Abstract
Background
Retinopathy of prematurity (ROP) is a major complication of preterm birth and has been associated with later visual and non-visual impairment
Objectives
To evaluate relationships between any stage of ROP, brain volumes and developmental outcome.
Methods
The study included 52 very preterm infants, mean (SD) GA 26.4 (1.9) weeks. Total brain (TBV), gray matter (GMV), unmyelinized white matter (UWMV) and cerebellar volumes were estimated in 51/52 infants by MRI at term equivalent age. Bayley Scales of Infant Development were used to assess developmental outcome in 49/52 infants at mean 24.6 months corrected age.
Results
19/52 infants developed any stage of ROP. Infants with ROP had lower median [interquartile range (IQR)] UWMV 173 [156–181] vs. 204 [186–216] ml, p<0.001) and cerebellar volume (18.3 [16.5–20] vs. 22.3 [20.3–24.7] ml, p<0.001) than infants without ROP. They also had a lower median [IQR] mental developmental index (72 [56–83] vs. 100 [88–104], p<0.001) and lower psychomotor developmental index (80 [60–85] vs. 92 [81–103], p=0.002). Brain volumes and developmental outcome did not differ among infants with different stages of ROP.
Conclusion
Any stage of ROP in preterm infants was associated with reduced brain volume and impaired developmental outcome. These results suggest that common pathways may lead to impaired neural and neurovascular development in the brain and retina and that all stages of ROP may be considered in future studies on ROP and development.
Keywords: brain volume, developmental outcome, magnetic resonance imaging, mental developmental index, psychomotor developmental index, preterm birth, retinopathy of prematurity
Introduction
With increasing survival of extremely preterm infants, a proportionately greater number of infants are affected by major complications of prematurity, where neurodevelopmental disability and visual problems are among the most common [1] [2]. Preterm birth coincides with a critical period of brain development but also with that of vascular development [3].
The retina is incompletely developed at preterm birth. ROP is considered to be a consequence of a primary arrest of retinal vascularization and a neurovascular disease involving both vascular and neural components [4, 5]. Postnatal head growth retardation, can be regarded as a proxy of brain growth and coincides with suppression of retinal vascular growth and with low levels of IGF-1, an important vascular and neural growth factor [6]. These events can be further related to development of severe ROP and low brain volumes at term equivalent age [6, 7]. Preterm infants who develop severe ROP appear to be at increased risk of visual [2] and nonvisual neurodevelopmental comorbidities and of delayed white matter maturation as estimated by MRI at early postnatal age and at term equivalent age [8– 11]. These findings suggest presence of common mechanisms in the development of these complications [6].
Although ROP is defined according to different stages, the disease is a continuous process of impaired vascular and neuronal retinal development. Therefore, our aim in this study was to evaluate the relationship between the presence of any stage of ROP, brain growth and later developmental outcome at 2 years of corrected age.
Material and methods
Study population
The original prospective study included 64 very preterm infants born at the neonatal intensive care unit (Lund, Sweden) between Jan 2005 and May 2007, where IGF-1 concentrations from birth until term age in relation to growth, MRI-estimated brain volume, and developmental outcome has been evaluated [7, 12, 13]. Fifty-two infants completed the study until term age, 51/52 underwent MRI at term age and 49/52 received follow-up examination at 2 years corrected age. Inclusion criteria were GA < 31 weeks, absence of major congenital anomalies, and written informed parental consent. Of the 64 recruited infants, 9 did not survive until term age and the parents of 3 infants chose to leave the study.
The study was approved by the regional ethical review board of Lund, Sweden and adhered to the tenets of the Declaration of Helsinki. All pregnancies were dated by ultrasound at 17–18 GW.
Clinical data
Weight standard deviation score (SDS) at birth was calculated from a Scandinavian intrauterine growth curve based on fetal weights estimated by ultrasound [14]. Cumulative dose of administered (mg/kg) hydrocortisone and betamethasone were registered until PMA 35 weeks. Total steroid exposure was estimated by converting the betamethasone dosage into hydrocortisone equivalents (1:40). Bronchopulmonary dysplasia was defined as a requirement for supplemental oxygen at PMA 36 weeks. Septicemia was defined as the presence of positive blood culture and concomitant increased levels of C-reactive protein.
Nutritional regime and calculation of intake
The nutritional strategy used for the infants has been described previously [7] and was based on individualized enteral nutrition using maternal or donor breast milk (fortified if required) and additional parenteral nutrition, initiated as soon as possible after birth. Maternal and donor breast milk was analyzed weekly for protein and energy content and enteral and parenteral daily intakes of protein (g/kg/d) and energy (kcal/kg/d) were prospectively calculated from birth until at least PMA 35 weeks.
Cerebral ultrasound
Cerebral ultrasound was performed on days 1, 3, and 7; at 3 and 6 weeks of age and at term. Severe intracranial hemorrhage was defined as the presence of IVH grade III or parenchymal hemorrhage. White matter damage was defined as the presence of periventricular echodensities or cysts that persisted for >7 days. Severe brain damage was defined as severe intracranial hemorrhage and/or white matter damage.
ROP examination
ROP screening followed the Swedish national protocol and began at 5–6 weeks of age, but not before PMA 31 weeks. The infants underwent retinal examinations through dilated pupils biweekly to once weekly depending on ROP severity, either until the retina was fully vascularized or the condition was considered stable. ROP was classified according to the International Classification of Retinopathy of Prematurity [15], and treatment followed the recommendations of the Early Treatment for Retinopathy of Prematurity Cooperative Group [16].
MRI
MRI was performed on a 3-Tesla Siemens Magnetom Allegra head scanner (Siemens AG Medical Solutions, Erlangen, Germany) in 51/52 infants at term age, mean (SD), 40.1 (0.6) gestational weeks. The protocol for image acquisition and image processing has previously been described in detail [7]. We calculated tissue volumes of TBV, GMV, UWMV in 46 infants and cerebellar volume in 51 infants.
Assessment at 2 years corrected age
A psychologist assessed developmental outcome in 49/52 infants at the mean (SD) corrected age of 24.6 (0.8) months by means of the Bayley Scales of Infant Development (BSID-II), with two different index scales, the MDI and the PDI, as previously described [13].
Statistical analysis
Statistical analysis was performed with SPSS 23 for Microsoft Windows (IBM, Armonk, NY, USA). P values < 0.05 were considered significant. Univariate analyses of differences between groups were assessed with the Mann–Whitney U test or Chi- square test as appropriate. Correlations between continuous variables were evaluated with the Spearman rank correlation coefficient.
Adjustment for other variables was performed with multiple linear regression analysis. In all models GA, BW and gender were included as independent variables. Additionally, all variables exhibiting significant univariate associations with the respective outcome variables (Table 2) were entered into the multivariate model as independent variables. Thus, independent variables entered into the analysis for cerebellar volume and UWMV respectively were GA (days), BW, gender, septicemia and total steroid intake (hydrocortisone equivalents mg/kg) from birth until PMA 35 weeks. Independent variables entered into the analysis for MDI were GA, BW, gender, Apgar score < 7 at 5 min and total steroid intake from birth until PMA 35 weeks. Independent variables entered into the analysis for PDI were GA (days), BW, gender and severe brain damage.
Table 2.
Relationships between clinical characteristics and cerebellar volume and unmyelinated white matter volume at a postmenstrual age of 40 weeks and Mental Developmental Index (MDI) and Psychomotor Developmental Index (PDI) at 2 years of corrected age (n = 52).
| Cerebellar volume |
Unmyelinated white matter volume |
MDI | PDI | |
|---|---|---|---|---|
| p-value | p-value | p-value | p-value | |
| GA, days | <0.001 | <0.001 | 0.001 | 0.015 |
| Birth weight, g | <0.001 | <0.001 | 0.004 | 0.191 |
| Apgar < 7 at 5 min | 0.992 | 0.78 | 0.047 | 0.344 |
| Male/female | 0.178 | 0.379 | 0.312 | 0.13 |
| Severe brain damagea | 0.298 | 0.9 | 0.111 | 0.025 |
| Septicemia | 0.022 | 0.021 | 0.199 | 0.294 |
| Total steroid intake, mg/kgb | 0.001 | <0.001 | 0.006 | 0.079 |
| Bronchopulmonary dysplasia | 0.063 | 0.076 | 0.362 | 0.656 |
| Energy intake, kcal/kg/dayc | 0.72 | 0.723 | 0.961 | 0.787 |
| Protein intake, g/kg/dayc | 0.138 | 0.907 | 0.496 | 0.957 |
| Maternal educational leveld | - | - | 0.099 | 0.352 |
| Paternal educational leveld | - | - | 0.131 | 0.223 |
Intraventricular hemorrhage grade III, periventricular hemorrhagic infarction, and/or white matter disease as defined by cerebral ultrasound.
Calculated hydrocortisone equivalents (mg/kg) from birth until postmenstrual age 35 weeks.
From birth until postmenstrual age 35 weeks
University degree vs. no university degree.
Results
Clinical characteristics and development of ROP
Table 1 presents clinical characteristics in infants with and without ROP. Infants with any ROP (n = 19) had lower GA, lower BW, higher frequency of septicemia and a higher total steroid intake compared to infants without ROP.
Table 1.
Clinical characteristics according to presence of retinopathy of prematurity (n = 52).
| No ROP (n = 33) | ROP (n = 19) | P value | |
|---|---|---|---|
| GA, weeks, median (range) | 27.4 (24.3–30.6) | 25.0 (23.0–27.1) | <0.001 |
| Birth weight, g, median (range) | 970 (592–1716) | 634 (348–854) | <0.001 |
| SDS weight at birth, median (range) | −0.7 (−4.4–0.8) | −1.8 (−4.7–0.6) | 0.1 |
| Apgar < 7 at 5 min, n (%) | 11 (33) | 10 (53) | 0.172 |
| Male/female, n (%) | 15/18 (45/55) | 10/9 (53/47) | 0.62 |
| Severe brain damage, n (%)a | 2 (6) | 3 (16) | 0.342 |
| Septicemia, n (%) | 7 (21) | 12 (63) | 0.002 |
| Total steroid intake, mg/kg, median (range)b | 0 (0–112) | 34 (0–105) | <0.001 |
| Bronchopulmonary dysplasia, n (%) | 22 (67) | 16 (84) | 0.206 |
| Energy intake, kcal/kg/day, median (range)c | 120 (104–140) | 121 (93–134) | 0.653 |
| Protein intake, g/kg/day, median (range)c | 3.2 (2.6–3.7) | 3.0 (2.6–3.6) | 0.082 |
Intraventricular hemorrhage grade III, parenchymal hemorrhage, and/or white matter damage as defined by cerebral ultrasound.
Calculated hydrocortisone equivalents (mg/kg) from birth until postmenstrual age 35 weeks.
From birth until postmenstrual age 35 weeks.
Clinical characteristics in relation to cerebellar volume, UWMV, MDI, and PDI
Table 2 presents relationships between clinical characteristics and cerebellar volume, UWMV, MDI, and PDI respectively. GA correlated with cerebellar volume (rs = 0.56, p < 0.001), UWMV (rs = 0.71, p < 0.001) and both MDI (rs= 0.46, p = 0.001), and PDI (rs = 0.34, p = 0.015). BW correlated with cerebellar volume (rs = 0.71, p < 0.001), UWMV (rs = 0.82, p < 0.001), and MDI (rs = 0.41, p = 0.004). Apgar score < 7 at 5 min was associated with lower MDI (p = 0.047), and severe brain damage was associated with lower PDI (p = 0.025). Any septicemia was associated with cerebellar volume (p=0.022) and UWMV (p=0.021). Higher total steroid intake correlated with lower cerebellar volume (rs = −0.45, p = 0.001), UWMV (rs = −0.50, p < 0.001), and MDI (rs = −0.39, p = 0.006).
Stages of ROP in relation to brain volume and neuro-developmental outcome
Out of 52 infants, 33 had no ROP, 9 had ROP stage 1 or 2, and 10 had ROP stage 3 (9 of these 10 infants received laser treatment). None of the infants had ROP > stage 3. Infants with ROP stages 1 or 2 had a lower mean TBV, GMV, UWMV, cerebellar volume, MDI, and PDI as compared to infants without ROP (p = 0.001, p = 0.007, p < 0.001, p < 0.001, p = 0.008 and p = 0.024 respectively). Infants with treated ROP had lower TBV, UWMV, cerebellar volume and MDI (p=0.03, p=0.002, p = 0.005, p= 0.002), whereas no significant difference could be shown for GMV or PDI, as compared to infants without ROP.
Mean values of brain volumes and developmental outcome did not differ between infants with ROP (stages 1–3) and those with treated ROP.
Any ROP in relation to brain volumes and developmental outcome
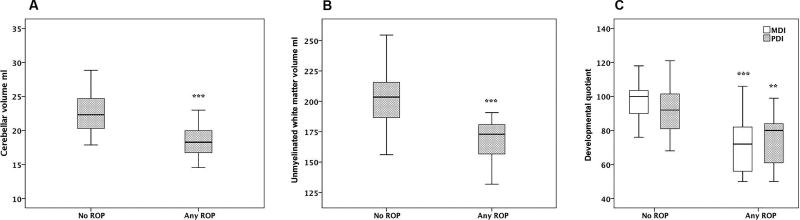
Table 3 presents median (IQR) values of brain volumes (TBV, GMV, UWMV and cerebellar volume) and of developmental outcome (MDI and PDI) in infants with and without ROP. In univariate analysis infants with any ROP had significantly lower TBV, UWMV, cerebellar volumes, MDI and PDI than infants without ROP. Multiple linear regression analysis was performed in order to further evaluate the relationship between any ROP and brain volumes at term-equivalent age and neuro-developmental outcome at 2 years of age. After adjustment for GA the relationships between any ROP and TBV and GMV, respectively, did not remain significant and were not further evaluated in multivariate models. Fig 1 A–C show the relationships between any ROP and cerebellar volume, UWMV, MDI and PDI, respectively. Table 4 presents the contribution of any ROP to cerebellar volume, UWMV, MDI and PDI following multivariate regression analysis. The relationships between any ROP and cerebellar and UWMV, respectively remained significant after adjustment for GA, BW, gender, septicemia and total steroid intake. The relationship between any ROP and MDI remained significant after adjustment for GA, BW, gender, Apgar score < 7 at 5 min and total steroid intake. The relationship between any ROP and PDI remained significant after adjustment for GA, BW, gender and severe brain damage.
Table 3.
Median (interquartile range) brain volumes (ml), at a postmenstrual age of 40 weeks and mental developmental index (MDI) and psychomotor developmental index (PDI) at 2 years of corrected age in infants with any ROP (n=19) or no ROP (n=32).
| Any ROP | No ROP |
p- value |
|
|---|---|---|---|
| Total brain volume | 371 (329–390) | 416 (377–445) | <0.001 |
| Gray matter volume | 193 (161–201) | 204 (186–231) | 0.054 |
| Unmyelinated white matter volume | 173 (156–181) | 204 (186–216) | <0.001 |
| Cerebellar volume | 18.3 (16.5–20) | 22.3 (20.3–24.7) | <0.001 |
| Mental Developmental Index | 72 (56–83) | 100 (88–104) | <0.001 |
| Psychomotor Developmental Index | 80 (60–85) | 92 (81–103) | 0.002 |
Fig 1.
Median and interquartile ranges of cerebellar volume (ml) (A), unmyelinated white matter volume (ml) (B), and Mental Developmental Index (MDI) and Psychomotor Developmental Index (PDI) (C) in relation to presence or absence of retinopathy of prematurity (ROP).
***p ≤ 0.001, **p ≤ 0.01
Table 4.
Contribution of any ROP (y/n) to cerebellar volume and to unmyelinized white matter volume (UWMV) at a postmenstrual age of 40 weeks and to mental developmental index (MDI) and psychomotor developmental index (PDI) at 2 years of corrected age in a linear regression model.
| Any ROP (y/n) | |||
|---|---|---|---|
|
| |||
| Outcome variable | P-value | R2 | Adj R2 |
| Cerebellar volumea | 0.029 | 0.50 | 0.43 |
| UWMVa | 0.030 | 0.58 | 0.52 |
| MDIb | 0.007 | 0.38 | 0.29 |
| PDIc | 0.03 | 0.42 | 0.35 |
Included independent variables: any ROP (y/n), gestational age (days), birth weight (g), gender, septicemia (y/n) and total steroid intake (calculated hydrocortisone equivalents, mg/kg) from birth to a postmenstrual age of 35 weeks.
Included independent variables: any ROP (y/n), gestational age (days), birth weight (g), gender, Apgar score <7 at 5 min and total steroid intake (calculated hydrocortisone equivalents, mg/kg) from birth to a postmenstrual age of 35 weeks.
Included independent variables: any ROP (y/n), gestational age (days), birth weight (g), gender, and severe brain damage (IVH grade III, and/or parenchymal hemorrhage, and/or WMD)
Discussion
Our main findings were that the presence of any stage of ROP was associated with lower cerebellar volume and UWMV at term equivalent age and with impaired cognitive and motor development at 2 years of corrected age. Thus, infants with less severe ROP (stages 1 or 2) exhibited a similar degree of brain volume reduction and neurodevelopmental impairment as did infants with more severe ROP (stage 3).
Several studies have shown an association between ROP and impaired later developmental outcome [2, 9–11]. The majority of studies compare severe ROP to no ROP and do not include less severe stages of ROP. A recent case control study comparing severe ROP with no or stage 1 ROP found severe ROP to be associated with reduced cerebellar and brainstem volumes at term and with neurodevelopmental deficits at 15 months and 2 years of age [17]. One study considered different stages of ROP and found the severity of neonatal ROP to be a marker for functional disability at 5.5 years of age. They found high rates of disability in VLBW infants who develop severe ROP and subsequently have unfavorable visual outcome [18].
Several features of retinal and brain development have links to common pathways that in turn may be affected by insults associated with very preterm birth. This is supported by similar risk factors being associated with ROP, lower brain volumes, and impaired neurodevelopment, which suggests common antecedent events that affect migration and proliferation in both the retina and the brain. The signaling and mitogenic protein and transcription factor sonic hedgehog is a key factor in both cerebellar and retinal proliferation during early development [19, 20].
In this study, ROP was primarily associated with lower cerebellar volume and UWMV. These respective brain regions and the retina may present a common vulnerability due to depletion of the same trophic factors. IGF-1 is an anabolic hormone that is critical to both vascular and neural development. Low IGF-1 levels in preterm infants have been associated with ROP [21], decreased brain volumes and impaired neurodevelopmental outcome at 2 years corrected age [7, 13].
The relationship between ROP and poor white matter development as determined by diffusion tensor imaging (DTI) and tractography has been evaluated in one study where severe ROP predicted white matter maturational delay in the optic radiations, but also in other regions of posterior white matter. The delayed white matter maturation was present independently of other signs of brain injury [11]. The retinal nerve fiber layer as estimated by spectral-domain optical coherence tomography at term equivalent age is thinner in very preterm infants and has been shown to correlate with white matter injury at term equivalent age and later impaired neurodevelopment [22].
The presence of supratentorial brain injury has previously been associated with impaired cerebellar growth [23]. Presence of severe brain damage, as defined by cerebral ultrasound was neither related to any ROP nor to estimated cerebellar volume in the current study although the small number of infants with severe brain damage could have obscured such an association.
In addition to lower cerebellar volume and UWMV, infants with any ROP had significantly lower MDI and PDI scores at 2 years of corrected age. Stage 1 ROP is sometimes difficult to diagnose and subtle forms may be missed at examination. However similar findings were reported by another study, in which the stage of ROP per se did not determine the subsequent presence of neurodevelopmental impairment [9]. Several studies have also documented the importance of cerebellar volume, particularly with respect to cognitive function in preterm infants at 2 years of age [13, 24, 25].
The accumulated dose of steroid intake displayed a significant association with any ROP, lower cerebellar and UWMV and lower MDI. Postnatal corticosteroid exposure in preterm infants has previously been related to increased risk of ROP, altered optic radiation structure and impaired cerebellar growth [26–28]. The neonatal cerebellum has the highest number of glucocorticoid receptors [29]. In mice, glucocorticoids inhibit proliferation of cerebellar granular neuron precursors [30].
In conclusion, we found that the development of any stage of ROP in very preterm infants was associated with reduced brain volumes and impaired developmental outcome at 2 years corrected age. Although based on a small cohort of infants our results suggests that any type of ROP, not just severe, should be considered in future studies on ROP and developmental outcome. These findings also raise the possibility of common pathways essential for the development of the retina and brain. Therefore strategies aiming at preventing any stage of ROP may also have neuroprotective effects on the developing brain.
Acknowledgments
We thank all of the families who participated in the study and the study nurses Eva Hammarstrand and Ann-Cathrine Berg for their excellent assistance.
Statement of financial support: This work was supported by the Swedish Medical Research Council (2014-3140); the European Commission FP7 project 305485 PREVENT-ROP; ALF government grants to Lund University; VINNOVA; the Skåne Council Foundation for Research and Development; the Linnéa and Josef Carlsson Foundation for Research and Development; and NIH EY024864, EY017017, P01 HD18655, and Lowy Medical Research Institute.
List of abbreviations
- BW
birth weight
- GW
gestational week
- GA
gestational age
- PMA
postmenstrual age
- IVH
intraventricular hemorrhage
- ROP
retinopathy of prematurity
- IGF-1
Insulin-like growth factor 1
- MRI
magnetic resonance imaging
- TBV
total brain volume
- GMV
gray matter volume
- UWMV
unmyelinated white matter volume
- MDI
Mental Developmental Index
- PDI
Psychomotor Developmental Index
- IQR
Interquartile range
Footnotes
Disclosure statement: Prevention of retinopathy of prematurity by administering Insulin-like growth factor 1 are covered by the patent owned by or licensed to Premacure AB, Uppsala, Sweden. A.H., D.L. and I.H.P. own shares in the company with financial interest in Premacure AB. A.H., D.L., L.E.H.S. and I.H.P. work as consultants for Shire Pharmaceuticals (Shire, Lexington, MA).
References
- 1.Laptook AR, O´Shea TM, Shankaran S, Bhaskar B NICHD Neonatal Network. Adverse Neurodevelopmental Outcomes Among Extremely Low Birth Weight Infants With a Normal Head Ultrasound: Prevalence and Antecedents. Pediatrics. 2005 Mar;115:673–680. doi: 10.1542/peds.2004-0667. [DOI] [PubMed] [Google Scholar]
- 2.Hellgren KM, Tornqvist K, Jakobsson PG, Lundgren P, Carlsson B, Källén K, Serenius F, Hellström A, Holmström G. Ophthalmologic Outcome of Extremely Preterm Infants at 6.5 Years of Age: Extremely Preterm Infants in Sweden Study (EXPRESS) JAMA Ophthalmol. 2016 doi: 10.1001/2016.0391. [DOI] [PubMed] [Google Scholar]
- 3.Volpe JJ. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J Child Neurol. 2009;24(9):1085–104. doi: 10.1177/0883073809338067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007;10(2):133–40. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 5.Hellström A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet. 2013;26(382):1445–1457. doi: 10.1016/S0140-6736(13)60178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Löfqvist C, Engström E, Sigurdsson J, Hård AL, Niklasson A, Ewald U, Holmström G, Smith LE, Hellström A. Postnatal head growth deficit among premature infants parallels retinopathy of prematurity and insulin-like growth factor-1 deficit. Pediatrics. 2006;117(6):1930–8. doi: 10.1542/peds.2005-1926. [DOI] [PubMed] [Google Scholar]
- 7.Hansen-Pupp I, Hövel H, Hellström A, Hellström-Westas L, Löfqvist C, Larsson EM, Lazeyras F, Fellman V, Hüppi PS, Ley D. Postnatal decrease in circulating insulin-like growth factor-I and low brain volumes in very preterm infants. J Clin Endocrinol Metab. 2011;96(4):1129–1135. doi: 10.1210/jc.2010-2440. [DOI] [PubMed] [Google Scholar]
- 8.Allred EN, Capone A, Jr, Fraioli A, Dammann O, Droste P, Duker J, Gise R, Kuban K, Leviton A, O'Shea TM, Paneth N, Petersen R, Trese M, Stoessel K, Vanderveen D, Wallace DK, Weaver G. Retinopathy of prematurity and brain damage in the very preterm newborn. J AAPOS. 2014;18(3):241–247. doi: 10.1016/j.jaapos.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beligere N, Perumalswamy V, Tandon M, Mittal A, Floora J, Vijayakumar B, Miller MT. Retinopathy of prematurity and neurodevelopmental disabilities in premature infants. Semin Fetal Neonatal Med. 2015;20:346–353. doi: 10.1016/j.siny.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt B, Davis PG, Asztalos EV, Solimano A, Roberts R. Association between severe retinopathy of prematurity and nonvisual disabilities at age 5 years. JAMA. 2014;311(5):523–5. doi: 10.1001/jama.2013.282153. [DOI] [PubMed] [Google Scholar]
- 11.Glass TJA, Chau V, Gardiner J, Foong J, Vinall J, Zwicker JG, Grunau RE, Synnes A, Poskitt KJ, Miller SP. Severe retinopathy of prematurity predicts delayed white matter maturation and poorer neurodevelopment. Arch Dis Child Fetal Neonatal Ed. 2017;102(6):F532–F537. doi: 10.1136/archdischild-2016-312533. [DOI] [PubMed] [Google Scholar]
- 12.Hansen-Pupp I, Löfqvist C, Polberger S, Niklasson A, Fellman V, Hellström A, Ley D. Influence of insulin-like growth factor i and nutrition during phases of postnatal growth in very preterm infants. Pediatric Research. 2011;69:448–53. doi: 10.1203/PDR.0b013e3182115000. [DOI] [PubMed] [Google Scholar]
- 13.Hansen-Pupp I, Hövel H, Lofqvist C, Hellström-Westas L, Cilio CM, Andersson S, Fellman V, Ley D. Circulatory insulin-like growth factor-I and brain volumes in relation to neurodevelopmental outcome in very preterm infants. Pediatr Res. 2013;74(5):564–569. doi: 10.1038/pr.2013.135. [DOI] [PubMed] [Google Scholar]
- 14.Marsal K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85(7):843–8. doi: 10.1111/j.1651-2227.1996.tb14164.x. [DOI] [PubMed] [Google Scholar]
- 15.Prematurity, ICftCoRo. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123(7):991–9. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 16.Group, ETFROPC. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121(12):1684–94. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 17.Drost FJ, Keunen K, Moeskops P, Claessens N, van Kalken F, Isgum I, Voskuil-Kerkhof E, Groenendaal F, de Vries LS, Benders MJ, Termote J. Severe retinopathy of prematurity is associated with reduced cerebellar and brainstem volumes at term and neurodevelopmental deficits at 2 years. Pediatric Res. 2018 doi: 10.1038/2018.2. [DOI] [PubMed] [Google Scholar]
- 18.Msall ME, Phelps DL, DiGaudio KM, Dobson V, Tung B, McClead RE, Quinn GE, Reynolds JD, Hardy RJ, Palmer EA. Severity of neonatal retinopathy of prematurity is predictive of neurodevelopmental functional outcome at age 5.5 years. Behalf of the Cryotherapy for Retinopathy of Prematurity Cooperative Group. Pediatrics. 2000;106(5):998–1005. doi: 10.1542/peds.106.5.998. [DOI] [PubMed] [Google Scholar]
- 19.Haldipur P, Bharti U, Govindan S, Sarkar C, Iyengar S, Gressens P, Mani S. Expression of Sonic Hedgehog During Cell Proliferation in the Human Cerebellum. Stem cells dev. 2012;21(7):1059–1068. doi: 10.1089/scd.2011.0206. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Dakubo G, Thurig S, Mazerolle CJ, Wallace VA. Retinal ganglion cell-derived sonic hedgehog locally controls proliferation and the timing of RGC development in the embryonic mouse retina. Development. 2005;132(22):5103–13. doi: 10.1242/dev.02096. [DOI] [PubMed] [Google Scholar]
- 21.Jensen AK, Ying GS, Huang J, Quinn GE, Binenbaum G. Postnatal serum Insulin-like growth factor I and retinopathy of prematurity. Retina. 2017;37(5):867–872. doi: 10.1097/IAE.0000000000001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothman AL, Sevilla MB, Mangalesh S, Gustafsson KE, Edwards L, Cotten CM, Shimony JS, Pizoli CE, El-Dairi MA, Freedman SF, Toth CA. Thinner Retinal Nerve Fiber Layer in Very Preterm Versus Term Infants and Relationship to Brain Anatomy and Neurodevelopment. Am J Ophthalmol. 2015;160(6):1296–1308. doi: 10.1016/j.ajo.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tam EW, Miller SP, Studholme C, Chau V, Glidden D, Poskitt KJ, Ferriero DM, Barkovich AJ. Differential effects of intraventricular hemorrhage and white matter injury on preterm cerebellar growth. J Pediatr. 2011;158(3):366–71. doi: 10.1016/j.jpeds.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lind A, Parkkola R, Lehtonen L, Munck P, Maunu J, Lapinleimu H, Haataja L PIPARI Study Group. Associations between regional brain volumes at term- equivalent age and development at 2 years of age in preterm children. Pediatr Radiol. 2011;41:953–961. doi: 10.1007/s00247-011-2071-x. [DOI] [PubMed] [Google Scholar]
- 25.Van Kooij BJ, Benders MJ, Anbeek P, Van Haastert IC, De Vries LS, Groenendaal F. Cerebellar volume and proton magnetic resonance spectroscopy at term, and neurodevelopment at 2 years of age in preterm infants. Developmental Medicine & Child Neurology. 2012;54:260–266. doi: 10.1111/j.1469-8749.2011.04168.x. [DOI] [PubMed] [Google Scholar]
- 26.Movsas TZ, Spitzer A, Gewolb IH. Postnatal corticosteroids and risk of retinopathy of prematurity. J AAPOS. 2016;20(4):348–52. doi: 10.1016/j.jaapos.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Kelly CE, Cheong JL, Molloy C, Anderson PJ, Lee KJ, Burnett AC, Connelly A, Doyle LW, Thompson DK Victorian Infant Collaborative Study Group. Neural Correlates of Impaired Vision in Adolescents Born Extremely Preterm and/or Extremely Low Birthweight. PLoS One. 2014;9(3):e93188. doi: 10.1371/journal.pone.0093188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tam EW, Chau V, Ferriero DM, Barkovich AJ, Poskitt KJ, Studholme C, Fok ED, Grunau RE, Glidden DV, Miller SP. Preterm cerebellar growth impairment after postnatal exposure to glucocorticoids. Sci Transl Med. 2011;3(105):105ra105. doi: 10.1126/scitranslmed.3002884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pavlik A, Buresova M. The neonatal cerebellum: the highest level of glucocorticoid receptors in the brain. Brain Res. 1984;314(1):13–20. doi: 10.1016/0165-3806(84)90171-8. [DOI] [PubMed] [Google Scholar]
- 30.Heine VM, Rowitch DH. Hedgehog signaling has a protective effect in glucocorticoid-induced mouse neonatal brain injury through an 11betaHSD2- dependent mechanism. J Clin Invest. 2009;119(2):267–77. doi: 10.1172/JCI36376. [DOI] [PMC free article] [PubMed] [Google Scholar]