Abstract
Background
It is debatable whether treating multimorbid nursing home patients with antihypertensive drugs produces beneficial effects. Most cardiovascular guidelines promote treatment; few have advice on how to deprescribe when treatment may no longer be necessary. We investigated the effect of medication review on antihypertensive drug use and the association between cognition, blood pressure, and prescribing.
Methods
From August 2014 to December 2015, 765 patients from 72 units (clusters) in 32 Norwegian nursing homes were included in a 4-month, multicentre, cluster-randomized, controlled trial, with 9-month follow-up. Patients ≥ 65 years old with antihypertensive treatment (n = 295, 39%) were randomized to systematic medication review where the physician received support from peers (collegial mentoring) or were given care as usual (control condition). Outcome measures were the number of antihypertensive drugs, systolic blood pressure, and pulse. We used hospitalizations and deaths as criteria to assess harm.
Results
At baseline, each patient used 9.2 ± 3.5 regular drugs, and 1.6 ± 0.7 antihypertensives. Mean blood pressure was 128/71 mmHg and 9% had a systolic pressure ≥ 160 mmHg. Between baseline and month four, antihypertensives were deprescribed to a significantly higher extent in the intervention group (n = 43, 32%) compared to control (n = 11, 10%); Incidence Rate Ratio = 0.8, 95% CI = 0.7–0.9. In the intervention group, there was an immediate increase in systolic blood pressure when antihypertensives were reduced, from baseline 128 ± 19.5 mmHg to 143 ± 25.5 mmHg at month four. However, at month nine, the blood pressure had reverted to baseline values (mean 134 mmHg). Deprescription did not affect pulse and systolic pressure. The number of hospitalizations was higher in control patients at month four (P = 0.031) and nine (P = 0.041).
Conclusion
A systematic medication review supported by collegial mentoring significantly decreased the use of antihypertensive drugs in nursing home patients without an effect on the systolic blood pressure over time.
Keywords: Antihypertensive drugs, Deprescribing, Hypertension, Long-term care, Medication review
1. Introduction
Cardiovascular diseases such as hypertension, heart failure, and atrial fibrillation affect almost half the residents living in a nursing home.[1],[2] Dementia affects over 80%.[3] While previous treatment with antihypertensives, beta-blocker or diuretics may have been beneficial, this treatment is often no longer necessary, or may even be harmful, when frail old patients approach the end of life. Multimorbidity combined with polypharmacy and increased risk of adverse events requires a patient-centred, individually adapted approach.
International guidelines by the European Society of Hypertension and the American Eight Joint National Committee state that only the treatment of hypertension grade two and above (systolic blood pressure ≥ 160 mmHg) is shown to reduce cardiovascular events in fit elderly people over 80 years old.[4],[5] Meanwhile, these recommendations do not cover the treatment of hypertension in the more fragile group of nursing home patients and people with dementia, who are often excluded from clinical studies[6] and are high-consumers of antihypertensives.[7] Treatment may lead to orthostatic hypotension, falls, and increased anticholinergic burden in this population.[8]
Nursing home patients were excluded from the two major trials exploring effects of antihypertensive treatment in the oldest population,[9],[10] however some smaller studies have included these patients: a prospective study on 406 Swedish nursing home patients shows that lower systolic blood pressure was associated with increased mortality.[11] Another non-randomized controlled trial of deprescribing in general included 190 Israeli nursing home patients found successful deprescribing of cardiovascular drugs without increased blood pressure or mortality.[12]
Given that antihypertensives are used to treat a range of different indications beyond hypertension, such as angina pectoris, rhythm control in atrial fibrillation and renal failure, treatment requires individual diagnostic and clinical evaluation of the patient that also includes his or her values and preferences on treatment-goals.[13] For patients with dementia, involving the relatives as the patient's spokesperson is relevant. In a person-centred approach, deprescribing also entails close monitoring of the blood pressure, pulse and other adverse events before and after the change of the treatment to avoid potential harm.[14]
The current study aimed to optimize the antihypertensive treatment for nursing home patients through systematic medication reviews supported by collegial mentoring. The primary objective of this paper is to investigate how individual person-centred care affects the use of antihypertensives. Secondary objectives were to assess the effect of changes in antihypertensive drugs on blood pressure and pulse after four and nine months, and to investigate whether baseline blood pressure or cognitive status could predict change.
2. Methods
2.1. Study design
This paper presents data from the COSMOS study, a multicentre, cluster-randomized controlled trial executed in Norwegian nursing homes between August 2014 and December 2015. COSMOS is a complex intervention study where the interventions are denoted by the acronym COSMOS: COmmunication, Systematic pain assessment and treatment, Medication review, Organization of activities, and Safety. The implementation process and sample-size analyses are described in detail in the published protocol.[15] The COSMOS study aimed to improve patients' quality of life. It also sought to improve neuropsychiatric symptoms such as agitation, depression and sleep. Other secondary aims were to improve the activities of daily living, pain, prescribing routines and costs for nursing home patients. In this paper, we focus on the effect of the systematic medication review involving collegial mentoring, especially antihypertensive drug prescription.
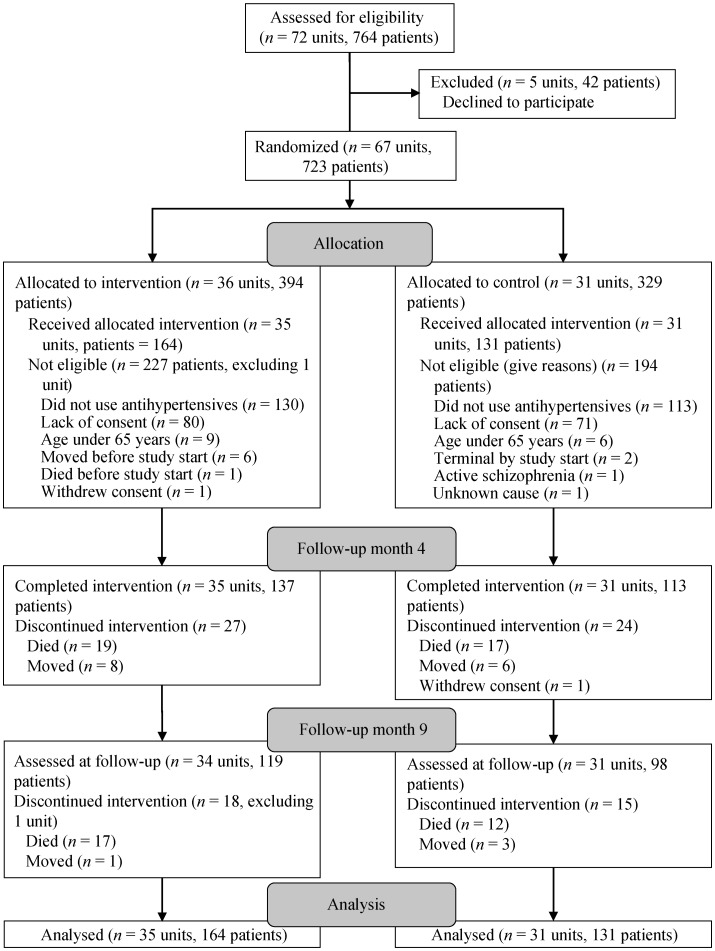
We initially invited 37 Norwegian nursing homes located in eight municipalities to participate in this study. Municipalities were of different size and location and the nursing homes housed between 16 and 150 patients living in 72 units (clusters) (Figure 1). Only nursing home units with long-term care patients were included. To be included in the present study, the patients had to use antihypertensive drugs at baseline and be 65 years or older. We excluded dying patients and those with schizophrenia.
Figure 1. Flow chart of the study.
We chose a cluster design to account for patients in the same units receiving comparable treatment. Each unit was defined as a cluster and was randomized with a random number sequence in SPSS 18 (IBM, Armork, NY), stratified by geographical location. The nature of the intervention prevented blinding of staff or researchers.
2.2. Intervention and control conditions
To implement the complex COSMOS intervention, a two-day educational seminar gathered all nursing home units randomized to the intervention group. The units sent at least two nurses elected to be the unit's COSMOS ambassadors. In addition, other nurses from the unit, the unit manager, the responsible physicians and a pharmacist were invited. The education program covered research-based knowledge about communication with relatives and patients through advance care planning, pain assessment and treatment, the rationale and method for multidisciplinary medication review, and organization of activities for all included patients. The education program also included role-playing and patient-centred discussions. At the seminar, the COSMOS ambassadors received educational material to train the other staff in the unit.
Regarding the medication review, we especially emphasized that the patient's clinical test results pertaining to blood pressure, pulse, blood tests, neuropsychiatric symptoms, cognitive status, activities of daily living function, pain, well-being, and symptom relief should inform drug-decision-making. In addition, the patient's and relatives wishes were considered. For example, if a patient was evaluated as not being in pain, the physician was encouraged to temporarily suspend the pain medication. Treatment changes should be communicated to the staff and patient (if possible). The staffs were also instructed to also inform the relatives. Every change had to be followed by re-assessment.
After the seminar, the COSMOS ambassadors trained the rest of the staff in their units.[16] The training sessions had a duration of approximately 15 min every day and included all staff in the unit. The ambassadors were instructed to focus on one component per week: communication, systematic pain assessment and treatment, medication reviews, and organization of activities. For the medication review in particular, the physicians received a separate booklet with a short description of the assessment tools used in the medication reviews, the Norwegian guidelines for medication reviews,[17],[18] the updated STOPP/START criteria for potentially inappropriate prescribing in older people,[19] and a list of anticholinergic drugs.[20]
In addition to the seminars, experienced researchers visited each unit and trained at least two nurses in the necessary clinical assessments in four-hour sessions.[15] The nurses learned how to use the instruments to evaluate their patients and how to interpret the results. These assessments informed the medication reviews, in addition to data from medical records, blood samples, and diagnoses.
The researchers scheduled a meeting with the physician and nurse in each unit to perform medication reviews on the patients. Before the medication reviews, the staff assessed cognitive function, neuropsychiatric symptoms, pain, activities of daily living, and quality of life in all patients, using tools validated for people with dementia.[15] This information, together with information on diagnoses, drug-drug interactions, blood pressure, pulse, and lab tests were used to assess medication lists. We used the START/STOPP 2 criteria and the available clinical observations to guide revisions.[19] The criteria recommend treating with antihypertensives if systolic blood pressure is ≥ 160 mmHg. We recommended dose tapering or withdrawal of drugs if the blood pressure was below the recommended limit. We also recommended deprescribing if the patients overall condition was deteriorating, if the patient was unwilling to allow monitoring of treatment, or if the patient had a short life-expectancy.[21] The physician ordered relevant blood tests, and the nurse checked the drugs in an interaction database.[22] The physician was in charge of all medical decisions in the meeting; the nurse provided updated knowledge about the patients' situation, and the researcher gave provided decision support by initiating discussions, giving support in difficult decisions and supplementing with relevant research on the field (collegial mentoring). The discussions around treatment had a patient-centred focus.
The control units continued treatment and provided care as usual. After data collection at month nine, the control units underwent the same education programme as the intervention units.
2.3. Data collection
All patients were assessed at baseline, month four and month nine. Data on prescribed drugs, diagnoses, blood pressure, pulse and hospital admissions were obtained from the medical records. The nurses in the units performed the data extraction from medical records and measured blood pressures and pulse. The researchers tested all patients with Mini Mental Status Examination (MMSE).[23] Drugs were coded to the fifth level of the Anatomical Therapeutic Chemical Index (ATC) classes and presented as the number of drugs in each fourth level class.[24] The diagnoses collected from the medical records were coded according to The International Classification of Primary Care (ICPC).[25]
2.4. Outcome measures
Hypertension was defined as the ICPC diagnoses K85 Elevated blood pressure, K86 Uncomplicated hypertension, and K87 Hypertension with organ complications. The number of antihypertensives comprised the sum of drugs used within the following five drug classes: C03C High Ceiling Diuretics (loop-diuretics), C07A Beta Blockers, C09A Plain Angiotensin-Converting-Enzyme Inhibitors, C09C Plain Angiotensin II Antagonists, and C08C Calcium Channel Blockers with mainly vascular effect. These drugs were the most prescribed drugs with hypertension as one of their main indications, but may also be prescribed for other indications such as heart failure, angina pectoris, rhythm control in atrial fibrillation, and renal failure. Twelve or fewer patients individually used other antihypertensive drug classes. The nurses in the units measured blood pressure in mmHg according to the local procedure. In one analysis, the blood pressure at baseline was dichotomized into high or normal/low. The systolic and diastolic cutoffs for high blood pressure were ≥ 160 mmHg and ≥ 90 mmHg, respectively. Deprescribing antihypertensive drugs was defined as using fewer antihypertensive drugs at month four than at baseline. Cognitive function was divided into four groups according to MMSE results at baseline―severe dementia, < 11; moderate, 11–20; mild, 21–25; and no dementia: > 25.[26]
2.5. Statistical analyses
For regression analyses with total number of antihypertensive drugs as the outcome, we used multilevel mixed effects Poisson regression. These analyses were: (1) antihypertensive drug use over time for intervention and control, with random effects of time and group; (2) the effect of a high vs. low/normal systolic or diastolic blood pressure on antihypertensive drug use, with fixed effect for blood pressure at baseline, and random effect of time; and (3) the effect of baseline cognitive function on antihypertensive drug use, with fixed effect for cognitive function at baseline, and random effect of time. In analyses 1−3, we used robust standard errors to account for deviations from assumptions.[27] Analyses 2 and 3 were stratified by group allocation due to different change over time in these two groups. Linear mixed models were used to investigate the effect of reducing antihypertensive drugs on (4) systolic blood pressure, (5) diastolic blood pressure, and (6) pulse. Analyses 4–6 used fixed effect for deprescribing and random effects of time. Analyses 4–6 were only performed in the intervention group because of too few deprescriptions in the control group. All analyses used random intercepts for patients and units, and patients were nested within units. Chi square tests were used to test potential differences in hospital admissions. All P-values were two-sided and significance was set at < 0.05. STATA 14.2 (StataCorp, Texas, USA) was used for all analyses.
3. Results
In total, 295 patients used antihypertensive drugs at baseline, 164 in the intervention group and 131 in the control group (Figure 1). Age, number of recorded diagnoses, the total number of drugs and types of antihypertensive drug used were similar across the groups (Table 1). Sixty-four (39%) patients in the intervention and 55 (42%) of the control group had a diagnosis of hypertension. A cardiovascular diagnosis was recorded in 216 (79%) patients. In addition to hypertension, the most frequent diagnoses were atrial fibrillation in 109 (23%) patients, heart failure in 50 (17%) patients, and stroke in 46 (16%) patients. The patients were treated by 36 different physicians in 20 units; six of the physicians attended both intervention and control units. No pharmacists attended the study.
Table 1. Demographics of included patients at baseline.
| Variables | Intervention n = 164 |
Control n = 131 |
||||||
| Min to Max | Min to Max | |||||||
| Females | 115 (70%) | 90 (69%) | ||||||
| Age | 86.9 ± 7.6 | 66–104 | 87.5 ± 7.2 | 67–102 | ||||
| *Mini mental status examination | 11.7 ± 7.4 | 0–28 | 13.2 ± 7.6 | 0–29 | ||||
| Severe dementia | 72 (53%) | 44 (43%) | ||||||
| Moderate dementia | 46 (34%) | 37 (36%) | ||||||
| Mild dementia | 10 (7%) | 11 (11%) | ||||||
| No dementia | 8 (6%) | 11 (11%) | ||||||
| Number of diagnoses | 4.8 ± 3.5 | 0–14 | 5.2 ± 3.8 | 0–27 | ||||
| Number of regular prescriptions | 9.2 ± 3.4 | 2–19 | 9.1 ± 3.6 | 3–21 | ||||
| Number of antihypertensive drugs | 1.6 ± 0.7 | 1–4 | 1.6 ± 0.7 | 1–4 | ||||
| C03C high-ceiling diuretics | 97 (59%) | 68 (52%) | ||||||
| C07A beta-blockers | 81 (49%) | 67 (51%) | ||||||
| C09C plain angiotensin II antagonists | 35 (21%) | 27 (21%) | ||||||
| C09A plain ACE inhibitors | 29 (18%) | 26 (20%) | ||||||
| C08C calcium channel blockers, mainly vascular effect | 21 (13%) | 19 (15%) | ||||||
| Blood pressure | ||||||||
| High systolic blood pressure | 14 (8%) | 9 (7%) | ||||||
| Systolic, mean mmHg | 128 ± 20.4 | 77–196 | 128 ± 20.3 | 90–190 | ||||
| Diastolic, mean mmHg | 71 ± 12.3 | 38–103 | 71 ± 12.9 | 40–117 | ||||
| Pulse, beats per minute | 70 ± 12.0 | 40–110 | 71 ± 12.1 | 44–109 | ||||
Data are presented as mean ± SD or n (%). *Mini Mental Status Examination range: 0–30. ACE: angiotensin converting enzyme.
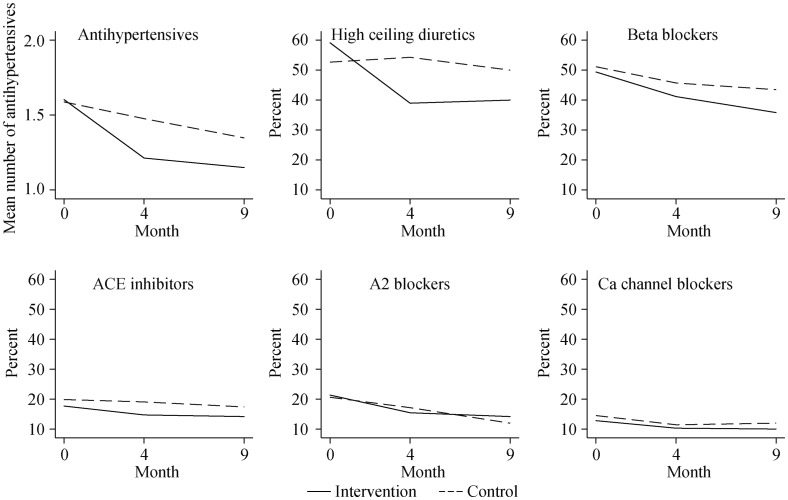
Both intervention and control patients used on average 1.6 ± 0.7 antihypertensives. In the intervention group, 14 (8%) had hypertension (systolic pressure ≥ 160 mmHg), and nine (5%) had hypotension (systolic pressure < 100 mmHg); in the control patients, these figures were 9 (7%) and 5 (4%), respectively. The number of antihypertensive drugs were reduced in 43 (32%) (Table 2) intervention group patients [incidence rate-ratio (IRR) = 0.8, 95% CI = 0.7–0.9] from baseline to month four, compared to a reduction in 11 (10%) control group patients (IRR = 0.9, 95% CI = 0.9–1.0) (Table 3). The reduction was greater in intervention than in control patients (IRR = 0.8, 95% CI = 0.7–0.9) (Figure 2). Three of 37 intervention patients with a reduction in antihypertensives from baseline to month four had antihypertensive drugs reinstated (Table 2). There were no significant changes in use of antihypertensives between month four and month nine (Table 3). Diuretics and beta-blockers were reduced most (Figure 2).
Table 2. Use of antihypertensives in patients with data at all three time points.
| Development | Intervention | Control |
| Decrease between baseline and month four | 37 | 6 |
| Reduction to month nine | 0 | 1 |
| No change month nine | 34 | 5 |
| Increase to month nine | 3 | 0 |
| No change between baseline and month four | 80 | 83 |
| Reduction to month nine | 10 | 11 |
| No change month nine | 68 | 72 |
| Increase to month nine | 2 | 0 |
| Increase between baseline and month four | 1 | 1 |
| Reduction to month nine | 0 | 0 |
| No change month nine | 1 | 1 |
| Increase to month nine | 0 | 0 |
Table 3. Tests of change in antihypertensive prescribing, blood pressure and pulse over nine months.
| Variables | Change between baseline and month four |
Change between month four and month nine |
|||||||||||
| Intervention | Control | Intervention | Control | ||||||||||
| *Antihypertensive drugs, IRR (95% CI) | 0.8 (0.7–0.9) | 0.9 (0.9–1.0) | 0.9 (0.9–1.0) | 0.9 (0.8–1.0) | |||||||||
| #High blood pressure, IRR (95% CI) | 1.0 (0.9–1.2) | 0.7 (0.5–1.0) | 1.0 (0.9–1.2) | 1.1 (0.5–2.5) | |||||||||
| †Cognitive status, IRR (95% CI) | |||||||||||||
| Severe dementia | 1 | 1 | 1 | 1 | |||||||||
| Moderate dementia | 1.1 (1.0–1.4) | 1.0 (0.9–1.2) | 1.0 (0.8–1.2) | 1.1 (0.8–1.3) | |||||||||
| Mild dementia | 1.0 (0.8–1.3) | 1.0 (0.9–1.2) | 1.1 (0.8–1.7) | 1.1 (0.8–1.4) | |||||||||
| No dementia | 1.2 (1.0–1.5) | 1.0 (0.9–1.2) | 1.1 (0.9–1.4) | 0.7 (0.4–1.3) | |||||||||
| §Systolic blood pressure, beta (95% CI) | |||||||||||||
| No antihypertensives described, n = 93 | –2 (–6.3 to 2.6) | 2 (–2.9 to 6.4) | 2 | ||||||||||
| Antihypertensives deprescribed, n = 43 | 14 (7.7–21.2) | –8 (–15.2 to –1.2) | –8 | ||||||||||
| ¤Pulse, beta (95% CI) | |||||||||||||
| No antihypertensives described, n = 93 | 0 (–2.7–3.5) | 0 (–3.1 to 3.5) | |||||||||||
| Antihypertensives deprescribed, n = 43 | 2 (–2.9–6.5) | 0 (–5.4 to 4.4) | |||||||||||
*Multilevel Poisson regression with IRR representing change in antihypertensive drug use; #Multilevel Poisson regression with IRR representing change in antihypertensive drug use for patients with high blood pressure compared to low/normal blood pressure; †Multilevel Poisson regression with IRR representing change in antihypertensive drug use between the different levels of cognitive function at baseline; §Multilevel linear regression with betas representing change in blood pressure between baseline and month four; ¤Multilevel linear regression with betas representing change in pulse between baseline and month four. IRR: incidence rate ratios.
Figure 2. Use of antihypertensives at baseline, month four, and month nine.
Percentages describe proportion of patients in each group using the drug. ACE: angiotensin converting enzyme.
Patients in the intervention group with a reduction in antihypertensive drugs from baseline to month four had an increase in their systolic blood pressure from 128 ± 19.5 to 143 ± 25.5 mmHg (mean difference = 14, 95% CI: 7.7–21.2) (Table 3). By month nine, the mean blood pressure had returned to its initial level, with no significant difference between baseline and month nine assessments (mean difference = 6, 95% CI: –1.9 to 14.6). There were no significant changes in blood pressure for patients staying on antihypertensive drugs (Table 3). The trends were similar for diastolic blood pressure, data not shown. Pulse was not affected by deprescribing (Table 3).
Patients with a systolic blood pressure ≥ 160 mmHg at baseline had the same reduction in number of antihypertensive drugs as patients with blood pressure < 160 mmHg (Table 3). There were no differences in deprescribing according to the different levels of cognitive function (Table 3).
Between baseline and month four, seven (5%) patients in the intervention group were admitted to the hospital and 19 (12%) died, while in the control group, 14 (13%) were admitted to the hospital and 17 (13%) died. Between month four and month nine, seven (6%) intervention group patients were hospitalized, and 17 (12%) died; corresponding figures for control group patients were 12 (13%) and 12 (11%) respectively. Hospitalization was significantly higher for controls at month four (P = 0.031) and month nine (P = 0.041).
4. Discussion
To our knowledge, this is the largest study on deprescribing antihypertensive drugs in nursing home patients with follow-up assessment of blood pressure over time. Independently of cognitive impairment, participants used 1.6 antihypertensive and in 32%, the drug regime was reduced following a person-centred care intervention. Over the next four months, the systolic blood pressure increased by 14 mmHg, however, at month nine, blood pressure had returned to baseline levels. The findings are of key importance for the clinician since collegial mentoring and re-assessment of the patient's blood pressure may decrease critical harms such as orthostatic hypotension, falls, and anticholinergic side effects. Especially, when keeping in mind that over 40% of Norwegian nursing home residents die in the first six months after admission, only 30% live more than two years, and over 80% have dementia.[3]
The mean baseline blood pressure in our patients was comparable to earlier findings,[11],[28] and the deprescribing procedures were conducted irrespective of hyper-, hypo-, or normotensive states. Not surprisingly, and in line with earlier observations,[29],[30] the blood pressure increased after discontinuation, but mean pressure was still below the indication for treatment.[4],[5] We also observed that the blood pressure decreased to initial levels after nine months, but the causality for this development is still uncertain. A Swedish nursing home study following 180 participants over 18 months found that the systolic blood pressure decreased by about 5 mmHg during the study period irrespective of any changes in cardiovascular drug prescriptions.[11] A steady decrease over time might therefore be expected in nursing home patients, and can explain the fall we experienced from month four to nine.
Beta-blockers and diuretics were the two drug groups that were most frequently deprescribed. Importantly, the reduction did not affect pulse throughout the study period. These drugs are also indicated in other cardiovascular diagnoses like atrial fibrillation and heart failure. However, more than 20% of the participants lacked a cardiovascular diagnosis. This is particularly alarming because beta-blockers and diuretics cause hypotension in 34% of elderly people at daytime and in 9% during the night, and they might even increase mortality.[31],[32] As discussed in the introduction, the guidelines are vague in their recommendations for antihypertensive treatment of the fragile old.[4],[5] Hence, effect of antihypertensive treatment for this group can be debated. Antihypertensive drugs are a major contributor to polypharmacy,[2] and polypharmacy is related to inappropriate prescribing, hospitalizations and side effects.[33] Reducing drugs with debatable potential benefits is a sensible approach when addressing polypharmacy. Another important element is to reduce drugs before the patient is too debilitated to swallow, by doing this we can reduce harmful effects of tapering.[34]
Our systematic medication review included the standardized training of nursing home staff, collegial support of nursing home physicians, and drug optimizing by person-centred care.[13] Contrary to other researchers, we did not introduce a new profession or recruit external aid from outside the nursing homes after the study period.[33] Consequently, we expected that the effect of collegial mentoring, multidisciplinary collaboration between physicians and nursing staff, and education would last beyond the four-month period. However, our results demonstrated that the main effects were concentrated between baseline and month four, and that significant differences between the intervention and control units diminished over time. Sustained collaboration, education, and learning are crucial prerequisites for person-centred care.[13]
This was a cluster-randomized trial, implying that total blinding of the healthcare staff is not possible. This may also explain a reduction of antihypertensives in the control groups. Another limitation may be that randomization of units―rather than physicians (six doctors were working in both intervention and control units)―possibly decreased the differences between the two groups. Furthermore, standard routines for measuring blood pressure are not well established in nursing homes. Our reliance on local routines may suggest an amount of uncertainty in the blood pressure results. Our study did not consider change in dosages that may have affected the clinical outcomes. The hospitalization rate was slightly higher in the control group, without significant differences in death rates; this might be a result of the advance care planning part of COSMOS.[15] At the same time, we did not systematically register adverse events like strokes or heart failure, so it is unclear whether the reductions may have caused additional events.
The transferability of the results is strengthened by a comprehensive sample size and the variety of units involved, as well as the individualized approach to medication optimization. Future research should focus on method development and validation of implementation processes.
4.1. Conclusions
Nursing home patients use numerous antihypertensive drugs with potential side effects and uncertain indication. The implementation of an individualized medication review decreased the use of antihypertensives. The change in drug prescription led to a temporary increase in the systolic blood pressure, which returned to initial readings over the study period. Multidisciplinary staff education, collegial mentoring, and person-centred care are crucial prerequisites for decision-making in connection with the medication review procedure.
4.2. Compliance with Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. We provided information about the study both orally and in writing to all patients and their next of kin. Patients who were able signed informed consent forms. When patients lacked the competence to provide consent, the next of kin provided presumed written consent on behalf of the patient. The Helsinki declaration was followed and the Regional Ethics Committee, West Norway approved the approach (2013/1765). The article follows the CONSORT guidelines on reporting cluster-randomized trials.[35], [36] The trial was registered at clinicaltrials.gov (NCT02238652), 7 July 2014.
Acknowledgments
We would like to give a special thanks to all participating nursing homes including patients, staff and physicians who made this study possible. BSH would like to thank the G.C. Rieber Foundation and the Norwegian Government for supporting our work at the Centre for Elderly and Nursing Home Medicine, University of Bergen, Norway. Statistician Dagrun Slettebo at the Biostatistics and Data analysis core facility (BIOS), University of Bergen, carried out parts of the work. We would also like to thank Irene Aasmul, Torstein Habiger, and Tony Elvegaard for helping with implementation of the study and data collection. This study was sponsored by the Norwegian Research Council, Oslo, Norway (Sponsor's Protocol Code: 222113/H10) and Rebekka Ege Hegermann's Legat, Bergen, Norway (R.E. Hegermann's Endowment). The sponsors had no say in the study concept and design, acquisition of subjects, analysis and interpretation of data and preparation of the manuscript. The authors declare no conflicts of interests. All authors contributed substantially in setting the aims and drafting the manuscript. All authors approved the final version.
References
- 1.Gordon AL, Franklin M, Bradshaw L, et al. Health status of UK care home residents: a cohort study. Age Ageing. 2014;43:97–103. doi: 10.1093/ageing/aft077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Onder G, Liperoti R, Foebel A, et al. Polypharmacy and mortality among nursing home residents with advanced cognitive impairment: results from the SHELTER study. J Am Med Dir Assoc. 2013;14:450 e457–412. doi: 10.1016/j.jamda.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Helvik AS, Engedal K, Benth JS, et al. Prevalence and Severity of Dementia in Nursing Home Residents. Dement Geriatr Cogn Disord. 2015;40:166–177. doi: 10.1159/000433525. [DOI] [PubMed] [Google Scholar]
- 4.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 5.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 6.Rich MW, Chyun DA, Skolnick AH, et al. Knowledge gaps in cardiovascular care of older adults: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society: Executive Summary. J Am Geriatr Soc. 2016;64:2185–2192. doi: 10.1111/jgs.14576. [DOI] [PubMed] [Google Scholar]
- 7.Onder G, Liperoti R, Fialova D, et al. Polypharmacy in nursing home in Europe: results from the SHELTER study. J Geront A Biol Sci Med. 2012;67:698–704. doi: 10.1093/gerona/glr233. [DOI] [PubMed] [Google Scholar]
- 8.Harrison JK, Van Der Wardt V, et al. New horizons: the management of hypertension in people with dementia. Age Ageing. 2016;45:740–746. doi: 10.1093/ageing/afw155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 10.Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged >/=75 years: a randomized clinical trial. JAMA. 2016;315:2673–2682. doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radholm K, Festin K, Falk M, et al. Blood pressure and all-cause mortality: a prospective study of nursing home residents. Age Ageing. 2016;45:826–832. doi: 10.1093/ageing/afw122. [DOI] [PubMed] [Google Scholar]
- 12.Garfinkel D, Zur-Gil S, Ben-Israel J. The war against polypharmacy: a new cost-effective geriatric-palliative approach for improving drug therapy in disabled elderly people. Israel Med Assoc J. 2007;9:430–434. [PubMed] [Google Scholar]
- 13.American Geriatrics Society Expert Panel on Person-Centered C. Person-centered care: a definition and essential elements. J Am Geriatr Soc. 2016;64:15–18. doi: 10.1111/jgs.13866. [DOI] [PubMed] [Google Scholar]
- 14.Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827–834. doi: 10.1001/jamainternmed.2015.0324. [DOI] [PubMed] [Google Scholar]
- 15.Husebo BS, Flo E, Aarsland D, et al. COSMOS―improving the quality of life in nursing home patients: protocol for an effectiveness-implementation cluster randomized clinical hybrid trial. Implement Sci. 2015;10:131. doi: 10.1186/s13012-015-0310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orfaly RA, Frances JC, Campbell P, et al. Train-the-trainer as an educational model in public health preparedness. J Public Health Manag Pract. 2005;Suppl:S123–S127. doi: 10.1097/00124784-200511001-00021. [DOI] [PubMed] [Google Scholar]
- 17.Norwegian Directorate of Health. [Guidelines for medication reviews] 2012;vol IS-1998 [Google Scholar]
- 18.[Check list for medication review] [(accessed March 22, 2017)]. Http://www.kunnskapssenteret.no/verktoy/sjekkliste-for-legemiddelgjennomgang#begreper.
- 19.O'Mahony D, O'Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44:213–218. doi: 10.1093/ageing/afu145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duran CE, Azermai M, Vander Stichele RH. Systematic review of anticholinergic risk scales in older adults. Eur J Clin Pharmacol. 2013;69:1485–1496. doi: 10.1007/s00228-013-1499-3. [DOI] [PubMed] [Google Scholar]
- 21.Lynn J. Perspectives on care at the close of life. Serving patients who may die soon and their families: the role of hospice and other services. JAMA. 2001;285:925–932. doi: 10.1001/jama.285.7.925. [DOI] [PubMed] [Google Scholar]
- 22.Interaksjoner.no [database on the Internet] The Norwegian Medicines Agency. 2014. [(accessed on 23 February 2017)]. Http://interaksjoner.
- 23.MMSE-NR The Standardized Norwegian MMSE. [(accessed on February 23 2017)]. http://www.aldringoghelse.no/ViewFile.aspx?ItemID=1495.
- 24.ATC/DDD Index [database on the Internet] WHO Collaborating Centre for Drug Statistics Methodology. 2015. [(accessed on February 23, 2017)]. Http://www.whocc.no/atc_ddd_index/
- 25.Wood M, Lamberts H, Hofmans-Okkes I. The International classification of primary care in the European community: with a multi-language layer. Oxford University Press; 1993. [Google Scholar]
- 26.Perneczky R, Wagenpfeil S, Komossa K, et al. Mapping scores onto stages: mini-mental state examination and clinical dementia rating. Am J Geriatr Psychiatry. 2006;14:139–144. doi: 10.1097/01.JGP.0000192478.82189.a8. [DOI] [PubMed] [Google Scholar]
- 27.Cameron AC, Trivedi PK. Regression analysis of count data. Cambridge university press; 2013. [Google Scholar]
- 28.Lochner S, Kirch W, Schindler C. Managing hypertension among nursing-home residents and community-dwelling elderly in Germany: a comparative pharmacoepidemiological study. Eur J Clin Pharmacol. 2012;68:867–875. doi: 10.1007/s00228-011-1195-0. [DOI] [PubMed] [Google Scholar]
- 29.Medical research council working party on mild hypertension. Course of blood pressure in mild hypertensives after withdrawal of long term antihypertensive treatment. Medical Research Council Working Party on Mild Hypertension. Br Med J (Clin Res Ed) 1986;293:988–992. doi: 10.1136/bmj.293.6553.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moonen JE, Foster-Dingley JC, de Ruijter W, et al. Effect of discontinuation of antihypertensive medication on orthostatic hypotension in older persons with mild cognitive impairment: the DANTE Study Leiden. Age Ageing. 2016;45:249–255. doi: 10.1093/ageing/afv199. [DOI] [PubMed] [Google Scholar]
- 31.Divison-Garrote JA, Ruilope LM, de la Sierra A, et al. Magnitude of hypotension based on office and ambulatory blood pressure monitoring: results from a cohort of 5066 treated hypertensive patients aged 80 years and older. J Am Med Dir Assoc. 2017;18:452 e451–452 e456. doi: 10.1016/j.jamda.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Delgado J, Masoli JAH, Bowman K, et al. Outcomes of treated hypertension at age 80 and older: cohort analysis of 79,376 individuals. J Am Geriatr Soc. 2017;65:995–1003. doi: 10.1111/jgs.14712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hovstadius B, Petersson G. Factors leading to excessive polypharmacy. Clin Geriatr Med. 2012;28:159–172. doi: 10.1016/j.cger.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Lundgren C. In: FAS UT 3. 3rd edition. Question AB, editor. Umeå, Sweden: 2010. [Google Scholar]
- 35.Loganathan M, Singh S, Franklin BD, et al. Interventions to optimise prescribing in care homes: systematic review. Age Ageing. 2011;40:150–162. doi: 10.1093/ageing/afq161. [DOI] [PubMed] [Google Scholar]
- 36.Campbell MK, Piaggio G, Elbourne DR, et al. Consort 2010 statement: extension to cluster randomised trials. BMJ. 2012;345:e5661. doi: 10.1136/bmj.e5661. [DOI] [PubMed] [Google Scholar]
- 37.Altman DG, Schulz KF, Moher D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134:663–694. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]