Abstract
Background
Heart failure is a significant problem leading to repeated hospitalizations. Telemonitoring and hemodynamic monitoring have demonstrated success in reducing hospitalization rates, but not all studies reported significant effects. The aim of this systematic review and meta-analysis is to examine the effectiveness of telemonitoring and wireless hemodynamic monitoring devices in reducing hospitalizations in heart failure.
Methods & Results
PubMed and Cochrane Library were searched up to 1st May 2017 for articles that investigated the effects of telemonitoring or hemodynamic monitoring on hospitalization rates in heart failure. In 31,501 patients (mean age: 68 ± 12 years; 61% male; follow-up 11 ± 8 months), telemonitoring reduced hospitalization rates with a HR of 0.73 (95% CI: 0.65–0.83; P < 0.0001) with significant heterogeneity (I2 = 94%). These effects were observed in the short-term (≤ 6 months: HR = 0.77, 95% CI: 0.65–0.89; P < 0.01) and long-term (≥ 12 months: HR = 0.73, 95% CI: 0.62–0.87; P < 0.0001). In 4831 patients (mean age 66 ± 18 years; 66% male; follow-up 13 ± 4 months), wireless hemodynamic monitoring also reduced hospitalization rates with a HR of 0.60 (95% CI: 0.53–0.69; P < 0.001) with significant heterogeneity (I2 = 64%).This reduction was observed both in the short-term (HR = 0.55, 95% CI: 0.45–0.68; P < 0.001; I2 = 72%) and long-term (HR = 0.64, 95% CI: 0.57–0.72; P < 0.001; I2 = 55%).
Conclusions
Telemonitoring and hemodynamic monitoring reduce hospitalization in both short- and long-term in heart failure patients.
Keywords: Heart failure, Hemodynamic monitoring, Hospitalization, Telemedicine, Telemonitoring
1. Introduction
Heart failure is characterized by structural abnormalities of left ventricular dysfunction and dilatation, a compensatory rise in systemic vascular resistance secondary to activation of neurohumoral pathways,[1] inflammation,[2] and metabolic adaptations to energy substrate utilization.[3] It is a major public health problem globally, causing significant mortality and morbidity and placing a significant burden on healthcare systems. Hospitalization rate, a measure of healthcare resource utilization, is estimated to be 20% at one month and 50% at 6 months.[4] A history of hospitalization is itself an independent predictor of long-term mortality. Therefore, measures to reduce hospitalization are likely beneficial in this patient population.[5]
Telemonitoring can be used to track patients' symptoms, adherence to medications and objective parameters such as blood pressure, heart rate, body weight and urine output.[6] However, the effectiveness of body weight monitoring has been disputed, as the largest randomized controlled trials to date failed to demonstrate a reduction in heart failure-related hospitalizations. The reasons behind this are complex, but can be partly explained by the fact that body weight and symptoms may not provide sufficient warning of impending decompensation of cardiac function.[7],[8] Patient data from implantable hemodynamic monitoring studies have shown that weight is not a good measure of filling pressures that may be important determinants of decompensation.[9] Moreover, hospitalization in heart failure may be related to not only abnormal physiological factors, but also social factors.[10]
In addition to tele-monitoring, recent interests have focused on the roles of implantable hemodynamic monitors. Three devices, CardioMEMS, Chronicle and HeartPOD are commercially available to monitor pulmonary arterial pressure, right ventricular pressure and left atrial pressure, respectively. Several meta-analyses have been performed on remote monitoring for heart failure. For example, in 2009, the impact of remote monitoring on mortality and hospitalization rates was examined.[11] Recently, two meta-analyses of randomized controlled trials were performed.[12],[13] This study complements these previous studies by providing an updated meta-analysis of both randomized controlled trials and observational studies on hospitalization rates.
2. Methods
2.1. Search strategy, inclusion and exclusion criteria
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.[14] It has been registered with PROSPERO (CRD42017073934). PubMed and Cochrane Library were searched up to 1st May 2017, with no language restriction, for studies that investigated the hospitalization rates in heart failure. The following search terms were used for PubMed and Cochrane Library: “telemonitoring heart failure hospitalization” and “hemodynamic monitoring heart failure hospitalization).
The following inclusion criteria were applied: (1) the design was a case-control, prospective or retrospective observational study or randomized controlled trial in humans, (2) patients with heart failure (both preserved and reduced ejection fraction included) were analyzed, (3) hospitalization rates, whether heart failure-specific, cardiovascular-related or all-cause, were reported or could be calculated from the published data; (3) and (4) hazard ratios (HRs) or relative risks (RRs) and their corresponding 95% CIs or data necessary to calculate these were available.
Quality assessment of case-control and cohort studies included in our meta-analysis was performed using the Newcastle–Ottawa Quality Assessment Scale (NOS) (Tables 1S and 2S for telemonitoring, Tables 3S and 4S for hemodynamic monitoring),[15] and of randomized controlled trials using the Jadad scale (Oxford quality scoring system) (Table 5S and 6S for telemonitoring and hemodynamic monitoring, respectively). The NOS evaluated the categories of study participant selection, comparability of the results, and quality of the outcomes. The following characteristics were assessed: (1) representativeness of the exposed cohort; (2) selection of the non-exposed cohort; (3) ascertainment of exposure; (4) demonstration that outcome of interest was not present at the start of study; (5) comparability of cohorts on the basis of the design or analysis; (6) assessment of outcomes; (7) follow-up period sufficiently long for outcomes to occur; and (8) adequacy of follow-up of cohorts. This scale varied from zero to nine stars, which indicated that studies were graded as poor quality if they met < 5 criteria, fair if they met 5 to 7 criteria, and good if they met > 8 criteria. The Jadad score assessed the quality by the following criteria of (1) randomization, (2) allocation concealment, (3) double blinding and (4) withdrawal and dropouts. The total score is 7, scores 1 to 3 indicate low quality and 4 to 7 high quality.
2.2. Data extraction and statistics
Data from the different studies were entered in pre-specified spreadsheet in Microsoft Excel. All potentially relevant reports were retrieved as complete manuscripts and assessed for compliance with the inclusion criteria. In this meta-analysis, the extracted data elements consisted of: (1) publication details: last name of first author, publication year and locations; (2) study design (cohort study or randomized controlled trial); (3) follow-up duration; (4) endpoints; (5) the quality score; and (6) the characteristics of the population including sample size, gender, age and number of subjects. Meta-analyses of observational studies are challenging due to differences in study designs and inherent biases. Two reviewers independently reviewed each included study and disagreements were resolved by adjudication with input from a third reviewer.
The endpoints for this meta-analysis were hospitalization rates. Where different types of hospitalization rates were reported, heart failure-specific rates were used preferentially, followed by cardiovascular-related hospitalization rates, and finally all-cause hospitalization rates. Multivariate adjusted hazard ratios (HRs) or relative risks (RRs) with 95% CI were extracted for each study. When values from multivariate analysis were not available, those from univariate analysis were used.
When HRs were not provided, they were calculated using raw data. The pooled adjusted risk estimates from each study as the HR values with 95% CI were presented. Different types of hospitalization rates were pooled together.
Heterogeneity between studies was determined using Cochran's Q, which is the weighted sum of squared differences between individual study effects and the pooled effect across studies, and the I2 statistic from the standard chi-square test, which is the percentage of the variability in effect estimates resulting from heterogeneity. I2 > 50% was considered to reflect significant statistical heterogeneity. A fixed effects model was used if I2 < 50%, otherwise the random-effects model using the inverse variance heterogeneity method was selected. To find the origin of the heterogeneity, sensitivity analysis excluding one study at a time was performed. Subgroup analyses based on time-points or type of telemonitoring or hemodynamic monitoring were performed. Short-term was defined as those occurring within 6 months, whereas long-term was defined as 12 months or longer. Where a study reported effective estimates at successive time points, the longer time point was used. Funnel plots, Begg and Mazumdar rank correlation test and Egger's test[16] were used to assess for possible publication bias.
3. Results
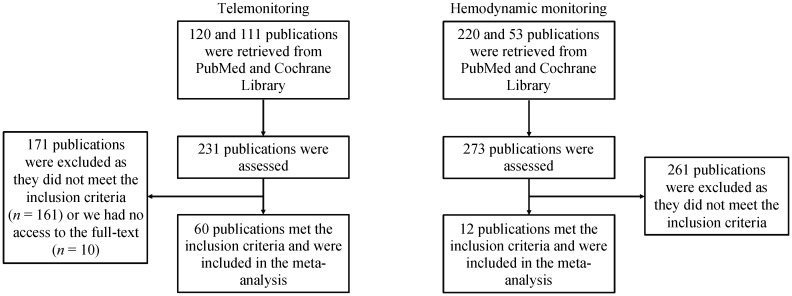
Figure 1 shows a flow diagram detailing the search strategy and study selection process. For telemonitoring, a total of 120 and 111 entries were retrieved from PubMed and Cochrane Library, with 60 articles included in our final meta-analysis.[6],[17]–[75] For hemodynamic monitoring, a total of 220 and 53 entries were retrieved from the same databases, with 12 articles included in our final meta-analysis.[4],[76]–[86]
Figure 1. A flow diagram detailing the search strategy and study selection process for this systematic review and meta-analysis on the effects of telemonitoring and hemodynamic monitoring on hospitalization rates in heart failure.
3.1. Telemonitoring
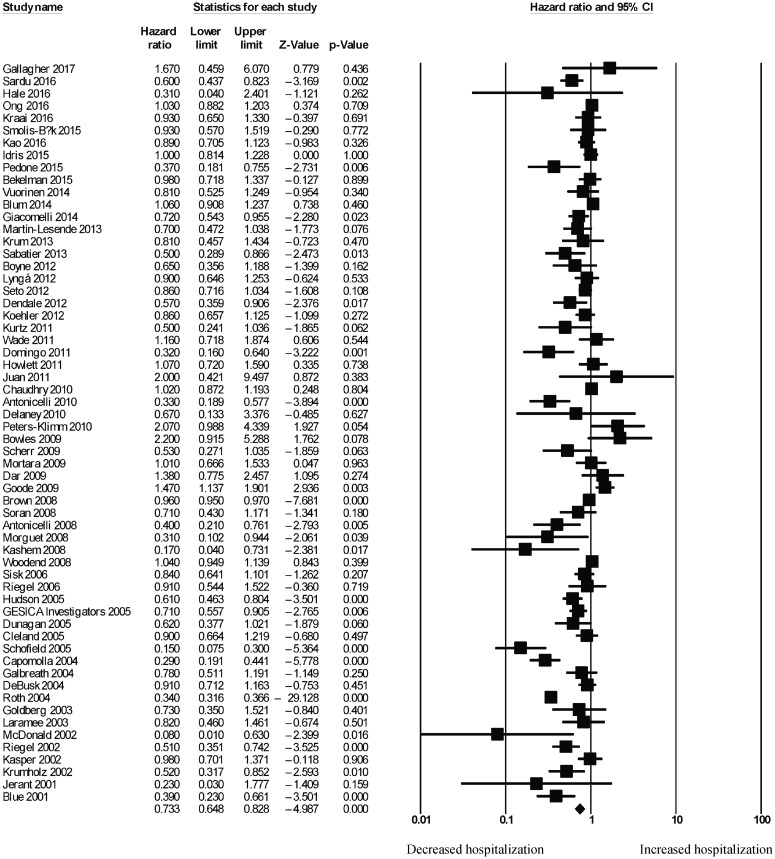
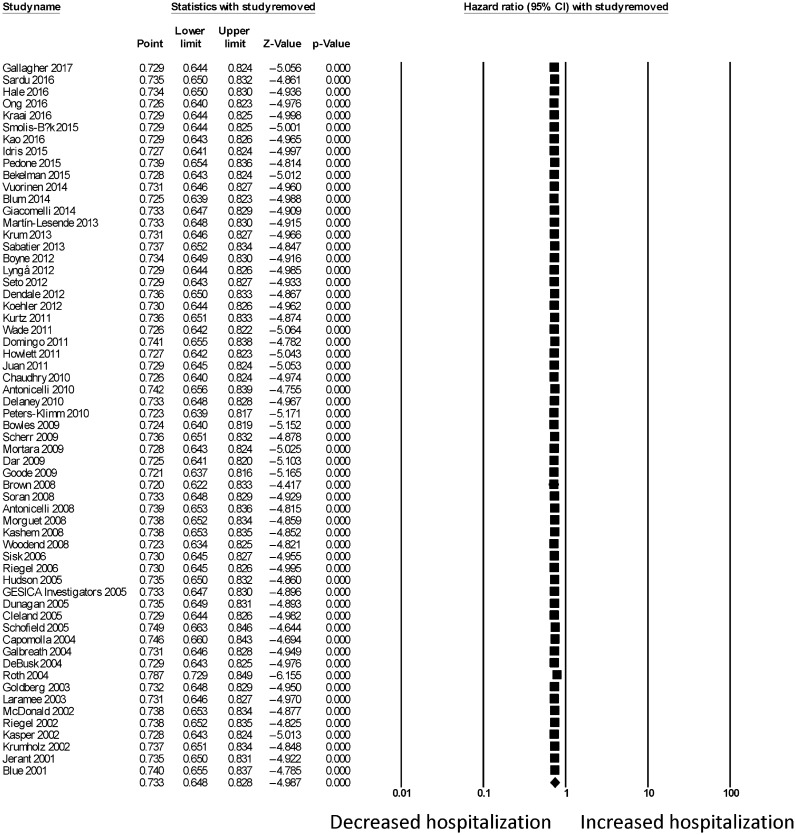
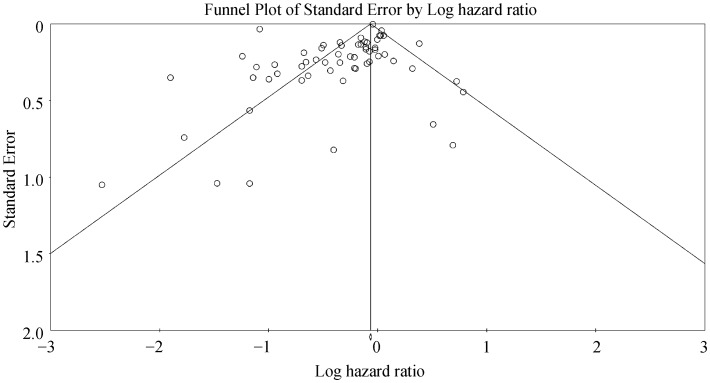
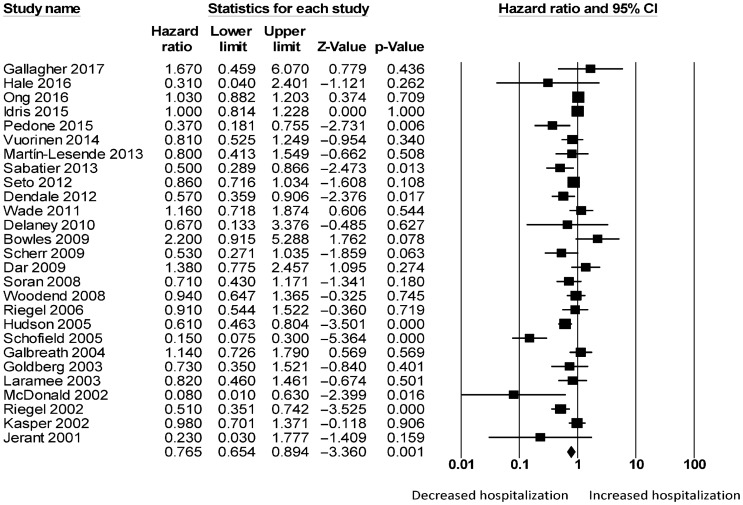
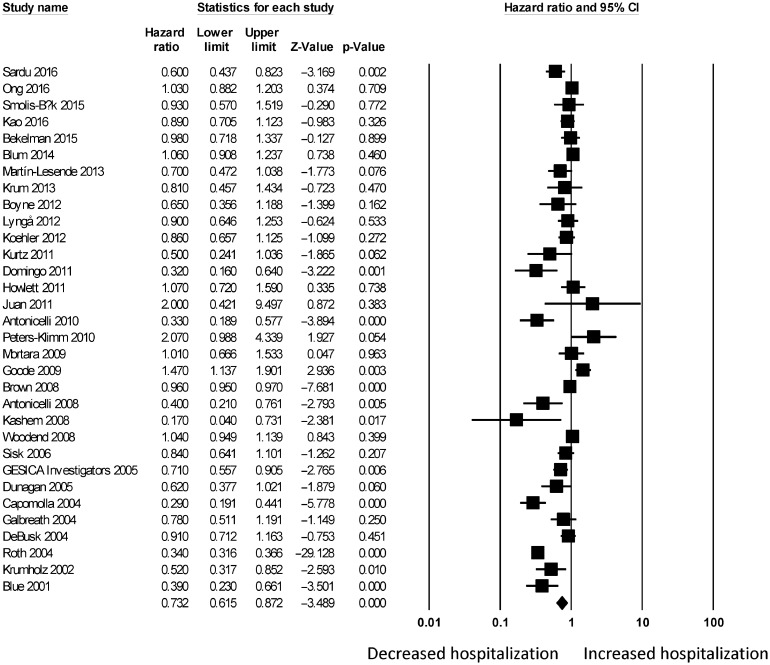
For telemonitoring, a total of 31,501 patients (mean age: 68 ± 12 years old; 61% male) were included. The baseline characteristics of these studies are listed in Table 1. Six were cohort studies and 55 were randomized controlled trials. The mean follow-up duration was 11 ± 8 months. Telemonitoring reduced hospitalization rates with a HR of 0.73 (95% CI: 0.65–0.83; P < 0.0001, Figure 2). The Cochran's Q value was greater than the degrees of freedom (994 vs. 59), suggesting the true effect size was different among the various studies. Moreover, I2 took a value of 94%, indicating the presence of significant heterogeneity. Sensitivity analysis by leaving out one study at a time did not significantly alter the pooled HR (Figure 1S). Funnel plot plotting standard errors or precision against the logarithms of the odds ratio are shown in Figures 2S and 3S, respectively. Begg and Mazumdar rank correlation suggested a significant publication bias (Kendal's Tau value = –0.2, P < 0.05); Egger's test demonstrated significant asymmetry (intercept: –1.4, t-value: 2.6; P < 0.05).
Table 1. Characteristics of the 60 studies on telemonitoring included in this meta-analysis.
| First author / Year | Study design | Sample size (n) | Age | SD | % Male | Ejection fraction, % | Endpoints | Follow-up (months) | Variables in multivariate model |
| Gallagher 2017 | RCT | 40 | 64 | 20 | 75 | 25 | All-cause, HF | 1 | (Univariate) |
| Sardu 2016 | RCT | 183 | 72 | 7 | 76 | < 35 | HF | 12 | Age, chronic kidney disease, hypercholesterolaemia, LVEF, NYHA class |
| Hale 2016 | RCT | 25 | 72 | 11 | 64 | - | All-cause, HF | 3 | (Univariate) |
| Ong 2016 | RCT | 1437 | 73 | - | 54 | 43 | All-cause | 3, 6 | Age, sex, race/ethnicity, insurance, comorbidities based on the Health Care Utilization Project methods, 6 year and quarter of enrollment, social isolation as measured by the Lubben Social Network Scale score, 31 and income level |
| Kraai 2016 | RCT | 177 | 69 | 16 | 37 | 27 | HF | 9 | (Univariate) |
| Smolis-Bąk 2015 | Cohort | 52 | 62 | 9 | 90 | 25 | All-cause | 18 | (Univariate) |
| Kao 2016 | Cohort | 1246 | 78 | 12 | 54 | - | All-cause | 36 | (Univariate) |
| Idris 2015 | RCT | 28 | 63 | - | 39 | 23 | Cardiac | 3, 6 | (Univariate) |
| Pedone 2015 | RCT | 90 | 80 | 7 | 39 | 46 | All-cause, HF | 6 | (Univariate) |
| Bekelman 2015 | RCT | 384 | 68 | 14 | 97 | - | All-cause | 12 | (Univariate) |
| Vuorinen 2014 | RCT | 94 | 58 | 17 | 83 | 28 | HF | 6 | (Univariate) |
| Blum 2014 | RCT | 203 | 73 | 13 | 71 | 29 | All-cause | 48 | Age, gender, practice region (RRMA), and baseline NYHA class |
| Giacomelli 2014 | RCT | 285 | 80 | - | 60 | - | All-cause | 9 | (Univariate) |
| Martín-Lesende 2013 | RCT | 58 | 81 | 8 | 59 | - | All-cause, cause-specific | 6, 12 | (Univariate) |
| Krum 2013 | RCT | 405 | 73 | 15 | 63 | 36 | All-cause, HF | 12 | Age, gender, practice region (RRMA), and baseline NYHA class |
| Sabatier 2013 | RCT | 90 | - | - | - | - | HF | 3 | (Univariate) |
| Boyne 2012 | RCT | 382 | 71 | 11 | 59 | 36 | All-cause, HF | 12 | Ischaemia, blood urea, haemoglobin level, heart rate, NYHA class, and systolic blood pressure |
| Lyngå 2012 | RCT | 319 | 73 | 10 | 75 | - | All-cause, cardiac | 12 | (Univariate) |
| Seto 2012 | RCT | 84 | 54 | 19 | 59 | 38 | All-cause | 6 | (Univariate) |
| Dendale 2012 | RCT | 160 | 76 | 10 | 65 | 35 | All-cause, HF | 6 | (Univariate) |
| Koehler 2012 | RCT | 670 | 67 | 15 | 86 | 267 | All-cause, cardiac, HF | 26 | (Univariate) |
| Kurtz 2011 | Cohort | 138 | 68 | 17 | 78 | 32 | HF | 12 | Age, state of residence, presence of various comorbid conditions, and prior cardiac events including coronary artery bypass surgery |
| Wade 2011 | RCT | 316 | 77 | 10 | 53 | - | All-cause, cardiac | 6 | (Univariate) |
| Domingo 2011 | RCT | 92 | 66 | 12 | 71 | 36 | Cardiac excluding HF, HF | 12 | (Univariate) |
| Howlett 2011 | RCT | 122 | 67 | - | 65 | 46 | All-cause | 12 | (Univariate) |
| Juan 2011 | Cohort | 120 | 76 | - | - | - | All-cause | 30 | (Univariate) |
| Chaudhry 2010 | RCT | 1653 | 61 | 16 | 58 | - | All-cause, HF | 9 | (Univariate) |
| Antonicelli 2010 | RCT | 57 | 78 | 7 | 58 | - | HF | 12 | (Univariate) |
| Delaney 2010 | RCT | 24 | 79 | 12 | 42 | - | All-cause, HF | 3 | (Univariate) |
| Peters-Klimm 2010 | RCT | 199 | 70 | 14 | 72 | - | All-cause, HF | 12 | (Univariate) |
| Bowles 2009 | RCT | 303 | 75 | 37 | - | HF | 2 | (Univariate) | |
| Scherr 2009 | RCT | 108 | 66 | 11 | 79 | 25 | All-cause | 6 | (Univariate) |
| Mortara 2009 | RCT | 461 | 60 | 17 | 86 | 29 | All-cause, HF | 12 | New York Heart Association class, β-blocker use at baseline, sex, and Na levels |
| Dar 2009 | RCT | 182 | 71 | 16 | 66 | - | All-cause, HF | 6 | (Univariate) |
| Goode 2009 | RCT | 201 | 70 | 11 | 70 | 24 | All-cause | 16 | (Univariate) |
| Brown 2008 | RCT | 14663 | - | - | - | - | All-cause | 12 | (Univariate) |
| Soran 2008 | RCT | 315 | 76 | 10 | 31 | 24 | All-cause, HF | 6 | New York Heart Association class, β-blocker use at baseline, sex, and Na levels |
| Antonicelli 2008 | RCT | 57 | 78 | 10 | 58 | 36 | HF | 12 | (Univariate) |
| Morguet 2008 | Case-control | 128 | 60 | 14 | 88 | 44 | All-cause, cardiac | 10 | (Univariate) |
| Kashem 2008 | RCT | 48 | 54 | 15 | 73 | 26 | All-cause, HF | 12 | (Univariate) |
| Woodend 2008 | RCT | 121 | 67 | 17 | 72 | - | All-cause, HF | 3, 12 | (Univariate) |
| Sisk 2006 | RCT | 406 | 59 | 19 | 54 | All-cause | 12 | (Univariate) | |
| Riegel 2006 | RCT | 134 | 72 | 11 | 46 | 43 | All-cause | 6 | (Univariate) |
| Hudson 2005 | Cohort | 91 | 74 | 11 | 53 | - | All-cause | 6 | (Univariate) |
| GESICA Investigators 2005 | RCT | 1518 | 65 | 13 | 71 | - | All-cause, cardiac, HF | 16 | NYHA class, age, baseline treatment, comorbidity, and systolic dysfunction |
| Dunagan 2005 | RCT | 151 | - | - | 47 | All-cause, HF | 12 | Severely impaired LV function, NYHA class, use of target or high doses of ACE inhibitor | |
| Cleland et al. (2005) | RCT | 253 | 67 | 16 | 53 | 25 | All-cause, cardiac, HF | 8 | Age, NT proBNP, body mass index, systolic and diastolic blood pressure, hemoglobin, sodium, urea, creatinine, NYHA functional classification, loop and potassium-sparing diuretics, ACE inhibitors, beta blockers |
| Schofield 2005 | Cohort | 73 | 67 | 11 | 99 | 23 | All-cause | 6 | (Univariate) |
| Capomolla 2004 | RCT | 133 | 57 | 10 | 47 | 29 | All-cause, cardiac, HF | 12 | (Univariate) |
| Galbreath 2004 | RCT | 1069 | 71 | 10 | 71 | 54 | All-cause, HF | 6, 18 | (Univariate) |
| DeBusk 2004 | RCT | 462 | 72 | 11 | 51 | - | All-cause, cardiac, HF | 12 | (Univariate) |
| Roth 2004 | Cohort | 118 | 74 | 9 | 69 | 24 | All-cause | 12 | (Univariate) |
| Goldberg 2003 | RCT | 208 | 59 | 15 | 68 | < 35 | All-cause, cardiac | 6 | (Univariate) |
| Laramee 2003 | RCT | 287 | 71 | 12 | 54 | - | All-cause, HF | 1.5 | (Univariate) |
| McDonald 2002 | RCT | 98 | 71 | 10 | 66 | 37 | HF | 3 | (Univariate) |
| Riegel 2002 | RCT | 358 | 72 | 12 | 49 | 43 | All-cause, HF | 3, 6 | (Univariate) |
| Kasper 2002 | RCT | 200 | 62 | 20 | 33 | 27 | HF | 6 | (Univariate) |
| Krumholz 2002 | RCT | 88 | 76 | 13 | 57 | 38 | All-cause, cardiac, HF | 12 | (Univariate) |
| Jerant 2001 | RCT | 25 | 70 | 16 | 48 | - | All-cause, HF | 2 | (Univariate) |
| Blue 2001 | RCT | 165 | 75 | 12 | 58 | - | All-cause, HF | 12 | (Univariate) |
ACE: angiotensin converting enzyme; HF: heart failure; LV: left ventricular; NT proBNP: N-terminal pro brain natriuretic peptide; RCT: randomized controlled trial.
Figure 2. Pooled hazard ratios for studies examining the effects of telemonitoring on hospitalization rates in heart failure.
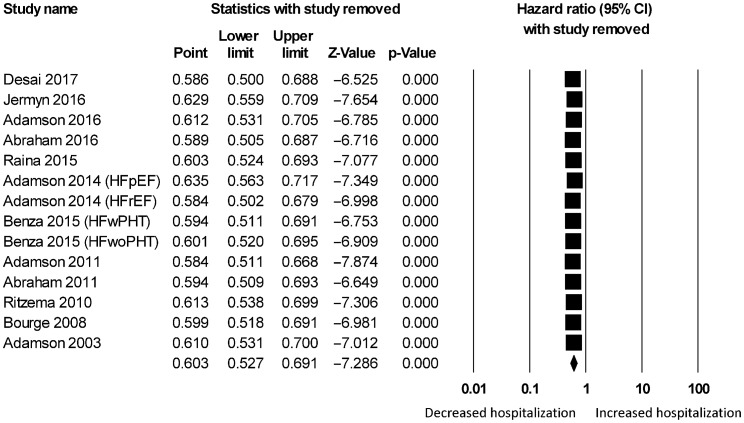
Figure 1S. Sensitivity analysis for hazard ratio on hospitalizations using telemonitoring.
Figure 2S. Funnel plot of standard error against the logarithm of hazard ratio for hospitalizations using telemonitoring.
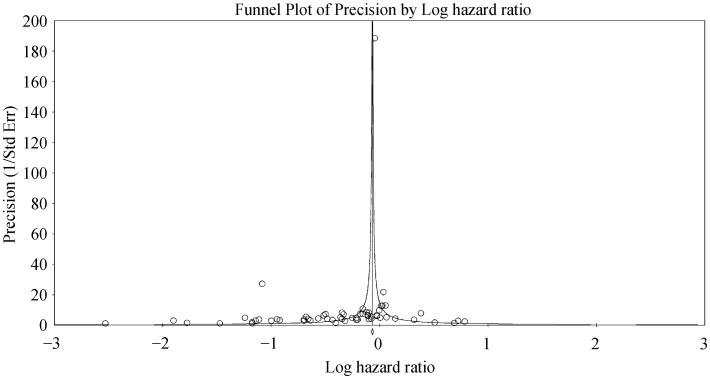
Figure 3S. Funnel plot of precision against the logarithm of hazard ratio for hospitalizations using telemonitoring.
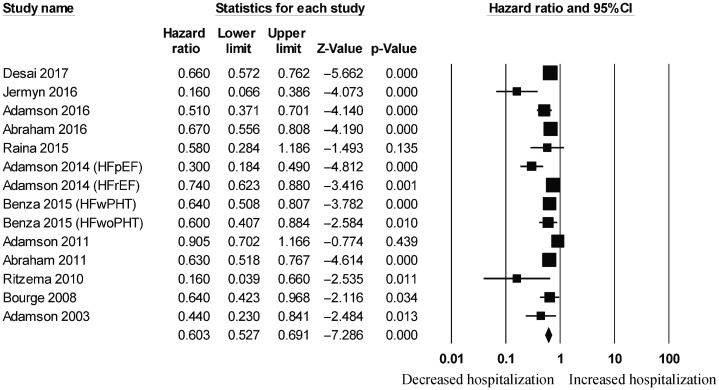
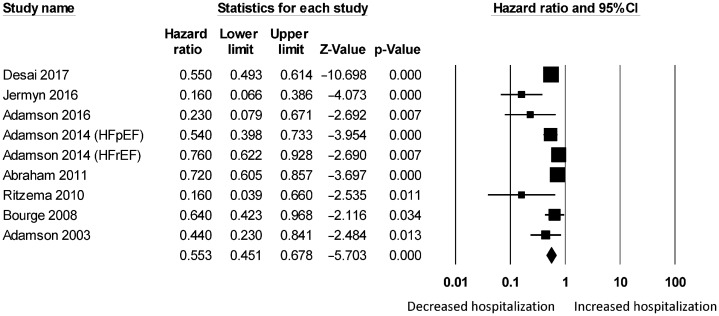
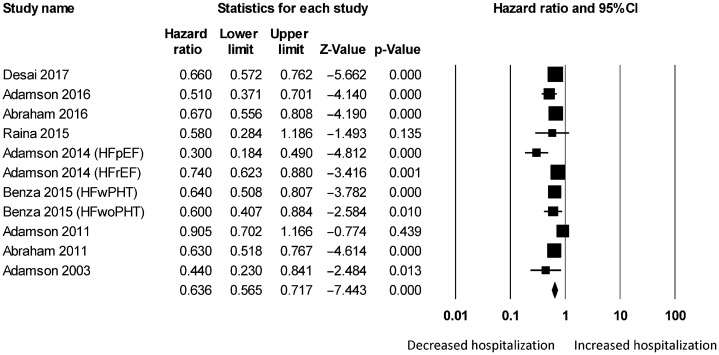
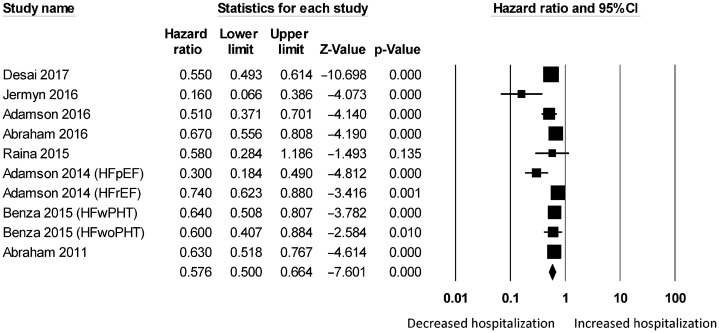
Figure 3. Pooled hazard ratios for studies examining the effects of hemodynamic monitoring on hospitalization rates in heart failure.
Because of the substantial heterogeneity present, we explored its possible origins. As we initially combined mortality assessed at different durations, univariate and multivariate HRs, and study design, the following subgroup analyses were performed. Firstly, we found that telemonitoring reduced hospitalization rates in the short-term (n = 27; ≤ 6 months; HR = 0.77, 95% CI: 0.65–0.89; P < 0.01; I2 = 67%; Figure 4S) and long-term (n = 32; ≥ 12 months: HR = 0.73, 95% CI: 0.62–0.87; P < 0.0001; I2 = 97%; Figure 5S). Secondly, subgroup analysis was performed for the type of HR. Meta-analysis of univariate HRs produced a pooled effect estimate of 0.94 (95% CI: 0.93–0.95; P < 0.0001) without significantly affecting heterogeneity (I2 = 95%, vs. 94% previously). By contrast, meta-analysis of multivariate HRs produced a similar pooled effect estimate of 0.91 (95% CI: 0.84–0.99; P < 0.05) whilst reducing I2 to 71%. Thirdly, subgroup analysis was performed for study design. Meta-analysis of randomized controlled trials (RCTs) yielded a pooled effect estimate of 0.96 (95% CI: 0.95–0.97; P < 0.0001) whilst reducing I2 to 72%. By contrast, meta-analysis of cohort studies yielded a significantly lower HR of 0.38 (95% CI: 0.36–0.41; P < 0.0001) whilst preserving I2 at 94%. Together, these findings suggest the duration over which mortality was assessed, type of HRs and study design to be possible sources of heterogeneity.
Figure 4S. Subgroup analysis for hazard ratio on short-term hospitalizations using telemonitoring.
Figure 5S. Subgroup analysis for hazard ratio on long-term hospitalizations using telemonitoring.
3.2. Hemodynamic monitoring
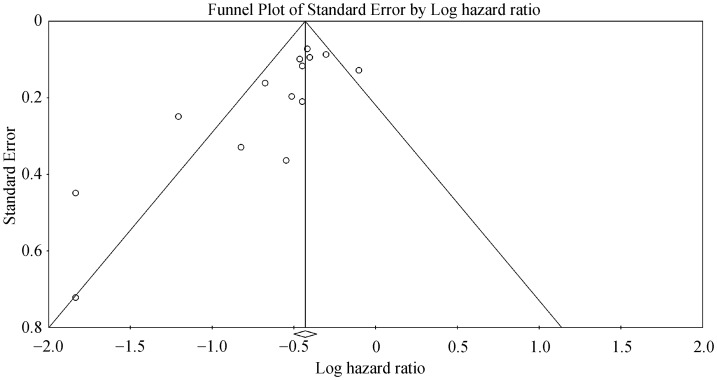
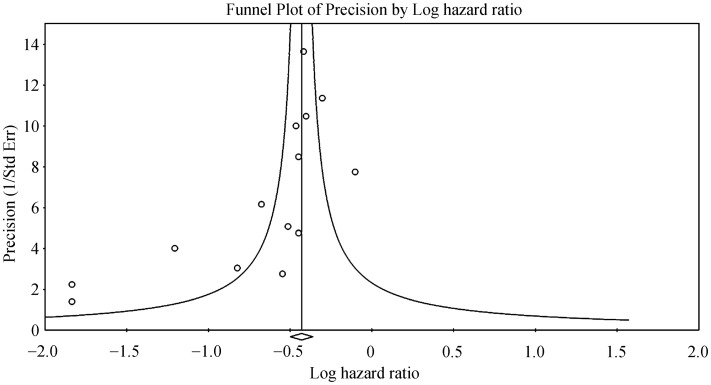
For wireless hemodynamic monitoring, a total of 4831 patients were included. The baseline characteristics of these studies are listed in Table 2. Four publications were cohort studies and eight publications were based on data from three randomized controlled trials (CHAMPION, COMPASS-HF and REDUCEhf). The mean follow-up duration was 13 ± 4 months. The mean age was 66 ± 18 years) of whom 66% were male. Wireless hemodynamic monitoring significantly reduced hospitalization rates with a HR of 0.60 (95% CI: 0.53–0.69; P < 0.001). The Cochran's Q value was greater than the degrees of freedom (36 vs. 13), suggesting the true effect size was different among the various studies. I2 took a value of 64%, indicating the presence of significant heterogeneity. Sensitivity analysis by leaving out one study at a time did not significantly alter the pooled HR (Figure 6S). Funnel plot plotting standard errors or precision against the logarithms of the odds ratio are shown in Figures 7S and 8S, respectively. Begg and Mazumdar rank correlation suggested a significant publication bias (Kendal's Tau value = –0.5, P < 0.05). Egger's test demonstrated significant asymmetry (intercept: –2.2, t-value = 3.2; P < 0.01).
Table 2. Characteristics of the 12 studies on hemodynamic monitoring included in this meta-analysis.
| First author/Year | Study design | Population | Type of hemodynamic monitoring | Sample size (n) | Age, yrs | SD | % Male | Ejection fraction, % | Endpoints | Follow-up (months) | Variables in multivariate model |
| Desai 2017 | Cohort | HF | Pulmonary arterial pressure | 1114 | 71 | 11 | 64 | - | All-cause, HF | 6 | (Univariate) |
| Jermyn 2016 | Cohort | HF | Pulmonary arterial pressure | 77 | - | - | - | - | HF | 12 | (Univariate) |
| Adamson 2016 | RCT | HF | Pulmonary arterial pressure | 245 | 73 | 8 | - | - | HF | 17 | (Univariate) |
| Abraham 2016 | RCT | HF | Pulmonary arterial pressure | 347 | 62 | 18 | - | - | All-cause, HF | 17 | (Univariate) |
| Raina 2015 | RCT | HF | Pulmonary arterial pressure | 537 | 62 | 18 | - | - | HF | 18 | (Univariate) |
| Adamson 2014 | RCT | HF with preserved ejection fraction | Pulmonary arterial pressure | 119 | 66 | 12 | 60 | 51 | HF | 18 | (Univariate) |
| HF with reduced ejection fraction | 66 | 60 | 13 | 76 | 23 | 18 | (Univariate) | ||||
| Benza 2015 | RCT | HF with pulmonary hypertension | Pulmonary arterial pressure | 314 | 62 | 13 | 72 | - | HF | 15 | (Univariate) |
| HF without pulmonary hypertension | 236 | 61 | 13 | 74 | - | HF | 15 | (Univariate) | |||
| Adamson 2011 | RCT | HF | Right ventricular pressure | 400 | 55 | 21 | 34 | 23 | All-cause, HF | 12 | (Univariate) |
| Abraham 2011 | RCT | HF | Pulmonary arterial pressure | 550 | 62 | 18 | 73 | 60 | HF | 6 | (Univariate) |
| Ritzema 2010 | Cohort | HF | Left atrial pressure | 40 | 66 | 10 | 78 | 32 | Combined HF hospitalization and all-cause mortality | 3 | (Univariate) |
| Bourge 2008 | RCT | HF | Right ventricular pressure | 274 | 58 | 19 | 65 | 33 | HF | 6 | (Univariate) |
| Adamson 2003 | Cohort | HF | Right ventricular pressure | 32 | 59 | 10 | 38 | 29 | HF | 17 | (Univariate) |
HF: heart failure; RCT: randomized controlled trial.
Figure 6S. Sensitivity analysis for hazard ratio on hospitalizations using heomdynamic monitoring.
Figure 7S. Funnel plot of standard error against the logarithm of hazard ratio for hospitalizations using heomdynamic monitoring.
Figure 8S. Funnel plot of precision against the logarithm of hazard ratio for hospitalizations using heomdynamic monitoring.
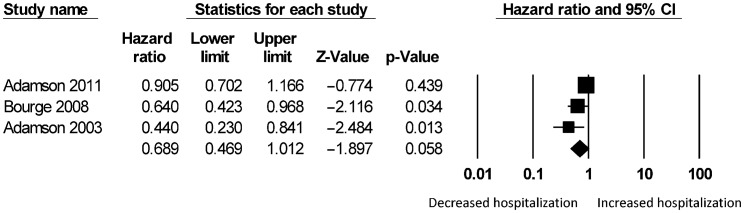
Significant reductions in hospitalization rates were observed in both short-term (HR: 0.55, 95% CI: 0.45–0.68; P < 0.001; I2 = 72%; Figure 9S) and long-term (HR: 0.64, 95% CI: 0.57–0.72; P < 0.001; I2 = 55%; Figure 10S). For the different types of hemodynamic devices, hospitalization rates were significantly reduced using pulmonary pressure monitoring (HR: 0.58, 95% CI: 0.50–0.66; P < 0.001; I2 = 67%; Figure 11S) or left atrial pressure monitoring (HR: 0.16, 95% CI: 0.04–0.68; P < 0.05). It was not possible to perform a meta-analysis for left atrial pressure monitoring because this was only assessed by one study. Right ventricular pressure monitoring tended to reduce hospitalization rates (HR: 0.69, 95% CI: 0.47–1.01; I2 = 61%; Supplementary Figure 12S) but this did not reach statistical significance (P = 0.058).
Figure 9S. Subgroup analysis for hazard ratio on short-term hospitalizations using heomdynamic monitoring.
Figure 10S. Subgroup analysis for hazard ratio on long-term hospitalizations using heomdynamic monitoring.
Figure 11S. Subgroup analysis for hazard ratio on long-term hospitalizations using pulmonary pressure monitoring.
Figure 12S. Subgroup analysis for hazard ratio on long-term hospitalizations using right ventricular pressure monitoring.
Table 1S. Quality ratings for included case-control studies using the Newcastle-Ottawa quality assessment scale for telemonitoring.
| Number | First Author | Selection (score) | Comparability (score) | Total Score | ||||||
| Case definition | Representative of cases | Selections of controls | Definition of controls | Comparability of cases and controls on the basis of the design or analysis | Ascertainment of exposure | Same method ascertainment participants | Nonresponse rate | |||
| 1 | Morguet 2008 | - | 1 | 1 | 1 | 2 | - | - | 1 | 6 |
Table 2S. Quality ratings for included cohort studies using the Newcastle-Ottawa quality assessment scale for telemonitoring.
| Number |
First Author |
Selection (score) |
Comparability (score) |
Exposure (score) |
Total Score |
|||||
| Representative of exposed cohort | Selections of non-exposed cohort | Assessment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Ascertainment of outcome | Was follow-up long enough for outcomes to occur? | Adequacy of follow up of cohorts | |||
| 1 | Kao 2016 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| 2 | Smolis-Bąk 2015 | 1 | 1 | 1 | 1 | 2 (age, comorbidities) | 1 | 1 | 1 | 9 |
| 3 | Kurtz 2011 | 1 | 1 | 1 | 1 | 2 (age, LVEF) | 1 | 1 | 1 | 9 |
| 4 | Hudson 2005 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| 5 | Schofield 2005 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| 6 | Roth 2004 | 1 | 1 | 1 | 1 | 2 | - | 1 | 1 | 8 |
LVEF: left ventricular ejection fraction.
Table 3S. Quality ratings for included case-control studies using the Newcastle-Ottawa quality assessment scale for hemodynamic monitoring.
| Number |
First Author |
Selection (score) |
Comparability (score) |
Total Score |
||||||
| Case definition | Representative of cases | Selections of controls | Definition of controls | Comparability of cases and controls on the basis of the design or analysis | Ascertainment of exposure | Same method ascertainment participants | Nonresponse rate | |||
| 1 | Jermyn 2016 | 1 | 1 | - | 1 | 2 | 1 | 1 | 1 | 8 |
| 2 | Abraham 2016 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| 3 | Raina 2015 | 1 | 1 | - | 1 | 2 | 1 | 1 | 1 | 8 |
| 4 | Benza 2015 | 1 | 1 | - | 1 | 2 | 1 | 1 | 1 | 8 |
| 5 | Abraham 2011 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
Table 4S. Quality ratings for included cohort studies using the Newcastle-Ottawa quality assessment scale for hemodynamic monitoring.
| Number |
First Author |
Selection (score) |
Comparability (score) |
Exposure (score) |
Total Score |
|||||
| Representative of exposed cohort | Selections of non-exposed cohort | Assessment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Ascertainment of outcome | Was follow-up long enough for outcomes to occur? | Adequacy of follow up of cohorts | |||
| 1 | Desai 2017 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| 2 | Ritzema 2010 | - | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 |
| 3 | Adamson 2003 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
Table 5S. Quality ratings for included randomized controlled trials using the Jadad quality assessment scale for telemonitoring.
| Number | Study | Randomization | Allocation concealment | Double blinding | Withdrawals and dropouts | Total score |
| 1 | Gallagher 2017 | 2 | 1 | 1 | 1 | 5 |
| 2 | Sardu 2016 | 2 | 2 | 2 | 0 | 6 |
| 3 | Hale 2016 | 1 | 0 | 0 | 1 | 2 |
| 4 | Ong 2016 | 2 | 2 | 2 | 1 | 7 |
| 5 | Kraai 2016 | 2 | 1 | 1 | 1 | 5 |
| 6 | Idris 2015 | 1 | 0 | 0 | 1 | 2 |
| 7 | Pedone 2015 | 1 | 1 | 2 | 1 | 5 |
| 8 | Bekelman 2015 | 2 | 0 | 0 | 1 | 3 |
| 9 | Vuorinen 2014 | 1 | 0 | 0 | 1 | 2 |
| 10 | Blum 2014 | 1 | 0 | 0 | 1 | 2 |
| 11 | Giacomelli 2014 | 1 | 0 | 0 | 1 | 2 |
| 12 | Martín-Lesende 2013 | 2 | 0 | 1 | 1 | 4 |
| 13 | Krum 2013 | 1 | 1 | 1 | 1 | 4 |
| 14 | Sabatier 2013 | 1 | 0 | 0 | 1 | 2 |
| 15 | Boyne 2012 | 2 | 0 | 2 | 1 | 5 |
| 16 | Lynga° 2012 | 1 | 1 | 2 | 1 | 5 |
| 17 | Seto 2012 | 2 | 2 | 2 | 1 | 7 |
| 18 | Dendale 2012 | 1 | 2 | 2 | 1 | 4 |
| 19 | Koehler 2012 | 2 | 1 | 1 | 1 | 5 |
| 20 | Wade 2011 | 1 | 0 | 0 | 1 | 2 |
| 21 | Domingo 2011 | 1 | 0 | 0 | 1 | 2 |
| 22 | Howlett 2011 | 1 | 0 | 0 | 1 | 2 |
| 23 | Chaudhry 2010 | 2 | 2 | 2 | 1 | 7 |
| 24 | Antonicelli 2010 | 1 | 0 | 0 | 1 | 2 |
| 25 | Delaney 2010 | 1 | 0 | 0 | 1 | 2 |
| 26 | Peters-Klimm 2010 | 2 | 2 | 2 | 1 | 7 |
| 27 | Bowles 2009 | 1 | 2 | 2 | 1 | 6 |
| 28 | Scherr 2009 | 1 | 0 | 2 | 1 | 4 |
| 29 | Mortara 2009 | 2 | 2 | 2 | 1 | 7 |
| 30 | Dar 2009 | 2 | 1 | 2 | 1 | 6 |
| 31 | Goode 2009 | 1 | 0 | 0 | 1 | 2 |
| 32 | Brown 2008 | 2 | 1 | 0 | 1 | 4 |
| 33 | Soran 2008 | 1 | 1 | 2 | 1 | 5 |
| 34 | Antonicelli 2008 | 1 | 0 | 0 | 1 | 2 |
| 35 | Kashem 2008 | 2 | 0 | 0 | 1 | 3 |
| 36 | Woodend 2008 | 1 | 0 | 0 | 1 | 2 |
| 37 | Sisk 2006 | 2 | 2 | 2 | 1 | 7 |
| 38 | Riegel 2006 | |||||
| 39 | GESICA Investigators 2005 | 1 | 0 | 0 | 1 | 2 |
| 40 | Dunagan 2005 | 2 | 0 | 0 | 1 | 3 |
| 41 | Cleland 2005 | 1 | 1 | 2 | 1 | 5 |
| 42 | Capomolla 2004 | 1 | 0 | 0 | 1 | 2 |
| 43 | Galbreath 2004 | 1 | 0 | 0 | 1 | 2 |
| 44 | DeBusk 2004 | 2 | 1 | 1 | 1 | 5 |
| 45 | Goldberg 2003 | 1 | 0 | 0 | 1 | 2 |
| 46 | Laramee 2003 | 2 | 1 | 2 | 1 | 6 |
| 47 | McDonald 2002 | 1 | 1 | 2 | 1 | 5 |
| 48 | Riegel 2002 | 1 | 2 | 2 | 1 | 6 |
| 49 | Kasper 2002 | 1 | 0 | 1 | 1 | 3 |
| 50 | Krumholz 2002 | 1 | 0 | 0 | 1 | 2 |
| 51 | Jerant 2001 | 2 | 2 | 0 | 1 | 5 |
| 52 | Blue 2001 | 1 | 1 | 1 | 1 | 4 |
Table 6S. Quality ratings for included randomized controlled trials using the Jadad quality assessment scale for hemodynamic monitoring.
| Number | Study | Randomization | Allocation concealment | Double blinding | Withdrawals and dropouts | Total score |
| 1 | Adamson 2016 | 1 | 1 | 1 | 1 | 4 |
| 2 | Adamson 2014 | 1 | 1 | 1 | 1 | 4 |
| 3 | Adamson 2011 | 1 | 1 | 1 | 1 | 4 |
| 4 | Bourge 2008 | 1 | 1 | 2 | 1 | 5 |
4. Discussion
This is a systematic review and meta-analysis of randomized controlled trials and real-world studies on the effects of remote patient monitoring on hospitalization rates in heart failure, complementing previous meta-analyses.[11]–[13] The main findings are the following: (1) hospitalization rates can be reduced by remote patient monitoring using either telemonitoring or hemodynamic monitoring by 26% (95% CI: 17%–35%) and 40% (95% CI: 31%–47%), respectively; (2) telemonitoring reduced hospitalization rates by 24% in the short-term (≤ 6 months) and 27% in the long-term (≥ 12 months); and (3) hemodynamic monitoring reduced hospitalization rates by 45% in the short-term and 37% in the long-term.
Telemonitoring is a broad term referring to the making telephone contact with patients to enquire about symptoms, adherence to pharmacotherapy, and obtain information on clinically important parameters such as heart rate, blood pressure, body weight and urine output. This in turn enables appropriate advice to be offered to patients.[17] The benefits of home monitoring systems on hospitalization are possibly due to its good potential for detecting early signs of decompensation and reinforcement of patient's self-care education, and are especially useful for those who needs extra support, such as older and more frail patients.[87],[88] Telemonitoring appears to have limited potential in early detection of worsening heart failure, but most effective when patient education toward medical adherence and patient self-care efficacy are reinforced. These different effects of telemonitoring could be attributable to the wide distribution or the disparate outcome of the effects on hospitalization, and to the heterogeneity observed. There are different vital signs that could be used to provide a warning for heart failure decompensation. These are heart rate, heart rate variability,[89] blood pressure, body weight and urine output.[6],[89]–[91] For example, increases in body weight can predict acute decompensation requiring hospitalization.[91] However, a study found that diastolic blood pressure, systolic blood pressure x heart rate and diastolic blood pressure x heart rate, but not heart rate or systolic blood pressure by itself, predicted 3-month major adverse cardiac events.[90]
Hemodynamic monitoring refers to the continuous measurement of cardiac chamber or vascular pressures. Three devices are available: CardioMEMS (pulmonary arterial pressure),[92] Chronicle (right ventricular pressure)[93] and HeartPOD (left atrial pressure).[94] The rationale behind hemodynamic monitoring is that increases in intracardiac and pulmonary arterial pressures were detectable several weeks prior to worsening of clinical symptoms and signs.[4],[9] Subgroup analyses were performed for the different hemodynamic parameter measured. The evidence for pulmonary artery pressure monitoring is the strongest, with a 42% reduction in hospitalization rates. Right ventricular pressure monitoring tended to reduce hospitalization rates by around 31% but this was not statistically significant. It was not possible to perform a meta-analysis for left atrial monitoring, as only one study has been published to date. Nevertheless The LAPTOP-HF trial is currently ongoing and when completed will provide important data for determining whether left atrial monitoring will similarly reduce hospitalization rates in heart failure.[95]
Theoretically, hemodynamic monitoring should reduce hospitalization rates to greater extents than usual care or telemonitoring if patients were offered appropriate advice to mitigate abnormal cardiac physiology, such as fluid overload or bradycardia, by altering medication regimens at home so that hospitalization would not be necessary. Our meta-analysis found that the risk reduction for hospitalization using hemodynamic monitoring was slightly higher at 40% compared to 27% using telemonitoring, but this was not significantly different. This meta-analysis provides data that less-invasive remote monitoring by telemedicine is equally effective as more invasive forms of hemodynamic monitoring. The former approach may be more cost-effective and yet able to prevent hospitalizations. Therefore, healthcare resources can be focused on the patients who do require hospital admission, who can be offered additional investigations such as quantification of blood biomarkers and echocardiography for guiding their management.[96],[97]
4.1. Limitations
There are some limitations of this study that must be recognized. Firstly, we had observed a substantial heterogeneity for the HRs for the effects of telemonitoring on hospitalization rates. In our study, hazard ratios of randomized controlled trials and cohort studies, which are different study designs, were initially pooled together. A recent Cochrane review showed that there were no significant difference in the effective estimates between observational studies and randomized controlled trials, suggesting that factors other than study design are responsible for differences in outcomes.[98] However, in our subgroup analysis, we found that the pooled HR was significantly lower for cohort studies when compared to the HR for RCT. Therefore, meta-analysis should combine the effect estimates separately based on trial design. Moreover, this subgroup analysis resulted in a reduction of I2 to 72% for RCTs, suggesting that this contributed to the heterogeneity observed. Other sources, as assessed by our subgroup analyses, were the duration over which mortality was assessed (short-term versus long-term mortality) and whether the HRs were univariate or multivariate HRs. Secondly, we detected significant bias using both Begg and Mazumdar rank correlation test and Egger's test, in that the reported HRs skewed towards reduced hospitalization by telemonitoring. In other words, fewer HRs were from the studies reporting a lack of effect on hospitalization. Therefore, this may represent publication bias in which only positive findings were published by the journals, with negative results possibly not published. Thirdly, there were only four cohort studies that assessed hemodynamic monitoring. As only three RCTs with a limited number of subjects were conducted, future RCTs are needed for different types of hemodynamic monitoring systems, especially left atrial pressure monitoring, for which the HR was only available in one study and it was therefore not possible to conduct a subgroup analysis for this system. Finally, there is a lack of studies that directly compare hemodynamic monitoring to telemonitoring, which needs to be investigated in the future, especially given the invasive nature of hemodynamic monitoring systems.
4.2. Conclusions
This meta-analysis demonstrates that both telemonitoring and hemodynamic monitoring are equally effective approaches to reduce hospitalization rates in heart failure. Telemonitoring should be used more widely, since it is less invasive than hemodynamic monitoring and may be more cost-effective. However, direct comparisons between these modes of monitoring are needed in the future.
Acknowledgments
There are no conflicts of interests to be declared.
References
- 1.Teerlink JR. Neurohumoral mechanisms in heart failure: a central role for the renin-angiotensin system. J Cardiovasc Pharmacol. 1996;27(Suppl 2):S1–S8. doi: 10.1097/00005344-199600002-00002. [DOI] [PubMed] [Google Scholar]
- 2.Tse G, Yan BP, Chan YW, Tian XY, Huang Y. Reactive oxygen species, endoplasmic reticulum stress and mitochondrial dysfunction: the link with cardiac arrhythmogenesis. Front Physiol. 2016;7:313. doi: 10.3389/fphys.2016.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 4.Ritzema J, Troughton R, Melton I, et al. Physician-directed patient self-management of left atrial pressure in advanced chronic heart failure. Circulation. 2010;121:1086–1095. doi: 10.1161/CIRCULATIONAHA.108.800490. [DOI] [PubMed] [Google Scholar]
- 5.Kommuri NVA, Koelling TM, Hummel SL. The impact of prior heart failure hospitalizations on long-term mortality differs by baseline risk of death. Am J Med. 2012;125:209.e9–.e15. doi: 10.1016/j.amjmed.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Antonicelli R, Testarmata P, Spazzafumo L, et al. Impact of telemonitoring at home on the management of elderly patients with congestive heart failure. J Telemed Telecare. 2008;14:300–305. doi: 10.1258/jtt.2008.071213. [DOI] [PubMed] [Google Scholar]
- 7.Desai AS. Home monitoring heart failure care does not improve patient outcomes: looking beyond telephone-based disease management. Circulation. 2012;125:828–836. doi: 10.1161/CIRCULATIONAHA.111.031179. [DOI] [PubMed] [Google Scholar]
- 8.Desai AS, Stevenson LW. Connecting the circle from home to heart-failure disease management. N Engl J Med. 2010;363:2364–2367. doi: 10.1056/NEJMe1011769. [DOI] [PubMed] [Google Scholar]
- 9.Zile MR, Bennett TD, St John Sutton M, et al. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.783910. [DOI] [PubMed] [Google Scholar]
- 10.Barnett ML, Hsu J, McWilliams JM. Patient characteristics and differences in hospital readmission rates. JAMA Intern Med. 2015;175:1803–1812. doi: 10.1001/jamainternmed.2015.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klersy C, De Silvestri A, Gabutti G, et al. A meta-analysis of remote monitoring of heart failure patients. J Am Coll Cardiol. 2009;54:1683–1694. doi: 10.1016/j.jacc.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura N, Koga T, Iseki H. A meta-analysis of remote patient monitoring for chronic heart failure patients. J Telemed Telecare. 2014;20:11–17. doi: 10.1177/1357633X13517352. [DOI] [PubMed] [Google Scholar]
- 13.Adamson PB, Ginn G, Anker SD, et al. Remote haemodynamic-guided care for patients with chronic heart failure: a meta-analysis of completed trials. Eur J Heart Fail. 2017;19:426–33. doi: 10.1002/ejhf.638. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall SC, Molnar F, Man-Son-Hing M, et al. Predictors of driving ability following stroke: a systematic review. Top Stroke Rehabil. 2007;14:98–114. doi: 10.1310/tsr1401-98. [DOI] [PubMed] [Google Scholar]
- 16.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonicelli R, Mazzanti I, Abbatecola AM, Parati G. Impact of home patient telemonitoring on use of beta-blockers in congestive heart failure. Drugs & Aging. 2010;27:801–805. doi: 10.2165/11538210-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Bekelman DB, Plomondon ME, Carey EP, et al. Primary results of the patient-centered disease management (PCDM) for heart failure study: a randomized clinical trial. JAMA Intern Med. 2015;175:725–732. doi: 10.1001/jamainternmed.2015.0315. [DOI] [PubMed] [Google Scholar]
- 19.Blue L, Lang E, McMurray JJ, et al. Randomised controlled trial of specialist nurse intervention in heart failure. BMJ. 2001;323:715–718. doi: 10.1136/bmj.323.7315.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blum K, Gottlieb SS. The effect of a randomized trial of home telemonitoring on medical costs, 30-day readmissions, mortality, and health-related quality of life in a cohort of community-dwelling heart failure patients. J Card Fail. 2014;20:513–521. doi: 10.1016/j.cardfail.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Bowles KH, Holland DE, Horowitz DA. A comparison of in-person home care, home care with telephone contact and home care with telemonitoring for disease management. J Telemed Telecare. 2009;15:344–350. doi: 10.1258/jtt.2009.090118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyne JJ, Vrijhoef HJ, Crijns HJ, et al. Tailored telemonitoring in patients with heart failure: results of a multicentre randomized controlled trial. Eur J Heart Fail. 2012;14:791–801. doi: 10.1093/eurjhf/hfs058. [DOI] [PubMed] [Google Scholar]
- 23.Brown R, Peikes D, Chen A, Schore J. 15-site randomized trial of coordinated care in Medicare FFS. Health Care Financ Rev. 2008;30:5–25. [PMC free article] [PubMed] [Google Scholar]
- 24.Capomolla S, Pinna G, La Rovere MT, et al. Heart failure case disease management program: a pilot study of home telemonitoring versus usual care. Eur Heart J Suppl. 2004;6(Suppl_F):F91–F8. [Google Scholar]
- 25.Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363:2301–2309. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cleland JG, Louis AA, Rigby AS, et al. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: the Trans-European Network-Home-Care Management System (TEN-HMS) study. J Am Coll Cardiol. 2005;45:1654–1664. doi: 10.1016/j.jacc.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 27.Dar O, Riley J, Chapman C, et al. A randomized trial of home telemonitoring in a typical elderly heart failure population in North West London: results of the Home-HF study. Eur J Heart Fail. 2009;11:319–325. doi: 10.1093/eurjhf/hfn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeBusk RF, Miller NH, Parker KM, et al. Care management for low-risk patients with heart failure: a randomized, controlled trial. Ann Intern Med. 2004;141:606–613. doi: 10.7326/0003-4819-141-8-200410190-00008. [DOI] [PubMed] [Google Scholar]
- 29.Delaney C, Apostolidis B. Pilot testing of a multicomponent home care intervention for older adults with heart failure: an academic clinical partnership. J Cardiovasc Nurs. 2010;25:E27–E40. doi: 10.1097/JCN.0b013e3181da2f79. [DOI] [PubMed] [Google Scholar]
- 30.Dendale P, De Keulenaer G, Troisfontaines P, et al. Effect of a telemonitoring-facilitated collaboration between general practitioner and heart failure clinic on mortality and rehospitalization rates in severe heart failure: the TEMA-HF 1 (TElemonitoring in the MAnagement of Heart Failure) study. Eur J Heart Fail. 2012;14:333–340. doi: 10.1093/eurjhf/hfr144. [DOI] [PubMed] [Google Scholar]
- 31.Domingo M, Lupon J, Gonzalez B, et al. [Noninvasive remote telemonitoring for ambulatory patients with heart failure: effect on number of hospitalizations, days in hospital, and quality of life. CARME (CAtalan Remote Management Evaluation) study] Rev Esp Cardiol. 2011;64:277–285. doi: 10.1016/j.recesp.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 32.Dunagan WC, Littenberg B, Ewald GA, et al. Randomized trial of a nurse-administered, telephone-based disease management program for patients with heart failure. J Card Fail. 2005;11:358–365. doi: 10.1016/j.cardfail.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Galbreath AD, Krasuski RA, Smith B, et al. Long-term healthcare and cost outcomes of disease management in a large, randomized, community-based population with heart failure. Circulation. 2004;110:3518–3526. doi: 10.1161/01.CIR.0000148957.62328.89. [DOI] [PubMed] [Google Scholar]
- 34.Gallagher BD, Moise N, Haerizadeh M, et al. Telemonitoring adherence to medications in heart failure patients (TEAM-HF): a pilot randomized clinical trial. J Card Fail. 2017;23:345–349. doi: 10.1016/j.cardfail.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giacomelli S, Marcon C, Penzo M, et al. The clinical impact of telemonitoring for chronic heart failure: the RENEWING HEALTH project. Eur Heart J. 2014;35:1132–1133. [Google Scholar]
- 36.Goldberg LR, Piette JD, Walsh MN, et al. Randomized trial of a daily electronic home monitoring system in patients with advanced heart failure: the Weight Monitoring in Heart Failure (WHARF) trial. Am Heart J. 2003;146:705–712. doi: 10.1016/S0002-8703(03)00393-4. [DOI] [PubMed] [Google Scholar]
- 37.Goode KM, Zhang J, Rigby AS, et al. Home-telemonitoring or nurse telephone support improves short-term outcomes in patients at greatest risk of death or hospitalisation compared to usual care: Evidence from the TEN-HMS Study. Eur J Heart Fail. 2009;8:ii195. [Google Scholar]
- 38.Hale TM, Jethwani K, Kandola MS, Saldana F, Kvedar JC. A Remote medication monitoring system for chronic heart failure patients to reduce readmissions: a two-arm randomized pilot study. J Med Internet Res. 2016;18:e91. doi: 10.2196/jmir.5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howlett JG, Marr D, Palmer K, O'Neill BJ, Rajda M. Abstract 17680: A Comparison of Primary Care, Home-Based Telemonitoring, Telemonitoring with Video, or Specialist Directed Heart Failure Clinic Care for Patients with High Risk Heart Failure. Circulation. 2011;124(Suppl 21):A17680. [Google Scholar]
- 40.Hudson LR, Hamar GB, Orr P, et al. Remote physiological monitoring: clinical, financial, and behavioral outcomes in a heart failure population. Dis Manag. 2005;8:372–381. doi: 10.1089/dis.2005.8.372. [DOI] [PubMed] [Google Scholar]
- 41.Idris S, Degheim G, Ghalayini W, et al. Home telemedicine in heart failure: a pilot study of integrated telemonitoring and virtual provider appointments. Rev Cardiovasc Med. 2015;16:156–162. doi: 10.3909/ricm0760. [DOI] [PubMed] [Google Scholar]
- 42.Investigators G. Randomised trial of telephone intervention in chronic heart failure: DIAL trial. BMJ. 2005;331:425. doi: 10.1136/bmj.38516.398067.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jerant AF, Azari R, Nesbitt TS. Reducing the cost of frequent hospital admissions for congestive heart failure: a randomized trial of a home telecare intervention. Med Care. 2001;39:1234–1245. doi: 10.1097/00005650-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Juan C, Sanjoaquin AC, Lopez M, Romero D, Pinilla R, Ochoa P. Presentation of a frail elderly telemonitoring service lessons learned in the Barbarstro health care area. European Geriatric Medicine. 2011;2(Suppl 1):S128. [Google Scholar]
- 45.Kao DP, Lindenfeld J, Macaulay D, et al. Impact of a telehealth and care management program on all-cause mortality and healthcare utilization in patients with heart failure. Telemed J E Health. 2016;22:2–11. doi: 10.1089/tmj.2015.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kashem A, Droogan MT, Santamore WP, Wald JW, Bove AA. Managing heart failure care using an internet-based telemedicine system. J Card Fail. 2008;14:121–126. doi: 10.1016/j.cardfail.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 47.Kasper EK, Gerstenblith G, Hefter G, et al. A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission. J Am Coll Cardiol. 2002;39:471–480. doi: 10.1016/s0735-1097(01)01761-2. [DOI] [PubMed] [Google Scholar]
- 48.Koehler F, Winkler S, Schieber M, et al. Telemedicine in heart failure: pre-specified and exploratory subgroup analyses from the TIM-HF trial. Int J Cardiol. 2012;161:143–150. doi: 10.1016/j.ijcard.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Kraai I, de Vries A, Vermeulen K, et al. The value of telemonitoring and ICT-guided disease management in heart failure: Results from the IN TOUCH study. Int J Med Inform. 2016;85:53–60. doi: 10.1016/j.ijmedinf.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 50.Krum H, Forbes A, Yallop J, et al. Telephone support to rural and remote patients with heart failure: the Chronic Heart Failure Assessment by Telephone (CHAT) study. Cardiovasc Ther. 2013;31:230–237. doi: 10.1111/1755-5922.12009. [DOI] [PubMed] [Google Scholar]
- 51.Krumholz HM, Amatruda J, Smith GL, et al. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. J Am Coll Cardiol. 2002;39:83–89. doi: 10.1016/s0735-1097(01)01699-0. [DOI] [PubMed] [Google Scholar]
- 52.Kurtz B, Lemercier M, Pouchin SC, et al. Automated home telephone self-monitoring reduces hospitalization in patients with advanced heart failure. J Telemed Telecare. 2011;17:298–302. doi: 10.1258/jtt.2011.100901. [DOI] [PubMed] [Google Scholar]
- 53.Laramee AS, Levinsky SK, Sargent J, et al. Case management in a heterogeneous congestive heart failure population: a randomized controlled trial. Arch Intern Med. 2003;163:809–817. doi: 10.1001/archinte.163.7.809. [DOI] [PubMed] [Google Scholar]
- 54.Lynga P, Persson H, Hagg-Martinell A, et al. Weight monitoring in patients with severe heart failure (WISH). A randomized controlled trial. Eur J Heart Fail. 2012;14:438–444. doi: 10.1093/eurjhf/hfs023. [DOI] [PubMed] [Google Scholar]
- 55.Martin-Lesende I, Orruno E, Bilbao A, et al. Impact of telemonitoring home care patients with heart failure or chronic lung disease from primary care on healthcare resource use (the TELBIL study randomised controlled trial) BMC Health Serv Res. 2013;13:118. doi: 10.1186/1472-6963-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McDonald K, Ledwidge M, Cahill J, et al. Heart failure management: multidisciplinary care has intrinsic benefit above the optimization of medical care. J Card Fail. 2002;8:142–148. doi: 10.1054/jcaf.2002.124340. [DOI] [PubMed] [Google Scholar]
- 57.Morguet AJ, Kuhnelt P, Kallel A, et al. Impact of telemedical care and monitoring on morbidity in mild to moderate chronic heart failure. Cardiology. 2008;111:134–139. doi: 10.1159/000119701. [DOI] [PubMed] [Google Scholar]
- 58.Mortara A, Pinna GD, Johnson P, et al. Home telemonitoring in heart failure patients: the HHH study (Home or Hospital in Heart Failure) Eur J Heart Fail. 2009;11:312–318. doi: 10.1093/eurjhf/hfp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ong MK, Romano PS, Edgington S, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the Better Effectiveness After Transition -- Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern Med. 2016;176:310–318. doi: 10.1001/jamainternmed.2015.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pedone C, Rossi FF, Cecere A, et al. Efficacy of a physician-led multiparametric telemonitoring system in very old adults with heart failure. J Am Geriatr Soc. 2015;63:1175–1180. doi: 10.1111/jgs.13432. [DOI] [PubMed] [Google Scholar]
- 61.Peters-Klimm F, Campbell S, Hermann K, et al. Case management for patients with chronic systolic heart failure in primary care: the HICMan exploratory randomised controlled trial. Trials. 2010;11:56. doi: 10.1186/1745-6215-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Riegel B, Carlson B, Glaser D, Romero T. Randomized controlled trial of telephone case management in Hispanics of Mexican origin with heart failure. J Card Fail. 2006;12:211–219. doi: 10.1016/j.cardfail.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 63.Riegel B, Carlson B, Kopp Z, et al. Effect of a standardized nurse case-management telephone intervention on resource use in patients with chronic heart failure. Arch Intern Med. 2002;162:705–712. doi: 10.1001/archinte.162.6.705. [DOI] [PubMed] [Google Scholar]
- 64.Roth A, Kajiloti I, Elkayam I, Sander J, Kehati M, Golovner M. Telecardiology for patients with chronic heart failure: the ‘SHL’ experience in Israel. Int J Cardiol. 2004;97:49–55. doi: 10.1016/j.ijcard.2003.07.030. [DOI] [PubMed] [Google Scholar]
- 65.Sabatier R, Coutance G, Belin A, et al. Three months educational remote telemonitoring in elderly patients with heart failure reduces hospitalizations for acute heart failure at one year: a randomized trial. Eur J Heart Fail. 2013;15(S1):S313. [Google Scholar]
- 66.Sardu C, Santamaria M, Rizzo MR, et al. Telemonitoring in heart failure patients treated by cardiac resynchronisation therapy with defibrillator (CRT-D): the TELECART Study. Int J Clin Pract. 2016;70:569–576. doi: 10.1111/ijcp.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scherr D, Kastner P, Kollmann A, et al. Effect of home-based telemonitoring using mobile phone technology on the outcome of heart failure patients after an episode of acute decompensation: randomized controlled trial. J Med Internet Res. 2009;11:e34. doi: 10.2196/jmir.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schofield RS, Kline SE, Schmalfuss CM, et al. Early outcomes of a care coordination-enhanced telehome care program for elderly veterans with chronic heart failure. Telemed J E Health. 2005;11:20–27. doi: 10.1089/tmj.2005.11.20. [DOI] [PubMed] [Google Scholar]
- 69.Seto E, Leonard KJ, Cafazzo JA, et al. Mobile phone-based telemonitoring for heart failure management: a randomized controlled trial. J Med Internet Res. 2012;14:e31. doi: 10.2196/jmir.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sisk JE, Hebert PL, Horowitz CR, et al. Effects of nurse management on the quality of heart failure care in minority communities: a randomized trial. Ann Intern Med. 2006;145:273–283. doi: 10.7326/0003-4819-145-4-200608150-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smolis-Bak E, Dabrowski R, Piotrowicz E, et al. Hospital-based and telemonitoring guided home-based training programs: effects on exercise tolerance and quality of life in patients with heart failure (NYHA class III) and cardiac resynchronization therapy. A randomized, prospective observation. Int J Cardiol. 2015;199:442–447. doi: 10.1016/j.ijcard.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 72.Soran OZ, Pina IL, Lamas GA, et al. A randomized clinical trial of the clinical effects of enhanced heart failure monitoring using a computer-based telephonic monitoring system in older minorities and women. J Card Fail. 2008;14:711–717. doi: 10.1016/j.cardfail.2008.06.448. [DOI] [PubMed] [Google Scholar]
- 73.Vuorinen AL, Leppanen J, Kaijanranta H, et al. Use of home telemonitoring to support multidisciplinary care of heart failure patients in Finland: randomized controlled trial. J Med Internet Res. 2014;16:e282. doi: 10.2196/jmir.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wade MJ, Desai AS, Spettell CM, et al. Telemonitoring with case management for seniors with heart failure. Am J Manag Care. 2011;17:e71–e79. [PubMed] [Google Scholar]
- 75.Woodend AK, Sherrard H, Fraser M, et al. Telehome monitoring in patients with cardiac disease who are at high risk of readmission. Heart Lung. 2008;37:36–45. doi: 10.1016/j.hrtlng.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 76.Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 77.Abraham WT, Stevenson LW, Bourge RC, et al. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet. 2016;387:453–461. doi: 10.1016/S0140-6736(15)00723-0. [DOI] [PubMed] [Google Scholar]
- 78.Adamson PB, Abraham WT, Bourge RC, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7:935–944. doi: 10.1161/CIRCHEARTFAILURE.113.001229. [DOI] [PubMed] [Google Scholar]
- 79.Adamson PB, Abraham WT, Stevenson LW, et al. Pulmonary artery pressure-guided heart failure management reduces 30-day readmissions. Circ Heart Fail. 2016;9:e002600. doi: 10.1161/CIRCHEARTFAILURE.115.002600. [DOI] [PubMed] [Google Scholar]
- 80.Adamson PB, Gold MR, Bennett T, et al. Continuous hemodynamic monitoring in patients with mild to moderate heart failure: results of The Reducing Decompensation Events Utilizing Intracardiac Pressures in Patients With Chronic Heart Failure (REDUCEhf) trial. Congest Heart Fail. 2011;17:248–254. doi: 10.1111/j.1751-7133.2011.00247.x. [DOI] [PubMed] [Google Scholar]
- 81.Adamson PB, Magalski A, Braunschweig F, et al. Ongoing right ventricular hemodynamics in heart failure: clinical value of measurements derived from an implantable monitoring system. J Am Coll Cardiol. 2003;41:565–571. doi: 10.1016/s0735-1097(02)02896-6. [DOI] [PubMed] [Google Scholar]
- 82.Benza RL, Raina A, Abraham WT, et al. Pulmonary hypertension related to left heart disease: insight from a wireless implantable hemodynamic monitor. J Heart Lung Transplant. 2015;34:329–337. doi: 10.1016/j.healun.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 83.Bourge RC, Abraham WT, Adamson PB, et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol. 2008;51:1073–1079. doi: 10.1016/j.jacc.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 84.Desai AS, Bhimaraj A, Bharmi R, et al. Reduction in heart failure hospitalizations with ambulatory hemodynamic monitoring seen in clinical trials is maintained in the ‘Real World’. J Am Coll Cardiol. 2017;69:2357–2365. doi: 10.1016/j.jacc.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 85.Jermyn R, Alam A, Kvasic J, et al. Hemodynamic-guided heart-failure management using a wireless implantable sensor: Infrastructure, methods, and results in a community heart failure disease-management program. Clin Cardiol. 2017;40:170–176. doi: 10.1002/clc.22643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raina A, Abraham WT, Adamson PB, et al. Limitations of right heart catheterization in the diagnosis and risk stratification of patients with pulmonary hypertension related to left heart disease: insights from a wireless pulmonary artery pressure monitoring system. J Heart Lung Transplant. 2015;34:438–447. doi: 10.1016/j.healun.2015.01.983. [DOI] [PubMed] [Google Scholar]
- 87.Tse G, Gong M, Wong SH, et al. Frailty and clinical outcomes in advanced heart failure patients undergoing left ventricular assist device implantation: a systematic review and meta-analysis. J Am Med Dir Assoc. 2018;19:255–261. doi: 10.1016/j.jamda.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 88.Tse G, Gong M, Nunez J, et al. Frailty and mortality outcomes after percutaneous coronary intervention: a systematic review and meta-analysis. J Am Med Dir Assoc. 2017;18:1097 e1–e10. doi: 10.1016/j.jamda.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 89.Santos N, Oliveira M, Da Silva N, et al. Utility of continuous monitoring of heart rate variability in the early detection of heart failure decompensation. Rev Port Cardiol. 2011;30:559–563. [PubMed] [Google Scholar]
- 90.Kowalczys A, Bohdan M, Gruchala M. Prognostic value of daytime heart rate, blood pressure, their products and quotients in chronic heart failure. Cardiol J. doi: 10.5603/CJ.a2017.0130. Published Online First: Nov 13, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gudmundsson K, Lynga P, Rosenqvist M, Braunschweig F. Monitoring of daily body weight and intrathoracic impedance in heart failure patients with a high risk of volume overload decompensation. Clin Cardiol. 2016;39:446–452. doi: 10.1002/clc.22547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Verdejo HE, Castro PF, Concepcion R, et al. Comparison of a radiofrequency-based wireless pressure sensor to swan-ganz catheter and echocardiography for ambulatory assessment of pulmonary artery pressure in heart failure. J Am Coll Cardiol. 2007;50:2375–2382. doi: 10.1016/j.jacc.2007.06.061. [DOI] [PubMed] [Google Scholar]
- 93.Steinhaus D, Reynolds DW, Gadler F, et al. Implant experience with an implantable hemodynamic monitor for the management of symptomatic heart failure. Pacing Clin Electrophysiol. 2005;28:747–753. doi: 10.1111/j.1540-8159.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- 94.Walton AS, Krum H. The Heartpod implantable heart failure therapy system. Heart Lung Circ. 2005;14(Suppl 2):S31–S33. doi: 10.1016/j.hlc.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 95.Maurer MS, Adamson PB, Costanzo MR, et al. Rationale and design of the Left Atrial Pressure Monitoring to Optimize Heart Failure Therapy Study (LAPTOP-HF) J Card Fail. 2015;21:479–88. doi: 10.1016/j.cardfail.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 96.Xu TY, Sun JP, Lee AP, et al. Three-dimensional speckle strain echocardiography is more accurate and efficient than 2D strain in the evaluation of left ventricular function. Int J Cardiol. 2014;176:360–366. doi: 10.1016/j.ijcard.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 97.Dong M, Liao JK, Fang F, et al. Increased Rho kinase activity in congestive heart failure. Eur J Heart Fail. 2012;14:965–973. doi: 10.1093/eurjhf/hfs068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anglemyer A, Horvath HT, Bero L. Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials. Cochrane Database Syst Rev. 2014:MR000034. doi: 10.1002/14651858.MR000034.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1S. Quality ratings for included case-control studies using the Newcastle-Ottawa quality assessment scale for telemonitoring.
| Number | First Author | Selection (score) | Comparability (score) | Total Score | ||||||
| Case definition | Representative of cases | Selections of controls | Definition of controls | Comparability of cases and controls on the basis of the design or analysis | Ascertainment of exposure | Same method ascertainment participants | Nonresponse rate | |||
| 1 | Morguet 2008 | - | 1 | 1 | 1 | 2 | - | - | 1 | 6 |
Table 2S. Quality ratings for included cohort studies using the Newcastle-Ottawa quality assessment scale for telemonitoring.
| Number |
First Author |
Selection (score) |
Comparability (score) |
Exposure (score) |
Total Score |
|||||
| Representative of exposed cohort | Selections of non-exposed cohort | Assessment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Ascertainment of outcome | Was follow-up long enough for outcomes to occur? | Adequacy of follow up of cohorts | |||
| 1 | Kao 2016 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 |
| 2 | Smolis-Bąk 2015 | 1 | 1 | 1 | 1 | 2 (age, comorbidities) | 1 | 1 | 1 | 9 |
| 3 | Kurtz 2011 | 1 | 1 | 1 | 1 | 2 (age, LVEF) | 1 | 1 | 1 | 9 |
| 4 | Hudson 2005 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| 5 | Schofield 2005 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| 6 | Roth 2004 | 1 | 1 | 1 | 1 | 2 | - | 1 | 1 | 8 |
LVEF: left ventricular ejection fraction.
Table 3S. Quality ratings for included case-control studies using the Newcastle-Ottawa quality assessment scale for hemodynamic monitoring.
| Number |
First Author |
Selection (score) |
Comparability (score) |
Total Score |
||||||
| Case definition | Representative of cases | Selections of controls | Definition of controls | Comparability of cases and controls on the basis of the design or analysis | Ascertainment of exposure | Same method ascertainment participants | Nonresponse rate | |||
| 1 | Jermyn 2016 | 1 | 1 | - | 1 | 2 | 1 | 1 | 1 | 8 |
| 2 | Abraham 2016 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| 3 | Raina 2015 | 1 | 1 | - | 1 | 2 | 1 | 1 | 1 | 8 |
| 4 | Benza 2015 | 1 | 1 | - | 1 | 2 | 1 | 1 | 1 | 8 |
| 5 | Abraham 2011 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
Table 4S. Quality ratings for included cohort studies using the Newcastle-Ottawa quality assessment scale for hemodynamic monitoring.
| Number |
First Author |
Selection (score) |
Comparability (score) |
Exposure (score) |
Total Score |
|||||
| Representative of exposed cohort | Selections of non-exposed cohort | Assessment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Ascertainment of outcome | Was follow-up long enough for outcomes to occur? | Adequacy of follow up of cohorts | |||
| 1 | Desai 2017 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| 2 | Ritzema 2010 | - | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 |
| 3 | Adamson 2003 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
Table 5S. Quality ratings for included randomized controlled trials using the Jadad quality assessment scale for telemonitoring.
| Number | Study | Randomization | Allocation concealment | Double blinding | Withdrawals and dropouts | Total score |
| 1 | Gallagher 2017 | 2 | 1 | 1 | 1 | 5 |
| 2 | Sardu 2016 | 2 | 2 | 2 | 0 | 6 |
| 3 | Hale 2016 | 1 | 0 | 0 | 1 | 2 |
| 4 | Ong 2016 | 2 | 2 | 2 | 1 | 7 |
| 5 | Kraai 2016 | 2 | 1 | 1 | 1 | 5 |
| 6 | Idris 2015 | 1 | 0 | 0 | 1 | 2 |
| 7 | Pedone 2015 | 1 | 1 | 2 | 1 | 5 |
| 8 | Bekelman 2015 | 2 | 0 | 0 | 1 | 3 |
| 9 | Vuorinen 2014 | 1 | 0 | 0 | 1 | 2 |
| 10 | Blum 2014 | 1 | 0 | 0 | 1 | 2 |
| 11 | Giacomelli 2014 | 1 | 0 | 0 | 1 | 2 |
| 12 | Martín-Lesende 2013 | 2 | 0 | 1 | 1 | 4 |
| 13 | Krum 2013 | 1 | 1 | 1 | 1 | 4 |
| 14 | Sabatier 2013 | 1 | 0 | 0 | 1 | 2 |
| 15 | Boyne 2012 | 2 | 0 | 2 | 1 | 5 |
| 16 | Lynga° 2012 | 1 | 1 | 2 | 1 | 5 |
| 17 | Seto 2012 | 2 | 2 | 2 | 1 | 7 |
| 18 | Dendale 2012 | 1 | 2 | 2 | 1 | 4 |
| 19 | Koehler 2012 | 2 | 1 | 1 | 1 | 5 |
| 20 | Wade 2011 | 1 | 0 | 0 | 1 | 2 |
| 21 | Domingo 2011 | 1 | 0 | 0 | 1 | 2 |
| 22 | Howlett 2011 | 1 | 0 | 0 | 1 | 2 |
| 23 | Chaudhry 2010 | 2 | 2 | 2 | 1 | 7 |
| 24 | Antonicelli 2010 | 1 | 0 | 0 | 1 | 2 |
| 25 | Delaney 2010 | 1 | 0 | 0 | 1 | 2 |
| 26 | Peters-Klimm 2010 | 2 | 2 | 2 | 1 | 7 |
| 27 | Bowles 2009 | 1 | 2 | 2 | 1 | 6 |
| 28 | Scherr 2009 | 1 | 0 | 2 | 1 | 4 |
| 29 | Mortara 2009 | 2 | 2 | 2 | 1 | 7 |
| 30 | Dar 2009 | 2 | 1 | 2 | 1 | 6 |
| 31 | Goode 2009 | 1 | 0 | 0 | 1 | 2 |
| 32 | Brown 2008 | 2 | 1 | 0 | 1 | 4 |
| 33 | Soran 2008 | 1 | 1 | 2 | 1 | 5 |
| 34 | Antonicelli 2008 | 1 | 0 | 0 | 1 | 2 |
| 35 | Kashem 2008 | 2 | 0 | 0 | 1 | 3 |
| 36 | Woodend 2008 | 1 | 0 | 0 | 1 | 2 |
| 37 | Sisk 2006 | 2 | 2 | 2 | 1 | 7 |
| 38 | Riegel 2006 | |||||
| 39 | GESICA Investigators 2005 | 1 | 0 | 0 | 1 | 2 |
| 40 | Dunagan 2005 | 2 | 0 | 0 | 1 | 3 |
| 41 | Cleland 2005 | 1 | 1 | 2 | 1 | 5 |
| 42 | Capomolla 2004 | 1 | 0 | 0 | 1 | 2 |
| 43 | Galbreath 2004 | 1 | 0 | 0 | 1 | 2 |
| 44 | DeBusk 2004 | 2 | 1 | 1 | 1 | 5 |
| 45 | Goldberg 2003 | 1 | 0 | 0 | 1 | 2 |
| 46 | Laramee 2003 | 2 | 1 | 2 | 1 | 6 |
| 47 | McDonald 2002 | 1 | 1 | 2 | 1 | 5 |
| 48 | Riegel 2002 | 1 | 2 | 2 | 1 | 6 |
| 49 | Kasper 2002 | 1 | 0 | 1 | 1 | 3 |
| 50 | Krumholz 2002 | 1 | 0 | 0 | 1 | 2 |
| 51 | Jerant 2001 | 2 | 2 | 0 | 1 | 5 |
| 52 | Blue 2001 | 1 | 1 | 1 | 1 | 4 |
Table 6S. Quality ratings for included randomized controlled trials using the Jadad quality assessment scale for hemodynamic monitoring.
| Number | Study | Randomization | Allocation concealment | Double blinding | Withdrawals and dropouts | Total score |
| 1 | Adamson 2016 | 1 | 1 | 1 | 1 | 4 |
| 2 | Adamson 2014 | 1 | 1 | 1 | 1 | 4 |
| 3 | Adamson 2011 | 1 | 1 | 1 | 1 | 4 |
| 4 | Bourge 2008 | 1 | 1 | 2 | 1 | 5 |