Abstract
Introduction
The purpose of this study was to investigate the extent and morphology of the choriocapillaris’ density defect in patients with drusen in non-neovascular age-related macular degeneration (AMD).
Methods
Participants in this study were 36 patients with non-neovascular AMD and drusen. All patients underwent best-corrected visual acuity, slit-lamp examination, spectral domain-optical coherence tomography (SD-OCT), and optical coherence tomography angiography (OCTA).
Results
In all studied cases, the presence of drusen was associated with choriocapillaris’ reduced blood flow signal of different extent and severity. Three types of choriocapillaris’ non-perfusion were observed, along with an association between the size of drusen and the morphology of choriocapillaris’ density defect. Moreover, the extent of choriocapillaris’ density change has been related to ellipsoid zone disruption and therefore to visual impairment.
Conclusions
Our study showed that in patients with drusen due to non-neovascular AMD, there is choriocapillaris’ impairment of different morphology in OCTA, which is mainly related to the size and location of the drusen.
Keywords: Choriocapillaris, Drusen, Ellipsoid zone, OCTA
Introduction
Age-related macular degeneration (AMD) is the leading cause of visual impairment in adults over 50 years in developed countries [1, 2]. Globally, the prevalence of AMD has been estimated at about 8.7% and is predicted to affect 288 million in 2040 [3]. According to the Age-Related Eye Disease Study Group, AMD has been classified into two major categories as dry or neovascular AMD, based on the presence of choroidal neovascularization (CNV) [4]. Dry AMD, especially at early and intermediate stages, has been characterized by the presence of retinal pigment epithelium (RPE) changes, Bruch’s membrane alterations, and drusen, which represent deposition of lipofuscin, photoreceptor debris, and inflammatory components between the RPE and Bruch’s membrane [5]. The material of which drusen is composed has a wide range of components and is thought to be derived from immune-mediated and metabolic processes in the RPE [5]. Additionally, there are studies showing a potential implication of choroidal involvement in AMD [6–8].
Optical coherence tomography angiography (OCTA) is a non-invasive dye-less imaging modality which generates depth-resolved images of the ocular vasculature by acquiring repeated B-scans from the same location, describing the pixel-by-pixel changes that occur as a result of erythrocyte movement [9]. In that way, OCTA allows imaging from internal limiting membrane (ILM) to the choroid, providing segmentation of the retina into different layers, as well as imaging of the choriocapillaris and of the choroid [9]. Since choriocapillaris has been reported to supply oxygen to the RPE and photoreceptors, the extent of its flow abnormality may correlate with visual acuity impairment in patients with AMD. Alten et al. recently found that choriocapillaris’ decorrelation signal index in OCTA was reduced compared to healthy controls and depended on drusen morphology [10], in line with Moult et al., who demonstrated that the focal choriocapillaris’ impairment was associated with areas of drusen and correspondent geographic atrophy [11].
In light of the above, the purpose of this study was to investigate qualitatively the extent and morphology of the choriocapillaris’ density defect using OCTA, in patients with drusen in non-neovascular AMD, and to study their impact on the ellipsoid zone.
Methods
Participants in this cross-sectional study were patients with soft drusen secondary to early non-neovascular AMD, who were examined at the Macula Center, Athens, Greece between June and December 2016. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 1964 Declaration of Helsinki, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
The diagnosis of dry AMD was confirmed by a retinal specialist on the basis of a complete ophthalmic examination, including best-corrected visual acuity (BCVA) measurement, slit-lamp biomicroscopy, spectral domain-OCT (SD-OCT), and OCTA. Patients with neovascular AMD, diabetic retinopathy, uveitis, or other retinal pathology were excluded. Cases in which drusen coexisted with reticular pseudodrusen (subretinal drusenoid deposits) were also excluded.
OCTA was performed to assess the retinal and choroidal vasculature, using Zeiss AngioPlex, Cirrus HD-OCT 5000 (Carl Zeiss Meditech, Dublin, USA), and the resulting 6 × 6 mm OCT angiograms were recorded for each patient. Only images with signal quality greater than 6 were included in the analysis. The segmentation of the choriocapillaris and of the superficial and deep capillary plexus was automatically done by the software of the machine. All 6 × 6 OCTA images were exported from the system as a Joint Photographic Experts Groupfile (jpeg) and were analyzed. Two trained retina specialists (IC and GT) independently performed the qualitative analysis of the images for segmentation.
Descriptive statistics for demographics and main clinical records were performed using SPSS 22.0 (SPSS Inc, Chicago, USA). BCVA was converted to the logarithm of the minimum angle of resolution (LogMAR).
Results
A total of 36 consecutive patients (36 eyes) with early dry AMD and presence of soft drusen, as defined by Ferris et al. [5], were included in the study. The mean age of patients was 76.8 ± 6.6 years. Fourteen patients were male (38.9%) and 22 female (61.1%). The mean BCVA was 0.21 ± 0.17 logMAR at baseline.
In all cases, the presence of drusen was associated with choriocapillaris’ reduced blood flow signal (non-perfusion) of different extent and severity. We observed three types of choriocapillaris’ non-perfusion appearance: (1) the almost total absence of choriocapillaris’ perfusion (approximately “absolute black” appearance), showing generalized lacking flow signal. This type of dysfunction corresponded to the existing large, successive drusen without any interval of normal area between consecutive drusen (Fig. 1); (2) the “honeycomb” type of choriocapillaris’ defect, which was noticed in the presence of medium and large drusen with intervals of normal areas in between (Fig. 2); and (3) the almost normal choriocapillaris’ blood flow appearance, interrupted by small, focal, black holes, which corresponded to the small drusen location (Fig. 3).
Fig. 1.
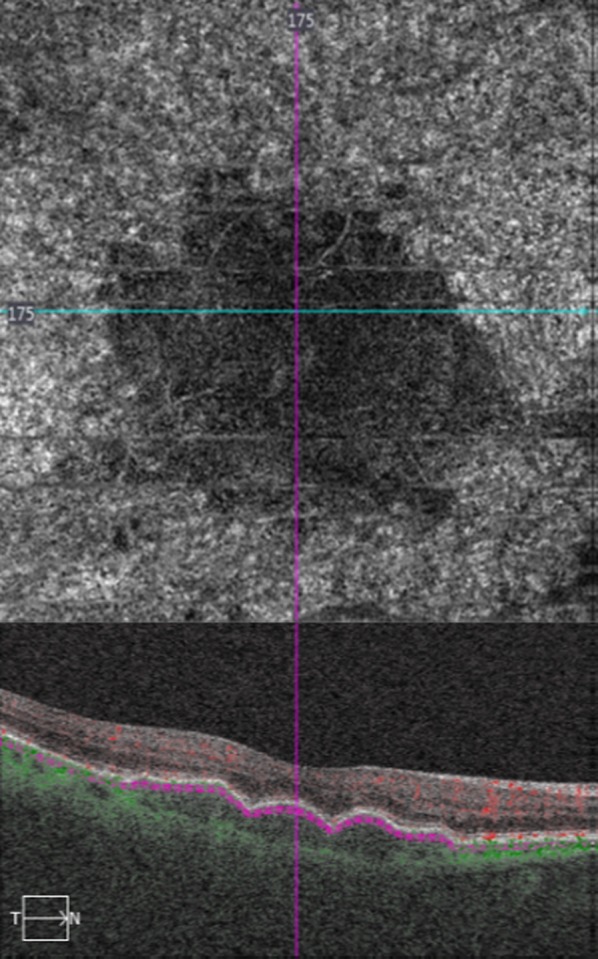
(Top) Optical coherence tomography angiography (OCTA) and (bottom) spectral domain optical coherence tomography (OCT) in a female patient with large, successive drusen. OCTA shows choriocapillaris’ vascular density defect in an almost “total black” appearance. In OCT, note also the ellipsoid zone disruption at the area of choriocapillaris’ vascular density defect, which corresponded to drusen
Fig. 2.
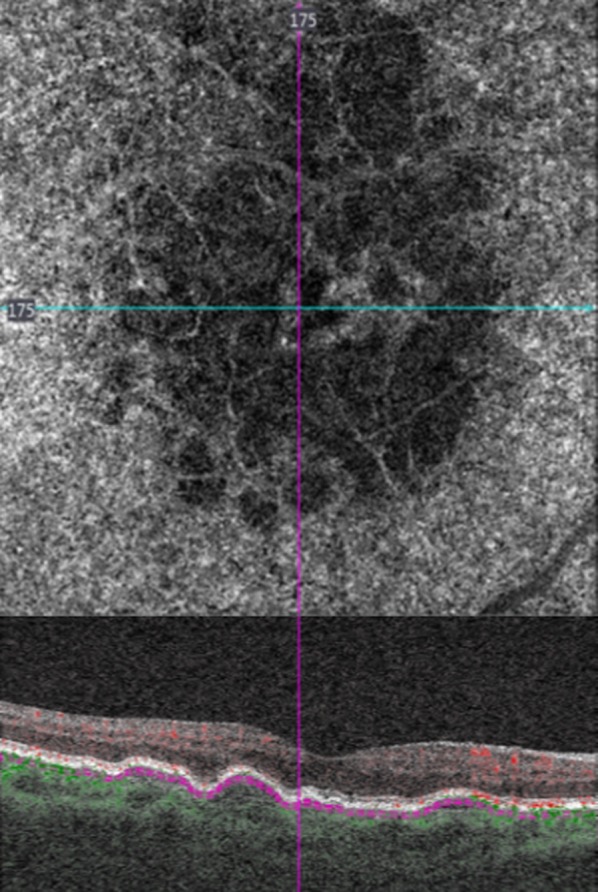
(Top) Optical coherence tomography angiography and (bottom) spectral domain optical coherence tomography in a female patient with drusen in a “honeycomb” choriocapillaris’ appearance, showing areas of non-perfusion and areas of normal blood flow signal. The ellipsoid zone integrity is related to the size of drusen
Fig. 3.
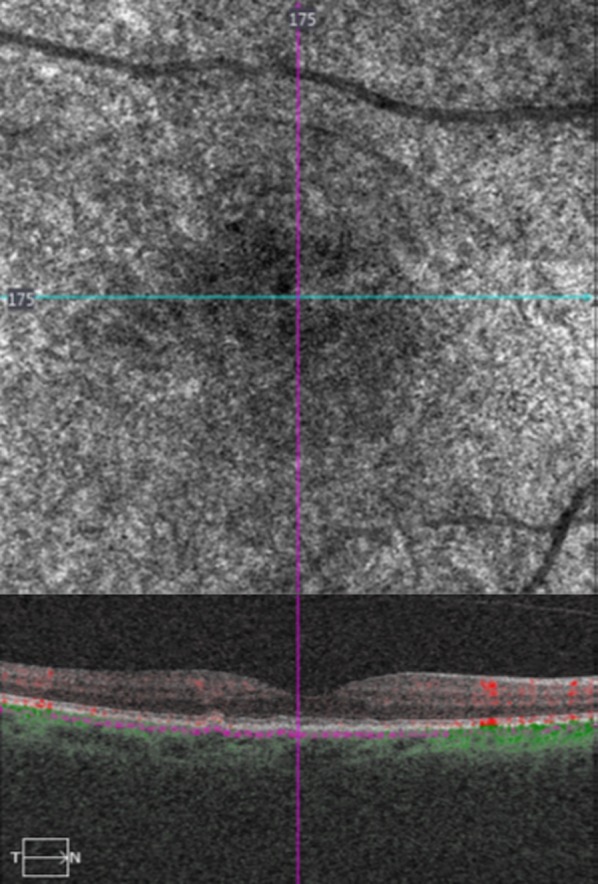
(Top) Optical coherence tomography angiography (OCTA) and (bottom) spectral domain optical coherence tomography (OCT) in a female patient with small drusen, showing almost normal choriocapillaris’ density, which was interrupted by small, black holes, correspondent to the small drusen area
In addition, the degree of choriocapillaris’ density defect, depicted as very low or absent/moderate/almost normal flow signal, has been associated with ellipsoid zone alteration on B-scan SD-OCT. More specifically, in the presence of continuous, large drusen with “absolute” choriocapillaris’ non-perfusion, there was a continuous ellipsoid zone disruption. The “honeycomb” type of choriocapillaris’ defect was accompanied by local interruption of ellipsoid zone, which corresponds to the location of drusen, while the mild and restricted in small areas choriocapillaris’ defect was consistent with the uninterrupted ellipsoid zone in small drusen.
Regarding the location of the choriocapillaris damage, in 30 out of 36 eyes (83.3%) the choriocapillaris’ non-perfusion was correspondent to the location of the existing drusen, as was confirmed on infrared fundus photo. It has also to be noted that in 6 out of 36 eyes (16.7%) the choriocapillaris’ impairment, except for the drusen coexistence, was extended covering the adjacent area of AMD outside drusen.
Discussion
Many factors have been implicated in AMD pathogenesis and progression, such as inflammation, oxidative damage, ageing, genetic predisposition, and environmental elements. Currently there is growing evidence that choroidal and retinal blood flows are diminished in AMD [4, 12]. The choroid is an extensive vascular network, consisting of an outer macrovascular layer and an inner capillary layer called the choriocapillaris, which lie immediately below Bruch’s membrane. The choroidal circulation provides about two-thirds of the total blood flow to the eye, providing nutrients and removing waste products from the outer retinal structures [12]. Previous studies, especially histopathologic ones, have shown that the drusen formation is affected by choriocapillaris’ dysfunction. This view suggests that drusen are more likely to develop at sites of insufficient choroidal perfusion, secondary to vascular endothelial cell loss [6, 13, 14]. Moreover, it enhances the aspect that drusen could be considered as a surrogate marker for choriocapillaris’ dysfunction.
Clinical investigations based on multimodal imaging, especially swept source OCT and enhanced depth imaging-OCT, have revealed a general thinning of the choroid and enlarged avascular areas between choroidal vessels as a result of increased stroma deposition [6–8]. Mullins et al., using quantitative morphometric analysis of choriocapillaris in patients with AMD, showed that the vascular density of the choriocapillaris presented a trend toward decreasing in association with AMD status. This finding supports the concept that microvascular changes are related to the pathogenesis of AMD and enhances the thought that vascular endothelial cell loss occurs in association with sub-RPE deposit formation [6]. Of note, the observation mentioned above was also supported by Corvi et al., who found not only the thinning of the choroid in patients with drusen but also a progressive loss of vascularity in a long-term follow-up of 2 years [15]. Moreover, Cicinelli et al. used OCTA in patients with drusen, showing a significant choroidal vascular depletion and fibrotic replacement compared to controls, which may suggest a potential role of choroid in the pathogenesis and progression of the disease [16].
The principal message of our study is that in all studied cases the presence of drusen was associated with choriocapillaris’ non-perfusion of different extent and severity. This finding is in agreement with the theory and histopathological findings, which claim that choriocapillaris’ defect influences the presence and growth of drusen [6]. OCTA studies related to the choriocapillaris’ dysfunction in the presence of drusen have shown the quantitative association between choroidal thinning or choriocapillaris’ dysfunction and drusen in AMD [6–8, 12–16]. Our study focused on the morphology of choriocapillaris’ density defects in association with the size and location of the coexisting drusen, instead of quantitative analysis. An interesting finding of the studied cases was that the size and type of consecutive drusen, continuous or interrupted, was associated with the shape and extent of choriocapillaris’ dysfunction. Specifically, we noticed that the almost total absence of choriocapillaris’ perfusion was mainly observed in the existing large, successively located drusen; the “honeycomb” type of choriocapillaris’ defect was noticed in the presence of large drusen with intervals of normal areas in between, while the almost normal choriocapillaris’ appearance, interrupted by small, focal, black holes corresponded to the existing small drusen.
Furthermore, the choriocapillaris’ non-perfusion may be extended in the area outside of well-defined drusen. This may be explained by the fact that in the area of the dry AMD adjacent to a large drusen the nutritional need is also affected.
It is also worth mentioning that our results revealed an existing correlation between the OCTA choriocapillaris’ dysfunction and the overlying of the drusen ellipsoid zone disruption, as it is depicted on SD-OCT. Since the ellipsoid zone has been previously found to affect visual acuity [17, 18], the choriocapillaris’ dysfunction may be related to the visual impairment in patients with drusen and ellipsoid zone interruption. However, it should be noted that the association between choriocapillaris’ density defect and ellipsoid zone alteration was based on subjective qualitative analysis and not on statistical correlation, as the data were mainly descriptive.
A potential limitation of our study was the relatively small sample size. Our study was based on morphological interpretation instead of quantitative analysis of choriocapillaris’ density changes, even though we know that in some cases OCTA images are subject to artifacts. In addition, the segmentation of the choriocapillaris was done automatically by the software of the machine; therefore, in some cases segmentation lines are difficult to identify and are localized behind the hyperreflective line of the RPE, leading to potential change in flow signal. Moreover, projection artifacts or false-negative attenuation of OCTA signal in the choroid and choriocapillaris may have affected our results, since they may occur especially in cases with pigment RPE detachment [19, 20].
Conclusion
Our study qualitatively investigated the extent and morphology of choriocapillaris’ flow density in patients with drusen. Impairment of the choriocapillaris in OCTA was noticed in patients with drusen due to non-neovascular AMD. The morphology of the choriocapillaris’ changes, which also influences the condition of ellipsoid zone and visual acuity impairment, is mainly related to the size and location of the drusen.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Irini Chatziralli, George Theodossiadis, Dimitrios Panagiotidis, Paraskevi Pousoulidi, and Panagiotis Theodossiadis have nothing to disclose.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/AE89D3601697ABAF.
References
- 1.Klein R, Chou CF, Klein BE, Zhang X, Meuer SM, Saaddine JB. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011;129:75–80. doi: 10.1001/archophthalmol.2010.318. [DOI] [PubMed] [Google Scholar]
- 2.Bourne RR, Jonas JB, Flaxman SR, et al. Vision loss expert group of the global burden of disease study. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe: 1990–2010. Br J Ophthalmol. 2014;98:629–638. doi: 10.1136/bjophthalmol-2013-304033. [DOI] [PubMed] [Google Scholar]
- 3.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 4.Davis MD, Gangnon RE, Lee LY, et al. Age-related eye disease study group. the age-related eye disease study severity scale for age-related macular degeneration: AREDS report no. 17. Arch Ophthalmol. 2005;123:1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferris FL, 3rd, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–851. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:1606–1612. doi: 10.1167/iovs.10-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueda-Arakawa N, Ooto S, Ellabban AA, et al. Macular choroidal thickness and volume of eyes with reticular pseudodrusen using swept-source optical coherence tomography. Am J Ophthalmol. 2014;157:994–1004. doi: 10.1016/j.ajo.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Grewal DS, Chou J, Rollins SD, Fawzi AA. A pilot quantitative study of topographic correlation between reticular pseudodrusen and the choroidal vasculature using en face optical coherence tomography. PLoS One. 2014;9:e92841. doi: 10.1371/journal.pone.0092841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Carlo TE, Romano A, Waheed NK, Duker JS. A review of optical coherence tomography angiography (OCTA) Int J Retina Vitreous. 2015;1:5. doi: 10.1186/s40942-015-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alten F, Lauermann JL, Clemens CR, Heiduschka P, Eter N. Signal reduction in choriocapillaris and segmentation errors in spectral domain OCT angiography caused by soft drusen. Graefes Arch Clin Exp Ophthalmol. 2017;255:2347–2355. doi: 10.1007/s00417-017-3813-8. [DOI] [PubMed] [Google Scholar]
- 11.Moult EM, Waheed NK, Novais EA, et al. Swept-source optical coherence tomography angiography reveals choriocapillaris alterations in eyes with nascent geographic atrophy and drusen-associated geographic atrophy. Retina. 2016;36(Suppl 1):2–11. doi: 10.1097/IAE.0000000000001287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol Aspects Med. 2012;33:295–317. doi: 10.1016/j.mam.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutty G, Grunwald J, Majji AB, Uyama M, Yoneya S. Changes in choriocapillaris and retinal pigment epithelium in age-related macular degeneration. Mol Vis. 1999;5:35. [PubMed] [Google Scholar]
- 14.Lengyel I, Tufail A, Hosaini HA, Luthert P, Bird AC, Jeffery G. Association of drusen deposition with choroidal intercapillary pillars in the aging human eye. Invest Ophthalmol Vis Sci. 2004;45:2886–2892. doi: 10.1167/iovs.03-1083. [DOI] [PubMed] [Google Scholar]
- 15.Corvi F, Souied EH, Capuano V, et al. Choroidal structure in eyes with drusen and reticular pseudodrusen determined by binarisation of optical coherence tomographic images. Br J Ophthalmol. 2017;101:348–352. doi: 10.1136/bjophthalmol-2016-309115. [DOI] [PubMed] [Google Scholar]
- 16.Cicinelli MV, Rabiolo A, Marchese A, et al. Choroid morphometric analysis in non-neovascular age-related macular degeneration by means of optical coherence tomography angiography. Br J Ophthalmol. 2017;101:1193–1200. doi: 10.1136/bjophthalmol-2016-309481. [DOI] [PubMed] [Google Scholar]
- 17.Coscas F, Coscas G, Lupidi M, et al. Restoration of outer retinal layers after aflibercept therapy in exudative AMD: prognostic value. Invest Ophthalmol Vis Sci. 2015;56:4129–4134. doi: 10.1167/iovs.15-16735. [DOI] [PubMed] [Google Scholar]
- 18.Theodossiadis GP, Grigoropoulos VG, Liarakos VS, Rouvas A, Emfietzoglou I, Theodossiadis PG. Restoration of the photoreceptor layer and improvement of visual acuity in successfully treated optic disc pit maculopathy: a long follow-up study by optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2012;250:971–979. doi: 10.1007/s00417-011-1918-z. [DOI] [PubMed] [Google Scholar]
- 19.Louzada RN, de Carlo TE, Adhi M, et al. Optical coherence tomography angiography artifacts in retinal pigment epithelial detachment. Can J Ophthalmol. 2017;52:419–424. doi: 10.1016/j.jcjo.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Lane M, Moult EM, Novais EA, et al. Visualizing the choriocapillaris under drusen: comparing 1050-nm swept-source versus 840-nm spectral-domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:585–590. doi: 10.1167/iovs.15-18915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.


