Abstract
Matrix metalloproteinases (MMPs) are extracellular matrix (ECM) remodelling enzymes involved in developmental processes, tissue remodelling and repair, inflammatory and immune diseases and cancer. In a recent issue of Bioscience Reports (vol. 37, issue 6, BSR20170973), Liu and colleagues investigated the expression of MMPs such as MMP-1 (interstitial collagenase), MMP-3 (stromelysin 1) and MMP-13 (collagenase 3) in human periodontal ligament fibroblasts (hPDLFs) regulated by interleukin-12 (IL-12), a cytokine implicated in inflammatory and immune responses. They showed that IL-12 activates canonical nuclear factor-κB (NF-κB) signalling leading to increased expression of MMP-1, MMP-3 and MMP-13, and to a smaller reduction in the expression of MMP-2 (gelatinase A) and MMP-9 (gelatinase B) at both mRNA and protein levels, with corresponding changes in the secreted levels of these ECM-remodelling and immune regulatory metalloproteinases. While canonical NF-κB signalling regulates these MMPs, it also interacts with additional factors to determine whether some of these MMPs are induced or downregulated, in response to IL-12. Here, we comment on the possible mechanisms of IL-12-mediated transcriptional regulation of MMPs.
Keywords: Cytokines, IL-12, Matrix metalloproteinases, nuclear factor kappaB
Cytokines are the key regulators of inflammation and immunity, and modulation of their function may have enormous potential for therapeutic benefit in chronic inflammatory and autoimmune diseases. Type-I cytokines include the interleukin-6 (IL-6) and IL-12 families, which consist of structurally related four-helix bundle proteins. Unlike the members of the IL-6 family, which are secreted as single-subunit monomers, the IL-12 family members form heterodimeric complexes.
The IL-12 family is unique in comprising the only heterodimeric cytokines. IL-12 is a heterodimeric protein with a molecular weight of 70 kDa composed of two covalently linked subunits. Co-expression of the ligand-binding α subunit IL-12p35 (35 kDa) and the β subunit IL-12p40 (40 kDa), encoded by different genes localized on human chromosomes 3 and 5, leads to the formation of the biologically active p70 cytokine. The sequence of the p40 chain has a homology to the soluble extracellular domain of the membrane-bound receptors for IL-6 cytokines (IL-6 receptor; IL-6R) α-chain. This explains some of the redundant actions of these cytokines. IL-12 family subunits lack a transmembrane domain and are thus secreted as soluble α/β heterodimers. The IL-12 family consists of four cytokines with unique α/β subunit pairings: IL-12 (p35/p40), IL-23 (p19/p40), IL-27 (p28/Ebi3) and IL-35 (p35/Ebi3). Although structurally similar, IL-12 family members vary in function. Chain sharing, a characteristic of the IL-12 cytokine family, may also extend to the receptor usage with several cytokines utilizing the same receptor chains [1,2].
IL-12 (IL-12p70) is implicated in inflammatory and immune responses. IL-12 is secreted by antigen-presenting cells (APCs) such as macrophages, monocytes, dendritic cells (DCs), granulocytes and B cells in response to pathogenic microorganisms. IL-12 secretion is tightly regulated by several transcription factors. The IL-12p35 gene is constitutively transcribed at low levels, but not translated. Following stimulation with microbial pathogen components, it is transcribed and its expression is amplified by NF-κB and interferon regulatory factors (IRFs) [2,3]. The IL-12p40 gene promoter contains a number of transcription factor binding sites including NF-κB and Ets [4]. Microbial pathogen components are sensed by APCs such as DCs through toll-like receptors (TLRs). Importantly, selective production of each of IL-12 family member is regulated by triggering specific TLRs. TLR4 activation induces the production of both IL-12 and IL-23, whereas TLR2 activation induces IL-23 but not IL-12 [2,5].
Signalling through TLRs involves binding of TLRs to the adapter molecule MyD88 resulting in canonical NF-κB activation, which then induces the expression of genes encoding the subunits of IL-12 [2,6]. TLRs also activate the IRFs, IRF1, IRF3 and IRF7. Canonical NF-κB and IRF activation induce the transcription of IL-12p35 and IL-12p40. Subsequently, IL-12p70 is released and recognized by the IL-12 receptor (IL-12R) on natural killer (NK) and T cells [2].
The biological activities of IL-12 are mediated via binding to a membrane receptor complex (IL-12R) which is also composed of two subunits, IL12Rβ1 and IL12Rβ2, which are members of the class I cytokine receptor family, which includes IL-6, IL-11 and leucocyte inhibitory factor related to glycoprotein gp130 [2,7]. IL-12R is predominantly found on NK and T cells. IL12Rβ1 is required for high-affinity binding to the IL-12p40 subunit and it is associated with the Janus kinase (Jak) family member Tyk-2, while IL-12p35 binds to the IL12Rβ2 chain, associates with Jak-2 and mediates signal transduction via three tyrosine residues that act as a docking site for signal transducer and activator of transcription (STAT) 4 (STAT4), which is phosphorylated by JAK2. Thus, binding of IL-12 to the IL-12R complex, activates the JAK-STAT signalling pathway, with STAT4 being the predominant mediator of cellular responses activated by IL-12 [8–10]. Upon homodimerization and translocation to the nucleus STAT4 activates IFN-γ transcription. A positive feedback loop is established whereby IL-12-induced IFN-γ production by NK/T cells primes additional APCs for IL-12 production through IRF1 and IRF8 and IFN-γ-induced activation of T-bet, a T-box transcription factor expressed in CD4+ T cell promotes their differentiation to type 1 T helper (TH1) cells which express IL-12Rβ2 [2,3,11]. Moreover, IL-12-dependent binding of the transcription factor, activator protein-1 (AP-1) has also been shown [2].
Since IL-12 stimulates TH1 differentiation, it has been suggested that enhancing IL-12 activity in cancer may lead to an increased TH1 response and augmentation of the antitumour activity of the immune system. Activation of cytotoxic cells like NK cells and cytotoxic T lymphocytes (CTLs) is thought to result in increased extinction of tumour cells. IL-12 can also inhibit neo-angiogenesis and might therefore reduce the vascularization of growing tumours resulting in tumour cell necrosis [2].
IL-12 has also been implicated in the regulation of matrix metalloproteinases (MMPs) [10]. MMPs constitute a multigene family of zinc- and calcium-dependent ECM remodelling endopeptidases and chemokine regulators involved in several physiological and pathological processes. These include morphogenesis and developmental processes, tissue remodelling and repair, wound healing, inflammatory and autoimmune diseases such as periodontal diseases, arthritis, cardiovascular diseases and cancer [12–17].
Studies showed that synovial MMP-1 [18] and MMP-3 [19] levels correlated with IL-12 expression in canine rheumatoid arthritis. Expression levels of MMP-13 and IL-12 were also found to be correlated in experimental osteoarthritis [20].
Previous studies showed that IL-12 did not affect either the MMP-2 or MMP-9 mRNA or protein expression in the human monocytic U-937 cell line [21]. Injection of recombinant murine IL-18 or IL-12 alone or in combination significantly increased the levels of MMP-9 in mouse lung tissues, but no mechanistic details were provided [22]. However others, employing the human choriocarcinoma cell line, JEG-3, showed that IL-12 reduced the mRNA and protein expression levels, and also the enzymic activity of both MMP-2 and MMP-9, leading to suppression of tumour cell motility and invasion [23,24]. IL-12 treatment increased IFN-γ production in this setting [23]. In a murine model of breast cancer, IL-12 treatment reduced the levels of MMP-9, but not MMP-2, and it also reduced tumour cell production of VEGF by up-regulation of IFN-γ production leading to the suppression of tumour angiogenesis [10,25,26]. In an in vivo tumour model, it was shown that that the protein, mRNA expression and/or activity of MMP-2, MMP-3, MMP-7 and MMP-9 were significantly higher in UVB-exposed skin and tumours of IL-12 knockout mice compared with wild-type mice [27]. Collectively, these studies showed that IL-12-mediated production of IFN-γ led to the suppression of the expression of MMP-2 and MMP-9 in different cell types. However, whether NF-κB signalling was activated was not investigated. In line with this, it was also shown by employing IKK-null mouse embryo fibroblasts, that a subset of IFN-γ-responsive genes was dependent on the upstream activating NF-κB kinases, IKKα and IKKβ, but independent of NF-κB activation. In this setting, there was no defect in IFN-γ-stimulated STAT1α activation [28].
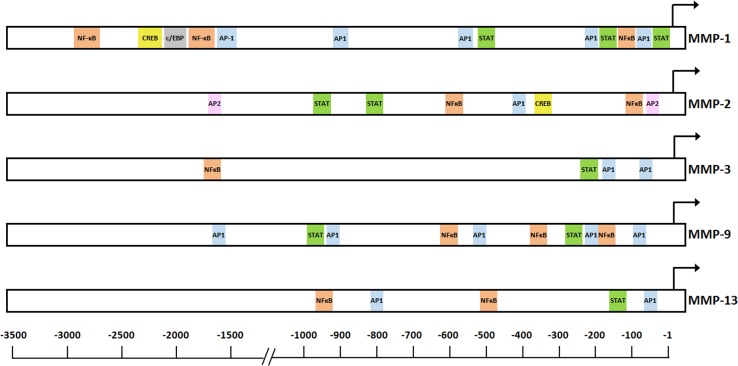
Studies have shown that cytokine-mediated regulation of MMP expression is complex involving the interplay of several transcription factors including mainly AP-1, NF-κB and STAT, and also activator protein-2 (AP-2), CREB and CCAAT/enhancer binding protein (C/EBP) [29–32] (Figure 1).
Figure 1. cis-Regulatory elements in the promoter regions of human MMP-1, MMP-2, MMP-3, MMP-9 and MMP-13 genes.
Transcription start sites are indicated with an angled arrow and the relevant cis-regulatory elements are represented within boxes. Data are compiled from the following references ([29,31,32] and references in the text).
The induction of the expression of several MMPs including MMP-1, MMP-3 and MMP-13 by pro-inflammatory cytokines in several cell types including fibroblasts [33,34–37] was shown to depend on extracellular signal-regulated kinase 1 and 2 (ERK1/2) and mitogen-activated kinase (MAPK) p38 mediated activation of canonical NF-κB, and to a lesser extent on the activation of other transcription factors such as AP-1, although these transcription factors co-operate to enhance MMP transcription [33,37–46].
In a different study employing articular chondrocytes, it was shown that MMP-1 and MMP-13 are differentially regulated by IL-1. IL-1 induction of MMP-13 required both AP-1 and NF-κB activation, while MMP-1 required only NF-κB activation [40,47–49].
Several studies have shown that the production of many inflammatory mediators and the production of MMPs depends on cytokine-mediated activation of MAP kinase leading to AP-1 transcription factor and of IKKβ-mediated canonical NF-κB activation [29–31,50,51]. For example, stimulation with LPS results in the activation of ERK1/2 and MAPK p38. Inhibition of MAPK p38 suppressed MMP-1 expression, but increased ERK activity and MMP-9 expression. This was because inhibition of MAPK p38 resulted in decreased binding of CREB and SP1 transcription factors to MMP-1 promoter, and to increased binding of NF-κB transcription factor to MMP-9 promoter. In contrast, inhibition of ERK1/2 suppressed MMP-1 and MMP-9 expression by inhibiting the binding of all AP-1, SP1 and NF-κB transcription factors to the promoters of both MMP-1 and MMP-9. Thus, LPS-induced production of MMP-1 is regulated by both ERK1/2 and p38, whereas MMP-9 production depends mainly on the ERK1/2-mediated activation of NF-κB [41,50].
In addition to the involvement of several transcription factors including SP1, AP-1 and NF-κB in the regulation of MMP gene expression, the MMP family members are also regulated by pro-inflammatory cytokines via STAT signalling [52,53]. For example, MMP-1 regulation involves binding of STAT3 to a proximal STAT-binding element (SBE) in the MMP-1 human gene promoter [54,55]. MMP-3 regulation by IL-6 involves a STAT3 binding to distal SBE element [56,57]. It was recently shown that IL-6 regulates MMP-1 expression, including MMP-1, MMP-2, MMP-3 and MMP-9, via proximal IFN-γ-activated site (GAS)-like SBEs involving binding of STAT1 and AP-1 but not STAT-3 [58–60]. Importantly, IFN-γ treatment resulted in the inhibition of MMP expression and also antagonized IL-6-dependent induction of MMP1 and MMP-3 gene expression, by reducing STAT1 binding to the respective MMP gene promoters [58]. IL-12 also leads to the activation of MAPK p38 and ERK1/2 and STAT phosphorylation [61–63].
Suppression of the expression of MMP-2 and MMP-9 by IL-12-mediated production of IFN-γ [10,23–27] may be due to STAT binding to GAS-SBEs in their respective gene promoters [61,62,64]. Previous studies showed that IFN-γ suppresses PMA-induced MMP-9 gene expression by activating the JAK-STAT pathway with p-STAT1α (Ser727) to bind to GAS, which is present in the promoters of IFN-γ-responsive genes. Genes that are negatively regulated by IFN-γ are some of the MMPs such as MMP-1, MMP-3, MMP-9 and MMP-13 [65–67]. Mechanistically, it was shown that IFN-γ-activated STAT1α suppresses MMP-9 gene transcription [68] by sequestration of the coactivators CBP/p300, without affecting binding of other transcription factors such as AP-1, SP1 and NF-κB to MMP-9 gene promoter [65,69]. Additional studies have shown a competition between IRF factors and NF-κB. For example, it was shown that IRF1 acts as competitive inhibitor of NF-κB binding to the MMP-9 promoter [70]. IL-12 was shown to induce the expression of IRF1 via STAT4 [71].
In summary, the regulation of MMP gene expression in response to pro-inflammatory cytokines involves the interaction of many transcription factors on MMP gene promoters. IL-12 can induce the activation of STATs leading to enhanced transcription of IFN-γ, which then suppresses MMP expression via GAS-SBEs such as in the case of MMP-2 and MMP-9, but it can also activate canonical NF-κB leading to the induction of MMP gene expression such as in the case of MMP-1, MMP-3 and MMP13. This differential expression of MMPs by IL-12-mediated IFN-γ production may be due to different STATs involved in MMP gene expression including STAT1, STAT3 and STAT4, and which of these co-operate with NF-κB to increase MMP gene expression or lead to sequestration of coactivators without affecting NF-κB binding to the MMP gene promoters. Alternatively, certain IL-12-induced IRF factors compete with NF-κB for binding to a MMP gene promoter.
Abbreviations
- APC
antigen-presenting cell
- AP-1
activator protein-1
- CREB
cAMP response element-binding protein
- DC
dendritic cell
- ECM
extracellular matrix
- ERK1/2
extracellular signal-regulated kinase 1 and 2
- Ets
E26 trasformation-specific
- GAS
growth arrest specific
- IKK
IκB kinase
- IL-6
interleukin-6
- IL-12
interleukin-12
- IL-12R
IL-12 receptor
- IFN-γ
interferon gamma
- IRF
interferon regulatory factor
- Jak
Janus kinase
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MMP
matrix matalloproteinase
- MyD88
myeloid differentiation primary response 88
- NF-κB
nuclear factor-κB
- NK
natural killer
- SBE
STAT-binding element
- Sp1
specificity protein 1
- STAT
signal transducer and activator of transcription
- TH1
type 1 T helper
- TLR
toll-like receptor
Funding
This work was supported by the Fondation Santé, Stavros Niarchos Foundation (Archers) [grant number Ref#SNF0031]; the project – ‘Advanced Research Activities in Biomedical and Agroalimentary Technologies’ - which is implemented under the - ‘Action for the Strategic Development on the Research and Technological Sector’ - funded by the Operational Programme - ‘Competitiveness, Entrepreneurship and Innovation’ [grant number NSRF 2014-2020]; and the Greece and the European Union (European Regional Development Fund) (co-financer) [grant number KRHPIS-2].
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Vignali D.A. and Kuchroo V.K. (2012) IL-12 family cytokines: immunological playmakers. Nat. Immunol. 13, 722–728 10.1038/ni.2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zundler S. and Neurath M.F. (2015) Interleukin-12: Functional activities and implications for disease. Cytokine Growth Factor Rev. 26, 559–568 10.1016/j.cytogfr.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 3.Goriely S., Neurath M.F. and Goldman M. (2008) How microorganisms tip the balance between interleukin-12 family members. Nat. Rev. Immunol. 8, 81–86 10.1038/nri2225 [DOI] [PubMed] [Google Scholar]
- 4.Becker C., Wirtz S., Ma X., Blessing M., Galle P.R. and Neurath M.F. (2001) Regulation of IL-12 p40 promoter activity in primary human monocytes: roles of NF-kappaB, CCAAT/enhancer-binding protein beta and PU.1 and identification of a novel repressor element (GA-12) that responds to IL-4 and prostaglandin E(2). J. Immunol. 167, 2608–2618 10.4049/jimmunol.167.5.2608 [DOI] [PubMed] [Google Scholar]
- 5.Re F. and Strominger J.L. (2001) Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 276, 37692–37699 10.1074/jbc.M105927200 [DOI] [PubMed] [Google Scholar]
- 6.Grumont R., Hochrein H., O’Keeffe M., Gugasyan R., White C., Caminschi I. et al. (2001) c-Rel regulates interleukin 12 p70 expression in CD8(+) dendritic cells by specifically inducing p35 gene transcription. J. Exp. Med. 194, 1021–1032 10.1084/jem.194.8.1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Presky D.H., Yang H., Minetti L.J., Chua A.O., Nabavi N., Wu C.Y. et al. (1996) A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc. Natl. Acad. Sci. U.S.A. 93, 14002–14007 10.1073/pnas.93.24.14002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trinchieri G. (2003) Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3, 133–146 10.1038/nri1001 [DOI] [PubMed] [Google Scholar]
- 9.Trinchieri G., Pflanz S. and Kastelein R.A. (2003) The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity 19, 641–644 10.1016/S1074-7613(03)00296-6 [DOI] [PubMed] [Google Scholar]
- 10.Vecchio M.D., Emilio B., Canova S., Lotze M., Wesa. A., Parmiani G. et al. (2007) Interleukin-12: biological properties and clinical application. Clin. Cancer Res. 13, 4677–4685 10.1158/1078-0432.CCR-07-0776 [DOI] [PubMed] [Google Scholar]
- 11.Thierfelder W.E., van Deursen J.M., Yamamoto K., Tripp R.A., Sarawar S.R., Carson R.T. et al. (1996) Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature 382, 171–174 10.1038/382171a0 [DOI] [PubMed] [Google Scholar]
- 12.Parks W.C., Wilson C.L. and Lopez-Boado Y.S. (2004) Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 4, 617–629 10.1038/nri1418 [DOI] [PubMed] [Google Scholar]
- 13.Gialeli C., Theocharis A.D. and Karamanos N.K. (2011) Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 278, 16–27 10.1111/j.1742-4658.2010.07919.x [DOI] [PubMed] [Google Scholar]
- 14.Hadler-Olsen E., Fadnes B., Sylte I., Uhlin-Hansen L. and Winberg J.O. (2011) Regulation of matrix metalloproteinase activity in health and disease. FEBS J. 278, 28–45 10.1111/j.1742-4658.2010.07920.x [DOI] [PubMed] [Google Scholar]
- 15.Dufour A. and Overall C.M. (2013) Missing the target: matrix metalloproteinase antitargets in inflammation and cancer. Trends Pharmacol. Sci. 34, 233–242 10.1016/j.tips.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 16.Franco C., Patricia H.R., Timo S., Claudia B. and Marcela H. (2017) Matrix metalloproteinases as regulators of periodontal inflammation. Int. J. Mol. Sci. 18, 10.3390/ijms18020440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fingleton B. (2017) Matrix metalloproteinases as regulators of inflammatory processes. Biochim. Biophys. Acta 1864, 2036–2042 10.1016/j.bbamcr.2017.05.010 [DOI] [PubMed] [Google Scholar]
- 18.Inomoto M., Miyakawa S., Mishima H. and Ochiai N. (2000) Elevated interleukin-12 in pseudosynovial fluid in patients with aseptic loosening of hip prosthesis. J. Orthop. Sci. 5, 369–373 10.1007/s007760070045 [DOI] [PubMed] [Google Scholar]
- 19.Hegemann N., Wondimu A., Ullrich K. and Schmidt M.F. (2003) Synovial MMP-3 and TIMP-1 levels and their correlation with cytokine expression in canine rheumatoid arthritis. Vet. Immunol. Immunopathol. 91, 199–204 10.1016/S0165-2427(03)00005-9 [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro M., Lopez de Figueroa P., Nogueira-Recalde U., Centeno A., Mendes A.F., Blanco F.J. et al. (2016) Diabetes-accelerated experimental osteoarthritis is prevented by autophagy activation. Osteoarthritis Cartilage 24, 2116–2125 10.1016/j.joca.2016.06.019 [DOI] [PubMed] [Google Scholar]
- 21.Abraham M., Shapiro S., Lahat N. and Miller A. (2002) The role of IL-18 and IL-12 in the modulation of matrix metalloproteinases and their tissue inhibitors in monocytic cells. Int. Immunol. 14, 1449–1457 10.1093/intimm/dxf108 [DOI] [PubMed] [Google Scholar]
- 22.Cero F.T., Hillestad V., Loberg E.M., Christensen G., Larsen K.O. and Skjonsberg O.H. (2012) IL-18 and IL-12 synergy induces matrix degrading enzymes in the lung. Exp. Lung Res. 38, 406–419 10.3109/01902148.2012.716903 [DOI] [PubMed] [Google Scholar]
- 23.Karmakar S., Dhar R. and Das C. (2004) Inhibition of cytotrophoblastic (JEG-3) cell invasion by interleukin 12 involves an interferon gamma-mediated pathway. J. Biol. Chem. 279, 55297–55307 10.1074/jbc.M407013200 [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z., Xu Q., Shi C. and Li Y. (2012) Interleukin-12 inhibits cell invasion in choriocarcinoma. Int. J. Mol. Med. 30, 57–62 [DOI] [PubMed] [Google Scholar]
- 25.Dias S., Boyd R. and Balkwill F. (1998) IL-12 regulates VEGF and MMPs in a murine breast cancer model. Int. J. Cancer 78, 361–365 10.1002/(SICI)1097-0215(19981029)78:3%3c361::AID-IJC17%3e3.0.CO;2-9 [DOI] [PubMed] [Google Scholar]
- 26.Strasly M., Cavallo F., Geuna M., Mitola S., Colombo M.P., Forni G. et al. (2001) IL-12 inhibition of endothelial cell functions and angiogenesis depends on lymphocyte-endothelial cell cross-talk. J. Immunol. 166, 3890–3899 10.4049/jimmunol.166.6.3890 [DOI] [PubMed] [Google Scholar]
- 27.Meeran S.M., Katiyar S., Elmets C.A. and Katiyar S.K. (2007) Interleukin-12 deficiency is permissive for angiogenesis in UV radiation-induced skin tumors. Cancer Res. 67, 3785–3793 10.1158/0008-5472.CAN-06-3134 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Sizemore N., Agarwal A., Das K., Lerner N., Sulak M., Rani S. et al. (2004) Inhibitor of kappaB kinase is required to activate a subset of interferon gamma-stimulated genes. Proc. Natl. Acad. Sci. U.S.A. 101, 7994–7998 10.1073/pnas.0401593101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan C. and Boyd D.D. (2007) Regulation of matrix metalloproteinase gene expression. J. Cell. Physiol. 211, 19–26 10.1002/jcp.20948 [DOI] [PubMed] [Google Scholar]
- 30.Vincenti M.P. and Brinckerhoff C.E. (2007) Signal transduction and cell-type specific regulation of matrix metalloproteinase gene expression: can MMPs be good for you? J. Cell. Physiol. 213, 355–364 10.1002/jcp.21208 [DOI] [PubMed] [Google Scholar]
- 31.Clark I.M., Swingler T.E., Sampieri C.L. and Edwards D.R. (2008) The regulation of matrix metalloproteinases and their inhibitors. Int. J. Biochem. Cell Biol. 40, 1362–1378 10.1016/j.biocel.2007.12.006 [DOI] [PubMed] [Google Scholar]
- 32.Fanjul-Fernandez M., Folgueras A.R., Cabrera S. and Lopez-Otin C. (2010) Matrix metalloproteinases: evolution, gene regulation and functional analysis in mouse models. Biochim. Biophys. Acta 1803, 3–19 10.1016/j.bbamcr.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 33.Miao L., Zhan S. and Liu J. (2017) Interleukin-12-mediated expression of matrix metalloproteinases in human periodontal ligament fibroblasts involves in NF-kappaB activation. Biosci. Rep. 37, 10.1042/BSR20170973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiBattista J.A., Pelletier J.P., Zafarullah M., Fujimoto N., Obata K. and Martel-Pelletier J. (1995) Coordinate regulation of matrix metalloproteases and tissue inhibitor of metalloproteinase expression in human synovial fibroblasts. J. Rheumatol. Suppl. 43, 123–128 [PubMed] [Google Scholar]
- 35.Nakaya H., Oates T.W., Hoang A.M., Kamoi K. and Cochran D.L. (1997) Effects of interleukin-1 beta on matrix metalloproteinase-3 levels in human periodontal ligament cells. J. Periodontol. 68, 517–523 10.1902/jop.1997.68.6.517 [DOI] [PubMed] [Google Scholar]
- 36.Kiili M., Cox S.W., Chen H.Y., Wahlgren J., Maisi P., Eley B.M. et al. (2002) Collagenase-2 (MMP-8) and collagenase-3 (MMP-13) in adult periodontitis: molecular forms and levels in gingival crevicular fluid and immunolocalisation in gingival tissue. J. Clin. Periodontol. 29, 224–232 10.1034/j.1600-051x.2002.290308.x [DOI] [PubMed] [Google Scholar]
- 37.Bond M., Baker A.H. and Newby A.C. (1999) Nuclear factor kappaB activity is essential for matrix metalloproteinase-1 and -3 upregulation in rabbit dermal fibroblasts. Biochem. Biophys. Res. Commun. 264, 561–567 10.1006/bbrc.1999.1551 [DOI] [PubMed] [Google Scholar]
- 38.Barchowsky A., Frleta D. and Vincenti M.P. (2000) Integration of the NF-kappaB and mitogen-activated protein kinase/AP-1 pathways at the collagenase-1 promoter: divergence of IL-1 and TNF-dependent signal transduction in rabbit primary synovial fibroblasts. Cytokine 12, 1469–1479 10.1006/cyto.2000.0743 [DOI] [PubMed] [Google Scholar]
- 39.Chase A.J., Bond M., Crook M.F. and Newby A.C. (2002) Role of nuclear factor-kappa B activation in metalloproteinase-1, -3, and -9 secretion by human macrophages in vitro and rabbit foam cells produced in vivo. Arterioscler. Thromb. Vasc. Biol. 22, 765–771 10.1161/01.ATV.0000015078.09208.92 [DOI] [PubMed] [Google Scholar]
- 40.Vincenti M.P. and Brinckerhoff C.E. (2002) Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 4, 157–164 10.1186/ar401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma Z., Shah R.C., Chang M.J. and Benveniste E.N. (2004) Coordination of cell signaling, chromatin remodeling, histone modifications, and regulator recruitment in human matrix metalloproteinase 9 gene transcription. Mol. Cell. Biol. 24, 5496–5509 10.1128/MCB.24.12.5496-5509.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bond M., Fabunmi R.P., Baker A.H. and Newby A.C. (1998) Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-kappa B. FEBS Lett. 435, 29–34 10.1016/S0014-5793(98)01034-5 [DOI] [PubMed] [Google Scholar]
- 43.Elsharkawy A.M., Oakley F., Lin F., Packham G., Mann D.A. and Mann J. (2010) The NF-kappaB p50:p50:HDAC-1 repressor complex orchestrates transcriptional inhibition of multiple pro-inflammatory genes. J. Hepatol. 53, 519–527 10.1016/j.jhep.2010.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Kane C.M., Elkington P.T., Jones M.D., Caviedes L., Tovar M., Gilman R.H. et al. (2010) STAT3, p38 MAPK, and NF-kappaB drive unopposed monocyte-dependent fibroblast MMP-1 secretion in tuberculosis. Am. J. Respir. Cell Mol. Biol. 43, 465–474 10.1165/rcmb.2009-0211OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Epanchintsev A., Shyamsunder P., Verma R.S. and Lyakhovich A. (2015) IL-6, IL-8, MMP-2, MMP-9 are overexpressed in Fanconi anemia cells through a NF-kappaB/TNF-alpha dependent mechanism. Mol. Carcinog. 54, 1686–1699 10.1002/mc.22240 [DOI] [PubMed] [Google Scholar]
- 46.Faour W.H., He Q., Mancini A., Jovanovic D., Antoniou J. and Di Battista J.A. (2006) Prostaglandin E2 stimulates p53 transactivational activity through specific serine 15 phosphorylation in human synovial fibroblasts. Role in suppression of c/EBP/NF-kappaB-mediated MEKK1-induced MMP-1 expression. J. Biol. Chem. 281, 19849–19860 10.1074/jbc.M601293200 [DOI] [PubMed] [Google Scholar]
- 47.Vincenti M.P., Coon C.I. and Brinckerhoff C.E. (1998) Nuclear factor kappaB/p50 activates an element in the distal matrix metalloproteinase 1 promoter in interleukin-1beta-stimulated synovial fibroblasts. Arthritis Rheum. 41, 1987–1994 10.1002/1529-0131(199811)41:11%3c1987::AID-ART14%3e3.0.CO;2-8 [DOI] [PubMed] [Google Scholar]
- 48.Mengshol J.A., Vincenti M.P., Coon C.I., Barchowsky A. and Brinckerhoff C.E. (2000) Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 43, 801–811 10.1002/1529-0131(200004)43:4%3c801::AID-ANR10%3e3.0.CO;2-4 [DOI] [PubMed] [Google Scholar]
- 49.Schmucker A.C., Wright J.B., Cole M.D. and Brinckerhoff C.E. (2012) Distal interleukin-1beta (IL-1beta) response element of human matrix metalloproteinase-13 (MMP-13) binds activator protein 1 (AP-1) transcription factors and regulates gene expression. J. Biol. Chem. 287, 1189–1197 10.1074/jbc.M111.264077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lai W.C., Zhou M., Shankavaram U., Peng G. and Wahl L.M. (2003) Differential regulation of lipopolysaccharide-induced monocyte matrix metalloproteinase (MMP)-1 and MMP-9 by p38 and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases. J. Immunol. 170, 6244–6249 10.4049/jimmunol.170.12.6244 [DOI] [PubMed] [Google Scholar]
- 51.Huang W.C., Sala-Newby G.B., Susana A., Johnson J.L. and Newby A.C. (2012) Classical macrophage activation up-regulates several matrix metalloproteinases through mitogen activated protein kinases and nuclear factor-kappaB. PLoS ONE 7, e42507 10.1371/journal.pone.0042507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mauviel A. (1993) Cytokine regulation of metalloproteinase gene expression. J. Cell. Biochem. 53, 288–295 10.1002/jcb.240530404 [DOI] [PubMed] [Google Scholar]
- 53.Gordon G.M., Ledee D.R., Feuer W.J. and Fini M.E. (2009) Cytokines and signaling pathways regulating matrix metalloproteinase-9 (MMP-9) expression in corneal epithelial cells. J. Cell. Physiol. 221, 402–411 10.1002/jcp.21869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Korzus E., Nagase H., Rydell R. and Travis J. (1997) The mitogen-activated protein kinase and JAK-STAT signaling pathways are required for an oncostatin M-responsive element-mediated activation of matrix metalloproteinase 1 gene expression. J. Biol. Chem. 272, 1188–1196 10.1074/jbc.272.2.1188 [DOI] [PubMed] [Google Scholar]
- 55.Sundararaj K.P., Samuvel D.J., Li Y., Sanders J.J., Lopes-Virella M.F. and Huang Y. (2009) Interleukin-6 released from fibroblasts is essential for up-regulation of matrix metalloproteinase-1 expression by U937 macrophages in coculture: cross-talking between fibroblasts and U937 macrophages exposed to high glucose. J. Biol. Chem. 284, 13714–13724 10.1074/jbc.M806573200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsareva S.A., Moriggl R., Corvinus F.M., Wiederanders B., Schutz A., Kovacic B. et al. (2007) Signal transducer and activator of transcription 3 activation promotes invasive growth of colon carcinomas through matrix metalloproteinase induction. Neoplasia 9, 279–291 10.1593/neo.06820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zugowski C., Lieder F., Muller A., Gasch J., Corvinus F.M., Moriggl R. et al. (2011) STAT3 controls matrix metalloproteinase-1 expression in colon carcinoma cells by both direct and AP-1-mediated interaction with the MMP-1 promoter. Biol. Chem. 392, 449–459 10.1515/bc.2011.038 [DOI] [PubMed] [Google Scholar]
- 58.Cutler S.J., Doecke J.D., Ghazawi I., Yang J., Griffiths L.R., Spring K.J. et al. (2017) Novel STAT binding elements mediate IL-6 regulation of MMP-1 and MMP-3. Sci. Rep. 7, 8526 10.1038/s41598-017-08581-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hughes K., Wickenden J.A., Allen J.E. and Watson C.J. (2012) Conditional deletion of Stat3 in mammary epithelium impairs the acute phase response and modulates immune cell numbers during post-lactational regression. J. Pathol. 227, 106–117 10.1002/path.3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Itoh M., Murata T., Suzuki T., Shindoh M., Nakajima K., Imai K. et al. (2006) Requirement of STAT3 activation for maximal collagenase-1 (MMP-1) induction by epidermal growth factor and malignant characteristics in T24 bladder cancer cells. Oncogene 25, 1195–1204 10.1038/sj.onc.1209149 [DOI] [PubMed] [Google Scholar]
- 61.Gollob J.A., Murphy E.A., Mahajan S., Schnipper C.P., Ritz J. and Frank D.A. (1998) Altered interleukin-12 responsiveness in Th1 and Th2 cells is associated with the differential activation of STAT5 and STAT1. Blood 91, 1341–1354 [PubMed] [Google Scholar]
- 62.Gollob J.A., Schnipper C.P., Murphy E.A., Ritz J. and Frank D.A. (1999) The functional synergy between IL-12 and IL-2 involves p38 mitogen-activated protein kinase and is associated with the augmentation of STAT serine phosphorylation. J. Immunol. 162, 4472–4481 [PubMed] [Google Scholar]
- 63.Gollob J.A., Veenstra K.G., Jyonouchi H., Kelly A.M., Ferrieri P., Panka D.J. et al. (2000) Impairment of STAT activation by IL-12 in a patient with atypical mycobacterial and staphylococcal infections. J. Immunol. 165, 4120–4126 10.4049/jimmunol.165.7.4120 [DOI] [PubMed] [Google Scholar]
- 64.Qin H., Moellinger J.D., Wells A., Windsor L.J., Sun Y. and Benveniste E.N. (1998) Transcriptional suppression of matrix metalloproteinase-2 gene expression in human astroglioma cells by TNF-alpha and IFN-gamma. J. Immunol. 161, 6664–6673 [PubMed] [Google Scholar]
- 65.Ma Z., Qin H. and Benveniste E.N. (2001) Transcriptional suppression of matrix metalloproteinase-9 gene expression by IFN-gamma and IFN-beta: critical role of STAT-1alpha. J. Immunol. 167, 5150–5159 10.4049/jimmunol.167.9.5150 [DOI] [PubMed] [Google Scholar]
- 66.Ihle J.N. (2001) The Stat family in cytokine signaling. Curr. Opin. Cell Biol. 13, 211–217 10.1016/S0955-0674(00)00199-X [DOI] [PubMed] [Google Scholar]
- 67.Schindler C.W. (2002) Series introduction. JAK-STAT signaling in human disease. J. Clin. Invest. 109, 1133–1137 10.1172/JCI0215644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitola S., Strasly M., Prato M., Ghia P. and Bussolino F. (2003) IL-12 regulates an endothelial cell-lymphocyte network: effect on metalloproteinase-9 production. J. Immunol. 171, 3725–3733 10.4049/jimmunol.171.7.3725 [DOI] [PubMed] [Google Scholar]
- 69.Ma Z., Chang M.J., Shah R.C. and Benveniste E.N. (2005) Interferon-gamma-activated STAT-1alpha suppresses MMP-9 gene transcription by sequestration of the coactivators CBP/p300. J. Leukoc. Biol. 78, 515–523 10.1189/jlb.0205112 [DOI] [PubMed] [Google Scholar]
- 70.Sanceau J., Boyd D.D., Seiki M. and Bauvois B. (2002) Interferons inhibit tumor necrosis factor-alpha-mediated matrix metalloproteinase-9 activation via interferon regulatory factor-1 binding competition with NF-kappa B. J. Biol. Chem. 277, 35766–35775 10.1074/jbc.M202959200 [DOI] [PubMed] [Google Scholar]
- 71.Coccia E.M., Passini N., Battistini A., Pini C., Sinigaglia F. and Rogge L. (1999) Interleukin-12 induces expression of interferon regulatory factor-1 via signal transducer and activator of transcription-4 in human T helper type 1 cells. J. Biol. Chem. 274, 6698–6703 10.1074/jbc.274.10.6698 [DOI] [PubMed] [Google Scholar]