Abstract
There is a great variation in the prevalence of cervical HPV infection worldwide with some of the highest rates being found in African women. Early onset of sexual activity (≤ 15 age), multiparity and sexual promiscuity have been recognized as some of the significant risk factors for HPV infection. In Nigeria, there is scarcity of data on the degree of relationship between these factors and the prevalence of HPV infection. Thus, this study was designed to determine the prevalence of genital HPV infection with its potential risk factors among women in Southwest Nigeria. Cervical swab specimen was collected from 295 consenting women including those presenting for routine cervical cancer screening, STI clinic attendees and women who attended community based outreach programmes. Viral DNA was extracted from the swab samples using commercially available DNA extraction Kit and amplified by PCR using two set of consensus primers (PGMY09/11 and degenerate GP-E6/E7). Fifty-five samples were positive to HPV DNA giving a prevalence of 18.6%. Risk factors such as lack of formal education (P-value: 0.003), divorcee (P-value: 0.019), polygamy (P-value: 0.027), unemployment (P-value: 0.023), low income earnings (P-value: 0.018), younger age (<18years) at sexual debut (P-value: 0.039) and passive smoking (P-value: 0.017) were significantly associated with HPV infection. High HPV prevalence and associated risk factors observed in this study shows the continuous transmission of the virus in Southwest Nigeria. Hence, enlarged monitoring including intense public awareness and cervical cancer screening is urgently needed for prevention and control strategies.
Keywords: Human papillomavirus, prevalence, risk factors, E6/E7 genes, Southwest Nigeria
INTRODUCTION
Globally, genital Human papillomavirus (HPV) infection is the most common sexually transmitted viral infection (zur Hausen, 2000) and about 75% of sexually active women and men will acquire a genital HPV infection at some time in their lifetime (Aral and Holmes, 2008). Human papillomaviruses of the Papillomaviridae family are small, non-enveloped, epitheliotropic, double-stranded DNA viruses (Woodman et al., 2007). They have been shown to be associated with benign and malignant epithelial lesions in humans (Zheng and Baker, 2006). The link between HPV infection and cervical cancer has been well established; HPV is found in 99.7% of cervical cancers specimens (Walboomers et al., 1999; Denny et al., 2014). Cervical cancer represents the fourth most common malignancy in women around the world with estimated 527,624 new cases per year (Ferlay et al., 2015), and the second most common cancer, i.e. next to breast cancer in Nigeria (WHO/ICO HPV information centre, 2012).
There is a great variation in the prevalence of cervical HPV infection worldwide with some of the highest rates being found in African women. About 291 million women worldwide are infected with HPV DNA, of whom 32% are infected with HPV16 or HPV18, or both (de Sanjose et al., 2007). Bruni et al. (2010) reported an estimated HPV prevalence of 11.7% globally and 24% in Sub-Saharan Africa. However, approximately 85% of the global HPV burden has been reported in the less developed countries, where it accounts for almost 12% of all cancers in females (WHO, 2016).
Global data on HPV infection shows that Africa has the highest prevalence of 22.1% (de Sanjose et al., 2007). HPV prevalence of 2.2% was reported in Sudan (Salih et al., 2010); 16.3% in rural Uganda (Serwadda et al., 1999); 20.4% in South African women (Allan et al., 2008); 23.5% in the Republic of Congo (Boumba et al., 2013); 25.4% in Burkina Faso (Traore et al., 2016); 33.2% in Benin Republic (Piras et al., 2011); 34% in Rwanda (Ngabo et al., 2016); 50.8% in Guinea (Keita et al., 2009) and 76% in Morocco (Birrou et al., 2015). In Nigeria, different HPV prevalence have been reported which ranged from 10% in Port Harcourt (Kennedy et al., 2016), 26.3% in Ibadan, Nigeria (Thomas et al., 2004) to 37% in Abuja (Akarolo-Anthony et al., 2014).
Some risk factors such as young age, early age (≤15years) at first sexual intercourse, sexual promiscuity and immunosuppression have been consistently associated with HPV infection in women (Das et al., 2000; Vinodhini et al., 2012; CDC, 2010). The risk increases with increasing number of recent and lifetime sexual partners. However, some other factors like long-term hormonal contraceptives usage, tobacco smoking, low socioeconomic status and poor nutrition have been less consistently associated with HPV infection (Das et al., 2000; CDC, 2013). In Nigeria, there is paucity of data on the degree of relationship between these factors and the prevalence of HPV infection. Thus, the aim of this study was to determine the prevalence of HPV infection with its potential risk factors among women in South-west Nigeria.
MATERIALS AND METHODS
Ethical approval for the study was obtained from UI/UCH IRC as well as the Ethical Committee of the various institutions where samples were collected. Samples were collected from two health facilities including, University College Hospital, Ibadan, Baptist Medical Centre, Saki and Molete community in Ibadan all located in Oyo State, Southwest Nigeria. Informed consent was obtained from each woman before participation in this study. A total of 295 sexually active individuals from age 15 years and above were enrolled from March, 2014 to November, 2015. These include women presenting for routine cervical cancer screening (Pap smear), sexually transmitted infection (STI) clinic attendees and women who attended community-based outreach programmes. Women with or without cytological abnormalities within the period of this study were recruited. Pregnant women or women who have undergone hysterectomy or menstruating women at the time of sample collection were excluded from the study. Structured questionnaire was used to obtain socio-demographic, behavioural and sexual information from each participant. Two cervical swab samples were collected from the endocervix of each participant and placed into labelled screw-capped tubes containing 0.5mL of viral transport medium. The specimens were transported to the laboratory in the Department of Virology, University College Hospital, Ibadan on ice packs and stored at −80°C until analysed.
Viral DNA was extracted from the cervical swab samples using commercially available DNA Extraction Kit (Jena Bioscience, Jena, Germany) according to the manufacturers’ instructions. The quality of the extracted DNA was ascertained by amplifying the human beta-globin gene with PC04 and GH20 primers. The extracted DNA that was positive for beta-globin gene was amplified by PCR using two set of consensus primers to detect HPV DNA; PGMY09/11 primer as described by Winder et al. (2009) and degenerate GP-E6/E7 primer as described by Sotlar et al. (2004). These primers target the highly conserved region of the viral L1 major capsid gene and the E6/E7 oncogenes respectively. The amplified DNA was detected on 2% agarose gel by electrophoresis. Data collectedwere analysed using IBM SPSS statistic version 21 software. Chi square statistics was used to estimate the degree of correlation between variables with p values of <0.05 considered as statistically significant.
RESULTS
Out of the 295 individuals that participated in this study, 178 (60.3%) were women who visited clinic for cervical cancer screening (Pap smear), 93 (31.5%) were STI clinic attendees and 24 (8.1%) were women who attended the community-based outreach programme. More individuals were recruited in Ibadan (75.9%) than in Saki (24.1%).
Socio-Demographic Characteristics of participants
The age of individuals for this study ranged from 23 to 77years with a mean age of 42.5±11.5 years. Majority of participants were in the age group 25-54years (81.8%), were married (84.7%) and in a monogamous marriage (76.3%). Most of the participants have tertiary education (60.3%) while only 8.8% have no formal education. Although 93.9% were employed including private and self-employment, more than half (55.6%) were average income earners. The Yorubas (87.1%) predominated among the participants while about half of the participants (51.2%) were resident in Oyo state. The age of participants at sexual debut ranged from 9 to 51years with a mean age of 23.7±8.6 years. Nearly all (91.2%) have given birth at least once while 27.5% were in their post-menopausal age.
Prevalence of HPV infection
Out of the 295 genital swab samples analysed, 55 samples were positive to HPV DNA giving an overall prevalence of 18.6%. Fifty-one of the samples (17.3%) were HPV DNA positive using the GP-E6/E7 primers while only 27 samples (9.2%) were positive with PGMY09/11 primers. The agarose gel electrophoresis images of HPV DNAs’ amplification are shown in Figures 1 and 2. The size of the PCR products generated with GP-E6/E7 primers ranged from 602 to 666bp due to sequence variations in the HPV DNA (Figure 1) while the length of amplicons generated with PGMY09/11 is 450bp in size (Figure 2). The prevalence of HPV infection was highest (36.6%) among STI clinic attendees (Figure 3) and lowest (4.2%) among women in the community-based outreach programmes (p-value: 0.001).
Figure 1.
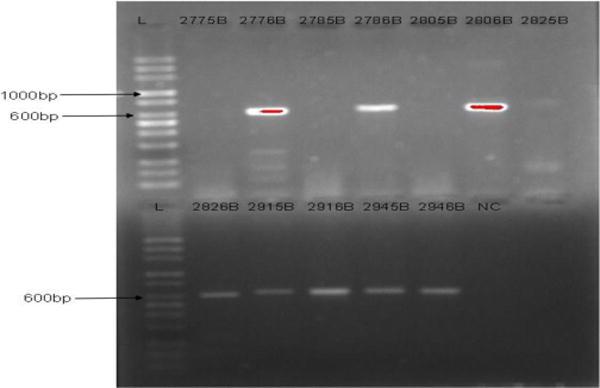
Agarose gel electrophoresis Image of HPV DNA amplification with GP-E6/E7 Consensus Primers
Figure 2.
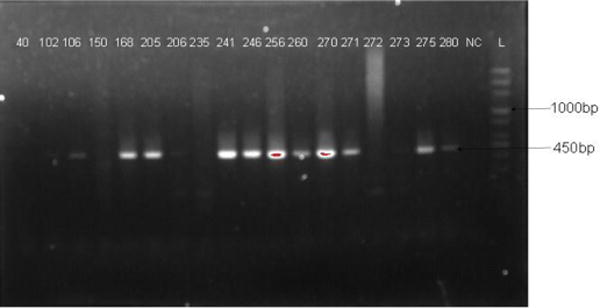
Agarose gel electrophoresis Image of HPV DNA amplification with PGMY09/11 Consensus Primers
Figure 3.
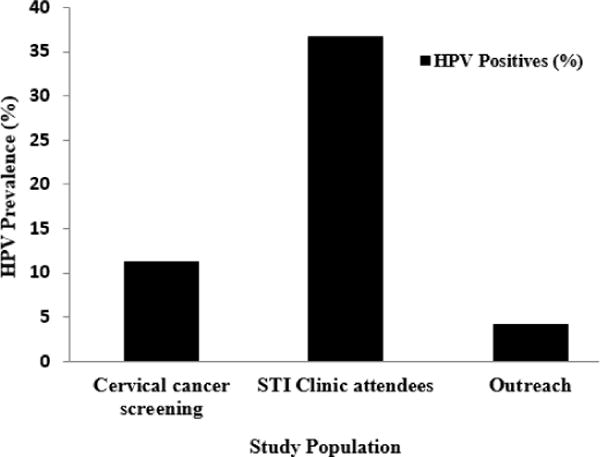
Prevalence of HPV infection by study population
Table 1 shows the prevalence of HPV infection according to socio-demographic characteristics of the study participants. There was a progressive decrease in the prevalence of HPV infection by age with the highest rate found among the 15-24 age-group (25.0%). However, this difference was not significant (P-value: 0.490).
Table 1.
Prevalence of HPV infection according to socio-demographic characteristics of study participants
| Characteristics | Categories | No. (%) screened | No. (%) positive | p-value |
|---|---|---|---|---|
| Age (years) | <25 | 4 (1.4) | 1 (25.0) | |
|
|
||||
| 25-34 | 76 (25.8) | 18 (23.7) | ||
|
|
||||
| 35-44 | 102 (34.6) | 21 (20.6) | 0.490 | |
|
|
||||
| 45-54 | 63 (21.4) | 10 (15.9) | ||
|
|
||||
| 55-64 | 39 (13.2) | 4 (10.3) | ||
|
|
||||
| ≥65 | 11 (3.7) | 1 (9.1) | ||
|
| ||||
| Single | 16 (5.4) | 2 (12.5) | 0.019 | |
|
|
||||
| Marital status | Married | 250 (84.7) | 44 (17.6) | |
|
|
||||
| Widow | 18 (6.1) | 3 (16.7) | ||
|
|
||||
| Divorced | 11 (3.7) | 6 (54.5) | ||
|
| ||||
| Marriage type | Monogamy | 225 (76.3) | 37 (16.4) | 0.027 |
|
|
||||
| Polygamy | 54 (18.3) | 16 (29.6) | ||
|
| ||||
| Level of Education | No Education | 26 (8.8) | 10 (38.5) | 0.003 |
|
|
||||
| Primary | 34 (11.5) | 8 (23.5) | ||
|
|
||||
| Secondary | 57 (19.3) | 15 (26.3) | ||
|
|
||||
| Tertiary | 178 (60.3) | 22 (12.4) | ||
|
| ||||
| Employment status | Employed | 277 (93.9) | 48 (17.3) | 0.023 |
|
|
||||
| Unemployed | 18 (6.1) | 7 (38.9) | ||
|
| ||||
| Income earners | High | 48 (16.3) | 7 (14.6) | 0.018 |
|
|
||||
| Average | 164 (55.6) | 24 (14.6) | ||
|
|
||||
| Low | 83 (28.1) | 24 (28.9) | ||
|
| ||||
| State of residence | Oyo | 151 (51.2) | 36 (23.8) | 0.345 |
|
|
||||
| Ekiti | 17 (5.8) | 1 (5.9) | ||
|
|
||||
| Ondo | 10 (3.4) | 2 (20.0) | ||
|
|
||||
| Osun | 32 (10.8) | 5 (15.6) | ||
|
|
||||
| Lagos | 4 (1.4) | 0 (0.0) | ||
|
|
||||
| Ogun | 29 (9.8) | 2 (6.9) | ||
|
|
||||
| Other states | 41 (13.9) | 7 (17.1) | ||
|
|
||||
| No Response | 8 (2.7) | 1 (12.5) | ||
HPV: Human papillomavirus
The prevalence of HPV infection was significantly higher among the divorcee (p-value: 0.019), polygamous women (p- value: 0.027), illiterates (no formal education) (p-value: 0.003), unemployed (p-value: 0.023) and low income earners (p-value: 0.018). There was no significant association of HPV infection with state of residence.
Table 2 shows the prevalence of HPV infection based on sexual and smoking history of study participants. HPV infection was significantly associated with age at sexual debut (P-value: 0.039) and living with cigarette smokers (passive smoking) (P-value: 0.017). On the other hand, HPV infection was not associated with cigarette smoking and the use of any form of contraceptive. Although the difference was not significant, the rate of infection was higher among women with more than one life time sexual partner than those that have only one (P-value: 0.086).
Table 2.
Distribution of HPV infection by sexual and smoking history of study participants
| Characteristics | Categories | No. (%) Screened | No. (%) Positive | P-Value |
|---|---|---|---|---|
| Age at sexual debut (Years) | <18 | 36 (12.2) | 12 (33.3) | 0.039 |
| 18-25 | 188 (63.7) | 31 (16.5) | ||
| 26-35 | 50 (16.9) | 7 (14.0) | ||
| Lifetime sexual Partner | 1 | 151 (51.2) | 23 (15.2) | 0.086 |
| ≥2 | 124 (42.0) | 29 (23.4) | ||
| Condom use as contraceptives | Yes | 41 (13.9) | 11 (26.8) | 0.147 |
| No | 254 (86.1) | 44 (17.3) | ||
| Hormonal contraceptive use | Yes | 51 (17.3) | 6(11.8) | 0.165 |
| No | 244 (82.7) | 49 (20.1) | ||
| Intrauterine device use | Yes | 84 (28.5) | 11(13.1) | 0.123 |
| No | 211 (71.5) | 44 (20.9) | ||
| Direct smoking | Yes | 3 (1.0) | 1 (33.3) | 0.511 |
| No | 292 (99.0) | 54 (18.5) | ||
| Passive smoking | Yes | 5 (1.7) | 3 (60.0) | 0.017 |
| No | 290 (98.3) | 52 (17.9) |
Table 3 shows the prevalence of HPV infection based on the clinical characteristics of study participants. No significant association of HPV infection was found with the number of times the women had given birth (parity), post-menopausal age, genital warts, symptoms of STI and its duration. Participants that have never been screened for cervical cancer had a higher preponderance of HPV infection but with low significance (P-value: 0.063). Two (18.2%) among 11 participants who reported abnormal result had HPV infection.
Table 3.
Prevalence of HPV infection by clinical characteristics of study participants
| Characteristics | Categories | No. (%) screened | No. (%) positive | p |
|---|---|---|---|---|
| Parity | 0 | 19 (6.4) | 3 (15.8) | 0.456 |
| 1 | 32 (10.9) | 9 (28.1) | ||
| 2 | 49 (16.6) | 10 (20.4) | ||
| ≥3 | 189 (64.1) | 33 (17.5) | ||
| Post-menopausal age | 1-5 | 48 (16.3) | 8 (16.7) | 0.685 |
| 6-10 | 23 (7.8) | 3 (13.0) | ||
| ≥11 | 10 (3.3) | 3 (30.0) | ||
| Symptoms of STI | Yes | 121 (41.0) | 25 (20.7) | 0.458 |
| No | 174 (59.0) | 30 (17.2) | ||
| STI duration (years) | 1-10 | 111 (37.6) | 23 (20.7) | 0.970 |
| 11-20 | 5 (1.7) | 1 (20.0) | ||
| Long time | 5 (1.7) | 1 (20.0) | ||
| Genital warts | Yes | 24 (8.1) | 5 (20.8) | 0.774 |
| No | 271 (91.9) | 50 (18.5) | ||
| Ever had Pap smear | Yes | 72 (24.4) | 8 (11.1) | 0.059 |
| No | 223 (75.6) | 47 (21.1) | ||
| Cervical cancer screening result | Normal | 61 (20.7) 6 (9.8) | 0.063 | |
| LSIL | 9 (3.1) 1 (11.1) | |||
| HSIL | 1 (0.3) 0 (0.0) | |||
| Cervical cancer | 1 (0.3) | 1 (100.0) |
HPV: Human papillomavirus
LSIL: Low-grade Squamous Intraepithelial Lesion HSIL: High-grade Squamous Intraepithelial Lesion
DISCUSSION
The HPV prevalence of 18.6% obtained in this study is high, compared to the adjusted global HPV prevalence of 10.41%, 10.4% and 11.7% reported by Burchell et al. (2006), de Sanjose et al. (2007) and Bruni et al. (2010) respectively. This high rate is an indication of continuous transmission of the infection and hence the importance of implementation of measures for the control of the spread of the virus and its resultant sequel in Nigeria. The result of this study was also higher than some previous reports on HPV prevalence in Nigeria; 14.7% in Irun (Gage et al., 2013) and 10% in Port Harcourt (Kennedy et al., 2016) but comparable with 17.0% and 18% reported among Western Africa women (Xi et al., 2003; de Sanjose et al., 2007).
However, some studies on HPV infection in Nigeria have reported higher prevalence of 21.6%-44.9% (Thomas et al., 2004; Schnatz et al., 2008; Akarolo-Anthony et al., 2014; Nweke et al., 2013). The difference in the reported HPV rates in Nigeria may be due to various factors such as sensitivity of HPV assay used, different study population with varying exposures to different risk factors based on diverse socio-cultural differences.
Although there was no significant association between HPV and age, the highest prevalence was found among individuals younger than 25 years of age, and lowest among the 65 years and above age group. This pattern has been reported by some previous studies in Nigeria (Akarolo-Anthony et al., 2014; Kennedy et al., 2016). The highest prevalence among younger age group may be an indication of sexual transmission, as it coincides with the initiation of sexual activity. Some biological mechanisms such as cervical immaturity, inadequate production of protective cervical mucus and increased cervical ectopy in younger women and adolescents could make them more susceptible to HPV infection (Kahn et al., 2002).
The highest prevalence of HPV infection was found among the divorcee women. According to Idso et al. (2009), one frequently accepted postulation is that divorced and separated women tend to return into dating act and new sexual partners thus increasing their risk of HPV infection. This may be an explanation for the high level of HPV infection found among this group of individuals in this study. On the other hand, Akarolo-Anthony et al. (2014) reported a higher prevalence of HPV among the married (61%) over the unmarried (39%), but a higher positivity among singles than married was reported by Thomas et al. (2004); these differences were however not significant.
Although there was no significant association between HPV and age, the highest prevalence was found among individuals younger than 25 years of age, and lowest among the 65 years and above age group. This pattern has been reported by some previous studies in Nigeria (Akarolo-Anthony et al., 2014; Kennedy et al., 2016). The highest prevalence among younger age group may be an indication of sexual transmission, as it coincides with the initiation of sexual activity. Some biological mechanisms such as cervical immaturity, inadequate production of protective cervical mucus and increased cervical ectopy in younger women and adolescents could make them more susceptible to HPV infection (Kahn et al., 2002).
The highest prevalence of HPV infection was found among the divorcee women. According to Idso et al. (2009), one frequently accepted postulation is that divorced and separated women tend to return into dating act and new sexual partners thus increasing their risk of HPV infection. This may be an explanation for the high level of HPV infection found among this group of individuals in this study. On the other hand, Akarolo-Anthony et al. (2014) reported a higher prevalence of HPV among the married (61%) over the unmarried (39%), but a higher positivity among singles than married was reported by Thomas et al. (2004); these differences were however not significant.
The higher rate of HPV infection found among women in a polygamous relationship in this study may be because polygamy has been reported to be a factor in the spread of sexually transmitted infections like HPV (Bayo et al., 2002; Rousseau et al., 2003). The risk of HPV infection has been reported to increase with increase in the number of wives within a family (Bayo et al., 2002). The result of this study is consistent with the findings of Xi et al. (2003).
In this study, there was no association between HPV infection and parity. This is in agreement with the previous findings of Thomas et al. (2004) and Sarma et al. (2013). However, Kennedy et al. (2016) found that patients with higher parity (˃3) had about two times higher risk of HPV infection, a report similar to that of Xi et al. (2003) and Fadahunsi et al. (2013). The differences in the findings of these various studies is significant but according to CDC (2015), there is still insufficient data to give final conclusions about the effect of number of births on the risk of HPV infections.
Result further shows that individuals with no formal education (illiterate) are at higher risk of acquiring HPV infection. This is similar to the findings of Thomas et al. (2004) with significant association between HPV positivity and illiteracy; an indicator of poverty. Kennedy et al. (2016) also confirms a statistically significant relationship between lack of education and the presence of oncogenic HPV. Lack of education has been associated with high risk sexual practices and a poor health seeking attitude which has resulted to increased STIs like HPV (Esere, 2008).
The rate of HPV infection was higher among the unemployed and an association was found between HPV infection and low income earners. It is more likely that most of these unemployed are low income earners and this could increase their level of poverty as well as high risk sexual practices resulting to acquiring STIs like HPV. Some other studies however did not find any association between HPV infection and employment status (Baloch et al., 2016; Traore et al., 2016).
Several cross-sectional studies have reported that earlier sexual initiation is a risk factor for HPV infection (Kahn et al., 2002a, 2002b; Collins et al., 2005). An association between HPV positivity and early age at first sexual intercourse (<18 years) was obtained in this study. This could be because earlier intercourse exposes young adults to other risky sexual behaviour, such as greater numbers of lifetime sexual partners and coexisting partnerships (Aral and Holmes, 1999). Similarly, there have been strong and consistent associations between numbers of new and recent sexual partners and HPV infection in female genital tract (Koutsky and Kiviat, 1999; Bayo et al., 2002; Winer and Koutsky, 2004). In agreement with previous findings by Thomas et al. (2004), the prevalence of HPV infection increases with increasing numbers of life time sexual partners though not statistically significant. Rivera et al. (2012) and Clarke et al. (2011) however showed a significantly higher HPV incidence among women with history of more than one sexual partner. Reason have been well explained by data supporting sexual intercourse as the primary route of genital HPV infection (Oriel, 1971; Partridge and Koutsky, 2006), and increased risk of HPV acquisition from new and recent sexual partners (Winer and Koutsky, 2004)
Although the rate of HPV infection was relatively higher among participants who reported symptoms of STI than those without STI symptoms, the difference was not statistically significant. Previous study reported that cervical infection with other STIs, such as Chlamydia trachomatis, Neisseria gonorrhoeae, HSV and Trichomonas vaginalis may increase susceptibility to genital HPV infection by microwound or cervical inflammation, or aid persistence of HPV infection through immunological mechanisms (Samoff et al., 2005). Nonetheless, this study did not test for any other genital infections giving no evidence to support the role of other STIs in HPV infection.
The result of this study showed no significant association of HPV infection with contraceptives use and duration of use. This result contrasts the findings of Clarke et al. (2011) which recorded a significant association between HPV infection and birth control use. Intrauterine contraceptive device was mostly used among the women in this study but higher HPV infection was found among those who use condom which could mean that this population is not consistent with condom use. Similar findings by a previous study showed that HPV infection is unrelated to the type of contraceptives used (Xi et al., 2003). Cigarette smoking has been associated to be risk factor for cervical HPV infection. In this study, only 1% of individuals have ever smoked cigarette (direct smokers) and HPV infection rate was higher among them, although not significantly. However, it is worthy to note that those who lived with smokers (passive smokers/second-hand smokers) had significantly higher HPV prevalence. In a previous study in China, a significantly higher prevalence of HPV infection was found among smokers in urban women and also a non-significant higher prevalence among smokers in rural women (Baloch et al., 2016). In addition, a 2015 overview of systematic reviews found that exposure to second-hand smoke increased the risk of cervical cancer (Cao et al., 2015). Cigarette smoking influences epithelial immunity by decreasing the numbers of antigen-presenting Langerhans cells in the genital epithelium. Such depletion could favour HPV infection, viral persistence thus contributing to malignant transformation.
Among participants with cervical screening result, higher HPV prevalence was found among those with abnormal result but not significantly (P-value: 0.063). Similar findings were obtained from the result of past studies (Xi et al., 2003; Thomas et al., 2004 and Meloni et al., 2014). Among those with abnormal result, the only one with cervical cancer was positive for HPV infection. This is expected because HPV has been recognized as the main causal agent of cervical cancer (Walboomers et al., 1999).
In conclusion, high prevalence of HPV obtained in this study is an indication of continuous transmission of HPV infection among women in Southwest Nigeria. Some risk factors such as divorce, polygamy, illiteracy, unemployment, low income earnings, younger age at sexual debut were identified in this study. In addition, low level of awareness about HPV infections and cervical screening was observed among the study participants despite high level of educational background, hence, a campaign to create awareness on HPV infection is urgently needed for prevention and control of the infection in Nigeria. Current HPV vaccines especially the 9-valent vaccine should have a huge possibility of reducing HPV infection and cervical cancer in Nigeria and hence should be licenced and incorporated into the routine immunization programme in the country.
Acknowledgments
We gratefully acknowledge all the participants who took part in this study. We also thank the Staff of the cytology unit of the Obstetrics and Gynaecology Department in University College Hospital, Ibadan, and of the Baptist Medical Centre, Saki, Oyo State for their assistance in sample collection in the course of this study. Dr Adedayo Faneye is also acknowledged for her useful advice in this study. Data analysis and writing of this paper was supported by the University of Ibadan Medical Education Partnership Initiative Junior Faculty Training Programme (UI-MEPI-J) project funded by Fogarty International Center, National Institute of Health under Award Number D43TW010140. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations.
References
- Akarolo-Anthony SN, Famooto AO, Dareng EO, Olaniyan OB, Offiong R, Wheeler CM, Adebamowo CA. Age-specific prevalence of human papilloma virus infection among Nigerian women. BMC Public Health. 2014;14(1):656. doi: 10.1186/1471-2458-14-656. https://doi.org/10.1186/1471-2458-14-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan B, Marais DJ, Hoffman M, Shapiro S, Williamson AL. Cervical human papillomavirus (HPV) infection in South African women: Implications for HPV screening and vaccine strategies. Journal of Clinical Microbiology. 2008;46:740–742. doi: 10.1128/JCM.01981-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aral SO, Holmes KK. The epidemiology of STIs and their social and behavioural determinants: industrialized and developing countries. In: Holmes KK, Sparling PF, Stamm WE, Piot P, Wasserheit JN, Corey L, Cohen MS, Watts DH, editors. Sexually Transmitted Diseases. 4th. New York: McGraw-Hill; 2008. 2008. pp. 53–92. [Google Scholar]
- Baloch Z, Yuan T, Yindi S, Feng Y, Tai W, Liu Y, Liu L, Zhang A, Wang B, Wu X, Xia X. Prevalence of genital human papillomavirus among rural and urban populations in southern Yunnan province, China. Brazilian Journal of Medical and Biological Research. 2016;49:1–7. doi: 10.1590/1414-431X20165254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayo S, Bosch FX, de Sanjosé S, Muñoz N, Combita AL, Coursaget P, Diaz M, Dolo A, van den Brule AJ, Meijer CJ. Risk factors of invasive cervical cancer in Mali. International Journal Epidemiology. 2002;31(1):202–209. doi: 10.1093/ije/31.1.202. [DOI] [PubMed] [Google Scholar]
- Borena W, Grünberger M, Widschwendter A, Kraxner KH, Marth E, Mayr P, Holm-von Laer D. Pre-vaccine era cervical Human papillomavirus infection among screening population of women in west Austria. BMC Public Health. 2016;16(1):889. doi: 10.1186/s12889-016-3581-0. http://doi.org/10.1186/s12889-016-3581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumba LMA, Assoumou SZ, Hilali L, Mambou JV, Moukassa D, Ennaji MM. Genetic variability in E6 and E7 oncogenes of human papillomavirus Type 16 from Congolese cervical cancer isolates. Infectious Agents and Cancer. 2015;10(1):15. doi: 10.1186/s13027-015-0010-4. https://doi.org/10.1186/s13027-015-0010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni L, Barrionuevo-Rosas L, Albero G, Serrano B, Mena M, Gomez Z, Muñoz J, Bosch FX, de SS. Human Papillomavirus and related diseases report. ICO Information Centre on HPV and Cancer (HPV Information Centre); 2016. [Google Scholar]
- Bruni L, Barrionuevo-Rosas L, Albero G, Aldea M, Serrano B, Valencia S, Brotons M, Mena M, Cosano R, Muñoz J, Bosch FX, Sanjosé S de, Castellsagué X. ICO Information Centre on HPV and Cancer (HPV Information Centre) 2015. (Human Papillomavirus and Related Diseases in Nigeria: Summary Report 2015-12-23). [Google Scholar]
- Burchell AN, Winer RL, de Sanjosé S, Franco EL. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006:24. doi: 10.1016/j.vaccine.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Cao S, Yang C, Gan Y, Lu Z. The Health Effects of Passive Smoking: An Overview of Systematic Reviews Based on Observational Epidemiological Evidence. PloS One. 2015;10(10):e0139907. doi: 10.1371/journal.pone.0139907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellsague X, Menendez C, Loscertales MP, Kornegay JR, dos Santos F, Gomez-Olive FX, Lloveras B, Abarca N, Vaz N, Barreto A, Bosch FX, Alonso P. Human papillomavirus genotypes in rural Mozambique. Lancet. 2001;358:1429–1430. doi: 10.1016/S0140-6736(01)06523-0. [DOI] [PubMed] [Google Scholar]
- Castle PE, Schiffman M, Herrero R, Hildesheim A, Rodriguez AC, Bratti MC, Sherman ME, Wacholder S, Tarone R, Burk RD. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis. 2005;191:1808–1816. doi: 10.1086/428779. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. STD curruculum for clinical educators. Genital Human papillomavirus. 2013:1–37. [Google Scholar]
- Centers for Disease Control and Prevention. Division of STD Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Centers for Disease Control and Prevention; 2015. Jun 12, [Google Scholar]
- Clarke MA, Gage JC, Ajenifuja KO, Wentzensen NA, Adepiti AC, Wacholder S, Burk RD, Schiffman M. A population-based, cross-sectional study of age-specific risk factors for high risk human papillomavirus prevalence in rural Nigeria. Infect Agent Cancer. 2011;6:12. doi: 10.1186/1750-9378-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SI, Mazloomzadeh S, Winter H, Rollason TP, Blomfield P, Young LS, Woodman CB. Proximity of first intercourse to menarche and the risk of human papillomavirus infection: A longitudinal study. International Journal of Cancer. 2005;114(3):498–500. doi: 10.1002/ijc.20732. [DOI] [PubMed] [Google Scholar]
- Das BC, Gopalkrishna V, Hedau S, Katiyar S. Cancer of the uterine cervix and human papillomavirus infection. CURRENT SCIENCE, 2000. 2000;78(1):10. [Google Scholar]
- de Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, Muñoz N, Bosch FX. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7(7):453–59. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- De Vuyst H, Steyaert S, Van Renterghem L, Claeys P, Muchiri L, Sitati S, Vansteelandt S, Quint W, Kleter B, Van Marck E, Temmerman M. Distribution of human papillomavirus in a family planning population in nairobi, kenya. Sex Transm Dis. 2003;30:137–142. doi: 10.1097/00007435-200302000-00009. [DOI] [PubMed] [Google Scholar]
- Denny L, Adewole I, Anorlu R, Dreyer G, Moodley M, Smith T, Snyman L, Wiredu E, Molijn A, Quint W, Ramakrishnan G, Schmidt J. Human papillomavirus prevalence and type distribution in invasive cervical cancer in sub-Saharan Africa. Int J Cancer. 2014;134:1389–1398. doi: 10.1002/ijc.28425. [DOI] [PubMed] [Google Scholar]
- Esere MO. Effect of sex education programme on at-risk sexual behavior of school-going adolescents in Ilorin Nigeria. Afr Health Sci. 2008;8(2):120–125. [PMC free article] [PubMed] [Google Scholar]
- Fadahunsi OO, Omoniyi-Esan GO, Banjo AA, Esimai OA, Osiagwu D, Clement F, Adeteye OV, Bejide RA, Iyiola S., 1 Prevalence of High Risk oncogenic HPV types in cervical smears of women attending well women clinic in Ile-Ife. Gynaecol Obstet. 2013;3(6):1000185. [Google Scholar]
- Fonseca AJ, Taeko D, Chaves TA, Amorim LDC, Murari RSW, Miranda AE, Chen Z, Burk RD, Ferreira LCL. HPV Infection and Cervical Screening in Socially Isolated Indigenous Women Inhabitants of the Amazonian Rainforest. PLoS ONE. 2015;10(7):e0133635. doi: 10.1371/journal.pone.0133635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage JC, Ajenifuja KO, Wentzensen N, Adepiti AC, Stoler M, Eder PS, Bell L, Shrestha N, Eklund C, Reilly M, Hutchinson M, Wacholder S, Castle PE, Burk RD, Schiffman M. Effectiveness of a simple rapid human papillomavirus DNA test in rural Nigeria. International Journal of Cancer. 2013 doi: 10.1002/ijc.27563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero R, Hildesheim AC, Bratti ME, Sherman M, Hutchinson J, Morales I, Balmaceda M, Greenberg D, Alfaro M, Burk RD, Wacholder S, Plummer M, Schiffman M. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst. 2000;92:464–74. doi: 10.1093/jnci/92.6.464. [DOI] [PubMed] [Google Scholar]
- Idso C. Sexually transmitted infection prevention in newly single older women: a forgotten health promotion need. Journal for Nurse Practitioners. 2009;5(6):440–6. [Google Scholar]
- Jiang Y, Brassard P, Severini A, Mao Y, Li Y, Laroche J, Chatwood S, Corriveau A, Kandola K, Hanley B, Sobol I, Ar-Rushdi M, Johnson G, Lo J, Ratnam S, Wong T, Demers A, Jayaraman G, Totten S, Morrison H. The prevalence of human papillomavirus and its impact on cervical dysplasia in Northern Canada. Infectious Agents and Cancer. 2013;8:25. doi: 10.1186/1750-9378-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn JA, Rosenthal SL, Succop PA, Ho GY, Burk RD. Mediators of the association between age of first sexual intercourse and subsequent Human papillomavirus infection. Paediatrics. 2002a;109(1):E5. doi: 10.1542/peds.109.1.e5. [DOI] [PubMed] [Google Scholar]
- Kahn JA, Rosenthal SL, Succop PA, Ho GY, Burk RD. The interval between menarche and age of first sexual intercourse as a risk factor for subsequent HPV infection in adolescent and young adult women. Journal of Paediatrics. 2002b;141(5):718–723. doi: 10.1067/mpd.2002.128893. [DOI] [PubMed] [Google Scholar]
- Keita N, Clifford GM, Koulibaly M, Douno K, Kabba I, Haba M, Franceschi S. HPV infection in women with and without cervical cancer in Conakry, Guinea. British Journal of Cancer. 2009;101(1):202–208. doi: 10.1038/sj.bjc.6605140. http://doi.org/10.1038/sj.bjc.6605140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy NT, Ikechukwu D, Goddy B. Risk factors and distribution of oncogenic strains of human papilloma virus in women presenting for cervical cancer screening in Port Harcourt, Nigeria. The Pan African medical journal. 2016;23:85. doi: 10.11604/pamj.2016.23.85.8510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsky LA, Kiviat NB. Genital human papillomavirus. In: Holmes KK, Mardh P-A, Sparling PF, et al., editors. Sexually transmitted diseases. New York: McGraw-Hill; 1999. pp. 347–59. [Google Scholar]
- Kuhn L, Denny L, Pollack A, Lorincz A, Richart RM, Wright TC. Human papillomavirus DNA testing for cervical cancer screening in low-resource settings. J Natl Cancer Inst. 2000;92:818–825. doi: 10.1093/jnci/92.10.818. [DOI] [PubMed] [Google Scholar]
- Lazcano-Ponce E, Herrero R, Munoz N, Cruz A, Shah KV, Alonso P, Hernandez P, Salmeron J, Hernandez M. Epidemiology of HPV infection among Mexican women with normal cervical cytology. Int J Cancer. 2001;91:412–20. doi: 10.1002/1097-0215(20010201)91:3<412::aid-ijc1071>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Meloni A, Pilia R, Campagna M, Usai A, Masia G, Caredda V, Coppola RC. Prevalence and molecular epidemiology of human papillomavirus infection in Italian women with cervical cytological abnormalities. Journal of Public Health Research. 2014;3 doi: 10.4081/jphr.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngabo F, Franceschi S, Baussano I, Umulisa MC, Snijders PJF, Uyterlinde AM, Lazzarato F, Tenet V, Gatera M, Binagwaho A, Clifford MG. Human papillomavirus infection in Rwanda at the moment of implementation of a national HPV vaccination programme. BMC Infectious Diseases (2016) 2016;16:225. doi: 10.1186/s12879-016-1539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nweke IG, Banjo AAF, Abdulkareem FB, Nwadike VU. British Microbiology Research Journal. New Delhi: 2013. Prevalence of Human Papilloma virus DNA in HIV positive women in Lagos University Teaching Hospital (LUTH) Lagos, Nigeria. Retrieved from http://www.sciencedomain.org/abstract.php?iid=219&id=8&aid=1525. [Google Scholar]
- Oriel JD. Natural history of genital warts. Br J Vener Dis. 1971;47(1):1–13. doi: 10.1136/sti.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JM, Koutsky LA. Genital human papillomavirus infection in men. Lancet Infect Dis. 2006;6(1):21–31. doi: 10.1016/S1473-3099(05)70323-6. [DOI] [PubMed] [Google Scholar]
- Piras F, Piga M, De Montis A, Zannou AR, Minerba L, Perra MT, Murtas D, Atzori M, Pittau M, Maxia C, Sirigu P. Prevalence of human papillomavirus infection in women in Benin, West Africa. Virology Journal. 2011;8:514. doi: 10.1186/1743-422X-8-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau MC, Abrahamowicz M, Villa LL, Costa MC, Thomas E, Rohan TE, Franco EL. Predictors of Cervical Co-infection with Multiple Human Papillomavirus Types. Cancer Epidemiology, Biomarkers & Prevention. 2003;12(10):1029–1037. [PubMed] [Google Scholar]
- Salih MM, Safi ME, Hart K, Tobi K, Adam I. Genotypes of human papilloma virus in Sudanese women with cervical pathology. Infectious Agents and Cancer. 2010;5:26. doi: 10.1186/1750-9378-5-26. http://doi.org/10.1186/1750-9378-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoff E, Koumans EH, Markowitz LE, Sternberg M, Sawyer MK, Swan D, Papp JR, Black CM, Unger ER. Association of Chlamydia trachomatis with persistence of high-risk types of human papillomavirus in a cohort of female adolescents. Am J Epidemiol. 2005;162(7):668–75. doi: 10.1093/aje/kwi262. [DOI] [PubMed] [Google Scholar]
- Sarma U, Mahanta J, Borkakoty BJ, Talukdar KL, Gogoi R, Yadav K. Demographic Characteristic of HPV Infection in Women - A Hospital Based Study from Guwahati, India. National Journal of Medical Research. 2013;3(1):1–4. [Google Scholar]
- Schnatz PF, Markelova NV, Holmes D, Mandavilli SR, O’Sullivan DM. The prevalence of cervical HPV and cytological abnormalities in association with reproductive factors of rural Nigerian women. J Womens Health (Larchmt) 2008;17:279–285. doi: 10.1089/jwh.2006.0295. [DOI] [PubMed] [Google Scholar]
- Serwadda D, Wawer MJ, Shah KV, Sewankambo NK, Daniel R, Li C, Lorincz A, Meehan MP, Wabwire-Mangen F, Gray RH. Use of a hybrid capture assay of self-collected vaginal swabs in rural Uganda for detection of human papillomavirus. J Infect Dis. 1999;180:1316–1319. doi: 10.1086/315026. [DOI] [PubMed] [Google Scholar]
- Smith JS, Melendy A, Rana RK, Pimenta JM. Age-specific prevalence of infection with human papillomavirus in females: a global review. J Adolesc Health. 2008;43:S5–S25. S25e21–41. doi: 10.1016/j.jadohealth.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Sotlar K, Diemer D, Dethleffs A, Hack Y, Stubner A, Vollmer N, Bültmann B. Detection and typing of human papillomavirus by E6 nested multiplex PCR. Journal of Clinical Microbiology. 2004;42(7):3176–3184. doi: 10.1128/JCM.42.7.3176-3184.2004. https://doi.org/10.1128/JCM.42.7.3176-3184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotlar K, Diemer D, Dethleffs A, Hack Y, Stubner A, Vollmer N, Menton S, Menton M, Dietz K, Wallwiener D, Kandolf R, Bültmann B. Detection and typing of human papillomavirus by E6 nested multiplex PCR. Journal of Clinical Microbiology. 2004;42:3176–3184. doi: 10.1128/JCM.42.7.3176-3184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temmerman M, Tyndall MW, Kidula N, Claeys P, Muchiri L, Quint W. Risk factors for human papillomavirus and cervical precancerous lesions, and the role of concurrent HIV-1 infection. Int J Gynaecol Obstet. 1999;65:171–181. doi: 10.1016/s0020-7292(99)00043-0. [DOI] [PubMed] [Google Scholar]
- Thomas JO, Herrero R, Omigbodun AA, Ojemakinde K, Ajayi IO, Fawole A, Oladepo O, Smith JS, Arslan A, Munoz N, Snijders PJ, Meijer CJ, Franceschi S. Prevalence of papillomavirus infection in women in Ibadan, Nigeria: a population-based study. Br J Cancer. 2004;90:638–645. doi: 10.1038/sj.bjc.6601515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traore IMA, Zohoncon TM, Dembele A, Djigma FW, Obiri-Yeboah D, Traore G, Simpore J. Molecular Characterization of High-Risk Human Papillomavirus in Women in Bobo-Dioulasso, Burkina Faso. BioMed Research International. 2016;2016:7092583. doi: 10.1155/2016/7092583. http://doi.org/10.1155/2016/7092583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarella S, Herrero R, Snijders PJF, Dai M, Thomas JO, Hieu NTC, Ferreccio E, Matos H, Posso S, de Sanjosé HR, Shin S, Sukvirach E, Lazcano-Ponce N, Muñoz CJ, Meijer LM, Franceschi S, the IARC HPV Prevalence Surveys (IHPS) Study Group Smoking and human papillomavirus infection: pooled analysis of the International Agency for Research on Cancer HPV Prevalence Surveys. Int J Epidemiol. 2008;37:536–46. doi: 10.1093/ije/dyn033. [DOI] [PubMed] [Google Scholar]
- Vinodhini K, Shanmughapriya S, Das BC, Natarajaseenivasan K. Prevalence and risk factors of HPV infection among women from various provinces of the world. Archives of Gynecol Obstet. 2012;2012285(3):771–7. doi: 10.1007/s00404-011-2155-8. [DOI] [PubMed] [Google Scholar]
- Walboomers JMM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Wiley DJ, Wiesmeier E, Masongsong E, Gylys KH, Koutsky LA, Ferris DG, Barr E, Yu RJ. Smokers at higher risk for undetected antibody for oncogenic human papillomavirus type 16 infection. Cancer Epidemiol Biomarkers Prev. 2006;15:915–20. doi: 10.1158/1055-9965.EPI-05-0963. [DOI] [PubMed] [Google Scholar]
- Winder DM, Ball SL, Vaughan K, Hanna N, Woo YL, Fränzer JT, Sterling JC, Stanley MA, Sudhoff H, Goon PK. Sensitive HPV detection in oropharyngeal cancers. BMC Cancer. 2009;9:440. doi: 10.1186/1471-2407-9-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer RL, Koutsky LA. The epidemiology of human papillomavirus infections. In: Rohan Dordrecht T, Shah K., editors. Cervical cancer: from etiology to prevention. The Netherlands: Kluwer Academic Publishers; 2004. pp. 143–87. [Google Scholar]
- Woodman CBJ, Stuart I, Collins SI, Young LS. The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer. 2007;7:11–22. doi: 10.1038/nrc2050. [DOI] [PubMed] [Google Scholar]
- Xi LF, Toure P, Critchlow CW, Hawes SE, Dembele B, Sow PS, Kiviat NB. Prevalence of specific types of human papillomavirus and cervical squamous intraepithelial lesions in consecutive, previously unscreened, West-African women over 35 years of age. Int J Cancer. 2003;103:803–809. doi: 10.1002/ijc.10876. [DOI] [PubMed] [Google Scholar]
- Yang L, Xie S, Feng X, Chen Y, Zheng T, Dai M, Zhou CK, Hu Z, Li N, Hang D. Worldwide Prevalence of Human Papillomavirus and Relative Risk of Prostate Cancer: A Meta-analysis. Nature Publishing Group. 2015:1–10. doi: 10.1038/srep14667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng ZM, Baker CC. Papillomavirus genome structure, expression, and post-transcriptional regulation. Front Biosci. 2006;11(1):2286–2302. doi: 10.2741/1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst. 2000;3(92(9)):690–8. doi: 10.1093/jnci/92.9.690. [DOI] [PubMed] [Google Scholar]


