et al. Peripheral nerve defect repair with epineural tubes supported with bone marrow stromal cells: a preliminary report. Ann Plast Surg. 2011;67(1):73–84. doi: 10.1097/SAP.0b013e318223c2db. [DOI] [PubMed] [Google Scholar]
- 28.Lin YC, Ramadan M, Van Dyke M, et al. Keratin gel filler for peripheral nerve repair in a rodent sciatic nerve injury model. Plast Reconstr Surg. 2012;129(1):67–78. doi: 10.1097/PRS.0b013e3182268ae0. [DOI] [PubMed] [Google Scholar]
- 29.Hussl H, Papp Ch, Koepfel-Kreiner I. Resorption time and tissue reactions with magnesium rods in rats and rabbits. Chir Plastica. 1981;6(2):117–126. [Google Scholar]
- 30.Witte F, Fischer J, Nellesen J, et al. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials. 2006;27(7):1013–1018. doi: 10.1016/j.biomaterials.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 31.Witte F, Hort N, Vogt C, et al. Degradable biomaterials based on magnesium corrosion. Curr Opin Solid State Mater Sci. 2008;12(5–6):63–72. [Google Scholar]
- 32.Witte F, Fischer J, Nellesen J, et al. Microtomography of magnesium implants in bone and their degradation. In: Bonse Ulrich., editor. Developments in X-ray tomography. San Diego, CA, USA: Aug 13, 2006. (Proceedings SPIE volume 6318). [Google Scholar]
- 33.Apel PJ, Garrett JP, Sierpinski P, et al. Peripheral nerve regeneration using a keratin-based scaffold: long-term functional and histological outcomes in a mouse model. J Hand Surg Am. 2008;33(9):1541–1547. doi: 10.1016/j.jhsa.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 34.Sierpinski P, Garrett J, Ma J, et al. The use of keratin biomaterials derived from human hair for the promotion of rapid regeneration of peripheral nerves. Biomaterials. 2008;29(1):118–128. doi: 10.1016/j.biomaterials.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 35.Williams LR, Longo FM, Powell HC. Spatial-temporal progress of peripheral nerve regeneration within a silicone chamber: parameters for a bioassay. J Comp Neurol. 1983;218(4):460–470. doi: 10.1002/cne.902180409. [DOI] [PubMed] [Google Scholar]
- 36.Anderson JM. Biological responses to materials. Annu Rev Mater Sci. 2001;31:81–110. [Google Scholar]


