Abstract
Background
Neuropsychiatric disorders are a leading source of disability and require novel treatments that target mechanisms of disease. As such disorders are thought to result from aberrant neuronal circuit activity, neuromodulation approaches are of increasing interest given their potential for manipulating circuits directly. Low intensity transcranial electrical stimulation (tES) with direct currents (transcranial direct current stimulation, tDCS) or alternating currents (transcranial alternating current stimulation, tACS) represent novel, safe, well-tolerated, and relatively inexpensive putative treatment modalities.
Objective
This report seeks to promote the science, technology and effective clinical applications of these modalities, identify research challenges, and suggest approaches for addressing these needs in order to achieve rigorous, reproducible findings that can advance clinical treatment.
Methods
The National Institute of Mental Health (NIMH) convened a workshop in September 2016 that brought together experts in basic and human neuroscience, electrical stimulation biophysics and devices, and clinical trial methods to examine the physiological mechanisms underlying tDCS/tACS, technologies and technical strategies for optimizing stimulation protocols, and the state of the science with respect to therapeutic applications and trial designs.
Results
Advances in understanding mechanisms, methodological and technological improvements (e.g., electronics, computational models to facilitate proper dosing), and improved clinical trial designs are poised to advance rigorous, reproducible therapeutic applications of these techniques. A number of challenges were identified and meeting participants made recommendations made to address them.
Conclusions
These recommendations align with requirements in NIMH funding opportunity announcements to, among other needs, define dosimetry, demonstrate dose/response relationships, implement rigorous blinded trial designs, employ computational modeling, and demonstrate target engagement when testing stimulation-based interventions for the treatment of mental disorders.
Keywords: Neuromodulation, Transcranial direct current stimulation (tDCS), Transcranial alternating current stimulation (tACS), Transcranial electrical stimulation (tES), Reproducibility
Introduction
Neuropsychiatric disorders are a leading source of disability that require novel treatments targeting mechanisms of disease. Historically, the predominant focus in psychiatry has been psychopharmacology and psychosocial treatments. As the pathophysiology of mental disorders is poorly understood, clinical trials were often pragmatic comparisons of new therapeutic interventions vs. placebo. By virtue of their design, clinical trials, particularly failed trials, yielded little knowledge of mechanisms of disease. Accordingly, the National Institute of Mental Health (NIMH) recently adopted an experimental medicine approach to clinical trials that uses interventions as probes of specific therapeutic targets and disease mechanisms. NIMH funded trials now require an explicit target, dose optimization, and demonstration of adequate target engagement, as specified in go/no-go criteria, prior to testing clinical efficacy [1]. This phased approach is expected to help validate or invalidate the targets being tested and ensure that both positive and negative trials are scientifically informative.
Disordered circuitry has been increasingly implicated in the pathoetiology of neuropsychiatric disorders. This has increased interest in neurostimulation approaches, given their potential for manipulating circuits directly, either alone or by enhancing the effects of other interventions [2]. Extending the experimental medicine approach to these modalities has raised issues of how best to define and optimize dose, demonstrate target engagement, and achieve rigorous design.
In recent years, a dramatic increase in the number of studies employing transcranial direct current stimulation (tDCS) to alter brain excitability and behavior [3] has stimulated interest in developing therapeutic applications of these techniques. tDCS is of particular interest given its high level of safety and tolerability [4], low cost, and portability. Because of an increased recognition of the involvement of neural oscillations in cognition and behavioral states [5], transcranial alternating current stimulation (tACS) has attracted interest as an approach for manipulating oscillations and synchronizing neural activity underlying cognition [6]. Ensuring the reproducibility and veracity of research findings involving these techniques is essential to their development for therapeutic application [7].
To promote the development of these approaches, NIMH sponsored a workshop “Transcranial Electrical Stimulation (tES): Mechanisms, Technologies and Therapeutic Applications” held on September 29–30, 2016 at the NIH in Bethesda, Maryland. The primary focus was on contemporary forms of low intensity electrical stimulation used in research and clinical applications over the last decade—specifically, tDCS and tACS. The agenda was organized around NIMH’s strategic research priorities which include the development of novel interventions for reducing the burden of mental illness and furthering an understanding of mechanisms through which these interventions impact behavior. An organizing committee comprised of NIMH staff and tES experts was formed and input from program officials in NIMH’s divisions of translational and basic research solicited to develop a list of speakers and major themes for the workshop. Experts in basic and clinical neuroscience, noninvasive brain stimulation technologies, and clinical trials met in a public forum to examine the physiological mechanisms of tDCS/tACS, the technologies and technical strategies for optimizing treatment protocols, and the state of the science with respect to therapeutic applications and trial designs.
Each section of the workshop included several presentations followed by discussion sessions during which issues raised by both speakers and a broad audience were considered. Questions and comments were solicited during the discussion session following each panel from those attending in person and online via publicly accessible video webcast. Following this, each speaker was invited to submit a brief writeup reflecting his/her presentation and its discussion for consolidation into a workshop report. Discussions focused on identifying research gaps, obstacles and opportunities, and establishing rigor and reproducibility. A draft report was reviewed by all authors. Issues raised went back to the larger group of authors in an iterative process until the group reached concurrence on the version that was submitted for publication. This report represents the state-of-the-science in the areas considered, identifies research challenges, and suggests avenues for addressing them.
Physiological mechanisms
The two common modalities of transcranial electrical stimulation (tES) are constant current (tDCS) or charge-balanced, alternating currents (tACS). The mechanisms of actions of these modalities are likely different. tDCS is thought to affect neuronal excitability [7,8] and the main outstanding question is how effects extend beyond the period of stimulation, perhaps via synaptic plasticity. tACS is thought to interact acutely with ongoing oscillatory activity in the brain and the main research question is how the stimulation parameters should be chosen to achieve optimal efficacy and ensure that changes in oscillations persist after stimulation [5,6].
Effects of tDCS on synaptic plasticity
Long term effects of tDCS have often been attributed to synaptic plasticity. A number of human and animal studies provide support for this hypothesis [7,9,10], but the underlying cellular mechanisms have yet to be established. In animal studies, direct current stimulation (DCS) has been shown to modulate long-term potentiation (LTP) and long-term depression (LTD) effects on synaptic efficacy, either by boosting ongoing plasticity (e.g., produced with specific pulsed stimulation protocols) [11,12] or, in some reports, de novo induction of LTP/LTD, even when applied to inactive brain slices (10,13). What is not clear is whether such de novo non-specific effects can explain the apparently specific effects of tDCS reported in behavioral and clinical studies [14]. tDCS can also modulate the efficacy of LTP when applied either concurrent with or before (pre) the specific pulsed stimulation protocols [15–17]. However, in these paradigms where tDCS preceded LTP induction, it is not clear how this priming effect operates. One hypothesis is that tDCS increases slow-acting brain-derived neurotrophic factor (BDNF) release [12,16,17]. The effects on LTP may also be mediated by glial function [18]. Some DCS effects depend on the N-methyl-D-aspartate (NMDA) receptor in the case of LTP [11,12,17,19] and on the metabotropic glutamate (mGlu) receptor in the case of LTD [13]. A similar dependence on NMDA receptor has been found for LTD-like plasticity observed in human studies (e.g., [10,20]) although one should be careful not to over-interpret these similarities. The net effects of stimulation in humans are likely to have more complex causes than what is observed in reduced animal experiments. MR spectroscopy has documented polarity-sensitive effects of tDCS on gamma-aminobutyric acid (GABA) such that anodal tDCS reduces GABA locally, while cathodal stimulation reduces glutamatergic activity [21]. In total, while the involvement of various signaling pathways has been demonstrated, it is not clear exactly how electric stimulation engages synaptic signaling.
One detailed mechanistic hypothesis posits that tDCS polarizes the cellular membrane [22], affecting LTP through the voltage-dependent NMDA channel. Consistent with this, even brief pairing of DCS with concurrent pulsed stimulation protocols can affect LTP and LTD, with the polarity of the effect depending on the specific neuronal compartment and stimulation protocol [11]. Contrary to some previous work (above), here tDCS was not able to act alone, but required plasticity induction. Additional in vitro and in vivo experiments are needed, along with computational modeling to reconcile the apparent conflicts in the current literature and to elaborate detailed mechanistic hypotheses at the cellular and network level. Based on the available data, the effect of tDCS in humans is postulated to be task specific because of the need for activation in the targeted pathway to produce synaptic modulation. Thus, it is expected that the most effective tDCS interventions in humans will be those that pair stimulation with a concurrent adaptation or learning protocol.
Interaction of tACS with ongoing brain rhythms
tACS employs sine-wave stimulation waveforms motivated by the rhythmic structure of endogenous brain activity [5,23–25]. The resulting periodic modulation of the neuronal membrane voltage is hypothesized to synergistically interact with the rhythmic depolarization associated with network oscillations in the brain. Thus, the strongest enhancement of brain rhythms is expected for tACS waveforms that match the frequency of the targeted endogenous oscillation. As a corollary to this presumed mechanism of action, tACS offers a degree of specificity in terms of target engagement by choice of the stimulation frequency that tDCS inherently lacks. Dynamical systems theory provides support for this mechanism of action since it suggests that even weak time-locked periodic stimulation can affect the rhythmic behavior of the targeted system [26]. Indeed, animal model studies support such interaction between weak periodic fields and endogenous oscillations (e.g., [24,25,27]). Specifically, the so-called “Arnold tongue” [23,28,29] predicts that if the frequencies of the endogenous activity and the stimulation input are similar, very low stimulation amplitudes can achieve synchronization of the system with the applied perturbation [5,30]. Many tACS studies are implicitly based on this model by using (individual) peak EEG frequencies as the stimulation frequency. It is noteworthy that the Arnold tongue has yet to be confirmed as the target engagement mechanism of tACS in experimental studies in animal models and human participants [6]. Of note, low-amplitude periodic stimulation can also enhance oscillations at the intrinsic oscillation frequency (in addition or instead of synchronization of the stimulation frequency). This suggests that mechanisms other than the Arnold tongue are involved in shaping target engagement of network oscillations by tACS. Most importantly, the effects of stimulation are state dependent [31,32]; in particular, the presence of a strong endogenous oscillation may alter or even limit the effect of stimulation [31–34]. Furthermore, the timing of firing of individual action potentials and the modulation of rhythms coupled to the targeted oscillation have also been observed in reduced animal preparations [5,29].
Methods and technology
Reproducibility
Reproducibility is critical to research. Several common technical issues can undermine the reproducibility of tDCS effects within and across studies, including: 1) variability in electrode location and placement, 2) inconsistencies in electrode preparation, 3) insufficient operator training, and 4) insufficient protocol reporting. For a comprehensive technical guide to tES, please see Woods et al., 2016 [35]. Examples of reporting sheets for tES have been proposed ([36]; http://www.neurologie.uni-goettingen.de/downloads.html).
Electrode location and placement
Variation in location of electrodes can result in significant differences in where and how much current is delivered to the brain [37–40]. Nitsche and Paulus (2000) demonstrated that differences in electrode placement determined whether or not tDCS affected transcranial magnetic stimulation (TMS)-generated motor-evoked potentials (MEPs) [40]. Numerous modeling studies have demonstrated that electrode placement determines where stimulation occurs with results varying from stimulation of the whole brain (including brain stem and subcortical structures) to more selective stimulation of particular areas of cortex [37–39]. In some cases, as little as 1 cm of change in electrode position significantly altered the distribution of predicted current flow in the brain, as well as the intensity of stimulation in specific brain regions [39]. Thus, careful selection of electrode sites and stable placement of the electrodes throughout the stimulation session is central to reproducibility of tDCS effects [35]. For repeated studies within subjects, careful placement will help maintain consistency of stimulation across time. However, the current delivered to the scalp does not provide sufficient information about the electric fields generated in the brain nor does careful placement of scalp electrodes, e.g., via the 10–20 system, guarantee consistency in the electric fields generated across subjects. (See section on Computational models and tES dose optimization below.)
The proportional International Electrode Placement system [41] provides a quick method for consistent placement of electrodes across different head sizes and shapes and serves as a current standard for placement of recording electrodes on the scalp. This method uses a series of measurements taken from common anatomical locations (e.g., inion, nasion, intraocular notch), applies percentage values of the measured distance between these landmarks (e.g., 5, 10, or 20%), and uses subsequent measurements along a grid to identify specific locations on the head (e.g., F3, F4, etc.). This method can take as little as a few minutes to identify a pair of desired locations on the head.
Once these locations are identified, the electrode assembly must be affixed to the head for delivery of current. For tES using sponge-covered electrodes, elastic straps are the most commonly used head-gear for electrode placement [42]. If these straps are under- or over-tightened, electrodes tend to move over the course of a tDCS session. Thus, the distribution of current delivery can change over the duration of a tDCS session [39]. This directly undermines tDCS replicability. Furthermore, if electrode straps are over-tightened, there is an increased probability of evacuation of saline from the electrode sponges [35] which may affect both efficacy and tolerability [43].
Electrode preparation
Saline is the most commonly used conducting contact medium (electrolyte) for delivering current to the scalp through an electrode, typically a sponge-based electrode. Oversaturation of the sponge, one of the most common mistakes in tES electrode preparation, significantly undermines the reproducibility of tES application and effects [35]. When sponges are over-saturated, saline is evacuated from the sponge and covers an area of the scalp outside of the electrode-sponge surface area. Rather than delivering current through a specified surface area on the scalp under the electrode (e.g., 5 × 5 cm), the area of current delivery now encompasses the entire area of the scalp that is covered in saline. This creates an unreproducible amorphous area of current delivery within and between subjects. This can be avoided with careful measurement and application of saline using a plastic disposable syringe with mL/cc measurements present on the syringe. Using this method, an exact amount of saline can be delivered to the electrode sponge and calibrated to optimize impedance but avoid evacuation of saline from the sponge. This exact measure of saline should be reported in manuscripts to improve reproducibility across laboratories [35]. Attention to the headgear used (e.g., designed for tES rather than ad hoc straps) also helps control this phenomenon. The use of a thick electrode conductance paste (e.g., Ten20 paste) applied directly to the biocarbon electrodes is an alternative preparation approach that avoids issues associated with saline and oversaturation. Thickness of the application of paste should be sufficient to not allow the electrode to directly contact the skin, which could result in skin burns. Impedance levels ≤1 kOhm can be obtained consistently and maintained over several hours. However, unlike saline, paste must be placed on the skin approximately ½ hour prior to stimulation delivery, as paste requires a longer period of time to saturate the skin and reach appropriately low impedance levels. Practically, High Definition (HD) approaches have been shown to offer comparable or superior tolerability [44–47] with unique features for sham control [48]. With regard to reproducibility, issues such as position-drift, saline-leak, and atypical skin irritation [35,39] can also be mitigated by precise positioning of and use of gel with HD electrodes in specialized caps equipped with electrode holders. Ultimately, selection of contact medium and electrode type (sponge-encased vs. HD) depends on the desired goals and treatment targets of the study or trial, as well as the design limitations inherent within a given application. Regardless, the approaches described above provide important considerations for rigorous electrode preparation.
Operator training
Although tES is, in principle, a simple technique and the operation of the device is relatively easy, developing skills to administer tES requires comprehensive, multiple-step training. As tES has not yet been integrated into routine medical practice, it is not included in medical graduate or postgraduate education. Well-trained tES personnel should be proficient in the following aspects of tES application i) the theoretical background of tES, ii) principles and rationale of tES use in specific populations, iii) dose, target, and stimulation protocol determination, iv) selection of subjects, v) safety evidence and safety precautions pertaining to tES delivery, vi) preparation and positioning of the electrodes, preparation and operation of the tES unit, vii) outcome monitoring and recording, including recording and reporting adverse events. Exposing subjects to tES delivered by personnel lacking sufficient practice and training would not be in keeping with best practices and may significantly hinder replicability [4,35].
Protocol reporting
Insufficient reporting of protocol parameters and procedures in the methods of published tES studies is unfortunately common, reducing the potential for study replication. For studies to be reproducible across labs, authors must report, at a minimum, key features of dose [49] and electrode preparation: number of electrodes, location of electrodes and method of placement, electrode size, contact medium type, amount of contact medium applied, duration of stimulation, intensity of stimulation, stimulation frequency/waveform, current ramp up/down period, subject’s activity during stimulation (engaged in activity vs. at rest), and the timing of outcome assessment relative to tES [35].
Masking
Masking (aka ‘blinding’) refers to the techniques used to keep participants and study personnel unaware of the intervention administered. Masking is critical for avoiding observer bias and resultant exaggeration of treatment effects. Just as placebo-controlled trials are fundamental for proving drug effectiveness in pharmacological research, masking both experimenters and subjects to tES condition is important for establishing study validity and preventing false positive conclusions regarding the efficacy of tES. For tDCS/tACS interventions, placebo control generally consists of sham stimulation in which an electrical current that can be felt is applied (ramping up and down) at the beginning of a session. Due to sensory adaptation and other unknown factors, this approach is thought to be effective in maintaining the mask since participants may be less likely to distinguish the active treatment from a sham condition in which no current is delivered and no attempts at mimicking scalp sensations are present.
A review was conducted to determine the frequency with which masking is reported in the tDCS intervention literature. Relying upon reporting guidelines available through the Enhancing the Quality and Transparency Of Health Research (EQUATOR) Network, the Cochrane Collaboration tool for assessing Risk Of Bias [50], and guidelines from the tDCS community (e.g., [35,51,52]), the review focused on the following: utilization of a sham or other control condition and masking of participants, tDCS administrators, assessors, and raters. Binary coding (reported = 1, not reported = 0) was used, and when a study reported upon one of the areas of interest, details were recorded to allow for adequate description.
Of the 206 articles (published at the time of this submission) reviewed, 84% (N = 173) reported using a sham or other masking condition. Of those 173 articles, 84% reported use of the approach suggested by Gandiga, Hummel, & Cohen [53] - an initial brief presentation of the experimental current, though occurrence and parameters for ramping were inconsistently reported. Other approaches involved different combinations of duration (initial, partial, full, intermittent), current level, opposite polarity, and/or off-target locations or were not specified. Administrator-level masking was reported for 39% of this study subset via device characteristics (e.g., built-in sham capability) or low-/no-tech approaches (e.g., covering device screen). Effectiveness of these masking approaches was assessed in 25% and 1.2% of studies at the participant- and administrator-level, respectively. There was minimal reporting of masking of assessors (8%) and raters (3.4%). Finally, despite repeated recommendations in the tDCS community to record sensations and adverse events (AEs), only 33% of the studies reviewed reported collection of these variables.
There remains inconsistency in the protocols used for the sham arm including the use of one ramp up-down (e.g., 10–30 s linear current ramp to the target intensity immediately followed by a 10–30 s linear current ramp down) at the start of stimulation, two ramp up-downs at the start and end, or ramp up-downs randomized during the session [54–57]. The rate of ramp slope, peak ramp value, and current used during the sham off-phase (which cannot be zero if impedance is monitored and in some cases, is intentionally not minimized) should be reported and carefully considered. Design and preparation of electrodes (see above) determines sensation in the active and sham arms such that control and reporting electrode details is needed for reproducibility of trial outcomes.
We recommend the use of a masking checklist in study design, reporting, and assessment of study validity. The checklist should include the following items: rationale for and description of sham condition; participant characteristics relevant to sham effectiveness (i.e., naive/experienced, old/young); description of masking procedures for participants, administrators, assessors, and raters; and procedures for monitoring masking/unmasking, followed by a report of when and for whom unmasking occurred and why. We reiterate various EQUATOR recommendations and discourage authors from using the uninformative terms “single blind” and “double blind” without providing details about which individuals were masked and how the masking was implemented. Given the limited effectiveness of sham conditions at higher currents delivered through single electrodes, further encouraged is the development of sham and experimental conditions that leverage high-definition (HD)-tDCS capabilities, where the smaller electrodes may reduce sensations and activate overall fewer receptive fields (e.g., [58,59]), and reducing intensity delivered through these electrodes corresponds to reductions in sensations [48]. Modeling demonstrates that total current can be delivered across functional sets [48,56,60,61], still delivering the intended current to brain areas of interest while effectively reducing the voltage through a single electrode and resultant scalp sensations. While it may not be possible or feasible to incorporate all recommendations, this review suggests that masking methods can be improved substantially and that the reporting of masking efforts should increase in information and precision. It should be noted that this is particularly critical in single-session, crossover designs where the same subject will be exposed to both active and sham stimulations [54,62].
Computational models and tES dose optimization
Computational models of Tes—including tDCS, tACS, and elec-troconvulsive therapy (ECT), —can help to address two key issues for rigor and reproducibility, namely spatial targeting and individualization of dosing. Regarding spatial targeting of specific brain regions, tES is often rationalized based on modulating the activity of a specific brain region implicated in the illness, with the assumption that stimulating this brain region will bring about desired benefits. A majority of tES studies approach this challenge by placing a large (compared to the brain region) electrode on a scalp location broadly “over” the brain target. The second issue aided by computational models is the individualization of electrode placement. A majority of tDCS/tACS do not vary stimulation dose with the subject/patient, which may result in varied target modulation [63]. Electroconvulsive therapy (ECT) typically individualizes dosage by varying the duration and frequency of the stimulus train. However, the ECT pulse current amplitude and pulse width—key determinants of the induced stimulation strength in the brain—remain fixed across individuals. The fixed stimulus current amplitude results in differential dosing in the brain, potentially contributing to variability in outcome [64]. Without consistent modulation of clinical targets, the efficacy and reproducibility of tES trials may be suboptimal.
The strategies for addressing these limitations are both doctrinal and practical. The continued use of large electrodes placed on opposite sides of the head, which may result in current flow through extensive volumes of the cortex and deep brain [65,66] is encouraged by experience (e.g., positive outcomes from prior trials) and the simplicity of using two-electrode devices (e.g. sponges positioned with rubber straps for tDCS, or large steel disc electrodes for ECT [35]). Relatively few studies adopt High-Definition (HD) montages wherein arrays of smaller electrodes can steer current during tDCS [46,67–70] and tACS [71,72] for presumed increased focality [73]. Even with two large electrodes, there is significant sophistication in the use and optimization of approaches using two large electrodes either to intentionally engage a broad network [74,75] or maximally stimulate a given brain region without necessarily optimized focality [76–80]. Nonetheless, computational models are important to rationalize and quantify the stated hypothesis of a tES trial. Given this ubiquitous need, access to robust and simple-to-use modeling software, including software that can automatically process imaging data in a manner that is suited for current flow modeling, represents a gap, in contrast to the ready availability of conventional image segmentation tools.
Over a decade, significant progress has been made in translating computational models to practice [81,82]. With regard to model validation, numerous studies [73,83–85] have confirmed the general model predictions illustrated in Fig. 1–that large electrodes produce diffuse current flow, while small electrode arrays may yield categorical increases in focality. Notably, intracranial recordings in humans demonstrate that models are fairly accurate in predicting distribution of electric fields across the brain (with correlation of predicted and measured fields around r = 0.81) [86]. Neurophysiological studies have also confirmed that individual differences can be predicted and controlled through the use of models [73]. In pediatric studies, computational models have suggested a need for reduced stimulation intensity [38,87]. Computational models have been used to design montages to direct current flow through lesioned brains following stroke [60]. Ongoing efforts to increase access to computational models include basic graphical-user interfaces (GUI) [88], packaged engineering tools [89,90], the development of standards [91], and importantly, algorithms that will reduce the computational burden [78] and automate image processing for individual electric field modeling [92,93].
Fig. 1. Common tDCS/tACS montages and corresponding simulated electric field distribution.
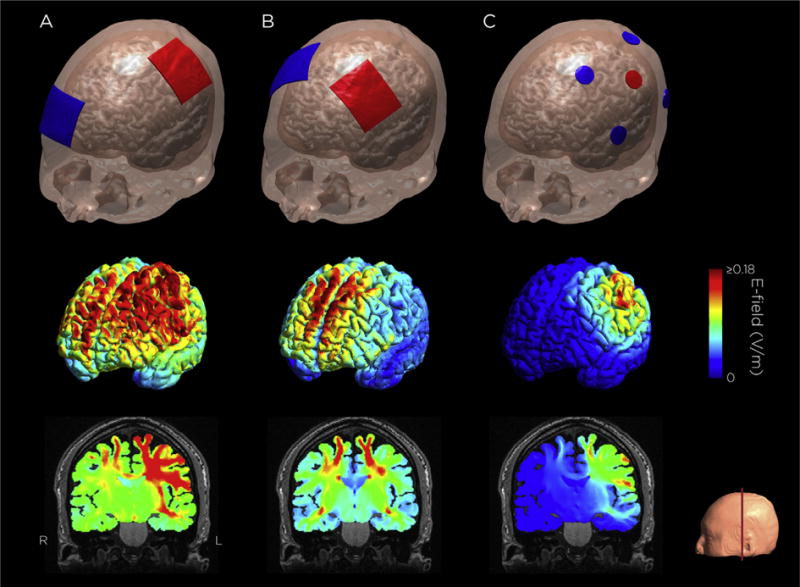
A. M1-SO configuration: Sponge electrodes, one over left primary motor cortex, one over the contralateral supraorbital ridge. B. Bilateral dorsolateral prefrontal cortex configuration: Sponge electrodes over the F3 and F4 EEG sites. C. 4 × 1 HD-tDCS M1 configuration: High-definition electrodes, one over M1, four return electrodes surrounding the center electrode. The electric field was simulated with a current amplitude of 1 mA. Electric field simulation was performed using SimNIBS 2.0.1 [191].
There are straightforward strategies for addressing remaining gaps in translating computational models into practice: educate the scientific community (e.g., journal and grant reviewers) regarding the role of computational models in hypothesis-driven tES research, support initiatives to create new tools, and promote the use of enhanced methodology. Failure to leverage computational models in tES research for pragmatic reasons can be addressed by providing and enhancing access to easy-to-use computational models that can design individualized and optimized montages for a given target region. Continued use of ad hoc electrode montages can be justified, for example, based on prior empirical success with a given montage, but claims that prior outcomes reflect modulation of a specific brain region may be hard to justify. “Functional targeting” [14] allows for modulation of an active network without targeted brain current flow, but the selection of stimulation dose should always be rationalized. Additional important innovations relate to computational neurostimulation, where models of current flow are linked to neuronal and, ultimately, behavioral models [94,95], and new algorithms link neurophysiological data with stimulation strategies (e.g., EEG-guided tES) [91,96–98]. Rising concerns about rigor and reproducibility render the adoption of computational models imperative, supporting consideration of when the use of conventional pad or HD montages are appropriate. Uninformed and misguided electric fields are one of the many possible causes of variability in tDCS/tACS research [99–103] that can be readily constrained with the use of computational models. Importantly, recognizing that computational models are an evolving tool to support rational hypothesis-driven experimentation (not ends in themselves) makes these models pivotal in enhancing the rigor and reproducibility of tES research.
Important questions remain about the utility of models either for montage design for a trial or individualizing current per subject [104]; however, these unknowns are not an excuse to not use models to the extent practical. For example, an important challenge is relating regional brain current flow with resulting changes in neuronal information processing and ultimately behavior. Efforts to bridge dose to behavior, also called computational neurostimulation, are ongoing. At the moment, the (implicit) assumption across applications using models is that brain regions respond in a monotonic/linear fashion with local current flow (electric field) intensity [105], such that increasing current delivered to a given brain region increases efficacy regardless of brain state and disregarding connectivity with other brain regions. Although this assumption is increasingly challenged by dose-response studies [106,107], at a more basic level one can assume brain regions receiving little current flow are spared direct effects of stimulation. For all these open questions on how to leverage models, they remain readily accessible and useful tools to support hypothesis-driven trials and indeed address questions on dose-response.
Remotely-supervised tDCS: at-home use for clinical trials
A growing number of potential clinical applications of tDCS are under investigation. To guide optimal clinical use, trials with repeated administration over multiple sessions are needed to understand tDCS behavioral effects. To enable trial designs with larger sample sizes and extended treatment sessions, a protocol for remotely-supervised or “RS” tDCS administration has been developed to meet pre-established guidelines for home use [108]. This RS-tDCS protocol [109] provides treatment to participants at home using real-time monitoring through videoconferencing. Procedures include baseline screening and tolerabilty testing, followed by training in device operation. Participants are then sent home with study equipment for remote operation. Headgear is designed for easy and uniform placement (currently, dorsolateral prefrontal cortex montage) with markers to guide consistent electrode location custom-designed for self-administration. tDCS devices are pre-programmed to deliver a preset “session” of a specific current “dose” (or sham), activated with a one-time use code that is provided by the study technician. Extensive safety and stop criteria are followed to prevent any adverse events or misuse, and safety and tolerability are measured before, during, and after each session. Stimulation can be paired with tele-rehabilitation such as cognitive remediation via computer or other cognitive or physical exercises. Discontinuation criteria include the experience of pain or adverse events above a predefined intensity (e.g., seven out of 10) at any point.
The RS-tDCS protocol has been validated for use in individuals with mutliple sclerosis (MS) [110] and Parkinson’s disease (PD) across a wide range of ages (18–73 years) and levels of neurologic disability, incuding those who are wheelchair-dependent, and with the use of a caregiver-proxy for headset placement and device operation. In total, 624 sessions have been completed using the RS-tDCS protocol. No session has been discontinued. Across studies, three participants have been discontinued and one has voluntarily withdrawn from the study resulting in an overall completion rate of 93%. The RS-tDCS protocol is safe and tolerable in both MS and PD participants, at both 1.5 and 2.0 mA stimulation intensity, and including sham. The most common side effects reported are skin tingling and itching.
A challenge to the uniformity of the set-up and reliance on self-placement is the potential for slight variance in electrode location across individuals. Further precision for electrode placement within individuals across uses is also needed to ensure reproducible behavioral effects. In addition, neuroimaging-based modeling of current flow is important to inform further headset design and checks to guide ensure location accuracy across individuals.
Remote supervision may be appropriate for clinical study of tDCS across central nervous system disorders for varying symptoms, as well as for pairing with telerehabilitation. It allows for a larger number of tDCS treatments to be administered in a study and offers overall scalability to answer key questions concerning appropriate and effective use. Future clinical trials may utilize this approach to increase the rate of recruitment with faster trial completion. Adapting the RS-tDCS protocol for use across a range of conditions (e.g., different montages, alternate activities during or following stimulation) will be important.
Neuroimaging in neuromodulation studies
Functional neuroimaging can be used to enhance the effectiveness of stimulation and to gain new information useful for inferring its mechanisms of action, both of which are needed to enhance rigor and reproducibility in tES research. As described below, the effectiveness of stimulation has been enhanced by identifying candidate regions and networks that are involved with specific behavioral effects and targeting these areas with tES. It has also been suggested that imaging may be useful for addressing individual differences in brain anatomy and function. Various neuroimaging methods exist that can be used to examine hemo-dynamic, electromagnetic or neurochemical changes associated with neurostimulation at different levels of spatial and temporal precision.
Electrical activity can be measured using electroencephalography (EEG) and magnetic activity measured using magnetoen-cephalography (MEG). Both are direct measures of brain activity with sub-millisecond temporal resolution [111]. As some examples, EEG has been used to assess changes in neural activity during the administration of tDCS [112] and following administration of tDCS [33,103]. tDCS modified the strength of specific event-related potential (ERP) components, suggesting a change in neuro-cognitive responses to stimuli. More in-depth comparison is needed to understand the relationship between tES effects, changes in ERPs, and related changes in cognition.
Successful measurement of brain activity with EEG during the application of tACS is a contentious subject with widely different opinions. The main problem is that the signal of interest, the brain-derived electric field measured by the EEG, is orders of magnitude smaller than electrical artifact resulting from the stimulation. Algorithms of various complexity have been devised and successfully tested in simulations, head phantoms, and different human data-sets [1]. Yet, none of these approaches can directly prove that the artifact and only the artifact is removed by this process, since the ground truth is inherently unknown. Some recent studies argue that successful artifact removal is not feasible with the current methods due to nonlinearities introduced by the stimulation hardware and other biological processes such as the heartbeat [2]. Final resolution of these conflicting perspectives has not yet been reached.
Other studies have recorded EEG before and after the administration of tDCS [35,113], avoiding the potential problem of artifacts induced by simultaneous tES and EEG. Findings include that stimulation of the medial frontal cortex modulates EEG indices of error monitoring [114] and that tDCS can modulate slow EEG activity (<3 Hz) [115]. EEG has also been used to optimize tDCS protocols, such as electrode placement for tinnitus [116] and for matching individual alpha frequencies with tACS [117]. MEG has been used to localize tDCS effects [118] and to show changes in network activation during rest [119] and task [120] and changes in EEG frequency during tACS [121]. The combination of transcranial magnetic stimulation (TMS) with EEG has been utilized to probe immediate and long-term effects of tDCS on TMS-evoked potentials (TEPs) and brain oscillations. The TMS-EEG approach can be used to shed light on the neurophysiological processes underlying behavioral changes induced by tDCS [122].
Magnetic resonance imaging (MRI) and positron emission tomography (PET) can provide information on structural, hemodynamic and chemical changes associated with stimulation. For example, tDCS over motor cortex has been found to alter fractional anisotropy (FA) [123], which correlates with scores of motor function. Resting-state functional MRI (fMRI) in schizophrenia patients receiving tDCS showed reduced connectivity of the left temporoparietal junction and the left anterior insula that correlated with reductions in hallucinations [124]. A series of studies [125–127] used results from fMRI to predict the effects of tDCS. These studies identified the magnitude of change in BOLD fMRI responses associated with learning to detect target objects in complex images and then applied anodal or cathodal tDCS to regions showing the greater changes in a separate group of participants. It was found that applying anodal tDCS to brain regions that showed an increase in BOLD fMRI response after training led to an acceleration of learning, while targeting regions that reduced their response or showed no significant changes had no effect relative to sham control stimulation. Conversely, applying cathodal tDCS over regions that showed a significantly reduced BOLD response after training also accelerated learning on this task. When taken together, these studies suggest that changes in BOLD fMRI associated with learning may be useful in optimizing protocols to enhance tDCS effects on learning rate and guiding electrode placements to accelerate learning. FMRI has also been used to show changes in the stimulated region concurrently with tDCS [128–130]. Magnetic resonance spectroscopy (MRS) with tDCS has been performed [21,131–133] and demonstrated a variety of neurochemical effects using different tDCS protocols. Such neurochemical changes were also correlated with changes in network connectivity when tES was performed in between imaging sessions [134].
Another means by which neuroimaging could be made useful for enhancing the effects of tDCS is by using imaging as indicators of target engagement [1,2,30]. Examples of this include imaging changes in EEG [30] and event related potentials [1] induced by tES that are associated with specific cognitive effects. Another example are newly-described methods using MRI to image current flow induced by tES [2,3]. Given that noise and other issues inherent in these methods can be overcome, these methods may be useful for quantifying the magnitude of field effects in specific anatomical regions.
While methods for applying neuroimaging to benefit stimulation such as quantifying target engagement are still being developed, this should not be used as a reason to avoid their combined use at present. Uncertainty regarding the relationship between neuroimaging measures and specific neural or cognitive processes are present to some extent for all neuroimaging studies. Even given this uncertainty, neuroimaging is useful for gaining a more complete understanding of the effects of stimulation on neurocognitive processes. There are a variety of ways that neuroimaging can be applied to examine the effects and mechanisms of tES and other forms of neurostimulation. Ultimately this could lead to safer and more effective treatments for mental illnesses.
Clinical trial design and implementation
TES has been applied at rest (without engagement in a behavioral task) as a monotherapy, during tasks to augment performance, and combined with behavioral therapies. In addition, patients may be on concurrent pharmacotherapies whose effects on tDCS are not known. The first approach has been applied primarily in depression, following the establishment of repetitive transcranial magnetic stimulation (rTMS) as a therapy for treatment-resistant depression, also generally applied at rest. The lessons learned from the considerable work in depression apply broadly to trials focused on other conditions, as well as to applications of tDCS in conjunction with tasks and other therapies.
tDCS depression trials: design considerations
Neuroimaging studies have identified altered activity in brain networks in major depressive disorder that are linked to key nodes in prefrontal cortex [135]. A growing body of evidence suggests that tDCS with the anode applied over the left dorsolateral prefrontal cortex has antidepressant effects, but the overall effect size from randomized controlled trials to date is small to moderate, with variable findings across studies [136]. While small sample sizes partly account for this variability, other important contributory factors, which should be closely examined to more accurately gauge the efficacy of tDCS and improve the treatment approach, are patient variability, differences in tDCS treatment methods, and differences in clinical trial design.
Patients have typically been selected for trials based on a DSM-defined diagnosis such as major depressive disorder. While this confers a structured evaluation of the illness being treated, the DSM diagnostic categories, based on clusters of symptoms, are heterogeneous, encompassing a mix of phenotypes (e.g., for depression–melancholic, psychotic, anxious) and genotypes. Precise characterization of the individual patients and/or selection of more biologically homogeneous samples would likely reduce the variability of treatment response. For example, tDCS response may be contingent on biological factors such as inflammatory status, level of neuroplasticity, genetic risk (evaluated by family history or even genotype) or, as recently found in depression, pre-treatment frontal-dependent neuropsychological function [137]. Failure to account for these factors may obscure the overall treatment effect of tDCS. Adopting a standardized approach to patient evaluation across different centers would facilitate meta-analyses based on individual patient data, allowing for more precise understanding of the efficacy of tDCS in different subtypes of depression or other disorders, identification of those patients most likely to respond, and, perhaps, customizing the tDCS treatment approach to the individual patient.
Lastly, trial design and methodology are important factors which can result in apparent contradictions in findings between studies. Several trial designs can be employed to verify tDCS effects. Open label (uncontrolled) trials are often employed in pilot studies to test the effects of novel tDCS montages [138] or effects in different patient populations (e.g., bipolar depression) [139,140]. Controlled trials usually employ a parallel [54] or cross-over [141] design to compare active vs. sham tDCS. Although cross-over designs are more efficient than parallel designs, they risk carry-over effects from the active to sham condition during the trial, as well as the risk of unmasking, and, therefore, this design should be used with caution.
Other designs permit combining tDCS with another pharmacological or non-pharmacological intervention. In a factorial trial, for instance, tDCS can be compared or combined with a pharmacological treatment [142]. Finally, a non-inferiority trial is the preferred design for determining whether tDCS is at least as efficacious as a standard pharmacological intervention with respect to a specified endpoint [62]. For designs comparing tDCS vs. drugs, it is crucial to use a double-dummy approach, i.e., participants should receive both active interventions along with an appropriate placebo for each (e.g., drug and placebo, tDCS and sham) to maintain masking. In fact, a recent non-inferiority trial using this approach [143] showed that tDCS was not non-inferior to the antidepressant drug escitalopram. Secondary analyses demonstrated that escitalopram was superior to tDCS and placebo and tDCS was superior to placebo. This reflects the clinical importance of comparing tDCS not only to a placebo but also to an active comparator.
Attrition, the premature discontinuation of participation in a trial, is an important issue in tDCS clinical trials, as subjects may need to return daily to the research setting to receive tDCS. Attrition can be minimized by using flexible schedules and conceding a few missed visits, which can be replaced after the treatment acute phase [144]. A “run in” period can also be employed. In this approach, participants receive a short period (one to two weeks) of sham stimulation before the trial onset. This allows placebo-responders and non-adherent participants to be excluded. However, the run-in approach also has some disadvantages, such as deception (participants do not know they will receive placebo before trial onset) and higher costs. Statistical approaches for handling attrition include “per-protocol” (PP) and intention-to-treat (ITT) analyses. Other approaches are “modified ITT” that include in the analyses only participants who complete a predetermined number of sessions and/or the first post-baseline assessment or those with no more than one missing, incomplete or rescheduled visit, as used in pivotal rTMS trials (e.g., [145]).
Clinical outcomes are usually measured with standard rating scales such as, in the case of depression, the Hamilton (HDRS) and the Montgomery-Åsberg scales (MADRS). Clinical response is defined as a ≥50% improvement from baseline to endpoint; although remission definition has varied across studies [136]. To ensure standardization across depression studies, cut-off points of ≤10 or ≤7 for MADRS and HDRS scales, respectively, are recommended. Safety outcomes include acceptability (number of dropouts) [146] and presence of treatment-emergent mania (preferentially assessed by an accepted clinical scale) [32]. Definitions of treatment response will vary for other conditions, and thus standardization or reporting outcomes as continuous or quantitative may provide data with which to better understand and improve treatment effects.
Another consideration is that there is growing evidence that tDCS effects may take several weeks to fully manifest, as seen with other treatments for depression. In some previous randomized trials for depression, tDCS had significantly greater efficacy over placebo only several weeks after the acute treatment phase, with null or modest effects immediately after this phase [142,147]. Use of the end of treatment or follow up score as the primary endpoint may also account for some of the discrepancy between meta-analyses which did [134,148] and did not [149,150] show efficacy of tDCS. Moreover, meta-analyses of depression scores immediately after the end of stimulation sessions did not show any efficacy of tDCS [151,152], in contrast to those that evaluated them at the study endpoint [136,153]. Furthermore, the placebo effects might be greater in the initial study phase, when patients return to the clinic and interact with the staff daily. Thus, future tDCS trials should include clinical assessments in the post-acute treatment phase to enhance the detection of a tDCS versus placebo signal and to test the durability and time course of treatment response.
Thus, it is recommended that further trials give careful attention to patient and illness characterization, evaluation of biological substrates which have been implicated in depressive pathophysiology, stimulation parameters, and overall treatment approach, as well as clinical trial methods, in both the design and reporting of trials. Greater precision and consistency across researchers in these aspects of methodology will enable the field to move beyond the first phase of mostly small trials which overall indicate a positive signal to fully exploring the potential of tDCS as an antidepressant treatment.
tDCS augmentation trials
Whereas experimental and therapeutic non-invasive neuromodulation of the brain has historically been given at rest (without the brain engaged in a goal-oriented manner), there has been an increasing shift toward applying tDCS in conjunction with a task or as a supplement to a behavioral therapy in order to augment learning or the effects of the behavioral therapy. The distinction here is that the use of a task provides a specific learning or behavioral performance context, but is not in itself a therapy. Administrating tDCS in conjunction with a task is a strategy that can be used to probe effects on relevant circuits, identify neurophysiological correlates of behavioral effects, and identify target engagement measures and biomarkers. Combining tDCS with a therapy seeks to enhance the benefits of learning-based therapies, e.g., cognitive, motor.
Task-based studies
tDCS has the potential to increase cortical plasticity [12], which, in turn, may improve learning and the ability of patients [154] to benefit from targeted remediation approaches. As noted above under Mechanisms, the effects of tDCS in humans may be task specific because of the requirement for activation of the targeted pathway to produce synaptic modulation. However, generalization to untrained tasks could occur [155] and requires future investigation. Thus, the most specific and effective tDCS interventions in humans may be those that pair stimulation with a concurrent learning task. Use of a task in conjunction with stimulation allows one to assess the effects on behavior and learning [156]. Neuromodulatory effects seen on learning tasks may help formulate clinically relevant hypotheses designed to enhance training-based neurorehabilitation. This paradigm also provides a basis for identifying objective neurophysiological/neuroimaging correlates of behavioral effects, thus facilitating the identification of mechanisms, biomarkers, and target engagement measures in general and for clinical trials in particular, and may ultimately provide a basis for optimizing protocols and improving treatments.
Studies of motor learning provide an exemplar of such an approach. The serial reaction time task (SRTT) is a classic paradigm involving the learning of complex motor sequences, which has been used to study mechanisms of motor learning and one in which the behavioral effects of tDCS have been well characterized [157]. This task is known to be affected by tDCS, albeit with mild to moderate effect sizes [158]. The motor system physiological signatures known as the Bereitschaftspotential and Motor Potential [159] are well characterized in the EEG. The study of the power changes of EEG oscillatory activity associated with motor activity has also characterized these components in the frequency domain with clearly observable signatures within the 12 Hz–24 Hz frequency band. A variant of SRTT in which the participant is required to follow a series of visually-cued key presses [160] necessitates the cooperative engagement of the motor and visual systems. As such, this variant allows investigation of the functional interactions between motor cortex, the supplementary motor area (SMA), and visual regions. This, in turn, provides the opportunity to study how stimulation of one task-relevant cortical region might modulate the activity of another functionally engaged region and their dynamic interaction.
The SRTT has documented limitations as a behavioral model of motor learning that have been addressed using more sophisticated tasks like visuomotor learning. Limitations of task-based approaches used in clinical trials might include insufficient use of double-blind designs (only 25 out of 60 published studies of tDCS effects on motor learning in healthy adults in a recent review utilized double-blind designs) [161] and failure to include positive controls (i.e., active stimulation of control cortical regions).
Other strengths of such task-based paradigms are that they also provide a platform for elucidating the differential neural substrates underlying different forms of learning (i.e., use-dependent, error-based, reinforcement, strategic learning) [149]. Such paradigms also allow for the investigation of potential selective influences of tDCS on specific stages of learning (online, offline, retention, consolidation, reconsolidation [150]). They also provide a platform to investigate, at a cortical network-level interaction, if implementation of multifocal tES would provide specific beneficial effects, and if so, how. Understanding which specific stages of learning are affected will help determine when to assess learning/behavioral outcomes and ultimately when to assess clinical effects [148]. Thus, an improved understanding of motor (or other) learning processes and the tasks used to assess them, as well as generalization to untrained tasks, is critical to determining whether tDCS can or cannot modulate learning [162] in daily living in healthy subjects or patient populations. Establishing predictive links from physiological markers to behavioral markers and ultimately to clinical effects may allow early signals to serve as surrogates.
Combined, multimodal therapies
Combined therapies are defined here as those in which a behavioral intervention (i.e., not drug therapy, surgery, or other neuromodulation intervention) is the principal therapy that when combined with a second therapy (i.e., an established tDCS protocol [4]) is expected to augment its effects. The behavioral intervention’s practice of goal-directed, repetitive behavior, known to endogenously activate functional neural circuits over time, leads to sustained behavioral improvement or symptom reduction, putatively augmented by the second therapy (e.g., tDCS), which typically has transient modest effects alone. The logic is ill-defined in the literature; however, the rationale appears to be that adaptive behavioral consequences and reduced symptoms from each intervention alone will be synergistic when combined and thus provide a stronger clinical effect [163]. There is some momentum with this combined approach, indicated by an increasing number of registered clinical trials2 and published studies where brain stimulation is intentionally given with temporal proximity to a behavioral therapy, such as cognitive-behavioral therapy (CBT) in depression [164], working memory training in schizophrenia [165], cognitive training in Alzheimer’s disease [166], speech/language therapy in post-stroke aphasia [167], and physical therapies in post-stroke hemiparesis [168–171].
The notion of a simple additive effect is challenged by a number of studies indicating an interaction effect when non-invasive neuromodulation (tDCS/rTMS) is followed, preceded or concurrent with brain activation through volition or a separate neuromodulation protocol. For example, Siebner and colleagues showed that excitatory tDCS priming (anode over M1) of 1Hz rTMS, reduced MEP amplitude relative to baseline, while sham tDCS prior to 1Hz rTMS showed no change in MEP amplitude. Conversely, inhibitory tDCS priming (cathode over M1) reversed the effect, with an MEP amplitude increase post-1Hz rTMS [172]. Giacobbe et al. reported that tDCS can modify a motor practice effect (in hemiparesis) only when preceding (not when given concurrently) and that the effect was not magnified or reduced, but rather the practice effect was transformed to a different clinically relevant effect than occurred with practice alone [173]. Lezzi and colleagues [174] showed that priming neuromodulation with voluntary muscle activity can reverse the effects of both inhibitory and excitatory theta-burst stimulation. These studies are examples of diverse lines of evidence pointing toward interaction effects of cortical neuro-modulation with synaptic cortical activity subserving behavior, whereby both physiological and behavioral data show effect modification. This is not an exhaustive account of the literature in the area, but raises the possibility that interaction effects of combined therapies could be accessed for superior clinical benefit, such as greater magnitude effect, more sustained effect, or need for fewer treatment sessions, and thus provides an exciting and worthwhile pursuit of optimization. These examples also indicate that effects are not always predictable. Depending on the circumstances, the observed effects could be increased, decreased, unchanged or transformed. The example of Giacobbe et al. [173] also illustrates that change may occur in unpredicted variables and may or not be clinically advantageous. Thus, sampling a range of clinically relevant variables would be important in systematic optimization trials.
In order to systematically approach the scientific evaluation of combination therapies, the working space should be defined. This would include well-defined stimulation parameters [49], a well-defined and reproducible behavioral intervention, and a well-characterized and (ideally) homogenous patient group. The details of the relationship of neuromodulation to the behavioral therapy should also be considered in the experimental design and reported [175]. Given that inter-individual variability in response to neuromodulation is a clear issue [176,177], it is possible that mean group differences may not show an effect, but that careful patient characterization may identify predictors (e.g. genotype, clinical history, prior neuromodulation exposure, brain-state, clinical status), and a host of currently unknown features that will become evident with more study. It is also becoming clearer that approaches and results derived from the healthy brain may not translate to disease states.
Despite knowledge of poor tDCS targeting to date, uncontrolled environmental and state-dependency factors, and a limited understanding of all the sources of individual differences, available evidence indicates some effectiveness for tDCS (for reviews in Neurology; https://paperpile.com/c/QniLBm/QanO Ubpw [178,179]) and Psychiatry [180]. While there are likely insufficient data available for reasonable meta-analyses of combined therapies or an evaluation of the relative merits of combined interventions versus those employing independent treatments, the early studies of combined therapies look promising.
Future aims include reduced publication bias, publication of negative results of well-designed studies, reproduction of study findings where possible, mechanistic studies and rationale based on underlying circuitry abnormality, use of imaging and computational models to select the optimal targets, and investigations of treatment response per disease state and conditions. With further refinements, goals are (1) to establish predictive biomarkers of treatment response such that prescriptive treatment algorithms can be developed and (2) to optimize protocols for greater individual and more consistent effects (less inter-individual variability). As the effects of tDCS are harnessed to augment behavioral therapies, vigilance in monitoring, interpreting, and reporting potential maladaptive plasticity effects (e.g., migraine, dystonia, spasticity) is needed in addition to general adverse event reporting.
tACS trials: targeting brain oscillations
In contrast to the case of tDCS, only a few clinical trials studying tACS have been performed. The rationale for the use of tACS to achieve therapeutic benefits derives from the growing understanding of how specific changes in (cortical) network oscillations relate to disorders such as schizophrenia and depression [181]. The vast literature of electroencephalography (EEG) and, to a smaller extent, also magnetoencephalography (MEG) delineate treatment targets, where “target” is defined as a specific temporal activity structure, commonly a change in oscillatory power or frequency at a specific location or a change in functional interaction between two sites. tACS therefore may have the potential to transform our body of knowledge about brain dynamics in disease states into an actionable map of treatment targets. Yet, it is not known if and how pathologically altered networks respond to stimulation, since the study of mechanism has almost exclusively focused on “intact” networks and healthy control participants.
As with tDCS, for tACS to become a clinically useful therapy, it needs to induce sustained changes. Likely, some type of treatment schedule with multiple treatments and perhaps additional maintenance sessions may be required. Despite some evidence for outlasting effects of tACS on the order of magnitude of minutes and hours [182], longer-lasting changes have yet to be studied. For the case of tACS to directly target cortical networks, no results are available based on reports in clinicaltrials.org (at the time of this submission). Several ongoing studies in the group of one of the authors (FF) aim to demonstrate target engagement in psychiatric patient populations and improvements in symptoms, including in patients with major depressive disorder (NCT02339285) and schizophrenia (NCT02360228).2 Importantly, these studies are randomized clinical trials for which the stimulation condition is masked for all participants, study personnel, and investigators. As with tDCS, these studies include a placebo arm (“sham stimulation”), which consists of a brief epoch of stimulation to mimic the initial skin sensation during stimulation, Yet, it is unclear if masking of tACS is successful, in particular for electrode montages that include frontal electrodes that tend to trigger phosphenes via stimulation of the optic nerve.
It can be expected that the number of tACS clinical trials will rapidly grow, particularly in the domain of psychiatric illnesses given the limitations of medication therapies. It will be crucial to (1) advance in parallel mechanistic work to further refine the currently very basic target engagement strategies, (2) advance the development of the next generation of tACS that will employ feedback based on EEG signals to provide personalized and adaptive stimulation [183], and (3) develop and disseminate device technology that enables high-quality double-blind trials to ensure the field avoids some of the typical pitfalls of a rapidly growing field.
Transparency
Several common practices limit transparency. There is an underreporting of negative effect studies [161] due to publication bias [153,184]. Failure to distinguish exploratory (hypothesis-generating) versus confirmatory (hypothesis-driven) research can result in inappropriate claims. Exploratory studies suggest trends and provide data for prospective power analyses. Hypothesis-driven, confirmatory research, strengthened by preregistration [185], permits conclusions regarding particular effects. Few studies preregister their hypotheses, design, data analyses, and power analyses, although NIMH now requires all clinical trials to be preregistered at clinicaltrials.gov (please see NIMH Support of Clinical Trials section below for further details). It would be important that future studies state in their Methods sections the exploratory (hypothesis-generating) or hypothesis-driven (confirmatory) characteristics of the methodology and design. More emphasis should be placed on a detailed description of methods (encompassing all relevant information to enable experimental replication, which includes computational modeling of induced currents in the target, in non-target regions, and the target-to-nontarget ratio which is a measure of focality of the stimulation). To ease reporting and reviewing, as well as to improve efforts to evaluate reproducibility, reporting checklists might be encouraged.
The field could improve substantially with the use of post-publication open data repositories. Data sharing can help provide a more complete record of parameters used in data acquisition, provide data for secondary analyses that add value to publications resulting from primary analyses, and allow for re-analyses using novel or alternate analytic tools. Data sharing may allow data to be combined or more directly compared across projects, thus clarifying how robust or reproducible findings are across platforms. Sources of disparate or variable findings might be examined across or within datasets.
The NIMH Data Archive (https://data-archive.nimh.nih.gov) is one such resource available to support data sharing [186]. Current expectations are that all NIMH-supported clinical research studies (not only clinical trials) will deposit and share data through this resource. The National Institutes of Health (NIH) espouses the sharing and reporting of the results of clinical trials [187] and several NIH initiatives (e.g., Brain Research through Advancing Innovative Neurotechnologies, BRAIN) have focused on data standards and sharing. The recent passage by Congress of the 21st Century Cures Act allows the NIH Director to require that data from NIH-supported research be shared [188]. While the sociology of science has at times resisted data sharing efforts, a culture-shift seems to be occurring with the development of bioinformatics and “big data” initiatives and emerging shared databases [189].
NIMH support of clinical trials
NIMH supports human device research ranging from exploratory biomarker discovery studies to the pivotal device trials required for Food and Drug Administration (FDA) approval. The requirements and goals of recently-issued NIMH funding opportunity announcements are based on an experimental medicine approach to clinical trials and address the need for clearly defined targets, dosimetry, and measures of target engagement. Applications must include a complete description of the delivered dose based on computational modeling of the electric-field (for example, Fig. 1). Additionally, the spatial and temporal parameters, as well as the context of dose delivery (context here means brain state at the time of stimulation, which may be resting or may involve active engagement with a cognitive task or psychosocial intervention), must be specified and a thorough description of the sham condition (demonstrating both its plausibility and its biological inactivity) included. These requirements will focus research on both stimulation-dependent and network-activity-dependent aspects of delivered dosage. Ultimately, the goal is to achieve rigorous, reproducible, and informative findings that support impactful device-based interventions.
NIH has recently begun to enforce a wider definition of clinical trials: “A research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes.” Many (if not most) studies with human subjects that were not previously considered clinical trials will now be so classified. Applicants are encouraged to explore material online (https://grants.nih.gov/policy/clinical-trials/definition.htm) and highly encouraged to reach out to program staff to determine the nature of their study. As part of this of this oversight, clinical trials will be required to register at clinicaltrials.gov. This will enforce preregistration of study details which will encourage the publication of null results [190] and increase reproducibility.
Conclusions
Clinical applications of tES remain at an early stage of development. Advances in understanding mechanisms, biomarkers of responsiveness, and technology (electronics, montages supported by computational models) are helping to inform protocols and therapeutic applications, but many needs remain.
Therapeutic use must be grounded in an improved understanding of physiological mechanisms at multiple levels. A broad approach spanning model systems to computer simulations to in vivo human trials is needed for rational design, target identifi-cation, and engagement to validation. Individual variability in response needs to be understood at multiple levels, including anatomy, physiology, and genetic heterogeneity. Methods are needed for individualized dosing, particularly in the absence of a motor threshold, as available in TMS. Improved masking and monitoring of masking of subjects and staff are needed, as are validation of sham interventions as biologically inactive. Tools for selecting montages and stimulation parameters and for more direct measurement of currents in the brain are needed. As stimulation is increasingly combined with cognitive or behavioral interventions, guidelines for determining the optimal timing in multimodal interventions (e.g., online, offline, pre-priming, etc.) would be helpful. Well-rationalized outcome measures should span the levels of physiology, behavior and clinical effects. Optimal measures of target engagement must be defined for various applications.
Reporting standards for publications are needed to provide the level of reporting needed to achieve reproducibility. Transparency can be achieved by prospective registration of trials, including data analytic plans, and providing access to raw individual-level data through data repositories. Progress in these areas promises to advance therapeutic applications of these methods.
Acknowledgments
This work was supported in part by grants P20GM109089 from the National Institute of General Medical Medical Sciences to JDR and by R01HD069776 from the National Institute of Child Health and Human Development to DJE.
Footnotes
Source: clinicaltrials.gov 9/29/2016; 524 hits with the following terms; tDCS/transcranial direct current stimulation, and; rehabilitation, cognitive behavioral therapy, cognitive training, physical therapy, occupational therapy, speech therapy, motor practice, task training, balance.
Financial disclosures
Lucas Parra and Marom Bikson are co-founders of Soterix Medical Inc. and co-inventors in patents held by the City College of New York (CCNY). The goal of these efforts is to make High-Definition tDCS broadly available. Flavio Frohlich is the founder, majority owner, and chief scientific officer of Pulvinar Neuro LLC, which markets devices for tDCS/tACS research. The University of North Carolina has filed several patents based on his inventions. Andre Russowsky Brunoni receives a CAPES-Humboldt research fellowship for experienced researchers. Colleen Loo received tDCS equipment from Soterix for conducting independent, investigator-initiated clinical trials. Zhi-De Deng and Sarah H. Lisanby are co-inventors on TMS technology, unrelated to the topics presented here. Drs. Charvet, Clark, Cohen, Dmochowski, Edwards, Kappen-man, Lim, Mantovani, McMullen, Richardson, Rumsey, Sehatpour, Sommers, Wassermann and Woods, Ms. Unal, and Ms. Pearson report no conflicts.
Disclaimer
The views expressed herein do not necessarily represent the official views of the National Institute of Mental Health, the National Institutes of Health, the US Department of Health and Human Services, or any other agency of the US Government.
References
- 1.Insel TR, Gogtay N. National Institute of Mental Health clinical trials: new opportunities, new expectations. JAMA Psychiatry. 2014;71:745–6. doi: 10.1001/jamapsychiatry.2014.426. https://doi.org/10.1001/jamapsychiatry.2014.426. [DOI] [PubMed] [Google Scholar]
- 2.Krystal JH, State MW. Psychiatric disorders: diagnosis to therapy. Cell. 2014;157:201–14. doi: 10.1016/j.cell.2014.02.042. https://doi.org/10.1016/j.cell.2014.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filmer HL, Dux PE, Mattingley JB. Applications of transcranial direct current stimulation for understanding brain function. Trends Neurosci. 2014;37:742–53. doi: 10.1016/j.tins.2014.08.003. https://doi.org/10.1016/j.tins.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of transcranial direct current stimulation: evidence based update. Brain Stimulat. 2016;9:641–61. doi: 10.1016/j.brs.2016.06.004. https://doi.org/10.1016/j.brs.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reato D, Rahman A, Bikson M, Parra LC. Effects of weak transcranial alternating current stimulation on brain activity—a review of known mechanisms from animal studies. Front Hum Neurosci. 2013;7:687. doi: 10.3389/fnhum.2013.00687. https://doi.org/10.3389/fnhum.2013.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fröhlich F. Experiments and models of cortical oscillations as a target for noninvasive brain stimulation. Prog Brain Res. 2015;222:41–73. doi: 10.1016/bs.pbr.2015.07.025. https://doi.org/10.1016/bs.pbr.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 7.Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. https://doi.org/10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- 8.Player MJ, Taylor JL, Weickert CS, Alonzo A, Sachdev PS, Martin D, et al. Increase in PAS-induced neuroplasticity after a treatment course of transcranial direct current stimulation for depression. J Affect Disord. 2014;167:140–7. doi: 10.1016/j.jad.2014.05.063. https://doi.org/10.1016/j.jad.2014.05.063. [DOI] [PubMed] [Google Scholar]
- 9.Jackson MP, Rahman A, Lafon B, Kronberg G, Ling D, Parra LC, et al. Animal models of transcranial direct current stimulation: methods and mechanisms. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2016;127:3425–54. doi: 10.1016/j.clinph.2016.08.016. https://doi.org/10.1016/j.clinph.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–47. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- 11.Kronberg G, Bridi M, Abel T, Bikson M, Parra LC. Direct current stimulation modulates LTP and LTD: activity dependence and dendritic effects. Brain Stimulat. 2017;10:51–8. doi: 10.1016/j.brs.2016.10.001. https://doi.org/10.1016/j.brs.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. https://doi.org/10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y, Lipton JO, Boyle LM, Madsen JR, Goldenberg MC, Pascual-Leone A, et al. Direct current stimulation induces mGluR5-dependent neocortical plasticity. Ann Neurol. 2016;80:233–46. doi: 10.1002/ana.24708. https://doi.org/10.1002/ana.24708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bikson M, Name A, Rahman A. Origins of specificity during tDCS: anatomical, activity-selective, and input-bias mechanisms. Front Hum Neurosci. 2013;7:688. doi: 10.3389/fnhum.2013.00688. https://doi.org/10.3389/fnhum.2013.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Márquez-Ruiz J, Leal-Campanario R, Sánchez-Campusano R, Molaee-Ardekani B, Wendling F, Miranda PC, et al. Transcranial direct-current stimulation modulates synaptic mechanisms involved in associative learning in behaving rabbits. Proc Natl Acad Sci U S A. 2012;109:6710–5. doi: 10.1073/pnas.1121147109. https://doi.org/10.1073/pnas.1121147109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Podda MV, Cocco S, Mastrodonato A, Fusco S, Leone L, Barbati SA, et al. Anodal transcranial direct current stimulation boosts synaptic plasticity and memory in mice via epigenetic regulation of Bdnf expression. Sci Rep. 2016;6:22180. doi: 10.1038/srep22180. https://doi.org/10.1038/srep22180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ranieri F, Podda MV, Riccardi E, Frisullo G, Dileone M, Profice P, et al. Modulation of LTP at rat hippocampal CA3-CA1 synapses by direct current stimulation. J Neurophysiol. 2012;107:1866–80. doi: 10.1152/jn.00319.2011. https://doi.org/10.1152/jn.00319.2011. [DOI] [PubMed] [Google Scholar]
- 18.Monai H, Ohkura M, Tanaka M, Oe Y, Konno A, Hirai H, et al. Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nat Commun. 2016;7:11100. doi: 10.1038/ncomms11100. https://doi.org/10.1038/ncomms11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rohan JG, Carhuatanta KA, McInturf SM, Miklasevich MK, Jankord R. Modulating hippocampal plasticity with in vivo brain stimulation. J Neurosci Off J Soc Neurosci. 2015;35:12824–32. doi: 10.1523/JNEUROSCI.2376-15.2015. https://doi.org/10.1523/JNEUR-OSCI.2376-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553:293–301. doi: 10.1113/jphysiol.2003.049916. https://doi.org/10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stagg CJ, Best JG, Stephenson MC, O’Shea J, Wylezinska M, Kincses ZT, et al. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci Off J Soc Neurosci. 2009;29:5202–6. doi: 10.1523/JNEUROSCI.4432-08.2009. https://doi.org/10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radman T, Ramos RL, Brumberg JC, Bikson M. Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Sci. 2009;2(215–28):228 e1–3. doi: 10.1016/j.brs.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali MM, Sellers KK, Fröhlich F. Transcranial alternating current stimulation modulates large-scale cortical network activity by network resonance. J Neurosci. 2013;33:11262–75. doi: 10.1523/JNEUROSCI.5867-12.2013. https://doi.org/10.1523/JNEUROSCI.5867-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fröhlich F, McCormick DA. Endogenous electric fields may guide neocortical network activity. Neuron. 2010;67:129–43. doi: 10.1016/j.neuron.2010.06.005. https://doi.org/10.1016/j.neuron.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozen S, Sirota A, Belluscio MA, Anastassiou CA, Stark E, Koch C, et al. Transcranial electric stimulation entrains cortical neuronal populations in rats. J Neurosci. 2010;30:11476–85. doi: 10.1523/JNEUROSCI.5252-09.2010. https://doi.org/10.1523/JNEUROSCI.5252-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pikovsky A, Rosenblum M, Kurths J. Synchronization: a universal concept in nonlinear sciences. xix. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- 27.Deans JK, Powell AD, Jefferys JG. Sensitivity of coherent oscillations in rat hippocampus to AC electric fields. J Physiol. 2007;583:555–65. doi: 10.1113/jphysiol.2007.137711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrmann CS, Murray MM, Ionta S, Hutt A, Lefebvre J. Shaping intrinsic neural oscillations with periodic stimulation. J Neurosci. 2016;36:5328–37. doi: 10.1523/JNEUROSCI.0236-16.2016. https://doi.org/10.1523/JNEUROSCI.0236-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reato D, Rahman A, Bikson M, Parra LC. Low-intensity electrical stimulation affects network dynamics by modulating population rate and spike timing. J Neurosci. 2010;30:15067–79. doi: 10.1523/JNEUROSCI.2059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thut G, Schyns PG, Gross J. Entrainment of perceptually relevant brain oscillations by non-invasive rhythmic stimulation of the human brain. Front Psychol. 2011;2:170. doi: 10.3389/fpsyg.2011.00170. https://doi.org/10.3389/fpsyg.2011.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuling T, Rach S, Herrmann CS. Orchestrating neuronal networks: sustained after-effects of transcranial alternating current stimulation depend upon brain states. Front Hum Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00161. https://doi.org/10.3389/fnhum.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alagapan S, Schmidt SL, Lefebvre J, Hadar E, Shin HW, Frӧhlich F. Modulation of cortical oscillations by low-frequency direct cortical stimulation is state-dependent. PLoS Biol. 2016;14:e1002424. doi: 10.1371/journal.pbio.1002424. https://doi.org/10.1371/journal.pbio.1002424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt SL, Iyengar AK, Foulser AA, Boyle MR, Frӧhlich F. Endogenous cortical oscillations constrain neuromodulation by weak electric fields. Brain Stimulat. 2014;7:878–89. doi: 10.1016/j.brs.2014.07.033. https://doi.org/10.1016/j.brs.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reato D, Bikson M, Parra LC. Lasting modulation of in vitro oscillatory activity with weak direct current stimulation. J Neurophysiol. 2015;113:1334–41. doi: 10.1152/jn.00208.2014. https://doi.org/10.1152/jn.00208.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. 2016:1031–48. doi: 10.1016/j.clinph.2015.11.012. https://doi.org/10.1016/j.clinph.2015.11.012. [DOI] [PMC free article] [PubMed]
- 36.Antal A, Alekseichuk I, Bikson M, Brockmӧller J, Brunoni AR, Chen R, et al. Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2017;128:1774–809. doi: 10.1016/j.clinph.2017.06.001. https://doi.org/10.1016/j.clinph.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minhas P, Bikson M, Woods AJ, Rosen AR, Kessler SK. Transcranial direct current stimulation in pediatric brain: a computational modeling study. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:859–62. doi: 10.1109/EMBC.2012.6346067. https://doi.org/10.1109/EMBC.2012.6346067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kessler SK, Minhas P, Woods AJ, Rosen A, Gorman C, Bikson M. Dosage considerations for transcranial direct current stimulation in children: a computational modeling study. PLos One. 2013;8:e76112. doi: 10.1371/journal.pone.0076112. https://doi.org/10.1371/journal.pone.0076112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woods AJ, Bryant V, Sacchetti D, Gervits F, Hamilton R. Effects of electrode drift in transcranial direct current stimulation. Brain Stimulat. 2015;8:515–9. doi: 10.1016/j.brs.2014.12.007. https://doi.org/10.1016/j.brs.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. doi:PHY_1055 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klem GH, Lüders HO, Jasper HH, Elger C. The ten-twenty electrode system of the international federation. The international federation of clinical neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:3–6. [PubMed] [Google Scholar]
- 42.DaSilva AF, Volz MS, Bikson M, Fregni F. Electrode positioning and montage in transcranial direct current stimulation. J Vis Exp JoVE. 2011 doi: 10.3791/2744. https://doi.org/10.3791/2744. [DOI] [PMC free article] [PubMed]
- 43.Kronberg G, Bikson M. Electrode assembly design for transcranial Direct Current Stimulation: a FEM modeling study. Annu Int Conf IEEE Eng Med Biol Soc EMBC. 2012;2012:891–5. doi: 10.1109/EMBC.2012.6346075. https://doi.org/10.1109/EMBC.2012.6346075. [DOI] [PubMed] [Google Scholar]
- 44.Shen B, Yin Y, Wang J, Zhou X, McClure SM, Li J. High-definition tDCS alters impulsivity in a baseline-dependent manner. Neuroimage. 2016;143:343–52. doi: 10.1016/j.neuroimage.2016.09.006. https://doi.org/10.1016/j.neuroimage.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Gözenman F, Berryhill ME. Working memory capacity differentially influences responses to tDCS and HD-tDCS in a retro-cue task. Neurosci Lett. 2016;629:105–9. doi: 10.1016/j.neulet.2016.06.056. https://doi.org/10.1016/j.neulet.2016.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuo H-I, Bikson M, Datta A, Minhas P, Paulus W, Kuo M-F, et al. Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: a neurophysiological study. Brain Stimulat. 2013;6:644–8. doi: 10.1016/j.brs.2012.09.010. https://doi.org/10.1016/j.brs.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 47.Zito GA, Senti T, Cazzoli D, Müri RM, Mosimann UP, Nyffeler T, et al. Cathodal HD-tDCS on the right V5 improves motion perception in humans. Front Behav Neurosci. 2015;9:257. doi: 10.3389/fnbeh.2015.00257. https://doi.org/10.3389/fnbeh.2015.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richardson JD, Fillmore P, Datta A, Truong D, Bikson M, Fridriksson J. Toward development of sham protocols for high-definition transcranial direct current stimulation (HD-tDCS) Neuromodulation. 2014;1:62. https://doi.org/10.15540/nr.1.1.62. [Google Scholar]
- 49.Peterchev AV, Wagner TA, Miranda PC, Nitsche MA, Paulus W, Lisanby SH, et al. Fundamentals of transcranial electric and magnetic stimulation dose: definition, selection, and reporting practices. Brain Stimulat. 2012;5:435–53. doi: 10.1016/j.brs.2011.10.001. https://doi.org/10.1016/j.brs.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Higgins J, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0. 2011 [updated March 2011] Available from: http://handbook.cochrane.org.
- 51.Brunoni AR, Fregni F. Clinical trial design in non-invasive brain stimulation psychiatric research. Int J Meth Psychiatr Res. 2011;20:e19–30. doi: 10.1002/mpr.338. https://doi.org/10.1002/mpr.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horvath JC, Carter O, Forte JD. Transcranial direct current stimulation: five important issues we aren’t discussing (but probably should be) Front Syst Neurosci. 2014;8:2. doi: 10.3389/fnsys.2014.00002. https://doi.org/10.3389/fnsys.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2006;117:845–50. doi: 10.1016/j.clinph.2005.12.003. https://doi.org/10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Alonzo A, Aaronson S, Bikson M, Husain M, Lisanby S, Martin D, et al. Study design and methodology for a multicentre, randomised controlled trial of transcranial direct current stimulation as a treatment for unipolar and bipolar depression. Contemp Clin Trials. 2016;51:65–71. doi: 10.1016/j.cct.2016.10.002. https://doi.org/10.1016/j.cct.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Wallace D, Cooper NR, Paulmann S, Fitzgerald PB, Russo R. Perceived comfort and blinding efficacy in randomised sham-controlled transcranial direct current stimulation (tDCS) trials at 2 mA in young and older healthy adults. PLos One. 2016;11:e0149703. doi: 10.1371/journal.pone.0149703. https://doi.org/10.1371/journal.pone.0149703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garnett EO, den Ouden D-B. Validating a sham condition for use in high definition transcranial direct current stimulation. Brain Stimulat. 2015;8:551–4. doi: 10.1016/j.brs.2015.01.399. https://doi.org/10.1016/j.brs.2015.01.399. [DOI] [PubMed] [Google Scholar]
- 57.Brunyé TT, Cantelon J, Holmes A, Taylor HA, Mahoney CR. Mitigating cutaneous sensation differences during tDCS: comparing sham versus low intensity control conditions. Brain Stimulat. 2014;7:832–5. doi: 10.1016/j.brs.2014.09.015. https://doi.org/10.1016/j.brs.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 58.Fertonani A, Ferrari C, Miniussi C. What do you feel if I apply transcranial electric stimulation? Safety, sensations and secondary induced effects. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2015;126:2181–8. doi: 10.1016/j.clinph.2015.03.015. https://doi.org/10.1016/j.clinph.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 59.Turi Z, Ambrus GG, Ho K-A, Sengupta T, Paulus W, Antal A. When size matters: large electrodes induce greater stimulation-related cutaneous discomfort than smaller electrodes at equivalent current density. Brain Stimulat. 2014;7:460–7. doi: 10.1016/j.brs.2014.01.059. https://doi.org/10.1016/j.brs.2014.01.059. [DOI] [PubMed] [Google Scholar]
- 60.Dmochowski JP, Datta A, Huang Y, Richardson JD, Bikson M, Fridriksson J, et al. Targeted transcranial direct current stimulation for rehabilitation after stroke. Neuroimage. 2013;75:12–9. doi: 10.1016/j.neuroimage.2013.02.049. https://doi.org/10.1016/j.neuroimage.2013.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richardson J, Datta A, Dmochowski J, Parra LC, Fridriksson J. Feasibility of using high-definition transcranial direct current stimulation (HD-tDCS) to enhance treatment outcomes in persons with aphasia. NeuroRehabilitation. 2015;36:115–26. doi: 10.3233/NRE-141199. https://doi.org/10.3233/NRE-141199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brunoni AR, Sampaio-Junior B, Moffa AH, Borrione L, Nogueira BS, Aparício LVM, et al. The Escitalopram versus Electric Current Therapy for Treating Depression Clinical Study (ELECT-TDCS): rationale and study design of a non-inferiority, triple-arm, placebo-controlled clinical trial. Sao Paulo Med J Rev Paul Med. 2015;133:252–63. doi: 10.1590/1516-3180.2014.00351712. https://doi.org/10.1590/1516-3180.2014.00351712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Datta A, Truong D, Minhas P, Parra LC, Bikson M. Inter-individual variation during transcranial direct current stimulation and normalization of dose using MRI-derived computational models. Front Psychiatry Front Res Found. 2012;3:91. doi: 10.3389/fpsyt.2012.00091. https://doi.org/10.3389/fpsyt.2012.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deng Z-D, Lisanby SH, Peterchev A. Effect of anatomical variability on electric field characteristics of electroconvulsive therapy and magnetic seizure therapy: a parametric modeling study. IEEE Trans Neural Syst Rehabil Eng. 2015;23:22–31. doi: 10.1109/TNSRE.2014.2339014. https://doi.org/10.1109/TNSRE.2014.2339014. Early Access Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimulat. 2009;2:201–207.e1. doi: 10.1016/j.brs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deng Z-D, Lisanby SH, Peterchev AV. Controlling stimulation strength and focality in electroconvulsive therapy via current amplitude and electrode size and spacing: comparison with magnetic seizure therapy. J ECT. 2013;29:325–35. doi: 10.1097/YCT.10.1097/YCT.0b013e3182a4b4a7. https://doi.org/10.1097/YCT.10.1097/YCT.0b013e3182a4b4a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borckardt JJ, Bikson M, Frohman H, Reeves ST, Datta A, Bansal V, et al. A pilot study of the tolerability and effects of high-definition transcranial direct current stimulation (HD-tDCS) on pain perception. J Pain Off J Am Pain Soc. 2012;13:112–20. doi: 10.1016/j.jpain.2011.07.001. https://doi.org/10.1016/j.jpain.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 68.Caparelli-Daquer EM, Zimmermann TJ, Mooshagian E, Parra LC, Rice JK, Datta A, et al. A pilot study on effects of 4×1 High-Definition tDCS on motor cortex excitability. 2012 Annu Int Conf IEEE Eng Med Biol Soc. 2012:735–8. doi: 10.1109/EMBC.2012.6346036. https://doi.org/10.1109/EMBC.2012.6346036. [DOI] [PMC free article] [PubMed]
- 69.Heimrath K, Breitling C, Krauel K, Heinze H-J, Zaehle T. Modulation of preattentive spectro-temporal feature processing in the human auditory system by HD-tDCS. Eur J Neurosci. 2015;41:1580–6. doi: 10.1111/ejn.12908. https://doi.org/10.1111/ejn.12908. [DOI] [PubMed] [Google Scholar]
- 70.Nikolin S, Loo CK, Bai S, Dokos S, Martin DM. Focalised stimulation using high definition transcranial direct current stimulation (HD-tDCS) to investigate declarative verbal learning and memory functioning. Neuroimage. 2015;117:11–9. doi: 10.1016/j.neuroimage.2015.05.019. https://doi.org/10.1016/j.neuroimage.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 71.Helfrich RF, Knepper H, Nolte G, Strüber D, Rach S, Herrmann CS, et al. Selective modulation of interhemispheric functional connectivity by HD-tACS shapes perception. PLoS Biol. 2014;12:e1002031. doi: 10.1371/journal.pbio.1002031. https://doi.org/10.1371/journal.pbio.1002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heise K-F, Kortzorg N, Saturnino GB, Fujiyama H, Cuypers K, Thielscher A, et al. Evaluation of a modified high-definition electrode montage for transcranial alternating current stimulation (tACS) of pre-central areas. Brain Stimulat. 2016;9:700–4. doi: 10.1016/j.brs.2016.04.009. https://doi.org/10.1016/j.brs.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 73.Edwards D, Cortes M, Datta A, Minhas P, Wassermann EM, Bikson M. Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: a basis for high-definition tDCS. Neuroimage. 2013;74:266–75. doi: 10.1016/j.neuroimage.2013.01.042. https://doi.org/10.1016/j.neuroimage.2013.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ruffini G, Fox MD, Ripolles O, Miranda PC, Pascual-Leone A. Optimization of multifocal transcranial current stimulation for weighted cortical pattern targeting from realistic modeling of electric fields. Neuroimage. 2014;89:216–25. doi: 10.1016/j.neuroimage.2013.12.002. https://doi.org/10.1016/j.neuroimage.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bai S, Galvez V, Dokos S, Martin D, Bikson M, Loo C. Computational models of Bitemporal, Bifrontal and Right Unilateral ECT predict differential stimulation of brain regions associated with efficacy and cognitive side effects. Eur Psychiatry J Assoc Eur Psychiatr. 2017;41:21–9. doi: 10.1016/j.eurpsy.2016.09.005. https://doi.org/10.1016/j.eurpsy.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 76.Ho K-A, Taylor JL, Chew T, Galvez V, Alonzo A, Bai S, et al. The effect of transcranial direct current stimulation (tDCS) electrode size and current intensity on motor cortical excitability: evidence from single and repeated sessions. Brain Stimulat. 2016;9:1–7. doi: 10.1016/j.brs.2015.08.003. https://doi.org/10.1016/j.brs.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 77.Seibt O, Brunoni AR, Huang Y, Bikson M. The pursuit of DLPFC: non-neuronavigated methods to target the left dorsolateral pre-frontal cortex with symmetric bicephalic transcranial direct current stimulation (tDCS) Brain Stimulat. 2015;8:590–602. doi: 10.1016/j.brs.2015.01.401. https://doi.org/10.1016/j.brs.2015.01.401. [DOI] [PubMed] [Google Scholar]
- 78.Dmochowski JP, Datta A, Bikson M, Su Y, Parra LC. Optimized multi-electrode stimulation increases focality and intensity at target. J Neural Eng. 2011;8:046011. doi: 10.1088/1741-2560/8/4/046011. https://doi.org/10.1088/1741-2560/8/4/046011. [DOI] [PubMed] [Google Scholar]
- 79.Im CH, Jung HH, Choi JD, Lee SY, Jung KY. Determination of optimal electrode positions for transcranial direct current stimulation (tDCS) Phys Med Biol. 2008;53:N219–25. doi: 10.1088/0031-9155/53/11/N03. [DOI] [PubMed] [Google Scholar]
- 80.Teichmann M, Lesoil C, Godard J, Vernet M, Bertrand A, Levy R, et al. Direct current stimulation over the anterior temporal areas boosts semantic processing in primary progressive aphasia. Ann Neurol. 2016;80:693–707. doi: 10.1002/ana.24766. https://doi.org/10.1002/ana.24766. [DOI] [PubMed] [Google Scholar]
- 81.Bikson M, Rahman A, Datta A. Computational models of transcranial direct current stimulation. Clin EEG Neurosci Off J EEG Clin Neurosci Soc ENCS. 2012;43:176–83. doi: 10.1177/1550059412445138. https://doi.org/10.1177/1550059412445138. [DOI] [PubMed] [Google Scholar]
- 82.Galletta EE, Cancelli A, Cottone C, Simonelli I, Tecchio F, Bikson M, et al. Use of computational modeling to inform tDCS electrode montages for the promotion of language recovery in post-stroke aphasia. Brain Stimulat. 2015;8:1108–15. doi: 10.1016/j.brs.2015.06.018. https://doi.org/10.1016/j.brs.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 83.Jog MV, Smith RX, Jann K, Dunn W, Lafon B, Truong D, et al. In-vivo imaging of magnetic fields induced by transcranial direct current stimulation (tDCS) in human brain using MRI. Sci Rep. 2016;6:34385. doi: 10.1038/srep34385. https://doi.org/10.1038/srep34385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Opitz A, Falchier A, Yan C-G, Yeagle EM, Linn GS, Megevand P, et al. Spatiotemporal structure of intracranial electric fields induced by transcranial electric stimulation in humans and nonhuman primates. Sci Rep. 2016;6:31236. doi: 10.1038/srep31236. https://doi.org/10.1038/srep31236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Datta A, Zhou X, Su Y, Parra LC, Bikson M. Validation of finite element model of transcranial electrical stimulation using scalp potentials: implications for clinical dose. J Neural Eng. 2013;10:036018. doi: 10.1088/1741-2560/10/3/036018. https://doi.org/10.1088/1741-2560/10/3/036018. [DOI] [PubMed] [Google Scholar]
- 86.Huang Y, Liu AA, Lafon B, Friedman D, Dayan M, Wang X, et al. Measurements and models of electric fields in the in vivo human brain during transcranial electric stimulation. eLife. 2017:6. doi: 10.7554/eLife.18834. https://doi.org/10.7554/eLife.18834. [DOI] [PMC free article] [PubMed]
- 87.Gillick BT, Kirton A, Carmel JB, Minhas P, Bikson M. Pediatric stroke and transcranial direct current stimulation: methods for rational individualized dose optimization. Front Hum Neurosci. 2014;8:739. doi: 10.3389/fnhum.2014.00739. https://doi.org/10.3389/fnhum.2014.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Truong DQ, Hüber M, Xie X, Datta A, Rahman A, Parra LC, et al. Clinician accessible tools for GUI computational models of transcranial electrical stimulation: BONSAI and SPHERES. Brain Stimulat. 2014;7:521–4. doi: 10.1016/j.brs.2014.03.009. https://doi.org/10.1016/j.brs.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Opitz A, Paulus W, Will S, Antunes A, Thielscher A. Determinants of the electric field during transcranial direct current stimulation. Neuroimage. 2015;109:140–50. doi: 10.1016/j.neuroimage.2015.01.033. https://doi.org/10.1016/j.neuroimage.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 90.Lee C, Jung Y-J, Lee SJ, Im C-H. COMETS2: an advanced MATLAB toolbox for the numerical analysis of electric fields generated by transcranial direct current stimulation. J Neurosci Meth. 2017;277:56–62. doi: 10.1016/j.jneumeth.2016.12.008. https://doi.org/10.1016/j.jneumeth.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 91.Huang Y, Parra LC, Haufe S. The New York Head–a precise standardized volume conductor model for EEG source localization and tES targeting. Neuroimage. 2016;140:150–62. doi: 10.1016/j.neuroimage.2015.12.019. https://doi.org/10.1016/j.neuroimage.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang Y, Parra LC. Fully automated whole-head segmentation with improved smoothness and continuity, with theory reviewed. PLos One. 2015;10:e0125477. doi: 10.1371/journal.pone.0125477. https://doi.org/10.1371/journal.pone.0125477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang Y, Dmochowski JP, Su Y, Datta A, Rorden C, Parra LC. Automated MRI segmentation for individualized modeling of current flow in the human head. J Neural Eng. 2013;10:066004. doi: 10.1088/1741-2560/10/6/066004. https://doi.org/10.1088/1741-2560/10/6/066004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bestmann S. Computational neurostimulation in basic and translational research. Prog Brain Res. 2015;222(xv–xx) doi: 10.1016/S0079-6123(15)00159-4. https://doi.org/10.1016/S0079-6123(15)00159-4. [DOI] [PubMed] [Google Scholar]
- 95.Rahman A, Lafon B, Bikson M. Multilevel computational models for predicting the cellular effects of noninvasive brain stimulation. Prog Brain Res. 2015;222:25–40. doi: 10.1016/bs.pbr.2015.09.003. https://doi.org/10.1016/bs.pbr.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 96.Fernández-Corazza M, Turovets S, Luu P, Anderson E, Tucker D. Transcranial electrical neuromodulation based on the reciprocity principle. Front Psychiatry. 2016;7:87. doi: 10.3389/fpsyt.2016.00087. https://doi.org/10.3389/fpsyt.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cancelli A, Cottone C, Tecchio F, Truong DQ, Dmochowski J, Bikson M. A simple method for EEG guided transcranial electrical stimulation without models. J Neural Eng. 2016;13:036022. doi: 10.1088/1741-2560/13/3/036022. https://doi.org/10.1088/1741-2560/13/3/036022. [DOI] [PubMed] [Google Scholar]
- 98.Roh T, Song K, Cho H, Shin D, Yoo H-J. A wearable neuro-feedback system with EEG-based mental status monitoring and transcranial electrical stimulation. IEEE Trans Biomed Circuits Syst. 2014;8:755–64. doi: 10.1109/TBCAS.2014.2384017. https://doi.org/10.1109/TBCAS.2014.2384017. [DOI] [PubMed] [Google Scholar]
- 99.Strube W, Bunse T, Nitsche MA, Nikolaeva A, Palm U, Padberg F, et al. Bidirectional variability in motor cortex excitability modulation following 1 mA transcranial direct current stimulation in healthy participants. Physiol Rep. 2016;4 doi: 10.14814/phy2.12884. https://doi.org/10.14814/phy2.12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Madhavan S, Sriraman A, Freels S. Reliability and variability of tDCS induced changes in the lower limb motor cortex. Brain Sci. 2016;6 doi: 10.3390/brainsci6030026. https://doi.org/10.3390/brainsci6030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dyke K, Kim S, Jackson GM, Jackson SR. Intra-subject consistency and reliability of response following 2 mA transcranial direct current stimulation. Brain Stimulat. 2016;9:819–25. doi: 10.1016/j.brs.2016.06.052. https://doi.org/10.1016/j.brs.2016.06.052. [DOI] [PubMed] [Google Scholar]
- 102.Horvath JC, Vogrin SJ, Carter O, Cook MJ, Forte JD. Effects of a common transcranial direct current stimulation (tDCS) protocol on motor evoked potentials found to be highly variable within individuals over 9 testing sessions. Exp Brain Res. 2016;234:2629–42. doi: 10.1007/s00221-016-4667-8. https://doi.org/10.1007/s00221-016-4667-8. [DOI] [PubMed] [Google Scholar]
- 103.Labruna L, Jamil A, Fresnoza S, Batsikadze G, Kuo M-F, Vanderschelden B, et al. Efficacy of anodal transcranial direct current stimulation is related to sensitivity to transcranial magnetic stimulation. Brain Stimulat. 2016;9:8–15. doi: 10.1016/j.brs.2015.08.014. https://doi.org/10.1016/j.brs.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bestmann S, Ward N. Are current flow models for transcranial electrical stimulation fit for purpose? Brain Stimulat. 2017;10:865–6. doi: 10.1016/j.brs.2017.04.002. https://doi.org/10.1016/j.brs.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 105.Bikson M, Truong DQ, Mourdoukoutas AP, Aboseria M, Khadka N, Adair D, et al. Chapter 1-Modeling sequence and quasi-uniform assumption in computational neurostimulation. In: Bestmann S, editor. Prog Brain Res. Vol. 222. Elsevier; 2015. pp. 1–23. [DOI] [PubMed] [Google Scholar]
- 106.Giordano J, Bikson M, Kappenman ES, Clark VP, Coslett HB, Hamblin MR, et al. Mechanisms and effects of transcranial direct current stimulation. Dose-Response Publ Int Hormesis Soc. 2017;15 doi: 10.1177/1559325816685467. 1559325816685467. https://doi.org/10.1177/1559325816685467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jamil A, Batsikadze G, Kuo H-I, Labruna L, Hasan A, Paulus W, et al. Systematic evaluation of the impact of stimulation intensity on neuroplastic after-effects induced by transcranial direct current stimulation. J Physiol. 2017;595:1273–88. doi: 10.1113/JP272738. https://doi.org/10.1113/JP272738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Charvet LE, Kasschau M, Datta A, Knotkova H, Stevens MC, Alonzo A, et al. Remotely-supervised transcranial direct current stimulation (tDCS) for clinical trials: guidelines for technology and protocols. Front Syst Neurosci. 2015;9:26. doi: 10.3389/fnsys.2015.00026. https://doi.org/10.3389/fnsys.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kasschau M, Sherman K, Haider L, Frontario A, Shaw M, Datta A, et al. A protocol for the use of remotely-supervised transcranial direct current stimulation (tDCS) in multiple sclerosis (MS) J Vis Exp JoVE. 2015:e53542. doi: 10.3791/53542. https://doi.org/10.3791/53542. [DOI] [PMC free article] [PubMed]
- 110.Kasschau M, Reisner J, Sherman K, Bikson M, Datta A, Charvet LE. Trans-cranial direct current stimulation is feasible for remotely supervised home delivery in multiple sclerosis. Neuromodulation J Int Neuromodulation Soc. 2016;19:824–31. doi: 10.1111/ner.12430. https://doi.org/10.1111/ner.12430. [DOI] [PubMed] [Google Scholar]
- 111.Darvas F, Pantazis D, Kucukaltun-Yildirim E, Leahy RM. Mapping human brain function with MEG and EEG: methods and validation. Neuroimage. 2004;23(Suppl 1):S289–99. doi: 10.1016/j.neuroimage.2004.07.014. https://doi.org/10.1016/j.neuroimage.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 112.Coffman B. Increasing your brain potential: transcranial direct current stimulation for enhancement of behavior and event-related potentials in tests of attention and impulsivity. 2014 http://digitalrepository.unm.edu/cgi/viewcontent.cgi?article=1024&context=psy_etds.
- 113.Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol. 2003;114:2220–2. doi: 10.1016/s1388-2457(03)00235-9. https://doi.org/10.1016/S1388-2457(03)00235-9. [DOI] [PubMed] [Google Scholar]
- 114.Reinhart RMG, Woodman GF. Causal control of medial–frontal cortex governs electrophysiological and behavioral indices of performance monitoring and learning. J Neurosci. 2014;34:4214–27. doi: 10.1523/JNEUROSCI.5421-13.2014. https://doi.org/10.1523/JNEUR-OSCI.5421-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marshall L, Molle M, Hallschmid M, Born J. Transcranial direct current stimulation during sleep improves declarative memory. J Neurosci. 2004;24:9985–92. doi: 10.1523/JNEUROSCI.2725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.De Ridder D, Vanneste S. EEG driven tDCS versus bifrontal tDCS for tinnitus. Front Psychiatry. 2012;3:84. doi: 10.3389/fpsyt.2012.00084. https://doi.org/10.3389/fpsyt.2012.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zaehle T, Rach S, Herrmann CS. Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PLos One. 2010;5:e13766. doi: 10.1371/journal.pone.0013766. https://doi.org/10.1371/journal.pone.0013766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sugawara K, Onishi H, Yamashiro K, Kojima S, Miyaguchi S, Kirimoto H, et al. The effect of anodal transcranial direct current stimulation over the primary motor or somatosensory cortices on somatosensory evoked magnetic fields. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2015;126:60–7. doi: 10.1016/j.clinph.2014.04.014. https://doi.org/10.1016/j.clinph.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 119.Venkatakrishnan A, Contreras-Vidal JL, Sandrini M, Cohen LG. Independent component analysis of resting brain activity reveals transient modulation of local cortical processing by transcranial direct current stimulation. Conf Proc IEEE Eng Med Biol Soc 2011. 2011:8102–5. doi: 10.1109/IEMBS.2011.6091998. https://doi.org/10.1109/IEMBS.2011.6091998. [DOI] [PubMed]
- 120.Suntrup S, Teismann I, Wollbrink A, Winkels M, Warnecke T, Flöel A, et al. Magnetoencephalographic evidence for the modulation of cortical swallowing processing by transcranial direct current stimulation. Neuroimage. 2013;83:346–54. doi: 10.1016/j.neuroimage.2013.06.055. https://doi.org/10.1016/j.neuroimage.2013.06.055. [DOI] [PubMed] [Google Scholar]
- 121.Neuling T, Ruhnau P, Fuscà M, Demarchi G, Herrmann CS, Weisz N. Friends, not foes: magnetoencephalography as a tool to uncover brain dynamics during transcranial alternating current stimulation. Neuroimage. 2015;118:406–13. doi: 10.1016/j.neuroimage.2015.06.026. https://doi.org/10.1016/j.neuroimage.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hill AT, Rogasch NC, Fitzgerald PB, Hoy KE. Effects of prefrontal bipolar and high-definition transcranial direct current stimulation on cortical reactivity and working memory in healthy adults. Neuroimage. 2017;152:142–57. doi: 10.1016/j.neuroimage.2017.03.001. https://doi.org/10.1016/j.neuroimage.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 123.Zheng X, Schlaug G. Structural white matter changes in descending motor tracts correlate with improvements in motor impairment after undergoing a treatment course of tDCS and physical therapy. Front Hum Neurosci. 2015;9:229. doi: 10.3389/fnhum.2015.00229. https://doi.org/10.3389/fnhum.2015.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mondino M, Brunelin J, Palm U, Brunoni AR, Poulet E, Fecteau S. Transcranial direct current stimulation for the treatment of refractory symptoms of schizophrenia. Current evidence and future directions. Curr Pharm Des. 2015;21:3373–83. doi: 10.2174/1381612821666150619093648. https://doi.org/10.2174/1381612821666150619093648. [DOI] [PubMed] [Google Scholar]
- 125.Clark VP, Coffman BA, Mayer AR, Weisend MP, Lane TDR, Calhoun VD, et al. TDCS guided using fMRI significantly accelerates learning to identify concealed objects. Neuroimage. 2012;59:117–28. doi: 10.1016/j.neuroimage.2010.11.036. https://doi.org/10.1016/j.neuroimage.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Coffman BA, Trumbo MC, Flores RA, Garcia CM, van der Merwe AJ, Wassermann EM, et al. Impact of tDCS on performance and learning of target detection: interaction with stimulus characteristics and experimental design. Neuropsychologia. 2012;50:1594–602. doi: 10.1016/j.neuropsychologia.2012.03.012. https://doi.org/10.1016/j.neuropsychologia.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 127.Falcone B, Coffman BA, Clark VP, Parasuraman R. Transcranial direct current stimulation augments perceptual sensitivity and 24-hour retention in a complex threat detection task. PLos One. 2012;7:e34993. doi: 10.1371/journal.pone.0034993. https://doi.org/10.1371/journal.pone.0034993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stagg CJ, Lin RL, Mezue M, Segerdahl A, Kong Y, Xie J, et al. Widespread modulation of cerebral perfusion induced during and after transcranial direct current stimulation applied to the left dorsolateral prefrontal cortex. J Neurosci Off J Soc Neurosci. 2013;33:11425–31. doi: 10.1523/JNEUROSCI.3887-12.2013. https://doi.org/10.1523/JNEUROSCI.3887-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Weber MJ, Messing SB, Rao H, Detre JA, Thompson-Schill SL. Prefrontal transcranial direct current stimulation alters activation and connectivity in cortical and subcortical reward systems: a tDCS-fMRI study. Hum Brain Mapp. 2014;35:3673–86. doi: 10.1002/hbm.22429. https://doi.org/10.1002/hbm.22429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zheng X, Alsop DC, Schlaug G. Effects of transcranial direct current stimulation (tDCS) on human regional cerebral blood flow. Neuroimage. 2011;58:26–33. doi: 10.1016/j.neuroimage.2011.06.018. https://doi.org/10.1016/j.neuroimage.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Clark VP, Coffman BA, Trumbo MC, Gasparovic C. Transcranial direct current stimulation (tDCS) produces localized and specific alterations in neurochemistry: a 1H magnetic resonance spectroscopy study. Neurosci Lett. 2011;500:67–71. doi: 10.1016/j.neulet.2011.05.244. https://doi.org/10.1016/j.neulet.2011.05.244. [DOI] [PubMed] [Google Scholar]
- 132.Rango M, Cogiamanian F, Marceglia S, Barberis B, Arighi A, Biondetti P, et al. Myoinositol content in the human brain is modified by transcranial direct current stimulation in a matter of minutes: a 1H-MRS study. Magn Reson Med. 2008;60:782–9. doi: 10.1002/mrm.21709. https://doi.org/10.1002/mrm.21709. [DOI] [PubMed] [Google Scholar]
- 133.Stagg CJ, O’Shea J, Kincses ZT, Woolrich M, Matthews PM, Johansen-Berg H. Modulation of movement-associated cortical activation by transcranial direct current stimulation. Eur J Neurosci. 2009;30:1412–23. doi: 10.1111/j.1460-9568.2009.06937.x. https://doi.org/10.1111/j.1460-9568.2009.06937.x. [DOI] [PubMed] [Google Scholar]
- 134.Hunter MA, Coffman BA, Gasparovic C, Calhoun VD, Trumbo MC, Clark VP. Baseline effects of transcranial direct current stimulation on glutamatergic neurotransmission and large-scale network connectivity. Brain Res. 2015;1594:92–107. doi: 10.1016/j.brainres.2014.09.066. https://doi.org/10.1016/j.brainres.2014.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107:11020–5. doi: 10.1073/pnas.1000446107. https://doi.org/10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brunoni AR, Moffa AH, Fregni F, Palm U, Padberg F, Blumberger DM, et al. Transcranial direct current stimulation for acute major depressive episodes: meta-analysis of individual patient data. Br J Psychiatry J Ment Sci. 2016;208:522–31. doi: 10.1192/bjp.bp.115.164715. https://doi.org/10.1192/bjp.bp.115.164715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Martin DM, Yeung K, Loo CK. Pre-treatment letter fluency performance predicts antidepressant response to transcranial direct current stimulation. J Affect Disord. 2016;203:130–5. doi: 10.1016/j.jad.2016.05.072. https://doi.org/10.1016/j.jad.2016.05.072. [DOI] [PubMed] [Google Scholar]
- 138.Ho K-A, Bai S, Martin D, Alonzo A, Dokos S, Loo CK. Clinical pilot study and computational modeling of bitemporal transcranial direct current stimulation, and safety of repeated courses of treatment, in major depression. J ECT. 2015;31:226–33. doi: 10.1097/YCT.0000000000000230. https://doi.org/10.1097/YCT.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 139.de Pereira Junior BS, Tortella G, Lafer B, Nunes P, Benseñor IM, Lotufo PA, et al. The bipolar depression electrical treatment trial (BETTER): design, rationale, and objectives of a randomized, sham-controlled trial and data from the pilot study phase. Neural Plast. 2015;2015:684025. doi: 10.1155/2015/684025. https://doi.org/10.1155/2015/684025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brunoni AR, Ferrucci R, Bortolomasi M, Vergari M, Tadini L, Boggio PS, et al. Transcranial direct current stimulation (tDCS) in unipolar vs. bipolar depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:96–101. doi: 10.1016/j.pnpbp.2010.09.010. https://doi.org/10.1016/j.pnpbp.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 141.Palm U, Schiller C, Fintescu Z, Obermeier M, Keeser D, Reisinger E, et al. Transcranial direct current stimulation in treatment resistant depression: a randomized double-blind, placebo-controlled study. Brain Stimulat. 2012;5:242–51. doi: 10.1016/j.brs.2011.08.005. https://doi.org/10.1016/j.brs.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 142.Brunoni AR, Valiengo L, Baccaro A, Zanao TA, Oliveira AC, Goulart AC, et al. The sertraline versus electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry. 2013;70:383–91. doi: 10.1001/2013.jamapsychiatry.32. [DOI] [PubMed] [Google Scholar]
- 143.Brunoni AR, Moffa AH, Sampaio-Junior B, Borrione L, Moreno ML, Fernandes RA, et al. Trial of electrical direct-current therapy versus escitalopram for depression. N Engl J Med. 2017;376:2523–33. doi: 10.1056/NEJMoa1612999. https://doi.org/10.1056/NEJMoa1612999. [DOI] [PubMed] [Google Scholar]
- 144.Zanão TA, Moffa AH, Shiozawa P, Lotufo PA, Benseñor IM, Brunoni AR. Impact of two or less missing treatment sessions on tDCS clinical efficacy: results from a factorial, randomized, controlled trial in major depression. Neuromodulation J Int Neuromodulation Soc. 2014;17:737–42. doi: 10.1111/ner.12167. https://doi.org/10.1111/ner.12167. discussion 742. [DOI] [PubMed] [Google Scholar]
- 145.George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatr. 2010;67:507–16. doi: 10.1001/archgenpsychiatry.2010.46. https://doi.org/10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- 146.Aparício LVM, Guarienti F, Razza LB, Carvalho AF, Fregni F, Brunoni AR. A systematic review on the acceptability and tolerability of transcranial direct current stimulation treatment in neuropsychiatry trials. Brain Stimulat. 2016;9:671–81. doi: 10.1016/j.brs.2016.05.004. https://doi.org/10.1016/j.brs.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 147.Valiengo LCL, Goulart AC, de Oliveira JF, Benseñor IM, Lotufo PA, Brunoni AR. Transcranial direct current stimulation for the treatment of post-stroke depression: results from a randomised, sham-controlled, double-blinded trial. J Neurol Neurosurg Psychiatry. 2017;88:170–5. doi: 10.1136/jnnp-2016-314075. https://doi.org/10.1136/jnnp-2016-314075. [DOI] [PubMed] [Google Scholar]
- 148.Reis J, Robertson E, Krakauer JW, Rothwell J, Marshall L, Gerloff C, et al. Consensus: “Can tDCS and TMS enhance motor learning and memory formation?”. Brain Stimulat. 2008;1:363–9. doi: 10.1016/j.brs.2008.08.001. https://doi.org/10.1016/j.brs.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72:443–54. doi: 10.1016/j.neuron.2011.10.008. https://doi.org/10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Censor N, Horovitz SG, Cohen LG. Interference with existing memories alters offline intrinsic functional brain connectivity. Neuron. 2014;81:69–76. doi: 10.1016/j.neuron.2013.10.042. https://doi.org/10.1016/j.neuron.2013.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinical utility of transcranial direct current stimulation (tDCS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. J Psychiatr Res. 2013;47:1–7. doi: 10.1016/j.jpsychires.2012.09.025. https://doi.org/10.1016/j.jpsychires.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 152.Meron D, Hedger N, Garner M, Baldwin DS. Transcranial direct current stimulation (tDCS) in the treatment of depression: systematic review and meta-analysis of efficacy and tolerability. Neurosci Biobehav Rev. 2015;57:46–62. doi: 10.1016/j.neubiorev.2015.07.012. https://doi.org/10.1016/j.neubiorev.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 153.Shiozawa P, Fregni F, Benseñor IM, Lotufo PA, Berlim MT, Daskalakis JZ, et al. Transcranial direct current stimulation for major depression: an updated systematic review and meta-analysis. Int J Neuropsychopharmacol. 2014;17:1443–52. doi: 10.1017/S1461145714000418. https://doi.org/10.1017/S1461145714000418. [DOI] [PubMed] [Google Scholar]
- 154.Remillard G. The study of sequence learning in individuals with schizophrenia: a critical review of the literature. J Neuropsychol. 2014;8:231–45. doi: 10.1111/jnp.12022. https://doi.org/10.1111/jnp.12022. [DOI] [PubMed] [Google Scholar]
- 155.Martin DM, Liu R, Alonzo A, Green M, Player MJ, Sachdev P, et al. Can transcranial direct current stimulation enhance outcomes from cognitive training? A randomized controlled trial in healthy participants. Int J Neu-ropsychopharmacol. 2013;16:1927–36. doi: 10.1017/S1461145713000539. https://doi.org/10.1017/S1461145713000539. [DOI] [PubMed] [Google Scholar]
- 156.Buch ER, Santarnecchi E, Antal A, Born J, Celnik PA, Classen J, et al. Effects of tDCS on motor learning and memory formation: a consensus and critical position paper. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2017;128:589–603. doi: 10.1016/j.clinph.2017.01.004. https://doi.org/10.1016/j.clinph.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 157.Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, et al. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci. 2003;15:619–26. doi: 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- 158.Hashemirad F, Zoghi M, Fitzgerald PB, Jaberzadeh S. The effect of anodal transcranial direct current stimulation on motor sequence learning in healthy individuals: a systematic review and meta-analysis. Brain Cogn. 2016;102:1–12. doi: 10.1016/j.bandc.2015.11.005. https://doi.org/10.1016/j.bandc.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 159.Deecke L, Scheid P, Kornhuber HH. Distribution of readiness potential, premotion positivity, and motor potential of the human cerebral cortex preceding voluntary finger movements. Exp Brain Res. 1969;7:158–68. doi: 10.1007/BF00235441. [DOI] [PubMed] [Google Scholar]
- 160.Manoach DS, White NS, Lindgren KA, Heckers S, Coleman MJ, Dubal S, et al. Hemispheric specialization of the lateral prefrontal cortex for strategic processing during spatial and shape working memory. Neuroimage. 2004;21:894–903. doi: 10.1016/j.neuroimage.2003.10.025. https://doi.org/10.1016/j.neuroimage.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 161.Horvath JC, Forte JD, Carter O. Evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: a systematic review. Neuropsychologia. 2015;66:213–36. doi: 10.1016/j.neuropsychologia.2014.11.021. https://doi.org/10.1016/j.neuropsychologia.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 162.Waters-Metenier S, Husain M, Wiestler T, Diedrichsen J. Bihemispheric transcranial direct current stimulation enhances effector-independent representations of motor synergy and sequence learning. J Neurosci Off J Soc Neurosci. 2014;34:1037–50. doi: 10.1523/JNEUROSCI.2282-13.2014. https://doi.org/10.1523/JNEUROSCI.2282-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.D’Urso G, Mantovani A, Micillo M, Priori A, Muscettola G. Transcranial direct current stimulation and cognitive-behavioral therapy: evidence of a synergistic effect in treatment-resistant depression. Brain Stimulat. 2013;6:465–7. doi: 10.1016/j.brs.2012.09.003. https://doi.org/10.1016/j.brs.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 164.Brunoni AR, Boggio PS, De Raedt R, Benseñor IM, Lotufo PA, Namur V, et al. Cognitive control therapy and transcranial direct current stimulation for depression: a randomized, double-blinded, controlled trial. J Affect Disord. 2014;162:43–9. doi: 10.1016/j.jad.2014.03.026. https://doi.org/10.1016/j.jad.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 165.Nienow TM, MacDonald AW, Lim KO. TDCS produces incremental gain when combined with working memory training in patients with schizophrenia: a proof of concept pilot study. Schizophr Res. 2016;172:218–9. doi: 10.1016/j.schres.2016.01.053. https://doi.org/10.1016/j.schres.2016.01.053. [DOI] [PubMed] [Google Scholar]
- 166.Rabey JM, Dobronevsky E, Aichenbaum S, Gonen O, Marton RG, Khaigrekht M. Repetitive transcranial magnetic stimulation combined with cognitive training is a safe and effective modality for the treatment of Alzheimer’s disease: a randomized, double-blind study. J Neural Transm Vienna Austria. 1996;2013(120):813–9. doi: 10.1007/s00702-012-0902-z. https://doi.org/10.1007/s00702-012-0902-z. [DOI] [PubMed] [Google Scholar]
- 167.Fridriksson J, Richardson JD, Baker JM, Rorden C. Transcranial direct current stimulation improves naming reaction time in fluent aphasia: a double-blind, sham-controlled study. Stroke. 2011;42:819–21. doi: 10.1161/STROKEAHA.110.600288. https://doi.org/10.1161/STROKEAHA.110.600288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Allman C, Amadi U, Winkler AM, Wilkins L, Filippini N, Kischka U, et al. Ipsilesional anodal tDCS enhances the functional benefits of rehabilitation in patients after stroke. Sci Transl Med. 2016;8:330. doi: 10.1126/scitranslmed.aad5651. https://doi.org/10.1126/scitranslmed.aad5651.re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bolognini N, Vallar G, Casati C, Latif LA, El-Nazer R, Williams J, et al. Neurophysiological and behavioral effects of tDCS combined with constraint-induced movement therapy in poststroke patients. Neurorehabilitation Neural Repair. 2011;25:819–29. doi: 10.1177/1545968311411056. https://doi.org/10.1177/1545968311411056. [DOI] [PubMed] [Google Scholar]
- 170.Edwards DJ, Krebs HI, Rykman A, Zipse J, Thickbroom GW, Mastaglia FL, et al. Raised corticomotor excitability of M1 forearm area following anodal tDCS is sustained during robotic wrist therapy in chronic stroke. Restor Neurol Neurosci. 2009;27:199–207. doi: 10.3233/RNN-2009-0470. https://doi.org/10.3233/RNN-2009-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75:2176–84. doi: 10.1212/WNL.0b013e318202013a. https://doi.org/10.1212/WNL.0b013e318202013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, et al. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci Off J Soc Neurosci. 2004;24:3379–85. doi: 10.1523/JNEUROSCI.5316-03.2004. https://doi.org/10.1523/JNEUROSCI.5316-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Giacobbe V, Krebs HI, Volpe BT, Pascual-Leone A, Rykman A, Zeiarati G, et al. Transcranial direct current stimulation (tDCS) and robotic practice in chronic stroke: the dimension of timing. NeuroRehabilitation. 2013;33:49–56. doi: 10.3233/NRE-130927. https://doi.org/10.3233/NRE-130927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Iezzi E, Conte A, Suppa A, Agostino R, Dinapoli L, Scontrini A, et al. Phasic voluntary movements reverse the aftereffects of subsequent theta-burst stimulation in humans. J Neurophysiol. 2008;100:2070–6. doi: 10.1152/jn.90521.2008. https://doi.org/10.1152/jn.90521.2008. [DOI] [PubMed] [Google Scholar]
- 175.Tsagaris KZ, Labar DR, Edwards DJ. A framework for combining rTMS with behavioral therapy. Front Syst Neurosci. 2016;10:82. doi: 10.3389/fnsys.2016.00082. https://doi.org/10.3389/fnsys.2016.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li LM, Uehara K, Hanakawa T. The contribution of interindividual factors to variability of response in transcranial direct current stimulation studies. Front Cell Neurosci. 2015;9:181. doi: 10.3389/fncel.2015.00181. https://doi.org/10.3389/fncel.2015.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Strube W, Bunse T, Malchow B, Hasan A. Efficacy and interindividual variability in motor-cortex plasticity following anodal tDCS and paired-associative stimulation. Neural Plast. 2015;2015:530423. doi: 10.1155/2015/530423. https://doi.org/10.1155/2015/530423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bastani A, Jaberzadeh S. Does anodal transcranial direct current stimulation enhance excitability of the motor cortex and motor function in healthy individuals and subjects with stroke: a systematic review and meta-analysis. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2012;123:644–57. doi: 10.1016/j.clinph.2011.08.029. https://doi.org/10.1016/j.clinph.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 179.Butler AJ, Shuster M, O’Hara E, Hurley K, Middlebrooks D, Guilkey K. A meta-analysis of the efficacy of anodal transcranial direct current stimulation for upper limb motor recovery in stroke survivors. J Hand Ther Off J Am Soc Hand Ther. 2013;26:162–70. doi: 10.1016/j.jht.2012.07.002. https://doi.org/10.1016/j.jht.2012.07.002. quiz 171. [DOI] [PubMed] [Google Scholar]
- 180.Lefaucheur J-P, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS) Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2016;128:56–92. doi: 10.1016/j.clinph.2016.10.087. https://doi.org/10.1016/j.clinph.2016.10.087. [DOI] [PubMed] [Google Scholar]
- 181.Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron. 2012;75:963–80. doi: 10.1016/j.neuron.2012.09.004. https://doi.org/10.1016/j.neuron.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 182.Kasten FH, Dowsett J, Herrmann CS. Sustained aftereffect of a-tACS lasts up to 70 min after stimulation. Front Hum Neurosci. 2016;10:245. doi: 10.3389/fnhum.2016.00245. https://doi.org/10.3389/fnhum.2016.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lustenberger C, Boyle MR, Alagapan S, Mellin JM, Vaughn BV, Fröhlich F. Feedback-controlled transcranial alternating current stimulation reveals a functional role of sleep spindles in motor memory consolidation. Curr Biol. 2016;26:2127–36. doi: 10.1016/j.cub.2016.06.044. https://doi.org/10.1016/j.cub.2016.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vannorsdall TD, van Steenburgh JJ, Schretlen DJ, Jayatillake R, Skolasky RL, Gordon B. Reproducibility of tDCS results in a randomized trial: failure to replicate findings of tDCS-induced enhancement of verbal fluency. Cogn Behav Neurol Off J Soc Behav Cogn Neurol. 2016;29:11–7. doi: 10.1097/WNN.0000000000000086. https://doi.org/10.1097/WNN.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 185.Finkel EJ, Eastwick PW, Reis HT. Best research practices in psychology: illustrating epistemological and pragmatic considerations with the case of relationship science. J Pers Soc Psychol. 2015;108:275–97. doi: 10.1037/pspi0000007. https://doi.org/10.1037/pspi0000007. [DOI] [PubMed] [Google Scholar]
- 186.Barch DM, Gotlib IH, Bilder RM, Pine DS, Smoller JW, Brown CH, et al. Common measures for National Institute of Mental Health funded research. Biol Psychiatr. 2016;79:e91–96. doi: 10.1016/j.biopsych.2015.07.006. https://doi.org/10.1016/j.biopsych.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hudson KL, Collins FS. Sharing and reporting the results of clinical trials. J Am Med Assoc. 2015;313:355–6. doi: 10.1001/jama.2014.10716. https://doi.org/10.1001/jama.2014.10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hudson KL, Collins FS. The 21st Century Cures act - a view from the NIH. N Engl J Med. 2017;376:111–3. doi: 10.1056/NEJMp1615745. https://doi.org/10.1056/NEJMp1615745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Margolis R, Derr L, Dunn M, Huerta M, Larkin J, Sheehan J, et al. The National Institutes of Health’s Big Data to Knowledge (BD2K) initiative: capitalizing on biomedical big data. J Am Med Inform Assoc JAMIA. 2014;21:957–8. doi: 10.1136/amiajnl-2014-002974. https://doi.org/10.1136/amiajnl-2014-002974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kaplan RM, Irvin VL. Likelihood of null effects of large NHLBI clinical trials has increased over time. PLos One. 2015;10:e0132382. doi: 10.1371/journal.pone.0132382. https://doi.org/10.1371/journal.pone.0132382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Thielscher A, Antunes A, Saturnino GB. Field modeling for transcranial magnetic stimulation: a useful tool to understand the physiological effects of TMS? Conf Proc IEEE Eng Med Biol Soc 2015. 2015:222–5. doi: 10.1109/EMBC.2015.7318340. https://doi.org/10.1109/EMBC.2015.7318340. [DOI] [PubMed]


