Abstract
Polycystic ovary syndrome (PCOS) affects approximately 7% of reproductive age women. Although its etiology is unknown, in animals, excess prenatal testosterone (T) exposure induces PCOS-like phenotypes. While measuring fetal T in humans is infeasible, demonstrating in utero androgen exposure using a reliable newborn biomarker, anogenital distance (AGD), would provide evidence for a fetal origin of PCOS and potentially identify girls at risk. Using data from a pregnancy cohort (The Infant Development and the Environment Study), we tested the novel hypothesis that infant girls born to women with PCOS have longer AGD, suggesting higher fetal T exposure, than girls born to women without PCOS. During pregnancy, women reported whether they ever had a PCOS diagnosis. After birth, infant girls underwent two AGD measurements: anofourchette distance [AGD-AF] and anoclitoral distance [AGD-AC]. We fit adjusted linear regression models to examine the association between maternal PCOS and girls’ AGD. In total, 300 mother-daughter dyads had complete data and 23 mothers reported PCOS. AGD was longer in the daughters of women with a PCOS diagnosis compared to daughters of women with no diagnosis (AGD-AF:β=1.21, p=0.05; AGD-AC:β=1.05, p=0.18). Results were stronger in analyses limited to term births (AGD-AF:β=1.65, p=0.02; AGD-AC:β=1.43, p=0.09). Our study is the first to examine AGD in offspring of women with PCOS. Our results are consistent with findings that women with PCOS have longer AGD and suggest that during PCOS pregnancies, daughters may experience elevated T exposure. Identifying the underlying causes of PCOS may facilitate early identification and intervention for those at risk.
Keywords: PCOS, AGD, testosterone, pregnancy, fetal programming
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders in premenopausal women, affecting approximately 7% of reproductive age women1, 2. It contributes to numerous morbidities ranging from metabolic dysfunction (including type 2 diabetes, glucose intolerance, and obesity) to ovulatory disorders and infertility3–5. Excess testosterone (T) is a hallmark of the disorder6, 7 and it has been hypothesized that excess prenatal T may be a key etiologic factor. Extensive evidence from animal models supports this hypothesis8, 9, 10. In nonhuman primates, sheep, rats, and mice, administration of T during critical gestational windows results in PCOS-like features when female offspring mature9, 11, 12. In humans, women with clinical conditions characterized by T excess during pregnancy (e.g. congenital adrenal hyperplasia, congenital virilizing tumors, or certain sex steroid pathway mutations) have daughters who are at increased risk of PCOS later in life13–15.
Typically, PCOS diagnosis is made after menarche, when menstrual cycle dysregulation and symptoms of T excess (e.g. hirsutism and acne) emerge. Even then, however, diagnosis is confounded and delayed by highly irregular menstrual cycles and hormone profiles common to normal female pubertal development16. Diagnosis of PCOS before or during adolescence has therefore proven difficult, and as a result, the estimated prevalence of PCOS in adolescent girls is uncertain17. In theory, early identification of girls at risk of developing PCOS would be useful because it could facilitate interventions to prevent or ameliorate symptoms. For example, lifestyle modifications aimed at weight reduction, improved cardiovascular fitness and insulin resistance, or better nutrition, all diminish PCOS symptomology in adolescence18–21.
If excess prenatal T exposure also contributes to PCOS risk, as the animal models suggest, identifying biomarkers of prenatal T excess early in life would be invaluable. Although there are currently no direct methods for safely indexing the fetal hormonal milieu in normal human pregnancies, evidence has increasingly supported the use of anogenital distance (AGD), or the distance from the anus to the genitals, as a reliable biomarker of prenatal T exposure. In humans and other mammalian species, AGD is 50–100 percent longer in males than females22–25. In animal models, AGD can be elongated in females by experimentally increasing the prenatal T environment. For example, female rodents26, rabbits27, sheep28, 29, and nonhuman primates30 exposed to fetal male T concentrations during gestation have longer AGD than controls.
Human data on this topic is limited, however interest is growing. Supporting the hypothesis that excess prenatal androgen exposure contributes to PCOS, two recent studies (in Chinese and Mediterranean women) have found that AGD is longer in adult women with PCOS compared to controls31, 32. One of the two studies additional found positive associations between AGD measurements and circulating T levels32. In healthy young women, longer AGD has been linked to multi-follicular ovaries and higher serum testosterone levels33, 34. However studies in adults cannot rule out postnatal influences on AGD or ovarian function. Indeed, cross-sectional studies in adult women have found that AGD is positively related to age35 and inversely associated with use of hormonal contraception33, suggesting that there may be some plasticity in adulthood. Examining newborns circumvents this issue and may yield important clues. In a small study of infants, girls with congenital adrenal hyperplasia, who experienced excess adrenal androgen production during gestation, had longer AGD than controls36. Here we extend this research to examine the daughters of women with PCOS, who may be exposed to elevated T during fetal development. Women with PCOS have elevated T during pregnancy compared to controls37, and it is plausible that the female fetuses they carry may also produce excess T. Our objective in this study was to translate these lines of fetal programming research to humans, and test the previously unexplored hypothesis that AGD is elongated in infant girls born to mothers with PCOS. To this end, we used data from a large pregnancy cohort study, classifying women as having: (1) diagnosed PCOS; (2) hirsutism, a diagnostic criterion for PCOS, without diagnosis; or (3) neither PCOS diagnosis nor symptoms.
Materials and methods
Study overview and population
The Infant Development and Environment Study (TIDES) was designed to examine environmental exposures during pregnancy in relation to infant reproductive system development. Study recruitment and methods have been described elsewhere38, 39. Briefly, pregnant women were recruited at prenatal clinics affiliated with four U.S. academic medical centers (University of California- San Francisco [UCSF]; University of Minnesota [UMN]; University of Rochester [URMC]; and University of Washington [UW]) between 2010–2012. Eligibility criteria included: less than 13 weeks pregnant, age 18 or older, ability to read and write in English (or Spanish at the UCSF center), no major medical conditions, pregnancy non-medically threatened, and planning to deliver at an affiliated study hospital. Subjects participated in study visits in each trimester, during which they completed questionnaires and gave urine samples. Prior to implementation, TIDES was approved by The University of Rochester Research Subjects Review Board, The University of Minnesota Human Research Protection Program Institutional Review Board, The University of Washington Human Subjects Review Committee, and the University of California San Francisco Human Research Protection Program Committee on Human Research. When the TIDES Coordinating Center moved from the University of Rochester to the Icahn School of Medicine at Mount Sinai, the latter’s Human Research Protection Program Institutional Review Board also approved the study. All subjects signed written informed consent.
Characterization of maternal PCOS status
In the first trimester, subjects completed a questionnaire including items on basic demographic information including race, ethnicity, age, height, and body weight immediately prior to pregnancy. In the second trimester, subjects completed a questionnaire detailing their reproductive health and history. Women were asked whether they were ever given a PCOS diagnosis. They were also asked a series of questions aimed at assessing possible undiagnosed PCOS, including an item on hirsutism, defined on the questionnaire as “excess hair growth on the upper lip, chin, chest, or abdomen prior to pregnancy”. An additional item on oligomenorrhea (another diagnostic criterion for PCOS, defined in the questionnaire as having “fewer than eight menstrual periods per year when not taking hormonal contraception, pregnant, or breast-feeding”). In other cohorts, self-reported symptoms have been reliably associated with PCOS diagnosis40–44. Based on their responses to these items, women were categorized as: (1) reporting ever having received a PCOS diagnosis (irrespective of hirsutism and menstrual regularity); (2) reporting hirsutism but no history of PCOS diagnosis (irrespective of menstrual regularity); or (3) non-PCOS (comparison group), with neither PCOS, hirsutism, nor oligomenorrhea. We did not pursue analyses specific to the group reporting oligomenorrhea because it became clear that it was a heterogeneous group that did not represent women with probable PCOS.
Birth exams
Shortly after birth (median and 75th percentile at 1 day old) trained teams of study coordinators administered standardized physical examinations to infants born to TIDES participants. These methods have been described elsewhere45 and are similar to those used in our previous study of infant reproductive development46. Briefly, for infant girls, two measurements were made using Vernier dial calipers: (1) the distance from the center of the anus to the anterior tip of the clitoral hood (AGD-AC); and (2) the distance from the center of the anus to the base of the posterior fourchette (AGD-AF). All measurements were taken with the infant lying flat on her back with the legs held in a “frog leg” position by the mother or a study assistant. Each measurement was repeated three times (with the calipers zeroed between measurements) and the average of those three values was used in analyses39,45. Finally, the study team measured infant weight and length at exam and recorded the infant’s gestational age at birth from the medical record.
Statistical methods
We calculated descriptive statistics (mean, standard deviation, maximum, minimum, frequencies) for the combined study population as well as stratified by PCOS status. We examined differences between the two groups in bivariate analyses, using analysis of variance for continuous variables and chi-square tests for categorical variables. We fit two sets of multivariable linear regression models to examine infant AGD in relation to maternal PCOS status. In both sets of models, our outcomes were infant AGD (AGD-AF or AGD-AC). In the first set of models, we examined AGD in daughters of mothers with diagnosed PCOS compared to daughters of mothers without PCOS. In the second set of models, we examined AGD in daughters of mothers with diagnosed PCOS or hirsutism (possibly indicating undiagnosed PCOS, or at least elevated T levels) compared to daughters of mothers without PCOS.
We selected a number of covariates for a priori inclusion based on our previous work demonstrating that they predict AGD in infants39, 45, 47, 48. These covariates were gestational age at birth, infant age at physical examination, weight for length z-score, maternal age, and study center. Because of their associations with PCOS, we considered the inclusion of race (dichotomized as white versus all others) and pre-pregnancy body mass index (BMI) as additional covariates. Race, but not BMI, changed estimates by more than 10% and was retained in final models. Thus the final set of covariates included in models was: infant age at exam, weight-for-length z-score, gestational age at birth, study center, maternal age, and race.
Finally, we performed a set of sensitivity analyses limited to term infants (≥37 weeks gestation) because it has been hypothesized that delays in measuring preterm infants after birth (due to their fragility) may make their AGD measurements fundamentally different than those of term babies. All analyses were performed in SAS Enterprise Version 5.1 (SAS Institute, Cary, NC) and results for which p≤0.05 were considered statistically significant.
Results
In total, 737 women in the TIDES study completed 2nd trimester questionnaires and had infants who underwent genital measurements shortly after birth. Of those, 376 gave birth to female infants and 85% had complete data needed for the current analyses. Among those missing data, 17 were lacking data on covariates and the remainder had not fully answered all questions on PCOS and related symptoms. Of the 300 subjects who were therefore included in this analysis, 23 were categorized as having PCOS, 45 were categorized as having hirsutism without a PCOS diagnosis, and 232 were categorized as having neither PCOS nor diagnostic criteria (Figure I). Compared to women not included in the analysis, those included were slightly older (31.4 vs 30.1 years) and had a lower pre-pregnancy BMI (27.1 vs 28.9 kg/m2) (not shown).
Fig I.
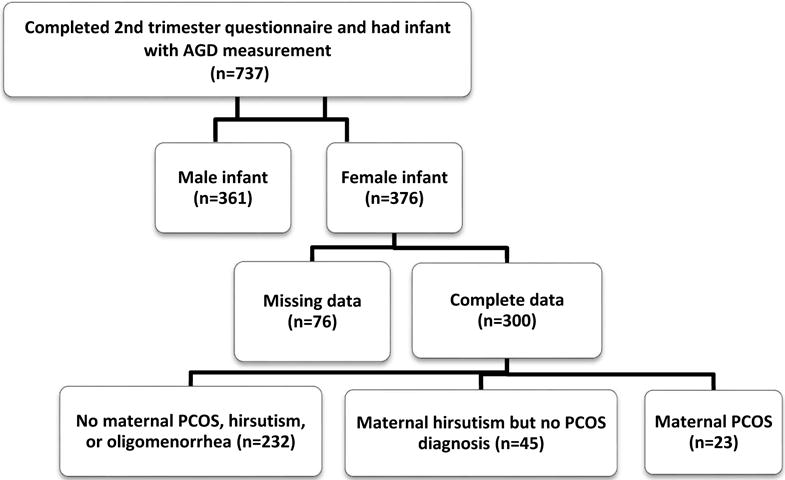
Determination of final sample for current TIDES analyses.
Focusing on the women included in the main analyses, in bivariate analyses, women with PCOS and the non-PCOS comparison group were similar in age and gestational age at delivery. Women with PCOS (or related symptoms) tended to have a higher pre-pregnancy BMI and were more likely to self-identify as white, and less likely to identify as black or Asian (Table 1). Women with hirsutism, but not a PCOS diagnosis, were more likely to report smoking during pregnancy. Not surprisingly, women who reported having PCOS or hirsutism, were more likely to also report oligomenorrhea and a history of infertility than the non-PCOS comparison group. Infants born to the three groups were similar in size at birth and crude AGD measurements.
Table I.
Characteristics of TIDES mothers and daughters (n=300)a.
| All (n=300) |
Maternal PCOS diagnosis (n=23) |
Hirsutism, but no PCOS diagnosis (n=45) |
No maternal PCOS, hirsutism, or oligomenorrhea (n=232) |
p-valueb | |
|---|---|---|---|---|---|
| Continuous variables; mean ±SD | |||||
|
| |||||
| Maternal age (yrs) | 31.4±5.5 | 30.6±4.5 | 31.0±5.3 | 31.5±5.7 | 0.64 |
| Pre-pregnancy BMI (kg/m2) | 27.1±6.2 | 27.3±3.7 | 29.6±9.1 | 26.6±5.6 | 0.02 |
| Gestational age at delivery (wks) | 39.5±1.6 | 39.1±1.8 | 39.3±1.6 | 39.5±1.6 | 0.39 |
| Birth weight (kg) | 3.3±0.5 | 3.2±0.4 | 3.4±0.5 | 3.3±0.5 | 0.39 |
| Genital measurements- female infants | |||||
| AGD-AF (mm) | 16.0±3.1 | 16.9±4.0 | 16.2±2.9 | 15.9±3.0 | 0.30 |
| AGD-AC (mm) | 36.8±3.8 | 37.1±4.8 | 37.6±3.9 | 36.6±3.7 | 0.20 |
|
| |||||
| Categorical variables; N(%) | |||||
|
| |||||
| Study Center | |||||
| San Francisco, CA | 78 (26.0) | 7 (30.4) | 11(24.4) | 130 (27.7) | |
| Minneapolis, MN | 79 (26.3) | 8 (35.8) | 14 (31.1) | 128 (27.3) | 0.44 |
| Rochester, NY | 80 (26.7) | 4 (17.4) | 15 (33.3) | 118 (25.2) | |
| Seattle, WA | 63 (21.0) | 4 (17.4) | 5 (11.1) | 93 (19.8) | |
| Race | |||||
| White | 197 (65.7) | 21 (91.3) | 32 (71.1) | 144(62.1) | 0.10 |
| Black | 37 (12.3) | 1 (4.3) | 7 (15.6) | 29 (12.5) | |
| American Indian/Alaska Native | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Asian | 24 (8.0) | 0 (0.0) | 2 (4.4) | 22 (9.5) | |
| Other/unknown | 42 (14.0) | 1 (4.3) | 4 (8.9) | 37 (16.0) | |
| Smoking during pregnancy | 13 (4.3) | 0 (0.0) | 5 (11.1) | 8 (3.4) | 0.04 |
| Fewer than eight periods a year | 13 (3.2) | 10 (43.5) | 3 (6.7) | 0 (0.0) | <0.0001 |
| Excess hair growth (hirsutism) | 56 (18.7) | 11 (47.8) | 45 (100) | 0 (0.0) | <0.0001 |
| History of infertility | 45 (15.0) | 11 (47.8) | 9 (20.0) | 25 (10.8) | <0.0001 |
| Parous (prior to index pregnancy) | 135 (45.1) | 7 (30.4) | 21 (46.7) | 107 (46.3) | 0.33 |
exact n may vary due to missing data
p-values based on results of analysis of variance and chi-square tests
In multivariable linear regression models, the daughters of mothers with PCOS had longer AGD-AF (β=1.21, 95% CI −0.01, 2.43, p=0.05) but not AGD-AC (β=1.05, 95% CI −0.49, 2.60, p=0.18) than daughters born to women without PCOS (Table II). In the second set of analyses (which grouped women with PCOS and those reporting hirsutism but no PCOS diagnosis), both AGD-AF and AGD-AC were longer among the daughters of possible PCOS cases compared to daughters of women without PCOS (AGD-AF: β=0.82, 95% CI 0.06, 1.58, p=0.03; AGD-AC: β=1.15, 95% CI 0.19, 2.11, p=0.02).
Table II.
Regression models comparing AGD in infant girls born to women with (A) PCOS or (B) PCOS or hirsutism; to infant girls born to a non-PCOS comparison group (β coefficient, 95% CI); p-value.
| All Infant Girls | ||||
|---|---|---|---|---|
|
| ||||
| (A) Diagnosed PCOS (23 cases; 232 non-casesa) |
(B) PCOS or hirsutism (68 cases; 232 non-casesa) |
|||
|
| ||||
| Unadjusted models | Adjusted modelsb | Unadjusted models | Adjusted modelsb | |
| AGD-AF | 0.99 (−0.36, 2.34); p=0.15 | 1.21 (−0.01, 2.43); p=0.05 | 0.57 (−0.27, 1.41); p=0.18 | 0.82 (0.06, 1.58); p=0.03 |
| AGD-ACc | 0.57 (−1.07, 2.20); p=0.49 | 1.05 (−0.49, 2.60); p=0.18 | 0.91 (−0.12, 1.94); p=0.08 | 1.15 (0.19, 2.11); p=0.02 |
|
| ||||
| Term births only (>37 weeks) | ||||
|
| ||||
|
(A) Diagnosed PCOS (19 cases; 218 non-casesa) |
(B) PCOS or hirsutism (58 cases; 218 non-casesa) |
|||
|
| ||||
| Unadjusted models | Adjusted modelsb | Unadjusted models | Adjusted modelsb | |
|
| ||||
| AGD-AF | 1.68 (0.22, 3.16); p=0.02 | 1.65 (0.30, 2.99); p=0.02 | 0.93 (0.05, 1.81); p=0.04 | 0.99 (0.19, 1.79); p=0.02 |
| AGD-ACc | 1.20 (−0.58, 2.98); p=0.19 | 1.43 (−0.27, 3.12); p=0.09 | 1.16 (0.07, 2.25); p=0.04 | 1.22 (−0.15, 2.91); p=0.08 |
The comparison group (“non-cases”) includes women who reported: (1) not having PCOS; (2) not being hirsute; (3) not having 8 or fewer menstrual periods per year.
Adjusting for infant age at exam, weight-for-length z-score, gestational age at delivery, study center, maternal age, race.
Due to one missing AGD-AC value, the sample size is one less than for the AGD-AF analysis.
In sensitivity analyses, we restricted our main models to only include term births. In these analyses, effect sizes were larger and p-values smaller, despite the smaller sample size. Among the term daughters of women diagnosed with PCOS (n=19), AGD-AF (β=1.65, 95% CI 0.30, 2.99; p=0.02) and AGD-AC (β=1.43, 95% CI −0.27, 3.12; p=0.09) were longer than in daughters of non-PCOS women. Similarly, in the term daughters of women with PCOS or hirsutism, both AGD measures were longer (AGD-AF: β=0.99, 95% CI: 0.19, 1.79; p=0.02; AGD-AC: β=1.22, 95% CI: −0.15, 2.91, p=0.08) than among daughters of women without PCOS (Table II).
Discussion
In this pregnancy cohort, we demonstrated for the first time that women with PCOS gave birth to daughters with longer AGD, a measure of prenatal T exposure, than daughters born to women without PCOS. These results support previous work indicating that during PCOS pregnancies, female fetuses may encounter elevated T levels49, 50 and suggest that AGD may provide a postnatal “read-out” of their prior intrauterine hormonal environment. They are also consistent with recent findings from two studies comparing AGD in adult women with PCOS and controls. In both studies, PCOS cases had significantly longer AGD than controls. In fact, the odds of having PCOS was 3–19 fold greater among women in the longest AGD tertile compared to women in the shortest AGD tertile31, 32. One of the two studies, furthermore, showed that AGD was positively associated with circulating T levels, particularly among PCOS cases32. Similar results have been found in a cross-sectional study of healthy, adult women. Women in the longest tertile of AGD were 3–6 times as likely to have multi-follicular ovaries (≥6 follicles per ovary) as women in the shortest tertile of AGD33. In that study, too few women had diagnosed PCOS to draw conclusions, however other work has indicated that multi-follicular ovaries may be indicative of hyperandrogenic ovarian dysregulation51, 52. In the same study population, moreover, AGD-AF was positively correlated with serum T concentrations in the early follicular phase34.
This body of work is also consistent with non-human primates (both the well-characterized prenatally androgenized model and naturally occurring high T females), whereby T exposure is positively correlated with AGD30, 53. For these reasons, AGD should be explored further as a possible early indicator of PCOS risk. Although an extensive literature has examined AGD as a marker of prenatal T in boys (particularly in relation to anti-androgenic environmental chemicals)39, 46, 54, 55, this is one of the first studies to demonstrate that AGD may also be a useful biomarker in girls, particularly in the context of a hyperandrogenic hormonal milieu.
These results are also noteworthy in relation to the growing body of research on health and development among the children of women with PCOS. Several studies have noted neurodevelopmental alterations among offspring of PCOS mothers that, as in our study, may be indicative of excess prenatal androgen exposure. For example, prenatal androgens organize the male brain and it has been hypothesized that excess androgens may contribute to autism spectrum disorders56, 57. In a recent nationwide case-control study in Sweden, the odds of autism spectrum disorders, were nearly 60% higher in the offspring of mothers with PCOS compared to controls58. In separate work on typically developing children, girls born to women with PCOS scored significantly higher than controls on “systematizing” scales (which measure conscious focus on working out the rules of a social/communication system) and lower on empathy scales, a pattern more common in males and indicative of non-clinical autism spectrum-like development59, 60. As further evidence of the effects of prenatal androgens on the female brain, in a macaque model, prenatally androgenized females exhibit masculinized patterns of play and mounting behavior concomitant with decreases in female-typical mating behavior61, 62. Finally, recent evidence from a rodent PCOS model suggests that prenatally androgenized animals display alterations in sex steroid pathways in key brain regions (e.g. the amygdala and hippocampus) leading to an anxiety phenotype characteristic of women with PCOS63, 64. Whether AGD at birth is predictive of any of these neurodevelopmental alterations is unknown. There is, however, evidence that AGD at birth is associated with sex-typed play behavior in boys later in childhood65 and similar studies are needed in girls.
Our results suggest that female fetuses in PCOS pregnancies experience elevated T exposure, however the course of that exposure is unclear. It is conventionally believed that during pregnancy, barrier mechanisms (including high levels of sex hormone binding globulin and aromatase) protect the fetus from maternal T exposure and that only in exceptional cases is the placental capacity for androgen metabolism exceeded66, 67. It is plausible that in PCOS pregnancies, these mechanisms are altered or insufficient. At term, placental steroidogenesis, particularly 3βHSD-1 and P450 aromatase activity, are altered in PCOS placentae compared to controls, in patterns that may promote increased placental androgen production68. In addition, morphometric and vascular differences between PCOS and non-PCOS placentae have been noted, further implicating placental dysfunction69, 70. Perhaps most plausibly, PCOS daughters may themselves produce excess ovarian or adrenal androgens during gestation, possibly due to a genetic predisposition71–73. While our results cannot distinguish among the possible sources of excess T in PCOS pregnancies (i.e. mother, fetus, placenta), this is clearly an area of great interest that merits additional research74, 75.
Our results also contribute to the small literature on sex steroid activity and ovarian function in the daughters of women with PCOS. In one study, mid-gestation amniotic testosterone levels were elevated among women with PCOS than controls, however this was only true for women carrying female fetuses59. This fits with evidence that by mid-gestation, much as in non-human primates, the human fetal ovary has androgen receptors and can produce androgens76–78. In infancy, daughters born to women with untreated PCOS have higher anti-Mullerian hormone (AMH) and stimulated estradiol levels (suggestive of greater ovarian mass and/or a larger growing follicular pool) than daughters from non-PCOS or metformin-treated PCOS women79. These endocrine differences in PCOS daughters appear to persist over time. In adolescence, daughters of women with PCOS have higher free androgen index, testosterone, and insulin levels (two hours after a glucose tolerance test), as well as larger ovarian volumes than controls, all symptoms suggestive of an emergent PCOS phenotype80. Thus maternal PCOS predicts a subsequent PCOS-like phenotype in daughters81 and incorporating AGD measurements into evaluations at birth (or even later in childhood) may ultimately help to predict which daughters are most likely to go on to develop PCOS, facilitating early intervention.
Our study has several notable strengths including a diverse cohort recruited from four medical centers. Our AGD measurement protocols were particularly rigorous, and included extensive centralized training of study examiners as well as regular quality control measures45. Importantly, our protocols were specifically designed to facilitate AGD measurement shortly after birth to minimize the possibility that postnatal factors might affect AGD as the infant ages. Although several cohorts have now measured infant AGD (mostly in the context of maternal environmental exposures), to our knowledge, no other study has examined AGD in relation to PCOS or other hyperandrogenic conditions in the mother. Because this analysis was situated in the context of an ongoing epidemiological cohort study, we will continue to follow these mother-daughter dyads forward and have the potential to track the development of PCOS-like symptoms in the daughters in the future.
There are also several notable limitations to our study. Because this large cohort study was not designed to examine PCOS, although our overall prevalence of PCOS was similar to national estimates, the absolute number of PCOS women was relatively small and replication in a larger cohort is needed. Lacking medical records, furthermore, we relied upon self-reported PCOS and we did not explicitly query women about other relevant conditions, such as familial hirsutism, congenital adrenal hyperplasia, and ovarian tumors that may have contributed to hirsutism and other PCOS-like symptoms. However given that this was a healthy pregnant population without any known major medical conditions, such disorders are unlikely. In addition, recent reports suggest that for research purposes, self-reported PCOS may be as good as clinical diagnosis of PCOS43, 44. To address this possibility of under-reporting of PCOS (due to the reliance on self-report), our second set of analyses additionally considered women reporting hirsutism but not PCOS. The strength of the associations was reduced when women with hirsutism (without a PCOS diagnosis) were included, which may indicate that it was a heterogeneous group. Future work examining this question should thoroughly assess all possible causes of PCOS-like symptoms and confirm with clinical records to avoid misclassification. Finally we did not collect data on use of metformin or other ovulation inducing drugs during pregnancy. Metformin can lower testosterone levels in non-pregnant women with PCOS82 and is sometimes used through the first trimester when the fetal reproductive system is forming. Although studies in pregnant women with PCOS have shown no differences in androgen levels in mothers in the metformin and placebo groups83,84, some endocrine differences have been noted in infant daughters of PCOS women who did and did not take metformin during pregnancy79. The same may be true of other ovulation inducing drugs that were not assessed. Importantly, these medications would be used more widely among the women with PCOS in our study, thus if there were effects on T levels, they would likely be in the direction of reducing fetal androgen exposure and attenuate associations with infant AGD. Nevertheless, future studies on this topic should consider the use of metformin and other hormonal treatments in relation to androgen-sensitive outcomes in offspring.
In conclusion, our results suggest that among infant girls, AGD may be a biomarker of excess T exposure in utero and may be worth further investigation as a possible predictor of subsequent PCOS risk. Because AGD can be easily measured in all children at birth (and throughout childhood), it could prove particularly useful as an early risk phenotype identifying girls who could benefit from early lifestyle modification programs aimed at reducing obesity, and thereby PCOS incidence. We will continue to follow this cohort as they age to study downstream sequelae of longer AGD in girls.
Acknowledgments
We wish to acknowledge the contributions of the TIDES Study Team: Coordinating Center: Fan Liu, Erica Scher; UCSF: Marina Stasenko, Erin Ayash, Melissa Schirmer, Jason Farrell, Mari-Paule Thiet, Laurence Baskin; UMN: Heather L. Gray Chelsea Georgesen, Brooke J. Rody, Carrie A. Terrell, Kapilmeet Kaur; URMC: Erin Brantley, Heather Fiore, Lynda Kochman, Lauren Parlett, Jessica Marino, William Hulbert, Robert Mevorach, Eva Pressman; UW/SCH: Kristy Ivicek, Bobbie Salveson, Garry Alcedo and the families who participated in the study. We thank the TIDES families for their participation and the residents at URMC and UCSF who assisted with birth exams.
Financial support: Funding for this research was provided by the following grants: NIH R01ES016863-04, R01ES016863-02S4, P30ES005022.
Footnotes
Conflicts of Interest: None.
Ethical standards: The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975 as revised in 2008, and has been approved by the institutional committees at the University of Rochester, the University of California-San Francisco, the University of Washington, the University of Minnesota, and the Icahn School of Medicine at Mount Sinai.
References
- 1.ACOG. Polycystic Ovary Syndrome. Obstet Gynecol. 2009;114(4):936–949. doi: 10.1097/AOG.0b013e3181bd12cb. [DOI] [PubMed] [Google Scholar]
- 2.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clinical epidemiology. 2013;6:1–13. doi: 10.2147/CLEP.S37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hart R, Doherty DA. The potential implications of a PCOS diagnosis on a woman’s long-term health using data linkage. J Clin Endocrinol Metab. 2015;100(3):911–919. doi: 10.1210/jc.2014-3886. [DOI] [PubMed] [Google Scholar]
- 4.Joham AE, Ranasinha S, Zoungas S, Moran L, Teede HJ. Gestational diabetes and type 2 diabetes in reproductive-aged women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99(3):E447–452. doi: 10.1210/jc.2013-2007. [DOI] [PubMed] [Google Scholar]
- 5.Nader S. Infertility and pregnancy in women with polycystic ovary syndrome. Minerva Endocrinol. 2010;35(4):211–225. [PubMed] [Google Scholar]
- 6.Group TREA-sPCW. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19(1):41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 7.Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91(2):456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 8.Abbott DH, Dumesic DA, Franks S. Developmental origin of polycystic ovary syndrome - a hypothesis. J Endocrinol. 2002;174(1):1–5. doi: 10.1677/joe.0.1740001. [DOI] [PubMed] [Google Scholar]
- 9.Abbott DH, Nicol LE, Levine JE, Xu N, Goodarzi MO, Dumesic DA. Nonhuman primate models of polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373(1–2):21–28. doi: 10.1016/j.mce.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padmanabhan V, Veiga-Lopez A. Animal models of the polycystic ovary syndrome phenotype. Steroids. 2013;78(8):734–740. doi: 10.1016/j.steroids.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Houten EL, Kramer P, McLuskey A, Karels B, Themmen AP, Visser JA. Reproductive and metabolic phenotype of a mouse model of PCOS. Endocrinology. 2012;153(6):2861–2869. doi: 10.1210/en.2011-1754. [DOI] [PubMed] [Google Scholar]
- 12.Comim FV, Hardy K, Robinson J, Franks S. Disorders of follicle development and steroidogenesis in ovaries of androgenised foetal sheep. J Endocrinol. 2015;225(1):39–46. doi: 10.1530/JOE-14-0150. [DOI] [PubMed] [Google Scholar]
- 13.Barnes RB, Rosenfield RL, Ehrmann DA, et al. Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: evidence for perinatal masculinization of neuroendocrine function in women. J Clin Endocrinol Metab. 1994;79(5):1328–1333. doi: 10.1210/jcem.79.5.7962325. [DOI] [PubMed] [Google Scholar]
- 14.Hague WM, Adams J, Rodda C, et al. The prevalence of polycystic ovaries in patients with congenital adrenal hyperplasia and their close relatives. Clin Endocrinol (Oxf) 1990;33(4):501–510. doi: 10.1111/j.1365-2265.1990.tb03887.x. [DOI] [PubMed] [Google Scholar]
- 15.Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab. 1995;80(12):3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- 16.Rosenfield RL. The Diagnosis of Polycystic Ovary Syndrome in Adolescents. Pediatrics. 2015;136(6):1154–1165. doi: 10.1542/peds.2015-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamangar F, Okhovat JP, Schmidt T, et al. Polycystic Ovary Syndrome: Special Diagnostic and Therapeutic Considerations for Children. Pediatr Dermatol. 2015;32(5):571–578. doi: 10.1111/pde.12566. [DOI] [PubMed] [Google Scholar]
- 18.Harden KA, Cowan PA, Velasquez-Mieyer P, Patton SB. Effects of lifestyle intervention and metformin on weight management and markers of metabolic syndrome in obese adolescents. J Am Acad Nurse Pract. 2007;19(7):368–377. doi: 10.1111/j.1745-7599.2007.00236.x. [DOI] [PubMed] [Google Scholar]
- 19.Harris-Glocker M, Davidson K, Kochman L, Guzick D, Hoeger K. Improvement in quality-of-life questionnaire measures in obese adolescent females with polycystic ovary syndrome treated with lifestyle changes and oral contraceptives, with or without metformin. Fertil Steril. 2010;93(3):1016–1019. doi: 10.1016/j.fertnstert.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoeger K, Davidson K, Kochman L, Cherry T, Kopin L, Guzick DS. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J Clin Endocrinol Metab. 2008;93(11):4299–4306. doi: 10.1210/jc.2008-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lass N, Kleber M, Winkel K, Wunsch R, Reinehr T. Effect of lifestyle intervention on features of polycystic ovarian syndrome, metabolic syndrome, and intima-media thickness in obese adolescent girls. J Clin Endocrinol Metab. 2011;96(11):3533–3540. doi: 10.1210/jc.2011-1609. [DOI] [PubMed] [Google Scholar]
- 22.Barrett ES, Parlett LE, Redmon JB, Swan SH. Evidence for sexually dimorphic associations between maternal characteristics and anogenital distance, a marker of reproductive development. Am J Epidemiol. 2014;179(1):57–66. doi: 10.1093/aje/kwt220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salazar-Martinez E, Romano-Riquer P, Yanez-Marquez E, Longnecker MP, Hernandez-Avila M. Anogenital distance in human male and female newborns: a descriptive, cross-sectional study. Environmental health: a global access science source. 2004;3(1):8. doi: 10.1186/1476-069X-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banszegi O, Altbacker V, Bilko A. Intrauterine position influences anatomy and behavior in domestic rabbits. Physiol Behav. 2009;98(3):258–262. doi: 10.1016/j.physbeh.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Fouqueray TD, Blumstein DT, Monclus R, Martin JG. Maternal effects on anogenital distance in a wild marmot population. PloS one. 2014;9(3):e92718. doi: 10.1371/journal.pone.0092718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hotchkiss AK, Lambright CS, Ostby JS, Parks-Saldutti L, Vandenbergh JG, Gray LE., Jr Prenatal testosterone exposure permanently masculinizes anogenital distance, nipple development, and reproductive tract morphology in female Sprague-Dawley rats. Toxicol Sci. 2007;96(2):335–345. doi: 10.1093/toxsci/kfm002. [DOI] [PubMed] [Google Scholar]
- 27.Banszegi O, Altbacker V, Ducs A, Bilko A. Testosterone treatment of pregnant rabbits affects sexual development of their daughters. Physiol Behav. 2010;101(4):422–427. doi: 10.1016/j.physbeh.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 28.Recabarren SE, Padmanabhan V, Codner E, et al. Postnatal developmental consequences of altered insulin sensitivity in female sheep treated prenatally with testosterone. American journal of physiology Endocrinology and metabolism. 2005;289(5):E801–806. doi: 10.1152/ajpendo.00107.2005. [DOI] [PubMed] [Google Scholar]
- 29.Manikkam M, Crespi EJ, Doop DD, et al. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145(2):790–798. doi: 10.1210/en.2003-0478. [DOI] [PubMed] [Google Scholar]
- 30.Abbott AD, Colman RJ, Tiefenthaler R, Dumesic DA, Abbott DH. Early-to-mid gestation fetal testosterone increases right hand 2D:4D finger length ratio in polycystic ovary syndrome-like monkeys. PloS one. 2012;7(8):e42372. doi: 10.1371/journal.pone.0042372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez-Ferrer ML, Mendiola J, Hernandez-Penalver AI, et al. Presence of polycystic ovary syndrome is associated with longer anogenital distance in adult Mediterranean women. Hum Reprod. 2017;32(11):2315–2323. doi: 10.1093/humrep/dex274. [DOI] [PubMed] [Google Scholar]
- 32.Wu Y, Zhong G, Chen S, Zheng C, Liao D, Xie M. Polycystic ovary syndrome is associated with anogenital distance, a marker of prenatal androgen exposure. Hum Reprod. 2017;32(4):937–943. doi: 10.1093/humrep/dex042. [DOI] [PubMed] [Google Scholar]
- 33.Mendiola J, Roca M, Minguez-Alarcon L, et al. Anogenital distance is related to ovarian follicular number in young Spanish women: a cross-sectional study. Environmental Health. 2012;11:90. doi: 10.1186/1476-069X-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mira-Escolano MP, Mendiola J, Minguez-Alarcon L, et al. Longer anogenital distance is associated with higher testosterone levels in women: a cross-sectional study. BJOG: an international journal of obstetrics and gynaecology. 2014;121(11):1359–1364. doi: 10.1111/1471-0528.12627. [DOI] [PubMed] [Google Scholar]
- 35.Wainstock T, Shoham-Vardi I, Sheiner E, Walfisch A. Fertility and anogenital distance in women. Reprod Toxicol. 2017 doi: 10.1016/j.reprotox.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Callegari C, Everett S, Ross M, Brasel JA. Anogenital ratio: measure of fetal virilization in premature and full-term newborn infants. J Pediatr. 1987;111(2):240–243. doi: 10.1016/s0022-3476(87)80075-6. [DOI] [PubMed] [Google Scholar]
- 37.Caanen MR, Kuijper EA, Hompes PG, et al. Mass spectrometry methods measured androgen and estrogen concentrations during pregnancy and in newborns of mothers with polycystic ovary syndrome. Eur J Endocrinol. 2016;174(1):25–32. doi: 10.1530/EJE-15-0699. [DOI] [PubMed] [Google Scholar]
- 38.Barrett ES, Sathyanarayana S, Janssen S, et al. Environmental health attitudes and behaviors: findings from a large pregnancy cohort study. Eur J Obstet Gynecol Reprod Biol. 2014;176:119–125. doi: 10.1016/j.ejogrb.2014.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swan SH, Sathyanarayana S, Barrett ES, et al. First-trimester phthalate exposure is linked to shorter anogenital distance in newborn boys. Human Reproduction. 2015 doi: 10.1093/humrep/deu363. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taponen S, Ahonkallio S, Martikainen H, et al. Prevalence of polycystic ovaries in women with self-reported symptoms of oligomenorrhoea and/or hirsutism: Northern Finland Birth Cohort 1966 Study. Hum Reprod. 2004;19(5):1083–1088. doi: 10.1093/humrep/deh214. [DOI] [PubMed] [Google Scholar]
- 41.Teede HJ, Joham AE, Paul E, et al. Longitudinal weight gain in women identified with polycystic ovary syndrome: results of an observational study in young women. Obesity (Silver Spring, Md) 2013;21(8):1526–1532. doi: 10.1002/oby.20213. [DOI] [PubMed] [Google Scholar]
- 42.West S, Lashen H, Bloigu A, et al. Irregular menstruation and hyperandrogenaemia in adolescence are associated with polycystic ovary syndrome and infertility in later life: Northern Finland Birth Cohort 1986 study. Hum Reprod. 2014;29(10):2339–2351. doi: 10.1093/humrep/deu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Day FR, Hinds DA, Tung JY, et al. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nature communications. 2015;6:8464. doi: 10.1038/ncomms9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunaif A. Perspectives in Polycystic Ovary Syndrome: From Hair to Eternity. J Clin Endocrinol Metab. 2016;101(3):759–768. doi: 10.1210/jc.2015-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sathyanarayana S, Grady R, Redmon JB, et al. Anogenital distance and penile width measurements in The Infant Development and the Environment Study (TIDES): Methods and Predictors. Journal of Pediatric Urology. 2015 doi: 10.1016/j.jpurol.2014.11.018. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008;108(2):177–184. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barrett E, Hoeger K, Sathyanarayana S, Redmon JB, Nguyen RH, Swan SH. In Endocrine Society (ENDO) Boston, MA: 2016. Anogenital distance, a biomarker of prenatal androgen exposure, is longer among newborn daughters of women with polycystic ovary syndrome (PCOS) [Google Scholar]
- 48.Barrett E, Parlett LE, Sathyanarayana S, et al. Prenatal life events stress modifies associations between phthalate exposure and male reproductive development. In preparation. 2015 doi: 10.1111/ppe.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Perez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17(10):2573–2579. doi: 10.1093/humrep/17.10.2573. [DOI] [PubMed] [Google Scholar]
- 50.Barry JA, Kay AR, Navaratnarajah R, et al. Umbilical vein testosterone in female infants born to mothers with polycystic ovary syndrome is elevated to male levels. J Obstet Gynaecol. 2010;30(5):444–446. doi: 10.3109/01443615.2010.485254. [DOI] [PubMed] [Google Scholar]
- 51.Adams J, Franks S, Polson DW, et al. Multifollicular ovaries: clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet. 1985;2(8469–70):1375–1379. doi: 10.1016/s0140-6736(85)92552-8. [DOI] [PubMed] [Google Scholar]
- 52.Padmanabhan V, Veiga-Lopez A. Reproduction Symposium: developmental programming of reproductive and metabolic health. J Anim Sci. 2014;92(8):3199–3210. doi: 10.2527/jas.2014-7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abbott DH, Dumesic DA, Lewis KC, et al. Naturally occurring hyperandrogenism and intermittent menstrual cycles in female rhesus monkeys. Endocr Rev. 2012;33(3 Suppl) MON-16. [Google Scholar]
- 54.Bornehag CG, Carlstedt F, Jonsson BA, et al. Prenatal Phthalate Exposures and Anogenital Distance in Swedish Boys. Environ Health Perspect. 2014 doi: 10.1289/ehp.1408163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki Y, Yoshinaga J, Mizumoto Y, Serizawa S, Shiraishi H. Foetal exposure to phthalate esters and anogenital distance in male newborns. Int J Androl. 2012;35(3):236–244. doi: 10.1111/j.1365-2605.2011.01190.x. [DOI] [PubMed] [Google Scholar]
- 56.Baron-Cohen S, Auyeung B, Norgaard-Pedersen B, et al. Elevated fetal steroidogenic activity in autism. Mol Psychiatry. 2015;20(3):369–376. doi: 10.1038/mp.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: implications for explaining autism. Science. 2005;310(5749):819–823. doi: 10.1126/science.1115455. [DOI] [PubMed] [Google Scholar]
- 58.Kosidou K, Dalman C, Widman L, et al. Maternal polycystic ovary syndrome and the risk of autism spectrum disorders in the offspring: a population-based nationwide study in Sweden. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palomba S, Marotta R, Di Cello A, et al. Pervasive developmental disorders in children of hyperandrogenic women with polycystic ovary syndrome: a longitudinal case-control study. Clin Endocrinol (Oxf) 2012;77(6):898–904. doi: 10.1111/j.1365-2265.2012.04443.x. [DOI] [PubMed] [Google Scholar]
- 60.Baron-Cohen S. The essential difference: the truth about the male and female brain. Basic Books; New York: 2003. [Google Scholar]
- 61.Goy RW, Bercovitch FB, McBrair MC. Behavioral masculinization is independent of genital masculinization in prenatally androgenized female rhesus macaques. Horm Behav. 1988;22(4):552–571. doi: 10.1016/0018-506x(88)90058-x. [DOI] [PubMed] [Google Scholar]
- 62.Thornton J, Goy RW. Female-typical sexual behavior of rhesus and defeminization by androgens given prenatally. Horm Behav. 1986;20(2):129–147. doi: 10.1016/0018-506x(86)90012-7. [DOI] [PubMed] [Google Scholar]
- 63.Dokras A, Clifton S, Futterweit W, Wild R. Increased prevalence of anxiety symptoms in women with polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril. 2012;97(1):225–230 e222. doi: 10.1016/j.fertnstert.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 64.Hu M, Richard JE, Maliqueo M, et al. Maternal testosterone exposure increases anxiety-like behavior and impacts the limbic system in the offspring. Proc Natl Acad Sci U S A. 2015;112(46):14348–14353. doi: 10.1073/pnas.1507514112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pasterski V, Acerini CL, Dunger DB, et al. Postnatal penile growth concurrent with mini-puberty predicts later sex-typed play behavior: Evidence for neurobehavioral effects of the postnatal androgen surge in typically developing boys. Horm Behav. 2015;69:98–105. doi: 10.1016/j.yhbeh.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 66.Kanova N, Bicikova M. Hyperandrogenic states in pregnancy. Physiol Res. 2011;60(2):243–252. doi: 10.33549/physiolres.932078. [DOI] [PubMed] [Google Scholar]
- 67.Xita N, Tsatsoulis A. Genetic variants of sex hormone-binding globulin and their biological consequences. Mol Cell Endocrinol. 2010;316(1):60–65. doi: 10.1016/j.mce.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 68.Maliqueo M, Lara HE, Sanchez F, Echiburu B, Crisosto N, Sir-Petermann T. Placental steroidogenesis in pregnant women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2013;166(2):151–155. doi: 10.1016/j.ejogrb.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 69.Palomba S, Russo T, Falbo A, et al. Macroscopic and microscopic findings of the placenta in women with polycystic ovary syndrome. Hum Reprod. 2013;28(10):2838–2847. doi: 10.1093/humrep/det250. [DOI] [PubMed] [Google Scholar]
- 70.Palomba S, Russo T, Falbo A, et al. Decidual endovascular trophoblast invasion in women with polycystic ovary syndrome: an experimental case-control study. J Clin Endocrinol Metab. 2012;97(7):2441–2449. doi: 10.1210/jc.2012-1100. [DOI] [PubMed] [Google Scholar]
- 71.Govind A, Obhrai MS, Clayton RN. Polycystic ovaries are inherited as an autosomal dominant trait: analysis of 29 polycystic ovary syndrome and 10 control families. J Clin Endocrinol Metab. 1999;84(1):38–43. doi: 10.1210/jcem.84.1.5382. [DOI] [PubMed] [Google Scholar]
- 72.Hague WM, Adams J, Reeders ST, Peto TE, Jacobs HS. Familial polycystic ovaries: a genetic disease? Clin Endocrinol (Oxf) 1988;29(6):593–605. doi: 10.1111/j.1365-2265.1988.tb03707.x. [DOI] [PubMed] [Google Scholar]
- 73.Lunde O, Magnus P, Sandvik L, Hoglo S. Familial clustering in the polycystic ovarian syndrome. Gynecol Obstet Invest. 1989;28(1):23–30. doi: 10.1159/000293493. [DOI] [PubMed] [Google Scholar]
- 74.Puttabyatappa M, Cardoso RC, Padmanabhan V. Effect of maternal PCOS and PCOS-like phenotype on the offspring’s health. Mol Cell Endocrinol. 2015 doi: 10.1016/j.mce.2015.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abbott DH, Levine JE, Dumesic DA. Translational Insight Into Polycystic Ovary Syndrome (PCOS) From Female Monkeys With PCOS-like Traits. Curr Pharm Des. 2016 doi: 10.2174/1381612822666160715133437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cole B, Hensinger K, Maciel GA, Chang RJ, Erickson GF. Human fetal ovary development involves the spatiotemporal expression of p450c17 protein. J Clin Endocrinol Metab. 2006;91(9):3654–3661. doi: 10.1210/jc.2006-0641. [DOI] [PubMed] [Google Scholar]
- 77.Ellinwood WE, McClellan MC, Brenner RM, Resko JA. Estradiol synthesis by fetal monkey ovaries correlates with antral follicle formation. Biol Reprod. 1983;28(2):505–516. doi: 10.1095/biolreprod28.2.505. [DOI] [PubMed] [Google Scholar]
- 78.Fowler PA, Anderson RA, Saunders PT, et al. Development of steroid signaling pathways during primordial follicle formation in the human fetal ovary. J Clin Endocrinol Metab. 2011;96(6):1754–1762. doi: 10.1210/jc.2010-2618. [DOI] [PubMed] [Google Scholar]
- 79.Crisosto N, Echiburu B, Maliqueo M, et al. Improvement of hyperandrogenism and hyperinsulinemia during pregnancy in women with polycystic ovary syndrome: possible effect in the ovarian follicular mass of their daughters. Fertil Steril. 2012;97(1):218–224. doi: 10.1016/j.fertnstert.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 80.Crisosto N, Codner E, Maliqueo M, et al. Anti-Mullerian hormone levels in peripubertal daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92(7):2739–2743. doi: 10.1210/jc.2007-0267. [DOI] [PubMed] [Google Scholar]
- 81.Dumesic DA, Abbott DH, Eisner JR, Goy RW. Prenatal exposure of female rhesus monkeys to testosterone propionate increases serum luteinizing hormone levels in adulthood. Fertil Steril. 1997;67(1):155–163. doi: 10.1016/s0015-0282(97)81873-0. [DOI] [PubMed] [Google Scholar]
- 82.Stadtmauer LA, Wong BC, Oehninger S. Should patients with polycystic ovary syndrome be treated with metformin? Benefits of insulin sensitizing drugs in polycystic ovary syndrome–beyond ovulation induction. Hum Reprod. 2002;17(12):3016–3026. doi: 10.1093/humrep/17.12.3016. [DOI] [PubMed] [Google Scholar]
- 83.Carlsen SM, Vanky E. Metformin influence on hormone levels at birth, in PCOS mothers and their newborns. Hum Reprod. 2010;25(3):786–790. doi: 10.1093/humrep/dep444. [DOI] [PubMed] [Google Scholar]
- 84.Vanky E, Salvesen KA, Heimstad R, Fougner KJ, Romundstad P, Carlsen SM. Metformin reduces pregnancy complications without affecting androgen levels in pregnant polycystic ovary syndrome women: results of a randomized study. Hum Reprod. 2004;19(8):1734–1740. doi: 10.1093/humrep/deh347. [DOI] [PubMed] [Google Scholar]


