Abstract
Objective
At the population level, obesity is associated with prostate cancer (PC) mortality; however, some studies found higher body mass index (BMI) is associated with better long-term PC outcomes among men with metastatic castration-resistant PC (mCRPC).
Patients and Methods
We tested whether obesity was associated with progression to metastasis, PC-specific mortality (PCSM), and all-cause mortality (ACM) among 1192 non-metastatic CRPC patients from the SEARCH Database. BMI was calculated from height and weight abstracted from the medical records at the time of but prior to CRPC diagnosis and categorized as underweight (<21 kg/m2), normal weight (21–24.9 kg/m2), overweight (25–29.9kg/m2), and obese (≥30kg/m2). Competing risks regression and Cox models were used to test associations between obesity and progression to metastasis, PCSM, and ACM, accounting for confounders.
Results
Overall, 51 (4%) men were underweight, 239 (25%) were normal weight, 464 (39%) were overweight, and 438 (37%) were obese. In adjusted analysis, higher BMI was significantly associated with reduced ACM (HR=0.98, p=0.012) but not PCSM (HR=1.00, p=0.737) or metastases (HR=0.99, p=0.225). Likewise, when BMI was treated as a categorical variable in adjusted models, obesity was not associated with PCSM (HR=1.11, p=0.436) or metastases (HR=1.06, p=0.647), but was associated with decreased ACM (HR=0.79, p=0.016) compared to normal weight. No data were available on treatments received after CRPC diagnosis.
Conclusions
Among non-metastatic CRPC patients, obesity was associated with better overall survival. Although this result mirrors evidence from men with mCRPC, obesity was not associated with PC outcomes. Larger studies are needed to confirm these findings.
Keywords: obesity, prostate cancer, castration-resistant prostate cancer
Introduction
Obesity is associated with aggressive prostate cancer (PC) at diagnosis [1–3]. We and others [4, 5] also found that obese men who underwent radical prostatectomy had a significantly increased risk of PC-specific mortality (PCSM) despite being more likely to receive postoperative radiation and ADT [4]. Thus, the literature suggests obesity in PC patients is linked with greater PCSM after surgery. However, among men with PC who progress and are treated with hormones, such as androgen deprivation therapy (ADT), the link between obesity and long-term PC outcomes is not clear yet.
Although few studies analyzed the effects of obesity and PC outcomes among metastatic castration resistant PC (mCRPC) patients [6–10], there is not a single published study on obesity and long-term PC outcomes among non-metastatic CRPC cases. Halabi et al. [6] analyzed data from 1296 men with advanced metastatic PC who were enrolled in nine prospective clinical trials conducted by the Cancer and Leukemia Group B (CALGB). These men had progressed despite castrate levels of testosterone, i.e. CRPC. In these men, the authors found that an elevated body mass index (BMI), as a measure of obesity, was associated with lower risks of both overall mortality and PCSM [6]. The authors speculate that obesity may lower the risk because these men had not developed cachexia yet. Similar results were seen among 55 men with mCRPC in Korea [9] and 63 men with mCRPC in Ireland [8]. However, a later study conducted among 1006 men with mCRPC participating on a phase III international clinical trial, found BMI was not associated with overall survival [7].
In this study, for the first time, we analyzed the association between obesity and metastasis, all-cause mortality (ACM), and PCSM among men with non-metastatic CPRC. We hypothesized that while obesity in mCRPC may be a marker of lack of cachexia (a favorable prognostic sign), in men with non-metastatic CRPC, where cachexia is rare, obesity would be associated with worse long-term PC outcomes, analogous to results seen in localized PC [3].
Patients and Methods
Study Population and Design
After obtaining Institutional Review Board approval, we identified 1,292 non-metastatic CRPC (M0/Mx) patients from the SEARCH database regardless of primary treatment modality at eight Veterans Affairs Medical Centers (Durham and Asheville, NC; Palo Alto, San Francisco, West LA, and San Diego, CA; Augusta, Georgia; Portland, OR). Data collection methods have been previously reported [11]. CRPC was defined as ≥25% PSA increase and an absolute ≥2 ng/mL increase from the post-ADT nadir while being castrate [12]. Castration was defined as testosterone <50ng/dL, bilateral orchiectomy, or continuous receipt of LHRH agonist or antagonist. Men were required to have no evidence of metastatic disease defined as the absence of a positive imaging test for distant metastases at or before CRPC diagnosis. All imaging tests after CRPC diagnosis were assessed for metastases. Time zero was time of CRPC diagnosis and patients were followed up to (1) the first positive imaging test, (2) ACM, and (3) PCSM, or otherwise censored at last known follow-up. PCSM was defined as having progressive, metastatic prostate cancer at the time of death and no other obvious cause of death.
Study outcomes
Our exposure, BMI, was calculated from height and weight abstracted from the medical records at the time closest to but prior to CRPC diagnosis. We were concerned about reverse causation in that men who were previously overweight/obese may have become cachexic from their cancer resulting in weight loss, which would make the lower weight groups appear to have increased risk of adverse outcomes. While the WHO defines underweight as <18.5 kg/m2, few men in our cohort were underweight (n=8). Therefore, we used an expanded definition of underweight (BMI <21 kg/m2) to ensure sufficient number of men in this category to analyze and to decrease the likelihood that the normal weight group was confounded by reverse causation. Though we explored alternative cut-points (BMI <20 and <22kg/m2), results were similar. Therefore, BMI was categorized as underweight (<21 kg/m2), normal weight (21–24.9 kg/m2), overweight (25–29.9kg/m2), and obese (≥30kg/m2). We excluded patients with missing data on race (n=43) or BMI (n=57) leaving a study cohort of 1,192 patients.
Statistical analyses
Patient demographic and clinical characteristics were compared among BMI groups. Categorical variables were summarized using count and percentage, while continuous variables were summarized using median, 25th percentile (Q1), and 75th percentile (Q3). Characteristics were compared between BMI groups using Kruskal-Wallis for continuous variables and chi-squared test for categorical variables.
Competing risks regression was used to test the association between BMI and progression to metastases with death as the competing risk. Similarly, competing risks regression was used to model hazard of PCSM. Cox proportional hazards models were used to test the association between BMI and ACM. BMI was treated as both a continuous and categorical variable with normal weight as the reference category. When BMI was treated as a continuous variable, underweight patients (BMI <21 kg/m2) were excluded to avoid concerns about reverse causation. We fit both crude models and models adjusted for age at CRPC (continuous), race (black vs. nonblack), year of CRPC diagnosis (continuous), treatment center, primary localized prostate cancer treatment (none vs. radical prostatectomy ± radiation vs. radiation alone), biopsy grade group (1 vs. 2–3 vs. 4–5 vs. unknown), Charlson comorbidity index [13] (0, 1, 2, ≥3) and PSA at CRPC (continuous, log-transformed). Cumulative incidence curves were created to show the incidence of metastases or death in different BMI groups and differences in survival were tested using the log-rank test (in the absence of competing risks). All statistical analyses were performed using STATA 14.2 (Stata Corp., College Station, TX). Statistical significance was defined as p<0.05.
Results
Patient characteristics
Overall, 51 (4%) men were underweight, 239 (20%) were normal weight, 464 (39%) were overweight, and 438 (37%) were obese (Table 1). As BMI group increased, age decreased (p<0.001) and primary treatment became more likely (p=0.011). Underweight and obese patients had more recent year of CRPC (p<0.001). There was no association between BMI group and race, biopsy grade group, PSA at CRPC, or length of follow-up. During the follow-up period, 686 patients developed metastases, 848 died, and 548 died from PC. The median follow-up time among patients who did not die during the study was 34 months (Q1–Q3: 20–59 months).
Table 1.
Clinical and pathological features of men with M0 CRPC by BMI group
| Underweight BMI <21 kg/m2 |
Normal Weight BMI 21–24.9 kg/m2 |
Overweight BMI 25–29.9 kg/m2 |
Obese BMI ≥30 kg/m2 |
p-value | |
|---|---|---|---|---|---|
| No. patients, n (%) | 51 (4) | 239 (20) | 464 (39) | 438 (37) | - |
| BMI (kg/m2), Median (Q1–Q3) | 20.0 (19.0–20.6) | 23.7 (22.7–24.3) | 27.5 (26.3–28.8) | 33.7 (31.5–37.1) | - |
| Age, Median (Q1–Q3) | 79 (71–87) | 80 (73–85) | 77 (70–83) | 73 (66–79) | <0.001K |
| Race, n (%) | 0.209C | ||||
| Non-black | 32 (63) | 172 (72) | 349 (75) | 312 (71) | |
| Black | 19 (37) | 67 (28) | 115 (25) | 126 (29) | |
| Year of CRPC, Median (Q1–Q3) | 2009 (2004–2011) | 2006 (2004–2009) | 2006 (2003–2010) | 2008 (2005–2011) | <0.001 K |
| Primary Localized Treatment | 0.011C | ||||
| None | 28 (55) | 109 (46) | 222 (48) | 165 (38) | |
| Radical Prostatectomy ± Radiation | 8 (16) | 41 (17) | 93 (20) | 114 (26) | |
| Radiation Alone | 15 (29) | 89 (37) | 149 (32) | 159 (36) | |
| Biopsy grade group, n (%) | 0.453C | ||||
| 1 | 4 (8) | 41 (18) | 76 (16) | 79 (18) | |
| 2 | 10 (20) | 24 (10) | 64 (14) | 51 (12) | |
| 3 | 3 (6) | 23 (10) | 33 (7) | 34 (10) | |
| 4 | 6 (12) | 27 (12) | 71 (15) | 60 (14) | |
| 5 | 22 (43) | 34 (15) | 57 (12) | 69 (16) | |
| Unknown | 34 (39) | 85 (36) | 160 (35) | 137 (32) | |
| PSA at CRPC (ng/mL),Median (Q1–Q3) | 7.5 (3.2–15.1) | 4.4 (3.0–8.3) | 4.7 (3.1–9.5) | 4.6 (3.1–9.4) | 0.228K |
| Developed Metastases, n (%) | 27 (53) | 122 (51) | 273 (59) | 264 (60) | 0.101C |
| Died, n (%) | 37 (73) | 174 (73) | 339 (73) | 298 (68) | 0.353C |
| Died from PC, n (%) | 21 (41) | 97 (41) | 226 (49) | 204 (47) | 0.193C |
| Follow-up (months)#, Median (Q1–Q3) | 28.8 (8.4–44.8) | 30.6 (12.9–56.6) | 36.8 (22.2–65.1) | 35.7 (21.9–58.9) | 0.208K |
P-value calculated using C chi-square test or K Kruskal-Wallis test
Reported among patients who did not die
BMI and metastases
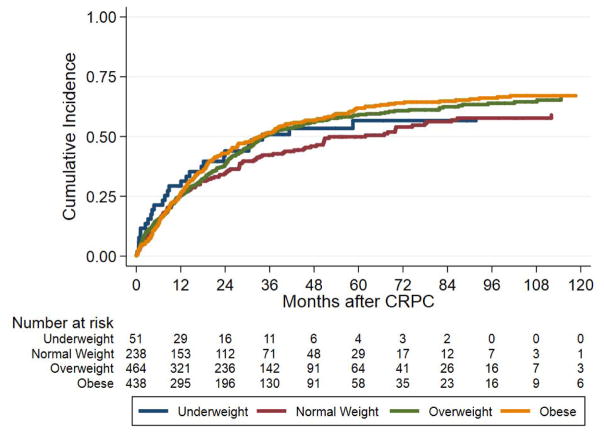
After excluding underweight men, there was no association between BMI as a continuous variable and metastases on univariable (p=0.360) or multivariable analysis (p=0.225; Table 2). When BMI was treated as a categorical variable, on univariable analysis, obese patients were more likely to develop metastases compared to normal weight patients (HR 1.27, 95% CI 1.03–1.58, p=0.028), but there was no difference in risk for underweight or overweight men. After adjusting for clinical and demographic characteristics there was no longer an association between obesity and risk of metastases (p=0.647), with age being the major factor weakening this association when covariates were added to the model one at a time. The cumulative incidence curve of BMI group and metastasis is shown in Figure 1A.
Table 2.
Hazard ratios for association between BMI group and CRPC outcomes
| Metastases | All-cause mortality | Prostate cancer-specific mortality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| N | HR | 95% CI | P | N | HR | 95% CI | P | N | HR | 95% CI | P | |
|
|
|
|
||||||||||
| Unadjusted | ||||||||||||
|
|
|
|
||||||||||
| BMI (continuous)^ | 659/1140 | 1.01 | 0.99–1.02 | 0.360 | 809/1138 | 0.98 | 0.97–0.99 | 0.026 | 527/1138 | 1.00 | 0.97–1.02 | 0.798 |
| BMI Category | ||||||||||||
| Underweight | 27/51 | 1.15 | 0.73–1.80 | 0.554 | 37/51 | 1.35 | 0.95–1.93 | 0.097 | 21/51 | 1.19 | 0.71–1.97 | 0.512 |
| Normal | 122/239 | 1.00 | Ref | - | 174/239 | 1.00 | Ref | - | 97/239 | 1.00 | Ref | - |
| Overweight | 273/464 | 1.19 | 0.96–1.48 | 0.107 | 339/464 | 0.90 | 0.75–1.08 | 0.454 | 226/464 | 1.20 | 0.95–1.53 | 0.129 |
| Obese | 264/438 | 1.27 | 1.03–1.58 | 0.028 | 298/438 | 0.81 | 0.67–0.97 | 0.025 | 204/438 | 1.18 | 0.92–1.50 | 0.185 |
| Adjusted* | ||||||||||||
| BMI (continuous)^ | 659/1140 | 0.99 | 0.98–1.01 | 0.225 | 809/1138 | 0.98 | 0.97–0.99 | 0.012 | 527/1138 | 1.00 | 0.98–1.01 | 0.737 |
| BMI Category | ||||||||||||
| Underweight | 27/51 | 1.20 | 0.75–1.90 | 0.450 | 37/51 | 1.38 | 0.96–1.98 | 0.083 | 21/51 | 1.14 | 0.67–1.95 | 0.629 |
| Normal | 122/239 | 1.00 | Ref | - | 174/239 | 1.00 | Ref | - | 97/239 | 1.00 | Ref | - |
| Overweight | 273/464 | 1.17 | 0.93–1.46 | 0.178 | 339/464 | 0.90 | 0.74–1.08 | 0.248 | 226/464 | 1.17 | 0.92–1.50 | 0.203 |
| Obese | 264/438 | 1.06 | 0.84–1.33 | 0.647 | 298/438 | 0.79 | 0.65–0.96 | 0.016 | 204/438 | 1.11 | 0.86–1.43 | 0.436 |
Adjusted for VA center and clinical characteristics: age at CRPC, race, year of CRPC diagnosis, treatment center, primary prostate cancer treatment, biopsy grade group, PSA at CRPC, and Charlson comorbidity index
The interaction between BMI (continuous) and Charlson comorbidity index (0, 1, 2, ≥3) was p=0.849 for metastases, p=0.672 for all-cause mortality, and p=0.942 for prostate cancer-specific mortality.
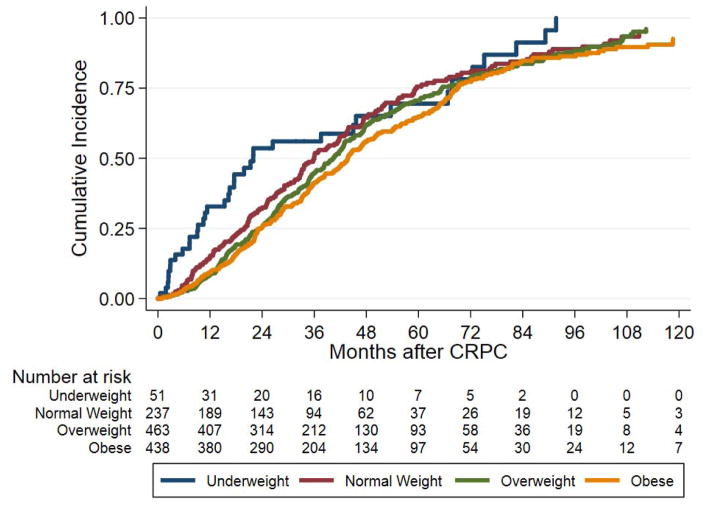
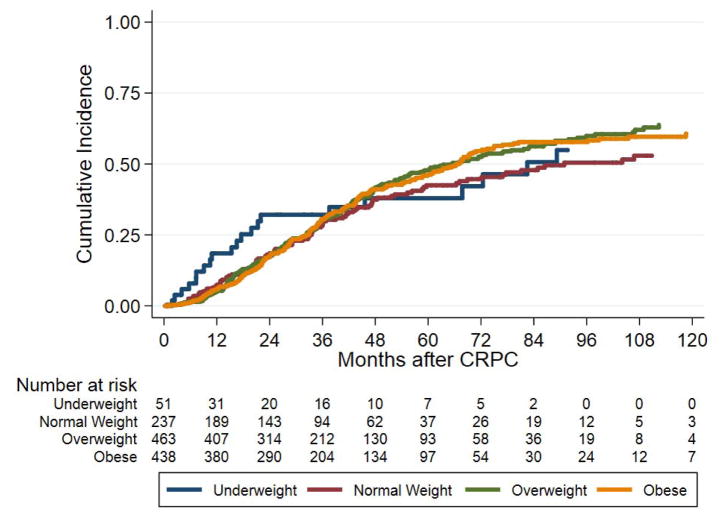
Figure 1.
BMI and all-cause mortality
On both crude and adjusted analyses, even after excluding underweight men, higher BMI, as a continuous variable, was associated with reduced risk of ACM (crude HR 0.98, 95% CI 0.97–0.99, p=0.026; adjusted HR 0.98, 95% CI 0.97–0.99, p=0.012) (Table 2). While not statistically significant, on crude analysis, underweight patients showed a trend towards increased risk of ACM compared to normal weight men (HR 1.35, 95% CI 0.95–1.93, p=0.097). In contrast, obese patients had a reduced risk of ACM compared to normal weight men on crude analysis (HR 0.81, 95% CI 0.67–0.97, p=0.025). After adjusting for multiple covariates, results were similar in that underweight patients showed a trend towards increased risk of ACM (HR 1.38, 95% CI 0.96–1.98, p=0.083) while the significant association between obesity and reduced risk of ACM remained (HR 0.79, 95% CI 0.65–0.96, p=0.016) (Table 2). Figure 1B shows the cumulative incidence of ACM by BMI group (log-rank; p=0.009).
BMI and prostate cancer-specific mortality
On both crude and adjusted analysis, BMI as a continuous variable was not associated with risk of PCSM (crude HR 1.00, 95% CI 0.97–1.02, p=0.798; adjusted HR 1.00, 95% CI 0.98–1.01, p=0.737) (Table 2). After categorizing BMI, overweight and obese men did not have increased risk of PCSM (p≥0.129) (Table 2). Figure 1C shows the cumulative incidence of PCSM by BMI group.
Discussion
It is now well established that obesity is associated with aggressive PC and worse long-term PC outcomes among patients with localized PC [3, 5, 10, 14]. However, only a few studies assessed the link between BMI and PC outcomes among men with metastatic CRPC [6–9], and all found BMI was associated with similar or better survival. However, no study to date has investigated the association between obesity and long-term PC outcomes among men with non-metastatic CRPC. Among these patients, we found a higher BMI was associated with reduced ACM. No associations were observed between obesity and metastasis or PCSM. These data suggest that among men with non-metastatic CRPC, obesity may be unrelated to PC aggressiveness and progression, but is an overall favorable prognostic sign. This is in sharp contrast to men with localized PC, wherein obesity clearly portends a worse PC and overall prognosis [1, 10]. Whether these results reflect that obese men are merely not cachexic or some underlying aspect of obesity that is associated with improved non-cancer outcomes, requires further study though our results are consistent with the general concept of the obesity paradox noted in other cancer types as well [15].
In men with metastatic CRPC, one study found obesity as measured by BMI ≥25 kg/m2, was associated with lower risks of overall mortality and PCSM, compared to normal weight mCRPC men [6]. A second study found no link between obesity and PC outcomes, however these were patients participating in a phase III clinical trial of docetaxel vs. mitoxantrone and thus all were treated with chemotherapy [7]. In a small study of 55 mCRPC Korean patients who received docetaxel, a higher BMI (≥23 kg/m2) was associated with decreased PCSM [9]. A report from a small study of 63 mCRPC Irish patients where BMI was cut at 25 kg/m2, found that higher BMI was a significant predictor of longer overall survival [8]. Thus, the literature to date suggests that unlike localized disease, for men with mCRPC, obesity is associated with similar or better overall survival. To our knowledge, however, our present study is the first to analyze the association of obesity on PC outcomes among non-metastatic CRPC patients. Our results, analogous to prior studies of men with mCRPC [6, 8, 9], showed that obesity is associated with lower ACM risk, though we found no association with prostate cancer specific outcomes.
Although obesity was linked with improved overall survival, in our study among non-metastatic CRPC patients, obese men were at equal risk of PCSM and metastases, compared to non-obese men. As such, obesity was selectively associated with improved non-PC outcomes and unrelated to PC outcomes. We propose several possible explanations for our findings. First, while tumor-related cachexia is unlikely in non-metastatic CRPC, patients may have cachexia from other causes such as heart disease, chronic obstructive pulmonary disease, and/or other types of cancer. Thus, greater BMI may demonstrate a current lack of cachexia but also perhaps provide some protection from future cachexia allowing patients to live longer. We also observed that risks of metastasis, ACM and PCSM, although not statistically significant, were greatest in underweight men compared to normal weight men, arguing this could be in part cachexia-related. Second, the null associations of obesity with PC outcomes could be related to competing biological factors of obesity that both may drive and inhibit PC. Obese men are prone to insulin resistance and hyperinsulinemia conditions, which drive a pro-insulin and pro-inflammation environment which could promote PC tumors [16]. However, obesity also alters hormone levels. Specifically, testosterone is converted to estrogen in the fat. It is well-known that obese men have higher estrogen levels. Though these would be reduced dramatically in castrated men, as testosterone levels are not zero in castrated men, estrogens levels are also not zero and presumably higher in obese men. Given estrogen may suppress CRPC growth [17], it is possible the anti-PC effects of higher estrogen off-set the pro-PC effects of higher insulin leading to an overall null effect of obesity on PC in men with CRPC.
This study has several strengths including the large number of patients with non-metastatic CRPC and PC events which allowed us to both stratify analysis by BMI categories and adjust for clinicopathological features. However, the study had a few limitations including not having data on further treatments after CRPC diagnosis though with a median year of non-metastatic CRPC diagnosis of 2006–2009 and median follow-up of 2–3 years, most men were managed in an era prior to modern mCRPC drugs and these agents were unlikely to have largely influenced our results. Likewise, no data reflecting changes in nutritional and lifestyle regimens prior to CRPC were available. No data were available on serum hormones to relate to obesity to test whether circulating estrogen and/or testosterone influenced PC outcomes in our cohort. Height and weight data were abstracted from the medical record, which was recorded in real-time. While we do not know if the values were measured or self-reported, we have previously shown very high correlation between measured and self-reported heights and weights within the VA system [18]. However, not all men had height and weight data at the exact time of CRPC diagnosis. As such, it is possible that some men may have gained or lost weight, though we have shown for men on hormonal therapy after the first year, weight tends to be stable [19]. How this may have influenced our results is unknown. Also, as few men in our cohort were underweight according to the WHO definition (BMI<18.5 kg/m2; n=8), we used an expanded definition of underweight (BMI <21 kg/m2) to ensure sufficient number of men in this category to analyze. Although this could be seen as a limitation, by doing this we decreased the likelihood that the normal weight group was confounded by reverse causation. In addition, when we explored alternative cut-points (BMI <20 and <22kg/m2), results were similar. Moreover, results were derived from men from a few VA centers in the US – to what degree these data are representative of all men with PC requires further study. Furthermore, ACM and PC deaths were hand abstracted from medical records based upon individual level chart reviews, supervised by a senior prostate cancer clinician (SF). As there is no reason to believe BMI should influence the accuracy of the determination of the cause of death from the chart reviews, inaccuracies in capturing these data should bias the results to the null, not create positive associations. Finally, our results require validation in other datasets and if validated, then future studies are needed to explain why obesity is associated with more aggressive cancers at diagnosis, but better outcomes in late stage disease.
Conclusions
This is the first study to investigate PC outcomes among men with non-metastatic CRPC. We found obesity to be associated with better overall survival similar to results from men with metastatic CRPC, although no associations were found with PC-specific outcomes, such as metastases and PCSM. While it is possible that higher BMI merely reflects a lack of tumor-induced cachexia, the degree of tumor-related cachexia for men with non-metastatic CRPC is likely low. Alternative explanations are needed to better understand the paradox that in men with early stage disease, obesity portends a poor prognosis, while in men with CRPC obesity is either unrelated to PC outcomes or associated with lower risk of ACM, this latter observation deserves further study.
Acknowledgments
Financial support: Supported by National Institutes of Health; Grant number: K24 CA160653 (SJF), NIH R01CA100938 (WJA), NIH Specialized Programs of Research Excellence Grant P50 CA92131-01A1 (WJA)
Footnotes
Conflicts of Interest: None declared
References
- 1.Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer--a dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23:1665–71. doi: 10.1093/annonc/mdr603. [DOI] [PubMed] [Google Scholar]
- 2.Moller H, Roswall N, Van Hemelrijck M, et al. Prostate cancer incidence, clinical stage and survival in relation to obesity: a prospective cohort study in Denmark. Int J Cancer. 2015;136:1940–7. doi: 10.1002/ijc.29238. [DOI] [PubMed] [Google Scholar]
- 3.Vidal AC, Howard LE, Moreira DM, et al. Obesity increases the risk for high-grade prostate cancer: results from the REDUCE study. Cancer Epidemiol, Biomarkers Prev. 2014;23:2936–42. doi: 10.1158/1055-9965.EPI-14-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vidal AC, Howard LE, Sun SX, et al. Obesity and prostate cancer-specific mortality after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Prostate Cancer and Prostatic Diseases. 2017;20:72–8. doi: 10.1038/pcan.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddiqui SA, Inman BA, Sengupta S, et al. Obesity and survival after radical prostatectomy: A 10-year prospective cohort study. Cancer. 2006;107:521–9. doi: 10.1002/cncr.22030. [DOI] [PubMed] [Google Scholar]
- 6.Halabi S, Ou SS, Vogelzang NJ, Small EJ. Inverse correlation between body mass index and clinical outcomes in men with advanced castration-recurrent prostate cancer. Cancer. 2007;110:1478–84. doi: 10.1002/cncr.22932. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong AJ, Halabi S, de Wit R, et al. The relationship of body mass index and serum testosterone with disease outcomes in men with castration-resistant metastatic prostate cancer. Prostate Cancer and Prostatic Diseases. 2009;12:88–93. doi: 10.1038/pcan.2008.36. [DOI] [PubMed] [Google Scholar]
- 8.Cushen SJ, Power DG, Murphy KP, et al. Impact of body composition parameters on clinical outcomes in patients with metastatic castrate-resistant prostate cancer treated with docetaxel. Clinical Nutrition. 2016;13:e39–e45. doi: 10.1016/j.clnesp.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Park JM, Nam JS, Na W, et al. Prognostic value of body mass index in Korean patients with castration-resistant prostate cancer. Korean Journal of Urology. 2012;53:761–5. doi: 10.4111/kju.2012.53.11.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalfin HJ, Lee SB, Jeong BC, et al. Obesity and long-term survival after radical prostatectomy. The Journal of Urology. 2014;192:1100–4. doi: 10.1016/j.juro.2014.04.086. [DOI] [PubMed] [Google Scholar]
- 11.Howard LE, De Hoedt AM, Aronson WJ, et al. Do skeletal-related events predict overall survival in men with metastatic castration-resistant prostate cancer? Prostate Cancer and Prostatic Diseases. 2016;19:380–4. doi: 10.1038/pcan.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scher HI, Morris MJ, Basch E, et al. End points and outcomes in castration-resistant prostate cancer: from clinical trials to clinical practice. Journal of Clinical Oncology. 2011;29:3695–704. doi: 10.1200/JCO.2011.35.8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. Journal of Clinical Epidemiology. 1994;47:1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 14.Vidal AC, Freedland SJ. Obesity and Prostate Cancer: A Focused Update on Active Surveillance, Race, and Molecular Subtyping. European Urology. 2017;72:78–83. doi: 10.1016/j.eururo.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lennon H, Sperrin M, Badrick E, et al. The Obesity Paradox in Cancer: a Review. Current Oncology Reports. 2016;18:56. doi: 10.1007/s11912-016-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S, Zhang Q, Chen C, et al. Hyperinsulinemia enhances interleukin-17-induced inflammation to promote prostate cancer development in obese mice through inhibiting glycogen synthase kinase 3-mediated phosphorylation and degradation of interleukin-17 receptor. Oncotarget. 2016;7:13651–66. doi: 10.18632/oncotarget.7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montgomery B, Nelson PS, Vessella R, et al. Estradiol suppresses tissue androgens and prostate cancer growth in castration resistant prostate cancer. BMC Cancer. 2010;10:244. doi: 10.1186/1471-2407-10-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerrios-Rivera L, Howard L, Frank J, et al. Is Body Mass Index the Best Adiposity Measure for Prostate Cancer Risk? Results From a Veterans Affairs Biopsy Cohort. Urology. 2017;105:129–35. doi: 10.1016/j.urology.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HS, Moreira DM, Smith MR, et al. A natural history of weight change in men with prostate cancer on androgen-deprivation therapy (ADT): results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. BJU International. 107:924–8. doi: 10.1111/j.1464-410X.2010.09679.x. 201. [DOI] [PMC free article] [PubMed] [Google Scholar]