Abstract
The mechanical environment can influence cell behaviour, including changes to transcriptional and proteomic regulation, morphology and, in the case of stem cells, commitment to lineage. However, current tools for characterizing substrates’ mechanical properties, such as atomic force microscopy (AFM), often do not fully recapitulate the length and time scales over which cells ‘feel’ substrates. Here, we show that an immortalised, clonal line of human mesenchymal stem cells (MSCs) maintains the responsiveness to substrate mechanics observed in primary cells, and can be used as a reporter of stiffness. MSCs were cultured on soft and stiff polyacrylamide hydrogels. In both primary and immortalised MSCs, stiffer substrates promoted increased cell spreading, expression of lamin-A/C and translocation of mechano-sensitive proteins YAP1 and MKL1 to the nucleus. Stiffness was also found to regulate transcriptional markers of lineage. A GFP-YAP/RFP-H2B reporter construct was designed and virally delivered to the immortalised MSCs for in situ detection of substrate stiffness. MSCs with stable expression of the reporter showed GFP-YAP to be colocalised with nuclear RFP-H2B on stiff substrates, enabling development of a cellular reporter of substrate stiffness. This will facilitate mechanical characterisation of new materials developed for applications in tissue engineering and regenerative medicine.
Introduction
Mechanical homeostasis is a fundamental property inherent to all tissues of the adult body. Establishment of the right stiffness for each tissue and stage in development is vital for the correct function of various organs1: bones, for example, must be stiff, while skin must be reversibly deformable. In order to maintain homeostasis in surrounding tissue, cells have mechanisms that allow them to ‘feel’ the mechanical properties of the extracellular matrix (ECM) and respond accordingly. Cells process physical stimuli through a set of mechanotransduction pathways2,3, such as mechanically-regulated ion channels4 or focal adhesion (FA) complexes that assemble at the plasma membrane where cells pull on the surrounding ECM5. Mechanical signals are propagated within cells through pathways such as RhoA (Ras homolog gene family, member A) and ROCK (Rho-associated protein kinase) signalling6, and through regulation of transcription factors (TFs). Stiff substrates cause TFs such as YAP1 (yes-associated protein 1)7 and MKL1 (myocardin-like protein 1, also known as MRTF-A or MAL)8 to translocate to the nucleus, thus modulating their activity. Mechanical signals may also be transmitted through cells by a system of interlinked structural proteins that connect the ECM through FAs to the cytoskeleton, and then to the nucleus through the linker of nucleo- and cyto- skeleton (LINC) complex9. Mechanical inputs can therefore be passed from substrate to nucleus where they can affect chromatin conformation and thus influence how genes are regulated10.
A broad range of cellular processes have been shown to be influenced by mechanical inputs. Adherent cells pull on and probe the surrounding microenvironment11, activating signalling pathways in FA complexes1 and prompting reorganisation of the actin cytoskeleton12. Mechanical signals are propagated to regulate aspects of cell morphology13, such as the extent to which cells spread when adhering to a two-dimensional substrate, and the amount of force that cells apply to deform their surroundings14. Changes to cell morphology and contractility require regulation of protein content within the cells, and this has been characterised in the cytoskeleton and the nuclear lamina15. Apoptosis pathways and the rate of proliferation are also influenced by substrate stiffness16, and cells such as fibroblasts have been shown to migrate along gradients of increasing stiffness, a process called durotaxis17. Mesenchymal stem cells (MSCs) have been used as a model system to examine a number of mechanotransduction processes6,7,15,18, with sensitivity to mechanical stimulation noted in even seminal characterisations19. MSCs are multipotent cells with lineage potential that can be influenced by substrate mechanics15,20: culture on soft substrates favours adipogenesis, while stiff substrates favour osteogenesis. Previous work has also shown that characteristics of MSC morphology, assessed through ‘high-content’ analysis of cells imaged by fluorescence microscopy, can serve as early predictors of lineage specification21.
The multipotent nature of MSCs combined with a capacity to modulate immune responses22 have led to investigations of their suitability for regenerative medicine, and the possibility of replacing damaged tissues with engineered scaffolds repopulated with stem cells23,24. James et al. have reported the generation of immortalised MSCs through overexpression of telomerase reverse transcriptase (TERT)25. Clonal lines were selected for expansion that showed exponential growth, matched the expected cell-surface marker profile for MSCs and presented no evidence of tumorigenicity in immuno-compromised mice. Endogenous populations of MSCs are typically heterogeneous, but the selected clonal lines exhibited specific characteristics of MSC behaviour. Some of the clones exhibited immuno-modulatory behaviour, while the clonal line “Y201” maintained potential for chemical induction of adipogenic, chondrogenic and osteogenic lineages25.
Here we compared the responses of primary and Y201 MSCs to culture on soft and stiff collagen-I coated polyacrylamide (PA) hydrogels to determine the extent to which mechanosensitive behaviour was maintained in the immortalised line. We found that the Y201 MSCs preserved the mechanosensitive features of primary cells: morphological responses such as cell spreading on stiffer substrates, the ability to remodel the nucleoskeleton, translocate TFs and regulate genes associated with adipogenesis and osteogenesis.
Recent efforts in the field of tissue engineering have sought to harness the multipotent nature of stem cells to rebuild or replace damaged tissues. This has required the development of substrates and scaffolds on which cells can be cultured for use in therapy or to produce synthetic tissues that can ultimately be implanted into patients. Substrates have been generated using diverse chemistries, enabling properties such as tuneable stiffness26–29 or the incorporation of ECM molecules30. Scaffolds have been constructed using a variety of technologies, from bio-inspired, synthetic polymers31 to decellularised tissues32. The behaviour of cells seeded onto such materials is directed by the combined chemical and mechanical characteristics of the microenvironment33. It is therefore often desirable to characterise the mechanical properties of culture substrates and scaffolds, as well as the tissues that they are engineered to mimic. The mechanical properties of biological tissues are best characterised by a combination of viscous and elastic (‘viscoelastic’) characteristics34 which vary with deformation over time. Atomic force microscopy (AFM), nanoindentation and rheometry offer means of measuring material parameters, such as the elasticity (Young’s modulus, E), but each have limitations. Measurements of time-dependent viscoelastic mechanical properties are challenging to make by indentation methods, requiring careful selection of the loading regime and indentation tip35. Rheometry methods can report on viscoelastic properties (loss and storage moduli), but lack spatial resolution and are restricted to measuring bulk material properties (see Canovic et al. for a comparison of indentation and rheometry methods applied to soft, viscoelastic brain tissue36). Cell behaviour within a tissue or synthetic matrix may be influenced by deformations over specific length or time scales, by complex viscoelastic material properties, by heterogeneity, or by dimensionality and topologies that would be difficult to examine with a mechanical probe (such as an AFM tip) or bulk measurement method (such as rheometry). We reasoned that the best predictor of how cells would experience and respond to a particular mechanical environment may therefore be to use the intrinsic behaviours of cells themselves to report on the properties of their surroundings. With this aim, having established the mechanosensitivity of the Y201 MSCs, we used them as a platform to develop a fluorescent stiffness probe. Here we demonstrate the use of immortalised MSCs stably expressing a red/green fluorescent reporter construct that can be analysed by microscopy to differentiate soft and stiff substrates.
Results
Immortalised MSCs maintain a morphological response to substrate stiffness
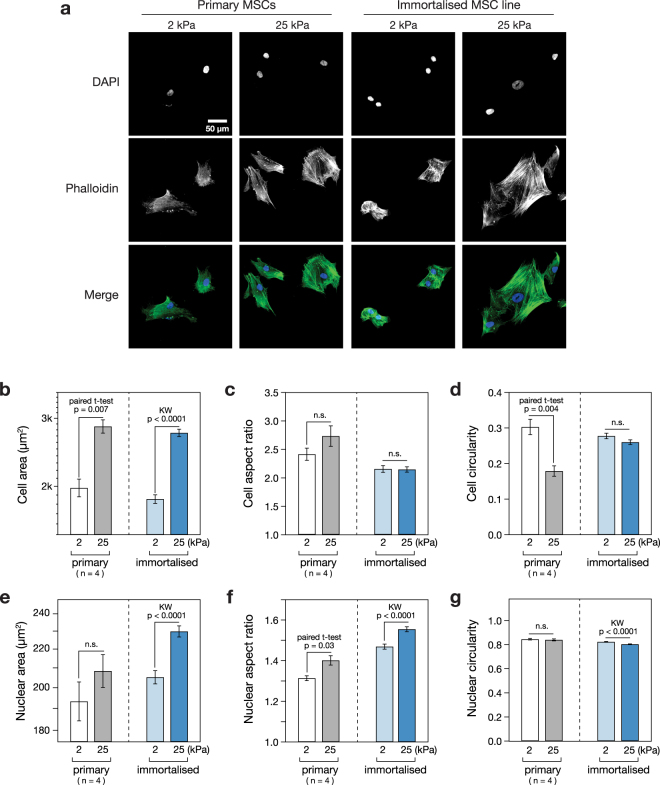
In order to determine whether an immortalised MSC cell line, previously reported to show the multipotent capacity of primary cells25, also maintained sensitivity to mechanical inputs, the response of the cells to culture on soft or stiff substrates was characterised. Immortalised MSCs, along with a comparison set of primary cells from four human donors, were cultured on soft (2 kPa) or stiff (25 kPa) collagen-I coated PA hydrogels for three days, before being fixed and stained with DAPI and phalloidin (Fig. 1a). Cells on stiffer substrates appeared larger with more pronounced and ordered stress fibres. Quantitative analysis of cell morphology showed that the mean spread area of primary MSCs was increased 1.4-fold (p = 0.007; n = 4 donors) on stiff versus soft hydrogels; the spread area of the immortalised line was increased 1.5-fold under the same conditions (p < 0.0001; Fig. 1b). Cell aspect ratio (defined as the ratio of long to short side lengths of the smallest rectangle that can enclose the perimeter of a cell) was not significantly affected by substrate stiffness in either primary or immortalised cells (Fig. 1c). Cell circularity (proportional to the ratio between the area and the square of the perimeter of a cell) was significantly lower on the stiff substrate for primary cells (p = 0.004; n = 4 donors), but not significantly altered in immortalised cells (Fig. 1d). Analysis of nuclear morphology in the same samples yielded a parallel set of characteristics. The spread areas of the nuclei of primary cells were increased 1.1-fold in both primary and immortalised cells, although this effect was only significant in the immortalised line (p < 0.0001), perhaps reflecting the greater variation amongst cells from primary donors (Fig. 1e). Nuclear aspect ratio was significantly increased on stiff substrates in both primary and immortalised MSCs (p = 0.003 and p < 0.0001, respectively; Fig. 1f). Nuclear circularity was not affected by stiffness in primary cells, although it was fractionally reduced on stiff substrates in the immortalised line (p < 0.0001; Fig. 1g). These results established that immortalised MSCs broadly replicated the characteristic morphological responses of primary cells when subjected to modulated substrate mechanics.
Figure 1.
Cellular and nuclear morphology are stiffness-responsive in primary and immortalised MSCs. (a) MSC morphology was assessed using phalloidin and DAPI staining following 3 days culture on soft (2 kPa) or stiff (25 kPa) collagen-I coated PA hydrogels. (b) The spread areas of both primary and immortalised MSCs were significantly greater on stiff substrates than on soft. (c) Cell aspect ratios were not significantly affected by stiffness. (d) Cell circularity was significantly decreased in primary MSCs on stiff substrates; this effect was not observed in immortalised cells. (e) Nuclear area was increased on stiff substrates, although this effect was only significant in immortalised MSCs. (f) Nuclear aspect ratio was significantly increased on stiff substrates in both primary and immortalised MSCs. (g) Nuclear circularity was not significantly affected by increased stiffness in primary MSCs, but was lowered in immortalised MSCs (p-values from paired t-tests and Kruskal-Wallis (KW) tests as indicated; n.s. = not significant; n = 4 primary donors; a minimum of 38 cells were analysed per condition for each primary donor; a minimum of 363 immortalised cells were analysed per condition).
The nuclear lamina of primary and immortalised MSCs is stiffness-responsive
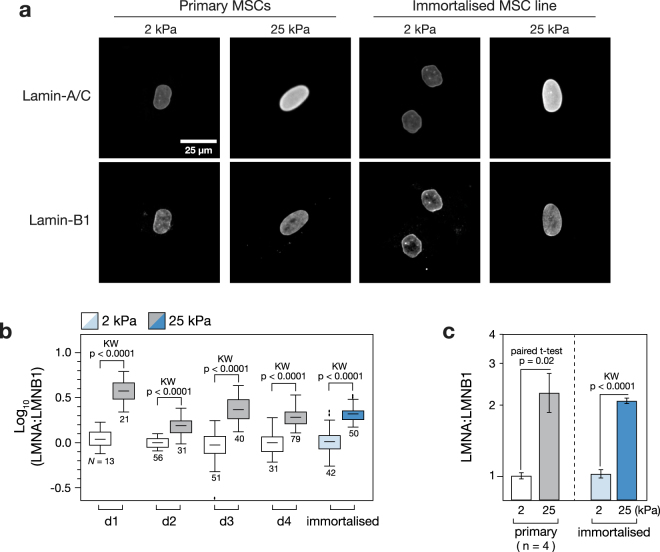
The composition of the nuclear lamina has been shown to be affected by substrate stiffness15. Primary and immortalised MSCs were cultured on soft (2 kPa) and stiff (25 kPa) collagen-I coated PA hydrogels for three days before being fixed and imaged with immuno-staining against lamins A/C and B1 (Fig. 2a). In order to compensate for changes in nuclear spread area, the ratio of lamin A/C to B1 stain intensity within the nucleus was calculated. Lamin B1 was used for normalisation as it has been reported to be relatively unresponsive to substrate stiffness15. Analysis of MSCs from four primary donors, in comparison to the immortalised line, showed that the response to stiffness was maintained: the LMNA:B1 ratio was significantly greater on the stiffer substrate (p < 0.0001; Fig. 2b). The mean LMNA:B1 ratio was increased 2.2-fold on stiff versus soft substrates in the primary MSCs (p = 0.02; n = 4 donors); in the immortalised MSCs, the increase was 2-fold (p < 0.0001; Fig. 2c). This result suggests that the mechanosensitivity of cell adhesions, and consequent cell spreading with increased substrate stiffness, was propagated to the nucleus in primary MSCs, consistent with earlier reports15, and that the mechanism was preserved in the immortalised cell line.
Figure 2.
Composition of the nuclear lamina is stiffness-responsive in primary and immortalised MSCs. (a) Immunofluorescence imaging was used to quantify lamins -A/C and -B1 (LMNA and LMNB1) in primary and immortalised MSCs cultured on soft (2 kPa) or stiff (25 kPa) collagen-I coated PA hydrogels. Lamin-B1 expression appeared constant in all conditions, consistent with earlier reports15. (b) Quantification of lamin A/C to B1 ratio (LMNA:LMNB1) from immunofluorescence images showed that lamin-A/C was consistently upregulated on stiff substrates, relative to lamin-B1, in each of four primary donors and the immortalised MSCs (N indicates number of cells analysed per condition). (c) LMNA:LMNB1 was significantly increased on stiff substrates (p-values from paired t-tests and Kruskal-Wallis (KW) tests as indicated; n = 4 primary donors).
Immortalised MSCs maintain the mechano-sensitive translocation of transcription factors YAP1 and MKL1
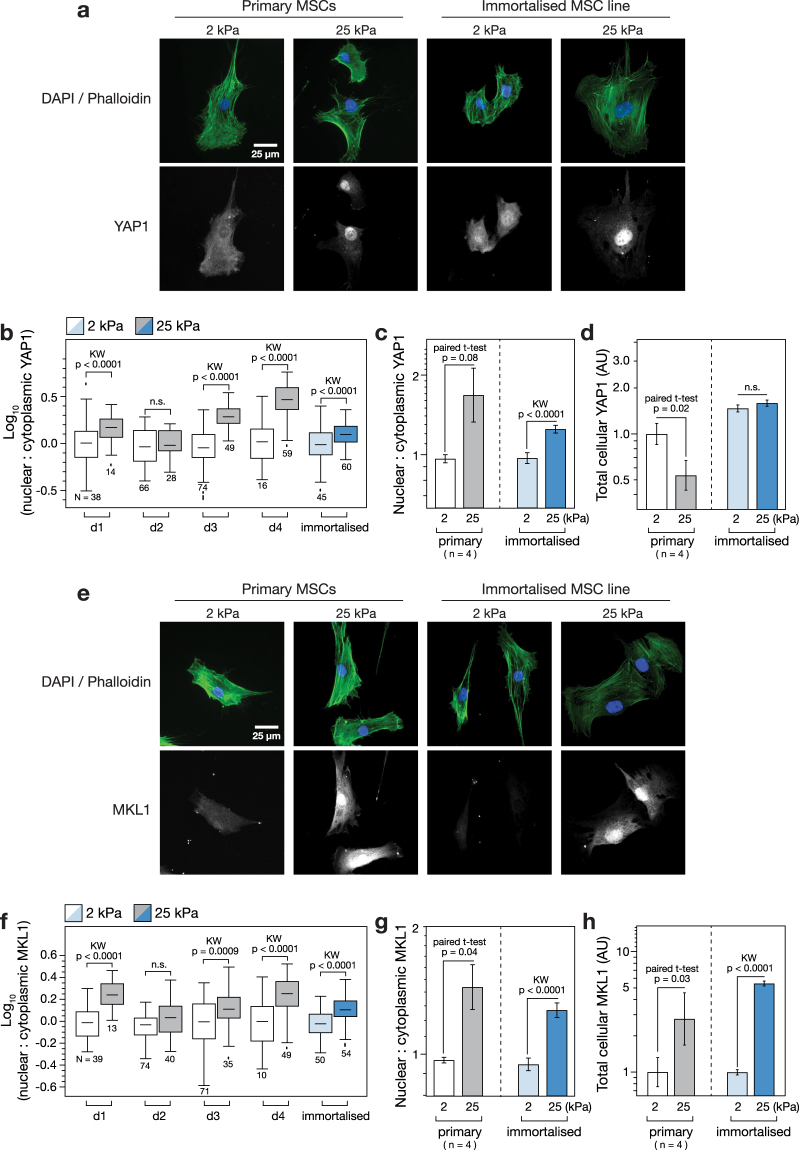
Primary and immortalised MSCs cultured on soft (2 kPa) and stiff (25 kPa) hydrogels for three days were also examined with immunofluorescence microscopy to determine the subcellular localisation of TFs YAP1 and MKL1, previously reported to translocate to the nucleus on stiff substrates7,8. YAP1 was found to be increasingly localised to the nucleus on the stiffer hydrogel in both primary and immortalised MSCs (Fig. 3a). Quantification of the ratio of nuclear to cytoplasmic YAP1 (determined by using DAPI and phalloidin channels to define cell and nuclear boundaries) showed donor-to-donor variation across four samples (Fig. 3b). Nonetheless, YAP1 was significantly more localised to the nucleus in three of the four samples (p < 0.0001). The mean nuclear:cytoplasmic ratio of YAP1 increased 1.7-fold on stiff versus soft substrates in the primary MSCs (not significant, p = 0.08; n = 4 donors) while the increase was 1.3-fold (p < 0.0001) in the immortalised cells (Fig. 3c). Mean total YAP1 (the sum of nuclear and cytoplasmic protein) was significantly downregulated on stiff substrates in the primary MSCs (p = 0.02), but was not affected in the immortalised cells (Fig. 3d). Examination of samples prepared in parallel showed the distribution of MKL1 to be similarly affected by substrate mechanics (Fig. 3e). The nuclear:cytoplasmic ratio of MKL1 was significantly higher on stiff than soft substrates in three of the four primary donor samples (p < 0.0009; Fig. 3f). Interestingly, the cells from donor 2 (d2) that showed no mechano-responsiveness in YAP1 cellular location also showed no significant regulation of MKL1. This reflects the variability among primary MSCs from different donors, and suggests the potential experimental utility of an immortalised line that reproduces canonical mechanosensitive features. The mean nuclear:cytoplasmic ratio of MKL1 was increased 1.5-fold in the primary cells (p = 0.04; n = 4 donors) and 1.3-fold in the immortalised line (p < 0.0001; Fig. 3g). In contrast to YAP1, total MKL1 was highly responsive to substrate stiffness, increasing 2.7-fold on stiff substrates in the primary cells (p = 0.03; n = 4 donors) and 5.5-fold in the immortalised cells (p < 0.0001; Fig. 3h). Both primary and immortalised MSCs thus exhibited substrate-directed regulation of TF subcellular localization.
Figure 3.
Transcription factors YAP1 and MKL1 respond to stiffness in primary and immortalised MSCs. (a) Immunofluorescence imaging was used to examine the location of yes-associated protein 1 (YAP1) in primary and immortalised MSCs cultured on soft (2 kPa) or stiff (25 kPa) collagen-I coated PA hydrogels. The nucleus and extent of the cytoplasm were identified by DAPI and phalloidin staining respectively. (b) YAP1 became increasingly localised in the nucleus on stiff substrates in MSCs from three of four primary donors, and in immortalised MSCs (N indicates number of cells analysed per condition). (c) Relative nuclear localisation of YAP1 was significantly increased in immortalised MSCs on stiff substrates. (d) The total amount of YAP1 (integrated signal from the whole cell) was significantly lower on stiff substrates in primary cells, but unchanged in immortalised cells. (e) Cellular location of myocardin-like protein 1 (MKL1, also known as MRTF-A or MAL) was imaged by immunofluorescence in primary and immortalised MSCs on soft and stiff substrates. (f) MKL1 was increasingly localised in the nucleus on stiff substrates in MSCs from three of four primary donors, and in immortalised MSCs (N indicates number of cells analysed per condition). (g) MKL1 was significantly more localised to the nucleus on stiff substrates in primary and immortalised cells. (h) Total levels of MKL1 were also highly dependent on substrate stiffness in both primary and immortalised cells: in both cases, MKL1 was significantly higher on stiff substrates (p-values from paired t-tests and Kruskal-Wallis (KW) tests as indicated; n.s. = not significant; n = 4 primary donors).
Substrate stiffness modulates lineage markers CEBPA and RUNX2 in immortalised MSCs
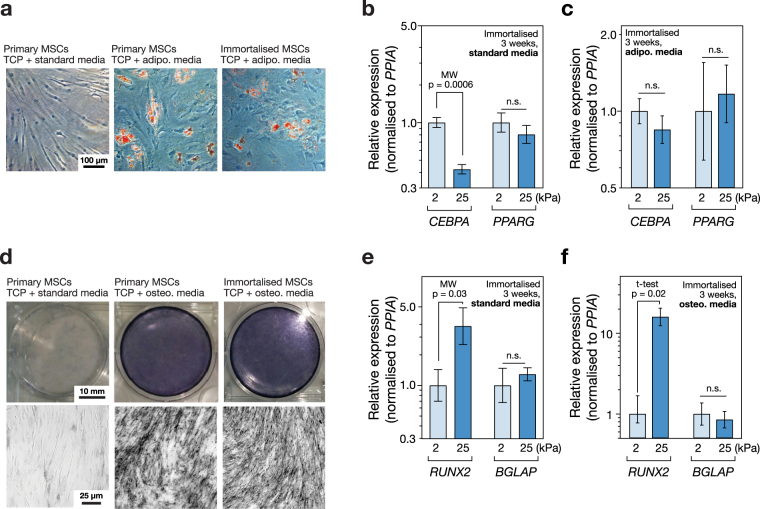
Primary MSCs cultured for three weeks on tissue culture plastic (TCP) in media supplemented with an adipo-induction cocktail formed lipid droplets that could be imaged by Oil Red O staining. Consistent with previous reports25, the immortalised MSCs also formed lipid droplets under the same conditions (Fig. 4a). The influence of substrate stiffness alone was sufficient to alter transcript levels of adipogenic marker CEBPA (CCAAT/enhancer-binding protein alpha) in immortalised MSCs: CEBPA was 2.4-fold higher in cells cultured on soft (2 kPa) versus stiff (25 kPa) collagen-I coated PA hydrogels in standard media for three weeks (p = 0.0006; Fig. 4b). Regulator of adipocyte differentiation PPARG (Peroxisome proliferator-activated receptor gamma) was also 1.3-fold higher on soft than stiff substrates, but this effect was not significant. Modulation of substrate stiffness did not significantly affect levels of CEBPA or PPARG transcripts in immortalised cells treated with adipo-induction media (Fig. 4c). Culture of primary MSCs on TCP for three weeks in media supplemented with an osteo-induction cocktail showed positive staining for alkaline phosphatase (ALP) activity. As reported previously25, the immortalised line also showed ALP activity under the same conditions (Fig. 4d). The influence of substrate stiffness was again found to be sufficient to affect genes indicative of lineage in the absence of chemical induction: culture of immortalised MSCs on stiff substrates caused a 4-fold increase in RUNX2 (runt-related transcription factor 2) relative to culture on soft (p = 0.03; Fig. 4e). BGLAP (osteocalcin) was also increased, but the change was not significant. RUNX2 transcript was increased 16-fold on stiff versus soft in immortalised MSCs cultured with osteo-induction media (p = 0.02), suggesting a synergistic relationship between chemical and mechanical stimuli (Fig. 4f). These results reproduce earlier observations of differentiation potential in the clonal, immortalised Y201 MSC line and furthermore show that genes associated with adipogenesis and osteogenesis are mechanically regulated.
Figure 4.
Substrate stiffness modulates lineage markers in immortalised MSCs. (a) Images of MSCs cultured on tissue culture plastic (TCP) for three weeks in the presence of adipogenic induction media, with Oil Red O staining (in red). Both primary and immortalised MSCs showed positive staining of lipid droplets, indicating adipogenic potential and confirming observations reported previously25. (b) Culture on soft (2 kPa) collagen-I coated PA hydrogels significantly increased the adipogenic marker CEBPA, relative to culture on stiff (25 kPa) hydrogels, measured by RT-qPCR in immortalised MSCs after three weeks in standard media. A second adipogenic marker PPARG was also higher on soft substrates, although this effect was not significant. (c) Combining substrate stiffness cues with adipogenic induction media did not significantly change levels of CEBPA or PPARG. (d) MSCs cultured on TCP for three weeks in the presence of osteogenic induction media and stained for alkaline phosphatase activity (ALP). Both primary and immortalised MSCs showed positive ALP staining, indicating an osteogenic potential in agreement with earlier reports25. (e) Culture on stiff PA hydrogels significantly increased the osteogenic marker RUNX2, relative to culture on soft, measured in immortalised MSCs after three weeks in standard media. Osteogenic marker BGLAP was also higher on stiff substrates, although this effect was not significant. (f) Stiff substrate significantly amplified the effect of chemical osteogenic induction on RUNX2 in immortalised MSCs; BGLAP levels were not significantly altered. A synergistic interaction between mechanical and chemical inputs in influencing lineage potential has previously been reported in primary MSCs15 (p-values from Mann-Whitney (MW) and un-paired t-tests as indicated; n.s. = not significant).
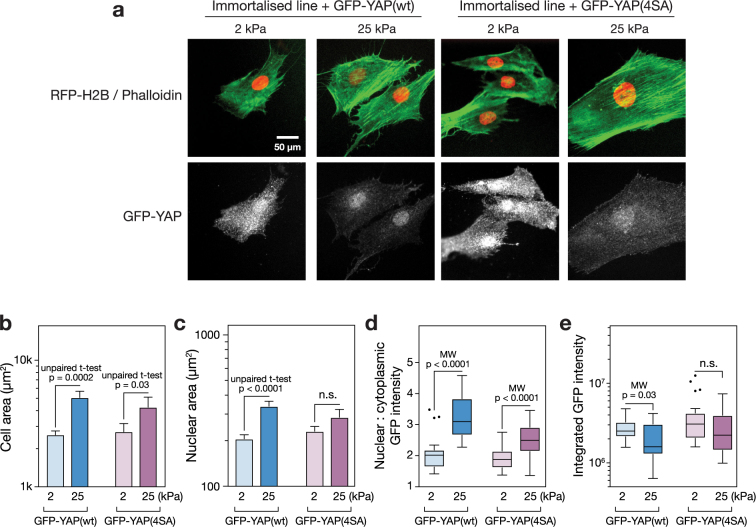
A stably expressed reporter construct responds to substrate stiffness
The immortalised MSC line was transformed to stably express histone H2B labelled with red fluorescent protein (RFP-H2B) and either wildtype YAP1 tagged with green fluorescent protein (GFP-YAP(wt)) or a tagged YAP1 with four point mutations to serine residues (GFP-YAP(4SA)). The construct with the mutant YAP1 was considered here to investigate whether point mutations affecting protein turnover could produce a more stable reporter system. The reporter cells were cultured on soft (2 kPa) or stiff (25 kPa) substrates for three days under standard conditions, fixed and imaged (Fig. 5a). The RFP-H2B was localised to the nuclei of the MSCs; the GFP-YAP constructs appeared throughout the cell, but were observed with increasing intensity in the nuclei of MSCs on stiff substrates, particularly in the case of the wildtype YAP construct. The mechanosensitivity of cell spreading was maintained in MSCs with GFP-YAP(wt) and GFP-YAP(4SA) constructs (p = 0.0002 and 0.03, respectively; Fig. 5b), reflecting the observations made of primary and non-transformed cells (Fig. 1). Nuclear area also showed sensitivity to the stiffness of the substrate in the GFP-YAP(wt) MSCs (Fig. 5c). Quantification of the nuclear:cytoplasmic ratio of GFP-YAP(wt) showed the construct to be 1.5-fold more intensely localised to the nucleus in MSCs cultured on stiff (25 kPa) than on soft (2 kPa) hydrogels (p < 0.0001; Fig. 5d). The GFP-YAP(4SA) construct also maintained significant mechanosensitivity (p < 0.0001), although the fold change in nuclear:cytoplasmic ratio was decreased. The integrated, whole-cell intensity of GFP-YAP(wt) was significantly lower on stiff substrates (p = 0.03), but levels of the GFP-YAP(4SA) construct showed no significant sensitivity to stiffness (Fig. 5e).
Figure 5.
Application of a GFP-YAP/RFP-H2B fluorescent construct to report on substrate stiffness. (a) Immortalised MSCs were virally transformed to express RFP-H2B and either GFP-YAP(wt) (wild type) or GFP-YAP(4SA) (YAP1 modified with mutations to four serine residues49). Cells were cultured on soft (2 kPa) or stiff (25 kPa) hydrogels for three days, fixed and stained with phalloidin and anti-GFP. (b) Analysis of cell areas of the transformed immortalised MSCs showed that responsiveness to substrate mechanics was maintained: spread area was significantly greater on stiff (25 kPa) versus soft (2 kPa) hydrogels with both the GFP-YAP(wt) and GFP-YAP(4SA) construct. (c) Nuclear spread area of the GFP-YAP(wt) transformed cells was significantly greater on stiffer hydrogels; nuclear spread of GFP-YAP(4SA) was also greater, although not significant. (d) Image analysis showed that nuclear localisation of the YAP reporter constructs was higher on stiff (25 kPa) than soft (2 kPa) substrates. Translocation was significant for both GFP-YAP(wt) and GFP-YAP(4SA) constructs, although the magnitude of the effect was greater with the wildtype construct. (e) Total GFP-YAP(wt) was significantly lower on stiff substrates. Total GFP-YAP(4SA) was greater than in the wildtype construct, although not significantly so, and responsiveness to substrate stiffness was lost. (p-values from Mann-Whitney (MW) and un-paired t-tests as indicated; n.s. = not significant; outliers in box-whisker plots were identified by the Tukey method; a minimum of 17 cells were analysed under each condition).
Discussion
An immortalised MSC line maintains mechanosensitive characteristics of primary cells
Since the earliest characterisations, MSCs have been reported to be responsive to mechanical input19, and have subsequently been used as a model system to study a wide range of mechanosensitive phenomena6,7,15,18. The immortalised MSCs (clonal line Y201, initially isolated from human bone marrow) generated by James et al. have been shown to maintain the adipogenic, chondrogenic and osteogenic potential observed in primary MSCs when treated with standard, differentiation-inducing chemical cocktails25. However, the extent to which immortalised MSCs preserved their responsiveness to substrate mechanics – and, by extension, could be used as a platform to develop mechanosensing tools – had not been established. Here, primary and immortalised cells were cultured on soft and stiff collagen-I coated PA hydrogels and aspects of recognised mechanosensing pathways compared. Hydrogel stiffnesses were chosen to be representative of the bone marrow microenvironment: fat and marrow tissues are soft while precalcified bone is comparatively stiff, modelled by 2 and 25 kPa gels, respectively15,37.
The immortalised MSCs reproduced the characteristic morphological responses to substrate stiffness observed in primary MSCs (Fig. 1). Primary and immortalised MSCs were found to spread more on the stiffer substrate (Fig. 1b), although cellular aspect ratio and circularity were more sensitive to substrate in primary cells (Fig. 1c,d). Cell elongation has previously been reported for MSCs cultured on substrates of around 10 kPa38. The ECM, cytoskeleton and nucleoskeleton are considered to be part of a continuous, mechanically linked system9,39. Changes in cellular morphology were matched by coincident changes in nuclear morphology (Fig. 1e), suggesting that the LINC complex that tethers the cytoskeleton to the nucleoskeleton remained functional in the immortalised cells. The nucleoskeleton, and specifically an increase in the ratio of A-type to B-type lamins, has been shown to report on a more contractile cell phenotype consequent of a stiffer substrate15. The ratio of lamin A/C to B1 was found here to be significantly higher on stiff substrates in both primary and immortalised cells (Fig. 2). This result again suggested that mechano-transmission pathways remained functional in the immortalised line. This is important as a range of signalling pathways, such as serum response factor (SRF), have been shown to be downstream of lamin-A/C regulation40.
Translocation of TFs between cytoplasm and nucleoplasm is a common motif within mechanosensing pathways, allowing TF activity to be modulated and thus providing control over specific transcriptionally regulated programs, such as lineage selection41,42. Regulation of the subcellular localization of TFs YAP1 and MKL1 as a function of substrate stiffness was found to be maintained in the immortalised MSCs (Fig. 3). Stiffness-directed regulation of YAP1 has been shown to be necessary for mechanically influenced lineage determination in MSCs, with nuclear YAP1 driving osteogenesis7. A mechanism that explains the mechanosensitivity of YAP translocation was recently reported, based on mechanical regulation of transport through the nuclear pores43; however, the universality of this mechanism, both in terms of the cell types and cellular factors it affects, remains to be established. The mechanical regulation of MKL1 and its influence on SRF have also been well characterised44, including through interactions with lamin-A/C and emerin8. MKL1 has also been shown to be important for PPARG mediated adipogenesis in pre-adipocyte cell lines45.
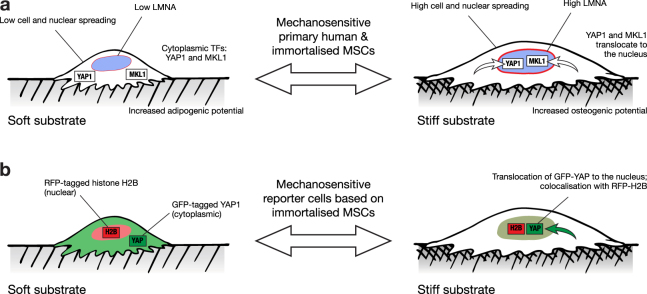
The differentiation potential of MSCs has been shown to be influenced by the mechanical properties of the microenvironment, with soft substrates favouring soft tissue specification, such as adipogenesis, and stiff substrates favouring osteogenesis7,15,20. Seminal characterisation of the Y201 immortalised MSC line showed that adipogenic lineage was induced by treatment with a standard adipo-induction cocktail, evidenced by positive Oil Red O staining and increased levels of LPL (lipoprotein lipase) and PPARG transcript after 14 and 21 days25. Here, chemically-induced adipogenesis was confirmed in immortalised MSCs cultured on TCP (Fig. 4a). Additionally, levels of the adipogenic reporter gene CEBPA were found to be increased in cells cultured on soft versus stiff substrates for 21 days in the absence of chemical stimulation (Fig. 4b). James et al. also reported chemically-induced osteogenesis in the Y201 line on TCP, coincident with increased levels of ALP and RUNX2 transcripts25. In addition to reproducing positive ALP staining on TCP (Fig. 4d), RUNX2 transcript was found here to be increased in the immortalised MSCs cultured on stiff versus soft hydrogels for 21 days in the absence of chemical stimulation (Fig. 4e). Furthermore, RUNX2 was more substantially affected by substrate stiffness when the immortalised MSCs were cultured in the presence of the osteo-inducing chemical cocktail, suggesting a synergistic relationship between chemical and physical inputs (Fig. 4f). Taken together, these results show that the mechano-responsive nature of MSC behaviour was maintained in an immortalised clonal line, from initial responses in cell morphology driven by ECM-FA interactions, through mechano-transmission to the nuclear skeleton and modulation of TF activity, and to influence the gene regulation that can affect cell fate (Fig. 6a).
Figure 6.
Mechanosensitive features of primary MSCs maintained in the immortalised MSC line. (a) Mechano-regulatory pathways observed in both primary and immortalised cells included: modulation of cell spreading and morphology, regulation of the nuclear lamina, translocation of transcription factors YAP1 and MKL1, and changes to differentiation potential. (b) The maintained sensitivity of YAP1 location to substrate stiffness enabled development of a reporter cell line: cytoplasmic GFP-YAP was indicative of a soft substrate, while colocalisation with nuclear RFP-H2B indicated a stiff substrate.
Generation of a stable cell line with a fluorescent stiffness reporter
Here we showed that cells could be used as tools to report on substrate stiffness (Fig. 5). Using MSCs as a platform on which to base such a technology is rational given their well-characterised mechanoresponse and long-established use in regenerative medicine research23,46. However, we found the extent of mechanosensitivity to vary between cells from different primary donors (Figs 2 and 3b,f) and variation has been described within heterogeneous populations derived from single donors47. Furthermore, a tool based on primary MSCs would have a limited lifespan as these cells typically show senescence over 8–12 passages48. The availability of an immortalised clonal MSC line (Y201)25 with maintained mechanosensing properties therefore represented an opportunity to develop a more reproducible measurement system. Two mechanosensitive TFs were identified as candidates on which to base a fluorescent stiffness probe. Both YAP1 and MKL1 maintained mechanosensitivity in the immortalised MSC line, translocating to the nucleus on stiff substrates (Fig. 3c,g). However, YAP1 was considered a better basis for a reporter construct as its mechanoresponse in the immortalised cells manifested only in its subcellular localisation, whereas both total MKL1 and its cellular compartment were affected by substrate stiffness (Fig. 3d,h).
The immortalised MSCs were transformed to express nuclear-localised, RFP-tagged histone H2B and wildtype YAP1 labelled with GFP. In addition, immortalised MSCs were transformed to express the RFP-H2B plus a GFP-tagged YAP1 with point mutations to four serine residues (S61A, S109A, S164A and S381G; “GFP-YAP(S4A)”). The intracellular location of both the GFP-YAP(wt) and GFP-YAP(S4A) probes was sensitive to substrate stiffness, being significantly more localised to the nucleus on a stiff (25 kPa) versus soft (2 kPa) hydrogel in both wildtype and mutant cases (Fig. 5a,d). YAP is phosphorylated by the serine/threonine protein kinase LATS (large tumour suppressor) at five serine residues as part of the Hippo signalling cascade. Phosphorylation at serine-127 – not mutated in the GFP-YAP(S4A) construct – is thought to regulate translocation between cytoplasm and the nucleus, while phosphorylation at serine-381 – mutated to a glycine in the GFP-YAP(S4A) construct – heads a cascade leading to proteasomal degradation of YAP49. It was therefore reasoned that the GFP-YAP(S4A) construct might retain the ability to report on stiffness because of its location within the cell, but be more stable and less liable to disturb other cellular functions. This was partially borne out by an increased stability of cytoplasmic GFP-YAP(S4A) that resulted in no significant change to total levels of the construct on soft and stiff substrates (Fig. 5e). However, the sensitivity and dynamic range of GFP-YAP(4SA) translocation was also reduced with respect to the wildtype construct (Fig. 5d). Previous reports have shown that overexpression of YAP can override the influence that substrate stiffness has over MSC lineage7. However, here the presence of the reporter constructs was found not to affect the initial mechanosensitive cell and nuclear spreading responses (Fig. 5b,c), found in the primary and untransformed immortalised MSCs (Fig. 1) and interpreted as being indicative of intact mechanosensing mechanisms at FAs. The GFP-YAP(wt) construct was thus found to translocate such that the nuclear:cytoplasmic ratio could be used to report on substrate stiffness (Fig. 6b).
In conclusion, we have shown that an immortalised, clonal line of human-derived MSCs maintained the mechanosensitive features of primary MSCs. Furthermore, when stably transformed with a YAP-based fluorescent construct, the immortalised cells maintained sensitivity to the mechanical properties of the microenvironment and could be used to distinguish soft from stiff surroundings. Given the continued interest in MSCs in tissue engineering and regenerative medicine, and the growing appreciation of the roles of mechanical signalling in determining a broad range of cell behaviours, these cells and derivative technologies have potential to benefit scientists wishing to understand the mechanical properties of substrates and scaffolds from a cellular perspective.
Methods
Cell culture
Experiments were performed in accordance with relevant guidelines and regulations, and with relevant National Research Ethics Service and University of Manchester approvals. Primary human MSCs were harvested from the bone marrow of male and female donors aged 58–80 undergoing knee and hip surgery, under provision of informed written consent. MSCs were isolated according to established methodology50. Primary MSCs were used at passages 3–5. Immortalised human MSCs (line Y201) were used as described previously25. MSCs were cultured in low glucose (1 g/L) DMEM (Gibco). 293 T cells were cultured in high glucose (4.5 g/L) DMEM (Lonza). All media was supplemented with 10% fetal bovine serum (FBS, Labtech) and 1% penicillin/streptomycin cocktail (P/S, Sigma). Investigations into the effects of substrate stiffness were performed on collagen-I coated PA gels (Matrigen).
Immunofluorescence
Cells were cultured on polyacrylamide hydrogels of defined stiffnesses (Matrigen) at a density of 450 cells/cm2 for 3 days. Cells were fixed with 4% paraformaldehyde (PFA, VWR International) in phosphate buffered saline (PBS) for 10 min at room temperature (RT), followed by 2 × 5 min washing in PBS. Cells were permeabilized using 1% Triton X-100 (Sigma-Aldrich) in PBS and blocked with 2% bovine serum albumin (BSA, Sigma-Aldrich), 0.25% Triton-X in PBS at RT for 30 mins. Cells were incubated with primary antibodies against YAP (from rabbit; Proteintech; used at 1:100 dilution overnight at 4 °C), MKL1 (from rabbit; Abcam; used at 1:300 dilution overnight at 4 °C), lamin-A/C (from mouse; Santa Cruz Biotechnology, sc-7292; used at 1:200 dilution overnight at 4 °C), lamin-B1 (from rabbit; Proteintech; used at 1:300 dilution overnight at 4 °C) and GFP (from rabbit; Invitrogen; used at 1:150 dilution for 1 hour at 37 °C). Following 3 × 5 min PBS washes, cells were incubated with secondary antibodies (Life Technologies, used at 1:1000 dilution) for 1 hour at 37 °C: AlexaFluor-488 donkey anti-rabbit, AlexaFluor-594 donkey anti-rabbit, AlexaFluor-594 donkey anti-mouse and AlexaFluor-647 donkey anti-rabbit. Following further 3 × 5 min PBS washes, DAPI (1:500; Sigma Aldrich, D9542) was used to stain cells at 37 °C for 1 hour; AlexaFluor-488 or 647 Phalloidin (1:100; Cell Signaling Technology) was added with the DAPI stain. Samples were washed in PBS for 3 × 5 mins prior to imaging. Secondary-only controls for antibody staining are shown in Supplemental Fig. 1.
Cells were imaged using an Axio Examiner A1 microscope (Zeiss) with an EC Epiplan-Neofluar 20 × /0.5 NA dipping objective lens (Zeiss). Images were processed in ImageJ (version 2.0.0, National Institutes of Health, USA)51; CellProfiler (version 2.1.1, Broad Institute, USA)52 was used to characterize cell morphometric parameters: cell area; nuclear area; ratios of nuclear to cytoplasmic protein intensities; aspect ratio (ratio of major to minor axes of an ellipse enclosing the cell or nucleus); and circularity (ratio of (4π x area) to square of the perimeter). Multiple random fields of view were analysed in order to characterise cell morphologies and immunofluorescence intensities. When making quantitative comparisons between images from different conditions, the same procedures and parameters were used in the image analysis software. Images were corrected for background fluorescence by subtracting the mean intensity of a cell-free area from each pixel; all images under comparison in the same experiment had matched exposure and contrast settings.
Differentiation assays
Osteogenesis was chemically induced by culture in StemXVivo Osteogenic/Adipogenic Base Media (R&D Systems), supplemented with StemXVivo Human Osteogenic Supplement (R&D Systems) and 1% P/S. Adipogenesis was chemically induced in high glucose (4.5 g/L) DMEM with 1 µM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX), 10 µg/mL insulin and 100 µM indomethacin (all reagents from Sigma), supplemented with 10% FBS and 1% P/S as described previously53. MSCs were grown in osteogenic, adipogenic or control media for three weeks on collagen-I coated PA hydrogels for RT-qPCR assays, with seeding densities of 2000 and 1000 cells/cm2, for primary and immortalised MSCs respectively (seeding rates were adjusted to account for a greater rate of proliferation in the immortalised cells). For cytochemistry assays, cells were seeded on tissue culture plastic (TCP) at densities of 1200 or 650 cells/cm2, for primary and immortalised MSCs respectively.
Cytochemistry
Adipogenesis was quantified by positive staining of lipid droplets with Oil Red O (Sigma) and osteogenesis by alkaline phosphatase activity (Sigma, following the manufacturer’s protocol). Adipogenic cells were imaged using an EVOS XL Core microscope (Life-Tech) with a Plan PH2 achromatic infinity-corrected 20 × lens.
RT-qPCR
Total RNA was extracted with miRNeasy Mini Kit (Qiagen) and its concentration was measured with a NanoDrop 2000 spectrophotometer (Thermo Scientific). mRNA was reverse transcribed using miScript II RT Kit with the HiFlex buffer (Qiagen) in a Verity Thermal Cycler (Applied Biosystems). Adipogenic (PPARG and CEBPA) and osteogenic markers (RUNX2 and BGLAP) were quantified with SYBR Select Master Mix (Applied Biosystems) according to the manufacturer’s instructions, with normalisation against PPIA (Sigma), in a StepOnePlus Real-Time PCR System (Applied Biosystems). Experiments were performed in technical triplicates and relative gene expression was calculated using the 2-ΔΔCt method54. Primers were purchased from PrimerDesign:
PPARG (Forward, AACACTAAACCACAAATATACAACAAG; Reverse, GGCATCTCTGTGTCAACCAT)
CEBPA (CGGCAACTCTAGTATTTAGGATAAC; CAAATAAAATGACAAGGCACGATT)
RUNX2 (TTCTCCCCTTTTCCCACTGA; CAAACGCAATCACTATCTATACCAT)
BGLAP (CAGCGAGGTAGTGAAGAGACC; TCAGCCAACTCGTCACAGTC)
PPIA (ATGCTGGACCCAACACAAA; TTTCACTTTGCCAAACACCA)
Preparation of reporter constructs
A construct expressing GFP-YAP/RFP-H2B (“GFP-YAP(wt)”) was cloned from a plasmid provided by the Discher laboratory (University of Pennsylvania, USA), with inclusion of peptide T2A to obtain a bicistronic vector (see Supplemental Fig. 2). A second construct expressing the same, but with four point mutations to serine residues in YAP (S61A, S109A, S164A and S381G; “GFP-YAP(4SA)”) was generated by site-directed mutagenesis (Agilent QuikChange Lightning kit, used according to the manufacturer’s instructions). Plasmid identities were confirmed by sequencing (GATC Biotech) and analysis with DNADynamo (Blue Tractor Software Ltd.) and BLAST (NCBI, National Institutes of Health, USA). Plasmids were amplified in competent E. coli cells, harvested and purified with a HiSpeed Plasmid Maxi Kit (Qiagen), following the manufacturer’s instructions.
Viral delivery of reporter constructs
Lentiviral envelope plasmid pMD2.G and packaging plasmid psPAX2 were gifts from Didier Trono (Addgene plasmids #12259 and #12260). 293T cells were transiently transfected with the reporter, packaging and envelope plasmids (at a ratio 2:1.5:1) using polyethylenimine (PEI; Merck-Millipore). Virus production was enhanced by addition of 10 mM sodium butyrate (Merck-Millipore) and viral particles concentrated using Vivaspin 20 ultracentrifugation column (Sartorius). Y201 cells were transduced with the viral particles and 8 µg/mL polybrene (Merck-Millipore). Two weeks following infection RFP-positive cells were selected by FACS-sorting.
Statistical analysis
All data is presented as mean ± SEM. All experiments were performed with four biological primary donors. Data was checked for normal distribution using D’Agostino-Pearson or Shapiro-Wilk tests. Differences in means of normally distributed data were analysed with t-tests (paired when applied to donor-matched samples), otherwise Kruskal-Wallis (KW) or Mann-Whitney (MW) non-parametric tests were used. Analysis was performed using Prism 7 (GraphPad Software Inc.) and Mathematica (Wolfram Research Inc.).
Electronic supplementary material
Acknowledgements
HTJG and JS were funded by a Biotechnology and Biological Sciences Research Council (BBSRC) David Phillips Fellowship (BB/L024551/1). OD was supported by a Wellcome Trust Institutional Strategic Support Fund (097820/Z/11/B). PG was funded by the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) and Arthritis Research UK (ARUK). Microscopy was carried out at the Wellcome Centre for Cell-Matrix Research (WTCCMR; 203128/Z/16/Z) Imaging Core Facility. The authors thank: Tim Board (Wrightington Hospital) for provision of tissue samples for MSC isolation; Mike Jackson and Gareth Howell (University of Manchester Flow Cytometry Core Facility); and Robert Pedley for assistance with viral protocols.
Author Contributions
Investigation, A.G.T., J.E.S., A.W., O.D., H.T.J.G.; Formal Analysis, A.G.T., J.E.S. and J.S.; Writing – Original Draft, A.G.T.; Writing – Review & Editing, A.G.T., J.E.S., A.W., O.D., H.T.J.G., P.G., K.B., S.M.R. and J.S.; Visualization, Project Administration and Funding Acquisition, P.G., K.B., S.M.R. and J.S.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-27346-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014;15:802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho S, Irianto J, Discher DE. Mechanosensing by the nucleus: From pathways to scaling relationships. J. Cell Biol. 2017;216:305–315. doi: 10.1083/jcb.201610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Athirasala A, Hirsch N, Buxboim A. Nuclear mechanotransduction: sensing the force from within. Curr. Opin. Cell Biol. 2017;46:119–127. doi: 10.1016/j.ceb.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Matthews BD, et al. Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface beta 1 integrins. Integr. Biol. 2010;2:435–442. doi: 10.1039/c0ib00034e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 6.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell. 2004;6:483–495. doi: 10.1016/S1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 7.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–U212. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 8.Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature. 2013;497:507–511. doi: 10.1038/nature12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl. Acad. Sci. USA. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iyer KV, Pulford S, Mogilner A, Shivashankar GV. Mechanical activation of cells induces chromatin remodeling preceding MKL nuclear transport. Biophys. J. 2012;103:1416–1428. doi: 10.1016/j.bpj.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang B, et al. Mechanosensing controlled directly by tyrosine kinases. Nano Lett. 2016;16:5951–5961. doi: 10.1021/acs.nanolett.6b02995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghibaudo M, et al. Traction forces and rigidity sensing regulate cell functions. Soft Matter. 2008;4:1836–1843. doi: 10.1039/b804103b. [DOI] [Google Scholar]
- 13.Pelham RJ, Wang YL. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 15.Swift J, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang HB, Dembo M, Wang YL. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am. J. Physiol.-Cell Ph. 2000;279:C1345–C1350. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- 17.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raab M, et al. Crawling from soft to stiff matrix polarizes the cytoskeleton and phosphoregulates myosin-II heavy chain. J. Cell Biol. 2012;199:669–683. doi: 10.1083/jcb.201205056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 20.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 21.Treiser MD, et al. Cytoskeleton-based forecasting of stem cell lineage fates. Proc. Natl. Acad. Sci. USA. 2010;107:610–615. doi: 10.1073/pnas.0909597107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Chen XD, Cao W, Shi YF. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat. Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 23.Richardson SM, et al. Mesenchymal stem cells in regenerative medicine: Opportunities and challenges for articular cartilage and intervertebral disc tissue engineering. J. Cell. Physiol. 2010;222:23–32. doi: 10.1002/jcp.21915. [DOI] [PubMed] [Google Scholar]
- 24.Benders KEM, et al. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol. 2013;31:169–176. doi: 10.1016/j.tibtech.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 25.James S, et al. Multiparameter analysis of human bone marrow stromal cells identifies distinct immunomodulatory and differentiation-competent subtypes. Stem Cell Rep. 2015;4:1004–1015. doi: 10.1016/j.stemcr.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee I-N, et al. Photo-responsive hydrogels with photoswitchable mechanical properties allow time-resolved analysis of cellular responses to matrix stiffening. ACS Appl. Mater. Interfaces. 2018;10:7765–7776. doi: 10.1021/acsami.7b18302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosales AM, Vega SL, DelRio FW, Burdick JA, Anseth KS. Hydrogels with reversible mechanics to probe dynamic cell microenvironments. Angew. Chem. Int. Ed. 2017;56:12132–12136. doi: 10.1002/anie.201705684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillette BM, Jensen JA, Wang MX, Tchao J, Sia SK. Dynamic hydrogels: Switching of 3D microenvironments using two-component naturally derived extracellular matrices. Advanced Materials. 2010;22:686–+. doi: 10.1002/adma.200902265. [DOI] [PubMed] [Google Scholar]
- 29.Yoshikawa HY, et al. Quantitative evaluation of mechanosensing of cells on dynamically tunable hydrogels. J. Am. Chem. Soc. 2011;133:1367–1374. doi: 10.1021/ja1060615. [DOI] [PubMed] [Google Scholar]
- 30.Dingal PCDP, et al. Fractal heterogeneity in minimal matrix models of scars modulates stiff-niche stem-cell responses via nuclear exit of a mechanorepressor. Nat. Mater. 2015;14:951–960. doi: 10.1038/nmat4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinderer S, Layland SL, Schenke-Layland K. ECM and ECM-like materials - Biomaterials for applications in regenerative medicine and cancer therapy. Adv. Drug Deliv. Rev. 2016;97:260–269. doi: 10.1016/j.addr.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Chen FM, Liu XH. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016;53:86–168. doi: 10.1016/j.progpolymsci.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dingal PCDP, Discher DE. Combining insoluble and soluble factors to steer stem cell fate. Nat. Mater. 2014;13:532–537. doi: 10.1038/nmat3997. [DOI] [PubMed] [Google Scholar]
- 34.Levental I, Georges PC, Janmey PA. Soft biological materials and their impact on cell function. Soft Matter. 2007;3:299–306. doi: 10.1039/B610522J. [DOI] [PubMed] [Google Scholar]
- 35.Lu H, Wang B, Ma J, Huang G, Viswanathan H. Measurement of creep compliance of solid polymers by nanoindentation. Mech. Time-Depend. Mater. 2003;7:189–207. doi: 10.1023/B:MTDM.0000007217.07156.9b. [DOI] [Google Scholar]
- 36.Canovic, E. P. et al. Characterizing multiscale mechanical properties of brain tissue using atomic force microscopy, impact indentation, and rheometry. J. Vis. Exp., 12 (2016). [DOI] [PMC free article] [PubMed]
- 37.Ivanovska, I. L. et al. Cross-linked matrix rigidity and soluble retinoids synergize in nuclear lamina regulation of stem cell differentiation. Mol. Biol. Cell28, 2010–2022 (2017). [DOI] [PMC free article] [PubMed]
- 38.Zemel A, Rehfeldt F, Brown AEX, Discher DE, Safran SA. Optimal matrix rigidity for stress-fibre polarization in stem cells. Nat. Phys. 2010;6:468–473. doi: 10.1038/nphys1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tajik A, et al. Transcription upregulation via force-induced direct stretching of chromatin. Nat. Mater. 2016;15:1287–1296. doi: 10.1038/nmat4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buxboim A, et al. Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin. Curr. Biol. 2014;24:1909–1917. doi: 10.1016/j.cub.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janmey PA, Wells RG, Assoian RK, McCulloch CA. From tissue mechanics to transcription factors. Differentiation. 2013;86:112–120. doi: 10.1016/j.diff.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho I, Jackson MR, Swift J. Roles of cross-membrane transport and signaling in the maintenance of cellular homeostasis. Cellular and Molecular Bioengineering. 2016;9:234–246. doi: 10.1007/s12195-016-0439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elosegui-Artola A, et al. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell. 2017;171:1397. doi: 10.1016/j.cell.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Connelly JT, et al. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat. Cell Biol. 2010;12:711–U177. doi: 10.1038/ncb2074. [DOI] [PubMed] [Google Scholar]
- 45.Nobusue H, et al. Regulation of MKL1 via actin cytoskeleton dynamics drives adipocyte differentiation. Nat. Commun. 2014;5:12. doi: 10.1038/ncomms4368. [DOI] [PubMed] [Google Scholar]
- 46.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013;45:16. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whitfield MJ, Lee WCJ, Van Vliet KJ. Onset of heterogeneity in culture-expanded bone marrow stromal cells. Stem Cells Res. 2013;11:1365–1377. doi: 10.1016/j.scr.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Wagner W, et al. Replicative senescence of mesenchymal stem cells: A continuous and organized process. Plos One. 2008;3:12. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF beta-TRCP. Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strassburg S, Richardson SM, Freemont AJ, Hoyland JA. Co-culture induces mesenchymal stem cell differentiation and modulation of the degenerate human nucleus pulposus cell phenotype. Regen. Med. 2010;5:701–711. doi: 10.2217/rme.10.59. [DOI] [PubMed] [Google Scholar]
- 51.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamentsky L, et al. Improved structure, function and compatibility for CellProfiler: modular high-throughput image analysis software. Bioinformatics. 2011;27:1179–1180. doi: 10.1093/bioinformatics/btr095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cai R, Nakamoto T, Hoshiba T, Kawazoe N, Chen G. Control of simultaneous osteogenic and adipogenic differentiation of mesenchymal stem cells. J. Stem Cell Res. Ther. 2014;4:1000223. [Google Scholar]
- 54.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.