Abstract
Background
Alzheimer’s disease (AD) is a complex neurodegenerative disorder characterized by the progressive loss of neurons, which typically leads to severe impairments in cognitive functions including memory and learning. Key pathological features of this disease include the deposition of highly insoluble amyloid β peptides and the formation of neurofibrillary tangles (NFTs) in the brain. Mounting evidence also implicates sustained glial-mediated inflammation as a major contributor of the neurodegenerative processes and cognitive deficits observed in AD.
Methods
This paper provides an overview of findings from both human and animal studies investigating the role of microglia and astrocytes in AD, and discusses potential avenues for therapeutic intervention.
Results
Glial-mediated inflammation is a ‘double-edged sword’, performing both detrimental and beneficial functions in AD. Despite tremendous effort in elucidating the molecular and cellular mechanisms underlying AD pathology, to date, there is no treatment that could prevent or cure this disease. Current treatments are only useful in slowing down the progression of AD and helping patients manage some of their behavioral and cognitive symptoms.
Conclusion
A better understanding of the role of microglia and astrocytes in the regulation of AD pathology is needed as this could pave the way for new therapeutic strategies.
Keywords: Alzheimer’s disease, astrocytes, cytokines, glial cells, inflammation, microglia
1. INTRODUCTION
Alzheimer’s disease (AD) is the leading cause of dementia in the elderly population, affecting approximately 24 million people worldwide [1]. First described by Alois Alzheimer in the early 20th century, AD is characterized by severe cognitive deficits including memory loss and language impairment, leading to increasing dependence in everyday life [2]. Neuropsychiatric symptoms such as depression, apathy and hallucinations are also frequently observed in the AD population, thus imposing even heavier burdens on affected individuals and families [3]. However, even if there are indubitably psychiatric symptoms in AD, this disease is more commonly characterized as a neurological or neurodegenerative disorder inasmuch as its key pathological hallmarks include neuronal loss and cellular dysfunction [4]. The amyloid cascade hypothesis, which was formulated in 1992, posits that the main pathological event leading to neuronal loss and dementia in AD is the formation of β-amyloid (Aβ) in the brain, which ultimately leads to the deposition of extracellular amyloid plaques [5]. The production of Aβ is the result of the proteolytic cleavage of β-amyloid precursor protein (APP) by two enzymes: Beta-secretase 1 (BACE1) and γ-secretase [6]. In addition to exerting detrimental effects to surrounding neurons, the accumulation of Aβ in the brain can lead to a series of events including the hyperphosphorylation of the microtubule-associated protein tau and the formation of neurofibrillary tangles (NFTs), both of which highly contribute to the neurodegenerative processes in AD [7, 8].
Over the past few years, the amyloid cascade hypothesis has been the prevailing concept for explaining AD pathogenesis. However, several lines of investigation are now supporting the view that inflammation may be the key neuropathological event leading to neurodegeneration in AD. Indeed, a number of studies have observed elevated cytokine levels in the brain of individuals with AD [9, 10] and animal models of the disease [11-13]. There is also abundant evidence showing that activation of glial cells, including microglia and astrocytes, plays an important role in eliciting the inflammatory signalling pathway involved in neurodegeneration [14, 15]. Also noteworthy are studies showing that reactive astrocytes and microglia are particularly found in high number near senile plaques of individuals with AD [16-18], suggesting a role for these immune cells in the pathogenesis of AD. However, despite significant progress in research, whether the glial-mediated inflammatory response observed in AD is a consequence or a cause of neurodegeneration is still a subject of debate.
Given the high burden of AD to the affected individual and to the society in terms of the ensued health care costs, a better understanding of the pathophysiological mechanisms of this disorder is urgently needed as this could help promote the development of new therapeutic strategies. The overarching aim of this paper is to provide a thorough overview of the contribution of microglia and astrocytes in inflammation and AD pathogenesis by illustrating key findings from animal and human studies. Potential avenues for therapeutic interventions are also discussed.
2. MICROGLIA DURING HEALTHY AND INFLAMMATORY CONDITIONS
Microglia are resident macrophages that represent approximately 10% of all the cells in the central nervous system (CNS) [19]. Being one of the first immune cells that get immunologically active during an inflammatory reaction, they constitute the first line of cellular defence against invading pathogens and other types of brain injury [20]. Despite being extensively studied, their true origin remains a subject of debate. Early evidence suggested that microglia differentiate in the bone marrow from embryonic hematopoietic precursor cells, whereas more recently, studies have shown that these cells may in fact arise from progenitors in the embryonic yolk sac early during development [21]. Under normal conditions, microglia exist in a quiescent (or resting) state, and are morphologically characterized by small-shaped soma and highly ramified processes [22]. One of the main functions of resting state microglia is to vigilantly monitor the CNS for the detection of pathogens and host-derived ligands, including pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) [20, 23]. The expression of pattern recognition receptors (PRRs) on their molecular surface makes them well equipped for this purpose [24]. In response to invading pathogens, microglia get activated and undergo morphological changes including enlargement of their soma and shortening of their cellular processes [25]. Activated microglia play an important role in the phagocytosis of pathogens and in the clearance of cellular debris and degenerating cells at the lesion site [26]. In addition to their phagocytic activity, activated microglia participate in the presentation of antigens to T cells, thereby coordinating the dialogue between the innate and adaptive immune systems during an inflammatory response [27, 28].
Mounting evidence points to the fact that microglia-mediated inflammatory response is a “double-edged sword”, executing both detrimental and beneficial functions [29, 30]. When activated, microglia produce inflammatory mediators including cytokines, chemokines, inducible nitric oxide synthase (NOS), cyclooxygenase-2 (COX-2) and free radicals like reactive oxygen species (ROS), which may disturb neuronal functions and produce cellular damage [20, 31]. Activated microglia also produce a wide array of neuroprotective factors that help prevent neuronal injury, including brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor (GDNF) and nerve growth factor (NGF) [31-33]. This duality in the effect of microglia on immune-mediated inflammation suggests that these immune cells adopt different functional phenotypes based on their surrounding environment. Depending on their activation state, microglial cells have been broadly classified into a proinflammatory M1 phenotype or an anti-inflammatory M2 phenotype [34]. However, it should be noted that this classification is not universally accepted by research findings, and there are still a lot to learn about the mechanisms underlying microglia functions during these different activation states [35].
The M1 phenotype is “classically activated” by Toll-like receptors or interferon-gamma (IFNγ), and plays a vital role in destroying invading pathogens by producing proinflammatory cytokines, ultimately causing neuronal damage in local tissues [14, 20]. In contrast, the M2 phenotype is “alternatively activated” by interleukin 4 (IL-4) or IL-13, and is involved in the release of high levels of anti-inflammatory cytokines, thus playing fundamental roles in tissue repair and angiogenesis [14]. Interestingly, microglia that have been polarized into an M1 or M2 state can rapidly switch their phenotype in order to adapt to their surrounding microenvironment, thus providing researchers with the possibility of targeting imbalances of macrophage polarization for various therapeutic applications [36]. For instance, M1 macrophages can be polarized to M2-like macrophages following experimental manipulations that inhibit the PI3K/AKT signalling pathway [37] or the NF-κB, MAPK and AKT pathways [38], whereas M2 macrophages can be reprogrammed into an M1 phenotype in response to lipopolysaccharide (LPS) and IFNγ [39, 40].
3. MICROGLIA IN ALZHEIMER’S DISEASE
There is an extensive number of studies indicating that inflammatory pathways are altered in AD owing to exacerbated immune response [41, 42]. The observation that inflammatory processes may promote neuronal loss and cognitive decline [43, 44], together with evidence associating polymorphic variations of inflammatory cytokines with AD [45-47], argue for a potential role of microglia in AD pathogenesis. Microglia are one of the first immune cells that get activated and recruited to the site of injury during an inflammatory response. Understanding how they are involved in AD could not only help decipher the cellular and molecular mechanisms underlying neurodegeneration, but could also open up new avenues for therapeutic interventions. One of the first ground-breaking findings implicating microglia in AD date from studies in the early 1990s showing that these immune cells are highly engaged in the formation of Aβ plaques in the brains of AD patients [41, 42]. More recently, data from studies utilizing animal models of AD have also demonstrated the presence of activated microglia at sites of Aβ deposition, suggesting that these glial cells might physically interact with Aβ and regulate their levels in the brain [43, 44].
Further evidence providing a link between microglia dysfunction and AD pathogenesis comes from genetic studies showing that a null mutation in TREM2 (Triggering Receptor Expressed on Myeloid cells 2) gene, which is specifically expressed by microglia in the CNS, is associated with severe neuritic tau hyperphosphorylation and reduced ability of microglia to envelop amyloid deposit [48]. In addition, genetic deletion of the complement factors C1q and C3, or the microglial complement receptor CR3, reduces the number of phagocytic microglia and the degree of early synapse loss, suggesting that complement activation can act as an early mediator of plaque associated synapse loss in AD by triggering the activation of phagocytic microglia [49]. Microglia and CR3 also play a crucial role in Aβ homeostasis inasmuch as ablation of CR3 in APP-transgenic mice leads to decreased Aβ accumulation, most likely as a result of increased secretion of Aβ-degrading enzymes and increased ability of microglia to degrade extracellular Aβ [50]. However, despite significant progress in the understanding of the interaction between microglia and Aβ in AD, whether the accumulation of Aβ in the brain precedes microglia activation still remains a subject of debate [51-54].
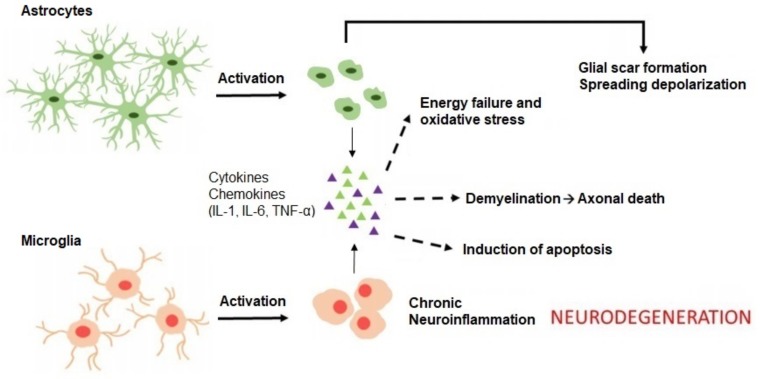
Microglia interact with Aβ, but also with APP, through specific PRRs, including CD14, CD36 and Toll-like receptors, which are highly expressed on their surface [55-58]. This interaction is required for phenotypic activation of microglia and induction of phagocytosis, and results in the clearance of Aβ from the brain [20, 59]. Inductors of inflammation, such as LPS, also activate microglia to promote the degradation of Aβ [60]. Consistent with the view that microglia are involved in Aβ clearance, impairment of microglia function in transgenic mice facilitates the progression of AD and results in increased Aβ accumulation in the brain [61, 62]. Besides providing beneficial effects to the host, activation of microglia by Aβ or APP also results in an upregulation of inflammatory mediators including inducible nitric oxide synthase (iNOS), tumor necrosis factor-alpha (TNF-α), interleukin-1beta (IL-1β) and IL-6, ultimately leading to exacerbated inflammatory response and severe neuronal loss [63-65] (Fig. 1).
Fig. (1).
Detrimental effects of glial-mediated inflammation. Activation of microglia and astrocytes by Aβ or following a signal of damage leads to the secretion and release of inflammatory chemokines and cytokines, including IL-1, IL-6, and TNF-α. These pro-inflammatory elements trigger a cascade of events, such as oxidative stress, demyelination and apoptosis, which eventually lead to neurodegeneration and cognitive decline. Reactive astrocytes also contribute to scar formation around injured tissue by accumulating around amyloid plaques. Adapted with permission from [65]. (The color version of the figure is available in the electronic copy of the article).
Taken together, the aforementioned studies suggest that microglia exert dual functions in AD in a context-dependent manner. While moderate activation of microglia provides protective effects by facilitating the clearance of Aβ in the brain, overactivation of these cells by Aβ or APP could trigger an exaggerated inflammatory response that may worsen the neurodegenerative processes in AD. However, despite significant progress in research, very few reports have investigated the relationship between microglia and the formation of NFTs in AD. Although studies thus far seem to point for a role of microglia in the internalization and degradation of tau, the major component of NFTs [66, 67], further investigations are warranted for a better understanding of the molecular mechanisms underlying the role of microglia in AD pathogenesis.
4. ASTROCYTES DURING HEALTHY AND INFLAMMATORY CONDITIONS
Astrocytes are the most abundant glial subtype in the CNS, and similar to microglia, play a crucial role in the regulation of neuroinflammation [68]. Also referred to as astroglia, astrocytes exhibit a star-shaped morphology with cellular processes extending from the soma [69]. In the healthy CNS, astrocytes perform several physiological functions involved in ion homeostasis, neurotransmitter transmission, growth factor secretion, synaptic remodeling, and oxidative stress regulation [42, 70]. In addition, astrocytes play a fundamental role in the protection and differentiation of dopaminergic neurons [71, 72], and have been associated with CNS pathologies like schizophrenia [73, 74] and Parkinson’s disease [75, 76], where dopamine neurotransmission is incriminated.
Because of their close proximity to blood vessels and their interaction with endothelial cells, astrocytes also participate in the maintenance and permeability of the blood-brain barrier (BBB), a multi-cellular unit involved in the exchange of molecules in and out of the brain [77, 78]. Anatomically, astrocytic terminal processes, known as endfeet, almost completely cover the outer surface of the endothelium, forming a lacework of fine lamellae [77]. Through the release of soluble factors such as GDNF, transforming growth factor-beta (TGFβ), basic fibroblast growth factor (bFGF) and angiopoetin-1 (ANG-1), astrocytic endfeet participate in the regulation of angiogenesis and in the formation of endothelial cell-to-cell junctions, thus preserving the function and structural integrity of the BBB [79, 80]. Astrocytic expression of growth factors and cytokines also tightly regulates the permeability of the BBB during inflammatory conditions, and in doing so help control the passage of immune cells into the CNS [81, 82].
Upon activation by pathogens, astrocytes produce a wide array of inflammatory cytokines that can have beneficial or detrimental consequences. In addition, astrocytes express major histocompatibility complex (MHC) class II molecules on their surface, thus acting as antigen-presenting cells for T cells [83, 84]. Depending on their surrounding environment and activation state, astrocytes either suppress [85, 86] or enhance [87, 88] T-cell functions. Although astrocytes are mainly neuroprotective [89], they participate in perpetuating the self-destructive environment by secreting various chemokines and proinflammatory cytokines, including IL-1β and TNF-α [90, 91]. In addition, astrocytes have the capacity to physically interact with microglia, thereby exerting a significant control over their activation [92], phagocytic capacity [93], and ability to secrete inflammatory mediators such as TNF-α [94], IL-12 [95] and iNOS [96].
5. ASTROCYTES IN ALZHEIMER’S DISEASE
Early evidence implicating astrocytes in the pathological processes of AD comes from the observation that these glial cells are associated with senile plaques in the brains of AD patients [97]. More recently, studies have reported profound astrogliosis in the brain of animal models of AD [98] and AD patients [99], where reactive astrocytes accumulate around amyloid plaques via phagocytosis of local degenerated dendrites and synapses, encircling Aβ deposits in a manner reminiscent of glial scarring [42, 65] (Fig. 1). Upon activation by Aβ or following a signal of damage or injury, astrocytes also participate in the secretion of inflammatory cytokines including IL-1, IL-6, and TNF-α, thereby promoting the neurodegenerative processes in AD [65] (Fig. 1). Although the mechanisms by which astrocytes react with Aβ remain largely elusive, astrocytes express a wide array of receptors, including the receptor for advanced glycation endproducts (RAGE), lipoprotein receptor-related proteins (LRPs), membrane-associated proteoglycans and scavenger receptor-like receptors, which recognize and bind to Aβ [42, 100]. On the other hand, Aβ aggregates can stimulate the production of chemotactic molecules including monocyte chemoattractant protein-1 (MCP-1), which help mediate the recruitment of astrocytes to the site of lesion [101, 102]. In addition to promoting the accumulation of immune cells in and around senile plaques, Aβ also contributes substantially to the inflammatory processes mediated by astrocytes. For instance, isolated senile plaques or Aβ aggregates from human AD brains lead to reactive astrogliosis when co-cultured with glial cells [93]. Aβ also activates astroglial nuclear factor-kappa B (NFκB) and complement signalling to impair synaptic density and dendritic morphology [103], and potentiates the production of inflammatory mediators by astrocytes in response to scavenger receptors ligands [104] and LPS [105], thereby contributing to the neurodegenerative changes observed in AD.
The effect of astrocytes on Aβ in AD remains a subject of controversy. Numerous studies have indicated that reactive astrocytes participate in the clearance of Aβ in vitro, suggesting a direct role for these glial cells in the attenuation of the neurodegenerative processes in AD [102, 106, 107]. In transgenic mice with AD-like pathology, the astrocyte-mediated clearance of Aβ is mediated by the increased expression of neprilysin [108] and insulin-degrading enzyme [109]. Extracellular brain clearance of Aβ is also promoted by the secretion of matrix metalloproteinase (MMP)-2 and MMP-9 by astrocytes [110]. However, despite being effective in mediating the degradation of amyloid plaques, astrocytes could also produce Aβ under certain inflammatory conditions. For instance, TGF-β1 alone [111] or IFN-γ in combination with TNF-α [112, 113] or IL-1β [113] drives the production of Aβ by astrocytes. Astrocytes could also engulf large amounts of Aβ that are partly digested, eventually leading to astrocytic defects and neuronal apoptosis [114]. Moreover, astrocytes can release many trophic factors that may exert either beneficial or detrimental functions in AD. For instance, GDNF secreted from astrocytes improves neuronal function and cognitive performance in aged rats [115], whereas overexpression of NGF by astrocytes leads to neurotoxicity and the degenerative loss of hippocampal neurons in-vitro [116].
Last but not least are studies implicating astrocytes and other glial cells in the evolution of NFTs in AD. In the parahippocampal cortex of AD patients, the number of activated astrocytes correlates with the number of tangles and the stage of NFTs formation, suggesting a role for astrocyte activation in the progression of NFTs in AD [117]. In addition, thrombin, a serine protease expressed by astrocytes and microglia, accumulates in NFTs [118] and participates in the cleavage of tau [119]. Although these studies propose a potential mechanistic pathway by which activated astrocytes may dampen the neurodegenerative processes in AD, more work is required to better understand the cellular mechanisms underlying the formation and progression of NFTs.
6. CLINICAL AND THERAPEUTIC IMPLICATIONS
Based on the compelling evidence implicating glial-mediated inflammation in AD, numerous studies have explored the possibility of using anti-inflammatory drugs to prevent or halt neurodegeneration. In particular, non-steroidal anti-inflammatory drugs (NSAIDs) have shown beneficial effects in reducing glial cell activation and slowing the progression of AD in animal models of the disease [120, 121]. Although the mechanisms of actions of NSAIDs in AD remain to be fully determined, these drugs bind to and activate the peroxisome proliferator-activated receptor-gamma (PPAR-γ) [122, 123] leading to reduced glial cells activation [124, 125] and cytokine-mediated inflammation [126, 127]. In mice overexpressing APP, a transgenic model of AD, treatment with the NSAID ibuprofen results in reduced microglial activation and amyloid plaque load [128, 129]. Consistently, a number of other NSAIDs were shown to selectively lower Aβ42 levels in the brain of transgenic mice as a result of decreased activity of γ-secretase, the enzyme responsible for the generation of Aβ from APP [130]. The view that NSAIDs delay some forms of AD pathology is also supported by in-vitro studies showing that these anti-inflammatory drugs selectively prevent the accumulation of Aβ peptides in culture cells, likely through a decrease in APP production or metabolism [131, 132]. Similar to NSAIDs, PPAR-γ agonists such as pioglitazone or GFT1803 show beneficial effects in attenuating the neurodegenerative processes of AD, namely by reducing Aβ plaque deposition and glial cells activation [133, 134]. However, despite robust preclinical evidence highlighting the protective effects of NSAIDs in AD, clinical trials of these drugs for the treatment of AD have mostly been disappointing so far [135-138], most likely due to the fact that NSAIDs’ effect may differ depending on whether they are used in early or late stages of disease [120] (Fig. 2). Glucocorticoids, a class of corticosteroids, have also been investigated for the treatment of AD due to their anti-inflammatory and immunosuppressive properties. In-vitro studies show that glucocorticoids inhibit cortical astrocyte proliferation [139] and exert neuroprotective functions against inflammation by down-regulating the production of nitric oxide (NO) from microglia [140]; effects that are reversed upon the addition of RU-486, a glucocorticoid receptor blocker [139, 140]. Glucocorticoids were also shown to inhibit both Aβ and LPS-induced pro-inflammatory cytokine and chemokine production in mice [141]. However, notwithstanding the beneficial effects of glucocorticoids in-vitro and in-vivo, clinical trials have failed to observed notable differences in cognitive decline between glucocorticoid-treated and placebo-treated patients [142], thus urging the need for more efficient therapeutic approaches.
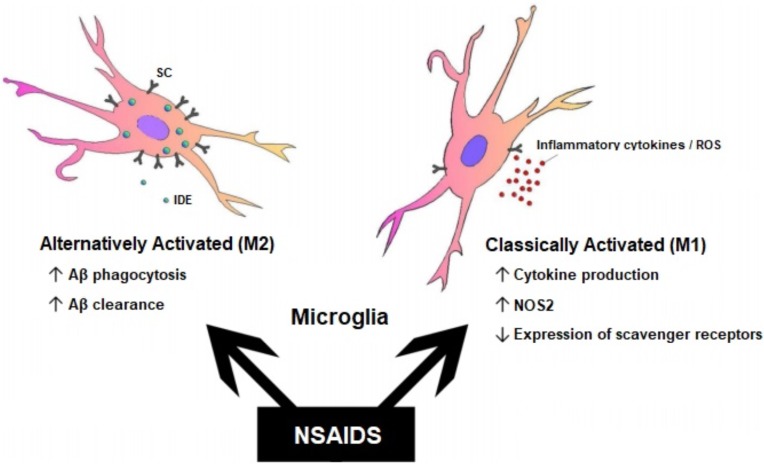
Fig. (2).
Differential effects of NSAIDs on microglia and AD pathogenesis. The therapeutic effects of NSAIDs may differ depending on the stage of AD. Alternatively activated (M2) microglia are present during the early stage of the disease, whereas classically activated (M1) microglia are present during the late stage of the disease. Furthermore, different subsets of NSAIDs have different affinity for immune and inflammatory targets in the brain, thus resulting in a range of effects including reduced inflammatory mediators and altered Aβ production. Abbreviations: insulin degrading enzyme (IDE); scavenger receptors (SC). Adapted with permission from [120].
As discussed earlier, many inflammatory responses mediated by microglia and astrocytes exert protective functions in AD. Therefore, directing or instructing the machinery responsible for the activation of these glial cells may prove more beneficial than supressing it. Notably, studies employing mouse models of AD have shown that injection of LPS [143] or delivery of gamma oscillations [144] in the hippocampus increases the activation of resident microglial cells and significantly reduces the cerebral Aβ load within the brain parenchyma. The view that microglial activation may be beneficial in AD is also bolstered by studies showing that stimulation of microglia with macrophage colony-stimulating factor (M-CSF) increases the phagocytosis of opsonized aggregated Aβ in culture medium [145], and improves cognitive functions in mice with AD-like pathology [146]. However, under other circumstances, glial cells activation can have deleterious roles in AD, and experimental manipulations that inhibit their activation or signalling may prove more effective in ameliorating cognitive functions. In APP/PS1 mice, a well-established model of AD, inhibition of astrocytes signalling with adeno-associated virus vectors [147] and selective suppression of astrocytic gamma-Aminobutyric acid (GABA) synthesis resulted in improved cognitive functions including learning and memory [148]. In another study, Heneka and colleagues showed that NLRP3 inflammasome inhibition in the APP/PS1 model of AD skews microglial cells to an M2 phenotype and results in enhanced spatial memory and decreased deposition of Aβ [149]. In addition, inhibition of Aβ-induced microglial activation resulted in increased protection against cell injury and toxicity [150], decreased proinflammatory genes expression [151], and enhanced level of neurotrophic factors [63] in-vitro. Together, these findings concur with the view that glial cells exert both protective and detrimental functions in AD, and suggest that the regulation of their activity and function might be an appealing way to promote neuroprotection and prevent cognitive decline.
Although the strategy to modulate glial cells activation has shown great potential in promoting neuroprotection in AD, its proper use has been limited by the fact that the microenvironment surrounding microglia and astrocytes during chronic neuroinflammation may impair their function. Another avenue of therapeutic intervention that might be more appealing in AD is the transplantation of bone marrow (BM)-derived precursor cells from healthy donors. The rationale behind this approach is based from the observation that microglia derived from BM progenitor cells are more competent in eliminating amyloid plaques compared to their resident counterparts [152, 153]. In a mouse model of AD, intracerebral transplantation of BM-derived mesenchymal stem cells (MSCs) restored defective microglial function and resulted in reduced Aβ deposition, decreased tau hyperphosphorylation, and improved cognitive functions [154]. Consistent with these findings, intracerebral transplantation of BM-MSCs in APP/PS1 mice promoted the differentiation of resident microglia into an M2 phenotype, which resulted in marked reductions of Aβ deposition and memory impairments [155]. MSCs derived from other sources, including adipose tissues [156] and human umbilical cord blood [157], have also been shown to provide beneficial effects in experimental AD in terms of promoting learning and memory recovery. The finding that transplanted stem cells or neural precursor cells survive and exert beneficial properties in-vivo constitutes a major step towards the development of novel and more efficient approaches for the treatment of AD. However, notwithstanding the beneficial effects of cell therapy in animal models of AD, further studies are needed to investigate its safety profile and long-term efficiency, notably in clinical settings.
CONCLUSION
The studies showcased in the present review support the notion that glial-mediated inflammation is a double-edged sword, performing both detrimental and beneficial functions. The response of microglia and astrocytes to CNS insults is regulated in a context-dependent manner by specific inflammatory mediators that dictate their functional phenotype. While some studies have indicated that glial activation prevents the progression of AD by facilitating the clearance of Aβ in the brain, others have shown that impaired or exacerbated glial activation increases the production of proinflammatory cytokines and Aβ in the brain. This duality in the effects of glial-mediated inflammation on the progression of AD-related pathologies have prompted investigators to explore different—and sometimes opposite—strategies for the treatment of AD. However, despite significant progress towards the development of new therapeutic approaches in animal models of AD, there is still no cure for this disease in humans, and patients are left with the same choices and disappointing prognosis they faced decades ago. It is therefore essential for future studies to continue characterizing the mechanisms of glial-mediated inflammation in AD, including potential cross-talk between different cellular signalling. A better interpretation of data from animal studies and their relevance in the context of human health is also needed, as this could open the way to numerous opportunities in terms of potential implications in the clinic.
Acknowledgements
The author is recipient of an award from the Natural Sciences and Engineering Research Council (NSERC) of Canada.
Consent for Publication
Not applicable.
Conflict of Interest
The author declares no conflict of interest, financial or otherwise.
References
- 1.Mayeux R., Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2(8):a006239. doi: 10.1101/cshperspect.a006239. [http://dx. doi.org/10.1101/cshperspect.a006239]. [PMID: 22908189]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarawneh R., Holtzman D.M. The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment. Cold Spring Harb. Perspect. Med. 2012;2(5):a006148. doi: 10.1101/cshperspect.a006148. [http://dx.doi. org/10.1101/cshperspect.a006148]. [PMID: 22553492]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyketsos C.G., Carrillo M.C., Ryan J.M., Khachaturian A.S., Trzepacz P., Amatniek J., Cedarbaum J., Brashear R., Miller D.S. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement. 2011;7(5):532–539. doi: 10.1016/j.jalz.2011.05.2410. [http://dx.doi.org/10.1016/j. jalz.2011.05.2410]. [PMID: 21889116]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gzil F. Alzheimer’s disease: psychiatric or neurological disorder? Poiesis Prax. 2009;6(1):13–26. [http://dx.doi.org/10.1007/s10202-008-0061-3]. [Google Scholar]
- 5.Hardy J.A., Higgins G.A. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [http://dx. doi.org/10.1126/science.1566067]. [PMID: 1566067]. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien R.J., Wong P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [http://dx.doi.org/10.1146/annurev-neuro-061010-113613]. [PMID: 21456963]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohandas E., Rajmohan V., Raghunath B. Neurobiology of Alzheimer’s disease. Indian J. Psychiatry. 2009;51(1):55–61. doi: 10.4103/0019-5545.44908. [http://dx.doi.org/10.4103/0019-5545.44908]. [PMID: 19742193]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niedowicz D.M., Nelson P.T., Murphy M.P. Alzheimer’s disease: pathological mechanisms and recent insights. Curr. Neuropharmacol. 2011;9(4):674–684. doi: 10.2174/157015911798376181. [http://dx.doi.org/10.2174/ 157015911798376181]. [PMID: 22654725]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ojala J., Alafuzoff I., Herukka S.K., van Groen T., Tanila H., Pirttilä T. Expression of interleukin-18 is increased in the brains of Alzheimer’s disease patients. Neurobiol. Aging. 2009;30(2):198–209. doi: 10.1016/j.neurobiolaging.2007.06.006. [http://dx.doi.org/10.1016/j.neurobiolaging.2007.06.006]. [PMID: 17658666]. [DOI] [PubMed] [Google Scholar]
- 10.Morimoto K., Horio J., Satoh H., Sue L., Beach T., Arita S., Tooyama I., Konishi Y. Expression profiles of cytokines in the brains of Alzheimer’s disease (AD) patients compared to the brains of non-demented patients with and without increasing AD pathology. J. Alzheimers Dis. 2011;25(1):59–76. doi: 10.3233/JAD-2011-101815. [PMID: 21368376]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benzing W.C., Wujek J.R., Ward E.K., Shaffer D., Ashe K.H., Younkin S.G., Brunden K.R. Evidence for glial-mediated inflammation in aged APP(SW) transgenic mice. Neurobiol. Aging. 1999;20(6):581–589. doi: 10.1016/s0197-4580(99)00065-2. [http://dx.doi.org/10.1016/S0197-4580(99) 00065-2]. [PMID: 10674423]. [DOI] [PubMed] [Google Scholar]
- 12.Apelt J., Schliebs R. Beta-amyloid-induced glial expression of both pro- and anti-inflammatory cytokines in cerebral cortex of aged transgenic Tg2576 mice with Alzheimer plaque pathology. Brain Res. 2001;894(1):21–30. doi: 10.1016/s0006-8993(00)03176-0. [http://dx.doi.org/10.1016/S0006-8993(00)03176-0]. [PMID: 11245811]. [DOI] [PubMed] [Google Scholar]
- 13.Patel N.S., Paris D., Mathura V., Quadros A.N., Crawford F.C., Mullan M.J. Inflammatory cytokine levels correlate with amyloid load in transgenic mouse models of Alzheimer’s disease. J. Neuroinflammation. 2005;2(1):9. doi: 10.1186/1742-2094-2-9. [http://dx.doi.org/10.1186/1742-2094-2-9]. [PMID: 15762998]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W.Y., Tan M.S., Yu J.T., Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015;3(10):136. doi: 10.3978/j.issn.2305-5839.2015.03.49. [PMID: 26207229]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agostinho P., Cunha R.A., Oliveira C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr. Pharm. Des. 2010;16(25):2766–2778. doi: 10.2174/138161210793176572. [http://dx.doi.org/10.2174/ 138161210793176572]. [PMID: 20698820]. [DOI] [PubMed] [Google Scholar]
- 16.McGeer P.L., Itagaki S., Tago H., McGeer E.G. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci. Lett. 1987;79(1-2):195–200. doi: 10.1016/0304-3940(87)90696-3. [http://dx.doi.org/10.1016/0304-3940(87)90696-3]. [PMID: 3670729]. [DOI] [PubMed] [Google Scholar]
- 17.Zotova E., Holmes C., Johnston D., Neal J.W., Nicoll J.A., Boche D. Microglial alterations in human Alzheimer’s disease following Aβ42 immunization. Neuropathol. Appl. Neurobiol. 2011;37(5):513–524. doi: 10.1111/j.1365-2990.2010.01156.x. [http://dx.doi.org/10.1111/j.1365-2990.2010.01156.x]. [PMID: 21166690]. [DOI] [PubMed] [Google Scholar]
- 18.Shao Y., Gearing M., Mirra S.S. Astrocyte-apolipoprotein E associations in senile plaques in Alzheimer disease and vascular lesions: a regional immunohistochemical study. J. Neuropathol. Exp. Neurol. 1997;56(4):376–381. doi: 10.1097/00005072-199704000-00006. [http://dx.doi.org/10.1097/ 00005072-199704000-00006]. [PMID: 9100668]. [DOI] [PubMed] [Google Scholar]
- 19.Lawson L.J., Perry V.H., Dri P., Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39(1):151–170. doi: 10.1016/0306-4522(90)90229-w. [http://dx.doi. org/10.1016/0306-4522(90)90229-W]. [PMID: 2089275]. [DOI] [PubMed] [Google Scholar]
- 20.Solito E., Sastre M. Microglia function in Alzheimer’s disease. Front. Pharmacol. 2012;3:14. doi: 10.3389/fphar.2012.00014. [http://dx.doi.org/10.3389/fphar. 2012.00014]. [PMID: 22363284]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ginhoux F., Lim S., Hoeffel G., Low D., Huber T. Origin and differentiation of microglia. Front. Cell. Neurosci. 2013;7:45. doi: 10.3389/fncel.2013.00045. [http://dx.doi.org/10.3389/fncel.2013.00045]. [PMID: 23616747]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hristovska I., Pascual O. Deciphering resting microglial morphology and process motility from a synaptic prospecT. Front. Integr. Nuerosci. 2016;9:73. doi: 10.3389/fnint.2015.00073. [http://dx.doi.org/10.3389/fnint.2015.00073]. [PMID: 26834588]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fakhoury M. Immune-mediated processes in neurodegeneration: where do we stand? J. Neurol. 2016;263(9):1683–1701. doi: 10.1007/s00415-016-8052-0. [http://dx. doi.org/10.1007/s00415-016-8052-0]. [PMID: 26872669]. [DOI] [PubMed] [Google Scholar]
- 24.Kigerl K.A., de Rivero V.J.P., Dietrich W.D., Popovich P.G., Keane R.W. Pattern recognition receptors and central nervous system repair. Exp. Neurol. 2014;258:5–16. doi: 10.1016/j.expneurol.2014.01.001. [http://dx.doi.org/10. 1016/j.expneurol.2014.01.001]. [PMID: 25017883]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Town T., Nikolic V., Tan J. The microglial “activation” continuum: from innate to adaptive responses. J. Neuroinflammation. 2005;2:24. doi: 10.1186/1742-2094-2-24. [http://dx.doi.org/10.1186/1742-2094-2-24]. [PMID: 16259628]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sierra A., Beccari S., Diaz-Aparicio I., Encinas J.M., Comeau S., Tremblay M.E. Surveillance, phagocytosis, and inflammation: how never-resting microglia influence adult hippocampal neurogenesis. Neural Plast. 2014;2014:610343. doi: 10.1155/2014/610343. [http://dx.doi.org/10. 1155/2014/610343]. [PMID: 24772353]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaked I., Porat Z., Gersner R., Kipnis J., Schwartz M. Early activation of microglia as antigen-presenting cells correlates with T cell-mediated protection and repair of the injured central nervous system. J. Neuroimmunol. 2004;146(1-2):84–93. doi: 10.1016/j.jneuroim.2003.10.049. [http://dx.doi. org/10.1016/j.jneuroim.2003.10.049]. [PMID: 14698850]. [DOI] [PubMed] [Google Scholar]
- 28.Perry V.H., Nicoll J.A., Holmes C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010;6(4):193–201. doi: 10.1038/nrneurol.2010.17. [http://dx.doi. org/10.1038/nrneurol.2010.17]. [PMID: 20234358]. [DOI] [PubMed] [Google Scholar]
- 29.Hanisch U.K., Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. [http://dx.doi.org/10.1038/nn1997]. [PMID: 17965659]. [DOI] [PubMed] [Google Scholar]
- 30.Sierra A., Abiega O., Shahraz A., Neumann H. Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front. Cell. Neurosci. 2013;7:6. doi: 10.3389/fncel.2013.00006. [http://dx.doi.org/ 10.3389/fncel.2013.00006]. [PMID: 23386811]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzumura A. Neuron-microglia interaction in neuroinflammation. Curr. Protein Pept. Sci. 2013;14(1):16–20. doi: 10.2174/1389203711314010004. [http://dx.doi.org/10. 2174/1389203711314010004]. [PMID: 23544747]. [DOI] [PubMed] [Google Scholar]
- 32.Glezer I., Simard A.R., Rivest S. Neuroprotective role of the innate immune system by microglia. Neuroscience. 2007;147(4):867–883. doi: 10.1016/j.neuroscience.2007.02.055. [http://dx.doi.org/10.1016/j.neuroscience.2007.02.055]. [PMID: 17459594]. [DOI] [PubMed] [Google Scholar]
- 33.Ding Y.M., Jaumotte J.D., Signore A.P., Zigmond M.J. Effects of 6-hydroxydopamine on primary cultures of substantia nigra: specific damage to dopamine neurons and the impact of glial cell line-derived neurotrophic factor. J. Neurochem. 2004;89(3):776–787. doi: 10.1111/j.1471-4159.2004.02415.x. [http://dx.doi.org/10.1111/j.1471-4159.2004.02415.x]. [PMID: 15086533]. [DOI] [PubMed] [Google Scholar]
- 34.Tang Y., Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 2016;53(2):1181–1194. doi: 10.1007/s12035-014-9070-5. [http://dx.doi.org/10.1007/s12035-014-9070-5]. [PMID: 25598354]. [DOI] [PubMed] [Google Scholar]
- 35.Ransohoff R.M. A polarizing question: do M1 and M2 microglia exist? Nat. Neurosci. 2016;19(8):987–991. doi: 10.1038/nn.4338. [http://dx.doi.org/10. 1038/nn.4338]. [PMID: 27459405]. [DOI] [PubMed] [Google Scholar]
- 36.Wang N., Liang H., Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [http://dx.doi.org/10.3389/fimmu.2014.00614]. [PMID: 25506346]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hyam S.R., Lee I.A., Gu W., Kim K.A., Jeong J.J., Jang S.E., Han M.J., Kim D.H. Arctigenin ameliorates inflammation in vitro and in vivo by inhibiting the PI3K/AKT pathway and polarizing M1 macrophages to M2-like macrophages. Eur. J. Pharmacol. 2013;708(1-3):21–29. doi: 10.1016/j.ejphar.2013.01.014. [http://dx.doi.org/10.1016/j.ejphar.2013. 01.014]. [PMID: 23375938]. [DOI] [PubMed] [Google Scholar]
- 38.Jang S.E., Hyam S.R., Han M.J., Kim S.Y., Lee B.G., Kim D.H. Lactobacillus brevis G-101 ameliorates colitis in mice by inhibiting NF-κB, MAPK and AKT pathways and by polarizing M1 macrophages to M2-like macrophages. J. Appl. Microbiol. 2013;115(3):888–896. doi: 10.1111/jam.12273. [http://dx.doi.org/10.1111/jam.12273]. [PMID: 23742179]. [DOI] [PubMed] [Google Scholar]
- 39.Mylonas K.J., Nair M.G., Prieto-Lafuente L., Paape D., Allen J.E. Alternatively activated macrophages elicited by helminth infection can be reprogrammed to enable microbial killing. J. Immunol. 2009;182(5):3084–3094. doi: 10.4049/jimmunol.0803463. [http://dx.doi.org/10.4049/ jimmunol.0803463]. [PMID: 19234205]. [DOI] [PubMed] [Google Scholar]
- 40.Stout R.D., Jiang C., Matta B., Tietzel I., Watkins S.K., Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J. Immunol. 2005;175(1):342–349. doi: 10.4049/jimmunol.175.1.342. [http://dx.doi.org/10.4049/jimmunol. 175.1.342]. [PMID: 15972667]. [DOI] [PubMed] [Google Scholar]
- 41.Meraz-Ríos M.A., Toral-Rios D., Franco-Bocanegra D., Villeda-Hernández J., Campos-Peña V. Inflammatory process in Alzheimer’s Disease. Front. Integr. Nuerosci. 2013;7:59. doi: 10.3389/fnint.2013.00059. [http://dx. doi.org/10.3389/fnint.2013.00059]. [PMID: 23964211]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wyss-Coray T., Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb. Perspect. Med. 2012;2(1):a006346. doi: 10.1101/cshperspect.a006346. [http://dx.doi.org/ 10.1101/cshperspect.a006346]. [PMID: 22315714]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cunningham C., Wilcockson D.C., Campion S., Lunnon K., Perry V.H. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J. Neurosci. 2005;25(40):9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [http://dx.doi.org/10.1523/JNEUROSCI.2614-05.2005]. [PMID: 16207887]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holmes C., El-Okl M., Williams A.L., Cunningham C., Wilcockson D., Perry V.H. Systemic infection, interleukin 1beta, and cognitive decline in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry. 2003;74(6):788–789. doi: 10.1136/jnnp.74.6.788. [http://dx.doi.org/10.1136/ jnnp.74.6.788]. [PMID: 12754353]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayes A., Green E.K., Pritchard A., Harris J.M., Zhang Y., Lambert J.C., Chartier-Harlin M.C., Pickering-Brown S.M., Lendon C.L., Mann D.M. A polymorphic variation in the interleukin 1A gene increases brain microglial cell activity in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry. 2004;75(10):1475–1477. doi: 10.1136/jnnp.2003.030866. [http://dx.doi.org/10.1136/jnnp.2003.030866]. [PMID: 15377701]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicoll J.A., Mrak R.E., Graham D.I., Stewart J., Wilcock G., MacGowan S., Esiri M.M., Murray L.S., Dewar D., Love S., Moss T., Griffin W.S. Association of interleukin-1 gene polymorphisms with Alzheimer’s disease. Ann. Neurol. 2000;47(3):365–368. [http://dx.doi.org/10.1002/1531-8249(200003)47:3<365:AID-ANA13>3.0.CO;2-G]. [PMID: 10716257]. [PMC free article] [PubMed] [Google Scholar]
- 47.McCusker S.M., Curran M.D., Dynan K.B., McCullagh C.D., Urquhart D.D., Middleton D., Patterson C.C., McIlroy S.P., Passmore A.P. Association between polymorphism in regulatory region of gene encoding tumour necrosis factor alpha and risk of Alzheimer’s disease and vascular dementia: a case-control study. Lancet. 2001;357(9254):436–439. doi: 10.1016/s0140-6736(00)04008-3. [http://dx.doi.org/10.1016/ S0140-6736(00)04008-3]. [PMID: 11273064]. [DOI] [PubMed] [Google Scholar]
- 48.Yuan P., Condello C., Keene C.D., Wang Y., Bird T.D., Paul S.M., Luo W., Colonna M., Baddeley D., Grutzendler J. TREM2 haplodeficiency in mice and humans impairs the microglia barrier function leading to decreased amyloid compaction and severe axonal dystrophy. Neuron. 2016;92(1):252–264. doi: 10.1016/j.neuron.2016.09.016. [http://dx.doi. org/10.1016/j.neuron.2016.09.016]. [PMID: 27710785]. [DOI] [PubMed] [Google Scholar]
- 49.Hong S., Beja-Glasser V.F., Nfonoyim B.M., Frouin A., Li S., Ramakrishnan S., Merry K.M., Shi Q., Rosenthal A., Barres B.A., Lemere C.A., Selkoe D.J., Stevens B. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352(6286):712–716. doi: 10.1126/science.aad8373. [http://dx.doi.org/10.1126/ science.aad8373]. [PMID: 27033548]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Czirr E., Castello N.A., Mosher K.I., Castellano J.M., Hinkson I.V., Lucin K.M., Baeza-Raja B., Ryu J.K., Li L., Farina S.N., Belichenko N.P., Longo F.M., Akassoglou K., Britschgi M. [DOI] [PMC free article] [PubMed]; Cirrito J.R., Wyss-Coray T. Microglial complement receptor 3 regulates brain Aβ levels through secreted proteolytic activity. J. Exp. Med. 2017;214(4):1081–1092. doi: 10.1084/jem.20162011. [http://dx.doi.org/10.1084/ jem.20162011]. [PMID: 28298456]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Itagaki S., McGeer P.L., Akiyama H., Zhu S., Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J. Neuroimmunol. 1989;24(3):173–182. doi: 10.1016/0165-5728(89)90115-x. [http://dx. doi.org/10.1016/0165-5728(89)90115-X]. [PMID: 2808689]. [DOI] [PubMed] [Google Scholar]
- 52.Heneka M.T., Sastre M., Dumitrescu-Ozimek L., Dewachter I., Walter J., Klockgether T., Van Leuven F. Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J. Neuroinflammation. 2005;2:22. doi: 10.1186/1742-2094-2-22. [http://dx.doi.org/10.1186/1742-2094-2-22]. [PMID: 16212664]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jung C.K., Keppler K., Steinbach S., Blazquez-Llorca L., Herms J. Fibrillar amyloid plaque formation precedes microglial activation. PLoS One. 2015;10(3):e0119768. doi: 10.1371/journal.pone.0119768. [http://dx.doi.org/10. 1371/journal.pone.0119768]. [PMID: 25799372]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodríguez J.J., Witton J., Olabarria M., Noristani H.N., Verkhratsky A. Increase in the density of resting microglia precedes neuritic plaque formation and microglial activation in a transgenic model of Alzheimer’s disease. Cell Death Dis. 2010;1:e1. doi: 10.1038/cddis.2009.2. [http://dx.doi.org/10.1038/cddis.2009.2]. [PMID: 21364611]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jana M., Palencia C.A., Pahan K. Fibrillar amyloid-beta peptides activate microglia via TLR2: implications for Alzheimer’s disease. J. Immunol. 2008;181(10):7254–7262. doi: 10.4049/jimmunol.181.10.7254. [http://dx.doi.org/10.4049/ jimmunol.181.10.7254]. [PMID: 18981147]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richard K.L., Filali M., Préfontaine P., Rivest S. Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1-42 and delay the cognitive decline in a mouse model of Alzheimer’s disease. J. Neurosci. 2008;28(22):5784–5793. doi: 10.1523/JNEUROSCI.1146-08.2008. [http://dx. doi.org/10.1523/JNEUROSCI.1146-08.2008]. [PMID: 18509040]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reed-Geaghan E.G., Savage J.C., Hise A.G., Landreth G.E. CD14 and toll-like receptors 2 and 4 are required for fibrillar Abeta-stimulated microglial activation. J. Neurosci. 2009;29(38):11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [http://dx.doi.org/10.1523/JNEUROSCI.3158-09.2009]. [PMID: 19776284]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bamberger M.E., Harris M.E., McDonald D.R., Husemann J., Landreth G.E. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J. Neurosci. 2003;23(7):2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [PMID: 12684452]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee C.Y., Landreth G.E. The role of microglia in amyloid clearance from the AD brain. J. Neural Transm. (Vienna) 2010;117(8):949–960. doi: 10.1007/s00702-010-0433-4. [http://dx.doi.org/10.1007/s00702-010-0433-4]. [PMID: 20552234]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiu W.Q., Ye Z., Kholodenko D., Seubert P., Selkoe D.J. Degradation of amyloid beta-protein by a metalloprotease secreted by microglia and other neural and non-neural cells. J. Biol. Chem. 1997;272(10):6641–6646. doi: 10.1074/jbc.272.10.6641. [http://dx.doi.org/10.1074/jbc.272.10. 6641]. [PMID: 9045694]. [DOI] [PubMed] [Google Scholar]
- 61.El Khoury J., Toft M., Hickman S.E., Means T.K., Terada K., Geula C., Luster A.D. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med. 2007;13(4):432–438. doi: 10.1038/nm1555. [http://dx.doi.org/10.1038/ nm1555]. [PMID: 17351623]. [DOI] [PubMed] [Google Scholar]
- 62.Krabbe G., Halle A., Matyash V., Rinnenthal J.L., Eom G.D., Bernhardt U., Miller K.R., Prokop S., Kettenmann H., Heppner F.L. Functional impairment of microglia coincides with Beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS One. 2013;8(4):e60921. doi: 10.1371/journal.pone.0060921. [http://dx.doi.org/10.1371/journal.pone. 0060921]. [PMID: 23577177]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cai H., Liang Q., Ge G. Gypenoside attenuates β amyloid-induced inflammation in N9 microglial cells via SOCS1 signaling. Neural Plast. 2016;2016:6362707. doi: 10.1155/2016/6362707. [http://dx.doi.org/10.1155/ 2016/6362707]. [PMID: 27213058]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Combs C.K., Karlo J.C., Kao S.C., Landreth G.E. beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J. Neurosci. 2001;21(4):1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [PMID: 11160388]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sajja V.S., Hlavac N., VandeVord P.J. Role of glia in memory deficits following traumatic brain injury: Biomarkers of glia dysfunction. Front. Integr. Nuerosci. 2016;10:7. doi: 10.3389/fnint.2016.00007. [http://dx.doi.org/ 10.3389/fnint.2016.00007]. [PMID: 26973475]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luo W., Liu W., Hu X., Hanna M., Caravaca A., Paul S.M. Microglial internalization and degradation of pathological tau is enhanced by an anti-tau monoclonal antibody. Sci. Rep. 2015;5:11161. doi: 10.1038/srep11161. [http://dx.doi.org/10.1038/srep11161]. [PMID: 26057852]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bolós M., Llorens-Martín M., Jurado-Arjona J., Hernández F., Rábano A., Avila J. Direct evidence of internalization of tau by microglia in vitro and in vivo. J. Alzheimers Dis. 2016;50(1):77–87. doi: 10.3233/JAD-150704. [http://dx.doi.org/10.3233/JAD-150704]. [PMID: 26638867]. [DOI] [PubMed] [Google Scholar]
- 68.Colombo E., Farina C. Astrocytes: Key regulators of neuroinflammation. Trends Immunol. 2016;37(9):608–620. doi: 10.1016/j.it.2016.06.006. [http://dx. doi.org/10.1016/j.it.2016.06.006]. [PMID: 27443914]. [DOI] [PubMed] [Google Scholar]
- 69.Placone A.L., McGuiggan P.M., Bergles D.E., Guerrero-Cazares H., Quiñones-Hinojosa A., Searson P.C. Human astrocytes develop physiological morphology and remain quiescent in a novel 3D matrix. Biomaterials. 2015;42:134–143. doi: 10.1016/j.biomaterials.2014.11.046. [http://dx.doi.org/ 10.1016/j.biomaterials.2014.11.046]. [PMID: 25542801]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carson M.J., Thrash J.C., Walter B. The cellular response in neuroinflammation: The role of leukocytes, microglia and astrocytes in neuronal death and survival. Clin. Neurosci. Res. 2006;6(5):237–245. doi: 10.1016/j.cnr.2006.09.004. [http://dx.doi.org/10.1016/j.cnr.2006.09.004]. [PMID: 19169437]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Segura-Aguilar J. A new mechanism for protection of dopaminergic neurons mediated by astrocytes. Neural Regen. Res. 2015;10(8):1225–1227. doi: 10.4103/1673-5374.162750. [http://dx.doi.org/10.4103/1673-5374.162750]. [PMID: 26487845]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.'Episcopo, F.L.; Tirolo, C.; Testa, N.; Caniglia, S.; Morale, M.C.; Marchetti, B. Reactive astrocytes are key players in nigrostriatal dopaminergic neurorepair in the MPTP mouse model of Parkinson’s disease: focus on endogenous neurorestoration. Curr. Aging Sci. 2013;6(1):45–55. doi: 10.2174/1874609811306010007. [http://dx.doi.org/10.2174/ 1874609811306010007]. [PMID: 23895521]. [DOI] [PubMed] [Google Scholar]
- 73.Catts V.S., Wong J., Fillman S.G., Fung S.J., Shannon Weickert C. Increased expression of astrocyte markers in schizophrenia: Association with neuroinflammation. Aust. N. Z. J. Psychiatry. 2014;48(8):722–734. doi: 10.1177/0004867414531078. [http://dx.doi.org/10.1177/0004867414531078]. [PMID: 24744400]. [DOI] [PubMed] [Google Scholar]
- 74.McCullumsmith R.E., O’Donovan S.M., Drummond J.B., Benesh F.S., Simmons M., Roberts R., Lauriat T., Haroutunian V., Meador-Woodruff J.H. Cell-specific abnormalities of glutamate transporters in schizophrenia: sick astrocytes and compensating relay neurons? Mol. Psychiatry. 2016;21(6):823–830. doi: 10.1038/mp.2015.148. [http://dx.doi.org/10.1038/mp.2015.148]. [PMID: 26416546]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gu X.L., Long C.X., Sun L., Xie C., Lin X., Cai H. Astrocytic expression of Parkinson’s disease-related A53T alpha-synuclein causes neurodegeneration in mice. Mol. Brain. 2010;3:12. doi: 10.1186/1756-6606-3-12. [http://dx.doi.org/10.1186/1756-6606-3-12]. [PMID: 20409326]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Solano R.M., Casarejos M.J., Menéndez-Cuervo J., Rodriguez-Navarro J.A., García de Yébenes J., Mena M.A. Glial dysfunction in parkin null mice: effects of aging. J. Neurosci. 2008;28(3):598–611. doi: 10.1523/JNEUROSCI.4609-07.2008. [http://dx.doi.org/10.1523/JNEUROSCI.4609-07.2008]. [PMID: 18199761]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abbott N.J. Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat. 2002;200(6):629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [http://dx. doi.org/10.1046/j.1469-7580.2002.00064.x]. [PMID: 12162730]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abbott N.J., Rönnbäck L., Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [http://dx.doi.org/10.1038/nrn1824]. [PMID: 16371949]. [DOI] [PubMed] [Google Scholar]
- 79.Haseloff R.F., Blasig I.E., Bauer H.C., Bauer H. In search of the astrocytic factor(s) modulating blood-brain barrier functions in brain capillary endothelial cells in vitro. Cell. Mol. Neurobiol. 2005;25(1):25–39. doi: 10.1007/s10571-004-1375-x. [http://dx.doi.org/10.1007/s10571-004-1375-x]. [PMID: 15962507]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cabezas R., Avila M., Gonzalez J., El-Bachá R.S., Báez E., García-Segura L.M., Jurado C.J.C., Capani F., Cardona-Gomez G.P., Barreto G.E. Astrocytic modulation of blood brain barrier: perspectives on Parkinson’s disease. Front. Cell. Neurosci. 2014;8:211. doi: 10.3389/fncel.2014.00211. [http://dx.doi.org/10.3389/fncel.2014.00211]. [PMID: 25136294]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Argaw A.T., Asp L., Zhang J., Navrazhina K., Pham T., Mariani J.N., Mahase S., Dutta D.J., Seto J., Kramer E.G., Ferrara N., Sofroniew M.V., John G.R. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J. Clin. Invest. 2012;122(7):2454–2468. doi: 10.1172/JCI60842. [http://dx.doi.org/10.1172/ JCI60842]. [PMID: 22653056]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Argaw A.T., Zhang Y., Snyder B.J., Zhao M.L., Kopp N., Lee S.C., Raine C.S., Brosnan C.F., John G.R. IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J. Immunol. 2006;177(8):5574–5584. doi: 10.4049/jimmunol.177.8.5574. [http://dx.doi.org/10.4049/jimmunol.177.8.5574]. [PMID: 17015745]. [DOI] [PubMed] [Google Scholar]
- 83.Constantinescu C.S., Tani M., Ransohoff R.M., Wysocka M., Hilliard B., Fujioka T., Murphy S., Tighe P.J., Das Sarma J., Trinchieri G., Rostami A. Astrocytes as antigen-presenting cells: expression of IL-12/IL-23. J. Neurochem. 2005;95(2):331–340. doi: 10.1111/j.1471-4159.2005.03368.x. [http://dx.doi.org/10.1111/j.1471-4159.2005.03368.x]. [PMID: 16086689]. [DOI] [PubMed] [Google Scholar]
- 84.Gimsa U., Mitchison N.A., Brunner-Weinzierl M.C. Immune privilege as an intrinsic CNS property: astrocytes protect the CNS against T-cell-mediated neuroinflammation. Mediators Inflamm. 2013;2013:320519. doi: 10.1155/2013/320519. [http://dx.doi.org/10.1155/2013/320519]. [PMID: 24023412]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sun D., Coleclough C., Whitaker J.N. Nonactivated astrocytes downregulate T cell receptor expression and reduce antigen-specific proliferation and cytokine production of myelin basic protein (MBP)-reactive T cells. J. Neuroimmunol. 1997;78(1-2):69–78. doi: 10.1016/s0165-5728(97)00083-0. [http://dx.doi.org/10.1016/S0165-5728(97)00083-0]. [PMID: 9307229]. [DOI] [PubMed] [Google Scholar]
- 86.Gimsa U. ØRen, A.; Pandiyan, P.; Teichmann, D.; Bechmann, I.; Nitsch, R.; Brunner-Weinzierl, M.C. Astrocytes protect the CNS: antigen-specific T helper cell responses are inhibited by astrocyte-induced upregulation of CTLA-4 (CD152). J. Mol. Med. (Berl.) 2004;82(6):364–372. doi: 10.1007/s00109-004-0531-6. [http://dx.doi.org/10.1007/s00109-004-0531-6]. [PMID: 15007511]. [DOI] [PubMed] [Google Scholar]
- 87.Xiao B.G., Diab A., Zhu J., van der Meide P., Link H. Astrocytes induce hyporesponses of myelin basic protein-reactive T and B cell function. J. Neuroimmunol. 1998;89(1-2):113–121. doi: 10.1016/s0165-5728(98)00123-4. [http://dx.doi.org/10.1016/S0165-5728(98)00123-4]. [PMID: 9726833]. [DOI] [PubMed] [Google Scholar]
- 88.Saikali P., Antel J.P., Pittet C.L., Newcombe J., Arbour N. Contribution of astrocyte-derived IL-15 to CD8 T cell effector functions in multiple sclerosis. J. Immunol. 2010;185(10):5693–5703. doi: 10.4049/jimmunol.1002188. [http://dx.doi.org/10.4049/jimmunol.1002188]. [PMID: 20926794]. [DOI] [PubMed] [Google Scholar]
- 89.Bélanger M., Magistretti P.J. The role of astroglia in neuroprotection. Dialogues Clin. Neurosci. 2009;11(3):281–295. doi: 10.31887/DCNS.2009.11.3/mbelanger. [PMID: 19877496]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deng Y., Xie D., Fang M., Zhu G., Chen C., Zeng H., Lu J., Charanjit K. Astrocyte-derived proinflammatory cytokines induce hypomyelination in the periventricular white matter in the hypoxic neonatal brain. PLoS One. 2014;9(1):e87420. doi: 10.1371/journal.pone.0087420. [http://dx.doi.org/10.1371/journal.pone.0087420]. [PMID: 24498101]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Choi S.S., Lee H.J., Lim I., Satoh J., Kim S.U. Human astrocytes: secretome profiles of cytokines and chemokines. PLoS One. 2014;9(4):e92325. doi: 10.1371/journal.pone.0092325. [http://dx.doi.org/10.1371/journal.pone. 0092325]. [PMID: 24691121]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hailer N.P., Wirjatijasa F., Roser N., Hischebeth G.T., Korf H.W., Dehghani F. Astrocytic factors protect neuronal integrity and reduce microglial activation in an in vitro model of N-methyl-D-aspartate-induced excitotoxic injury in organotypic hippocampal slice cultures. Eur. J. Neurosci. 2001;14(2):315–326. doi: 10.1046/j.0953-816x.2001.01649.x. [http://dx. doi.org/10.1046/j.0953-816x.2001.01649.x]. [PMID: 11553282]. [DOI] [PubMed] [Google Scholar]
- 93.DeWitt D.A., Perry G., Cohen M., Doller C., Silver J. Astrocytes regulate microglial phagocytosis of senile plaque cores of Alzheimer’s disease. Exp. Neurol. 1998;149(2):329–340. doi: 10.1006/exnr.1997.6738. [http://dx.doi.org/10.1006/exnr.1997.6738]. [PMID: 9500964]. [DOI] [PubMed] [Google Scholar]
- 94.Smits H.A., van Beelen A.J., de Vos N.M., Rijsmus A., van der Bruggen T., Verhoef J., van Muiswinkel F.L., Nottet H.S. Activation of human macrophages by amyloid-beta is attenuated by astrocytes. J. Immunol. 2001;166(11):6869–6876. doi: 10.4049/jimmunol.166.11.6869. [http://dx.doi.org/10.4049/jimmunol.166.11.6869]. [PMID: 11359847]. [DOI] [PubMed] [Google Scholar]
- 95.Aloisi F., Penna G., Cerase J., Menéndez Iglesias B., Adorini L. IL-12 production by central nervous system microglia is inhibited by astrocytes. J. Immunol. 1997;159(4):1604–1612. [PMID: 9257819]. [PubMed] [Google Scholar]
- 96.Solà C., Casal C., Tusell J.M., Serratosa J. Astrocytes enhance lipopolysaccharide-induced nitric oxide production by microglial cells. Eur. J. Neurosci. 2002;16(7):1275–1283. doi: 10.1046/j.1460-9568.2002.02199.x. [http://dx.doi.org/10.1046/j.1460-9568.2002.02199.x]. [PMID: 12405988]. [DOI] [PubMed] [Google Scholar]
- 97.Alzheimer A. Beiträge zur Kenntnis der pathologischen Neuroglia und ihrer Beziehung zu den Abbauvorgängen im Nervengewebe. In: Nissl F., Alzheimer A., editors. His-tologische und Histopathologische Arbeiten über die Grosshirnrinde mit besonderer Berücksichtigung der pathologischen Anatomie der Geisteskrankheiten. Vol. 1-3. Jena: Gustav Fisch-er; 1910. pp. 401–562. [Google Scholar]
- 98.Matsuoka Y., Picciano M., Malester B., LaFrancois J., Zehr C., Daeschner J.M., Olschowka J.A., Fonseca M.I., O’Banion M.K., Tenner A.J., Lemere C.A., Duff K. Inflammatory responses to amyloidosis in a transgenic mouse model of Alzheimer’s disease. Am. J. Pathol. 2001;158(4):1345–1354. doi: 10.1016/S0002-9440(10)64085-0. [http://dx.doi.org/10. 1016/S0002-9440(10)64085-0]. [PMID: 11290552]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nagele R.G., D’Andrea M.R., Lee H., Venkataraman V., Wang H.Y. Astrocytes accumulate A beta 42 and give rise to astrocytic amyloid plaques in Alzheimer disease brains. Brain Res. 2003;971(2):197–209. doi: 10.1016/s0006-8993(03)02361-8. [http://dx.doi.org/10.1016/S0006-8993(03)02361-8]. [PMID: 12706236]. [DOI] [PubMed] [Google Scholar]
- 100.Ries M., Sastre M. Mechanisms of Aβ clearance and degradation by glial cells. Front. Aging Neurosci. 2016;8:160. doi: 10.3389/fnagi.2016.00160. [http://dx. doi.org/10.3389/fnagi.2016.00160]. [PMID: 27458370]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smits H.A., Rijsmus A., van Loon J.H., Wat J.W., Verhoef J., Boven L.A., Nottet H.S. Amyloid-beta-induced chemokine production in primary human macrophages and astrocytes. J. Neuroimmunol. 2002;127(1-2):160–168. doi: 10.1016/s0165-5728(02)00112-1. [http://dx.doi.org/10.1016/ S0165-5728(02)00112-1]. [PMID: 12044988]. [DOI] [PubMed] [Google Scholar]
- 102.Wyss-Coray T., Loike J.D., Brionne T.C., Lu E., Anankov R., Yan F., Silverstein S.C., Husemann J. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat. Med. 2003;9(4):453–457. doi: 10.1038/nm838. [http://dx.doi.org/10.1038/nm838]. [PMID: 12612547]. [DOI] [PubMed] [Google Scholar]
- 103.Lian H., Yang L., Cole A., Sun L., Chiang A.C., Fowler S.W., Shim D.J., Rodriguez-Rivera J., Taglialatela G., Jankowsky J.L., Lu H.C., Zheng H. NFκB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer’s disease. Neuron. 2015;85(1):101–115. doi: 10.1016/j.neuron.2014.11.018. [http://dx.doi.org/10.1016/j.neuron.2014.11.018]. [PMID: 25533482]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Murgas P., Godoy B., von Bernhardi R. Aβ potentiates inflammatory activation of glial cells induced by scavenger receptor ligands and inflammatory mediators in culture. Neurotox. Res. 2012;22(1):69–78. doi: 10.1007/s12640-011-9306-3. [http://dx.doi.org/10.1007/s12640-011-9306-3]. [PMID: 22237943]. [DOI] [PubMed] [Google Scholar]
- 105.Forloni G., Mangiarotti F., Angeretti N., Lucca E., De Simoni M.G. Beta-amyloid fragment potentiates IL-6 and TNF-alpha secretion by LPS in astrocytes but not in microglia. Cytokine. 1997;9(10):759–762. doi: 10.1006/cyto.1997.0232. [http://dx.doi.org/10.1006/cyto.1997.0232]. [PMID: 9344508]. [DOI] [PubMed] [Google Scholar]
- 106.Koistinaho M., Lin S., Wu X., Esterman M., Koger D., Hanson J., Higgs R., Liu F., Malkani S., Bales K.R., Paul S.M. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat. Med. 2004;10(7):719–726. doi: 10.1038/nm1058. [http://dx.doi.org/10.1038/nm1058]. [PMID: 15195085]. [DOI] [PubMed] [Google Scholar]
- 107.Liu R.X., Huang C., Bennett D.A., Li H., Wang R. The characteristics of astrocyte on Aβ clearance altered in Alzheimer’s disease were reversed by anti-inflammatory agent (+)-2-(1-hydroxyl-4-oxocyclohexyl) ethyl caffeate. Am. J. Transl. Res. 2016;8(10):4082–4094. [PMID: 27829994]. [PMC free article] [PubMed] [Google Scholar]
- 108.Apelt J., Ach K., Schliebs R. Aging-related down-regulation of neprilysin, a putative beta-amyloid-degrading enzyme, in transgenic Tg2576 Alzheimer-like mouse brain is accompanied by an astroglial upregulation in the vicinity of beta-amyloid plaques. Neurosci. Lett. 2003;339(3):183–186. doi: 10.1016/s0304-3940(03)00030-2. [http://dx.doi.org/10.1016/ S0304-3940(03)00030-2]. [PMID: 12633883]. [DOI] [PubMed] [Google Scholar]
- 109.Leal M.C., Dorfman V.B., Gamba A.F., Frangione B., Wisniewski T., Castaño E.M., Sigurdsson E.M., Morelli L. Plaque-associated overexpression of insulin-degrading enzyme in the cerebral cortex of aged transgenic tg2576 mice with Alzheimer pathology. J. Neuropathol. Exp. Neurol. 2006;65(10):976–987. doi: 10.1097/01.jnen.0000235853.70092.ba. [http://dx.doi.org/10.1097/01.jnen.0000235853.70092.ba]. [PMID: 17021402]. [DOI] [PubMed] [Google Scholar]
- 110.Yin K.J., Cirrito J.R., Yan P., Hu X., Xiao Q., Pan X., Bateman R., Song H., Hsu F.F., Turk J., Xu J., Hsu C.Y., Mills J.C., Holtzman D.M., Lee J.M. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J. Neurosci. 2006;26(43):10939–10948. doi: 10.1523/JNEUROSCI.2085-06.2006. [http://dx.doi.org/10.1523/JNEUROSCI.2085-06.2006]. [PMID: 17065436]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lesné S., Docagne F., Gabriel C., Liot G., Lahiri D.K., Buée L., Plawinski L., Delacourte A., MacKenzie E.T., Buisson A., Vivien D. Transforming growth factor-beta 1 potentiates amyloid-beta generation in astrocytes and in transgenic mice. J. Biol. Chem. 2003;278(20):18408–18418. doi: 10.1074/jbc.M300819200. [http://dx.doi.org/10.1074/ jbc.M300819200]. [PMID: 12626500]. [DOI] [PubMed] [Google Scholar]
- 112.Zhao J., O’Connor T., Vassar R. The contribution of activated astrocytes to Aβ production: implications for Alzheimer’s disease pathogenesis. J. Neuroinflammation. 2011;8:150. doi: 10.1186/1742-2094-8-150. [http://dx. doi.org/10.1186/1742-2094-8-150]. [PMID: 22047170]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Blasko I., Veerhuis R., Stampfer-Kountchev M., Saurwein-Teissl M., Eikelenboom P., Grubeck-Loebenstein B. Costimulatory effects of interferon-gamma and interleukin-1beta or tumor necrosis factor alpha on the synthesis of Abeta1-40 and Abeta1-42 by human astrocytes. Neurobiol. Dis. 2000;7(6 Pt B):682–689. doi: 10.1006/nbdi.2000.0321. [http://dx.doi.org/10.1006/nbdi.2000.0321]. [PMID: 11114266]. [DOI] [PubMed] [Google Scholar]
- 114.Söllvander S., Nikitidou E., Brolin R., Söderberg L., Sehlin D., Lannfelt L., Erlandsson A. Accumulation of amyloid-β by astrocytes result in enlarged endosomes and microvesicle-induced apoptosis of neurons. Mol. Neurodegener. 2016;11(1):38. doi: 10.1186/s13024-016-0098-z. [http://dx.doi.org/10.1186/s13024-016-0098-z]. [PMID: 27176225]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pertusa M., García-Matas S., Mammeri H., Adell A., Rodrigo T., Mallet J., Cristòfol R., Sarkis C., Sanfeliu C. Expression of GDNF transgene in astrocytes improves cognitive deficits in aged rats. Neurobiol. Aging. 2008;29(9):1366–1379. doi: 10.1016/j.neurobiolaging.2007.02.026. [http://dx.doi.org/10.1016/j.neurobiolaging.2007.02.026]. [PMID: 17399854]. [DOI] [PubMed] [Google Scholar]
- 116.Sáez E.T., Pehar M., Vargas M.R., Barbeito L., Maccioni R.B. Production of nerve growth factor by beta-amyloid-stimulated astrocytes induces p75NTR-dependent tau hyperphosphorylation in cultured hippocampal neurons. J. Neurosci. Res. 2006;84(5):1098–1106. doi: 10.1002/jnr.20996. [http://dx.doi.org/10.1002/jnr.20996]. [PMID: 16862561]. [DOI] [PubMed] [Google Scholar]
- 117.Sheng J.G., Mrak R.E., Griffin W.S. Glial-neuronal interactions in Alzheimer disease: progressive association of IL-1alpha+ microglia and S100beta+ astrocytes with neurofibrillary tangle stages. J. Neuropathol. Exp. Neurol. 1997;56(3):285–290. [http://dx.doi.org/10.1097/00005072-199703000-00007]. [PMID: 9056542]. [PubMed] [Google Scholar]
- 118.Arai T., Miklossy J., Klegeris A., Guo J.P., McGeer P.L. Thrombin and prothrombin are expressed by neurons and glial cells and accumulate in neurofibrillary tangles in Alzheimer disease brain. J. Neuropathol. Exp. Neurol. 2006;65(1):19–25. doi: 10.1097/01.jnen.0000196133.74087.cb. [http://dx. doi.org/10.1097/01.jnen.0000196133.74087.cb]. [PMID: 16410745]. [DOI] [PubMed] [Google Scholar]
- 119.Olesen O.F. Proteolytic degradation of microtubule associated protein tau by thrombin. Biochem. Biophys. Res. Commun. 1994;201(2):716–721. doi: 10.1006/bbrc.1994.1759. [http://dx.doi.org/10.1006/bbrc.1994.1759]. [PMID: 8003007]. [DOI] [PubMed] [Google Scholar]
- 120.Sastre M., Gentleman S.M. NSAIDs: How they work and their prospects as therapeutics in alzheimer’s disease. Front. Aging Neurosci. 2010;2:20. doi: 10.3389/fnagi.2010.00020. [PMID: 20589102]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gasparini L., Ongini E., Wenk G. Non-steroidal anti-inflammatory drugs (NSAIDs) in Alzheimer’s disease: old and new mechanisms of action. J. Neurochem. 2004;91(3):521–536. doi: 10.1111/j.1471-4159.2004.02743.x. [http://dx.doi.org/10.1111/j.1471-4159.2004.02743.x]. [PMID: 15485484]. [DOI] [PubMed] [Google Scholar]
- 122.Wick M., Hurteau G., Dessev C., Chan D., Geraci M.W., Winn R.A., Heasley L.E., Nemenoff R.A. Peroxisome proliferator-activated receptor-gamma is a target of nonsteroidal anti-inflammatory drugs mediating cyclooxygenase-independent inhibition of lung cancer cell growth. Mol. Pharmacol. 2002;62(5):1207–1214. doi: 10.1124/mol.62.5.1207. [http://dx.doi.org/10.1124/mol.62.5.1207]. [PMID: 12391285]. [DOI] [PubMed] [Google Scholar]
- 123.Jaradat M.S., Wongsud B., Phornchirasilp S., Rangwala S.M., Shams G., Sutton M., Romstedt K.J., Noonan D.J., Feller D.R. Activation of peroxisome proliferator-activated receptor isoforms and inhibition of prostaglandin H(2) synthases by ibuprofen, naproxen, and indomethacin. Biochem. Pharmacol. 2001;62(12):1587–1595. doi: 10.1016/s0006-2952(01)00822-x. [http://dx.doi.org/10.1016/S0006-2952(01)00822-X]. [PMID: 11755111]. [DOI] [PubMed] [Google Scholar]
- 124.Combs C.K., Johnson D.E., Karlo J.C., Cannady S.B., Landreth G.E. Inflammatory mechanisms in Alzheimer’s disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. J. Neurosci. 2000;20(2):558–567. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [PMID: 10632585]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bernardo A., Minghetti L. PPAR-gamma agonists as regulators of microglial activation and brain inflammation. Curr. Pharm. Des. 2006;12(1):93–109. doi: 10.2174/138161206780574579. [http://dx.doi.org/10.2174/138161206780574579]. [PMID: 16454728]. [DOI] [PubMed] [Google Scholar]
- 126.De Nuccio C., Bernardo A., Cruciani C., De Simone R., Visentin S., Minghetti L. Peroxisome proliferator activated receptor-γ agonists protect oligodendrocyte progenitors against tumor necrosis factor-alpha-induced damage: Effects on mitochondrial functions and differentiation. Exp. Neurol. 2015;271:506–514. doi: 10.1016/j.expneurol.2015.07.014. [http://dx. doi.org/10.1016/j.expneurol.2015.07.014]. [PMID: 26210873]. [DOI] [PubMed] [Google Scholar]
- 127.Jiang C., Ting A.T., Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391(6662):82–86. doi: 10.1038/34184. [http://dx.doi.org/10.1038/34184]. [PMID: 9422509]. [DOI] [PubMed] [Google Scholar]
- 128.Yan Q., Zhang J., Liu H., Babu-Khan S., Vassar R., Biere A.L., Citron M., Landreth G. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer’s disease. J. Neurosci. 2003;23(20):7504–7509. doi: 10.1523/JNEUROSCI.23-20-07504.2003. [PMID: 12930788]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lim G.P., Yang F., Chu T., Chen P., Beech W., Teter B., Tran T., Ubeda O., Ashe K.H., Frautschy S.A., Cole G.M. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J. Neurosci. 2000;20(15):5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [PMID: 10908610]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Eriksen J.L., Sagi S.A., Smith T.E., Weggen S., Das P., McLendon D.C., Ozols V.V., Jessing K.W., Zavitz K.H., Koo E.H., Golde T.E. NSAIDs and enantiomers of flurbiprofen target gamma-secretase and lower Abeta 42 in vivo. J. Clin. Invest. 2003;112(3):440–449. doi: 10.1172/JCI18162. [http://dx.doi.org/10.1172/JCI18162]. [PMID: 12897211]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weggen S., Eriksen J.L., Das P., Sagi S.A., Wang R., Pietrzik C.U., Findlay K.A., Smith T.E., Murphy M.P., Bulter T., Kang D.E., Marquez-Sterling N., Golde T.E., Koo E.H. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414(6860):212–216. doi: 10.1038/35102591. [http://dx.doi.org/10.1038/35102591]. [PMID: 11700559]. [DOI] [PubMed] [Google Scholar]
- 132.Blasko I., Apochal A., Boeck G., Hartmann T., Grubeck-Loebenstein B., Ransmayr G. Ibuprofen decreases cytokine-induced amyloid beta production in neuronal cells. Neurobiol. Dis. 2001;8(6):1094–1101. doi: 10.1006/nbdi.2001.0451. [http://dx.doi.org/10.1006/nbdi.2001.0451]. [PMID: 11741404]. [DOI] [PubMed] [Google Scholar]
- 133.Kummer M.P., Schwarzenberger R., Sayah-Jeanne S., Dubernet M., Walczak R., Hum D.W., Schwartz S., Axt D., Heneka M.T. Pan-PPAR modulation effectively protects APP/PS1 mice from amyloid deposition and cognitive deficits. Mol. Neurobiol. 2015;51(2):661–671. doi: 10.1007/s12035-014-8743-4. [http://dx.doi.org/10.1007/s12035-014-8743-4]. [PMID: 24838579]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Heneka M.T., Sastre M., Dumitrescu-Ozimek L., Hanke A., Dewachter I., Kuiperi C., O’Banion K., Klockgether T., Van Leuven F., Landreth G.E. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1-42 levels in APPV717I transgenic mice. Brain. 2005;128(Pt 6):1442–1453. doi: 10.1093/brain/awh452. [http://dx.doi.org/10.1093/brain/awh452]. [PMID: 15817521]. [DOI] [PubMed] [Google Scholar]
- 135.de Jong D., Jansen R., Hoefnagels W., Jellesma-Eggenkamp M., Verbeek M., Borm G., Kremer B. No effect of one-year treatment with indomethacin on Alzheimer’s disease progression: a randomized controlled trial. PLoS One. 2008;3(1):e1475. doi: 10.1371/journal.pone.0001475. [http://dx. doi.org/10.1371/journal.pone.0001475]. [PMID: 18213383]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Scharf S., Mander A., Ugoni A., Vajda F., Christophidis N. A double-blind, placebo-controlled trial of diclofenac/misoprostol in Alzheimer’s disease. Neurology. 1999;53(1):197–201. doi: 10.1212/wnl.53.1.197. [http://dx. doi.org/10.1212/WNL.53.1.197]. [PMID: 10408559]. [DOI] [PubMed] [Google Scholar]
- 137.Aisen P.S., Schmeidler J., Pasinetti G.M. Randomized pilot study of nimesulide treatment in Alzheimer’s disease. Neurology. 2002;58(7):1050–1054. doi: 10.1212/wnl.58.7.1050. [http://dx.doi.org/10.1212/WNL.58.7.1050]. [PMID: 11940691]. [DOI] [PubMed] [Google Scholar]
- 138.Sainati S.M., Ingram D.M., Talwalker S., Geis G.S. Results of a double-blind, randomized, placebocontrolled study of celecoxib in the treatment of progression of Alzhei-mer’s disease.; Proceedings of the Sixth International Stock-holm/Springfield Symposium on Advances in Alzheimer Thera-py; 2000. [Google Scholar]
- 139.Crossin K.L., Tai M.H., Krushel L.A., Mauro V.P., Edelman G.M. Glucocorticoid receptor pathways are involved in the inhibition of astrocyte proliferation. Proc. Natl. Acad. Sci. USA. 1997;94(6):2687–2692. doi: 10.1073/pnas.94.6.2687. [http://dx.doi.org/10.1073/pnas.94.6.2687]. [PMID: 9122257]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Golde S., Coles A., Lindquist J.A., Compston A. Decreased iNOS synthesis mediates dexamethasone-induced protection of neurons from inflammatory injury in vitro. Eur. J. Neurosci. 2003;18(9):2527–2537. doi: 10.1046/j.1460-9568.2003.02917.x. [http://dx.doi.org/10.1046/j.1460-9568.2003. 02917.x]. [PMID: 14622153]. [DOI] [PubMed] [Google Scholar]
- 141.Szczepanik A.M., Ringheim G.E. IL-10 and glucocorticoids inhibit Abeta(1-42)- and lipopolysaccharide-induced pro-inflammatory cytokine and chemokine induction in the central nervous system. J. Alzheimers Dis. 2003;5(2):105–117. doi: 10.3233/jad-2003-5205. [http://dx.doi.org/10.3233/ JAD-2003-5205]. [PMID: 12719628]. [DOI] [PubMed] [Google Scholar]
- 142.Aisen P.S., Davis K.L., Berg J.D., Schafer K., Campbell K., Thomas R.G., Weiner M.F., Farlow M.R., Sano M., Grundman M., Thal L.J. A randomized controlled trial of prednisone in Alzheimer’s disease. Alzheimer’s Disease Cooperative Study. Neurology. 2000;54(3):588–593. doi: 10.1212/wnl.54.3.588. [http://dx.doi.org/10.1212/WNL.54.3. 588]. [PMID: 10680787]. [DOI] [PubMed] [Google Scholar]
- 143.DiCarlo G., Wilcock D., Henderson D., Gordon M., Morgan D. Intrahippocampal LPS injections reduce Abeta load in APP+PS1 transgenic mice. Neurobiol. Aging. 2001;22(6):1007–1012. doi: 10.1016/s0197-4580(01)00292-5. [http://dx.doi.org/10.1016/S0197-4580(01)00292-5]. [PMID: 11755009]. [DOI] [PubMed] [Google Scholar]
- 144.Iaccarino H.F., Singer A.C., Martorell A.J., Rudenko A., Gao F., Gillingham T.Z., Mathys H., Seo J., Kritskiy O., Abdurrob F., Adaikkan C., Canter R.G., Rueda R., Brown E.N., Boyden E.S., Tsai L.H. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature. 2016;540(7632):230–235. doi: 10.1038/nature20587. [http://dx.doi.org/10.1038/nature20587]. [PMID: 27929004]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mitrasinovic O.M., Murphy G.M., Jr Microglial overexpression of the M-CSF receptor augments phagocytosis of opsonized Abeta. Neurobiol. Aging. 2003;24(6):807–815. doi: 10.1016/s0197-4580(02)00237-3. [http://dx.doi.org/10.1016/ S0197-4580(02)00237-3]. [PMID: 12927763]. [DOI] [PubMed] [Google Scholar]
- 146.Boissonneault V., Filali M., Lessard M., Relton J., Wong G., Rivest S. Powerful beneficial effects of macrophage colony-stimulating factor on beta-amyloid deposition and cognitive impairment in Alzheimer’s disease. Brain. 2009;132(Pt 4):1078–1092. doi: 10.1093/brain/awn331. [http://dx.doi.org/10.1093/brain/awn331]. [PMID: 19151372]. [DOI] [PubMed] [Google Scholar]
- 147.Furman J.L., Sama D.M., Gant J.C., Beckett T.L., Murphy M.P., Bachstetter A.D., Van Eldik L.J., Norris C.M. Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer’s disease. J. Neurosci. 2012;32(46):16129–16140. doi: 10.1523/JNEUROSCI.2323-12.2012. [http://dx.doi.org/10.1523/JNEUROSCI.2323-12.2012]. [PMID: 23152597]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jo S., Yarishkin O., Hwang Y.J., Chun Y.E., Park M., Woo D.H., Bae J.Y., Kim T., Lee J., Chun H., Park H.J., Lee D.Y., Hong J., Kim H.Y., Oh S.J., Park S.J., Lee H., Yoon B.E., Kim Y., Jeong Y., Shim I., Bae Y.C., Cho J., Kowall N.W., Ryu H., Hwang E., Kim D., Lee C.J. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat. Med. 2014;20(8):886–896. doi: 10.1038/nm.3639. [http://dx.doi.org/10.1038/nm. 3639]. [PMID: 24973918]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Heneka M.T., Kummer M.P., Stutz A., Delekate A., Schwartz S., Vieira-Saecker A., Griep A., Axt D., Remus A., Tzeng T.C., Gelpi E., Halle A., Korte M., Latz E., Golenbock D.T. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493(7434):674–678. doi: 10.1038/nature11729. [http://dx.doi.org/10.1038/nature11729]. [PMID: 23254930]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu Y.Y., Bian J.S. Hydrogen sulfide protects amyloid-β induced cell toxicity in microglia. J. Alzheimers Dis. 2010;22(4):1189–1200. doi: 10.3233/JAD-2010-101002. [http://dx.doi.org/10.3233/JAD-2010-101002]. [PMID: 20930302]. [DOI] [PubMed] [Google Scholar]
- 151.Fleisher-Berkovich S., Filipovich-Rimon T., Ben-Shmuel S., Hülsmann C., Kummer M.P., Heneka M.T. Distinct modulation of microglial amyloid β phagocytosis and migration by neuropeptides (i). J. Neuroinflammation. 2010;7:61. doi: 10.1186/1742-2094-7-61. [http://dx.doi.org/10. 1186/1742-2094-7-61]. [PMID: 20937084]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Simard A.R., Soulet D., Gowing G., Julien J.P., Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49(4):489–502. doi: 10.1016/j.neuron.2006.01.022. [http://dx.doi.org/10.1016/j.neuron.2006.01.022]. [PMID: 16476660]. [DOI] [PubMed] [Google Scholar]
- 153.Malm T.M., Koistinaho M., Pärepalo M., Vatanen T., Ooka A., Karlsson S., Koistinaho J. Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to beta-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol. Dis. 2005;18(1):134–142. doi: 10.1016/j.nbd.2004.09.009. [http://dx.doi.org/10.1016/j.nbd. 2004.09.009]. [PMID: 15649704]. [DOI] [PubMed] [Google Scholar]
- 154.Lee J.K., Jin H.K., Endo S., Schuchman E.H., Carter J.E., Bae J.S. Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-beta deposition and rescues memory deficits in Alzheimer’s disease mice by modulation of immune responses. Stem Cells. 2010;28(2):329–343. doi: 10.1002/stem.277. [PMID: 20014009]. [DOI] [PubMed] [Google Scholar]
- 155.Lee J.K., Schuchman E.H., Jin H.K., Bae J.S. Soluble CCL5 derived from bone marrow-derived mesenchymal stem cells and activated by amyloid β ameliorates Alzheimer’s disease in mice by recruiting bone marrow-induced microglia immune responses. Stem Cells. 2012;30(7):1544–1555. doi: 10.1002/stem.1125. [http://dx.doi.org/10.1002/stem.1125]. [PMID: 22570192]. [DOI] [PubMed] [Google Scholar]
- 156.Ma T., Gong K., Ao Q., Yan Y., Song B., Huang H., Zhang X., Gong Y. Intracerebral transplantation of adipose-derived mesenchymal stem cells alternatively activates microglia and ameliorates neuropathological deficits in Alzheimer’s disease mice. Cell Transplant. 2013;22(Suppl. 1):S113–S126. doi: 10.3727/096368913X672181. [http://dx.doi.org/10. 3727/096368913X672181]. [PMID: 24070198]. [DOI] [PubMed] [Google Scholar]
- 157.Lee H.J., Lee J.K., Lee H., Carter J.E., Chang J.W., Oh W., Yang Y.S., Suh J.G., Lee B.H., Jin H.K., Bae J.S. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer’s disease mouse model through modulation of neuroinflammation. Neurobiol. Aging. 2012;33(3):588–602. doi: 10.1016/j.neurobiolaging.2010.03.024. [http://dx.doi.org/10.1016/j. neurobiolaging.2010.03.024]. [PMID: 20471717]. [DOI] [PubMed] [Google Scholar]