Abstract
Introduction
Primary open-angle glaucoma is estimated to affect 3% of the population aged 40–80 years. Trabeculectomy is considered the gold standard in surgical management of glaucoma; however, it is a technically complex procedure that may result in a range of adverse outcomes. Device-augmented, minimally invasive procedures (micro-invasive glaucoma surgeries, MIGS) have been developed aiming for safer and less invasive intraocular pressure (IOP) reduction compared with traditional surgery.
Methods
This paper presents results from a systematic literature review conducted in accordance with National Institute for Health and Care Excellence requirements for the Medical Technology Evaluation Programme via multiple databases from 2005 to 2016. For clinical outcomes, randomized clinical trials (RCTs) comparing MIGS with trabeculectomy or other therapies, observational studies, and other non-RCTs were included. Clinical outcomes reviewed were the change from baseline in mean IOP levels and change in topical glaucoma medication. Safety was assessed by reported harm and adverse events. For economic evidence, trials on cost-effectiveness, cost-utility, cost-benefit, cost-consequences, cost-minimization, cost of illness, and specific procedure costs were included. Risk of bias was assessed for clinical studies using the Cochrane Risk of Bias tool.
Results
A total of nine RCTs (seven iStents®, one Hydrus®, and one CyPass®), seven non-RCTs (three iStent®, three CyPass®, and one Hydrus®), and 23 economic studies were analyzed. While various forms of trabeculectomy can achieve postoperative IOP of between 11.0 and 13.0 mmHg, MIGS devices described in this review were typically associated with higher postoperative IOP levels. In addition, MIGS devices may result in increased hypotony rates or bleb needling in subconjunctival placed devices, requiring additional medical resources to manage. There is limited available evidence on the cost-effectiveness of MIGS and therefore it remains unclear whether the cost of using MIGS is outweighed by cost savings through decreased medication and need for further interventions.
Conclusion
Larger randomized trials and real-world observational studies are needed for MIGS devices to better assess clinical and economic effectiveness. Given the shortage of published data and increasing use of such procedures, living systematic reviews may help to provide ongoing and timely evidence-based direction for clinicians and decision makers. This review highlights the current unmet need for treatments that are easy to implement and reduce long-term IOP levels without increasing postoperative aftercare and cost.
Funding
Santen GmbH, Germany.
Electronic supplementary material
The online version of this article (10.1007/s40123-018-0131-0) contains supplementary material, which is available to authorized users.
Keywords: CyPass, Hydrus, Intraocular pressure (IOP), iStent, Micro-invasive, MIGS, Open-angle glaucoma, Trabeculectomy, XEN
Introduction
Glaucoma is the leading global cause of irreversible blindness. It is estimated that 44.1 million people, or 3% of the population aged between 40 and 80 years, have primary open-angle glaucoma (POAG) [1]. The incidence of POAG is expected to rise to 65.5 million in 2020 because of an aging population [2]. POAG, a progressive ophthalmic disease which causes damage to the optic nerve and nerve fiber layer resulting in visual field and acuity loss [3, 4], can be caused by either elevated intraocular pressure (IOP; IOP-related) or alternative mechanisms (non-IOP-related) [5, 6]. In the IOP-related pathway, treatment requires a decrease in IOP achieved through various methods including topical ocular hypotensive treatments, laser trabeculoplasty, and invasive surgical management [7].
Topical ocular hypotensive medication can delay or prevent POAG in patients with elevated IOP [8]; however, patient adherence and ocular surface toxicity are major issues with medical management [9]. When topical medications or other interventions (such as laser) do not adequately reduce IOP, incisional surgery (trabeculectomy) is considered. Although trabeculectomy is considered the gold standard in the surgical management of glaucoma, it is a technically complex procedure that can result in failure due to scarring, decreased quality of life due to bleb-related foreign body sensation, induced astigmatism, and secondary cataracts [10]. Apart from incisional surgery and topical medication, various devices have been developed for the treatment of POAG including tube-based Molteno, Baerveldt, and Ahmed implants [11–13]. However, the failure rate of these is approximately 50% after 5 years [14], and the rate of re-operation in both trabeculectomy and tube-based devices is relatively high, at 29% and 9%, respectively [15]. Consequently, there have been further developments in the biomaterials, shape, and drainage technique in newer devices, collectively referred to as micro-invasive glaucoma surgeries (MIGS); available MIGS include the iStent® [16], Hydrus Micro-Stent® [17], CyPass Micro-Stent® [18], and XEN® (XEN gel stent) [19].
The main mechanisms by which IOP is lowered with MIGS devices include increasing trabecular outflow by bypassing the trabecular meshwork, increasing uveoscleral outflow via suprachoroidal pathways, or creating a subconjunctival drainage pathway [20]. These devices aim to provide a safer and less invasive means of achieving IOP reduction compared with traditional surgery. However, the clinical efficacy as measured by IOP reduction tends to be less pronounced; hence, to date MIGS are currently targeted at patients with mild to moderate glaucoma [21].
In addition, there is an economic burden of glaucoma attributable to ocular hypotensive medications, health care consultations, and glaucoma-related procedures (e.g., trabeculectomy, laser surgery, combined cataract/glaucoma surgery), and direct medical costs generally increase with glaucoma severity [22].
Since the launch of MIGS devices, as of December 2016 (the date of this literature review), little evidence had been summarized on their clinical and economic outcomes. Our full literature study also studied clinical and economic outcomes from device-augmented trabeculectomy using EX-PRESS® shunts. However, the aim of this present paper is to share findings on the analysis set of clinical outcomes and safety of commercially available MIGS devices compared with trabeculectomy, and findings for EX-PRESS-augmented incisional procedures (which are not classified as MIGS) have been excluded from the results presented herein. Economic outcomes are also reviewed to assess the positioning of MIGS devices in POAG treatment.
Methods
Search Methods for Identifying Studies
The systematic literature review was conducted in accordance with National Institute for Health and Care Excellence (NICE) requirements for the Medical Technology Evaluation Programme [23]. Searches for clinical and economic outcomes were carried out in MEDLINE, EMBASE, and the Cochrane Library (CENTRAL and Cochrane Database of Systematic Reviews). Additional searches for economic evidence were carried out in the National Health Service Economic Evaluation Database (NHS EED) and National Institute of Health Research Health Technology Assessment (NIHR-HTA) database. Trials published in English between 2005 and 2016 were included using specific search terms for each of the databases, as detailed in supplementary online material Table 1.
Ethics Statement
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Eligibility Criteria
Studies included in the analysis were based on PICO inclusion criteria. Specifically, a population of adults at least 18 years old with POAG, an intervention of MIGS in at least one treatment arm vs any other glaucoma treatment, inclusion of all comparators, and primary outcomes of (1) IOP reduction (absolute or relative) and (2) mean reduction in ocular medicated drops, and secondary outcomes of (3) visual prognosis and (4) quality of life. For the clinical outcomes and effectiveness, randomized clinical trials (RCTs) comparing MIGS (e.g., iStent®, CyPass®, Hydrus®, and XEN®) and non-MIGS procedures specifically using EX-PRESS®, with trabeculectomy (or other therapies), as well as observational studies or other non-RCTs were included in the full analysis set. For economic evidence, trials on cost-effectiveness, cost-utility, cost-benefit, cost-consequences, cost-minimization, and cost of illness, as well as trials on specific costs for procedures from the payer perspective were included. For this paper, which focuses on MIGS, clinical and economic analyses of EX-PRESS® procedures have been excluded.
RCTs published only as abstracts were excluded as it was not possible to appraise quality. In addition, reviews/editorials, studies from low-income countries (where factors independent of the devices are likely to influence results, such as surgical facilities and training), and those not reporting the two primary outcomes were excluded.
Study Selection
Titles and abstracts of all electronically identified studies were reviewed independently by two reviewers. Data from studies were extracted and assessed by one reviewer. Results were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews [24]. Selected studies were typically RCTs, although non-RCTs and gray literature were also assessed where information was lacking. For a list of sources used for gray literature searches, please see supplementary online material Table 3.
Risk-of-Bias Assessment and Data Collection
Risk-of-bias assessments provide a methodological way of analyzing whether the true effect of interventions is reported correctly, misdirected, or underreported. The risk of bias across studies was assessed using the Cochrane Collaboration Risk of Bias tool (CCRBT) and Review Manager 5.3 (RevMan) [25]. The CCRBT addresses the following six domains of potential bias that could compromise the integrity or credibility of a study: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias (e.g., conflict of interest and financial disclosures declared). Assessments were made within each domain for one or more areas of potential bias towards each individual study outcome. For each domain assessment, the risk of bias was divided into two sections, the first providing support for judgement using free text by reviewers to document evidence or judgements inferred upon the paper, and the second assessing bias risk from a three-tiered approach: low risk, high risk, or unclear risk (if study information was insufficient) selected as relevant to each study [26]. To further reduce bias risk, two reviewers used the tool independently [26–28].
Clinical evidence grading was performed by two reviewers and disagreements resolved through discussion and agreement. To allow comparability of the economic evidence, costs were converted to pounds sterling (£) using Organisation for Economic Co-operation and Development exchange rates [29], and inflated to 2016 values (the most recent index year) using the Hospital and Community Health Services (HCHS) pay and price inflation index [30] (where the cost year was not reported, original currencies were used).
Outcomes
Clinical outcomes reviewed were the change from baseline in mean IOP levels described as mean IOP level at longest follow-up, or as a relative reduction (from baseline) in IOP. Change in topical glaucoma medication was also reviewed, described as a reduction in number of eye drops used by patients at longest follow-up. Safety was assessed by harm and adverse events (AEs) reported as a sum of all events per MIGS device. Economic outcomes were described by cost-effectiveness and cost of treatment (MIGS, trabeculectomy, medication) sourced from various trials and HTAs.
Results
Study Selection
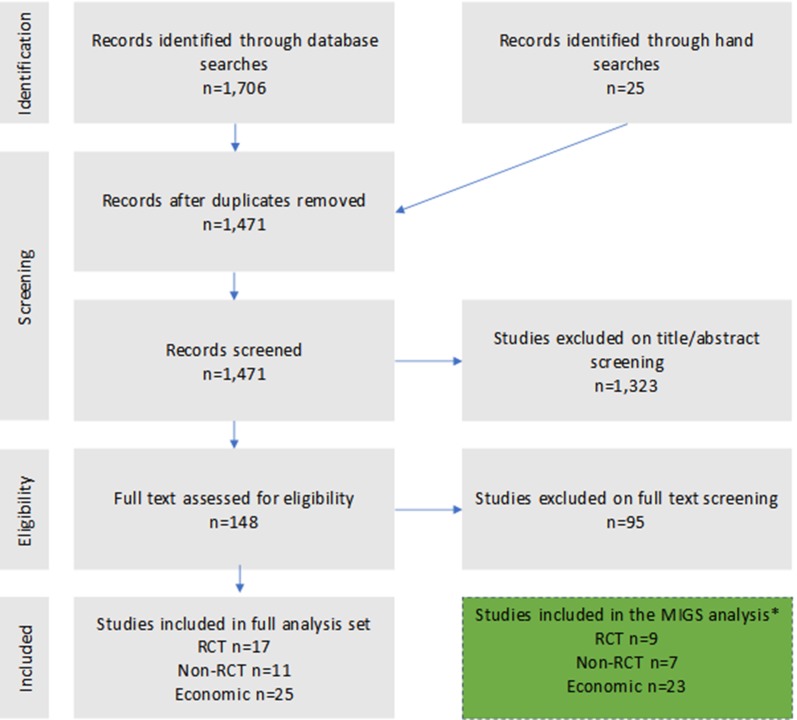
The initial search yielded 1706 unique references which were de-duplicated to 1471 records. These were then screened by title or abstract to 148 records, which were further assessed on full text for relevance. This full analysis set included results for non-MIGS procedures with EX-PRESS (eight RCTs, four non-RCTs, and two economic publications) which have been excluded from this present analysis which focuses on MIGS. A total of nine RCTs, seven non-RCTs, and 23 economic studies were analyzed specifically for MIGS, which are presented in this paper. A flow diagram depicting the study selection for both the full set and that specific to MIGS (i.e., without EX-PRESS studies) is shown in Fig. 1.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram for the literature screening. *Please note that this publication presents results only for MIGS devices and we have excluded our separate analysis of device-augmented trabeculectomy using EX-PRESS®. In total seven RCTs and two economic studies for EX-PRESS® have been excluded from the above final set for this present analysis
Study Characteristics
All the nine RCTs included in this review, shown in Table 1, reported IOP-lowering interventions in patients with POAG. There were seven RCTs on iStents® [31–37] of which three reported the clinical effectiveness of one iStent implantation combined with cataract surgery compared with cataract surgery alone [31, 33, 35], three reported the clinical effectiveness of two iStent implantation devices [32, 34, 37], and one reported clinical effectiveness in three intervention arms using different quantities of implanted iStent devices [36]. There was a single RCT for the Hydrus® Micro-Stent [38], and one on the CyPass® Micro-Stent [39], both of which compared MIGS device combined with cataract surgery vs cataract surgery alone.
Table 1.
Study characteristics of RCT studies with MIGS devices
| Study | Study design | IOP-lowering intervention (n) | Subgroup | Intervention | Mean baseline IOP (mmHg; ± SD) | Country | Longest follow-up |
|---|---|---|---|---|---|---|---|
| iStent® | |||||||
| Craven et al. [33] | Prospective, multicenter | MIGS (n = 98) | iStent | Single trabecular micro-bypass stent with concomitant cataract surgery | 25.4 ± 3.6 | USA | 24 months |
| Cataract surgery alone (n = 101) | Phacoemulsification | Cataract surgery | 25.2 ± 3.6 | ||||
| Fea et al. [31] | Prospective, double-masked | Cataract surgery with MIGS (n = 12) | iStent | Phacoemulsification with iStent® implantation (combined group) | 17.9 ± 2.6 | Italy | 15 months |
| Cataract surgery (n = 24) | Phacoemulsification | Phacoemulsification alone (control group) | 17.3 ± 3.0 | ||||
| Fea et al. [34] | Prospective unmasked randomized evaluation | MIGS (n = 94) | iStent (inject) × 2 stents | iStent® inject device | 25.2 ± 1.4 | Italy, Spain, Poland, Germany, UK, and Armenia | 12 months |
| Medication (n = 98) | Meds fixed combination of latanoprost/timolol | Two medications | 24.8 ± 1.7 | ||||
| Fea et al. [35] | Prospective | MIGS (n = 10) | iStent micro-bypass | iStent® implantation and cataract surgery (combined group) | 17.8 ± 2.7 | Italy | 48 months |
| Cataract surgery alone (n = 14) | Phacoemulsification | Cataract surgery alone (control group) | 16.7 ± 3.0 | ||||
| Fernández-Barrientos et al. [32] | Prospective | Cataract surgery with MIGS (n = 17) | iStent × 2 | iStents and cataract surgery | 24.2 ± 1.8 | Spain | 12 months |
| Cataract surgery alone (n = 16) | Phacoemulsification | Cataract surgery alone | 23.6 ± 1.5 | ||||
| Katz et al. [36] | Prospective | MIGS (n = 38) | iStent × 1 | One trabecular microbypass stent | 19.8 ± 1.3 | USA | 18 months |
| MIGS (n = 41) | iStent × 2 | Two stents | 20.1 ± 1.6 | ||||
| MIGS (n = 40) | iStent × 3 | Three stents | 20.4 ± 1.8 | ||||
| Vold et al. [37] | Cohort study | MIGS (n = 54) | iStent × 2 | Two trabecular bypass stents | 25.5 ± 2.5 | Armenia | 36 months |
| Medication (n = 47) | Topical travoprost | Topical travoprost | 25.1 ± 4.6 | ||||
| Hydrus® Micro-Stent | |||||||
| Pfeiffer et al. [38] | Prospective, multicenter, single-masked | Cataract surgery with MIGS (n = 50) | Hydrus Micro-Stent plus Phacoemulsification | Hydrus Micro-Stent and cataract surgery group | 18.9 ± 3.3 | Germany, Spain, Netherlands, and Italy | 24 months |
| Cataract surgery alone (n = 50) | Phacoemulsification | Cataract surgery group | 18.6 ± 3.8 | ||||
| CyPass® Micro-Stent | |||||||
| Vold et al. [39] | Prospective, multicenter | MIGS (n = 54) | CyPass Micro-Stent | Micro-stent implantation via an ab interno approach | 26.3 ± 4.4 | USA | 24 months |
| Cataract surgery (n = 47) | Phacoemulsification | Phacoemulsification alone (control group) | 26.6 ± 4.2 | ||||
A total of seven non-RCTs (three iStent®, three CyPass®, and one Hydrus®) and 23 economic trials were also assessed for MIGS. The characteristics of the non-RCTs are shown in the supplementary online material Table 2.
Risk of Bias
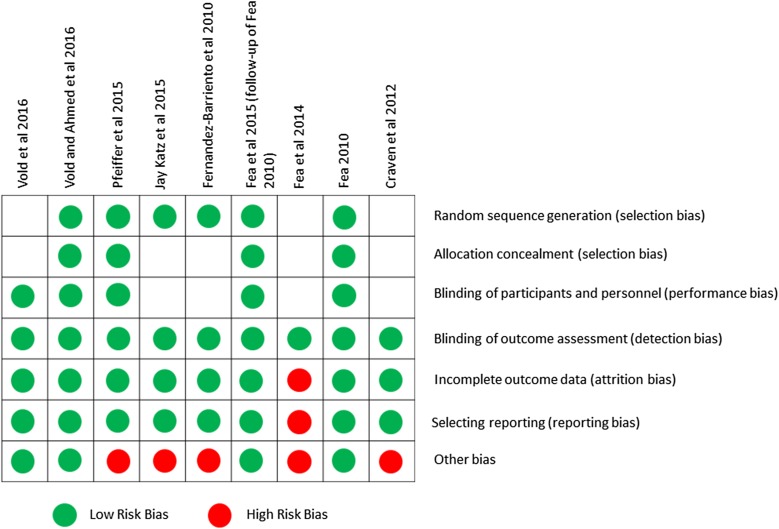
All RCTs were analyzed for potential risk of bias. Given the objective nature of IOP measurements, reviewers judged that a lack of blinding to outcome assessment would be unlikely to increase the risk of bias for IOP measurements. All outcomes for the detection bias domain were therefore judged to be of low risk as shown in supplementary online material Fig. 1. A summary of the reviewers’ judgements on risk of bias for each of the nine MIGS RCTs is shown in Fig. 2. RCTs were judged to have a risk of bias for issues such as financial matters or conflicts of interest [32–34, 36, 40] and lack of evidence of allocation concealment and blinding, or lack of evidence of sequence generation of randomization and allocation concealment [37]. In one study [34], reviewers identified three domains of bias; hence interpretation of outcomes from this study was with caution.
Fig. 2.
Reviewers’ judgement for each risk-of-bias item per RCT
Overall, RCTs with the iStent and CyPass devices were judged to have the lowest risk of bias; two Hydrus Micro-Stent RCTs were judged to have six out of seven low-risk domain outcomes, although these had conflicts of interest and financial affiliation potential bias; and eight RCTs on the iStent were judged to have potentially high risk of bias to study outcomes.
Clinical Effectiveness and Outcomes of MIGS Devices
Evidence from RCTs
An overview of the clinical effectiveness and outcomes from RCTs using the various MIGS devices is shown in Table 2.
Table 2.
Clinical effectiveness and outcomes of MIGS devices from RCTs
| Study | Study design | IOP-lowering intervention, (n) | Subgroup | Mean baseline IOP (mmHg ± SD) | Mean IOP level at longest follow-up mmHg (± SD) | Relative reduction in IOP (mean/median IOP reduction ± SD) | Reduction in number of eye drops used by patients at longest follow-up (mean reduction ± SD) | |
|---|---|---|---|---|---|---|---|---|
| MIGS | Comparator | |||||||
| Craven et al. [33] | Prospective, multicenter, longest follow-up 24 months |
MIGS (n = 98) Single trabecular micro-bypass stent with concomitant cataract surgery |
iStent | 25.4 ± 3.6 | 17.1 ± 2.9 |
8.3 mmHg 33% reduction from baseline |
Baseline: 1.6 ± 0.8 ocular hypotensive medications. At 24 months: mean of 0.3 ± 0.6 medications | |
| Cataract surgery alone (n = 101) | Phacoemulsification | 25.2 ± 3.6 | 17.8 ± 3.3 |
7.4 mmHg 29% reduction from baseline |
Baseline: 1.5 ± 0.6 ocular hypotensive medications. At 24 months: mean of 0.5 ± 0.7 medications | |||
| Fea et al. [31] | Prospective, double-masked, longest follow-up 15 months |
Cataract surgery with MIGS (n = 12) Phacoemulsification with iStent® implantation (combined group) |
iStent | 17.9 ± 2.6 | 14.8 ± 1.2 mmHg at 15 months and 16.6 ± 3.1 mmHg after washout |
3.1 mmHg 17% reduction from baseline |
Baseline ocular hypotensive medications used was 2.0 ± 0.9. After 15 months: 0.4 ± 0.7 | |
|
Cataract surgery (n = 24) Phacoemulsification alone (control group) |
Phacoemulsification | 17.3 ± 3.0 | 15.7 ± 1.1 mmHg at 15 months and 19.2 + 3.5 mmHg after washout |
1.6 mmHg 9% reduction from baseline |
Baseline ocular hypotensive medications used was 1.9 ± 0.7. After 15 months: 1.3 ± 1.0 | |||
| Fea et al. [34] | Prospective unmasked randomized evaluation, longest follow-up 12 months |
MIGS (n = 94) iStent® inject device |
iStent (inject) × 2 stents | 25.2 ± 1.4 | 13.0 ± 2.3 mmHg |
12.2 mmHg 48% reduction from baseline |
N/A | |
|
Medication (n = 98) Two medications |
Meds fixed combination of latanoprost/timolol | 24.8 ± 1.7 | 13.2 ± 2.0 mmHg |
11.6 mmHg 46% reduction from baseline |
N/A | |||
| Fea et al. [35] | Prospective, longest follow-up 48 months |
MIGS (n = 10) iStent® implantation and cataract surgery (combined group) |
iStent micro-bypass | 17.8 ± 2.7 |
15.9 ± 2 3 mmHg |
1.9 mmHg 11% reduction from baseline |
Baseline: 1.9 ± 0.9 At 12 months: 0.4 ± 0.7 |
|
|
Cataract surgery alone (n = 14) (control group) |
Phacoemulsification | 16.7 ± 3.0 | 17 ± 2.5 mmHg |
− 0.3 mmHg 2% increase from baseline |
Baseline: 1.8 ± 0.7 At 12 months: 1 ± 1 |
|||
| Fernández-Barrientos et al. [34] | Prospective, longest follow-up 12 months |
Cataract surgery with MIGS (n = 17) iStents and cataract surgery |
iStent × 2 | 24.2 ± 1.8 | 17.6 ± 2.8 |
6.6 mmHg 27% reduction from baseline |
N/A | |
| Cataract surgery alone (n = 16) | Phacoemulsification | 23.6 ± 1.5 | 19.8 ± 2.3 |
3.8 mmHg 16% reduction from baseline |
N/A | |||
| Katz et al. [36] | Prospective, longest follow-up 18 months |
MIGS (n = 38) One trabecular microbypass stent |
iStent × 1 | 19.8 ± 1.3 | 15.6 ± 1.5 |
1 × stent = 4.2 mmHg 21% reduction from baseline |
N/A | After 18 months, 4 (11.1%) patients were on medication |
|
MIGS (n = 41) Two stents |
iStent × 2 | 20.1 ± 1.6 | 13.8 ± 1.3 |
2 × stent = 6.3 mmHg 31% reduction from baseline |
4 (9.8%) | |||
|
MIGS (n = 40) Three stents |
iStent × 3 | 20.4 ± 1.8 | 12.1 ± 1.2 |
3 × stent = 8.3 mmHg 41% reduction from baseline |
3 (7.9%) | |||
| Vold et al. [37] | Cohort study, longest follow-up 36 months |
MIGS (n = 54) 2 trabecular bypass stents |
iStent × 2 | 25.5 ± 2.5 | 14.6 |
10.9 mmHg 43% reduction from baseline |
N/A | |
|
Medication (n = 47) Topical travoprost |
Topical travoprost | 25.1 ± 4.6 | 15 .3 |
9.8 mmHg 39% reduction from baseline |
N/A | |||
| Hydrus® Micro-Stent | ||||||||
| Pfeiffer et al. [38] | Prospective, multicenter, single-masked, longest follow-up 24 months |
Cataract surgery with MIGS (n = 50) Hydrus micro-stent and cataract surgery group |
Hydrus micro-stent plus Phacoemulsification | 18.9 ± 3.3 | 16.9 ± 3.3 |
2 mmHg 11% reduction from baseline |
Baseline medication: 2.0 ± 1.0 Follow-up: 0.5 ± 1.0 |
|
| Cataract surgery alone (n = 50) | Phacoemulsification | 18.6 ± 3.8 | 19.2 ± 4.7 |
− 0.6 mmHg 3% increase from baseline |
Baseline medication: 2.0 ± 1.0 Follow-up: 0.5 ± 1.1 |
|||
| CyPass® Micro-Stent | ||||||||
| Vold et al. [39] | Prospective, multicenter, longest follow-up 24 months |
MIGS (n = 54) Micro-stent implantation via an ab interno approach |
CyPass Micro-Stent | 26.3 ± 4.4 | 16.9 ± 3.3 |
9.4 mmHg 36% reduction from baseline |
Microstenting significantly reduced hypotensive ocular medication use. From 1.4 ± 0.9 to 0.2 ± 0.6 | |
|
Cataract surgery (n = 47) Phacoemulsification alone (control group) |
Phacoemulsification | 26.6 ± 4.2 | 19.2 ± 4.7 |
7.4 mmHg 28% reduction from baseline |
Medication use in the control group was 1.3 ± 1.0 medications at baseline and 0.6 ± 0.8 at follow-up |
|||
In the three RCTs using the iStent combined with cataract surgery vs cataract surgery alone [31, 33, 35], the mean relative reduction in IOP levels from baseline ranged from 8.3 to 1.9 mmHg in the iStent combined with cataract surgery groups vs 7.4 mmHg to an increase of 0.3 mmHg in the cataract surgery alone groups. However, cataract surgery alone was associated with greater reductions in the use of eye drops compared with iStent plus cataract surgery. In two RCTs using two iStent devices plus cataract surgery vs cataract surgery alone, Fea et al. and Vold et al. reported mean relative IOP reductions of between 12.2 and 10.9 mmHg with the iStents vs 11.6 to 9.8 mmHg in the cataract surgery alone groups, which received a fixed combination of latanoprost and timolol or travoprost [34, 37]. Increasing clinical effectiveness by increasing the number of iStent devices implanted was reported by Katz et al. whereby, at 18 months, implantation of one, two, or three iStents resulted in a mean relative reduction of IOP of 4.2 mmHg (21% from baseline), 6.3 mmHg (31%), and 8.3 mmHg (41%), respectively [36].
For the Hydrus Micro-Stent, Pfeiffer et al. reported a 2 mmHg relative reduction in IOP level (11% reduction from baseline) in the Hydrus group at 24 months’ follow-up vs an increase of 0.6 mmHg in the cataract surgery alone group. Both arms showed a 25% reduction in use of medicated ocular drops from baseline [38]. Similarly, with the CyPass Micro-Stent plus cataract surgery vs cataract surgery alone Vold et al. reported a greater overall IOP reduction of 9.4 mmHg (36% reduction from baseline) in the CyPass group compared with 7.4 mmHg (28% reduction from baseline) in the cataract surgery group, at 24 months. Furthermore, the CyPass Micro-Stent reduced the use of topical glaucoma medication (eye drops) from 1.4 drops at baseline to 0.9 drops at 24 months’ follow-up [39].
Evidence from Non-RCTs and Gray Literature
Non-RCTs and gray literature were used, where possible, to provide further clinical effectiveness evidence. Non-RCTs were identified for MIGS devices, and in some cases gray literature was identified and reviewed where information was lacking. For example, as no RCTs or non-RCTs were available for the XEN device, gray literature from posters and abstracts were used. Details of these sources are shown in the supplementary online material in Table 2 for non-RCTs and Table 3 for XEN-related gray literature.
Non-RCTs using the iStent included various combinations such as iStent with cataract surgery [41], iStent alone [42], and iStent plus cataract surgery compared with trabeculectomy plus cataract surgery [43]. The mean baseline IOP in these trials ranged from 17.5 to 22.3 mmHg. Kurji et al. reported the lowest follow-up IOP of 13.6 mmHg (22% relative reduction from baseline) [43], while the greatest decrease in mean baseline IOP of 21.3 to 14.0 mmHg (34% decrease) for two iStents with micro-incision cataract surgery (MICS) was reported by Gonnerman et al. at 12-month follow-up [41]. The greatest reduction in medicated hypotensive drops was reported by Khan et al. with a reduction in number of drops from 2.86 preoperative/at baseline to 1.22 at 12 months [42]. A number of case series were also identified for the iStent. In one comparative series based in Canada, the implantation of two iStents vs three iStents resulted in a similar (20%) reduction from baseline in IOP levels after 12-month follow-up [44]. The differential in IOP reduction using two vs three iStents was less pronounced than in the RCT reported by Katz et al. in which follow-up was at 18 months [36]. Similarly, in the UK-based Manchester iStent study, a prospective uncontrolled interventional case series, Tan and Au reported a 19% reduction in IOP levels from baseline after 36 months (baseline IOP, 21.2 mmHg; mean IOP at longest follow-up, 17.1 mmHg) [45].
In a non-RCT case series with the CyPass a relative reduction in IOP of 26%, over 6 months, was reported with mean IOP levels decreasing from a baseline of 21.2 to 15.6 mmHg at follow-up [18]. At 24-month follow-up the reduction in IOP from baseline was 37% [46]. Furthermore, in patients who had a baseline IOP below 21 mmHg and who achieved IOP of 15.8 mmHg, there was a reduction in the use of medicated ocular drops from an average 2.0 to 1.1 [46]. Similar results were reported in another open-label interventional study across five European countries [47].
In the single identified non-RCT for the Hydrus by Fea et al., the mean IOP at baseline was similar for the Hydrus Micro-Stent vs selective laser trabeculoplasty groups at 23.1 vs 23.2 mmHg, decreasing to 16.5 vs 15.9 mmHg at 12-month follow-up, respectively [48].
Evidence for the XEN (XEN gel stent) device was obtained from posters and abstracts summarized in the online supplementary material Table 3. In these abstracts, the mean preoperative (best-medicated) IOP ranged from 20.8 to 22.7 mmHg. Various reductions in IOP levels from preoperative levels have been reported for various follow-up times using the XEN device. Kersten-Gomez et al. presented an abstract in 2012 reporting an IOP decrease from 21.3 to 12.2 mmHg at 1 week [40]. In the longest follow-up period of 4 years an IOP reduction from a baseline of 22.3 to 13.5 mmHg (reduction of 39.5%) was reported [49]. Although initially produced and studied with three different lumen diameters (140, 63, and 45 nm), the 45-nm lumen size is the only device now recommended for implantation, as the dimensions of this device aimed to prevent postoperative hypotony [50]. Little published data exist for the XEN 45 implant. The pilot study by Sheybani et al. was on the XEN 63 and XEN 140 implants and showed a reduction in IOP from 22.4 to 15.4 mmHg at 12-month follow-up, with reduction in eye drops from 2.5 to 0.9 [51].
Considerations and Adverse Events from MIGS RCTs
Although MIGS devices have proven “successful”, depending on the definition of success, which varies between clinicians, patients, and studies, they can be associated with various complications and AEs that require care. For example, implantation of MIGS devices may result in increased hypotony rates or bleb needling in subconjunctival placed devices. Such procedures require additional resources in outpatient clinics and potentially additional theater time.
In this review AEs for each of the MIGS devices were extracted from the 17 RCTs, non-RCTs, and various case studies (gray literature).
The iStent, Hydrus, and CyPass devices generally have favorable safety profiles with few reported AEs. Hyphema is common with iStent and Hydrus, with rates of 19.04% for Hydrus and a few cases reported for iStent [52, 53]. High rates of hyphema are unsurprising for these MIGS devices considering they are implanted into a highly vascular region. Other harm and AEs reported with the iStent included stent malpositioning or occlusion early in the postoperative period, affecting 4–18% of cases [32, 33, 44, 53]. Corneal erosion has also been reported in one study, attributed to repeated intraoperative gonioscopy [54]: these types of risks are only relevant for MIGS that require gonioscopy.
Hypotony has also been reported for the CyPass with rates of between 2.9% and 15.4%, most cases being mild and not requiring intervention [39, 46].
For the CyPass, in addition to hypotony, other ocular harm and AEs reported have included iritis (8.6%), secondary ocular surgical intervention (5.5%), corneal edema (3.5%), and hyphema (2.7%). However, most of these were transient and did not affect visual acuity [39]. Pfeiffer et al. reported a statistically significant (p = 0.0077) increase in focal peripheral anterior synechiae (18.8%) after 2 years in patients implanted with the Hydrus combined with cataract surgery [38].
Limited information is currently available on the safety profile of XEN; the manufacturer’s website states that postoperative adverse events have included hypotony (defined as IOP below 6 mmHg at any time) in 24.6% of subjects (with no associated clinically significant consequences, no cases of persistent hypotony, and no surgical intervention required), an IOP increase of at least 10 mmHg from baseline in 21.5% of patients, and needling procedure rates of 32.3% [55]. The high rates of bleb needling reported after XEN insertion potentially offset the economic value of the XEN because of the extra surgical time and patient investment required to address.
Economic Outcomes with MIGS Devices
Cost-Effectiveness
As there is limited available evidence on the cost-effectiveness of MIGS as primary interventions for glaucoma [21, 56], it remains unclear whether the cost of using MIGS is outweighed by cost savings through decreased medication and need for further interventions. The few available studies are either retrospective case studies or industry-sponsored RCTs with short follow-up times [57].
Medical management, stand-alone cataract surgery, and cataract surgery with iStent implantation were compared over 5 years in patients with cataract and glaucoma but inadequately controlled IOP with two medications. The study used a Markov model and a public third-party payer perspective (Ontario Health Insurance Plan). Compared with medical management, the incremental cost-effectiveness ratio (ICER) of iStent plus cataract surgery was CA$6824/quality-adjusted life year compared with $4179/quality-adjusted life year (cost year not stated) for cataract surgery alone. The ICER for iStent plus cataract surgery compared with cataract surgery alone was not reported [58].
Cost-effectiveness evidence for CyPass and Hydrus Micro-Stents or the XEN device was not available. A summary of the studies identified for economic evidence and reported costs is shown in Table 3.
Table 3.
Glaucoma treatment costs
| Reference | Study | Time horizon | Country | Costs reported | Cost | Currency | Cost year | GBP (2016) | Disaggregation |
|---|---|---|---|---|---|---|---|---|---|
| Tan and Au [45] | Prospective uncontrolled interventional case-series of iStent implantation in combination with cataract surgery for glaucoma patients | 3 years | UK | All patients 3 years, cost of iStent plus drops (branded) | £36,598.02 | GBP | Not stated | N/A | Cost calculations included cost of iStent implantation (incl. disposables, viscoelastic materials; excl. reusable instruments, surgeon time, theater time) and of drops in the two treatment strategies |
| All patients 3 years, cost of iStent plus drops (generic) | £31,893.12 | GBP | Not stated | N/A | As above | ||||
| All patients 3 years—drops (branded) | £12,812.10 | GBP | Not stated | N/A | As above | ||||
| All patients 3 years—drops (generic) | £8107.20 | GBP | Not stated | N/A | As above | ||||
| Kaplan et al. [62] | Markov cohort model, hypothetical cohort of 100,000 patients requiring glaucoma surgery, managed medically, with trabeculectomy or with Baerveldt implant (latter not shown) | 5 years | USA | Medication mean total cost | $6172.00 | USD | 2013 | £4036.32 | Total costs were not disaggregated; however, unit costs reported for surgeon, facility, office visit, Humphrey visual field, optical coherence tomography, bleb leak (multiple items), diplopia (multiple items) |
| Trabeculectomy mean total cost | $7872.00 | USD | 2013 | £5148.08 | |||||
| Yep et al. [88] | Claims database analysis to estimate the pre/post index diagnosis cost of care for 8575 glaucoma patients | 1 year | USA | Mean annual total direct glaucoma-specific costs per patient—before diagnosis | $107.00 | USD | Not stated | N/A | Costs not disaggregated but based on inpatient stays, emergency department visits, general and vision-related office visits, glaucoma diagnostic tests, glaucoma surgeries, and medications |
| Mean annual total direct glaucoma-specific costs per patient—after diagnosis | $487.00 | USD | Not stated | N/A | |||||
| Iordanous et al. [58] | Direct cost projection of glaucoma treatment with trabectome, iStent, and endoscopic cytophotocoagulation | 6 years | Canada | Cost of stent materials per patient—iStent | $1044.00 | CAD | Not stated | N/A | Based only on cost of device obtained from local distributors (2× iStent, total CA$1000), plus cost of disposable materials during surgery (CA$44). Surgeon’s fees were not included, and no follow-up health care utilization was included |
| Olsen et al. [59] | Danish National Register cross-sectional study of 27,380 new glaucoma/ocular hypertension patients | 5.5 years | Denmark | Total mean annual direct glaucoma-specific health care costs of “average patient” | €369.00 | EUR | 2007 | £291.69 | Not disaggregated but includes primary care (visits, examinations, laser treatment) and secondary care (inpatient/outpatient) |
| Rahman et al. [60] | Retrospective register study at the Glasgow Royal Infirmary Glaucoma Clinic | Lifetime | UK | Total mean annual direct glaucoma-specific health care cost per patient | £475.00 | GBP | 2011 | £499.38 | Disaggregated by (over the lifetime) outpatient costs, surgical cost, procedure costs, inpatient costs |
| Orme et al. [63] | Cost-effectiveness (Markov) model examining different sequences of medical management (latonoprost, bimatoprost, or travoprost as first line) | 10 years | UK | Total mean cumulative direct glaucoma-specific cost per patient over 10 years (latonoprost first line) | £6086.40 | GBP | 2008/2009 | £6770.27 | Disaggregated by medical therapy (first, second, and third line), scheduled and additional follow-up visits, surgery, long-term cost of low vision. Results were comparable for all first-line drugs |
| Sharma et al. [66] | Costing study of hospital-based glaucoma clinic with community optometrists | 1 year | UK | Mean annual clinic cost per patient—hospital | £102.25 | GBP | Not stated | N/A | Disaggregated as staff costs, non-pay costs (facilities, patient transport, domestics, interpreter, deprecation, sundries as lump sum), overhead |
| Mean annual clinic cost per patient—community | £254.17 | GBP | Not stated | N/A | Based on opportunity cost of running normal clinic (not disaggregated) | ||||
| Stein et al. [64] | Cost-effectiveness (Markov) model comparing observation only, prostaglandin medical therapy, and laser trabeculectomy in the US setting | 25 years | USA | Total estimated glaucoma-specific direct cost per patient over 25 years—observation only | $2700.00 | USD | 2010 | £1875.58 | Not disaggregated, but based on medication, laser surgery, trabeculectomy, initial and follow-up evaluations, diagnostics, low vision services (uni- and bilateral) |
| Total estimated glaucoma-specific direct cost per patient over 25 years—prostaglandin | $18,101.00 | USD | 2010 | £12,574.02 | As above | ||||
| Stein, Niziol et al. [71] | Longitudinal cohort study examining managed care claims data from 19,927 newly diagnosed OAG 2001 to 2009 with at least 2 years’ follow-up data | 2 years | USA | Cumulative mean total direct glaucoma-specific costs at 2 years | $2515.61 | USD | 2009 | £1785.56 | Total costs for all enrolled patients disaggregated by eye care providers (32%), glaucoma medications (31%), glaucoma diagnostic tests (16%), laser or incisional glaucoma surgeries (20%) |
| Holtzer-Goor et al. [67] | Economic evaluation alongside RCT. Monitoring of 815 stable glaucoma patients in glaucoma follow-up unit (GFU—optometrist and ophthalmic technicians) compared with usual care (glaucoma specialist). Costs reported from hospital, patient, health care, and societal perspective | 30 months | Netherlands | Mean annual direct cost of glaucoma-specific health care per patient (health care perspective)—GFU | €138.85 | EUR | 2007 | £109.76 | Breakdown by hospital visits, tests (HFA, refraction, pachymetry etc.), laser treatment, glaucoma surgery, advice |
| Mean annual direct cost of glaucoma-specific health care per patient (health care perspective) -usual care | €161.43 | EUR | 2007 | £127.61 | Breakdown by hospital visits, tests (HFA, refraction, pachymetry etc.), laser treatment, glaucoma surgery, advice | ||||
| Van Gestel et al. [65] | Discrete event simulation of the pathway of care for OHT and glaucoma patients across the life course, incl. medication switches, laser treatment, trabeculectomy, and care related to impaired vision. Comparison of no treatment and three strategies (A–C) with different IOP targets and visual field measurement frequencies | Lifetime | Netherlands | Mean total glaucoma-specific cost per patient over life course—no treatment | €41,618.00 | EUR | 2006 | £33,736.44 | Total costs not disaggregated, but unit costs extensively reported for medications, ophthalmologists, procedures (trabeculectomy, laser surgery, Baerveldt implant, cataract extraction etc.), low-vision aids, home care etc. in supplementary online material |
| Mean total glaucoma-specific cost per patient over life course—strategy A | €25,648.00 | EUR | 2006 | £20,790.82 | As above. Strategies B and C did not differ substantially from strategy A | ||||
| Kobelt et al. [61] | Prospective cohort of 602 patients enrolled through specialized hospitals or private practices in France, with uncontrolled IOP and treated with prostaglandin alone or in combination with other medications | 4 years | France | Total mean direct glaucoma-specific health care costs per patient over 4 years | €2204.00 | EUR | 2008 | £1953.51 | Disaggregated by medications, consultations/examinations, inpatient admissions, surgery (trabeculectomy, cataract, trabeculectomy/cataract, other), outpatient surgery |
| Pasquale et al. [89] | Retrospective cohort of 72,412 glaucoma patients from an insurance claims database | 1 year | USA | Mean annual total glaucoma-specific direct costs per patient | $1449 | USD | Not stated | N/A | Costs not disaggregated, but resource utilization disaggregated by visual field examination, scanning or laser ophthalmoscopy, optic nerve photographs, eye examination, trabeculoplasty, trabeculectomy, visual acuity, gonioscopy, serial tonometry or tonography, provocative test for glaucoma, laser iridotomy, and other ophthalmologic services and procedures |
| Lee et al. [68] | Cross-sectional cohort of 151 patients with glaucoma or OHT from 12 sites in the USA | 5 years | USA | Average annual glaucoma-specific direct costs per patient—stage 0 | $623 | USD | Not stated | N/A | Disaggregated (graphically) by office visits, visual field tests, medications, surgery, low-vision services, other services |
| Average annual glaucoma-specific direct costs per patient—stage 5 | $2511 | USD | Not stated | N/A | |||||
| Lindblom et al. [90] | Review of medical records of 267 patients in Sweden and France with glaucoma and ocular hypertension | 2 years | Sweden, France | Average annual glaucoma-specific direct costs per patient | €467.00 | EUR | 2002 | £407.56 | Disaggregated by consultations (26%), diagnostics/monitoring (16%), medications (49%), surgical procedures (5%), hospitalization (4%) |
| Denis et al. [70] | Cross-sectional retrospective study of resource utilization of 337 patients enrolled by 88 ophthalmologists in France | N/A | France | Mean annual glaucoma-specific direct and indirect cost (productivity loss)—no switches | €314.66 | EUR | 2001 | £281.32 | Breakdown by exam/outpatient surgery, drugs (majority of costs), visits, inpatient stays, indirect costs |
GFU glaucoma follow-up unit
Cost of Treatment
Of the studies reporting economic evidence for glaucoma treatment, the majority reported total, average direct costs for management strategies based on bottom-up costing or retrospective claims and registry review. Most studies included established care pathways, such as medical management, trabeculectomy, and laser surgery. Where currencies and base years were reported, current prices (GBP 2016) were calculated for costs.
As part of the Manchester iStent study, Tan et al. reported that in 36 patients who completed the 3-year follow-up the overall cost of combined cataract surgery and iStent implantation was £829.32 more in total than conservative management with branded eye drops and £14,176.90 more if generic drops were used [45]. No cost-effectiveness was reported and costs did not include follow-up care after iStent insertion or other downstream health care utilization [45].
Economic evidence suggests that iStent implantation and follow-up costs are higher than trabeculectomy costs, but the incremental cost-effectiveness of these implants remains unknown.
The majority of studies on the treatment cost of trabeculectomy and other routine procedures have reported mean direct health care costs per patient with glaucoma, rather than for specific treatments. Per patient costs include consultations, procedures (trabeculectomy, laser surgery, etc.), and medications, generally averaged across the study population. On the basis of this criterion, Olsen et al. reported the total mean annual direct glaucoma-specific health care costs per patient to be £261.69, which included both primary (visits, examinations, laser treatment) and secondary care (in- and outpatient episodes) costs [59]. This approach has also been used in other studies in which the mean annual cost per patient was below £500 [60, 61]. Some studies have addressed the cost of specific treatment strategies, such as medical management vs trabeculectomy [62], different medical management strategies [63], observation only vs medical therapy or laser treatment [64], or different treatment targets for IOP and visual field measurement frequencies [65]. A small number of studies have considered the impact of treatment setting on cost [66, 67]. In a study reported by Sharma et al. community clinics were more expensive to run than hospital-based glaucoma clinics, over the course of a year, when implementation and opportunity costs but not health care or follow-up costs were considered; the authors concluded that this was due to higher overhead costs in the community setting [66]. In another study a glaucoma follow-up unit (GFU), staffed with optometrists and ophthalmic technicians, was compared with usual care provided by glaucoma specialists. Findings from this study showed the mean direct annual cost per patient to be lower in the GFU arm (£109.76), accounting for hospital visits, tests, interventions (laser treatment, trabeculectomy, etc.), and other costs, vs usual care (£127.61) [67].
Studies have been conducted to examine the variation in cost in patients with POAG. A number of factors have been identified that are associated with higher or lower mean costs of care. Disease stage has been identified as a predictor of higher cost [68, 69]. Long-term direct cost of 194 glaucoma patients in France, Germany, Italy, and the UK was linearly associated with disease stage, estimated at €455/person-year at disease stage 0 vs €969/person-year at disease stage 4 across the four countries [69]. Other studies have highlighted treatment changes/switches as an important predictor of costs [59, 61, 70]. For example, Danish registry data show higher costs associated with treatment changes, longer treatment duration, and age [59]. Finally, Stein et al. [71] examined factors associated with higher treatment costs and identified comorbidities as significant covariates (diabetic retinopathy, age-related macular degeneration, cataract, pseudophakia/aphakia) associated with increased cost. In the UK, the use of glaucoma medications has been analyzed on the basis of Prescription Cost Analysis data [72]. In 2009 NICE introduced clinical guidance on ocular hypertension and glaucoma, recommending prostaglandin analogues as first-line medication, and beta-blockers as first-line medication for patients with IOP levels between 26 and 32 mmHg, pachymetry 555–590 μm, and age below 60 years [73]. Between 2000 and 2012, prescriptions in the UK increased from 4.76 million to 7.96 million (up 67%), with drug costs almost doubling from £55.2 million to £103.7 million. During this period, the overall use of prostaglandin increased while the use of beta-blockers decreased. There was significant heterogeneity in the drugs dispensed, with 40 medications being prescribed at a rate of more than 10,000 prescriptions per year. Latanoprost was prescribed approximately three times more frequently than the second most frequently prescribed drug; however, this cost has decreased following the availability of generic latanoprost [72].
HTA appraisals may be a potential source for economic data; however, research for this review identified records only for XEN in the NIHR-HTA database. Furthermore, the XEN HTA had no appraisal of the clinical or economic benefits of the device.
Discussion
Clinical and Economic Outcomes
Primary open-angle glaucoma is a major public health problem with its increasing prevalence and substantial impact on quality of life for patients, their families, and caregivers. MIGS procedures are a heterogenous group of techniques that seek to reduce IOP with lower risk than more established filtration surgery procedures: they may increase trabecular outflow by bypassing the trabecular meshwork, increase uveoscleral outflow via suprachoroidal pathways, or create a subconjunctival drainage pathway. Although clinical experience with MIGS is increasing, and they may provide safety advantages over trabeculectomy, issues remain such as surgical difficulty, limited efficacy, complications, and the absence of long-term data.
In this review MIGS were linked to clinical disadvantages such as insufficient IOP reduction, surgical complexity, device failure, and other potential risks and AEs. For MIGS devices in which bleb management (such as needling, most commonly, and treatment of leakage) is frequent practice (i.e., Xen), many studies do not classify these as an AE and thus the true impact of bleb management remains unclear. These types of AEs require postoperative interventions which can have an impact on time and outpatient resources/costs. While various forms of trabeculectomy can achieve postoperative IOP of 11.0–13.0 mmHg [15, 74, 75], MIGS devices described in this review were typically associated with higher postoperative IOP levels. This therefore suggests that MIGS devices are best suited for patients with mild to moderate disease in which lower target IOPs are not necessary, or as a method by which patients can reduce their topical hypotensive load. For example, mean IOP at the longest follow-up (36 months) reported for the iStent as a separate glaucoma intervention was 14.6 mmHg [37], while the iStent combined with cataract surgery resulted in IOP levels of 15.9 mmHg at 48 months [35], 17.1 mmHg at 24 months [33], and 14.8 mmHg at 15 months [31]. The lowest IOP of 13.0 mmHg was reported with the shortest follow-up of 12 months using the iStent. This was achieved by implanting two stents; however, this was on a slight up-trend from month 6 (12.7 mmHg) onwards [34]. Although the iStent is associated with fewer risks and AEs compared to other MIGS devices, it has limited effectiveness in IOP lowering, which is dictated by the episcleral venous pressure. In addition, iStent implantations, which require tilting microscopes, in-theater gonioscopy, and lens extraction, tend to be restricted to elderly patients with cataracts, and it is not possible to ascertain blockage of an iStent as there is no bleb.
Clinical effectiveness of the Hydrus Micro-Stent appeared to be similar to the iStent with a mean IOP level of 16.9 mmHg at 24-month follow-up [38]. However, it is unknown whether surgically implantation of the Hydrus may be more challenging than the iStent, or if complications vary: there are a lack of published data. The CyPass RCT showed a reduction in IOP by 36% at 24 months, plus reduction in topical glaucoma medications [39]. Similar findings are also seen in real-world observational studies [47]. The suprachoroidal space is highly vascular, which in theory potentially increases the risk of an intraoperative suprachoroidal hemorrhage with suprachoroidal devices; however, there is currently no evidence to substantiate this fear.
Details of clinical evidence of the XEN device are currently unavailable and evidence from abstracts is limited on safety data. From available information, IOP reduction with the XEN device may be comparable to other MIGS devices with the lowest reported follow-up IOP of 13.0 mmHg at 12 months [76] and the highest of 15.9 mmHg at 12 months [77]. These preliminary reports are based on non-peer-reviewed materials and are subject to significant uncertainty. A potential concern with the XEN device is that it is porcine gelatin-based; implantation of porcine-derived material may be an issue in patients with certain religious and personal beliefs [78].
Economic outcomes were challenging to assess in this review because of limited availability of information on cost-effectiveness and cost of treatment of all MIGS devices. Although there was one economic study with the iStent [58], there was no cost-effectiveness evidence. As disease stage has been identified as a predictor of higher management costs, devices aimed at advanced glaucoma patients or those with high IOP may have higher cost-savings potential [68, 69].
MIGS Devices and Unmet Need
The comparative effectiveness of a MIGS device is dependent on implantation site, device material, and design. A key challenge is in using materials that induce minimal tissue reaction and scarring. Despite antimetabolite use, the subconjunctival space is prone to fibrosis, hence a reduction in efficacy or late failure. This is certainly the case in glaucoma patients using long-term preserved drop therapy, as the conjunctiva has been shown to be pro-inflammatory and primed for scarring in the case of further insult [79]. Using the suprachoroidal route (i.e., CyPass and iStent Supra) avoids subconjunctival filtration bleb-related complications including hypotony, leakage, bleb failure, bleb-related infection (short- and long-term), and discomfort with foreign body sensation or pain [80]. The suprachoroidal space also offers the opportunity for significant reductions in IOP. Evidence suggests that a negative pressure gradient exists between the anterior chamber and the suprachoroidal space, promoting aqueous outflow through a vacuum-like effect [81]. However, it is more invasive and intraocular than ab externo procedures, and although suprachoroidal hemorrhage [82] has not been reported, lack of long-term data makes it difficult to confirm clinical benefits and safety. Furthermore, implants placed in the suprachoroidal space do not escape tissue reaction and implant failure through fibrosis [83].
Larger, multicenter, randomized trials and real-world observations are needed for all MIGS devices to better assess their clinical and economic effectiveness. In addition, this review highlights the unmet need for better treatment options for patients with open-angle glaucoma; MIGS devices should be simple for surgeons to use and provide sustained long-term IOP-reducing effect, with postoperative management suitable to the general ophthalmologist and few potential complications (e.g., ideally with minimal hypotony and easy-to-manage blebs). In addition, rapid visual recovery would be ideal. Such qualities would be better for patients as well as for busy outpatient settings where the management of complications can place a burden on economic resources. The use of MIGS devices may also benefit from defined treatment options for specific patient groups (such as those with high IOP), and guidelines for surgeons as to which device should be used in which patient population.
Since completion of this systematic review study in December 2016, a number of commentaries and reviews have been published discussing the efficacy and safety of MIGS devices [84–87]. Our findings agree with other reports in that current data indicate a balance between potential IOP lowering and AEs while acknowledging the lack of comparable long-term data [84–87]. A common theme, expanded upon in this systematic review, is the importance of understanding the characteristics of each MIGS device (e.g., mode of action and safety profile, as well as the IOP-lowering potential) and how these relate to the specific target population profile [85, 87]. However, none have reviewed or discussed in detail the economic data, which further highlights the need for additional information on which to distinguish between the various MIGS devices available.
Study Strengths and Limitations
A major strength of this research is the comprehensive, structured, and systematic approach in searching the literature to identify all studies that report clinical and or economic outcomes in the glaucoma surgery segment. To the best of our knowledge this is the first systematic review to include economic outcomes in currently available MIGS devices. Possible limitations may be the difficulty in making direct comparisons either between studies or MIGS devices as well as the limited availability of suitable economic data on MIGS devices. A further limitation is that the search and analysis are based on published literature up to December 2016. Systematic reviews are universally limited in scope by providing a snapshot of evidence in time based on tight inclusion and exclusion criteria, and cutoff dates for literature inclusion: a trade-off for the methodological robustness. As real-world experience grows with MIGS we suggest conducting “living” systematic reviews that are continually updated, incorporating new relevant RCT and non-RCT evidence as it becomes available to best inform evidence-based practice.
Conclusion
Despite the increasing prevalence of POAG as a leading cause of blindness, and the availability of treatments such as hypotensive medicated ocular drops, trabeculectomy, or, more recently, MIGS devices, there still remains a need for treatments that are easy to implement and reduce IOP levels without increasing postoperative aftercare and cost.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This systematic review and article processing charges were funded by Santen GmbH, Germany. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Editorial Assistance
Writing and editorial support has been provided by Valid Insight. This support was funded by Santen GmbH, Germany. We also acknowledge Nik Prowse of Valid Insight for editorial assistance.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ contributions
SB was involved in designing and conducting the systematic literature review (SLR), developing the concept, and drafting and reviewing the manuscript. PA provided advice on scope of the SLR, publication concept development, drafting, reviewing, and editing the manuscript. Both authors have read and approved the final manuscript.
Disclosures
Valid Insight, the company of which Steven E Bradshaw is a director, received funding for this systematic review and its publication from Santen GmbH, Germany. Pavi Agrawal has nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain studies with human participants or animals performed by any of the authors.
Data availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced digital features
To view enhanced digital features for this article go to 10.6084/m9.figshare.6165725.
References
- 1.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Kapetanakis VV, Chan MP, Foster PJ, Cook DG, Owen CG, Rudnicka AR. Global variations and time trends in the prevalence of primary open angle glaucoma (POAG): a systematic review and meta-analysis. Br J Ophthalmol. 2016;100(1):86–93. doi: 10.1136/bjophthalmol-2015-307223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmid Biggerstaff K. Primary open-angle glaucoma (POAG). http://emedicine.medscape.com/article/1206147-overview. Accessed 4 Aug 2017.
- 4.EGS. European Glaucoma Society guidelines, 4th ed. EGS; 2014. http://www.eugs.org. Accessed Aug 2017.
- 5.Ren R, Jonas JB, Tian G, et al. Cerebrospinal fluid pressure in glaucoma. Ophthalmology. 2010;117:259–266. doi: 10.1016/j.ophtha.2009.06.058. [DOI] [PubMed] [Google Scholar]
- 6.Wang N, Xie X, Yang D, et al. Orbital cerebrospinal fluid space in glaucoma: the Beijing intracranial and intraocular pressure (iCOP) study. Ophthalmology. 2012;119(2065–2073):e2061. doi: 10.1016/j.ophtha.2012.03.054. [DOI] [PubMed] [Google Scholar]
- 7.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma. JAMA. 2014;311(18):1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma (Chicago, IL: 1960) Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 9.Stolz J, Lemij H, Hoevenaars J, van der Windt C, Baudouom C. Patient satisfaction with glaucoma therapy: reality or myth? Clin Ophthalmol. 2015;9:785–793. doi: 10.2147/OPTH.S78918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gedde SJ, Herndon LW, Brandt JD, et al. Postoperative complications in the tube versus trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(804–814):e801. doi: 10.1016/j.ajo.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molteno AC. New implant for drainage in glaucoma. Clinical trial. Br J Ophthalmol. 1969;53:606–615. doi: 10.1136/bjo.53.9.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lloyd MA, Baerveldt G, Heuer DK, Minckler DS, Martone JF. Initial clinical experience with the Baerveldt implant in complicated glaucomas. Ophthalmology. 1994;101:640–650. doi: 10.1016/S0161-6420(94)31283-8. [DOI] [PubMed] [Google Scholar]
- 13.Coleman AL, Hill R, Wilson MR, et al. Initial clinical experience with the Ahmed glaucoma valve implant. Am J Ophthalmol. 1995;120:23–31. doi: 10.1016/S0002-9394(14)73755-9. [DOI] [PubMed] [Google Scholar]
- 14.Patel S, Pasquale LR. Glaucoma drainage devices: a review of the past, present, and future. Semin Ophthalmol. 2010;25:265–270. doi: 10.3109/08820538.2010.518840. [DOI] [PubMed] [Google Scholar]
- 15.Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the tube versus trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153(789–803):e782. doi: 10.1016/j.ajo.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spiegel D, García-Feijoó J, García-Sánchez J, Lamielle H. Coexistent primary open-angle glaucoma and cataract: preliminary analysis of treatment by cataract surgery and the iStent trabecular micro-bypass stent. Adv Ther. 2008;25:453–464. doi: 10.1007/s12325-008-0062-6. [DOI] [PubMed] [Google Scholar]
- 17.Shareef S, Fea A, Ahmed IIK. The Hydrus micro-stent. In: Samples JR, Ahmed IIK, editors. Surgical innovations in glaucoma. New York: Springer; 2014. pp. 171–174. [Google Scholar]
- 18.Höh H, Ahmed IIK, Grisanti S, et al. Early postoperative safety and surgical outcomes after implantation of a suprachoroidal micro-stent for the treatment of open-angle glaucoma concomitant with cataract surgery. J Cataract Refract Surg. 2013;39:431–437. doi: 10.1016/j.jcrs.2012.10.040. [DOI] [PubMed] [Google Scholar]
- 19.Lewis RA. Ab interno approach to the subconjunctival space using a collagen glaucoma stent. J Cataract Refract Surg. 2014;40:1301–1306. doi: 10.1016/j.jcrs.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 20.Gazzard G. Minimally invasive glacuoma surgery: MIGS. Focus. London: Royal College of Ophthalmologists; 2016. [Google Scholar]
- 21.Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol. 2016;10:189–206. doi: 10.2147/OPTH.S80490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varma R, Lee PP, Goldberg I, Kotak S. An assessment of the health and economic burdens of glaucoma. Am J Ophthalmol. 2011;152:515–522. doi: 10.1016/j.ajo.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NICE. The guidelines manual. https://www.nice.org.uk/process/pmg6/chapter/introduction. Accessed Aug 2017.
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cochrane Collaboration. RevMan 5. http://community.cochrane.org/tools/review-production-tools/revman-5. Accessed Aug 2017.
- 26.Higgins JP, Altman DG. Assessing risk of bias in included studies. In: Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions. Chichester: Wiley; 2008. pp. 187–241. [Google Scholar]
- 27.Jadad AR, Enkin MW. Bias in randomized controlled trials. In: Jadad AR, Enkin MW, editors. Randomized controlled trials. Oxford: Blackwell; 2007. pp. 29–47. [Google Scholar]
- 28.CRD . Systematic reviews: CRD’s guidance for undertaking reviews in health care. York: Centre for Reviews and Dissemination, University of York; 2009. [Google Scholar]
- 29.OECD . Exchange rates. Paris: Organisation for Economic Co-operation and Development; 2017. [Google Scholar]
- 30.PSSRU . PSSRU | Unit costs of health and social care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016. [Google Scholar]
- 31.Fea AM. Phacoemulsification versus phacoemulsification with micro-bypass stent implantation in primary open-angle glaucoma. Randomized double-masked clinical trial. J Cataract Refract Surg. 2010;36:407–412. doi: 10.1016/j.jcrs.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 32.Fernández-Barrientos Y, García-Feijoo J, Martínez-de-la-Casa JM, Pablo LE, Fernandez-Perez C, Sanchez JG. Fluorophotometric study of the effect of the Glaukos trabecular microbypass stent on aqueous humor dynamics. Invest Ophthalmol Vis Sci. 2010;51:3327–3332. doi: 10.1167/iovs.09-3972. [DOI] [PubMed] [Google Scholar]
- 33.Craven ER, Katz LJ, Wells JM, Giamporcaro JE. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38:1339–1345. doi: 10.1016/j.jcrs.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 34.Fea AM, Belda JI, Rekas M, et al. Prospective unmasked randomized evaluation of the iStent inject® versus two ocular hypotensive agents in patients with primary open-angle glaucoma. Clin Ophthalmol. 2014;8:875–882. doi: 10.2147/OPTH.S59932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fea AM, Consolandi G, Zola M, et al. Micro-bypass implantation for primary open-angle glaucoma combined with phacoemulsification: 4-year follow-up. J Ophthalmol. 2015; 4. Article ID 795357. 10.1155/2015/795357. [DOI] [PMC free article] [PubMed]
- 36.Katz JL, Erb C, Guillamet AC, et al. Prospective, randomized study of one, two, or three trabecular bypass stents in open-angle glaucoma subjects on topical hypotensive medication. Clin Ophthalmol. 2015;9:2313–2320. doi: 10.2147/OPTH.S96695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vold SD, Voskanyan L, Tetz M, et al. Newly diagnosed primary open-angle glaucoma randomized to 2 trabecular bypass stents or prostaglandin: outcomes through 36 months. Ophthalmol Ther. 2016;5:161–172. doi: 10.1007/s40123-016-0065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfeiffer N, Garcia-Feijoo J, Martinez-De-La-Casa JM, et al. A randomized trial of a Schlemm’s canal microstent with phacoemulsification for reducing intraocular pressure in open-angle glaucoma. Ophthalmology. 2015;122:1283–1293. doi: 10.1016/j.ophtha.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 39.Vold S, Ahmed IIK, Craven ER, et al. Two-year COMPASS trial results: supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology. 2016;123:2103–2112. doi: 10.1016/j.ophtha.2016.06.032. [DOI] [PubMed] [Google Scholar]
- 40.Kersten-Gomez I, Dick H. First results of the innovative minimal-invasive glaucoma surgery technique: the AqueSys Aquecentesis procedure. Eur Soc Cataract Refractive Surgeons At: Glaucoma II; 2012. [Google Scholar]
- 41.Gonnermann J, Bertelmann E, Pahlitzsch M, Torun N, Klamann MKJ. Contralateral eye comparison study in MICS & MIGS: Trabectome® vs iStent inject®. Graefe’s Arch Clin Exp Ophthalmol. 2016;255(2):359–365. doi: 10.1007/s00417-016-3514-8. [DOI] [PubMed] [Google Scholar]
- 42.Khan M, Saheb H, Neelakantan A, et al. Efficacy and safety of combined cataract surgery with 2 trabecular microbypass stents versus ab interno trabeculotomy. J Cataract Refract Surg. 2015;41:1716–1724. doi: 10.1016/j.jcrs.2014.12.061. [DOI] [PubMed] [Google Scholar]
- 43.Kurji K, Rudnisky CJ, Rayat JS, et al. Phaco-trabectome versus phaco-iStent in patients with open-angle glaucoma. Can J Ophthalmol. 2015;52(1):99–106. doi: 10.1016/j.jcjo.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 44.Belovay GW, Naqi A, Chan BJ, Rateb M, Ahmed IIK. Using multiple trabecular micro-bypass stents in cataract patients to treat open-angle glaucoma. J Cataract Refract Surg. 2012;38:1911–1917. doi: 10.1016/j.jcrs.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 45.Tan SZ, Au L. Manchester iStent study: 3-year results and cost analysis. Eye (Lond) 2016 doi: 10.1038/eye.2016.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Höh H, Grisanti S, Grisanti S, Rau M, Ianchulev S. Two-year clinical experience with the CyPass micro-stent: safety and surgical outcomes of a novel supraciliary micro-stent. Klin Monbl Augenheilkd. 2014;231:377–381. doi: 10.1055/s-0034-1368214. [DOI] [PubMed] [Google Scholar]
- 47.Höh H, Vold SD, Ahmed IK, et al. Initial clinical experience with the CyPass micro-stent. J Glaucoma. 2016;25:106–112. doi: 10.1097/IJG.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 48.Fea AM, Ahmed IIK, Lavia C, et al. Hydrus microstent compared to selective laser trabeculoplasty in primary open angle glaucoma: one year results. Clin Exp Ophthalmol. 2016;45(2):120–127. doi: 10.1111/ceo.12805. [DOI] [PubMed] [Google Scholar]
- 49.Lenzhofer M, Kersten-Gomez IS, Sheybani A, et al. Four-year follow-up results after transscleral glaucoma gel stent implantation in a prospective multicentre trial. ASCRS—ASOA Symposium and Congress; 2016.
- 50.Sheybani A, Reitsamer H, Ahmed II. Fluid dynamics of a novel micro-fistula implant for the surgical treatment of glaucoma. Invest Ophthalmol Vis Sci. 2015;56(8):4789–4795. doi: 10.1167/iovs.15-16625. [DOI] [PubMed] [Google Scholar]
- 51.Sheybani A, Lenzhofer M, Hohensinn M, Reitsamer H, Ahmed IIK. Phacoemulsification combined with a new ab interno gel stent to treat open-angle glaucoma: pilot study. J Cataract Refract Surg. 2015;41:1905–1909. doi: 10.1016/j.jcrs.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 52.Gandolfi SA, Ungaro N, Ghirardini S, Tardini MG, Mora P. Comparison of surgical outcomes between canaloplasty and Schlemm’s canal scaffold at 24 months’ follow-up. J Ophthalmol. 2016; 5. Article ID 3410469. 10.1155/2016/3410469. [DOI] [PMC free article] [PubMed]
- 53.Le K, Saheb H. iStent trabecular micro-bypass stent for open-angle glaucoma. Clin Ophthalmol. 2014;8:1937–1945. doi: 10.2147/OPTH.S45920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vandewalle E, Zeyen T, Stalmans I. The iStent trabecular micro-bypass stent: a case series. Bull Soc Belge Ophtalmol. 2009;311:23–29. [PubMed] [Google Scholar]
- 55.XEN® gel stent. https://hcp.xengelstent.com/. Accessed Aug 2017.
- 56.Kerr NM, Wang J, Barton K. Minimally invasive glaucoma surgery as primary stand-alone surgery for glaucoma. Clin Exp Ophthalmol. 2016;45(4):393–400. doi: 10.1111/ceo.12888. [DOI] [PubMed] [Google Scholar]
- 57.Page RD, Johnson SJ. Minimally invasive glaucoma surgeries. In: Topics in cateract surgery. Avid Science; 2016. p.2–45.
- 58.Iordanous Y, Kent JS, Hutnik CM, Malvankar-Mehta MS. Projected cost comparison of trabectome, iStent, and endoscopic cyclophotocoagulation versus glaucoma medication in the Ontario Health Insurance Plan. J Glaucoma. 2013;23:112–118. doi: 10.1097/IJG.0b013e31829d9bc7. [DOI] [PubMed] [Google Scholar]
- 59.Olsen J, Berdeaux G, Skov J. Glaucoma costs in Denmark in treatment naive patients. Acta Ophthalmol. 2013;91:25–31. doi: 10.1111/j.1755-3768.2011.02212.x. [DOI] [PubMed] [Google Scholar]
- 60.Rahman MQ, Beard SM, Discombe R, Sharma R, Montgomery DMI. Direct healthcare costs of glaucoma treatment. Br J Ophthalmol. 2013;97:720–724. doi: 10.1136/bjophthalmol-2012-302525. [DOI] [PubMed] [Google Scholar]
- 61.Kobelt G, Texier-Richard B, Buchholz P, et al. Treatment of glaucoma in clinical practice. J Glaucoma. 2010;19:199–206. doi: 10.1097/IJG.0b013e3181af31d6. [DOI] [PubMed] [Google Scholar]
- 62.Kaplan RI, De Moraes CG, Cioffi GA, Al-Aswad LA, Blumberg DM. Comparative cost-effectiveness of the Baerveldt implant, trabeculectomy with mitomycin, and medical treatment. JAMA Ophthalmol. 2015;133:560–567. doi: 10.1001/jamaophthalmol.2015.44. [DOI] [PubMed] [Google Scholar]
- 63.Orme M, Collins S, Loftus J. Long-term medical management of primary open-angle glaucoma and ocular hypertension in the UK. J Glaucoma. 2012;21:433–449. doi: 10.1097/IJG.0b013e31821dac2a. [DOI] [PubMed] [Google Scholar]
- 64.Stein J, Kim D, Peck W, Gianetti S, Hutton D. Cost-effectiveness of medications compared with laser trabeculoplasty in patients with newly diagnosed open-angle glaucoma. Arch Ophthalmol. 2012;130:497. doi: 10.1001/archophthalmol.2012.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Gestel A, Severens JL, Webers CAB, Beckers HJM, Jansonius NM, Schouten JSAG. Modeling complex treatment strategies: construction and validation of a discrete event simulation model for glaucoma. Value Health. 2010;13:359–367. doi: 10.1111/j.1524-4733.2009.00678.x. [DOI] [PubMed] [Google Scholar]
- 66.Sharma A, Jofre-Bonet M, Panca M, Lawrenson JG, Murdoch I. An economic comparison of hospital-based and community-based glaucoma clinics. Eye. 2012;26:967–971. doi: 10.1038/eye.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holtzer-Goor KM, van Sprundel E, Lemij HG, Plochg T, Klazinga NS, Koopmanschap MA. Cost-effectiveness of monitoring glaucoma patients in shared care: an economic evaluation alongside a randomized controlled trial. BMC Health Serv Res. 2010;10:312. doi: 10.1186/1472-6963-10-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee P, Walt J, Doyle J, et al. A multicenter, retrospective pilot study of resource use and costs associated with severity of disease in glaucoma. Arch Ophthalmol. 2006;124:12–19. doi: 10.1001/archopht.124.1.12. [DOI] [PubMed] [Google Scholar]
- 69.Traverso CE, Walt JG, Kelly SP, et al. Direct costs of glaucoma and severity of the disease: a multinational long term study of resource utilisation in Europe. Br J Ophthalmol. 2005;89:1245–1249. doi: 10.1136/bjo.2005.067355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Denis P, Lafuma A, Berdeaux G. Medical predictive factors of glaucoma treatment costs. J Glaucoma. 2004;13:283–290. doi: 10.1097/00061198-200408000-00005. [DOI] [PubMed] [Google Scholar]
- 71.Stein JD, Niziol LM, Musch DC, et al. Longitudinal trends in resource use in an incident cohort of open-angle glaucoma patients: resource use in open-angle glaucoma. Am J Ophthalmol. 2012;154(452–459):e452. doi: 10.1016/j.ajo.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Connor AJ, Fraser SG. Glaucoma prescribing trends in England 2000 to 2012. Eye. 2014;28:863–869. doi: 10.1038/eye.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.NICE . CG85: Glaucoma diagnosis and management. London: National Institute for Health and Care Excellence; 2009. [Google Scholar]
- 74.Saeed AM. Comparative study between trabeculectomy with photodynamic therapy (BCECF-AM) and trabeculectomy with antimetabolite (MMC) in the treatment of primary open angle glaucoma. Clin Ophthalmol. 2012;6:1651–1664. doi: 10.2147/OPTH.S29909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matlach J, Dhillon C, Hain J, Schlunck G, Grehn F, Klink T. Trabeculectomy versus canaloplasty (TVC study) in the treatment of patients with open-angle glaucoma: a prospective randomized clinical trial. Acta Ophthalmol. 2015;93:753–761. doi: 10.1111/aos.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lewis R, Reitsamer H. The Xen procedure: 1-year results of an ab-interno gelatin stent along with cataract surgery for the treatment of glaucoma. ASCRS—ASOA Symposium and Congress; 2016.
- 77.Rekas M, Lewczuk K, Jablonska J, Rudowicz J. Two year follow-up data with a soft and permanent, minimally-invasive ab-interno subconjunctival implant in open-angle glaucoma subjects. Eur Soc Cataract Refractive Surgeons. 2014:P291.
- 78.Eriksson A, Burcharth J, Rosenberg J. Animal derived products may conflict with religious patients’ beliefs. BMC Med Ethics. 2013;14:48. doi: 10.1186/1472-6939-14-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hopes M, Broadway D. Preservative-free treatment in glaucoma is a sensible and realistic aim for the future. Eur Ophthalmic Rev. 2010;4(1):23–28. doi: 10.17925/EOR.2010.04.01.23. [DOI] [Google Scholar]
- 80.Azuara-Blanco A, Katz LJ. Dysfunctional filtering blebs. Surv Ophthalmol. 1998;43:93–126. doi: 10.1016/S0039-6257(98)00025-3. [DOI] [PubMed] [Google Scholar]
- 81.Emi K, Pederson JE, Toris CB. Hydrostatic pressure of the suprachoroidal space. Invest Ophthalmol Vis Sci. 1989;30:233–238. [PubMed] [Google Scholar]
- 82.Cunliffe I. Suprachoroidal haemorrhage. Eye (Lond) 1998;12:755–756. doi: 10.1038/eye.1998.197. [DOI] [PubMed] [Google Scholar]
- 83.Gigon A, Shaarawy T. The suprachoroidal route in glaucoma surgery. J Curr Glaucoma Pract. 2016;10(1):13–20. doi: 10.5005/jp-journals-10008-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ansari E. An update on implants for minimally invasive glaucoma surgery (MIGS) Ophthalmol Ther. 2017;6:1–9. doi: 10.1007/s40123-017-0077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen DZ, Sng CCA. Safety and efficacy of microinvasive glaucoma surgery. J Ophthalmol. 2017; 13. Article ID 3182935. 10.1155/2017/3182935. [DOI] [PMC free article] [PubMed]
- 86.Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12(8):e0183142. doi: 10.1371/journal.pone.0183142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pillunat LE, Erb C, Junemann AG, Kimmich F. Micro-invasive glaucoma surgery (MIGS): a review of surgical procedures using stents. Clin Ophthalmol. 2017;11:1583–1600. doi: 10.2147/OPTH.S135316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yep T, Patel V, Slejko JF, Devine B. Comparing total and disease specific healthcare costs for glaucoma patients before and after their index diagnosis: a retrospective claims database analysis. Value Health. 2015;18:A181. doi: 10.1016/j.jval.2015.03.1047. [DOI] [Google Scholar]
- 89.Pasquale LR, Dolgitser M, Wentzloff JN, et al. Health care charges for patients with ocular hypertension or primary open-angle glaucoma. Ophthalmology. 2008;115(4):633–638. doi: 10.1016/j.ophtha.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 90.Lindblom B, Nordmann JP, Sellem E, et al. A multicentre, retrospective study of resource utilization and costs associated with glaucoma management in France and Sweden. Acta Ophthalmol Scand. 2006;84:74–83. doi: 10.1111/j.1600-0420.2005.00560.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.