Abstract
The somatostatin-secreting δ-cells comprise ~5% of the cells of the pancreatic islets. The δ-cells have complex morphology and may, via cellular process, interact with many more islet cells than suggested by their low number. δ-cells are equipped with ATP-sensitive potassium channels (KATP channels). These channels are open at low glucose, but close in response to glucose stimulation. This results in membrane depolarisation, initiation of electrical activity, and somatostatin secretion. Glucose also stimulates somatostatin secretion by KATP channel-independent mechanisms. The Ca2+ signal initiated by electrical activity leads to further mobilization by intracellular Ca2+ stores. Factors released by neighbouring β-cells (like GABA and urocortin-3) amplify the glucose-induced effects on δ-cell electrical activity/somatostatin secretion. Somatostatin secreted from the δ-cell acts locally within the islets as a paracrine inhibitor of insulin and glucagon secretion. The effects of somatostatin are mediated by activation of somatostatin receptors that are coupled to the inhibitory G protein, which culminates in transient suppression of α- and β-cell electrical activity and exocytosis. There is evidence that somatostatin secretion is perturbed in diabetes. This may explain the loss of appropriate hypoglycaemia-induced glucagon secretion in diabetic animals, which can be mitigated by SSTR2 antagonists. Somatostatin secretion is stimulated by hypokalaemia, a well-known by-product of insulin therapy, and this effect may, via inhibition of glucagon secretion, increase the risk of hypoglycaemia in insulin-treated patients. It is proposed that somatostatin antagonists or agents that suppress somatostatin secretion should be considered as an adjunct to insulin therapy.
Introduction
A human pancreas contains 1–3 million pancreatic islets1,2. These are complex micro-organs that consist of several types of endocrine cell that play a key role in the regulation of whole-body energy metabolism3. Whereas insulin (secreted by the β-cells) is the body’s only hormone capable of lowering blood glucose, glucagon (secreted by the α-cells) is the principal plasma glucose-increasing hormone. In general, insulin and glucagon levels vary reciprocally and the insulin/glucagon ratio determines the balance between anabolism (glucose and fat storage) and catabolism (glycogen, fat breakdown and gluconeogenesis)3.
The severe metabolic disturbances associated with diabetes that culminate in hyperglycaemia result from the combination of lack of insulin and excess of glucagon4,5. Most therapeutic interventions focus on insulin: they stimulate release of endogenous insulin (i.e. by administration of sulphonylureas or GLP-1 agonists), promote insulin action, or involve administration of exogenous insulin. A serious (potentially fatal) complication of insulin therapy is hypoglycaemia. It has been estimated that up to 10% of insulin-treated diabetes patients die of ‘iatrogenic hypoglycaemia’ (iatros, Greek for healer/physician)6.
Because of their key roles in diabetes, the α- and β-cells have been the focus of much research during the last 30–40 years. We now have a very good understanding of the regulation of insulin secretion7,8. A consensus model for the regulation of glucagon secretion remains to be formulated but there is extensive information on α-cell gene expression, ultrastructure and electrical activity9,10. By comparison, the δ-cell has not received much attention from islet physiologist of late and our understanding of the cell physiology of somatostatin secretion remains fragmentary. Recent findings implicate increased somatostatin signalling as a cause of the reduced counter-regulatory glucagon secretion during insulin-induced hypoglycaemia in diabetic animals11–13. Conversely, insulin and glucagon secretion during high glucose are both tonically inhibited by somatostatin14 and there is evidence that hyperglucagonemia in poorly controlled diabetes can be suppressed by somatostatin15–17. Indeed, an early study demonstrated that somatostatin infusion in type 1 and type 2 diabetic patients led to improved glycaemic control despite lower insulin requirements by reducing glucagon secretion and it was proposed that a long-acting somatostatin agonist may be useful as an adjunct to insulin therapy18. Collectively, these observations merit renewed interest in the δ-cell. A review on δ-cell physiology and somatostatin secretion in health and disease is therefore timely. Here we will present a model for the cellular regulation of somatostatin secretion in δ-cells and discuss the crucial role that the δ-cells play by controlling the α- and β-cells via inhibitory paracrine crosstalk. We will then turn to the impact of diabetes on somatostatin secretion and its role in the pathophysiology of diabetes. Finally, we will consider the δ-cell and somatostatin signalling as pharmacological targets.
Discovery of somatostatin and the pancreatic δ-cell
Somatostatin was originally isolated from the hypothalamus and found to inhibit the release of growth hormone (GH) in the pituitary19. Initially named as ‘GH release-inhibiting hormone’ it was renamed somatostatin to reflect this growth-inhibiting effect. Shortly after its discovery, somatostatin was found to be produced and secreted in the islets of Langerhans20.
Insulin and glucagon are released by the β- (B-) and α- (A-) cells of the endocrine pancreas of the pancreatic islets. The A- and B-cells were identified based on the staining properties following alcohol- and aqueous-based fixation methods. In addition, a third type of islet cell type was left unstained and referred to a ‘clear’ or C-cell. Subsequently another granulated cell type was identified and referred to as D-cell (or δ-cells). They are identical to the argyrophilic islet cells originally referred to as A1/α1-cells (to distinguish them from the argyrophobic glucagon-containing A2/α2-cells). It now seems clear that the C-cells were in fact D-cells (reviewed by REF21). With hindsight, it seems likely that somatostatin is the factor in lysates from the α1 cells (=δ-cells) responsible for the inhibition of insulin release22,23. In this review we will refer to the different islet cells using the Greek letter nomenclature.
Somatostatin
There are two types of somatostatin: somatostatin-14 and somatostatin-28. Both forms of somatostatin are derived from the precursor pre-prosomatostatin (116 amino acids) which is cleaved into prosomatostatin (92 amino acids). Prosomatostatin undergoes C-terminal post-translational processing to generate somatostatin-14 and somatostatin-28. Both peptides are very short-lived and have a half-life of 1min in circulation. While somatostatin-28 is the dominant isoform elsewhere in the gastrointestinal tract, the pancreatic δ-cells secrete somatostatin-14, which is stored in secretory granules24 and released by Ca2+-dependent exocytosis.
The pancreatic δ-cell
The δ-cells comprise only 5% of the islet cell number25. In mouse islets, where β-cells occupy the islet core, most of the δ-cells are located in the islet ‘cortex’ with few δ-cells found in the islet centre. Whereas most α- and β-cells are rounded or rhomboid, the δ-cells show more complex morphology and have long, neurite-like processes (FIG. 1A) that make close contact with α-, β-, and δ-cells at some distance from the cell body, thereby enabling an extensive paracrine network. In human islets, where islet architecture is less defined, δ-cells occur throughout the islet. Direct δ- to α-cell contacts are increased in islets from patients with type 2 diabetes24, raising the interesting possibility that the α-cells may be subject to stronger somatostatin-mediated paracrine inhibition in diabetic patients. The δ-cell neurite-like processes can be >20μm long (one-third of the average islet diameter26) and may make contact with multiple α- and β-cells. This may explain the stimulation of both glucagon and insulin secretion seen in the presence of somatostatin receptor antagonists27–30. Moreover, β-cells are connected via connexin36 gap junctions that ensure synchronized calcium responses and insulin secretion within each islet31–33. Thus, β-cells not in direct contact with a δ-cell may be indirectly affected via gap-junctional signalling. Recently direct evidence for β- to δ-cell electrical coupling was reported34.
Figure 1.
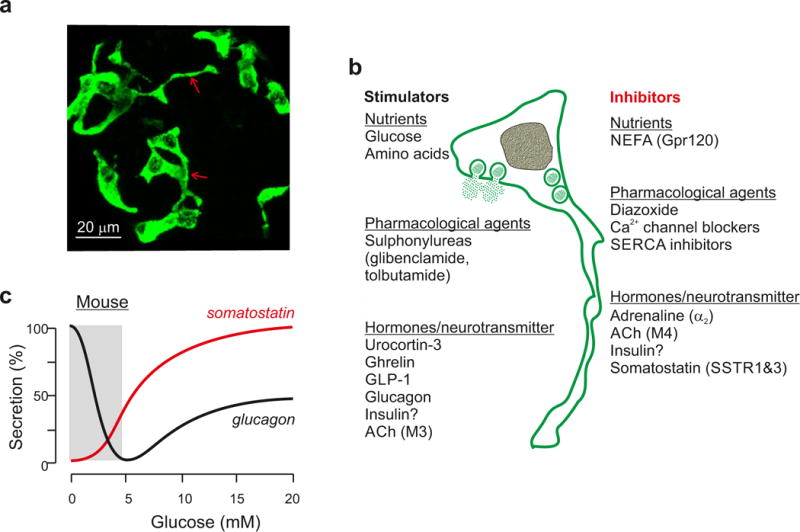
Somatostatin secretion and δ-cell histology. a| δ-cells in mouse islets. Note that some δ-cells possess processes (arrows) that extend for tens of microns. Image supplied by Dr Q Zhang (Oxford) (Methods as in REF44). b| Regulation of somatostatin secretion by nutrients, hormones/neurotransmitters and pharmacological agents. Compounds that (have been reported to) stimulate somatostatin secretion shown left in black and those that inhibit release to the right in red. c| Parallel measurements of somatostatin (red) and glucagon (black) secretion. Data taken from (REF43). 100% and 0% correspond to the maximum and the minimum secretion, respectively. The grey rectangle highlights that glucagon secretion is regulated at glucose concentrations that have little effect on somatostatin secretion.
Gastric and enteric somatostatin-secreting D-cells
In addition to the pancreatic δ-cells, somatostatin is also expressed throughout the gastrointestinal (GI) tract. It has been estimated that the gastrointestinal D-cells contain 65% of total body somatostatin and that the pancreatic islets only account for 5% and the rest found in the CNS35. In fact, most of circulating somatostatin originates from the D-cells36. Although these cells are notoriously difficult to study (scattered among the enterocytes as they are), by expressing fluorescent protein under the somatostatin promoter it is now possible to isolate them in sufficient numbers to perform more comprehensive transcriptomic and physiological characterization37. However, the regulation of somatostatin secretion from the gastrointestinal D-cells is beyond the scope of this review and interested readers are instead referred to two recent reviews that cover aspects of this topic38,39.
Pancreatic islet somatostatin secretion
FIG. 1B shows schematically the regulation of somatostatin secretion by nutrients, pharmacological agents, hormones and neurotransmitters. Agents have been divided into stimulators and inhibitors. TABLE 1 provides further details on the membrane receptor complement. In general, the regulation of somatostatin secretion in response to nutrients resembles that of insulin secretion. This is perhaps not surprising given that β- and δ-cells share an immediate common progenitor cell40. In this section we consider how δ-cells sense nutrients. We correlate the regulation of glucagon secretion against recently published information on δ-cell transcriptomes41,42.
TABLE 1.
Overview of receptors that are expressed by pancreatic delta cells and their effect on somatostatin secretion.
| Ligand | Receptor name | Gene symbol | Expression level (RPKM)a, b | Effect on secretion |
|---|---|---|---|---|
| Ucn3 | CRHR2α | Crhr2 | 5.14 | stimulates |
| ghrelin | GHSR | Ghsr | 48.44 | stimulates |
| GLP1 | GLP1R | Glp1r | 39.91 | stimulates |
| GIP | GIPR | Gipr | 13.70 | stimulates |
| GABA | Ionotropic (GABAA) and metabotropic (GABAB) | Gabra1–5, Gabrb1–3, Gabrd, Gabre, Gabrg1–3, Garbrp, Gabrq, Gabrr1,2 subunits | Multiple genes, expression invariably low. | stimulates |
| Acetylcholine | Muscarinic M3 | Chrm3 | 2.71 | stimulates |
| Muscarinic M4 | Chrm4 | 22.05 | inhibits | |
| Adrenaline | α2a adrenergic receptor | Adra2α | 9.72 | inhibits |
| Somatostatin | SSTR1 | Sstr1 | 18.28 | inhibits |
| Palmitate/NEFA | GPR120 | Ffar4 | 60.89 | inhibits |
| insulin | INSR | Insr | 12.16 | conflicting reports |
| Glucagon | GCGR | Gcgr | 5.62 | stimulates |
reads per kilobase of gene model per million reads sequenced (data from REF42).
RPKM values provide a useful approximation of actual receptor expression levels, but many post-transcriptional processes contribute to the actual cell-surface expression of receptor protein.
Glucose
Glucose-induced somatostatin secretion is initiated in mouse islets somewhat lower than those required in mouse islets (3 vs 6mM) and saturates at concentrations above 10mM (FIG. 1C). In human islets, somatostatin secretion is initiated at 3mM and then increases linearly with the glucose concentration (in parallel with insulin secretion)43. δ-cells express high levels of the high-affinity glucose transporters Glut1 (Slc2a1) and Glut3 (Slc2a3) instead of the beta cell-specific Glut2 (Slc2a2). δ-cells also express high levels of the glucokinase (Gck), which probably explains the inhibition of somatostatin secretion by the glucokinase inhibitor mannoheptulose44,45. It seems likely that glucose (via its metabolism) stimulates somatostatin secretion via an increased cytoplasmic ATP/ADP ratio and closure of ATP-sensitive K+ channels (KATP channels) in a way similar to what has been described in β-cells46 (see below). It is therefore surprising that the non-metabolisable glucose analogue 3-O-methyl-D-glucose also stimulates somatostatin secretion47, i.e. without an increase in the intracellular ATP/ADP ratio. Thus, it appears that glucose, in at least some δ-cells, can stimulate somatostatin secretion by a mechanism not involving closure of KATP channels. There is some evidence for low expression of SGLT1 (Slc5a1) in δ-cells41,42 and the ability of 3-O-methyl-D-glucose to stimulate somatostatin secretion may reflect sufficiently strong electrogenic (i.e. ability to affect the membrane potential) operation of this transporter to stimulate somatostatin release even without stimulation of ATP production.
Amino acids
Somatostatin secretion can be stimulated by the amino acids leucine and arginine48–52. The ability of arginine to stimulate somatostatin secretion probably reflects the expression of the cationic amino acid transporters Slc7a1 and Slc7a2 (that encode CAT-1 and CAT-2, respectively)41,42. In β-cells, these transporters mediate electrogenic uptake of amino acids like arginine and lysine53 and thereby produce membrane depolarisation and initiate action potential firing when KATP channel activity is low (for example, in the presence of glucose). It is likely that arginine stimulates somatostatin secretion by the same mechanism. Leucine is transported via the neutral amino acid transporter Slc7a5, which is expressed in δ-cells41,42. Leucine is, following deamidation and formation of α-ketoisocarproic acid54, is metabolized by the Krebs cycle and probably stimulates somatostatin secretion via closure of the KATP channels.
Fatty acids
The plasma concentration of non-esterified free fatty acids (NEFA: mainly palmitate, oleate, stearate and lineoleate55) oscillates between <0.1 mM after a meal and 0.5 mM in the fasted state3. The free fatty acid palmitate inhibits glucose-induced somatostatin secretion56. Mouse δ-cells express high levels of the free fatty acid receptor GPR120 (Ffar4). Interestingly, GPR120-specific agonists inhibit somatostatin secretion and these effects are not seen in islets from Ffar4 knockout mice57. This selective inhibition of the δ-cell can be expected to result in relief from paracrine suppression of α- and β-cells, which may contribute to the acute palmitate-induced stimulation of both insulin and glucagon secretion56,58.
δ-cell electrical activity
Like β- and α-cells, δ-cells are electrically excitable and experimental conditions that stimulate somatostatin secretion are generally associated with increased action potential firing in the δ-cells59–61 (FIG. 2A) The δ-cells are equipped with KATP channels of exactly the same type as those found in β- and α-cells. Expression of the KATP subunits Kir6.2 (Kcnj11) and Sur1 (Abcc8) in δ-cells is at least as high as in β-cells41,42. The KATP channels are active (open) at low glucose and this maintains a negative (hyperpolarized) membrane potential in the δ-cell. Increasing glucose or application of sulphonylureas (such as tolbutamide and glibenclamide) reduces KATP channel activity, depolarizes the δ-cell and initiates action potential firing, thus accounting for the stimulation of somatostatin secretion44,49,62–64. Conversely, the KATP channel activator diazoxide prevents depolarization and thereby inhibits somatostatin secretion59,65.
Figure 2.
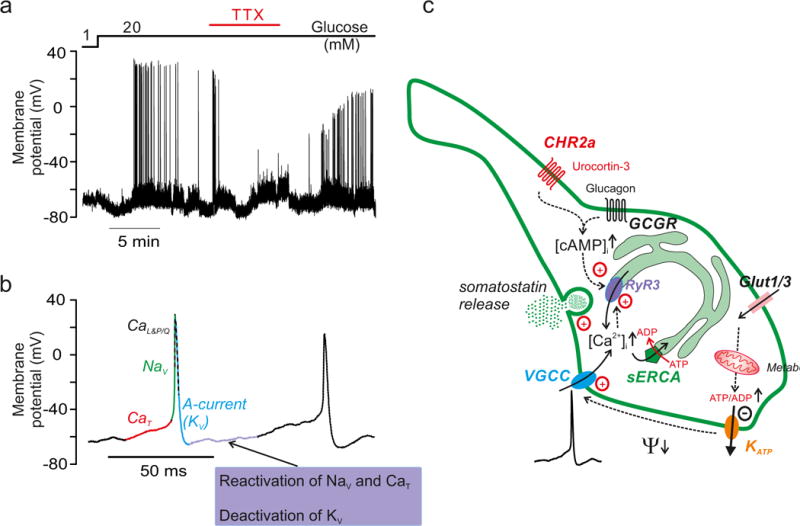
Regulation of somatostatin secretion by δ-cell electrical activity. a| Electrical activity at 1 and 20mM glucose recorded from a δ-cell in an intact mouse δ-cell. Cell type identified as described in (REF66). The Na+ channel blocker tetrodotoxin (TTX) was added as indicated. Data taken from (REF61). Note suppression of action potential firing in the presence of TTX. b| Two successive action potential recorded from mouse δ-cell (left) and contribution of different voltage-gated ion channels to depolarization, repolarization and interval between two successive action potentials as deduced from electrophysiological66 and transcriptomic analyses41,42. The action potential is initiated by opening of ‘low-threshold’ T-type Ca2+ channels (CaT; specifically CaV3.2). The upstroke of the action potential involves opening of voltage-gated Na+ channels (NaV; NaV1.3 and/or NaV1.7) and high-threshold voltage-gated L- (CaL; specifically CaV1.3) and P/Q-type (CaP/Q; CaV2.1) Ca2+ channels. The downstroke (repolarising phase) of the action potential involves opening of A-type K+ currents (specifically KV4.1/4.2) with some contribution of delayed rectifying K+ channels (KV; specifically KV1.5 and KV2.1). In the interval during two successive action potential, the KV channels that activated during the action potential deactivates slowly and this, together with the recovery from inactivation of NaV and CaT explains the ‘pacemaker’ depolarization. Single-cell transcriptomics112 indicates a similar ion channel complement in human δ-cells. c| Stimulus-secretion coupling in a (mouse) δ-cell. Glucose uptake (via Glut1 and Glut3) leads to stimulation of glucose metabolism (glycolysis and mitochondria) and an increased cytoplasmic ATP/ADP ratio. This closes KATP channels in the plasma membrane, producing membrane depolarization (Ψ↓) and action potential firing, culminating in Ca2+ influx through voltage-gated Ca2+ channels (VGCC). The KATP channel activity is lower than in β-cells, which might explain why somatostatin secretion is initiated at lower glucose concentrations than insulin secretion. Ca2+ influx associated with electrical activity triggers further increase in cytoplasmic Ca2+ ([Ca2+]i) by Ca2+-induced Ca2+ release (CICR) in the sarco/endoplasmic reticulum (sER) by activation of ryanodine receptor 3 (Ryr3) Ca2+ release channels. The resultant increase in [Ca2+]i triggers somatostatin secretion. Glucose stimulation is also associated with elevation of cAMP, which may be produced be secondary to the glucose-induced [Ca2+]i increase and/or stimulation by glucagon (released by neighbouring α-cells following activation of the glucagon receptor: GCGR), urocortin-3 (released by β-cells and following activation of CRHR2a) or circulating GLP-1. Cyclic AMP sensitises the Ryr3 channels to the increase in [Ca2+]i and thereby facilitates CICR. Inhibitors of the Ca2+ ATPase (‘Ca2+ pump’) of the sER (SERCA inhibitors; e.g. thapsigargin) inhibit somatostatin secretion by depleting sER of Ca2+. Ca2+ channel blockers inhibit somatostatin secretion by reducing the Ca2+ influx that triggers CICR. Stimulation and inhibition indicated by dashed arrows and + and −, respectively. Upward and downward arrows indicate an increase in concentration or membrane hyperpolarisation.
In mouse δ-cells, the action potential originates from a membrane potential as negative as −60mV, may reach +30mV and have a duration of only a few milliseconds66 (FIG. 2B). The voltage-gated membrane currents underlying these action potentials have been characterized in some detail66 (See Legend FIG. 2B).
Local and systemic modulators of somatostatin secretion
In addition to the direct effects of glucose (and other nutrients) on the δ-cell, somatostatin secretion is modulated by paracrine factors (released from neighbouring α- and β-cells), circulating hormones, and neurotransmitters released by intra-islet nerve endings (FIG. 1B and TABLE 1).
Intra-islet factors
Pancreatic δ-cells express insulin receptors (Insr), but the action of insulin on somatostatin secretion is unclear. Anterograde infusion (i.e. in the direct of the normal blood flow) of an insulin antibody into perifused rat pancreata leads to dramatic (20-fold) stimulation of somatostatin secretion67. Administration of exogenous insulin has variably been reported not to affect68, inhibit52 or even stimulate somatostatin secretion69. However, it should be remembered that the intra-islet interstitial insulin concentration is likely to very high: the release of a single insulin granule (1.6 amol insulin) is sufficient to increase the insulin concentration to ~10nM, >100-fold higher than circulating levels of insulin26.
The δ-cells also express low levels of the glucagon receptor (Gcgr) and respond to glucagon with increased somatostatin secretion50,70.
Urocortin 3 (Ucn3) is co-released with insulin from β-cells and stimulates somatostatin secretion65. Urocortin-3 acts via activation of the α-isoform of the CRH receptor 2 (Crhr2), which is selectively expressed by δ-cells within the islet65. Genetic ablation of Crhr2 or Ucn3 leads to a 50–60% reduction of glucose-induced somatostatin secretion, an effect that was paralleled by a corresponding decrement in islet somatostatin content. Collectively, these observations indicate that islet somatostatin secretion is modulated by local release of urocortin 3 from β-cells.
The neurotransmitter GABA is also co-released with insulin from β-cells and stimulates somatostatin secretion in human islets71. Thus, GABA co-released with insulin and urocortin 3 may contribute to glucose-induced somatostatin secretion. In addition, there is evidence that GABA released from human δ-cells stimulates δ-cell electrical activity in an autocrine fashion71. Expression of GABA receptor subunits is low in mouse δ-cells (TABLE 1) but expression in human δ-cells is likely to be higher71.
In addition to the paracrine stimulation of the δ-cell by β-cell-derived factors (as exemplified by urocortin 3 and GABA) there is (as mentioned above) also evidence that the β-cells stimulate δ-cells by electrical coupling via gap junctions34.
In mouse islets, acetylcholine (ACh) is released by cholinergic nerve endings46,72. ACh has variously been reported to either stimulate44 or inhibit57,63 somatostatin secretion. Mouse δ-cells express muscarinic M3 (Chrm3) and M4 (Chrm4) receptors. Whereas M3 receptors are coupled to Gq (leading to Ca2+ mobilization and somatostatin exocytosis), M4 receptors are coupled to Gi (resulting in suppression of somatostatin secretion). Thus, expression of these two muscarinic receptors, coupled to different canonical signalling cascades, may explain the discrepant reported effects of ACh action on somatostatin secretion.
Human α-cells release ACh in response and that this potentiates insulin secretion by activation of M3 receptors in β-cells73. However, this is unlikely to be the only source of ACh within the islet. Although innervation of the human islet may be sparse74, the existence of a ‘cephalic phase’ of insulin secretion (that is partially dependent on cholinergic inactivation and that cannot be accounted for by an elevation of plasma glucose75) is well established.
Endocrine modulators
δ-cells express GLP-1 (Glp1r) as well as lower levels of GIP receptors (Gipr). Predictably, somatostatin secretion is stimulated by the incretin hormones GLP-1 (and agonists) and GIP and agents that increase cAMP (such as forskolin)48,68,76,77.
Of the adrenergic receptors, only α2A receptors (encoded by Adra2a) are expressed at significant levels. This is the same receptor that is responsible for the direct inhibition of insulin secretion in response to direct adrenergic inputs to the β-cell and likely accounts for the reported inhibitory effects of adrenaline on somatostatin secretion63,78.
The hunger hormone ghrelin has long been known to inhibit insulin secretion, although various and sometimes conflicting mechanisms had been proposed to explain how a receptor that acts via Gαq (and thus predicted to stimulate intracellular Ca2+ release) inhibits insulin release. This conundrum was resolved with the discovery that ghrelin receptors (Ghsr) are selectively expressed in δ-cells where they mediate robust and selective secretion of somatostatin from mouse and human islets in response to ghrelin, which inhibits insulin release by a paracrine mechanism41,42.
Ca2+-regulated somatostatin secretion
Glucose-induced somatostatin secretion is associated with an elevation of [Ca2+]i. In isolated δ-cells, large [Ca2+]i oscillations are observed at glucose levels as low as 3mM79. Glucose-induced [Ca2+]i oscillations are also observed in δ-cells in intact islets at 3mM glucose80. These were suppressed by lowering glucose to 0.5mM or addition of the KATP channel activator diazoxide81.
Membrane depolarization (produced by supraphysiological extracellular K+ concentration (see BOX 1) increases [Ca2+]i and stimulates somatostatin secretion42,44,79,82.
BOX 1.
The potassium (K+) equilibrium potential (EK) is given by the Nernst equation
where [K+]i and [K+]o represent the intra- and extracellular K+ concentrations, respectively.
Assuming that the intra- ([K+]i) and extracellular K+ concentrations ([K+]o) are normally 110 and 5mM, EK can be estimated to be −81mV (close to the normal resting potential).
Increasing [K+]o to 50mM (as commonly used experimentally) will change EK to −21mV (thus opening Ca2+ channels and stimulating somatostatin secretion).
Conversely, a drop in [K+]o) to 2.5mM shifts EK to −100mV (thus inhibiting electrical activity and somatostatin secretion).
The nature of these [Ca2+]i oscillations has not been conclusively established, but it is possible that they in part involve mobilization of intracellular Ca2+. The role of intracellular Ca2+ stores in δ-cells and somatostatin secretion is suggested by the strong inhibitory effects of thapsigargin, dantrolene and ryanodine on glucose-induced somatostatin secretion44. Dantrolene and ryanodine are inhibitors of the intracellular ryanodine receptor (RyR) Ca2+ release channels and thapsigargin inhibit the Ca2+ ATPase of the sarco-endoplasmic reticulum. A role for intracellular Ca2+ release is not in conflict with the observation that inhibitor that Ca2+ channel inhibitors (including the L-type Ca2+ channel blocker isradipine) inhibit somatostatin secretion65; plasmalemmal Ca2+ entry may result in further Ca2+ release by Ca2+-induced Ca2+ release (CICR) mediated by RyR Ca2+ release channels (FIG. 2C). Transcription profiling reveals that in mouse islet, RyR expression is almost exclusively restricted to δ-cells and that RyR3 is the dominant RyR subtype41,42. RyR expression in both α- and β-cells is very low. Thus, any effects of ryanodine on insulin and glucagon secretion may reflect a paracrine effect (see FIG. 3A).
Figure 3.
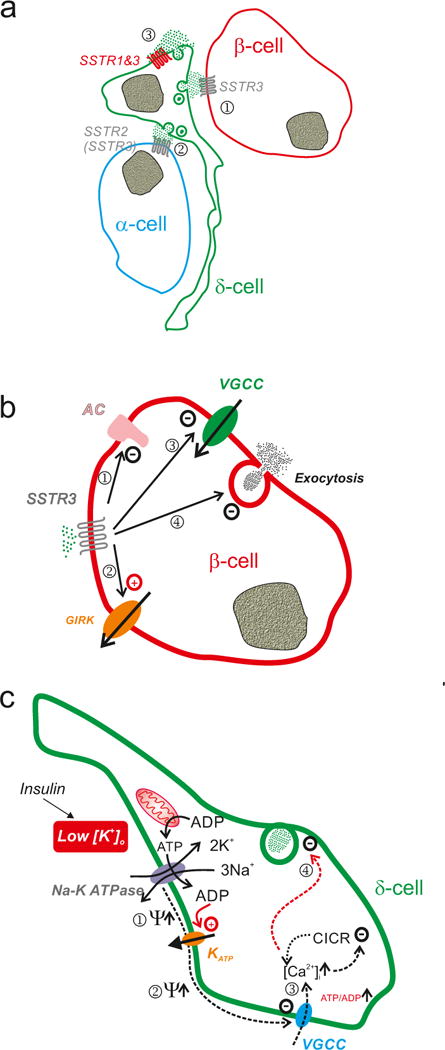
Somatostatin signalling in pancreatic islets. a| Schematic of somatostatin signalling in the islet. Somatostatin released from δ-cells activates SSTR2 (but also some SSTR3) in adjacent α- (1) and SSTR3 in β-cells (2) that are in close proximity to the δ-cell. In addition, somatostatin released from the δ-cells may exert an autocrine inhibitory effect by activation of SSTR1 or SSTR3 in the δ-cell itself (3). b| Effects of somatostatin in α- and β-cells. Activation of SSTR2 leads to: i) inhibition of adenylate cyclase, thereby resulting in lower cytoplasmic cAMP ([cAMP]i)113 and less cAMP-induced exocytosis; ii) inhibition of voltage-gated Ca2+ channels: iii) activation of G protein-regulated GIRK channels (producing membrane repolarization and inhibition of electrical activity)114,115; and iv) a direct inhibitory effect on exocytosis independent of [cAMP]i and that may involve activation of the protein phosphatase calcineurin115. c| Schematic how hypokalaemia (associated with insulin therapy) may stimulate somatostatin secretion. The Na-K pump is electrogenic and for every ATP hydrolysed 3 Na+ and 2 K+ are transported across the cell membrane in opposing directions, leading to a net loss of a positive charge inside the cell. Thus, the activity of the pump tends to repolarise the membrane potential (Ψ↑) (1). In addition, the activity of the pump may lower the submembrane ATP/ADP ratio, which maintains the KATP channels in the open state (potentially leading to further repolarization) (2). Inhibition of the pump (by lowering [K+]o) leads to membrane depolarisation by removal of the repolarising influence of the Na-K pump and exerting an ATP-sparing effect (the Na-K pump accounts for up to 50% of energy expenditure116) leading to closure of KATP channels. This leads to the initiation of electrical activity and Ca2+ entry/CICR (3) and stimulation of somatostatin release (4). Reduced expression of the Na-K pump (following hypokalaemia) may exert similar effects, thus increasing somatostatin secretion under hypoglycaemic conditions. This may account for the increased risk of recurrent hypoglycaemia (via reduced counter-regulatory glucagon secretion) in some insulin-treated patients.
The CICR-dependent component of somatostatin secretion is small at low glucose but increases with glucose with an EC50 of ~10mM44. Glucose-induced somatostatin release is nearly abolished by inhibitors of protein kinase A and the cAMP sensor Epac2 (own unpublished). Cyclic AMP (cAMP) may promote CICR by sensitising the RyR3s to Ca2+ (REF83). How glucose increases intracellular cAMP and promotes CICR is not known, but may be the result of paracrine signaling within the islets. For example, release of urocortin-3 from neighbouring β-cells can be expected to activate adenylate cyclase and increase cAMP upon binding to the CRHR2 receptor.
Somatostatin as a paracrine regulator
Once somatostatin has been released from the δ-cell, it will exert local paracrine effects by activation of somatostatin receptors (SSTRs) in islet cells. Whether pancreatic islet somatostatin also exerts more systemic effects is not known but quantitative considerations suggest that such effects are unlikely to be of major functional significance. of somatostatin in plasma, >90% is somatostatin-28 and only 5–10% represents somatostatin-14 (the δ-cell variety of somatostatin)84. Furthermore, pancreatectomy does not lower plasma somatostatin levels85.
There are five different somatostatin receptors (SSTR1–5)86. They are all coupled to an inhibitory G protein (Gi) and the effects of somatostatin can be prevented by pretreatment of pertussis toxin. It was formerly believed that β- and α-cells expression SSTR5 and SSTR2, respectively. More recent transcriptomic analyses using RNAseq on FACS-purified islet cell populations confirm that SSTR2 is the predominant receptor type in mouse and human α-cells87 SSTR3 is expressed by mouse α- and β-cells41,42 (FIG. 3A). Somatostatin exerts a plethora of effects on both α- and β-cells, which collectively result in reduction of insulin and glucagon secretion (summarized in FIG. 3B). Interestingly, mouse δ-cells express SSTR1 as well as SSTR341,42, possibly suggestive of autocrine feedback control of its release. Indeed, somatostatin secretion is strongly stimulated in the presence of somatostatin receptor antagonists76,88,89.
In addition to the inhibitory effects of somatostatin on hormone release, somatostatin also influences β-cell mass. Somatostatin agonists are potent suppressors of neuroendocrine tumour growth90 and inhibit the proliferation of MIN6 insulinoma cells91 as well as mouse and human β-cells92, in agreement with the observation that Gi signalling inhibits β-cell proliferation93. However, mice in which the somatostatin gene was ablated do not demonstrate increased β-cell mass63. This argues that somatostatin does not normally suppress β-cell proliferation but it should be noted that δ-cells contribute to β-cell mass by direct transdifferentiation94. The latter finding fits into an emerging picture of cellular plasticity and that transdifferentiation between α-, β- and δ-cells (formerly considered to be terminally differentiated) is a feature of the adjustments of islets during physiological and pathophysiological conditions. Whether somatostatin contributes to this islet cell plasticity and the extent to which these observations apply to human islets remain open questions.
Diabetes – a somatostatin secretion disorder
Diabetes is considered a ‘bihormonal disorder’ (involving both insulin and glucagon secretion defects)95. Given the inhibitory effects of somatostatin in both insulin and glucagon secretion, the question arises whether diabetes may involve all three major islet hormones.
Studies in perfused rat and dog pancreases indicate that somatostatin secretion at low glucose is higher than in non-diabetic control animals9697,98. Studies in rats further indicate that diabetes is associated with a reduced counter-regulatory stimulation of glucagon secretion in response to insulin-induced hypoglycaemia13. To our knowledge, there is no published information on the impact of diabetes on somatostatin secretion in isolated human islets. Measurements of circulating somatostatin-like immunoreactivity (SLI) and indicate that a mixed meal increases plasma SLI by 10% in healthy and 20% in type 2 diabetic patients, respectively99. How much of this stimulation that reflects δ-cell somatostatin-14 is unclear but it is clear that it only represents a small (5–10%) fraction of SLI100. Studies in dogs indicate that a glucose-induced stimulation of somatostatin secretion can only be detected in the pancreatic vein and that changes in vena cava as well as the mesenteric, gastroepiploic and short gastric veins are small101.
Somatostatin and δ-cells – therapeutic implications
Of course, the most important question whether the δ-cell and somatostatin secretion can be pharmacologically targeted in a way that provides benefits to diabetic patients. The risk of hypoglycaemia constitutes a barrier to good glycaemic control and many insulin-dependent diabetic patients are treated less aggressively with insulin than would otherwise be the case102. There is in fact a U-shaped relationship between plasma glucose and mortality in diabetic patients with the lowest mortality in diabetic patients at an HbA1C of 7.5% (a surrogate marker of long-term glycaemic control)103, well above that of non-diabetic individuals (5%).
As discussed above, experiments in rat models of diabetes are suggestive of impaired counter-regulatory glucagon secretion during insulin-induced hypoglycaemia due to increased somatostatin signalling11–13. The consequences of somatostatin oversecretion under hypoglycaemic conditions may be corrected by preventing its biological effects. Indeed, SSTR2 antagonists restore counter-regulatory glucagon secretion during insulin-induced hypoglycaemia in diabetic rats11–13 but these receptors are widely expressed (stomach, adrenal medulla, cerebral cortex, hypothalamus)104 and translation to human studies therefore requires safety testing. A radiolabelled SSTR2 antagonist (JR11) is used in clinical trials105, which will provide information on the safety and tolerability.
Patients who have experienced one hypoglycaemic episode (due to impaired counter-regulatory glucagon secretion) have an increased risk of another106. The underlying reasons for both phenomena remain unestablished. In this context, it is worth remembering that insulin not only produces hypoglycaemia but also lowers the plasma concentration of K+ (hypokalaemia)107. Hypokalaemia is associated with increased mortality108, possibly via cardiac effects109. In general, hypokalaemia decreases electrical excitability. This because lowered is [K+]o generally leads to membrane hyperpolarization (BOX 1), resultant reduction of cellular activity (secretion, nerve activity, with muscle contraction etc.). Paradoxically, hypokalaemia (low ([K+]o) stimulates rather than inhibits somatostatin secretion110. The stimulation of somatostatin secretion was attributed to inhibition of the Na-K pump. The expression of the Na-K ATPase subunit Atp1a2 is reduced by hypokalemia111. If hypokalemia influences Na-K pump expression in δ-cells (δ-cells express Atp1a1) similarly, it can be expected to increase somatostatin release (summarized in FIG. 3C) that in turn leads to an attenuation of counterregulatory glucagon secretion and persists even after normokalaemia has been restored.
Coda
Here we have attempted to illustrate the important roles played by the δ-cells and somatostatin in ‘health and disease’. It is clear that the islets are very complex structures and that they, via the paracrine cross-talk’ are much more than the ‘sum of the parts’. The δ-cells is emerging as a master regulator within the islet and represents an interesting and novel pharmacological target through which dysregulated insulin and glucagon secretion in diabetes may be corrected. Indeed, by virtue of their capacity of restoring counter-regulatory glucagon secretion SSTR2 antagonists should be considered as an adjunct to insulin therapy, thereby enabling more aggressive insulin treatment by minimising the risk of hypoglycaemia.
Key points.
The δ-cells of the pancreatic islets secrete somatostatin, a powerful paracrine inhibitor of both insulin and glucagon secretion from islet β- and α-cells
δ-cells are electrically excitable and glucose stimulates action potential firing and somatostatin secretion by both metabolic and non-metabolic effects in δ-cells
Factors released by the β-cells stimulate somatostatin secretion, thereby providing a mechanism for feedback control of insulin and glucagon secretion during hyperglycemia.
Diabetes is associated with impaired glucagon secretion in response to hypoglycaemia; an effect corrected by somatostatin antagonists, suggesting that diabetes may involve hypersecretion of somatostatin during hypoglycaemia.
Agents that inhibit somatostatin secretion/action may reduce the risk of insulin-induced hypoglycaemia and should be considered as an adjunct to insulin therapy
Acknowledgments
We thank Dr Q Zhang for providing the confocal image of δ-cells in an intact islet. Work discussed in this review was supported by a Wellcome Trust Senior Investigator Award, the Knut and Alice Wallenberg Foundation, the Swedish Research Council, the Hartwell Foundation for Biomedical Research (201500731), the Juvenile Diabetes Research Foundation (CDA-2-2013-54) and the National Institutes of Health (DK110276).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Hellman B. Actual distribution of the number and volume of the islets of Langerhans in different size classes in non-diabetic humans of varying ages. Nature. 1959;184(Suppl 19):1498–9. doi: 10.1038/1841498a0. [DOI] [PubMed] [Google Scholar]
- 2.Ionescu-Tirgoviste C, et al. A 3D map of the islet routes throughout the healthy human pancreas. Sci Rep. 2015;5:14634. doi: 10.1038/srep14634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frayn KN. Metabolic regulation : a human perspective. Vol. 384. Oxford Wiley-Blackwell; 2010. [Google Scholar]
- 4.Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973;182:171–3. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- 5.Muller WA, Faloona GR, Aguilar-Parada E, Unger RH. Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med. 1970;283:109–15. doi: 10.1056/NEJM197007162830301. [DOI] [PubMed] [Google Scholar]
- 6.Cryer PE. Hypoglycemia-Associated Autonomic Failure in Diabetes: Maladaptive, Adaptive, or Both? Diabetes. 2015;64:2322–3. doi: 10.2337/db15-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashcroft FM, Rorsman P. Diabetes mellitus and the beta cell: the last ten years. Cell. 2012;148:1160–71. doi: 10.1016/j.cell.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol. 2013;75:155–79. doi: 10.1146/annurev-physiol-030212-183754. [DOI] [PubMed] [Google Scholar]
- 9.Rorsman P, Ramracheya R, Rorsman NJ, Zhang Q. ATP-regulated potassium channels and voltage-gated calcium channels in pancreatic alpha and beta cells: similar functions but reciprocal effects on secretion. Diabetologia. 2014;57:1749–61. doi: 10.1007/s00125-014-3279-8. [DOI] [PubMed] [Google Scholar]
- 10.Gylfe E, Gilon P. Glucose regulation of glucagon secretion. Diabetes Res Clin Pract. 2014;103:1–10. doi: 10.1016/j.diabres.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Karimian N, et al. Somatostatin receptor type 2 antagonism improves glucagon counterregulation in biobreeding diabetic rats. Diabetes. 2013;62:2968–77. doi: 10.2337/db13-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yue JT, et al. Amelioration of hypoglycemia via somatostatin receptor type 2 antagonism in recurrently hypoglycemic diabetic rats. Diabetes. 2013;62:2215–22. doi: 10.2337/db12-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yue JT, et al. Somatostatin receptor type 2 antagonism improves glucagon and corticosterone counterregulatory responses to hypoglycemia in streptozotocin-induced diabetic rats. Diabetes. 2012;61:197–207. doi: 10.2337/db11-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, et al. Submembrane ATP and Ca2+ kinetics in alpha-cells: unexpected signaling for glucagon secretion. FASEB J. 2015;29:3379–88. doi: 10.1096/fj.14-265918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobbs R, et al. Glucagon: role in the hyperglycemia of diabetes mellitus. Science. 1975;187:544–7. doi: 10.1126/science.1089999. [DOI] [PubMed] [Google Scholar]
- 16.Gerich JE, et al. Prevention of human diabetic ketoacidosis by somatostatin. Evidence for an essential role of glucagon. N Engl J Med. 1975;292:985–9. doi: 10.1056/NEJM197505082921901. [DOI] [PubMed] [Google Scholar]
- 17.Raskin P, Unger RH. Hyperglucagonemia and its suppression. Importance in the metabolic control of diabetes. N Engl J Med. 1978;299:433–6. doi: 10.1056/NEJM197808312990901. [DOI] [PubMed] [Google Scholar]
- 18.Gerich JE, Schultz TA, Lewis SB, Karam JH. Clinical Evaluation of Somatostatin as a Potential Adjunct to Insulin in Management of Diabetes- Mellitus. Diabetologia. 1977;13:537–544. doi: 10.1007/BF01234510. [DOI] [PubMed] [Google Scholar]
- 19.Brazeau P, et al. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–9. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- 20.Guillemin R. Somatostatin: the early days. Metabolism. 1992;41:2–4. doi: 10.1016/0026-0495(92)90022-3. [DOI] [PubMed] [Google Scholar]
- 21.Baskin DG. A Historical Perspective on the Identification of Cell Types in Pancreatic Islets of Langerhans by Staining and Histochemical Techniques. J Histochem Cytochem. 2015;63:543–58. doi: 10.1369/0022155415589119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hellman B, Lernmark A. Inhibition of in Vitro Secretion of Insulin by an Extract of Pancreatic Alpha1 Cells. Endocrinology. 1969;84:1484. doi: 10.1210/endo-84-6-1484. [DOI] [PubMed] [Google Scholar]
- 23.Hellman B, Lernmark A. Evidence for an Inhibitor of Insulin Release in Pancreatic Islets. Diabetologia. 1969;5:22. doi: 10.1007/BF01212214. &. [DOI] [PubMed] [Google Scholar]
- 24.Brereton MF, Vergari E, Zhang Q, Clark A. Alpha-, Delta- and PP-cells: Are They the Architectural Cornerstones of Islet Structure and Coordination? J Histochem Cytochem. 2015;63:575–91. doi: 10.1369/0022155415583535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cabrera O, et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A. 2006;103:2334–9. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rorsman P, Ashcroft FM. Pancreatic β-Cell Electrical Activity and Insulin Secretion: Of Mice and Men. Physiological Reviews. 2018;98:117–214. doi: 10.1152/physrev.00008.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Q, et al. Na+ current properties in islet alpha- and beta-cells reflect cell-specific Scn3a and Scn9a expression. Journal of Physiology-London. 2014;592:4677–4696. doi: 10.1113/jphysiol.2014.274209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Q, et al. Role of K-ATP Channels in Glucose-Regulated Glucagon Secretion and Impaired Counterregulation in Type 2 Diabetes. Cell Metabolism. 2013;18:871–882. doi: 10.1016/j.cmet.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vieira E, Salehi A, Gylfe E. Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic alpha cells. Diabetologia. 2007;50:370–379. doi: 10.1007/s00125-006-0511-1. [DOI] [PubMed] [Google Scholar]
- 30.Adriaenssens A, et al. A Transcriptome-Led Exploration of Molecular Mechanisms Regulating Somatostatin-Producing D-Cells in the Gastric Epithelium. Endocrinology. 2015;156:3924–3936. doi: 10.1210/en.2015-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benninger RKP, Zhang M, Head WS, Satin LS, Piston DW. Gap Junction Coupling and Calcium Waves in the Pancreatic Islet. Biophysical Journal. 2008;95:5048–5061. doi: 10.1529/biophysj.108.140863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ravier MA, et al. Loss of connexin36 channels alters beta-cell coupling, islet synchronization of glucose-induced Ca2+ and insulin oscillations, and basal insulin release. Diabetes. 2005;54:1798–807. doi: 10.2337/diabetes.54.6.1798. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Q, et al. Cell coupling in mouse pancreatic beta-cells measured in intact islets of Langerhans. Philos Trans A Math Phys Eng Sci. 2008;366:3503–23. doi: 10.1098/rsta.2008.0110. [DOI] [PubMed] [Google Scholar]
- 34.Briant LJB, et al. delta-cells and beta-cells are electrically coupled and regulate alpha-cell activity via somatostatin. Journal of Physiology-London. 2018;596:197–215. doi: 10.1113/JP274581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez V, Tauche U. Somatostatin. 2004:426–433. [Google Scholar]
- 36.Mani BK, Zigman JM. A Strong Stomach for Somatostatin. Endocrinology. 2015;156:3876–3879. doi: 10.1210/en.2015-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adriaenssens A, et al. A Transcriptome-Led Exploration of Molecular Mechanisms Regulating Somatostatin-Producing D-Cells in the Gastric Epithelium. Endocrinology. 2015;156:3924–36. doi: 10.1210/en.2015-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gribble FM, Reimann F. Enteroendocrine Cells: Chemosensors in the Intestinal Epithelium. Annual Review of Physiology. 2016;78:277–299. doi: 10.1146/annurev-physiol-021115-105439. [DOI] [PubMed] [Google Scholar]
- 39.Mace OJ, Tehan B, Marshall F. Pharmacology and physiology of gastrointestinal enteroendocrine cells. Pharmacology Research & Perspectives. 2015;3 doi: 10.1002/prp2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- 41.Adriaenssens AE, et al. Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia. 2016;59:2156–65. doi: 10.1007/s00125-016-4033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DiGruccio MR, et al. Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. Mol Metab. 2016;5:449–58. doi: 10.1016/j.molmet.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker JN, et al. Regulation of glucagon secretion by glucose: paracrine, intrinsic or both? Diabetes Obes Metab. 2011;13(Suppl 1):95–105. doi: 10.1111/j.1463-1326.2011.01450.x. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Q, et al. R-type Ca(2+)-channel-evoked CICR regulates glucoseinduced somatostatin secretion. Nat Cell Biol. 2007;9:453–60. doi: 10.1038/ncb1563. [DOI] [PubMed] [Google Scholar]
- 45.Hermansen K. Pancreatic D-cell recognition of D-glucose: studies with Dglucose, D-glyceraldehyde, dihydroxyacetone, D-mannoheptulose, Dfructose, D-galactose, and D-ribose. Diabetes. 1981;30:203–10. doi: 10.2337/diab.30.3.203. [DOI] [PubMed] [Google Scholar]
- 46.Rorsman P, Ashcroft FM. Pancreatic beta-Cell Electrical Activity and Insulin Secretion: Of Mice and Men. Physiol Rev. 2018;98:117–214. doi: 10.1152/physrev.00008.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hermansen K, Lindskog S, Ahren B. Stimulation of somatostatin secretion by 3-O-methylglucose in the perfused dog pancreas. Int J Pancreatol. 1996;20:103–7. doi: 10.1007/BF02825508. [DOI] [PubMed] [Google Scholar]
- 48.Orgaard A, Holst JJ. The role of somatostatin in GLP-1-induced inhibition of glucagon secretion in mice. Diabetologia. 2017 doi: 10.1007/s00125-017-4315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sako Y, Wasada T, Umeda F, Ibayashi H. Effect of glibenclamide on pancreatic hormone release from isolated perifused islets of normal and cysteamine-treated rats. Metabolism. 1986;35:944–9. doi: 10.1016/0026-0495(86)90059-4. [DOI] [PubMed] [Google Scholar]
- 50.Patton GS, et al. Pancreatic immunoreactive somatostatin release. Proc Natl Acad Sci U S A. 1977;74:2140–3. doi: 10.1073/pnas.74.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ipp E, et al. Release of immunoreactive somatostatin from the pancreas in response to glucose, amino acids, pancreozymin-cholecystokinin, and tolbutamide. J Clin Invest. 1977;60:760–5. doi: 10.1172/JCI108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerber PP, Trimble ER, Wollheim CB, Renold AE. Effect of insulin on glucose- and arginine-stimulated somatostatin secretion from the isolated perfused rat pancreas. Endocrinology. 1981;109:279–83. doi: 10.1210/endo-109-1-279. [DOI] [PubMed] [Google Scholar]
- 53.Ashcroft FM, Coles B, Gummerson N, Sakura H, Smith PA. 2 Cationic Amino-Acid Transporters Expressed in Pancreatic Beta-Cells. Journal of Physiology-London. 1995;487p:192–193. [Google Scholar]
- 54.Panten U, Kriegstein E, Poser W, Schonborn J, Hasselblatt A. Effects of L-leucine and alpha-ketoisocaproic acid upon insulin secretion and metabolism of isolated pancreatic islets. FEBS Lett. 1972;20:225–228. doi: 10.1016/0014-5793(72)80801-9. [DOI] [PubMed] [Google Scholar]
- 55.Richieri GV, Kleinfeld AM. Unbound Free Fatty-Acid Levels in Human Serum. Journal of Lipid Research. 1995;36:229–240. [PubMed] [Google Scholar]
- 56.Olofsson CS, Salehi A, Gopel SO, Holm C, Rorsman P. Palmitate stimulation of glucagon secretion in mouse pancreatic alpha-cells results from activation of L-type calcium channels and elevation of cytoplasmic calcium. Diabetes. 2004;53:2836–43. doi: 10.2337/diabetes.53.11.2836. [DOI] [PubMed] [Google Scholar]
- 57.Stone VM, et al. GPR120 (FFAR4) is preferentially expressed in pancreatic delta cells and regulates somatostatin secretion from murine islets of Langerhans. Diabetologia. 2014;57:1182–91. doi: 10.1007/s00125-014-3213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olofsson CS, Salehi A, Holm C, Rorsman P. Palmitate increases L-type Ca2+ currents and the size of the readily releasable granule pool in mouse pancreatic beta-cells. J Physiol. 2004;557:935–48. doi: 10.1113/jphysiol.2004.066258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Braun M, et al. Somatostatin release, electrical activity, membrane currents and exocytosis in human pancreatic delta cells. Diabetologia. 2009;52:1566–78. doi: 10.1007/s00125-009-1382-z. [DOI] [PubMed] [Google Scholar]
- 60.Briant LJB, et al. delta-cells and beta-cells are electrically coupled and regulate alpha-cell activity via somatostatin. J Physiol. 2017 doi: 10.1113/JP274581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Q, et al. Na+ current properties in islet alpha- and beta-cells reflect cell-specific Scn3a and Scn9a expression. J Physiol. 2014;592:4677–96. doi: 10.1113/jphysiol.2014.274209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Efendic S, Enzmann F, Nylen A, Uvnas-Wallensten K, Luft R. Effect of glucose/sulfonylurea interaction on release of insulin, glucagon, and somatostatin from isolated perfused rat pancreas. Proc Natl Acad Sci U S A. 1979;76:5901–4. doi: 10.1073/pnas.76.11.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hauge-Evans AC, et al. Somatostatin secreted by islet delta-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes. 2009;58:403–11. doi: 10.2337/db08-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hermansen K. Tolbutamide, glucose, calcium, and somatostatin secretion. Acta Endocrinol (Copenh) 1982;99:86–93. doi: 10.1530/acta.0.0990086. [DOI] [PubMed] [Google Scholar]
- 65.van der Meulen T, et al. Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nat Med. 2015;21:769–76. doi: 10.1038/nm.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Briant LJ, et al. Functional identification of islet cell types by electrophysiological fingerprinting. J R Soc Interface. 2017;14 doi: 10.1098/rsif.2016.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Samols E, Stagner JI, Ewart RBL, Marks V. The Order of Islet Microvascular Cellular Perfusion Is B-]a-]D in the Perfused Rat Pancreas. Journal of Clinical Investigation. 1988;82:350–353. doi: 10.1172/JCI113593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hauge-Evans AC, Anderson RL, Persaud SJ, Jones PM. Delta cell secretory responses to insulin secretagogues are not mediated indirectly by insulin. Diabetologia. 2012;55:1995–2004. doi: 10.1007/s00125-012-2546-9. [DOI] [PubMed] [Google Scholar]
- 69.Honey RN, Fallon MB, Weir GC. Effects of exogenous insulin, glucagon, and somatostatin on islet hormone secretion in the perfused chicken pancreas. Metabolism. 1980;29:1242–6. doi: 10.1016/0026-0495(80)90152-3. [DOI] [PubMed] [Google Scholar]
- 70.Weir GC, Samols E, Day JA, Jr, Patel YC. Glucose and glucagon stimulate the secretion of somatostatin from the perfused canine pancreas. Metabolism. 1978;27:1223–6. doi: 10.1016/0026-0495(78)90047-1. [DOI] [PubMed] [Google Scholar]
- 71.Braun M, et al. Gamma-aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic beta-cells. Diabetes. 2010;59:1694–701. doi: 10.2337/db09-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rodriguez-Diaz R, et al. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 2011;14:45–54. doi: 10.1016/j.cmet.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodriguez-Diaz R, et al. Alpha cells secrete acetylcholine as a nonneuronal paracrine signal priming beta cell function in humans. Nat Med. 2011;17:888–92. doi: 10.1038/nm.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodriguez-Diaz R, et al. Innervation Patterns of Autonomic Axons in the Human Endocrine Pancreas. Cell Metabolism. 2011;14:45–54. doi: 10.1016/j.cmet.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahren B, Holst JJ. The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes. 2001;50:1030–1038. doi: 10.2337/diabetes.50.5.1030. [DOI] [PubMed] [Google Scholar]
- 76.de Heer J, Rasmussen C, Coy DH, Holst JJ. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia. 2008;51:2263–2270. doi: 10.1007/s00125-008-1149-y. [DOI] [PubMed] [Google Scholar]
- 77.Gerber PP, Trimble ER, Wollheim CB, Renold AE, Miller RE. Glucose and cyclic AMP as stimulators of somatostatin and insulin secretion from the isolated, perfused rat pancreas: a quantitative study. Diabetes. 1981;30:40–4. doi: 10.2337/diab.30.1.40. [DOI] [PubMed] [Google Scholar]
- 78.Sorenson RL, Elde RP, Seybold V. Effect of norepinephrine on insulin, glucagon, and somatostatin secretion in isolated perifused rat islets. Diabetes. 1979;28:899–904. doi: 10.2337/diab.28.10.899. [DOI] [PubMed] [Google Scholar]
- 79.Berts A, Ball A, Dryselius G, Gylfe E, Hellman B. Glucose stimulation of somatostatin-producing islet cells involves oscillatory Ca2+ signaling. Endocrinology. 1996;137:693–7. doi: 10.1210/endo.137.2.8593819. [DOI] [PubMed] [Google Scholar]
- 80.Nadal A, Quesada I, Soria B. Homologous and heterologous asynchronicity between identified alpha-, beta- and delta-cells within intact islets of Langerhans in the mouse. J Physiol. 1999;517(Pt 1):85–93. doi: 10.1111/j.1469-7793.1999.0085z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Quesada I, Nadal A, Soria B. Different effects of tolbutamide and diazoxide in alpha, beta-, and delta-cells within intact islets of Langerhans. Diabetes. 1999;48:2390–7. doi: 10.2337/diabetes.48.12.2390. [DOI] [PubMed] [Google Scholar]
- 82.Hermansen K, Christensen SE, Orskov H. Characterization of somatostatin release from the pancreas: the role of potassium. Scand J Clin Lab Invest. 1979;39:717–22. doi: 10.3109/00365517909108162. [DOI] [PubMed] [Google Scholar]
- 83.Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- 84.D’Alessio DA, Ensinck JW. Fasting and postprandial concentrations of somatostatin-28 and somatostatin-14 in type II diabetes in men. Diabetes. 1990;39:1198–202. doi: 10.2337/diab.39.10.1198. [DOI] [PubMed] [Google Scholar]
- 85.Taborsky GJ, Jr, Ensinck JW. Contribution of the pancreas to circulating somatostatin-like immunoreactivity in the normal dog. J Clin Invest. 1984;73:216–23. doi: 10.1172/JCI111194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20:157–98. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 87.Blodgett DM, et al. Novel Observations From Next-Generation RNA Sequencing of Highly Purified Human Adult and Fetal Islet Cell Subsets. Diabetes. 2015;64:3172–81. doi: 10.2337/db15-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Orgaard A, Holst JJ. The role of somatostatin in GLP-1-induced inhibition of glucagon secretion in mice. Diabetologia. 2017;60:1731–1739. doi: 10.1007/s00125-017-4315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Adriaenssens AE, et al. Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia. 2016;59:2156–2165. doi: 10.1007/s00125-016-4033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reubi JC, Schonbrunn A. Illuminating somatostatin analog action at neuroendocrine tumor receptors. Trends in Pharmacological Sciences. 2013;34:676–688. doi: 10.1016/j.tips.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yoshitomi H, et al. Involvement of MAP kinase and c-fos signaling in the inhibition of cell growth by somatostatin. American Journal of Physiology- Endocrinology and Metabolism. 1997;272:E769–E774. doi: 10.1152/ajpendo.1997.272.5.E769. [DOI] [PubMed] [Google Scholar]
- 92.Vivot K, et al. The regulator of G-protein signaling RGS16 promotes insulin secretion and beta-cell proliferation in rodent and human islets. Molecular Metabolism. 2016;5:988–996. doi: 10.1016/j.molmet.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Berger M, et al. G alpha(i/o)-coupled receptor signaling restricts pancreatic beta-cell expansion. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:2888–2893. doi: 10.1073/pnas.1319378112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chera S, et al. Diabetes recovery by age-dependent conversion of pancreatic delta-cells into insulin producers. Nature. 2014;514:503–+. doi: 10.1038/nature13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Unger RH. The Banting Memorial Lecture 1975. Diabetes and the alpha cell. Diabetes. 1976;25:136–51. doi: 10.2337/diab.25.2.136. [DOI] [PubMed] [Google Scholar]
- 96.Hermansen K, Orskov H, Christensen SE. Streptozotocin diabetes: a glucoreceptor dysfunction affecting D cells as well as B and A cells. Diabetologia. 1979;17:385–9. doi: 10.1007/BF01236274. [DOI] [PubMed] [Google Scholar]
- 97.Abdel-Halim SM, Guenifi A, Efendic S, Ostenson CG. Both somatostatin and insulin responses to glucose are impaired in the perfused pancreas of the spontaneously noninsulin-dependent diabetic GK (Goto-Kakizaki) rats. Acta Physiol Scand. 1993;148:219–26. doi: 10.1111/j.1748-1716.1993.tb09551.x. [DOI] [PubMed] [Google Scholar]
- 98.Weir GC, Clore ET, Zmachinski CJ, Bonner-Weir S. Islet secretion in a new experimental model for non-insulin-dependent diabetes. Diabetes. 1981;30:590–5. doi: 10.2337/diab.30.7.590. [DOI] [PubMed] [Google Scholar]
- 99.Conlon JM, Mcculloch AJ, Alberti KGMM. Circulating Somatostatin Concentrations in Healthy and Non-Insulin-Dependent (Type-Ii) Diabetic Subjects. Diabetes. 1983;32:723–729. doi: 10.2337/diab.32.8.723. [DOI] [PubMed] [Google Scholar]
- 100.Dalessio DA, Ensinck JW. Fasting and Postprandial Concentrations of Somatostatin-28 and Somatostatin-14 in Type-Ii Diabetes in Men. Diabetes. 1990;39:1198–1202. doi: 10.2337/diab.39.10.1198. [DOI] [PubMed] [Google Scholar]
- 101.Schusdziarra V, Dobbs RE, Harris V, Unger RH. Immunoreactive Somatostatin Levels in Plasma of Normal and Alloxan Diabetic Dogs. Febs Letters. 1977;81:69–72. doi: 10.1016/0014-5793(77)80930-7. [DOI] [PubMed] [Google Scholar]
- 102.Cryer PE. Glycemic goals in diabetes: trade-off between glycemic control and iatrogenic hypoglycemia. Diabetes. 2014;63:2188–95. doi: 10.2337/db14-0059. [DOI] [PubMed] [Google Scholar]
- 103.Currie CJ, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375:481–9. doi: 10.1016/S0140-6736(09)61969-3. [DOI] [PubMed] [Google Scholar]
- 104.Taleb N, Rabasa-Lhoret R. Can somatostatin antagonism prevent hypoglycaemia during exercise in type 1 diabetes? Diabetologia. 2016;59:1632–5. doi: 10.1007/s00125-016-3978-4. [DOI] [PubMed] [Google Scholar]
- 105.Nicolas GP, et al. Safety, Biodistribution, and Radiation Dosimetry of 68Ga-OPS202 (68Ga-NODAGA-JR11) in Patients with Gastroenteropancreatic Neuroendocrine Tumors: A Prospective Phase I Imaging Study. J Nucl Med. 2017 doi: 10.2967/jnumed.117.199737. [DOI] [PubMed] [Google Scholar]
- 106.Arbelaez AM, Powers WJ, Videen TO, Price JL, Cryer PE. Attenuation of counterregulatory responses to recurrent hypoglycemia by active thalamic inhibition: a mechanism for hypoglycemia-associated autonomic failure. Diabetes. 2008;57:470–5. doi: 10.2337/db07-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Caduff A, et al. Dynamics of blood electrolytes in repeated hyper- and/or hypoglycaemic events in patients with type 1 diabetes. Diabetologia. 2011;54:2678–89. doi: 10.1007/s00125-011-2210-9. [DOI] [PubMed] [Google Scholar]
- 108.Jensen HK, Brabrand M, Vinholt PJ, Hallas J, Lassen AT. Hypokalemia in acute medical patients: risk factors and prognosis. Am J Med. 2015;128:60–7 e1. doi: 10.1016/j.amjmed.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 109.Kacheva S, et al. QT prolongation caused by insulin-induced hypoglycaemia - An interventional study in 119 individuals. Diabetes Res Clin Pract. 2017;123:165–172. doi: 10.1016/j.diabres.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 110.Hermansen K, Christensen SE, Orskov H. The significance of the Na+/K+ pump for somatostatin release. Horm Metab Res. 1980;12:23–5. doi: 10.1055/s-2007-996187. [DOI] [PubMed] [Google Scholar]
- 111.Norgaard A, Kjeldsen K, Clausen T. Potassium depletion decreases the number of 3H-ouabain binding sites and the active Na-K transport in skeletal muscle. Nature. 1981;293:739–41. doi: 10.1038/293739a0. [DOI] [PubMed] [Google Scholar]
- 112.Segerstolpe A, et al. Single-Cell Transcriptome Profiling of Human Pancreatic Islets in Health and Type 2 Diabetes. Cell Metab. 2016;24:593–607. doi: 10.1016/j.cmet.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Braun M. The somatostatin receptor in human pancreatic beta-cells. Vitam Horm. 2014;95:165–93. doi: 10.1016/B978-0-12-800174-5.00007-7. [DOI] [PubMed] [Google Scholar]
- 114.Kailey B, et al. SSTR2 is the functionally dominant somatostatin receptor in human pancreatic beta- and alpha-cells. Am J Physiol Endocrinol Metab. 2012;303:E1107–16. doi: 10.1152/ajpendo.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Renstrom E, Ding WG, Bokvist K, Rorsman P. Neurotransmitterinduced inhibition of exocytosis in insulin-secreting beta cells by activation of calcineurin. Neuron. 1996;17:513–22. doi: 10.1016/s0896-6273(00)80183-x. [DOI] [PubMed] [Google Scholar]
- 116.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–58. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]


