Abstract
Childhood socioeconomic status (SES) is an important aspect of early life environment associated with later life health/health behaviors, including alcohol misuse. However, alcohol misuse is modestly heritable and involves differing etiological pathways. Externalizing disorders show significant genetic overlap with substance use, suggesting an impulsivity pathway to alcohol misuse. Alcohol misuse also overlaps with internalizing disorders, suggesting alcohol is used to cope. These differing pathways could lead to different patterns over time and/or differential susceptibility to environmental conditions, such as childhood SES. We examine whether: 1) genetic risk for externalizing and internalizing disorders influence trajectories of alcohol problems across adolescence to adulthood, 2) childhood SES alters genetic risk these disorders on trajectories of alcohol problems, and 3) these patterns are consistent across sex. We find modest evidence of gene-environment interaction. Higher childhood SES increases the risk of alcohol problems in late adolescence/early adulthood, while lower childhood SES increases the risk of alcohol problems in later adulthood, but only among males at greater genetic risk of externalizing disorders. Females from lower SES families with higher genetic risk of internalizing or externalizing disorders have greater risk of developing alcohol problems.
Keywords: Gene-environment interaction, Alcohol misuse, Life course, Childhood socioeconomic status
Alcohol misuse remains a serious threat to public health, resulting in increased mortality/morbidity, globally (World Health Organization, 2014). However, the risks for alcohol misuse stem from a variety of social, psychological, and genetic influences. Fortunately, there is growing recognition in the benefits of integrating these levels of analysis within health research (Boardman et al., 2013; Harris, 2010). We use a population-based sample of twins to examine whether trajectories of alcohol problems vary across genetic liability for different types of psychiatric disorders (internalizing vs. externalizing), and whether genetic risk is moderated by childhood socioeconomic status (SES). Additionally, we consider the possibility of sex differences in the influence of genetic risk and childhood SES.
Childhood SES is an important aspect of the early life environment, consistently linked to later life physical and mental health (Cohen et al., 2010). This has generally been referred to as the “long arm” of early life conditions (Hayward & Gorman, 2004). Socioeconomic disadvantage during childhood is associated with a variety of adverse experiences, such as poor environmental conditions (Evans & Kantrowitz, 2002), greater levels of neighborhood violence (Harding, 2009), and greater family stress (Conger et al., 2010), among others. These adverse conditions and experiences are thought, in part, to be why disadvantage experienced early in the life course is associated with a variety of negative health outcomes in adulthood (Elo et al., 2014; Goosby, 2013; Montez & Hayward, 2014).
Childhood SES is also related to health behaviors, such as smoking, physical activity, or alcohol use (Gilman et al., 2003; van de Mheen et al., 1998). In terms of alcohol misuse, childhood SES appears to have differing influences when we look across levels in severity of use. Childhood SES is positively related to both consumption and binge drinking in adolescence and early adulthood (Kendler et al., 2014; Patrick et al., 2012), though this relationship appears to reverse in mid-to-later life (Ferraro et al., 2016). While those from higher SES families consume more, those from more disadvantaged backgrounds suffer more negative consequences, such as alcohol-related problems, in young adulthood (Kendler et al., 2014).
Individual predispositions are also important in alcohol misuse. Approximately 50% of the variance in alcohol misuse is attributable to genetic influences (Verhulst et al., 2015). The importance of genetic influences changes across environmental conditions (gene-environment interaction or GxE), such as peer influences (Cooke et al., 2015), relationship status (Barr et al., 2017) or neighborhood stability (Dick et al., 2009). Developmental context is also important, as genetic influences become stronger as individuals age while shared environmental influences are more important earlier in the life course (Dick, 2011).
Genetic influences can also manifest through differing etiological pathways related to alcohol misuse. Broadly conceptualized these pathways encompass an externalizing pathway, characterized by high impulsivity and sensation seeking, and an internalizing pathway related to anxiety and negative affect. Additionally, because genes and environments do not exist independently of one another, it is possible that different environmental conditions associated with childhood SES may influence alcohol misuse differently depending on the etiology of genetic risk.
Externalizing type disorders (e.g. Conduct Disorder, Antisocial Personality Disorder) generally reflect a common predisposition towards problems in behavioral disinhibition (Iacono et al., 2008). Externalizing problems show significant genetic overlap with substance use, broadly (Kendler et al., 2003), and genetic risk for externalizing problems is related to trajectories of alcohol use behaviors across adolescence (Kendler et al., 2011; Meyers et al., 2014). Heritability for externalizing problems is also greater under conditions of higher SES during childhood (Middeldorp et al., 2014; Tuvblad et al., 2006). Alongside this increased heritability for externalizing disorders, individuals from higher SES families have greater access to (Swahn et al., 2002) and consume more alcohol (Patrick et al., 2012). Following the social control/opportunity model of GxE (Shanahan & Hofer, 2005), where environments that afford greater opportunity/less control (such as easy access to alcohol) allow individuals to realize underlying genetic liability, living in a higher SES household reflects an environment of increased opportunity to express underlying genetic liability through an externalizing pathway.
In addition to externalizing, there are internalizing type disorders (e.g. Major Depressive Disorder, Generalized Anxiety Disorder). Substance use shows genetic overlap with these internalizing disorders, especially those related to anxiety (Kendler et al., 2003) and depression (Edwards et al., 2011). For some, the use of alcohol is intended to cope with anxiety or limit negative affect. Internalizing problems are generally associated with more severe outcomes such as alcohol-related problems (Hussong et al., 2011), rather than consumption. Though there is some evidence that heritability for internalizing problems is greater among children from higher SES families (Middeldorp et al., 2014), there is also evidence of greater heritability under conditions associated with low childhood SES. Genetic influences on anxiety (Lau et al., 2007), and internalizing problems (Hicks et al., 2009) were greater among children exposed to negative life events. Because exposure to psychosocial stressors is an important mechanism connecting low childhood SES to later psychopathology (Cohen et al., 2010), lower childhood SES likely increases the risk of alcohol misuse among those with a greater predisposition towards internalizing disorders. This follows the contextual triggering model, where genetic risk for alcohol misuse will be stronger among those exposed to adverse conditions (Shanahan & Hofer, 2005).
Finally, these differing pathways may vary across sex/gender (Salvatore et al., 2017). The prevalence of internalizing disorders is greater among females, while the prevalence of externalizing disorders is greater among males (Kessler et al., 2005). These differences reflect, in part, differences in social conditions, especially those related to stress. There is some evidence that men tend to respond to stress with alcohol misuse more than women (Elliott, 2013). Others have found both men and women tend to react to stress with increased internalizing and externalizing type problems (Slopen et al., 2011) or that stress does little to explain group differences (McDonough & Walters, 2001). This has led some to reject the idea that externalizing for men/internalizing for women reflect functionally equivalent reactions to stress (Hill & Needham, 2013). Beyond social influences, difference in prevalence and patterns over time may also be due, in part, to underlying genetic differences. Twin studies have identified qualitative sex differences in the genetic influences on major depression (Kendler et al., 2006) and alcohol use disorders (Kendler et al., 2016). A careful consideration of sex differences in environmental and genetic risk is an important part of any research into GxE related to mental health outcomes.
In the current analysis, we examine three specific research questions. 1) Does genetic risk for externalizing disorders and internalizing disorders influence trajectories of alcohol problems across adolescence and into young adulthood? We expect both externalizing and internalizing risk to be associated with trajectories of alcohol problems over time. 2) Does childhood SES moderate the influence of genetic risk for internalizing or externalizing disorders on trajectories of alcohol problems? We expect that higher childhood SES will exacerbate the relationship between externalizing risk and alcohol problems (e.g. the social control/social opportunity model), while lower childhood SES will exacerbate the relationship between internalizing risk and alcohol problems (e.g. the contextual triggering model). 3) Are the influences of these genetic and environmental risk factors consistent across sex? We expect externalizing risk to be stronger among males, while internalizing risk is stronger among females.
Methods
Sample
Our sample comes from the Virginia Twin Study of Adolescent Behavioral Development (VTSABD). The VTSABD is a longitudinal study of twins born between 1974–1983 ascertained through the Virginia public and private school system. The focus of the VTSABD was to understand the trajectories of environmental and genetic influences on adolescent psychopathology across development (Meyer et al., 1996). Participation was limited to twins between the ages of 8 and 16. Of the families identified, 1,412 participated (~75%, N = 2,775, 1,364 complete twin pairs with known zygosity). Follow-up assessments were completed up to three times as long as the twins were under the age of 18 and a resident of Virginia. Assessments collected information on a variety of risk factors, as well clinical measures of psychopathology, completed by twins and their parents. A separate young adult follow up was conducted when twins were approximately between the ages of 18-28 on 2,376 individuals. One final assessment was completed on 1,084 individuals who participated when twins were between the ages of 22 and 32, resulting in 6 waves of data collection.
We limited our analyses to those who completed the initial young adult survey. We focused on alcohol problems after age 12, due to the lack of individuals who had initiated use before that point. Each participant contributed 1 to 5 data points across time, with an average of 3.1 per person. Because the ABD only contains a limited number of non-white twin pairs, we excluded them from the current analysis along with those who did not have a confirmed zygosity. This resulted in a final sample size of 2,315 individuals. Comparisons of the full sample to those who completed the analytic sample revealed no differences in either sex or childhood SES. Those in the analytic sample were significantly older during the first assessment.
Measures
Alcohol Problems
During the first 4 assessments, alcohol problems were measured based on responses to questions regarding the degree to which respondents ‘had a desire to cut down’, ‘been advised to cut down’, ‘had concern over their drinking’, or ‘engaged in morning drinking’ ever or in the previous three months. In addition, respondents were rated on whether their alcohol use was negatively influencing various social domains (e.g. family, peers, work, school). Items were coded to reflect whether respondents had incapacity in social relationships or responsibilities. The result was a measure of alcohol problems ranging from 0 to 10, with items that are similar to those used to assess symptoms of alcohol dependence (AD). Both self-report and parent observations were collected. Problems were counted if reported by either the twin or parent. In the final two waves, we used symptoms counts of DSM-IIIR criteria for AD, ranging from 0 to 9. These include both criteria for physical dependence (such as withdrawal), and the degree to which alcohol interferes with an individual’s ability to function (interference with responsibilities at school or work). Alcohol Problems (AP) was log transformed for the analyses.
Genetic Risk Scores
We indexed genetic risk for either externalizing or internalizing disorders using a modified ridit score approach (Kendler et al., 2011; Meyers et al., 2014), which provides a way of combining information from binary and ordinal variables into a composite score. Each level in an ordinal variable is assigned a score based on the midpoint of a uniform distribution of 0–1. For example, if the prevalence of depression among parents in the sample was .50, those whose parent met criteria for depression would receive a score of .75 and those whose parents did not would receive a score of .25. Scores from the monozygotic (MZ) co-twins were unmodified from the 0–1 scale, as MZ twins share all of their genetic variants, identical-by-descent. Scores from the dizygotic (DZ) co-twins and parents were adjusted half way back to the center of the uniform distribution (0.5) because both parents and DZ co-twins share only half of their genetic variants on average.
Externalizing scores are based on parental diagnosis of Alcohol Dependence (AD) or Antisocial Personality Disorder (ASD) from a clinical interview using DSM III-R criteria, and symptom counts of Conduct Disorder (retrospective) and ASD using DSM-IV criteria in the co-twins. Internalizing scores were based on parental diagnosis of DSM-IIIR Major Depressive Disorder (MDD) and Generalized Anxiety Disorder (GAD) and symptom counts of MDD and GAD from the co-twins during the young adult follow-up of the ABD. Parents and co-twin scores were combined and averaged to create a risk score for each individual. Internalizing and externalizing risk scores were modestly correlated (r = .263).
Childhood Socioeconomic Status
Childhood SES was comprised of data from two different sources. First, we used self-reported information from the parent interview on parents’ education, occupation, and income. Mother’s and father’s education were measured in total years of education (constructed from a 13-item ordinal measure of the highest completed degree/grade). Parents’ occupations were recoded to reflect the average occupational prestige scores corresponding to scores generated from the 1989 General Social Survey (Davis, 1991). Possible values ranged from 0-100. Finally, income was reported using a 31-item ordinal measure for the previous years household income (lowest category = Less than $2500; highest category $250,000 or more).
Second, we used census information from the respondent’s home of record, which allowed us to retain individuals with missing items on the parent’s survey. We included percentage of unemployed males, percentage of individuals over 25 with a college degree, and median household income (in thousands of US dollars) at the tract level. We created a composite measure of childhood SES from factor scores using all 6 of the above measures, taking missing data into account using full information maximum likelihood (FIML). Childhood SES scores were standardized for interpretation.
Other Covariates
All models included sex and age as covariates. We also included dichotomous indicators for whether the mother smoked during pregnancy, whether the mother consumed alcohol during pregnancy, and whether or not the child was low birth-weight (<5.5lbs) as these are related to neurocognitive development and may contribute to later alcohol misuse and behavioral problems (Aarnoudse-Moens et al., 2009; Huizink & Mulder, 2006). Finally, we included the mother’s age when the twins were born as this may reflect other aspects of a disadvantaged background (López Turley, 2003).
Analytic Plan
We fit a series of multilevel growth models, with the data structured on age rather than wave (Singer & Willett, 2003) to assess change in AP over time. These models account for the correlated nature of repeated measurements and allow one to estimate the influence of covariates on initial status and change over time. The initial within-individual change model for AP for time i of respondent j would appear as:
where β0j represents respondents’ baseline AP, β1j models the change in AP as a function of age. The level 2 model, consisting of the between-individuals portion would appear as:
The k number of X’s represent the between individual differences in other covariates included in the model. We include the effects of these covariates on initial status only to reduce the number of interactions. Genetic risk (GENRISK), childhood SES (SES), and sex (FEMALE) model the influence of these factors on initial status and change over time, and the model includes random effects for age and baseline status at the between-person (μ00j, μ10j) level. Continuous covariates were standardized and centered at zero.
We first fit models to determine the functional form of change in AP. We then fit a series of models to determine the best structure and number of random effects. This provided the base model for change over time. Model selection was based on improvements in model fit using a likelihood ratio (LR) test in the case of nested models, based on a chi-square distribution of 2*(log-likelihood of reduced model – log-likelihood of full model), and Akaike’s Information Criterion (AIC) in the case of non-nested models. We then tested our specific hypotheses, beginning with the separate influence of genetic risk, childhood SES, and sex on initial status and change over time, followed by the models with interactions between genetic risk and childhood SES (GxE models). The final models tested whether there were sex-specific influences of genetic risk, childhood SES, and their interaction. We determined whether or not parameters were significant by examining improvements in model fit (a global test), using a likelihood ratio test, and examining the specific parameter estimates and their standard errors. We highlight estimates that do not meet the established threshold for statistical significance but are close to this threshold (p < .10), as these may be of interest for future research. We fit all models using the mixed command in Stata14 and the cluster option to adjust for family clustering.
Results
Table 1 provides descriptive statistics. Overall, the sample was split evenly according to sex. Descriptive statistics for the measure of childhood SES and its component items are also listed. The overall prevalence of smoking during pregnancy was high (23.68%) compared to recent estimates, where only 8.4% of mothers smoked any time during pregnancy (Curtin & Mathews, 2016), likely reflecting the time period in which these data were collected. Finally, while the percentage of low-weight births seems high, low-weight births are much more prevalent among twins and the prevalence of low birth weight twins in the VTSABD is comparable to recent estimates (Martin et al., 2017). The mean level of AP increased across each wave.
Table 1.
VTSABD Descriptive Statistics (N = 2,315*)
| Female | N/Mean | %/SD | Range |
|---|---|---|---|
| 1257 | 54.30% | – | |
| Age: | |||
| Wave I | 12.15 | 2.58 | 8 – 18 |
| Wave II | 13.11 | 2.46 | 9 – 18 |
| Wave III | 15.05 | 1.56 | 12 – 17 |
| Wave IV | 15.71 | 0.95 | 14 – 17 |
| Wave V | 21.91 | 2.04 | 18 – 28 |
| Wave VI | 26.53 | 2.51 | 22 – 32 |
| Alcohol Problems: | |||
| Wave I | 0.12 | 0.60 | 0 – 10 |
| Wave II | 0.14 | 0.68 | 0 – 10 |
| Wave III | 0.24 | 0.89 | 0 – 10 |
| Wave IV | 0.21 | 0.73 | 0 – 10 |
| Wave V | 0.97 | 1.66 | 0 – 9 |
| Wave VI | 1.01 | 1.81 | 0 – 9 |
| Childhood SES | 0.00 | 1.00 | −2.76 – 3.42 |
| Parental Education | 14.56 | 2.32 | 8 – 21 |
| Parental Occupation Prestige | 48.35 | 9.98 | 23.95 – 64.38 |
| Household Income | 16.88 | 5.80 | 1 – 31 |
| % Unemployment | 3.56 | 3.89 | 0 – 22.53 |
| % With College Degree | 26.39 | 18.95 | 0 – 80.67 |
| Median HH Income† | 41.45 | 19.33 | 5.98 – 150.00 |
| Externalizing Genetic Risk | .46 | .14 | .27 – .99 |
| Internalizing Genetic Risk | .48 | .11 | .32 – .96 |
| Mother Smoked During Pregnancy | 515 | 23.68% | – |
| Mother Drank During Pregnancy | 54 | 2.46% | – |
| Low Birth-weight | 944 | 44.30% | – |
| Maternal Age at Birth | 27.83 | 4.61 | 15.47 – 44.28 |
All percentages based on valid percentages
Sample size across the VTSABD (Wave I = 2,315; Wave II = 1,788; Wave III = 1,091; Wave IV = 329; Wave V = 2,315; Wave VI = 1,056)
In thousands of US dollars
Baseline Model Fitting
Table 2 provides estimates for determining the functional form of change in AP over time and the random effects structure. We constrained all random effects to be independent of one another. In the unconditional model, AP demonstrated modest clustering within person (intraclass correlation = .177). Inclusion of age (Δχ2 = 947.16, Δdf = 1, p < .001) and age2 (Δχ2 = 89.12, Δdf = 1, p < .001) each resulted in a better fitting model. A quadratic function best described the change over time in AP, similar to patterns from visual inspection of the data. Additionally, the estimate for baseline AP was no longer significantly different from zero once accounting for the effects of age or age2.
Table 2.
Models for Functional Form and Random Effects
| Functional Form | AIC | LL | DF | Intercept | Age | Age2 |
|---|---|---|---|---|---|---|
| Unconditional Model | 10,598.33 | −5,296.17 | 3 | 0.241*** | – | – |
| Linear Growth Model | 9,653.17 | −4,822.59 | 4 | 0.016 | 0.035*** | – |
| Quadratic Growth Model | 9,566.05 | −4,778.03 | 5 | −0.060 | 0.068*** | −0.002*** |
| Random Effects | AIC | LL | DF | Δ –2LL | Δ DF | P |
| Random Intercept | 9,600.87 | −4,795.44 | 5 | – | – | – |
| Random Intercept & Slope for Age | 7,915.61 | −3,951.80 | 6 | 1,687.27 | 1 | 0.000 |
| Random Intercept, Slope for Age, & Slope for Age2 | 7,917.10 | −3,951.55 | 7 | 0.50 | 1 | 0.479 |
| Random Slope for Age & Age2 | 7,915.10 | −3,951.55 | 6 | 0.00 | 1 | 0.999 |
p < .05
p < .01
p < .001
For determining the random effects, we fit models including random intercepts and random slopes for age and age2 using restricted maximum likelihood (REML). The inclusion of random slopes for age resulted in significant improvement in model fit over the random intercepts model (Δχ2 = 1,687.27, Δdf = 1, p < .001), but including a random slope for age2 did not further improve model fit (Δχ2 = 0.50, Δdf = 1, p = .479). We also evaluated model fit for including only random slopes for age and age2. We relied on model AIC to compare this to other models, as it was not nested within the other models. The model with random slopes for age and age-squared had the lowest AIC (7915.10). This provided the baseline model for evaluating change over time in AP, allowing us to determine the impact of risk factors on initial status, the linear slope (age), and the quadratic slope (age2).
Externalizing Risk
Table 3 presents results for models externalizing genetic risk on trajectories of AP. Model 1 presents the independent influences of childhood SES, genetic risk, and sex on initial status (I) and change over time (S and Q). Inclusion of these terms significantly improved model fit (Δχ2 = 150.97, Δdf = 9, p < .001) over the model that included only the additional covariates (not listed). Childhood SES was not related to either initial status or change over time. Genetic risk for externalizing was positively associated with the linear slope for AP (b = 0.0164, p < .001), but not initial status or the quadratic term for age. Females had significantly higher initial levels of AP (b = 0.0388, p < .01), but slower linear growth (b = −0.0236, p < .001).
Table 3.
Growth Models for Alcohol Problems by Externalizing Genetic Risk
| Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|
| b | se | b | se | b | se | ||
| Age | I | −0.1528*** | (0.0420) | −0.1503*** | (0.0422) | −0.1521*** | (0.0425) |
| S | 0.0744*** | (0.0064) | 0.0745*** | (0.0064) | 0.0738*** | (0.0063) | |
| Q | −0.0020*** | (0.0004) | −0.0020*** | (0.0004) | −0.0020*** | (0.0004) | |
| Genetic Risk | I | −0.0076 | (0.0072) | −0.0069 | (0.0073) | −0.0016 | (0.0112) |
| S | 0.0164*** | (0.0043) | 0.0165*** | (0.0043) | 0.0071 | (0.0067) | |
| Q | −0.0003 | (0.0003) | −0.0003 | (0.0003) | 0.0006 | (0.0004) | |
| Childhood SES | I | −0.0071 | (0.007) | −0.0069 | (0.007) | −0.0219* | (0.0102) |
| S | 0.0018 | (0.0038) | 0.0026 | (0.0039) | 0.0139* | (0.0061) | |
| Q | 0.0000 | (0.0003) | −0.0000 | (0.0003) | −0.0006 | (0.0004) | |
| Female | I | 0.0388** | (0.0148) | 0.0392** | (0.0148) | 0.0346* | (0.0150) |
| S | −0.0236** | (0.0079) | −0.0231** | (0.0079) | −0.0201* | (0.0079) | |
| Q | 0.0005 | (0.0005) | 0.0005 | (0.0005) | 0.0003 | (0.0005) | |
| Family SES × Genetic Risk | I | – | – | 0.0009 | (0.0071) | 0.0064 | (0.0112) |
| S | – | – | 0.0083† | (0.0045) | 0.0080 | (0.0069) | |
| Q | – | – | −0.0006† | (0.0003) | −0.0006 | (0.0004) | |
| Female × Genetic Risk | I | – | – | – | – | −0.0072 | (0.0143) |
| S | – | – | – | – | 0.0163† | (0.0084) | |
| Q | – | – | – | – | −0.0015** | (0.0006) | |
| Female × Childhood SES | I | – | – | – | – | 0.0262* | (0.0132) |
| S | – | – | – | – | −0.0206** | (0.0077) | |
| Q | – | – | – | – | 0.0010† | (0.0005) | |
| Female × Childhood SES × Genetic Risk | I | – | – | – | – | −0.0077 | (0.0142) |
| S | – | – | – | – | −0.0012 | (0.0089) | |
| Q | – | – | – | – | 0.0000 | (0.0006) | |
| Log-likelihood (df) | −3,061.34 (15) | −3,054.90 (18) | −3,040.54 (27) | ||||
| AIC | 6,160.69 | 6,153.79 | 6,143.09 | ||||
All models include low birth weight, maternal smoking during pregnancy, maternal drinking during pregnancy, and maternal age at birth on initial status.
p < .10
p < .05
p < .01
p < .001
I = initial status; S = linear slope; Q = quadratic slope
N = 1,845 (Person observations = 5,808)
Model 2 includes the interaction between genetic risk and childhood SES on initial status and change over time (GxE). Including the effect of this interaction resulted in a significant improvement in overall model fit (Δχ2 = 12.90, Δdf = 3, p < .01). Significant associations from Model 1 remained virtually unchanged. The interaction between childhood SES and genetic risk was not significantly related to initial status or change over time in AP. However, estimates for the relationship between this interaction and the linear (b = 0.0083, p = .066) and quadratic (b = −0.0006, p = .057) slopes were just above the established threshold.
Model 3 includes the interaction between all parameters with sex, in order to assess whether the effects of childhood SES or externalizing genetic risk, and their interaction, vary across sex. Including these interactions resulted in a large improvement in model fit (Δχ2= 28.71, Δdf = 9, p < .001). However, the interaction between sex, childhood SES, and genetic risk was not associated with any aspect of alcohol problem trajectories. Rather, the interaction between sex and childhood SES was related to initial status (b = 0.0262, p < .05) and linear change (b = −0.0206, p < .01). The interaction between sex and genetic risk was significantly related to the quadratic slope (b = −0.0015, p < .01) and just above the threshold for the linear slope (b = 0.0163, p < .053). In addition the effect of childhood SES on the linear slope (b = −0.0219, p < .05) and quadratic slope (b = 0.0139, p < .05) became significant.
Internalizing Risk
Table 4 presents the models for internalizing genetic risk. In Model 1, inclusion of the effects of sex, childhood SES, and genetic risk on initial status and change over time improved overall model fit (Δχ2 = 89.45, Δdf = 9, p < .001). Childhood SES was unrelated to initial status, linear growth, or quadratic growth in AP. Genetic risk for internalizing was positively associated with the linear term for age (b = 0.0151, p < .001) and negatively associated with the quadratic term for age (b = −0.0006, p < .05). Genetic risk for internalizing disorders was not related to lower initial levels of AP (b = −0.0124, p = .097), though this was again just over the traditional threshold. Females had significantly higher initial levels of AP (b = 0.0480, p < .01), but slower growth over time (b = −0.0315, p < .001).
Table 4.
Growth Models for Alcohol Problems by Internalizing Genetic Risk
| Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|
| b | se | b | se | b | se | ||
| Age | I | −0.1691*** | (0.0455) | −0.1657*** | (0.0450) | −0.1697*** | (0.0455) |
| S | 0.0815*** | (0.0065) | 0.0810*** | (0.0065) | 0.0797*** | (0.0066) | |
| Q | −0.0023*** | (0.0004) | −0.0023*** | (0.0004) | −0.0022*** | (0.0004) | |
| Genetic Risk | I | −0.0124† | (0.0075) | −0.0115 | (0.0073) | −0.0203† | (0.0122) |
| S | 0.0151*** | (0.0042) | 0.0145*** | (0.0041) | 0.0169** | (0.0063) | |
| Q | −0.0006* | (0.0003) | −.00006* | (0.0003) | −0.0005 | (0.0004) | |
| Childhood SES | I | −0.0069 | (0.0071) | −0.0058 | (0.0071) | −0.0148 | (0.0105) |
| S | −0.0014 | (0.0039) | −0.0018 | (0.0039) | 0.0090 | (0.0064) | |
| Q | 0.0002 | (0.0003) | 0.0002 | (0.0003) | −0.0004 | (0.0004) | |
| Female | I | 0.0480** | (0.0148) | 0.0474** | (0.0148) | 0.0438** | (0.0151) |
| S | −0.0315*** | (0.0080) | −0.0313*** | (0.0079) | −0.0290*** | (0.0081) | |
| Q | 0.0008 | (0.0005) | 0.0008 | (0.0005) | 0.0006 | (0.0005) | |
| Childhood SES × Genetic Risk | I | – | – | 0.0160* | (0.008) | 0.0305* | (0.0125) |
| S | – | – | −0.0103* | (0.0045) | −0.0131† | (0.0076) | |
| Q | – | – | 0.0006* | (0.0003) | 0.0007 | (0.0005) | |
| Female × Genetic Risk | I | – | – | – | – | 0.0122 | (0.0149) |
| S | – | – | – | – | −0.0036 | (0.0082) | |
| Q | – | – | – | – | −0.0002 | (0.0005) | |
| Female × Childhood SES | I | – | – | – | – | 0.0170 | (0.0133) |
| S | – | – | – | – | −0.0201* | (0.0078) | |
| Q | – | – | – | – | 0.0010† | (0.0005) | |
| Female × Childhood SES × Genetic Risk | I | – | – | – | – | −0.0243 | (0.0159) |
| S | – | – | – | – | 0.0057 | (0.0092) | |
| Q | – | – | – | – | −0.0003 | (0.0006) | |
| Log-likelihood (df) | −3,191.93 (15) | −3,188.08 (18) | −3,178.47 (27) | ||||
| AIC | 6,421.87 | 6,420.15 | 6,418.94 | ||||
All models include low birth weight, maternal smoking during pregnancy, maternal drinking during pregnancy, and maternal age at birth on initial status.
p < .10
p < .05
p < .01
p < .001
I = intercept; S = linear slope; Q = quadratic slope
N = 1,868 (Person observations = 5,888)
Including the interaction between childhood SES and internalizing risk (Model 2), did not result in a significant improvement in model fit, though the LR test was just over the threshold (Δχ2 = 7.71, Δdf = 3, p = .052). While overall model fit did not improve, the interaction between childhood SES and internalizing risk was significantly related to initial status (b = 0.0160, p < .05), linear change (b = −0.0103, p < .05), and quadratic change (b = 0.0006, p < .05). The effects of sex from Model 2 remained essentially unchanged.
Model 3 again includes all parameters and their interactions with sex. These interactions lead to a significant improvement in overall model fit (Δχ2 = 19.22, Δdf = 9, p < .05), though the interaction between sex, childhood SES, and genetic risk was not significantly related to initial status or change over time. Of the interactions entered in Model 3, only the effect of the interaction between sex and childhood SES on linear change was significant (b = −0.0201, p < .05). In comparison to Model 2, the effect of female again remained unchanged. However, the effect of genetic risk on the quadratic slope and the effect of the interaction between genetic risk and childhood SES on the linear slope were no longer significant, though the latter was just above the p < .05 threshold (b = −0.0131, p = .085).
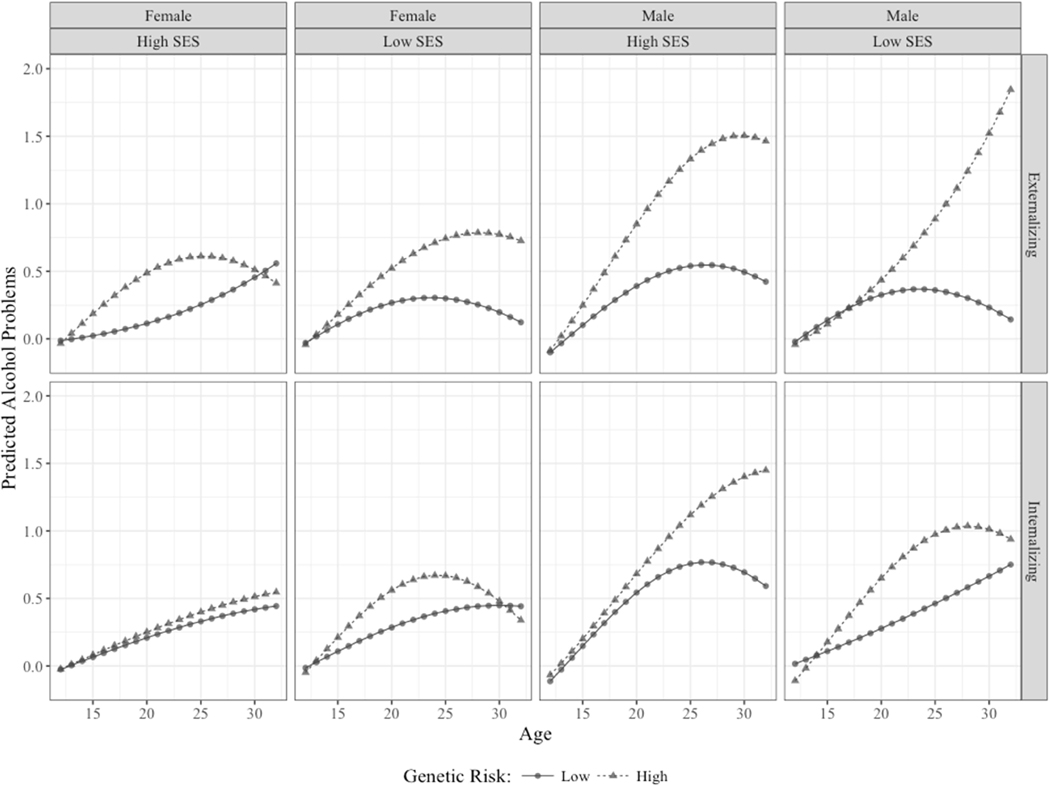
Figure 1 presents results from the Model 3 for both externalizing genetic risk and internalizing genetic risk, broken down by sex (Male vs. Female), childhood SES (±2 SD), and level of genetic risk (± 1 SD). We see several patterns that conform to the significant parameter estimates in the model. First, those at higher genetic risk are consistently at greater risk of AP. Second, males have consistently higher levels of AP. Third, low SES seems to be a risk factor for females at genetic risk, while high SES seems to be a risk factor for males at genetic risk, regardless of the etiological pathway. The one exception to this appears to be the lack of a drop off in AP among low SES males at high externalizing risk.
Figure 1.
Predicted values for alcohols problems across time, genetic risk, levels of childhood SES, and sex. Genetic risk values were set to ± 1 SD from the mean. SES values set to ± 2 SD from the mean.
Discussion
Our goal was to determine whether genetic risk for either internalizing and externalizing disorders, estimated from parental and co-twin reports of these disorders, was associated with trajectories of alcohol problems across adolescence and young adulthood. Genetic risk for both internalizing and externalizing disorders were associated with a significant increase in AP over time and followed a similar pattern. Though it appeared that risk for externalizing disorders exerted a stronger effect, overall.
GxE in Trajectories of Alcohol Problems
We were interested in whether childhood SES moderated genetic risk on alcohol problem trajectories. We expected that externalizing genetic risk would be stronger among those from higher SES families based on the social control/opportunity model of GxE, a consistent finding in GxE for alcohol misuse (Barr et al., 2017; Cooke et al., 2015; Dick et al., 2009). Because of the greater heritability of externalizing problems under conditions of greater SES (Middeldorp et al., 2014; Tuvblad et al., 2006) and the greater access to alcohol (Swahn et al., 2002) these individuals may be more susceptible to genetic predispositions. We found modest evidence that childhood SES moderated the effect of genetic risk for externalizing disorders on AP. While the individual parameters were just below the traditional threshold for significance, inclusion of the gene-environment interaction significantly improved the overall model fit. Those from high SES backgrounds at high externalizing genetic risk experienced greater increase in levels of AP followed by a faster decline compared to those at genetic risk from low SES backgrounds. This switch among those at high genetic risk mimics previous main effects of childhood SES, in that those from higher SES families misuse alcohol at greater rates during young adulthood (Kendler et al., 2014; Patrick et al., 2012), but those from lower SES families misuse alcohol at greater rates during mid-to-later life (Ferraro et al., 2016).
One possible mechanism for this shift is educational attainment. Those from higher SES backgrounds are more likely to attend college settings, where genetic influences on alcohol misuse become more pronounced (Timberlake et al., 2007), placing them at risk during emerging adulthood. However, those from disadvantaged backgrounds generally have truncated educational attainment (Duncan et al., 1998). While educational attainment does not alter genetic influences on alcohol misuse during young adulthood (Barr et al., 2016), lower educational attainment is related to greater genetic influences on alcohol use in the broader population (Hamdi et al., 2015). Those from more advantaged backgrounds with a higher genetic load may bare the initial risk of alcohol misuse, through increased exposure to settings where alcohol is available, while genetic risk among those from disadvantaged backgrounds becomes important later in the life course.
For internalizing genetic risk, we expected results to conform to the contextual triggering model of GxE, where stressful conditions (lower SES) increase the strength of genetic influences. Individuals from more disadvantaged backgrounds typically face greater exposure to stressful and adverse conditions (Cohen et al., 2010). Greater exposure to these conditions is associated with increased heritability in many internalizing disorders (Hicks et al., 2009; Lau et al., 2007). We found partial evidence to support this model. Those from higher SES experienced a slower growth in AP over time. However, these higher SES individuals started with higher initial levels, and those at greater internalizing risk from higher SES backgrounds did not experience a decrease in AP. These differences in patterns of problems across life course context suggest this relationship is not as straightforward as we initially proposed.
Sex Differences in the Role of Childhood SES
Finally, we expected externalizing genetic risk to have a stronger influence on trajectories of AP in males and internalizing genetic risk to have a stronger influence in females, as the prevalence of internalizing and externalizing disorders differs by sex (Kessler et al., 2005) and previous evidence of qualitative differences in the additive genetic variance contributing to each across sex (Kendler et al., 2006; Kendler et al., 2016). We found evidence of sex differences when we allowed the trajectories for AP to vary as a function of sex, childhood SES, and genetic risk.
However, sex differences were primarily the result of differences in the effect of childhood SES rather than different effects of genetic risk. Low SES seemed to be a greater risk for females. A supplemental analysis revealed childhood SES was positively associated with AP for males and negatively for females in young adulthood. We found little evidence of sex differences in the effect of genetic risk, and internalizing or externalizing risk seems to equally risky in relation to AP across sex, though there were some mean differences between males and females (internalizing risk mean difference = .016, p < .001; externalizing risk mean difference = −.044, p < .001). While there may be differences in genetic liability for certain disorders, we found that sex differences in trajectories of alcohol misuse primarily reflect differences in the influence of early life socioeconomic conditions. Future work will need to identify the sex-specific mechanisms through which childhood SES influences later patterns of alcohol misuse.
These analyses reiterate the “long arm” of early life socioeconomic conditions as a risk factor for future health-related outcomes, including alcohol problems (Kendler et al., 2014). The burden of early life conditions is not shared equally across genetic risk. Being at genetic risk for externalizing disorders and coming from a low SES background sets individuals on a trajectory for problem alcohol use later in the life course. Those from higher SES families at high genetic risk had higher levels of AP throughout adolescence and the transition to adulthood. For internalizing risk, lower SES increased the likelihood of AP during adolescence and young adulthood. Future research should examine the mechanisms related to both childhood SES, alcohol misuse, and comorbid behaviors, such as truncated educational attainment (Breslau et al., 2008), exposure to stressful or traumatic experiences (Boynton-Jarrett et al., 2013; Lloyd & Turner, 2008), or cognitive development (Noble et al., 2006).
Our analyses have several limitations. First, because of sample attrition and missing data, we may be underestimating the influence of genetic and environmental risk factors associated with alcohol problems. Early alcohol problems and childhood SES were associated with lower likelihood of participating in the following waves of data collection, and we may be underestimating the effect of these risk factors. Second, our measure of alcohol problems may underestimate the amount of risky drinking individuals engage in early in life (such as heavy episodic drinking) that does not meet criteria for problems. The measure of alcohol problems also differed between the first four waves and the final two. However, we see a strong pattern of autocorrelation across time points, even from when the measures of alcohol problems switched. Third, our analyses were limited to white twins due to the small number of African-Americans twins ascertained in the ABD. As others have noted, research using genetic designs must do better to recruit non-European samples (Dick et al., 2017). Finally, our design used inferred genetic risk, rather than measured genetic risk, such as polygenic risk scores. However, the approach used in these analyses has been validated previously (Kendler et al., 2011; Meyers et al., 2014). To date, twin studies remain a robust way to study aggregate genetic risk.
Using a genetically informed design, we examined different pathways of risk for alcohol problem trajectories. Genetic risk for internalizing or externalizing disorders was associated with increased alcohol problems over time. Childhood SES moderated genetic risk for externalizing disorders, such that those at genetic risk from higher SES families had greater risk of problems during early adulthood while lower SES families demonstrated the most problematic trajectories later in adulthood. The effect was reversed when considering internalizing disorders. We found evidence of sex differences in trajectories, though these were the result of differential influences of childhood SES rather than differences in the effect of genetic risk. This work demonstrates the importance of considering the intersection between individual predispositions and socially patterned environments.
Research Highlights.
High externalizing genetic risk/high SES increase risk for alcohol problems across adolescence.
High externalizing genetic risk/low SES increase risk for alcohol problems in adulthood.
The opposite pattern occurs for internalizing genetic risk, but to a lesser degree.
We find sex differences in the effect of childhood SES, but not genetic risk.
Lower SES increases risk for females. Higher SES increases risk for males.
Acknowledgments
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award numbers R01AA015416 and K02AA018755. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarnoudse-Moens CSH, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-Analysis of Neurobehavioral Outcomes in Very Preterm and/or Very Low Birth Weight Children. Pediatrics. 2009;124:717. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- Barr PB, Salvatore JE, Maes HH, Aliev F, Latvala A, Viken RJ, et al. Education and alcohol use: A study of gene-environment interaction in young adulthood. Social Science & Medicine. 2016;162:158–167. doi: 10.1016/j.socscimed.2016.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr PB, Salvatore JE, Maes HH, Korhonen T, Latvala A, Aliev F, et al. Social Relationships Moderate Genetic Influences on Heavy Drinking in Young Adulthood. J Stud Alcohol Drugs. 2017;78:817–826. doi: 10.15288/jsad.2017.78.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman JD, Daw J, Freese J. Defining the Environment in Gene–Environment Research: Lessons From Social Epidemiology. American Journal of Public Health. 2013;103:S64–S72. doi: 10.2105/AJPH.2013.301355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton-Jarrett R, Hair E, Zuckerman B. Turbulent times: effects of turbulence and violence exposure in adolescence on high school completion, health risk behavior, and mental health in young adulthood. Social Science & Medicine. 2013;95:77–86. doi: 10.1016/j.socscimed.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Breslau J, Lane M, Sampson N, Kessler RC. Mental disorders and subsequent educational attainment in a US national sample. Journal of psychiatric research. 2008;42:708–716. doi: 10.1016/j.jpsychires.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Chen E, Matthews KA. Childhood socioeconomic status and adult health. Annals of the New York Academy of Sciences. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- Conger RD, Conger KJ, Martin MJ. Socioeconomic Status, Family Processes, and Individual Development. Journal of Marriage and Family. 2010;72:685–704. doi: 10.1111/j.1741-3737.2010.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke ME, Meyers JL, Latvala A, Korhonen T, Rose RJ, Kaprio J, et al. Gene-Environment Interaction Effects of Peer Deviance, Parental Knowledge and Stressful Life Events on Adolescent Alcohol Use. Twin Research and Human Genetics. 2015;18:507–517. doi: 10.1017/thg.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin SC, Mathews TJ. National Center for Health Statistics. 2016. Smoking Prevalence and Cessation Before and During Pregnancy: Data From the Birth Certificate, 2014. [PubMed] [Google Scholar]
- Davis JA. Occupational prestige ratings from the 1989 general social survey. Inter-University Consortium for Political and Social Research; 1991. [Google Scholar]
- Dick DM. Developmental Changes in Genetic Influences on Alcohol Use and Dependence. Child Development Perspectives. 2011;5:223–230. [Google Scholar]
- Dick DM, Barr P, Guy M, Nasim A, Scott D. Review: Genetic research on alcohol use outcomes in African American populations: A review of the literature, associated challenges, and implications. The American Journal on Addictions. 2017;26:486–493. doi: 10.1111/ajad.12495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Bernard M, Aliev F, Viken R, Pulkkinen L, Kaprio J, et al. The role of socioregional factors in moderating genetic influences on early adolescent behavior problems and alcohol use. Alcoholism: Clinical and Experimental Research. 2009;33:1739–1748. doi: 10.1111/j.1530-0277.2009.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GJ, Yeung WJ, Brooks-Gunn J, Smith JR. How Much Does Childhood Poverty Affect the Life Chances of Children? American sociological review. 1998;63:406–423. [Google Scholar]
- Edwards AC, Sihvola E, Korhonen T, Pulkkinen L, Moilanen I, Kaprio J, et al. Depressive symptoms and alcohol use are genetically and environmentally correlated across adolescence. Behavior genetics. 2011;41:476–487. doi: 10.1007/s10519-010-9400-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M. Gender Differences in the Determinants of Distress, Alcohol Misuse, and Related Psychiatric Disorders. Society and Mental Health. 2013;3:96–113. [Google Scholar]
- Elo IT, Martikainen P, Myrskyla M. Socioeconomic status across the life course and all-cause and cause-specific mortality in Finland. Social Science & Medicine. 2014;119:198–206. doi: 10.1016/j.socscimed.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Kantrowitz E. Socioeconomic status and health: the potential role of environmental risk exposure. Annual Review of Public Health. 2002;23:303–331. doi: 10.1146/annurev.publhealth.23.112001.112349. [DOI] [PubMed] [Google Scholar]
- Ferraro KF, Schafer MH, Wilkinson LR. Childhood Disadvantage and Health Problems in Middle and Later Life: Early Imprints on Physical Health? American sociological review. 2016;81:107–133. doi: 10.1177/0003122415619617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SE, Abrams DB, Buka SL. Socioeconomic status over the life course and stages of cigarette use: initiation, regular use, and cessation. Journal of epidemiology and community health. 2003;57:802–808. doi: 10.1136/jech.57.10.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosby BJ. Early life course pathways of adult depression and chronic pain. Journal of Health and Social Behavior. 2013;54:75–91. doi: 10.1177/0022146512475089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdi NR, Krueger RF, South SC. Socioeconomic status moderates genetic and environmental effects on the amount of alcohol use. Alcohol Clin Exp Res. 2015;39:603–610. doi: 10.1111/acer.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding DJ. Collateral Consequences of Violence in Dsadvantaged Neighborhoods. Social forces. 2009;88:757–784. doi: 10.1353/sof.0.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM. An Integrative Approach to Health. Demography. 2010;47:1–22. doi: 10.1353/dem.0.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward MD, Gorman BK. The Long Arm of Childhood: The Influence of Early-Life Social Conditions on Men’s Mortality. Demography. 2004;41:87–107. doi: 10.1353/dem.2004.0005. [DOI] [PubMed] [Google Scholar]
- Hicks BM, DiRago AC, Iacono WG, McGue M. Gene–environment interplay in internalizing disorders: consistent findings across six environmental risk factors. Journal of Child Psychology and Psychiatry. 2009;50:1309–1317. doi: 10.1111/j.1469-7610.2009.02100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill TD, Needham BL. Rethinking gender and mental health: a critical analysis of three propositions. Soc Sci Med. 2013;92:83–91. doi: 10.1016/j.socscimed.2013.05.025. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Mulder EJH. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neuroscience & Biobehavioral Reviews. 2006;30:24–41. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Hussong AM, Jones DJ, Stein GL, Baucom DH, Boeding S. An internalizing pathway to alcohol use and disorder. Psychology of Addictive Behaviors. 2011;25:390–404. doi: 10.1037/a0024519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral Disinhibition and the Development of Early-Onset Addiction: Common and Specific Influences. Annual Review of Clinical Psychology. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner C, Dick D. Predicting alcohol consumption in adolescence from alcohol-specific and general externalizing genetic risk factors, key environmental exposures and their interaction. Psychological medicine. 2011;41:1507–1516. doi: 10.1017/S003329171000190X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Hickman M, Heron J, Macleod J, Lewis G, et al. Socioeconomic Status and Alcohol-Related Behaviors in Mid- to Late Adolescence in the Avon Longitudinal Study of Parents and Children. J Stud Alcohol Drugs. 2014;75:541–545. doi: 10.15288/jsad.2014.75.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish National Twin Study of Lifetime Major Depression. American Journal of Psychiatry. 2006;163:109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Pirouzi Fard MN, Lönn S, Edwards AC, Maes HH, Lichtenstein P, et al. A National Swedish Twin-Sibling Study of Alcohol Use Disorders. Twin Research and Human Genetics. 2016;19:430–437. doi: 10.1017/thg.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of general psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of dsm-iv disorders in the national comorbidity survey replication. Archives of general psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Lau JYF, Gregory AM, Goldwin MA, Pine DS, Eley TC. Assessing gene–environment interactions on anxiety symptom subtypes across childhood and adolescence. Development and Psychopathology. 2007;19:1129–1146. doi: 10.1017/S0954579407000582. [DOI] [PubMed] [Google Scholar]
- Lloyd DA, Turner RJ. Cumulative lifetime adversities and alcohol dependence in adolescence and young adulthood. Drug and alcohol dependence. 2008;93:217–226. doi: 10.1016/j.drugalcdep.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López Turley RN. Are Children of Young Mothers Disadvantaged Because of Their Mother’s Age or Family Background? Child Development. 2003;74:465–474. doi: 10.1111/1467-8624.7402010. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Mathews TJ. National Center for Health Statistics. 2017. Births: Final Data for 2015. [PubMed] [Google Scholar]
- McDonough P, Walters V. Gender and health: reassessing patterns and explanations. Social Science & Medicine. 2001;52:547–559. doi: 10.1016/s0277-9536(00)00159-3. [DOI] [PubMed] [Google Scholar]
- Meyer JM, Silberg JL, Simonoff E, Kendler KS, Hewitt JK. The Virginia Twin-Family Study of Adolescent Behavioral Development: assessing sample biases in demographic correlates of psychopathology. Psychological medicine. 1996;26:1119–1133. doi: 10.1017/s0033291700035844. [DOI] [PubMed] [Google Scholar]
- Meyers JL, Salvatore JE, Vuoksimaa E, Korhonen T, Pulkkinen L, Rose RJ, et al. Genetic Influences on Alcohol Use Behaviors Have Diverging Developmental Trajectories: A Prospective Study Among Male and Female Twins. Alcoholism: Clinical and Experimental Research. 2014;38:2869–2877. doi: 10.1111/acer.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp CM, Lamb DJ, Vink JM, Bartels M, van Beijsterveldt CEM, Boomsma DI. Child Care, Socio-economic Status and Problem Behavior: A Study of Gene–Environment Interaction in Young Dutch Twins. Behavior genetics. 2014;44:314–325. doi: 10.1007/s10519-014-9660-z. [DOI] [PubMed] [Google Scholar]
- Montez JK, Hayward MD. Cumulative Childhood Adversity, Educational Attainment, and Active Life Expectancy Among U.S. Adults. Demography. 2014;51:413–435. doi: 10.1007/s13524-013-0261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Farah MJ, McCandliss BD. Socioeconomic background modulates cognition-achievement relationships in reading. Cognitive Development. 2006;21:349–368. doi: 10.1016/j.cogdev.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Wightman P, Schoeni RF, Schulenberg JE. Socioeconomic Status and Substance Use Among Young Adults: A Comparison Across Constructs and Drugs. J Stud Alcohol Drugs. 2012;73:772–782. doi: 10.15288/jsad.2012.73.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore JE, Cho SB, Dick DM. Genes, Environments, and Sex Differences in Alcohol Research. J Stud Alcohol Drugs. 2017;78:494–501. doi: 10.15288/jsad.2017.78.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan MJ, Hofer SM. Social context in gene–environment interactions: Retrospect and prospect. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2005;60:65–76. doi: 10.1093/geronb/60.special_issue_1.65. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford university press; 2003. [Google Scholar]
- Slopen N, Williams DR, Fitzmaurice GM, Gilman SE. Sex, stressful life events, and adult onset depression and alcohol dependence: are men and women equally vulnerable? Soc Sci Med. 2011;73:615–622. doi: 10.1016/j.socscimed.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Swahn MH, Hammig BJ, Ikeda RM. Prevalence of youth access to alcohol or a gun in the home. Injury Prevention. 2002;8:227. doi: 10.1136/ip.8.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timberlake DS, Hopfer CJ, Rhee SH, Friedman NP, Haberstick BC, Lessem JM, et al. College attendance and its effect on drinking behaviors in a longitudinal study of adolescents. Alcohol Clin Exp Res. 2007;31:1020–1030. doi: 10.1111/j.1530-0277.2007.00383.x. [DOI] [PubMed] [Google Scholar]
- Tuvblad C, Grann M, Lichtenstein P. Heritability for adolescent antisocial behavior differs with socioeconomic status: gene–environment interaction. Journal of Child Psychology and Psychiatry. 2006;47:734–743. doi: 10.1111/j.1469-7610.2005.01552.x. [DOI] [PubMed] [Google Scholar]
- van de Mheen H, Stronks K, Looman CWN, Mackenbach JP. Does childhood socioeconomic status influence adult health through behavioural factors? International Journal of Epidemiology. 1998;27:431–437. doi: 10.1093/ije/27.3.431. [DOI] [PubMed] [Google Scholar]
- Verhulst B, Neale MC, Kendler KS. The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychological medicine. 2015;45:1061–1072. doi: 10.1017/S0033291714002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Global status report on alcohol and health, 2014 2014 [Google Scholar]