Abstract
The goal of this work was to investigate the tumor mutational burden (TMB) in Chinese patients with gynecologic cancer. In total, 117 patients with gynecologic cancers were included in this study. Both tumor DNA and paired blood cell genomic DNA were isolated from formalin-fixed paraffin-embedded (FFPE) specimens and blood samples, and next-generation sequencing was performed to identify somatic mutations. TP53, PTEN, ARID1A, and PIK3CA alterations were significantly different in various types of gynecologic cancers (p = 0.001, 1.15E-07, 0.004, and 0.009, respectively). The median TMB of all 117 gynecologic tumor specimens was 0.37 mutations/Mb, with a range of 0–41.45 mutations/Mb. Despite the lack of significant difference, endometrial cancer cases had a higher median TMB than cervical and ovarian cancer cases. Younger gynecologic cancer patients (age <40 years) had a significantly lower TMB than older patients (age ≥40 years) (p = 0.04). In addition, TMB was significantly increased with increasing clinical stage of disease (p = 0.001). PTEN alterations were commonly observed in patients with a moderate to high TMB (n = 8, 38.10%, p = 9.95E-04). Although limited by sample size, all of the patients with TSC2 (n = 3, p = 3.83E-11) or POLE (n = 2, p = 0.005) mutations had a moderate to high TMB. Further large-scale, prospective studies are needed to validate our findings.
Introduction
Gynecologic oncology is a specialized field of medicine that focuses on cancers of the female reproductive system, including cancer of the cervix, ovaries, uterus, fallopian tubes, vagina and vulva. Gynecologic cancers annually affect approximately 100 million people worldwide and account for approximately 18% of all female cancers1. Cervical cancer is the most common cancer of the female reproductive system in China2. According to the latest data3, 100 thousand new cases of cervical cancer occurred in 2013. Additionally, an estimated 98.9 thousand new cervical cancer cases and an estimated 30.5 thousand associated deaths were predicted in 20154. The overall five-year relative survival rate for cervical cancer is 45.4% in China5. Endometrial cancer is the second-most common cancer of the female reproductive system in China2. In 2013, 61.9 thousand new cases of endometrial cancer were reported. Furthermore, endometrial cancer was estimated to account for 63.4 thousand new cancer cases in 20154. Moreover, the number of disease-specific deaths was estimated to increase from 17.9 thousand in 2013 to 21.8 thousand in 20153,4. The overall five-year relative survival rate for endometrial cancer is 55.1% in China5. Ovarian cancer is the third-most common cancer of the female reproductive system in China2. Approximately 50 thousand new ovarian cancer cases occurred in 2013, while 52.1 thousand new cases were estimated to have occurred in 20153,4. In 2013, 21.3 thousand patients died of ovarian cancer5. Ovarian cancer was estimated to account for 22.5 thousand deaths in 20154. The overall five-year relative survival rate for ovarian cancer is 38.9% in China5. The survival rate for ovarian cancer is lower than that for cervical and endometrial cancer. The major reason for this poor survival is that ovarian cancer is commonly diagnosed at an advanced stage due to its anatomic location. Survival rates decrease sharply with increasing stage of the disease. The five-year relative survival rates of ovarian cancer were 90.2%, 68.3%, 32.9%, and 16.1% for stage I, II, III, and IV, respectively, according to data from a historical cohort study in Hong Kong6; however, the data at the national level are still not available. Traditionally, the management of women with gynecologic cancer is predominantly based on surgery, cytotoxic chemotherapy and radiotherapy, either alone or in combination, as dictated by the clinical circumstances, with the stage of disease largely determining the need for adjuvant or first-line chemotherapy or radiation. However, approximately 70% of patients experience disease relapse after varying disease-free intervals. PEGylated liposomal doxorubicin, topotecan, and gemcitabine are among the cytotoxic agents used in the platinum-resistant setting, with generally low response rates (RRs)7–9. More recently, an increasing number of targeted therapies directed against a variety of molecular targets in gynecologic cancers and their microenvironments have been developed and used in women with these malignancies10. Among these targeted therapies, increasing evidence supports a potential role for immune checkpoint inhibitors as a viable therapeutic strategy in gynecologic cancers11–17. Although encouraging results have been reported in early clinical trials, immune checkpoint inhibitors exhibit limited response in cancers, and none of the new drugs is being studied in prospective phase III trials16,18,19. Given the potential that these agents have shown for the treatment of refractory disease and durable responses in some cases, there is great interest in identifying patients who are most likely to benefit from these therapies. In addition to PD-1/PD-L1 expression, there are other factors that can affect responses to immunotherapy, including mismatch repair deficiency, microsatellite instability (MSI), POLE mutations and tumor mutational burden (TMB)15,20–24. In fact, in one clinical trial, TMB was more significantly associated with RR than was the expression of PD-L1 by immunohistochemistry25. Neoantigen load has also been correlated with response to immunotherapy26.
To date, no study has reported on TMB in Chinese populations with gynecologic cancer. Here, we used a designed 1.15-megabase (Mb) panel to analyze the range and frequency of hypermutations among and within different types of gynecologic cancers. Furthermore, we characterized and identified specific genes associated with an increased TMB in Chinese patients with gynecologic cancer.
Results
Patient characteristics
In this study, the patient age ranged from 16 to 80 years with a median of 55 years (Table 1). Most tumors originated in the ovary: 68 cases were ovarian cancer including 1 case of primary peritoneal cancer and 3 cases of fallopian tube cancer; 32 cases were cervical cancer; and 17 cases were endometrial cancer. Of the 117 patients, 17.95% had stage I, 29.06% had stage II, 36.75% had stage III, and 12.82% had stage IV disease, and we were unable to determine the stage for 4 cases. According to the 2014 WHO Classification of Tumors of the Female Reproductive Organs27, 60 (51.28%) cases were serous adenocarcinoma including 2 cases with minor clear cell adenocarcinoma components, of which 59 cases occurred in the ovary and 1 case was endometrial; 3 (2.56%) cases were clear cell adenocarcinoma in the ovary; 17 (14.53%) cases were endometrioid adenocarcinoma, of which one case occurred in the ovary whereas the remainder were endometrial; 31 (26.5%) cases were squamous cell carcinoma in the cervix; 1 (0.85%) case was mucinous adenocarcinoma in the cervix; and 5 (4.27%) cases were of other histologic types in the ovary, including one case with osteosarcoma, one case with immature teratoma, one case with sex cord-mesenchymal tumor, and two cases with dysgerminoma. For cervical cancer, HPV status was checked in 14 patients. Nine patients were HPV positive, whereas five patients were HPV negative. Among the patients with endometrial carcinoma included in this study, most (16/17, 94.12%) were classified as type I, including one case with squamous differentiation. One patient with serous adenocarcinoma was identified as type II endometrial carcinoma.
Table 1.
Characteristics of studied group.
| Numbers of patients (n = 117) | |
|---|---|
| Age, median (range), years | 55 (16–80) |
| <40 | 14 (11.97%) |
| 40–49 | 26 (22.22%) |
| 50–59 | 43 (36.75%) |
| >=60 | 34 (29.06%) |
| Site of Disease | |
| Ovary (include primary peritoneal and fallopian tube) | 68 (58.12%) |
| Cervix | 32 (27.35%) |
| Endometrial | 17 (14.53%) |
| Stage of Disease | |
| stage I | 21 (17.95%) |
| stage II | 34 (29.06%) |
| stage III | 43 (36.75%) |
| stage IV | 15 (12.82%) |
| NA | 4 (3.42%) |
| HPV status (Cervix cancer) | |
| positive | 9 (28.13%) |
| negative | 5 (15.63%) |
| uncertain | 18 (56.25%) |
| Subtype of Endometrial Carcinoma | |
| Type I | 16 (94.12%) |
| Type II | 1 (5.88%) |
| Tissue types (in each cancer types) | |
| Ovarian cancer | |
| Serous adenocarcinoma | 59 (86.76%) |
| Clear cell adenocarcinoma | 3 (4.41%) |
| Endometroid adenocarcinoma | 1 (1.47%) |
| Other | 5 (7.35%) |
| Cervical cancer | |
| Squamous cell carcinoma | 31 (96.88%) |
| Mucinous adenocarcinoma | 1 (3.12%) |
| Endometrial cancer | |
| Endometroid adenocarcinoma | 16 (94.12%) |
| Serous adenocarcinoma | 1 (5.88%) |
Genomic profiles of gynecologic cancers
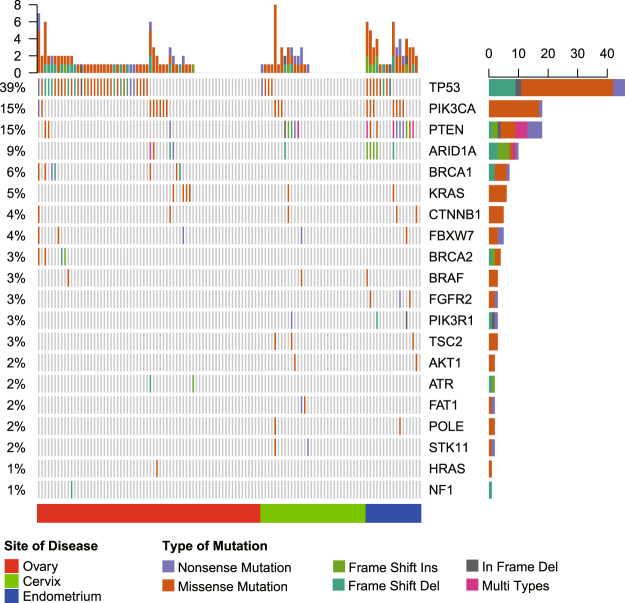
The genomic profiles of the 117 gynecologic cancer specimens were analyzed by using a designed panel which covered 1086 cancer-related genes (Supplemental Table S1). Out of the 117 cases, genomic alterations were identified in 79 (67.52%) including 48 (70.59%) ovarian cancer cases, 15 (46.88%) cervical cancer cases, and 16 (94.12%) endometrial cancer cases. The genomic profiles of the three types of cancers were distinct. In the cases with ovarian cancer (including primary peritoneal cancer and fallopian tube cancer), the most frequently altered genes were TP53 (50%), PIK3CA (12%), BRCA1 (10%), ARID1A (6%), and BRCA2 (6%) (Fig. 1). In the cases with cervical cancer, the most frequently altered genes were PTEN (16%), TP53 (12%), PIK3CA (9%), FAT1 (6%), STK11 (6%), and TSC2 (6%) (Fig. 1). Finally, in the cases with endometrial cancer, the most frequently altered genes were PTEN (59%), TP53 (47%), PIK3CA (41%), ARID1A (29%), and FGFR2 (18%) (Fig. 1). TP53 alterations were significantly more common in ovarian and endometrial cancer cases than in cervical cancer cases (50% and 47% vs 12%, p = 0.001). Additionally, ARID1A, PIK3CA and PTEN alterations were significantly more common in endometrial cancer cases than in ovarian and cervical cancer cases (ARID1A: 29% vs 6% and 3%, p = 0.004; PIK3CA: 41% vs 12% and 9%, p = 0.009; PTEN: 59% vs 4% and 16%, p = 1.15E-07), whereas BRCA1 and BRCA2 somatic mutations were found only in patients with ovarian cancer.
Figure 1.
Heat map showing somatic mutation profiles of gynecologic cancers.
TMB of gynecologic cancers
We next investigated the TMB of gynecologic cancers and their association with clinical status. Across the entire dataset, the median TMB was 0.37 mutations/Mb, with a range of 0–41.45 mutations/Mb. Out of the 117 patients, 96 (82.05%) had a low TMB, 14 (11.97%) had a moderate TMB, and 7 (5.98%) had a high TMB. Although the TMB of different age groups was not significantly different (p = 0.37), younger patients with gynecologic cancer (age <40 years) had a significantly lower TMB than older patients (age ≥40 years) (Table 2 and Supplementary Table S2, p = 0.04). In addition to age, different cancer types also showed various TMB distribution patterns. High TMB values were found in all gynecologic cancer types – 8 (11.76%) ovarian cancer cases had a moderate to high TMB value including one case with a high TMB; 4 (23.53%) endometrial cancer cases had a moderate to high TMB including 2 cases with a high TMB; and 9 (28.13%) cervical cancer cases had a moderate to high TMB including 4 cases with a high TMB. Despite the lack of statistically significant difference, patients with endometrial cancer had a higher median TMB than patients with cervical and ovarian cancer (including primary peritoneal and fallopian tube cancer) (2.30 vs 0.67 and 0.36 Fig. 2 and Table 2), indicating that immune checkpoint blockade may be a potential treatment for endometrial cancer patients. In cervical cancer, TMB did not differ significantly according to HPV status (Table 2). Additionally, TMB was significantly associated with the clinical stage of gynecologic cancer (Table 2, p = 0.001). Increasing stage of tumors was associated with increasing median TMB – 0 mutations/Mb for stage I, 0 mutations/Mb for stage II, 0.83 mutations/Mb for stage III, and 2.92 mutations/Mb for stage IV disease. Although endometrioid adenocarcinoma cases had a relatively increased median TMB, there was no statistically significant difference in TMB values among gynecologic cancers with different histologies (Table 2 and Supplementary Table S2, p = 0.34).
Table 2.
The association of TMB levels and clinical status.
| TMB, median (range), mutations/Mb | P valuea | TMB level | P valueb | |||
|---|---|---|---|---|---|---|
| Low (%) | Moderate to high (%) | |||||
| Age, median (range), years | 0.04 | 0.26 | ||||
| <40 | 14 | 0 (0–4.80) | 13 (92.86%) | 1 (7.14%) | ||
| >=40 | 103 | 0.67 (0–41.45) | 83 (80.58%) | 20 (19.42%) | ||
| Site of Disease | 0.81 | 0.11 | ||||
| Ovary (include primary peritoneal and fallopian tube) | 68 | 0.36 (0–22.26) | 60 (88.24%) | 8 (11.76%) | ||
| Cervix | 32 | 0.67 (0–40.81) | 23 (71.87%) | 9 (28.13%) | ||
| Endometrial | 17 | 2.30 (0–41.45) | 13 (76.47%) | 4 (23.53%) | ||
| stage | 0.001c | 0.03 | ||||
| Stage I | 21 | 0 (0–3.20) | 21 (100%) | 0 (0%) | ||
| Stage II | 34 | 0 (0–4.80) | 30 (88.24%) | 4 (11.76%) | ||
| Stage III | 43 | 0.83 (0–22.26) | 34 (79.07%) | 9 (20.93%) | ||
| Stage IV | 15 | 2.92 (0–41.45) | 8 (53.33%) | 7 (46.67%) | ||
| NA | 4 | 2.62 (0.45–13.80) | 3 (75%) | 1 (25%) | ||
| HPV status (Cervix cancer) | 0.506 | 0.255 | ||||
| positive | 9 | 0 (0–9) | 7 (77.78%) | 2 (22.22%) | ||
| negative | 5 | 2 (0–7) | 5 (100%) | 0 (0%) | ||
| uncertain | 18 | 2.28 (0–40.81) | 11 (61.11%) | 7 (38.89%) | ||
| Subtype of Endometrial Carcinoma | — | — | ||||
| Type I | 16 | 2.30 (0–41.45) | 12 (75%) | 4 (25%) | ||
| Type II | 1 | 0.95 | 1 (100%) | 0 (0%) | ||
| Tissue types | 0.34 | 0.18 | ||||
| Serous adenocarcinoma | 60 | 0.36 (0–22.26) | 52 (86.67%) | 8 (13.33%) | ||
| Clear cell adenocarcinoma | 3 | 0.38 (0–3.20) | 3 (100%) | 0 (0%) | ||
| Endometroid adenocarcinoma | 17 | 2.30 (0–41.45) | 13 (76.47%) | 4 (23.53%) | ||
| Squamous cell carcinoma | 31 | 0.99 (0–40.81) | 22 (70.97%) | 9 (29.03%) | ||
| Mucinous adenocarcinoma | 1 | 0 | 1 (100%) | 0 (0%) | ||
| Other | 5 | 0 (0–2.30) | 5 (100%) | 0 (0%) | ||
Note: acalculated using Mann-Whitney test/Kruskal-Wallis test followed by Dunn’s multiple comparison as post-hoc test. bCalculated using Pearson’s chi-square test. cCalculated using Kruskal-Wallis test followed by Dunn’s multiple comparison as post-hoc test, the Dunn’s multiple comparison showed that the difference between stage I and stage IV, stage II and stage IV, satge I and stage III were statistical significant (P = 0.0007, 0.019, and 0.009 respectively).
Figure 2.
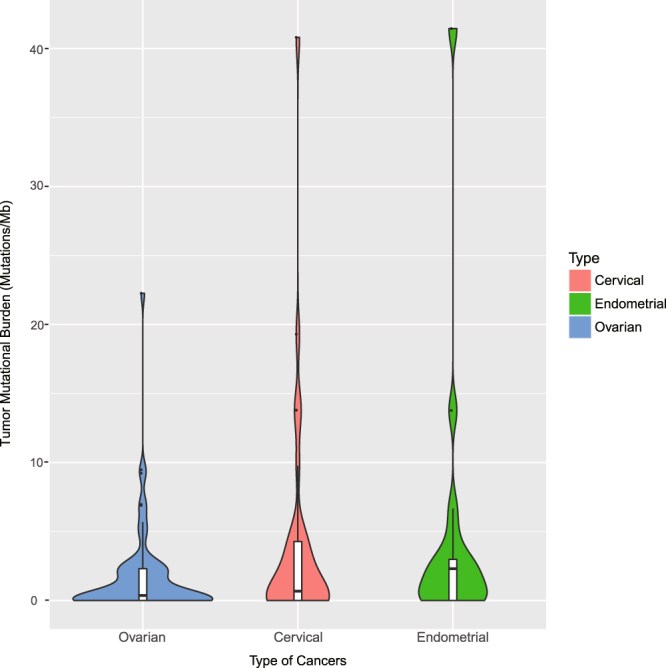
TMB levels of gynecologic cancers.
Genomic alterations associated with TMB
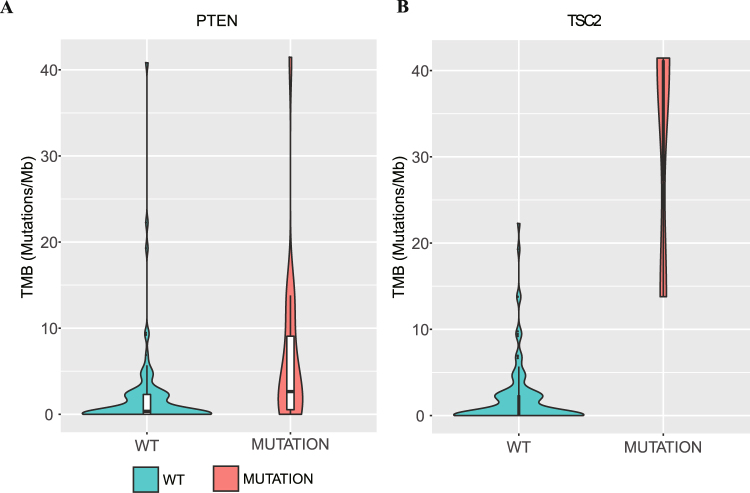
To explore the association between genomic alterations and TMB, we investigated the molecular profiles of moderate to high TMB across our samples and performed statistical analysis to identify the specific genetic alterations related to increased TMB. Previous studies have shown that POLE alterations are associated with TMB in multiple cancer types28. In line with previous research, we found that patients with POLE mutations had a significantly higher TMB (Table 3, p = 0.005). In addition to POLE, other genomic alterations associated with TMB were identified in our study. Indeed, somatic mutations in PTEN were commonly found in patients with a moderate to high TMB (n = 8, 38.10%) (Fig. 3A and Table 3). Moreover, limited by the sample size, TSC2 mutations were detected in only 3 patients (2.56%). However, all three of these patients had a high TMB (Fig. 3B, Table 3 and Supplementary Fig. S1). In addition to PTEN, POLE and TSC2, the gene mutation status of BRCA1, FBXW7, PIK3R1 and STK11 differed significantly according to TMB (Table 3 and Supplementary Fig. S1). Patients with mutated BRCA1 (4, 57.14%), FBXW7 (3, 60%), PIK3R1 (2, 66.67%) and STK11 (1, 50%) tended to have higher TMB levels. However, due to the limited sample size, these four genes need to be further confirmed in a large study. Although TP53 alterations are commonly seen in gynecologic cancer, it was observed in only five patients with a moderate to high TMB in our cohort (Table 3). Furthermore, there was no significant difference in TMB between patients with or without TP53 mutations (p = 0.26).
Table 3.
The association of TMB levels and genomic alterations.
| mutation status | TMB, median (range), mutations/Mb | P valuea | TMB level | P valueb | ||
|---|---|---|---|---|---|---|
| Low (%) | Moderate to high (%) | |||||
| BRCA1 | mutated | 5.68 (0–22.26) | 0.015 | 3 (42.86%) | 4 (57.14%) | 0.005 |
| wild type | 0.36 (0–41.45) | 93 (84.55%) | 17 (15.45%) | |||
| FBXW7 | mutated | 4.80 (0.45–22.26) | 0.003 | 2 (40%) | 3 (60%) | 0.012 |
| wild type | 0.36 (0–41.45) | 94 (83.93%) | 18 (16.07%) | |||
| PIK3R1 | mutated | 13.77 (2.30–13.79) | 4.46E-05 | 1 (33.33%) | 2 (66.67%) | 0.026 |
| wild type | 0.36 (0–41.45) | 95 (83.33%) | 19 (16.67%) | |||
| POLE | mutated | 23.73 (6.66–40.81) | 0.005 | 0 (0%) | 2 (100%) | 0.002 |
| wild type | 0.36 (0–41.45) | 96 (83.48%) | 19 (16.52%) | |||
| PTEN | mutated | 2.64 (0–41.45) | 0.000995 | 10 (55.56%) | 8 (44.44%) | 0.001 |
| wild type | 0.35 (0–40.81) | 86 (86.87%) | 13 (13.13%) | |||
| STK11 | mutated | 20.41 (0–40.81) | 0.028 | 1 (50%) | 1 (50%) | 0.234 |
| wild type | 0.37 (0–41.45) | 95 (82.61%) | 20 (17.39%) | |||
| TSC2 | mutated | 40.81 (13.78–41.45) | 3.83E-11 | 0 (0%) | 3 (100%) | 0.005 |
| wild type | 0.36 (0–22.26) | 96 (84.21%) | 18 (15.79%) | |||
| AKT1 | mutated | 1.15 (0–2.30) | 0.802 | 2 (100%) | 0 (0%) | 0.505 |
| wild type | 0.37 (0–41.45) | 94 (81.74%) | 21 (18.26%) | |||
| ARID1A | mutated | 1.15 (0–9.77) | 0.135 | 7 (70%) | 3 (30%) | 0.299 |
| wild type | 0.37 (0–41.45) | 89 (83.18%) | 18 (16.82%) | |||
| ATR | mutated | 2.84 (0–5.68) | 0.245 | 1 (50%) | 1 (50%) | 0.234 |
| wild type | 0.37 (0–41.45) | 95 (82.61%) | 20 (17.39%) | |||
| BRAF | mutated | 2.98 (0.69–4.80) | 0.488 | 2 (66.67%) | 1 (33.33%) | 0.482 |
| wild type | 0.37 (0–41.45) | 94 (82.46%) | 20 (17.54%) | |||
| BRCA2 | mutated | 4.25 (0–22.26) | 0.166 | 2 (50%) | 2 (50%) | 0.089 |
| wild type | 0.36 (0–41.45) | 94 (83.19%) | 19 (16.81%) | |||
| CTNNB1 | mutated | 0 (0–22.26) | 0.313 | 4 (80%) | 1 (20%) | 0.903 |
| wild type | 0.38 (0–41.45) | 92 (82.14%) | 20 (17.86%) | |||
| FAT1 | mutated | 2.40 (0–4.80) | 0.245 | 1 (50%) | 1 (50%) | 0.234 |
| wild type | 0.37 (0–41.45) | 95 (82.61%) | 20 (17.39%) | |||
| FGFR2 | mutated | 2.30 (0–6.66) | 0.488 | 2 (66.67%) | 1 (33.33%) | 0.482 |
| wild type | 0.37 (0–41.45) | 94 (82.46%) | 20 (17.54%) | |||
| HRAS | mutated | 3.2 | 0.896 | 1 (100%) | 0 (0%) | 0.639 |
| wild type | 0.37 (0–41.45) | 95 (81.90%) | 21 (18.10%) | |||
| KRAS | mutated | 0.22 (0–4.53) | 0.782 | 5 (83.33%) | 1 (16.67%) | 0.933 |
| wild type | 0.37 (0–41.45) | 91 (81.98%) | 20 (18.02%) | |||
| NF1 | mutated | 0.37 | 0.896 | 1 (100%) | 0 (0%) | 0.639 |
| wild type | 0.37 (0–41.45) | 95 (81.90%) | 21 (18.10%) | |||
| PIK3CA | mutated | 1.34 (0–40.81) | 0.114 | 12 (66.67%) | 6 (33.33%) | 0.061 |
| wild type | 0.36 (0–41.45) | 84 (84.85%) | 15 (15.15%) | |||
| TP53 | mutated | 0.76 (0–22.26) | 0.265 | 41 (89.13%) | 5 (10.87%) | 0.108 |
| wild type | 0 (0–41.45) | 55 (77.46%) | 16 (22.54%) | |||
Note: acalculated using Mann-Whitney test/Kruskal-Wallis test. bCalculated using Pearson’s chi-square test.
Figure 3.
Association of mutations in cancer genes with TMB. (A) Plot of mutation burden in specimens with known or likely driver mutations in PTEN (n = 18) and specimens without such mutations (n = 99). (B) Plot of mutation burden in specimens with known or likely driver mutations in TSC2 (n = 3) and specimens without such mutations (n = 114).
Discussion
To the best of our knowledge, the present study is the first to assess TMB in Chinese patients with gynecologic cancers and to describe the mutational profiles that are associated with an increased TMB. Tumor mutations are a key mechanism for the generation of anticancer immunity29. It is hypothesized that highly mutated tumors are more likely to harbor neoantigens that make them targets of activated immune cells22–24. In fact, in one clinical trial, TMB was more significantly associated with RR than was PD-L1 expression by immunohistochemistry25. Neoantigen load has also been linked with response to immunotherapy26. Whole exome sequencing (WES) has been previously used to measure TMB; however, smaller gene panels have been recently used to measure TMB with equal accuracy as that of WES and to determine the association of TMB with response to immunotherapy25,26. In this study, we used a 1.15-Mb panel representing 1086 cancer-related genes to measure TMB in Chinese patients with gynecologic cancers. Both WES and our designed small 1.15-Mb panel were used to calculate the TMB of 25 cancer specimens. The TMB values based on the designed small panel were consistent with those determined by WES (R2 = 0.734, data not shown), indicating that the designed small panel is an accurate, cost-effective and clinically available tool for measuring TMB.
Overall the median TMB in gynecologic cancers is 0.37 mutations/Mb, with a range of 0–41.45 mutations/Mb. Most of the patients (82.05%) had a low TMB, while only a few patients (5.98%) had a high TMB. Although high TMB values were observed in all types of gynecologic cancers, endometrial cancer cases had a higher median TMB than ovarian and cervical cancer cases, and 23.53% of patients with endometrial cancer had a moderate to high TMB, indicating that immune checkpoint blockade may be a potential treatment for endometrial cancer. In previous clinical studies, pembrolizumab showed durable anti-tumor activity in a subset of endometrial cancer patients16,30. In contrast, the median TMB in ovarian cancer was lower than that in endometrial and cervical cancer. In addition, most of the ovarian cancer patients (88.24%) had a low TMB, and only one patient had a high TMB. These results were consistent with clinical findings that immune checkpoint inhibitors result in limited tumor response in ovarian cancer19,31. Previous researchers have reported PD-L1 expression in 95% of cervical intraepithelial neoplasia and 80% of squamous cell carcinomas11, and lymph nodes harboring metastatic cervical cancer were characterized by high levels of PD-L1+ antigen-presenting cells (APCs) and FOXP3+ regulatory T (Treg) cells12. Although the median TMB in cervical cancer cases was not high, moderate to high TMB values were observed in a subset of patients (28.13%), which may be a potential group that can benefit from immune checkpoint inhibitor therapy. Several ongoing trials are investigating immune checkpoint inhibitors alone or in combination with chemotherapy or other biological agents in patients with advanced and recurrent cervical cancer, but the results have not been published yet32.
Understanding the factors associated with genomic instability is also important to better comprehend carcinogenesis and progression. Previous researchers have shown that patients with POLE mutations have a significantly higher TMB than patients without POLE mutations28. We investigated the molecular profiles of patients with a moderate to high TMB and characterized the distribution of somatic mutations in known genes. Similar to previous studies, we observed two patients with POLE mutations – one patient had a moderate TMB, and one patient had a high TMB – and TMB values were significantly increased in patients with POLE mutations. In addition to POLE, we identified several other genes associated with high TMB. Alterations in TSC2 were associated with a large increase in TMB, although we identified only three cases with single nucleotide variants (SNVs) in this gene. There was another gene, PTEN, that was significantly associated with TMB. PTEN mutations were commonly seen in patients with a moderate to high TMB. Both PTEN and TSC2 are tumor suppressor genes involved in the PI3K/MTOR/AKT pathway, which is commonly activated in gynecologic cancers33–35. According to previous studies, PTEN is correlated with PD-1/PD-L1 expression in lung cancer, and it also plays a role in maintaining genomic integrity during processes such as DNA replication and chromosome segregation36. In addition to PTEN, POLE, and TSC2, the gene mutation status of BRCA1, FBXW7, PIK3R1, and STK11 was related to TMB level. Both FBXW7 and PIK3R1 are involved in the PI3K/MTOR/AKT pathway37,38. The relationship among FBXW7, PIK3R1, and immune check point inhibitors remains to be elucidated. BRCA1, a tumor suppressor gene, plays an important role in gynecologic cancers, especially ovarian cancer39. In 2016, Strickland et al. reported that high grade serous ovarian cancers with BRCA1/2 mutations and a high number of tumor-infiltrating lymphocytes (TILs) were associated with elevated expression of PD-1/PD-L140. Loss of STK11 or STK11 somatic mutations may affect the progression of gynecologic cancers41,42. According to previous studies, STK11 deficiency in patients with lung cancer is associated with decreased expression of PD-1/PD-L1 and a reduced response rate to immune check point inhibitors43,44. Due to the limited sample size, the associations of the BRCA1, FBXW7, PIK3R1, and STK11 genes with TMB must be further verified in a larger cohort.
In conclusion, we investigated the landscape of TMB in Chinese patients with gynecologic cancers. Patients with mutations in the PTEN, TSC2 or POLE gene have an increased TMB. Further large-scale, prospective studies are needed to validate our findings.
Materials and Methods
Patient cohort
Formalin-fixed paraffin-embedded (FFPE) tumor specimens were collected at the Department of Gynecology at Chinese People’s Liberation Army (PLA) General Hospital. Clinical data regarding age at first diagnosis, diagnosis, and histology were collected from patient records. The clinical characteristics of the patients are listed in Table 1. The cohort consisted of 64 patients with ovarian cancer, 1 patient with primary peritoneal cancer, 3 patients with fallopian tube cancer, 32 patients with cervical cancer, and 17 patients with endometrial cancer.
Ethics statement
The study protocol was approved by the Committee of Medical Ethics of the Chinese PLA General Hospital and carried out according to the principles of the Declaration of Helsinki. All patients provided written informed consent to participate in the study prior to their enrollment. All experiments were carried out in accordance with relevant guidelines and regulations.
DNA extraction, library construction and next-generation sequencing (NGS) analysis
FFPE tissue specimens and matched blood samples were collected from 117 patients who were diagnosed with gynecologic cancer. Tumor DNA was isolated from FFPE specimens with the blackPREP FFPE DNA Kit (Analytik Jena AG, Jena, Germany) according to the manufacturer’s instructions. Each DNA sample was obtained from four 2-μm tissue specimens and quantified with the Qubit dsDNA HS Assay kit (Life Technologies, USA) according to the manufacturer’s recommended protocol. Blood lymphocytes were isolated by centrifugation of whole blood at 1600 g for 10 min at room temperature. Tiangen whole blood DNA kits (Tiangen, Beijing, PRC) were used to extract DNA from peripheral blood lymphocytes according to the manufacturer’s instruction. Tumor DNA and matched genomic DNA were sheared into 150–200-bp fragments by a Covaris M220 Focused-Ultrasonicator (Covaris, Massachusetts, USA). Fragmented DNA libraries were constructed with a KAPA HTP Library Preparation Kit (Illumina Platform) (KAPA Biosystems, Massachusetts, USA) according the manufacturer’s instruction. DNA libraries were captured with a designed 1086-gene panel, NimbleGen SeqCap EZ Library (Roche, Wisconsin, USA), that includes major tumor-related genes. The captured samples were then subjected to Illumina HiSeq X-Ten for paired-end sequencing.
Identification of somatic mutations and assessment of tumor mutational burden (TMB)
FFPE tumor samples and blood lymphocytes samples were submitted for NGS. The NimbleDesign assay was used (1086 genes) to identify mutations. We used VarScan2 with the following filters: (i) located in intergenic regions or intronic regions; (ii) synonymous SNVs; (iii) allele frequency >= 0.002 in the database exac03 or gnomad_exome; (iv) the value of ljb2_pp2hdiv = “B” and ljb2_pp2hvar = “B”; (v) allele frequency <0.05 in the tumor sample; and (vi) allele depth <5. To identify somatic mutations, the mutations in FFPE tumor samples were blanked by matched blood lymphocyte samples from patients. For the determination of TMB, the number of somatic nonsynonymous SNVs (with depth >100X and allele frequency ≥ 0.05) detected on NGS (interrogating Mb of the genome) were quantified, and the value was extrapolated to the whole exome using a validated algorithm. Alterations likely or known to be bona fide oncogenic drivers were excluded. TMB was measured in mutations per Mb. TMB values were divided into the following three groups: low (<3.24 mutations/Mb), intermediate (3.24–12.94 mutations/Mb), and high (≥12.94 mutations/Mb).
Statistical methods
Both the Mann-Whitney test/Kruskal-Wallis test followed by Dunn’s multiple comparison as post-hoc test and Pearson’s chi-square test were used to analyze the significance of the association of TMB with disease site, patient age and tumor type. The significance of associations of the number of gene mutations with TMB and disease site was also analyzed with Kruskal-Wallis tests. All tests were two-sided, and statistical significance was set at p < 0.05. All statistical analyses were performed with SPSS version 22.0 software (SPSS Inc., Chicago, IL, USA).
Data avaliability statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
This work was supported by grant from the National Natural Science Foundation of China (No. 81272867) and supported in part by grants from the Scientific Research Common Program of Beijing Municipal Commission of Education (No. KM201510025024).
Author Contributions
Min Wang, Mingxia Ye, Chen Tian, Henghui Zhang, Yuanguang Meng designed the project. Min Wang and Wensheng Fan wrote the paper. Min Wang, Wen Yang, Chenglei Gu, Mingxia Li, Zhe Zhang, and Yongjun Wang collected the samples and clinical data. Jianfei Wang, Chen Tian, and Wenbo Han conducted experiments. Lili Zhao did the data analysis. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Min Wang, Wensheng Fan and Mingxia Ye contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-25583-6.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Henghui Zhang, Email: zhhbao@ccmu.edu.cn.
Yuanguang Meng, Email: meng6512@vip.sina.com.
References
- 1.Carmen MGD, Rice LW, Schmeler KM. Global health perspective on gynecologic oncology. J Gynecol Oncol. 2015;13:329–34. doi: 10.1016/j.ygyno.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Jiang X, Tang H, Chen T. Epidemiology of gynecologic cancers in China. J Gynecol Oncol. 2018;1:e7. doi: 10.3802/jgo.2018.29.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, et al. Cancer incidence and mortality in China, 2013. Cancer Lett. 2017;401:63–71. doi: 10.1016/j.canlet.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 5.Zeng H, et al. Cancer survival in China, 2003–2005: a population-based study. Int J Cancer. 2015;136:1921–30. doi: 10.1002/ijc.29227. [DOI] [PubMed] [Google Scholar]
- 6.Wong KH, Mang OW, Au KH, Law SC. Incidence, mortality, and survival trends of ovarian cancer in Hong Kong, 1997 to 2006: a population-based study. Hong Kong Med J. 2012;18:466–74. [PubMed] [Google Scholar]
- 7.Gordon AN, Tonda M, Sun S. Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. J Gynecol Oncol. 2004;95:1–8. doi: 10.1016/j.ygyno.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Sehouli J, et al. Topotecan weekly versus conventional 5-day schedule in patients with platinum-resistant ovarian cancer: a randomized multicenter phase II trial of the North-Eastern German Society of Gynecological Oncology Ovarian Cancer Study Group. J Clin Oncol. 2011;29:242–8. doi: 10.1200/JCO.2009.27.8911. [DOI] [PubMed] [Google Scholar]
- 9.Mutch DG, et al. Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2007;25:2811–8. doi: 10.1200/JCO.2006.09.6735. [DOI] [PubMed] [Google Scholar]
- 10.Murali R, Grisham RN, Soslow RA. The roles of pathology in targeted therapy of women with gynecologic cancers. J Gynecol Oncol. 2018;148(1):213–221. doi: 10.1016/j.ygyno.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mezache L, Paniccia B, Nyinawabera A, Nuovo GJ. Enhanced expression of PD L1 in cervical intraepithelial neoplasia and cervical cancers. Mod Pathol. 2015;28:1594–602. doi: 10.1038/modpathol.2015.108. [DOI] [PubMed] [Google Scholar]
- 12.Heeren AM, et al. Nodal metastasis in cervical cancer occurs in clearly delineated fields of immune suppression in the pelvic lymph catchment area. Oncotarget. 2015;6:32484–93. doi: 10.18632/oncotarget.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 14.Sato E, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howitt BE, et al. Association of polymerase e-mutated and microsatellite-instable endometrial cancers with Neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol. 2015;1:1319–1323. doi: 10.1001/jamaoncol.2015.2151. [DOI] [PubMed] [Google Scholar]
- 16.Mehnert JM, et al. Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer. J Clin Invest. 2016;126:2334–2340. doi: 10.1172/JCI84940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gatalica Z, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol. Biomark. Prev. 2014;23:2965–2970. doi: 10.1158/1055-9965.EPI-14-0654. [DOI] [PubMed] [Google Scholar]
- 18.Barra F, et al. Investigational drugs for the treatment of cervical cancer. Expert Opin Investig Drugs. 2017;26(4):389–402. doi: 10.1080/13543784.2017.1302427. [DOI] [PubMed] [Google Scholar]
- 19.Heong V, Ngoi N, Tan DS. Update on immune checkpoint inhibitors in gynecological cancers. J Gynecol Oncol. 2017;28(2):e20. doi: 10.3802/jgo.2017.28.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Church DN, et al. DNA polymerase ε and δ exonuclease domain mutations in endometrial cancer. Hum Mol Genet. 2013;22:2820–8. doi: 10.1093/hmg/ddt131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldsby RE, et al. Defective DNA polymerase-delta proofreading causes cancer susceptibility in mice. Nat Med. 2001;7:638–9. doi: 10.1038/88963. [DOI] [PubMed] [Google Scholar]
- 22.Le DT, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rizvi NA, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snyder A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg JE, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–20. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Rooij N, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol. 31. 2013;42:e439. doi: 10.1200/JCO.2012.47.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurman, R. J., Carcangiu, M. L., Herrington, C. S. & Young, R. H. WHO classification of tumours of female reproductive organs (2014).
- 28.Chalmers ZR, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gubin MM, Artyomov MN, Mardis ER, Schreiber RD. Tumor neoantigens: building a framework for personalized cancer immunotherapy. J Clin Invest. 2015;125:3413–21. doi: 10.1172/JCI80008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ott PA, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: results from the KEYNOTE-028 study. J Clin Oncol. 2017;35:2535–2541. doi: 10.1200/JCO.2017.72.5952. [DOI] [PubMed] [Google Scholar]
- 31.Gaillard SL, Secord AA, Monk B. The role of immune checkpoint inhibition in the treatment of ovarian cancer. Gynecol Oncol Res Pract. 2016;3:11. doi: 10.1186/s40661-016-0033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gadducci A, Guerrieri ME. Immune Checkpoint Inhibitors in Gynecological Cancers: Update of Literature and Perspectives of Clinical Research. Anticancer Res. 2017;37(11):5955–5965. doi: 10.21873/anticanres.12042. [DOI] [PubMed] [Google Scholar]
- 33.Salvesen, H. B., Werner, H. M. & Krakstad, C. PI3K pathway in gynecologic malignancies. Am Soc Clin Oncol Educ Book (2013). [DOI] [PubMed]
- 34.Slomovitz BM, Coleman RL. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin Cancer Res. 2012;18(21):5856–64. doi: 10.1158/1078-0432.CCR-12-0662. [DOI] [PubMed] [Google Scholar]
- 35.Spaans VM, et al. The landscape of somatic mutations in Indonesian cervical cancer is predominated by the PI3K pathway. J Gynecol Oncol. 2018;148(1):189–196. doi: 10.1016/j.ygyno.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Hou, S. Q. et al. PTEN in the maintenance of genome integrity: From DNA replication to chromosome segregation. Bioessays. 39(10) (2017). [DOI] [PMC free article] [PubMed]
- 37.Jardim, D. L. et al. FBXW7 mutations in patients with advanced cancers: clinical and molecular characteristics and outcomes with mTOR inhibitors. PLoS One. 9(2) (2014). [DOI] [PMC free article] [PubMed]
- 38.Cheung LW, et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov. 2011;1(2):170–85. doi: 10.1158/2159-8290.CD-11-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nesic, K. et al. Targeting DNA repair: the genome as a potential biomarker. J Pathol. (2017). [DOI] [PubMed]
- 40.Strickland KC, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7(12):13587–98. doi: 10.18632/oncotarget.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Co NN, et al. Loss of LKB1 in high-grade endometrial carcinoma: LKB1 is a novel transcriptional target ofp53. Cancer. 2014;120(22):3457–68. doi: 10.1002/cncr.28854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wingo SN, et al. Somatic LKB1 mutations promote cervical cancer progression. PLoS One. 2009;4(4):e5137. doi: 10.1371/journal.pone.0005137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koyama S, et al. STK11/LKB1 Deficiency Promotes Neutrophil Recruitment and Proinflammatory Cytokine Production to Suppress T-cell Activity in the Lung Tumor Microenvironment. Cancer Res. 2016;76(5):999–1008. doi: 10.1158/0008-5472.CAN-15-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rizvi, H. et al. Molecular Determinants of Response to Anti-Programmed Cell Death (PD)-1 and Anti-Programmed Death-Ligand (PD-L)-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer Profiled With Targeted Next-Generation Sequencing. J Clin Oncol. JCO2017753384 (2018). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.