SUMMARY
Besides their function in limiting blood loss and promoting wound healing, experimental evidence has highlighted platelets as active players in all steps of tumorigenesis including tumor growth, tumor cell extravasation and metastasis. Additionally, thrombocytosis in cancer patients is associated with adverse patient survival. Due to the secretion of large amounts of microparticles and exosomes, platelets are well positioned to coordinate both local and distant tumor-host crosstalk. Here, we present a review of recent discoveries in the field of platelet biology and the role of platelets in cancer progression as well as challenges in targeting platelets for cancer treatment.
1. INTRODUCTION
Significant platelet involvement in cancer growth and metastasis is a longstanding concept. Historically, thrombocytosis in association with solid tumors was first noted over a century ago by Leopold Riess (Riess, 1872). Given the short life span of circulating platelets (average of 7 days), the adult human must produce an estimated one-hundred billion platelets daily to maintain a platelet count within normal range. This massive level of baseline platelet production can potentially increase via thrombocytosis as much as 20-fold in response to various tumor derived and systemic factors. Correlations between high platelet counts and shorter disease-specific survival are often described for lung, colon, breast, pancreatic, kidney and gynecologic cancers. Platelets affect disease burden and treatment efficacy in cancer patients, and participate in several steps of cancer metastasis. Platelets also play an important role in protecting cancer cells against chemotherapy-induced apoptosis and in maintaining integrity of tumor vasculature. Recognition of the pro-tumor effects of platelets has led to incorporating anti-platelet agents more frequently into cancer prevention and treatment strategies. Nevertheless, crucial challenges remain in identifying and treating cancer patients who would derive the most benefit from anti-platelet therapy. Furthermore, incorporation of platelet-based biomarkers into emerging “liquid biopsy” platforms for cancer patients has significant potential for improving diagnostic accuracy and predicting therapeutic response.
2. ROLE OF PLATELETS IN MALIGNANCY
2.1. Importance of thrombocytosis and thromboembolism in cancer
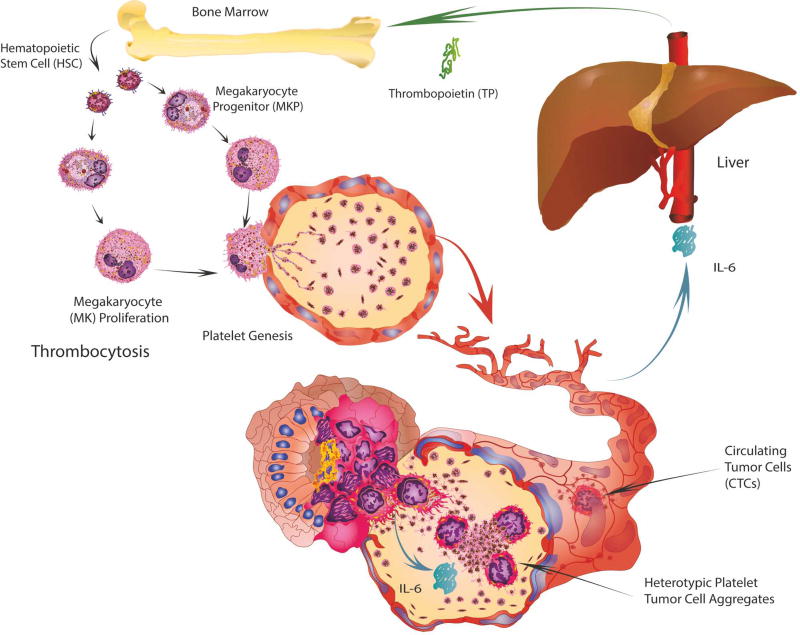
Over 100 years ago, Armand Trousseau diagnosed patients, including himself, with excessive blood clotting, which was caused by an occult carcinoma (Trousseau, 1865). Gasic first ascertained that tumors in mice with neuraminidase-induced thrombocytopenia exhibited reduced metastasis (Gasic et al., 1968). Clinically, from approximately 140,000 patients with thrombocytosis (>400,000 platelets/µl blood) and devoid of inflammatory condition or iron deficiency, almost 40% exhibited occult malignancy, mainly of the stomach, colon, lung, breast or ovary (Levin and Conley, 1964). Increased platelet counts were revealed as a predictor of cancer in patients with an occult malignancy (Bailey et al., 2016) and are consistently related to a worse progression-free and/or overall survival in ovarian (Stone et al., 2012), lung (Pedersen and Milman, 1996), colorectal (Sasaki et al., 2012), gastric (Ikeda et al., 2002) and breast cancers (Taucher et al., 2003). We discovered that in ovarian cancer, tumor-derived interleukin 6 (IL-6) stimulated thrombopoietin (TPO) production by the liver, thereby stimulating megakaryopoiesis and thrombocytosis (Stone et al., 2012) (Figure 1).
Figure 1. Mechanisms of Trousseau’s Syndrome.
Paracrine secretion of interleukin-6 (IL-6) from tumor cells stimulates the production of thrombopoietin (TP) by the liver. In turn, this fuels megakaryopoiesis and platelet genesis. The combination of these events contributes to thrombocytosis and hypercoagulability in cancer patients known as Trousseau’s Syndrome.
Up to 20% of cancer patients suffer from vascular thromboembolism including pulmonary embolism (PE) and deep vein thrombosis (DVT) (Khorana et al., 2007). This associated risk is especially high in patients with brain, pancreatic or gastric cancer along with hematological malignancies (Sallah et al., 2002; Stein et al., 2006). A case-control study of 3,220 participants demonstrated that cancer patients have a 4 to 7.5 times greater risk of venous thrombosis or embolus compared to those without a malignancy (Blom et al., 2005). Risk factors for venous thrombosis in cancer patients include anatomical location of the tumor and cancer-related treatments including surgery, chemotherapy, hormonal or anti-angiogenic therapy (Khorana and Connolly, 2009). Identifying cancer patients who are at risk for developing venous thrombosis is an important, but challenging task. Clinical risk-assessment systems considering cancer type, pre-chemotherapy platelet and leukocyte counts, hemoglobin level and body mass index (BMI) may identify patients with a specificity of almost 90% (Khorana and Connolly, 2009). Alternatively, the Vienna VTE Risk Assessment Score (Eichinger et al., 2014) may predict the risk of recurrent VTE, which considers soluble P-selectin, D-dimer levels primarily before chemotherapy and pro-thrombin fragment 1 and 2. The possible contribution of platelets to the increased risk of venous thrombosis in malignancy is not only due to increased platelet numbers (Khorana et al., 2005), but might also be related to altered platelet function. For example, platelets from subjects with late-stage metastatic disease have increased reactivity to ADP and epinephrine (Cooke et al., 2013). However, other studies found either no hyper-reactivity (Feng et al., 2016) or even an overall decreased platelet reactivity, most likely as a consequence of continuous platelet activation (Riedl et al., 2016). These variances may be due to differences in cancer type and stage, time of blood draw and cancer-related treatments and require further study. Despite a knowledge base of platelet and VTE involvement in cancer, few routinely used clinical standard of care laboratory assessments are applied in this space. There is a clear need for more effective standardized assessments involving malignancy related thrombogenic potential of cancer. These should be coupled with clinically validated platelet/lymphocyte ratio, platelet function, and platelet/plasma molecular profiling and biomarker assays that more effectively identify and help to monitor cancer patients with a high risk of venous thrombosis.
2.2. Platelet – tumor cell interactions and their role in cancer dissemination
A prerequisite for cancer metastasis is the survival of cancer cells in circulation. While a substantial amount of circulating tumor cells (CTCs) are rapidly destroyed, a mechanism of CTC survival involves interactions of tumor cells with platelets, shielding them from TNFα (Philippe et al., 1993) and natural killer (NK) cell-induced cell death (Nieswandt et al., 1999). Indeed, preclinical evidence suggests that mice lacking functional NK cells have increased susceptibility for experimental metastasis (Wiltrout et al., 1985), independent of the number of circulating platelets (Nieswandt et al., 1999). This physical interaction of cancer cells with platelets is mainly mediated via GPIb-IX-V, GPIIb-IIIa and tumor cell integrin αvβ3 as well as P-Selectin, which can bind to mucins on the surface of cancer cells (Kim et al., 1998). Besides helping to protect cancer cells by building a partial physical barrier towards NK cells, platelets may also interfere with the recognition of cancer cells by NK cells. Platelets can transfer ‘normal’ MHC-class I molecules onto the surface of tumor cells, which makes them unrecognizable as foreign cells and impairs cytotoxicity and IFN-γ production by NK cells (Placke et al., 2012). Moreover, platelet-derived TGF-β diminishes NK cell activity by NKG2D downregulation and inhibition of NK cell antitumor reactivity (Kopp et al., 2009). TGF-β from platelets not only weakens NK cell function, but also increases tumor cell survival by activating the TGF-β/Smad and NF-κB pathways, which induce an EMT-like phenotype in cancer cells and increase metastases in vivo (Labelle et al., 2011), and increases proliferation of cancer cells (Cho et al., 2012). Mice with selective deficiency in TGF-β develop less metastasis (Labelle et al., 2011) and smaller tumors (Hu et al., 2017). Our data suggest that YAP activation in tumor cells by platelets provides a platform of increased cancer cell survival in circulation and ascites (Haemmerle et al., 2017). Finally, mice with platelets lacking platelet integrin GPIIb-IIIa (Bakewell et al., 2003) or having quantitative or qualitative defects in platelets (Camerer et al., 2004) have reduced numbers of metastases to the bone and lungs, respectively. Moreover, platelet-secreted 12-lipoxygenase induces MMP-9 expression and thereby cellular invasion in prostate cancer (Dilly et al., 2013). Breast cancer metastasis to bone was enhanced by platelet-derived autotaxin (Leblanc et al., 2014) and lysophosphatidic acid (Boucharaba et al., 2004). Similar results were found for fibrinogen (Palumbo et al., 2005) or the fibrin crosslinker FXIII (Palumbo et al., 2008) with diminished experimental and spontaneous metastasis when these genes were knocked out. Table 1 summarizes the receptors expressed on the surface of platelets and reported to have influence on cancer metastasis. The impact of platelets on cancer cell dissemination and metastasis is depicted in Figure 2.
Table 1.
Platelet receptors with known roles in metastasis
| Molecule | Effect on metastasis | References |
|---|---|---|
| GPIbα | Reduced experimental metastasis of melanoma cells in GPIbα−/− mice | (Jain et al., 2007) |
| GPVI | Reduced experimental metastasis of melanoma and Lewis lung carcinoma in GPVI−/− mice | (Jain et al., 2009) |
| P-Selectin | Reduced metastasis of B16 cells in P-sel−/− mice | (Becker et al., 2017; Qi et al., 2014) |
| NFE2 | Fewer lung metastases after intravenous injection of melanoma cells | (Camerer et al., 2004) |
| Par4 | Fewer lung metastases after intravenous injection of melanoma cells | (Camerer et al., 2004) |
| Gαq | Reduced experimental metastasis of B16-BL6 melanoma and Lewis lung carcinoma cells following intravenous injection | (Palumbo et al., 2005) |
| LPA | Reduced bone metastasis of breast cancer after LPA deprivation or LPAR overexpression in tumor cells | (Boucharaba et al., 2004; Leblanc et al., 2014) |
| β3 integrin | Reduced bone metastasis of B16 melanoma cells after intracardiac injection | (Bakewell et al., 2003) |
| α6β1 integrin | Reduced metastasis of AT-3, B16F10, MC38 and E0771 cancer cells in α6β1 integrin−/− mice | (Mammadova-Bach et al., 2016) |
| CLEC2 | Reduced metastasis of CHO cells by inhibition of Podoplanin-CLEC-2 binding | (Takagi et al., 2013) |
| P2Y12 | Reduced metastasis of Lewis lung carcinoma and B16 melanoma cells | (Wang et al., 2013) |
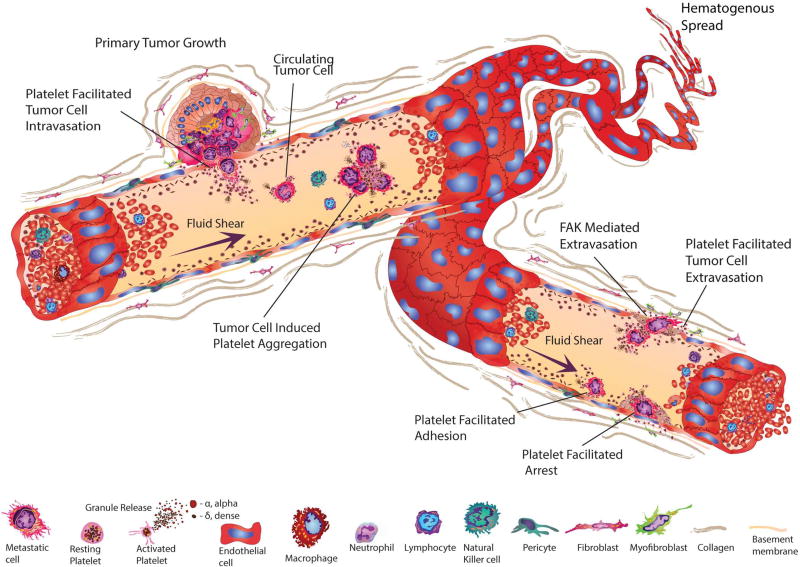
Figure 2. Platelet involvement in cancer growth and metastasis.
Platelet activation releases growth factors and small molecules that facilitate growth and invasion. Once tumor cells enter the blood stream, platelet-tumor cell aggregates form and platelets protect CTCs from NK-cell and TNFα-induced cell death. Additionally, platelets stimulate and aid cancer cell adhesion and extravasation thereby supporting cancer cell transmigration and metastasis formation. On the other hand, tumor cells mediate platelet activation leading to platelet aggregation and granule release. Activated platelets are also able to extravasate into the tumor microenvironment via focal adhesion kinase (FAK) to fuel tumor growth.
In mice, tumor cells injected intravenously lead to platelet aggregation within minutes, followed by degranulation and entrapment of tumor cells by additional platelet recruitment that walls off these cells rapidly. Extensive membrane interactions ensue between these heterotypic clusters that include platelet engulfment by tumor cells (Menter et al., 1987b). Tumor-cell induced platelet aggregation (TCIPA) triggers secretion of a plethora of pro-angiogenic and protumorigenic factors from platelets depending on the type of stimulus (Italiano et al., 2008; Ma et al., 2001). TCIPA has been shown in vitro for various cancer cells (Egan et al., 2011) and platelets are generally present at the core of the heterotypic aggregates that rarely fully engulf the tumor cells (Menter et al., 1987a). This is likely due to the significant differences in surface to volume ratios of the much larger tumor cell versus the very small platelets. These heterotypic aggregates could serve as a more effective target for isolating CTC subsets that represent a greater risk for triggering prothrombogenic properties and exhibiting EMT associated with metastasis. The molecular basis of TCIPA remains to be fully characterized. Proposed mechanisms include expression of podoplanin on cancer cells, which binds to CLEC-2 on the surface of platelets (Suzuki-Inoue et al., 2007). Developmentally, CLEC-2 and podoplanin knockout mice fail to separate the lymphatic and blood vascular system due to lack of platelet aggregate formation at the separation zone of lymph sacs and the cardinal veins (Uhrin et al., 2010). Moreover, podoplanin-CLEC-2 interaction might be important for vessel integrity in vivo (Herzog et al., 2013). In cancer, platelet CLEC-2 promotes tumor growth and metastasis via interaction with podoplanin on cancer cells (Takagi et al., 2013). TCIPA might also be triggered by MMP-2 (Jurasz et al., 2003), thromboxane A2 (de Leval et al., 2003; Pacchiarini et al., 1991), and thrombin (Danckwardt et al., 2013). After platelets are activated, they undergo a shape change and release ADP, which further enforces activation in an autocrine and paracrine fashion. ADP is not only secreted by platelets, but also by cancer cells, especially under hypoxic conditions in vitro and in vivo (Haemmerle et al., 2016). Platelet activation via ADP is mainly dependent on platelet expression of P2Y1 and P2Y12 (Kahner et al., 2006). P2Y1-knockout mice display increased bleeding time and are resistant to ADP-induced thromboembolism (Fabre et al., 1999). Similar, although less pronounced phenotypes are reported for P2Y12 knockout mice (Wang et al., 2013). We have recently shown that P2Y12 receptor on platelets plays an important role in the interaction between platelets and cancer cells. Blocking or deficiency of P2Y12 on platelets significantly reduced growth of orthotopic tumors in murine models of ovarian cancer (Cho et al., 2017). Aside from being blood-based, platelet-tumor cell interaction may occur in ascites and the tumor microenvironment. There is growing evidence that platelets can extravasate into the tumor microenvironment which is, in part, dependent on platelet expression of focal adhesion kinase (FAK) (Haemmerle et al., 2016). Moreover, platelet accumulation specifically increases around tumor cells in various types of human cancer (Qi et al., 2015b) and might even precede development of colorectal cancer (Qi et al., 2015a).
Interaction between tumor cells and microvascular endothelium occurs prior to successful extravasation and transmigration to form secondary metastases. In vivo experiments indicate that tumor cell adhesion to vessels was significantly increased by platelets (Zhang et al., 2011). Intriguingly, platelet-secreted ATP induced breakdown of the endothelial barrier and increased subsequent tumor cell transmigration and extravasation, which was dependent on endothelial P2Y2 (Schumacher et al., 2013). Most interestingly, this study provided insight into a new approach to interfere with platelet-dependent tumor metastasis without affecting the physiological function of platelets. Important for metastasis is the development of a suitable microenvironment to support engraftment of tumor cells. In addition to myeloid cells, platelets might guide the formation of a pre-metastatic niche in lung by secretion of CXCL5 and CXCL7 and thereby increasing granulocyte recruitment (Labelle et al., 2014) and in bone by secretion of TGF-β and MMP-1 (Kerr et al., 2013). Additionally, stromal derived factor 1 (SDF-1) secreted from platelet granules supports the homing of hematopoietic progenitor cells to sites of neovascularization in tumors and ischemic tissues (Jin et al., 2006). Cancer cells can also exhibit “platelet mimicry”, in which tumor cells express platelet or megakaryocyte specific gene products such as GPIIb-IIIa, protease-activated receptors (PARs) and platelet endothelial cell adhesion molecule 1 (PECAM1) that facilitates dissemination (Bambace and Holmes, 2011). Figure 3 summarizes the interactions between platelets and cancer cells during transmigration of cancer cell across endothelium (invasion into and extravasation out of vasculature) and circulation of cancer cells inside blood vessels.
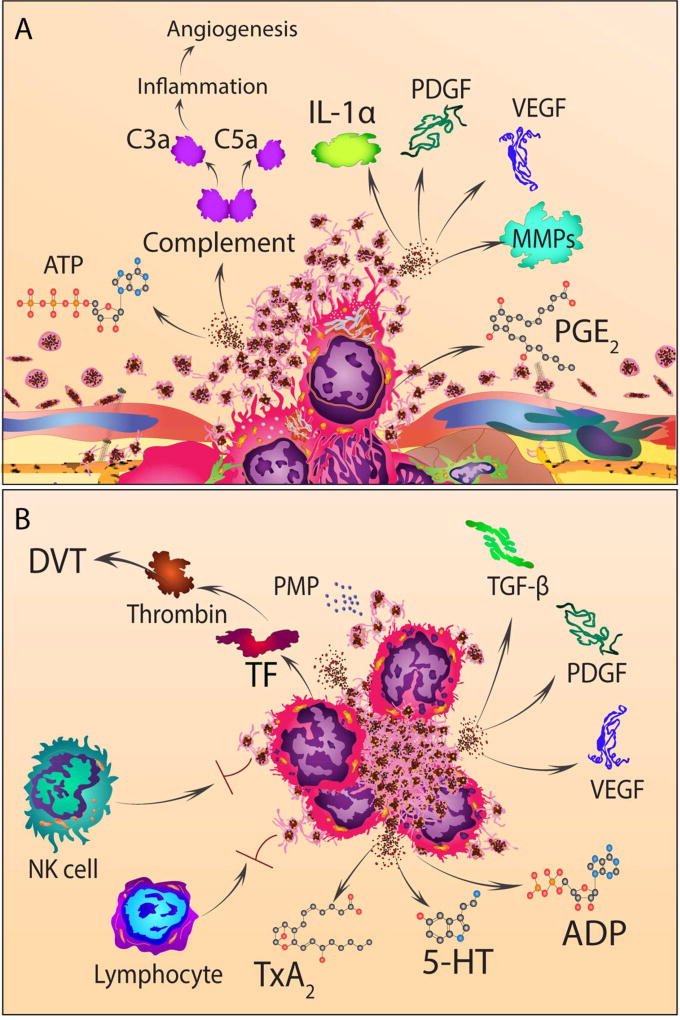
Figure 3. The interactions between platelets and cancer cells during transmigration and circulation.
(A) Tumor cells release complement (enzymatically processed to anaphylatoxin, C3a and C5a), prostaglandin E2 (PGE2), IL-1alpha (IL-1α), and matrix metalloproteases that assist transmigration of cancer cells across the endothelium (both into and out of blood vessels). Concurrently, ATP secreted from dense granules of platelets activates P2Y2 on endothelial cells, increasing permeability of the endothelium and promoting transmigration of cancer cells. Furthermore, activated platelets release the content of their alpha granules that contain numerous growth factors such as platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) that promote tumor growth and angiogenesis.
(B) Tumor cell-induced platelet aggregation leads to formation of heterotypic platelet-cancer cell aggregates, activation and degranulation of platelets. Platelet dense granules release G-protein coupled receptor agonists such as adenosine diphosphate (ADP) and serotonin (5-HT). Alpha granules release growth factors including VEGF and PDGF. Through a multistep synthetic process, arachidonic acid in activated platelets is converted to thromboxane A2 (TXA2) that in turn activates TXA2 receptors on other platelets and endothelial cells. Tissue factor (TF) expressed on cancer cells and platelet microparticles (PMP) further magnifies the procoagulant milieu generated by the interaction between platelets and cancer cells and result in thrombin generation and venous thrombosis.
2.3. Impact of platelets on tumor angiogenesis
Tumor angiogenesis results from a complex network between tumor cells and cells of the tumor microenvironment as well as cells recruited to the tumor from the bone marrow (Kerbel, 2008). This intercellular communication is provided by secreted growth factors (e.g. VEGF, FGF, PDGF, etc.) and their corresponding receptors (e.g. Notch signaling receptors, integrins, etc., (Carmeliet and Jain, 2011)). As a key source of circulating angiogenesis-related factors, platelets may regulate tumor angiogenesis and vascular integrity. One of the most important angiogenic proteins is vascular endothelial growth factor (VEGF) that is transported and released by platelets (Mohle et al., 1997). Platelet alpha (α) and dense (δ) granules contain and release proteins, lipids, growth factors, cytokines and proteases that impact angiogenesis directly or indirectly (Table 2, (Carmeliet and Jain, 2000; Carmeliet and Jain, 2011)). Platelets can actively take up these factors via endocytosis and sequester them (Klement et al., 2009) or megakaryocytes selectively transfer a subset of mRNAs or proteins to platelets (Cecchetti et al., 2011). Moreover, once stimulated, platelets can actively synthesize proteins (Schwertz et al., 2012). This compartmentalization and organization in distinct granules in platelets leads to selective release of factors depending upon specific stimuli; for example, a selective PAR4 agonist stimulated the release of endostatin-containing granules, whereas VEGF-containing granules were released after stimulation with a selective PAR1 agonist (Italiano et al., 2008). VEGF is also released from platelets by ADP stimulation, which is inhibited by antagonizing P2Y1 and P2Y12 receptors and does not affect endostatin release (Bambace et al., 2010). In addition, release of CXCL12 (SDF-1) from platelets induces revascularization by recruitment of hematopoietic progenitors (Jin et al., 2006). Besides regulating angiogenesis, platelets can regulate vascular integrity and thereby prevent tumor hemorrhage (Ho-Tin-Noe et al., 2009b). Maintaining vascular integrity can result from secretion of granules that contain ANGPT1 and serotonin as well as, which stabilizes tumor blood vessels by counteracting tumor cell-derived VEGF (Ho-Tin-Noe et al., 2008). By regulating vascular integrity, platelets might also reduce tissue damage by dampening tumor infiltration of immune cells and thereby controlling cytokine release (Ho-Tin-Noe et al., 2009a).
Table 2.
Platelet released factors important for tumor angiogenesis
| Effect on angiogenesis | Molecule | |
|---|---|---|
| Alpha granules | Pro-angiogenic | SERPINE1 |
| EGF | ||
| PDGF | ||
| bFGF | ||
| IGF | ||
| VEGF | ||
| TP (PD-ECGF) | ||
| IL-1β | ||
| SDF-1 | ||
| Anti-angiogenic | ANGTP1 | |
| S1P | ||
| PF4 | ||
| TSP1 | ||
| PGK | ||
| Endostatin | ||
| TIMPs (TIMP-1, -2, -4) | ||
| Pro- and anti-angiogenic | MMPs (MMP-1, -2, -3, -9, -14) | |
| TGFβ | ||
| HGF | ||
| Dense granules | Pro-angiogenic | Epinephrine |
| ATP, ADP | ||
| Anti-angiogenic | TFIP | |
| Pro- and anti-angiogenic | Histamine | |
| Serotonin |
2.4. Platelet interactions with other cells of the tumor microenvironment
Platelet-mediated secretion of TGF-β and other growth factors including PDGF and ANGPT1 are important for pericyte differentiation, proliferation, recruitment and interaction with endothelial cells (Armulik et al., 2011). Interestingly, thrombocytopenia in mice led to decreased pericyte coverage in B16/F10 tumors accompanied with reduced tumor angiogenesis, increased vascular leakage and decreased metastasis (Li et al., 2014). Platelet-induced growth of fibroblasts is mediated by an increase in 5-lipoxygenase activity (Berg et al., 2006). Furthermore, platelets, via membrane-bound FasL, induce apoptosis in fibroblasts (Schleicher et al., 2015). Similar to their effect on pericytes, platelets impact fibroblast proliferation and contribute to tumorigenesis and metastasis. Little is known about the impact of platelets on the function of immune cells other than NK cells. Recently, it was shown that platelet-derived TGF-β inhibits T-cell proliferation and interferon-γ production in vitro. Furthermore, a combination of anti-platelet agents including aspirin and clopidogrel with adoptive T-cell transfer (ACT) in vivo significantly increased ACT efficacy (Rachidi et al., 2017). Likewise, aspirin increases T-cell infiltration into ovarian tumors in mice and might be used in combination with checkpoint inhibitors (Motz et al., 2014). However, whether aspirin induced effects on T-cell infiltration are mediated by its inhibitory impact on platelets is not known. Figure 4 summarizes the involvement of platelets in inflammation and immunity.
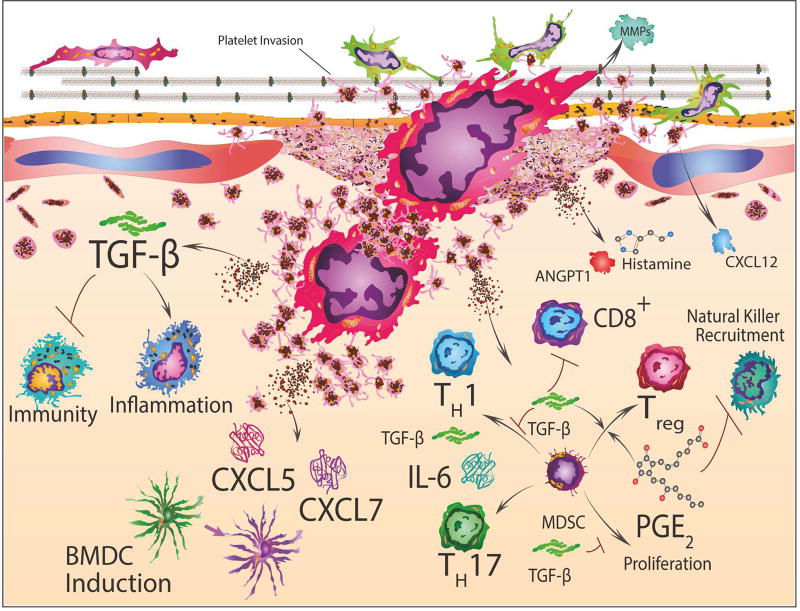
Figure 4. Platelet involvement in inflammation and immunity.
Bone marrow derived cell (BMDC) induction and differentiation is stimulated by platelet secretion of CXCL5 and CXCL7 which promotes granulocyte recruitment to tumor cells. Transforming growth factor-beta (TGF-β) released from platelets and microenvironmental PGE2 accumulation also stimulate marrow derived stem cells (MDSC) and T-cell differentiation or inhibition, which encompasses CD8+ cytotoxic T-cells, T-helper1 (TH1), T-helper17 (TH17) and T-regulatory cells (Treg). Secreted TGF-β induces epithelial-mesenchymal-transition (EMT) genes and also facilitates myeloid polarization of macrophages and neutrophils towards immunosuppressive phenotypes. This can generate microenvironmental niches at platelet facilitated arrest sites for cancer cells during the establishment of metastasis. Thus, these platelet-tumor cell microenvironmental niches may direct tumor-associated immune cells to convert from a pro-inflammatory to an immunosuppressive phenotype.
2.5. Platelets and chronic wound healing related to cancer
Platelets are derived from bone marrow stem cell lineages and play a key role in normal wound healing, which occurs in four stages including hemostasis, inflammation, proliferation and remodelling (Baum and Arpey, 2005; Nami et al., 2016). After tissue injury, platelets recognize vascular breaches and in coordination with the coagulation cascade limit blood loss by forming fibrin mesh that traps and activates additional platelets, and through multiple rounds of this process lead to fibrin clot formation. Platelets initially secrete multiple growth factors, chemokines and cytokines into the wound microenvironment that promote inflammation and cell proliferation, and subsequently promote resolution of inflammation and repair of injured tissue (Gawaz and Vogel, 2013). In physiological wound healing, the inflammatory process is carefully regulated to start and stop cell proliferation and the epithelial mesenchymal transition (EMT) in a timely manner. In cancer, the inflammatory response is not well regulated, which results in a pathogenic chronic wound healing process. Whether platelet interaction with tumor cells initiates EMT in circulating cancer cells remains unknown, but TGF-β may also affect cancer cells and immune milieu inside the tumor microenvironment (Labelle et al., 2011). TGF-β is released from platelet aggregates in a complex with latent TGF-β binding protein (LTBP) and latency associated peptide (LTB) that undergoes processing and slow chronic release into the circulation (Grainger et al., 2000). Persistent TGF-β release could directly and/or indirectly result in an immune suppressive tumor microenvironment. Platelets may be recruited inadvertently through chronic wounding found in solid tumors and co-opted to release TGF-β, thereby enabling tumor cells to avoid the immune system.
3. PLATELETS AND CHEMOTHERAPY
Multiple studies have shown that cancer patients who receive chemotherapy have a three- to five fold higher risk for developing VTE compared to those with no chemotherapy (Blom et al., 2006; Heit et al., 2000). In turn, platelets can influence efficacy of chemotherapy. In vitro experiments demonstrated that platelets increase resistance of colon and ovarian cancer cell lines to 5-fluorouracil and paclitaxel (Radziwon-Balicka et al., 2012). Moreover, lower blood platelet counts in a murine model of breast or lung carcinoma strongly increased sensitivity to paclitaxel (Demers et al., 2011). In cancer patients and mouse models of cancer, high platelet counts have been shown to be associated with a poor response to chemotherapy (Bottsford-Miller et al., 2015; Kim et al., 2015). We have shown that number and infiltration of platelets into the tumor microenvironment can influence rebound growth of orthotopic ovarian tumors in mice after cessation of anti-angiogenic therapy, including bevacizumab or pazopanib (Haemmerle et al., 2016). This was mediated by hypoxia-induced ADP production by cancer cells and FAK expression by platelets. Treatment with a FAK inhibitor reduced platelet infiltration into the tumor tissue and prevented tumor rebound growth after discontinuation of anti-angiogenic therapy (Hutchinson, 2016).
4. PLATELET-DERIVED MICROPARTICLES
Platelet activation, complement, high shear forces or apoptosis generates platelet-derived microparticles (PMP) from the plasma membrane (Goubran et al., 2015). Platelet activation increases intracellular calcium which in turn activates calpain, cytoskeleton proteins and Rho kinase resulting in cellular contraction and membrane blebbing (Fox et al., 1990). Alternatively, microparticles can also be released by resting platelets involving αIIβ3 integrin-mediated destabilization of the actin cytoskeleton independent of calpain activation (Cauwenberghs et al., 2006). Initially considered as “platelet dust” (Wolf, 1967), these microparticles represent a heterogeneous group of vesicles that are 0.05–1 µm in diameter and differ in their composition. In general, plasma microparticles are mainly derived from platelets (Flaumenhaft et al., 2009) although other cell types including endothelial cells, leukocytes, erythrocytes or cancer cells also release them. Depending on the cell type of origin, microparticles are characterized by their surface antigens, identifying those derived from platelets by surface expression of CD41 or CD42b (George et al., 1986; George et al., 1982). In general, microparticles differ from exosomes, which are derived from endocytosis and are released from multivesicular bodies (Heijnen et al., 1999). It has been shown that microparticles derived from platelets are important for various physiological and pathophysiological signaling pathways. As a source of tissue factor (TF) and phosphatidylserine, PMPs participate in coagulation (Freyssinet and Toti, 2010). In cancer patients with VTE, increased MP procoagulant activity was present already at baseline, suggesting that this could be used as a predictive marker for VTE in cancer patients (van Doormaal et al., 2012). In other studies, increased numbers of tumor derived TF-containing microparticles correlated with venous thrombosis in cancer patients (Hron et al., 2007; Tesselaar et al., 2009) and high PMP levels were associated with aggressive tumors, poor prognosis and metastasis in prostate cancer patients (Helley et al., 2009). In gastric cancer, PMP levels were significantly higher in stage IV compared to stage I or stage II/III tumors and correlated with plasma levels of IL-6, VEGF and RANTES. Intriguingly, PMP levels had the highest diagnostic accuracy and could predict existence of distant metastasis with specificity and sensitivity rates of over 90% (Kim et al., 2003). Functionally, PMPs promote the expression of adhesion molecules (Nomura et al., 2001), regulate the release of cytokines such as IL-17 and IFN-γ (Dinkla et al., 2016) and induce angiogenesis (Kim et al., 2004). Their tumor promoting capacity is likely mediated by VEGF, PDGF, TGF-β and bFGF, which also have angiogenic activity (Burnouf et al., 2014). Furthermore, they might transfer platelet surface receptors, including GPIIb-IIIa and P-Selectin (Salanova et al., 2007) to different cell types and thereby enhance engraftment of hematopoietic stem/progenitor cells (Janowska-Wieczorek et al., 2001). Moreover, PMPs can deliver miRNAs and other RNA molecules to cancer cells which can either promote or inhibit tumor growth. Interestingly, platelet microparticles can infiltrate into solid tumors of various origins and suppress tumor growth by delivering tumor suppressive miRNA-24 which targets mitochondrial mt-Nd2 and Snora75 in cancer cells (Michael et al., 2017). Oncogenic miR-223 derived from PMPs was shown to promote tumor cell invasion by downregulating tumor suppressor EPB41L3 (Liang et al., 2015). Besides RNA molecules and proteins, platelet microparticles also harbor lipids. Lipid profiling of platelets and PMPs showed increased procoagulant (i.e. phosphatidylinositol) and decreased anti-coagulant (i.e. lysophosphatidylcholine) lipids in ovarian cancer patients compared to healthy controls, suggesting a possible role for this class of lipids in hypercoagulability related to ovarian cancer (Hu et al., 2016).
5. ROLE OF ANTI-PLATELET THERAPY IN CANCER TREATMENT
Numerous drugs have been developed that target platelet receptors, interfere with platelet granule release or inhibit platelet-specific enzymes. These drugs are either used clinically or are still in pre-clinical development. For some of these drugs, survival advantage in cancer patients has been shown. However, obvious limitations for implementation in cancer patient care arise from their interference with hemostasis and also include challenges due to our limited understanding of the importance of platelet interaction with other cell types including cancer cells. Figure 5 and Table 3 summarize the most important drugs including depiction of the relevant pathways.
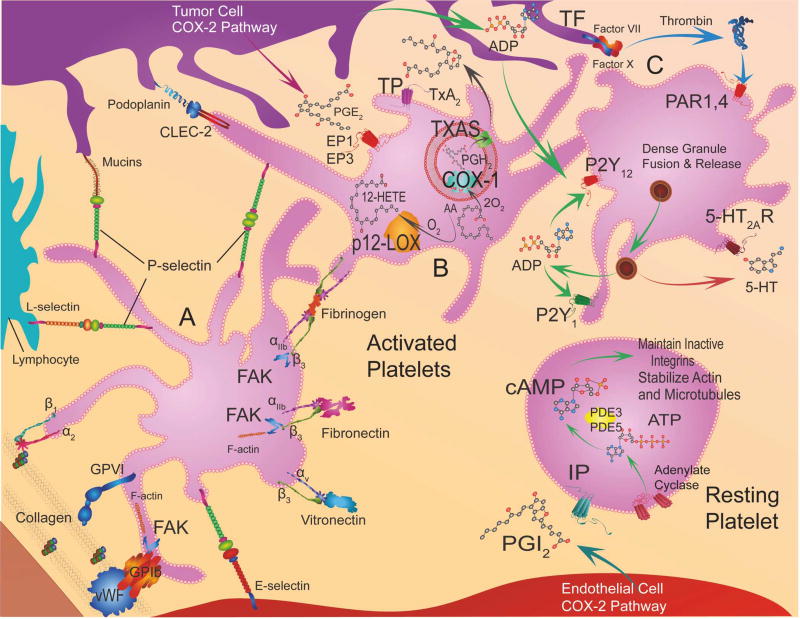
Figure 5. Platelet receptors, intracellular signaling, and targeted therapeutics.
This figure is accompanied by table 3, which lists the targeted receptors or enzymes, ligands or substrates/products, and signal pathways in platelets and the relevant drugs.
Adhesion receptors, Platelet A: Adhesion molecules on platelets bind platelets to the vessel wall at sites of damaged endothelium, these interactions result in intracellular signaling, actin polymerization, and shape change in platelets. The key interactions are between Von Willebrand factor (VWF) in the subendothelium and Glycoprotein (GP) Ib-IX-V complex and integrin αIIbβ3 on platelets, and between collagen in the in the subendothelium and GPVI and integrin α2β1. Focal adhesion kinase (FAK) facilitates GPVI binding to collagen. Tumor cell mucins interact with P-selectin on platelets, E-selectin on endothelial cells and L-selectin on leukocytes. Eicosanoid Pathway, Resting Platelet: Prostacylin (PGI2) activates Gαs-protein coupled receptor (IP) on platelets that stimulates cyclic adenosine monophosphate (cAMP) production by adenylate cyclase (AC) and prevents aggregation. Eicosanoid Pathway, Platelet B: One key platelet prostaglandin is the potent pro-aggregatory (TX)A2 synthesized by TXA2 synthase (TXAS) from PGG2 and then PGH2 that in turn are generated by cyclooxygenase-1 (COX-1) from arachidonic acid. TXA2 stimulates G-protein coupled TP receptors. Cyclooxygenase 2 (COX- 2) in cancer cells generates prostaglandin E2 (PGE2) that activates EP1 and EP3 receptors on platelets stimulating calcium release. Another abundant eicosanoid produced from arachidonic acid is 12(S)-HETE [12-(S)-hydroxyeicosatetraenoic acid] via the activity of platelet-type lipoxygenase (p12-LOX). Tumor cell podoplanin interacts with platelet C-type lectin domain family 2 (CLEC-2). GPCR, Platelet C: Platelet activation occurs through protease-activated receptors (PAR1 and 4) via Tissue factor (TF)-Factor VII-Factor X-mediated thrombin production. ADP (adenosine diphosphate) released from cancer cells and from dense granules of platelets stimulates P2Y1 or P2Y12 receptors. Serotonin (5-hydroxytryptamine) is also released from dense granules that act through 5-hydroxytryptamine receptors (5HT2AR).
Table 3.
List of specific pathways relevant for platelet effects on tumor growth, respective receptors and ligands as well as candidate drugs to target those.
| Target Type | Receptor | Ligand | Pathway | Drug |
|---|---|---|---|---|
| ADHESION RECEPTORS | ||||
| Platelet A | GPIa/IIa (α2β1) | Collagen | FAK/f-actin, Rac1/p38/MAPK | Secretion |
| GPVI | Collagen | CaM/Lyn/Syk/SLP76/Btk/PI3K/PLCγ2 | RevaceptC | |
| GPIb, (GPIX,GPIbβ,GPI bα,GPV complex) | vWF | FAK/F-actin, CaM/Lyn/Syk/SLP76/Btk/PI3K/PLCγ2 | GSK2256098C, PF-562271PC, DefactinibC, PF-573228PC, Y15 & Y11PC, CEP37440PC | |
| GPIIb/IIIa (αIIb/β3) | Fibrinogen | FAK/CIB-1/actin, ARP 2/3/actin Polymerization, FAK/Paxillin/RhoGEF | AbciximabC, EptifibatideC, TirofibanC, XV454PC | |
| αv/β3 | Vitronectin | c-Src, FAK, Paxillin, PI3K | SB-273005PC, SC-68448C, EMD121974C, vitaxinC | |
| Signal Transduction | ROCK | Rho Kinase activity | Y27632PC | |
| Myosin II | ATPase activity | BlebbistatinPC | ||
| CLEC-2 | Podoplanin | Syk/PLCγ2 | 2CPPC, Mabs | |
| P-Selectin | Mucins, P-,E-,L-Selectins | Shc·Grb2·Sos1 | RivipanselC, CrizanlizumabC, HeparinsC | |
| EICOSANOID PATHWAY | ||||
| Resting Platelet | IP, Prostacyclin receptor Agonists | PGI2 | GPCR/αGs/adenylate cyclase/cAMP | IloprostC, TreprostinilC, SelexipagC |
| PDE3-PDE5 | cAMP | Phosphodiesterase activity | CilostazolC, DipyridamoleC, | |
| Platelet B | TP, Thromboxane A2 receptor Antagonists | TxA2 | GPCR/G12/13/RhoGEF/Rho/ROCK/LIMK/Cofilin/actin MLCK/Myosin | TerutrobanPC, DaltrobanPC, PicotamideC, SulotrobanPC, CAY10535PC, IfetrobanC, SQ29548PC, BM 567PC, Pinane TXA2PC |
| Thromboxane Synthase (TXAS) Inhibitors | TxA2 | AA-2O2/COX-1 /PGG2/PGH2/TXAS/ TxA2 | OKY-046C, RidogrelC | |
| Cyclooxygenase 1 (COX-1) | AA-2O2 | AA-2O2/COX-1 /PGG2/PGH2 | AspirinC, ASP6537PC, SC560PC, FR122047PC, MofezolacPC, FluorofuranonesPC | |
| EP3 Prostaglandin E2 receptor Antagonist | PGE2 | GPCR/Gαq/PLCγ/P/IP2/IP3/IP3-R/Ca+2 release/DAG/PKC/CalDAGGEF1/Rap1B/RIA M/actin | DG 041PC | |
| 12-lipoxygenase (12-LOX) Inhibitors | 12-HETE | AA-O2/12-LOX/12-HETE | ML355PC, NCTT-956 | |
| GPCR | ||||
| Platelet C | PAR1 Antagonist | ADP | GPCR/Gq/RhoGEF/Rho/ROCK/LIM-K/Cofilin/actin/MLCK/Myosin, β-arrestin | VoraxoparC, CH 79797C, RWJ 56110PC |
| P2Y1 Receptor Antagonists | ADP | GPCR/Gαq/PLCγ/P/IP2/IP3/IP3-R/Ca+2 release/DAG/PKC/CalDAG-GEF1/Rap1B/RIAM/actin | A2P5PPC, A3P5PPC, MRS2179PC, MRS2500PC | |
| P2Y12 Receptor Antagonists | ADP | Gai2/AC | ClopidogrelC, TiclopidineC, PrasugrelC, TicagrelorC, CangrelorC, ElinogrelC | |
| 5HT2A Receptor Antagonists | Serotonin (5-hydroxy tryptamine) | GPCR/Gαq/PLCγ/P/IP2/IP3/IP3-R/Ca+2 release/DAG/PKC/CalDAG-GEF1/Rap1B/RIAM/actin | TemanogrelC, NaftidrofurylC, SarpogrelatC, AT-1015PC |
PC: preclinical, C: clinical
5.1. Integrin glycoprotein receptors
Platelet glycoprotein (GP) receptors mediate platelet adhesion, activation and aggregation. Many GP receptors exist as non-covalently linked heterodimers, including those belonging to the α and β subunit integrin family. Under physiologic conditions, local generation of thrombin by tissue factor or exposure of the underlying subendothelial matrix upon endothelial cell activation/injury triggers platelet adhesion and activation. Early phase platelet adhesion is coordinated by GPIbα interactions with von Willebrand factor (VWF) and integrin binding to collagen. Engagement of these GP receptors in concert with thrombin signaling via PARs (see below) activates platelets. Upon platelet activation, GPIIb/IIIa (integrin αIIbβ3) conformation changes from a low to high affinity state. High affinity GPIIb-IIIa binds fibrinogen, accelerating the formation of inter-platelet bridges and thrombus formation. Given that all of these GP receptors coordinate platelet-tumor cell interactions, blocking them could be an effective anti-cancer strategy. Drugs designed to block platelet collagen GPVI/α2β1 receptors are being evaluated in clinical trials for cardiovascular disease and GPIIb/IIIa antagonists are registered drugs with wide clinical application. There are also several trials evaluating the clinical safety and effectiveness of blocking platelet aggregation with antibodies directed against VWF and GPIbα, bivalent humanized nanobodies targeting the GPIbα binding site of VWF and aptamers with high binding affinity for VWF (Firbas et al., 2010). Blocking these GP receptors can abrogate the tumor promoting effects of platelets. For example, revacept (AdvanceCor) is a soluble fusion protein consisting of the human immunoglobulin Fc domain linked to the extracellular domain of the platelet collagen receptor GPVI. In contrast to anti-GPVI antibodies, revacept does not reduce platelet GPVI receptor expression or impact platelet counts (Ungerer et al., 2011). At clinically relevant concentrations, this drug inhibits platelet adhesion to HT29 colon cancer cells. This effectively prevents platelet-induced upregulation of cyclooxygenase-2 (COX-2) as well as markers of epithelial-mesenchymal transition (EMT) in cancer cells (Dovizio et al., 2013). There are currently three FDA approved GPIIb/IIIa inhibitors including abciximab (Reopro), tirofiban (Aggrastat) and eptifibatide (Integrilin). Tirofiban and eptifibatide are highly selective, reversible small molecule GPIIb/IIIa inhibitors, whereas abciximab is a chimeric human/murine antibody directed against GPIIb/IIIa receptor. It is unique among the three available GPIIb/IIIa inhibitors in that it also binds to integrin αVβ3 on tumor and endothelial cells, as well as the integrin αMβ2 expressed on leukocytes (Trikha et al., 2002). This cross reactivity on both platelets and endothelial cells may help suppress tumor angiogenesis (Yan et al., 2014). Recently, a humanized single-chain variable fragment antibody (scFv Ab) was developed against integrin GPIIIa49–66 (named A11) capable of inducing oxidative platelet fragmentation and selective lysis of activated platelets. Experimental findings suggest that scFv primarily exerts its anti-platelet activity when activated platelets are bound to tumor cells (Zhang et al., 2012). A potential use of A11 can be during surgical resection of a primary tumor, when scFv administration can reduce the likelihood of platelet mediated tumor cell embolization (Ware, 2012).
5.2. P-Selectin
Malignant transformation of cells is associated with abnormal glycosylation, resulting in altered carbohydrate structures (Varki et al., 2009). Enhanced expression of sialyl-Lewisx and sialyl-Lewisa structures is frequently associated with cancer progression and poor prognosis. These carbohydrates act as ligands for selectins, which are vascular cell adhesion molecules involved in interactions between leukocytes, platelets, and the endothelium. P-selectin is stored in platelet α granules and is expressed on activated platelets and endothelium, while E-selectin and L-selectin are constitutively expressed on endothelial cells and leukocytes, respectively. Upon activation, P-selectin is rapidly transferred to the cell membrane through exocytosis. Experimental evidence suggests that P-selectin dependent binding of platelets to sialyl-Lewis carbohydrates on cancer cells contributes to platelet-tumor microemboli formation and tumor cell extravasation through a process that resembles that of leukocyte transendothelial migration (Laubli and Borsig, 2010). Given that P-selectin dependent platelet-tumor cell interactions appear to be important for hematogenous metastasis during the intravascular phase of cancer cells, new compounds that target selectins are now emerging for cancer therapy. The glycomimetic small molecule rivipansel has inhibitory activity against P-, L-, and E-selectins. This agent has been shown to prevent homing of malignant plasma cells to the bone marrow in multiple myeloma (MM) and to increase their sensitivity to bortezomib (Azab et al., 2012). Crizanlizumab is a monoclonal antibody that selectively binds to P-selectin and can reduce vaso-occlusive crises in patients with sickle cell anemia (Ataga et al., 2017). The utility of this new drug in cancer treatment remains unknown.
5.3. Protease-activated Thrombin Receptors and other Anti-Thrombin Approaches
Protease-activated receptors (PARs) are a family of 4 transmembrane G-protein-coupled receptors that are activated by the most potent platelet agonist, thrombin, (PAR-1, PAR-3 and PAR-4) or by trypsin-like serine proteases (PAR-2) (Zhao et al., 2014). PARs are expressed on platelets, neutrophils, macrophages and on nearly all cell types in the blood vessel wall (endothelial cells, fibroblasts, myocytes). Human platelets express two types of thrombin-triggered PARs, the high-affinity PAR-1 and low-affinity PAR-4. In addition to triggering platelet aggregation, PAR-1 and PAR-4 activation may induce selective release of platelet angiogenic and mitogenic regulators. Some experimental evidence suggests that release of VEGF-containing granules is elicited by PAR-1 activation, whereas PAR-4 signaling preferentially releases endostatin-containing granules (Italiano et al., 2008). However, other quantitative proteomic analyses have shown that PAR-1 and PAR-4 stimulation leads to non-differential release of VEGF, endostatin and other angiogenesis mediators from human platelets (Etulain et al., 2015; van Holten et al., 2014). Furthermore, the observation that spontaneous mammary tumor development is delayed in PAR-2-null, but not in PAR-1-null mice, suggests that PAR-2, rather than PAR-1 may play a more dominant role in the angiogenic switch at least in breast cancer development (Versteeg et al., 2008).
Anti-tumor efficacy is also possible by direct blockade of PARs on tumor cells, platelets, fibroblasts and the endothelium (e.g., with SCH79797, vorapaxar, atopaxar) or by administering tissue factor or thrombin inhibitors (e.g., hirudin, argatroban). For example, the thrombin inhibitor hirudin decreased platelet-derived VEGF at the site of thrombus by almost 50% and inhibited metastasis in experimental models (Sierko and Wojtukiewicz, 2007). Although preclinical results remain promising, inhibition of PAR activity on both host and tumor cells may cause serious side effects, such as hemorrhage, making PAR-tailored drug discovery a great challenge. Vorapaxar and atopaxar are orally active, reversible selective small molecule inhibitors of PAR-1 activation. Vorapaxar is first-in class and has an FDA indication for the reduction of thrombotic cardiovascular events in patients with a history of myocardial infarction or with peripheral arterial disease. Clinical development of atopaxar is currently limited to phase 1 and 2 trials and abnormalities in liver enzymes and cardiac conduction detected with its use may negatively impact the future of this drug. Non-competitive modulators of PAR activity include pepducins and parmodulins. Pepducins are cell-penetrating lipidated peptide mimetics that target the intracellular loops of G-protein receptors. The PAR-1 pepducin antagonist PZ-128 is currently being tested in a trial of patients undergoing non-emergent percutaneous coronary intervention. The recently published safety study of PZ-128 in patients with coronary risk factors suggests that PZ-128 may be associated with decreased bleeding risk compared to vorapaxar (Gurbel et al., 2016). Adverse bleeding events are also expected to be less with the parmodulin ML-161, a reversible allosteric PAR-1 inhibitor that targets the intracellular receptor domains. However, its clinical value as an anti-platelet agent remains undetermined (Cunningham et al., 2016).
5.4. Selective Inhibition of Platelet Storage Granule Release
While controlling differential release of pro- and anti-angiogenic factors from platelet α-granules by manipulating PARs is feasible, the exact mechanisms of granule release and whether they can be selectively manipulated requires more study. For example, platelet α granule release and aggregation are regulated, in part, via protein kinase C (PKC) α and β signaling. PKCα inhibition with Ro32-0432, a small molecule inhibitor with greater selectivity for PKCα than other isoforms, preferentially suppresses angiogenesis-regulator secretion from α granules but not platelet aggregation, limiting platelet-stimulated angiogenesis. These data suggest that it may be possible to pharmacologically abate the effects of platelets on tumor angiogenesis while maintaining hemostasis (Moncada de la Rosa et al., 2013). Aspirin may block the differential release of angiogenic regulators from platelets and inhibit angiogenesis (Battinelli et al., 2011). In fact, a recent prospective study in women with breast cancer on tamoxifen showed that platelet and serum thrombospondin-1 (TSP-1) levels rise upon initiation of aspirin therapy. Conversely, platelet and serum VEGF levels decreased following the completion of a 45-day course (Holmes et al., 2013).
5.5. Aspirin and Cyclooxygenase Inhibition
While aspirin therapy may suppress angiogenesis-dependent tumor growth, many other aspirin-acetylation based mechanisms can impact malignancy including disruption of platelet-tumor cell interactions, cyclooxygenase inhibition, promotion of apoptosis, restoration of DNA mismatch repair, and inhibition of tumor cell proliferation by blockade of mitochondrial calcium uptake. Aspirin irreversibly acetylates cyclooxygenase (COX) and prevents the conversion of arachidonic acid to thromboxane A2 (TXA2). This is particularly important in platelets because they lack the ability to resynthesize COX-1. Thus, long-term aspirin use results in a durable decrease in TXA2 production and platelet aggregation. Pharmacologic data suggest that low dose aspirin (81–162 mg orally daily) is relatively selective for COX-1 inhibition, blocking platelet aggregation and interaction with circulating tumor cells. Higher or more frequent dosing likely also inhibits the inducible COX-2 pathway in other cells. This may also inhibit the proaggregation role of PGE2 on platelets (Vezza et al., 1993). Presently, there is a significant body of preclinical, epidemiologic, and randomized trial data supporting the anticancer effects of aspirin. There is consistent evidence that regular low-dose aspirin reduces the incidence and death rates from colon and other cancers (Algra and Rothwell, 2012; Rothwell et al., 2012). Reduction in the incidence of cancer has a latency period of around 5 to 10 years, suggesting an effect on early carcinogenesis. Emerging data also indicate that aspirin as an adjuvant treatment following a diagnosis of cancer may mitigate metastatic spread and prolong survival. A comprehensive meta-analysis examining cause-specific mortality for patients taking aspirin regularly after a cancer diagnosis revealed that aspirin, in addition to treatment for cancer, is associated with a 24% reduction in colon cancer mortality as well a 13% and an 11% decrease in deaths due to breast or prostate cancer, respectively (Elwood et al., 2016). The most pronounced reductions in mortality have been observed in proximal colon (HR 0.55; 95% CI 0.37 to 0.82), compared to distal colon or rectal cancer (HR 1.00; 95% CI 0.72 to 1.41) (Coghill et al., 2011). Given that two-thirds of Lynch syndrome associated colon cancers originate in the proximal colon, the question of a selective benefit of aspirin prevention in this patient population has been raised. The Colorectal Adenoma/carcinoma Prevention Programme 2 (CAPP2) was the first large-scale genetically targeted chemoprevention trial, focused on individuals diagnosed with Lynch syndrome. The 861 participants were randomly assigned to aspirin (600 mg daily) or placebo. An intention-to-treat, time-to-event analysis accounting for participants with multiple colon primaries (mean follow-up of 55.7 months) demonstrated a compelling protective effect [Incidence Rate Ratio (IRR) 0.56; 95% CI 0.32 to 0.99, p=0.05]. The effect size was significant for participants completing 2 years of intervention (IRR 0.37; 95% CI 0.18 to 0.78, p=0.008) (Burn et al., 2011). Aspirin benefit in colorectal cancer, and possibly other cancers, may be restricted to patients with tumors containing certain genetic alterations (e.g., high COX-2 or HLA class 1 antigen expression and PIK3CA mutation). In two large observational cohorts, the Nurses’ Health Study (NHS) and Health Professionals Follow-Up Study (HPFS), regular aspirin use after a diagnosis of colorectal cancer reduced disease-specific mortality in patients with COX-2 overexpressing tumors (Chan et al., 2009) and in patients with cancers harboring activating mutations in PIK3CA, with a multivariate hazards ratio for cancer death of 0.18 and for all-cause mortality of 0.54 (Liao et al., 2012). The survival benefit associated with low-dose aspirin use after a diagnosis of colon cancer may be associated with the presence of HLA class I antigen-positive tumors (Reimers et al., 2014). While platelets are known to transfer HLA-1 to tumor cells (Placke et al., 2012), a connection between platelets, tumor cell HLA-1 expression and aspirin has not been described.
5.6. Heparins
The impact of heparin and its low molecular weight derivatives (LMWH) on survival in solid tumors is uncertain. The inhibitory effects of heparins on tumors seem to be less dependent on a direct anticoagulant effect and more dependent on modulation of cellular interactions, as well as modulation of platelet function. Heparins block the conversion of prothrombin to thrombin, thereby leading to impaired platelet PAR-1 activation. For example, LMWH inhibits tumor cell-mediated release of platelet angiogenic proteins (Battinelli et al., 2014). While heparins also inhibit P- and L-selectin, their variable effects on integrin GPIIb-IIIa can result in platelet activation in some cases (Gao et al., 2011; Lever and Page, 2002). The effect of heparin on platelet-tumor cell interactions mediated by these adhesion receptors has been demonstrated in in vivo models of metastasis (Borsig et al., 2001). A number of early studies showed improved patient survival with LMWH for the initial treatment of VTE and for perioperative VTE prophylaxis (Hettiarachchi et al., 1999; Kakkar et al., 1995; Prandoni et al., 1992). In the prospective MALT trial of a 6-week course of therapeutic dose nadroparin versus placebo in advanced cancer patients without clotting history, the hazards ratio for mortality was 0.75 in favor of nadroparin over placebo (Klerk et al., 2005). The Fragmin Advanced Malignancy Outcome Study (the FAMOUS trial) assessed the effect of extended duration LMWH in cancer patients without VTE on 1-, 2- and 3-yr survival. Patients with advanced malignancy were randomized to receive either dalteparin (5,000 IU daily) or placebo for 1 year. Despite the primary endpoint not being met, the subgroup of patients still alive 17 months after randomization to dalteparin had a significant survival advantage at 2 and 3 years (Kakkar et al., 2004). In contrast, results from larger scale randomized prospective trials are mixed without a significant survival benefit (the FRAGMATIC and the CLOT trials) (Lee et al., 2005; Macbeth et al., 2016). Patient selection, dose and duration of LMWH administration and the selection of warfarin for the control arm may account for the discrepancy in the outcomes of these trials. For example, in the CLOT trial, beneficial effect of LMWH was greater in patients with early-stage cancers who undergo surgical resection and adjuvant chemotherapy over those with metastatic disease. Additionally, treatment of VTE with warfarin is associated with higher rates of recurrent thromboembolism in cancer patients, resulting in cross-over to LMWH that could impact survival analyses (Lee et al., 2003). Currently, LMWH remains under further study as an adjunct to standard anti-cancer therapies (Zhang et al., 2016).
5.7. ADP P2Y12 Receptor Antagonists
The P2Y12 receptor is the predominant receptor involved in the ADP-stimulated activation of the platelet glycoprotein IIb/IIIa receptor. While clopidogrel (Plavix) is the most widely used P2Y12 receptor antagonist for the treatment of vascular disease, the newly developed P2Y12 inhibitors such as prasugrel (Effient) and ticagrelor (Brilinta) are more potent and have a faster onset of action (Damman et al., 2012). Since the 2010 analysis of the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel (TRITON-TIMI 38), concern has grown that these drugs may actually increase the incidence and accelerate the growth of existing solid malignancies. This study randomly assigned 13,608 patients with moderate-to-high-risk acute coronary syndromes to prasugrel or clopidogrel (Wiviott et al., 2007). Excluding non-melanoma skin cancers and brain tumors, there were 112 treatment-emergent cancers in the prasugrel arm and 69 treatment-emergent cancers in the clopidogrel-treated patients, a difference that translated into a significant 62% increase in the risk of new or worsening solid tumors. In addition to these findings, there was a non-significant 57% increase in deaths from cancer among patients treated with prasugrel (Floyd and Serebruany, 2010). The association between clopidogrel and cancer risk remains controversial. In the Dual Anti-Platelet Therapy (DAPT) study for the prevention of thrombotic complications following drug-eluting stent placement, the risks for non-cardiovascular deaths were predominantly attributed to cancer, and emerged 24 months after therapy (Mauri et al., 2014). This outcome was similar to that seen with the CAPRIE trial (clopidogrel versus aspirin in patients at risk of ischemic events) in which the cancer curves diverged at about 26 months, suggesting a potentially delayed cancer risk emerging after 2 years of clopidogrel treatment (Committee, 1996; Serebruany et al., 2015). However, the recent large-scale cohort study examining the long-term use (≥ 5 years) of dual anti-platelet therapy with clopidogrel and aspirin showed that combined aspirin and clopidogrel use is at least as safe as prolonged aspirin use with regard to cancer incidence (Leader et al., 2017). Risk is similarly uncertain with PAR-1 inhibitors. The excess of solid cancers after vorapaxar in the TRACER trial (Thrombin-Receptor Antagonist Vorapaxar in Acute Coronary Syndromes) appeared similar to that for prasugrel in TRITON. However, cancer incidence did not differ by study arm in the TRA2P-TIMI study (Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events). Since cancer rates were only reported until 60 days after the last dose of study drug in both trials, they may be inaccurate if the risk is time-dependent. The mechanisms by which P2Y12 receptor antagonists and PAR-1 inhibitors might increase cancer risk are unknown. One possible explanation is that platelet-tumor cell trapping in the microvasculature, is to some extent, anti-metastatic and platelet inhibition with these agents impairs the natural barrier function of platelets (Floyd and Serebruany, 2010). While experimental evidence supporting this theory is lacking, it reflects our current understanding of the important role platelets play in maintaining normal endothelial barrier function. In some circumstances, this protective function of platelets may be overcome, resulting in increased vascular permeability and tumor cell entry into circulation and/or extravasation at metastatic sites.
5.8. Other Potential Therapeutic Approaches
Successful development of therapeutics that interfere with platelet-cancer cell interactions must balance inhibiting platelet specific targets that play a role in cancer progression against maintaining normal platelet function in hemostasis. With the continued evolution of anti-platelet agents, there is an added challenge of critically appraising their risk-benefit profiles within the context of oncology. Given the high proportion of cancer patients with thrombocytosis, approaches aimed at long-term maintenance of normal platelet counts and turnover may provide a solution. Challenges persist though since our understanding of the drivers of paraneoplastic thrombocytosis in various malignancies remains limited. The interplay between tumor IL-6, thrombopoietin and paraneoplastic thrombocytosis was described in ovarian cancer. IL-6 neutralization with an anti-IL-6 antibody (e.g., siltuximab) safely lowered platelet counts and improved disease control in patients with recurrent, platinum-resistant ovarian cancer (Stone et al., 2012). Clinical benefit was also observed for metastatic renal cell and castration-resistant prostate cancer (Dorff et al., 2010; Rossi et al., 2010), but changes in platelet counts with anti-IL-6 treatment were not assessed in these studies. An alternative strategy, inspired by the adhesion of activated platelets to circulating tumor cells, involves using biocompatible silica (Si) particles with membrane-derived vesicles from activated platelets. This biomimetic coating of platelet membrane-derived vesicles is designed to allow for targeted drug delivery to circulating tumor cells. Conjugation of tumor necrosis factor-related apoptosis inducing ligand (TRAIL) to the platelet membrane-coated Si particles was an effective method for arresting hematogenous tumor spread in experimental models of metastasis (Li et al., 2016).
5.9. Challenges of Targeting Platelets in Cancer Treatment
The most obvious challenge in incorporating anti-platelet agents into the treatment of cancer is our limited understanding of the conditions that differentially elicit the mitogenic, pro- or anti-angiogenic, pro-inflammatory, anti-inflammatory, and adhesive functions of platelets. We also do not know which specific platelet activities are most relevant to the malignant process and to what extent such an intervention will impair the necessary physiologic role of platelets in maintaining hemostasis. Current anti-platelet drugs target platelet receptors, their binding partners, signaling proteins or soluble mediators of platelet function. While drugs that block cyclooxygenase (e.g., aspirin), the ADP receptor P2Y12 (clopidogrel, prasugrel, ticagrelor) and GPIIb/IIIa (abciximab, eptifibatide, and tirofiban) are most widely used, their effects on cells other than platelets make it difficult to conclude with certainty that their anti-neoplastic properties are solely due to platelet inhibition. For example, the off-target effects of small molecule GPIIb/IIIa receptor inhibitors are predominantly related to their suppressive influence on the inflammatory response and this might be as beneficial as platelet GPIIb/IIIa receptor blockade in some patients. The limited distribution of GPIbα and GPVI/α2β1 to megakaryocytes and platelets offers some advantage for studying platelet-specific events, compared to targets with more diverse expression profiles. However, these glycoprotein receptors are probably most important in the high shear rate conditions of the blood stream. Blockade of platelet granule release or neutralization of specific factors in platelet releasate, as opposed to inhibition of these adhesion receptors, in the slowed blood flow of local tumor microenvironments may be an attractive approach for preventing cancer growth. There is also wide inter-individual variation in response to anti-platelet agents like aspirin and clopidogrel. In fact, observational studies suggest that at standard doses of aspirin (100 mg) and clopidogrel (75 mg), over one-third of patients have predefined ex vivo resistance. Reasons for anti-platelet drug resistance are manifold and include gene polymorphisms that influence drug metabolism, increased baseline platelet reactivity such as that found in diabetes mellitus and smoking, and increased platelet turnover (Metharom et al., 2015). Presently, we have little insight into how these factors impact anti-platelet drug activity in patients with active cancer or even how to best assess this pharmacodynamically. Variable response to and the off-target effects of anti-platelet agents could explain the conflicting observations that have been made about their influence on cancer incidence and survival. To date, this has significantly decreased enthusiasm for cancer management protocols reliant on anti-platelet strategies. Forward progress depends on better understanding of the mechanisms of paraneoplastic thrombocytosis and of the cancer-promoting interactions between platelets and other host cells. Applied in the right context, this has potential to add to our growing success with molecular- and immune-based cancer treatments. For example, genetic and pharmacologic targeting of platelets can enhance adoptive T cell therapy of cancer, suggesting that there may be unique opportunity to optimize cancer immunotherapy by simultaneously blocking platelets and immune checkpoint molecules (Rachidi et al., 2017).
6. PLATELETS AS BIOMARKERS
Interest in developing blood-based biomarker platforms that reliably detect cancer has intensified within the context of “liquid biopsies.” These approaches invoke findings that all cells, including cancer cells, release small fragments of DNA into the bloodstream, known as cell-free DNA (cfDNA). Blood cfDNA can be isolated and rapidly sequenced by advanced methods for the presence of circulating tumor DNA (ctDNA). Alternatives to filtering cfDNA from blood plasma include capturing whole CTCs or small vesicles ejected by tumor cells called exosomes and analyzing their nucleic acid content. While “liquid biopsies” hold great promise as a convenient, minimally invasive means for early cancer detection, monitoring therapeutic response and forecasting recurrence, concerns exist about specificity and accurate quantitation. The stability of cfDNA is not well understood and the isolation of cfDNA requires highly sensitive techniques due to the small fraction of tumor-specific DNA masked by background levels of wild-type cfDNA. The low frequency of CTCs and the associated problems of their reliable detection also remains a challenge. Isolation of tumor-derived exosomes, which are variable in size, and their separation from normal exosomes, presents technical challenges as well. Thus, looking beyond traditional body fluids at other less obvious biomarker sources is of great interest.
Cancer associated platelets represent a compelling addition to our liquid biopsy arsenal. Emerging data suggest that platelet-based analytics have the potential to add to the diagnostic utility of other biomarker strategies (Chi, 2016). Platelets are the second most abundant cell type in peripheral blood and are easily isolated and counted in blood tests. Platelets are anucleate and derive most of their genetic material and proteins, in part, from megakaryocytes. Platelets also actively uptake and accumulate additional biomass during their lifespan in circulation, which may include that shed from tumor cells as exosome cargo. In addition to sequestering both solubilized tumor-derived proteins as well as spliced and unspliced mRNA transcripts, platelets are also the largest storage pool of oncogenic and angiogenic growth factors, including VEGF, PDGF, and TGF-β in the human body. Thus, platelets represent a massive, concentrated biorepository of tumor-derived and bioactive molecules. It is known that platelets scavenge tumor-derived transcripts. For example, the cancer-associated RNA biomarkers epidermal growth factor variant III (EGFRvIII) and prostate cancer antigen 3 (PCA3) have been recovered from platelets isolated from patients with glioma and prostate cancer, respectively. Further, analysis of platelet RNA profiles from healthy control subjects and glioma patients using gene-expression arrays reveals distinctly different expression profiles. Differences were significant enough that a glioma-associated gene-expression signature could be formulated solely based on information gleaned from platelets (Nilsson et al., 2011). Recently, Best and colleagues expanded upon this finding by characterizing the differential expression patterns between healthy donor and cancer patient platelet transcriptomes (Best et al., 2015). They prospectively isolated, amplified and sequenced RNA from blood platelets obtained from large cohorts of healthy donors and both treated and untreated patients with early, localized or advanced, metastatic cancer. They detected 5,003 differentially expressed protein coding and non-coding RNAs between healthy donors and cancer patients. Using this readout, they were able to distinguish patients with localized and metastatic tumors from healthy individuals with 96% accuracy. Further, platelet profiles appeared to be tumor-type specific enough that the primary origin of disease was correctly pinpointed in the majority of cases. In many instances, platelet RNA profiles precisely predicted the oncogene status of many tumors including MET or HER2-positivity and the existence of KRAS, EGFR, or PIK3CA mutations (Best et al., 2015). Consequently, these findings suggest that profiling the platelet transcriptome may enrich the clinical utility of blood-based “liquid biopsies”.
Extending this platelet liquid biopsy notion may even help predict response to targeted therapies. For example, in ALK-rearranged non-small-cell lung cancer, exosome-mediated transfer of tumor-derived EML4-ALK rearranged RNA from cancer cells to platelets is an early predictor of resistance to ALK inhibitors (Nilsson et al., 2016). The platelet proteome may be an equally opportune source of both diagnostic and predictive biomarkers. Platelets contain 40 to 100 times as much TGF-β as other cells (Meyer et al., 2012). It is also well known that platelets are the largest reservoir of VEGF in the human body and contain over 95% of the VEGF in healthy adults. The amount of free circulating VEGF in the liquid fraction of blood is miniscule by comparison. In cancer patients, platelet VEGF is markedly higher (6.5 to 28.2 fold) than VEGF concentrations in other relevant body compartments, even compared to the tumor itself. It has been suggested that platelets scavenge VEGF produced by other cellular sources, including tumor cells, since the VEGF concentration in platelets increases over time as long as the VEGF source is present (Kut et al., 2007). Thus, thrombocytosis may be an ominous prognosticator because it represents a massive expansion of the carrying capacity for VEGF in the body. Further research is needed to determine how platelets contribute to VEGF upregulation in cancer and whether the concentration of tumor-derived platelet VEGF impacts therapeutic response to anti-VEGF therapy. Interestingly, thrombocytosis has been identified as an independent adverse prognostic factor for overall survival among patients with metastatic renal cell carcinoma who were treated with VEGF-targeted agents including sunitinib, sorafenib, and bevacizumab (Heng et al., 2009). Platelets from patients receiving biweekly bevacizumab for the treatment of renal cell or colorectal carcinoma take up bevacizumab in a dose-dependent and durable manner (Verheul et al., 2007). These and related findings could explain some of the variability observed in the therapeutic index of and the unique toxicities associated with bevacizumab based therapy. Experiments in mice also demonstrated that platelet levels of angiogenic factors, while relatively constant and stable under physiologic conditions, selectively increase in the presence of even microscopic tumors (Klement et al., 2009). Clinical studies also revealed that cancer patients (breast, colorectal, prostate, renal, ovarian, lymphoma) have elevated platelet angiogenesis regulator levels compared to healthy controls. These factors include: VEGF, ANGPT-1, MMP2, platelet factor-4 (PF-4), and PDGF (Yan et al., 2014). Accordingly, fluctuations in the platelet proteome may serve as surrogate markers of tumor activity. The finding that the platelet VEGF load positively correlates with tumor growth kinetics among breast cancer patients may provide one example of this relationship (Salgado et al., 2001). However, anti-VEGF therapies have only shown modest improvements in time-to-progression and response rates in metastatic breast cancer, underscoring the fact that biomarkers and therapeutic targets are often paradoxically different.
Mindful of the cancer-platelet role, global ultrastructural changes in platelet morphology in cancer may coincide with alterations in the platelet transcriptome and proteome. Using electron cryotomography to examine platelets from healthy donors and from patients with either a benign ovarian mass or ovarian cancer, we discovered major morphological alterations in the microtubules, mitochondria, storage granules and open canalicular system of platelets isolated from ovarian cancer patients compared to healthy donors as well as women with benign adnexal pathology (Wang et al., 2015). Collectively, these data provide substantial basis for incorporating platelet biomarkers into “liquid biopsy” development. Their concentration of tumor-derived proteins as well as spliced and unspliced mRNA transcripts may improve on some cfDNA-based shortcomings (e.g., high levels of normal DNA during inflammation and injury could dilute ctDNA or result in detection of non-progressing benign lesions). However, as with ctDNA, appropriate analytic and clinical validity will need to be established. Foremost, isolation of pure platelet samples from whole blood is crucial for the study of platelet gene expression. The main obstacles to overcome include (1) platelet activation; (2) leukocyte and red blood cell contamination, and (3) time-dependent platelet mRNA degradation (Amisten, 2012). Methodology will need to ensure inhibition of platelet activation during leukocyte removal by filtration followed by magnetic bead-depletion of residual contaminating leukocytes and red blood cells. Additionally, considering that leukocytes have 10,000 times more RNA than platelets, protocols will need to set high standards for platelet purity along with bioinformatics analysis (Rolf et al., 2005). Only once analytic validity is established should we move forward with incorporating platelet- and other blood-based biomarkers into future prospective clinical trials.
7. CONCLUDING REMARKS
This review highlights the vital role of platelets in cancer. Our knowledge of the mechanisms by which platelets and thrombocytosis contribute to tumor growth and metastasis has substantially increased since the first experimental in vivo evidence by Gasic in the 1960s (Gasic et al., 1968). It has become evident that platelets are not merely bystander cells in the circulation, but rather functional players in primary tumor growth and in all steps of the metastatic process. They infiltrate into the tumor microenvironment to directly interact with cancer cells. In the circulation, platelets protect CTCs from the deadly attack of the immune system and other pro-apoptotic stimuli. Platelets help CTCs to attach to the endothelium and provide signals to establish a pre-metastatic niche. They even influence the sensitivity of chemo- and other targeted therapies in cancer patients. Approaches to target platelet-tumor cell interaction or reduce platelet counts have been undertaken, but these often interfere with their normal physiological role and can result in life-threatening bleeding complications. However, developing methods to specifically target platelet interaction with tumor cells without interfering with normal platelet functions could provide an exciting advance in the treatment of cancer patients, especially in the metastatic setting. Therefore, investigation of platelets and their influences on the coagulation system, their surface receptors and their uptake and release functions will aid to elucidate their roles in metastatic dissemination and tumor angiogenesis. Moreover, the role of platelets in intratumoral inflammation and impairing drug response offers unique opportunities for improving clinical outcomes. The inclusion of bioinformatics approaches that account for both beneficial and negative platelet influences on cancer will undoubtedly help improve selectively targeted interventions moving forward. Ultimately, the lifeline that platelets provide to cancer deserves significant attention in order to fully reveal their complex roles and contributions and any associated selective targeting challenges.
Acknowledgments
Portions of this work were supported by the National Institutes of Health (CA177909, P30 CA016672, CA109298, UH3TR000943, P50 CA083639, P50 CA098258, and R35 CA209904), Cancer Prevention and Research Institute of Texas (RP110595, RP120214), the Ovarian and Colorectal Cancer Moon Shots, Ovarian Cancer Research Fund, Inc. (Program Project Development Grant), The Judi A Rees ovarian cancer research fund, Mr. and Mrs. Daniel P. Gordon, the Blanton-Davis Ovarian Cancer Research Program, American Cancer Society Research Professor Award, and the Frank McGraw Memorial Chair in Cancer Research. M.H. was supported by a fellowship of the Deutsche Forschungsgemeinschaft (DFG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. The Lancet Oncology. 2012;13:518–527. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- Amisten S. A rapid and efficient platelet purification protocol for platelet gene expression studies. Methods in molecular biology. 2012;788:155–172. doi: 10.1007/978-1-61779-307-3_12. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Developmental cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Ataga KI, Kutlar A, Kanter J, Liles D, Cancado R, Friedrisch J, Guthrie TH, Knight-Madden J, Alvarez OA, Gordeuk VR, et al. Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. The New England journal of medicine. 2017;376:429–439. doi: 10.1056/NEJMoa1611770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azab AK, Quang P, Azab F, Pitsillides C, Thompson B, Chonghaile T, Patton JT, Maiso P, Monrose V, Sacco A, et al. P-selectin glycoprotein ligand regulates the interaction of multiple myeloma cells with the bone marrow microenvironment. Blood. 2012;119:1468–1478. doi: 10.1182/blood-2011-07-368050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey SE, Ukoumunne OC, Shephard E, Hamilton W. How useful is thrombocytosis in predicting an underlying cancer in primary care? a systematic review. Family practice. 2016 doi: 10.1093/fampra/cmw100. [DOI] [PubMed] [Google Scholar]
- Bakewell SJ, Nestor P, Prasad S, Tomasson MH, Dowland N, Mehrotra M, Scarborough R, Kanter J, Abe K, Phillips D, Weilbaecher KN. Platelet and osteoclast beta3 integrins are critical for bone metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14205–14210. doi: 10.1073/pnas.2234372100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambace NM, Holmes CE. The platelet contribution to cancer progression. Journal of thrombosis and haemostasis : JTH. 2011;9:237–249. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- Bambace NM, Levis JE, Holmes CE. The effect of P2Y-mediated platelet activation on the release of VEGF and endostatin from platelets. Platelets. 2010;21:85–93. doi: 10.3109/09537100903470298. [DOI] [PubMed] [Google Scholar]
- Battinelli EM, Markens BA, Italiano JE., Jr Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood. 2011;118:1359–1369. doi: 10.1182/blood-2011-02-334524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battinelli EM, Markens BA, Kulenthirarajan RA, Machlus KR, Flaumenhaft R, Italiano JE., Jr Anticoagulation inhibits tumor cell-mediated release of platelet angiogenic proteins and diminishes platelet angiogenic response. Blood. 2014;123:101–112. doi: 10.1182/blood-2013-02-485011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg. 2005;31:674–686. doi: 10.1111/j.1524-4725.2005.31612. discussion 686. [DOI] [PubMed] [Google Scholar]
- Becker KA, Beckmann N, Adams C, Hessler G, Kramer M, Gulbins E, Carpinteiro A. Melanoma cell metastasis via P-selectin-mediated activation of acid sphingomyelinase in platelets. Clinical & experimental metastasis. 2017;34:25–35. doi: 10.1007/s10585-016-9826-6. [DOI] [PubMed] [Google Scholar]
- Berg C, Hammarstrom S, Herbertsson H, Lindstrom E, Svensson AC, Soderstrom M, Tengvall P, Bengtsson T. Platelet-induced growth of human fibroblasts is associated with an increased expression of 5-lipoxygenase. Thrombosis and haemostasis. 2006;96:652–659. [PubMed] [Google Scholar]
- Best MG, Sol N, Kooi I, Tannous J, Westerman BA, Rustenburg F, Schellen P, Verschueren H, Post E, Koster J, et al. RNA-Seq of Tumor-Educated Platelets Enables Blood-Based Pan-Cancer, Multiclass, and Molecular Pathway Cancer Diagnostics. Cancer cell. 2015;28:666–676. doi: 10.1016/j.ccell.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. Jama. 2005;293:715–722. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- Blom JW, Vanderschoot JP, Oostindier MJ, Osanto S, van der Meer FJ, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. Journal of thrombosis and haemostasis : JTH. 2006;4:529–535. doi: 10.1111/j.1538-7836.2006.01804.x. [DOI] [PubMed] [Google Scholar]
- Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3352–3357. doi: 10.1073/pnas.061615598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottsford-Miller J, Choi HJ, Dalton HJ, Stone RL, Cho MS, Haemmerle M, Nick AM, Pradeep S, Zand B, Previs RA, et al. Differential platelet levels affect response to taxane-based therapy in ovarian cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21:602–610. doi: 10.1158/1078-0432.CCR-14-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucharaba A, Serre CM, Gres S, Saulnier-Blache JS, Bordet JC, Guglielmi J, Clezardin P, Peyruchaud O. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. The Journal of clinical investigation. 2004;114:1714–1725. doi: 10.1172/JCI22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn J, Gerdes AM, Macrae F, Mecklin JP, Moeslein G, Olschwang S, Eccles D, Evans DG, Maher ER, Bertario L, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081–2087. doi: 10.1016/S0140-6736(11)61049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnouf T, Goubran HA, Chou ML, Devos D, Radosevic M. Platelet microparticles: detection and assessment of their paradoxical functional roles in disease and regenerative medicine. Blood reviews. 2014;28:155–166. doi: 10.1016/j.blre.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood. 2004;104:397–401. doi: 10.1182/blood-2004-02-0434. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauwenberghs S, Feijge MA, Harper AG, Sage SO, Curvers J, Heemskerk JW. Shedding of procoagulant microparticles from unstimulated platelets by integrin-mediated destabilization of actin cytoskeleton. FEBS Lett. 2006;580:5313–5320. doi: 10.1016/j.febslet.2006.08.082. [DOI] [PubMed] [Google Scholar]
- Cecchetti L, Tolley ND, Michetti N, Bury L, Weyrich AS, Gresele P. Megakaryocytes differentially sort mRNAs for matrix metalloproteinases and their inhibitors into platelets: a mechanism for regulating synthetic events. Blood. 2011;118:1903–1911. doi: 10.1182/blood-2010-12-324517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. Jama. 2009;302:649–658. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi KR. The tumour trail left in blood. Nature. 2016;532:269–271. doi: 10.1038/532269a. [DOI] [PubMed] [Google Scholar]
- Cho MS, Bottsford-Miller J, Vasquez HG, Stone R, Zand B, Kroll MH, Sood AK, Afshar-Kharghan V. Platelets increase the proliferation of ovarian cancer cells. Blood. 2012;120:4869–4872. doi: 10.1182/blood-2012-06-438598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MS, Noh K, Haemmerle M, Li D, Park H, Hu Q, Hisamatsu T, Mitamura T, Mak SLC, Kunapuli S, et al. Role of ADP receptors on platelets in the growth of ovarian cancer. Blood. 2017;130:1235–1242. doi: 10.1182/blood-2017-02-769893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill AE, Newcomb PA, Campbell PT, Burnett-Hartman AN, Adams SV, Poole EM, Potter JD, Ulrich CM. Prediagnostic non-steroidal anti-inflammatory drug use and survival after diagnosis of colorectal cancer. Gut. 2011;60:491–498. doi: 10.1136/gut.2010.221143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee CS. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- Cooke NM, Egan K, McFadden S, Grogan L, Breathnach OS, O'Leary J, Hennessy BT, Kenny D. Increased platelet reactivity in patients with late-stage metastatic cancer. Cancer medicine. 2013;2:564–570. doi: 10.1002/cam4.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M, McIntosh K, Bushell T, Sloan G, Plevin R. Proteinase-activated receptors (PARs) as targets for antiplatelet therapy. Biochemical Society transactions. 2016;44:606–612. doi: 10.1042/BST20150282. [DOI] [PubMed] [Google Scholar]
- Damman P, Woudstra P, Kuijt WJ, de Winter RJ, James SK. P2Y12 platelet inhibition in clinical practice. Journal of thrombosis and thrombolysis. 2012;33:143–153. doi: 10.1007/s11239-011-0667-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danckwardt S, Hentze MW, Kulozik AE. Pathologies at the nexus of blood coagulation and inflammation: thrombin in hemostasis, cancer, and beyond. Journal of molecular medicine. 2013;91:1257–1271. doi: 10.1007/s00109-013-1074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leval X, Benoit V, Delarge J, Julemont F, Masereel B, Pirotte B, Merville MP, David JL, Dogne JM. Pharmacological evaluation of the novel thromboxane modulator BM-567 (II/II). Effects of BM-567 on osteogenic sarcoma-cell-induced platelet aggregation. Prostaglandins, leukotrienes, and essential fatty acids. 2003;68:55–59. doi: 10.1016/s0952-3278(02)00235-1. [DOI] [PubMed] [Google Scholar]
- Demers M, Ho-Tin-Noe B, Schatzberg D, Yang JJ, Wagner DD. Increased efficacy of breast cancer chemotherapy in thrombocytopenic mice. Cancer research. 2011;71:1540–1549. doi: 10.1158/0008-5472.CAN-10-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilly AK, Ekambaram P, Guo Y, Cai Y, Tucker SC, Fridman R, Kandouz M, Honn KV. Platelet-type 12-lipoxygenase induces MMP9 expression and cellular invasion via activation of PI3K/Akt/NF-kappaB. International journal of cancer. 2013;133:1784–1791. doi: 10.1002/ijc.28165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkla S, van Cranenbroek B, van der Heijden WA, He X, Wallbrecher R, Dumitriu IE, van der Ven AJ, Bosman GJ, Koenen HJ, Joosten I. Platelet microparticles inhibit IL-17 production by regulatory T cells through P-selectin. Blood. 2016;127:1976–1986. doi: 10.1182/blood-2015-04-640300. [DOI] [PubMed] [Google Scholar]
- Dorff TB, Goldman B, Pinski JK, Mack PC, Lara PN, Jr, Van Veldhuizen PJ, Jr, Quinn DI, Vogelzang NJ, Thompson IM, Jr, Hussain MH. Clinical and correlative results of SWOG S0354: a phase II trial of CNTO328 (siltuximab), a monoclonal antibody against interleukin-6, in chemotherapy-pretreated patients with castration-resistant prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:3028–3034. doi: 10.1158/1078-0432.CCR-09-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovizio M, Maier TJ, Alberti S, Di Francesco L, Marcantoni E, Munch G, John CM, Suess B, Sgambato A, Steinhilber D, Patrignani P. Pharmacological inhibition of platelet-tumor cell cross-talk prevents platelet-induced overexpression of cyclooxygenase-2 in HT29 human colon carcinoma cells. Molecular pharmacology. 2013;84:25–40. doi: 10.1124/mol.113.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan K, Crowley D, Smyth P, O'Toole S, Spillane C, Martin C, Gallagher M, Canney A, Norris L, Conlon N, et al. Platelet adhesion and degranulation induce pro-survival and pro-angiogenic signalling in ovarian cancer cells. PloS one. 2011;6:e26125. doi: 10.1371/journal.pone.0026125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichinger S, Heinze G, Kyrle PA. D-dimer levels over time and the risk of recurrent venous thromboembolism: an update of the Vienna prediction model. Journal of the American Heart Association. 2014;3:e000467. doi: 10.1161/JAHA.113.000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwood PC, Morgan G, Pickering JE, Galante J, Weightman AL, Morris D, Kelson M, Dolwani S. Aspirin in the Treatment of Cancer: Reductions in Metastatic Spread and in Mortality: A Systematic Review and Meta-Analyses of Published Studies. PloS one. 2016;11:e0152402. doi: 10.1371/journal.pone.0152402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etulain J, Mena HA, Negrotto S, Schattner M. Stimulation of PAR-1 or PAR-4 promotes similar pattern of VEGF and endostatin release and pro-angiogenic responses mediated by human platelets. Platelets. 2015;26:799–804. doi: 10.3109/09537104.2015.1051953. [DOI] [PubMed] [Google Scholar]
- Fabre JE, Nguyen M, Latour A, Keifer JA, Audoly LP, Coffman TM, Koller BH. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nature medicine. 1999;5:1199–1202. doi: 10.1038/13522. [DOI] [PubMed] [Google Scholar]
- Feng S, Kroll MH, Nick AM, Sood AK, Afshar-Kharghan V. Platelets are not hyperreactive in patients with ovarian cancer. Platelets. 2016;27:716–718. doi: 10.3109/09537104.2016.1174204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firbas C, Siller-Matula JM, Jilma B. Targeting von Willebrand factor and platelet glycoprotein Ib receptor. Expert review of cardiovascular therapy. 2010;8:1689–1701. doi: 10.1586/erc.10.154. [DOI] [PubMed] [Google Scholar]
- Flaumenhaft R, Dilks JR, Richardson J, Alden E, Patel-Hett SR, Battinelli E, Klement GL, Sola-Visner M, Italiano JE., Jr Megakaryocyte-derived microparticles: direct visualization and distinction from platelet-derived microparticles. Blood. 2009;113:1112–1121. doi: 10.1182/blood-2008-06-163832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd JS, Serebruany VL. Prasugrel as a potential cancer promoter: review of the unpublished data. Archives of internal medicine. 2010;170:1078–1080. doi: 10.1001/archinternmed.2010.154. [DOI] [PubMed] [Google Scholar]
- Fox JE, Austin CD, Boyles JK, Steffen PK. Role of the membrane skeleton in preventing the shedding of procoagulant-rich microvesicles from the platelet plasma membrane. J Cell Biol. 1990;111:483–493. doi: 10.1083/jcb.111.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyssinet JM, Toti F. Formation of procoagulant microparticles and properties. Thrombosis research. 2010;125(Suppl 1):S46–48. doi: 10.1016/j.thromres.2010.01.036. [DOI] [PubMed] [Google Scholar]
- Gao C, Boylan B, Fang J, Wilcox DA, Newman DK, Newman PJ. Heparin promotes platelet responsiveness by potentiating alphaIIbbeta3-mediated outside-in signaling. Blood. 2011;117:4946–4952. doi: 10.1182/blood-2010-09-307751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasic GJ, Gasic TB, Stewart CC. Antimetastatic effects associated with platelet reduction. Proceedings of the National Academy of Sciences of the United States of America. 1968;61:46–52. doi: 10.1073/pnas.61.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawaz M, Vogel S. Platelets in tissue repair: control of apoptosis and interactions with regenerative cells. Blood. 2013;122:2550–2554. doi: 10.1182/blood-2013-05-468694. [DOI] [PubMed] [Google Scholar]
- George JN, Pickett EB, Saucerman S, McEver RP, Kunicki TJ, Kieffer N, Newman PJ. Platelet surface glycoproteins. Studies on resting and activated platelets and platelet membrane microparticles in normal subjects, and observations in patients during adult respiratory distress syndrome and cardiac surgery. The Journal of clinical investigation. 1986;78:340–348. doi: 10.1172/JCI112582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George JN, Thoi LL, McManus LM, Reimann TA. Isolation of human platelet membrane microparticles from plasma and serum. Blood. 1982;60:834–840. [PubMed] [Google Scholar]
- Goubran HA, Burnouf T, Stakiw J, Seghatchian J. Platelet microparticle: a sensitive physiological "fine tuning" balancing factor in health and disease. Transfus Apher Sci. 2015;52:12–18. doi: 10.1016/j.transci.2014.12.015. [DOI] [PubMed] [Google Scholar]
- Grainger DJ, Mosedale DE, Metcalfe JC. TGF-beta in blood: a complex problem. Cytokine Growth Factor Rev. 2000;11:133–145. doi: 10.1016/s1359-6101(99)00037-4. [DOI] [PubMed] [Google Scholar]
- Gurbel PA, Bliden KP, Turner SE, Tantry US, Gesheff MG, Barr TP, Covic L, Kuliopulos A. Cell-Penetrating Pepducin Therapy Targeting PAR1 in Subjects With Coronary Artery Disease. Arteriosclerosis, thrombosis, and vascular biology. 2016;36:189–197. doi: 10.1161/ATVBAHA.115.306777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemmerle M, Bottsford-Miller J, Pradeep S, Taylor ML, Choi HJ, Hansen JM, Dalton HJ, Stone RL, Cho MS, Nick AM, et al. FAK regulates platelet extravasation and tumor growth after antiangiogenic therapy withdrawal. The Journal of clinical investigation. 2016;126:1885–1896. doi: 10.1172/JCI85086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemmerle M, Taylor ML, Gutschner T, Pradeep S, Cho MS, Sheng J, Lyons YM, Nagaraja AS, Dood RL, Wen Y, et al. Platelets reduce anoikis and promote metastasis by activating YAP1 signaling. Nat Commun. 2017;8:310. doi: 10.1038/s41467-017-00411-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Archives of internal medicine. 2000;160:809–815. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- Helley D, Banu E, Bouziane A, Banu A, Scotte F, Fischer AM, Oudard S. Platelet microparticles: a potential predictive factor of survival in hormone-refractory prostate cancer patients treated with docetaxel-based chemotherapy. Eur Urol. 2009;56:479–484. doi: 10.1016/j.eururo.2008.06.038. [DOI] [PubMed] [Google Scholar]
- Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- Herzog BH, Fu J, Wilson SJ, Hess PR, Sen A, McDaniel JM, Pan Y, Sheng M, Yago T, Silasi-Mansat R, et al. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature. 2013;502:105–109. doi: 10.1038/nature12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettiarachchi RJ, Smorenburg SM, Ginsberg J, Levine M, Prins MH, Buller HR. Do heparins do more than just treat thrombosis? The influence of heparins on cancer spread. Thrombosis and haemostasis. 1999;82:947–952. [PubMed] [Google Scholar]
- Ho-Tin-Noe B, Carbo C, Demers M, Cifuni SM, Goerge T, Wagner DD. Innate immune cells induce hemorrhage in tumors during thrombocytopenia. The American journal of pathology. 2009a;175:1699–1708. doi: 10.2353/ajpath.2009.090460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho-Tin-Noe B, Goerge T, Cifuni SM, Duerschmied D, Wagner DD. Platelet granule secretion continuously prevents intratumor hemorrhage. Cancer research. 2008;68:6851–6858. doi: 10.1158/0008-5472.CAN-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho-Tin-Noe B, Goerge T, Wagner DD. Platelets: guardians of tumor vasculature. Cancer research. 2009b;69:5623–5626. doi: 10.1158/0008-5472.CAN-09-1370. [DOI] [PubMed] [Google Scholar]
- Holmes CE, Jasielec J, Levis JE, Skelly J, Muss HB. Initiation of aspirin therapy modulates angiogenic protein levels in women with breast cancer receiving tamoxifen therapy. Clinical and translational science. 2013;6:386–390. doi: 10.1111/cts.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hron G, Kollars M, Weber H, Sagaster V, Quehenberger P, Eichinger S, Kyrle PA, Weltermann A. Tissue factor-positive microparticles: cellular origin and association with coagulation activation in patients with colorectal cancer. Thrombosis and haemostasis. 2007;97:119–123. [PubMed] [Google Scholar]
- Hu Q, Hisamatsu T, Hammerle M, Cho MS, Pradeep S, Rupaimoole R, Rodriguez-Aguayo C, Lopez-Berestein G, Wong ST, Sood AK, Afshar-Kharghan V. Role of platelet-derived Tgfbeta1 in the progression of ovarian cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017 doi: 10.1158/1078-0432.CCR-16-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Wang M, Cho MS, Wang C, Nick AM, Thiagarajan P, Aung FM, Han X, Sood AK, Afshar-Kharghan V. Lipid profile of platelets and platelet-derived microparticles in ovarian cancer. BBA Clin. 2016;6:76–81. doi: 10.1016/j.bbacli.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson L. Ovarian cancer: FAK - new target for antiangiogenic therapy. Nature reviews Clinical oncology. 2016;13:328. [Google Scholar]
- Ikeda M, Furukawa H, Imamura H, Shimizu J, Ishida H, Masutani S, Tatsuta M, Satomi T. Poor prognosis associated with thrombocytosis in patients with gastric cancer. Annals of surgical oncology. 2002;9:287–291. doi: 10.1007/BF02573067. [DOI] [PubMed] [Google Scholar]
- Italiano JE, Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement GL. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Russell S, Ware J. Platelet glycoprotein VI facilitates experimental lung metastasis in syngenic mouse models. Journal of thrombosis and haemostasis : JTH. 2009;7:1713–1717. doi: 10.1111/j.1538-7836.2009.03559.x. [DOI] [PubMed] [Google Scholar]
- Jain S, Zuka M, Liu J, Russell S, Dent J, Guerrero JA, Forsyth J, Maruszak B, Gartner TK, Felding-Habermann B, Ware J. Platelet glycoprotein Ib alpha supports experimental lung metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9024–9028. doi: 10.1073/pnas.0700625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowska-Wieczorek A, Majka M, Kijowski J, Baj-Krzyworzeka M, Reca R, Turner AR, Ratajczak J, Emerson SG, Kowalska MA, Ratajczak MZ. Platelet-derived microparticles bind to hematopoietic stem/progenitor cells and enhance their engraftment. Blood. 2001;98:3143–3149. doi: 10.1182/blood.v98.10.3143. [DOI] [PubMed] [Google Scholar]
- Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nature medicine. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurasz P, North S, Venner P, Radomski MW. Matrix metalloproteinase-2 contributes to increased platelet reactivity in patients with metastatic prostate cancer: a preliminary study. Thrombosis research. 2003;112:59–64. doi: 10.1016/j.thromres.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Kahner BN, Shankar H, Murugappan S, Prasad GL, Kunapuli SP. Nucleotide receptor signaling in platelets. Journal of thrombosis and haemostasis : JTH. 2006;4:2317–2326. doi: 10.1111/j.1538-7836.2006.02192.x. [DOI] [PubMed] [Google Scholar]
- Kakkar A, Hedges R, Williamson R, Kakkar V. Perioperative heparin-therapy inhibits late death from metastatic cancer. International journal of oncology. 1995;6:885–888. doi: 10.3892/ijo.6.4.885. [DOI] [PubMed] [Google Scholar]
- Kakkar AK, Levine MN, Kadziola Z, Lemoine NR, Low V, Patel HK, Rustin G, Thomas M, Quigley M, Williamson RC. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (FAMOUS) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:1944–1948. doi: 10.1200/JCO.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Kerbel RS. Tumor angiogenesis. The New England journal of medicine. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr BA, McCabe NP, Feng W, Byzova TV. Platelets govern pre-metastatic tumor communication to bone. Oncogene. 2013;32:4319–4324. doi: 10.1038/onc.2012.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:4839–4847. doi: 10.1200/JCO.2009.22.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. Journal of thrombosis and haemostasis : JTH. 2007;5:632–634. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- Khorana AA, Francis CW, Culakova E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104:2822–2829. doi: 10.1002/cncr.21496. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Choi GS, Park JS, Park S, Kawai K, Watanabe T. Clinical significance of thrombocytosis before preoperative chemoradiotherapy in rectal cancer: predicting pathologic tumor response and oncologic outcome. Annals of surgical oncology. 2015;22:513–519. doi: 10.1245/s10434-014-3988-8. [DOI] [PubMed] [Google Scholar]
- Kim HK, Song KS, Chung JH, Lee KR, Lee SN. Platelet microparticles induce angiogenesis in vitro. British journal of haematology. 2004;124:376–384. doi: 10.1046/j.1365-2141.2003.04773.x. [DOI] [PubMed] [Google Scholar]
- Kim HK, Song KS, Park YS, Kang YH, Lee YJ, Lee KR, Kim HK, Ryu KW, Bae JM, Kim S. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: possible role of a metastasis predictor. Eur J Cancer. 2003;39:184–191. doi: 10.1016/s0959-8049(02)00596-8. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Borsig L, Varki NM, Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9325–9330. doi: 10.1073/pnas.95.16.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement GL, Yip TT, Cassiola F, Kikuchi L, Cervi D, Podust V, Italiano JE, Wheatley E, Abou-Slaybi A, Bender E, et al. Platelets actively sequester angiogenesis regulators. Blood. 2009;113:2835–2842. doi: 10.1182/blood-2008-06-159541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerk CP, Smorenburg SM, Otten HM, Lensing AW, Prins MH, Piovella F, Prandoni P, Bos MM, Richel DJ, van Tienhoven G, Buller HR. The effect of low molecular weight heparin on survival in patients with advanced malignancy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:2130–2135. doi: 10.1200/JCO.2005.03.134. [DOI] [PubMed] [Google Scholar]
- Kopp HG, Placke T, Salih HR. Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer research. 2009;69:7775–7783. doi: 10.1158/0008-5472.CAN-09-2123. [DOI] [PubMed] [Google Scholar]
- Kut C, Mac Gabhann F, Popel AS. Where is VEGF in the body? A meta-analysis of VEGF distribution in cancer. British journal of cancer. 2007;97:978–985. doi: 10.1038/sj.bjc.6603923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle M, Begum S, Hynes RO. Platelets guide the formation of early metastatic niches. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E3053–3061. doi: 10.1073/pnas.1411082111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubli H, Borsig L. Selectins promote tumor metastasis. Seminars in cancer biology. 2010;20:169–177. doi: 10.1016/j.semcancer.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Leader A, Zelikson-Saporta R, Pereg D, Spectre G, Rozovski U, Raanani P, Hermoni D, Lishner M. The Effect of Combined Aspirin and Clopidogrel Treatment on Cancer Incidence. The American journal of medicine. 2017;130:826–832. doi: 10.1016/j.amjmed.2017.01.022. [DOI] [PubMed] [Google Scholar]
- Leblanc R, Lee SC, David M, Bordet JC, Norman DD, Patil R, Miller D, Sahay D, Ribeiro J, Clezardin P, et al. Interaction of platelet-derived autotaxin with tumor integrin alphaVbeta3 controls metastasis of breast cancer cells to bone. Blood. 2014;124:3141–3150. doi: 10.1182/blood-2014-04-568683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, Rickles FR, Julian JA, Haley S, Kovacs MJ, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. The New England journal of medicine. 2003;349:146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- Lee AY, Rickles FR, Julian JA, Gent M, Baker RI, Bowden C, Kakkar AK, Prins M, Levine MN. Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:2123–2129. doi: 10.1200/JCO.2005.03.133. [DOI] [PubMed] [Google Scholar]
- Lever R, Page CP. Novel drug development opportunities for heparin. Nature reviews Drug discovery. 2002;1:140–148. doi: 10.1038/nrd724. [DOI] [PubMed] [Google Scholar]
- Levin J, Conley CL. Thrombocytosis Associated with Malignant Disease. Archives of internal medicine. 1964;114:497–500. doi: 10.1001/archinte.1964.03860100079008. [DOI] [PubMed] [Google Scholar]
- Li J, Ai Y, Wang L, Bu P, Sharkey CC, Wu Q, Wun B, Roy S, Shen X, King MR. Targeted drug delivery to circulating tumor cells via platelet membrane-functionalized particles. Biomaterials. 2016;76:52–65. doi: 10.1016/j.biomaterials.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Ren M, Chen N, Luo M, Deng X, Xia J, Yu G, Liu J, He B, Zhang X, et al. Presence of intratumoral platelets is associated with tumor vessel structure and metastasis. BMC cancer. 2014;14:167. doi: 10.1186/1471-2407-14-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Yan X, Pan Y, Wang Y, Wang N, Li L, Liu Y, Chen X, Zhang CY, Gu H, Zen K. MicroRNA-223 delivered by platelet-derived microvesicles promotes lung cancer cell invasion via targeting tumor suppressor EPB41L3. Mol Cancer. 2015;14:58. doi: 10.1186/s12943-015-0327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, Imamura Y, Qian ZR, Baba Y, Shima K, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. The New England journal of medicine. 2012;367:1596–1606. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Elliott SN, Cirino G, Buret A, Ignarro LJ, Wallace JL. Platelets modulate gastric ulcer healing: role of endostatin and vascular endothelial growth factor release. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6470–6475. doi: 10.1073/pnas.111150798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macbeth F, Noble S, Evans J, Ahmed S, Cohen D, Hood K, Knoyle D, Linnane S, Longo M, Moore B, et al. Randomized Phase III Trial of Standard Therapy Plus Low Molecular Weight Heparin in Patients With Lung Cancer: FRAGMATIC Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34:488–494. doi: 10.1200/JCO.2015.64.0268. [DOI] [PubMed] [Google Scholar]
- Mammadova-Bach E, Zigrino P, Brucker C, Bourdon C, Freund M, De Arcangelis A, Abrams SI, Orend G, Gachet C, Mangin PH. Platelet integrin alpha6beta1 controls lung metastasis through direct binding to cancer cell-derived ADAM9. JCI insight. 2016;1:e88245. doi: 10.1172/jci.insight.88245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. The New England journal of medicine. 2014;371:2155–2166. doi: 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menter DG, Harkins C, Onoda J, Riorden W, Sloane BF, Taylor JD, Honn KV. Inhibition of tumor cell induced platelet aggregation by prostacyclin and carbacyclin: an ultrastructural study. Invasion Metastasis. 1987a;7:109–128. [PubMed] [Google Scholar]
- Menter DG, Hatfield JS, Harkins C, Sloane BF, Taylor JD, Crissman JD, Honn KV. Tumor cell-platelet interactions in vitro and their relationship to in vivo arrest of hematogenously circulating tumor cells. Clinical & experimental metastasis. 1987b;5:65–78. doi: 10.1007/BF00116627. [DOI] [PubMed] [Google Scholar]
- Metharom P, Berndt MC, Baker RI, Andrews RK. Current state and novel approaches of antiplatelet therapy. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:1327–1338. doi: 10.1161/ATVBAHA.114.303413. [DOI] [PubMed] [Google Scholar]
- Meyer A, Wang W, Qu J, Croft L, Degen JL, Coller BS, Ahamed J. Platelet TGF-beta1 contributions to plasma TGF-beta1, cardiac fibrosis, and systolic dysfunction in a mouse model of pressure overload. Blood. 2012;119:1064–1074. doi: 10.1182/blood-2011-09-377648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael JV, Wurtzel JGT, Mao GF, Rao AK, Kolpakov MA, Sabri A, Hoffman NE, Rajan S, Tomar D, Madesh M, et al. Platelet microparticles infiltrating solid tumors transfer miRNAs that suppress tumor growth. Blood. 2017;130:567–580. doi: 10.1182/blood-2016-11-751099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohle R, Green D, Moore MA, Nachman RL, Rafii S. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:663–668. doi: 10.1073/pnas.94.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada de la Rosa C, Radziwon-Balicka A, El-Sikhry H, Seubert J, Ruvolo PP, Radomski MW, Jurasz P. Pharmacologic protein kinase Calpha inhibition uncouples human platelet-stimulated angiogenesis from collagen-induced aggregation. The Journal of pharmacology and experimental therapeutics. 2013;345:15–24. doi: 10.1124/jpet.112.200881. [DOI] [PubMed] [Google Scholar]
- Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, Lal P, Feldman MD, Benencia F, Coukos G. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nature medicine. 2014;20:607–615. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nami N, Feci L, Napoliello L, Giordano A, Lorenzini S, Galeazzi M, Rubegni P, Fimiani M. Crosstalk between platelets and PBMC: New evidence in wound healing. Platelets. 2016;27:143–148. doi: 10.3109/09537104.2015.1048216. [DOI] [PubMed] [Google Scholar]
- Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer research. 1999;59:1295–1300. [PubMed] [Google Scholar]
- Nilsson RJ, Balaj L, Hulleman E, van Rijn S, Pegtel DM, Walraven M, Widmark A, Gerritsen WR, Verheul HM, Vandertop WP, et al. Blood platelets contain tumor-derived RNA biomarkers. Blood. 2011;118:3680–3683. doi: 10.1182/blood-2011-03-344408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson RJ, Karachaliou N, Berenguer J, Gimenez-Capitan A, Schellen P, Teixido C, Tannous J, Kuiper JL, Drees E, Grabowska M, et al. Rearranged EML4-ALK fusion transcripts sequester in circulating blood platelets and enable blood-based crizotinib response monitoring in non-small-cell lung cancer. Oncotarget. 2016;7:1066–1075. doi: 10.18632/oncotarget.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura S, Tandon NN, Nakamura T, Cone J, Fukuhara S, Kambayashi J. High-shear-stress-induced activation of platelets and microparticles enhances expression of cell adhesion molecules in THP-1 and endothelial cells. Atherosclerosis. 2001;158:277–287. doi: 10.1016/s0021-9150(01)00433-6. [DOI] [PubMed] [Google Scholar]
- Pacchiarini L, Zucchella M, Milanesi G, Tacconi F, Bonomi E, Canevari A, Grignani G. Thromboxane production by platelets during tumor cell-induced platelet activation. Invasion Metastasis. 1991;11:102–109. [PubMed] [Google Scholar]
- Palumbo JS, Barney KA, Blevins EA, Shaw MA, Mishra A, Flick MJ, Kombrinck KW, Talmage KE, Souri M, Ichinose A, Degen JL. Factor XIII transglutaminase supports hematogenous tumor cell metastasis through a mechanism dependent on natural killer cell function. Journal of thrombosis and haemostasis : JTH. 2008;6:812–819. doi: 10.1111/j.1538-7836.2008.02938.x. [DOI] [PubMed] [Google Scholar]
- Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirouskova M, Degen JL. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–185. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- Pedersen LM, Milman N. Prognostic significance of thrombocytosis in patients with primary lung cancer. Eur Respir J. 1996;9:1826–1830. doi: 10.1183/09031936.96.09091826. [DOI] [PubMed] [Google Scholar]
- Philippe C, Philippe B, Fouqueray B, Perez J, Lebret M, Baud L. Protection from tumor necrosis factor-mediated cytolysis by platelets. The American journal of pathology. 1993;143:1713–1723. [PMC free article] [PubMed] [Google Scholar]
- Placke T, Orgel M, Schaller M, Jung G, Rammensee HG, Kopp HG, Salih HR. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer research. 2012;72:440–448. doi: 10.1158/0008-5472.CAN-11-1872. [DOI] [PubMed] [Google Scholar]
- Prandoni P, Lensing AW, Buller HR, Carta M, Cogo A, Vigo M, Casara D, Ruol A, ten Cate JW. Comparison of subcutaneous low-molecular-weight heparin with intravenous standard heparin in proximal deep-vein thrombosis. Lancet. 1992;339:441–445. doi: 10.1016/0140-6736(92)91054-c. [DOI] [PubMed] [Google Scholar]
- Qi C, Li B, Guo S, Wei B, Shao C, Li J, Yang Y, Zhang Q, Li J, He X, et al. P-Selectin-Mediated Adhesion between Platelets and Tumor Cells Promotes Intestinal Tumorigenesis in Apc(Min/+) Mice. International journal of biological sciences. 2015a;11:679–687. doi: 10.7150/ijbs.11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi C, Wei B, Zhou W, Yang Y, Li B, Guo S, Li J, Ye J, Li J, Zhang Q, et al. P-selectin-mediated platelet adhesion promotes tumor growth. Oncotarget. 2015b;6:6584–6596. doi: 10.18632/oncotarget.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi CL, Wei B, Ye J, Yang Y, Li B, Zhang QQ, Li JC, He XD, Lan T, Wang LJ. P-selectin-mediated platelet adhesion promotes the metastasis of murine melanoma cells. PloS one. 2014;9:e91320. doi: 10.1371/journal.pone.0091320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachidi S, Metelli A, Riesenberg B, Wu BX, Nelson MH, Wallace C, Paulos CM, Rubinstein MP, Garrett-Mayer E, Hennig M, et al. Platelets subvert T cell immunity against cancer via GARP-TGF[beta] axis. Sci Immunol. 2017;2:eaai7911. doi: 10.1126/sciimmunol.aai7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziwon-Balicka A, Medina C, O'Driscoll L, Treumann A, Bazou D, Inkielewicz-Stepniak I, Radomski A, Jow H, Radomski MW. Platelets increase survival of adenocarcinoma cells challenged with anticancer drugs: mechanisms and implications for chemoresistance. British journal of pharmacology. 2012;167:787–804. doi: 10.1111/j.1476-5381.2012.01991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers MS, Bastiaannet E, Langley RE, van Eijk R, van Vlierberghe RL, Lemmens VE, van Herk-Sukel MP, van Wezel T, Fodde R, Kuppen PJ, et al. Expression of HLA class I antigen, aspirin use, and survival after a diagnosis of colon cancer. JAMA internal medicine. 2014;174:732–739. doi: 10.1001/jamainternmed.2014.511. [DOI] [PubMed] [Google Scholar]
- Riedl J, Kaider A, Marosi C, Prager GW, Eichelberger B, Assinger A, Pabinger I, Panzer S, Ay C. Decreased platelet reactivity in patients with cancer is associated with high risk of venous thromboembolism and poor prognosis. Thrombosis and haemostasis. 2016;117 doi: 10.1160/TH16-02-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riess L. Zur pathologischen Anatomie des Blutes. Arch Anat Physiol Wissensch Med. 1872:237–249. [Google Scholar]
- Rolf N, Knoefler R, Suttorp M, Kluter H, Bugert P. Optimized procedure for platelet RNA profiling from blood samples with limited platelet numbers. Clinical chemistry. 2005;51:1078–1080. doi: 10.1373/clinchem.2005.049486. [DOI] [PubMed] [Google Scholar]
- Rossi JF, Negrier S, James ND, Kocak I, Hawkins R, Davis H, Prabhakar U, Qin X, Mulders P, Berns B. A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. British journal of cancer. 2010;103:1154–1162. doi: 10.1038/sj.bjc.6605872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, Lee R, Belch JF, Wilson M, Mehta Z, Meade TW. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–1612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- Salanova B, Choi M, Rolle S, Wellner M, Luft FC, Kettritz R. Beta2-integrins and acquired glycoprotein IIb/IIIa (GPIIb/IIIa) receptors cooperate in NF-kappaB activation of human neutrophils. The Journal of biological chemistry. 2007;282:27960–27969. doi: 10.1074/jbc.M704039200. [DOI] [PubMed] [Google Scholar]
- Salgado R, Benoy I, Bogers J, Weytjens R, Vermeulen P, Dirix L, Van Marck E. Platelets and vascular endothelial growth factor (VEGF): a morphological and functional study. Angiogenesis. 2001;4:37–43. doi: 10.1023/a:1016611230747. [DOI] [PubMed] [Google Scholar]
- Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: determination of frequency and characteristics. Thrombosis and haemostasis. 2002;87:575–579. [PubMed] [Google Scholar]
- Sasaki K, Kawai K, Tsuno NH, Sunami E, Kitayama J. Impact of preoperative thrombocytosis on the survival of patients with primary colorectal cancer. World journal of surgery. 2012;36:192–200. doi: 10.1007/s00268-011-1329-7. [DOI] [PubMed] [Google Scholar]
- Schleicher RI, Reichenbach F, Kraft P, Kumar A, Lescan M, Todt F, Gobel K, Hilgendorf I, Geisler T, Bauer A, et al. Platelets induce apoptosis via membrane-bound FasL. Blood. 2015;126:1483–1493. doi: 10.1182/blood-2013-12-544445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer cell. 2013;24:130–137. doi: 10.1016/j.ccr.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Schwertz H, Rowley JW, Tolley ND, Campbell RA, Weyrich AS. Assessing protein synthesis by platelets. Methods in molecular biology. 2012;788:141–153. doi: 10.1007/978-1-61779-307-3_11. [DOI] [PubMed] [Google Scholar]
- Serebruany VL, Cherepanov V, Golukhova EZ, Kim MH. The Dual Antiplatelet Therapy Trial after the FDA Update: Noncardiovascular Deaths, Cancer and Optimal Treatment Duration. Cardiology. 2015;132:74–80. doi: 10.1159/000431356. [DOI] [PubMed] [Google Scholar]
- Sierko E, Wojtukiewicz MZ. Inhibition of platelet function: does it offer a chance of better cancer progression control? Seminars in thrombosis and hemostasis. 2007;33:712–721. doi: 10.1055/s-2007-991540. [DOI] [PubMed] [Google Scholar]
- Stein PD, Beemath A, Meyers FA, Skaf E, Sanchez J, Olson RE. Incidence of venous thromboembolism in patients hospitalized with cancer. The American journal of medicine. 2006;119:60–68. doi: 10.1016/j.amjmed.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, Rupairmoole R, Armaiz-Pena GN, Pecot CV, Coward J, et al. Paraneoplastic thrombocytosis in ovarian cancer. The New England journal of medicine. 2012;366:610–618. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki-Inoue K, Kato Y, Inoue O, Kaneko MK, Mishima K, Yatomi Y, Yamazaki Y, Narimatsu H, Ozaki Y. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. The Journal of biological chemistry. 2007;282:25993–26001. doi: 10.1074/jbc.M702327200. [DOI] [PubMed] [Google Scholar]
- Takagi S, Sato S, Oh-hara T, Takami M, Koike S, Mishima Y, Hatake K, Fujita N. Platelets promote tumor growth and metastasis via direct interaction between Aggrus/podoplanin and CLEC-2. PloS one. 2013;8:e73609. doi: 10.1371/journal.pone.0073609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taucher S, Salat A, Gnant M, Kwasny W, Mlineritsch B, Menzel RC, Schmid M, Smola MG, Stierer M, Tausch C, et al. Impact of pretreatment thrombocytosis on survival in primary breast cancer. Thrombosis and haemostasis. 2003;89:1098–1106. [PubMed] [Google Scholar]
- Tesselaar ME, Romijn FP, van der Linden IK, Bertina RM, Osanto S. Microparticle-associated tissue factor activity in cancer patients with and without thrombosis. Journal of thrombosis and haemostasis : JTH. 2009;7:1421–1423. doi: 10.1111/j.1538-7836.2009.03504.x. [DOI] [PubMed] [Google Scholar]
- Trikha M, Zhou Z, Timar J, Raso E, Kennel M, Emmell E, Nakada MT. Multiple roles for platelet GPIIb/IIIa and alphavbeta3 integrins in tumor growth, angiogenesis, and metastasis. Cancer research. 2002;62:2824–2833. [PubMed] [Google Scholar]
- Trousseau A. Lectures on clinical medicine, delivered at the Hotel-Dieu. Paris: 1865. pp. 281–332. [Google Scholar]
- Uhrin P, Zaujec J, Breuss JM, Olcaydu D, Chrenek P, Stockinger H, Fuertbauer E, Moser M, Haiko P, Fassler R, et al. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood. 2010;115:3997–4005. doi: 10.1182/blood-2009-04-216069. [DOI] [PubMed] [Google Scholar]
- Ungerer M, Rosport K, Bultmann A, Piechatzek R, Uhland K, Schlieper P, Gawaz M, Munch G. Novel antiplatelet drug revacept (Dimeric Glycoprotein VI-Fc) specifically and efficiently inhibited collagen-induced platelet aggregation without affecting general hemostasis in humans. Circulation. 2011;123:1891–1899. doi: 10.1161/CIRCULATIONAHA.110.980623. [DOI] [PubMed] [Google Scholar]
- van Doormaal F, Kleinjan A, Berckmans RJ, Mackman N, Manly D, Kamphuisen PW, Richel DJ, Buller HR, Sturk A, Nieuwland R. Coagulation activation and microparticle-associated coagulant activity in cancer patients. An exploratory prospective study. Thrombosis and haemostasis. 2012;108:160–165. doi: 10.1160/TH12-02-0099. [DOI] [PubMed] [Google Scholar]
- van Holten TC, Bleijerveld OB, Wijten P, de Groot PG, Heck AJ, Barendrecht AD, Merkx TH, Scholten A, Roest M. Quantitative proteomics analysis reveals similar release profiles following specific PAR-1 or PAR-4 stimulation of platelets. Cardiovascular research. 2014;103:140–146. doi: 10.1093/cvr/cvu113. [DOI] [PubMed] [Google Scholar]
- Varki A, Kannagi R, Toole BP. Glycosylation Changes in Cancer. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. Cold Spring Harbor (NY): 2009. [PubMed] [Google Scholar]
- Verheul HM, Lolkema MP, Qian DZ, Hilkes YH, Liapi E, Akkerman JW, Pili R, Voest EE. Platelets take up the monoclonal antibody bevacizumab. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:5341–5347. doi: 10.1158/1078-0432.CCR-07-0847. [DOI] [PubMed] [Google Scholar]
- Versteeg HH, Schaffner F, Kerver M, Ellies LG, Andrade-Gordon P, Mueller BM, Ruf W. Protease-activated receptor (PAR) 2, but not PAR1, signaling promotes the development of mammary adenocarcinoma in polyoma middle T mice. Cancer research. 2008;68:7219–7227. doi: 10.1158/0008-5472.CAN-08-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezza R, Roberti R, Nenci GG, Gresele P. Prostaglandin E2 potentiates platelet aggregation by priming protein kinase C. Blood. 1993;82:2704–2713. [PubMed] [Google Scholar]
- Wang R, Stone RL, Kaelber JT, Rochat RH, Nick AM, Vijayan KV, Afshar-Kharghan V, Schmid MF, Dong JF, Sood AK, Chiu W. Electron cryotomography reveals ultrastructure alterations in platelets from patients with ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:14266–14271. doi: 10.1073/pnas.1518628112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sun Y, Li D, Zhang L, Wang K, Zuo Y, Gartner TK, Liu J. Platelet P2Y12 is involved in murine pulmonary metastasis. PloS one. 2013;8:e80780. doi: 10.1371/journal.pone.0080780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware J. Fragmenting the platelet to reduce metastasis. Blood. 2012;120:2779–2780. doi: 10.1182/blood-2012-08-450072. [DOI] [PubMed] [Google Scholar]
- Wiltrout RH, Herberman RB, Zhang SR, Chirigos MA, Ortaldo JR, Green KM, Jr, Talmadge JE. Role of organ-associated NK cells in decreased formation of experimental metastases in lung and liver. Journal of immunology. 1985;134:4267–4275. [PubMed] [Google Scholar]
- Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. The New England journal of medicine. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- Wolf P. The nature and significance of platelet products in human plasma. British journal of haematology. 1967;13:269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- Yan M, Lesyk G, Radziwon-Balicka A, Jurasz P. Pharmacological regulation of platelet factors that influence tumor angiogenesis. Seminars in oncology. 2014;41:370–377. doi: 10.1053/j.seminoncol.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Zhang N, Lou W, Ji F, Qiu L, Tsang BK, Di W. Low molecular weight heparin and cancer survival: clinical trials and experimental mechanisms. Journal of cancer research and clinical oncology. 2016;142:1807–1816. doi: 10.1007/s00432-016-2131-6. [DOI] [PubMed] [Google Scholar]
- Zhang N, Zhang WJ, Cai HQ, Liu HL, Peng L, Li CH, Ye LY, Xu SQ, Yang ZH, Lou JN. Platelet adhesion and fusion to endothelial cell facilitate the metastasis of tumor cell in hypoxia-reoxygenation condition. Clinical & experimental metastasis. 2011;28:1–12. doi: 10.1007/s10585-010-9353-9. [DOI] [PubMed] [Google Scholar]
- Zhang W, Dang S, Hong T, Tang J, Fan J, Bu D, Sun Y, Wang Z, Wisniewski T. A humanized single-chain antibody against beta 3 integrin inhibits pulmonary metastasis by preferentially fragmenting activated platelets in the tumor microenvironment. Blood. 2012;120:2889–2898. doi: 10.1182/blood-2012-04-425207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Metcalf M, Bunnett NW. Biased signaling of protease-activated receptors. Frontiers in endocrinology. 2014;5:67. doi: 10.3389/fendo.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]