Abstract
Amyotrophic lateral sclerosis (ALS) is characterized by a progressive degeneration of upper and lower motor neurons, resulting in fatal paralysis due to denervation of the muscle. Due to genetic, pathological and symptomatic overlap, ALS is now considered a spectrum disease together with frontotemporal dementia (FTD), the second most common cause of dementia in individuals under the age of 65. Interestingly, in both diseases, there is a large prevalence of RNA binding proteins (RBPs) that are mutated and considered disease-causing, or whose dysfunction contribute to disease pathogenesis. The most common shared genetic mutation in ALS/FTD is a hexanucleuotide repeat expansion within intron 1 of C9ORF72 (C9). Three potentially overlapping putative toxic mechanisms causing disease pathogenesis have been proposed: loss of function due to haploinsufficient expression of the C9ORF72 mRNA, gain of function of the repeat RNA aggregates, or RNA foci, and repeat-associated non-ATG-initiated translation (RAN) of the repeat RNA into toxic dipeptide repeats (DPRs). Regardless of the causative mechanism, disease symptoms are ultimately caused by a failure of neurotransmission in three regions: the brain, the spinal cord, and the neuromuscular junction. Here, we review C9 ALS/FTD-associated synaptic dysfunction and aberrant neuronal excitability in these three key regions, focusing on changes in morphology and synapse formation, excitability, and excitotoxicity in patients, animal models, and in vitro models. We compare these deficits to those seen in other forms of ALS and FTD in search of shared pathways, and discuss the potential targeting of synaptic dysfunctions for therapeutic intervention in ALS and FTD patients.
Introduction
The mutation found in the C9ORF72 (C9) gene is characterized by an expanded GGGGCC (G4C2) hexanucleotide repeat expansion (HRE) in its non-coding region on chromosome 9p21 and represents the most common genetic abnormality in frontotemporal dementia (FTD; 10–30%) and amyotrophic lateral sclerosis (ALS; 20–50%) to date (DeJesus-Hernandez et al., 2011; Renton et al., 2011). This discovery strengthened the already known-yet largely neglected-symptomatic and genetic overlap between a uniformly fatal motor neuron disease and the second most common form of early-onset dementia. Extensive research over the past six years on the role of the (G4C2)n repeat expansion in C9ORF72, has led to the proposal of different disease-causing mechanisms for mutant C9: (1) protein loss-of-function due to haploinsufficient expression of C9ORF72 mRNA (Ciura et al., 2013; Therrien et al., 2013; Xiao et al., 2015); (2) toxic RNA gain-of-function (Almeida et al., 2013; Donnelly et al., 2013; Lagier-Tourenne et al., 2013; Lee et al., 2013; Sareen et al., 2013; Zhang et al., 2015); and (3) toxicity caused by repeat-associated non-ATG initiated (RAN) translation, which leads to the accumulation of dipeptide repeat (DPR) proteins (Ash et al., 2013; Gendron et al., 2015; Jovicic et al., 2015; Kwon et al., 2014; May et al., 2014; Mizielinska et al., 2014; Mori et al., 2013; Tran et al., 2015; Wen et al., 2014; Yang et al., 2015; Zhang et al., 2014; Zu et al., 2013). Ongoing studies are investigating the cellular consequences of these causative pathways. As an example, multiple recent studies suggest that there are deficits in nuclear-cytoplasmic trafficking of RNAs and/or proteins due to C9 RNA toxicity or DPR formation (Boeynaems et al., 2016; Freibaum et al., 2015; Jovicic et al., 2015; Khosravi et al., 2017; Kim and Taylor, 2017; Xiao et al., 2015; Zhang et al., 2015). A major consensus of these studies is that the C9 repeat expansion leads to aberrant RNA-processing events, critical for overall cellular health, but also important for the organization of the synapse and its cytoskeletal components, as well as for the regulation of scaffolding and signaling complexes that modulate synaptic strength and function (Iacoangeli and Tiedge, 2013; Liu-Yesucevitz et al., 2011).
The primary physiologic function of neurons is the transmission of information and commands via electrical potentials created by ion gradients. The propagation of those potentials from one neuron to another occurs at the synapse, where a variety of excitatory and inhibitory inputs combine to drive a tightly regulated release of neurotransmitters. Synapse homeostasis is maintained in part by glia, which contribute to neuronal function by mediating neurotransmitter concentrations, eliminating damaged synapses, and maintaining myelin insulation (Barres and Barde, 2000; von Bernhardi et al., 2016). Disruption of the electrochemical gradient has a negative effect not only on the proper propagation of information and neuronal network activities, but on the health of neurons themselves, eventually leading to motor and memory deficits and neuronal cell death.
Cognitive dysfunction observed during normal ageing is known to parallel selective loss of synapses and changes in spine density and morphology (Morrison and Baxter, 2012). Progressive synapse loss and dysfunction (i.e. synaptopathy) is believed to occur early in disease progression of cognitive disorders including Alzheimer’s disease (AD) (DeKosky and Scheff, 1990; Selkoe, 2002; Terry et al., 1991) and Frontotemporal Dementia (FTD) (Gong and Lippa, 2010; Petkau et al., 2012), and in other neurodegenerative diseases such as Huntington’s disease and Parkinson’s disease (Day et al., 2006; Picconi et al., 2012; Raymond et al., 2011; Villalba et al., 2015)). It is therefore no surprise that synaptic dysfunction is also implicated in ALS, the most common motor neuron disease (Belzil et al., 2016; Cleveland and Rothstein, 2001). It is now believed that ALS and FTD exist on the same spectrum; they share hereditable genetic causes, including mutations in C9, and defining protein aggregation pathologies, including cytoplasmic mislocalization and aggregation of TAR DNA-binding protein 43 (TDP-43) (Gitler and Tsuiji, 2016). In addition, they can exist together or independently in individuals carrying familial mutations (Belzil et al., 2016). These diseases are ultimately defined by the degeneration of neurons; in ALS, the motor neurons of the motor cortex and spinal cord, and, in FTD, the cortical neurons of the frontal and anterior temporal lobes. The plasticity of synapses has led to the belief that synapse loss may be reversible, which raises the possibility of therapeutic intervention early in disease progression prior to neuronal cell death, and warrants studies aimed at identifying the underlying mechanisms of synaptopathy in conjunction with the discovery of novel therapeutic targets (Freeman and Mallucci, 2016).
Here, we review observations regarding synaptic dysfunction in the C9ORF72 ALS-FTD spectrum. We will discuss changes of overall neuronal morphology, including dendritic arborization and spine density, as well as defects in membrane excitability, such as hyperexcitability. We will examine how these alterations lead to neuronal dysfunction of both cortical and motor neurons, the two major neuronal subtypes affected by degeneration in FTD and ALS. We will compare these findings to reports of synaptic deficits in non-C9 ALS/FTD to see whether there are potential overlapping mechanisms between subsets of ALS and FTD patients. Finally, we will briefly discuss putative therapies targeted towards modulating synaptic deficits in ALS/FTD.
Aberrant Neuronal Morphology and Synapse Formation
The length and complexity of dendrites are common measurements of neuronal health and maturity, yet few studies to date have examined the effects of the C9 repeat expansion on neuronal morphology. Overexpression of poly-GA DPRs in primary mouse cortical neuron cultures led to decreased dendritic arborization, caused by the co-aggregation of the transport factor Unc119, which supports the concept of synaptic dysfunction in C9 disease pathogenesis (May et al., 2014). In a second study, overexpression of a short hexanucleotide repeat construct ((G4C2)48-MS2) in rat spinal cord neurons reduced the number of primary dendritic branches specifically for those neurons that showed neuritic expression of repeat RNA foci as determined by RNA FISH analysis (Schweizer Burguete et al., 2015). In the same report, similar dendritic branching deficits were also found in a Drosophila model of (G4C2)48 supporting the hypothesis of synaptic dysfunction as a result of the C9 repeat expansion.
As for non-C9 ALS, a very recent study reported significant synapse loss in the prefrontal cortex of sporadic ALS patients using high-resolution imaging of postmortem brain tissue samples (Henstridge et al., 2017). Most interestingly, the degree of synapse loss correlated with the severity of cognitive impairment of the individual patients, and was not due to cortical atrophy, further supporting the idea that synapse loss occurs before neurodegeneration. This is in strong agreement with observations of synapse loss in the ventral thalamus of a mouse model deficient of the FTD gene progranulin (Grn−/−) (Lui et al., 2016). Further, mutant superoxide dismutase 1 (SOD1)G93A mature mouse cortical neurons and spinal motor neurons showed reduced dendritic length and complexity, as determined by Sholl analysis, in addition to a reduced spine density (Fogarty et al., 2016; Fogarty et al., 2017; Sgobio et al., 2008; Spalloni et al., 2011). In contrast, early postnatal SOD1G85R mouse spinal cord motor neurons elongate and branch more rapidly than control motor neurons (Filipchuk and Durand, 2012) suggesting differing synaptopathic mechanisms between the two mutations, or age dependent morphological aberrations, do exist. Interestingly, overexpressing an ALS-associated profilin 1 mutation, PFN1C71G, in mouse hippocampal neurons increases dendritic length, dendritic arborization and spine density (Brettle et al., 2015) while TDP-43A315T mouse cortical neurons show increased blebbing of dendrites without changes in length (Zhang et al., 2016). Genetic manipulations of other disease-associated proteins in different in vitro models support a loss of synaptic integrity in ALS/FTD. For example, mutations of the mitochondrial protein coiled-coil-helix-coiled-coil-helix domain-containing 10 (CHCHD10) have been linked to sporadic and familial ALS/FTD spectrum disease (Bannwarth et al., 2014). Knockdown or overexpression of mutant CHCHD10 in primary hippocampal neurons reduced expression of synaptic marker proteins synaptophysin and drebrin (Woo et al., 2017). Transgenic mice overexpressing mutant fused in sarcoma (FUSR521G), a well-characterized RNA binding protein with known mutations causing ALS and FTD (Kapeli et al., 2017), have severely reduced dendritic arborization in spinal motor neurons and reduced spine density in cortical layers IV–V (Sephton et al., 2014). Similar changes were observed in a transgenic FTD mouse model overexpressing ΔNLS-FUS, which exhibits decreased dendritic spine and synapse density in the frontal cortex in addition to a decrease in synaptic protein expression(Shiihashi et al., 2017). Similarly, knockdown of FUS in primary hippocampal neurons leads to decreased spine density and spine maturity, and reduces the subcellular synaptic expression of post-synaptic density protein 95 (PSD-95) and its interacting protein SynGAP (Yokoi et al., 2017). Similar to FUS, mutations in ubiquilin2 (UBQLN2) can cause both ALS and FTD-like symptoms. Consequently, a transgenic mouse overexpressing mutant UBQLN2 (UBQLN2P497H) showed behavioral cognitive impairments and significant morphological synaptic deficits as demonstrated by protein aggregates in dendritic spines of the hippocampus and frontal cortex and decreased spine density in the molecular layer of the dentate gyrus (Gorrie et al., 2014). Furthermore, there is reduced synapse formation in mutant valosin-containing protein (VCP) ALS patient-derived, induced pluripotent stem cells differentiated into spinal motor neurons (iPSC-MNs) (Hall et al., 2017). These examples highlight the complexity of synaptic dysfunctions and suggest that dendritic phenotypes may differ between subtypes of ALS, between ALS and FTD and between different brain regions and neuronal cell types. More qualitative assessments of dendrite morphologies may be important to discerning specific disease mechanisms (Figure 1).
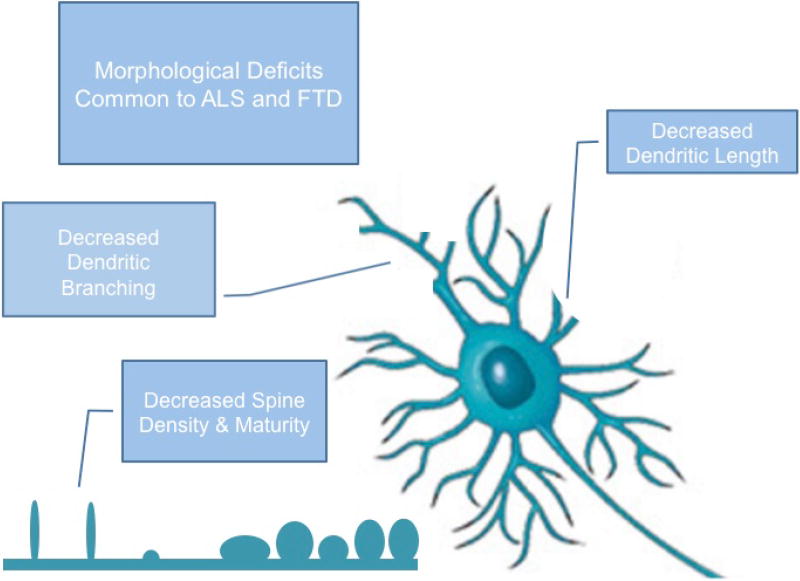
Figure 1. Schematic depiction of morphological deficits contributing to synaptic dysfunction in ALS/FTD.
Key features include alterations of dendritic arborization and changes in spine density and spine shape. These deficits have been commonly observed in cortical neurons, hippocampal neurons, and spinal motor neurons of ALS, FTD, and ALS/FTD models.
In addition to alterations of dendritic morphology, changes in synapse formation and maintenance may be observed by looking at the expression and subcellular distribution of synaptic proteins. Local control and regulation of RNA translation and protein synthesis at the synapse are known to be crucial for fast, efficient reaction to synaptic activity and plasticity. Regulation of the temporal and spatial synthesis of synaptodendritic proteins (e.g. cytoskeletal components, transmembrane receptors and transporters, anchoring proteins, and kinases) requires the translation of the corresponding mRNAs to be tightly regulated by RNA regulators such as mRNA binding proteins (RBPs) (Iacoangeli and Tiedge, 2013; Liu-Yesucevitz et al., 2011; Sephton and Yu, 2015). RBPs also play a role in dendritic and axonal mRNA transport and storage via RNA granules. Recent studies link stress granules, processing bodies and transport RNP granules to ALS pathogenesis (Buchan, 2014; Li et al., 2013; Sephton and Yu, 2015). These observed deficits can be triggered by mutations of the actual RBPs of familial ALS patients (e.g. TDP-43, FUS, and Ataxin-2) (Alami et al., 2014; Belly et al., 2005; Bosco et al., 2010; Coyne et al., 2015; Nonhoff et al., 2007; Sellier et al., 2016), but also have been discovered in C9 patients and C9 disease models (Dafinca et al., 2016; Daigle et al., 2016; Maharjan et al., 2016; Schweizer Burguete et al., 2015; Sellier et al., 2016), further supporting the idea that either of the above proposed C9 disease mechanisms may lead to dysfunctional RNA processing and RNA granule dynamics which could cause degradation of the integrity of synapses and synaptic function (see also discussion of aberrant excitability below).
The ultimate cause of symptoms in ALS is denervation of muscles, resulting in loss of muscle control, paralysis, and atrophy. The morphology of the neuromuscular junction (NMJ) can be assessed by the number and size of cholinergic boutons (C-boutons), as well as by their degree of fractionation. Most studies aimed at examining alterations of the NMJ have been performed in ALS mouse models, namely the SOD1G93A transgenic mouse. Conflicting data have been presented on whether the number of C-boutons increased or decreased with disease progression in this mouse model (Casas et al., 2013) (Milan et al., 2015) (Lasiene et al., 2016). Dukkipati and colleagues noticed the apparent discrepancies between previous reports of bouton size and count in ALS and performed a reanalysis of the data, which revealed that changes in C-bouton size and density in pre-end stage SOD1G93A mice are not reproducible (Dukkipati et al., 2017). In support of the apparent difficulties in quantifying morphological changes at the motor neuron-muscle synapse, Tremblay and colleagues find that, in the SOD1G37R mouse model, NMJ morphological deficits vary with age and muscle location (Tremblay et al., 2017). The authors suggest that reporting methods must be standardized to truly determine the significance of structural changes at the NMJ.
Mouse models of C9orf72 ALS/FTD have shown considerable variation in their ability to recapitulate motor disease. No structural changes or denervation of the neuromuscular junction were observed in either of two mouse models carrying a bacterial artificial chromosome (BAC) containing human C9ORF72 with expanded repeats (O'Rourke et al., 2015; Peters et al., 2015); however, Liu and colleagues report that a third BAC C9 model shows substantial NMJ denervation in mice with fast-progressing disease and subtle morphological abnormalities in mildly affected mice (Liu et al., 2016). Mice injected with an AAV9 virus containing 102 interrupted hexanucleuotide repeats show cytoskeletal abnormalities and decreased postsynaptic acetylcholine receptor expression (Herranz-Martin et al., 2017). Finally, NMJs remain intact despite inducible knockout of C9orf72 in neuronal and glial cells of adult mice (Koppers et al., 2015).
Drosophila C9 models more consistently capture motor dysfunction. Most overexpress only the hexanucleotide repeat, which is sufficient to disrupt NMJ structure and function. Developmentally expressing 30 or 58 repeats in motor neurons causes a reduction in the number of active zones (Freibaum et al., 2015; Zhang et al., 2015). 58 repeats also decreased bouton counts and total muscle area (Freibaum et al., 2015; Perry et al., 2017). In addition to the development of structural abnormalities, HRE overexpression can disrupt functional NMJ homeostasis. In the NMJ of ALS fly models expressing mutant TDP-43G298S or (G4C2)36 repeat expansions, expression of the synaptic vesicle trafficking protein Hsc-70 is post-transcriptionally decreased, resulting in impaired FM1-43 dye uptake, which suggests a common mechanism of synaptic dysfunction between TDP-43 and C9 ALS (Coyne et al., 2017). Flies overexpressing the poly-GR DPRs without the presence of repeat RNA show even more severe NMJ structural deterioration than do flies expressing repeat RNA, and also show increased levels of postsynaptic glutamate receptor subunits (Perry et al., 2017). Thus, flies reveal potential structural and functional mechanisms of NMJ deterioration in C9 ALS.
As for other non-C9 genetic models of ALS, knockdown of the TDP-43 fly-homolog, TBPH, in Drosophila leads to NMJ structural deficits, which can be rescued by inducible TBPH expression at specific time points (Romano et al., 2014). In addition, Drosophila NMJs expressing mutant FUS revealed disorganized presynaptic active zones via electron microscopy, but did not show gross changes in morphology when analyzed via confocal microscopy (Shahidullah et al., 2013). At the same time, mutant and wild type FUS overexpression in mice induces denervation of the NMJ (Sephton et al., 2014) and decreased dendritic spine and synaptic density in the frontal cortex, which was observed before neuronal loss had occurred (Shiihashi et al., 2017). Similarly, mutant Ubiquilin 2 (UBQLN2) mice, a model for both ALS and FTD, show decreased spine density and impaired synaptic plasticity (Gorrie et al., 2014) while mutant VAPB mice NMJs show increased bouton counts, increased fragmentation, and increased total area (Larroquette et al., 2015). Interestingly, synaptic overgrowth at the NMJ is also observed in a Drosophila model of ALS/FTD, overexpressing Charged Multivesicular Body Protein 2B (CHMP2B) Intron5, which was rescued by overexpression of the small endosomal GTPase Rab8 (West et al., 2015). Mutations in CHMP2B are found in both ALS and FTD patients (van Blitterswijk et al., 2012), and a transgenic mouse overexpressing mutant CHMP2Bintron5 in neurons shows both ALS and FTD behavioral phenotypes, including a decreased number of fully innervated NMJs, albeit no significant spinal motor neuron loss (Vernay et al., 2016).
Excitotoxicity
Excitotoxicity is broadly defined as toxicity induced by excessive exposure of the brain to the excitatory neurotransmitter glutamate (Olney, 1969). It is considered a synaptic event during which excess glutamate in the synaptic cleft leads to overactivation of post-synaptically localized glutamate receptors, which in turn triggers intracellular cell death cascades. Excitotoxicity is one of the major disease mechanisms ascribed to contributing to the degeneration of motor neurons in ALS (Rothstein et al., 1991; Taylor et al., 2016) (Figure 2), while less is known about excitotoxic mechanisms in FTD.
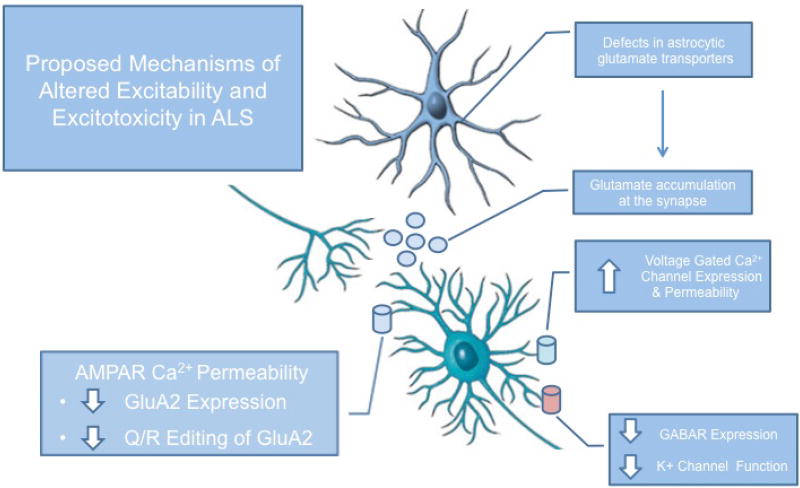
Figure 2. Schematic depiction of proposed mechanisms of altered excitability and excitotoxicty in ALS.
Major contributing factors to altered excitability and excitotoxicty in spinal motor neurons and cortical neurons in ALS include reduced levels of astrocytic glutamate transporters, changes in AMPA receptors composition and function, and decreased inhibitory activity – all of which are hypothesized to cause excessive calcium load and subsequent neuronal cell death.
One of the underlying mechanisms of excitotoxicity in ALS is the loss of glial glutamate transporters, which, under normal physiological conditions, are responsible for maintaining a tightly controlled glutamate homeostasis in the synaptic cleft, ensuring fast neuronal synaptic transmission (Danbolt et al., 1992; Danbolt, 1994; Danbolt et al., 1994). Rothstein and colleagues initially observed increased glutamate levels in ALS patient-derived cerebral spinal fluid and were then able to show selective loss of glutamate transporter EAAT2 in postmortem motor cortex brain tissue of ALS patients when compared to healthy control subjects (Rothstein et al., 1990; Rothstein et al., 1995). The authors proposed that dysregulation of glutamate transporters leads to increased extracellular glutamate levels, which in turn triggers excitotoxic cell death of motor neurons, without the actual loss of the glia cell itself. Subsequent in vitro studies confirmed that the loss of astroglia glutamate transporter function alone was sufficient to cause motor neuron degeneration (Rothstein et al., 1993). The concept of non-cell autonomous disease mechanisms in ALS via astrocyte dysfunction has since been demonstrated in numerous in vivo and in vitro studies, and has revolutionized the way ALS disease is studied and treated (see Therapeutics section, below; (Arbour et al., 2017; Boillee et al., 2006; Darman et al., 2004; Haidet-Phillips et al., 2011; Ilieva et al., 2009; Madill et al., 2017; Marchetto et al., 2008; Nagai et al., 2007)). While most of these studies were done working with mouse models or primary cultured cells from mutant SOD1 transgenic mice, recent data support the idea of astrocyte-mediated motor neuron cell death in other cases of familial ALS, as well as sporadic ALS (Pehar et al., 2017). For example, treatment of spinal motor neurons with mutant TDP-43-expressing astrocyte conditioned media increases levels of reactive oxygen species, which can be reversed using sodium channel blockers, such as riluzole (Rojas et al., 2015).
Additional contributing factors to excitotoxicity in ALS are thought to be via intrinsic neuronal deficits at the synapse caused by altered glutamate receptor expression or receptor dysfunction, leading to excess calcium influx and subsequent neuronal cell death. Many studies addressing this aspect of excitotoxicity have focused on the expression, distribution, and function of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) in the spinal cord of ALS patients. The AMPARs are ionotropic glutamate receptors responsible for fast synaptic transmission in the central nervous system (CNS) (Henley and Wilkinson, 2016). Functional properties of AMPARs depend greatly on the composition of their subunits, GluA1-4, which determines their effect on synapse formation, stabilization and synaptic plasticity (Huganir and Nicoll, 2013). Research has focused on the GluA2 subunit because of its ability to regulate the Ca2+-permeability and the trafficking of AMPARs (Bennett et al., 1996; Hollmann et al., 1991; Wright and Vissel, 2012). GluA2 renders GluA2-containing AMPARs Ca2+-impermeable, and it controls constitutive and activity-dependent AMPAR endocytosis and synaptic plasticity (Malinow and Malenka, 2002). Although most AMPARs in the adult brain contain the GluA2 subunit and thus are Ca2+-impermeable, the Ca2+-permeable AMPARs in mature neurons are most likely regulated during synaptic plasticity (long term potentiation (LTP) and long term depression (LTD)) and under stress (Henley and Wilkinson, 2016; Man, 2011; Sanderson et al., 2016; Shepherd, 2012). Most AMPARs become Ca2+-permeable by the removal of GluA2 (Wenthold et al., 1996). However, numerous reports suggest that GluA2-containing AMPARs become Ca2+-permeable due to unediting of GluA2 RNA at the Q/R site (Melcher et al., 1995; Melcher et al., 1996; Nishikura, 2010; Sommer et al., 1991; Washburn et al., 2014). GluA2 editing at this site is accomplished by the editing enzyme ADAR2, a member of the adenosine deaminase acting on RNA (ADAR) protein family (Keegan et al., 2004; Seeburg et al., 1998). ADARs deaminate adenosine in double-stranded RNA transcripts, thus changing the nucleotide from an adenosine to an inosine (Bass and Weintraub, 1987; Kim et al., 1994; Melcher et al., 1995; O'Connell et al., 1995). Under basal physiological conditions, GluA2 is fully edited, but under certain physiological or pathological conditions, it is unedited (Wright and Vissel, 2012). Lack of GluA2 editing and the subsequent increase in Ca2+ permeability of AMPARs has been associated with vulnerability of neurons to cell stressors, such as ischemia, and in neurodegenerative diseases, such as AD and ALS (Gaisler-Salomon et al., 2014; Grosskreutz et al., 2010; Hideyama et al., 2012b; Kawahara et al., 2004; Kwak and Kawahara, 2005; Peng et al., 2006; Takuma et al., 1999).
Kwak and colleagues have been instrumental in elucidating the role of ADAR2 function and GluA2 Q/R editing deficiencies in ALS and were able to show that, in laser captured spinal motor neurons of sporadic ALS patients, GluA2 Q/R has reduced editing efficiency, which is correlated with reduced levels of the editing enzyme ADAR2 (Kawahara et al., 2003; Takuma et al., 1999). Similar results were obtained in a recent study analyzing spinal motor neurons from a single mutant FUS ALS patient (Aizawa et al., 2016). Follow up studies using conditional ADAR2 knockout mice and human tissue propose that reduced GluA2 Q/R editing and subsequent increase in AMPAR calcium permeability is responsible for motor neuron deficits in the spinal cord, as well as TDP-43 pathology due to calcium-activated, calpain-induced fragmentation of full length TDP-43 (Aizawa et al., 2010; Hideyama et al., 2010; Hideyama et al., 2012a; Yamashita and Kwak, 2014). An alternative hypothesis proposed that calcium influx via NMDA receptors leads to calpain-mediated ADAR2 cleavage and subsequent changes in GluA2 Q/R editing efficiencies, which in turn increases AMPAR calcium permeability and neuronal cell death (Mahajan et al., 2011). Increased AMPAR calcium permeability was further suggested to be to due relatively lower levels of GluA2 mRNA, as well as the limited calcium buffering capacity of motor neurons in general (Carriedo et al., 1996; Van Damme et al., 2002; Virgo et al., 1996). Ongoing studies in our laboratory and by other research groups are examining the role of AMPARs in C9ORF72 ALS/FTD and will hopefully provide additional information on this important excitotoxic pathway. Pervious studies in our laboratory using C9 patient derived hiPSC motor neuron cultures showed increased susceptibility to glutamate toxicity, supporting a contribution of AMPARs to excitotoxic cell death in C9 ALS/FTD (Donnelly et al., 2013). Similar findings have been confirmed in a recent study looking at increased neuronal cell death upon specific AMPA-receptor activation using patient derived C9 ALS iPSCs with corresponding isogenic control lines (Selvaraj et al., 2018). Interestingly, this study shows that the C9 mutation leads to increased mRNA and protein expression of the GluA1 subunit of AMPA receptors concomitant with increased Ca2+ permeability of C9 iPSC motor neurons. No GluA2 Q/R editing deficits were found in these neurons, suggesting that the increased Ca2+ permeability and increased susceptibility to AMPA-mediated cell death, is due to an increase in GluA2 lacking AMPA receptors. Using RNAscope imaging technology the authors confirmed increased GluA1 transcript expression in C9 ALS spinal cord autopsy tissue, but not C9 patient prefrontal cortex tissue samples, suggesting that this change in AMPA receptor subunit composition is specific to motor neurons and might explain motor neuron susceptibility in ALS.
Additional studies support a role of AMPA receptors and excitotoxicity in ALS as well as FTD. For example, the gene linked to juvenile-onset, autosomal recessive ALS (ALS2), the ALS2 gene, binds to the glutamate receptor interacting protein 1 (GRIP1). Loss of ALS2, mimicking ALS2 disease, leads to decreased plasma membrane expression of GluA2 and subsequent increased neuronal susceptibility to glutamate excitotoxicity (Lai et al., 2006). Similarly, phosphatase and tensin homolog (PTEN), a negative regulator of the rapamycin pathway, is thought to be involved in motor neuron survival. PTEN knockdown decreases GluA1, GluA2, and GluA3 expression in induced pluripotent stem cells differentiated into spinal motor neurons and thereby decreases AMPA-induced toxicity (Yang et al., 2014). Misfolded SOD1 in SOD1G93A mouse spinal cord binds to Na(+)/K(+)ATPase-α3, leading to decreased GluA2 expression and increased size of cholinergic synapses (Ruegsegger et al., 2016). Altered Na(+)/K(+)ATPase-α3 expression was confirmed in spinal cord tissue of both sporadic and familial ALS patients (Ruegsegger et al., 2016). Changes in AMPA receptor subunit composition were also found in a mouse model of FTD, generated by forebrain-specific expression of mutant CHMP2B (Gascon et al., 2014). In a time-dependent manner and correlated to FTD-like behavioral and pathological features, the mice showed increased mRNA and protein levels of GluA2, GluA3 and GluA4, but not GluA1, a finding that was confirmed in behavioral variant FTD patient-derived iPSC cortical neurons. Via electrophysiological examination of acute mouse brain slices, the authors then showed that this altered AMPA receptor subunit composition led to more Ca2+-impermeable AMPA receptors in the mouse prefrontal cortex. These data strongly suggest that synaptic AMPA receptor function might be altered differently in motor neurons from ALS patients versus cortical neurons from FTD patients. It will be of great interest to understand whether such differences are present in C9 ALS/FTD patients, where both, motor neurons and cortical neurons are affected.
Despite its high Ca2+ permeability and significant involvement in excitotoxic events in other neurodegenerative diseases, especially stroke, the N-methyl-D-aspartate (NMDA) receptor has not been discussed to the same extend as AMPA receptors in ALS or FTD related disease mechanisms (Spalloni et al., 2013), and no studies have been published to date to link NMDA receptor dysfunction to C9-mediated disease pathogenesis. Initial studies by Couratier and colleagues found that CSF from ALS patients induced toxicity in cultured rat neurons and that this toxicity was reversible by treatment with AMPA receptor antagonists, but not NMDA receptor antagonists, suggesting a more prominent role of AMPA-mediated excitotoxicity in ALS (Couratier and Hugon, 1993). These early studies are likely one reason why NMDA receptors have not been studied extensively in regards to synaptic dysfunction in ALS. However, some studies do suggest that NMDA receptors play a role in motor neuron degeneration in ALS. For example, a mutation in D-amino acid oxidase (DAO) was found to co-segregate with familial ALS (Mitchell et al., 2010). Increases in D-serine levels and decreased in DAO activity were shown in SOD1 mouse models (Sasabe et al., 2007) and DAO knockout mice exhibit severe motor neuron degeneration (Sasabe et al., 2012). Mutant DAO activates autophagy and promotes apoptosis in cell culture models, both of which was rescued by an D-serine/glycine antagonist of the NMDA receptor, further suggesting that this pathway might contribute to ALS disease pathogenesis (Paul and de Belleroche, 2014; Paul and de Belleroche, 2015). Another study shows that disruption of lipid rafts in cortical neurons via cholesterol membrane depletion in SOD1G93A mice only affected NMDA receptor currents, but not AMPA receptor currents, suggesting that lipid raft composition is altered in disease and affects only select membrane receptors (Antonini et al., 2018). Finally, using the non-competitive NMDA receptor antagonist memantine in SOD1G93A mouse models did show prolonged survival in two independent studies (Joo et al., 2007; Wang and Zhang, 2005). Memantine was shown to be safe and well tolerated in a Phase II/III trial for ALS patients, but had no efficacy on functional decline of the patients, while survival was not included in the primary endpoints (de Carvalho et al., 2010).
Few studies have examined the role of NMDA receptors in FTD. When Gascon et al analyzed mutant CHMP2B mice, they did not find any changes in the RNA or protein levels of NMDA receptor subunits (Gascon et al., 2014). In addition, while intraperitoneal injection of AMPA receptor antagonist NBQX rescued the FTD-like behavioral sociability defects, injections of NMDA receptor antagonist AV5 did not, suggesting that in mutant progranulin FTD, NMDA receptors do not play a significant contribution to disease pathogenesis. However, a transgenic mouse model overexpressing human mutant Tau (hTauAT), which is considered a risk factor for FTD, exhibits increased intracellular Ca2+ levels via extrasynaptic NR2B containing NMDA receptors (Decker et al., 2016). Furthermore, a different mutant tau overexpressing transgenic mouse, TauV337M, showed decreased PSD-95 expression, smaller postsynaptic densities, and impaired synaptic NMDA receptor localization, resulting in reduced neuronal striatal neuronal firing (Warmus et al., 2014).
Finally, other receptors and ion channels have been implicated in an aberrant neuronal calcium homeostasis as ALS disease mechanisms. For example, mutations in the voltage-gated calcium channel subunit CACNA1H, which cause reduced voltage sensitivity and decreased thalamic neuron excitability, may be involved in disease pathogenesis (Rzhepetskyy et al., 2016). Further, Comley and colleagues suggested that differences in expression of the GABA receptors GABAA receptor α1 and α2 between spinal motor neurons and oculomotor neurons in ALS patients may indicate pathological differences in inhibitability, which might explain selective neurodegeneration of motor neurons (Comley et al., 2015). In addition, voltage gated calcium channel subunit mRNAs and high voltage activated calcium channels were found to be upregulated in SOD1G93A mouse spinal cord (Chang and Martin, 2016). Knocking down FUS in primary mouse neurons leads to reduced levels of GluA1 mRNA, which in turn reduced AMPA receptor surface expression and basal synaptic transmission as well as a reduction in mature mushroom-shaped spines, suggesting that loss of function of FUS greatly impacts synaptic function (Udagawa et al., 2015).
Altered Neuronal Excitability
The culmination of synaptic deficits is a change in the neuron’s ability to receive and propagate electrical signals. Therefore, alterations on the overall neurophysiological level are to be expected in diseases characterized by synaptopathy. Here we will summarize studies supporting the presence of altered intrinsic neuronal excitability and its contribution to disease pathogenesis in C9 ALS and FTD. Almost all ALS patients, familial and sporadic, present with neurophysiological alterations described as hyperexcitability, which is detected early during disease progression and often recedes at later stages of the disease (Bae et al., 2013b; Kiernan, 2009). Hyperexcitability in neurons is often described as a condition during which the neuron is unusually or excessively excitable, frequently reducing the threshold to fire action potentials upon stimulation. The mechanisms leading to hyperexcitability are multifactorial, and include altered inhibitory inputs from GABAergic interneurons, intrinsic changes of neuronal sodium (Na+) and potassium (K+) channel function, and extrinsic impairments of the extracellular potassium homeostasis due to the dysfunction of perisynaptic astrocytes (Do-Ha et al., 2017; Geevasinga et al., 2016). Cortical hyperexcitability is measured with the use of transcranial magnetic stimulation (TMS; (Hallett, 2000)) and can be detected in both sporadic and familial ALS patients, including C9ORF72 patients, as well as some subsets of FTD patients, suggesting that divergent mechanistic pathways lead to altered neuronal physiological function shared among all patients on this spectrum disorder (Bae et al., 2013a; Bae et al., 2013b; Geevasinga et al., 2015c; Vucic et al., 2008; Wainger and Cudkowicz, 2015). Interestingly, while cortical hyperexcitability was confirmed via TMS to be present in symptomatic C9ORF72 ALS patients, it was absent in asymptomatic C9ORF72 expansion repeat carriers (Geevasinga et al., 2015b), similar to what had been reported for SOD1 ALS patients (Vucic et al., 2010). Furthermore, cortical hyperexcitability detected by TMS was only associated with C9orf72 ALS patients but not with C9 behavioral variable FTD (bvFTD) or C9 ALS-FTD patients (Schanz et al., 2016). These data suggest that TMS measures of cortical excitability could be used to detect early onset of ALS disease and could potentially be used to facilitate detection of disease onset of asymptomatic familial patients. In addition, these studies provides evidence that, despite their commonalities, alterations in cortical excitability may differ between ALS and FTD, even in familial cases sharing the same genetic cause (Figure 3).
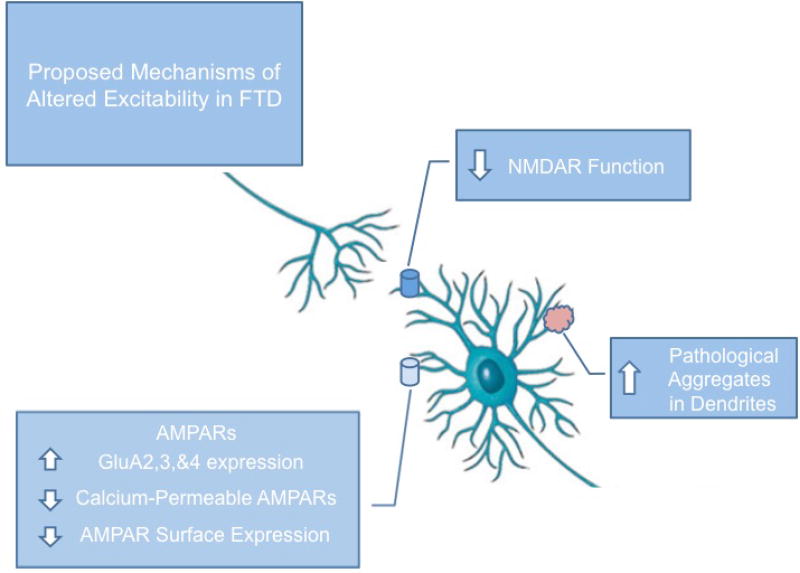
Figure 3. Schematic depiction of suggested mechanisms of altered excitability in FTD.
In FTD and ALS/FTD models with primarily cognitive phenotypes, alterations in cortical and hippocampal excitability typically result in reduced excitability, as opposed to the hyperexcitability observed in primarily motor ALS. This may be due to aberrant expression of AMPARs and changes in their calcium permeability, as well as NMDAR dysfunction and toxic protein aggregation in neurites.
In addition to cortical hyperexcitability, there is also axonal excitability, which is often referred to as peripheral nerve excitability, or peripheral hyperexcitability (Do-Ha et al., 2017; Park et al., 2017). Measurements of axonal excitability are performed via the generation of compound muscle action potential (CMAP) responses and the identification of a threshold necessary to produce a specific target response (Park et al., 2017). Changes in axonal excitability are known to occur in both familial and sporadic ALS patients and are thought to contribute to clinical symptoms such as fasciculation and muscle cramps (Layzer, 1994). Increased axonal excitability has been reported in symptomatic C9 ALS patients, similar to sporadic ALS patients, but was unchanged in asymptomatic C9 ALS patients, similar to what had been reported for cortical hyperexcitability (Geevasinga et al., 2015a).
In vitro studies using hiPSC-derived motor neurons confirmed the presence of altered neurophysiological properties in familial forms of ALS and validated the use of patient-derived neurons to study mechanisms of aberrant neuronal excitability. Specifically, hyperexcitability was found in young hiPSC-MNs derived from familial ALS patients (SOD1, FUS, C9orf72) (Wainger et al., 2014), and decreased excitability was detected in C9ORF72 hiPSC-MNs at an older age of differentiation (Sareen et al., 2013). These early findings were confirmed in a study of the physiological properties of hiPSC-MNs at different time points of differentiation that showed initial hyperexcitability followed by progressive loss of action potential output and decreased synaptic activity in both C9 and mutant TDP-43 hiPSC-MNs (Devlin et al., 2015). However, no apparent changes in basal cell survival were found, which further supports the notion that changes in excitability indicate early functional loss of motor neurons prior to cell death. This hypothesis was further confirmed by an in vivo study of 140 ALS patients comparing compound muscle action potentials (CMAP), as a measure of motor neuron loss to nerve excitability indices (Iwai et al., 2016).
Mouse models of C9orf72 haploinsufficiency have failed to recreate motor dysfunction or altered excitability at the NMJ. C9orf72 homozygous and hemizygous knockout mice do not differ from wild-type mice in the CMAP of the foot flexor muscle (O'Rourke et al., 2016), nor in resting EMGs and myogenic motor evoked potentials of the hind limbs (Jiang et al., 2016). Alternatively, C9 fly models overexpressing the C9 hexanucleotide repeat expansion show alterations in evoked junctional potentials (Zhang et al., 2015), indicating impaired neurotransmitter release at the NMJ, and overexpression of poly-GR DPRs suppresses both spontaneous and evoked responses (Perry et al., 2017). This suggests that a toxic gain of function of RNA foci or DPRs impairs neuromuscular junction transmission in C9 ALS.
Changes in neuronal excitability can also be detected in non-C9 ALS disease models. SOD1G93A mouse cortical neurons show impaired long term potentiation (LTP) (Spalloni et al., 2011), and, although hyperexcitability in this model is not specific to cortico-spinal neurons, increased calcium permeability is (Kim et al., 2017). SOD1G93A mouse diaphragm muscle NMJs were also found to be hyperexcitable pre-symptomatically (Rocha et al., 2013). This may be attributable to the same mechanisms by which oligomeric mutant SOD1 increases excitability through suppression of potassium currents in Aplysia californica ganglion (Zhang et al., 2017). An alternative cause of synaptic dysfunction at the muscle is that increased muscarinic sensitivity of perisynaptic Schwann cells in the SOD1G37R mouse soleus impairs NMJ plasticity and repair (Arbour et al., 2015). Similar to what was found in regards to the fact that NMJ morphological deficits vary with age and muscle location, changes in electrical propagation vary spatially and temporally (Tremblay et al., 2017). TDP-43A315T mouse cortical neurons also show disinhibition and hyperexcitability (Zhang et al., 2016), while Drosophila motor neurons expressing mutant FUS or FUS knockouts show enhanced firing, however, transmission across the NMJ is severely decreased (Shahidullah et al., 2013). Interestingly, hippocampal CA1 pyramidal cells examined in brain slices obtained from one month old UBQLN2P497H transgenic mice exhibited hypoexcitability (Radzicki et al., 2016), highlighting the fact that different brain regions may show different forms of aberrant neurophysiological properties and synaptic dysfunction. It would be interesting to see whether, in this model of FTD, cortical hyperexcitability or axonal excitability exists in addition to hippocampal hypoexcitability. Overall, more mechanistic studies are required to understand what leads to these neurophysiological changes and whether the mechanisms are shared between subtypes of ALS and FTD patients.
Therapeutic approaches aimed at synaptic dysfunction
Targeting synapse loss and synaptic dysfunction in clinical trials in ALS have mostly focused on the excitotoxic mechanisms of the disease. Riluzole, a sodium channel blocker, is one of only two FDA approved treatments for ALS to date. Although it does not reverse any symptoms, on average it delays disease progression and prolongs life span by approximately three months. Riluzole, which has been as ascribed many different routes of therapeutic action, has been extensively reviewed (Bellingham, 2011).
Other potential targets in this pathway were unsuccessfully tested in clinical trials as disease modifiers: memantine (NMDA receptors; (de Carvalho et al., 2010)), topiramate (brain vasculature; (Cudkowicz et al., 2003)), lamotrigine or mexiletine (sodium channels; (Ryberg et al., 2003) (Shibuya et al., 2015)) and gabapentin (GABA receptors; (Miller et al., 2001)). A phase 2 trial of the AMPA receptor antagonist talampanel was initially positive in slowing disease, but a larger phase 3 trial showed no benefit (Pascuzzi et al., 2010). Perampanel, an approved AMPAR antagonist, was effective in halting disease progression and reversing pathology in conditional ADAR2 knockout mice (Akamatsu et al., 2016) and is currently being tested in a clinical Phase 2 trial for sporadic ALS (https://clinicaltrials.gov/ct2/show/NCT03019419). Activation of the glutamate transporter was unsuccessful in improving disease progression when studied as a potential therapy using the FDA-approved beta lactam antibiotic ceftriaxone in a Phase 3 clinical trial, despite showing significant protection in pre-clinical studies by upregulation of glutamate transporter protein (Berry et al., 2013; Rothstein et al., 2005). The search for ALS therapeutics has extended to other types of ion channels. Retigabine, a Kv7 channel opener, has been shown to correct hyperexcitability and prolong survival in C9ORF72, SOD1, and FUS familial ALS patient-derived iPSC-MNs (Wainger et al., 2014). Based on these data, Retigabine is currently being tested in ALS patients in a Phase 2 trial (https://clinicaltrials.gov/ct2/show/NCT02450552).
In addition to targeting receptors or ion channels, recent clinical trials have been approved to study excitability in ALS patients, either as a prognostic marker of the disease or as a potential biomarker to accompany pharmacological clinical trials. As an example, patients are currently being recruited to measure excitability of spinal neurons by measuring electromyogram (EMG) activity in combination with electrical and/or magnetic stimulation of peripheral nerve and/or cortical structures (https://clinicaltrials.gov/ct2/show/NCT02429492?cond=ALS&draw=1&rank=8; (Turner and Kiernan, 2012)). Finally, a recent study described the use of a positron emission tomography (PET) ligand to quantify synaptic densities in patients with temporal lobe epilepsy (Finnema et al., 2016). The radioligand, which was targeting synaptic vesicle glycoprotein 2A (SV2A; [(11)C]UCB-J), showed good imaging properties and was sensitive enough to detect changes in the patient population. One could imagine that such pre-symptomatic approaches could assist as prognostic disease biomarker detecting early neuronal dysfunction before any behavioral symptoms appear.
Concluding Remarks
In conclusion, possibly due to the renewed awareness of the genetic, pathologic, and clinical overlap between ALS and FTD, more attention has been given to mechanisms of synaptic dysfunction and neuronal excitability in ALS/FTD patients, in particular those carrying the C9ORF72 hexanucleotide repeat expansion mutation. Other shared genetic mutations, including mutations in VCP, TBK1, SQSTM1, UBQLN2 and CHMP2B have already, or will likely in the near future, demonstrated overlapping disease mechanisms leading to cortical neuronal dysfunctions and/or axonal excitability of motor neuron axons connecting to the muscle via the NMJs. These dysfunctions are likely to occur early during disease progression, before any significant neuronal degeneration or loss. Because of this early appearance, a better understanding of the underlying mechanisms and a search for tools to detect and monitor these deficits will provide new opportunities to develop novel early stage-interfering therapeutics and to clinically diagnose ALS/FTD patients before the presence of any behavioral symptoms, respectively.
Highlights.
Aberrant RNA metabolism contributes to synaptic deficits in C9orf72 ALS/FTD
There are common mechanisms of synaptic dysfunction amongst different subtypes of ALS/FTD
Changes in dendritic morphology, neuronal excitability and excitotoxicity trigger ALS/FTD disease pathogenesis
Acknowledgments
We would like to thank the Sattler Laboratory for suggestions and comments towards the manuscript. We would also like to thank all ALS patients that have contributed to the summarized research. This work was support by the NIH (RO1 NS085207), the Muscular Dystrophy Association (MDA), the ALS Association (ALSA), the Robert Packard Center for ALS Research and the Barrow Neurological Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizawa H, et al. TDP-43 pathology in sporadic ALS occurs in motor neurons lacking the RNA editing enzyme ADAR2. Acta Neuropathol. 2010;120:75–84. doi: 10.1007/s00401-010-0678-x. [DOI] [PubMed] [Google Scholar]
- Aizawa H, et al. Deficient RNA-editing enzyme ADAR2 in an amyotrophic lateral sclerosis patient with a FUSP525L mutation. J Clin Neurosci. 2016 doi: 10.1016/j.jocn.2015.12.039. [DOI] [PubMed] [Google Scholar]
- Akamatsu M, et al. The AMPA receptor antagonist perampanel robustly rescues amyotrophic lateral sclerosis (ALS) pathology in sporadic ALS model mice. Sci Rep. 2016;6:28649. doi: 10.1038/srep28649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alami NH, et al. Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron. 2014;81:536–43. doi: 10.1016/j.neuron.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida S, et al. Modeling key pathological features of frontotemporal dementia with C9ORF72 repeat expansion in iPSC-derived human neurons. Acta Neuropathologica. 2013 doi: 10.1007/s00401-013-1149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A, et al. Membrane cholesterol depletion in cortical neurons highlights altered NMDA receptor functionality in a mouse model of amyotrophic lateral sclerosis. Biochim Biophys Acta. 2018;1864:509–519. doi: 10.1016/j.bbadis.2017.11.008. [DOI] [PubMed] [Google Scholar]
- Arbour D, et al. Early and persistent abnormal decoding by glial cells at the neuromuscular junction in an ALS model. J Neurosci. 2015;35:688–706. doi: 10.1523/JNEUROSCI.1379-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbour D, Vande Velde C, Robitaille R. New perspectives on amyotrophic lateral sclerosis: the role of glial cells at the neuromuscular junction. J Physiol. 2017;595:647–661. doi: 10.1113/JP270213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash PE, et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77:639–46. doi: 10.1016/j.neuron.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae JS, et al. Cortical excitability differences between flexor pollicis longus and APB. Neurosci Lett. 2013a;541:150–4. doi: 10.1016/j.neulet.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Bae JS, et al. The puzzling case of hyperexcitability in amyotrophic lateral sclerosis. J Clin Neurol. 2013b;9:65–74. doi: 10.3988/jcn.2013.9.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannwarth S, et al. A mitochondrial origin for frontotemporal dementia and amyotrophic lateral sclerosis through CHCHD10 involvement. Brain. 2014;137:2329–45. doi: 10.1093/brain/awu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Barde Y. Neuronal and glial cell biology. Curr Opin Neurobiol. 2000;10:642–8. doi: 10.1016/s0959-4388(00)00134-3. [DOI] [PubMed] [Google Scholar]
- Bass BL, Weintraub H. A developmentally regulated activity that unwinds RNA duplexes. Cell. 1987;48:607–13. doi: 10.1016/0092-8674(87)90239-x. [DOI] [PubMed] [Google Scholar]
- Bellingham MC. A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade? CNS Neurosci Ther. 2011;17:4–31. doi: 10.1111/j.1755-5949.2009.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belly A, et al. Delocalization of the multifunctional RNA splicing factor TLS/FUS in hippocampal neurones: exclusion from the nucleus and accumulation in dendritic granules and spine heads. Neurosci Lett. 2005;379:152–7. doi: 10.1016/j.neulet.2004.12.071. [DOI] [PubMed] [Google Scholar]
- Belzil VV, Katzman RB, Petrucelli L. ALS and FTD: an epigenetic perspective. Acta Neuropathol. 2016;132:487–502. doi: 10.1007/s00401-016-1587-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MV, et al. The GluR2 hypothesis: Ca(++)-permeable AMPA receptors in delayed neurodegeneration. Cold Spring Harb Symp Quant Biol. 1996;61:373–84. [PubMed] [Google Scholar]
- Berry JD, et al. Design and initial results of a multi-phase randomized trial of ceftriaxone in amyotrophic lateral sclerosis. PLoS One. 2013;8:e61177. doi: 10.1371/journal.pone.0061177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeynaems S, et al. Drosophila screen connects nuclear transport genes to DPR pathology in c9ALS/FTD. Sci Rep. 2016;6:20877. doi: 10.1038/srep20877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Bosco DA, et al. Mutant FUS proteins that cause amyotrophic lateral sclerosis incorporate into stress granules. Hum Mol Genet. 2010;19:4160–75. doi: 10.1093/hmg/ddq335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettle M, et al. Amyotrophic lateral sclerosis-associated mutant profilin 1 increases dendritic arborisation and spine formation in primary hippocampal neurons. Neurosci Lett. 2015;609:223–8. doi: 10.1016/j.neulet.2015.09.034. [DOI] [PubMed] [Google Scholar]
- Buchan JR. mRNP granules. Assembly, function, and connections with disease. RNA Biol. 2014;11:1019–30. doi: 10.4161/15476286.2014.972208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriedo SG, Yin HZ, Weiss JH. Motor neurons are selectively vulnerable to AMPA/Kainate receptor-mediated injury in vitro. J. Neuroscience. 1996;16:4069–4079. doi: 10.1523/JNEUROSCI.16-13-04069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas C, et al. Early presymptomatic cholinergic dysfunction in a murine model of amyotrophic lateral sclerosis. Brain Behav. 2013;3:145–58. doi: 10.1002/brb3.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Martin LJ. Voltage-gated calcium channels are abnormal in cultured spinal motoneurons in the G93A-SOD1 transgenic mouse model of ALS. Neurobiol Dis. 2016;93:78–95. doi: 10.1016/j.nbd.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciura S, et al. Loss of function of C9orf72 causes motor deficits in a zebrafish model of Amyotrophic Lateral Sclerosis. Annals of Neurology. 2013 doi: 10.1002/ana.23946. [DOI] [PubMed] [Google Scholar]
- Cleveland DW, Rothstein JD. From charcot to lou gehrig: deciphering selective motor neuron death in als. Nature Reviews Neuroscience. 2001;2:806+. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- Comley L, et al. Motor neurons with differential vulnerability to degeneration show distinct protein signatures in health and ALS. Neuroscience. 2015;291:216–29. doi: 10.1016/j.neuroscience.2015.02.013. [DOI] [PubMed] [Google Scholar]
- Couratier P, Hugon J. Cell culture evidence for neuronal degeneration in amyotrophic lateral sclerosis being. Lancet. 1993;341:265. doi: 10.1016/0140-6736(93)92615-z. [DOI] [PubMed] [Google Scholar]
- Coyne AN, et al. Fragile X protein mitigates TDP-43 toxicity by remodeling RNA granules and restoring translation. Hum Mol Genet. 2015 doi: 10.1093/hmg/ddv389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne AN, et al. Post-transcriptional Inhibition of Hsc70-4/HSPA8 Expression Leads to Synaptic Vesicle Cycling Defects in Multiple Models of ALS. Cell Rep. 2017;21:110–125. doi: 10.1016/j.celrep.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudkowicz ME, et al. A randomized, placebo-controlled trial of topiramate in amyotrophic lateral sclerosis. Neurology. 2003;61:456–464. doi: 10.1212/wnl.61.4.456. [DOI] [PubMed] [Google Scholar]
- Dafinca R, et al. C9orf72 Hexanucleotide Expansions are Associated with Altered ER Calcium Homeostasis and Stress Granule Formation in iPSC-Derived Neurons from Patients with Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Stem Cells. 2016 doi: 10.1002/stem.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle JG, et al. Pur-alpha regulates cytoplasmic stress granule dynamics and ameliorates FUS toxicity. Acta Neuropathol. 2016;131:605–20. doi: 10.1007/s00401-015-1530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC, Storm-Mathisen J, Kanner BI. An [Na+ + K+]coupled L-glutamate transporter purified from rat brain is located in glial cell processes. Neuroscience. 1992;51:295–310. doi: 10.1016/0306-4522(92)90316-t. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. The high affinity uptake system for excitatory amino acids in the brain. Prog. Neurobiol. 1994;44:377–396. doi: 10.1016/0301-0082(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Danbolt NC, Storm-Mathisen J, Ottersen OP. Sodium/potassium-coupled glutamate transporters, a "new" family of eukaryotic proteins: do they have "new" physiological roles and could they be new targets for pharmacological intervention? Prog. Brain Res. 1994;100:53–60. doi: 10.1016/s0079-6123(08)60768-2. [DOI] [PubMed] [Google Scholar]
- Darman J, et al. Viral-induced spinal motor neuron death is non-cell-autonomous and involves glutamate excitotoxicity. J. Neurosci. 2004;24:7566–7575. doi: 10.1523/JNEUROSCI.2002-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–9. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- de Carvalho M, et al. A randomized, placebo-controlled trial of memantine for functional disability in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2010;11:456–60. doi: 10.3109/17482968.2010.498521. [DOI] [PubMed] [Google Scholar]
- Decker JM, et al. The Tau/A152T mutation, a risk factor for frontotemporal-spectrum disorders, leads to NR2B receptor-mediated excitotoxicity. EMBO Rep. 2016;17:552–69. doi: 10.15252/embr.201541439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–56. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–64. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Devlin AC, et al. Human iPSC-derived motoneurons harbouring TARDBP or C9ORF72 ALS mutations are dysfunctional despite maintaining viability. Nat Commun. 2015;6:5999. doi: 10.1038/ncomms6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do-Ha D, Buskila Y, Ooi L. Impairments in Motor Neurons, Interneurons and Astrocytes Contribute to Hyperexcitability in ALS: Underlying Mechanisms and Paths to Therapy. Mol Neurobiol. 2017 doi: 10.1007/s12035-017-0392-y. [DOI] [PubMed] [Google Scholar]
- Donnelly CJ, et al. RNA Toxicity from the ALS/FTD C9ORF72 Expansion Is Mitigated by Antisense Intervention. Neuron. 2013;80:415–28. doi: 10.1016/j.neuron.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukkipati SS, et al. Experimental Design and Data Analysis Issues Contribute to Inconsistent Results of C-Bouton Changes in Amyotrophic Lateral Sclerosis. eNeuro. 2017;4 doi: 10.1523/ENEURO.0281-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipchuk AA, Durand J. Postnatal dendritic development in lumbar motoneurons in mutant superoxide dismutase 1 mouse model of amyotrophic lateral sclerosis. Neuroscience. 2012;209:144–54. doi: 10.1016/j.neuroscience.2012.01.046. [DOI] [PubMed] [Google Scholar]
- Finnema SJ, et al. Imaging synaptic density in the living human brain. Sci Transl Med. 2016;8:348ra96. doi: 10.1126/scitranslmed.aaf6667. [DOI] [PubMed] [Google Scholar]
- Fogarty MJ, et al. Marked changes in dendritic structure and spine density precede significant neuronal death in vulnerable cortical pyramidal neuron populations in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Acta Neuropathol Commun. 2016;4:77. doi: 10.1186/s40478-016-0347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MJ, et al. Motor Areas Show Altered Dendritic Structure in an Amyotrophic Lateral Sclerosis Mouse Model. Front Neurosci. 2017;11:609. doi: 10.3389/fnins.2017.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman OJ, Mallucci GR. The UPR and synaptic dysfunction in neurodegeneration. Brain Res. 2016 doi: 10.1016/j.brainres.2016.03.029. [DOI] [PubMed] [Google Scholar]
- Freibaum BD, et al. GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature. 2015;525:129–33. doi: 10.1038/nature14974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaisler-Salomon I, et al. Hippocampus-specific deficiency in RNA editing of GluA2 in Alzheimer's disease. Neurobiol Aging. 2014;35:1785–91. doi: 10.1016/j.neurobiolaging.2014.02.018. [DOI] [PubMed] [Google Scholar]
- Gascon E, et al. Alterations in microRNA-124 and AMPA receptors contribute to social behavioral deficits in frontotemporal dementia. Nat Med. 2014;20:1444–51. doi: 10.1038/nm.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geevasinga N, et al. Axonal ion channel dysfunction in c9orf72 familial amyotrophic lateral sclerosis. JAMA Neurol. 2015a;72:49–57. doi: 10.1001/jamaneurol.2014.2940. [DOI] [PubMed] [Google Scholar]
- Geevasinga N, et al. Cortical Function in Asymptomatic Carriers and Patients With C9orf72 Amyotrophic Lateral Sclerosis. JAMA Neurol. 2015b;72:1268–74. doi: 10.1001/jamaneurol.2015.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geevasinga N, et al. Cortical Function in Asymptomatic Carriers and Patients With C9orf72 Amyotrophic Lateral Sclerosis. JAMA Neurol. 2015c:1–7. doi: 10.1001/jamaneurol.2015.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geevasinga N, et al. Pathophysiological and diagnostic implications of cortical dysfunction in ALS. Nat Rev Neurol. 2016;12:651–661. doi: 10.1038/nrneurol.2016.140. [DOI] [PubMed] [Google Scholar]
- Gendron TF, et al. Cerebellar c9RAN proteins associate with clinical and neuropathological characteristics of C9ORF72 repeat expansion carriers. Acta Neuropathol. 2015;130:559–73. doi: 10.1007/s00401-015-1474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler AD, Tsuiji H. There has been an awakening: Emerging mechanisms of C9orf72 mutations in FTD/ALS. Brain Res. 2016;1647:19–29. doi: 10.1016/j.brainres.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Lippa CF. Review: disruption of the postsynaptic density in Alzheimer's disease and other neurodegenerative dementias. Am J Alzheimers Dis Other Demen. 2010;25:547–55. doi: 10.1177/1533317510382893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrie GH, et al. Dendritic spinopathy in transgenic mice expressing ALS/dementia-linked mutant UBQLN2. Proc Natl Acad Sci U S A. 2014;111:14524–9. doi: 10.1073/pnas.1405741111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosskreutz J, Van Den Bosch L, Keller BU. Calcium dysregulation in amyotrophic lateral sclerosis. Cell Calcium. 2010;47:165–74. doi: 10.1016/j.ceca.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Haidet-Phillips AM, et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol. 2011;29:824–8. doi: 10.1038/nbt.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CE, et al. Progressive Motor Neuron Pathology and the Role of Astrocytes in a Human Stem Cell Model of VCP-Related ALS. Cell Rep. 2017;19:1739–1749. doi: 10.1016/j.celrep.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406:147–50. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- Henley JM, Wilkinson KA. Synaptic AMPA receptor composition in development, plasticity and disease. Nat Rev Neurosci. 2016 doi: 10.1038/nrn.2016.37. [DOI] [PubMed] [Google Scholar]
- Henstridge CM, et al. Synapse loss in the prefrontal cortex is associated with cognitive decline in amyotrophic lateral sclerosis. Acta Neuropathol. 2017 doi: 10.1007/s00401-017-1797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz-Martin S, et al. Viral delivery of C9orf72 hexanucleotide repeat expansions in mice leads to repeat-length-dependent neuropathology and behavioural deficits. Dis Model Mech. 2017;10:859–868. doi: 10.1242/dmm.029892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideyama T, et al. Induced loss of ADAR2 engenders slow death of motor neurons from Q/R site-unedited GluR2. J Neurosci. 2010;30:11917–25. doi: 10.1523/JNEUROSCI.2021-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideyama T, et al. Co-occurrence of TDP-43 mislocalization with reduced activity of an RNA editing enzyme, ADAR2, in aged mouse motor neurons. PLoS One. 2012a;7:e43469. doi: 10.1371/journal.pone.0043469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideyama T, et al. Profound downregulation of the RNA editing enzyme ADAR2 in ALS spinal motor neurons. Neurobiol Dis. 2012b;45:1121–8. doi: 10.1016/j.nbd.2011.12.033. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Hartley M, Heinemann SF. Calcium permeability of KA-AMPA-gated glutamate receptor channels depnds on subunit composition. Science. 1991;252:851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013;80:704–17. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoangeli A, Tiedge H. Translational control at the synapse: role of RNA regulators. Trends Biochem Sci. 2013;38:47–55. doi: 10.1016/j.tibs.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–72. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai Y, et al. Axonal Dysfunction Precedes Motor Neuronal Death in Amyotrophic Lateral Sclerosis. PLoS One. 2016;11:e0158596. doi: 10.1371/journal.pone.0158596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, et al. Gain of Toxicity from ALS/FTD-Linked Repeat Expansions in C9ORF72 Is Alleviated by Antisense Oligonucleotides Targeting GGGGCC-Containing RNAs. Neuron. 2016;90:535–50. doi: 10.1016/j.neuron.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo IS, et al. Oral administration of memantine prolongs survival in a transgenic mouse model of amyotrophic lateral sclerosis. J Clin Neurol. 2007;3:181–6. doi: 10.3988/jcn.2007.3.4.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicic A, et al. Modifiers of C9orf72 dipeptide repeat toxicity connect nucleocytoplasmic transport defects to FTD/ALS. Nat Neurosci. 2015;18:1226–9. doi: 10.1038/nn.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapeli K, Martinez FJ, Yeo GW. Genetic mutations in RNA-binding proteins and their roles in ALS. Hum Genet. 2017;136:1193–1214. doi: 10.1007/s00439-017-1830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y, et al. Low editing efficiency of GluR2 mRNA is associated with a low relative abundance of ADAR2 mRNA in white matter of normal human brain. Eur J Neurosci. 2003;18:23–33. doi: 10.1046/j.1460-9568.2003.02718.x. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, et al. Glutamate receptors: RNA editing and death of motor neurons. Nature. 2004;427:801. doi: 10.1038/427801a. [DOI] [PubMed] [Google Scholar]
- Keegan LP, et al. Adenosine deaminases acting on RNA (ADARs): RNA-editing enzymes. Genome Biol. 2004;5:209. doi: 10.1186/gb-2004-5-2-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi B, et al. Cytoplasmic poly-GA aggregates impair nuclear import of TDP-43 in C9orf72 ALS/FTLD. Hum Mol Genet. 2017;26:790–800. doi: 10.1093/hmg/ddw432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan MC. Hyperexcitability, persistent Na+ conductances and neurodegeneration in amyotrophic lateral sclerosis. Exp Neurol. 2009;218:1–4. doi: 10.1016/j.expneurol.2009.03.039. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Taylor JP. Lost in Transportation: Nucleocytoplasmic Transport Defects in ALS and Other Neurodegenerative Diseases. Neuron. 2017;96:285–297. doi: 10.1016/j.neuron.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, et al. Changes in the Excitability of Neocortical Neurons in a Mouse Model of Amyotrophic Lateral Sclerosis Are Not Specific to Corticospinal Neurons and Are Modulated by Advancing Disease. J Neurosci. 2017;37:9037–9053. doi: 10.1523/JNEUROSCI.0811-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U, et al. Molecular cloning of cDNA for double-stranded RNA adenosine deaminase, a candidate enzyme for nuclear RNA editing. Proc Natl Acad Sci U S A. 1994;91:11457–61. doi: 10.1073/pnas.91.24.11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppers M, et al. C9orf72 ablation in mice does not cause motor neuron degeneration or motor deficits. Ann Neurol. 2015;78:426–38. doi: 10.1002/ana.24453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak S, Kawahara Y. Deficient RNA editing of GluR2 and neuronal death in amyotropic lateral sclerosis. J Mol Med (Berl) 2005;83:110–20. doi: 10.1007/s00109-004-0599-z. [DOI] [PubMed] [Google Scholar]
- Kwon I, et al. Poly-dipeptides encoded by the C9ORF72 repeats bind nucleoli, impede RNA biogenesis, and kill cells. Science. 2014 doi: 10.1126/science.1254917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagier-Tourenne C, et al. Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2013 doi: 10.1073/pnas.1318835110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C, et al. Amyotrophic lateral sclerosis 2-deficiency leads to neuronal degeneration in amyotrophic lateral sclerosis through altered AMPA receptor trafficking. J Neurosci. 2006;26:11798–806. doi: 10.1523/JNEUROSCI.2084-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larroquette F, et al. Vapb/Amyotrophic lateral sclerosis 8 knock-in mice display slowly progressive motor behavior defects accompanying ER stress and autophagic response. Hum Mol Genet. 2015;24:6515–29. doi: 10.1093/hmg/ddv360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasiene J, et al. Neuregulin 1 confers neuroprotection in SOD1-linked amyotrophic lateral sclerosis mice via restoration of C-boutons of spinal motor neurons. Acta Neuropathol Commun. 2016;4:15. doi: 10.1186/s40478-016-0286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzer RB. The origin of muscle fasciculations and cramps. Muscle Nerve. 1994;17:1243–9. doi: 10.1002/mus.880171102. [DOI] [PubMed] [Google Scholar]
- Lee YB, et al. Hexanucleotide Repeats in ALS/FTD Form Length-Dependent RNA Foci, Sequester RNA Binding Proteins, and Are Neurotoxic. Cell reports. 2013 doi: 10.1016/j.celrep.2013.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YR, et al. Stress granules as crucibles of ALS pathogenesis. J Cell Biol. 2013;201:361–72. doi: 10.1083/jcb.201302044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, et al. C9orf72 BAC Mouse Model with Motor Deficits and Neurodegenerative Features of ALS/FTD. Neuron. 2016;90:521–34. doi: 10.1016/j.neuron.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Liu-Yesucevitz L, et al. Local RNA translation at the synapse and in disease. J Neurosci. 2011;31:16086–93. doi: 10.1523/JNEUROSCI.4105-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui H, et al. Progranulin Deficiency Promotes Circuit-Specific Synaptic Pruning by Microglia via Complement Activation. Cell. 2016;165:921–35. doi: 10.1016/j.cell.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madill M, et al. Amyotrophic lateral sclerosis patient iPSC-derived astrocytes impair autophagy via non-cell autonomous mechanisms. Mol Brain. 2017;10:22. doi: 10.1186/s13041-017-0300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan SS, et al. Exposure of neurons to excitotoxic levels of glutamate induces cleavage of the RNA editing enzyme, adenosine deaminase acting on RNA 2, and loss of GLUR2 editing. Neuroscience. 2011;189:305–15. doi: 10.1016/j.neuroscience.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharjan N, et al. C9ORF72 Regulates Stress Granule Formation and Its Deficiency Impairs Stress Granule Assembly, Hypersensitizing Cells to Stress. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-9850-1. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–26. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Man HY. GluA2-lacking, calcium-permeable AMPA receptors--inducers of plasticity? Curr Opin Neurobiol. 2011;21:291–8. doi: 10.1016/j.conb.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto MC, et al. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–57. doi: 10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- May S, et al. C9orf72 FTLD/ALS-associated Gly-Ala dipeptide repeat proteins cause neuronal toxicity and Unc119 sequestration. Acta Neuropathol. 2014 doi: 10.1007/s00401-014-1329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher T, et al. Editing of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR-B pre-mRNA in vitro reveals site-selective adenosine to inosine conversion. J Biol Chem. 1995;270:8566–70. doi: 10.1074/jbc.270.15.8566. [DOI] [PubMed] [Google Scholar]
- Melcher T, et al. RED2, a brain-specific member of the RNA-specific adenosine deaminase family. J Biol Chem. 1996;271:31795–8. doi: 10.1074/jbc.271.50.31795. [DOI] [PubMed] [Google Scholar]
- Milan L, et al. Age-Related Changes in Pre- and Postsynaptic Partners of the Cholinergic C-Boutons in Wild-Type and SOD1G93A Lumbar Motoneurons. PLoS One. 2015;10:e0135525. doi: 10.1371/journal.pone.0135525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RG, et al. Phase III randomized trial of gabapentin in patients with amyotrophic lateral sclerosis. Neurology. 2001;56:843–848. doi: 10.1212/wnl.56.7.843. [DOI] [PubMed] [Google Scholar]
- Mitchell J, et al. Familial amyotrophic lateral sclerosis is associated with a mutation in D-amino acid oxidase. Proc Natl Acad Sci U S A. 2010;107:7556–61. doi: 10.1073/pnas.0914128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizielinska S, et al. C9orf72 repeat expansions cause neurodegeneration in Drosophila through arginine-rich proteins. Science. 2014 doi: 10.1126/science.1256800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science. 2013;339:1335–8. doi: 10.1126/science.1232927. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Baxter MG. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci. 2012;13:240–50. doi: 10.1038/nrn3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–22. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K. Functions and regulation of RNA editing by ADAR deaminases. Annu Rev Biochem. 2010;79:321–49. doi: 10.1146/annurev-biochem-060208-105251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonhoff U, et al. Ataxin-2 interacts with the DEAD/H-box RNA helicase DDX6 and interferes with P-bodies and stress granules. Mol Biol Cell. 2007;18:1385–96. doi: 10.1091/mbc.E06-12-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell MA, et al. Cloning of cDNAs encoding mammalian double-stranded RNA-specific adenosine deaminase. Mol Cell Biol. 1995;15:1389–97. doi: 10.1128/mcb.15.3.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke JG, et al. C9orf72 BAC Transgenic Mice Display Typical Pathologic Features of ALS/FTD. Neuron. 2015;88:892–901. doi: 10.1016/j.neuron.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke JG, et al. C9orf72 is required for proper macrophage and microglial function in mice. Science. 2016;351:1324–9. doi: 10.1126/science.aaf1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- Park SB, Kiernan MC, Vucic S. Axonal Excitability in Amyotrophic Lateral Sclerosis : Axonal Excitability in ALS. Neurotherapeutics. 2017;14:78–90. doi: 10.1007/s13311-016-0492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascuzzi RM, et al. A phase II trial of talampanel in subjects with amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2010;11:266–71. doi: 10.3109/17482960903307805. [DOI] [PubMed] [Google Scholar]
- Paul P, de Belleroche J. The role of D-serine and glycine as co-agonists of NMDA receptors in motor neuron degeneration and amyotrophic lateral sclerosis (ALS) Front Synaptic Neurosci. 2014;6:10. doi: 10.3389/fnsyn.2014.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul P, de Belleroche J. Experimental approaches for elucidating co-agonist regulation of NMDA receptor in motor neurons: Therapeutic implications for amyotrophic lateral sclerosis (ALS) J Pharm Biomed Anal. 2015;116:2–6. doi: 10.1016/j.jpba.2014.12.040. [DOI] [PubMed] [Google Scholar]
- Pehar M, et al. Role and Therapeutic Potential of Astrocytes in Amyotrophic Lateral Sclerosis. Curr Pharm Des. 2017 doi: 10.2174/1381612823666170622095802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng PL, et al. ADAR2-dependent RNA editing of AMPA receptor subunit GluR2 determines vulnerability of neurons in forebrain ischemia. Neuron. 2006;49:719–733. doi: 10.1016/j.neuron.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Perry S, et al. Homeostatic plasticity can be induced and expressed to restore synaptic strength at neuromuscular junctions undergoing ALS-related degeneration. Hum Mol Genet. 2017;26:4153–4167. doi: 10.1093/hmg/ddx304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters OM, et al. Human C9ORF72 Hexanucleotide Expansion Reproduces RNA Foci and Dipeptide Repeat Proteins but Not Neurodegeneration in BAC Transgenic Mice. Neuron. 2015;88:902–9. doi: 10.1016/j.neuron.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkau TL, et al. Synaptic dysfunction in progranulin-deficient mice. Neurobiology of disease. 2012;45:711–22. doi: 10.1016/j.nbd.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Picconi B, Piccoli G, Calabresi P. Synaptic dysfunction in Parkinson's disease. Adv Exp Med Biol. 2012;970:553–72. doi: 10.1007/978-3-7091-0932-8_24. [DOI] [PubMed] [Google Scholar]
- Radzicki D, et al. Early Impairment of Synaptic and Intrinsic Excitability in Mice Expressing ALS/Dementia-Linked Mutant UBQLN2. Front Cell Neurosci. 2016;10:216. doi: 10.3389/fncel.2016.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond LA, et al. Pathophysiology of Huntington's disease: time-dependent alterations in synaptic and receptor function. Neuroscience. 2011;198:252–73. doi: 10.1016/j.neuroscience.2011.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–68. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha MC, et al. Early changes of neuromuscular transmission in the SOD1(G93A) mice model of ALS start long before motor symptoms onset. PLoS One. 2013;8:e73846. doi: 10.1371/journal.pone.0073846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas F, et al. Reactive oxygen species trigger motoneuron death in non-cell-autonomous models of ALS through activation of c-Abl signaling. Front Cell Neurosci. 2015;9:203. doi: 10.3389/fncel.2015.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano G, et al. Chronological requirements of TDP-43 function in synaptic organization and locomotive control. Neurobiol Dis. 2014;71:95–109. doi: 10.1016/j.nbd.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, et al. Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann Neurol. 1990;28:18–25. doi: 10.1002/ana.410280106. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, et al. Excitatory amino acids in ALS: an update. Ann. Neurol. 1991;30:224–225. doi: 10.1002/ana.410300223. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, et al. Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proc Natl Acad Sci U S A. 1993;90:6591–6595. doi: 10.1073/pnas.90.14.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, et al. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–7. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Ruegsegger C, et al. Aberrant association of misfolded SOD1 with Na(+)/K(+)ATPase-alpha3 impairs its activity and contributes to motor neuron vulnerability in ALS. Acta Neuropathol. 2016;131:427–51. doi: 10.1007/s00401-015-1510-4. [DOI] [PubMed] [Google Scholar]
- Ryberg H, Askmark H, Persson LI. A double-blind randomized clinical trial in amyotrophic lateral sclerosis using lamotrigine: effects on CSF glutamate, aspartate, branched-chain amino acid levels and clinical parameters. Acta Neurol Scand. 2003;108:1–8. doi: 10.1034/j.1600-0404.2003.00111.x. [DOI] [PubMed] [Google Scholar]
- Rzhepetskyy Y, et al. CACNA1H missense mutations associated with amyotrophic lateral sclerosis alter Cav3.2 T-type calcium channel activity and reticular thalamic neuron firing. Channels (Austin) 2016;10:466–77. doi: 10.1080/19336950.2016.1204497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson JL, Gorski JA, Dell'Acqua ML. NMDA Receptor-Dependent LTD Requires Transient Synaptic Incorporation of Ca(2+)-Permeable AMPARs Mediated by AKAP150-Anchored PKA and Calcineurin. Neuron. 2016;89:1000–15. doi: 10.1016/j.neuron.2016.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareen D, et al. Targeting RNA Foci in iPSC-Derived Motor Neurons from ALS Patients with a C9ORF72 Repeat Expansion. Science translational medicine. 2013;5:208ra149. doi: 10.1126/scitranslmed.3007529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasabe J, et al. D-serine is a key determinant of glutamate toxicity in amyotrophic lateral sclerosis. EMBO J. 2007;26:4149–59. doi: 10.1038/sj.emboj.7601840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasabe J, et al. D-amino acid oxidase controls motoneuron degeneration through D-serine. Proc Natl Acad Sci U S A. 2012;109:627–32. doi: 10.1073/pnas.1114639109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanz O, et al. Cortical hyperexcitability in patients with C9ORF72 mutations: Relationship to phenotype. Muscle Nerve. 2016;54:264–9. doi: 10.1002/mus.25047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer Burguete A, et al. GGGGCC microsatellite RNA is neuritically localized, induces branching defects, and perturbs transport granule function. Elife. 2015;4:e08881. doi: 10.7554/eLife.08881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg PH, Higuchi M, Sprengel R. RNA editing of brain glutamate receptor channels: mechanism and physiology. Brain Res. Rev. 1998;26:217–229. doi: 10.1016/s0165-0173(97)00062-3. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–91. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Sellier C, et al. Loss of C9ORF72 impairs autophagy and synergizes with polyQ Ataxin-2 to induce motor neuron dysfunction and cell death. EMBO J. 2016 doi: 10.15252/embj.201593350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj BT, et al. C9ORF72 repeat expansion causes vulnerability of motor neurons to Ca(2+)-permeable AMPA receptor-mediated excitotoxicity. Nat Commun. 2018;9:347. doi: 10.1038/s41467-017-02729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton CF, et al. Activity-dependent FUS dysregulation disrupts synaptic homeostasis. Proc Natl Acad Sci U S A. 2014;111:E4769–78. doi: 10.1073/pnas.1406162111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton CF, Yu G. The function of RNA-binding proteins at the synapse: implications for neurodegeneration. Cell Mol Life Sci. 2015;72:3621–35. doi: 10.1007/s00018-015-1943-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgobio C, et al. Abnormal medial prefrontal cortex connectivity and defective fear extinction in the presymptomatic G93A SOD1 mouse model of ALS. Genes Brain Behav. 2008;7:427–34. doi: 10.1111/j.1601-183X.2007.00367.x. [DOI] [PubMed] [Google Scholar]
- Shahidullah M, et al. Defects in synapse structure and function precede motor neuron degeneration in Drosophila models of FUS-related ALS. J Neurosci. 2013;33:19590–8. doi: 10.1523/JNEUROSCI.3396-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD. Memory, plasticity and sleep - A role for calcium permeable AMPA receptors? Front Mol Neurosci. 2012;5:49. doi: 10.3389/fnmol.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya K, et al. A single blind randomized controlled clinical trial of mexiletine in amyotrophic lateral sclerosis: Efficacy and safety of sodium channel blocker phase II trial. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16:353–8. doi: 10.3109/21678421.2015.1038277. [DOI] [PubMed] [Google Scholar]
- Shiihashi G, et al. Dendritic Homeostasis Disruption in a Novel Frontotemporal Dementia Mouse Model Expressing Cytoplasmic Fused in Sarcoma. EBioMedicine. 2017;24:102–115. doi: 10.1016/j.ebiom.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B, et al. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–9. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Spalloni A, et al. Postsynaptic alteration of NR2A subunit and defective autophosphorylation of alphaCaMKII at threonine-286 contribute to abnormal plasticity and morphology of upper motor neurons in presymptomatic SOD1G93A mice, a murine model for amyotrophic lateral sclerosis. Cereb Cortex. 2011;21:796–805. doi: 10.1093/cercor/bhq152. [DOI] [PubMed] [Google Scholar]
- Spalloni A, Nutini M, Longone P. Role of the N-methyl-d-aspartate receptors complex in amyotrophic lateral sclerosis. Biochim Biophys Acta. 2013;1832:312–22. doi: 10.1016/j.bbadis.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Takuma H, et al. Reduction of GluR2 RNA editing, a molecular change that increases calcium influx through AMPA receptors, selective in the spinal ventral gray of patients with amyotrophic lateral sclerosis. ANN. NEUROL. 1999;46:806–815. doi: 10.1002/1531-8249(199912)46:6<806::aid-ana2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Taylor JP, Brown RH, Jr, Cleveland DW. Decoding ALS: from genes to mechanism. Nature. 2016;539:197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD, et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–80. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Therrien M, et al. Deletion of C9ORF72 Results in Motor Neuron Degeneration and Stress Sensitivity in C. elegans. PLoS One. 2013;8:e83450. doi: 10.1371/journal.pone.0083450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran H, et al. Differential Toxicity of Nuclear RNA Foci versus Dipeptide Repeat Proteins in a Drosophila Model of C9ORF72 FTD/ALS. Neuron. 2015;87:1207–14. doi: 10.1016/j.neuron.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay E, Martineau E, Robitaille R. Opposite Synaptic Alterations at the Neuromuscular Junction in an ALS Mouse Model: When Motor Units Matter. J Neurosci. 2017;37:8901–8918. doi: 10.1523/JNEUROSCI.3090-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MR, Kiernan MC. Does interneuronal dysfunction contribute to neurodegeneration in amyotrophic lateral sclerosis? Amyotroph Lateral Scler. 2012;13:245–50. doi: 10.3109/17482968.2011.636050. [DOI] [PubMed] [Google Scholar]
- Udagawa T, et al. FUS regulates AMPA receptor function and FTLD/ALS-associated behaviour via GluA1 mRNA stabilization. Nat Commun. 2015;6:7098. doi: 10.1038/ncomms8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Blitterswijk M, et al. Genetic overlap between apparently sporadic motor neuron diseases. PLoS One. 2012;7:e48983. doi: 10.1371/journal.pone.0048983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme P, et al. GluR2-dependent properties of AMPA receptors determine the selective vulnerability of motor neurons to excitotoxicity. J. Neurophysiol. 2002;88:1279–1287. doi: 10.1152/jn.2002.88.3.1279. [DOI] [PubMed] [Google Scholar]
- Vernay A, et al. A transgenic mouse expressing CHMP2Bintron5 mutant in neurons develops histological and behavioural features of amyotrophic lateral sclerosis and frontotemporal dementia. Hum Mol Genet. 2016;25:3341–3360. doi: 10.1093/hmg/ddw182. [DOI] [PubMed] [Google Scholar]
- Villalba RM, Mathai A, Smith Y. Morphological changes of glutamatergic synapses in animal models of Parkinson's disease. Front Neuroanat. 2015;9:117. doi: 10.3389/fnana.2015.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgo L, Samarasinghe S, de Belleroche J. Analysis of AMPA receptor subunit mRNA expression in control and ALS spinal cord. NeuroReport. 1996;7:2507–2512. doi: 10.1097/00001756-199611040-00021. [DOI] [PubMed] [Google Scholar]
- von Bernhardi R, et al. Glial Cells and Integrity of the Nervous System. Adv Exp Med Biol. 2016;949:1–24. doi: 10.1007/978-3-319-40764-7_1. [DOI] [PubMed] [Google Scholar]
- Vucic S, Nicholson GA, Kiernan MC. Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain. 2008;131:1540–50. doi: 10.1093/brain/awn071. [DOI] [PubMed] [Google Scholar]
- Vucic S, et al. Corticomotoneuronal function in asymptomatic SOD-1 mutation carriers. Clin Neurophysiol. 2010;121:1781–5. doi: 10.1016/j.clinph.2010.02.164. [DOI] [PubMed] [Google Scholar]
- Wainger BJ, et al. Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep. 2014;7:1–11. doi: 10.1016/j.celrep.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainger BJ, Cudkowicz ME. Cortical Hyperexcitability in Amyotrophic Lateral Sclerosis: C9orf72 Repeats. JAMA Neurol. 2015;72:1235–6. doi: 10.1001/jamaneurol.2015.2197. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhang D. Memantine prolongs survival in an amyotrophic lateral sclerosis mouse model. Eur J Neurosci. 2005;22:2376–80. doi: 10.1111/j.1460-9568.2005.04431.x. [DOI] [PubMed] [Google Scholar]
- Warmus BA, et al. Tau-mediated NMDA receptor impairment underlies dysfunction of a selectively vulnerable network in a mouse model of frontotemporal dementia. J Neurosci. 2014;34:16482–95. doi: 10.1523/JNEUROSCI.3418-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MC, et al. The dsRBP and Inactive Editor ADR-1 Utilizes dsRNA Binding to Regulate A-to-I RNA Editing across the C. elegans Transcriptome. Cell Rep. 2014 doi: 10.1016/j.celrep.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, et al. Antisense Proline-Arginine RAN Dipeptides Linked to C9ORF72-ALS/FTD Form Toxic Nuclear Aggregates that Initiate In Vitro and In Vivo Neuronal Death. Neuron. 2014;84:1213–25. doi: 10.1016/j.neuron.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, et al. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–9. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RJ, et al. Rab8, POSH, and TAK1 regulate synaptic growth in a Drosophila model of frontotemporal dementia. J Cell Biol. 2015;208:931–47. doi: 10.1083/jcb.201404066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo JA, et al. Loss of function CHCHD10 mutations in cytoplasmic TDP-43 accumulation and synaptic integrity. Nat Commun. 2017;8:15558. doi: 10.1038/ncomms15558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A, Vissel B. The essential role of AMPA receptor GluR2 subunit RNA editing in the normal and diseased brain. Front Mol Neurosci. 2012;5:34. doi: 10.3389/fnmol.2012.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, et al. Isoform-specific antibodies reveal distinct subcellular localizations of C9orf72 in amyotrophic lateral sclerosis. Ann Neurol. 2015;78:568–83. doi: 10.1002/ana.24469. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Kwak S. The molecular link between inefficient GluA2 Q/R site-RNA editing and TDP-43 pathology in motor neurons of sporadic amyotrophic lateral sclerosis patients. Brain Res. 2014;1584:28–38. doi: 10.1016/j.brainres.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Yang D, et al. FTD/ALS-associated poly(GR) protein impairs the Notch pathway and is recruited by poly(GA) into cytoplasmic inclusions. Acta Neuropathol. 2015;130:525–35. doi: 10.1007/s00401-015-1448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DJ, et al. PTEN regulates AMPA receptor-mediated cell viability in iPS-derived motor neurons. Cell Death Dis. 2014;5:e1096. doi: 10.1038/cddis.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi S, et al. 3'UTR Length-Dependent Control of SynGAP Isoform alpha2 mRNA by FUS and ELAV-like Proteins Promotes Dendritic Spine Maturation and Cognitive Function. Cell Rep. 2017;20:3071–3084. doi: 10.1016/j.celrep.2017.08.100. [DOI] [PubMed] [Google Scholar]
- Zhang K, et al. The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature. 2015;525:56–61. doi: 10.1038/nature14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, et al. Hyperactive somatostatin interneurons contribute to excitotoxicity in neurodegenerative disorders. Nat Neurosci. 2016;19:557–559. doi: 10.1038/nn.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, et al. An ALS-Associated Mutant SOD1 Rapidly Suppresses KCNT1 (Slack) Na(+)-Activated K(+) Channels in Aplysia Neurons. J Neurosci. 2017;37:2258–2265. doi: 10.1523/JNEUROSCI.3102-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, et al. Aggregation-prone c9FTD/ALS poly(GA) RAN-translated proteins cause neurotoxicity by inducing ER stress. Acta Neuropathol. 2014 doi: 10.1007/s00401-014-1336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu T, et al. RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc Natl Acad Sci U S A. 2013;110:E4968–77. doi: 10.1073/pnas.1315438110. [DOI] [PMC free article] [PubMed] [Google Scholar]