Abstract
Background: Mutant transactive response DNA-binding protein (TDP-43) is closely correlated to the inherited form of amyotrophic lateral sclerosis (ALS). TDP-43 transgenic rats can reproduce the core phenotype of ALS and constitutive expression of TDP-43 caused postnatal death.
Objective: The study aimed to understand whether neurologic deficiency caused by mutant TDP-43 is dependent on its temporal expression.
Method: Transgenic rats were established that express mutant human TDP-43 (M337V substitution) in neurons, then a Tet-off system was used to regulate its expression.
Results: TDP-43 mutant transgenic rats developed significant weakness after the transgene was activated. Rats with expression of mutant TDP-43 at 30 days showed a more aggressive phenotype. More severe pathological changes in neurogenic atrophy were observed in these rats.
Conclusion: Temporal expression of mutant TDP-43 in neurons promoted serious phenotype in rats. The dysfunction of TDP-43 had a profound impact on the development of motor neurons and skeletal muscles.
Keywords: Amyotrophic Lateral Sclerosis (ALS), TAR DNA-binding protein 43, motor neurons, transgenic rats, CAG, Tet-responsive transactivator
1. INTRODUCTION
Amyotrophic Lateral Sclerosis (ALS) is the most frequent form of adult-onset motor neuron disease, in which loss of motor neurons leads to weakness and denervation atrophy of voluntary muscles. The disease often develops around middle life and inexorably progresses to paralysis [1, 2]. Although most ALS cases are sporadic, Familiar ALS (FALS) accounts for 5 to 10% of cases with an autosomal dominant pattern of inheritance.
Transactive response DNA-binding Protein (TDP-43) is a heterogeneous nuclear RNA binding protein encoded by TDP-43 gene. TDP-43 plays important roles in multiprotein/RNA complex and regulating gene expression [3]. TDP-43 has been shown to promote neurodegeneration by impairing chromatin remodeling, accelerate age-dependent degen eration of interneurons, and cause defects in dendritic growth [4-6]. Accumulating evidence have implicated that the pathogenic mutation of TDP-43 causes an inherited form of ALS [7]. Mutation of TDP-43 gene has been identified responsible for some familiar ALS. Including M337V mutation, no less than 30 pathogenic TDP-43 mutants have been reported so far [8].
Using a Tet-off system, inducible expression of human TDP-43 with a pathogenic mutation (M337V substitution) successfully induced progressive paralysis in transgenic rats [9]. The removal of mutant TDP-43 partly restored motor function in ALS rats expressing the causative gene in neurons and muscles, indicating that the motor neuron degeneration is partially reversible [10]. However, it is unclear whether neurologic deficiency caused by mutant TDP-43 is correlated with the time of its expression. To address this question, we used Tet-off system to produce inducible gene expression in transgenic rats, and the consequence events including the onset age and severity of illness were assessed in transgenic models. Our findings support the hypothesis that earlier expression of mutant TDP-43 can cause a more aggressive phenotype of ALS in transgenic rats.
2. MATERIALS AND METHODS
2.1. Generation of Transgenic Rats
The animal use protocol was approved by the Ethical Review Board from Thomas Jefferson University, PA, USA. Transgenic rats were established and maintained in a Sprague Dawley background. The TDP-43 gene was isolated from BAC clone (RP11-829B14), and an M337V substitution was integrated into TDP gene by homologous recombination in Escherichia coli [11]. Tetracycline (Tet)-inducible transgenic rats carried a mutant human TDP-43 (TDP-43M337V) transgene at the control of a Tet-responsive Element (TRE) [12]. Selected Cytomegalovirus enhancer fused to chicken beta actin promoter (CAG) was used to drive Tet-responsive Transactivator (tTA) gene. TRE-TDP-43 M337V transgenic rats were crossed with CAG-tTA positive rats. To selectively induce human TDP-43 gene expressed in rats, breeding rats and transgenic offspring were drunk with Dox (50 ug/ml) to inhibit transgene expression during embryonic and postnatal phase. Then CAG-tTA/TRE-TDP-43M337V double transgenic rats were deprived of Dox to activate mutant TDP gene. The time of withdrawal of Dox (Dox-) was selected as 30-day-old and 70-day-old, to mimic the juvenile stage and adulthood of rats.
2.2. Behavioral Tests and Disease Stages
The onset and progression of the ALS phenotypes in transgenic rats were detected by mobility and grip strength. Mobility was surveyed by open field test (Med Associates), which measured the total distance a rat travelled within 10 min [13]. Grip strength was measured with a grip-strength meter (Columbus Instruments). We defined the disease onset as an irreversible reduction in travelled distance. Paralysis was defined as dragging of legs or an inability to retract individual legs. Disease end stage was defined as the inability to retract two or more legs, or inability to right itself when the rat was placed on its side, or as a severe weight loss. Related data were compared using unpaired t-tests. P-value < 0.05 was regarded as statistically significant.
2.3. Immunofluorescence, Immunohistochemistry and Cresyl Violet Staining
Coronal forebrain sections and cross-sections of spine were used for immunohistochemistry and Cresyl violet staining. The colocalization of human TDP-43 with NeuN was detected by double-labelling immunofluorescence. Primary antibodies used for immunofluorescence were; anti-human TDP-43 (Abnova, clone 2E2-D3), polyclonal antibody against NeuN from rabbits immunized with a peptide (MAQPYPPAQYPPC) and affinity purified. Immunostained tissues were visualized using a Nikon microscope, and images were acquired using a Nikon digital camera as previously reported [14]. Motor neurons in the spinal cord were observed by Cresyl violet staining and quantified as previously reported [14]. The motor neurons in a long segment of lumbar cord (L3-L5) were assessed to increase the accuracy of cell counting. The central segment of lumbar cord was cut into 30-um thick sections using a freezing microtome, and motor neurons were counted in every 10th section on both sides using a fractionator-based stereology software (Stereologer). Numbers of neurons were compared using unpaired t-tests. P-value < 0.05 was regarded as statistically significant.
2.4. Histology and Histochemistry in Skeletal Muscles
Fresh gastrocnemius muscles were quick-frozen in isopentane/liquid nitrogen, then cut into cross sections (10 µm) on a Cryostat Microtome. The structures of muscle were assessed by H&E staining, histochemistry for nonspecific esterase and ATPase. Nonspecific esterase activity was detected using a-napthyl acetate protocol. Myosin ATPase staining (pH 4.5) was used to discriminate three types of skeletal muscle fibers.
3. RESULTS
3.1. TDP-43 Transgenic Rats had Worse Phenotype
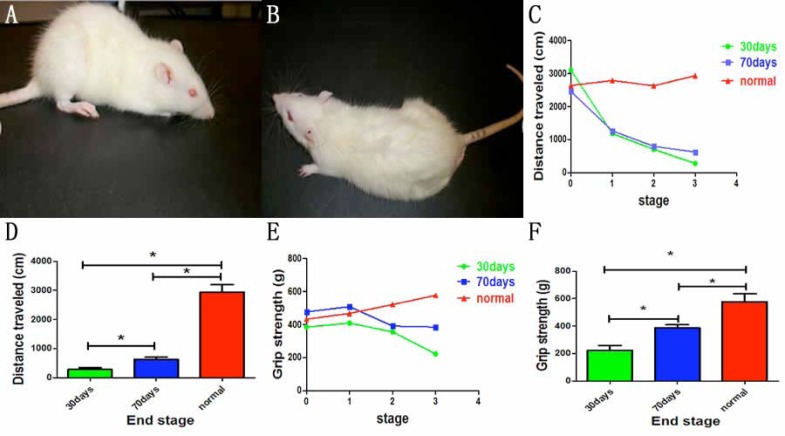
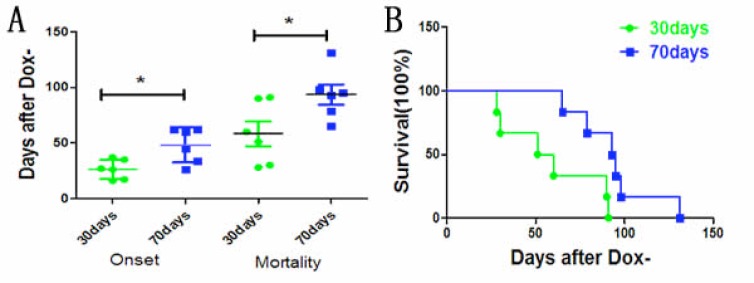
To examine whether mutant TDP-43 expressed in neurons could induce ALS-like phenotypes, we chose the regulatory promoter of CAG gene to control the activation of transgene and established CAG-tTA/TRE-TDP-43M337V double transgenic rats, with their mutant human TDP-43 gene under the control of a Tetracycline (Tet)-responsive Element (TRE). Then, we used the Tet-off system to regulate the expression of mutant TDP-43 in neurons. Dox was used to inhibit transgene expression during embryonic and postnatal phase. To mimic mutant TDP-43 expression in juvenile stage or adulthood, transgenic rats were deprived Dox (Dox-) at the age of 30 days or 70 days, respectively. Both the groups of CAG-tTA/TRE-TDP-43M337V double transgenic rats suffered from progressive weakness after Dox (Fig. 1A, B). In the group of Dox- at the age of 30 days (Dox- 30 days), transgenic rats began losing grip strength and mobility as early as 16 days after Dox- (26.33±3.575 days after Dox-), while the counterpart rats with Dox- at the age of 70 days (Dox- 70days) developed early signs of paralysis (disease onset) not until 26 days after Dox- (48.17±6.39 days after Dox-) (Fig. 1C-F). There were significant differences in the time of onset between the two groups (Fig. 2A, B).
Fig. (1).
Mutant human TDP-43 expressed on 30 days causes shorter onset and induces more severe paralysis in transgenic rats. Double transgenic rats developed paralysis (A): Dox- at the age of 30 days, (B): Dox- at the age of 70 days). Compared to control group, open-field assay (C) and the measured grip strength of paws (E) indicated an irreversible reduction of mobility in two groups of double-transgenic rats (*P<0.05). Compared with the rats deprived of Dox at the age of 70 days, the rats deprived of Dox at the age of 30 days developed more serious paralysis (D and F). Data are means ± SD (n=6). *P<0.05. Stage 0: the time of Dox-; stage 1: onset time; stage 2: middle stage; stage 3: end stage. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper).
3.2. Severe Loss of Neurons in Transgenic Rats
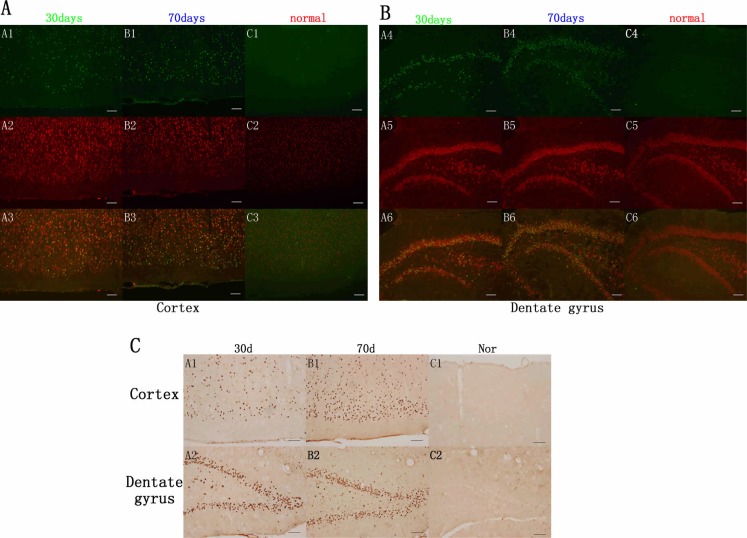
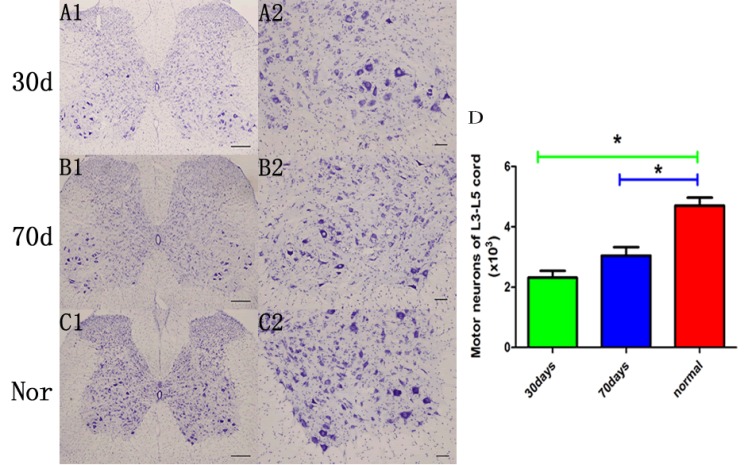
In the cortex and dentate gyrus, double-label fluorescence staining demonstrated that mutant human TDP-43 was colocalized with the neurons (Fig. 3A, B). CAG-tTA/TRE-TDP-43M337V double transgenic rats showed apparent expression of TDP-43 at the disease end stage, while TDP-43 was not detected in normal rats with matched age (Fig. 3C). To accurately estimate motor neuron death in rats, we used unbiased stereological cell counting to count the number of motor neurons in a long segment of spinal cord (L3-L5). Compared with normal rats, stereological cell counting identified more loss of neurons in transgenic rats with Dox- at the age of 30 days than in rats with Dox- at the age of 70 days (Fig. 4).
Fig. (3).
Overexpression of mutant human TDP-43 (hTDP-43) in neurons of transgenic rats. Immunofluorescence showed that mutant TDP-43 was expressed in the cortex (A) and dentate gyrus (B) of CAG-tTA/TRE-TDP43M337V double-transgenic rats after Dox was deprived (Dox-) at the age of 30 days and 70 days, respectively (green, A1, B1, A4, B4), but was not detected in the normal group (C1, C4). Neuronal marker NeuN was stained in the cortex and dentate gyrus in transgenic groups and control group (red, A2, B2, C2, A5, B5, C5). Double-label fluorescence staining demonstrated that mutant human TDP-43 was colocalized with NeuN in the cortex and dentate gyrus in transgenic rats (A3, B3, A6, B6), but not in control group (C3, C6). Scale bar: 100 µm. (C). Immunohistochemistry showed overexpression of TDP-43 in the cortex (A1, B1, C1) and dentate gyrus (A2, B2, C2). Scale bar: 100 µm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper).
Fig. (4).
Expression of mutant TDP-43 causes motor neuron death. (A-C) Representative images of the lumbar cord of transgenic rats at end stage (A): Dox- at the age of 30 days, (B): Dox- at the age of 70 days) and normal control subjects at matched ages (C). Scale bar: 200 µm (A1, B1, C1) and 50 µm (A2, B2, C2). (D) Stereological cell counting demonstrated a significant loss of motor neurons (>25 µm in diameter) in the L3-L5 cords of CAG-tTA/TRE-TDP43M337V double-transgenic rats compared with normal rats. Data are means ± SD (n=6). *P<0.05.
3.3. Neurogenic Damages in Transgenic Rats
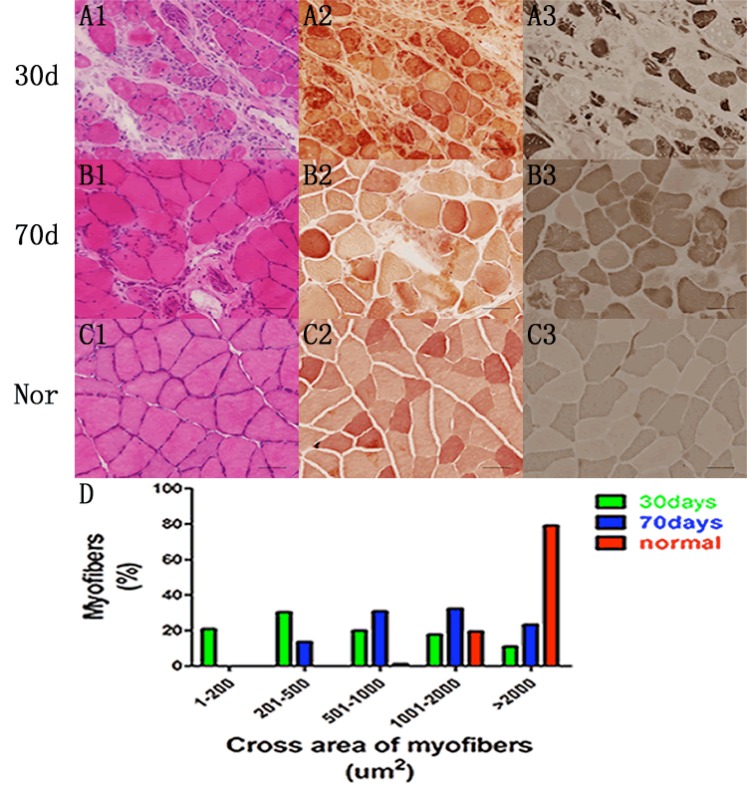
As an outcome of motor neuron degeneration, we examined skeletal muscle atrophy by H&E staining (Fig. 5A1, B1), and performed immunohistochemistry for nonspecific esterase (Fig. 5A2, B2) and ATPase (Fig. 5C1, C2) in mut-ant TDP-43 transgenic rats at end stage. In addition, CAG-
tTA/TRE-TDP-43M337V double transgenic rats with Dox- at the age of 30 days revealed a worse pathologic change than the rats of Dox- 70 days. In contrast, rats in control group with matched age had no sign of amyotrophy (Fig. 5C). We also counted the cross areas of myofibers in all groups of rats on cross sections of gastrocnemius stained by ATPase 4.5, and found that the size of fibers was remarkably reduced in TDP-43 rats (Fig. 5D).
Fig. (5).
Overexpression of mutant human TDP-43 in early stage results in more severe muscular atrophy. (A-C) H & E staining (A1, B1), immunohistochemistry for nonspecific esterase (A2, B2) and ATPase 4.5 (A3, B3) indicated substantial atrophy of skeletal muscle in two group of double transgenic rats at the end stage, while the normal rats with matched ages showed no sign of amyotrophy (C1, C2, C3). Scale bar: 30 µm. (D) The cross areas of myofibers in all groups of rats were measured on cross sections of gastrocnemius stained by ATPase 4.5. Data are means ± SD (n=6).
4. DISCUSSION
As a highly conserved ribonucleoprotein, TDP-43 plays an important role in gene expression. The level of TDP-43 is remarkably elevated in the brain and spine of patients in late stage ALS [15-17]. Moreover, overexpression of either wild-type or mutant TDP-43 could cause cell death, suggesting the gain-of-function of TDP-43 in disease progress [18, 19]. The previous study confirmed that mutant TDP-43 gene expressed in motor neurons is sufficient to induce ALS-like symptoms [8]. In this study, we used CAG-tTA/TRE-TDP-43M337V double transgenic rats that express mutant human TDP-43 in neurons. Dependent on Tet-responsive element, we regulated the expression of mutant TDP-43 by administration or withdrawal of Dox. We validated that CAG-tTA/TRE-TDP-43M337V double transgenic rats developed progressive severe weakness, atrophy and weight loss. In the end stage, transgenic rat models with Dox- showed significantly reduced motor neurons in spinal cord and apparent neurogenic atrophy, consistent with the pathologic changes in ALS.
Notably, transgenic rats that expressed mutant human TDP-43 at 30 days exhibited more devastating clinical phenotypes as (a) the interval between Dox- and disease onset was shorter, (b) there was an expedited proceeding to terminal stage, (c) their limb asthenia and muscle degeneration were more remarkable. It is unquestionable that the progressive loss of motor neurons plays a critical role in ALS, and significant decreases in motor neurons in spinal cord are observed in ALS transgenic rats. However, there was no significant difference between double transgenic rats. Small sample size may explain the results because there were less motor units in transgenic rats with Dox- 30 days.
It has been well established that the pathogenic mutation and elevated expression of TDP-43 are neurotoxic to motor neurons. Although the detailed mechanisms of mutant TDP-43 induced motor neuron death are unclear, ubiquitin aggregation, gliosis and cytoplasmic accumulation of TDP-43 are all correlated to motor neuron degeneration [10]. However, whether the adverse effect of mutant TDP-43 varied at different periods in developmental process of motor system remains a conundrum. Since the copies of mutant TDP-43 transgene are equal in both groups of transgenic rats, we speculate that normal function of TDP-43 is more crucial to the early development of motor neurons and skeletal muscles.
Increasing evidences suggested that the loss of TDP-43 function is involved in neurodegeneration and ALS symptoms [20, 21]. A motor neuron-specific TDP-43 knockout mice model demonstrated age-dependent motor impairment and morphological abnormalities in the motor neuron system, and the loss of TDP-43 function led to the pathogenesis of ALS [22]. In addition, TDP-43 gene deletion resulted in early embryonic lethality in mammals [23, 24]. Embryonic TDP-43 expression was colocalized with the neuroepithelium that contains neural progenitors [25]. Given that interneuron subtypes and motor neurons were derived of neural progenitors, it can be speculated that mutations in TDP-43 regulation may affect the development central nervous system, including the growth of motor neurons. Since the early development of motor neurons is crucial to motor function, it is possible that earlier disturbance to TDP-43 expression gives rise to developmental anomalies of motor systems and causes more severe ALS-like pathological changes. Therefore, our findings suggest that it is very important to perform early intervention to prevent or delay the progression of TDP-43 induced ALS pathological changes, providing new strategy for ALS treatment.
CONCLUSION
In summary, our study suggests that mutant TDP-43 expressed at juvenile stage of the rats can cause a more aggressive phenotype than mutant TDP-43 expressed at an adult stage of the rats. The normal function of TDP-43 is important to promote the development of motor neurons and skeletal muscles.
Ethics Approval and Consent to Participate
The animal use protocol was approved by the Ethical Review Board from Thomas Jefferson University, PA, USA.
Human and Animal Rights
No Humans were used for studies that are base of this research. All the animals used were in accordance with the standards set forth in the 8th Edition of Guide for the Care and Use of Laboratory Animals (http://grants.nih.gov/grants/olaw/Guide-for-thecare-and-use-of-laboratory-ani-mals.pdf) published by the National Academy of Sciences, The National Academies Press, Washington DC, United States of America.
Consent for Publication
Not applicable.
Fig. (2).
The survival of transgenic rats. (A). The disease onset in two groups of rats deprived of Dox at the age of 30 and 70 days, respectively. (B). The survival in two groups of rats deprived of Dox at the age of 30 and 70 days, respectively. Data are means ± SD (n=6).
Acknowledgements
We would like to thank all participants in this study and Dr. Xugang Xia from the Thomas Jefferson University for laboratory assistance. This work was supported by Natural Science Foundation of China (No. 81401065, 81760238, 81571256) and science fund for young scientists from Xiangya Hospital of China (No. 2013Q09).
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Boillee S., Yamanaka K., Lobsiger C.S., et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312(5778):1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 2.Lim M.A., Selak M.A., Xiang Z., et al. Reduced activity of AMP-activated protein kinase protects against genetic models of motor neuron disease. J. Neurosci. 2012;32(3):1123–1141. doi: 10.1523/JNEUROSCI.6554-10.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buratti E., Baralle F.E. The multiple roles of TDP-43 in pre-mRNA processing and gene expression regulation. RNA Biol. 2010;7(4):420–429. doi: 10.4161/rna.7.4.12205. [DOI] [PubMed] [Google Scholar]
- 4.Berson A., Sartoris A., Nativio R., et al. TDP-43 Promotes neurodegeneration by impairing chromatin remodeling. Curr. Biol. 2017;27(23):3579–3590. doi: 10.1016/j.cub.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herzog J.J., Deshpande M., Shapiro L., Rodal A.A., Paradis S. TDP-43 misexpression causes defects in dendritic growth. Sci. Rep. 2017;7(1):15656. doi: 10.1038/s41598-017-15914-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuiji H., Inoue I., Takeuchi M., et al. TDP-43 accelerates age-dependent degeneration of interneurons. Sci. Rep. 2017;7(1):14972. doi: 10.1038/s41598-017-14966-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braak H., Del Tredici K. Anterior cingulate cortex TDP-43 pathology in sporadic amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 2018;77(1):74–83. doi: 10.1093/jnen/nlx104. [DOI] [PubMed] [Google Scholar]
- 8.Sreedharan J., Blair I.P., Tripathi V.B., et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319(5870):1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou H., Huang C., Chen H., et al. Transgenic rat model of neurodegeneration caused by mutation in the TDP gene. PLoS Genet. 2010;6(3):e1000887. doi: 10.1371/journal.pgen.1000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C., Tong J., Bi F., Zhou H., Xia X.G. Mutant TDP-43 in motor neurons promotes the onset and progression of ALS in rats. J. Clin. Invest. 2012;122(1):107–118. doi: 10.1172/JCI59130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warming S., Costantino N., Court D.L., Jenkins N.A., Copeland N.G. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 2005;33(4):e36. doi: 10.1093/nar/gni035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou H., Huang C., Yang M., et al. Developing tTA transgenic rats for inducible and reversible gene expression. Int. J. Biol. Sci. 2009;5(2):171–181. doi: 10.7150/ijbs.5.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tong J., Huang C., Bi F., et al. Expression of ALS-linked TDP-43 mutant in astrocytes causes non-cell-autonomous motor neuron death in rats. EMBO J. 2013;32(13):1917–1926. doi: 10.1038/emboj.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C., Tong J., Bi F., Zhou H., Xia X.G. Mutant TDP-43 in motor neurons promotes the onset and progression of ALS in rats. J. Clin. Invest. 2012;122(1):107–118. doi: 10.1172/JCI59130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arai T., Hasegawa M., Akiyama H., et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006;351(3):602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 16.Neumann M., Sampathu D.M., Kwong L.K., et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 17.Neumann M., Kwong L.K., Lee E.B., et al. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol. 2009;117(2):137–149. doi: 10.1007/s00401-008-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bilican B., Serio A., Barmada S.J., et al. Mutant induced pluripotent stem cell lines recapitulate aspects of TDP-43 proteinopathies and reveal cell-specific vulnerability. Proc. Natl. Acad. Sci. USA. 2012;109(15):5803–5808. doi: 10.1073/pnas.1202922109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sephton C.F., Good S.K., Atkin S., et al. TDP-43 is a developmentally regulated protein essential for early embryonic development. J. Biol. Chem. 2010;285(9):6826–6834. doi: 10.1074/jbc.M109.061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee E.B., Lee V.M., Trojanowski J.Q. Gains or losses: Molecular mechanisms of TDP43-mediated neurodegeneration. Nat. Rev. Neurosci. 2012;13(1):38–50. doi: 10.1038/nrn3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halliday G., Bigio E.H., Cairns N.J., Neumann M., Mackenzie I.R., Mann D.M. Mechanisms of disease in frontotemporal lobar degeneration: gain of function versus loss of function effects. Acta Neuropathol. 2012;124(3):373–382. doi: 10.1007/s00401-012-1030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iguchi Y., Katsuno M., Niwa J., et al. Loss of TDP-43 causes age-dependent progressive motor neuron degeneration. Brain. 2013;136(Pt5):1371–1382. doi: 10.1093/brain/awt029. [DOI] [PubMed] [Google Scholar]
- 23.Kraemer B.C., Schuck T., Wheeler J.M., et al. Loss of murine TDP-43 disrupts motor function and plays an essential role in embryogenesis. Acta Neuropathol. 2010;119(4):409–419. doi: 10.1007/s00401-010-0659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu L.S., Cheng W.C., Hou S.C., Yan Y.T., Jiang S.T., Shen C.K. TDP-43: A neuro-pathosignature factor, is essential for early mouse embryogenesis. Genesis. 2010;48(1):56–62. doi: 10.1002/dvg.20584. [DOI] [PubMed] [Google Scholar]
- 25.Rowitch D.H. Glial specification in the vertebrate neural tube. Nat. Rev. Neurosci. 2004;5(5):409–419. doi: 10.1038/nrn1389. [DOI] [PubMed] [Google Scholar]