Abstract
Chain elongation is an open-culture fermentation process that facilitates conversion of organic residues with an additional electron donor, such as ethanol, into valuable n-caproate. Open-culture processes are catalyzed by an undefined consortium of microorganisms which typically also bring undesired (competing) processes. Inhibition of competing processes, such as syntrophic ethanol oxidation, will lead to a more selective n-caproate production process. In this study, we investigated the effect of n-caproate concentration on the specific activity of chain elongation and competing processes using batch inhibition assays. With “synthetic medium sludge” (originally operating at 3.4 g/L n-caproate), syntrophic ethanol oxidation was proportionally inhibited by n-caproate until 45% inhibition at 20 g/L n-caproate. Hydrogenotrophic methanogenesis was for 58% inhibited at 20 g/L n-caproate. Chain elongation of volatile fatty acids (volatile fatty acid upgrading; the desired process), was completely inhibited at 20 g/L n-caproate with all tested sludge types. “Adapted sludge” (operating at 23.2 g/L n-caproate) showed a 10 times higher volatile fatty acid upgrading activity at 15 g/L n-caproate compared to “nonadapted sludge” (operating at 7.1 g/L n-caproate). This shows that open cultures do adapt to perform chain elongation at high n-caproate concentrations which likely inhibits syntrophic ethanol oxidation through hydrogenotrophic methanogenesis. As such, we provide supporting evidence that the formation of n-caproate inhibits syntrophic ethanol oxidation which leads to a more selective medium chain fatty acid production process.
Keywords: Chain elongation, n-Caproate, Inhibition, Competing processes, Adapted sludge
Short abstract
Chain elongation with open-cultures is more effective at high n-caproate concentrations because this inhibits competitive processes.
Introduction
Organic residual streams, such as food waste, have great potential as alternative resource for production of fuels and chemicals. These residues are conventionally anaerobically digested to produce methane for electric power and/or heat production.1 Methane, however, has a low monetary value. Producing higher-value products than methane, therefore, is gaining increasing interest. Among these higher-value products, particularly medium chain fatty acids (MCFAs), such as caproic acid and heptanoic acid are interesting because MCFAs can be used for a wide variety of applications (e.g., aviation fuels, lubricants, feed additives). Moreover, like methane, MCFAs are also produced through open-culture anaerobic reactor microbiomes. Open-culture processes are catalyzed by an undefined consortium of microorganisms. This is different from pure culture or defined coculture processes, which are catalyzed by only one (or more) known species with known pathways. The advantage of open-culture processes is that they handle a mixture of residual streams without the need for sterilization. The technological challenge is to control the mixed microbial population within the microbiome to produce MCFAs selectively from the substrates.
MCFA production from organic residues by open-cultures occurs via two subsequent processes which can be combined or performed separately. First, organic residues are hydrolyzed by hydrolytic enzymes and acidified by acidogenic bacteria, resulting in production of volatile fatty acids (VFAs) such as acetate, propionate and butyrate. Second, these VFAs are converted together with an electron donor, such as ethanol, into MCFAs through chain elongation. This conversion is performed by chain elongating micro-organisms (e.g., Clostridium kluyveri) that use the reverse β-oxidation pathway (eq 1).2 Chain elongation with open cultures is an emerging application that can handle many organic feedstocks at various conditions and reactor configurations.3
| 1 |
Here, we focus on ethanol-based chain elongation which is catalyzed by anaerobic open-cultures. These open-cultures include not only chain elongating microorganisms but also other functional groups of microorganisms. These other functional groups can degrade the substrates and products into undesired metabolites. This makes their presence and activity detrimental to the MCFA selectivity of chain elongation processes. The challenge, therefore, is to find process conditions that reduce their activity through a selection pressure like inhibition. Inhibition is preferably performed without the use of bioactive chemicals (e.g., 2-bromoethanesulfonate or iodoform as methanogenic inhibitor) so that the nonconverted organics from the process can be used as soil fertilizer upon composting. We recognize the following competing processes in chain elongation:4,5
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
Understanding the role of competing processes and how they can be suppressed is essential to establish a selective MCFA production process. For example, acetotrophic methanogenesis degrades acetate into methane and CO2 (eq 2). This process is performed by acetotrophic methanogens. Acetotrophic methanogenesis was shown to be largely inhibited at slightly acidic pH (5.5) in chain elongating processes.6 When working at near-neutral pH, which is more favorable for these methanogens, they could be outselected by applying a combination of sufficient hydraulic shear force and a low hydraulic retention time (HRT) to respectively detach and wash out these slow-growing methanogens.7 Anaerobic oxidation of fatty acids occurs though β-oxidation of fatty acids into acetate and hydrogen (and also CO2 in the case of propionate oxidation). Acetate can be further oxidized into CO2 and hydrogen (eq 3). Anaerobic oxidation of fatty acids is performed by acetogenic bacteria and can be thermodynamically inhibited at a hydrogen partial pressure (pH2) of >0.007% at standard conditions.5 Excessive ethanol oxidation (EEO) occurs through direct anaerobic oxidation of ethanol into acetate and hydrogen (eq 4). This process is considered to be performed by ethanol-oxidizing microorganisms which do not perform chain elongation. Suppression of EEO is important not only because this process leads to an inefficient use of ethanol but also because it acidifies the fermentation broth. Because of this acidifying effect, EEO requires extra base addition which increases operating expenses.8 EEO can be thermodynamically inhibited at a hydrogen partial pressure (pH2) of ≥14% at standard conditions.5 Such high pH2 pressures, however, are not common in chain elongation processes because hydrogen is typically consumed through hydrogenotrophic methanogenesis (eq 5). In our previous study,5 we showed that EEO and hydrogenotrophic methanogenesis are coupled processes. The overall reaction can be referred to as syntrophic ethanol oxidation (eq 6). Syntrophic ethanol oxidation can be limited by reducing the available amount of CO2. This has been shown to limit hydrogenotrophic methanogenic activity and the resulting higher pH2 thermodynamically inhibits EEO.4,5,9 CO2 loading rate may be difficult to control, however, when working with (hydrolyzed and acidified) organic residues. This is because CO2 is produced during acidification of hydrolyzed residues and the presence of this CO2 in the feed may complicate the actual CO2 supply to the chain elongation process. Even though acidification and chain elongation can be separated, dissolved CO2 could still complicate the control over the actual CO2 supply. As such, alternative methods to limit EEO in chain elongation are needed.
In a previous study, an alternative method to inhibit EEO may have been shown; by operating a continuous chain elongation process at long HRT (4 d), EEO was limited to 0.9 g/L/d (14.7% of total ethanol consumption) while n-caproate was produced at a high concentration (23.4 g/L).8 In contrast, at short HRT (1 d), EEO occurred at a higher rate (5.6 g/L/d; 45.0% of total ethanol consumption) while n-caproate was produced at a lower concentration (7.1 g/L). A hypothesis is that the high n-caproate concentration may have had a selective inhibitory effect on one of the competing syntrophs (ethanol oxidizers or hydrogenotrophic methanogens). Note that we refer to n-caproate as both forms together, undissociated n-caproic acid and dissociated n-caproate, and that we refer to each specific form when appropriate.
It is well-known that MCFAs are toxic to microorganisms because they can damage the cell membrane.10 Gram-positive bacteria and methanogens tend to be more easily inhibited by long chain fatty acids (LCFAs) than Gram-negative bacteria.11 Selective inhibition of MCFAs on competing functional groups of microorganisms may be an alternative strategy to suppress competing processes to enhance the MCFA selectivity. High MCFAs concentrations, however, may also inhibit chain elongation itself. To circumvent potential MCFA toxicity, in situ MCFA extraction was applied which showed to enhance n-caproate production.12 These results were used to clarify that MCFAs inhibit chain elongating microorganisms. A systematic investigation of the inhibitory effect of n-caproate on chain elongation and competing processes in chain elongation microbiomes has not been reported to date.
In this study, we investigated the effect of n-caproate concentration on the specific activities of chain elongation microbiomes in two experiments. First, we studied the effect of n-caproate on the specific activity of five processes: (1) chain elongation (VFA upgrading), (2) syntrophic ethanol oxidation, (3) hydrogenotrophic methanogenesis, (4) acetotrophic methanogenesis, and (5) anaerobic acetate oxidation. This was performed using sludge from a continuous chain elongation process that operated at 3.4 g/L n-caproate. Second, we extended the experiment for chain elongation comparing two types of sludge to study the potential adaptation of chain elongation microbiomes to high n-caproate concentrations. One sludge type originally operated at 7.1 g/L caproate (nonadapted sludge) whereas the other sludge type originally operated at 23.2 g/L n-caproate (adapted sludge)
Experimental Section
Experimental Design of Batch Inhibition Assays
An overview of the experimental design is reported in Table 1.
Table 1. Experimental Design of Batch Inhibition Assays.
| Process studied in inhibition assay | Initial aqueous substrate(s) [g/L] | Initial headspace composition [vol %] | Activity based on production of | Inoculum | Initial n-caproate concentration [g/L] | Working volume/Total bottle volume [mL] |
|---|---|---|---|---|---|---|
| VFA Upgrading | Ethanol [11.5] | N2/CO2 [80/20] | n-valerate and n-heptanoate | Synthetic medium sludgec | 0, 10, 15, 20 | 50/125 |
| Propionate [3] | ||||||
| Syntrophic ethanol oxidation | Ethanol [11.5] | N2/CO2 [80/20] | Methane | Synthetic medium sludgec | 0, 10, 15, 20 | 50/125 |
| Hydrogenotrophic methanogenesis | None | H2/CO2 [80/20] | Methane | Synthetic medium sludgec | 0, 10, 15, 20 | 50/250 |
| Acetotrophic methanogenesis | Acetate [2] | N2/CO2 [80/20] | Methanea | Synthetic medium sludgec | 0, 10, 15, 20 | 50/125 |
| Anaerobic acetate oxidation | Acetate [2] | N2/CO2 [80/20] | Hydrogenb | Synthetic medium sludgec | 0, 10, 15, 20 | 50/125 |
| VFA Upgrading | Ethanol [11.5] | N2/CO2 [80/20] | n-valerate and n-heptanoate | Nonadapted sludged | 0, 10, 15, 20, 25 | 50/125 |
| Propionate [3] | ||||||
| VFA Upgrading | Ethanol [11.5] | N2/CO2 [80/20] | n-valerate and n-heptanoate | Adapted sludgee | 0, 10, 15, 20, 25 | 50/125 |
| Propionate [3] |
Can only be quantified when anaerobic acetate oxidation and hydrogenotrophic methanogenesis do not occur.
Can only be quantified when acetotrophic methanogenesis and hydrogenotrophic methanogenesis do not occur.
Synthetic medium sludge was grown converting ethanol and propionate into MCFAs at 3.4 g/L caproate.
Nonadapted sludge was grown converting acidified food waste and ethanol into MCFAs at 7.1 g/L n-caproate.
Adapted sludge was grown converting acidified food waste and ethanol into MCFAs at 23.2 g/L n-caproate.
Media Preparation
All liquid media were initially prepared the same way by adding salts, vitamins, trace elements and yeast extract to deoxygenated water.13 For NH4(H2)PO4, we used 1.8 g/L instead of 3.6 g/L. Buffering agents were also added: 200 mM MES (VWR, The Netherlands), 200 mM BISTRIS (Sigma-Aldrich, The Netherlands) and 10 mM PIPPS (98.4%, Merck Millipore, USA). For the first experiment, five different media were prepared. Thus, after buffers were added, the mixture was divided into five fractions. The fraction used to monitor chain elongation (VFA upgrading) was supplemented with 11.5 g/L ethanol (Absolute, VWR, France) and 3 g/L propionic acid (≥99.5%, Sigma-Aldrich). With this ethanol concentration, ethanol toxicity is avoided.14 The fraction used to monitor syntrophic ethanol oxidation was supplemented with 11.5 g/L ethanol. The fraction used to monitor acetotrophic methanogenesis and anaerobic acetate oxidation was supplemented with 2 g/L acetic acid (99.9%, VWR, France). The fraction used to monitor hydrogenotrophic methanogenesis was not supplemented with a substrate. Each fraction used to monitor a specific process was again divided into 4 smaller fractions to add n-caproic acid (≥98%, Sigma-Aldrich) to concentrations of 0 (control), 10, 15 and 20 g/L. For the second experiment, we added one assay in which n-caproic acid was added up to 25 g/L. Finally, the pH of all assays were adjusted to 6.8 with 5 M NaOH.
Methods
Batch inhibition assays were performed in 125 or 250 mL serum bottles. The bottles were filled with 45.0 g medium under anaerobic conditions. After filling, bottles were closed with a rubber stopper and an aluminum crimp seal. The headspaces of the bottles for chain elongation (VFA upgrading), syntrophic ethanol oxidation, acetotrophic methanogenesis and anaerobic acetate oxidation were flushed and pressurized to 1.5 bar with a mixture of N2/CO2 (80/20%). Likewise, the headspaces of the bottles for hydrogenotrophic methanogenesis were flushed and pressurized to 1.5 bar with a mixture of H2/CO2 (80/20%). After preparation, bottles were inoculated with a fresh inoculum (5 mL) using a needle and syringe.
In the first experiment, bottles were inoculated with “synthetic medium sludge”. Synthetic medium sludge was directly derived from a running continuous chain elongation process that converted synthetic substrates (propionate and ethanol) into MCFAs at 3.4 g/L n-caproate.13 Based on a carbon flux analysis (e.g., ref (5)), we determined that synthetic medium sludge contained chain elongating microorganisms, ethanol oxidizers and hydrogenotrophic methanogens.
In the second experiment, bottles were inoculated with “nonadapted sludge” or “adapted sludge”. Both sludge types were directly derived from a running continuous chain elongation process that converted acidified food waste and ethanol into MCFAs.8 Whereas nonadapted sludge operated at 7.1 g/L n-caproate (day 103), adapted sludge operated at 23.2 g/L n-caproate (day 124).8 Properties of the used inocula, including VSS concentrations, sodium concentrations, n-caproate concentrations and original average specific activities in reactor, are reported in Table 2. Specifications on process conditions that conditioned the inocula are reported in the Supporting Information.
Table 2. Inoculum Specifications, Average Specific Activities in Batch Assays and Observed Sodium and Undissociated n-Caproic Acid Concentrations in Batch Assays.
| Inoculum
name, compounds in inoculum [g/L] and
original average specific activity of inoculum in reactor [mmol C/gVSS/d] |
Average
specific activity in batch assays [mmol C/gVSS/d] at different initial n-caproate concentrations [g/L] |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Process | Inoculum name | VSS | Sodium | n-Caproate | Activityc | 0 | 10 | 15 | 20 | 25 |
| VFA Upgrading | Synthetic medium sludge | 0.6 | 6.1 ± 0.5e | 3.4 ± 0.3e | 1362 ± 106e | 798 | 77 | 0 | 0 | NDd |
| Syntrophic ethanol oxidation | Synthetic medium sludge | 0.6 | 6.1 ± 0.5e | 3.4 ± 0.3e | 59 ± 11e | 97 | 82 | 68 | 53 | NDd |
| Hydrogenotrophic methanogenesis | Synthetic medium sludge | 0.6 | 6.1 ± 0.5e | 3.4 ± 0.3e | 59 ± 11e | 295 | 319 | 301 | 124 | NDd |
| Acetotrophic methanogenesis | Synthetic medium sludge | 0.6 | 6.1 ± 0.5e | 3.4 ± 0.3e | NDd | 0 | 0 | 0 | 0 | 0 |
| Anaerobic acetate oxidation | Synthetic medium sludge | 0.6 | 6.1 ± 0.5e | 3.4 ± 0.3e | NDd | 0 | 0 | 0 | 0 | 0 |
| VFA Upgrading | Nonadapted sludge | 0.4 | 7.0 ± 0.0e | 7.1 ± 0.9e | 220 ± 95e | 1476 | 29 | 18 | 17 | 0 |
| VFA Upgrading | Adapted sludge | 0.5 | 9.1 ± 0.5e | 23.2 ± 1.9e | 386 ± 76e | 1056 | 411 | 181 | 0 | 0 |
| Solutes in batch assays [g/L] | ||||||||||
| Sodium concentrationa | 2.3–3.2 | 4.0–5.0 | 4.7–5.6 | 5.6–6.4 | 7.1–7.1 | |||||
| Undissociated n-caproic acid concentrationb | 0–0.1 | 0.1–0.2 | 0.1–0.3 | 0.2–0.4 | 0.3–0.4 | |||||
Observed range of initial sodium concentrations in batch assays for different initial n-caproate concentrations.
Observed range of undissociated n-caproic acid concentrations during batch inhibition assays for different initial n-caproate concentrations.
Original average reactor activity of inoculum; calculated from reactor data (see Supporting Information).
ND = Not determined.
± = Standard deviation based on 3 or more measurements.
After inoculation, bottles were incubated at 30 °C at 100 rpm in a rotary shaker (New Brunswick Scientific Innova 44). Contents of the bottles were daily analyzed on headspace pressure, headspace composition, fatty acids and alcohols. All assays were conducted in duplicate.
Determination of Specific Activity
The activity of syntrophic ethanol oxidation, acetotrophic methanogenesis and hydrogenotrophic methanogenesis was based on the maximum slope of methane production vs time divided by the initial VSS concentration.
The activity of anaerobic acetate oxidation was based on the maximum slope of hydrogen production vs time divided by the initial VSS concentration.
The activity of chain elongation was based on the maximum slope of n-valerate plus n-heptanoate production vs time divided by the initial VSS concentration. Chain elongation activity was based on the production of odd-numbered fatty acids and not on production of even-numbered fatty acids. This was performed to determine a measure of chain elongation activity that is independent of other active functional groups of microorganisms. Whereas odd-numbered fatty acids can only be produced through VFA upgrading (chain elongation of added VFAs), even-numbered fatty acids can also be produced through ethanol upgrading (in situ ethanol oxidation into acetate and subsequent chain elongation into even-numbered fatty acids). Because ethanol upgrading is not only mediated by chain elongating microorganisms but also by ethanol oxidizers and hydrogenotrophic methanogens,5 we quantified only the odd numbered chain elongation products (n-valerate and n-heptanoate) by VFA upgrading as a measure for chain elongation activity.
Undissociated n-caproic acid concentrations were calculated from the observed n-caproate concentrations and pH values (during the maximum specific activity) using the Henderson–Hasselbalch equation.
Analytical Techniques
Alcohols (C2–C6) and fatty acids (C2–C8) were analyzed by gas chromatography.15 Gaseous compounds were determined by gas chromatography.16 Sodium was measured by ion chromatography.17 VSS measurements were performed in the same way as before.13
Results
n-Caproate Concentration Affects Chain Elongation and Competing Processes Using “Synthetic Medium Sludge”
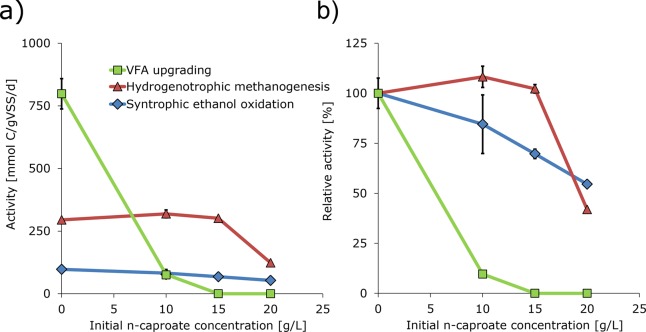
The effect of n-caproate concentration on the maximum specific activities of various processes was investigated with “synthetic medium sludge” as inoculum. Processes that were investigated were: VFA upgrading, syntrophic ethanol oxidation, hydrogenotrophic methanogenesis, acetotrophic methanogenesis and anaerobic acetate oxidation. Hereby we note that VFA upgrading is the desired chain elongation process that leads to a selective MCFA production process. Results of this batch inhibition assay that showed an effect of n-caproate are graphically summarized in Figure 1a,b. Numerical values of average specific activities are reported in Table 2 and profiles of product production are presented in the Supporting Information (Figures S1–S3).
Figure 1.
Results of batch inhibition assays; initial n-caproate concentration vs observed activity (a) and relative activity (b) of various processes using synthetic medium sludge as inoculum. Synthetic medium sludge was grown converting synthetic substrates (propionate and ethanol) at 3.4 g/L n-caproate. Values indicate averages of duplicates and bars indicate range of duplicates (often too small to be visual). T = 30 °C, pH = 6.8 (buffered).
The effect of n-caproate on both acetotrophic methanogenesis and anaerobic acetate oxidation could not be determined because these processes did not occur in the controls (0 g/L n-caproate) or in the experimental assays. Likely, acetotrophic methanogens and acetogenic bacteria were not substantially present in the sludge.
Evidently, n-caproate inhibited all other quantified processes in the tested range (Figure 1). The activity of VFA upgrading (i.e., specific n-valerate plus n-heptanoate activity) in the control was 798 mmol C/gVSS/d and was for 90% inhibited at 10 g/L n-caproate. Remarkably, at 15 and 20 g/L n-caproate, VFA upgrading was completely inhibited. VFA upgrading was the most sensitive process for n-caproate inhibition compared to syntrophic ethanol oxidation and hydrogenotrophic methanogenesis. The activity of syntrophic ethanol oxidation in the control was 97 mmol C/gVSS/d and was proportionally inhibited by n-caproate until 45% inhibition at 20 g/L n-caproate. Syntrophic ethanol oxidation was the least sensitive process to n-caproate inhibition. Hydrogenotrophic methanogenic activity in the control was 295 mmol C/gVSS/d and was not inhibited at 10 and 15 g/L n-caproate. However, at a higher 20 g/L n-caproate, hydrogenotrophic methanogenesis was indeed inhibited with 58%. Hydrogenotrophic methanogenic activity was always higher than the activity of syntrophic ethanol oxidation.
Up to 15 g/L n-caproate, the relative order of inhibition was VFA upgrading > syntrophic ethanol oxidation with no inhibition for hydrogenotrophic methanogenesis. Within this n-caproate concentration range, syntrophic ethanol oxidation was not limited by hydrogenotrophic methanogenesis. At 20 g/L n-caproate, the relative order of inhibition was VFA upgrading > hydrogenotrophic methanogenesis > syntrophic ethanol oxidation. At this n-caproate concentration, hydrogenotrophic methanogenesis was severely suppressed by n-caproate inhibition. This is in line with earlier work by Hajarnis et al. (1994) who found that pure cultures of hydrogenotrophic methanogens, Methanobacterium bryantii and Methanobacterium formicium, were inhibited for 96 and 95% respectively by 20 g/L n-caproate at pH 7.0.18 By extrapolating our data, we estimate that, at 25 g/L n-caproate, syntrophic ethanol oxidation may become rate-limited by hydrogenotrophic methanogenesis. This extrapolation provides supportive evidence for the hypothesis that a high n-caproate concentration is a control strategy to suppress syntrophic ethanol oxidation through hydrogenotrophic methanogenesis in chain elongation processes. At high n-caproate concentrations, however, chain elongation itself may also be inhibited; though ethanol may be used more efficiently, as was shown in our previous study.8
It was remarkable that at 15 and 20 g/L n-caproate, VFA upgrading was not observed, which shows that chain elongating microorganisms were completely inhibited by n-caproate. Previous studies, however, reported chain elongation activity up to considerably higher n-caproate concentrations (i.e., 21,19 23 g/L20 and even up to 25 g/L8 at similar pH). Probably, the sludge that we used as inoculum was not adapted to such high n-caproate concentrations. The actual inoculum, “synthetic medium sludge”, was indeed derived from a running chain elongation process with a lower n-caproate concentration (3.4 g/L) than that was present in the experimental assays at 15 and 20 g/L n-caproate. Therefore, the sudden increase of n-caproate during inoculation may have resulted in an acute toxic effect to the chain elongating microorganisms.
Comparing VFA Upgrading Using Nonadapted Sludge and Adapted Sludge
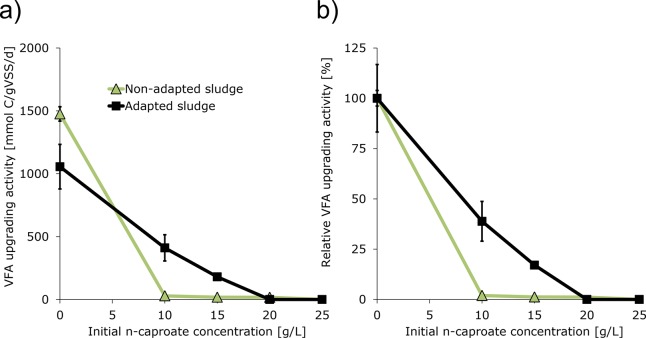
To investigate whether sludge can be adapted to perform VFA upgrading (i.e., chain elongation) at high n-caproate concentrations, a follow-up experiment was performed. In this experiment, VFA upgrading activity was compared using an inoculum that operated at low n-caproate concentration (7.1 g/L; nonadapted sludge) with an inoculum that operated at high n-caproate concentration (23.2 g/L; adapted sludge). Results of this experiment are graphically summarized in Figure 2. Numerical values on average specific activities are reported in Table 2 and profiles of product production are reported in the Supporting Information (Figures S4 and S5). At 10 g/L n-caproate, VFA upgrading activity was a factor 14 higher with adapted sludge (411 mmol C/gVSS/d) than with nonadapted sludge. At 15 g/L, VFA upgrading activity was a factor 10 higher with adapted sludge (181 mmol C/gVSS/d) than with nonadapted sludge. These figures show that the n-caproate tolerance of VFA upgrading activity was clearly increased with adapted sludge. However, at 20 g/L n-caproate the VFA upgrading process was completely inhibited with both adapted sludge and nonadapted sludge.
Figure 2.
Results of batch inhibition assays; initial n-caproate concentration vs observed activity (a) and relative activity (b) of VFA upgrading using nonadapted and adapted sludge as inoculum. Nonadapted sludge was grown converting acidified food waste and ethanol into MCFAs at 7.1 g/L n-caproate. Adapted sludge was grown converting acidified food waste and ethanol into MCFAs at 23.2 g/L n-caproate. Values indicate averages of duplicates and bars indicate range of duplicates (often too small to be visual). T = 30 °C, pH = 6.8 (buffered).
Discussion
n-Caproate Inhibits the Active Functional Groups in Chain Elongation Microbiomes
In this study, the effect of n-caproate concentration on the specific activity of chain elongation and competing processes was investigated. The specific activity of all active processes decreased with an increasing n-caproate concentration, though the degree in which these processes were inhibited was different for each process. The most plausible explanation for these decreased activities is that n-caproate is inhibitory to the active microorganisms. Such kind of inhibition depends on the MCFA concentration (in this case n-caproate), the chain length, the pH21 and also on the identity and the degree of adaptation of the microorganism.10 Evenly, in theory, n-caproate can thermodynamically inhibit its own production. However, we calculated that under the actual conditions, n-caproate did not induce a thermodynamic bottleneck for chain elongation.5
Both Undissociated n-Caproic Acid and Dissociated n-Caproate Are Inhibitory
Evidently, fatty acids in water can appear in their undissociated form or in their dissociated form. The distribution of these two forms depends on the pH and the pKa of the fatty acid. A low (i.e., acidic) pH yields a high fraction of undissociated fatty acids whereas a neutral and a high pH yields a low fraction of undissociated fatty acids. Undissociated MCFAs are considered to be more inhibitory to microorganisms than dissociated MCFAs.10 This seems also true for chain elongating microorganisms. Chain elongation studies that operate at slightly acidic pH, therefore, typically report lower MCFA production rates9,12 compared to studies that operate at near-neutral pH13,22,23. For details on mechanisms of (un)dissociated MCFA inhibition, we refer the interested reader to a review article.24
In this study, we were interested in the inhibitory effect of both n-caproate forms together at constant pH. As such, even though EEO and the reverse β-oxidation pathway release a proton (i.e., acidify the media), we had to minimize undissociation of n-caproate by keeping the pH constant during the assays. This was performed through the use of various buffers.
The assays in our study had an initial pH of 6.8; meaning that 1.1% of total n-caproate, with a pKa of 4.85, was undissociated. The lowest observed pH at the end of an assay was 6.5 (data not shown); meaning that, at most, 2% of total n-caproate was undissociated. Therefore, we conclude that the buffers worked well and the protocol used in this study could thus be used for other batch inhibition assays that require constant pH. We also conclude that VFA upgrading in underlying study was likely not solely inhibited by undissociated n-caproic acid alone. Rather, this process was likely inhibited by a combination of both forms together. This is because the highest observed undissociated n-caproic acid concentration in our study (∼0.4 g/L; Table 2) was always below the proposed toxic limit of undissociated n-caproic acid for chain elongation (∼0.87 g/L12). This would mean that, although dissociated MCFAs are considered less toxic than undissociated MCFAs, they are inhibitory too.
Role of Exogenous n-Caproate and Sodium
Before adapted sludge was used as inoculum in the batch inhibition assays, it was active in VFA upgrading (386 mmolC/gVSS/d) at an average n-caproate concentration of 23.2 g/L (Table 2). In the batch assays at 20 and 25 g/L n-caproate, however, this sludge showed no VFA upgrading activity (Figure 2). We have no mechanistic explanation for this. Possibly, exogenous (i.e., supplied) n-caproate may impose a different inhibitory effect than endogenous (i.e., produced) n-caproate. The exogenous n-caproate in the batch inhibition assays may thus be more toxic to chain elongating microorganisms than endogenous produced n-caproate in the continuous chain elongation process. Earlier work demonstrated a similar, but opposite effect; endogenous ethanol exerted a greater impact on yeast performance than exogenous ethanol.25 Another example is that Escherichia coli was associated with fewer membrane damage with exogenously supplied styrene than with endogenously produced styrene.26 Hence, these authors stressed the importance of considering the difference between exogenous and endogenous compounds when characterizing the effects of product inhibition.
All assays started with the same initial pH (6.8). Because n-caproic acid was added at different concentrations in the preparation of the media, however, also different amounts of sodium hydroxide were added to adjust the pH. This means that, besides n-caproate, also the sodium concentrations varied among the assays. Indeed, observed initial sodium concentrations were proportional to the initial n-caproate concentrations (Table 2). Sodium can be inhibitory to microorganisms through an increase of osmotic pressure or complete dehydration of microorganisms.27 To determine whether sodium was inhibitory, observed sodium concentrations were compared (Table 2).
Sodium had likely no substantial inhibitory effect on VFA upgrading in all assays. The used inocula were derived from environments with a similar, but often higher, sodium concentration than that was present in the assays. For example, adapted sludge was active in VFA upgrading in the reactor (386 mmolC/gVSS/d) at a high sodium concentration (9.1 g/L) but was completely inhibited in the 20 g/L n-caproate batch assay that contained a lower sodium concentration (6.4 g/L). Some strains of the chain elongating bacterium C. kluyveri were derived from brackish sediments,28 underlining that the active chain elongating microorganisms in our batch inhibition assays were likely not rigorously inhibited by sodium but by n-caproate. Also, the synthetic medium sludge was active in hydrogenotrophic methanogenesis in the reactor (59 mmolC/gVSS/d) at a sodium concentration of 6.1 g/L whereas sodium concentrations in the batch inhibition assays were lower or similar (2.3–7.1 g/L). Likewise, earlier work showed that hydrogenotrophic methanogens can remain active even up to high sodium chloride concentrations of 1.25 M (29 g/L sodium).29 This shows that hydrogenotrophic methanogenesis was likely also not rigorously inhibited by sodium but by n-caproate in our batch inhibition assays.
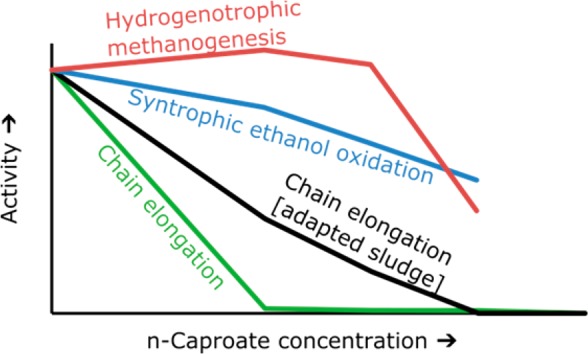
n-Caproate Formation May Adapt the Microbial Sludge To Perform Chain Elongation at Higher Concentrations
In an effective chain elongation process, chain elongation is stimulated while competing processes are suppressed. Whereas chain elongation can be stimulated by adding C. kluyveri,30 competing processes can be suppressed in various ways. Past research explained that pH,9 hydraulic shear force in combination with low hydraulic retention time7 and CO2 loading rate5 are control parameters to suppress competing processes. Also, the n-caproate concentration itself may have an effect since earlier work stated that undissociated n-caproic acid does have a microbial toxicity (at pH 5.5) which warranted the need of an continuous extraction unit.12 In our systematic study, it was shown that exogenous n-caproate does affect the different functional groups of microorganisms which may also be the case with endogenous n-caproate within bioreactors. With the activity tests, we show that adapted sludge is less inhibited than nonadapted sludge at higher n-caproate concentrations. This difference in n-caproate tolerance between adapted sludge and nonadapted sludge can be explained by a change in physiology of chain elongating microorganisms (e.g., membrane composition, fluidity, integrity and hydrophobicity).24 Another explanation is that the higher caproate concentration in the reactors selected for more n-caproate-tolerant chain elongating microorganisms. Our explanation (i.e., hypothesis) is that the formation of n-caproate in reactor microbiomes does acts as an in situ stimulatory agent to form higher n-caproate concentrations which leads toward a further accumulation and possible more selective production of n-caproate.
Conclusions
In this study, we showed that n-caproate inhibits chain elongation and competing processes in chain elongation microbiomes. Hydrogenotrophic methanogenesis was severely inhibited at 20 g/L n-caproate in batch. This inhibition probably rate-limits syntrophic ethanol oxidation at 25 g/L n-caproate. VFA upgrading was the most sensitive process to n-caproate inhibition but we demonstrated that the n-caproate tolerance of this process is higher with adapted sludge than with nonadapted sludge. Thus, microbiomes can be adapted to perform VFA upgrading at high n-caproate concentrations which likely limits syntrophic ethanol oxidation through hydrogenotrophic methanogenesis. n-Caproate formation is consequently another control parameter to inhibit competing processes to steer to higher n-caproate concentrations.
Acknowledgments
This work has been carried out with a grant from the BE-BASIC program FS 01.006 (www.be-basic.org)
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acssuschemeng.8b00200.
Profiles of product production and specifications on used inocula (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Weiland P. Biogas production: current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85 (4), 849–860. 10.1007/s00253-009-2246-7. [DOI] [PubMed] [Google Scholar]
- Seedorf H.; Fricke W. F.; Veith B.; Bruggemann H.; Liesegang H.; Strittmatter A.; Miethke M.; Buckel W.; Hinderberger J.; Li F.; Hagemeier C.; Thauer R. K.; Gottschalk G. The genome of Clostridium kluyveri, a strict anaerobe with unique metabolic features. Proc. Natl. Acad. Sci. U. S. A. 2008, 105 (6), 2128–2133. 10.1073/pnas.0711093105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angenent L. T.; Richter H.; Buckel W.; Spirito C. M.; Steinbusch K. J. J.; Plugge C. M.; Strik D. P. B. T. B.; Grootscholten T. I. M.; Buisman C. J. N.; Hamelers H. V. M Chain Elongation with Reactor Microbiomes: Open-Culture Biotechnology To Produce Biochemicals. Environ. Sci. Technol. 2016, 50 (6), 2796–2810. 10.1021/acs.est.5b04847. [DOI] [PubMed] [Google Scholar]
- Grootscholten T. I. M.; Strik D. P. B. T. B.; Steinbusch K. J. J.; Buisman C. J. N.; Hamelers H. V. M Two-stage medium chain fatty acid (MCFA) production from municipal solid waste and ethanol. Appl. Energy 2014, 116 (116), 223–229. 10.1016/j.apenergy.2013.11.061. [DOI] [Google Scholar]
- Roghair M.; Hoogstad T.; Strik D. P. B. T. B.; Plugge C. M.; Timmers P. H. A.; Weusthuis R. A.; Bruins M. E.; Buisman C. J. N. Controlling ethanol use in chain elongation by CO2 loading rate. Environ. Sci. Technol. 2018, 52 (3), 1496–1505. 10.1021/acs.est.7b04904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agler M. T.; Spirito C. M.; Usack J. G.; Werner J. J.; Angenent L. T. Chain elongation with reactor microbiomes: Upgrading dilute ethanol to medium-chain carboxylates. Energy Environ. Sci. 2012, 5 (8), 8189–8192. 10.1039/c2ee22101b. [DOI] [Google Scholar]
- Grootscholten T. I. M.; Steinbusch K. J. J.; Hamelers H. V. M; Buisman C. J. N. Chain elongation of acetate and ethanol in an upflow anaerobic filter for high rate MCFA production. Bioresour. Technol. 2013, 135, 440–445. 10.1016/j.biortech.2012.10.165. [DOI] [PubMed] [Google Scholar]
- Roghair M.; Liu Y.; Strik D. P. B. T. B.; Weusthuis R. A.; Bruins M. E.; Buisman C. J. N. Development of an effective chain elongation process from acidified food waste and ethanol into n-caproate. Front. Bioeng. Biotechnol. 2018, 10.3389/fbioe.2018.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agler M. T.; Spirito C. M.; Usack J. G.; Werner J. J.; Angenent L. T. Development of a highly specific and productive process for n-caproic acid production: applying lessons from methanogenic microbiomes. Water Sci. Technol. 2014, 69 (1), 62–68. 10.2166/wst.2013.549. [DOI] [PubMed] [Google Scholar]
- Royce L. A.; Liu P.; Stebbins M. J.; Hanson B. C.; Jarboe L. R. The damaging effects of short chain fatty acids on Escherichia coli membranes. Appl. Microbiol. Biotechnol. 2013, 97 (18), 8317–8327. 10.1007/s00253-013-5113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy F.; Albagnac G.; Samain E. Influence of Calcium Addition on Growth of Highly Purified Syntrophic Cultures Degrading Long-Chain Fatty Acids. Appl. Environ. Microbiol. 1985, 49 (3), 702–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S.; Usack J. G.; Spirito C. M.; Angenent L. T. Long-term n-caproic acid production from yeast-fermentation beer in an anaerobic bioreactor with continuous product extraction. Environ. Sci. Technol. 2015, 49 (13), 8012–8021. 10.1021/acs.est.5b00238. [DOI] [PubMed] [Google Scholar]
- Roghair M.; Strik D. P. B. T. B.; Steinbusch K. J. J.; Weusthuis R. A.; Bruins M. E.; Buisman C. J. N. Granular sludge formation and characterization in a chain elongation process. Process Biochem. 2016, 51 (10), 1594–1598. 10.1016/j.procbio.2016.06.012. [DOI] [Google Scholar]
- Lonkar S.; Fu Z.; Holtzapple M. Optimum alcohol concentration for chain elongation in mixed-culture fermentation of cellulosic substrate. Biotechnol. Bioeng. 2016, 113 (12), 2597–2604. 10.1002/bit.26024. [DOI] [PubMed] [Google Scholar]
- Sudmalis D.; Gagliano M. C.; Pei R.; Grolle K.; Plugge C. M.; Rijnaarts H. H. M.; Zeeman G.; Temmink H. Fast anaerobic sludge granulation at elevated salinity. Water Res. 2018, 128, 293–303. 10.1016/j.watres.2017.10.038. [DOI] [PubMed] [Google Scholar]
- Roman P.; Bijmans M. F. M.; Janssen A. J. H. Influence of methanethiol on biological sulphide oxidation in gas treatment system. Environ. Technol. 2016, 37 (13), 1693–1703. 10.1080/09593330.2015.1128001. [DOI] [PubMed] [Google Scholar]
- Rodríguez Arredondo M.; Kuntke P.; ter Heijne A.; Hamelers H. V. M; Buisman C. J. N. Load ratio determines the ammonia recovery and energy input of an electrochemical system. Water Res. 2017, 111 (111), 330–337. 10.1016/j.watres.2016.12.051. [DOI] [PubMed] [Google Scholar]
- Hajarnis S. R.; Ranade D. R. Inhibition of methanogens by n- and iso-volatile fatty acids. World J. Microbiol. Biotechnol. 1994, 10 (3), 350–351. 10.1007/BF00414879. [DOI] [PubMed] [Google Scholar]
- Liu Y.; He P.; Shao L.; Zhang H.; Lü F. Significant enhancement by biochar of caproate production via chain elongation. Water Res. 2017, 119, 150–159. 10.1016/j.watres.2017.04.050. [DOI] [PubMed] [Google Scholar]
- Zhu X.; Tao Y.; Liang C.; Li X.; Wei N.; Zhang W.; Zhou Y.; Yang Y.; Bo T. The synthesis of n-caproate from lactate: A new efficient process for medium-chain carboxylates production. Sci. Rep. 2015, 5, 14360. 10.1038/srep14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.; Chernyshov A.; Najdi T.; Fu Y.; Dickerson J.; Sandmeyer S.; Jarboe L. Membrane stress caused by octanoic acid in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2013, 97 (7), 3239–3251. 10.1007/s00253-013-4773-5. [DOI] [PubMed] [Google Scholar]
- Grootscholten T. I. M.; Steinbusch K. J. J.; Hamelers H. V. M; Buisman C. J. N. Improving medium chain fatty acid productivity using chain elongation by reducing the hydraulic retention time in an upflow anaerobic filter. Bioresour. Technol. 2013, 136 (136), 735–738. 10.1016/j.biortech.2013.02.114. [DOI] [PubMed] [Google Scholar]
- Grootscholten T. I. M.; Steinbusch K. J. J.; Hamelers H. V. M; Buisman C. J. N. High rate heptanoate production from propionate and ethanol using chain elongation. Bioresour. Technol. 2013, 136 (0), 715–718. 10.1016/j.biortech.2013.02.085. [DOI] [PubMed] [Google Scholar]
- Jarboe L. R.; Royce L. A.; Liu P. Understanding biocatalyst inhibition by carboxylic acids. Front. Microbiol. 2013, 4, 272. 10.3389/fmicb.2013.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.; Wu D.; Lin Y.; Wang X.; Kong H.; Tanaka S. Substrate and Product Inhibition on Yeast Performance in Ethanol Fermentation. Energy Fuels 2015, 29 (2), 1019–1027. 10.1021/ef502349v. [DOI] [Google Scholar]
- Lian J.; McKenna R.; Rover M. R.; Nielsen D. R.; Wen Z.; Jarboe L. R. Production of biorenewable styrene: utilization of biomass-derived sugars and insights into toxicity. J. Ind. Microbiol. Biotechnol. 2016, 43 (5), 595–604. 10.1007/s10295-016-1734-x. [DOI] [PubMed] [Google Scholar]
- Hierholtzer A.; Akunna J. C. Modelling sodium inhibition on the anaerobic digestion process. Water Sci. Technol. 2012, 66 (7), 1565–1573. 10.2166/wst.2012.345. [DOI] [PubMed] [Google Scholar]
- Kenealy W. R.; Waselefsky D. M. Studies on the substrate range of Clostridium kluyveri; the use of propanol and succinate. Arch. Microbiol. 1985, 141 (3), 187–194. 10.1007/BF00408056. [DOI] [Google Scholar]
- Liu Y.; Boone D. R. Effects of salinity on methanogenic decomposition. Bioresour. Technol. 1991, 35 (3), 271–273. 10.1016/0960-8524(91)90124-3. [DOI] [Google Scholar]
- Reddy M. V.; Hayashi S.; Choi D.; Cho H.; Chang Y.-C. Short chain and medium chain fatty acids production using food waste under non-augmented and bio-augmented conditions. J. Cleaner Prod. 2018, 176, 645–653. 10.1016/j.jclepro.2017.12.166. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.