Abstract
Highly luminescent CdS quantum dots capped with thioglycolic acid (TGA@CdS QDs) were synthesized from cadmium chloride and thiourea as cadmium and sulfur sources via simple hydrothermal method. The room temperature photoluminescence (RTPL) properties of TGA@CdS QDs were investigated. The results indicate that the polarity of the solvent and the surface trap state resulted in the broadness Stokes shift between the maximum absorption wavelength and the emission wavelength of TGA@CdS QDs. The Co2+ sensing properties of fluorescence determination were investigated using TGA@CdS QDs. The as-synthesized CdS QDs exhibits the excellent selectivity and sensitivity of fluorescence quenching for cobalt ion (Co2+). The limit of detection (LOD) is as low as 0.05 μM which is much lower than maximum limit of cobalt ions in drinking water. The linear response range of Co2+ was from 0.5 to 80 μM. The sensing system revealed the advantages of low detection limit, excellent selectivity, high sensitivity, convenience and low cost. The color change of CdS QDs shows potential applications in the detection of Co2+.
Introduction
Cobalt, as an important micronutrient, is an essential component of vitamin B12, and plays a vitally important role in humans and biological systems1–3. However, cobalt could be a toxic compound at high concentrations which could cause many diseases such as asthma, decreased cardiac output, cardiac enlargement, heart disease, lung disease, dermatitis and vasodilation4. With the widely applications of the cobalt salt5–7, large quantities of wastewater containing cobalt ions also arise. It will be caused serious harm to people, animals, plants and other microorganisms if the untreated wastewater discharged into the environment. The concentration of cobalt ions shall not exceed 1 mg/L (16.9 μM) in the wastewater8. The World Health Organization (WHO) recommends maximum limits of cobalt ions in drinking water of 1.7 μΜ9. For these reasons, it is important to detect trace amounts of Co2+ in the water.
So far a variety of sophisticated methods have been used to detect Co2+ concentrations on aqueous solution, such as atomic absorption spectrometry (AAS), high performance liquid chromatography (HPLC), inductively coupled plasma mass/emission spectroscopy, and voltammetry10–18. These methods are characterized by good accuracy while they in general are quite time-consuming and require expensive and complex equipment. The sample preparation and pretreatment could be cumbersome. These restrict its application range. Therefore, a rapid, simple, inexpensive and highly sensitive method for the detection of Co2+ is undoubtedly realistic significance.
More recently, optical sensing approaches have attracted the attention of many researchers due to their quick response, simplicity, high sensitivity and selectivity19–25. Luminescent semiconductor quantum dots (QDs) have high quantum yield, sharp emission spectra, broad absorption spectra, long life-times and tunable color which are superior to conventional fluorescent dyes. Recently, based on the Co2+–induced changes in fluorescence intensity of QDs, the various reports have been published on the using of QDs for fluorescence determination of Co2+19–24. The concentration of Co2+ can be measured through the change of fluorescence intensity of QDs caused by the interactions between QDs surface and Co2+. Besides, Some papers have reported that heavy metal ion, such as Hg2+, Ag+, Cu2+, Co2+and Ni2+, have been detected by surface-modified CdS QDs based on the gradual quench of the fluorescence intensity of the QDs25–28. Previous reports have demonstrated monitoring the optical changes of QDs fluorescence by Co2+ may provide a simple method to develop QD-based fluorescence probes. However, these studies revealed weak abilities of anti-interference and high detection limit. So, the sensitivity of selectivity of the related sensor based fluorescence QDs still had the large space to improve by the stability of CdS QDs. TGA could improve the performance of CdS QDs in aqueous solutions because of the passivation and hydrophilicity properties. Hence, TGA as the surface modification would enhance the stability of CdS QDs as well as the selectivity for metal ions in aqueous solutions.
In this work, the low-cost TGA-capped CdS QDs (TGA@CdS QDs) was prepared from cadmium chloride and thiourea as cadmium and sulfur sources by hydrothermal method without removing oxygen during the preparation process. The obtained TGA@CdS QDs exhibited excellent selectivity and sensitivity for Co2+ by fluorescence quenching. The fluorescence quenching mechanism and the fluorescence generation mechanism of the TGA@CdS QDs was also studied.
Experimental
Reagents and materials
The materials used in the experiments included cadmium chloride hydrate (CdCl2·2.5H2O 99%), thioglycolic acid (TGA, 90.0%), thiourea (99%), sodium hydroxide (NaOH, 99%) and various metal salts. All the chemical reagents used in the experiments were obtained from commercial sources as guaranteed-grade reagents and used without further purification. All solutions were prepared with deionized water. The concentration of the QDs was 0.012 M calculated based on the original cadmium source.
Characterization
Photoluminescence spectra were measured at room temperature with an imaging spectrometer (iHR320 HORIBA Jobin Yvon Inc., Edison, NJ, USA.). The slit width used for both excitation and emission was 2 nm. Transmission electron microscopy (TEM), high resolution transmission electron microscopy (HR-TEM), and selected area electron diffraction (SAED) with energy dispersive X-ray (EDX) measurements were performed by JEOL JEM-2100 with an acceleration voltage of 200 kV. The microstructure of the synthesis CdS QDs was analyzed by TEM and HR-TEM and the crystal structure was obtained by SAED. The surface topography was investigated using a SPA-400 SPM atomic force microscope (AFM). The UV-vis absorption spectra were obtained at an UV-1800 spectrophotometer of Jinghua Instruments with a wavelength range between 200 and 800 nm. Fourier transform infrared (FTIR) spectra were recorded on AVATAR 360 FT-IR spectrophotometer in the range of 4000–5000 cm−1. The time resolved photoluminescence lifetime decays were measured by Hamamatsu compact fluorescence lifetime spectrometer C11367 (Quantaurus-Tau), using an LED light source with λex = 365 nm. pH were measured by an inoLab pH Level 1 precision pH meter.
Synthesis of TGA@CdS QDs
TGA@CdS QDs were prepared via a classic hydrothermal method as previously described in report29. Briefly, 0.7458 g cadmium chloride hydrate (CdCl2·2.5H2O) and 0.4400 g thiourea (CH4N2S) were dissolved in 280 mL deionized water and stirring for 30 min. Then 15 μL thioglycolic acid (TGA) was slowly added to the aqueous solution stirring for 30 min. The pH value of the solution was adjusted by using 1 M NaOH aqueous solution. After stirring for 1 h, the mixture solution was transferred to a 300 mL Teflon-lined stainless steel autoclave. The autoclave was maintained at 120 °C for 2 h and cooled to room temperature. The pH value of the as-synthesized solution is 7.0.
Selective detection of Co2+
The fluorescence determination of Co2+ in aqueous solution at room temperature was achieved using the prepared TGA@CdS QDs aqueous solution based on fluorescence quenching. The QDs solution is stable and a distinct color change occurred by our eyes when the required amount of cobalt ion added to the QDs solution. The fluorescence quenching was analyzed by the maximum fluorescence intensity.
Results and Discussion
Characterization of the TGA@CdS QDs
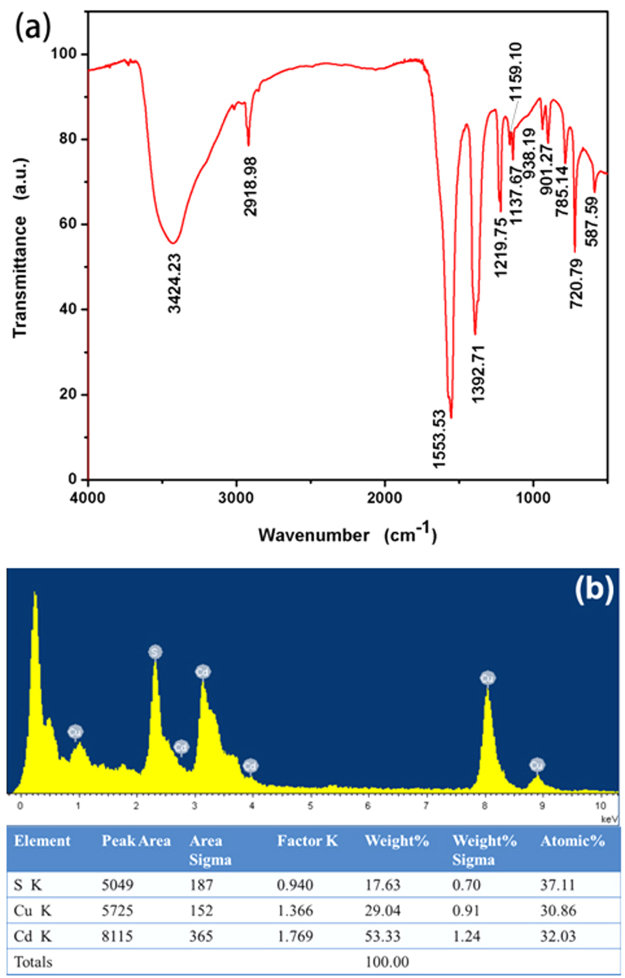
Figure 1(a) shows the FTIR spectrum of as-obtained TGA@CdS QDs in the range from 400 cm−1 to 4000 cm−1. The characteristic IR peaks appear at 3424, 2919, 1553, 1392, 1219, 1137 cm−1, respectively. The strong absorption peak is observed at 3424.23 cm−1 arisen from the hydroxyl bond stretching vibration of -COOH group and broaden hydroxyl bond can be ascribed to the solvent30. The peak at 2918.98 cm−1 corresponds to –CH2 asymmetric stretching vibration modes31. The strong peak at 1553.53 cm−1 and 1392.71 cm−1 correspond to asymmetric and symmetric stretching respectively in carboxylate group of TGA. The peaks at 1219.75 cm−1 and 1137.67 cm−1 are assigned to asymmetric and symmetric stretching vibrations of C-O, respectively. The absence of distinct S-H characteristic band of TGA at 2600–2550 cm−1 confirms the capping of CdS QDs which is due to covalent bond between cadmium of CdS and sulfur of TGA32. These results confirm that the synthesized QDs are well capped and stabilized with TGA molecules. The EDX spectrum (Fig. 1(b)) of the as-synthesized solution confirms the presence of CdS QDs. The atomic ratio of [Cd]:[S] is 1:1.16. One part sulfur forms CdS QDs with cadmium, the other belongs to TGA which capped on the CdS QDs surface. The Cu peak arises from the copper nets.
Figure 1.
FTIR spectrum (a) and EDX spectrum (b) of the as-synthesized CdS QDs.
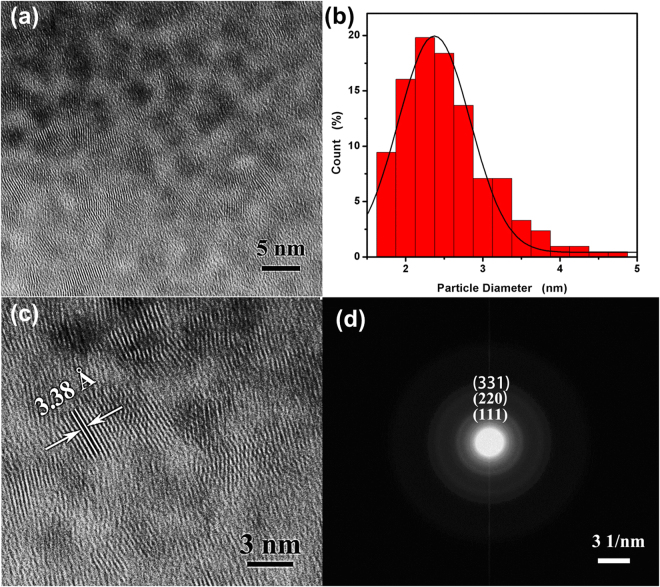
Figure 2 shows the HR-TEM images of the as-synthesized TGA@CdS QDs. From the HR-TEM image shown in Fig. 2(a), a number of uniformed-sized CdS QDs with distinct crystal lattice can be clearly observed. Figure 2(b) shows that the average size of stable CdS QDs is 2.37 ± 1.1 nm by measuring the diameters of more than 200 particles from the HR-TEM image. The as-synthesized quantum dots, with an interplanar spacing of 3.38 Å corresponding to the lattice plane (111) of CdS (JCPDS 10–454), as shown in Fig. 2(c). The SAED pattern (Fig. 2(d)) illustrates the presence of (111), (220), (311) planes of the cubic zinc blende structure of CdS. The EDX spectrum (Fig. 1(b)) also confirms the formation of TGA@CdS QDs with the atomic ratio of Cd to S being 1:1.16. All the analyses demonstrate that the CdS QDs was synthesized by the one-step hydrothermal method, which is simple and cost-effective.
Figure 2.
(a) HR-TEM image of as-synthesized CdS QDs. (b) statistical analysis of the sizes of as-synthesized CdS QDs. (c) HR-TEM images of as-synthesized CdS QDs with the lattice fringes. (d) The corresponding SAED pattern of the as-synthesized CdS QDs.
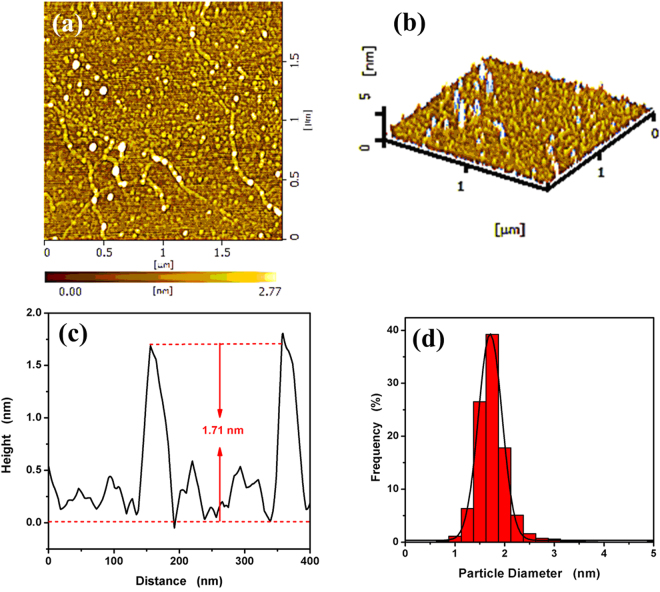
The topographic morphology of as-synthesized CdS QDs was examined further through AFM. Figure 3(a–c) show the typical two-dimensional (2D), three-dimensional (3D) AFM images, and height profile of as-synthesized CdS QDs, respectively. As shown in Fig. 3(a,b), many uniformly sized CdS QDs are observed and the height of CdS QDs is 1.71 nm approximately. The histogram of the height of the CdS QDs is exhibited in Fig. 3(d), which shows an average size of 1.71 ± 0.6 nm. In contrast of the size distribution of QDs by both TEM and AFM, it could be concluded that slightly different result. The size distribution of QDs by measured the lattice fringe image. Hence, the as-synthesized QDs is uniform and thin, which could be conductive to improve performance.
Figure 3.
(a) and (b) represent 2D and 3D AFM images of TGA@CdS QDs deposited on silicon substrate, respectively. (c) Height profiles along the line in (a). (d) Statistical analysis of the heights of TGA@CdS QDs measured by AFM.
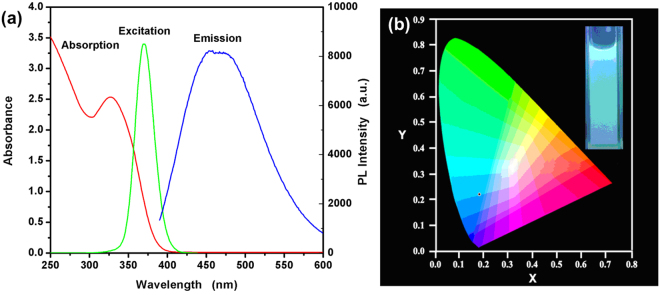
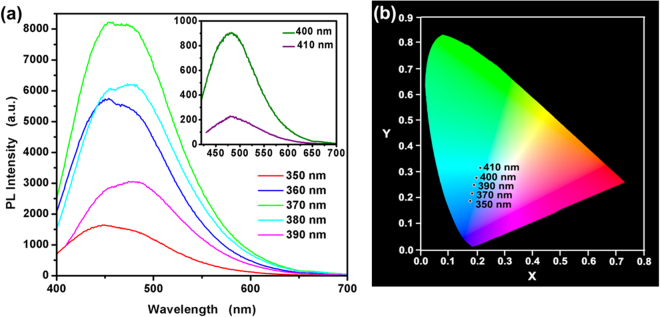
The absorption, emission and excitation spectra of the as-synthesized CdS QDs are show in Fig. 4(a). The broad absorption spectra can be observed in the ultraviolet range with the wavelength of 400 nm which is different from the bulk CdS30. This is attributed to the quantum size effects of the samples. The emission spectra indicate the maximum fluorescence intensity at 454 nm under the 370 nm excitation wavelength. Between the maximum absorption wavelength and the emission wavelength, a broadness and large Stokes shift was observed owing to the surface defect emission25. The characteristic is not always observed when using organic fluorophores which lead to the semiconductor quantum dots are better than the conventional organic fluorescent probes. The absorption edges (obtained by the intersection of the sharply decreasing region of the spectrum with the baseline) are located at 386 nm, which corresponds to band gap of 3.21 eV. According to the relationship between the absorption edge (λem) and the size of QDs (Eq. (1)), the mean size of the particles can be calculated using the following equation33:
| 1 |
Figure 4.
(a) The UV-vis absorbance (red line), photoluminescence spectra (green line) and the emission spectra (blue line) of the as-synthesized CdS QDs. (b) The CIE 1931 coordinates and picture of the samples under 365 nm UV lamp with a distance of 25 cm.
Regarding Eq. (1) and absorption spectra (red line), it can be estimated that the mean diameter size of TGA@CdS QDs is 2.31 nm, corresponding to the consequence of TEM. Using the band gap value of 3.21 eV, the particles diameter was calculated following the formula of Brus (Eq. (2)) based on the effective mass approximation34–36.
| 2 |
where Eg is the band gap of the bulk material (2.42 eV), h is the reduced Plank’s constant (6.625 × 10−34 J·s), R is the radius of spherical nanocrystals, e is the charge of the electron (1.6 × 10−19 C), is the vacuum dielectric constant ( = 8.854 × 10−12 F/m), is the semiconductor dielectric constant (5.7 ), me and are effective masses of the electrons and holes, respectively, and is the free electron mass ( = 9.108 × 10−31 kg). With the effective masses of electrons ( = 0.19 ) and holes ( = 0.8 ), the diameters of TGA@CdS QDs is found to be 1.76 nm, which agrees well with the AFM. The sample emitted greenish blue color was exhibited on the corresponding CIE coordinates (Fig. 4(b)) with a corresponding CIE coordinates are (0.18, 0.22) and the photographs of the emission under UV light (Fig. 4(b), inset) for the same samples.
Figure 5(a) shows that the emission peak almost unchanged with the variation of excitation wavelength in the range of 350–390 nm. The photoluminescence intensity firstly increases and then decreases with the increase of excitation wavelength. The change of fluorescence intensity can be explained that the long wave emission part of the as-synthesized CdS QDs is associated with the quantum confinement effect. The TEM and AFM images of as-synthesized CdS QDs indicate that the particle size is not identical versions. So, when the excitation wavelength gradually increases, the more CdS QDs are excited and the fluorescence intensity is also gradually enhanced after reaching the maximum. And then, the excited CdS QDs gradually reduce and the fluorescence intensity also gradually weakened. The photoluminescence spectrum shows two fluorescence peaks centered on 454 nm and 474 nm, respectively. It can be mainly viewed from two aspects: the surface defects formed in the as-synthesized CdS QDs and the size distribution of particles. There is small variation in color across the coordinates of the as-synthesized CdS QDs under the different excitation wavelength as shown in Fig. 5(b). The main reason for this is that the band-edge emission of large size CdS QDs and trap-stare emission are distinct increasingly.
Figure 5.
The excitation-dependent emission spectrum (a) and the CIE 1931 chromaticity chart (b) of TGA@CdS QDs.
Time-resolved PL experiment (Fig. 6) shows that the lifetime of TGA@CdS QDs. The lifetime measurements were carried out immediately after preparation as well as after 120 min of aging. The fluorescence decay curve was measured with an excitation of 365 nm. The decay curve can be best fitted to a two exponential kinetic programs. The average lifetime (τav) of as-synthesized TGA@CdS QDs is calculated to be 36.57 ns depending on the lifetimes are τ1 = 3.15, τ2 = 115.73 ns. The longer lifetime is generally assigned to the involvement of surface defects in the carrier recombination process. The shorter lifetime may be assigned to radiative depopulation due to band-edge recombination or to electro–hole recombination at the surface38–40.
Figure 6.
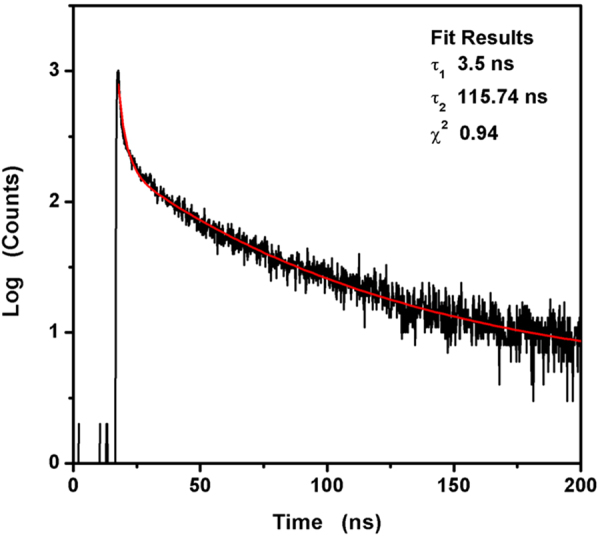
Fluorescence decay curves of as-synthesized CdS QDs (black) and the corresponding quadratic exponential fitting curve (red).
Selectivity and sensitivity of Co2+ by TGA@CdS QDs
To evaluate the selectivity and sensitivity of the present sensing system, 50 μM of various metal ions, including Cd2+, Zn2+, Na+, Al3+, K+, Mg2+, Ba2+, Ca2+, Mn2+, Cr2+, Fe2+, Fe3+, Cu2+, Ni2+ and Co2+ were added into the CdS QDs solution, and the PL responses were recorded, respectively. The PL spectra of TGA@CdS QDs before and after adding metal cation and the comparison of fluorescence intensity of TGA@CdS QDs in the absence and presence of interfering ions are shown in Fig. 7(a,b), respectively. Significant fluorescence quenching was observed with the addition of Co2+, while the other metal ions showed only a negligible quenching effect. Cu2+ and Ni2+ also have effect on the fluorescence, but the effect is relatively little compare with Co2+. The synthesized TGA@CdS QDs can be used for metal ions sensing according to metal–ligand interaction between the carboxyl acid group of TGA and metal ions21, and it has been observed that the CdS QDs have great sensitivity to Co2+.
Figure 7.
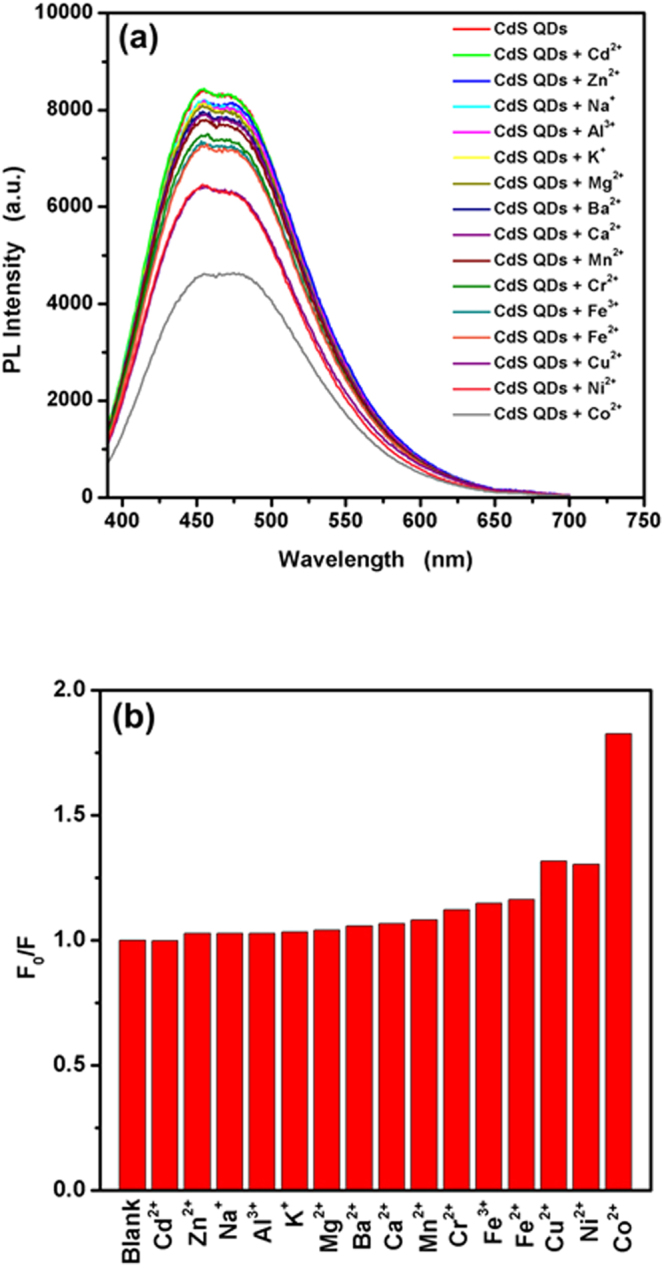
(a) Fluorescence spectra of TGA@CdS QDs in the presence of various cations, [Mn+] = 50 μM, (b) Comparison of degree of fluorescence quenching (F0/F) in the presence of each cation.
Fluorescence determination of Co2+ by TGA@CdS QDs
The fluorescence intensity of TGA@CdS QDs decreased greatly when Co2+ was added to the QDs solution. However, the pH value of the system plays an important role, because the deprotonation process could be influenced by changing the pH value. Thus, the effect of the system pH value was studied in the range of 5.0–9.0. The pH values were adjusted by 0.3 M Tris-Hcl buffer. The results are shown in Fig. 8. The fluorescence quenching was observed at all pH value as shown in Fig. 8(a). As shown in Fig. 8(b), the quenched fluorescence intensity increases a little as the pH value varies from 5.0 to 7.0, the quenched fluorescence intensity decreasing sharply from 7.0–9.0. The reasons for it may be partial Co2+ coordination with free hydroxyl rather than the carboxyl group on the surface of the QDs. The maximum fluorescence quenching was obtained at pH 7.0. Therefore, a Tris-Hcl buffer of pH 7.0 was used in this work.
Figure 8.
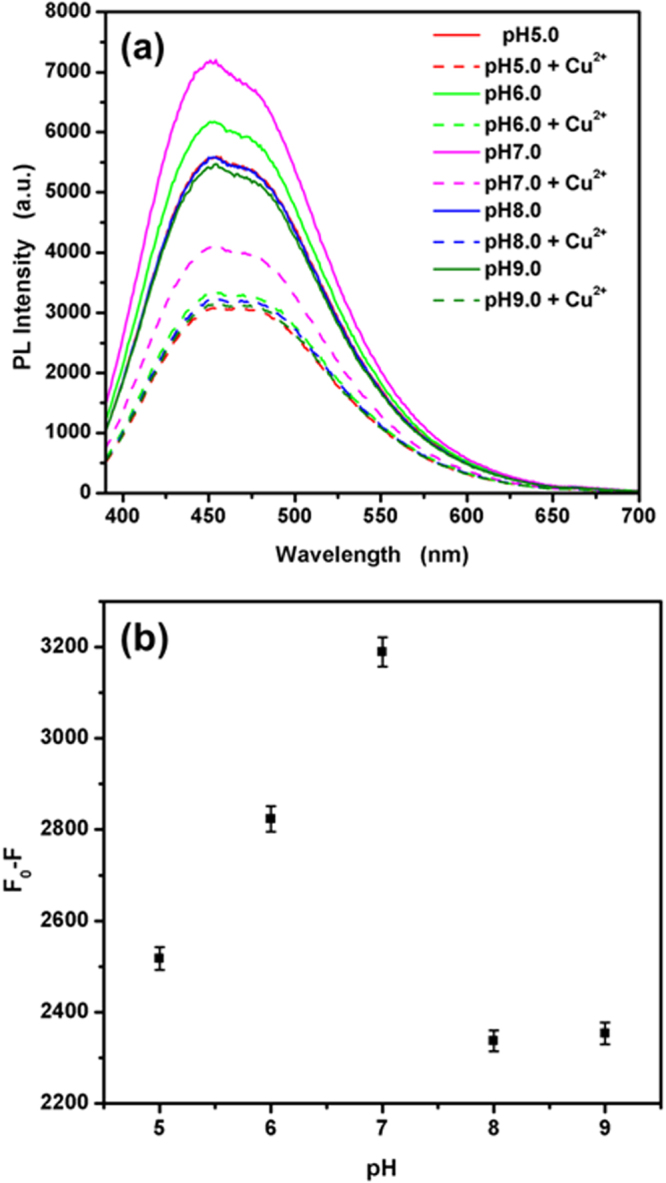
(a) Effect of pH on the fluorescence intensity of TGA@CdS QDs in the absence and the presence of 50 μM Co2+. (b) The fluorescence quenching in the presence of 50 μM Co2+.
In order to study TGA@CdS QDs for the quantitative analysis of Co2+, the analytical parameters were then evaluated. The fluorescence spectra of TGA@CdS QDs in the presence of different concentrations of Co2+ were recorded and the results are shown in Fig. 9. Figure 9(a) shows the emission peaks remain essentially unchanged and the PL intensity gradually decreased with the increasing the concentration of Co2+. Figure 9(b) shows a great linear correlation (R2 = 0.97927) between the PL intensity and the concentration of Co2+ in the range of 0.5 to 80 μM. The limit of detection (LOD) is estimated to be 0.05 μM, based on a signal-to-noise ratio (S/N) of 3. The results suggested that the as-synthesized TGA@CdS QDs can be used to fluorescence detection of Co2+ with high sensitivity and simplicity.
Figure 9.
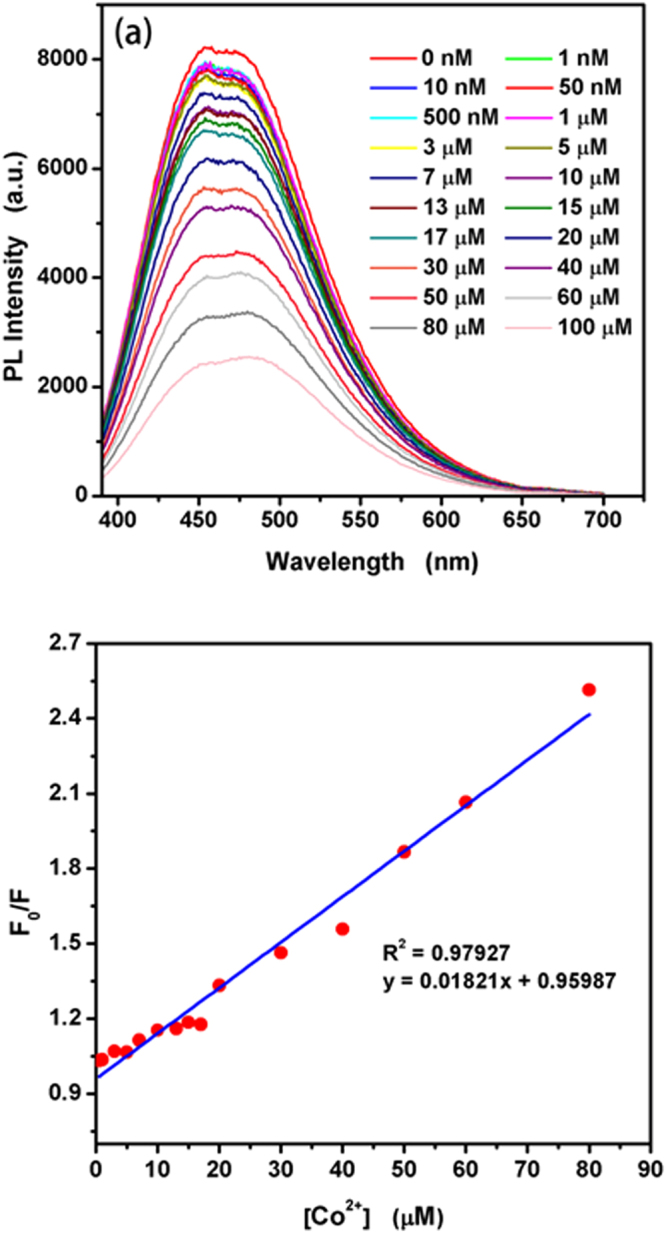
(a) Fluorescence spectra of as-synthesized CdS QDs solution upon addition of various concentrations of Co2+. (b) Relative emission intensity of CdS QDs in the presence of various concentrations of Co2+.
Study of the quenching mechanisms of Co2+ on PL emission of TGA@CdSQDs
The as-synthesized TGA@CdS QDs can be used for metal sensing based on metal–ligand interaction between the carboxyl acid group of TGA and metal ions, and it has been observed that these QDs have outstanding sensitivity towards Co2+25. In this work, the fluorescence decay curves (Fig. 10) and the UV-vis spectra (Fig. 11) of the as-synthesized CdS QDs in the absence and presence of Co2+ provide foundation for further study. As shown in Fig. 10, the fluorescence decay curves are does not change upon addition of Co2+. The fluorescence lifetime keeps constant indicates that the excitation and electron transition dynamics in CdS QDs are cannot affected by Co2+. Hence, there can exclude the possibility of the excited state of PL quenching and the electron transfer processes due to the bigger Ksp value of CoS than that of CdS (the pKsp values of CoS and CdS are 20.4 and 26.1, respectively). The coordination interaction between QDs and metal ions through the carboxyl group of TGA may form some non-radiative channels for energy dissipation resulting in weakened fluorescence of QDs upon addition of Co2+25,37,41. The UV-vis spectra of the as-synthesized TGA@CdS QDs reveal the same track in the presence of Co2+. But a gradual increase in the absorbance of TGA@CdS QDs occurs at 325 nm with a slight red shift about 10 μM in absorbance maxima. Besides, a visual color change from colorless to yellow was observed in Fig. 11 inset. Thus the coordination interaction between QDs and Co2+ through the carboxyl group of TGA may result in the change of QDs with different sizes.
Figure 10.
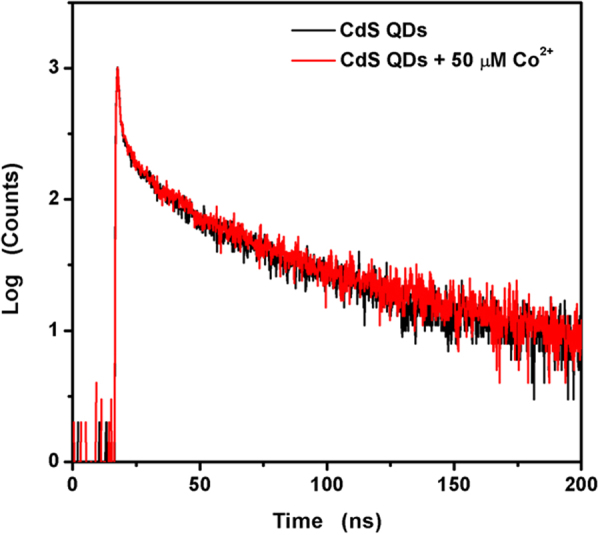
Photoluminescence lifetime decays of pure as-synthesized CdS QDs (black line) and presence of a concentration of Co2+ (red line).
Figure 11.
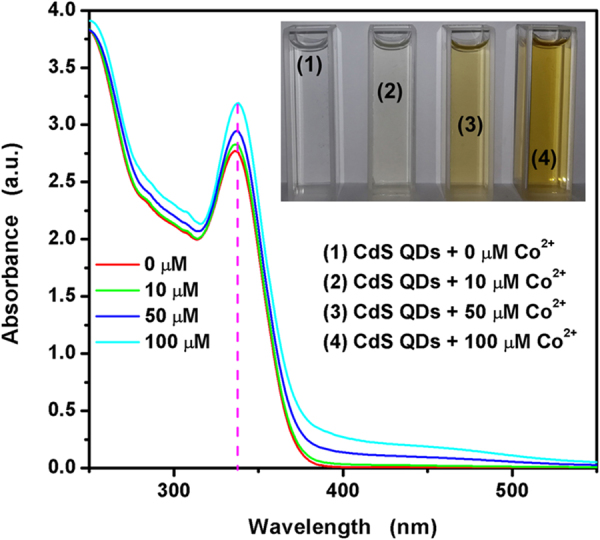
UV-vis spectra of Co2+ solution and as-synthesized CdS QDs with different Co2+ concentration.
The basic principle of as-synthesized QDs based Co2+ sensing system was shown in Fig. 12. In the presence of Co2+, the thiol groups of TGA attached to the surface of the QDs through Cd-S bonding and the negatively charged carboxyl group is easily coordination with Co2+. This means that the decrease of PL intensity was caused Co2+ could coordinate with two or more TGA@CdS QDs with a conspicuous change in absorbance and color of TGA@CdS QDs.
Figure 12.
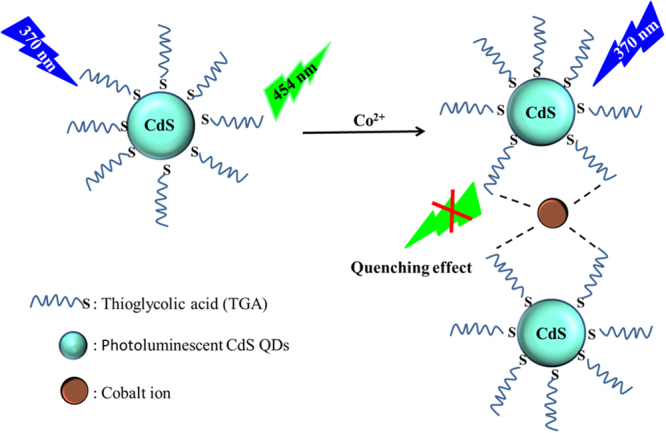
Schematic illustration of the process of Co2+-induced luminescence quenching of TGA@CdS QDs due to the coordination interaction between Co2+ and QDs through the carboxyl group of TGA.
Comparison the analytical merits to other Co2+ determination based QDs
Fluorescence determination based quantum dots for selective detection of Co2+ is an attractive research topic because their high sensitivity and selectivity. Several types of core quantum dots were synthesized with different surface-modified and used to as the Co2+ determination as summarized in Table 1. The results show that the present assay system exhibits superior selectivity and sensitivity to Co2+. Although the mode of detection is not different with other related determinations based fluorescence QDs, its detection limit (0.05 μM) is low which provide high sensitivity of the proposed sensor.
Table 1.
Comparison the analytical merits of the Co2+ determination based fluorescence quantum dots.
| Type of QDs | Surface-modified | Mode of detection | Linear range (μM) | Detection limit (μM) | Ref. |
|---|---|---|---|---|---|
| CdS QDs | N-thiophene-2-carboxamide | Fluorescence quenching | 10–150 | 0.83 | 20 |
| CdS QDs | L-Cysteine | Fluorescence quenching | 4–80 | 1.13 | 22 |
| CdS QDs | 3-Mercaptopropionic acid (MPA) | Fluorescence quenching | 0.01–1/1–50 | 0.01 | 25 |
| CdSe QDs | L-Cysteine | Fluorescence quenching | 0–20 | 0.1 | 21 |
| ZnO QDs | β-Cyclodextrin | Fluorescence quenching | 1–10 | 0.34 | 19 |
| CdS QDs | Thioglycolic acid | Fluorescence quenching | 0.5–80 | 0.05 | This work |
Conclusions
In summary, TGA capped CdS QDs have been synthesized via a simple hydrothermal method. The coordination interaction between QDs and Co2+ through the carboxyl group of TGA suggested that the as-synthesized CdS QDs could be used for detecting Co2+. After adding Co2+ into the solution of QDs, the change of fluorescence spectrum can be observed. At high concentration level (>10 μM), the Co2+ induced color changing also can be observed. The fluorescence intensity selectively quenched by Co2+ due to the coordination interaction between QDs and Co2+ through the carboxyl group of TGA. Mechanisms of fluorescence quenching are discussed in detail. The calibration plot was linear in the range between 0.5 and 80 μM. The detection limit of the present sensor is 0.05 μM, which is quite low as desired. Besides, the Co2+-induced color changing of the as-synthesized QDs is particularly outstanding. The TGA@CdS QDs could provide a low cost, simple, rapid and sensitive sensing strategy for detecting trace Co2+.
Acknowledgements
This work was supported by Department of Science and Technology of Yunnan Province via the Key Project for the Science and Technology (Grant No. 2017FA025) and the National Natural Science Foundation of China (Grant No. 61761047).
Author Contributions
The research was planned by Y.D.W. Experiments were performed by Z.Z.W., X.X.X., R.J.Z. and T.Z. Z.Z.W. and Y.D.W. prepared the manuscript. All the authors participated in discussing and reviewing of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li J, et al. A tricorn-rhodamine fluorescent chemosensor for detection of Co2+ ions. Luminescence. 2017;32:1448–1455. doi: 10.1002/bio.3344. [DOI] [PubMed] [Google Scholar]
- 2.Chen HJ, et al. Nitrogen doped graphene quantum dots based single-luminophor generated dual-potential electrochemiluminescence system for ratiometric sensing of Co2+ ion. Electrochim. Acta. 2016;214:94–102. doi: 10.1016/j.electacta.2016.08.028. [DOI] [Google Scholar]
- 3.Tvermoes BE, et al. Cobalt whole blood concentrations in healthy adult male volunteers following two-weeks of ingesting a cobalt supplement. Food Chem. Toxicol. 2013;53:432–439. doi: 10.1016/j.fct.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 4.Park GJ, et al. A dual chemosensor for Zn2+ and Co2+ in aqueous media and living cells: Experimental and theoretical studies. Sensor. Actuat. B. 2016;223:509–519. doi: 10.1016/j.snb.2015.09.129. [DOI] [Google Scholar]
- 5.Sharma Y, et al. Lithium recycling behavior of nano-phase-CuCo2O4 as anode for lithium-ion batteries. J. Power Sources. 2017;173:495–501. doi: 10.1016/j.jpowsour.2007.06.022. [DOI] [Google Scholar]
- 6.Jia BR, et al. Synthesis of mesoporous single crystal Co(OH)2 nanoplate and its topotactic conversion to dual-pore mesoporous single crystal Co3O4. ACS Appl. Mater. Inter. 2016;8:15582–15590. doi: 10.1021/acsami.6b02768. [DOI] [PubMed] [Google Scholar]
- 7.Shi N, et al. Facile synthesis of monodisperse Co3O4 quantum dots with efficient oxygen evolution activity. Chem. Commun. 2015;51:1338–1340. doi: 10.1039/C4CC08179J. [DOI] [PubMed] [Google Scholar]
- 8.Emission standard of pollutants for copper, nickel, cobalt industry. China 2010.
- 9.Mohandoss S, et al. A new fluorescent PET sensor probe for Co2+ ion detection: computational, logic device and living cell imaging applications. RSC Adv. 2017;7:16581–16593. doi: 10.1039/C6RA27497H. [DOI] [Google Scholar]
- 10.Ghaedi M, et al. Cloud point extraction for the determination of copper, nickel and cobalt ions in environmental samples by flame atomic absorption spectrometry. J. Hazard. Mater. 2008;150:533–540. doi: 10.1016/j.jhazmat.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 11.Beikzadeh E, et al. Determination of trace levels of cobalt ion in different real samples using dispersive liquid–liquid microextraction followed by flame atomic absorption spectrometry. J. Food Meas. Charact. 2017;11:994–1002. doi: 10.1007/s11694-017-9474-9. [DOI] [Google Scholar]
- 12.Felipe-Sotelo M, et al. Microwave-assisted extraction and ultrasonic slurry sampling procedures for cobalt determination in geological samples by electrothermal atomic absorption spectroscopy. Talanta. 2004;63:735–742. doi: 10.1016/j.talanta.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, et al. Simultaneous determination of copper, cobalt, and mercury ions in water samples by solid-phase extraction using carbon nanotube sponges as adsorbent after chelating with sodium diethyldithiocarbamate prior to high performance liquid chromatography. Anal. Bioanal. Chem. 2016;408:4445–4453. doi: 10.1007/s00216-016-9542-8. [DOI] [PubMed] [Google Scholar]
- 14.Bakkaus E, et al. Anion exchange liquid chromatography–inductively coupled plasma-mass spectrometry detection of the Co2+, Cu2+, Fe3+ and Ni2+ complexes of mugineic and deoxymugineic acid. J. Chromatogr. A. 2006;1192:208–215. doi: 10.1016/j.chroma.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Hutton EA, et al. Validation of bismuth film electrode for determination of cobalt and cadmium in soil extracts using ICP–MS. Talanta. 2004;63:849–855. doi: 10.1016/j.talanta.2003.12.038. [DOI] [PubMed] [Google Scholar]
- 16.Zhao LL, et al. Determination of cadmium(II), cobalt(II), nickel(II), lead(II), zinc(II), and copper(II) in water samples using dual-cloud point extraction and inductively coupled plasma emission spectrometry. J. Hazard. Mater. 2012;239-240:206–212. doi: 10.1016/j.jhazmat.2012.08.066. [DOI] [PubMed] [Google Scholar]
- 17.Korolczuk M, et al. Determination of traces of cobalt in zinc matrix by catalytic adsorptive stripping voltammetry at bismuth film electrode. Electroanal. 2007;19:2155–2158. doi: 10.1002/elan.200703924. [DOI] [Google Scholar]
- 18.Morfobos, M. et al. Simultaneous determination of nickel(II) and cobalt(II) by square wave adsorptive stripping voltammetry on a rotating-disc bismuth-film electrode. Anal. Chim. Acta 519, 57–64 (2004).
- 19.Geng S, et al. Determination of cobalt(II) using β-cyclodextrin-capped ZnO quantum dots as a fluorescent probe. Microchim. Acta. 2017;184:2533–2539. doi: 10.1007/s00604-017-2193-4. [DOI] [Google Scholar]
- 20.Faridbod F, et al. A Novel Cobalt-Sensitive Fluorescent Chemosensor Based on Ligand Capped CdS Quantum Dots. J. Fluoresc. 2015;25:613–619. doi: 10.1007/s10895-015-1544-y. [DOI] [PubMed] [Google Scholar]
- 21.Ben Brahima N, et al. Interaction of L-cysteine functionalized CdSe quantum dots with metallic cations and selective binding of cobalt in water probed by fluorescence. Sensor. Actuat. B. 2017;243:489–499. doi: 10.1016/j.snb.2016.12.003. [DOI] [Google Scholar]
- 22.Tedsana W, et al. A circular dichroism sensor for Ni2+ and Co2+ based on L-cysteine capped cadmium sulfide quantum dots. Anal. Chim. Acta. 2015;867:1–8. doi: 10.1016/j.aca.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Zi LL, et al. Thioglycolic acid-capped CuInS2/ZnS quantum dots as fluorescent probe for cobalt ion detection. J. Lumin. 2014;148:359–363. doi: 10.1016/j.jlumin.2013.12.051. [DOI] [Google Scholar]
- 24.Bian W, et al. A novel phosphorescence sensor for Co2+ ion based on Mn-doped ZnS quantum dots. Luminescence. 2014;29:151–157. doi: 10.1002/bio.2520. [DOI] [PubMed] [Google Scholar]
- 25.Mahapatra N, et al. A single source-precursor route for the one-pot synthesis of highly luminescent CdS quantum dots as ultra-sensitive and selective photoluminescence sensor for Co2+ and Ni2+ ions. J. Mater. Chem. C. 2014;2:7373–7384. doi: 10.1039/C4TC00887A. [DOI] [Google Scholar]
- 26.Butwong N, et al. Detection of silver(I) ion based on mixed surfactant-adsorbed CdS quantum dots. Microchim. Acta. 2013;180:1101–1107. doi: 10.1007/s00604-013-1031-6. [DOI] [Google Scholar]
- 27.Zhang KX, et al. Facile synthesis L-cysteine capped CdS:Eu quantum dots and their Hg2+ sensitive properties. Appl. Surf. Sci. 2013;276:333–339. doi: 10.1016/j.apsusc.2013.03.093. [DOI] [Google Scholar]
- 28.Boonmee C, et al. Cysteamine capped CdS quantum dots as a fluorescence sensor for the determination of copper ion exploiting fluorescence enhancement and long-wave spectral shifts. Spectrochim. Acta A. 2016;169:161–168. doi: 10.1016/j.saa.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Ganiga M, et al. An ascorbic acid sensor based on cadmium sulphide quantum dots. Anal. Bioanal. Chem. 2016;408:3699–3706. doi: 10.1007/s00216-016-9454-7. [DOI] [PubMed] [Google Scholar]
- 30.Kumar DS, et al. Optical properties of colloidal aqueous synthesized 3 Mercaptopropionic Acid stabilized CdS quantum dots. AIP. Conf. Proc. 2016;1728:020162-1–020162-4. [Google Scholar]
- 31.Hosseini SS, et al. Facile microwave approach for synthesis of CdS quantum dots as barrier layer for increasing dye-sensitized solar cells efficiency. J. Mater. Sci-Mater. El. 2016;27:12240–12246. doi: 10.1007/s10854-016-5380-x. [DOI] [Google Scholar]
- 32.Kaur G, et al. Size tuning of MAA capped CdSe and CdSe/CdS quantum dots and their stability in different pH environments. Mater. Chem. Phys. 2014;143:514–523. doi: 10.1016/j.matchemphys.2013.09.003. [DOI] [Google Scholar]
- 33.Samadi-maybodi A, et al. Aqueous synthesis and characterization of CdS quantum dots capped with some amino acids and investigations of their photocatalytic activities. Colloid. Surface. A. 2014;447:111–119. doi: 10.1016/j.colsurfa.2014.01.036. [DOI] [Google Scholar]
- 34.Brus LE, et al. Electron-electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J. Chem. Phys. 1984;80:4403–4407. doi: 10.1063/1.447218. [DOI] [Google Scholar]
- 35.Brus LE, et al. Electronic wave functions in semiconductor clusters: experiment and theory. J. Phys. Chem. 1986;90:2555–2560. doi: 10.1021/j100403a003. [DOI] [Google Scholar]
- 36.Aboulaich A, et al. One-Pot noninjection route to CdS quantum dots via hydrothermal synthesis. ACS Appl. Mater. Inter. 2012;4:2561–2569. doi: 10.1021/am300232z. [DOI] [PubMed] [Google Scholar]
- 37.Wu P, et al. Ni2+-modulated homocysteine-capped CdTe quantum dots as a turn-on photoluminescent sensor for detecting histidine in biological fluids. Biosens. Bioelectron. 2010;26:485–490. doi: 10.1016/j.bios.2010.07.068. [DOI] [PubMed] [Google Scholar]
- 38.Sanz M, et al. Femtosecond dynamics of CdTe quantum dots in water. J. Photochem. Photobio. A. 2008;196:51–58. doi: 10.1016/j.jphotochem.2007.11.012. [DOI] [Google Scholar]
- 39.Jagadeeswari S, et al. Photoinduced interaction between MPA capped CdTe QDs and certain anthraquinone dyes. J. Lumin. 2011;131:297–602. doi: 10.1016/j.jlumin.2010.10.037. [DOI] [Google Scholar]
- 40.Uchihara T, et al. Subpicosecond time-resolved photoluminescence of thioglycerol-capped CdS nanoparticles in water. J. Photoch. Photobio. A. 2006;181:86–93. doi: 10.1016/j.jphotochem.2005.11.005. [DOI] [Google Scholar]
- 41.Gan ZX, et al. Photoluminescence of MoS2 quantum dots quenched by hydrogen peroxide: A fluorescent sensor for hydrogen peroxide. J. Appl. Phys. 2016;120:104503. doi: 10.1063/1.4962318. [DOI] [Google Scholar]