Abstract
Serotonin (5-HT) is one of the best-studied modulatory neurotransmitters with ubiquitous presynaptic release and postsynaptic reception. 5-HT has been implicated in a wide variety of brain functions, ranging from autonomic regulation, sensory perception, feeding and motor function to emotional regulation and cognition. The role of this neuromodulator in neuropsychiatric diseases is unquestionable with important neuropsychiatric medications, e.g., most antidepressants, targeting this system. Importantly, 5-HT modulates neurodevelopment and changes in its levels during development can have life-long consequences. In this mini-review, we highlight that exposure to both low and high serotonin levels during the perinatal period can lead to behavioral deficits in adulthood. We focus on three exogenous factors that can change 5-HT levels during the critical perinatal period: dietary tryptophan depletion, exposure to serotonin-selective-reuptake-inhibitors (SSRIs) and poor early life care. We discuss the effects of each of these on behavioral deficits in adulthood.
Keywords: serotonin, development, SSRI, tryptophan depletion, perinatal, maternal separation
Introduction
Serotonin (5-hydroxytryptamine, 5-HT) is a classic and well-studied neuromodulator. In addition to being implicated in various functions ranging from autonomic regulation to emotions in the adult, 5-HT also has a key role in neurodevelopment. Indeed, in the mammalian brain, 5-HT is one of first neurotransmitters to emerge. In rodents, serotonergic neurons appear on embryonic day (E) 12 (Lauder and Bloom, 1974), and start releasing 5-HT at E13 (Lidov and Molliver, 1982a,b; Lambe et al., 2000). Levels of 5-HT peak during the first postnatal week, declining thereafter to adult levels by postnatal day (P) 15 (Hohmann et al., 1988). The expression of 5-HT receptors starts between E12 and E17 with variations and shifts across the different subtypes and regions (Waeber et al., 1994; Beique et al., 2004; Bonnin et al., 2006; for review: Booij et al., 2015).
In humans, 5-HT neurons start to appear when the embryo is 5 weeks old and proliferate until gestational week 10 (Sundstrom et al., 1993; Levallois et al., 1997; Gingrich et al., 2017). Levels of 5-HT increase during the first 2 years of age and slowly decline to reach adult levels by age 5 (Sodhi and Sanders-Bush, 2004).
Importantly, 5-HT is involved in neural crest stem cell migration and proliferation (Vichier-Guerre et al., 2017). Notably, 5-HT can shape neuronal microcircuitry by acting on Reelin secretion, a protein involved in neuronal migration and positioning during development (Chameau et al., 2009). 5-HT is also critical in cell survival, growth and differentiation (Lavdas et al., 1997; Fricker et al., 2005; Khozhai and Otellin, 2012) as well as synaptogenesis (Khozhai and Otellin, 2012). For reviews see (Gaspar et al., 2003; Daubert and Condron, 2010).
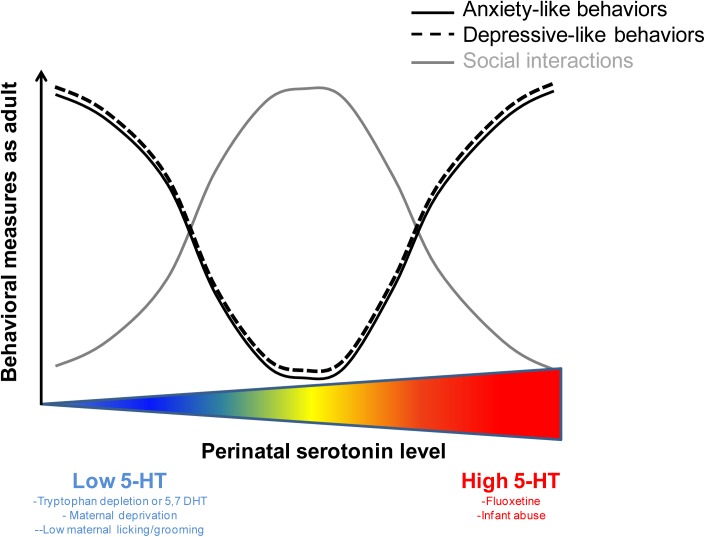
Plasticity during the perinatal period is essential for the developing brain to adapt to a changing environment but opens a window where external factors can derail neuronal circuits and lead to maladaptive behaviors. This perinatal time window is a critical period when serotonergic activity can shape the development of neuronal circuitry and specifically emotional neurocircuitry (Lauder, 1990; Brummelte et al., 2017). In addition to genetic variants in key serotonergic genes [e.g., SERT, MAO; see (Murphy et al., 2008; Suri et al., 2014)], several exogenous factors change 5-HT levels during development: stress (Papaioannou et al., 2002), physical abuse (Raineki et al., 2015; Rincon-Cortes et al., 2015), food intake (Zhang et al., 2006; Serfaty et al., 2008; Zoratto et al., 2013), pharmaceutical and recreational drugs (Xu et al., 2004; Suri et al., 2014) as well as maternal inflammation (Goeden et al., 2016; St-Pierre et al., 2016) and maternal separation (Shannon et al., 2005). Importantly, manipulations that either increase or decrease 5-HT levels during development have been shown to lead to behavioral deficits in the adult (Table 1). Together, these studies suggest that optimal levels of 5-HT must be maintained during development and that any deviations from these optimal levels, in either direction, can lead to long-lasting behavioral deficits (Figure 1). In this mini-review, we focus on three distinct exogenous factors, diet, pharmacological exposure and early-life care, which affect serotonin levels during the perinatal period and have behavioral consequences in adulthood.
Table 1.
Manipulations of the serotonergic system during development lead to behavioral deficits observed during adulthood: Examples of diet, pharmacological agents and early-life maternal care effects.
| Treatment | Effect on 5-HT signaling | Species and Sex | Age of exposure | Behavior change (measured in adulthood) | Reference | |
|---|---|---|---|---|---|---|
| Diet-induced | Tryptophan depletion | Decrease | Mice - 
|
P0-P8 | Low break point in progressive ratio Deficits in approach-avoidance conflict paradigms |
Zoratto et al., 2013 |
| Tryptophan depletion | Decrease | Rat - 
|
P1-P28 | Higher immobility in the forced-swim-test Less time in open arms of the elevated plus maze |
Zhang et al., 2006 | |
| Pharmacological: Neurotoxin or tryptophan hydroxylase inhibitor | 5,7 DHT | Decrease | Rat - 
|
P3 | Reduced locomotor activity Reduced social interaction Increased ultrasonic vocalization in fear-conditioning |
Rok-Bujko et al., 2012 |
| PCPA | Decrease | Rat - 
|
P10-20 | Delayed extinction in interchangeable mazes task Increased number of errors in eight-arm radial maze |
Mazer et al., 1997 | |
| Pharmacological: SSRI | Fluoxetine | Increase | Rat -  – – 
|
E6- E20 | Transient delay in motor development Improvements in the water maze and passive avoidance tests |
Bairy et al., 2007 |
| Fluoxetine | Increase | Rat -  – – 
|
E11-birth | Increased anxiety-like behavior in the novelty-suppressed feeding test, the footshock-induced conditioned place aversion test and in the elevated plus maze | Olivier et al., 2011 | |
| Fluoxetine | Increase | Mice -  – – 
|
P2/P4- P21 | Decreased exploratory behavior Increased anxiety-like behavior in the novelty-suppressed-feeding paradigm Impaired performance in active avoidance |
Ansorge et al., 2004; Yu et al., 2014 | |
| Fluoxetine | Increase | Mice -  – – 
|
P2- P21 | Reduced aggression | Yu et al., 2014 | |
| Fluoxetine | Increase | Rat - 
|
10 days during infancy | Increased immobility in the forced-swim-test | Rincon-Cortes et al., 2015 | |
| Fluoxetine | Increase | Mice -  – – 
|
P2-P11 | Exploratory deficits Increased latency to feed in the novelty-suppressed feeding test Increased escape latency in the shock-avoidance test Decreased sucrose consumption Increased immobility time in the forced-swim-test |
Rebello et al., 2014 | |
| Early-life maternal care Maternal care |
Low maternal licking/ grooming | Decrease | Rat -  – – 
|
Early-life | Impaired stress response | Francis et al., 1999; Weaver et al., 2004; Hellstrom et al., 2012 |
| Infant abuse (Odor-shock conditioning) | Increase | Rat - 
|
P8-P12 | Deficits in social behavior Increased immobility in the forced-swim-test |
Rincon-Cortes et al., 2015 | |
| Maternal deprivation (maternal separation) | Increase 5-HT2 receptor function | Rat - 
|
P2-P14 | Increased 5-HT2R agonist-induced head-shake behavior Increased anxiety-like behavior in open field test and the elevated plus maze (blocked by 5-HT2R antagonist) |
Benekareddy et al., 2010, 2011 | |
| Maternal deprivation (nursery reared) | Lower 5-HIAA levels | Monkey - 
|
From 24h post-birth | Increased alcohol consumption | Huggins et al., 2012 | |
| Maternal deprivation (maternal rejection) | Lower 5-HIAA levels | Monkey - 
|
From birth | Increased scratching (indicator of anxiety-like behaviors) Increased solitary play |
Maestripieri et al., 2006 | |
Postnatal day (P) – Embryonic day (E); 5,7-dihydroxytryptamine (5,7 DHT) and parachlorophenylalanine (PCPA).  = Female;
= Female;  = Male.
= Male.
FIGURE 1.
The relation between perinatal levels of serotonin and behavioral performance as an adult is non-linear. U-shapped and inversed U-shape curves illustrating the optimal levels of perinatal serotonin sustaining normal behavioral performance in adulthood. Deviation from these optimal levels (either above or below) can lead to behavioral deficits in the adult.
Diet-Induced Disruption of 5-Ht Synthesis: Tryptophan Depletion
Tryptophan is a 5-HT precursor and reducing its availability substantially reduces 5-HT synthesis (Biggio et al., 1974; Moja et al., 1989; Fadda et al., 2000). Since tryptophan cannot be internally synthesized, diets low in tryptophan have been used to decrease 5-HT synthesis.
For ethical reasons, tryptophan depletion studies in humans have only been performed in adults. Such diets have been found to induce depressive symptoms in healthy individuals with a family history of depression and lead to relapse in depressed patients who were previously treated with selective-serotonin-reuptake-inhibitors (SSRIs) (Benkelfat et al., 1994; Delgado et al., 1999; Klaassen et al., 1999; Quintin et al., 2001). For an in-depth review of the behavioral consequences of acute, adult, tryptophan depletion in humans see (Faulkner and Deakin, 2014).
In rodents, only a few studies have examined the long-term effects of low tryptophan diet during early development. Early studies (Segall and Timiras, 1976; Segall et al., 1983) found that female rats starting a diet low in tryptophan at an early developmental age (weaning age-3 weeks) displayed a delay in growth, ovarian function and sexual maturation. Those effects were suggested to be related to low 5-HT in the pituitary-pineal glands. More recently, administration of a low tryptophan diet to lactating dams was found to reduce maternal care. Importantly, this intervention also induced anxiety-like and anhedonia-related behaviors in the adult offspring (Zoratto et al., 2013). Moreover, early life administration of a maize-based diet, containing approximately 20% of tryptophan of regular diets led to increased immobility in the forced-swim-test, reduced time in the open arms of the elevated plus maze, decreased neurogenesis and abnormal dendritic development in the dentate gyrus at 4 weeks of age (Serfaty et al., 2008).
Tryptophan depletion and pharmacologically disrupting 5-HT synthesis using a selective neurotoxin, 5,7-dihydroxytryptamine (5,7 DHT), greatly differ given that the latter leads to permanent neuronal loss. However, the long-term effects of a low-tryptophan diet can be compared to the effects of disrupting 5-HT synthesis with 5,7 DHT. Indeed, administering this neurotoxin during development also disrupts behavior in the adult. 5,7 DHT administration at P3 in rats led to reduced locomotor activity, attenuated social interaction and increased ultrasonic vocalization during fear-conditioning (Rok-Bujko et al., 2012). In addition, depletion of serotonin (P10-20) using the tryptophan hydroxylase inhibitor (parachlorophenylalanine, PCPA) decreases dendritic density (microtubule-associated protein) in hippocampus up to P62 and produces deficits in spatial learning and delayed extinction interpreted as a deficit in normal response inhibition (Mazer et al., 1997). The effects in adulthood of a transient 5-HT disruption during development highlight the importance of precise regulation of serotonergic levels during critical developmental periods.
In summary, reducing 5-HT synthesis during perinatal life, through tryptophan depletion or 5,7 DHT administration, leads to maladaptive behavioral phenotypes including depressive-like and anxiety-like symptoms as well as defective social interactions.
Pharmacological Modifications of Perinatal 5-Ht Levels: Exposure to SSRIs
SSRIs are the most prescribed treatments for depression due to their safety and mild-to-moderate adverse effects, with several being approved for use in children and adolescents (Henry et al., 2012). Pregnancy is a risk factor for depression and SSRIs are taken by a substantial proportion of women (∼ 10%) during pregnancy (Cooper et al., 2007; Huybrechts et al., 2013) possibly exposing the fetus to increased 5-HT levels. SSRIs cross the placenta barrier (Hendrick et al., 2003; Rampono et al., 2009; Sit et al., 2011) and can also be passed to the baby via breast milk (Kristensen et al., 1999). Notably, due to the possibly devastating effects of depression, including potential suicide, discontinuation of antidepressant treatment during pregnancy is often problematic.
Because of the altricial nature of rodents, the 3rd trimester of pregnancy in humans corresponds in terms of development of the serotonergic system to the first postnatal weeks in rodents (Suri et al., 2014). This is the period during which the serotonergic system’s development is at its peak (Suri et al., 2014), which presumably makes it more vulnerable to disruption. To test the influence of SSRIs during the perinatal stage in rodents, two approaches have been taken. These are exposure to SSRIs in utero or during the early-postnatal period (Kepser and Homberg, 2015). In-depth reviews on the effects of developmental exposure to antidepressant medications can be found in (Olivier et al., 2013; Kepser and Homberg, 2015).
The offspring of pregnant rats exposed to the prototypic SSRI, fluoxetine, given orally at 12 mg/kg, from E6 to E20 showed a decrease in birth weight, a transient delay in motor development and improvements in performance in the water maze and passive avoidance tests tested post-weaning (Bairy et al., 2007). In Olivier et al. (2011), pregnant rats were injected daily with 12 mg/kg of fluoxetine from E11 until birth. Adult offspring exhibited increased anxiety-like behaviors in the novelty-suppressed feeding test, the footshock-induced conditioned place aversion test and in the elevated plus maze (Olivier et al., 2011). In Rincon-Cortes et al. (2015), daily administration of 8 mg/kg of fluoxetine to rat pups for 10 days during infancy produced depressive-like behaviors in the adult, i.e., increased immobility in the forced-swim-test (Rincon-Cortes et al., 2015). Postnatal exposure to 10 mg/kg fluoxetine from P2 to P21 in mice led to decreased exploratory behavior, increased anxiety-like behavior in the novelty-suppressed feeding paradigm and impaired active avoidance performance at adulthood (Ansorge et al., 2004; Yu et al., 2014). Furthermore, this treatment reduced aggression when the mice were tested as adults (Yu et al., 2014). Interestingly, the detrimental effects of fluoxetine were restricted to a critical period from P2 to P11 (Rebello et al., 2014). Indeed, fluoxetine administration during this period, but not after, was sufficient to cause exploratory deficits, increased latency to feed in the novelty-suppressed feeding test and increases in escape latency in the shock-avoidance test (Rebello et al., 2014). Furthermore, these mice showed depression-like behaviors with decreased sucrose consumption and increased immobility time in the forced-swim-test (Rebello et al., 2014). Interestingly, these deleterious effects were specific to SSRIs. Postnatal treatment with fluoxetine, clomipramine and citalopram all produced emotional behavioral deficits in mice while administration of the norepinephrine transporter inhibitor, desipramine, did not (Ansorge et al., 2008).
Concerning the possible neurobiological mechanisms underlying the lasting effects of fluoxetine administered during the critical P2-P11 period, modulation of the serotonergic system during this period has been shown to lead to changes in prefrontal cortex pyramidal neuron morphology and reduced excitability (Rebello et al., 2014). 5-HT2 receptors are major modulators of cortical serotonergic signaling. 5-HT2 activation results in neuronal depolarization and increases excitatory postsynaptic currents (Celada et al., 2013b). Interestingly, blocking 5-HT2 signaling concomitantly with fluoxetine administration in pups prevents the behavioral deficits observed with fluoxetine administration alone (Sarkar et al., 2014), suggesting that 5-HT2 receptors may mediate serotonergic effects during this period.
In humans, although fewer studies exist than in rodents, there is also some evidence that perinatal exposure to SSRIs can lead to behavioral deficits. In a study using Finnish national registry data, the incidence of depression by age 14.9 years in offspring exposed prenatally to SSRIs was 8.2% (N = 15,729) compared to 1.9% (N = 9,651) in the control group with psychiatric disorders but no medication (Malm et al., 2016). In addition to possible effects in mood-related development, exposure to SSRIs during development has been associated with an increased incidence of autism in children (Boukhris et al., 2015), which is congruent with the observation of hyperserotonemia in close to one third of autistic children (Hranilovic et al., 2009). An in depth review of the effects of perinatal SSRI administration in humans and animal models can be found in (Olivier et al., 2013).
In summary, increasing 5-HT signaling during a critical period of perinatal life, via exposure to SSRIs, has been shown to lead to emotional deficits in adulthood (e.g., increased anxiety-like and depressive-like behaviors).
Early-Life Maternal Care: Relationship With the Serotonergic System and Long-Term Behavioral Consequences
Emotional deficits related to poor quality of care during early-life are a major social issue. The prevalence of child maltreatment is alarmingly high (Finkelhor et al., 2009) with important societal consequences (U.S. Department of Health and Human Services, 2010). Indeed, early life experience is critical for life-long mental health as it is a period of high plasticity (Chaudhury et al., 2016). One of the major environmental inputs during early life is the caregiver. Importantly, in both humans and rodents, fear and stress responses (Routh et al., 1978; Hellstrom et al., 2012; Rincon-Cortes et al., 2015) as well as changes in mood (Vetulani, 2013) and drug consumption (Vetulani, 2013) are regulated by the quality of maternal care during early life.
Numerous studies have demonstrated the link between maternal care, serotonergic signaling and later vulnerability to mental health issues. Seminal work by Meaney and colleagues describe an increase in basal glucocorticoid levels, as well as in the secretion of glucocorticoids in response to stress in rats that were less groomed and licked by their mothers (Hellstrom et al., 2012). This effect was found to be mediated through the serotonergic system (Mitchell et al., 1990, 1992; Laplante et al., 2002; Hellstrom et al., 2012). Indeed, Hellstrom et al. (2012) suggest that 5-HT, resulting from licking/grooming, acts via the 5-HT7 receptor to activate a signaling cascade that in turn leads to the activation of transcription factors such as NGFI-A (nerve growth factor-inducible factor A) leading to demethylation of the glucocorticoid receptor promoter (Laplante et al., 2002; Hellstrom et al., 2012).
In monkeys, early-life maternal separation reduced serotonin transporter binding (Ichise et al., 2006) and lowered the levels of cerebrospinal 5-HIAA (a 5-HT metabolite) (Shannon et al., 2005; Maestripieri et al., 2006, 2009). Importantly, naturalistic maternal rejection, associated with lower cerebrospinal fluid 5-HIAA levels, was associated with anxiety-like behaviors later in life (Maestripieri et al., 2006). Furthermore, 5-HIAA in the putamen and 5-HIAA/5-HT ratio measures of 5-HT turnover in the hippocampus (and at trend levels in caudate, Substantia nigra and putamen) were significantly lower in nursery reared compared to mother-reared animals; ethanol drinking was also higher in nursery reared monkeys (Huggins et al., 2012).
In rodents, maternal separation has been shown to affect the tissue level of both 5-HT and 5-HIAA in different brain structures. Arborelius and Eklund (2007) found that maternal separation (P1-P13) modified the tissue levels of 5-HT and 5-HIAA in the dorsal raphe nucleus (both increased during long maternal separation) and in the amygdala (decreased during brief maternal separation) of adult female rats. Moreover, the 5-HIAA/5-HT ratio in the rat prefrontal cortex was decreased by maternal separation (Xue et al., 2013; Gonzalez-Mariscal and Melo, 2017). Analysis of the metabolite/monoamine turnover ratio is a useful estimator of serotonergic activity. An increase in this ratio indicates increased serotonergic turnover usually associated with higher rates of 5-HT release into the synapse (Shannon et al., 1986).
Maternal separation also affects 5-HT receptor expression in adulthood. Indeed, adult animals subjected to maternal separation display modifications of 5-HT1A receptor mRNA expression with a decrease in the dorsal raphe nucleus and an increase in the amygdala (Bravo et al., 2014). Postsynaptic 5-HT1A receptor activation in corticolimbic areas appears anxiolytic, while high density of presynaptic 5-HT1A receptors increases susceptibility to mood disorders and activation of these presynaptic receptors can reduce raphe signaling through a negative-feedback system (Celada et al., 2013a). However, an increase in post-synaptic 5-HT1A in the amygdala can also produce anxiogenic behaviors (Gonzalez et al., 1996). The behavioral endpoint of the two distinct regional alterations in 5-HT1A receptor levels may be dictated by the differential density of this receptor in each structure.
In addition, maternal separation also leads to a dysregulation of 5-HT2 receptor function in the prefrontal cortex (Benekareddy et al., 2010). Behaviorally, 5-HT2A agonist-induced head-twitch responses, a model for psychosis and hallucinations in rodents, are potentiated by maternal separation (Benekareddy et al., 2010). Moreover, maternal separation increases anxiety-like behaviors (measured in the open field and the elevated-plus-maze test) and 5-HT2 receptor expression. These effects have been shown to be blocked by treatment with the 5-HT2 antagonist, ketanserin (Benekareddy et al., 2011). Developmental physical abuse as modeled by paired odor-shock conditioning, has also been shown to increase 5-HT levels and lead to deficits in social behavior and increased immobility in the forced swim-test in the adult (Rincon-Cortes et al., 2015). Importantly, both deficits can be rescued by increasing amygdala 5-HT and suppressing corticosterone (Rincon-Cortes et al., 2015), an effect that may be paralleled to the aforementioned work of Hellstrom et al. (2012).
In summary, early life maternal separation or abuse induce modifications of serotonergic signaling (for example increased 5-HT level or increased 5-HT2 receptor expression) that are correlated with later-on maladaptive behaviors such as deficits in social interactions and anxiety-like and depressive-like behaviors in adulthood.
Discussion
In this mini-review, we highlighted the striking and enduring effects on adult behavior of both low and high 5-HT levels during the perinatal period. However, many questions remain. Notably, how do both low and high 5-HT levels during development lead to similar behavioral deficits? One hypothesis is that both can lead to a reduction in serotonergic signaling in the adult. For instance, tryptophan depletion may directly impair the development of the serotonergic system (Mazer et al., 1997) while excessive serotonergic signaling may reduce serotonergic activity/development through negative feedback. Serotonergic innervation peaks during the perinatal period. Perhaps excessive serotonin during this period falsely signals a sufficiency of serotonergic growth leading to stunted development. In fact, maternal inflammation was recently found to lead to increased levels of 5-HT in the fetal brain which subsequently inhibits serotonergic development (Goeden et al., 2016). Furthermore, the firing rate of serotonergic cells in the dorsal raphe of postnatal fluoxetine-treated animals decreases dramatically during adulthood (Teissier et al., 2015). Future experiments, taking advantage of the ability to apply opto- and chemo-genetics tools in neonatal models (Bitzenhofer et al., 2017) can further help dissect how both high and low 5-HT levels can disrupt the development of the serotonergic system and affect behavior in the adult.
This review highlights the critical period when any deviations of 5-HT levels can lead to later maladaptive behavior. For example, Rebello et al. (2014) demonstrated that vulnerability to fluoxetine is restricted to P2-P11. This apparent critical period can be explained by (1) the timeline of brain developmental processes such as proliferation of neurons and glia, migration, apoptosis, and synaptogenesis encompassing both the embryonic stages as well as the first postnatal weeks in rats (Rice and Barone, 2000) and (2) the ontogeny of serotonergic system development [for review see (Booij et al., 2015)]. Indeed, while the serotonergic system continues to mature after birth, there are also critical shifts of receptor expression and activity during this period. For example, from P6 to P19, pyramidal neurons change from being depolarized to being hyperpolarized by 5-HT through different 5-HT receptor subtypes (Beique et al., 2004).
Another question that remains unsettled related to whether the vulnerability to abnormal 5-HT during the perinatal period leading to maladaptive behaviors in adulthood differs in males and females. Indeed, as shown in Table 1, except for the early exposure to SSRIs, most prior studies have only used male animals. While sex differences in animal models of vulnerability to psychiatric disorder and early life stress are increasingly demonstrated (Dalla et al., 2010; Reincke and Hanganu-Opatz, 2017) and a sexual dimorphism in serotonergic system activity in adults is known (Carlsson and Carlsson, 1988; Haleem et al., 1990), the systematic study of the effects of 5-HT level variations during the perinatal period in both sexes remains a high priority.
In this review, we chose to present examples of three exogenous factors supporting the hypothesis of a non-linear relationship between serotonin-levels during early-life and adult behavior. In addition to the models on which we focused, other exogenous factors and genetic models/variants support the hypothesis that both high and low levels of 5-HT during development lead to behavioral deficits, and more specifically to emotional deficits. MAO-A/B-KO mice, which have increased monoamine levels, show a significantly decreased distance traveled in the open-field, and a reduction in the time spent in open-arms in the elevated-plus-maze (Chen et al., 2004). Similarly, constitutive SERT-KO mice, with elevated 5-HT levels, present anxiety-like and depression-like behaviors such as reduced locomotor activity in the open-field, increased latency-to-feed in the novelty-suppressed-feeding test and social-interaction deficits (Lira et al., 2003; Ansorge et al., 2004; Kalueff et al., 2007). Hyposerotonergic function in Lmx1b-raphe specific-KO mice, leads to increased freezing in the fear conditioning test and impaired water-maze memory retrieval (Dai et al., 2008). Constitutively reduced serotonergic levels in a model of SERT-overexpression resulted in reduced anxiety-like phenotypes in the elevated-plus-maze and in the hyponeophagia tests (Jennings et al., 2006). Although the latter study suggests that hyposerotonergic function may be protective of anxiety-like behaviors, other studies using mutant mice with severe 5-HT ablations point to the inverse. While Pet1-KO mice, which only retain residual serotonergic innervation, exhibited reduced anxiety-like behaviors and increased fear responses in one study (Kiyasova et al., 2011), Pet1-KO mice showed increased anxiety-like behaviors and increased aggression in another (Hendricks et al., 2003). Furthermore, Tph2- R441H-KI mice, in which 5-HT brain levels are approximately 80% of wildtype, exhibit increased immobility in the tail suspension test and increased latency to cross to the light chamber in the Dark-Light test (Beaulieu et al., 2008).
Finally, these studies raise the urgent question of whether the behavioral deficits caused by altered 5-HT levels during development can be reversed in adulthood. In Lmx1b-raphe specific-KO mice, plasticity deficits were rescued with a bath application of 5-HT (Dai et al., 2008). Interestingly, Tph2- R441H-KI behavioral deficits could be rescued by administration of a GSK3-β inhibitor (Beaulieu et al., 2008). However, it is striking that adult behavior is impaired in both the SERT-KO mouse (lifelong impaired serotonergic transporter) and the temporally restricted administration of a transporter blocker during perinatal-life (fluoxetine from P4-P21) (Ansorge et al., 2004). This demonstrates that increased serotonergic tone in the adult SERT-KO is not sufficient to rescue the deficits caused by increased serotonergic signaling during development.
Conclusion
High perinatal plasticity allows the brain to adapt to its environment but also marks a period of increased vulnerability. The regulation of serotonin levels during development is extremely important for typical brain development. Both low and high levels of serotonergic signaling seem to be detrimental, leading to multiple behavioral deficits. Many exogenous factors can affect 5-HT levels during development. Future studies need to address the boundary conditions and underlying mechanisms of optimal 5-HT levels during the critical perinatal period to optimize long-term behavioral and emotional outcomes.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by NICHD grant R03HD094978 to CT.
References
- Ansorge M. S., Morelli E., Gingrich J. A. (2008). Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J. Neurosci. 28 199–207. 10.1523/JNEUROSCI.3973-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge M. S., Zhou M., Lira A., Hen R., Gingrich J. A. (2004). Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 306 879–881. 10.1126/science.1101678 [DOI] [PubMed] [Google Scholar]
- Arborelius L., Eklund M. B. (2007). Both long and brief maternal separation produces persistent changes in tissue levels of brain monoamines in middle-aged female rats. Neuroscience 145 738–750. 10.1016/j.neuroscience.2006.12.007 [DOI] [PubMed] [Google Scholar]
- Bairy K. L., Madhyastha S., Ashok K. P., Bairy I., Malini S. (2007). Developmental and behavioral consequences of prenatal fluoxetine. Pharmacology 79 1–11. 10.1159/000096645 [DOI] [PubMed] [Google Scholar]
- Beaulieu J.-M., Zhang X., Rodriguiz R. M., Sotnikova T. D., Cools M. J., Wetsel W. C., et al. (2008). Role of GSK3β in behavioral abnormalities induced by serotonin deficiency. Proc. Natl. Acad. Sci. U.S.A. 105 1333–1338. 10.1073/pnas.0711496105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beique J. C., Campbell B., Perring P., Hamblin M. W., Walker P., Mladenovic L., et al. (2004). Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J. Neurosci. 24 4807–4817. 10.1523/JNEUROSCI.5113-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benekareddy M., Goodfellow N. M., Lambe E. K., Vaidya V. A. (2010). Enhanced function of prefrontal serotonin 5-HT(2) receptors in a rat model of psychiatric vulnerability. J. Neurosci. 30 12138–12150. 10.1523/JNEUROSCI.3245-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benekareddy M., Vadodaria K. C., Nair A. R., Vaidya V. A. (2011). Postnatal serotonin type 2 receptor blockade prevents the emergence of anxiety behavior, dysregulated stress-induced immediate early gene responses, and specific transcriptional changes that arise following early life stress. Biol. Psychiatry 70 1024–1032. 10.1016/j.biopsych.2011.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkelfat C., Ellenbogen M. A., Dean P., Palmour R. M., Young S. N. (1994). Mood-lowering effect of tryptophan depletion. Enhanced susceptibility in young men at genetic risk for major affective disorders. Arch. Gen. Psychiatry 51 687–697. 10.1001/archpsyc.1994.03950090019003 [DOI] [PubMed] [Google Scholar]
- Biggio G., Fadda F., Fanni P., Tagliamonte A., Gessa G. L. (1974). Rapid depletion of serum tryptophan, brain tryptophan, serotonin and 5-hydroxyindoleacetic acid by a tryptophan-free diet. Life Sci. 14 1321–1329. 10.1016/0024-3205(74)90440-8 [DOI] [PubMed] [Google Scholar]
- Bitzenhofer S. H., Ahlbeck J., Hanganu-Opatz I. L. (2017). Methodological approach for optogenetic manipulation of neonatal neuronal networks. Front. Cell. Neurosci. 11:239. 10.3389/fncel.2017.00239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A., Peng W., Hewlett W., Levitt P. (2006). Expression mapping of 5-HT1 serotonin receptor subtypes during fetal and early postnatal mouse forebrain development. Neuroscience 141 781–794. 10.1016/j.neuroscience.2006.04.036 [DOI] [PubMed] [Google Scholar]
- Booij L., Tremblay R. E., Szyf M., Benkelfat C. (2015). Genetic and early environmental influences on the serotonin system: consequences for brain development and risk for psychopathology. J. Psychiatry Neurosci. 40 5–18. 10.1503/jpn.140099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukhris T., Sheehy O., Mottron L., Berard A. (2015). Antidepressant use during pregnancy and the risk of autism spectrum disorder in children. JAMA Pediatr. 170 117–124. 10.1001/jamapediatrics.2015.3356 [DOI] [PubMed] [Google Scholar]
- Bravo J. A., Dinan T. G., Cryan J. F. (2014). Early-life stress induces persistent alterations in 5-HT1A receptor and serotonin transporter mRNA expression in the adult rat brain. Front. Mol. Neurosci. 7:24. 10.3389/fnmol.2014.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelte S., Mc Glanaghy E., Bonnin A., Oberlander T. F. (2017). Developmental changes in serotonin signaling: implications for early brain function, behavior and adaptation. Neuroscience 342 212–231. 10.1016/j.neuroscience.2016.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson M., Carlsson A. (1988). A regional study of sex differences in rat brain serotonin. Prog. Neuropsychopharmacol. Biol. Psychiatry 12 53–61. [DOI] [PubMed] [Google Scholar]
- Celada P., Bortolozzi A., Artigas F. (2013a). Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: rationale and current status of research. CNS Drugs 27 703–716. 10.1007/s40263-013-0071-0 [DOI] [PubMed] [Google Scholar]
- Celada P., Puig M. V., Artigas F. (2013b). Serotonin modulation of cortical neurons and networks. Front. Integr. Neurosci. 7:25 10.3389/fnint.2013.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chameau P., Inta D., Vitalis T., Monyer H., Wadman W. J., van Hooft J. A. (2009). The N-terminal region of reelin regulates postnatal dendritic maturation of cortical pyramidal neurons. Proc. Natl. Acad. Sci. U.S.A. 106 7227–7232. 10.1073/pnas.0810764106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury S., Sharma V., Kumar V., Nag T. C., Wadhwa S. (2016). Activity-dependent synaptic plasticity modulates the critical phase of brain development. Brain Dev. 38 355–363. 10.1016/j.braindev.2015.10.008 [DOI] [PubMed] [Google Scholar]
- Chen K., Holschneider D. P., Wu W., Rebrin I., Shih J. C. (2004). A spontaneous point mutation produces monoamine oxidase A/B knock-out mice with greatly elevated monoamines and anxiety-like behavior. J. Biol. Chem. 279 39645–39652. 10.1074/jbc.M405550200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper W. O., Willy M. E., Pont S. J., Ray W. A. (2007). Increasing use of antidepressants in pregnancy. Am. J. Obstet. Gynecol. 196 544.e1–544.e5. 10.1016/j.ajog.2007.01.033 [DOI] [PubMed] [Google Scholar]
- Dai J.-X., Han H.-L., Tian M., Cao J., Xiu J.-B., Song N.-N., et al. (2008). Enhanced contextual fear memory in central serotonin-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 105 11981–11986. 10.1073/pnas.0801329105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C., Pitychoutis P. M., Kokras N., Papadopoulou-Daifoti Z. (2010). Sex differences in animal models of depression and antidepressant response. Basic Clin. Pharmacol. Toxicol. 106 226–233. 10.1111/j.1742-7843.2009.00516.x [DOI] [PubMed] [Google Scholar]
- Daubert E. A., Condron B. G. (2010). Serotonin: a regulator of neuronal morphology and circuitry. Trends Neurosci. 33 424–434. 10.1016/j.tins.2010.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado P. L., Miller H. L., Salomon R. M., Licinio J., Krystal J. H., Moreno F. A., et al. (1999). Tryptophan-depletion challenge in depressed patients treated with desipramine or fluoxetine: implications for the role of serotonin in the mechanism of antidepressant action. Biol. Psychiatry 46 212–220. 10.1016/S0006-3223(99)00014-1 [DOI] [PubMed] [Google Scholar]
- Fadda F., Cocco S., Stancampiano R. (2000). A physiological method to selectively decrease brain serotonin release. Brain Res. Brain Res. Protoc. 5 219–222. 10.1016/S1385-299X(00)00016-7 [DOI] [PubMed] [Google Scholar]
- Faulkner P., Deakin J. F. (2014). The role of serotonin in reward, punishment and behavioural inhibition in humans: insights from studies with acute tryptophan depletion. Neurosci. Biobehav. Rev. 46(Pt 3), 365–378. 10.1016/j.neubiorev.2014.07.024 [DOI] [PubMed] [Google Scholar]
- Finkelhor D., Turner H., Ormrod R., Hamby S. L. (2009). Violence, abuse, and crime exposure in a national sample of children and youth. Pediatrics 124 1411–1423. 10.1542/peds.2009-0467 [DOI] [PubMed] [Google Scholar]
- Francis D., Diorio J., Liu D., Meaney M. J. (1999). Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science 286 1155–1158. 10.1126/science.286.5442.1155 [DOI] [PubMed] [Google Scholar]
- Fricker A. D., Rios C., Devi L. A., Gomes I. (2005). Serotonin receptor activation leads to neurite outgrowth and neuronal survival. Brain Res. Mol. Brain Res. 138 228–235. 10.1016/j.molbrainres.2005.04.016 [DOI] [PubMed] [Google Scholar]
- Gaspar P., Cases O., Maroteaux L. (2003). The developmental role of serotonin: news from mouse molecular genetics. Nat. Rev. Neurosci. 4 1002–1012. 10.1038/nrn1256 [DOI] [PubMed] [Google Scholar]
- Gingrich J. A., Malm H., Ansorge M. S., Brown A., Sourander A., Suri D., et al. (2017). New insights into how serotonin selective reuptake inhibitors shape the developing brain. Birth Defects Res. 109 924–932. 10.1002/bdr2.1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeden N., Velasquez J., Arnold K. A., Chan Y., Lund B. T., Anderson G. M., et al. (2016). Maternal inflammation disrupts fetal neurodevelopment via increased placental output of serotonin to the fetal brain. J. Neurosci. 36 6041–6049. 10.1523/JNEUROSCI.2534-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez L. E., Andrews N., File S. E. (1996). 5-HT1A and benzodiazepine receptors in the basolateral amygdala modulate anxiety in the social interaction test, but not in the elevated plus-maze. Brain Res. 732 145–153. 10.1016/0006-8993(96)00517-3 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal G., Melo A. I. (2017). Bidirectional effects of mother-young contact on the maternal and neonatal brains. Adv. Exp. Med. Biol. 1015 97–116. 10.1007/978-3-319-62817-2_6 [DOI] [PubMed] [Google Scholar]
- Haleem D. J., Kennett G. A., Curzon G. (1990). Hippocampal 5-hydroxytryptamine synthesis is greater in female rats than in males and more decreased by the 5-HT1A agonist 8-OH-DPAT. J. Neural Transm. Gen. Sect. 79 93–101. 10.1007/BF01251004 [DOI] [PubMed] [Google Scholar]
- Hellstrom I. C., Dhir S. K., Diorio J. C., Meaney M. J. (2012). Maternal licking regulates hippocampal glucocorticoid receptor transcription through a thyroid hormone–serotonin–NGFI-A signalling cascade. Philos. Trans. R. Soc. B 367 2495–2510. 10.1098/rstb.2012.0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick V., Stowe Z. N., Altshuler L. L., Hwang S., Lee E., Haynes D. (2003). Placental passage of antidepressant medications. Am. J. Psychiatry 160 993–996. 10.1176/appi.ajp.160.5.993 [DOI] [PubMed] [Google Scholar]
- Hendricks T. J., Fyodorov D. V., Wegman L. J., Lelutiu N. B., Pehek E. A., Yamamoto B., et al. (2003). Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron 37 233–247. 10.1016/S0896-6273(02)01167-4 [DOI] [PubMed] [Google Scholar]
- Henry A., Kisicki M. D., Varley C. (2012). Efficacy and safety of antidepressant drug treatment in children and adolescents. Mol. Psychiatry 17 1186–1193. 10.1038/mp.2011.150 [DOI] [PubMed] [Google Scholar]
- Hohmann C. F., Hamon R., Batshaw M. L., Coyle J. T. (1988). Transient postnatal elevation of serotonin levels in mouse neocortex. Brain Res. 471 163–166. 10.1016/0165-3806(88)90163-0 [DOI] [PubMed] [Google Scholar]
- Hranilovic D., Bujas-Petkovic Z., Tomicic M., Bordukalo-Niksic T., Blazevic S., Cicin-Sain L. (2009). Hyperserotonemia in autism: activity of 5HT-associated platelet proteins. J. Neural Transm. 116 493–501. 10.1007/s00702-009-0192-2 [DOI] [PubMed] [Google Scholar]
- Huggins K. N., Mathews T. A., Locke J. L., Szeliga K. T., Friedman D. P., Bennett A. J., et al. (2012). Effects of early life stress on drinking and serotonin system activity in rhesus macaques: 5-hydroxyindoleacetic acid in cerebrospinal fluid predicts brain tissue levels. Alcohol 46 371–376. 10.1016/j.alcohol.2011.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huybrechts K. F., Palmsten K., Mogun H., Kowal M., Avorn J., Setoguchi-Iwata S., et al. (2013). National trends in antidepressant medication treatment among publicly insured pregnant women. Gen. Hosp. Psychiatry 35 265–271. 10.1016/j.genhosppsych.2012.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichise M., Vines D. C., Gura T., Anderson G. M., Suomi S. J., Higley J. D., et al. (2006). Effects of early life stress on [11C]DASB positron emission tomography imaging of serotonin transporters in adolescent peer- and mother-reared rhesus monkeys. J. Neurosci. 26 4638–4643. 10.1523/JNEUROSCI.5199-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings K. A., Loder M. K., Sheward W. J., Pei Q., Deacon R. M., Benson M. A., et al. (2006). Increased expression of the 5-HT transporter confers a low-anxiety phenotype linked to decreased 5-HT transmission. J. Neurosci. 26 8955–8964. 10.1523/JNEUROSCI.5356-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff A. V., Fox M. A., Gallagher P. S., Murphy D. L. (2007). Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav. 6 389–400. 10.1111/j.1601-183X.2006.00270.x [DOI] [PubMed] [Google Scholar]
- Kepser L. J., Homberg J. R. (2015). The neurodevelopmental effects of serotonin: a behavioural perspective. Behav. Brain Res. 277 3–13. 10.1016/j.bbr.2014.05.022 [DOI] [PubMed] [Google Scholar]
- Khozhai L. I., Otellin V. A. (2012). [Synaptogenesis in the dorsal raphe nucleus of rat medulla oblongata in serotonin deficiency]. Morfologiia 142 20–24. [PubMed] [Google Scholar]
- Kiyasova V., Fernandez S. P., Laine J., Stankovski L., Muzerelle A., Doly S., et al. (2011). A genetically defined morphologically and functionally unique subset of 5-HT neurons in the mouse raphe nuclei. J. Neurosci. 31 2756–2768. 10.1523/JNEUROSCI.4080-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen T., Riedel W. J., van Someren A., Deutz N. E., Honig A., van Praag H. M. (1999). Mood effects of 24-hour tryptophan depletion in healthy first-degree relatives of patients with affective disorders. Biol. Psychiatry 46 489–497. 10.1016/S0006-3223(99)00082-7 [DOI] [PubMed] [Google Scholar]
- Kristensen J. H., Ilett K. F., Hackett L. P., Yapp P., Paech M., Begg E. J. (1999). Distribution and excretion of fluoxetine and norfluoxetine in human milk. Br. J. Clin. Pharmacol. 48 521–527. 10.1046/j.1365-2125.1999.00040.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe E. K., Krimer L. S., Goldman-Rakic P. S. (2000). Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkey. J. Neurosci. 20 8780–8787. 10.1523/JNEUROSCI.20-23-08780.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante P., Diorio J., Meaney M. J. (2002). Serotonin regulates hippocampal glucocorticoid receptor expression via a 5-HT7 receptor. Brain Res. Dev. Brain Res. 139 199–203. 10.1016/S0165-3806(02)00550-3 [DOI] [PubMed] [Google Scholar]
- Lauder J. M. (1990). Ontogeny of the serotonergic system in the rat: serotonin as a developmental signal. Ann. N. Y. Acad. Sci. 600 297–313; discussion314 10.1111/j.1749-6632.1990.tb16891.x [DOI] [PubMed] [Google Scholar]
- Lauder J. M., Bloom F. E. (1974). Ontogeny of monoamine neurons in the locus coeruleus, Raphe nuclei and substantia nigra of the rat. I. Cell differentiation. J. Comp. Neurol. 155 469–481. 10.1002/cne.901550407 [DOI] [PubMed] [Google Scholar]
- Lavdas A. A., Blue M. E., Lincoln J., Parnavelas J. G. (1997). Serotonin promotes the differentiation of glutamate neurons in organotypic slice cultures of the developing cerebral cortex. J. Neurosci. 17 7872–7880. 10.1523/JNEUROSCI.17-20-07872.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levallois C., Valence C., Baldet P., Privat A. (1997). Morphological and morphometric analysis of serotonin-containing neurons in primary dissociated cultures of human rhombencephalon: a study of development. Brain Res. Dev. Brain Res. 99 243–252. 10.1016/S0165-3806(97)00026-6 [DOI] [PubMed] [Google Scholar]
- Lidov H. G., Molliver M. E. (1982a). An immunohistochemical study of serotonin neuron development in the rat: ascending pathways and terminal fields. Brain Res. Bull. 8 389–430. [DOI] [PubMed] [Google Scholar]
- Lidov H. G., Molliver M. E. (1982b). Immunohistochemical study of the development of serotonergic neurons in the rat CNS. Brain Res. Bull. 9 559–604. [DOI] [PubMed] [Google Scholar]
- Lira A., Zhou M., Castanon N., Ansorge M. S., Gordon J. A., Francis J. H., et al. (2003). Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol. Psychiatry 54 960–971. 10.1016/S0006-3223(03)00696-6 [DOI] [PubMed] [Google Scholar]
- Maestripieri D., Hoffman C. L., Anderson G. M., Carter C. S., Higley J. D. (2009). Mother-infant interactions in free-ranging rhesus macaques: relationships between physiological and behavioral variables. Physiol. Behav. 96 613–619. 10.1016/j.physbeh.2008.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D., McCormack K., Lindell S. G., Higley J. D., Sanchez M. M. (2006). Influence of parenting style on the offspring’s behaviour and CSF monoamine metabolite levels in crossfostered and noncrossfostered female rhesus macaques. Behav. Brain Res. 175 90–95. 10.1016/j.bbr.2006.08.002 [DOI] [PubMed] [Google Scholar]
- Malm H., Brown A. S., Gissler M., Gyllenberg D., Hinkka-Yli-Salomaki S., McKeague I. W., et al. (2016). Gestational exposure to selective serotonin reuptake inhibitors and offspring psychiatric disorders: a national register-based study. J. Am. Acad. Child Adolesc. Psychiatry 55 359–366. 10.1016/j.jaac.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazer C., Muneyyirci J., Taheny K., Raio N., Borella A., Whitaker-Azmitia P. (1997). Serotonin depletion during synaptogenesis leads to decreased synaptic density and learning deficits in the adult rat: a possible model of neurodevelopmental disorders with cognitive deficits. Brain Res. 760 68–73. 10.1016/S0006-8993(97)00297-7 [DOI] [PubMed] [Google Scholar]
- Mitchell J. B., Betito K., Rowe W., Boksa P., Meaney M. J. (1992). Serotonergic regulation of type II corticosteroid receptor binding in hippocampal cell cultures: evidence for the importance of serotonin-induced changes in cAMP levels. Neuroscience 48 631–639. 10.1016/0306-4522(92)90407-S [DOI] [PubMed] [Google Scholar]
- Mitchell J. B., Rowe W., Boksa P., Meaney M. J. (1990). Serotonin regulates type II corticosteroid receptor binding in hippocampal cell cultures. J. Neurosci. 10 1745–1752. 10.1523/JNEUROSCI.10-06-01745.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moja E. A., Cipolla P., Castoldi D., Tofanetti O. (1989). Dose-response decrease in plasma tryptophan and in brain tryptophan and serotonin after tryptophan-free amino acid mixtures in rats. Life Sci. 44 971–976. 10.1016/0024-3205(89)90497-9 [DOI] [PubMed] [Google Scholar]
- Murphy D. L., Fox M. A., Timpano K. R., Moya P. R., Ren-Patterson R., Andrews A. M., et al. (2008). How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology 55 932–960. 10.1016/j.neuropharm.2008.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier J. D., Valles A., van Heesch F., Afrasiab-Middelman A., Roelofs J. J., Jonkers M., et al. (2011). Fluoxetine administration to pregnant rats increases anxiety-related behavior in the offspring. Psychopharmacology 217 419–432. 10.1007/s00213-011-2299-z [DOI] [PubMed] [Google Scholar]
- Olivier J. D. A., Åkerud H., Kaihola H., Pawluski J. L., Skalkidou A., Högberg U., et al. (2013). The effects of maternal depression and maternal selective serotonin reuptake inhibitor exposure on offspring. Front. Cell. Neurosci. 7:73 10.3389/fncel.2013.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou A., Dafni U., Alikaridis F., Bolaris S., Stylianopoulou F. (2002). Effects of neonatal handling on basal and stress-induced monoamine levels in the male and female rat brain. Neuroscience 114 195–206. 10.1016/S0306-4522(02)00129-X [DOI] [PubMed] [Google Scholar]
- Quintin P., Benkelfat C., Launay J. M., Arnulf I., Pointereau-Bellenger A., Barbault S., et al. (2001). Clinical and neurochemical effect of acute tryptophan depletion in unaffected relatives of patients with bipolar affective disorder. Biol. Psychiatry 50 184–190. 10.1016/S0006-3223(01)01140-4 [DOI] [PubMed] [Google Scholar]
- Raineki C., Sarro E., Rincon-Cortes M., Perry R., Boggs J., Holman C. J., et al. (2015). Paradoxical neurobehavioral rescue by memories of early-life abuse: the safety signal value of odors learned during abusive attachment. Neuropsychopharmacology 40 906–914. 10.1038/npp.2014.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampono J., Simmer K., Ilett K. F., Hackett L. P., Doherty D. A., Elliot R., et al. (2009). Placental transfer of SSRI and SNRI antidepressants and effects on the neonate. Pharmacopsychiatry 42 95–100. 10.1055/s-0028-1103296 [DOI] [PubMed] [Google Scholar]
- Rebello T. J., Yu Q., Goodfellow N. M., Caffrey Cagliostro M. K., Teissier A., Morelli E., et al. (2014). Postnatal day 2 to 11 constitutes a 5-HT-sensitive period impacting adult mPFC function. J. Neurosci. 34 12379–12393. 10.1523/JNEUROSCI.1020-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reincke S. A., Hanganu-Opatz I. L. (2017). Early-life stress impairs recognition memory and perturbs the functional maturation of prefrontal-hippocampal-perirhinal networks. Sci. Rep. 7:42042. 10.1038/srep42042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D., Barone S., Jr. (2000). Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 108(Suppl. 3), 511–533. 10.1289/ehp.00108s3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon-Cortes M., Barr G. A., Mouly A. M., Shionoya K., Nunez B. S., Sullivan R. M. (2015). Enduring good memories of infant trauma: rescue of adult neurobehavioral deficits via amygdala serotonin and corticosterone interaction. Proc. Natl. Acad. Sci. U.S.A. 112 881–886. 10.1073/pnas.1416065112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rok-Bujko P., Krzascik P., Szyndler J., Kostowski W., Stefanski R. (2012). The influence of neonatal serotonin depletion on emotional and exploratory behaviours in rats. Behav. Brain Res. 226 87–95. 10.1016/j.bbr.2011.08.030 [DOI] [PubMed] [Google Scholar]
- Routh D. K., Walton M. D., Padan-Belkin E. (1978). Development of activity level in children revisited: effects of mother presence. Dev. Psychol. 14 571–581. 10.1037/0012-1649.14.6.571 [DOI] [Google Scholar]
- Sarkar A., Chachra P., Vaidya V. A. (2014). Postnatal fluoxetine-evoked anxiety is prevented by concomitant 5-HT2A/C receptor blockade and mimicked by postnatal 5-HT2A/C receptor stimulation. Biol. Psychiatry 76 858–868. 10.1016/j.biopsych.2013.11.005 [DOI] [PubMed] [Google Scholar]
- Segall P. E., Timiras P. S. (1976). Patho-physiologic findings after chronic tryptophan deficiency in rats: a model for delayed growth and aging. Mech. Ageing Dev. 5 109–124. 10.1016/0047-6374(76)90012-9 [DOI] [PubMed] [Google Scholar]
- Segall P. E., Timiras P. S., Walton J. R. (1983). Low tryptophan diets delay reproductive aging. Mech. Ageing Dev. 23 245–252. 10.1016/0047-6374(83)90024-6 [DOI] [PubMed] [Google Scholar]
- Serfaty C. A., Oliveira-Silva P., Faria Melibeu Ada C., Campello-Costa P. (2008). Nutritional tryptophan restriction and the role of serotonin in development and plasticity of central visual connections. Neuroimmunomodulation 15 170–175. 10.1159/000153421 [DOI] [PubMed] [Google Scholar]
- Shannon C., Schwandt M. L., Champoux M., Shoaf S. E., Suomi S. J., Linnoila M., et al. (2005). Maternal absence and stability of individual differences in CSF 5-HIAA concentrations in rhesus monkey infants. Am. J. Psychiatry 162 1658–1664. 10.1176/appi.ajp.162.9.1658 [DOI] [PubMed] [Google Scholar]
- Shannon N. J., Gunnet J. W., Moore K. E. (1986). A comparison of biochemical indices of 5-hydroxytryptaminergic neuronal activity following electrical stimulation of the dorsal raphe nucleus. J. Neurochem. 47 958–965. 10.1111/j.1471-4159.1986.tb00704.x [DOI] [PubMed] [Google Scholar]
- Sit D., Perel J. M., Wisniewski S. R., Helsel J. C., Luther J. F., Wisner K. L. (2011). Mother-infant antidepressant concentrations, maternal depression, and perinatal events. J. Clin. Psychiatry 72 994–1001. 10.4088/JCP.10m06461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi M. S., Sanders-Bush E. (2004). Serotonin and brain development. Int. Rev. Neurobiol. 59 111–174. 10.1016/S0074-7742(04)59006-2 [DOI] [PubMed] [Google Scholar]
- St-Pierre J., Laurent L., King S., Vaillancourt C. (2016). Effects of prenatal maternal stress on serotonin and fetal development. Placenta 48(Suppl. 1), S66–S71. 10.1016/j.placenta.2015.11.013 [DOI] [PubMed] [Google Scholar]
- Sundstrom E., Kolare S., Souverbie F., Samuelsson E. B., Pschera H., Lunell N. O., et al. (1993). Neurochemical differentiation of human bulbospinal monoaminergic neurons during the first trimester. Brain Res. Dev. Brain Res. 75 1–12. 10.1016/0165-3806(93)90059-J [DOI] [PubMed] [Google Scholar]
- Suri D., Teixeira C. M., Cagliostro M. K., Mahadevia D., Ansorge M. S. (2014). Monoamine-sensitive developmental periods impacting adult emotional and cognitive behaviors. Neuropsychopharmacology 40 88–112. 10.1038/npp.2014.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teissier A., Chemiakine A., Inbar B., Bagchi S., Ray Russell S., Palmiter Richard D., et al. (2015). Activity of raphé serotonergic neurons controls emotional behaviors. Cell Rep. 13 1965–1976. 10.1016/j.celrep.2015.10.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (2010). Child Maltreatment 2009. Washington DC: US Government Printing Office. [Google Scholar]
- Vetulani J. (2013). Early maternal separation: a rodent model of depression and a prevailing human condition. Pharmacol. Rep. 65 1451–1461. 10.1016/S1734-1140(13)71505-6 [DOI] [PubMed] [Google Scholar]
- Vichier-Guerre C., Parker M., Pomerantz Y., Finnell R. H., Cabrera R. M. (2017). Impact of selective serotonin reuptake inhibitors on neural crest stem cell formation. Toxicol. Lett. 281 20–25. 10.1016/j.toxlet.2017.08.012 [DOI] [PubMed] [Google Scholar]
- Waeber C., Sebben M., Nieoullon A., Bockaert J., Dumuis A. (1994). Regional distribution and ontogeny of 5-HT4 binding sites in rodent brain. Neuropharmacology 33 527–541. 10.1016/0028-3908(94)90084-1 [DOI] [PubMed] [Google Scholar]
- Weaver I. C., Cervoni N., Champagne F. A., D’Alessio A. C., Sharma S., Seckl J. R., et al. (2004). Epigenetic programming by maternal behavior. Nat. Neurosci. 7 847–854. 10.1038/nn1276 [DOI] [PubMed] [Google Scholar]
- Xu Y., Sari Y., Zhou F. C. (2004). Selective serotonin reuptake inhibitor disrupts organization of thalamocortical somatosensory barrels during development. Brain Res. Dev. Brain Res. 150 151–161. 10.1016/j.devbrainres.2003.02.001 [DOI] [PubMed] [Google Scholar]
- Xue X., Shao S., Li M., Shao F., Wang W. (2013). Maternal separation induces alterations of serotonergic system in different aged rats. Brain Res. Bull. 95 15–20. 10.1016/j.brainresbull.2013.03.003 [DOI] [PubMed] [Google Scholar]
- Yu Q., Teixeira C. M., Mahadevia D., Huang Y., Balsam D., Mann J. J., et al. (2014). Dopamine and serotonin signaling during two sensitive developmental periods differentially impact adult aggressive and affective behaviors in mice. Mol. Psychiatry 19 688–698. 10.1038/mp.2014.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Guadarrama L., Corona-Morales A. A., Vega-Gonzalez A., Rocha L., Escobar A. (2006). Rats subjected to extended L-tryptophan restriction during early postnatal stage exhibit anxious-depressive features and structural changes. J. Neuropathol. Exp. Neurol. 65 562–570. 10.1097/00005072-200606000-00004 [DOI] [PubMed] [Google Scholar]
- Zoratto F., Fiore M., Ali S. F., Laviola G., Macri S. (2013). Neonatal tryptophan depletion and corticosterone supplementation modify emotional responses in adult male mice. Psychoneuroendocrinology 38 24–39. 10.1016/j.psyneuen.2012.04.015 [DOI] [PubMed] [Google Scholar]