Abstract
Background
Observational studies have shown inverse associations between fitness and physical activity, and cardiovascular disease. However, little is known about these associations in individuals with elevated genetic susceptibility for these diseases.
Methods
We estimated associations of grip strength, objective and subjective physical activity, and cardiorespiratory fitness with cardiovascular events and all-cause death in a large cohort of up to 502,635 individuals from the UK Biobank (median follow-up 6.1 years; interquartile range, 5.4–6.8 years). Then, we further examined these associations in individuals with different genetic burden by stratifying individuals based on their genetic risk scores for coronary heart disease and atrial fibrillation. We compared disease risk among individuals in different tertiles of fitness, physical activity and genetic risk, using lowest tertiles as reference.
Results
Grip strength, physical activity, and cardiorespiratory fitness showed inverse associations with incident cardiovascular events (for coronary heart disease; HR, 0.79, 95% CI, 0.77–0.81; HR, 0.95, 95% CI, 0.93–0.97, and HR, 0.68, 95% CI, 0.63–0.74, per SD change, respectively, and for atrial fibrillation: HR, 0.75, 95% CI 0.73–0.76; HR, 0.93, 95% CI 0.91–0.95; and HR, 0.60, 95% CI 0.56–0.65, per SD change, respectively). Higher grip strength and cardiorespiratory fitness were associated with lower risk of incident coronary heart disease and atrial fibrillation in each genetic risk score group (Ptrend < 0.001 in each genetic risk category). In particular, high levels of cardiorespiratory fitness were associated with 49% lower risk for coronary heart disease (HR, 0.51, 95% CI 0.38–0.69) and 60% lower risk for atrial fibrillation (HR, 0.40, 95% CI 0.30–0.55), among individuals at high genetic risk for these diseases.
Conclusions
Fitness and physical activity demonstrated inverse associations with incident cardiovascular disease, also in individuals with elevated genetic risk for these diseases.
Keywords: Physical activity, fitness, cardiovascular disease, epidemiology, genetics
Introduction
Cardiovascular disease (CVD) is a major public health issue and societal burden worldwide. Exercise has been highlighted as a cost-effective strategy for CVD prevention; it improves cardiorespiratory fitness (CRF) and muscular strength, which both have shown to be inversely associated with future CVD events in population-based studies1, 2. However, fitness and physical activity are hard to measure accurately and consistently on a large scale; and thus, observational analyses prospectively relating fitness and physical activity with new-onset CVD among healthy individuals have typically been limited to smaller study samples and self-reported measures. Moreover, the extent to which the genetic risk for CVD can be compensated with exercise is not known.
In this paper, we analyzed objective and subjective measures of fitness and physical activity together with information of CVD risk factors and genomics in relation to prospective CVD disease events and all-cause death in up to 502,635 individuals from the UK Biobank. We had two aims: 1) To evaluate associations of fitness and physical activity with incident cardiovascular disease and all-cause death; and 2) to assess whether these associations are modified by genetic risk.
Methods
Study sample
In 2006–2010, over 500,000 individuals aged 40–69 years were enrolled into the UK Biobank, a longitudinal cohort study based in the UK. Participants have undergone a range of physical measurements, detailed assessments about health-related factors, and sampling of blood, urine and saliva. The participants have also agreed to have their future health, including disease events, monitored. In our study, we utilized the data collected at the UK Biobank assessment centers at baseline, combined with the information on incident disease events from the hospital and death registry. After excluding individuals who had withdrawn consent at the time of the study and prevalent CVD events (N=19,933), 482,702 individuals remained in our study sample for observational analyses of CVD. In addition, 2,524 individuals reported too high or low reported values for physical activity variables according to data cleaning rules of International Physical Activity Questionnaire (IPAQ)3, and these were removed in analyses involving physical activity data. For analyses of CRF, we utilized a subset of 66,438 individuals free from CVD at the baseline that underwent a submaximal exercise test on a treadmill. In addition, we also analyzed a subset of 103,702 individuals with objectively measured physical activity with a wrist-worn accelerometer. To evaluate the gene-environment interaction effects of fitness and physical activity on disease incidence, we used 468,095 individuals with genome-wide genetic data available (19,311 prevalent CVD cases removed). The UK Biobank study was approved by the North West Multi-Centre Research Ethics Committee and all participants provided written informed consent to participate in the UK Biobank study. The study protocol is available online4. The data reported in this paper will be made available via application to the UK Biobank to other researchers for purposes of reproducing the results or replicating the procedure.
Baseline data
In this study, the exposures of interest were different measures of fitness and physical activity (grip strength, total physical activity and CRF). Grip strength was measured in a sitting position using a Jamar J00105 hydraulic hand dynamometer. The participants were asked to squeeze the device as hard as they could for three seconds, and the maximum value that was reached during that time was recorded. Both hands were measured in turn (UK Biobank field ID 46 for left and 47 for right hand). In line with prior studies5, 6, to adjust for confounding of strength by body mass, we calculated relative grip strength as an average of measurements of right and left hand divided by weight (ID 21002). Physical activity was assessed with a short form IPAQ questionnaire3, which includes six questions of frequency (IDs 864, 884, and 904) and duration (IDs 874, 894 and 914) of walking, moderate-intensity and vigorous exercise. The answer “Unable to walk” in 864 was recoded to 0 and “Prefer not to answer” and “Do not know” in all six variables were set missing. Objective assessment of physical activity was measured for a 7-day period using Axivity AX3 wrist-worn triaxial accelerometer. The non-wear time was detected and imputed by the expert working group and total physical activity was calculated by averaging all worn and imputed values7. CRF was assessed with net oxygen consumption (VO2), calculated from individuals’ body weight and maximum workload (ID 6032) during the cycle ergometry on a stationary bike (eBike, Firmware v1.7), with using the equation VO2=7+10.8(workload)/weight8.
In addition, we used information of potential confounders, specifically age (field ID 21022), sex (ID 31), region of the UK biobank assessment center (ID 54; recoded to three groups: UK, Scotland and Wales), ethnicity (ID 21000; recoded to four groups: white, black, Asian, mixed), Townsend index reflecting socioeconomic status (ID 189), smoking status (ID 20116; current, former, never), body mass index (ID 21001), diabetes (ID 2443), lipid medication (ID 20003; including following medications: simvastatin, pravastatin, fluvastatin, atorvastatin, rosuvastatin, ezetimibe, nicotinic acid product or fenofibrate), systolic blood pressure (ID 4080, but if missing ID 93), and height (ID 50) as covariates in our models. The details of these measurements can be found in the study protocol4.
Outcomes and follow-up
The disease outcomes were defined as primary or secondary events using in-patient hospital and death registry data that have been linked to the UK Biobank. Coronary heart disease (CHD) was defined as International Classification of Diseases (ICD) edition 9 codes 410–411, edition 10 codes I20.0, I21, and I22, and surgical codes for percutaneous transluminal coronary angioplasty and coronary artery bypass graft (codes K40–K46, K49–K50, and K75). Stroke was defined as ischemic (ICD-9: 433–434, ICD-10: I63) or hemorrhagic stroke (ICD-9: 430–432, ICD-10: I60–I62). Heart failure was defined as ICD-9 code 428 and ICD-10 code I50. Atrial fibrillation (AF) was defined as ICD-9 code 427.3, ICD-10 code I48, and surgical codes K50.1, K62.2–K62.4. The hospital registry -based follow-up ended on March 31, 2015 in England, August 31, 2014 in Scotland, and 28 February, 2015 in Wales. Individuals were censored on these dates, time of event in question or the time of death, whichever occured first. Death due to CVD was defined using the same ICD-10 codes for different endpoints from the death registry. Death registry included all deaths that occured before January 31, 2016 in England and Wales, and November 30, 2015 in Scotland.
Statistical analysis
Missing values of the baseline data were imputed with multivariate imputation by chained equation (MICE) by using predictive mean matching9. By using all variables of the final analysis model (frequency and duration of exercise, grip strength, BMI, smoking, lipid medication, systolic blood pressure, diabetes, height and Townsend index), Nelson-Aalen estimate of cumulative hazard, and the event indicator as the input, we selected predictors for each variable with missing values by using quickpred function from mice package in R. This function computes predictor matrix for each variable based on: 1) correlations between observed values of the variable of interest and other variables; and 2) correlations between an indicator of missingness of the variable of interest and other variables. We performed five repetitions of imputations. The imputed values were compared with the observed values to evaluate the performance of the imputation. We then performed data quality control for frequency and duration variables and calculated total physical activity (“IPAQ-PA”) as MET-hours per week according to IPAQ scoring protocol3. We did not perform imputation for the CRF and acceleration variables.
Associations between measures of fitness and physical activity, and CVD events were analyzed using Cox proportional hazards models. The distributions of subjective (IPAQ) and objective (accelerometer) measures of physical activity were skewed, whereas the distributions of grip strength and CRF were approximately normal (Supplementary Figure 1). Thus, to facilitate comparison between effect sizes of different measures, physical activity measures were first rank transformed and then, all measures were scaled to standard normal distribution. Analyses were conducted separately for CHD, AF, ischemic and hemorrhagic stroke, and heart failure, as well as for composite CVD events. In secondary analyses, we also analyzed associations with all-cause death. Accelerometer data was used for all-cause death analysis only, due to short follow up (data was collected from May 2013 until Dec 2015). For each endpoint, we ran three sets of multivariable-adjusted models: a) adjusting for age, sex and region of the UK Biobank assessment center; b) additional adjustment for possible confounders2 including ethnicity, BMI, smoking, lipid medication, systolic blood pressure, diabetes, height and Townsend index; and c) adjusting for IPAQ-PA and/or grip strength in addition to those in b). Proportional hazards assumption was assessed using Schoenfeld’s test, and when not fulfilled (P ≤ 0.001), we added interaction terms with time for those covariates for which proportional hazards assumption was not met. In addition, we stratified all models by region to allow different baseline hazard function for each stratum. All analyses were conducted separately for five imputed datasets and results were pooled with Rubin’s rule9.
Next, we evaluated the risk-modifying associations of fitness and physical activity in individuals with different genetic risk load for CHD and AF. First, we calculated a genetic risk scores (GRSs) for CHD and AF representing joint effects of individual and independent genetic markers. The genetic markers were selected from the largest (external from the UK Biobank) published GWAS for CHD10 and AF11, and the GRS was calculated as the weighted sum of the risk alleles by using effect sizes from the reference GWAS10, 11 as weights (Supplementary Tables 1–2). The GRS was then divided into tertiles to stratify individuals into high, intermediate and low genetic risk category. Similarly, we stratified grip strength, IPAQ-PA and CRF into tertiles to compare hazard ratios for subjects in different groups. Individuals at the lowest GRS and grip strength, IPAQ-PA and CRF tertile were used as reference group in each model. In addition, we conducted a subgroup analysis by estimating the hazard ratios in each genetic risk group separately. Further, to evaluate whether there was an interaction between exercise traits and genetic risk of CHD, we added interaction terms between the measures of fitness and physical activity, and the GRS. The models were adjusted for age, sex, ethnicity, genotype array and 10 principal components and stratified by region of the UK Biobank assessment center.
Results
The study characteristics are shown in Table 1. Mean age at baseline was 56.5 years (SD, 8.1 years) and 54% of subjects were females. During follow-up (median 6.1 years; interquartile range, 5.4–6.8 years; 2,899,342 person-years at risk), 20,914 incident CVD cases occurred in participants free from the disease at baseline (8,518 CHD, 9,836 AF, 2,222 ischemic stroke, 1,116 hemorrhagic stroke, and 3298 heart failure events).
Table 1.
Baseline characteristics of the UK Biobank (N=502,635)
| Gender | ||
| Females | 273,465 (54%) | |
| Males | 229,173 (46%) | |
| Baseline age, years | 56.5 (8.1) | |
| Ethnicity* | ||
| White | 475,378 (94.6%) | |
| Black | 8,152 (1.6%) | |
| Asian | 11,534 (2.3%) | |
| Mixed | 7,574 (1.5%) | |
| Smoking status* | ||
| Never | 275,221 (54.8%) | |
| Previous | 174,129 (34.6%) | |
| Current | 53,288 (10.6%) | |
| Body mass index*, kg/m2 | 27.4 (4.8) | |
| Blood pressure*, mmHg | ||
| Systolic | 139.8 (19.7) | |
| Diastolic | 82.3 (10.7) | |
| Diabetes* | 26,587 (5.3%) | |
| Lipid medication | 82,369 (16.4%) | |
| Cardiovascular disease at baseline | 17,017 (3.4%) | |
| Grip strength*, kg | 0.40 (0.13) | |
| Physical activity*, MET-hours / week | 43.8 (43.7) | |
| Cardiorespiratory fitness, 7+10.8(watts)/kg | 18.5 (3.3) |
Data are mean (SD) or N (%).
Missing values of the variable were imputed with predictive mean matching 9.
Observational analyses
The results from observational analyses are shown in Table 2. We found inverse associations between grip strength and all outcomes (hazard ratios [HR] between 0.59 for heart failure and 0.90 for hemorrhagic stroke) in our age, gender, and region-adjusted models (models a). The associations were slightly attenuated when adjusting for confounding factors, but still highly significant. Grip strength was associated with these endpoints also after adjusting for IPAQ-PA.
Table 2.
Incidence and hazard ratios for cardiovascular disease endpoints by different measures of fitness and physical activity.
| Coronary heart disease | Atrial fibrillation | Cardiovascular disease | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | N events | HR (95 % CI) | P-value | N events | HR (95 % CI) | P-value | N events | HR (95 % CI) | P-value | |
| Grip strength | 1 | 8518 | 0.79 (0.77, 0.81) | <0.001 | 9836 | 0.75 (0.73, 0.76) | <0.001 | 20914 | 0.76 (0.75, 0.77) | <0.001 |
| 2 | 8518 | 0.88 (0.85, 0.90) | <0.001 | 9836 | 0.88 (0.85, 0.90) | <0.001 | 20914 | 0.87 (0.85, 0.89) | <0.001 | |
| 3 | 8475 | 0.88 (0.85, 0.91) | <0.001 | 9780 | 0.88 (0.85, 0.90) | <0.001 | 20799 | 0.87 (0.85, 0.89) | <0.001 | |
| IPAQ-PA | 1 | 8475 | 0.95 (0.93, 0.97) | <0.001 | 9780 | 0.93 (0.91, 0.95) | <0.001 | 20799 | 0.93 (0.92, 0.94) | <0.001 |
| 2 | 8475 | 0.98 (0.96, 1.00) | 0.045 | 9780 | 0.99 (0.97, 1.01) | 0.196 | 20799 | 0.97 (0.95, 0.98) | <0.001 | |
| 3 | 8475 | 0.99 (0.97, 1.01) | 0.197 | 9780 | 0.99 (0.97, 1.01) | 0.554 | 20799 | 0.98 (0.96, 0.99) | <0.001 | |
| CRF | 1 | 749 | 0.68 (0.63, 0.74) | <0.001 | 904 | 0.60 (0.56, 0.65) | <0.001 | 1855 | 0.65 (0.62, 0.69) | <0.001 |
| 2 | 749 | 0.75 (0.67, 0.83) | <0.001 | 904 | 0.61 (0.55, 0.67) | <0.001 | 1855 | 0.68 (0.63, 0.72) | <0.001 | |
| 3 | 746 | 0.75 (0.68, 0.84) | <0.001 | 902 | 0.61 (0.55, 0.67) | <0.001 | 1850 | 0.68 (0.64, 0.73) | <0.001 | |
| Ischemic stroke | Hemorrhagic stroke | Heart failure | ||||||||
| Model | N events | HR (95 % CI) | P-value | N events | HR (95 % CI) | P-value | N events | HR (95 % CI) | P-value | |
| Grip strength | 1 | 2222 | 0.78 (0.74, 0.82) | <0.001 | 1116 | 0.90 (0.83, 0.96) | 0.003 | 3298 | 0.59 (0.56, 0.61) | <0.001 |
| 2 | 2222 | 0.83 (0.78, 0.88) | <0.001 | 1116 | 0.85 (0.78, 0.93) | <0.001 | 3298 | 0.74 (0.70, 0.78) | <0.001 | |
| 3 | 2204 | 0.83 (0.78, 0.88) | <0.001 | 1112 | 0.86 (0.79, 0.94) | <0.001 | 3285 | 0.75 (0.71, 0.79) | <0.001 | |
| IPAQ-PA | 1 | 2204 | 0.95 (0.91, 0.99) | 0.009 | 1112 | 0.93 (0.88, 0.99) | 0.020 | 3285 | 0.80 (0.77, 0.83) | <0.001 |
| 2 | 2204 | 0.97 (0.93, 1.01) | 0.151 | 1112 | 0.93 (0.87, 0.98) | 0.011 | 3285 | 0.87 (0.84, 0.90) | <0.001 | |
| 3 | 2204 | 0.98 (0.94, 1.02) | 0.374 | 1112 | 0.98 (0.94, 1.02) | 0.374 | 3285 | 0.89 (0.86, 0.92) | <0.001 | |
| CRF | 1 | 172 | 0.68 (0.56, 0.81) | <0.001 | 91 | 0.99 (0.76, 1.29) | 0.946 | 254 | 0.56 (0.49, 0.65) | <0.001 |
| 2 | 172 | 0.69 (0.56, 0.87) | 0.001 | 91 | 0.93 (0.66, 1.32) | 0.697 | 254 | 0.56 (0.47, 0.66) | <0.001 | |
| 3 | 171 | 0.71 (0.57, 0.89) | 0.003 | 91 | 0.96 (0.68, 1.37) | 0.829 | 254 | 0.58 (0.49, 0.68) | <0.001 | |
Associations are reported per SD-units in of fitness and physical activity traits. Model adjustments: 1. Age, gender and region. 2. Age, gender, region, diabetes, smoking, systolic blood pressure, body mass index, lipid medication, height, ethnicity and Townsend index. 3. All in 2) plus IPAQ-PA (grip strength analyses) or grip strength (IPAQ-PA analyses). Analyses of CRF were adjusted for both IPAQ-PA and grip strength.
Abbreviations: IPAQ-PA, physical activity assessed by international physical activity questionnaire; CRF, cardiorespiratory fitness; HR, hazard ratio; CI, confidence interval.
Higher levels of IPAQ-PA were associated with lower risk of CVD events and all-cause death, but the associations were more modest than for grip strength (Table 2–3). Associations with the composite CVD outcome, heart failure, and all-cause death remained significant after adjusting for confounding factors and grip strength. Physical activity assessed with a wrist-worn accelerometer had the strongest inverse association with all-cause death, when compared to all other measures (HR=0.52, 95% CI 0.46–0.58, Table 3). The hazard ratio for the subjective measure of physical activity, IPAQ-PA, was notably more modest than that of objective measurement (HR=0.83, 95% CI 0.82–0.84, Table 3) than the association of physical activity objectively measured by accelerometer. The correlation of these two measures was modest (R=0.20) indicating substantial measurement inaccuracy in self-reported physical activity.
Table 3.
Incidence and hazard rations for all-cause mortality by by different measures of fitness and physical activity.
| All-cause death | ||||
|---|---|---|---|---|
| Model | N events | HR (95 % CI) | P-value | |
| Grip strength | 1 | 14,419 | 0.75 (0.73, 0.76) | <0.001 |
| 2 | 14,419 | 0.76 (0.74, 0.78) | <0.001 | |
| 3 | 14,350 | 0.78 (0.76, 0.79) | <0.001 | |
| IPAQ-PA | 1 | 14,350 | 0.83 (0.82, 0.84) | <0.001 |
| 2 | 14,350 | 0.86 (0.84, 0.87) | <0.001 | |
| 3 | 14,350 | 0.87 (0.86, 0.89) | <0.001 | |
| CRF | 1 | 1,162 | 0.78 (0.72, 0.83) | <0.001 |
| 2 | 1,162 | 0.75 (0.69, 0.81) | <0.001 | |
| 3 | 1,157 | 0.76 (0.70, 0.83) | <0.001 | |
| PA | 1 | 348 | 0.52 (0.46, 0.58) | <0.001 |
| 2 | 348 | 0.56 (0.50, 0.63) | <0.001 | |
| 3 | 347 | 0.56 (0.50, 0.63) | <0.001 | |
Associations are reported per SD-units of fitness and physical activity traits. Model adjustments: 1. Age, sex and region. 2. Age, sex, region, diabetes, smoking, systolic blood pressure, body mass index, lipid medication, height, ethnicity and Townsend index. 3. All in 2) plus IPAQ-PA (grip strength analyses) or grip strength (IPAQ-PA analyses). Analyses of CRF and PA were adjusted for both IPAQ-PA and grip strength.
Abbreviations: IPAQ-PA, physical activity assessed by international physical activity questionnaire; CRF, cardiorespiratory fitness; PA, Physical activity assessed by wrist-worn accelerometer; HR, hazard ratio; CI, confidence interval.
In a subgroup analysis including 66,438 individuals that underwent a submaximal fitness test, CRF was inversely associated with all CVD events, except hemorrhagic stroke (no. of events=91). The strongest associations were observed for heart failure (HR=0.56, 95% CI 0.49–0.65) and AF (HR=0.60, 95% CI 0.56–0.65).
There were some evidence of nonlinear associations of fitness and physical activity on CVD events (Supplementary Figure 2–4) and all-cause death (Supplementary Figure 5). In particular, the association of IPAQ-PA was U-shaped for CHD, AF and CVD (Pnonlinearity < 0.0001). However, objectively measured of physical activity by accelerometry did not show a similar U-shaped association with mortality (Supplementary Figure 5).
Interactions between fitness, physical activity, strength and genetic determinants of CVD
Overall, individuals in the highest tertiles of the CHD- and AF-GRSs showed increased risk for incident CHD and AF when compared to those in the lowest tertile (HR, 1.77, 95% CI 1.67–1.87 and HR, 1.95, 95% CI 1.86–2.06, respectively). Further adjustment for traditional CVD risk factors (BMI, smoking, lipid medication, systolic blood pressure, and diabetes), grip strength and physical activity did not change the results notably (Table 4). To compare the hazard ratios for those at extreme ends of the GRS distributions, we divided the CHD- and AF-GRSs into 20 groups. Those at the highest 5% of the GRS distributions had 2.7- and 3.4-fold increased risk for CHD and AF, respectively, when compared to those at the lowest 5% (95% CI 2.38–3.17 and 2.97–3.93, respectively, Table 4).
Table 4.
Associations between genetic risk scores (GRSs) and cardiovascular events.
| Model | Outcome | N events | HR (95% CI) for top versus bottom categories of GRS | |
|---|---|---|---|---|
| 33% | 5% | |||
| 1 | CHD | 8,227 | 1.77 (1.67, 1.87) | 2.74 (2.38, 3.17) |
| 2 | CHD | 8,185 | 1.73 (1.64, 1.83) | 2.67 (2.31, 3.08) |
| 1 | AF | 9,498 | 1.95 (1.86, 2.06) | 3.42 (2.97, 3.93) |
| 2 | AF | 9,444 | 1.95 (1.86, 2.06) | 3.42 (2.97, 3.94) |
Associations are for highest versus lowest 33% and 5% of the genetic risk score distribution. Model adjustments: 1. Age, sex, ethnicity, genotype array and principal components. 2. All in 1) plus diabetes, smoking, systolic blood pressure, body mass index, lipid medication, physical activity assessed by international physical activity questionnaire and grip strength.
Abbreviations: CHD, coronary heart disease; AF, atrial fibrillation; HR, hazard ratio; CI, confidence interval.
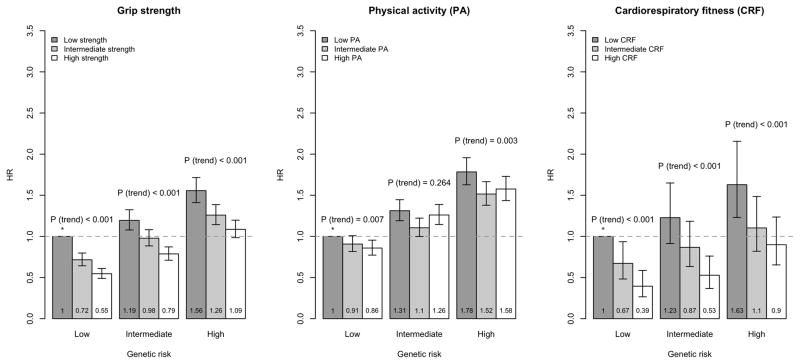
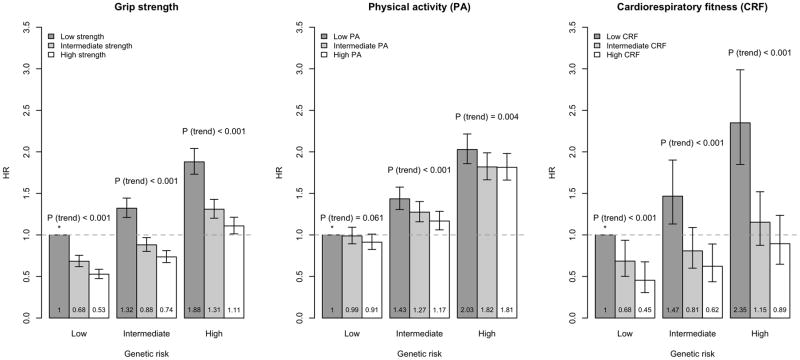
Grip strength and CRF demonstrated inverse associations with incident CHD and AF in each GRS group (Ptrend < 0.001 in each GRS group, Figures 1–2). High CRF was associated with 49% lower risk for CHD (HR=0.51, 95% CI 0.38–0.69) and 60% lower risk for AF (HR=0.40, 95% CI 0.30–0.55, compared to those at low CRF group) among individuals at the highest tertiles of CHD- and AF-GRSs (Supplementary Tables 3–4). Similarly, high grip strength was associated with lower risk for CHD (HR=0.69, 95% CI 0.62–0.75) and AF (HR=0.61, 95% CI 0.56–0.67, compared to those at low grip strength, Supplementary Tables 3–4).
Figure 1.
Hazard ratios with 95% confidence intervals for coronary heart disease (CHD) according to tertiles of genetic risk and grip strength (left), physical activity (middle) and cardiorespiratory fitness (right).
* denotes the reference group.
Figure 2.
Hazard ratios with 95% confidence intervals for atrial fibrillation (AF) according to tertiles of genetic risk and grip strength (left), physical activity (middle) and cardiorespiratory fitness (right).
* denotes the reference group.
IPAQ-PA showed inverse associations with CHD in the lowest and highest GRS group (Ptrend = 0.007 and Ptrend = 0.003, respectively), and with AF in the intermediate and highest GRS group (Ptrend < 0.001 and Ptrend = 0.004, respectively), but the inverse patterns of associations were more modest than for grip strength and CRF. The hazard ratio in the high CHD-GRS group was 0.89 (95% CI 0.82–0.96) and in the high AF-GRS group 0.90 (95% CI 0.83–0.97, compared to those at low IPAQ-PA, Supplementary Tables 3–4).
The overall inverse linear association of grip strength was strongest in the lowest GRS group (Pinteraction=0.0002 for CHD, Pinteraction=0.01 for AF). For CRF, the pattern was similar for CHD (Pinteraction =0.03); whereas for AF, there was no statistically significant interaction (Pinteraction =0.22). For IPAQ-PA, we observed no significant differences by GRS groups (Pinteraction=0.52 for CHD, Pinteraction=0.37 for AF).
Discussion
Principal Findings
In this study of up to 502,635 individuals from the UK Biobank, we analyzed the associations of objective and subjective measures of fitness and physical activity with six cardiovascular outcomes and total mortality, and explored these associations in individuals with different genetic burden for CVD. Our main findings were two-fold. First, in an observational study of unprecedented size, we established that fitness and physical activity demonstrated inverse associations with different types of incident CVD events, and that among all the measures of fitness and physical activity, accelerometry-based physical activity showed the strongest inverse association for the risk of premature death. Interestingly, the associations for questionnaire-based physical activity were weaker than those for objectively measured physical activity, and the correlation was low (R=0.20). Second, the inverse associations of grip strength and CRF with CHD and AF were seen in each category of genetic risk, indicating that maintaining good fitness can compensate for genetic risk of these diseases.
Comparison with Prior Literature
Exercise has been highlighted as a cost-effective prevention strategy for CVD. Both human and animal studies have demonstrated multifactorial effects of exercise12, including skeletal muscle growth, vascular remodeling, and beneficial effects on metabolism. Exercise also induces structural changes in cardiac muscle, which helps to protect against ischemic damage12. Intervention studies have reported that both aerobic and strength training have favorable effects on cardiovascular risk factors in subjects at high risk for CVD (e.g. subjects with dyslipidemia or type 2 diabetes)13–15.
Due to the challenges of measuring fitness and physical activity, studies relating these traits with prospective CVD in the general population have previously been limited by small sample size or lack of measurement accuracy. Attempts to combine data in meta-analyses have had to use broad categories of fitness and physical activity to harmonize the data across many small studies16, 17. Compared to these studies, the UK Biobank has a clear advantage as the traits were measured in the same way in over 500,000 individuals. Nevertheless, our results are consistent with previous meta-analyses reporting weaker associations for questionnaire-based physical activity assessment when compared to objective measures16. This finding suggests that associations of physical activity with outcome are most likely underestimated in studies using questionnaire data. Moreover, our results demonstrated a modest correlation between self-reported and objective physical activity; as well as a U-shaped association between self-reported, but not objective physical activity, and all-cause death, suggest that some additional factors might explain an increased risk in those individuals reporting very high values of self-reported physical activity.
Observational studies have reported contradictory results on the relationship between physical activity, fitness and AF. While moderate intensity exercise has been shown to decrease the risk of AF, especially long-term strenuous endurance exercise has been associated with an increased risk18. For example, in contrast to our results, a large study conducted in Swedish young men at military service reported a positive association between CRF and AF2. The contradictory results in the field might be explained by several methodological factors, such as the confounders used, age distribution and gender – majority of studies, including the study by Andersen and colleagues2 have been conducted only in men. Indeed, it has been suggested that the exercise-associated AF mostly affects male endurance athletes and the etiology might be different from that of general AF18, presumably examined in the present study. In the present study, we were able to adjust for a large set of potential confounders, and this strengthened the inverse association of CRF with AF.
Clinical Implications
Little is known about the risk-modifying effects of exercise among individuals with increased genetic risk for cardiovascular diseases. Our results showing inverse associations of grip strength and CRF with CVD outcomes across different categories of cardiovascular genetic risk is in line with a recent study19 reporting similar associations of healthy lifestyle (defined by four lifestyle factors; smoking, obesity, physical activity and diet) in each of genetic risk score strata for CHD. Although the direct comparison is not feasible due to differences in variable definitions, together these studies highlight the importance of lifestyle factors in prevention of CVD, also in genetically predisposed individuals, demonstrating non-deterministic nature of genetic risk. They also stress the potential advantages of genetic risk profiling for better detection of individuals at increased risk for CVD. Even though more information is needed to evaluate how people understand the genetic risks, the knowledge that lifestyle choices have substantial effects on the disease risks could encourage individuals to initiate a healthier lifestyle to reduce their overall risk.
Strengths and Limitations
Strengths of this study include a large study sample with objective and subjective measures of fitness and physical activity, and a prospective ascertainment of different types of CVD events. There are also limitations in our study. First, the trade-off for the large sample collection is weaker measurement accuracy. For example, grip strength is a simple proxy for general muscular strength level and it captures mainly upper body strength, especially when measured in sitting position. However, it is highly correlated with knee extension muscle strength (r= 0.772 to 0.805)20, which makes it a decent indicator of general strength level in large samples, where more detailed assessments of muscular strength are not feasible. Moreover, CRF was measured with a submaximal fitness test, which is less accurate, but more applicable and safer in large samples than laborious maximal fitness test. Further, there might be some unmeasured or inadequately measured confounders that could have had an effect on the results obtained in our analyses.
Second, as in any population-based cohort study, the disease prevalence and incidence rates derived in the UK Biobank are not broadly generalizable due to “healthy volunteer bias” among study participants. That is, when compared with the general population, the UK Biobank participants had fewer self-reported illnesses, were less likely to be obese, to smoke and drink alcohol21. However, valid assessment of associations between exposures and outcomes do not require participants to be representative of the population at large; and thus, associations between fitness, physical activity and disease events are still likely be generalizable to other populations21.
Finally, although our analyses were conducted in a cohort with different ethnicities, the majority of participants were of European ancestry. Hence, the generalizability of the results to other ethnicities is not fully understood.
Conclusions
In conclusion, different measures of fitness and physical activity demonstrated inverse associations with future CVD events and all-cause death in a large population-based sample. Among those at high, intermediate, or low genetic predisposition for CHD and AF, there was a graded inverse association with these parameters among each strata of genetic risk. Future studies evaluating the effects of strength versus aerobic training on subclinical or clinical cardiovascular outcomes could help to tailor exercise programs for individuals with elevated genetic risk for these diseases.
Supplementary Material
Clinical Perspective.
What is new?
In this study of ~500,000 individuals from the UK Biobank, we report and compare the associations of objective and subjective measures of fitness and physical activity with prospective CVD events and all-cause death.
We found consistent and robust inverse associations, particularly between objective measures of fitness and physical activity, and six cardiovascular outcomes and total mortality.
Using genetic risk scores for coronary heart disease and atrial fibrillation, we show that these inverse associations were present in each genetic risk category, suggesting that that elevated genetic risk for these diseases can be compensated for by exercise.
What are the clinical implications?
Little is known about the risk-modifying effects of exercise in individuals with increased genetic risk of cardiovascular disease.
Our results demonstrating the risk-decreasing associations of exercise across genetic risk strata have important public health impact; the knowledge that lifestyle choices have substantial effects on disease risk could encourage individuals to initiate a healthier lifestyle to reduce their overall risk.
In the longer term, identifying subgroups based on genetic risk that benefit most from lifestyle interventions could help personalizing prevention strategies of chronic diseases.
Further, personalized prevention and treatment strategies could help motivating individuals more efficiently compared with general guidelines.
Acknowledgments
This research has been conducted using the UK Biobank Resource under Application Number 13721. Data on coronary heart disease have been contributed by CARDIoGRAMplusC4D investigators and have been downloaded from www.cardiogramplusc4d.org. Data on atrial fibrillation has been contributed by AFGen Consortium.
Sources of Funding
The research was performed with support from National Institutes of Health (1R01HL135313-01; 1R01DK106236-01A1), Knut and Alice Wallenberg Foundation (2013.0126), Finnish Cultural Foundation, Finnish Foundation for Cardiovascular Research and Emil Aaltonen Foundation.
Footnotes
Disclosures
Erik Ingelsson is a scientific advisor for Precision Wellness and Olink Proteomics for work unrelated to the present project.
Authors’ contributions
Design of the study: ET, EI; Analysis and interpretation of data: ET, SG, EI; Drafting the manuscript: ET; Critical revision of the manuscript: all authors; Final approval of the version to be published: all authors.
References
- 1.Timpka S, Petersson IF, Zhou C, Englund M. Muscle strength in adolescent men and risk of cardiovascular disease events and mortality in middle age: A prospective cohort study. BMC Med. 2014;12:62. doi: 10.1186/1741-7015-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen K, Rasmussen F, Held C, Neovius M, Tynelius P, Sundstrom J. Exercise capacity and muscle strength and risk of vascular disease and arrhythmia in 1.1 million young swedish men: Cohort study. BMJ. 2015;351:h4543. doi: 10.1136/bmj.h4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. [Accessed: Jan 10 2017];International physical activity questionnaire. Available from: https://sites.google.com/site/theipaq/home.
- 4.Uk biobank. [Accessed: Jan 10 2017]; Available from: https://http://www.ukbiobank.ac.uk/
- 5.Choquette S, Bouchard DR, Doyon CY, Sénéchal M, Brochu M, Dionne I. Relative strength as a determinant of mobility in elders 67–84 years of age. A nuage study: Nutrition as a determinant of successful aging. J Nutr Health Aging. 2009:1–6. doi: 10.1007/s12603-009-0132-8. [DOI] [PubMed] [Google Scholar]
- 6.Lawman HG, Troiano RP, Perna FM, Wang CY, Fryar CD, Ogden CL. Associations of relative handgrip strength and cardiovascular disease biomarkers in u.S. Adults, 2011–2012. Am J Prev Med. 2016;50:677–683. doi: 10.1016/j.amepre.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doherty A, Jackson D, Hammerla N, Plotz T, Olivier P, Granat MH, White T, van Hees VT, Trenell MI, Owen CG, Preece SJ, Gillions R, Sheard S, Peakman T, Brage S, Wareham NJ. Large scale population assessment of physical activity using wrist worn accelerometers: The uk biobank study. PLoS One. 2017;12:e0169649. doi: 10.1371/journal.pone.0169649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swain DP. Energy cost calculations for exercise prescription: An update. Sports Med. 2000;30:17–22. doi: 10.2165/00007256-200030010-00002. [DOI] [PubMed] [Google Scholar]
- 9.Marshall A, Altman DG, Holder RL, Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: Current practice and guidelines. BMC Med Res Methodol. 2009;9:57. doi: 10.1186/1471-2288-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, Saleheen D, Kyriakou T, Nelson CP, Hopewell JC, Webb TR, Zeng L, Dehghan A, Alver M, Armasu SM, Auro K, Bjonnes A, Chasman DI, Chen S, Ford I, Franceschini N, Gieger C, Grace C, Gustafsson S, Huang J, Hwang SJ, Kim YK, Kleber ME, Lau KW, Lu X, Lu Y, Lyytikainen LP, Mihailov E, Morrison AC, Pervjakova N, Qu L, Rose LM, Salfati E, Saxena R, Scholz M, Smith AV, Tikkanen E, Uitterlinden A, Yang X, Zhang W, Zhao W, de Andrade M, de Vries PS, van Zuydam NR, Anand SS, Bertram L, Beutner F, Dedoussis G, Frossard P, Gauguier D, Goodall AH, Gottesman O, Haber M, Han BG, Huang J, Jalilzadeh S, Kessler T, Konig IR, Lannfelt L, Lieb W, Lind L, Lindgren CM, Lokki ML, Magnusson PK, Mallick NH, Mehra N, Meitinger T, Memon FU, Morris AP, Nieminen MS, Pedersen NL, Peters A, Rallidis LS, Rasheed A, Samuel M, Shah SH, Sinisalo J, Stirrups KE, Trompet S, Wang L, Zaman KS, Ardissino D, Boerwinkle E, Borecki IB, Bottinger EP, Buring JE, Chambers JC, Collins R, Cupples LA, Danesh J, Demuth I, Elosua R, Epstein SE, Esko T, Feitosa MF, Franco OH, Franzosi MG, Granger CB, Gu D, Gudnason V, Hall AS, Hamsten A, Harris TB, Hazen SL, Hengstenberg C, Hofman A, Ingelsson E, Iribarren C, Jukema JW, Karhunen PJ, Kim BJ, Kooner JS, Kullo IJ, Lehtimaki T, Loos RJ, Melander O, Metspalu A, Marz W, Palmer CN, Perola M, Quertermous T, Rader DJ, Ridker PM, Ripatti S, Roberts R, Salomaa V, Sanghera DK, Schwartz SM, Seedorf U, Stewart AF, Stott DJ, Thiery J, Zalloua PA, O’Donnell CJ, Reilly MP, Assimes TL, Thompson JR, Erdmann J, Clarke R, Watkins H, Kathiresan S, McPherson R, Deloukas P, Schunkert H, Samani NJ, Farrall M, Consortium CAD. A comprehensive 1,000 genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47:1121–1130. doi: 10.1038/ng.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.AFGen Consortium, Metastroke Consortium of the ISGC, Neurology Working Group of the Charge Consortium. Large-scale analyses of common and rare variants identify 12 new loci associated with atrial fibrillation. Nat Genet. 2017 doi: 10.1038/ng.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mann N, Rosenzweig A. Can exercise teach us how to treat heart disease? Circulation. 2012;126:2625–2635. doi: 10.1161/CIRCULATIONAHA.111.060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martins RA, Neves AP, Coelho-Silva MJ, Verissimo MT, Teixeira AM. The effect of aerobic versus strength-based training on high-sensitivity c-reactive protein in older adults. Eur J Appl Physiol. 2010;110:161–169. doi: 10.1007/s00421-010-1488-5. [DOI] [PubMed] [Google Scholar]
- 14.Cauza E, Hanusch-Enserer U, Strasser B, Ludvik B, Metz-Schimmerl S, Pacini G, Wagner O, Georg P, Prager R, Kostner K, Dunky A, Haber P. The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Arch Phys Med Rehabil. 2005;86:1527–1533. doi: 10.1016/j.apmr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 16.Nocon M, Hiemann T, Muller-Riemenschneider F, Thalau F, Roll S, Willich SN. Association of physical activity with all-cause and cardiovascular mortality: A systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil. 2008;15:239–246. doi: 10.1097/HJR.0b013e3282f55e09. [DOI] [PubMed] [Google Scholar]
- 17.Sofi F, Capalbo A, Cesari F, Abbate R, Gensini GF. Physical activity during leisure time and primary prevention of coronary heart disease: An updated meta-analysis of cohort studies. Eur J Cardiovasc Prev Rehabil. 2008;15:247–257. doi: 10.1097/HJR.0b013e3282f232ac. [DOI] [PubMed] [Google Scholar]
- 18.Sanchis-Gomar F, Perez-Quilis C, Lippi G, Cervellin G, Leischik R, Lollgen H, Serrano-Ostariz E, Lucia A. Atrial fibrillation in highly trained endurance athletes - description of a syndrome. Int J Cardiol. 2017;226:11–20. doi: 10.1016/j.ijcard.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 19.Khera AV, Emdin CA, Drake I, Natarajan P, Bick AG, Cook NR, Chasman DI, Baber U, Mehran R, Rader DJ, Fuster V, Boerwinkle E, Melander O, Orho-Melander M, Ridker PM, Kathiresan S. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. 2016;375:2349–2358. doi: 10.1056/NEJMoa1605086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bohannon RW, Magasi SR, Bubela DJ, Wang YC, Gershon RC. Grip and knee extension muscle strength reflect a common construct among adults. Muscle Nerve. 2012;46:555–558. doi: 10.1002/mus.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, Collins R, Allen NE. Comparison of sociodemographic and health-related characteristics of uk biobank participants with those of the general population. Am J Epidemiol. 2017;186:1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.