Abstract
Background and objective
The Brahma gene (BRM) encodes a catalytic ATPase subunit of the Switch/Sucrose non-fermentable (SWI/SNF) complex, which modulates gene expression and many important cellular processes. Two indel polymorphisms in the promoter region of BRM (BRM-741 and BRM-1321) are associated with its reduced expression and the risk of susceptibility or survival outcomes in multiple solid cancers. In this study, we have examined these variants in relation to susceptibility and survival outcomes in colorectal cancer.
Methods
Genotypes were obtained using TaqMan assays in 427 cases and 408 controls. Multivariate logistic and Cox regression models were fitted to examine the associations of the BRM-741 and BRM-1321 genotypes adjusting for relevant covariates. Sub-group analyses based on tumor location and patient sex were also performed. In all analyses, indels were examined individually as well as in combination.
Results
Our results showed that there was no association between the BRM polymorphisms and the risk of colorectal cancer. However, genotype combinations of the BRM-741 and BRM-1321 variants were associated with the risk of colon cancer. Particularly, patients having at least one variant allele had increased risk of colon cancer when compared to patients with the double wild-type genotype. In the survival analyses, BRM-741 heterozygosity was associated with longer progression-free survival time in the colorectal cancer patients. A stronger association was detected in the male patients under the recessive genetic model where the homozygosity for the variant allele of BRM-741 was associated with shorter progression-free survival time.
Conclusions
Our analyses suggest that BRM-741 and BRM-1321 indels are associated with the risk of developing colon cancer and the BRM-741 indel is associated with the disease progression in colorectal cancer patients, especially in the male patients. Although our results show a different relationship between these indels and colorectal cancer compared to other cancer sites, they also suggest that BRM and its promoter variants may have biological roles in susceptibility and survival outcomes in colorectal cancers. Performing further analyses in additional and larger cohorts are needed to confirm our conclusions.
Introduction
Each year, around 1.4 million people are diagnosed with colorectal cancer and about 700,000 deaths occur because of it [1]. In Canada, around one in 15 people are expected to be diagnosed with this disease in their lifetime [2]. Worldwide, ~40–66% colorectal cancer patients do not survive 5-years after diagnosis [3]. Understanding factors, including genetic factors, that influence the susceptibility to this disease and patient prognosis can help improve its control and patient survival outcomes. For this reason, many studies have examined the associations of genetic variations with the risk of developing colorectal cancer or clinically important events after diagnosis [4–11].
BRM encodes Brahma, one of the two mutually exclusive DNA-dependent ATPase subunits of the SWI/SNF complex [12]. The SWI/SNF complex includes several subunits, exists in multiple forms with different subunit compositions, facilitates transcriptional regulation through remodeling of the chromatin, and is known to play critical roles in many important biological processes, such as cell proliferation and differentiation [13, 14]. Not surprisingly, several alterations of the multiple SWI/SNF complex subunits (including of BRM) have been identified in cancer, linking them to carcinogenesis or disease progression [14, 15].
Loss of BRM is often observed in various types of tumors [16–20], which is mainly mediated through epigenetic silencing [16]. Two promoter polymorphisms, BRM-741 and BRM-1321, are highly correlated with the expression levels of BRM [21]. Both of these polymorphisms are indel/repeating sequence variants [21]. BRM-741 consists of two (deletion or wild-type allele = Del) or three (insertion or variant allele = Ins) copies of a 7 bp long sequence (TATTTTT) located 741 bp upstream of the BRM transcription start site. BRM-1321, on the other hand, exists as either one (deletion or wild-type allele = Del) or two copies (insertion or variant allele = Ins) of a 6 bp long sequence (TTTTAA) located further upstream of the BRM transcription start site [21]. Variant sequences of these two polymorphisms are highly homologous to the binding site for myocyte enhancer factor-2 (MEF-2), which together with histone deacetylases (HDACs) has been shown to epigenetically silence the BRM gene [21, 22]. In examination of tissue samples, Liu et al. [21] associated the homozygosity for the variant allele (Ins/Ins) in either or both of the indels with the absence of BRM protein in both lung tumor and unaffected tissues. Examination by Gao et al. [23] showed that in both hepatocellular carcinoma tumors and non-tumor tissues the BRM expression levels decreased similarly with each Ins allele of BRM-1321. It is not known at the time being whether the Ins allele of BRM-741 has a similar effect on BRM expression as in the case of BRM-1321 (i.e. expression levels decreasing similarly with each copy of the Ins allele), but considering the fact that the BRM silencing is mediated through the binding of the MEF-2 and HDACs to the Ins alleles [21,22], it is a plausible possibility. Last but not least, both indels are linked to each other to varying degrees in different populations (D’ = 0.39–0.86) [20, 21, 23–26] and are common in Caucasians with similar minor allele frequencies (MAFs) of 45% [21].
Because BRM-741 and BRM-1321 can affect the expression of BRM and thus the activity of SWI/SNF, it is reasonable to suspect that these two polymorphisms may influence the risk or prognosis of human cancers. Supporting this, specific genotypes of either -741, -1321, or their combinations have been reported to be associated with the risk of lung [21, 27], head and neck [20, 27], and liver [23] cancers. Similar associations with the survival outcomes of lung [24], esophageal [25], hepatocellular [26] and pancreatic cancer [28] patients have also been detected. However, these two indels were not evaluated for their potential associations with the risk or survival outcomes in colorectal cancer before. In this study, we tested these associations in colorectal cancer cases and controls from Newfoundland.
Methods
Ethical approval
This study was approved by the Health Research Ethics Authority (HREA) of Newfoundland and Labrador (Reference numbers 09.106 and 15.294). Since this was a secondary use of data, no patient consent specific for this study was required.
Study cohorts
Cases and controls recruited to Newfoundland Familial Colorectal Cancer Registry (NFCCR) were examined. NFCCR was described elsewhere in detail [29, 30]. In brief, participants (or their family members) provided consent to participate in NFCCR. A total of 750 stage 0-IV cases diagnosed between January 1999 and December 2003 were recruited. Age, sex and other related demographic information was collected at the time of recruitment. Access to medical records and blood or tissue samples were requested. Individuals free of colorectal cancer were enrolled as controls in the year of 2004 and 2005 by random-digit-dialing [31]. Controls were frequency-matched with the cases in terms of age and sex. In total, 720 controls were recruited. Blood samples and demographic information using questionnaires were collected at the time of recruitment. Cases who smoked cigarettes before the time of diagnosis were defined as ever-smokers while those did not smoke till this time point were defined as never-smokers. For controls, the time of recruitment was used as the time point to define ever-smoker and never-smoker status. Body mass index (BMI) was calculated based on the body mass and height information provided by the participants. For cases, these data were based on approximately one year before the diagnosis and for controls, these data were based on approximately two years before their recruitment.
Exclusion criteria for the study cohorts included: (1) cases and controls who were >75 years of age; (2) cases and controls who self-identified themselves as non-white or of mixed race; those who did not provide this information were also excluded; (3) cases who were diagnosed with stage 0 disease; (4) cases who were affected by Lynch syndrome, familial colorectal cancer type X (FCCX), or familial adenomatous polyposis (FAP); (5) cases who were the first, second, or third degree relatives with each other; in such a case one of the patients were randomly excluded. This information was based on a previously obtained genome-wide SNP genotype data [11] and was available for all but three patients; (6) controls who had a known first, second, or third degree relative in the case cohort; (7) controls who are known to have developed colorectal cancer after recruitment; (8) controls with no epidemiological/demographic data; and (9) cases or controls without genomic DNA extracted from blood samples. In the end, 427 cases and 408 controls passed these eligibility criteria. As for the survival analysis, one patient with no prognosis-related data was excluded. Characteristics of the cases and controls are summarized in Table 1.
Table 1. Distribution of baseline characteristics of the study cohorts.
| Characteristics | Cases N (%) |
Controls N (%) |
* P value |
|---|---|---|---|
| Total | 427 (100) | 408 (100) | |
| † Age | 0.40 | ||
| < 65 years | 268 (62.76) | 245 (60.05) | |
| ≥ 65 years | 158 (37.00) | 163 (39.95) | |
| Unknown | 1 (0.23) | - | |
| Sex | 0.91 | ||
| Female | 172 (40.28) | 166 (40.69) | |
| Male | 255 (59.72) | 242 (59.31) | |
| Number of FDR with colorectal cancer | 0.0004 | ||
| 0 | 304 (71.19) | 333 (81.62) | |
| At least 1 | 123 (28.81) | 75 (18.38) | |
| Smoking status | 0.09 | ||
| Never | 124 (29.04) | 143 (35.05) | |
| Ever | 296 (69.32) | 265 (64.95) | |
| Unknown | 7 (1.64) | - | |
| ‡ BMI | 0.35 | ||
| Underweight and normal | 119 (27.87) | 127 (31.13) | |
| Overweight and obese | 294 (68.85) | 272 (66.67) | |
| Unknown | 14 (3.28) | 9 (2.21) | |
| § Disease stage | - | ||
| I | 76 (17.84) | - | |
| II | 167 (39.20) | - | |
| III | 140 (32.86) | - | |
| IV | 43 (10.09) | - | |
| § Tumor location | - | ||
| colon | 280 (65.73) | - | |
| rectum | 146 (34.27) | - | |
| § MSI status | - | ||
| MSS\MSI-L | 368 (86.38) | - | |
| MSI-H | 40 (9.39) | - | |
| Unknown | 18 (4.23) | - | |
| § Treatment with adjuvant chemotherapy | - | ||
| No | 189 (44.37) | - | |
| Yes | 233 (54.69) | - | |
| Unknown | 4 (0.94) | - |
BMI, body mass index; FDR, first-degree relative(s); MSI, microsatellite instability; MSI-H, microsatellite instability-high; MSI-L, microsatellite instability-low; MSS, microsatellite stable; N, number. P values < 0.05 are bolded.
* Two-sided χ2 test for comparison between cases and controls with available data.
† Age is the age at diagnosis for cases, and age at recruitment for controls.
‡ Underweight, normal, overweight, and obese are defined as BMI <18.5, 18.5 ≤ BMI < 25, 25 ≤ BMI < 30, BMI ≥ 30, respectively. Categorization criterion was based on the information provided on the website of National Institutes of Health (https://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmicalc.htm).
§ Total number of cases is 426.
Follow-up
Patients were followed until the year 2010. The median follow up was 6.98 years (range: 2.00–10.88 years) with 95% confidence intervals (CIs) of 6.69–7.28 years (calculated based on reverse Kaplan-Meier method [32] using IBM SPSS Statistics-23). Data on vital status and occurrence of recurrence and metastasis were collected from various sources as explained in Negandhi et al. [33]. In brief, collection of prognostic data was performed by NFCCR. Clinical events of interest (i.e. recurrence/metastasis/death) were surveyed through and extracted/obtained from the patients’ medical records, the Newfoundland Cancer Treatment and Research Foundation database, or patient follow-up questionnaires.
DNA genotyping
All NFCCR cases and controls with available DNA samples were genotyped for the BRM promoter indel polymorphisms (n = 493 for cases and n = 448 for controls). Genotyping for BRM -741 and BRM -1321 promoter region polymorphisms was performed by two custom designed Taqman assays (BRM-741: forward primer: 5’ TGGCAGGAACGTTCTTTGTG 3’; reverse primer: 5’ TGCCGGCTGAAACTTTTTCT 3’; probe for insertion: /56-FAM/TCCCTTTTCTA/ZEN/TTTTTTATTTTTTATTTTTTTACCTGGAA/3IABkFQ/; probe for wild-type: /5HEX/CCTCCCTTTTC/ZEN/TATTTTTTATTTTTTTACCTGGAAT/3IABkFQ/; BRM-1321: forward primer: 5’ CATACTTTTCATAACACTACTGCATAGGAACA 3’; reverse primer: 5’ TTTTATGAAGTGTGAAAGAATGTTAGGAGACT 3’; probe for insertion: /56-FAM/A+CT+CTTA+AAAT+T+AAAA+CTGT/3IABkFQ/; probe for wild-type: /5HEX/T+G+CTT+GA+CT+CTTAA+AAC/3IABkFQ/. TaqMan assay reaction condition for BRM-741 and -1321 polymorphisms was: 95°C 2 min followed by 40 cycles of 95°C for 6 sec / 60°C for 20 sec. Reaction volume for each sample was 5μl. PCR master mix was obtained from Kapabiosystems (Kapa probe fast qPCR kit, Cat#kk4702). For BRM-741 and BRM-1321, 9.58% and 19.26% of the DNA samples were genotyped twice and concordance rate was 100%. These methods had been previously compared with Sanger sequencing, and two other sets of primers and probes in 190 patients with 100% concordance.
A total of 831 (n = 424 of 427 cases, n = 407 of 408 controls) and 832 (n = 425 of 427 cases, n = 407 of 408 controls) individuals included into the study were successfully genotyped for the BRM-741 and BRM-1321 variants, respectively.
Statistical analysis
Hardy-Weinberg equilibrium (HWE) calculations were performed using an online calculator (http://www.oege.org/software/hwe-mr-calc.shtml) [34]. D' and r2 for linkage disequilibrium (LD) between BRM-741 and BRM-1321 were calculated by using the LD function of genetics package [35] in R (ver3.2.4) [36]. Chi-squared test was used to examine the differences between cases and controls. All analyses were performed by using R (ver3.2.4) [36] unless otherwise specified.
Similar to other studies, deletions (Del) were assigned as wild-type alleles, and insertions (Ins) as variant alleles. Individual associations of the BRM-741 and BRM-1321 were analyzed under different genetic models (co-dominant, dominant, recessive and additive genetic models). Combination analyses involving both polymorphisms were performed as follows: Category A) the genotype categorizations used by the previous investigators [20, 21, 24, 25, 28]; Category B) double homozygous variant genotype (Ins/Ins+Ins/Ins) compared to others; Category C) double wild-type genotype (Del/Del+Del/Del) compared to others; and Category D) at least one homozygous variant genotype compared to others (shown in S1 Table; Categories A-D).
Association analyses
In the case-control study, unconditional logistic regression analyses were applied to test the associations between the indels and the risk of colorectal cancer. Known risk factors (age, sex, and number of first-degree relatives (FDR) with colorectal cancer) were included in multivariable models. Smoking status and BMI were sequentially examined using the log likelihood ratio test. We first examined smoking status and compared models with and without this variable. As the models were significantly different from each other (P values < 0.001) and the model with this variable had a smaller Akaike Information Criterion (AIC) value [37] (and, thus improved model's fit to data), smoking status was included as a covariate in the baseline model. We then examined BMI and obtained similar results. Thus, BMI was also included in the final baseline model as a covariate. Odds ratios (ORs) and 95% CIs for the genotypes were calculated under the multivariate logistic regression models adjusted for the baseline variables.
Cox Proportional Hazards (PH) regression method was used for survival analyses. The outcome of interest was progression-free survival that was defined as the time from diagnosis till the time of death, or, local or distant recurrence. Patients were censored if they experienced none of the events (death, recurrence or metastasis) till the last follow-up. The proportional hazard assumption was tested by using the cox.zph function [38] in R (ver3.2.4) [36]. Age was the only variable that violated the PH assumption (including genotypes), thus multivariate models were stratified by age. Other model covariates included disease stage, tumor location, microsatellite instability (MSI) status, and treatment status (adjuvant chemotherapy Yes/No). Their independent associations with the outcome were confirmed in a multivariable baseline model. Hazard ratios (HRs) and corresponding 95% CIs for the genotype categories were estimated under the age-stratified multivariate Cox models adjusting for these baseline variables.
Sub-cohort analyses
To explore whether the associations of these indels vary by sex and tumor location (colon, rectum), we also performed sub-cohort analyses separately (S2–S5 Tables). Adjustments in sub-cohort analyses were done by the covariates previously selected, except for the covariate sex in the risk analyses in male and female sub-cohorts, and tumor location in survival analyses in colon and rectal cancer sub-cohorts. In addition, MSI was not included as a covariate in survival analyses of rectal cancer cases because there were only two rectal cancer patients with microsatellite instability-high (MSI-H) tumors.
Results
Minor allele frequencies, Hardy-Weinberg equilibrium test, and linkage disequilibrium between the BRM-741 and BRM-1321 indels
Minor allele frequencies of BRM-741 and BRM-1321 were 48% and 44% in controls and 47% and 43% in cases, respectively. Both BRM-741 and BRM-1321 genotype frequencies satisfied the HWE in controls. D' and the r2 between the BRM-741 and BRM-1321 were lower than 0.8 in cases (D' = 0.48; r2 = 0.20) and controls (D' = 0.58; r2 = 0.29), indicating the two polymorphisms were not highly correlated with each other in this population.
Associations of the BRM-741 and BRM-1321 indels with the susceptibility to colorectal cancer
Case-control analyses in colorectal cases and controls
Cases (n = 427) and controls (n = 408) were comparable to each other in terms of frequency distribution of age, sex, smoking status, and BMI, except the number of FDR affected by colorectal cancer as expected (Table 1). After adjusting for age, sex, number of FDR, smoking status, and BMI, BRM indels were not associated with the risk of colorectal cancer when analyzed alone or together (Table 2).
Table 2. BRM promoter indels and colorectal cancer risk.
| Variable | Genotypes | Cases N (%) |
Controls N (%) |
OR (95% CI) | * P value |
|---|---|---|---|---|---|
| BRM-741 | |||||
| Co-dominant model | |||||
| Del/Del (wild-type) | 119 (27.87) | 113 (27.70) | 1 (reference) | ||
| Ins/Del | 215 (50.35) | 201 (49.26) | 1.09 (0.78, 1.52) | 0.61 | |
| Ins/Ins | 90 (21.08) | 93 (22.79) | 0.96 (0.64, 1.44) | 0.85 | |
| Unknown | 3 (0.70) | 1 (0.25) | |||
| Dominant model | |||||
| Del/Del | 119 (27.87) | 113 (27.70) | 1 (reference) | ||
| Ins/Ins + Ins/Del | 305 (71.43) | 294 (72.06) | 1.05 (0.77, 1.44) | 0.76 | |
| Unknown | 3 (0.70) | 1 (0.25) | |||
| Recessive model | |||||
| Ins/Del + Del/Del | 334 (78.22) | 314 (76.96) | 1 (reference) | ||
| Ins/Ins | 90 (21.08) | 93 (22.79) | 0.91 (0.65, 1.28) | 0.59 | |
| Unknown | 3 (0.70) | 1 (0.25) | |||
| † Additive model | |||||
| Del/Del | 119 (27.87) | 113 (27.70) | 0.99 (0.81, 1.21) | 0.90 | |
| Ins/Del | 215 (50.35) | 201 (49.26) | |||
| Ins/Ins | 90 (21.08) | 93 (22.79) | |||
| Unknown | 3 (0.70) | 1 (0.25) | |||
| BRM-1321 | |||||
| Co-dominant model | |||||
| Del/Del (wild-type) | 136 (31.85) | 135 (33.09) | 1 (reference) | ||
| Ins/Del | 213 (49.88) | 188 (46.08) | 1.20 (0.87, 1.65) | 0.27 | |
| Ins/Ins | 76 (17.80) | 84 (20.59) | 0.93 (0.62, 1.39) | 0.73 | |
| Unknown | 2 (0.47) | 1 (0.25) | |||
| Dominant model | |||||
| Del/Del | 136 (31.85) | 135 (33.09) | 1 (reference) | ||
| Ins/Ins + Ins/Del | 289 (67.68) | 272 (66.67) | 1.11 (0.83, 1.51) | 0.48 | |
| Unknown | 2 (0.47) | 1 (0.25) | |||
| Recessive model | |||||
| Ins/Del + Del/Del | 349 (81.73) | 323 (79.17) | 1 (reference) | ||
| Ins/Ins | 76 (17.80) | 84 (20.59) | 0.84 (0.58, 1.19) | 0.32 | |
| Unknown | 2 (0.47) | 1 (0.25) | |||
| † Additive model | |||||
| Del/Del | 136 (31.85) | 135 (33.09) | 0.99 (0.81, 1.21) | 0.93 | |
| Ins/Del | 213 (49.88) | 188 (46.08) | |||
| Ins/Ins | 76 (17.80) | 84 (20.59) | |||
| Unknown | 2 (0.47) | 1 (0.25) | |||
|
‡
BRM-741 and BRM-1321 genotype combinations |
|||||
| Category A. | |||||
| Double wild-type genotype | 73 (17.10) | 81 (19.85) | 1 (reference) | ||
| No homozygous variant genotype | 223 (52.22) | 196 (48.04) | 1.36 (0.92, 2.00) | 0.12 | |
| One homozygous variant genotype | 91 (21.31) | 81 (19.85) | 1.32 (0.84, 2.08) | 0.23 | |
| Double homozygous variant genotype | 37 (8.67) | 48 (11.76) | 0.90 (0.51, 1.56) | 0.70 | |
| Unknown | 3 (0.70) | 2 (0.49) | |||
| Category B. | |||||
| Other genotype combinations | 387 (90.63) | 358 (87.75) | 1 (reference) | ||
| Double homozygous variant genotype | 37 (8.67) | 48 (11.76) | 0.71 (0.44, 1.13) | 0.15 | |
| Unknown | 3 (0.70) | 2 (0.49) | |||
| Category C. | |||||
| Double wild-type genotype | 73 (17.10) | 81 (19.85) | 1 (reference) | ||
| Other genotype combinations | 351 (82.20) | 325 (79.66) | 1.28 (0.89, 1.84) | 0.19 | |
| Unknown | 3 (0.70) | 2 (0.49) | |||
| Category D. | |||||
| Other genotype combinations | 296 (69.32) | 277 (67.89) | 1 (reference) | ||
| At least one homozygous variant genotype | 128 (29.98) | 129 (31.62) | 0.93 (0.68, 1.26) | 0.63 | |
| Unknown | 3 (0.70) | 2 (0.49) |
CI, confidence interval; Del, deletion; Ins, insertion; N, number; OR, odds ratio.
* Adjusted for age, sex, number of first degree relatives with colorectal cancer, smoking status, and body mass index. Please note that final models include only the patients with the available covariate data. For further information on genotype combinations/categories, please refer to Methods/S1 Table.
† Ins/Ins vs Ins/Del vs Del/Del.
‡ Homozygous variant genotype is Ins/Ins genotype.
Case-control analyses in the sub-cohorts
In multivariate analyses, significant associations were found only in the colon cases and when the BRM-741 and BRM-1321 indel genotypes were analyzed together (Table 3). Specifically, compared to double wild-type genotype (Del/Del+Del/Del), no homozygous or one homozygous variant genotypes were associated with the increased risk of colon cancer (no homozygous variant genotype; OR [95% CI] = 1.65 [1.05–2.63]; P value = 0.03; one homozygous variant genotype; OR [95% CI] = 1.77 [1.05–3.01]; P value = 0.03; Category A). Additionally, compared to the double wild-type genotype (Del/Del+Del/Del), combined genotypes that included at least one variant allele were associated with increased risk of colon cancer (OR [95% CI] = 1.60 [1.04–2.50]; P value = 0.03; Category C). There were no associations detected in the rectal cancer, female, or male cancer sub-cohorts (S2 and S3 Tables).
Table 3. Associations between BRM promoter indels and colon cancer risk.
| Sub-cohort | Variable | Genotypes | Cases N (%) |
Controls N (%) |
OR (95% CI) | * P value |
|---|---|---|---|---|---|---|
| Colon cases + Controls | † BRM-741 and BRM-1321 genotype combination | |||||
| Category A. | ||||||
| Double wild-type genotype | 41 (14.64) | 81 (19.85) | 1 (reference) | |||
| No homozygous variant genotype | 148 (52.86) | 196 (48.04) | 1.65 (1.05, 2.63) | 0.03 | ||
| One homozygous variant genotype | 64 (22.86) | 81 (19.85) | 1.77 (1.05, 3.01) | 0.03 | ||
| Double homozygous variant genotype | 25 (8.93) | 48 (11.76) | 1.14 (0.60, 2.15) | 0.69 | ||
| Unknown | 2 (0.71) | 2 (0.49) | ||||
| Category C. | ||||||
| Double wild-type genotype | 41 (14.64) | 81 (19.85) | 1 (reference) | |||
| Other genotype combinations | 237 (84.64) | 325 (79.66) | 1.60 (1.04, 2.50) | 0.03 | ||
| Unknown | 2 (0.71) | 2 (0.49) |
CI, confidence interval; N, number; OR, odds ratio. P values < 0.05 are bolded.
* Adjusted for age, sex, number of first degree relatives with colorectal cancer, smoking status and body mass index. Please note that final models include only the patients with the available covariate data. For further information on genotype combinations/categories, please refer to Methods/S1 Table.
† Homozygous variant genotype is Ins/Ins genotype.
Only the results with P value less than 0.05 are shown in this table; all results obtained in the sub-cohort analyses are shown in S2 and S3 Tables.
Associations of the BRM-741 and BRM-1321 indels with progression-free survival in colorectal cancer
Survival analyses in the colorectal cancer cases
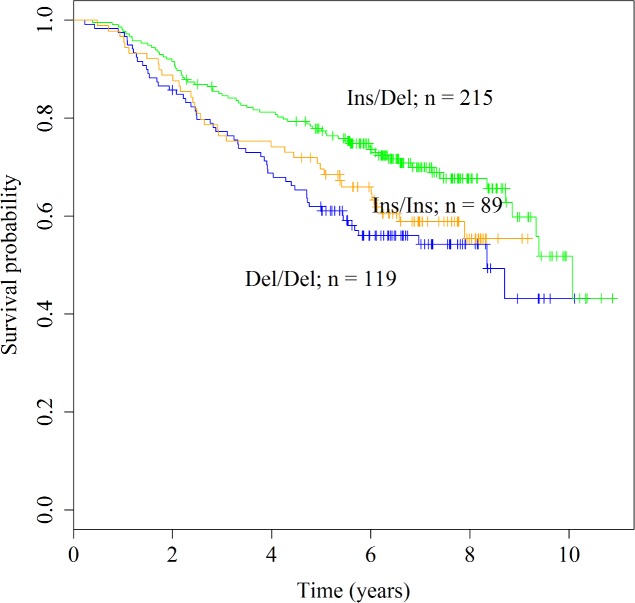
Results are summarized in Table 4. The only association was detected under the co-dominant genetic model where the heterozygosity for the BRM-741 indel was significantly associated with longer progression-free survival time when compared to wild-type genotype (Ins/Del vs Del/Del; HR [95% CI] = 0.67 [0.45, 0.98]; P value = 0.04; Fig 1). This association was independent of age, disease stage, tumor location, MSI and adjuvant chemotherapy status.
Table 4. BRM promoter indels and progression-free survival in colorectal cancer.
| Variable | Genotypes | Cases N (%) |
P value for PH assumption test | HR (95% CI) | * P value |
|---|---|---|---|---|---|
| BRM-741 | |||||
| Co-dominant model | |||||
| Del/Del (wild-type) | 119 (27.93) | 1 (reference) | |||
| Ins/Del | 215 (50.47) | 0.72 | 0.67 (0.45, 0.98) | 0.04 | |
| Ins/Ins | 89 (20.89) | 0.95 | 0.97 (0.62, 1.51) | 0.89 | |
| Unknown | 3 (0.70) | ||||
| Dominant model | |||||
| Del/Del | 119 (27.93) | 1 (reference) | |||
| Ins/Ins + Ins/Del | 304 (71.36) | 0.86 | 0.75 (0.53, 1.07) | 0.12 | |
| Unknown | 3 (0.70) | ||||
| Recessive model | |||||
| Ins/Del + Del/Del | 334 (78.40) | 1 (reference) | |||
| Ins/Ins | 89 (20.89) | 0.81 | 1.24 (0.85, 1.82) | 0.27 | |
| Unknown | 3 (0.70) | ||||
| † Additive model | |||||
| Del/Del | 119 (27.93) | ||||
| Ins/Del | 215 (50.47) | 0.96 | 0.96 (0.75, 1.21) | 0.72 | |
| Ins/Ins | 89 (20.89) | ||||
| Unknown | 3 (0.70) | ||||
| BRM-1321 | |||||
| Co-dominant model | |||||
| Del/Del (wild-type) | 136 (31.92) | 1 (reference) | |||
| Ins/Del | 212 (49.77) | 0.45 | 0.95 (0.66, 1.36) | 0.76 | |
| Ins/Ins | 76 (17.84) | 0.64 | 0.98 (0.61, 1.58) | 0.93 | |
| Unknown | 2 (0.47) | ||||
| Dominant model | |||||
| Del/Del | 136 (31.92) | 1 (reference) | |||
| Ins/Ins + Ins/Del | 288 (67.61) | 0.44 | 0.95 (0.68, 1.34) | 0.79 | |
| Unknown | 2 (0.47) | ||||
| Recessive model | |||||
| Ins/Del + Del/Del | 348 (81.69) | 1 (reference) | |||
| Ins/Ins | 76 (17.84) | 0.88 | 1.01 (0.66, 1.55) | 0.96 | |
| Unknown | 2 (0.47) | ||||
| † Additive model | |||||
| Del/Del | 136 (31.92) | ||||
| Ins/Del | 212 (49.77) | 0.54 | 0.98 (0.78, 1.24) | 0.88 | |
| Ins/Ins | 76 (17.84) | ||||
| Unknown | 2 (0.47) | ||||
|
‡
BRM-741 and BRM-1321 genotype combinations |
|||||
| Category A. | |||||
| Double wild-type genotype | 73 (17.14) | 1 (reference) | |||
| No homozygous variant genotype | 223 (52.35) | 0.56 | 0.66 (0.43, 1.00) | 0.05 | |
| One homozygous variant genotype | 90 (21.13) | 0.60 | 0.72 (0.44, 1.18) | 0.19 | |
| Double homozygous variant genotype | 37 (8.69) | 0.58 | 1.02 (0.55, 1.89) | 0.96 | |
| Unknown | 3 (0.70) | ||||
| Category B. | |||||
| Other genotype combinations | 386 (90.61) | 1 (reference) | |||
| Double homozygous variant genotype | 37 (8.69) | 0.60 | 1.39 (0.81, 2.38) | 0.24 | |
| Unknown | 3 (0.70) | ||||
| Category C. | |||||
| Double wild-type genotype | 73 (17.14) | 1 (reference) | |||
| Other genotype combinations | 350 (82.16) | 0.79 | 0.71 (0.48, 1.05) | 0.09 | |
| Unknown | 3 (0.70) | ||||
| Category D. | |||||
| Other genotype combinations | 296 (69.48) | 1 (reference) | |||
| At least one homozygous variant genotype | 127 (29.81) | 0.51 | 1.07 (0.76, 1.52) | 0.69 | |
| Unknown | 3 (0.70) |
CI, confidence interval; Del, deletion; HR, hazard ratio; Ins, insertion; N, number; PH, proportional hazard. P values < 0.05 are bolded. For further information on genotype combinations/categories, please refer to Methods/S1 Table.
* Results by age stratified Cox models adjusted for disease stage, tumor location, microsatellite instability (MSI) status, and treatment status (adjuvant chemotherapy Yes or No).
† Ins/Ins vs Ins/Del vs Del/Del.
‡ Homozygous variant genotype is Ins/Ins genotype.
Fig 1. Kaplan-Meier curves for the BRM-741 indel under the co-dominant genetic model in the colorectal cancer cases.
P value of the log-rank test is 0.017.
Survival analyses in the sub-cohorts
In the male colorectal cancer cohort, BRM-741 indel was associated with the progression-free survival time under the co-dominant and recessive genetic models (S4 Table). Similar to the results obtained in the entire patient cohort (Table 4), under the co-dominant genetic model heterozygosity for BRM-741 was associated with longer progression-free survival time compared to wild-type genotype (HR [95% CI] = 0.54 [0.34, 0.88]; P value = 0.01). This pattern was also evident in the Kaplan-Meier curves where the male patients with the wild-type Del/Del and the homozygous Ins/Ins genotypes had similar survival probabilities compared to heterozygous Ins/Del individuals who had better survival probability (S1 Fig). A stronger association was detected under the recessive genetic model, where the homozygosity for the BRM-741 Ins allele was associated with decreased progression-free survival time compared to other genotypes (HR [95% CI] = 1.84 [1.17, 2.90]; P value = 0.009; S2 Fig). These associations were restricted to the male patients and were not detected in female, colon, or rectal cancer cases (S4 and S5 Tables).
Discussion
In this study, for the first time we have investigated whether two functional variants (BRM-741 and BRM-1321) located in the promoter region of the BRM gene were associated with the susceptibility to develop colorectal cancer and survival times of the patients. Our results show that presence of at least one variant allele in both of these indels are associated with the increased risk of colon but not rectal cancer. Our results also show that BRM-741 may be associated with progression-free survival time in colorectal cancer patients, particularly in the male patients.
BRM codes for one of the two ATPase subunits of the SWI/SNF complex [12]. Two indel polymorphisms in the promoter region of BRM, BRM-741 and BRM-1321, have been shown to be associated with down-regulation of this gene [21], and therefore may affect the function of the SWI/SNF complex and cellular processes regulated by it. Some of these cellular processes are related to cancer, such as cell proliferation and differentiation [39], making these two genetic variants functionally interesting in cancer research. These variants are not included in many of the genotyping platforms and are not in high LD with other platform polymorphisms to be accurately imputed [21]. This means that the potential associations of these two BRM variants may have been missed in genome-wide studies, including one of ours in the NFCCR patient cohort [11]. A number of research groups genotyped and studied the associations of BRM-741 and BRM-1321 indels with the risk or survival outcomes in various solid cancers. As it is summarized below, while the particular genotypes that are associated with the risk of disease or clinical outcomes are not consistent across different cancer sites, it has been so far consistent that when an association is detected, the variant allele containing genotypes were associated with increased risk of disease/clinical events compared to the homozygous wild-type genotypes. These findings suggest that down-regulation of BRM may have a role in carcinogenesis or progression in these cancers.
Studies published so far have showed that either or both of the BRM-741 and BRM-1321 indels are associated with the risk of development of cancer in multiple, but not all, tissues examined. For example, in stage I-II lung and head and neck cancer patients, one study identified the association of the double homozygous variant genotype with increased disease risk [27]. Two other studies involving stage I-IV patients reported similar associations, in addition to associations of -741 (Ins/Ins genotype) and -1321 (Ins allele containing genotypes), with increased risks of lung cancer [21] as well as head and neck cancers [20]. Additionally, in two separate patient cohorts from Asia, BRM-741 was not found to be associated with the disease risk, whereas both the heterozygosity and homozygosity for the BRM-1321 Ins allele were associated with increased risk of liver cancer [23]. However, these associations were not detected in a Canadian cohort in a recent study [26]. Additionally, no associations were found between the two indels and the disease risk in pancreatic cancer (when analyzed either alone or in combination) [28], or in early stage esophageal cancer patients (when analyzed in combination) [27].
In colorectal cancer patients, including the male and female sub-cohorts, our multivariate analyses detected no associations of the BRM-741 and BRM-1321 indels with the disease risk when these variants were analyzed individually or in combination. However, when the analysis was restricted to the colon cancer patients, genotypes containing at least one variant allele were associated with increased risk of colon cancer compared to the double wild-type genotype (Del/Del+Del/Del) (Table 3 –Category C). These associations were independent of age, sex, number of first degree relatives with colorectal cancer, smoking status, and body-mass index. Additionally, as also shown in Table 3 (Category A), no homozygous and one homozygous genotypes were associated with increased risk of colon cancer compared to double wild type genotype. While we have not observed the association of the double homozygous variant genotype with increased cancer risk compared to double homozygous wild type genotype (likely because of the rarity of this genotype in our cases and controls; Table 3 –Category A), the fact that the presence of the variant alleles associates with increased colon cancer risk is biologically in line with the findings in other cancers. Therefore, similar to other cancer sites (e.g. lung and head and neck cancers [20, 21, 27]) our results suggest that the loss or reduced expression of BRM may increase the colon cancer risk. Interestingly, another gene coding for a subunit of the SWI/SNF complex (ARID1A/BAF250A) has been found to have frameshift or nonsense mutations in up to 10% of colon tumors [40], suggesting that abnormalities in ARID1A protein may have a role colon carcinogenesis. Together with our results, these findings suggest the possible involvement of the SWI/SNF complex in colon carcinogenesis. Overall, once confirmed in other patient cohorts our results may have significant implications for understanding the biological functions of the BRM gene and the SWI/SNF complex, and their potential roles in pathogenesis or treatment of colon cancer. In contrast, there was no evidence of associations of the BRM indels with the risk of rectal cancer. This may be attributed to insufficient power in the rectal cancer cohort (n = 146), or the fact that colon and rectal cancers are separate cancer sites arising in distinct tissues characterized with different pathogenesis and molecular alterations [41]. Further cohort and/or molecular studies can be valuable in addressing this hypothesis.
Similar to susceptibility studies, associations of the BRM-741 and BRM-1321 indels with survival outcomes have been reported in multiple cancer sites. For example, in pancreatic as well as in esophageal cancers, one or two copies of the indel variant alleles (Ins/Del, Ins/Ins) or double homozygous variant genotype (Ins/Ins+Ins/Ins) were associated with reduced overall survival time [25, 28]. Additionally, in two separate stage III-IV non-small cell lung cancer cohorts, homozygosity for the variant alleles of either indels as well as the double homozygous variant genotype were associated with shortened overall and progression-free survival time [24]. A recent study on liver cancer patients also showed similar associations between overall survival time and these indel variants [26]. In our study, no associations were detected between the progression-free survival time of the patients and the BRM-1321 genotypes or the genotype combinations of the BRM-741 and BRM-1321 indels. However, associations were detected for the BRM-741 genotypes. Specifically, when compared to the wild-type genotype (Del/Del), heterozygosity for the BRM-741 indel was associated with longer progression-free survival time in the colorectal cancer cohort independent of age, disease stage, tumor location, MSI and adjuvant treatment status (Table 4; Fig 1). A similar association was also detected in the male colorectal cancer patients (S4 Table; S1 Fig). Based on the previous studies on other cancers, we would expect the wild-type genotype to have better survival outcomes compared to the genotypes that include the variant allele. However, our results do not support this assumption. We also note that it is possible that the small sample size in the wild-type homozygous genotype group may have led to missing a possible association. In addition, under the recessive genetic model we found that the male patients who had the Ins/Ins genotype of BRM-741 had shorter survival times compared to the rest of the male patients (S4 Table; S2 Fig). This association was not detected in the female patients (p > 0.05: S4 Table). However, as shown in S3 Fig, while it did not reach significance, an opposite effect of the Ins/Ins allele in the female patients was observable, suggesting that the prognostic associations of the BRM-741 may be different between male and female colorectal cancer patients. This opens new research avenues for future studies that can help dissect the biological basis of sex-based differences in colorectal cancer outcomes.
Strengths/limitations of this study can be summarized as follows: replications in independent patient cohorts are required to rule out false-positive associations and to confirm our results; death from any cause was used as one of the endpoints as the cause of death information was not available for all patients; and the low frequency of the double homozygous variant genotype has possibly prevented examination/detection of its potential associations in our cohort, thus analysis of larger patient cohorts are needed. However, to our knowledge, this is the first study that investigated the association between the BRM-741 and BRM-1321 promoter variations and disease risk and patient survival outcomes in colorectal cancer; the patient cohort is a well described cohort with long follow-up time (median: 6.98 years); a comprehensive investigation has been conducted including application of multiple genetic models and sub-group analyses; and more importantly, in the survival analysis the proportional hazard assumption of the Cox regression method has been assessed and appropriate models have been constructed, which makes our estimations more reliable [42, 43].
Conclusions
In conclusion, our results suggest the potential involvement of BRM in colon cancer pathogenesis and colorectal cancer progression. Analyses in larger and additional patient cohorts are needed to verify our results.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(TIFF)
(TIFF)
(TIFF)
Acknowledgments
We are grateful for the patients and controls that participated in the NFCCR and made this study possible; Dr. Roger Green who was involved in the initial phases of this study, who passed away before the end of study; Andrea Kavanagh for extracting the epidemiological data from the NFCCR database; Drs. Elizabeth Dicks and Jane Green and many other investigators and staff for their efforts in collection of the participants and their information; and Megan Carey for helping with the tables. SS is a Beatrice Hunter Cancer Research Institute (BHCRI) senior investigator.
Data Availability
The data used in this study cannot be made publicly available as patients were not consented to make their data publicly available or accessible. Data are available from the Newfoundland Colorectal Cancer Registry (NFCCR; Patrick Parfrey (pparfrey@mun.ca) / Geoffrey Liu (genotype data only: geoffrey.liu@uhn.ca) for researchers who meet the criteria for access to confidential data. Permission to obtain the data can be requested from the Research Grant and Contract Services Memorial University of Newfoundland, St. John’s, NL, A1C 5S7, Canada (e-mail: rgcs@mun.ca), and the ethics approval shall be obtained from the Health Research Ethics Board (HREB), Ethics Office, Health Research Ethics Authority, Suite 200, 95 Bonaventure Avenue, St. John’s, NL, A1B 2X5, Canada (e-mail: info@hrea.ca).
Funding Statement
This study is supported by the Alan B. Brown Chair funds to GL. YY is supported by TPMI/NL Support educational funding fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65(2):87–108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2017. Canadian Cancer Society, Toronto; 2017. Available at: http://www.cancer.ca/~/media/cancer.ca/CW/publications/Canadian%20Cancer%20Statistics/Canadian-Cancer-Statistics-2017-EN.pdf. Last accessed on August 23, 2017.
- 3.Sankaranarayanan R1, Swaminathan R, Brenner H, Chen K, Chia KS, Chen JG, et al. Cancer survival in Africa, Asia, and Central America: a population-based study. Lancet Oncol 2010; 11(2):165–173. doi: 10.1016/S1470-2045(09)70335-3 [DOI] [PubMed] [Google Scholar]
- 4.Al-Tassan NA, Whiffin N, Hosking FJ, Palles C, Farrington SM, Dobbins SE, et al. A new GWAS and meta-analysis with 1000Genomes imputation identifies novel risk variants for colorectal cancer. Sci Rep 2015; 5:10442 doi: 10.1038/srep10442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang K, Sun Y, Wang C, Ji J, Li Y, Ye Y, et al. Genome-wide association study identifies two new susceptibility loci for colorectal cancer at 5q23. 3 and 17q12 in Han Chinese. Oncotarget 2015; 6(37):40327–40336. doi: 10.18632/oncotarget.5530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemire M, Qu C, Loo LWM, Zaidi SHE, Wang H, Berndt SI, et al. A genome-wide association study for colorectal cancer identifies a risk locus in 14q23. 1. Hum Genet 2015; 134(11–12):1249–1262. doi: 10.1007/s00439-015-1598-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phipps AI, Passarelli MN, Chan AT, Harrison TA, Jeon J, Hutter CM, et al. Common genetic variation and survival after colorectal cancer diagnosis: a genome-wide analysis. Carcinogenesis 2016; 37(1):87–95. doi: 10.1093/carcin/bgv161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schumacher FR, Schmit SL, Jiao S, Edlund CK, Wang H, Zhang B, et al. Genome-wide association study of colorectal cancer identifies six new susceptibility loci. Nat Commun 2015; 6:7138 doi: 10.1038/ncomms8138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomlinson I, Webb E, Carvajal-Carmona L, Broderick P, Kemp Z, Spain S, et al. A genome-wide association scan of tag SNPs identifies a susceptibility variant for colorectal cancer at 8q24. 21. Nat Genet 2007; 39(8):984–988. doi: 10.1038/ng2085 [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Schmit SL, Haiman CA, Keku TO, Kato I, Palmer JR, et al. Novel colon cancer susceptibility variants identified from a genome-wide association study in African Americans. Int J Cancer 2017; 140(12):2728–2733. doi: 10.1002/ijc.30687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu W, Xu J, Shestopaloff K, Dicks E, Green J, Parfrey P, et al. A genome wide association study on Newfoundland colorectal cancer patients’ survival outcomes. Biomark Res 2015; 3:6 doi: 10.1186/s40364-015-0031-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muchardt C, Yaniv M. A human homologue of Saccharomyces cerevisiae SNF2/SWI2 and Drosophila brm genes potentiates transcriptional activation by the glucocorticoid receptor. EMBO J 1993; 12(11):4279–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterson CL, Workman JL. Promoter targeting and chromatin remodeling by the SWI/SNF complex. Curr Opin Genet Dev 2000; 10(2):187–192. [DOI] [PubMed] [Google Scholar]
- 14.Reisman D, Glaros S, Thompson EA. The SWI/SNF complex and cancer. Oncogene 2009; 28(14):1653–1668. doi: 10.1038/onc.2009.4 [DOI] [PubMed] [Google Scholar]
- 15.Savas S, Skardasi G. The SWI/SNF complex subunit genes: Their functions, variations, and links to risk and survival outcomes in human cancers. Crit Rev Oncol Hematol 2018; 123: 114–131. doi: 10.1016/j.critrevonc.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 16.Glaros S, Cirrincione GM, Muchardt C, Kleer CG, Michael CW, Reisman D. The reversible epigenetic silencing of BRM: implications for clinical targeted therapy. Oncogene 2007; 26(49):7058–7066. doi: 10.1038/sj.onc.1210514 [DOI] [PubMed] [Google Scholar]
- 17.Herpel E, Rieker RJ, Dienemann H, Muley T, Meister M, Hartmann A, et al. SMARCA4 and SMARCA2 deficiency in non-small cell lung cancer: immunohistochemical survey of 316 consecutive specimens. Ann Diagn Pathol 2017; 26:47–51. doi: 10.1016/j.anndiagpath.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 18.Matsubara D, Kishaba Y, Ishikawa S, Sakatani T, Oguni S, Tamura T, et al. Lung cancer with loss of BRG1/BRM, shows epithelial mesenchymal transition phenotype and distinct histologic and genetic features. Cancer Sci 2013; 104(2):266–273. doi: 10.1111/cas.12065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reisman D, Sciarrotta J, Wang W, Funkhouser WK, Weissman BE. Loss of BRG1/BRM in human lung cancer cell lines and primary lung cancers: correlation with poor prognosis. Cancer Res 2003; 63(3):560–566. [PubMed] [Google Scholar]
- 20.Wang JR, Gramling SJ, Goldstein DP, Cheng D, Chen D, Azad AK, et al. Association of two BRM promoter polymorphisms with head and neck squamous cell carcinoma risk. Carcinogenesis 2013; 34(5):1012–1017. doi: 10.1093/carcin/bgt008 [DOI] [PubMed] [Google Scholar]
- 21.Liu G, Gramling S, Munoz D, Cheng D, Azad AK, et al. Two novel BRM insertion promoter sequence variants are associated with loss of BRM expression and lung cancer risk. Oncogene 2011; 30(29):3295–3304. doi: 10.1038/onc.2011.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahali B, Yu J, Marquez SB, Thompson KW, Liang SY, Lu L, et al. The silencing of the SWI/SNF subunit and anticancer gene BRM in Rhabdoid tumors. Oncotarget 2014; 5(10):3316–3332. doi: 10.18632/oncotarget.1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao X, Huang M, Liu L, He Y, Yu Q, Zhao H, et al. Insertion/deletion polymorphisms in the promoter region of BRM contribute to risk of hepatocellular carcinoma in Chinese populations. PLOS ONE 2013; 8(1):e55169 doi: 10.1371/journal.pone.0055169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu G, Cuffe S, Liang S, Azad AK, Cheng L, Brhane Y, et al. BRM promoter polymorphisms and survival of advanced non-small cell lung cancer patients in the Princess Margaret cohort and CCTG BR. 24 trial. Clin Cancer Res 2017; 23(10):2460–2470. doi: 10.1158/1078-0432.CCR-16-1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korpanty GJ, Eng L, Qiu X, Faluyi OO, Renouf DJ, Cheng D, et al. Association of BRM promoter polymorphisms and esophageal adenocarcinoma outcome. Oncotarget 2017; 8(17):28093–28100. doi: 10.18632/oncotarget.15890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasic I, Wong KM, Lee JJ, Espin-Garcia O, Brhane Y, Cheng D, et al. Two BRM promoter polymorphisms predict poor survival in patients with hepatocellular carcinoma. Mol Carcinog 2017; 1–8. doi: 10.1002/mc.22736 [DOI] [PubMed] [Google Scholar]
- 27.Wong KM, Qiu X, Cheng D, Azad AK, Habbous S, Palepu P, et al. Two BRM promoter insertion polymorphisms increase the risk of early-stage upper aerodigestive tract cancers. Cancer Med 2014; 3(2):426–433. doi: 10.1002/cam4.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segedi M, Anderson LN, Espin-Garcia O, Borgida A, Bianco T, Cheng D, et al. BRM polymorphisms, pancreatic cancer risk and survival. Int J Cancer 2016; 139(11):2474–2481. doi: 10.1002/ijc.30369 [DOI] [PubMed] [Google Scholar]
- 29.Green RC, Green JS, Buehler SK, Robb JD, Daftary D, Gallinger S, et al. Very high incidence of familial colorectal cancer in Newfoundland: a comparison with Ontario and 13 other population-based studies. Fam Cancer 2007; 6(1):53–62. doi: 10.1007/s10689-006-9104-x [DOI] [PubMed] [Google Scholar]
- 30.Woods MO, Younghusband HB, Parfrey PS, Gallinger S, McLaughlin J, Dicks E, et al. The genetic basis of colorectal cancer in a population-based incident cohort with a high rate of familial disease. Gut 2010; 59(10):1369–1377. doi: 10.1136/gut.2010.208462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang PP, Dicks E, Gong X, Buehler S, Zhao J, Squires J, et al. Validity of random-digit-dialing in recruiting controls in a case-control study. Am J Health Behav 2009; 33(5):513–520. [DOI] [PubMed] [Google Scholar]
- 32.Schemper M. and Smith TL. A note on quantifying follow-up in studies of failure time. Contemp Clin Trials 1996; 17(4):343–346. [DOI] [PubMed] [Google Scholar]
- 33.Negandhi AA, Hyde A, Dicks E, Pollett W, Younghusband BH, Parfrey P, et al. MTHFR Glu429Ala and ERCC5 His46His polymorphisms are associated with prognosis in colorectal cancer patients: analysis of two independent cohorts from Newfoundland. PLOS ONE 2013; 8(4):e61469 doi: 10.1371/journal.pone.0061469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez S, Gaunt TR, Day IN. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am J Epidemiol 2009; 169(4):505–514. doi: 10.1093/aje/kwn359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warnes G, with contributions from Gorjanc G, Leisch F and Man M. genetics: Population Genetics. R package version 1.3.8.1; 2013. Available at: http://CRAN.R-project.org/package=genetics. Last accessed on September 13, 2017.
- 36.R Development Core Team. R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria: ISBN 3-900051-07-0; 2008. Available at: https://www.R-project.org/. Last accessed on September 13, 2017. [Google Scholar]
- 37.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control 1974; 19(6):716–723. [Google Scholar]
- 38.Therneau TM. A package for survival analysis in S. version 2.38; 2015. Available at: http://CRAN.R-project.org/package=survival. Last accessed on September 13, 2017.
- 39.Wilson BG, Roberts CW. SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer 2011; 11(7):481–492. doi: 10.1038/nrc3068 [DOI] [PubMed] [Google Scholar]
- 40.Jones S, Li M, Parsons DW, Zhang X, Wesseling J, Kristel P, et al. Somatic mutations in the chromatin remodeling gene ARID1A occur in several tumor types. Hum Mutat 2012; 33(1):100–103. doi: 10.1002/humu.21633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li FY, Lai MD. Colorectal cancer, one entity or three. J Zhejiang Univ Sci B 2009; 10(3):219–229. doi: 10.1631/jzus.B0820273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Altman DG, De Stavola BL, Love SB, Stepniewska KA. Review of survival analyses published in cancer journals. Br J Cancer 1995; 72(2):511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bellera CA, MacGrogan G, Debled M, de Lara CT, Brouste V, Mathoulin-Pélissier S. Variables with time-varying effects and the Cox model: some statistical concepts illustrated with a prognostic factor study in breast cancer. BMC Med Res Methodol 2010; 10(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(TIFF)
(TIFF)
(TIFF)
Data Availability Statement
The data used in this study cannot be made publicly available as patients were not consented to make their data publicly available or accessible. Data are available from the Newfoundland Colorectal Cancer Registry (NFCCR; Patrick Parfrey (pparfrey@mun.ca) / Geoffrey Liu (genotype data only: geoffrey.liu@uhn.ca) for researchers who meet the criteria for access to confidential data. Permission to obtain the data can be requested from the Research Grant and Contract Services Memorial University of Newfoundland, St. John’s, NL, A1C 5S7, Canada (e-mail: rgcs@mun.ca), and the ethics approval shall be obtained from the Health Research Ethics Board (HREB), Ethics Office, Health Research Ethics Authority, Suite 200, 95 Bonaventure Avenue, St. John’s, NL, A1B 2X5, Canada (e-mail: info@hrea.ca).