Abstract
Background
HIV-1 CRF01_AE is dominant in Thailand where RV144 vaccine trial was conducted. To study immune correlates of protection in ongoing trials, CRF01_AE derived reagents are essential. Here we present a panel of 14 HIV-1 infectious molecular clones (IMC) identified from different stages of infection, and characterization of their neutralization sensitivity using two standard assays.
Methods
One full-length IMC was constructed using a transmitted-founder virus to express Renilla luciferase (LucR) reporter gene and full-length envelopes (envs) of exogenous HIV-1. A panel of IMCs was generated, expressing envs of viruses from acute (Fiebig stages I/II and I-IV) and chronic (>Febig VI) infection. Neutralization assays were performed using TZM-bl or A3R5 cell lines, and sera or monoclonal antibodies (mAbs). Wilcoxon matched-paired test was used to assess neutralization differences between assays and reagents; correlation coefficients were evaluated by linear regression.
Results
Neutralization potency observed was significantly higher in the A3R5 assay when testing mAbs and serum pools (p<0.0001); the stage of infection from which env was derived did not associate with IMC neutralization sensitivity. Neutralization values from A3R5 and TZM-bl assays were strongly correlated when mAbs were tested (R2=0.7, p<0.0001), but a weaker association was seen with serum pools (R2=0.17, p=0.03).
Conclusions
This novel panel of CRF01_AE reporter-IMC is useful for assessing vaccine-induced neutralizing antibodies in multiple assays, including those utilizing primary cell targets. The significant differences in TZM-bl and A3R5 neutralization sensitivity, as well as the poor association when using polyclonal sera indicates the need for caution in choosing one specific platform.
Keywords: HIV-1, CRF01_AE, Infectious Molecular Clone, Neutralization Assay, A3R5, TZM-bl
INTRODUCTION
Vaccine efficacy in the Thai RV144 vaccine trial was 31%1 and stimulated an intensive worldwide effort to identify the cellular and humoral immune responses associated with this protective effect2–7. CRF01_AE accounted for 91.7% of RV144 HIV-1 infections8 but when compared to the large panels of subtype B and C viruses available, the CRF01_AE HIV-1 isolates currently represent only ~10% of the LANL sequence database.
To assess neutralizing antibody responses, two standardized cell-based assays have been developed, the TZM-bl assay9–11, and, more recently, the A3R5 assay12,13. While both assays use cell lines as the target for HIV-1 infection and luminescence as a reporter for target cell infection and both cell lines express the receptors required for HIV-1 infection (CD4, CXCR4 and CCR5), important differences exist between these two models. The TZM-bl cell line, derived from epithelial HeLa cells, expresses Firefly luciferase (FF) upon infection and has been widely used with envelope (Env)-pseudotyped viruses (PSV)10,14,15. To facilitate infection, the TZM-bl cell line was engineered to express CD4 and CCR5 at higher than physiologic levels16 that are observed for CD4+ T lymphocytes found in vivo12,17.
With the development of HIV-1 full-length replication competent infectious molecular clones (IMCs) expressing the Renilla reneformis luciferase gene (LucR)18,19, a second cell-based assay using A3R5 lymphoblastoid target cells, that naturally express CD4 and CXCR4 and are engineered to express CCR5 in copy numbers similar to that observed on human PBMC (peripheral blood mononuclear cells) was developed13,20. However, with previous studies showing differences in neutralization sensitivity between the two cell-based assays, uncertainty remains regarding which assay best reflects the events that occur in vivo and might eventually serve to measure antibodies that correlate with protection.
Here, we present the development of 14 Thai CRF01_AE full-length HIV-1 constructs and their neutralization profiles. Thai CRF01_AE envelope genes (envs) were cloned into a novel and highly functional CRF01_AE IMC backbone expressing LucR, resulting in fully functional virions that can productively infect permissive cell lines and natural HIV-1 target cells. The neutralizing activities of monoclonal antibodies (mAbs) and polyclonal sera were measured in both TZM-bl and A3R5 assays. We found that the neutralization susceptibility of these IMCs was greater in A3R5 cells, as has been previously shown18. In addition, comparison of serum antibody-mediated neutralization in the TZM-bl versus A3R5 assays showed little or no correlation.
METHODS
Cells
The TZM-bl cell line10 and the 293T/17 Human kidney cell line (CRL-11268) were obtained through the NIH AIDS Research and Reference Program, and from the American Type Culture Collection (ATCC), respectively. Adherent cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (PAA laboratories), 1% L-Glutamine and 1% penicillin/streptomycin (Gibco/BRL). The A3R5.7 cell line20 was made in our laboratory and maintained in RPMI 1640 growth medium supplemented with 15% heat-inactivated fetal bovine serum (PAA laboratories), 1% L-Glutamine and 1% penicillin/streptomycin (Gibco/BRL) and 600 µg/ml (acitive) geneticin (G418, Gibco/BRL).
Viruses
With the exception of TH023, CM235 and CM244, isolated from infected PBMC co-cultures, CRF01_AE HIV-1 envelopes were retrieved by single genome amplification (SGA) and sequenced from plasma of Thai infected subjects21. Full viral genomes were also retrieved and cloned to generate full-length IMCs. One particular IMC, 40061, with the highest replicative capacity was selected to serve as CRF01_AE backbone and the entire gp160 coding sequences were then cloned as previously described18. Briefly, the cassette for the LucR gene was inserted in the full-length molecular clone, between the env and nef DNA sequences by multiple rounds of fusion PCR and unique enzyme restriction sites, AarI and BglI. The backbone was then further engineered to express the MluI restriction site which is not naturally present. For chimeric constructs, purified env inserts were amplified with primers containing the MluI and BsiWI sequences, digested with the required restriction enzymes, and ligated into the backbone.LucR. Final constructs were sequenced for verification.
Infection and tropism
Viral stocks were prepared in 293T cells and each stock was titrated in TZM-bl10,11 and A3R520 cell lines to determine TCID50 (50% tissue culture infectious dose). A cut-off value of 3-times over background (uninfected cells) relative light units (RLU) for FF was implemented, as described elsewhere14. For LucR, the cut-off was determined as being 3× [(average of negative wells)+3SD]18. RLUs were measured on a Victor X light Luminescence counter (Perkin-Elmer) with an exposure time of 0.1 s/well.
Neutralization assays
Inhibition of HIV-1 infection with different mAbs and sera was analyzed in TZM-bl and A3R5 cells using the 14 chimeric IMCs. TZM-bl and A3R5 neutralization assays were performed as previously described17,20. Neutralization was measured as the reduction in FF RLU and/or LucR RLU in the presence of serial antibody/serum dilutions. Viral input used in both assay platforms was normalized to a dilution expected to produce a level of RLU at least 10-times above cut off value17. The reciprocal titers were derived through averaging values from two independent assays for which the IC50 values were within 3-fold range. For our study, soluble CD4 (sCD4) and a total of eleven mAbs recognizing different Env regions were tested: VRC01, 3BNC117 and b12 (CD4 binding site (CD4bs)); PG9 and PG16 (V1/V2 region); PGT121 and PGT126 (V3 region); 2F5, 4E10, and 10E8 (MPER); and glycan-dependent mAb 2G12. All reagents were obtained through the NIH AIDS Research and Reference Program. Pools of sera from subjects infected with HIV-1 CRF01_AE or subtype B sera were also investigated17. Serum IgG was depleted using protein G sepharose 4 Fast Flow (GE Healthcare Life Sciences, Marlborough, MA).
Data analysis
DNA sequences were assembled and analyzed using Sequencher version 5.0 (Genecodes Inc., Ann Arbor, MI). Statistical analysis was carried out using GraphPad Prism 6.0 software. Wilcoxon matched-paired test was used to assess significant differences of the neutralization sensitivity. Correlation coefficients were evaluated using linear regression.
RESULTS
Newly developed IMC
We had previously developed IMCs to swap and express HIV-1 env genes as replication competent viruses in subtype matched- and non-matched HIV backbones, and have shown that non-env genes may impact neutralization profiles18. At the time these IMCs were engineered, only one CRF01_AE IMC was available, which had been isolated from multiple-passaged PBMCs22 and showed moderate replicative capacity. We have now developed several CRF01_AE full-length IMC, directly derived from plasma of infected Thai subjects using SGA. Among those, the construct showing the highest replicative capacity in TZM-bl and A3R5 cells, 40061, was selected (Supplemental Table 1) and engineered to encode (i) LucR and (ii) to express gp160 of exogenous HIV-1 env18, generating a panel of 14 CRF01_AE envs expressed into fully replicative 40061.LucR subtype-matched-backbone. Furthermore, 40061.LucR showed productive infection in human PBMCs and monocyte-derived macrophages expanding the scope of immunological assays to HIV-1 natural target cells (Supplemental Table 1).
The CRF01_AE envelope panel chosen was exclusively from Thailand and included mainly Envs derived from recently infected individuals. Among them, three were isolated in early Fiebig stage I/II, two were isolated in later Fiebig stage I/II and three were from Fiebig stage III–V (Table 1). Those envelopes were SGA-derived from plasma of subjects infected between 2005 and 2010. Finally, three Envs from subjects isolated early in HIV-1 Thai epidemic, between 1990 and 1992 were added to our panel (Table 1); these envelopes were isolated from PBMC co-cultures and have been previously referenced as chronic viruses23. All virus stocks demonstrated robust viral replication in TZM-bl and A3R5 cells. Most IMCs had higher titers in TZM-bl cells with an average TCID50 of 2×106 as compared with 1×105 in the A3R5 cell line (Supplemental Figure 1).
Table 1.
Envelope characterization
| Env | Subject | Year of isolation |
Stage1 | Co-receptor usage |
Co-receptor prediction |
Accession number |
|---|---|---|---|---|---|---|
| 40061 | 40061 | 2009 | early FI/II | CCR5 | CCR5 | KY580548 |
| 40100 Mj | 40100 | 2010 | early FI/II | CCR5 | CXCR4/CCR5 | KY580588 |
| 40100 mn | 40100 | 2010 | early FI/II | CCR5 | CXCR4/CCR5 | MF622080 |
|
| ||||||
| 356272 | AA047 | 2005 | late FI/II | CCR5 | CXCR4/CCR5 | JN944654 |
| 427299 | AA116 | 2006 | late FI/II | CCR5 | CCR5 | JN944655 |
| 620345 | AA081 | 2005 | late FI/II | CCR5 | CXCR4/CCR5 | JX12900 |
| 703357 | AA118 | 2005 | late FI/II | CCR5 | CCR5 | JN944658 |
| 816763 | AA119 | 2006 | late FI/II | CCR5 | CXCR4/CCR5 | JN944659 |
|
| ||||||
| 434239 | AA058 | 2006 | FIII-V | CCR5 | CXCR4/CCR5 | JX447346 |
| 731027 | AA104 | 2006 | FIII-V | CCR5 | CCR5 | JX447986 |
| 816769 | AA107 | 2006 | FIII-V | CCR5 | CCR5 | JX448026 |
|
| ||||||
| TH023 | 92TH023 | 1992 | C | CCR5 | CCR5 | KU562843 |
| CM244 | 1990 | C | CCR5 | CCR5 | AY13425 | |
| CM245 | 1990 | C | CCR5 | CXCR4/CCR5 | JN944662 | |
Stages of infection. Early FI/II: Fiebig stage I/II isolated within 7 days after the patient’s first nucleic acid positive test; late FI/II: Fiebig stage I/II; FIII-V: Fiebig stage III-V; C: chronic (after Fiebig stage VI).
Higher sensitivity of HIV-1 neutralization in A3R5-based assays
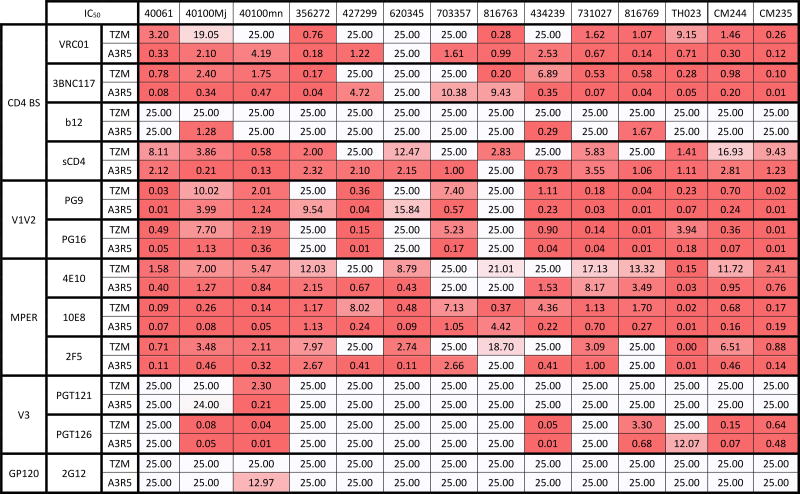
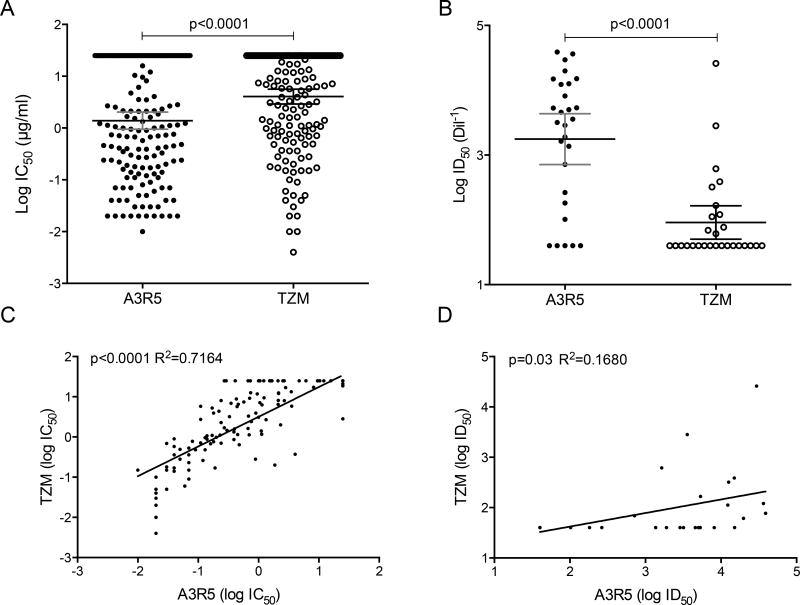
Neutralization sensitivity of the CRF01_AE IMC panel was tested in TZM-bl and in A3R5 cells against sCD4 and an array of mAbs targeting the four major antigenic regions on HIV-1 envelope as well as pooled HIV-1 positive sera; individual results are depicted in Table 2. We compared the neutralization results obtained in both assays by pairing all IC50 (mAbs) and ID50 (sera) data and found that neutralization sensitivity to mAbs (Figure 1A) and to sera (Figure 1B) was significantly higher in A3R5 than in TZM-bl cells (p<0.0001). While a strong correlation of neutralization susceptibility against mAbs between the two cell-based assays was found (R2=0.7 and p<0.0001 Figure 1C), a low to moderate correlation was observed with sera (R2=0.17 and p=0.03 Figure 1D). To investigate further if the increased sensitivity in the A3R5 assay is mediated by non-IgG factors, we measured neutralization sensitivity of three IMCs using untreated and IgG depleted sera in the A3R5 assay and observed a significant reduction in activity with the IgG-depleted sera (p=0.03; Supplemental Figure 2). The low levels of detectable neutralizing activity in some IgG-depleted serum assays could be due to: 1) the presence of residual IgG which was not quantified, 2) the presence of potentially neutralizing serum IgA which would not have been fully removed by the protein G sepharose beads, or 3) the presence of potentially neutralizing degraded IgG Fab fragments which would have also not been fully removed by the protein G sepharose beads. However, the neutralizing activity was reduced by 98.8% to 99.8% compared with the matched untreated serum, indicating that the majority of the activity was IgG-mediated.
Table 2.
Neutralization IC50 titers (µg/ml) of a panel of mAbs against CRF01_AE viruses in A3R5 versus TZM-bl cells
Figure 1.
Neutralization sensitivity of CRF01_AE viruses against mAbs (A) and sera (B) measured in A3R5 and TZM-bl cells by luminescence readouts. Linear regression to compare neutralization sensitivity against mAbs (C) and sera (D) between both cell lines were performed.
We then investigated if mAbs against any particular envelope domain were specifically responsible for the increased neutralization sensitivity observed in A3R5 vs TZM-bl cell-based assay. A panel of mAbs targeting the four different antigenic regions on HIV-1 envelope, chosen as described in the Methods and Materials, were significantly more potent in the A3R5 cell-based assay (Figure 2A; p-values range 0.0078 to <0.0001). HIV+ CRF01_AE and subtype B pooled sera were also evaluated (Figure 2B), with similarly higher neutralization observed using A3R5 when compared to TZM-bl cell targets (p=0.0005 and p=0.001, respectively). CRF01_AE viruses were more sensitive to neutralization by matched-subtype pooled sera in both cell-based assays, as seen in previous studies24,25.
Figure 2.
Neutralization sensitivity against the four different antigenic regions on CRF01_AE envelopes measured in A3R5 and TZM-bl cells using specific mAbs (A). Neutralization susceptibility against CRF01_AE and subtype B pooled sera was evaluated in A3R5 and TZM-bl cells (B). Neutralization sensitivity of CRF01_AE viruses isolated at different Fiebig stages against mAbs (C) and CRF01_AE and subtype B pooled sera (D) measured in A3R5 and TZM-bl cells by luminescence readouts.
Higher neutralization susceptibility in A3R5 cells is independent of the virus stage, year and mode of isolation
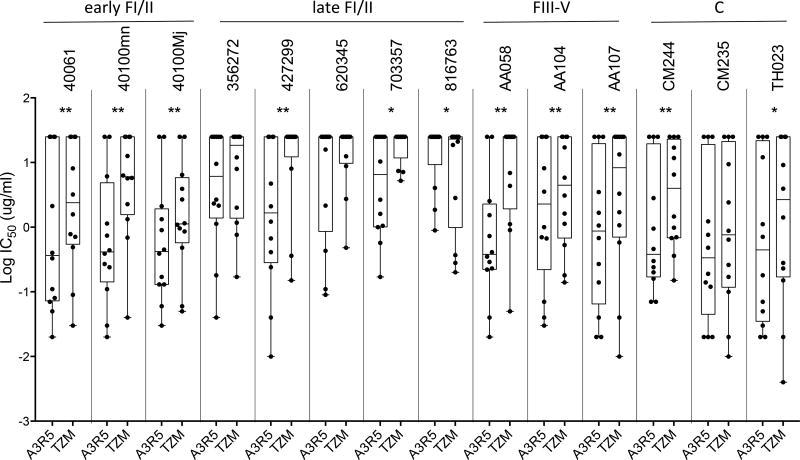
It has previously been shown that viruses isolated in the early years of the HIV epidemic were more sensitive to neutralization than recently isolated viruses26,27. Previous studies have yielded conflicting results regarding neutralization susceptibility of acute and chronic viruses24,28–30. As described in Table 1, our panel of CRF01_AE IMCs included isolates of different stages of the viral infection and from different times in the Thai HIV epidemic history, ranging from 1990 to 2010. Our panel can be categorized into three groups of viruses: early Fiebig stage I/II, late Fiebig stage I/II, Fiebig stage III-V, and one group of chronic viruses (Fiebig stage VI). In this study, we analyzed the combined neutralization data obtained in both cell-based assays against the 11 mAbs and sCD4. While higher sensitivity was observed in A3R5 cells, a similar trend of neutralization was observed among the 4 groups of viruses in TZM-bl cells (Figure 2C). Late Fiebig stage I/II viruses were less sensitive to neutralization than chronic (p<0.0001) and unexpectedly, less sensitive compared to early Fiebig stage I/II viruses (p=0.0002 and 0.0003, respectively). Although there was a trend towards higher neutralization sensitivity with HIV-1 from the early Thai epidemic (1990's) in TZM-bl and A3R5 cells, no statistical differences were found except between early Fiebig stage I/II and late Fiebig stage I/II viruses in A3R5 cells and between late Fiebig stage I/II and chronic viruses in TZM-bl cells (Figure 2D). The increased neutralization sensitivity observed in A3R5 was neither linked to the year of virus transmission nor to the stages of infection as represented in Figure 3, which depicts the neutralization sensitivities of individual viruses against the panel of mAbs. Interestingly, mAb-neutralization of 816763 revealed the unique case of higher TZM-bl sensitivity (Figure 3; p=0.03).
Figure 3.
Neutralization sensitivity of individual CRF01_AE virus against the panel of mAbs measured in A3R5 and TZM-bl cells.
DISCUSSION
The need for replication-competent IMC that can be used in a variety of cell types and standardized immune assays has become increasingly important. These molecular constructs express the entire gp160 Env, have full replicative capacity, and preliminary results have shown that they can be used in other immunological assays such as ADCC (antibody-dependent cell-mediated cytotoxicity)31 (Ferrari G, personal communication). Here we present a panel of novel reporter-IMC to assess neutralization susceptibility of CRF01_AE envelopes and to better evaluate the immune responses against CRF01_AE HIV-1 using TZM-bl and A3R5 cell-based assays. Since we have previously shown that non-env genes may affect neutralization sensitivity18, a CRF01_AE IMC was selected to serve as an HIV backbone for use with CRF01_AE envelopes for assessing vaccine responses in trials conducted in Thailand and countries where CRF01_AE HIV-1 is prevalent. This IMC was SGA-derived from a plasma sample of a Thai subject seven days after the last negative blood test, qualifying it as a transmitted/founder virus. This virus showed a high replicative capacity and was never passaged in the laboratory, making it a suitable backbone vector that should perform at a level similar to naturally transmitted virus to express heterologous CRF01_AE Thai Envs.
IMCs in this study were characterized for sensitivity to neutralization by mAbs and sera in the two standardized assays, TZM-bl and A3R5 cell-based assays. Greater neutralization sensitivity was observed in A3R5 compared to TZM-bl cells for all the reagents tested, supporting previous reports13,32. However a significant correlation between assays was only observed with monoclonal antibodies (R2=0.7, p<0.0001). These data imply that, despite the higher neutralization sensitivity of the A3R5 assay, both cell lines measure similar patterns or relationships between mAb activities against these IMCs. Similar results were reported in the analysis of the RV144 and Vax003 HIV-1 vaccine efficacy trials, where serum neutralizing activity was detected in the A3R5 assay but not the TZM-bl assay when using the same CRF01_AE IMC (16). Due to the heightened sensitivity of the A3R5 assay for detecting neutralizing antibodies (NAbs), discrepancies between assay platforms may be more apparent when neutralization-resistant, tier 2 virus stocks are utilized. Additionally, differences in monoclonal antibody neutralization of cell-free versus cell-associated HIV-1 have been reported for the TZM-bl and A3R5 assay33. In both assays cell-free virus was found to be more sensitive to neutralization when compared to cell-associated virus, however this was dependent on the specificity of the mAb tested.
No correlation (R2 = 0.002) was observed when comparing the serum titers obtained using TZM-bl cells versus PBMC, which express CCR5 levels similar to those of A3R5 cells20, and higher neutralization sensitivity was observed using PBMC compared with TZM-bl cells, using both mAbs34 as well as sera17. This is not surprising, particularly with respect to polyclonal sera, for which the full content of the antibody repertoire may be largely unknown, and polyclonal sera have varying epitope specificities with potentially differing affinities and/or valencies. Antibody-virus-host cell interactions may also be heavily influenced by target cell adherence, primary and coreceptor densities, and the presence or absence of other host cell molecules at the cell surface and/or incorporated into virions; all of these parameters may affect neutralization read-outs.
We confirmed that the more potent A3R5 activity observed in this study was due to IgG-mediated HIV neutralization, and not a result of an artifact registered only when using A3R5 cells. The increased neutralization sensitivity observed in the A3R5 assay was not linked to the year of virus transmission or to the stages of infection. Indeed, with the exception of 816763, all viruses were more susceptible to mAb-neutralization in A3R5 than in the TZM-bl cell-based assay. Chronic viruses from the years 1990–92 were more sensitive to neutralization than the more current viruses, in both assays. The findings on subtype B HIV-1 showing an increase in neutralization resistance over a period of 20 years have also been reported26,27. With the limited panel of viruses isolated early after transmission, we did observe some differences in neutralization sensitivities between early Fiebig stage I/II viruses, and Fiebig stage III-V viruses, which may in part contribute to conflicting results reported previously24,28–30. Our study has some limitations due to the small sample size and the differences seen here may be due to the features of the viruses from earlier versus later stages of infection, or due to novel aspects of subtype CRF01_AE24. We are now engineering IMCs expressing cognate chronic envelopes to longitudinally measure neutralization profiles of a cohort of subjects who have been intensively studied very early during acute infection35,36
Considering the RV144 trial results, which are being elaborated in ongoing studies, antibody-mediated protection against HIV-1 acquisition is a likely mechanism, with a component of that reduction attributable to non-neutralizing antibody6,37–39. Similar findings have been observed in protection from SIV acquisition induced by vaccines and passive immunization with mAbs36,40–42. To best assess the role of neutralizing antibody, future HIV-1 biomedical prevention modalities will continue to require introspection about which antibody neutralization test is employed. Understanding the relevance of humoral responses elicited by HIV-1 vaccines may require analysis with viruses being transmitted or found circulating in the population, most of which have a tier 2 phenotype.
In this study we present a novel panel of full-length CRF01_AE infectious molecular clones with a reporter gene, generated from different Feibig acute stages of infection and from chronic infection. These IMcs will be useful for immunological studies, to include different functional humoral responses to HIV-1, and may be used to detect responses to envelopes of differing antibody sensitivities.
Supplementary Material
Supplemental Figure 1. TCID50 measured for CRF01_AE IMCs in A3R5 and TZM-bl cells.
Supplemental Figure 2. Neutralization sensitivity of three IMCs (40061, 40100 Mj and 40100 mn) against CRF01_AE and subtype B pooled whole or IgG-depleted sera.
Acknowledgments
We are grateful to the RV144 and RV217 study team and volunteers from whom the HIV-1 sequences derived. We thank the laboratory and sequence analysis team at Laboratory of Molecular Virology and Pathogenesis for their technical assistance. We are thankful to Dr. David Montefiori, Dr. Hongmei Gao and Ms. Kelli Green for contributing some of the clones generated through the Collaboration of AIDS Vaccine Discovery/Comprehensive Antibody Immune Monitoring Consortium. We also appreciate other members of MHRP for their time, efforts and support.
Disclosure Statements: The study was supported by a cooperative agreement (W81XWH-07-2-0067 and W81XWH-11-2-0174) between the Henry M. Jackson Foundation for the Advancement of Military Medicine and the Department of Defense. Some of the HIV-1 sequences used to generate infectious molecular clones were derived from RV144 and RV217 clinical protocols. The remaining sequences were retrieved from GenBank. Some of the clones were contributed by the Collaboration of AIDS Vaccine Discovery/Comprehensive Antibody Immune Monitoring Consortium (OPP#38619) that funded by the Bill & Melinda Gates Foundation.
Footnotes
Disclaimer: Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70–25.
Conflicts of Interest: The authors have declared that no conflicts of interest exist.
References
- 1.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 2.Li SS, Gilbert PB, Tomaras GD, et al. FCGR2C polymorphisms associate with HIV-1 vaccine protection in RV144 trial. J Clin Invest. 2014;124(9):3879–3890. doi: 10.1172/JCI75539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollara J, Bonsignori M, Moody MA, et al. HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities. J Virol. 2014;88(14):7715–7726. doi: 10.1128/JVI.00156-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitney JB, Hill AL, Sanisetty S, et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. 2014;512(7512):74–77. doi: 10.1038/nature13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zolla-Pazner S, Edlefsen PT, Rolland M, et al. Vaccine-induced Human Antibodies Specific for the Third Variable Region of HIV-1 gp120 Impose Immune Pressure on Infecting Viruses. EBioMedicine. 2014;1(1):37–45. doi: 10.1016/j.ebiom.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottardo R, Bailer RT, Korber BT, et al. Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS One. 2013;8(9):e75665. doi: 10.1371/journal.pone.0075665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu P, Yates NL, Shen X, et al. Infectious virion capture by HIV-1 gp120-specific IgG from RV144 vaccinees. J Virol. 2013;87(14):7828–7836. doi: 10.1128/JVI.02737-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kijak GH, Tovanabutra S, Rerks-Ngarm S, et al. Molecular evolution of the HIV-1 Thai epidemic between the time of RV144 immunogen selection to the execution of the vaccine efficacy trial. J Virol. 2013;87(13):7265–7281. doi: 10.1128/JVI.03070-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montefiori DC. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol. 2009;485:395–405. doi: 10.1007/978-1-59745-170-3_26. [DOI] [PubMed] [Google Scholar]
- 10.Wei X, Decker JM, Liu H, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46(6):1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei X, Decker JM, Wang S, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422(6929):307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 12.Sarzotti-Kelsoe M, Bailer RT, Turk E, et al. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J Immunol Methods. 2014;409:131–146. doi: 10.1016/j.jim.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarzotti-Kelsoe M, Daniell X, Todd CA, et al. Optimization and validation of a neutralizing antibody assay for HIV-1 in A3R5 cells. J Immunol Methods. 2014;409:147–160. doi: 10.1016/j.jim.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozaki DA, Gao H, Todd CA, Greene KM, Montefiori DC, Sarzotti-Kelsoe M. International technology transfer of a GCLP-compliant HIV-1 neutralizing antibody assay for human clinical trials. PLoS One. 2012;7(1):e30963. doi: 10.1371/journal.pone.0030963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polonis VR, Brown BK, Rosa Borges A, et al. Recent advances in the characterization of HIV-1 neutralization assays for standardized evaluation of the antibody response to infection and vaccination. Virology. 2008;375(2):315–320. doi: 10.1016/j.virol.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Montefiori DC, Karnasuta C, Huang Y, et al. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J Infect Dis. 2012;206(3):431–441. doi: 10.1093/infdis/jis367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown BK, Wieczorek L, Sanders-Buell E, et al. Cross-clade neutralization patterns among HIV-1 strains from the six major clades of the pandemic evaluated and compared in two different models. Virology. 2008;375(2):529–538. doi: 10.1016/j.virol.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Chenine AL, Wieczorek L, Sanders-Buell E, et al. Impact of HIV-1 backbone on neutralization sensitivity: neutralization profiles of heterologous envelope glycoproteins expressed in native subtype C and CRF01_AE backbone. PLoS One. 2013;8(11):e76104. doi: 10.1371/journal.pone.0076104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edmonds TG, Ding H, Yuan X, et al. Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology. 2010;408(1):1–13. doi: 10.1016/j.virol.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLinden RJ, Labranche CC, Chenine AL, et al. Detection of HIV-1 neutralizing antibodies in a human CD4(+)/CXCR4(+)/CCR5(+) T-lymphoblastoid cell assay system. PLoS One. 2013;8(11):e77756. doi: 10.1371/journal.pone.0077756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salazar-Gonzalez JF, Salazar MG, Keele BF, et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med. 2009;206(6):1273–1289. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salminen MO, Ehrenberg PK, Mascola JR, et al. Construction and biological characterization of infectious molecular clones of HIV-1 subtypes B and E (CRF01_AE) generated by the polymerase chain reaction. Virology. 2000;278(1):103–110. doi: 10.1006/viro.2000.0640. [DOI] [PubMed] [Google Scholar]
- 23.McCutchan FE, Hegerich PA, Brennan TP, et al. Genetic variants of HIV-1 in Thailand. AIDS Res Hum Retroviruses. 1992;8(11):1887–1895. doi: 10.1089/aid.1992.8.1887. [DOI] [PubMed] [Google Scholar]
- 24.Hraber P, Korber BT, Lapedes AS, et al. Impact of clade, geography, and age of the epidemic on HIV-1 neutralization by antibodies. J Virol. 2014;88(21):12623–12643. doi: 10.1128/JVI.01705-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seaman MS, Janes H, Hawkins N, et al. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol. 2010;84(3):1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bunnik EM, Euler Z, Welkers MR, et al. Adaptation of HIV-1 envelope gp120 to humoral immunity at a population level. Nat Med. 2010;16(9):995–997. doi: 10.1038/nm.2203. [DOI] [PubMed] [Google Scholar]
- 27.Euler Z, Bunnik EM, Burger JA, et al. Activity of broadly neutralizing antibodies, including PG9, PG16, and VRC01, against recently transmitted subtype B HIV-1 variants from early and late in the epidemic. J Virol. 2011;85(14):7236–7245. doi: 10.1128/JVI.00196-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derdeyn CA, Decker JM, Bibollet-Ruche F, et al. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science. 2004;303(5666):2019–2022. doi: 10.1126/science.1093137. [DOI] [PubMed] [Google Scholar]
- 29.Wilen CB, Parrish NF, Pfaff JM, et al. Phenotypic and immunologic comparison of clade B transmitted/founder and chronic HIV-1 envelope glycoproteins. J Virol. 2011;85(17):8514–8527. doi: 10.1128/JVI.00736-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H, Rola M, West JT, et al. Functional properties of the HIV-1 subtype C envelope glycoprotein associated with mother-to-child transmission. Virology. 2010;400(2):164–174. doi: 10.1016/j.virol.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joachim A, Nilsson C, Aboud S, et al. Potent functional antibody responses elicited by HIV-I DNA priming and boosting with heterologous HIV-1 recombinant MVA in healthy Tanzanian adults. PLoS One. 2015;10(4):e0118486. doi: 10.1371/journal.pone.0118486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.deCamp A, Hraber P, Bailer RT, et al. Global panel of HIV-1 Env reference strains for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2014;88(5):2489–2507. doi: 10.1128/JVI.02853-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gombos RB, Kolodkin-Gal D, Eslamizar L, et al. Inhibitory Effect of Individual or Combinations of Broadly Neutralizing Antibodies and Antiviral Reagents against Cell-Free and Cell-to-Cell HIV-1 Transmission. J Virol. 2015;89(15):7813–7828. doi: 10.1128/JVI.00783-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choudhry V, Zhang MY, Harris I, et al. Increased efficacy of HIV-1 neutralization by antibodies at low CCR5 surface concentration. Biochem Biophys Res Commun. 2006;348(3):1107–1115. doi: 10.1016/j.bbrc.2006.07.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ananworanich J, Chomont N, Eller LA, et al. HIV DNA Set Point is Rapidly Established in Acute HIV Infection and Dramatically Reduced by Early ART. EBioMedicine. 2016;11:68–72. doi: 10.1016/j.ebiom.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robb ML, Eller LA, Kibuuka H, et al. Prospective Study of Acute HIV-1 Infection in Adults in East Africa and Thailand. N Engl J Med. 2016;374(22):2120–2130. doi: 10.1056/NEJMoa1508952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stieh DJ, King DF, Klein K, et al. Aggregate complexes of HIV-1 induced by multimeric antibodies. Retrovirology. 2014;11:78. doi: 10.1186/s12977-014-0078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corey L, Gilbert PB, Tomaras GD, Haynes BF, Pantaleo G, Fauci AS. Immune correlates of vaccine protection against HIV-1 acquisition. Sci Transl Med. 2015;7(310):310rv317. doi: 10.1126/scitranslmed.aac7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dugast AS, Chan Y, Hoffner M, et al. Lack of protection following passive transfer of polyclonal highly functional low-dose non-neutralizing antibodies. PLoS One. 2014;9(5):e97229. doi: 10.1371/journal.pone.0097229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barouch DH, Liu J, Li H, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482(7383):89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hessell AJ, Hangartner L, Hunter M, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449(7158):101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 42.Barouch DH, Stephenson KE, Borducchi EN, et al. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell. 2013;155(3):531–539. doi: 10.1016/j.cell.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. TCID50 measured for CRF01_AE IMCs in A3R5 and TZM-bl cells.
Supplemental Figure 2. Neutralization sensitivity of three IMCs (40061, 40100 Mj and 40100 mn) against CRF01_AE and subtype B pooled whole or IgG-depleted sera.