Abstract
This study aimed to evaluate the effects of ischemia-reperfusion injury (IRI) on the risk of hepatocellular carcinoma (HCC) recurrence after liver transplantation. Data of 195 patients were retrospectively analysed. Post-reperfusion aspartate (AST), alanine transaminase, and lactate dehydrogenase (LDH) levels were the primary measures of IRI. Tumour recurrence was the primary endpoint. Post-reperfusion AST was a continuous risk factor for tumour recurrence in patients within Milan criteria (p = 0.035), with an optimal cut-off of 1896 U/L. Recurrence-free survival of patients within Milan criteria and post-reperfusion AST of <1896 and ≥1896 U/L was 96.6% and 71.9% at 5 and 3.7 years, respectively (p = 0.006). Additionally, post-reperfusion AST and LDH exceeding the upper quartile significantly increased the risk of HCC recurrence in patients within Milan criteria (p = 0.039, hazard ratio [HR] = 5.99 and p = 0.040, HR = 6.08, respectively) and to a lesser extent, in patients within Up-to-7 criteria (p = 0.028, HR = 3.58 and p = 0.039, HR = 3.33, respectively). No other significant IRI effects were found in patients beyond the Up-to-7 criteria and in analyses stratified for independent risk factors for recurrence: tumour number and differentiation, alpha-fetoprotein, and microvascular invasion. Thus, IRI exerts major negative effects on the risk of HCC recurrence after liver transplantation in patients within standard and extended criteria.
Introduction
Hepatocellular carcinoma (HCC) remains one of the most common indications for liver transplantation1. The Milan criteria defined transplant eligibility for HCC patients for more than two decades; however, the limits are now being expanded according to morphological and biological tumour features2–6. Nevertheless, discussion on widening the pool of potential candidates is controversial owing to a major and relatively constant shortage of deceased donors. Further expansion of the selection criteria will inevitably lead to increased waiting times for both HCC and non-HCC populations. In HCC patients, markedly prolonged times on the waiting list are characterised by more common dropouts, possibly leading to the development of more aggressive tumours7. Owing to increased rates of listing and privileged positions of HCC patients under the current allocation policies, a higher number of HCC transplant candidates may have even more detrimental effects on non-HCC patients’ waiting times and pre-transplant mortality8,9. Because widening the donor pool with living donors and high-risk or extended criteria deceased donors is a common strategy, it appears to have major relevance, particularly for HCC patients.
Experimental studies demonstrate the increased risk of cancer recurrence associated with ischemia-reperfusion injury (IRI)10,11. Changes in hepatic microenvironment caused by IRI promote seeding and the development of metastases, whereas IRI-induced proinflammatory response, release of growth factors, mobilization of progenitor cells, and transformation of cancer cells to more aggressive phenotypes may potentiate the formation and growth of metastases at both local and remote sites12–16. Because grafts procured from high-risk deceased donors and to a lesser extent, partial grafts procured from living donors may be more susceptible to IRI, the use of these grafts may increase the risk of post-transplant HCC recurrence. This hypothesis was subject to numerous studies with inconsistent results. Although transplantations of grafts procured from living donors or high-risk grafts procured from deceased donors after cardiac death or those who were older and had hepatic steatosis or other risk factors were reported to have adverse effects on outcomes after liver transplantations for HCC in several studies, the results of available studies are not completely consistent17–23. However, recent reports found that prolonged ischemic times, directly related to the magnitude of IRI, increased the risk of post-transplant HCC recurrence24,25. Nevertheless, data on the direct effect of the magnitude of IRI on the risk of HCC recurrence after deceased donor liver transplantation are limited. Therefore, this study aimed to evaluate the association between the degree of graft IRI as indicated by post-reperfusion transaminase and lactate dehydrogenase (LDH) levels and the risk of post-transplant HCC recurrence after deceased donor liver transplantation with respect to patients’ initial risk profile.
Methods
This was a retrospective observational study. In total, 250 liver transplantations were performed for HCC patients between January 2001 and June 2016 at the Department of General, Transplant and Liver Surgery (Medical University of Warsaw). Patients with fibrolamellar HCCs and those with combined HCC/cholangiocarcinoma were not included. After exclusion of 55 patients with missing measurements of transaminase levels 2 h after reperfusion, the final study cohort comprised 195 liver transplant recipients. The study protocol was approved by the institutional review board of the Medical University of Warsaw. Informed consents were not obtained from the patients due to the retrospective nature of the study, which is in line with institutional review board and national regulations. All methods were performed in accordance with the relevant guidelines and regulations. No organs were procured from prisoners.
The degree of IRI was represented by three variables, namely, serum alanine transaminase (ALT), serum aspartate transaminase (AST), and serum LDH levels; each was assessed from a blood sample obtained 2 h after portal reperfusion. These variables were the primary factors of interest. Peak serum bilirubin concentration, international normalised ratio (INR), and gamma-glutamyl transpeptidase (GGTP) activity over the first 7 post-transplant days were additionally analysed as variables associated with IRI. The duration of cold and warm ischemia was defined as the time from clamping of the donor aorta until the removal of the graft from the preservation solution and that from the removal of the graft from the cold preservation solution until portal reperfusion, respectively. The sum of cold and warm ischemic times formed the total ischemic time. All grafts were procured from donors after brain death. Tumour recurrence over the 5-year post-transplant observation period was the primary end-point. Recurrence-free survival was calculated from the date of transplantation until tumour recurrence and censored at the date of last available follow-up, death for non-HCC related causes or 5 years post-transplantation (whichever occurred first). Details on the surgical technique, perioperative care, immunosuppression, and follow-up protocol are provided elsewhere26,27.
First, post-reperfusion ALT, AST, and LDH levels were assessed for their potential effect on the risk of post-transplant tumour recurrence in all patients. Other independent predictors of recurrence were also assessed, including peak post-transplant bilirubin concentration, INR, and GGTP activity. Furthermore, the analyses were adjusted for their potential confounding effects in bivariable analyses. Subgroup analyses were subsequently performed to determine the potential differences in associations between IRI degree and the risk of HCC recurrence according to patients’ initial risk profile, based on fulfilment of selection criteria and established independent predictors of recurrence.
Continuous and categorical variables are given as medians (interquartile ranges) and numbers (percentages). The Kaplan-Meier method was used for survival calculations, and log-rank test was used for intergroup comparisons. A Cox proportional hazards regression analysis was performed to evaluate the associations between particular factors and the risk of recurrence. A multivariable model was created using forward stepwise method with p thresholds of 0.05 and 0.150 for inclusion and exclusion of variables, respectively. An additional series of bivariable analyses were performed to adjust the effects of IRI to potential confounding effects of independent risk factors for tumour recurrence. Spearman correlation coefficients were calculated to evaluate the associations between ischemic times and donor age and post-reperfusion laboratory measurements. Post-reperfusion AST, ALT, and LDH levels; peak post-transplant bilirubin concentration; and peak post-transplant GGTP activity were transformed to their natural logarithms prior to their analyses as continuous variables. Additionally, they were assessed as categorical factors using the upper quartile for division. Receiver operating characteristic (ROC) curves were constructed to determine the optimal cut-offs of continuous factors in predicting recurrence. Hazard ratios (HRs) and c-statistics were presented with 95% confidence intervals (95% CIs). Significance threshold was set to two-tailed p values of 0.05. Analyses were computed in STATISTICA version 13 (Dell Inc., Tulsa, USA) software. The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Results
The characteristics of the 195 patients are shown in Table 1. Median AST, ALT, and LDH levels assessed 2 h post-reperfusion were 850 U/L (interquartile range: 486–1625 U/L; range 153–14375 U/L), 566 U/L (interquartile range: 304–935 U/L; range 102–9912 U/L), and 2240 U/L (interquartile range: 1322–4670 U/L; range 385–38207 U/L), respectively. Post-reperfusion AST and ALT levels were significantly, yet poorly correlated, with total (both p = 0.001) and cold ischemic times (both p < 0.001), whereas post-reperfusion LDH levels were poorly correlated with cold ischemic time (p = 0.031), intraoperative fresh frozen plasma transfusions (p = 0.002), and intraoperative packed red blood cell transfusions (p = 0.018, Table 2). Donor age and warm ischemic times were not correlated with post-reperfusion AST, ALT, and LDH levels.
Table 1.
Recipient, donor, and operative characteristics of 195 liver transplant recipients with hepatocellular carcinoma included in the study.
| Variables | Number (%) or median (IQR) |
|---|---|
| Post-reperfusion AST (U/L) | 850 (486–1625) |
| Post-reperfusion ALT (U/L) | 566 (304–935) |
| Post-reperfusion LDH (U/L) | 2240 (1322–4670) |
| Peak 7-day postoperative bilirubin concentration (mg/dL) | 3.6 (2.1–5.6) |
| Peak 7-day postoperative international normalized ratio | 1.5 (1.3–1.8) |
| Peak 7-day postoperative GGTP activity (U/L) | 663 (396–967) |
| Recipient sex | |
| male | 146 (74.9%) |
| female | 49 (25.1%) |
| Recipient age (years) | 58 (52–61) |
| Hepatitis C virus infection | 132 (67.7%) |
| Hepatitis B virus infection | 89 (45.6%) |
| Model for End-stage Liver Disease | 11 (8–13) |
| Within Milan criteria | 113 (57.9%) |
| Within UCSF criteria | 136 (69.7%) |
| Within Up-to-7 criteria | 144 (73.8%) |
| Number of tumors | 1 (1–3) |
| Diameter of the largest tumor (mm) | 30 (20–45) |
| Total tumor volume (cm3) | 22 (5–62) |
| Alpha-fetoprotein concentration (ng/ml) | 13.8 (5.7–112.8) |
| Microvascular invasion | 52 (26.7%) |
| Poor tumor differentiation | 19 (9.7%) |
| Neoadjuvant treatment | 102 (52.3%) |
| Total ischemic time (hours) | 9.0 (8.0–10.3) |
| Cold ischemic time (hours) | 8.0 (6.9–9.5) |
| Warm ischemic time (minutes) | 55 (44–68) |
| Intraoperative PRBC transfusions (units) | 3 (1–6) |
| Intraoperative FFP transfusions (units) | 6 (4–9) |
| Donor age | 51 (41–60) |
| Donor sex | |
| male | 120 (61.5%) |
| female | 75 (38.5%) |
IQR – interquartile range; AST – aspartate transaminase; ALT – alanine transaminase; LDH – lactate dehydrogenase; UCSF – University of California, San Francisco; PRBC – packed red blood cells; FFP – fresh frozen plasma.
Table 2.
Analyses of correlations between selected factors and activity of aspartate transaminase (AST), alaninę transaminase (ALT), and lactate dehydrogenase (LDH) at 2 hours after reperfusion in liver transplantation for hepatocellular carcinoma.
| AST activity | ALT activity | LDH activity | ||||
|---|---|---|---|---|---|---|
| R | p | R | p | R | p | |
| Total ischemic time | 0.241 | 0.001 | 0.244 | 0.001 | 0.132 | 0.082 |
| Cold ischemic time | 0.324 | <0.001 | 0.312 | <0.001 | 0.188 | 0.031 |
| Warm ischemic time | 0.083 | 0.324 | 0.102 | 0.222 | 0.124 | 0.155 |
| Intraoperative PRBC transfusions | 0.107 | 0.143 | 0.125 | 0.086 | 0.179 | 0.018 |
| Intraoperative FFP transfusions | 0.042 | 0.565 | 0.055 | 0.449 | 0.235 | 0.002 |
| Donor age | 0.010 | 0.892 | 0.017 | 0.815 | −0.120 | 0.108 |
Correlations were assessed with Spearman correlation coefficients. AST – aspartate transaminase; ALT – alanine transaminase; LDH – lactate dehydrogenase; PRBC – packed red blood cells; FFP – fresh frozen plasma.
The median follow-up period was 37.5 months. A total of 27 patients developed HCC recurrence with recurrence-free survival rates of 90.8%, 83.4%, and 81.0% at 1, 3, and 5 years, respectively. Univariable analyses revealed that post-reperfusion AST (p = 0.521), ALT (p = 0.773), and LDH (p = 0.575) levels and peak 7-day post-transplant bilirubin concentration (p = 0.592), INR (p = 0.553), and GGTP activity (p = 0.534) were not significantly associated with recurrence in all patients (Table 3). There were also no significant differences in recurrence-free survival depending on the quartile of AST (p = 0.725), ALT (p = 0.819), and LDH (p = 0.656) levels (Fig. 1). Similarly, no differences with respect to recurrence-free survival were observed depending on the quartile of peak 7-day postoperative bilirubin concentration (p = 0.849), INR (p = 0.309), and GGTP activity (p = 0.866; Fig. 2). In multivariable analysis, the independent risk factors comprised tumour number (p = 0.004), pre-transplant alpha-fetoprotein concentration (p < 0.001), presence of microvascular invasion (p = 0.014), and poor tumour differentiation (p = 0.007). No significant effects of post-reperfusion AST (all p > 0.250), ALT (all p > 0.403), and LDH (all p > 0.176) levels and peak 7-day postoperative bilirubin concentration (all p > 0.167), INR (all p > 0.230), and GGTP activity (all p > 0.123) on the risk of recurrence were found in analyses adjusted for the effects of these independent predictors. The corresponding series of bivariable analyses are presented in Tables 4 and 5. Additionally, fulfilment of the Milan (p < 0.001; HR 0.17, 95% CI 0.07–0.43); University of California, San Francisco (UCSF, p = 0.009; HR 0.37, 95% CI 0.17–0.78); and Up-to-7 (p < 0.001; HR 0.24, 95% CI 0.11–0.51) criteria significantly reduced the risk of recurrence.
Table 3.
Analyses of risk factors for hepatocellular carcinoma recurrence after deceased donor liver transplantation.
| Factors | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Post-reperfusion AST activity (continuous) | 1.17 (0.72–1.89) | 0.521 | ||
| Post-reperfusion AST activity (upper quartile) | 1.23 (0.52–2.91) | 0.638 | ||
| Post-reperfusion ALT activity (continuous) | 1.07 (0.67–1.72) | 0.773 | ||
| Post-reperfusion ALT activity (upper quartile) | 0.77 (0.29–2.04) | 0.602 | ||
| Post-reperfusion LDH activity (continuous) | 1.13 (0.73–1.76) | 0.575 | ||
| Post-reperfusion LDH activity (upper quartile) | 1.66 (0.73–3.78) | 0.226 | ||
| Peak postoperative bilirubin concentration (continuous) | 0.86 (0.51–1.47) | 0.592 | ||
| Peak postoperative bilirubin concentration (upper quartile) | 0.67 (0.25–1.78) | 0.424 | ||
| Peak postoperative INR (continuous) | 0.81 (0.41–1.60) | 0.553 | ||
| Peak postoperative INR (upper quartile) | 0.91 (0.34–2.42) | 0.854 | ||
| Post-reperfusion GGTP activity (continuous) | 1.20 (0.67–2.15) | 0.534 | ||
| Post-reperfusion GGTP activity (upper quartile) | 1.36 (0.59–3.12) | 0.474 | ||
| Total ischemic time | 1.02 (0.83–1.24) | 0.866 | ||
| Cold ischemic time | 1.07 (0.85–1.35) | 0.544 | ||
| Warm ischemic time | 1.00 (0.84–1.19) | 0.963 | ||
| Donor age | 1.00 (0.97–1.03) | 0.896 | ||
| Male donor sex | 0.52 (0.24–1.12) | 0.095 | ||
| Number of tumors | 1.25 (1.12–1.41) | <0.001 | 1.21 (1.06–1.39) | 0.004 |
| Diameter of the largest tumor | 1.02 (1.01–1.03) | 0.001 | ||
| Total tumor volume | 1.01 (1.00–1.03) | 0.170 | ||
| Alpha-fetoprotein concentration | 1.31 (1.15–1.50) | <0.001 | 1.29 (1.12–1.47) | <0.001 |
| Microvascular invasion | 4.28 (1.99–9.24) | <0.001 | 2.67 (1.22–5.84) | 0.014 |
| Poor tumor differentiation | 4.05 (1.71–9.59) | 0.002 | 3.35 (1.38–8.13) | 0.007 |
| Neoadjuvant treatment | 2.04 (0.91–4.54) | 0.082 | ||
| Male recipient sex | 0.64 (0.29–1.42) | 0.269 | ||
| Recipient age | 1.01 (0.96–1.05) | 0.809 | ||
| Hepatitis C virus infection | 0.89 (0.41–1.95) | 0.770 | ||
| Hepatitis B virus infection | 1.16 (0.54–2.47) | 0.703 | ||
| Model for End-stage Liver Disease | 0.93 (0.84–1.03) | 0.186 | ||
| Intraoperative PRBC transfusions | 0.99 (0.92–1.07) | 0.839 | ||
| Intraoperative FFP transfusions | 0.97 (0.89–1.05) | 0.470 | ||
Hazard ratios for continuous variables are given per: 1 loge (U/L) increase for AST, ALT, LDH, and GGTP activity; 1 increase for INR; 1 hour increase for total and cold ischemic times; 10 minute increase for warm ischemic time; 1 year increase for recipient and donor age; 1 increase for tumor number; 1 mm increase for diameter of the largest tumor; 10 cm3 increase for total tumor volume; 1 loge (ng/ml) increase for alpha-fetoprotein concentration; 1 point increase for Model for End-stage Liver Disease; and 1 unit increase for transfusions. HR - hazard ratio; 95% CI – 95% confidence interval; AST – aspartate transaminase; ALT – alanine transaminase; GGTP – gamma-glutamyl transpeptidase; INR – international normalized ratio; LDH – lactate dehydrogenase; PRBC – packed red blood cells; FFP – fresh frozen plasma
Figure 1.
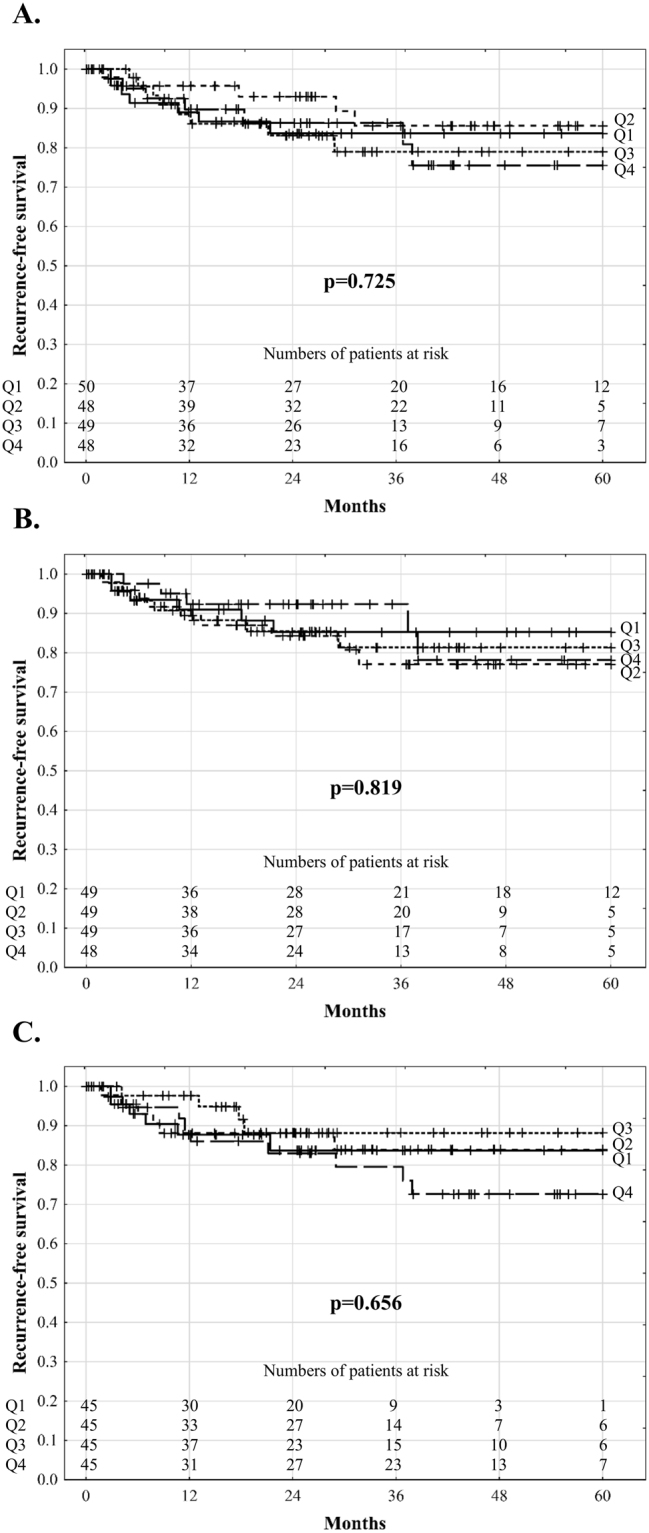
Recurrence-free survival of hepatocellular carcinoma patients after liver transplantation according to quartiles of aspartate transaminase (A), alanine transaminase (B), and lactate dehydrogenase (C) activity 2 hours after portal reperfusion.
Figure 2.
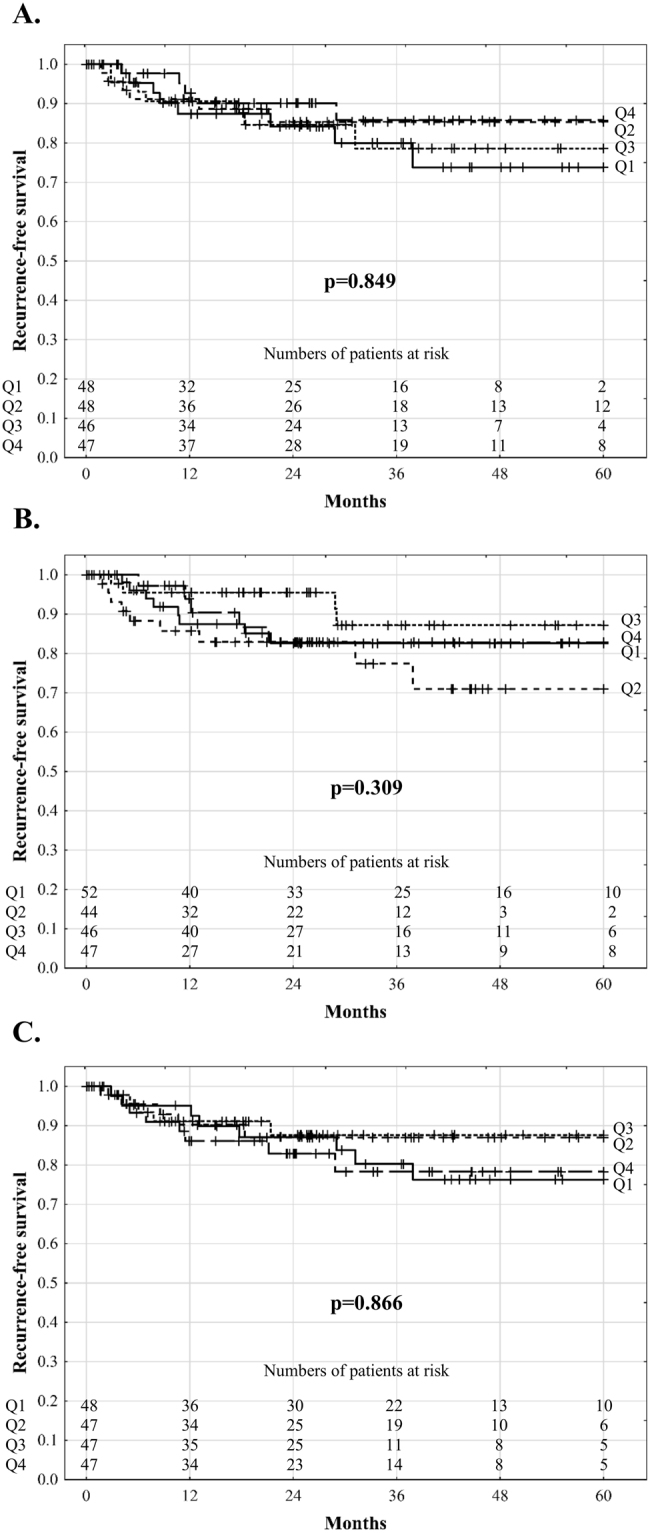
Recurrence-free survival of hepatocellular carcinoma patients after liver transplantation according to quartiles of peak 7-day postoperative bilirubin concentration (A), international normalized ratio (B), and gamma-glutamyl transpeptidase activity (C).
Table 4.
Analyses of the effects of the degree of ischemia-reperfusion injury on the risk of hepatocellular carcinoma recurrence after liver transplantation adjusted for the confounding influence of independent risk factors.
| Factor | HR | 95% CI | p | Adjusted for the effects of: |
|---|---|---|---|---|
| Post-reperfusion AST activity (continuous) | 1.10 | 0.70–1.74 | 0.683 | Tumor number |
| Post-reperfusion AST activity (categorical) | 1.20 | 0.51–2.85 | 0.677 | Tumor number |
| Post-reperfusion AST activity (continuous) | 1.11 | 0.70–1.74 | 0.667 | Alpha-fetoprotein concentration |
| Post-reperfusion AST activity (categorical) | 1.10 | 0.46–2.62 | 0.825 | Alpha-fetoprotein concentration |
| Post-reperfusion AST activity (continuous) | 1.31 | 0.76–2.25 | 0.332 | Microvascular invasion |
| Post-reperfusion AST activity (categorical) | 1.67 | 0.70–3.99 | 0.250 | Microvascular invasion |
| Post-reperfusion AST activity (continuous) | 1.25 | 0.75–2.09 | 0.398 | Poor tumor differentiation |
| Post-reperfusion AST activity (categorical) | 1.43 | 0.60–3.41 | 0.425 | Poor tumor differentiation |
| Post-reperfusion ALT activity (continuous) | 1.02 | 0.64–1.62 | 0.931 | Tumor number |
| Post-reperfusion ALT activity (categorical) | 0.71 | 0.27–1.87 | 0.483 | Tumor number |
| Post-reperfusion ALT activity (continuous) | 0.96 | 0.61–1.51 | 0.856 | Alpha-fetoprotein concentration |
| Post-reperfusion ALT activity (categorical) | 0.66 | 0.25–1.75 | 0.403 | Alpha-fetoprotein concentration |
| Post-reperfusion ALT activity (continuous) | 1.14 | 0.70–1.87 | 0.595 | Microvascular invasion |
| Post-reperfusion ALT activity (categorical) | 0.90 | 0.34–2.37 | 0.827 | Microvascular invasion |
| Post-reperfusion ALT activity (continuous) | 1.08 | 0.65–1.79 | 0.766 | Poor tumor differentiation |
| Post-reperfusion ALT activity (categorical) | 0.89 | 0.33–2.39 | 0.824 | Poor tumor differentiation |
| Post-reperfusion LDH activity (continuous) | 1.18 | 0.78–1.81 | 0.435 | Tumor number |
| Post-reperfusion LDH activity (categorical) | 1.69 | 0.74–3.85 | 0.211 | Tumor number |
| Post-reperfusion LDH activity (continuous) | 1.06 | 0.69–1.63 | 0.782 | Alpha-fetoprotein concentration |
| Post-reperfusion LDH activity (categorical) | 1.77 | 0.77–4.06 | 0.176 | Alpha-fetoprotein concentration |
| Post-reperfusion LDH activity (continuous) | 1.06 | 0.69–1.65 | 0.784 | Microvascular invasion |
| Post-reperfusion LDH activity (categorical) | 1.31 | 0.57–3.03 | 0.521 | Microvascular invasion |
| Post-reperfusion LDH activity (continuous) | 1.09 | 0.70–1.69 | 0.709 | Poor tumor differentiation |
| Post-reperfusion LDH activity (categorical) | 1.41 | 0.61–3.27 | 0.427 | Poor tumor differentiation |
HR – hazard ratio; 95% CI – 95% confidence interval; AST – aspartate transaminase; ALT – alanine transaminase; LDH – lactate dehydrogenase.
Table 5.
Analyses of the associations between peak 7-day postoperative bilirubin concentration, INR value, and GGTP activity and the risk of hepatocellular carcinoma recurrence after liver transplantation adjusted for the confounding influence of independent risk factors.
| Factor | HR | 95% CI | p | Adjusted for the effects of: |
|---|---|---|---|---|
| Peak postoperative bilirubin concentration (continuous) | 0.78 | 0.47–1.28 | 0.324 | Tumor number |
| Peak postoperative bilirubin concentration (categorical) | 0.49 | 0.18–1.35 | 0.167 | Tumor number |
| Peak postoperative bilirubin concentration (continuous) | 0.89 | 0.54–1.46 | 0.641 | Alpha-fetoprotein concentration |
| Peak postoperative bilirubin concentration (categorical) | 0.69 | 0.26–1.85 | 0.465 | Alpha-fetoprotein concentration |
| Peak postoperative bilirubin concentration (continuous) | 0.81 | 0.48–1.39 | 0.453 | Microvascular invasion |
| Peak postoperative bilirubin concentration (categorical) | 0.63 | 0.24–1.67 | 0.349 | Microvascular invasion |
| Peak postoperative bilirubin concentration (continuous) | 0.83 | 0.48–1.46 | 0.526 | Poor tumor differentiation |
| Peak postoperative bilirubin concentration (categorical) | 0.71 | 0.27–1.90 | 0.500 | Poor tumor differentiation |
| Peak postoperative INR (continuous) | 0.64 | 0.31–1.32 | 0.230 | Tumor number |
| Peak postoperative INR (categorical) | 0.75 | 0.28–2.01 | 0.564 | Tumor number |
| Peak postoperative INR (continuous) | 0.66 | 0.33–1.35 | 0.258 | Alpha-fetoprotein concentration |
| Peak postoperative INR (categorical) | 0.77 | 0.29–2.06 | 0.603 | Alpha-fetoprotein concentration |
| Peak postoperative INR (continuous) | 0.72 | 0.32–1.60 | 0.420 | Microvascular invasion |
| Peak postoperative INR (categorical) | 0.78 | 0.29–2.08 | 0.620 | Microvascular invasion |
| Peak postoperative INR (continuous) | 0.86 | 0.42–1.74 | 0.673 | Poor tumor differentiation |
| Peak postoperative INR (categorical) | 1.23 | 0.44–3.41 | 0.696 | Poor tumor differentiation |
| Peak postoperative GGTP activity (continuous) | 1.24 | 0.67–2.29 | 0.488 | Tumor number |
| Peak postoperative GGTP activity (categorical) | 1.42 | 0.61–3.26 | 0.415 | Tumor number |
| Peak postoperative GGTP activity (continuous) | 1.58 | 0.83–2.99 | 0.164 | Alpha-fetoprotein concentration |
| Peak postoperative GGTP activity (categorical) | 2.00 | 0.83–4.84 | 0.123 | Alpha-fetoprotein concentration |
| Peak postoperative GGTP activity (continuous) | 1.28 | 0.70–2.34 | 0.422 | Microvascular invasion |
| Peak postoperative GGTP activity (categorical) | 1.53 | 0.66–3.54 | 0.321 | Microvascular invasion |
| Peak postoperative GGTP activity (continuous) | 1.12 | 0.62–2.01 | 0.709 | Poor tumor differentiation |
| Peak postoperative GGTP activity (categorical) | 1.23 | 0.53–2.83 | 0.634 | Poor tumor differentiation |
Hazard ratios for continuous variables are given per: 1 mg/dL increase for bilirubin concentration; 1 increase for INR; 1 loge (U/L) increase for GGTP activity. HR – hazard ratio; 95% CI – 95% confidence interval; INR – international normalized ratio; GGTP – gamma-glutamyl transpeptidase
For further analyses, the patients were divided into subgroups based on the fulfilment of selection criteria and independent predictors of recurrence. Cut-offs for tumour number of ≥3 and alpha-fetoprotein concentration of ≥48.3 ng/ml were derived from the corresponding ROC curves. As a continuous variable, post-reperfusion AST significantly influenced the risk of HCC recurrence only in patients within the Milan criteria (p = 0.035, Table 6) with the optimal cut-off of ≥1896 U/L. Additionally, post-reperfusion AST and LDH levels exceeding the upper quartiles were significantly associated with increased risk of recurrence in patients either within the Milan (p = 0.039 and p = 0.040, respectively) or Up-to-7 (p = 0.028 and p = 0.039, respectively) criteria. The degree of IRI, as reflected by post-reperfusion AST, ALT, and LDH levels, did not significantly influence the HCC recurrence risk in patients within the UCSF criteria or in those beyond the Milan, UCSF, or Up-to-7 criteria. No other significant associations between post-reperfusion AST, ALT, and LDH levels and the risk of post-transplant tumour recurrence were observed in subgroups derived from divisions based on tumour number, alpha-fetoprotein concentration, presence of microvascular invasion, and degree of tumour differentiation. In contrast to the significant effects of IRI in patients within the Milan or Up-to-7 criteria, no effects were found for the duration of total ischemia (all p > 0.701), cold ischemia (all p > 0.417), warm ischemia (all p > 0.373), and donor age (all p > 0.276) in these subgroups (Table 7). No significant associations between peak 7-day postoperative bilirubin concentration (all p > 0.081), INR (all p > 0.205), and GGTP activity (p > 0.097) and HCC recurrence risk were identified in subgroup analyses (Table 8).
Table 6.
Subgroup analyses of the associations between post-reperfusion aspartate transaminase, alanine transaminase, and lactate dehydrogenase activity and the risk of hepatocellular carcinoma recurrence after liver transplantation according to fulfillment of selection criteria and independent risk factors.
| Factor | Subgroup of patients | Analyzed as continuous variable: per loge (U/L) increase | Analyzed as categorical variable: Q4 versus Q1-Q3 | ||
|---|---|---|---|---|---|
| AST activity | Within Milan criteria | 2.75 (1.07–7.03) | 0.035 | 5.99 (1.10–32.78) | 0.039 |
| AST activity | Beyond Milan criteria | 0.86 (0.47–1.55) | 0.606 | 0.71 (0.21–2.43) | 0.591 |
| AST activity | Within UCSF criteria | 1.67 (0.84–3.30) | 0.141 | 2.79 (0.94–8.33) | 0.065 |
| AST activity | Beyond UCSF criteria | 0.84 (0.40–1.75) | 0.640 | 0.36 (0.05–2.77) | 0.327 |
| AST activity | Within Up-to-7 criteria | 1.91 (0.94–3.90) | 0.073 | 3.58 (1.15–11.11) | 0.028 |
| AST activity | Beyond Up-to-7 criteria | 0.80 (0.41–1.55) | 0.500 | 0.24 (0.03–1.82) | 0.167 |
| AST activity | Tumor number <3 | 1.58 (0.81–3.09) | 0.183 | 2.22 (0.72–6.80) | 0.164 |
| AST activity | Tumor number ≥3 | 0.81 (0.38–1.70) | 0.572 | 0.62 (0.14–2.79) | 0.536 |
| AST activity | AFP < 48.3 ng/ml | 1.51 (0.68–3.33) | 0.309 | 1.06 (0.23–5.02) | 0.937 |
| AST activity | AFP ≥ 48.3 ng/ml | 0.92 (0.50–1.70) | 0.801 | 1.04 (0.37–2.97) | 0.936 |
| AST activity | Without MVI | 1.37 (0.67–2.80) | 0.386 | 2.58 (0.79–8.47) | 0.117 |
| AST activity | With MVI | 1.18 (0.51–2.71) | 0.694 | 0.89 (0.20–3.95) | 0.882 |
| AST activity | Well or moderately differentiated tumors | 1.22 (0.71–2.11) | 0.478 | 1.11 (0.40–3.05) | 0.842 |
| AST activity | Poorly differentiated tumors | 1.66 (0.34–8.03) | 0.526 | 3.95 (0.75–20.76) | 0.104 |
| ALT activity | Within Milan criteria | 2.41 (0.88–6.61) | 0.087 | 3.15 (0.63–15.78) | 0.163 |
| ALT activity | Beyond Milan criteria | 0.84 (0.47–1.50) | 0.551 | 0.43 (0.10–1.84) | 0.254 |
| ALT activity | Within UCSF criteria | 1.22 (0.62–2.41) | 0.571 | 1.02 (0.28–3.73) | 0.974 |
| ALT activity | Beyond UCSF criteria | 0.97 (0.48–1.94) | 0.924 | 0.62 (0.14–2.76) | 0.527 |
| ALT activity | Within Up-to-7 criteria | 1.37 (0.66–2.83) | 0.398 | 1.25 (0.34–4.66) | 0.735 |
| ALT activity | Beyond Up-to-7 criteria | 0.89 (0.48–1.68) | 0.728 | 0.40 (0.09–1.80) | 0.233 |
| ALT activity | Tumor number <3 | 1.14 (0.58–2.24) | 0.699 | 0.63 (0.14–2.87) | 0.555 |
| ALT activity | Tumor number ≥3 | 0.97 (0.48–1.97) | 0.934 | 0.89 (0.25–3.18) | 0.852 |
| ALT activity | AFP < 48.3 ng/ml | 1.35 (0.60–3.04) | 0.469 | 0.99 (0.21–4.65) | 0.986 |
| ALT activity | AFP ≥ 48.3 ng/ml | 0.85 (0.48–1.51) | 0.582 | 0.52 (0.15–1.82) | 0.308 |
| ALT activity | Without MVI | 1.31 (0.63–2.72) | 0.466 | 1.23 (0.33–4.64) | 0.761 |
| ALT activity | With MVI | 1.00 (0.52–1.96) | 0.989 | 0.63 (0.14–2.77) | 0.540 |
| ALT activity | Well or moderately differentiated tumors | 1.02 (0.59–1.75) | 0.942 | 0.79 (0.26–2.37) | 0.676 |
| ALT activity | Poorly differentiated tumors | 1.67 (0.34–8.13) | 0.526 | 1.80 (0.21–15.23) | 0.589 |
| LDH activity | Within Milan criteria | 1.93 (0.75–4.97) | 0.175 | 6.08 (1.09–33.95) | 0.040 |
| LDH activity | Beyond Milan criteria | 0.88 (0.54–1.43) | 0.602 | 0.85 (0.31–2.38) | 0.764 |
| LDH activity | Within UCSF criteria | 1.33 (0.72–2.45) | 0.363 | 2.75 (0.91–8.27) | 0.073 |
| LDH activity | Beyond UCSF criteria | 0.90 (0.49–1.66) | 0.737 | 0.74 (0.20–2.77) | 0.658 |
| LDH activity | Within Up-to-7 criteria | 1.68 (0.86–3.31) | 0.130 | 3.33 (1.06–10.40) | 0.039 |
| LDH activity | Beyond Up-to-7 criteria | 0.80 (0.47–1.37) | 0.420 | 0.57 (0.15–2.10) | 0.398 |
| LDH activity | Tumor number <3 | 1.21 (0.59–2.45) | 0.604 | 2.33 (0.76–7.15) | 0.140 |
| LDH activity | Tumor number ≥ 3 | 1.04 (0.61–1.76) | 0.889 | 1.03 (0.31–3.44) | 0.964 |
| LDH activity | AFP < 48.3 ng/ml | 1.16 (0.54–2.52) | 0.698 | 1.66 (0.42–6.67) | 0.472 |
| LDH activity | AFP ≥ 48.3 ng/ml | 1.01 (0.61–1.68) | 0.963 | 1.49 (0.53–4.14) | 0.449 |
| LDH activity | Without MVI | 1.15 (0.59–2.27) | 0.680 | 2.13 (0.62–7.35) | 0.231 |
| LDH activity | With MVI | 0.99 (0.56–1.75) | 0.976 | 0.90 (0.30–2.73) | 0.856 |
| LDH activity | Well or moderately differentiated tumors | 1.11 (0.66–1.85) | 0.703 | 1.93 (0.76–4.92) | 0.169 |
| LDH activity | Poorly differentiated tumors | 1.05 (0.43–2.54) | 0.922 | 0.59 (0.10–3.33) | 0.547 |
Q4 – fourth quartile; Q1-Q3 – first to third quartile; AST – aspartate transaminase; ALT – alanine transaminase; LDH – lactate dehydrogenase; UCSF – University of California, San Francisco; AFP – alpha-fetoprotein; MVI – microvascular invasion.
Table 7.
Analyses of the associations between allograft ischemia and donor age and the risk of hepatocellular carcinoma recurrence after liver transplantation in patient within Milan and Up-to-7 criteria.
| Factor | Subgroup of patients | Analyzed as continuous variable: | Analyzed as categorical variable: Q4 versus Q1-Q3 | ||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
| Total ischemia | Within Milan criteria | 1.03 (0.69–1.54) | 0.893 | 1.39 (0.26–7.62) | 0.701 |
| Total ischemia | Within Up-to-7 criteria | 1.05 (0.77–1.41) | 0.769 | 1.01 (0.27–3.72) | 0.991 |
| Cold ischemia | Within Milan criteria | 1.14 (0.69–1.87) | 0.611 | 2.25 (0.32–16.06) | 0.417 |
| Cold ischemia | Within Up-to-7 criteria | 1.08 (0.76–1.55) | 0.665 | 1.50 (0.36–6.30) | 0.577 |
| Warm ischemia | Within Milan criteria | 0.78 (0.42–1.48) | 0.452 | —a | 0.373a |
| Warm ischemia | Within Up-to-7 criteria | 0.98 (0.70–1.37) | 0.908 | 0.52 (0.06–4.26) | 0.544 |
| Donor age | Within Milan criteria | 1.04 (0.97–1.13) | 0.276 | 1.71 (0.31–9.54) | 0.541 |
| Donor age | Within Up-to-7 criteria | 1.01 (0.96–1.06) | 0.687 | 1.03 (0.28–3.84) | 0.960 |
Q4 – fourth quartile; Q1-Q3 – first to third quartile; HR – hazard ratio; 95% CI – 95% confidence interval. Hazard ratios for continuous variables are given per 1 loge(U/L) increase. Compared with log-rank test, 100% versus 89.4% recurrence free survival at 5 years in Q4 and Q1–Q3 patients, respectively.
Table 8.
Subgroup analyses of the associations between peak 7-day postoperative bilirubin concentration, INR value, and GGTP activity and the risk of hepatocellular carcinoma recurrence after liver transplantation according to fulfillment of selection criteria and independent risk factors.
| Factor | Subgroup of patients | Analyzed as continuous variable: per loge (U/L) increase | Analyzed as categorical variable: Q4 versus Q1-Q3 | ||
|---|---|---|---|---|---|
| Bilirubin | Within Milan criteria | 1.59 (0.50–5.10) | 0.436 | 1.37 (0.25–7.48) | 0.717 |
| Bilirubin | Beyond Milan criteria | 0.74 (0.41–1.34) | 0.318 | 0.50 (0.15–1.72) | 0.275 |
| Bilirubin | Within UCSF criteria | 1.23 (0.58–2.65) | 0.589 | 0.95 (0.26–3.46) | 0.940 |
| Bilirubin | Beyond UCSF criteria | 0.56 (0.26–1.22) | 0.142 | 0.42 (0.09–1.88) | 0.254 |
| Bilirubin | Within Up-to-7 criteria | 1.56 (0.67–3.61) | 0.300 | 0.98 (0.27–3.63) | 0.978 |
| Bilirubin | Beyond Up-to-7 criteria | 0.53 (0.26–1.08) | 0.081 | 0.42 (0.09–1.90) | 0.263 |
| Bilirubin | Tumor number <3 | 1.18 (0.55–2.54) | 0.666 | 0.95 (0.26–3.47) | 0.942 |
| Bilirubin | Tumor number ≥3 | 0.59 (0.28–1.24) | 0.163 | 0.42 (0.09–1.89) | 0.256 |
| Bilirubin | AFP < 48.3 ng/ml | 0.61 (0.26–1.44) | 0.262 | 0.68 (0.14–3.18) | 0.620 |
| Bilirubin | AFP ≥ 48.3 ng/ml | 1.02 (0.51–2.05) | 0.952 | 0.72 (0.20–2.56) | 0.617 |
| Bilirubin | Without MVI | 0.62 (0.28–1.41) | 0.259 | 0.31 (0.04–2.43) | 0.266 |
| Bilirubin | With MVI | 1.00 (0.50–2.01) | 0.997 | 0.87 (0.28–2.72) | 0.805 |
| Bilirubin | Well or moderately differentiated tumors | 1.01 (0.55–1.86) | 0.975 | 0.73 (0.24–2.20) | 0.577 |
| Bilirubin | Poorly differentiated tumors | 0.35 (0.08–1.45) | 0.149 | 0.68 (0.08–5.66) | 0.717 |
| INR | Within Milan criteria | 1.21 (0.23–6.21) | 0.823 | 1.16 (0.13–9.92) | 0.894 |
| INR | Beyond Milan criteria | 0.58 (0.25–1.35) | 0.205 | 0.55 (0.19–1.66) | 0.292 |
| INR | Within UCSF criteria | 0.82 (0.25–2.68) | 0.747 | 0.97 (0.21–4.38) | 0.968 |
| INR | Beyond UCSF criteria | 0.65 (0.25–1.69) | 0.380 | 0.61 (0.17–2.22) | 0.452 |
| INR | Within Up-to-7 criteria | 0.90 (0.26–3.08) | 0.861 | 1.04 (0.23–4.75) | 0.960 |
| INR | Beyond Up-to-7 criteria | 0.56 (0.22–1.46) | 0.240 | 0.48 (0.13–1.71) | 0.255 |
| INR | Tumor number <3 | 0.64 (0.14–2.92) | 0.569 | 0.80 (0.18–3.62) | 0.774 |
| INR | Tumor number ≥3 | 0.77 (0.37–1.59) | 0.474 | 0.91 (0.25–3.31) | 0.881 |
| INR | AFP < 48.3 ng/ml | 1.12 (0.36–3.50) | 0.843 | 1.92 (0.50–7.45) | 344 |
| INR | AFP ≥ 48.3 ng/ml | 0.53 (0.19–1.48) | 0.225 | 0.39 (0.09–1.73) | 0.218 |
| INR | Without MVI | 0.38 (0.06–2.52) | 0.318 | 0.46 (0.06–3.63) | 0.464 |
| INR | With MVI | 0.93 (0.37–2.30) | 0.869 | 1.01 (0.32–3.19) | 0.990 |
| INR | Well or moderately differentiated tumors | 0.93 (0.48–1.78) | 0.817 | 1.24 (0.45–3.45) | 0.678 |
| INR | Poorly differentiated tumors | 0.17 (0/01–7.11) | 0.351 | — | — |
| GGTP | Within Milan criteria | 1.55 (0.48–5.02) | 0.467 | 2.87 (0.58–14.24) | 0.196 |
| GGTP | Beyond Milan criteria | 1.09 (0.53–2.26) | 0.818 | 1.09 (0.40–3.02) | 0.862 |
| GGTP | Within UCSF criteria | 1.44 (0.66–3.14) | 0.361 | 1.97 (0.64–6.01) | 0.236 |
| GGTP | Beyond UCSF criteria | 0.84 (0.32–2.25) | 0.732 | 0.82 (0.22–2.97) | 0.758 |
| GGTP | Within Up-to-7 criteria | 1.41 (0.61–3.21) | 0.420 | 2.14 (0.68–6.75) | 0.193 |
| GGTP | Beyond Up-to-7 criteria | 1.00 (0.38–2.60) | 0.992 | 0.86 (0.24–3.11) | 0.823 |
| GGTP | Tumor number <3 | 1.76 (0.77–4.00) | 0.177 | 2.06 (0.67–6.29) | 0.207 |
| GGTP | Tumor number ≥3 | 0.63 (0.25–1.58) | 0.321 | 0.77 (0.21–2.79) | 0.689 |
| GGTP | AFP < 48.3 ng/ml | 2.47 (0.85–7.21) | 0.097 | 2.40 (0.69–8.28) | 0.167 |
| GGTP | AFP ≥ 48.3 ng/ml | 1.04 (0.48–2.25) | 0.915 | 1.55 (0.44–5.51) | 0.499 |
| GGTP | Without MVI | 0.95 (0.41–2.22) | 0.910 | 1.02 (0.27–3.84) | 0.979 |
| GGTP | With MVI | 1.78 (0.77–4.11) | 0.179 | 2.22 (0.74–6.65) | 0.154 |
| GGTP | Well or moderately differentiated tumors | 1.36 (0.68–2.69) | 0.385 | 1.47 (0.56–3.88) | 0.433 |
| GGTP | Poorly differentiated tumors | 0.71 (0.22–2.23) | 0.555 | 0.80 (0.15–4.14) | 0.789 |
Hazard ratios for continuous variables are given per: 1 mg/dL increase for bilirubin concentration; 1 increase for INR; 1 loge (U/L) increase for GGTP activity. Q4 – fourth quartile; Q1-Q3 – first to third quartile; INR – international normalized ratio; GGTP – gamma-glutamyl transpeptidase; AFP – alpha-fetoprotein; MVI – microvascular invasion.
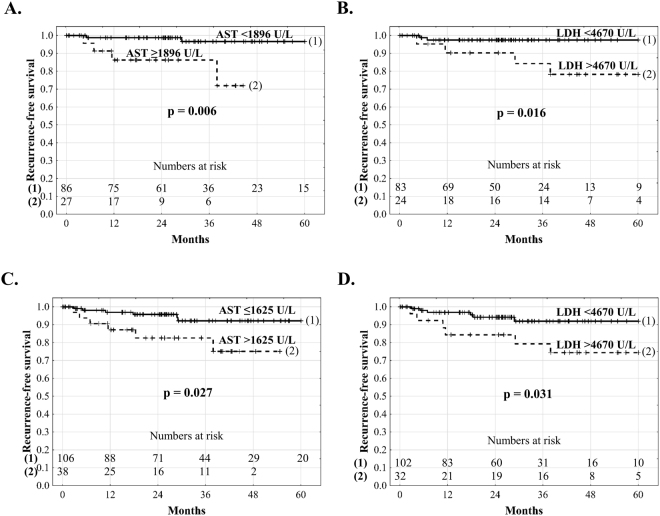
In patients within the Milan criteria, recurrence-free survival at 1, 3, and 5 years was 98.8%, 96.6%, and 96.6%, respectively, when post-reperfusion AST level was <1896 U/L as opposed to 86.2%, 86.2%, and 71.9% at 1, 3, and 3.7 years, respectively, when post-reperfusion AST level was ≥1896 U/L (p = 0.006, Fig. 3A). Similarly, patients within the Milan criteria and with post-reperfusion LDH level <4670 U/L exhibited 5-year recurrence-free survival of 97.4%, which was significantly higher (p = 0.016) than the 1-, 3-, and 5-year rates of 90.2%, 84.2%, and 78.2%, respectively, observed for those within the Milan criteria and with post-reperfusion LDH level ≥4670 U/L (Fig. 3B). Significant differences with respect to 5-year recurrence-free survival depending on post-reperfusion AST (p = 0.027) and LDH (p = 0.031) levels were also observed for patients within the Up-to-7 criteria (Fig. 3C,D).
Figure 3.
Recurrence-free survival after liver transplantation for hepatocellular carcinoma in patients within Milan criteria (A,B) and Up-to-7 criteria (C,D) according to post-reperfusion aspartate transaminase and lactate dehydrogenase activity.
Discussion
In the era of donor shortage and increasing utilization of high-risk grafts to partly ameliorate its negative effects, the problem of potential association between the degree of IRI and the risk of HCC recurrence after liver transplantation is of utmost importance. According to the available results of experimental studies, hepatic IRI, universally present in the setting of liver transplantation, increases the risk of metastasis formation both within the ischemic and remote sites through changes in the local microenvironment, induction of inflammatory response, induction of metastatic potential of circulating cancer cells, and systemic release of pro-tumourigenic cytokines12–16. Our study results demonstrate a major negative effect of IRI on the risk of post-transplant HCC recurrence, although limited to patients with low tumour burden.
Importantly, initial analyses performed in all patients failed to reveal any significant associations between post-reperfusion AST, ALT, and LDH levels and HCC recurrence risk, irrespective whether the factors were analysed as continuous or categorical variables. However, the study cohort comprised patients with a wide range of tumour burden due to a liberal selection policy utilised in the authors’ department before establishment of precise criteria5. Nevertheless, a major significant negative effect of post-reperfusion AST and LDH levels was observed for patients within the Milan criteria, which still determine the majority of liver transplant recipients28. Similar findings, although of remarkably lesser extent, were found for patients within the Up-to-7 criteria, whereas the magnitude of IRI did not influence the risk of recurrence in patients beyond the extended criteria. This indicates that the clinical relevance of IRI is limited to generally low-risk populations and diminishes with increasing tumour burden. This appears to be particularly importantly because it demonstrates the possibility of using high-risk grafts to expand the donor pool for high-risk HCC candidates in the context of discussion on widening the boundaries of existing selection criteria2–6,29. Notably, the safe use of extended criteria allografts preferentially for patients with advanced tumours was already reported30. Conversely, none of the subgroup analyses performed in high-risk patients, including those beyond particular selection criteria, with ≥3 tumours, alpha-fetoprotein concentration ≥48.3 ng/mL, or with tumours either poorly differentiated or with microvascular invasion, revealed a significant effect of IRI on the risk of HCC recurrence. Therefore, while these findings point toward the possibility of the utilization of grafts more prone to IRI for high-risk HCC patients, they also indicate limited clinical relevance of reducing IRI in these patients.
In contrast to the use of post-reperfusion transaminases and LDH levels as surrogates of IRI degree in the present study, previous studies focused on the negative effects of prolonged graft ischemia or donor characteristics17–25,31. However, the degree of IRI is driven by the interplay of several donor risk factors, of which a single component may not necessarily be an adequate measure of IRI32. In the present study, the laboratory measures of graft ischemia were significantly, yet poorly correlated to graft ischemic times, which in fact is consistent with the results presented by other authors25. This may partly explain the inconsistent results of studies on the effect of duration of graft ischemia and particular donor factors on HCC recurrence risk, as these may not always accurately reflect the magnitude of IRI17–25,31.
In contrast to the significant effects of IRI limited to low-risk patients found in the present study, two previous analyses specifically aimed at the effect of ischemic times on tumour recurrence revealed the presence of significant associations particularly in high-risk HCC patients24,25. These populations were characterised by 18F-fluorodeoxyglucose tumour avidness on pre-transplant positron emission tomography and vascular invasion, both of which are known surrogates of biological aggressiveness. Although positron emission tomography data were not available, categorization of patients based on pre-transplant alpha-fetoprotein concentration and tumour differentiation, which are important markers of tumour biology, did not reveal any significant effects of IRI and neither did the analyses stratified for microvascular invasion. The reason for this discrepancy is unclear, although it may be related to a wider spectrum of tumour burden in patients included in the present study. Of note, post-operative peak transaminases did not emerge as risk factors for HCC recurrence in these previous reports. However, we chose post-reperfusion AST, ALT, and LDH levels routinely assessed in our department and not peak levels over the postoperative period in order to minimise the effect of events other than IRI on these parameters.
The results of the present study point toward the importance of strategies aimed to decrease IRI particularly for patients within the standard selection criteria. A single retrospective study revealed decreased magnitude of IRI, as illustrated by low transaminase levels and decreased risk of HCC recurrence in patients receiving prostaglandin E1 analog alprostadil in the early period after liver transplantation33. The protective effects of ischemic preconditioning with respect to the development of metastases were also reported in a recent experimental study14. The use of machine perfusion devices has also been shown to decrease the magnitude of IRI and recently even enabled the development of a strategy to practically eliminate its negative consequences34–36. Although the present study does not provide any evidence for the effects of these measures in liver transplantation for HCC, it provides a rationale for prospective trials aimed at addressing this issue.
This study had several limitations besides those inherent to its retrospective nature. Donor characteristics other than a baseline variable of age were neither analysed for associations with post-reperfusion transaminase and LDH levels nor as predictors of tumour recurrence. However, such analyses were beyond the scope of this study, specifically aimed at the effect of IRI on post-transplant HCC recurrence rather than on its determinants. Because all recipients received grafts from donors after brain death, this study did not directly address the issue of using grafts from donors after cardiac death for HCC patients, which was recently shown not to increase the risk of post-transplant recurrence23. Although subject to additional warm ischemia and thus potentially increased magnitude of IRI, their use in HCC patients may be confounded by other factors, including but not limited to, non-random allocation and differences in other donor characteristics. Furthermore, the duration of warm ischemia was not identified as a significant predictor of HCC recurrence. Finally, the main findings of our study are based on the results of univariable subgroup analyses. Therefore, the findings may be confounded by the effects of other risk factors for tumour recurrence. Although there was no particular policy at the authors’ department for the allocation of high-risk grafts to higher-risk HCC patients, the results may also be confounded by non-random allocation of grafts more prone to IRI to patients within the Milan or Up-to-7 criteria, yet at higher initial recurrence risk.
In conclusion, the magnitude of IRI is strongly associated with the risk of tumour recurrence in patients within the Milan criteria and to a lesser extent, in patients within the extended criteria. Available measures to decrease IRI should be evaluated as a method to prevent HCC recurrence after liver transplantation, specifically in patients with low tumour burden.
Acknowledgements
Michał Grąt received a START 2018 stipend from the Foundation for Polish Science. The Authors would like to thank Editage (www.editage.com) for English language revision of the manuscript.
Author Contributions
Study concept: M.G.; Study design: M.G., M.K., Z.L., K.Z.; Acquisition of data: M.G., M.K., K.W., J.S., M.W., P.K., K.G., W.P., K.Z.; Analyses of data: M.G., Z.L.; Preparation of manuscript: M.G.; Critical revision of the manuscript: All remaining authors; Acceptance of the final version: All authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kim WR, et al. OPTN/SRTR 2015 Annual Data Report: Liver. Am. J. Transplant. 2017;17:174–251. doi: 10.1111/ajt.14126. [DOI] [PubMed] [Google Scholar]
- 2.Mazzaferro V, et al. Metroticket 2.0 Model for analysis of competing risks of death following liver transplantation for hepatocellular carcinoma. Gastroenterology. 2018;154:128–139. doi: 10.1053/j.gastro.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Notarpaolo A, et al. Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related cirrhosis who had received a liver transplant for HCC. J. Hepatol. 2017;66:552–559. doi: 10.1016/j.jhep.2016.10.038. [DOI] [PubMed] [Google Scholar]
- 4.Halazun KJ, et al. Recurrence After Liver Transplantation for Hepatocellular Carcinoma: A New MORAL to the Story. Ann. Surg. 2017;265:557–564. doi: 10.1097/SLA.0000000000001966. [DOI] [PubMed] [Google Scholar]
- 5.Grąt M, et al. The Warsaw Proposal for the Use of Extended Selection Criteria in Liver Transplantation for Hepatocellular Cancer. Ann. Surg. Oncol. 2017;24:526–534. doi: 10.1245/s10434-016-5500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai Q, et al. A Novel Prognostic Index in Patients With Hepatocellular Cancer Waiting for Liver Transplantation: Time-Radiological-response-Alpha-fetoprotein-INflammation (TRAIN) Score. Ann. Surg. 2016;264:787–796. doi: 10.1097/SLA.0000000000001881. [DOI] [PubMed] [Google Scholar]
- 7.Mehta N, et al. Wait Time of Less Than 6 and Greater Than 18 Months Predicts Hepatocellular Carcinoma Recurrence After Liver Transplantation: Proposing a Wait Time “Sweet Spot”. Transplantation. 2017;101:2071–2078. doi: 10.1097/TP.0000000000001752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg D, et al. Patients With Hepatocellular Carcinoma Have Highest Rates of Wait-listing for Liver Transplantation Among Patients With End-Stage Liver Disease. Clin. Gastroenterol. Hepatol. 2016;14:1638–1646. doi: 10.1016/j.cgh.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel MS, et al. The race to liver transplantation: a comparison of patients with and without hepatocellular carcinoma from listing to post-transplantation. J. Am. Coll. Surg. 2015;220:1001–1007. doi: 10.1016/j.jamcollsurg.2014.12.050. [DOI] [PubMed] [Google Scholar]
- 10.Orci LA, et al. The role of hepatic ischemia-reperfusion injury and liver parenchymal quality on cancer recurrence. Dig. Dis. Sci. 2014;59:2058–2068. doi: 10.1007/s10620-014-3182-7. [DOI] [PubMed] [Google Scholar]
- 11.Li CX, Man K, Lo CM. The Impact of Liver Graft Injury on Cancer Recurrence Posttransplantation. Transplantation. 2017;101:2665–2670. doi: 10.1097/TP.0000000000001844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim C, et al. Hepatic ischemia-reperfusion increases circulating bone marrow-derived progenitor cells and tumor growth in a mouse model of colorectal liver metastases. J. Surg. Res. 2013;184:888–897. doi: 10.1016/j.jss.2013.04.069. [DOI] [PubMed] [Google Scholar]
- 13.Oldani G, et al. Pre-retrieval reperfusion decreases cancer recurrence after rat ischemic liver graft transplantation. J. Hepatol. 2014;61:278–285. doi: 10.1016/j.jhep.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 14.Orci LA, et al. Effect of ischaemic preconditioning on recurrence of hepatocellular carcinoma in an experimental model of liver steatosis. Br. J. Surg. 2016;103:417–426. doi: 10.1002/bjs.10080. [DOI] [PubMed] [Google Scholar]
- 15.Hamaguchi Y, et al. Longer warm ischemia can accelerate tumor growth through the induction of HIF-1α and the IL-6-JAK-STAT3 signaling pathway in a rat hepatocellular carcinoma model. J. Hepatobiliary. Pancreat. Sci. 2016;23:771–779. doi: 10.1002/jhbp.406. [DOI] [PubMed] [Google Scholar]
- 16.Man K, et al. Ischemia-reperfusion of small liver remnant promotes liver tumor growth and metastases–activation of cell invasion and migration pathways. Liver Transpl. 2007;13:1669–1677. doi: 10.1002/lt.21193. [DOI] [PubMed] [Google Scholar]
- 17.Croome KP, et al. Inferior survival in liver transplant recipients with hepatocellular carcinoma receiving donation after cardiac death liver allografts. Liver Transpl. 2013;19:1214–1223. doi: 10.1002/lt.23715. [DOI] [PubMed] [Google Scholar]
- 18.Orci LA, et al. Donor characteristics and risk of hepatocellular carcinoma recurrence after liver transplantation. Br. J. Surg. 2015;102:1250–1257. doi: 10.1002/bjs.9868. [DOI] [PubMed] [Google Scholar]
- 19.Ninomiya M, et al. Comparative study of living and deceased donor liver transplantation as a treatment for hepatocellular carcinoma. J. Am. Coll. Surg. 2015;220:297–304. doi: 10.1016/j.jamcollsurg.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Salgia RJ, Goodrich NP, Marrero JA, Volk ML. Donor factors similarly impact survival outcome after liver transplantation in hepatocellular carcinoma and non-hepatocellular carcinoma patients. Dig. Dis. Sci. 2014;59:214–219. doi: 10.1007/s10620-013-2883-7. [DOI] [PubMed] [Google Scholar]
- 21.Azoulay D, et al. Living or Brain-dead Donor Liver Transplantation for Hepatocellular Carcinoma: A Multicenter, Western, Intent-to-treat Cohort Study. Ann. Surg. 2017;266:1035–1044. doi: 10.1097/SLA.0000000000001986. [DOI] [PubMed] [Google Scholar]
- 22.Khorsandi SE, et al. Does Donation After Cardiac Death Utilization Adversely Affect Hepatocellular Cancer Survival? Transplantation. 2016;100:1916–1924. doi: 10.1097/TP.0000000000001150. [DOI] [PubMed] [Google Scholar]
- 23.Croome KP, et al. The Use of Donation After Cardiac Death Allografts Does Not Increase Recurrence of Hepatocellular Carcinoma. Am. J. Transplant. 2015;15:2704–2711. doi: 10.1111/ajt.13306. [DOI] [PubMed] [Google Scholar]
- 24.Kornberg A, Witt U, Kornberg J, Friess H, Thrum K. Extended Ischemia Times Promote Risk of HCC Recurrence in Liver Transplant Patients. Dig. Dis. Sci. 2015;60:2832–2839. doi: 10.1007/s10620-015-3541-z. [DOI] [PubMed] [Google Scholar]
- 25.Nagai S, et al. Ischemia time impacts recurrence of hepatocellular carcinoma after liver transplantation. Hepatology. 2015;61:895–904. doi: 10.1002/hep.27358. [DOI] [PubMed] [Google Scholar]
- 26.Krawczyk M, et al. 1000 liver transplantations at the Department of General, Transplant and LiverSurgery, Medical University of Warsaw–analysis of indications and results. Pol. Przegl. Chir. 2012;84:304–312. doi: 10.2478/v10035-012-0051-y. [DOI] [PubMed] [Google Scholar]
- 27.Grąt M, et al. The impact of surgical technique on the results of liver transplantation in patients with hepatocellular carcinoma. Ann. Transplant. 2013;18:448–459. doi: 10.12659/AOT.884005. [DOI] [PubMed] [Google Scholar]
- 28.Geissler EK, et al. Sirolimus Use in Liver Transplant Recipients With Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation. 2016;100:116–125. doi: 10.1097/TP.0000000000000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aravinthan AD, et al. Liver Transplantation is a Preferable Alternative to Palliative Therapy for Selected Patients with Advanced Hepatocellular Carcinoma. Ann. Surg. Oncol. 2017;24:1843–1851. doi: 10.1245/s10434-017-5789-3. [DOI] [PubMed] [Google Scholar]
- 30.Facciuto ME, et al. Liver transplantation for hepatocellular carcinoma: defining the impact of using extended criteria liver allografts. Transplantation. 2011;92:446–452. doi: 10.1097/TP.0b013e3182252733. [DOI] [PubMed] [Google Scholar]
- 31.Vagefi PA, Dodge JL, Yao FY, Roberts JP. Potential role of the donor in hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl. 2015;21:187–194. doi: 10.1002/lt.24042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali JM, et al. Analysis of ischemia/reperfusion injury in time-zero biopsies predicts liver allograft outcomes. Liver Transpl. 2015;21:487–499. doi: 10.1002/lt.24072. [DOI] [PubMed] [Google Scholar]
- 33.Kornberg A, Witt U, Kornberg J, Friess H, Thrum K. Treating ischaemia-reperfusion injury with prostaglandin E1 reduces the risk of early hepatocellular carcinoma recurrence following liver transplantation. Aliment. Pharmacol. Ther. 2015;42:1101–1110. doi: 10.1111/apt.13380. [DOI] [PubMed] [Google Scholar]
- 34.van Rijn R, et al. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br. J. Surg. 2017;104:907–917. doi: 10.1002/bjs.10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dutkowski P, et al. First Comparison of Hypothermic Oxygenated PErfusion Versus Static Cold Storage of Human Donation After Cardiac Death Liver Transplants: An International-matched Case Analysis. Ann. Surg. 2015;262:764–770. doi: 10.1097/SLA.0000000000001473. [DOI] [PubMed] [Google Scholar]
- 36.He X, et al. The First Case of Ischemia-free Organ Transplantation in Humans: A Proof of Concept. Am. J. Transplant. 2018;18:737–744. doi: 10.1111/ajt.14583. [DOI] [PubMed] [Google Scholar]