Abstract
Objectives:
To compare the visual and anatomical outcomes after photodynamic therapy (PDT) and navigated microsecond laser (nMSL) for chronic central serous chorioretinopathy (CSCR).
Methods:
This retrospective study included eyes with chronic CSCR who underwent either PDT or nMSL with a minimum of 6 months’ follow-up. Eyes with a history of treatment with any other modalities in the past or during 6 months post PDT or microsecond laser follow-up were excluded. Primary outcome measures included change in best-corrected visual acuity (BCVA) and central macular thickness (CMT). Secondary outcome measures included changes in subretinal fluid, hyper-reflective foci, cystic spaces, subfoveal choroidal thickness, and outer retinal structure integrity.
Results:
Forty-five eyes of 39 subjects (PDT group—23 eyes, nMSL group—22 eyes) with chronic CSCR were analyzed. At 6 months’ follow-up, the nMSL group had significantly higher improvement in visual acuity compared to the PDT group (0.12 ± 0.24 vs −0.02 ± 0.20 (p = 0.039)). Reduction in central macular thickness was significantly higher in nMSL group compared to the PDT (85.5 ± 93.26 vs 24.47 ± 73.18 microns (p = 0.02)). Thirteen (59%) eyes in nMSL group had complete resolution of the SRF at 6 months compared to 5 (21.7%) eyes in PDT group. There was no significant difference in rest of the anatomical features between the groups.
Conclusions:
nMSL seems to be superior over PDT in improving visual and anatomical outcomes at 6 months and can be considered as a cheap alternative to PDT in treatment of CSCR.
Introduction
Chronic central serous chorioretinopathy (CSCR) is characterized by disseminated and extensive areas of granular hyperfluorescence on fundus fluorescein angiogram along with generalized areas of retinal pigment epithelial (RPE) atrophy, persistent subretinal fluid (SRF), unresolved intra-retinal cystic spaces, and extensive outer retinal damage on optical coherence tomography (OCT) [1–6]. In the last two decades, several treatment modalities for chronic CSCR have been investigated. These have included pharmacologic therapy, continuous-wave conventional laser thermal photocoagulation, photodynamic therapy (PDT), anti-vascular endothelial growth factor (anti-VEGF), and subthreshold micropulse diode laser [7–12].
PDT has been the mainstay of treatment for CSCR, especially for chronic CSCR. However, it is associated with various complications such as RPE atrophy, choroidal ischemia, and rarely choroidal neovascularization [13]. In presence of preexisting RPE atrophy and choroidal ischemia, PDT could be causing additional damage. Other associated limitations with PDT are unavailability and high cost.
In recent past, micropulse laser has been shown to be effective in acute as well as chronic CSCR, with stabilization of visual acuity, improvement in retinal sensitivity, and more importantly, without any complications [10, 12]. While there are comparison studies involving PDT, focal laser, and anti-VEGF efficiencies against one another [11, 14] there is a paucity of data relating the effectiveness of PDT and subthreshold laser in treatment of CSCR.
The purpose of this study was to compare anatomical outcomes using various spectral domain OCT (SD-OCT) parameters between PDT and micropulse laser in the management of chronic CSCR.
Methods
A retrospective study of patients who underwent either PDT or navigated subthreshold laser for the management of chronic CSCR, from January 2013 to February 2016 at the L. V. Prasad Eye Institute, Hyderabad, India was carried out. The study adhered to the Helsinki declaration, and Institutional Review Board approval (L. V. Prasad Eye Institute Ethics Committee) was obtained beforehand.
All clinically diagnosed cases of chronic CSC had additional SD-OCT (Carl Zeiss Meditec AG, Jena, Germany), fundus fluorescein angiography, indocyanine green angiography, and fundus autofluorescence imaging (Carl Zeiss Meditec AG), which helped confirm the condition and also gave additional information regarding the retinal and choroidal status. Patients who underwent microsecond subthreshold laser had their fluorescein angiographs on the Navilas® system (OD-OS GmbH, Teltow, Germany), which was used for treatment as well.
The inclusion criteria were as follows: (1) age ≥ 18 years; (2) vision complaints for more than 3 months’ duration due to persistent CSCR diagnosed by the presence of SRF at the fovea and verified by SD-OCT; (3) visual complaint for a duration of <3 months with clinically proven signs of chronicity like persistent intra-retina cystic and RPE changes provided that they had persistent SRF at the fovea verified by SD-OCT; (4) a minimum follow-up of 6 months.
The exclusion criteria were as follows: (1) a history of treatment with other modalities like anti-VEGF, focal laser, and eplerenone in past or during 6 months’ follow-up after PDT or microsecond laser; (2) incomplete or poor quality of data on the duration of disease, history of treatment, and/or OCT findings; (3) comorbidity due to other vitreo-retinal/macular disorders; (4) history of intraocular procedure 6 months before or after PDT/microsecond treatment; (5) patients on systemic medication such as pioglitazones, which could cause macular edema; and (6) spherical equivalent > ± 3 D.
A detailed ocular history (onset of symptoms and previous treatment), the demography (age and sex), laterality, and systemic comorbidities (diabetes and hypertension) were noted along with the best-corrected distance visual acuity (BCVA; Snellen’s chart), and spherical equivalent of refractive status of the eye.
Photodynamic therapy
Trained consultants at the institute had treated areas of choroidal hyper-permeability at the posterior pole on indocyanine angiography (ICG) patients with half fluence PDT (25 J/cm2) in which a normal dose of vertepofin (Visudyne; Novartis AG, Basel, Switzerland) had been infused to patients over 8 min followed by 83 s delivery of laser energy at 693 nm, 10 min after initiating the infusion. These data (i.e., dose, fluence, duration, and spot size) were retrieved and recorded for each eye.
Microsecond laser
Eyes for microsecond laser had been treated by the qualified consultants placing confluent spots over the area of focal leak on the earliest phase of fundus fluorescein angiography on Navilas® system using 5% duty cycle with 100 micron spot size with 200 ms envelope. Thirty percent of threshold laser burn power was used.
Spectral domain OCT
The SD-OCT scans for the baseline, 3, and 6 months post treatment obtained by using Zeiss Cirrus HD-OCT (Carl Zeiss Meditec AG) were reviewed. The scanning protocol used had included HD5 line raster, HD single line raster, enhanced depth imaging (EDI), and macular cube.
Central macular thickness (CMT) was determined and analyzed automatically by OCT software as the retinal thickness area in the central 1 mm circle of the ETDRS fields [15]. Using a virtual caliper the subfoveal choroidal thickness (SFCT) was measured from EDI scan by taking the vertical distance between the hyper-reflective line of Bruch’s membrane and the innermost hyper-reflective line of the chorioscleral interface, and the average of the two scans (vertical and horizontal) was regarded as the SFCT. Measurement of large choroidal vessel thickenss was made as the perpendicular distance from the innermost aspect of the large choroidal vessel layer to the chorioscleral interface at the fovea or close to 750 μm nasal and temporal to the fovea. In the same manner, neurosensory detachment (NSD) height was measured as the distance between the highest point of the NSD and the inner border of the RPE/Bruch membrane complex. The presence of photoreceptor inner and outer segment (IS/OS) granulation and external limiting membrane (ELM) disruption were graded independently from high-quality scans into percentages [16]. The IS/OS junction and ELM disruption was measured as a percentage (0–1000%) of disruption in 500 micron section in either direction from fovea, in both horizontal and vertical scans. An average of two measurements were considered for statistical analysis. Two masked observers performed this calculation with interobserver concordance of 0.94. Pigment epithelial detachment was defined as a dome-shaped protrusion of the RPE within the sub-RPE hyporeflective space and its height was measured with a caliper from the apex to the Bruch’s membrane border. Irregular RPE, RPE bump, and RPE defect all were considered as part of altered RPE integrity and were reported present or absent.
The existence of PED and altered RPE integrity were assessed and the number of hyper-reflective foci (HF) was counted at the leakage site. HF were manually counted using a horizontal scan within the 500 micron area from the center of the fovea. HF were defined as discrete and well-circumscribed dots whose HF was equal to or higher than the RPE band. HF clumps in the SD-OCT scan that were spatially matched to typical hard exudates and larger than 50 microns in size were excluded.
Statistical analysis
Descriptive statistics were calculated for each variable. Visual acuity was converted to the logarithm of the minimum angle of resolution (logMAR) for statistical analysis. Statistical analyses were calculated using GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, USA). To analyze the change in BCVA and CMT at baseline, 3 and 6 months’ follow-up, paired t-test was used. A P-value of 0.05 was considered statistically significant.
Outcome measures
Primary outcome measures included change in BCVA and CMT. Changes in SRF, HF, cystic spaces, CT, IS/OS, and ELM integrity were considered as the secondary outcome measures.
Results
Forty-five eyes of 39 subjects with chronic CSCR fulfilling the inclusion and exclusion criteria were analyzed in this study. Male patients were predominant, when compared to the females 7 (15.15%). Twenty-three eyes of 19 subjects were included in the PDT group, whereas 22 eyes of 20 subjects constituted the navigated microsecond laser (nMSL) group. The mean age (±SD) of the patients at presentation was 49.5 ± 19.8 (36–64) years. The PDT group was older than the nMSL group (50.1 ± 18.2 vs 48.9 ± 19.1 years; p = 0.04). There was no significant difference between the reported duration of the disease between the two groups (42.0 ± 79.9 vs 43.76 ± 101.1 months; p = 0.89). None of the treated eyes in the two groups had prior treatment. Clinical characteristics of both groups are shown in Table 1.
Table 1.
Clinical characteristics of study subjects in two groups
| Microsecond group | PDT group | |||||
|---|---|---|---|---|---|---|
| Age (years) | 48.9 ± 7.5 | 50.1 ± 7.5 | ||||
| Duration of symptoms (months) | 43.727 ± 46.4 | 39.0 ± 35.5 | ||||
| Number of eyes (patients) | 23 (19) | 22 (20) | ||||
| Baseline | 3 months | 6 months | Baseline | 3 months | 6 months | |
| BCVA (logMAR) | 0.5 ± 0.5 | 0.4 ± 0.4 (0.5) | 0.3 ± 0.5 (0.8) | 0.5 ± 0.3 | 0.4 ± 0.3 (0.5) | 0.4 ± 0.3 (0.8) |
| CMT (µm) | 326.5 ± 140.1 | 243.4 ± 84.4 (0.4) | 253.8 ± 91.2 (0.8) | 288.1 ± 100.4 | 265.2 ± 106.5 (0.4) | 281.6 ± 108.0 (0.8) |
| PED height (µm) | 38.0 ± 74.2 | 24.4 ± 44.1 (0.8) | 16.4 ± 34.2 (0.9) | 67.9 ± 99.4 | 63.9 ± 94.7 (0.8) | 67.8 ± 97.1 (0.9) |
| SRF height (µm) | 160.5 ± 117.1 | 77.9 ± 77.4 (0.1) | 71.3 ± 81.2 (0.5) | 121.3 ± 74.7 | 92.7 ± 66.9 (0.1) | 105.6 ± 81.1 (0.5) |
| HF number | 2.4 ± 1.9 | 2.0 ± 2.4 (0.3) | 1.2 ± 1.9 (0.2) | 5.3 ± 2.2 | 3.9 ± 2.1 (0.3) | 4.4 ± 2.6 (0.2) |
| SFCT (µm) | 377.4 ± 55.8 | 356.9 ± 55.6 (0.1) | 349.5 ± 50.5 (0.2) | 412.0 ± 71.8 | 378.7 ± 63.6 (0.1) | 382.3 ± 83.1 (0.2) |
| LCVT (µm) | 125.0 ± 30.7 | 113.9 ± 22.9 (0.1) | 110.2 ± 18.9 (0.5) | 156.6 ± 66.1 | 130.5 ± 45.5 (0.1) | 144.5 ± 60.3 (0.5) |
| IS/OS percentage integrity | 0.7 ± 0.3 | 0.7 ± 0.3 (0.7) | 0.78 ± 0.3 (0.6) | 0.5 ± 0.3 | 0.4 ± 0.3 (0.7) | 0.4 ± 0.3 (0.6) |
| ELM percentage integrity | 0.9 ± 0.02 | 0.9 ± 0.02 (0.3) | 0.99 ± 0.02 (0.9) | 0.8 ± 0.2 | 5.3 ± 21.1 (0.3) | 0.8 ± 0.2 (0.91) |
BCVA best-corrected visual acuity, PED pigment epithelial detachment, SRF subretinal fluid, HF hyper-reflective foci, CMT central macular thickness, SFCT subfoveal choroidal thickness, LCVT large choroidal vessel thickness, IS/OS inner segment and outer segment junction, ELM external limiting membrane
Change during follow-up within the group
Table 1 shows the changes in various parameters at 3 and 6 months’ follow-up in both the groups. There was slight improvement in visual acuity at the 3 and 6 months’ visit in comparison to baseline in both the groups, however, this was not significant. Change in CMT, SRF, outer retinal integrity, and many other parameters on OCT were not significant at 3 and 6 months in both groups.
Comparison between nMSL and PDT group
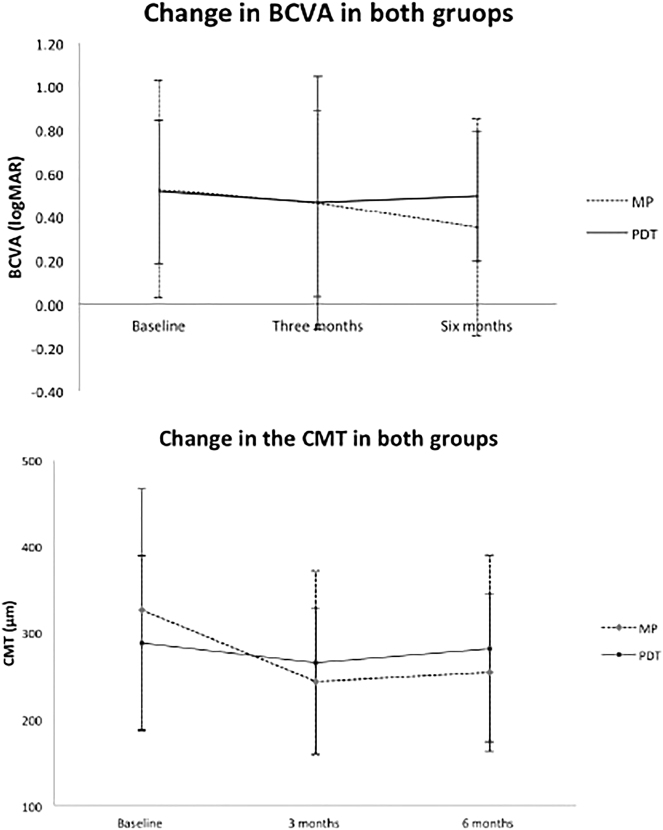
Table 2 shows the comparison between the groups in regard to the change of various parameters during follow-up visits. At the end of 6 months’ follow-up, the improvement in BCVA in the nMSL group was significantly higher as compared to the PDT group (logMAR 0.1 ± 0.2 vs −0.01 ± 0.20; p = 0.039; Fig. 1). The reduction in the CMT in eyes treated with nMSL (85.5 ± 93.26 microns) was significant when compared to eyes treated with PDT (24.47 ± 73.18 microns; p = 0.02; Fig. 1). Though the mean reduction in the SRF in the nMSL group by 46.2 ± 35.64 microns was higher when compared to 39.05 ± 68.99 microns in the PDT eyes, the difference was not significant statistically (p = 0.35). Thirteen (59.09%) eyes in nMSL group had complete resolution of the SRF at 6 months compared to 5 (21.7%) eyes in PDT group. Though nMSL group had slightly more decrease in SFCT, however, mean change in SFCT between the two groups at 6 months (46.2 ± 35.63 vs 39.05 ± 68.99 microns) was not statistically difference (p = 0.35). Neither was the reduction in the large choroidal vessel thickness significant between the PDT and nMSL eyes (20.52 ± 42.79 vs 16 ± 36.27 microns; p = 0.37) nor were the changes in the IS/OS and ELM in both groups at 6 months.
Table 2.
Comparison between two groups in regard to various outcome measures at 6 months follow-up
| Microsecond group | PDT group | P value | |
|---|---|---|---|
| Change in BCVA (logMAR) | −0.1 ± 0.2 | 0.01 ± 0.2 | 0.03 |
| Change in CMT (µm) | −85.5 ± 93.2 | −24.4 ± 73.1 | 0.02 |
| Change in SFCT (µm) | −46.2 ± 35.6 | −39.0 ± 68.9 | 0.3 |
| Change in LCVT (µm) | −16 ± 36.2 | −20.5 ± 42.7 | 0.3 |
| Change in IS/OS percentage integrity | −0.009 ± 0.06 | −0.02 ± 0.1 | 0.3 |
| Change in ELM percentage integrity | 0 ± 0 | 0.01 ± 0.05 | 0.1 |
BCVA best-corrected visual acuity, CMT central macular thickness, SFCT subfoveal choroidal thickness, LCVT large choroidal vessel thickness, IS/OS inner segment and outer segment junction, ELM external limiting membrane
Fig. 1.
Graphs showing changes in best-corrected visual acuity (BCVA) and central macular thickness (CMT) at different time points in both groups
Representative cases are shown as Figs. 2 and 3.
Fig. 2.
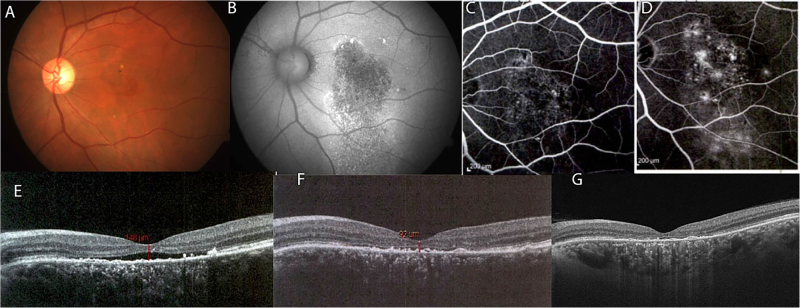
A 50-year-old male presented with a history of gradual vision loss in his right eye for last 8 years. On examination his best-corrected visual acuity in his right eyes was 20/200. Color fundus photograph showed areas of retinal pigment epithelial (RPE) atrophy with pigmentary changes (a). Autofluorescence (b) and fluorescein angiography confirmed diffuse loss of RPE in early phase (c) with later leakage near fovea in late phase (d). Spectral domain optical coherence tomography (SD-OCT) revealed subfoveal neurosensory detachment with intra-retinal cystic changes (e). Right eye underwent micropulse laser (100 mW, 100 microns size, 5% duty cycle). His visual acuity remained stable at 20/200 with improvement on SD-OCT with decrease in subretinal fluid with resolution of cystic changes at 3 (f) and 6 months (g)
Fig. 3.
A 54-year-old male presented with a history of gradual vision loss in his left eye for last 2 years. On examination his best-corrected visual acuity in his right eyes was 20/160. Color fundus photograph showed areas of retinal pigment epithelial (RPE) atrophy with pigmentary changes (a). Autofluorescence (b) and fluorescein angiography confirmed diffuse loss of RPE in early phase (c) with later leakage in late phase (d). Spectral domain optical coherence tomography (SD-OCT) revealed subfoveal neurosensory detachment (e). Right eye underwent half fluence photodynamic therapy. At 3 months’ follow-up, his visual acuity maintained at 20/160 with decrease in subretinal fluid on SD-OCT (f). At 6 months’ follow-up, there was foveal thinning with no subretinal fluid (g) with drop in visual acuity to 20/200
Discussion
Current study reports the anatomical outcomes on OCT comparing PDT and micropulse, and supports navigated micropulse laser equally or more effective. nMSL eyes had a statistically significant (p = 0.039) final BCVA of logMAR units 0.1 ± 0.2 as compared to PDT eyes −0.01 ± 0.20. CMT improved more in nMSL eyes over PDT by 85.5 ± 93.26 and 24.47 ± 73.18 (p = 0.02) microns.
Our results are slightly comparable to one similar comparison study by Ozmert et al. [17] who found subthreshold micropulse yellow wavelength laser more effective in the treatment of chronic CSC through better final BCVA as compared to PDT. In their study the micropulse laser group had a 66.7% improvement of at least five ETDRS letters as compared to 33.3% in the PDT group, however the difference was statistically insignificant (p = 0.101).
Considering the theories of pathophysiology of CSCR largely incriminating choroidal disorder with increased thickness, choriocapillary hyper-permeability, vascular congestion, and venous dilatation with exudation of serous fluid via weakened RPE to eventually cause SRF and visual loss [18–23], treatment should aim to interrupt those mechanism and cause resorption of SRF. Though there was no significant difference in choroidal parameters between the group, however, higher percentage of eyes had resolution of SRF in nMSL group compared to PDT group (59.09% vs 21.7%).
PDT with vertepofin has proven effective in causing choroidal vascular remodeling and the reduction of choroidal exudation [8, 9, 24, 25]. In an earlier study by Yanuzzi et al. [2] ICG-guided PDT had 12 of 20 (60%) eyes gaining complete resolution of SRF at 6 months mean follow-up and a study by Piccolino et al. [3] resulted in complete resolution of serous retinal detachment 1 month after treatment in 75% of the studied eyes. However, PDT is reported to have several potential serious adverse effects, including RPE changes, permanent choroidal ischemia, and secondary choroidal neovascularization [9, 26–28]. Moreover, it is a relatively invasive procedure that requires the intravenous injection of vertepofin and the ICG drugs all of which carry risk of anaphylaxis and urticarial reactions in patients [25].
Alternatively, the 810 or 577 nm subthreshold MP laser photostimulation employing low-intensity, high-density laser in a repetitive short pulses during 5–15% of duty cycle stimulate the production of intracellular anti-angiogenic and restorative biological factors without causing visible laser scars with appreciable resorption of SRF [11, 29–31]. Other studies have shown that subthreshold laser energy induces local regenerative effects, such as restoration of the RPE blood retinal barrier and increased cell adhesion [28].
The technique has proven effective in treating diabetic retinopathy and branch retinal vein occlusion [32–34], and of recent chronic CSCR with an added advantage of better retina sensitivity after treatment [24, 35]. One of the prospective non-comparative interventional study of 26 chronic CSCR with juxta-foveal leakage on FFA using subthreshold microdiode laser had 57% (n = 15) eyes gaining three or more lines of vision [36]. The treatment effect in CSCR is more robust with yellow wavelength (577 nm) laser, which produces combined absorption by both melanin and oxyhemoglobin with maximum absorption in the pigment epithelium and choriocapillaries. This carry negligible xanthophyll absorption with tissue preservation making the treatment safe to repeat in a case of recurrence or quick unresponsiveness which are common with this disease [30, 31]. A study by Chhablani et al. using Navilas® yellow laser found a complete resolution of SRF in 15 out of 16 (94%) eyes treated indicating high efficiency [37].
Limitations of the study include retrospective nature, small sample size, and only 6 months’ follow-up. Retinal sensitivity on microperimetry may have been another important parameter to compare the functional outcomes between the groups.
In conclusion, nMSL seems to be superior over PDT in improving visual and anatomical outcomes. Subthreshold microsecond laser is a cost-effective, less destructive with less potential adverse effects like RPE changes, choroidal ischemia, and neovascularization, therefore, it can be considered as a cheap alternative to PDT in treatment of CSCR. Further randomized prospective studies are warranted with larger sample size and longer follow-up are warranted to establish its role in this intriguing disease.
Summary
What was known before
PDT has been the standard of care for CSCR especially for chronic CSCR.
PDT is associated with various complications such as RPE atrophy, choroidal ischemia, and rarely choroidal neovascularization.
Subthreshold laser is effective in CSCR.
What this study adds
Microsecond laser achieved better visual and anatomical outcomes compared to PDT in chronic CSCR.
Microsecond laser can be considered as cheap alternative to PDT in treatment of CSCR.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Loo RH, Scott IU, FLYNN HW, Jr, Gass JDM, Murray TG, Lewis ML, et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina. 2002;22:19–24. doi: 10.1097/00006982-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Yannuzzi LA, Shakin JL, Fisher YL, Altomonte MA. Peripheral retinal detachments and retinal pigment epithelial atrophic tracts secondary to central serous pigment epitheliopathy. Ophthalmology. 1984;91:1554–72. doi: 10.1016/S0161-6420(84)34117-3. [DOI] [PubMed] [Google Scholar]
- 3.Piccolino FC, Borgia L. Central serous chorioretinopathy and indocyanine green angiography. Retina. 1994;14:231–42. doi: 10.1097/00006982-199414030-00008. [DOI] [PubMed] [Google Scholar]
- 4.Lee H, Lee J, Chung H, Kim HC. Baseline spectral domain optical coherence tomographic hyperreflective foci as a predictor of visual outcome and recurrence for central serous chorioretinopathy. Retina. 2016;36:1372–80. doi: 10.1097/IAE.0000000000000929. [DOI] [PubMed] [Google Scholar]
- 5.Maruko I, Iida T, Ojima A, Sekiryu T. Subretinal dot-like precipitates and yellow material in central serous chorioretinopathy. Retina. 2011;31:759–65. doi: 10.1097/IAE.0b013e3181fbce8e. [DOI] [PubMed] [Google Scholar]
- 6.Vasconcelos H, Marques I, Santos AR, Melo P, Pires I, Figueira J, et al. Long-term chorioretinal changes after photodynamic therapy for chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2013;251:1697–705. doi: 10.1007/s00417-013-2270-2. [DOI] [PubMed] [Google Scholar]
- 7.Chhablani J, Rani PK, Mathai A, Jalali S, Kozak I. Navigated focal laser photocoagulation for central serous chorioretinopathy. Clin Ophthalmol. 2014;8:1543–7. doi: 10.2147/OPTH.S67025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicoló M, Eandi CM, Alovisi C, Grignolo FM, Traverso CE, Musetti D, et al. Half-fluence versus half-dose photodynamic therapy in chronic central serous chorioretinopathy. Am J Ophthalmol. 2014;157:1033–7. doi: 10.1016/j.ajo.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Piccolino FC, Eandi CM, Ventre L, De La Longrais RCR, Grignolo FM. Photodynamic therapy for chronic central serous chorioretinopathy. Retina. 2003;23:752–63. doi: 10.1097/00006982-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Roisman L, Magalhaes FP, Lavinsky D, Moraes N, Hirai FE, Cardillo JA, et al. Micropulse diode laser treatment for chronic central serous chorioretinopathy: a randomized pilot trial. Ophthalmic Surg Lasers Imaging Retina. 2013;44:465–70. doi: 10.3928/23258160-20130909-08. [DOI] [PubMed] [Google Scholar]
- 11.Verma L, Sinha R, Venkatesh P, Tewari H. Comparative evaluation of diode laser versus argon laser photocoagulation in patients with central serous retinopathy: a pilot, randomized controlled trial [ISRCTN84128484] BMC Ophthalmol. 2004;4:15. doi: 10.1186/1471-2415-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yadav NK, Jayadev C, Mohan A, Vijayan P, Battu R, Dabir S, et al. Subthreshold micropulse yellow laser (577 nm) in chronic central serous chorioretinopathy: safety profile and treatment outcome. Eye (Lond) 2015;29:258–64. doi: 10.1038/eye.2014.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong KH, Lau KP, Chhablani J, Tao Y, Li Q, Wong IY. Central serous chorioretinopathy: what we have learnt so far. Acta Ophthalmol. 2016;94:321–5. doi: 10.1111/aos.12779. [DOI] [PubMed] [Google Scholar]
- 14.Chen SN, Hwang JF, Tseng LF, Lin CJ. Subthreshold diode micropulse photocoagulation for the treatment of chronic central serous chorioretinopathy with juxtafoveal leakage. Ophthalmology. 2008;115:2229–34. doi: 10.1016/j.ophtha.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Group ETDRSR. Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification: ETDRS report number 10. Ophthalmology. 1991;98:786–806. doi: 10.1016/S0161-6420(13)38012-9. [DOI] [PubMed] [Google Scholar]
- 16.Chhablani JK, Kim JS, Cheng L, Kozak I, Freeman W. External limiting membrane as a predictor of visual improvement in diabetic macular edema after pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2012;250:1415–20. doi: 10.1007/s00417-012-1968-x. [DOI] [PubMed] [Google Scholar]
- 17.Özmert E, Demirel S, Yanık Ö, Batıoğlu F. Low-Fluence Photodynamic Therapy versus Subthreshold Micropulse Yellow Wavelength Laser in the Treatment of Chronic Central Serous Chorioretinopathy. Journal of Ophthalmology. 2016;2016:3513794. doi: 10.1155/2016/3513794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donald J, Gass M. Pathogenesis of disciform detachment of the neuroepithelium: II. Idiopathic central serous choroidopathy. Am J Ophthalmol. 1967;63:587/515–615/543. doi: 10.1016/0002-9394(67)90028-1. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi K, Hasegawa Y, Tokoro T. Indocyanine green angiography of central serous chorioretinopathy. Int Ophthalmol. 1986;9:37–41. doi: 10.1007/BF00225936. [DOI] [PubMed] [Google Scholar]
- 20.Prünte C. Indocyanine green angiographic findings in central serous chorioretinopathy. Int Ophthalmol. 1995;19:77–82. doi: 10.1007/BF00133176. [DOI] [PubMed] [Google Scholar]
- 21.Prünte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996;121:26–34. doi: 10.1016/S0002-9394(14)70531-8. [DOI] [PubMed] [Google Scholar]
- 22.Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29:1469–73. doi: 10.1097/IAE.0b013e3181be0a83. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y, Kang S, Bai K. Choroidal thickness in both eyes of patients with unilaterally active central serous chorioretinopathy. Eye. 2011;25:1635–40. doi: 10.1038/eye.2011.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yannuzzi LA, Slakter JS, Gross NE, Spaide RF, Costa DL, Huang SJ, et al. Indocyanine green angiography‐guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2003;23:288–98. doi: 10.1097/00006982-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Chan W, Lam D, Lai T, Tam B, Liu D, Chan C. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol. 2003;87:1453–8. doi: 10.1136/bjo.87.12.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Özmert E, Demirel S, Yanık Ouml, Batıoğlu F. Low-fluence photodynamic therapy versus subthreshold micropulse yellow wavelength laser in the treatment of chronic central serous chorioretinopathy. J Ophthalmol. 2016;2016:3513794. doi: 10.1155/2016/3513794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colucciello M. Choroidal neovascularization complicating photodynamic therapy for central serous retinopathy. Retina. 2006;26:239–42. doi: 10.1097/00006982-200602000-00027. [DOI] [PubMed] [Google Scholar]
- 28.Breukink MB, Downes SM, Querques G, van Dijk EH, den Hollander AI, Blanco-Garavito R, et al. Comparing half-dose photodynamic therapy with high-density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy (the PLACE trial): study protocol for a randomized controlled trial. Trials. 2015;16:419. doi: 10.1186/s13063-015-0939-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Behnia M, Khabazkhoob M, Aliakbari S, Abadi AE, Hashemi H, Pourvahidi P. Improvement in visual acuity and contrast sensitivity in patients with central serous chorioretinopathy after macular subthreshold laser therapy. Retina. 2013;33:324–8. doi: 10.1097/IAE.0b013e3182670fa3. [DOI] [PubMed] [Google Scholar]
- 30.Chhablani J, Rani PK, Mathai A, Jalali S, Kozak I. Navigated focal laser photocoagulation for central serous chorioretinopathy. Clin Ophthalmol. 2014;8:1543. doi: 10.2147/OPTH.S67025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owens SL. Indocyanine green angiography. Br J Ophthalmol. 1996;80:263. doi: 10.1136/bjo.80.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ambiya V, Goud A, Mathai A, Rani PK, Chhablani J. Microsecond yellow laser for subfoveal leaks in central serous chorioretinopathy. Clin Ophthalmol. 2016;10:1513. doi: 10.2147/OPTH.S112431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inagaki K, Ohkoshi K, Ohde S, Deshpande GA, Ebihara N, Murakami A. Subthreshold micropulse photocoagulation for persistent macular edema secondary to branch retinal vein occlusion including best-corrected visual acuity greater than 20/40. J Ophthalmol. 2014;2014:251257. doi: 10.1155/2014/251257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohkoshi K, Yamaguchi T. Subthreshold micropulse diode laser photocoagulation for diabetic macular edema in Japanese patients. Am J Ophthalmol. 2010;149:133–9. doi: 10.1016/j.ajo.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Roisman L, Magalhães FP, Lavinsky D, Moraes N, Hirai FE, Cardillo JA, et al. Micropulse diode laser treatment for chronic central serous chorioretinopathy: a randomized pilot trial. Ophthalmic Surg Lasers Imaging Retina. 2013;44:465–70. doi: 10.3928/23258160-20130909-08. [DOI] [PubMed] [Google Scholar]
- 36.Lai TY, Chan WM, Li H, Lai RY, Liu DT, Lam DS. Safety enhanced photodynamic therapy with half dose verteporfin for chronic central serous chorioretinopathy: a short term pilot study. Br J Ophthalmol. 2006;90:869–74. doi: 10.1136/bjo.2006.090282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ambiya V, Goud A, Mathai A, Rani PK, Chhablani J. Microsecond yellow laser for subfoveal leaks in central serous chorioretinopathy. Clin Ophthalmol. 2016;10:1513–9. doi: 10.2147/OPTH.S112431. [DOI] [PMC free article] [PubMed] [Google Scholar]