Abstract
Introduction
The aim of this prospective crossover study was to evaluate the non-inferiority of PRO-122 (a preservative-free fixed combination) compared with 0.5% timolol + 0.2% brimonidine + 2.0% dorzolamide fixed combination (KOF) by evaluating its efficacy, tolerability and safety in subjects with controlled primary open-angle glaucoma (POAG) previously treated with KOF for at least 2 months.
Methods
In a prospective, crossover, randomized, double-masked multicenter study, patients previously treated with KOF were randomly assigned to receive either PRO-122 or KOF for 30 days. On day 31, the A sequence changed to KOF, while the B sequence received PRO-122. All patients remained in the protocol for 30 additional days for a total of 60 days. The main efficacy endpoint was maintaining the controlled intraocular pressure (IOP). The safety and tolerability of both products were assessed by the presence of adverse events (AEs), ocular findings, a questionnaire on ocular comfort and the VF-14 index.
Results
A total of 51 patients participated. After application of PRO-122 twice a day, its efficacy was demonstrated through maintenance of the controlled IOP in patients previously controlled with KOF. The crossover between PRO-122 and KOF and vice versa, after 30 days of use, did not affect IOP control. PRO-122 was shown not to be inferior to KOF in maintaining IOP at control levels. The safety of both drugs is similar, as neither presented drug-related AEs or differences regarding safety issues. The tolerability of the two medications—evaluated by ocular findings, the questionnaire on ocular comfort and the VF-14 index—was also determined to be similar.
Conclusions
The controlled IOP in patients with controlled POAG treated with PRO-122 was maintained both in relation to the initial controlled IOP of the study and when compared with KOF in the B sequence. Finally, the treatment with PRO-122 demonstrated similar safety and tolerability to KOF.
Funding
Laboratorios Sophia, S.A. de C.V. (Zapopan, Jalisco, México).
Trial Registration
ClinicalTrials.gov identifier: NCT03257813 (registered retrospectively).
Keywords: Benzalkonium chloride, Fixed combination preservative-free, Intraocular pressure, Primary open-angle glaucoma
Introduction
Glaucoma is a progressive optic neuropathy characterized by the loss of retinal ganglion cells and their respective axons that results in a distinctive appearance of the optic disc and loss of concomitant visual function [1]. Glaucoma is the second leading cause of blindness worldwide (following only cataracts among visual disorders), and it is estimated that by 2020 79.6 million people will develop this condition, 74% of them with primary open-angle glaucoma (POAG) [2]. Some recent studies seem to suggest that rates of POAG among Latin American populations, especially subjects of Mexican origin, exceed those of Caucasian, Australian or African-American populations [3–5].
Despite major advances in surgical filtering treatments, implants and laser procedures that improve trabecular drainage, pharmacologic therapy continues to be the initial treatment for most patients with glaucoma, typically including topical application of hypotensive agents [7].
Currently, topical pharmacologic options for reducing intraocular pressure (IOP) include prostaglandin analogs (PAGs), beta-blockers (βB), alpha adrenergic agonists (αA), carbonic anhydrase inhibitors (CAI) and parasympathomimetic drugs. Normally, pharmacotherapy begins with the application of a single hypotensive agent, usually one of the so-called first-line medications (PAGs or βB) [8]. However, monotherapy may be insufficient in many cases because of its inability to achieve the target IOP and/or prevent progression. In other cases, the drug applied may lose its effectiveness over time because of either tolerance or tachyphylaxis [9].
The Ocular Hypertensive Treatment Study (OHTS) reported that after 5 years, about 40% of patients required two medications to complete a 20% IOP reduction compared with baseline, while an additional 9% needed more than two medications to achieve this goal [6]. Therefore, two or more drugs are often required for adequate mid- and long-term IOP control. This can be achieved with the concurrent use of drugs of different classes, either in a fixed combination or through concomitant application [9].
In addition to their greater efficacy in lowering IOP, fixed combinations offer multiple benefits compared with concomitant application of their active ingredients [10]: (1) lower cost; (2) easy treatment regimen; (3) improved treatment adherence; (4) decreased washout risk [11, 12].
Fixed combinations also offer a decreased risk of corneal and ocular surface damage associated with cumulative exposure to preservative agents. For example, chronic exposure to the benzalkonium chloride (BAK) included in many ophthalmic medications has been associated with inflammation, conjunctival and corneal damage, tear film abnormalities and symptoms of ocular surface disease [13–15]. BAK concentrations under 0.003% produced a few corneal toxicities, but those containing over 0.005% BAK presented intensified corneal erosion and were associated with reduced tear break-up time.
Currently, the fixed combinations available include β-B timolol 0.5% combined with another drug from a different therapeutic class: a PAGs, αA or topical CAI [16]. In Mexico and some South American countries, fixed triple combinations are also available, including KOF and timolol + brimonidine + bimatoprost. The first treatment of this kind was a 0.5% timolol + 0.2% brimonidine + 2.0% dorzolamide fixed combination (Laboratorios Sophia, S.A. de C.V., Zapopan, Jalisco, Mexico), which has demonstrated its efficacy and safety during many years of clinical practice [17].
Based on the foregoing, the purpose of this study was to demonstrate the non-inferiority of PRO-122 (a preservative-free 0.5% timolol + 0.2% brimonidine + 2.0% dorzolamide fixed combination) compared with KOF by evaluating its efficacy, tolerability and safety in subjects with controlled POAG previously treated with KOF for at least 2 months.
Methods
Study Design
This prospective crossover AB:BA, 12-month, multicenter, double-blind phase III study (ClinicalTrials.gov registration number: NCT03257813, registered retrospectively) was conducted in eight centers in Mexico (see author details for a list). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. The study was approved by an Institutional Review Board at each research site (see Acknowledgments for a list). All patients who participated provided their written informed consent prior to the beginning of the study. They were recruited between May 2016 and June 2017.
Participants
Participants were adult patients with primary open-angle glaucoma (≥ 18 years old), classified as mild, moderate or severe glaucomatous damage (stage 1–4 according to Hodapp-Parrish-Anderson criteria, [18]) users of KOF for at least 2 months (1 drop twice daily) before inclusion, and with controlled IOP (range 7–19 mmHg); controlled IOP was defined at the investigator’s discretion in relation to the individualized therapeutic plan.
The primary exclusion criteria included patients legally blind in one eye, visual field loss indicative of end-stage glaucoma (stage 5 of the Hodapp–Parrish–Anderson criteria), subjects with a narrow-angle history without treatment, total or partial angle closure of either eye, a history of cataract surgery or any other intraocular surgery within 3 months prior to baseline, contraindication to any medication used in the study, pregnancy, risk of pregnancy and breastfeeding.
The primary efficacy endpoint consisted of maintaining the controlled IOP during the interval between the baseline and final visit.
Treatment and Evaluations
The control group selected—subjects treated with KOF—meant that the protocol was that of a non-inferiority study, since KOF contains the same active principles, and the two formulations differ only by the presence/absence of the preservative, BAK. Patients were assessed for eligibility during a screening visit scheduled 6–9 weeks before baseline, when their medical and ocular histories were obtained (n = 79). If the patient fulfilled all the inclusion criteria and presented none of the exclusion criteria, she/he was enrolled. Each patient was evaluated on five visits after enrollment (n = 68): baseline, safety 1 (15th day), crossover (30th day), safety 2 (45th day) and final visits. On each occasion, the IOP was measured in each eye using a calibrated Goldmann applanation tonometer. Two measurements were performed at hour 0 and hour 2 (i.e., 2 h after the first measurement). For each time point, two consecutive measurements were performed; if a difference > 1.5 mmHg was detected, a third measurement was made and the media reported. The highest value of the two pressures was considered the peak intraocular pressure, while the lowest was taken as the base intraocular pressure. We assessed the Snellen best-corrected visual acuity measurement and visual field using 24-2 SITA-Standard Humphrey automated perimetry (Carl Zeiss Meditec AG; Dublin, CA, USA). Abnormal findings upon slit lamp examination (biomicroscopy) of the ocular surface with fluorescein dye were graded as mild, moderate or severe. In addition, the VF-14 index and a questionnaire on ocular comfort were applied on the baseline, crossover (30th day) and final visits (60th day). The ocular comfort questionnaire consists of five questions, with answers graded 0–10 (0 = no inconvenience, 10 = unbearable inconvenience) exploring fatigue, burning, itching, dryness and pain. The demographic characteristics of the patients are shown in Table 1.
Table 1.
Baseline characteristics (n = 51 completed patients)
| Characteristics | |
|---|---|
| Female, n (%) | 37 (72.5) |
| Male, n (%) | 14 (27.5) |
| Mean age (years) ± SD | 65.6 ± 10.7 |
| Range | 22–93 |
| Mean baseline controlled IOP (mmHg) ± SD | 12.82 ± 2.5 |
| Mean baseline peak IOP (mmHg) ± SD | 13.02 ± 2.7 |
| Mean visual acuity (LogMAR) ± SD | 0.22 ± 0.23 |
| Mean Snellen 20/best-corrected visual acuity ± SD | 40 ± 30 |
| Pachymetry | 538.7 ± 29.1 |
IOP intraocular pressure, SD standard deviation
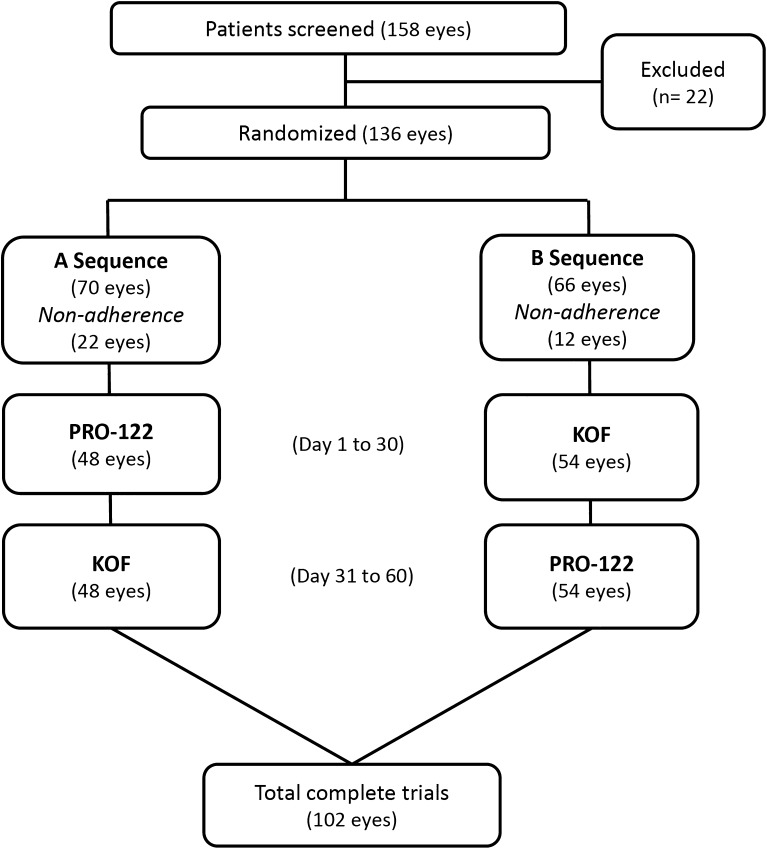
Using simple randomization, subjects were assigned to AB or BA, to receive the A or B sequence. To maintain masking, the labels on the bottles used in the study were covered. During the baseline visit (day 0), patients in the A sequence (n = 24) received PRO-122 (a preservative-free 0.5% timolol + 0.2% brimonidine + 2.0% dorzolamide fixed combination), while patients in the B sequence (n = 27) received KOF. The run-in period ended with the crossover visit (day 30), when the A sequence patients began to use KOF and the B patients changed to PRO-122. The patients enrolled did not have a washout period during crossover between the two drugs (PRO-122—KOF). All the researchers, patients and other sponsoring team members were blind to treatment assignment throughout the study. Patients were instructed to apply one drop of either treatment into each eye twice a day (9 a.m. ± 15 min and 9 p.m. ± 15 min). A safety call was carried out 2 weeks after the second treatment periods (75th day). The trial medication was discontinued if either the researcher or patient judged that it was not in the latter’s best interest to continue or if a female patient became pregnant. The flow chart of study participants is shown in Fig. 1.
Fig. 1.
Current diagram of patients enrolled in the study
Outcome Variables
Efficacy was evaluated by monitoring controlled IOP maintenance and for peak IOP obtained during the interval from baseline to day 60. The variables used to analyze tolerability and safety were: ocular findings (tear break-up time, conjunctival hyperemia, tearing, chemosis and burning sensation in the eye), questionnaire on ocular comfort, the VF-14 index and the incidence of adverse events.
Statistical Analysis
Data analysis was performed for the modified intent-to-treat population (ITT), defined as all randomized patients with at least one post-baseline efficacy evaluation, and then repeated for the per-protocol population (PP), established as a randomized patient with no major deviation from the protocol after performing a bivariate analysis. Before the study began, it was determined that at least 48 eyes were needed per group to detect a difference of at least 1.5 mmHg in mean controlled IOP maintenance between treatments using a significance level of 0.05 with a power of 0.80.
Base and peak IOPs were analyzed using a Student t test for repeated measures and a chi-square test. In case of multiple comparisons, a t test and chi-square test with Bonferroni post hoc correction were used to adjust the p value for individual time points. All categorical variables were analyzed with Pearson’s chi-squared test or Fisher’s exact test. All p values presented are two-tailed with p ≤ 0.05 considered significant. Analyses were conducted using SPSS 19.0 (SPSS Inc., Chicago, IL, USA).
Results
Characteristics of Subjects
Seventy-nine patients were screened and 68 enrolled. Mean age ± standard deviation (SD) was 65.6 ± 10.7 years (range 22–93); 72.5% of patients were female. Mean visual acuity ± SD (LogMAR) was 0.22 ± 0.23 in both sequences. The mean deviation (MD) of the visual field test was -12.0 ± 10.5 dB for the A sequence and − 9.9 ± 8.6 for the B sequence. No differences in patient demographic characteristics were observed; see Table 1.
Efficacy
Base Intraocular Pressure
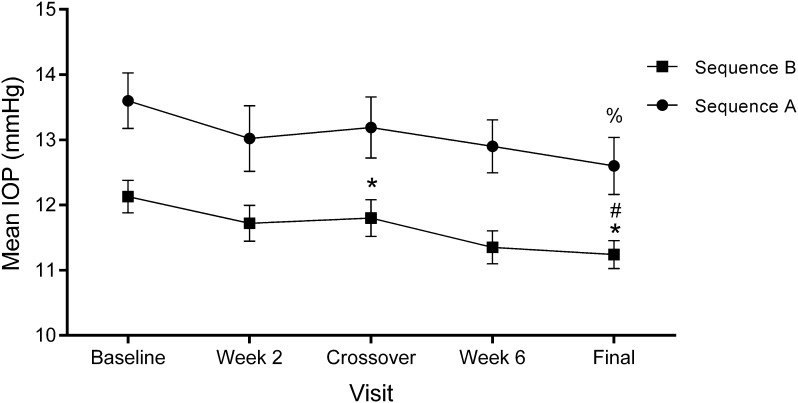
On each visit, intraocular pressure was measured in each eye at hour 0 and hour 2 (2 h later). The lower of these two values was considered the base intraocular pressure. IOP was analyzed for 51 patients (102 eyes); 48 eyes were assigned to the A sequence (PRO-122: day 1–30, KOF: day 31–60) and 54 to the B sequence (KOF day 1–30, PRO-122: day 31–60). Both products contained 0.5% timolol + 0.2% brimonidine + 2.0% dorzolamide, but differed in the presence/absence of BAK. For the A sequence, the baseline IOP ± SD was 13.60 ± 2.9 mmHg, a value that decreased to 13.19 ± 3.2 mmHg after 1 month of treatment with PRO-122, representing a reduction of 0.41 mmHg, which was not significant (t(47) = − 1.110, p = 0.272). After this period, the crossover to KOF took place. After 30 days of treatment, the IOP was 12.60 ± 3.0 mmHg, a decrease of 0.59 mmHg, which was also not significant (t(47) = − 1.79, p = 0.080). The patients in sequence A thus experienced a decrease of 1.0 mmHg by the end of the study. This difference was significant with respect to the initial value (t(47) = − 2.576, p = 0.013).
The mean IOP ± SD for the B sequence during the baseline visit was 12.13 ± 1.8 mmHg. After the first treatment period with KOF, this decreased to 11.80 ± 2.1 mmHg, 0.33 mmHg lower than their baseline, with no significant changes (t(53) = − 1.552, p = 0.127). After the crossover visit and the corresponding change to PRO-122, a decrease in IOP to 11.24 ± 1.6 mmHg was reported, 0.56 mmHg lower than during the KOF period (t(53) = − 2.007, p = 0.05). At the conclusion of the study, the patients in sequence B had a significant decrease of 0.89 mmHg with respect to their baseline IOP (t(53) = − 3.420, p = 0.001).
Thus, control of IOP was maintained throughout the 8 weeks of the study, regardless of period; see Fig. 2. In both sequences, the controlled IOP was lower on the final visit than at baseline; however, the adjusted difference between sequences for change in IOP for baseline vs. crossover (week 4) showed no statistically significant difference between groups (t(100) = − 1.98, p = 0.843) or on the final visit (week 8) vs. crossover (t(100) = − 0.065, p = 0.948). In this analysis, each eye is shown as one case.
Fig. 2.
Mean base intraocular pressure ± SEM on each experimental visit for the A (full circle) and B (full square) sequences. Statistical significance was determined using a two-sample t test. For the A sequence: IOP baseline vs. final, %p = 0.013. For the B sequence: IOP baseline vs. crossover, *p = 0.01, baseline vs. final, *p = 0.001, and crossover vs. final, #p = 0.05. A sequence: PRO-122 day 1 to crossover, KOF day 31 to final. B: KOF: day 1 to crossover, PRO-122 day 31 to final
Peak Intraocular Pressure
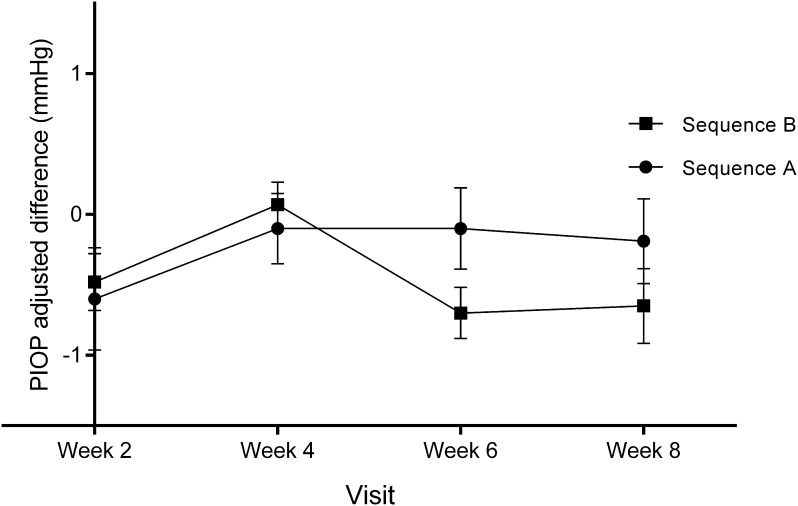
On each visit, intraocular pressure was measured in each eye at hour 0 and hour 2 (2 h later). The peak intraocular pressure was considered the highest value of the two readings. Mean peak IOP was higher for the B sequence than the A sequence on all visits regardless of the treatment assigned, but the adjusted difference between sequences for change in peak IOP for baseline vs. crossover (week 4) showed no statistically significant difference between groups (t(100) = − 0.767, p = 0.445) or on the final visit (week 8) vs. crossover (t(100) = 1.153, p = 0.522). In this analysis, each eye is shown as a separate case; see Fig. 3.
Fig. 3.
Comparison of changes in mean peak intraocular pressure between sequences for baseline vs. each visit. A sequence (full circle) n = 48 eyes; B sequence (full square) n = 54 eyes. A sequence: PRO-122 day 1 to crossover, KOF day 31 to final. B: KOF: day 1 to crossover, PRO-122 day 31 to final. Data are presented as mean ± SEM
Tolerability
Ocular Findings
The tear break-up time (TBUT), conjunctival hyperemia, tearing, chemosis, burning and foreign body sensation were evaluated in relation to the ocular findings. Regarding TBUT, no differences between treatments were found in either sequence, as this parameter was maintained during the entire 8 weeks, regardless of the study period. Conjunctival hyperemia was scored as “0” (no hyperemia) or “1” (hyperemia). During the baseline visit only, B showed less conjunctival hyperemia than A (X2(1) = 11.134, p = 0.001). Similarly, and again only on the baseline visit, the B sequence showed less presence of tearing than the A sequence (X2(1) = 5.979, p = 0.023). On the crossover and final visits, no between-sequence differences were observed. Finally, for chemosis, a burning sensation and foreign body sensation, no differences were observed between the sequences in any period of the study protocol. For ocular findings, each eye is also shown as a separate case; see Table 2.
Table 2.
Ocular findings between sequences
| A sequence (PRO-122 → KOF) | B sequence (KOF → PRO-122) | |||||
|---|---|---|---|---|---|---|
| Baseline | Week 4 | Week 8 | Baseline | Week 4 | Week 8 | |
| TBUT, Mean ± SD | 6.46 ± 3.3 | 6.65 ± 2.9 | 6.08 ± 1.7 | 7.30 ± 1.8 | 6.41 ± 1.4 | 6.74 ± 2.4 |
| Conjunctival hyperemia, n (%) | 19 (39.6) | 18 (37.5) | 10 (20.8) | 6 (11.1)* | 14 (25.9) | 7 (13.0) |
| Tearing, n (%) | 9 (18.8) | 8 (16.7) | 7 (14.6) | 2 (3.7)+ | 9 (16.7) | 9 (16.7) |
| Chemosis, n (%) | 0 (0) | 0 (0) | 0 (0) | 2 (3.7) | 2 (3.7) | 0 (0) |
| Eye burning, n (%) | 16 (33.3) | 12 (25.0) | 14 (29.2) | 18 (33.3) | 18 (33.3) | 19 (35.2) |
| Foreign body sensation, n (%) | 14 (29.2) | 14 (29.2) | 5 (10.4) | 14 (25.9) | 16 (29.6) | 7 (13.0) |
Comparisons between sequences, Fisher’s exact test for hyperemia at baseline, *p = 0.001, and tearing, +p=0.023. A: n = 48; B: n = 54
TBUT tear break-up time (s)
Questionnaire on Ocular Comfort
Participants were asked to score the discomfort they felt during the study protocol on a scale of 0–10, with 0 being ‘no inconvenience’ and 10 ‘unbearable inconvenience.’ The factors considered during the administration of each drug were: fatigue, burning, itching, dryness and pain. The scores reported for both drugs were low (always below 5). No differences were observed between treatments (p > 0.05); see Table 3.
Table 3.
Questionnaire on ocular comfort
| PRO-122 | KOF | p | |
|---|---|---|---|
| Fatigue | 1.84 (2.8) | 1.51 (2.7) | 0.379 |
| Burning | 2.06 (2.5) | 2.33 (2.8) | 0.454 |
| Itching | 1.37 (1.9) | 1.51 (1.9) | 0.676 |
| Dryness | 1.73 (2.5) | 2.00 (2.6) | 0.423 |
| Pain | 0.94 (1.8) | 0.88 (2.2) | 0.803 |
Mean (SD), 0: no inconvenience; 10: unbearable inconvenience. t test for repeated measures, n = 51 completed patients
VF-14 Index
Visual Function Index-14 (VF-14) is a questionnaire that evaluates the visual function in relation to daily-use vision in patients with ophthalmologic diseases. The questionnaire contains 14 items; each questionnaire was scored on a scale of 0–4. Average scores were then multiplied by 25 to give a scale of 0–100. No differences were observed between the sequences during the clinical trial on the initial visit (t(49) = 0.663, p = 0.510), the crossover visit (t(49) = 0.436, p = 0.665) or the final visit (t(49) = 1.860, p = 0.069). The average score (SD) after using PRO-122 was 84.13 (17.03) for the A sequence and 82.26 (13.47) for B, while after using KOF, it was 85.42 (18.62) for the A sequence and 76.04 (17.39) for B.
Safety
Adverse Events
A total of 29 AEs were reported by 32.3% (22/68) of subjects during the study, but there were no significant differences between treatments for their incidence (X2(2) = 4.931, p = 0.085); 41.3% were mild, 55.3% moderate and 3.4% severe. For PRO-122, 55.6% of the AEs (10/18) were mild and 44.4% (8/18) were moderate, whereas for KOF, 18.2% (2/11) were mild, 72.7% (8/11) were moderate, and 9.1 (1/11) were severe.
Only one serious adverse event was identified during this study; however, it was not related to the products evaluated. The subject involved, a 42-year-old male who suffered acute abdominal pain, was examined and hospitalized with a presumptive diagnosis of appendicitis. However, after a more thorough examination, appendicitis was ruled out and gastritis was diagnosed, with the respective treatment prescribed. That event was classified as serious because it required hospitalization, but was resolved without sequel, and no causal association was established with the medication tested in the study (KOF).
The most commonly reported non-ocular AE was rhinopharyngitis (27.6%), while the most frequent ocular AE was conjunctivitis (10.3%). There were no significant differences in AEs between the two fixed combination treatment groups (X2(5) = 7.692, p = 0.174). The adverse events are shown in Table 4.
Table 4.
Safety analysis: treatment-related adverse events (29 AEs/68 subjects)
| Treatment group | ||
|---|---|---|
| PRO-122 | KOF | |
| Patients with AEs, n (%) | 18 (26.5) | 11 (16.2) |
| Non-ocular AEs, n (%) | 15 (83.3) | 7 (63.6) |
| Rhinopharyngitis, n (%) | 6 (33.3) | 2 (18.2) |
| Headache, n (%) | 2 (11.1) | 0 (0) |
| Acid peptic disease, n (%) | 0 (0) | 2 (18.2) |
| Other, n (%) | 7 (38.9) | 3 (27.3) |
| Ocular AEs, n (%) | 3 (16.7) | 4 (36.4) |
| Conjunctivitis, n (%) | 2 (11.1) | 1 (9.1) |
| Other, n (%) | 1 (5.6) | 3 (27.3) |
No significant differences between groups, all p values (X2 Pearson) were > 0.05
Discussion
The primary goal of medical therapy in cases of glaucoma is to reduce the intraocular pressure (IOP) and so preserve visual function. For these aims to be achieved, treatment with a fixed combination of hypotensive drugs is sometimes necessary. Most fixed combination drugs include the topical βB timolol (0.5%) combined with a αA agonist or a topical CAI. These combinations can effectively lower IOP in patients with POAG [16, 19, 20]. PRO-122 is a preservative-free 0.5% timolol + 0.02% brimonidine + 2.0% dorzolamide fixed combination. As mentioned above, topical therapies are often necessary to prevent the progression of glaucoma-related optic nerve damage, and here fixed combinations may be more beneficial than therapy with concomitant single agents. In accordance with this, KOF proved to be well tolerated and to have minimal harmful effects on the ocular surface [21].
Because POAG is a progressive chronic condition, their management requires long-term treatment, perhaps even for a lifetime, so the benefits of the chosen treatment must be balanced against the possible risks and adverse reactions. In addition, the impact of tolerability on therapeutic adherence becomes a factor that contributes to the effectiveness of prolonged therapies. Previous clinical trials have demonstrated the efficacy of the triple-fixed combination KOF in treating POAG [17, 21, 22]. However, the preservative agent included in this formulation could potentially decrease tear film stability by altering the lipid component of the tear and perhaps produce dry eye syndrome by affecting the evaporative mechanism, producing irritation of the ocular surface [15, 23].
The present study proved that PRO-122 is effective in keeping IOP within the target range in patients with POAG previously controlled with KOF. The change from PRO-122 to KOF, and vice versa, after 30 days of use did not affect the control of IOP in our study cohort. Therefore, PRO-122 was shown not to be inferior to KOF in patients with POAG.
The mechanism described for BAK toxicity includes detergent effects that cause loss of tear film stability, toxic effects on the corneal and conjunctival epithelium, and immunoallergic reactions. BAK possesses high affinity to membrane proteins and may change the ionic resistance of the cornea by interacting with its cellular membranes [14]. Preservative-free medications may be useful in maintaining the integrity of the ocular surface, especially in patients with glaucoma, since about 50% of treated patients show ocular surface disease. But, of course, such preservative-free formulations must demonstrate the efficacy described for similar products that include a preservative [13].
One strength of our study was the use of a crossover design, since this minimizes the impact of confounding covariates and allows for each subject to be her/his own comparison. In addition, a crossover trial can theoretically achieve the same degree of precision as a parallel group trial, but with only half the sample size. Thus, the control group selected—treatment with KOF—allowed our work to be marked as a non-inferiority study because it involved the same active principles, as the only difference between the PRO-122 and KOF formulations is the presence of BAK. Hence, the crossover design may have facilitated the determination of the real efficacy of the two fixed combinations.
The theoretical advantages of a BAK-free formulation were not observed in this study, probably because of its 8-week duration; thus, to evaluate the tolerability of the BAK-free product, a longer follow-up period is needed. Rolle et al. found that preservative-free timolol produced a significantly higher ocular surface disease score, greater basal epithelial cell density and stromal reflectivity, lower Glaucoma Symptoms Scale scores and tear break-up times and effects on several sub-basal nerves compared with controls after 36 months [24].
In addition, the study design did not allow attesting the superiority of PRO-122. Crossover studies have some limitations, and the treatment order may affect the outcome (adverse effects, subjective satisfaction, etc.). For this reason, a crossover design usually includes a washout period between treatments; however, given the characteristics of our participants who needed the application of anti-glaucoma drugs, this made having a long washout period unfeasible.
In the present study, after 30 days of use, the safety of both medications was similar between groups since no AEs related to the medication were reported. Thus, because the preservative-free product enhanced satisfaction (according to the questionnaire on ocular comfort), recommending this medication to glaucoma patients would seem to be beneficial in terms of maintaining adherence to anti-glaucoma eye drop therapy for longer periods [13].
The absence of statistically significant differences between the sequences in relation to IOP, together with the maintenance of IOP control, indicate that the main objective of the study was met. Even considering peak IOP, the IOP in subjects treated with PRO-122 remained consistent with the baseline IOP. When compared with KOF in the B sequence, a difference of 0.41 mmHg was observed, which was neither a statistically significant difference nor clinically relevant. This variance remained well below the allowed non-inferiority limit of 1.5 mmHg.
The treatment with PRO-122 proved to be as safe as that of KOF. The presence of AEs in both groups showed no statistically significant differences. No relation to study drugs was established for any of the AEs reported, nor were there any clinically or statistically significant differences between the groups in terms of the safety.
Concerning tolerability, one of the strengths of the design of the study is that it allowed each subject to act as her/his own control, thus eliminating potential biases in the subjective variables. That being the case, no differences were observed, and subjects reported minimal annoyances associated with their use, as reflected in their responses to the questionnaire on ocular comfort. Indeed, the only difference between the two sequences was the conjunctival hyperemia and tearing on the baseline visit on ocular findings.
In other findings, the two formulations were similar in terms of the VF-14 index and in general were well tolerated. No statistically significant differences between the two sequences were found in terms of quality of life related to the use of these meditations.
Finally, this study had some limitations, including the lack of a washout period. This is relevant for crossover studies because the treatment during the first period may have a residual effect that persists through the subsequent stage—i.e., a “carryover effect”—especially when there is no “washout” between periods. Another limitation was the short treatment period of just 2-month duration. To evaluate the tolerability and safety of PRO-122, a longer follow-up period is needed.
Conclusion
KOF is a medication used to treat glaucoma cases that require more than one medication to control intraocular pressure. Fixed combinations of glaucoma medications are preferred over the separate use of their components to improve treatment adherence. The present study confirmed that PRO-122 was effective in maintaining controlled intraocular pressure in POAG patients.
The crossover between PRO-122 and KOF, and vice versa, after 30 days of use did not affect IOP control in patients diagnosed with POAG. Therefore, PRO-122 is not inferior to KOF for IOP maintenance in such patients. After 30 days, the tolerability and safety of both products proved to be similar, since no drug-related EAs were reported. Thus, PRO-122 is a formulation that may prove to be an effective treatment for POAG patients as it incorporates the benefits inherent in preservative-free ophthalmic medications.
Acknowledgements
The following institutions and researchers in Mexico participated: Global Glaucoma Institute. Mariano Azuela 37, Guadalajara, Jalisco, 44600; Unidad de Diagnóstico Temprano del Glaucoma. Boulevard Puerta de Hierro 5150, Interior 404-A, Zapopan, Jalisco, 45116; Instituto de Oftalmología y Ciencias Visuales, CUCS, Universidad de Guadalajara. Sierra Mojada 950, Edificio N, Guadalajara, Jalisco, 44340 (Paczka J.A.); Consultorio Privado F. Gómez-Aguayo. Eulogio Parra 3061, Guadalajara, Jalisco, 44670 (Gómez-Aguayo F.); Hospital San José para Enfermos de la Vista, A.C. Andrés Terán 261, Guadalajara, Jalisco, 44600 (Leñero-Córdova R.); APEC: Asociación para evitar la Ceguera. Vicente García Torres 46, Asociación para Evitar la Ceguera en México (APEC), Vicente García Torres 46, Mexico City, 04030, Mexico (Jiménez-Román J.); Servicios Médicos Quirúrgicos de Monterrey S.C. Privada Muguerza 756, Monterrey, Nuevo León, 66010 (Dávila-Villarreal J.); and Fundación de Asistencia Privada Conde de Valenciana I.A.P. Chimalpopoca 14, Fundación de Asistencia Privada Conde de Valenciana, I.A.P., Chimalpopoca 14, Mexico City, 06800, Mexico (Hartleben C.).
We thank the participants in the study.
Funding
Sponsorship for this study and article processing charges were funded by Laboratorios Sophia, S.A. de C.V. (Zapopan, Jalisco, México). All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Editorial assistance
The authors thank Alejandra Sánchez Rios and Ricardo Llamas Velazquez (Laboratorios Sophia, S.A. de C.V.) for their editorial assistance in the preparation of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work and have given their approval for this version to be published.
Authorship contributions
Gómez-Aguayo F., Paczka J.A., Leñero-Córdova R., Jiménez-Román J., Davila-Villarreal J. and Hartleben C. participated in data collection and study supervision. Paczka J.A., Baiza-Durán L., Olvera-Montaño O., García-Velez F. and Muñoz-Villegas P. participated in the study design, data analysis and interpretation, drafting the manuscript and its critical revision.
Disclosures
Baiza-Duran L. is an employee of Laboratorios Sophia, S.A. de C.V. Olvera-Montaño O. is an employee of Laboratorios Sophia, S.A. de C.V. Garcia-Velez F. is an employee of Laboratorios Sophia, S.A. de C.V. Muñoz-Villegas P. is an employee of Laboratorios Sophia, S.A. de C.V. Gómez-Aguayo F, Paczka J.A., Leñero-Córdova R., Jiménez-Román J. Davila-Villarreal J. and Hartleben C. have nothing to disclose.
Compliance with Ethic Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/for national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Data availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced digital features
To view enhanced digital features for this article go to 10.6084/m9.figshare.6081161.
References
- 1.Weinreb R, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 2.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim E, Varma R. Glaucoma in latinos/hispanics. Curr Opin Ophthalmol. 2010;21:100–105. doi: 10.1097/ICU.0b013e3283360b1e. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell P, et al. Prevalence of open-angle glaucoma in Australia. J Ophthalmol. 1996;103:1661–1669. doi: 10.1016/s0161-6420(96)30449-1. [DOI] [PubMed] [Google Scholar]
- 5.Tielsch J, et al. Racial variations in the prevalence of primary open-angle glaucoma. JAMA J Am Med Assoc. 1991;266:369–374. doi: 10.1001/jama.1991.03470030069026. [DOI] [PubMed] [Google Scholar]
- 6.Kass MA, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 7.Bhagat P, et al. Efficacy and safety of benzalkonium chloride-free fixed combination of latanosprot and timolol in patients with open-angle glaucoma or ocular hypertension. Clin Ophthalmol. 2014;8:1241–1252. doi: 10.2147/OPTH.S64584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Glaucoma Society. Terminology and Guidelines for Glaucoma, 4th Edition—Chapter 3. Italy: Publicomm. pp 1–72; 2014.
- 9.Sharma S, Trikha S, Perera SA, Aung T. Clinical effectivenes of brizolamine 1%-brimonide 0.2% fixed combination for primary open-angle glaucoma and ocular hypertension. Clin Ophthalmol. 2015;9:2201–2207. doi: 10.2147/OPTH.S72380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holló G, Topouzis F, Fechtner RD. Fixed-combination intraocular pressure-lowering therapy for glaucoma and ocular hypertension: advantages in clinical practice. Expert Opin Pharmacother. 2014;15:1737–1747. doi: 10.1517/14656566.2014.936850. [DOI] [PubMed] [Google Scholar]
- 11.Higginbotham EJ. Considerations in glaucoma therapy: fixed combinations versus their component medications. Clin Ophthalmol. 2009;4:1–9. doi: 10.2147/OPTH.S6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazcano-Gomez G, Hernandez-Oteyza A, Iriarte-Barbosa MJ, Hernandez-Garciadiego C. Topical glaucoma therapy cost in Mexico. Int Ophthalmol. 2014;34:241–249. doi: 10.1007/s10792-013-9823-6. [DOI] [PubMed] [Google Scholar]
- 13.Lee W, Lee S, Bae H, Kim CY, Seong GJ. Efficacy and tolerability of preservative-free 0.0015% tafluprost in glaucoma patients: a prospective crossover study. BMC Ophthalmol. 2017;17:61. doi: 10.1186/s12886-017-0453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Servat JJ, Bernardino CR. Effects of common topical antiglaucoma medications on the ocular surface, eyelids and periorbital tissue. Drugs Aging. 2011;28:267–282. doi: 10.2165/11588830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Pisella P, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86:418–423. doi: 10.1136/bjo.86.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng JW, Cheng SW, Gao LD, Lu GC, Wei RL. Intraocular pressure-lowering effects of commonly used fixed-combination drugs with timolol: a systematic review and meta-analysis. PLoS One. 2012;7:e45079. doi: 10.1371/journal.pone.0045079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baiza-Durán LM, Llamas-Moreno JF, Ayala-Barajas C. Comparison of timolol 0.5% + brimodine 0.2% + dorzolamide 2% versus timolol 0.5% + brimonidine 0.2% in a Mexican population with primary open-angle glaucoma or ocular hypertension. Clin Ophthalmol. 2012;6:1051–1055. doi: 10.2147/OPTH.S33578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson RD, Paterlla VM. Automated static perimetry. 2. St Louis: Mosby; 1999. [Google Scholar]
- 19.Budengeri P, Cheng JW, Cai JP, Wei RL. Efficacy and tolerability of fixed combination of brimonide 0.2% + timolol 0.5% compared with fixed combination of dorzolamide 2% + timolol 0.5% in the treatment of patients with elevated intraocular pressure: a meta-analysis of randomized controlled trials. J Ocul Pharmacol Ther. 2013;29:474–479. doi: 10.1089/jop.2012.0134. [DOI] [PubMed] [Google Scholar]
- 20.Konstas AG, et al. Twenty-four hour efficacy with the dorzolamide/timolol-fixed combination compared with the brimonide-timolol-fixed combination in primary open-angle glaucoma. Eye. 2012;26:80–87. doi: 10.1038/eye.2011.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.García-López A, Paczka JA, Jiménez-Román J, Hartleben C. Efficacy and tolerability of fixed-combination bimatoprost-timolol versus fixed-combination dorzolamide-brimonide-timolol in patients with primary open-angle glaucoma or ocular hypertension: a multicenter, prospective, crossover study. BMC Ophthalmol. 2014;14:161. doi: 10.1186/1471-2415-14-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baiza-Durán L, et al. The efficacy and safety of two fixed combinations: timolol-dorzolamide-brimonide versus timolol-dorzolamide. A prospective, randomized, double-masked, multi-center, 6-month clinical trial. Ann Ophthalmol (Skokie) 2009;41:174–178. [PubMed] [Google Scholar]
- 23.Jaenen N, et al. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthal. 2007;17:341–349. doi: 10.1177/112067210701700311. [DOI] [PubMed] [Google Scholar]
- 24.Rolle T, Spinetta R, Nuzzi R. Long-term safety and tolerability of tafluprost 0.0015% vs. timolol 0.1% preservative-free in ocular hypertensive and in primary open-angle glaucoma patients: a cross sectional study. BMC Ophthalmol. 2017;17:136. doi: 10.1186/s12886-017-0534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.