Abstract
Background
Development of arterial dissection is thought to be an important key factor for bailout stenting in femoropopliteal disease. We aimed to evaluate the difference in dissection rate and outcomes between the treatment group with rotational atherectomy and without it.
Methods
From January 2011 to October 2016, we compared the angiography after balloon angioplasty (BA) of de-novo, femoropopliteal, steno-occlusive lesions whether they were treated by rotational atherectomy prior to the BA or not. Fifty-nine lesions (8 occlusions; 3 involving popliteal segment; lesion length: 86.3 ± 66.8 mm) in 44 patients (29 males; mean age 66.9 ± 9.7 years) were enrolled for this review.
Results
Forty-two lesions were treated using rotational atherectomy, prior to BA while 17 were recanalized firstly by BA. Clinical and lesion characteristics were not different between the groups. However, the rate of significant arterial dissection (type C to F) was lower in the atherectomy group (88.2% vs. 42.9%; P = 0.001). In multivariate analysis, use of the atherectomy device was the only risk factor for prevention of development of significant dissection (P = 0.013; OR = 0.12; 95% CI: 0.025–0.642). Patients were treated either by the angioplasty alone, drug coated balloon or stent insertion. There was lower trend in target vessel revascularization and primary patency toward the atherectomy group (low rank P = 0.108 and 0.166), however secondary patency was significantly better (low rank P = 0.001).
Conclusions
Rotational atherectomy before BA reduced the rate of significant dissection and therefore, might be a valuable option for minimizing need of bailout stenting.
Keywords: Arterial dissection, Atherectomy, Femoropopliteal disease, Peripheral arterial disease
1. Introduction
In treating peripheral arterial diseases (PAD), stent placement to restore and maintain the patency is a viable treatment option and its results are superior compared to plain balloon angioplasty (BA) in terms of long-term patency and target-vessel revascularization (TVR).[1],[2] However, patency rate using stent for the femoropopliteal (FP) arterial disease was still disappointing despite the continuous remarkable improvement of stent technology.[3] Stent fatigue and fracture problem of implanted stents for the younger patients and longer requirement of antiplatelet therapy for elderly patients might be the important problems which could not be ignored. Consequently, there was a need for novel treatment option with better treatment outcome compared to stent angioplasty. Recently, drug coated balloon (DCB) have been introduced with very promising results that work without vascular irritation with additional anti-proliferative effect directly towards the vessels. However, bailout stenting rates were reported as high as approximately 60%[4] and most of them required stenting because of dissection. Recently, favorable outcome data was shown with several studies using atherectomy with reduction in bailout stenting rate.[5] Atherectomy before BA in FP segment was believed to be an adequate procedure for DCB era; however, the reason why it could reduce the rate of bailout stenting is not well known. We hypothesized that the types and severity of dissections after BA would be different and would affect the outcomes between the group with atherectomy and without atherectomy. Therefore, we conducted a retrospective comparison of our real clinical data.
2. Methods
2.1. Patient population and review parameters
From January 2011 to October 2016, consecutive patients diagnosed with and treated by endovascular procedures for FP steno-occlusive disease were analyzed retrospectively. The procedures were eligible for this study purpose if they were treated for de-novo FP steno-occlusive disease. The exclusion criteria were (1) intervention for aortoiliac, below the knee or multi-level peripheral arterial disease; (2) absence or incongruity of images after final balloon angioplasty; and (3) intentionally treated by the subintimal wire technique. A total of 59 lesions (8 occlusions; 3 involving popliteal segment; lesion length: 86.3 ± 66.8 mm) in 44 patients (29 males; mean age 66.9 ± 9.7) were enrolled for this review resulting in the comparison of 17 lesions treated without atherectomy and 42 with atherectomy. The institutional review board of the University of Connecticut Health Center approved the study and waived the requirement for informed consent for this retrospective study.
2.2. Intervention and atherectomy devices
Percutaneous transluminal balloon angioplasty (PTA) procedure was performed by the conventional treatment method in both groups under dual antiplatelet treatment (81–325 mg/day of aspirin and 75 mg/day of clopidogrel or 200 mg/day of cilostazol) and intra-arterial/venous heparin administration. After inserting a 6 French sheath retrogradely from contralateral limb or antegradely from ipsilateral limb at the common femoral artery, a 0.035 coated guidewire or 0.014/0.018 stiff guidewire was used to penetrate the steno-occlusive lesions by intraluminal wiring. BA was first performed after successful wire introduction in the group without atherectomy, while atherectomy procedure preceded the BA in the group with atherectomy. Three different types of atherectomy devices were utilized before BA in atherectomy group. The majority of lesions (36 lesion; 85.7%) were treated by orbital Diamondback 360° atherectomy system (CSI, St. Paul, MN, USA) and the burr sizes of this devices were 1.25 mm in 1 (2.8%), 1.5 mm in 9 (25%), and 2 mm in 26 lesions (72.2%). Four lesions (11.1%) were treated by the Phoenix atherectomy system (Volcano Corp. San Diego, CA, USA). Two lesions (5.6%) were treated by JetStream athetectomy system (Boston Scientific, Marlborough, MA, USA). Atherectomy was performed according to the protocol for the respective device. BA was similarly performed in both groups either by single balloon size or by the gradually increasing size of balloon by the physician's discretion. After performing over 2 min of balloon inflation, digital subtraction angiography (DSA) or conventional cine angiography was performed in order to make the next treatment strategy. Thus, with the angiography images after the BA, we compared the features and types of dissections in each group. Decision of BA alone or implantation of stent was conducted after the post balloon angiographic results by each physician's discretion.
2.3. Endpoint determination and clinical follow-up
Arterial dissection was classified followed by the National Heart, Lung, and Blood Institute (NHLBI) classification system for coronary intimal tears.[6] We provided the term “dissection with subintimal space filling” for type C to F dissection and we considered it as a significant dissection.[7] Two experienced peripheral interventionists separately classified type of dissection and later reached a consensus for the classification. Because the current study also enrolled the patients and lesions previously for the introduction of DCB, we considered a possible indication for stent implantation as the presence of > 30% recoil or dissection having persistent filling defect (type E, F) after the prolonged balloon inflation. Loss of patency was defined as either a drop in the ankle brachial index by ≥ 0.15, or a luminal narrowing on CT or angiography by ≥ 50%.[8] A significant (≥ 50%) restenosis was diagnosed using Duplex ultrasound, if a peak velocity ≥ 200 cm/s or a lesion velocity/adjacent segment velocity ratio of 2.0 was present.[9] A loss of primary patency was defined as symptom driven restenosis or the need for an additional target vessel (FP segment) revascularization procedure. Secondary patency was defined as patency of the target vessel after treatment of a re-occlusion of the index lesion.
2.4. Definitions and statistical analysis
Chronic kidney disease was defined as a glomerular filtration rate < 60 mL/min per 1.73 m2 for ≥ 3 months. Coronary artery disease was defined as any history of percutaneous coronary intervention or bypass surgery. Lesion calcification was classified followed by peripheral artery calcification scoring system,[10] where 0 to 1 was designated as none to minimal, 2 to 3 as mild to moderate, and 4 as severe calcifications.
Data are expressed as mean ± SD for continuous variables and as counts for categorical variables. Comparisons involving clinical, angiographic and procedure characteristics were performed using the Fisher exact test for categorical variables and Mann-Whitney U test for continuous variables. Odds ratio (OR) and 95% confidence interval (CI) were calculated with univariate logistic regression to correlate patient and angiographic or procedural with development of dissection having subintimal space filling. Variables with a P value ≤ 0.2 in the univariate model or known to be significantly associated were entered into a multivariate logistic regression model to calculate independent effects on significant dissection. The target vessel revascularization, primary and secondary patency rates during 1 year of follow-up were displayed with Kaplan-Meier event-free survival curves. A value of P ≥ 0.05 was defined statistically insignificant. All statistical calculations were performed with SPSS 18.0 software (SPSS Inc., Chicago, Ill, USA).
3. Results
Patient's age was varied from 49 years to 86 years (mean age 67.1 ± 9.4) and was not different in each group. A 52.9% of the patients in the non-atherectomy group and a 50.0% of the atherectomy group had age equal or more than 65 years. Younger patients (< 65 years) had trend to have less calcification (calcium score 0 = 37.9% in young vs. 20.0% in elderly; P = 0.158), while no difference to have moderate or severe calcification between young and elderly patients (P = 0.36 in moderate and P = 0.506 in severe calcification).
As shown in Table 1, baseline patients' clinical characteristics were not significantly different between the groups except for the number of occlusions. More occluded lesions were treated without atherectomy (29.4% vs. 7.1%; P = 0.037). However, in contrast, the populations having less than minimal calcification had a higher trend toward the group without atherectomy (76.5% vs. 50%; P = 0.062).
Table 1. Patients' clinical characteristics.
| Without atherectomy, n = 17 | Atherectomy, n = 42 | P-value | |
| Clinical variables | |||
| Age, yrs | 66.9 ± 10.9 | 67.1 ± 9.4 | 0.950 |
| Age over 65 | 9 (52.9%) | 21 (50.0%) | > 0.99 |
| Male | 11 (64.7%) | 27 (64.3%) | 0.976 |
| BMI | 27.3 ± 5.6 | 29.2 ± 7.3 | 0.367 |
| Diabetes | 6 (37.5%) | 17 (44.7%) | 0.623 |
| Hypertension | 14 (87.5%) | 27 (71.7%) | 0.197 |
| Smoking | 4 (25%) | 8 (21.1%) | 0.734 |
| Dyslipidemia | 12 (75%) | 30 (78.9%) | 0.734 |
| CAD | 8 (50%) | 23 (60.5%) | 0.475 |
| CKD | 3 (18.8%) | 7 (18.4%) | > 0.99 |
| CVA | 3 (18.8%) | 3 (7.9%) | 0.346 |
| Rutherford stage | |||
| 2 | 6 (35.3%) | 16 (38.1%) | 0.840 |
| 3 | 10 (62.5%) | 22 (52.4%) | 0.653 |
| 4 | 1 (6.3%) | 3 (7.9%) | > 0.99 |
| 5 | 0 | 1 (2.6%) | > 0.99 |
| Lesion character | |||
| TASC II class | |||
| A | 11 (64.7%) | 30 (71.4%) | 0.612 |
| B | 2 (11.8%) | 10 (23.8%) | 0.478 |
| C | 1 (5.9%) | 0 | 0.288 |
| D | 3 (17.6%) | 2 (4.8%) | 0.138 |
| Occlusion | 5 (29.4%) | 3 (7.1%) | 0.037 |
| Lesion calcification | |||
| None to minimal | 13 (76.5%) | 21 (50%) | 0.062 |
| Moderate | 2 (11.8%) | 12 (28.6%) | 0.310 |
| Severe | 2 (11.8%) | 9 (21.4%) | 0.484 |
| Lesion location | |||
| Focal lesion | |||
| Proximal | 2 (11.8%) | 3 (7.1%) | 0.450 |
| Mid | 5 (29.4%) | 14 (33.3%) | 0.770 |
| Distal | 4 (23.5%) | 16 (38.1%) | 0.284 |
| Popliteal segment | 1 (5.9%) | 2 (4.8%) | > 0.99 |
| Diffuse lesion | 5 (29.4%) | 7 (16.7%) |
Data are presented as mean ± SD or n (%). BMI: body mass index; CAD: coronary artery disease; CKD: chronic kidney disease; CVA: cerebrovascular disease; TASC: the trans-atlantic inter-society consensus document on management of peripheral arterial disease.
Table 2 showed angiographic lesion characteristics and procedural details. Lesion length (104.1 ± 74.2 vs. 83.3 ± 63.6 mm; P = 0.284) and minimal luminal diameters (0.80 ± 0.80 vs. 0.84 ± 0.68 mm; P = 0.845) were similar between two groups. Dissection of various types developed in both groups. Less than type A dissection developed in 26.2% of atherectomy group, however, there was no less than type A dissection in group without atherectomy (P = 0.024). Similarly, significant dissection (dissection with subintimal space filling; type C to F) was more in the group without atherectomy, while more than half of lesions had insignificant dissection when it was first treated by atherectomy (88.2% vs. 42.0%; P = 0.001). The atherectomy group had a significantly lower incidence of stent implantation (provisional stenting) compared with the non-atherectomy group (23.5 vs. 2.4%; P = 0.021).
Table 2. Lesion characteristics and procedural parameters.
| Without atherectomy, n = 17 | Atherectomy n = 42 | P-value | |
| Lesion character | |||
| Lesion length, mm | 104.1 ± 74.2 | 83.3 ± 63.6 | 0.284 |
| Reference diameter, mm | 5.05 ± 0.82 | 4.92 ± 0.67 | 0.533 |
| MLD, mm | 0.80 ± 0.80 | 0.84 ± 0.68 | 0.845 |
| Diameter stenosis | 83.5% ± 15.8% | 82.7% ± 13.1% | 0.852 |
| Dissection type | |||
| Less than type A | 0 | 11 (26.2%) | 0.024 |
| B | 2 (11.8%) | 13 (31.0%) | 0.190 |
| C | 9 (52.9%) | 12 (28.6%) | 0.077 |
| D | 3 (17.6%) | 5 (11.9%) | 0.678 |
| E | 1 (5.9%) | 1 (2.4%) | 0.497 |
| F | 2 (11.8%) | 0 | 0.079 |
| Dissection with subintimal space filling (type C-F dissection) | 15 (88.2%) | 18 (42.9%) | 0.001 |
| Recoil (> 20) after balloon | 7 (41.2%) | 8 (19.0%) | 0.103 |
| Possible indication for bail-out stenting (> 30% recoil or type E, F dissection) | 4 (23.5%) | 1 (2.4%) | 0.021 |
| Gradual ballooning | 9 (52.9%) | 23 (54.8%) | 0.899 |
| Dimeter of balloon (max), mm | |||
| 3 | 2 (11.8%) | 0 | 0.079 |
| 4 | 2 (11.8%) | 6 (14.3%) | > 0.99 |
| 5 | 1 (29.4%) | 3 (38.1%) | 0.528 |
| 6 | 8 (47.1%) | 2 (42.9%) | 0.768 |
| 7 | 0 | 2 (4.8%) | > 0.99 |
| Balloon ratio (balloon/reference) | 1.04 ± 0.31 | 1.1 ± 0.18 | 0.477 |
| Use of oversizing balloon | 11 (64.7%) | 35 (83.3%) | 0.166 |
| Final treatment for lesion | |||
| Plain angioplasty | 1 (5.9%) | 16 (38.1%) | 0.013 |
| DCB | 2 (11.8%) | 14 (33.3%) | 0.115 |
| Stent | 14 (82.4%) | 12 (28.6%) | < 0.001 |
Data are presented as mean ± SD or n (%). DCB: drug-coated balloon; MLD: minimal lesion diameter.
Table 3 identifies the univariate and multivariate analysis to the development of significant dissection. Among the possible factors for developing significant dissection, include occlusion, use of the oversized balloon and the use of the atherectomy devices. These factors meet a criterion (P > 0.2) for multivariate analysis. Finally, only the use of the atherectomy device was an independent predictor for development of significant dissection (P = 0.013; OR = 0.12; 95% CI: 0.025–0.642).
Table 3. Univariate and multivariate analysis for risk of significant dissection.
| P value | Odd ratio | 95% CI | |
|
Univariate analysis | |||
| Age | 0.236 | 0.968 | 0.91–1.02 |
| Diabetes | 0.319 | 1.758 | 0.57–5.33 |
| Occlusion | 0.084 | 6.731 | 0.77–58.7 |
| MLD | 0.896 | 0.953 | 0.46–1.97 |
| Minimal calcium | 0.294 | 1.750 | 0.61–4.98 |
| Severe calcium | 0.918 | 0.933 | 0.25–3.48 |
| Gradual ballooning | 0.637 | 0.779 | 0.27–2.19 |
| Balloon to reference vessel ratio | 0.242 | 0.244 | 0.023–2.59 |
| Oversize ballooning | 0.095 | 0.30 | 0.073–1.23 |
| Use of atherectomy device | 0.005 | 0.10 | 0.020–0.494 |
| Multivariate analysis | |||
| Occlusion | 0.292 | 3.53 | 0.33–36.8 |
| Oversize ballooning | 0.287 | 0.42 | 0.09–2.04 |
| Use of atherectomy device | 0.013 | 0.12 | 0.025-0.642 |
MLD: minimal lesion diameter.
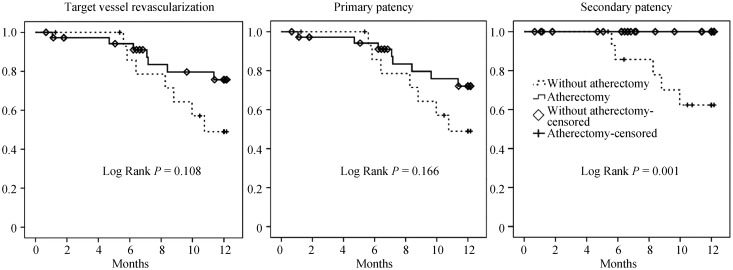
Clinically, there are 14 events during the one year follow-up period (Table 4; Figure 1). There was no procedure-related adverse event or death during the one year observation period. However, one in BA and two in the DCB with atherectomy group underwent TVR. There were 11 TVR in stent implantation without a significant difference in both groups, however occlusion was significantly more in the group without atherectomy [5 lesions out of 14 (35.7%) in the group without atherectomy; P = 0.039]. Although each groups' population were treated differently by the plain BA, DCB or stent implantation, there was a lower tendency toward the atherectomy group of TVR (Log rank P = 0.108) and primary patency (Log rank P = 0.166) during one year of follow-up period. Importantly, secondary patency was significantly better in the atherectomy group (no occlusion in atherectomy group during one year follow-up; Log rank P = 0.001).
Table 4. Patient's events within a year after index procedure.
| Atherectomy | Plain angioplasty (n = 17) |
DCB (n = 16) |
Stent implantation (n = 26) |
||||||
| No (n = 1) | Yes (n = 16) | P | No (n = 2) | Yes (n = 14) | P | No (n = 14) | Yes (n = 16) | P | |
| Death | 0 | 0 | 0 | 0 | 0 | 0 | |||
| TVR | 0 | 1 | > 0.99 | 0 | 2 | > 0.99 | 7 | 4 | 0.302 |
| TV-occlusion | 0 | 0 | 0 | 0 | 5 | 0 | 0.039 | ||
DCB: drug coated balloon; TV: target-vessel; TVR: target-vessel revascularization.
Figure 1. The Kaplan-Meier's curves for target vessel revascularization, primary and secondary patency within a year after index procedure.
4. Discussion
The core findings of the current investigation are that atherectomy prior to BA reduced development of significant dissection and thus, it could affect the outcome by preventing development of the occlusion.
4.1. Study design and patient selection
Although durability or long-term patency were the targeted end-point in the majority of comparison studies for PAD, it was difficult to achieve a difference from the results of the studies of an atherectomy device. Atherectomy is not regarded as a single definite procedure for recanalization or luminal gain and is usually combined with BA, DCB or stent implantation. Thus, no previous study was successful in revealing better outcomes of atherectomy than other treatment options. Therefore, we aimed to focus on a definite role and product from the atherectomy procedure. We set a development of significant dissection after the BA as primary end-point because several previous studies indicated a reduction in development of dissection or recoil in atherectomy procedures.[7],[11],[12] To differentiate from other studies, we tried to make a fair comparison between with-atherectomy and without-atherectomy groups. Importantly, sub-intimally treated long occlusions were not enrolled for this comparison, since atherectomy is usually not indicated or significantly associated with poorer outcomes.[13]
4.2. Treatment option for FP lesions
Although there was remarkable increase of stent technology, angioplasty using metallic stent has generally been suboptimal and frequently requiring re-intervention. Various treatment options were developed and DCB has been highlighted for eliminating stent related problems. Many physicians advocate the “no stent left behind” policy, especially for primary lesions or for short lesions. This policy is important to apply for joint (flexion area) lesion interventions or for the relatively young patients in terms of longevity of stent. On the other hand, elderly patients with bleeding tendency need to treat by the short duration of dual antiplatelet therapy for minimize the risk of major or fatal bleeding. Importantly, an occurrence of a flow-limiting dissection after conventional PTA could be a critical factor for failure of this strategy. Indeed, the rate of severe dissection was reported as high as 60%[4] and bailout stenting was performed in 11%–27% of the previous population.[4],[14]
4.3. Atherectomy devices for FP lesions
Theoretically, the atherectomy device can decrease intimal calcifications and high burden plaques and thus, may facilitate drug delivery by removing the barrier for DCB procedure. Additionally, atherectomy allows BA with low pressure to the wall of arteries, reducing the barotrauma or overstretch from high pressure ballooning.[15] Various atherectomy systems are available in many countries, however due to the complexity of mechanism of action in atherectomy devices, it can roughly be classified as the following: (1) laser atherectomy; (2) directional atherectomy; and (3) rotational atherectomy. Although atherectomy device have been introduced for quite some time,[16] definite favorable outcome results from comparison studies or indications for certain lesion characteristics are not clearly understood. From our research, we compared the results of rotational atherectomy from BA alone. Three types of rotational atherectomy were enrolled in our study purpose: (1) orbital diamondback 360° atherectomy system (CSI, St. Paul, MN, USA).This system uniquely consists of eccentric, diamond-grit-coated, crowns which create larger luminal gain than the size of the burr using centrifugal force as rotates. With this unique principle, some authors classified it as an independent category from rotational atherectomy. Recently, in a randomized trial for the infrapopliteal lesion intervention, OAS showed better results in terms of fewer dissections and a lower need for bailout stenting.[7] Additionally, luminal expansion without having a major dissection by the modification of superficial calcium was achieved in the intravascular ultrasound (IVUS) study. (2) Phoenix atherectomy systems (Volcano Corp. San Diego, CA, USA).This system has cutting blades at the distal tip and the plaque can be shaved and directly removed by the screw architecture inside the catheter. The debulked material removed into the catheter and thus, minimizes the chance of distal embolization. (3) JetStream atherectomy system (Boston Scientific, Marlborough, MA, USA). This atherectomy device uniquely has an aspiration capability and it can actively remove atherosclerotic debris or thrombus during the procedure.[17] It has showed significant lumen gain with calcium modification in the moderate to severe calcified lesions and decreased the amount of vessel dissection.[12]
4.4. Arterial dissections and its risk factors
The NHLBI classification system[6] was used for our study and almost every previous study classified dissection using this system. Generally, persistence contrast filling outside of the lumen which represented as type E and F was considered as indication for bailout stenting. Because of the introduction of DCB took place during the study period, we also used a term of possible indication of stenting when there presented more than type E dissection or more than 30% of recoil. However, we also used the term of a significant dissection if there was an extraluminal contrast filling or a presence of subintimal space filling (from type C to F dissection). Importantly, over 20% patients treated solely by BA needed bailout stenting in our registry. In contrast of that, only 2.4% of lesions treated by concomitant atherectomy procedure were classified as a possible indication for bailout stenting. Over half of the patients in each group were treated by gradually increased size of balloons (more than two sizes of the balloons) in order to prevent direct barotrauma. However, any benefit from gradual ballooning was not visible in risk analysis for dissection. Neither the occlusion of the vessel at the presentation nor the usage of the oversizing balloons predicted development of dissection. Interestingly, severe calcification is generally regarded as a high risk factor for development of significant dissection after ballooning, however it was not a risk factor in this study. One study in the literature indicated that a mild calcified lesion which comprised of fibrotic, soft plaques had a higher rate of total dissection, compared with moderate to severe calcified lesions.[18] It should be highlighted that only atherectomy procedures prior to the balloon angioplasty reduced the rate of development of significant dissection or bailout stenting.
4.5. Limitations
The primary limitations of this study are its retrospective design and the small numbers of patients in each group. Additionally, due to the introduction of DCB in the middle of the study period, the interventionists in our hospital incorporated this treatment modality into their practice. Outcomes are mainly affected according to the use of DCB or stent. However we could not compare the outcome difference in each group by this treatment option due to the small study population. To overcome these weaknesses, we exclusively included the lesion treated by the intraluminal wire technique for equal comparison between plain BA and concomitant atherectomy procedure.
4.6. Conclusion
Rotational atherectomy before BA reduced the rate of significant dissection and therefore might be a valuable option for minimizing need of bailout stenting. It needs to be more highlighted in DCB era.
Acknowledgments
The authors report no financial relationships or conflicts of interest regarding the content herein.
References
- 1.Laird JR, Katzen BT, Scheinert D, et al. Nitinol stent implantation vs. balloon angioplasty for lesions in the superficial femoral and proximal popliteal arteries of patients with claudication: three-year follow-up from the RESILIENT randomized trial. J Endovasc Ther. 2012;19:1–9. doi: 10.1583/11-3627.1. [DOI] [PubMed] [Google Scholar]
- 2.Acin F, de Haro J, Bleda S, Varela C, Esparza L. Primary nitinol stenting in femoropopliteal occlusive disease: a meta-analysis of randomized controlled trials. J Endovasc Ther. 2012;19:585–595. doi: 10.1583/JEVT-12-3898R.1. [DOI] [PubMed] [Google Scholar]
- 3.Sabeti S, Schillinger M, Amighi J, et al. Primary patency of femoropopliteal arteries treated with nitinol versus stainless steel self-expanding stents: propensity score-adjusted analysis. Radiology. 2004;232:516–521. doi: 10.1148/radiol.2322031345. [DOI] [PubMed] [Google Scholar]
- 4.Werk M, Albrecht T, Meyer DR, et al. Paclitaxel-coated balloons reduce restenosis after femoro-popliteal angioplasty: evidence from the randomized PACIFIER trial. Circ Cardiovasc Interv. 2012;5:831–840. doi: 10.1161/CIRCINTERVENTIONS.112.971630. [DOI] [PubMed] [Google Scholar]
- 5.Foley TR, Cotter RP, Kokkinidis DG, et al. Mid-term outcomes of orbital atherectomy combined with drug-coated balloon angioplasty for treatment of femoropopliteal disease. Catheter Cardiovasc Interv. 2017;89:1078–1085. doi: 10.1002/ccd.26984. [DOI] [PubMed] [Google Scholar]
- 6.Dorros G, Cowley MJ, Simpson J, et al. Percutaneous transluminal coronary angioplasty: report of complications from the National Heart, Lung, and Blood Institute PTCA Registry. Circulation. 1983;67:723–730. doi: 10.1161/01.cir.67.4.723. [DOI] [PubMed] [Google Scholar]
- 7.Shammas NW, Lam R, Mustapha J, et al. Comparison of orbital atherectomy plus balloon angioplasty vs. balloon angioplasty alone in patients with critical limb ischemia: results of the CALCIUM 360 randomized pilot trial. J Endovasc Ther. 2012;19:480–488. doi: 10.1583/JEVT-12-3815MR.1. [DOI] [PubMed] [Google Scholar]
- 8.Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–538. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 9.Kohler TR, Nance DR, Cramer MM, et al. Duplex scanning for diagnosis of aortoiliac and femoropopliteal disease: a prospective study. Circulation. 1987;76:1074–1080. doi: 10.1161/01.cir.76.5.1074. [DOI] [PubMed] [Google Scholar]
- 10.Okuno S, Iida O, Shiraki T, et al. Impact of calcification on clinical outcomes after endovascular therapy for superficial femoral artery disease: assessment using the peripheral artery calcification scoring system. J Endovasc Ther. 2016;23:731–737. doi: 10.1177/1526602816656612. [DOI] [PubMed] [Google Scholar]
- 11.Minko P, Buecker A, Jaeger S, Katoh M. Three-year results after directional atherectomy of calcified stenotic lesions of the superficial femoral artery. Cardiovasc Intervent Radiol. 2014;37:1165–1170. doi: 10.1007/s00270-014-0884-3. [DOI] [PubMed] [Google Scholar]
- 12.Maehara A, Mintz GS, Shimshak TM, et al. Intravascular ultrasound evaluation of JETSTREAM atherectomy removal of superficial calcium in peripheral arteries. EuroIntervention. 2015;11:96–103. doi: 10.4244/EIJV11I1A17. [DOI] [PubMed] [Google Scholar]
- 13.Indes JE, Shah HJ, Jonker FH, et al. Subintimal angioplasty is superior to SilverHawk atherectomy for the treatment of occlusive lesions of the lower extremities. J Endovasc Ther. 2010;17:243–250. doi: 10.1583/09-2821.1. [DOI] [PubMed] [Google Scholar]
- 14.Tepe G, Zeller T, Albrecht T, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med. 2008;358:689–699. doi: 10.1056/NEJMoa0706356. [DOI] [PubMed] [Google Scholar]
- 15.Lyden SP, Shimshak TM. Contemporary endovascular treatment for disease of the superficial femoral and popliteal arteries: an integrated device-based strategy. J Endovasc Ther. 2006;13(Suppl 2):II41–II51. doi: 10.1177/15266028060130S208. [DOI] [PubMed] [Google Scholar]
- 16.Smalling RW, Cassidy DB, Schmidt WA, et al. Effects of rotational atherectomy in normal canine coronary and diseased human cadaveric arteries: potential for plaque removal from distal, tortuous, and diffusely diseased vessels. Cathet Cardiovasc Diagn. 1991;24:300–307. doi: 10.1002/ccd.1810240418. [DOI] [PubMed] [Google Scholar]
- 17.Sixt S, Rastan A, Scheinert D, et al. The 1-year clinical impact of rotational aspiration atherectomy of infrainguinal lesions. Angiology. 2011;62:645–656. doi: 10.1177/0003319711403300. [DOI] [PubMed] [Google Scholar]
- 18.Adams GL, Das T, Lee MS, et al. Subanalysis of the CONFIRM registries: acute procedural outcomes in claudicant and critical limb ischemia patients with varying levels of calcification treated for peripheral arterial disease with orbital atherectomy. J Invasive Cardiol. 2015;27:516–520. [PubMed] [Google Scholar]