Abstract
Background
Glioblastoma is the most aggressive and deadly brain tumor, with low disease-free period even after surgery and combined radio and chemotherapies. Among the factors contributing to the devastating effect of this tumor in the brain are the elevated proliferation and invasion rate, and the ability to induce a local immunosuppressive environment. The intermediate-conductance Ca2+-activated K+ channel KCa3.1 is expressed in glioblastoma cells and in tumor-infiltrating cells.
Methods
We first describe the researches related to the role of KCa3.1 channels in the invasion of brain tumor cells and the regulation of cell cycle. In the second part we review the involvement of KCa3.1 channel in tumor-associated microglia cell behaviour.
Results
In tumor cells, the functional expression of KCa3.1 channels is important to substain cell invasion and proliferation. In tumor infiltrating cells, KCa3.1 channel activity is required to regulate their activation state. Interfering with KCa3.1 activity can be an adjuvant therapeutic approach in addition to classic chemotherapy and radiotherapy, to counteract tumor growth and prolong patient's survival.
Conclusion
In this mini-review we discuss the evidence of the functional roles of KCa3.1 channels in glioblastoma biology.
Keywords: Intermediate conductance Ca2+-activated K+ channel (KCa3.1), brain tumors, Glioblastoma Multiforme (GBM), invasion, microglia, proliferation, 1-[(2-Chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM-34)
1. INTRODUCTION
KCa3.1, the intermediate-conductance Ca2+-activated K+ channel, is a tetrameric trans-membrane protein, with all the subunits composed of six trans-membrane segments, with a pore between the fifth and sixth segments [1]. The Ca2+ sensitivity of the channel is due to the presence of a calmodulin binding site at the C terminus of each segment, being also responsible for assembly and surface expression of the channel [2]. K+ efflux through the channel helps to maintain a negative cell membrane potential that permits an electrochemical driving force for Ca2+ influx into the cells [3]. These changes in ion equilibrium help the cells to modify their shape and volume, both events necessary for cell migration. KCa3.1 is expressed, in physiological conditions, in different body districts, by many cells, such as epithelial cells of the gastro-intestinal regions, the lung, the endocrine and exocrine glands, as well as by vascular endothelial cells, fibroblasts and vascular smooth muscle cells (reviewed in [4]) and by cells of the hematopoietic lineage, such as erythrocytes, platelets, lymphocytes, mast cells, monocytes and macrophages [5]. Physiologically, the main role of the channel has been described in cell movement. During migration, cells increase their volume in the leading edge for protrusion and reduce their volume in the trailing one for the retraction. This localized change of volume is due to specific local K+ (mainly through BK channels), Cl- (ClC-3 channels) and water fluxes in the invadopodia, the dynamic actin-rich membrane structures, permitting cell migration and invasion in the neighbouring tissue; BK and ClC-3 co-localize in the lipid-raft domains of invadopodia, both contributing to ions efflux [6]. Channels involved in this process are voltage-gated K+ channels [7-9] and, mainly, Ca2+ activated K+ channels [9-11]. This mechanism is further strengthened by the activity of a Na+/H+ pumps at the leading edge and of aquaporin channels at the trailing one [12]. Deletion of KCa3.1 gene, KCNN4, inhibits the ability of lymphocytes and red blood cells to sense osmotic changes in mice [13].
KCa3.1 is also expressed in the brain, in particular, by CNS-resident immune cells (microglia/macrophages), where KCa3.1 regulates the activation state [14, 15]. Recently, KCa3.1 has been demonstrated to be expressed on amyloid beta oligomer treated astrocytes [16] and on human pyramidal neurons in brain edema [17] deducing an important role played by KCa3.1 in brain dysfunction. The most common brain tumors are gliomas; the high grade (IV) glioblastoma multiforme (GBM) leaves patients with a mean survival time of 14.6 months [18]. KCa3.1 channel is commonly expressed by GBM, and correlates with shorter patient survival [19]. KCa3.1 is necessary for GBM cell movement modulating cell invasion both in vitro and in vivo. This mini-review aims to recapitulate the evidence of the efficacy of KCa3.1 inhibition to counteract GBM progression.
1.1. KCa3.1 Roles in Brain Tumors
In the last few years, ion channels have been identified as promising therapeutic targets to reduce the invasiveness of brain tumor cells. Ion channels are indeed deeply involved in tumor cell functions such as migration, angiogenesis and proliferation [20-23]. Movement of ions causes cytoplasmic water to move across the membrane allowing for the robust shape and volume changes. Volume changes are necessary for migration and, if inhibited, block cell migration [24]. Calcium-activated potassium channels are involved in this process [25-29]. Several studies show that large conductance Ca2+-activated potassium channels (KCa1.1 or BK), ubiquitously expressed in the body, are required for tumor cell migration in pleural mesothelioma, glioma and breast cancer [30-33]. One additional promising target for new therapeutic approaches to glioma is the intermediate conductance Ca2+-activated K+ channel (KCa3.1). This channel is functionally expressed in virtually all normal and transformed migrating cells [25-29, 34] and its calcium dependency was shown in human GBM [22]. GBM is a deadly brain tumor [35]; its malignancy is mainly due to a high infiltrative behavior that renders complete surgical resection difficult. In 1938, Hans Joachim Scherer [36] first described the invasive patterns of GBM cells into human brain parenchyma. He detected, in over one hundred human GBM specimens, specific cell invasion pathways, named secondary structures today referred to as i) perineuronal and perivascular satellitosis, ii) subpial spread, and iii) invasion along the white matter tracts [37]. In 2008, Newcomb’s group [38] provided a possible explanation for the secondary Scherer’ structures, investigating the role of CXCR4, a G-protein coupled chemokine receptor overexpressed in most GBM cells (for a review [39]). He described that CXCR4 and its ligand CXCL12 are highly expressed in the GBM, providing an autocrine stimulatory loop for tumor cell proliferation, invasion and angiogenesis. In addition, he showed that invading glioma cells, positive for CXCR4, were closely organized around and along CXCL12 positive Scherer’ structures; this was demonstrated in mouse brain transplanted with the murine GL261 glioma cells. We have demonstrated [40] that KCa3.1 is involved in CXCL12-induced glioma cell migration. The role of KCa3.1 channels was confirmed by experiments in GBM cell lines and in cells obtained from patients, that were silenced for KCa3.1 channel expression. We showed the first in vivo evidence of KCa3.1 involvement in GBM cell invasion in severe combined immunodeficiency (SCID) mice xenografted with human GL-15 GBM cells [41]: these cells have a high infiltrative behavior and permit to study the effect of compounds affecting tumor spreading in the brain parenchyma [42]. Inhibiting KCa3.1 by shRNA or by pharmacological tools, significantly reduced the tumor infiltrated area, especially along the white matter tracts, and reduced the maximal antero-rostral spreading of GBM in cerebral parenchyma [41]. KCa3.1 inhibition also decreased astrogliosis and microglia/macrophages (M/Mϕ) activation at the boundary of the tumor, suppressing M/Mϕ phagocytosis and migration. In 2014, Turner et al. [19] also tested whether KCa3.1 channels contributed to glioma invasion using patient derived gliomas propagated in the flank of nude mice as well as GBM cell lines (U251 cells). They showed that the expression of KCa3.1 conferred an invasive phenotype to GBM, and its deletion significantly reduced tumor cell invasion both in vivo and ex vivo, in acute slices obtained from tumor bearing brain. Altogether these results confirmed a crucial role of KCa3.1 channels in tumor cell spreading. The current chemotherapeutic agent for newly diagnosed GBM is temozolomide (TMZ), which extends patient’s survival of about three months [18]. TMZ is a cytotoxic imidazotetrazine that leads to the formation of O6- methylguanine, which mismatches with thymine in subsequent DNA replication cycles, with effects on several cellular functions, such as autophagy [43], apoptosis and mitotic catastrophe [44]. A role for KCa3.1 in chemo and radio resistance has been recently observed in melanoma cells and in GBM. Cells with a blockade of KCa3.1 function are sensitized to this drug, potentiating the anti-tumor effects [45-47]. We recently demonstrated that treating GBM cells or cancer stem cells freshly dissected and isolated from patients, or human and murine glioma cell lines (GL261, U87MG), with TMZ and the selective KCa3.1 inhibitor, TRAM-34, induces a co-adjuvant effect on different tumor cell parameters such as cell invasion, proliferation and cell cycle progression [46]. We showed that channel inhibition forces TMZ treated cells, arrested in the G2 phase of cell cycle, to move toward the G0/G1 phases [46], a process that induces apoptotic death [48]. We demonstrated that KCa3.1 function is involved in the modulation of cdc2 G2/M check point protein, through cdc25C phosphatase activity; and that TRAM-34 and TMZ co-treatments increased the frequency of apoptotic cells and the mean survival time in a syngeneic GL261 glioma mouse model in comparison with single treatments. In line with this evidence, Stegen and co-authors [47] showed that ionizing radiation activates KCa3.1 channels in human GBM cells (T98G, U87MG) and that the pharmacological inhibition or the mRNA silencing of KCa3.1 channels reduced cell arrest in the G2-M phase and DNA repair, and reduced the clonogenicity of irradiated glioblastoma cells. In addition, they reported that pharmacologic targeting of KCa3.1 channels radiosensitized GBM cells grown ectopically in mice during fractionated radiation therapy. These in vitro findings and the in vivo outcomes in different mouse models highlighted the role of KCa3.1 channels in cell cycle regulation and are in accordance with retrospective clinical data where high levels of KCa3.1 gene transcription are correlated with reduced patient survival [19]. KCa3.1 channel is an attractive therapeutic target for brain tumors mainly because it is highly expressed in GBM cells [39] and tumor associated microglia [49] and is poorly expressed [50] in normal CNS. KCa3.1 targeting could be a ready to use therapeutic approach to treat GBM patients considering the strategy of drug repurposing. In fact a KCa3.1 inhibitor, structurally related to TRAM-34, named Senicapoc® already passed through clinical phase I to III trials for sickle cell disease and is considered safe and well-tolerated by patients [51].
1.2. KCa3.1 Role in Tumor Associated Microglia Cells
In many brain pathologies, such as Alzheimer disease, ischemia, traumatic brain injury, spinal cord injury, optic nerve transection and experimental autoimmune encephalomyelitis, the selective inhibition of KCa3.1 channel turned out to be beneficial [50, 52-59]. In all these cases, the effect of KCa3.1 inhibition results in an overall improvement and in the reduction of pathological symptoms. Glioma cells in the brain produce high level of glutamate that can produce local concentrations as high as 10 times more than normal [60]. This is induced by altered buffering by surrounding astrocytes [61], and dysfunction of the Na+-independent cystine/glutamate transporters Xc, expressed on glioma cells, and represents the main cause of neuron death (by excitotoxicity) in case of brain tumors [62]. Neuronal loss creates free spaces in the brain parenchyma, enabling tumor expansion [63]. Intriguingly, TRAM-34, in vitro, protects neurons from glioma-induced toxicity [46], and in vivo reduces the tumor-associated gliosis [41]. Altogether, these data suggest that KCa3.1 blockade might reduce tumor burden also reducing neuronal death induced by the presence of the tumor. KCa3.1 channels are functionally expressed in microglia, as shown by electrophysiological patch clamp experiments [64], by mRNA expression studies [65], that excluded the contribution of additional channels in Ca2+ activated K+ currents recorded in these cells [66, 67]. In microglia, KCa3.1 channels are also involved in cell migration, with Ca2+- [15, 68] and cAMP/PKA-dependent mechanisms. The effects of KCa3.1 channels in microglia also comprise the production of reactive oxygen species (ROS) through the p38/MAPK and cGMP/PKG pathways [69]. Migration and nitric oxide generation are closely associated with the inflammatory roles of these cells [70]; microglia exert both pro- and anti-inflammatory activities, in response to surrounding stimuli, that induce continuous shape and volume changes, enabling cells to monitor brain parenchyma. In vitro microglia stimulated with IL-4 assume an anti-inflammatory phenotype, producing cytokines such as IL-6 and TGFß [71, 72], and increasing the migratory ability by enhancing expression of MMP2, cathepsin S and cathepsin K [64]. This is often referred to as M2-like phenotype, in analogy with macrophages ([73], but see also [74]). IL-4 treatment of microglia also increased the functional expression of KCa3.1 channels, and channel inhibition blocks the intrinsic migratory ability of IL-4 stimulated cells [15, 68]. The M2-like phenotype is also induced in vivo by glioma, on tumor-associated myeloid cells (TAMs). Myeloid cells invading the tumor are mainly microglia and macrophages, and represent up to 30-50% of tumor mass [75, 76]. These cells are recruited in the tumor mass by cytokines and other soluble factors released by glioma [77-79]. In response to tumor factors, such as CXCL12, CCL2/MCP1, MCP3, GDNF, CSF-1 and GM-CSF [80-85], TAMs release a wide array of molecules that enhance tumor progression by multiple mechanisms that favour cell invasion [86]. Many of these factors, such as STI1, EGF, IL-6, TGFß, also stimulate migration of glioma cells [84, 87]. IL-6, in turn, increases CCL2 release by tumor cells, enhancing tumor invasion, while TGFß increases the expression of integrins on glioma cell lines, and favour the degradation of the extracellular matrix [88-90]. TAMs also increase the expression of membrane type 1–matrix metalloproteinase and reduce the expression of metalloproteinases inhibitors, further favouring extracellular matrix degradation, necessary for glioma invasion [91]. In murine glioma models, microglia depletion by clodronate treatment (ex vivo), reduced glioma cell invasion in surrounding parenchyma [92]. Specific depletion of CD11b+ cells in mice also decreased glioma cell proliferation in in vivo models [93, 94]. We demonstrated that KCa3.1 activity in TAMs (in cultures, as well as in vivo) reverts the pro-tumor phenotype toward a pro-inflammatory, anti-tumor state [49]. All these experiments demonstrated that TAMs are potentially appropriate therapeutic targets to fight against gliomas. Trying to interfere with the pro-tumor effects of TAMs, for example reverting the phenotype toward a pro-inflammatory one, could be a promising approach to counteract GBM progression [95-97].
CONCLUSION
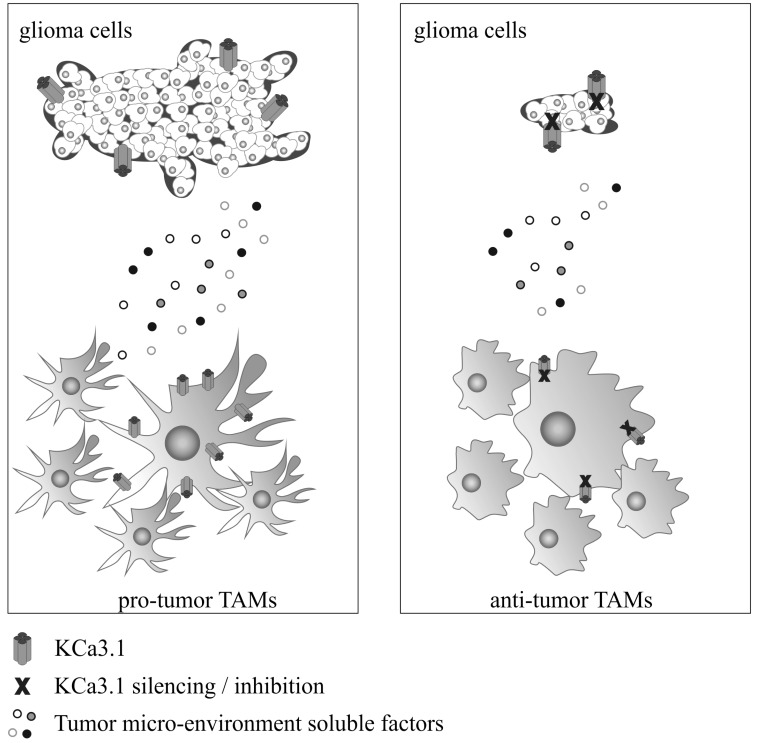
GBM, the most common primary brain tumor, presents a high level of cell infiltration and proliferation. Inside and around tumor mass, M/Mϕ represent the major infiltrating cell population. These TAMs are forced to assume an anti-inflammatory phenotype necessary to maintain and promote tumor progression. Current standard therapy, that includes surgical resection, radiotherapy plus chemotherapy, is insufficient to eradicate the tumor. New therapies are necessary to reduce invasion and proliferation of tumor cells as well as to reprogram the immune response of microglia and macrophages towards a pro-inflammatory, anti-tumor phenotype. KCa3.1 channel blockade by mRNA silencing or by pharmacological tools acts on these two fronts. In fact, the selective inhibition of the channel reduces tumor invasion and growth with direct effects on glioma, and switches the activation state of TAM towards an antitumor phenotype. Fig. (1) summarizes the actions of KCa3.1 on tumor cells and TAMs. Data obtained in in vitro and in vivo models of glioma delineate the possibility to target KCa3.1 as innovative therapeutic approach for GBM.
Fig. (1).
A schematic representation of tumor microenvironment composed by glioma cells and tumor-associated microglia/macrophages (TAMs) is reported on the left, with high level of tumor growth and invasion and TAMs oriented toward a pro-tumor phenotype. Right: effect of KCa3.1 channel silencing/inhibition, with a reduction of tumor growth and invasion, and TAMs acquiring an anti-tumor phenotype.
Acknowledgements
This work was supported by AIRC IG2015-16699 to C.L.
LIST OF ABBREVIATIONS
- cAMP
cyclic adenosine monophosphate
- CCL2
C-C motif ligand 2
- Cdc2
cell division control 2
- Cdc25C
cell division control protein 25 C
- cGMP
cyclic guanosine monophosphate
- CNS
central nervous system
- CSF-1
colony stimulating factor 1
- CXCR4
C-X-C motif receptor 4
- EGF
epidermal growth factor
- GDNF
glial cell line-derived neurutrophic factor
- GM-CSF
granulocyte macrophage colony stimulating factor
- IL-4
interleukin 4
- IL-6
interleukin 6
- MAPK
mitogen activated protein kinase
- MCP-1
monocyte chemoattractant protein-1
- MCP3
monocyte chemoattractant protein-3
- M/Mɸ
microglia/ macrophages
- MMP2
matrix metalloproteinases 2
- PKA
protein kinase A
- PKG
protein kinase G
- CXCL12
chemokine ligand 12
- STI1
stress inducible protein 1
- TGFß
transforming growth factor ß
- TRAM-34
1-[(2-Chlorophenyl)diphenylmethyl]-1H-pyrazole
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Morales P., Garneau L., Klein H., Lavoie M.F., Parent L., Sauvé R. Contribution of the KCa3.1 channel-calmodulin interactions to the regulation of the KCa3.1 gating process. J. Gen. Physiol. 2013;142(1):37–60. doi: 10.1085/jgp.201210933. [http://dx.doi.org/10.1085/jgp. 201210933]. [PMID: 23797421]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joiner W.J., Khanna R., Schlichter L.C., Kaczmarek L.K. Calmodulin regulates assembly and trafficking of SK4/IK1 Ca2+-activated K+ channels. J. Biol. Chem. 2001;276(41):37980–37985. doi: 10.1074/jbc.M104965200. [PMID: 11495911]. [DOI] [PubMed] [Google Scholar]
- 3.Ghanshani S., Wulff H., Miller M.J., Rohm H., Neben A., Gutman G.A., Cahalan M.D., Chandy K.G. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J. Biol. Chem. 2000;275(47):37137–37149. doi: 10.1074/jbc.M003941200. [http://dx.doi.org/10.1074/jbc.M003941200]. [PMID: 10961988]. [DOI] [PubMed] [Google Scholar]
- 4.Wulff H., Castle N.A. Therapeutic potential of KCa3.1 blockers: recent advances and promising trends. Expert Rev. Clin. Pharmacol. 2010;3(3):385–396. doi: 10.1586/ecp.10.11. [http://dx.doi.org/10.1586/ecp.10.11]. [PMID: 22111618]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Logsdon N.J., Kang J., Togo J.A., Christian E.P., Aiyar J. A novel gene, hKCa4, encodes the calcium-activated potassium channel in human T lymphocytes. J. Biol. Chem. 1997;272(52):32723–32726. doi: 10.1074/jbc.272.52.32723. [http://dx.doi.org/10.1074/jbc.272.52.32723]. [PMID: 9407042]. [DOI] [PubMed] [Google Scholar]
- 6.McFerrin M.B., Sontheimer H. A role for ion channels in glioma cell invasion. Neuron Glia Biol. 2006;2(1):39–49. doi: 10.1017/S17440925X06000044. [http://dx.doi. org/10.1017/S1740925X06000044]. [PMID: 16520829]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinhardt J., Golenhofen N., Pongs O., Oberleithner H., Schwab A. Migrating transformed MDCK cells are able to structurally polarize a voltage-activated K+ channel. Proc. Natl. Acad. Sci. USA. 1998;95(9):5378–5382. doi: 10.1073/pnas.95.9.5378. [http://dx.doi.org/10.1073/pnas. 95.9.5378]. [PMID: 9560284]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Artym V.V., Petty H.R. Molecular proximity of Kv1.3 voltage-gated potassium channels and β(1)-integrins on the plasma membrane of melanoma cells: effects of cell adherence and channel blockers. J. Gen. Physiol. 2002;120(1):29–37. doi: 10.1085/jgp.20028607. [http://dx.doi.org/ 10.1085/jgp.20028607]. [PMID: 12084773]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y.Y., Li G., Che H., Sun H.Y., Xiao G.S., Wang Y. ; Li G.R. Effects of BKCa and Kir2.1 channels on cell cycling progression and migration in human cardiac c-kit+ progenitor cells. PLoS One. 2015;10(9):e0138581. doi: 10.1371/journal.pone.0138581. [http://dx.doi.org/10.1371/ journal.pone.0138581]. [PMID: 26390131]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girault A., Brochiero E. Evidence of K+ channel function in epithelial cell migration, proliferation, and repair. Am. J. Physiol. Cell Physiol. 2014;306(4):C307–C319. doi: 10.1152/ajpcell.00226.2013. [http://dx.doi.org/10.1152/ ajpcell.00226.2013]. [PMID: 24196531]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner K.L., Sontheimer H. Cl- and K+ channels and their role in primary brain tumour biology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369(1638):20130095. doi: 10.1098/rstb.2013.0095. [http://dx.doi.org/10.1098/rstb. 2013.0095]. [PMID: 24493743]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McFerrin M.B., Sontheimer H. A role for ion channels in glioma cell invasion. Neuron Glia Biol. 2006;2(1):39–49. doi: 10.1017/S17440925X06000044. [http://dx.doi. org/10.1017/S1740925X06000044]. [PMID: 16520829]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Begenisich T., Nakamoto T., Ovitt C.E., Nehrke K., Brugnara C., Alper S.L., Melvin J.E.J. Physiological roles of the intermediate conductance, Ca2+-activated potassium channel Kcnn4. J. Biol. Chem. 2004;279(46):47681–47687. doi: 10.1074/jbc.M409627200. [http://dx.doi.org/10.1074/ jbc.M409627200]. [PMID: 15347667]. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen H.M., Singh V., Pressly B., Jenkins D.P., Wulff H., Yarov-Yarovoy V. Structural insights into the atomistic mechanisms of action of small molecule inhibitors targeting the KCa3.1 channel pore. Mol. Pharmacol. 2017;91(4):392–402. doi: 10.1124/mol.116.108068. [http://dx. doi.org/10.1124/mol.116.108068]. [PMID: 28126850]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira R., Lively S., Schlichter L.C. IL-4 type 1 receptor signaling up-regulates KCNN4 expression, and increases the KCa3.1 current and its contribution to migration of alternative-activated microglia. Front. Cell. Neurosci. 2014;8:183. doi: 10.3389/fncel.2014.00183. [http://dx.doi.org/10. 3389/fncel.2014.00183]. [PMID: 25071444]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei T., Yi M., Gu W., Hou L., Lu Q., Yu Z., Chen H. The potassium channel KCa3.1 represents a valid pharmacological target for astrogliosis-induced neuronal impairment in a mouse model of alzheimer’s disease. Front. Pharmacol. 2017;7:528. doi: 10.3389/fphar.2016.00528. [http://dx.doi.org/10.3389/fphar.2016.00528]. [PMID: 28105015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faragó N., Kocsis Á.K., Braskó C., Lovas S., Rózsa M., Baka J., Kovács B., Mikite K., Szemenyei V., Molnár G., Ozsvár A., Oláh G., Piszár I., Zvara Á., Patócs A., Barzó P., Puskás L.G., Tamás G. Human neuronal changes in brain edema and increased intracranial pressure. Acta Neuropathol. Commun. 2016;4(1):78. doi: 10.1186/s40478-016-0356-x. [http://dx.doi.org/10.1186/s40478-016-0356-x]. [PMID: 27487831]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J., Belanger K., Brandes A.A., Marosi C., Bogdahn U., Curschmann J., Janzer R.C., Ludwin S.K., Gorlia T., Allgeier A., Lacombe D., Cairncross J.G., Eisenhauer E., Mirimanoff R.O. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [http://dx.doi.org/10.1056/NEJMoa043330]. [PMID: 15758009]. [DOI] [PubMed] [Google Scholar]
- 19.Turner K.L., Honasoge A., Robert S.M., McFerrin M.M., Sontheimer H. A proinvasive role for the Ca(2+) -activated K(+) channel KCa3.1 in malignant glioma. Glia. 2014;62(6):971–981. doi: 10.1002/glia.22655. [http://dx.doi.org/10.1002/glia.22655]. [PMID: 24585442]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becchetti A. Ion channels and transporters in cancer. 1. Ion channels and cell proliferation in cancer. Am. J. Physiol. Cell Physiol. 2011;301(2):C255–C265. doi: 10.1152/ajpcell.00047.2011. [http://dx.doi.org/10.1152/ajpcell.00047. 2011]. [PMID: 21430288]. [DOI] [PubMed] [Google Scholar]
- 21.Prevarskaya N., Skryma R., Shuba Y. Ion channels and the hallmarks of cancer. Trends Mol. Med. 2010;16(3):107–121. doi: 10.1016/j.molmed.2010.01.005. [http:// dx.doi.org/10.1016/j.molmed.2010.01.005]. [PMID: 20167536]. [DOI] [PubMed] [Google Scholar]
- 22.Cuddapah V.A., Sontheimer H. Ion channels and transporters [corrected] in cancer. 2. Ion channels and the control of cancer cell migration. Am. J. Physiol. Cell Physiol. 2011;301(3):C541–C549. doi: 10.1152/ajpcell.00102.2011. [http://dx.doi.org/10.1152/ajpcell.00102.2011]. [PMID: 21543740]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuddapah V.A., Robel S., Watkins S., Sontheimer H. A neurocentric perspective on glioma invasion. Nat. Rev. Neurosci. 2014;15(7):455–465. doi: 10.1038/nrn3765. [http://dx.doi.org/10.1038/nrn3765]. [PMID: 24946761]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watkins S., Sontheimer H. Hydrodynamic cellular volume changes enable glioma cell invasion. J. Neurosci. 2011;31(47):17250–17259. doi: 10.1523/JNEUROSCI.3938-11.2011. [http://dx.doi.org/10.1523/JNEUROSCI.3938-11.2011]. [PMID: 22114291]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catacuzzeno L., Aiello F., Fioretti B., Sforna L., Castigli E., Ruggieri P., Tata A.M., Calogero A., Franciolini F. Serum-activated K and Cl currents underlay U87-MG glioblastoma cell migration. J. Cell. Physiol. 2011;226(7):1926–1933. doi: 10.1002/jcp.22523. [http://dx. doi.org/10.1002/jcp.22523]. [PMID: 21506123]. [DOI] [PubMed] [Google Scholar]
- 26.Chantome A., Girault A., Potier M., Collin C., Vaudin P., Pagès J.C., Vandier C., Joulin V. KCa2.3 channel-dependent hyperpolarization increases melanoma cell motility. Exp. Cell Res. 2009;315(20):3620–3630. doi: 10.1016/j.yexcr.2009.07.021. [http://dx.doi.org/10.1016/j.yexcr.2009.07.021]. [PMID: 19646982]. [DOI] [PubMed] [Google Scholar]
- 27.Potier M., Joulin V., Roger S., Besson P., Jourdan M.L., Leguennec J.Y., Bougnoux P., Vandier C. Identification of SK3 channel as a new mediator of breast cancer cell migration. Mol. Cancer Ther. 2006;5(11):2946–2953. doi: 10.1158/1535-7163.MCT-06-0194. [http://dx.doi.org/10.1158/ 1535-7163.MCT-06-0194]. [PMID: 17121942]. [DOI] [PubMed] [Google Scholar]
- 28.Schwab A., Reinhardt J., Schneider S.W., Gassner B., Schuricht B.K. (+) channel-dependent migration of fibroblasts and human melanoma cells. Cell. Physiol. Biochem. 1999;9(3):126–132. doi: 10.1159/000016309. [http://dx.doi.org/10.1159/000016309]. [PMID: 10494026]. [DOI] [PubMed] [Google Scholar]
- 29.Schwab A., Wulf A., Schulz C., Kessler W., Nechyporuk-Zloy V., Römer M., Reinhardt J., Weinhold D., Dieterich P., Stock C., Hebert S.C. Subcellular distribution of calcium-sensitive potassium channels (IK1) in migrating cells. J. Cell. Physiol. 2006;206(1):86–94. doi: 10.1002/jcp.20434. [http://dx.doi.org/10.1002/jcp.20434]. [PMID: 15965951]. [DOI] [PubMed] [Google Scholar]
- 30.Cheng Y.Y., Wright C.M., Kirschner M.B., Williams M., Sarun K.H., Sytnyk V., Leshchynska I., Edelman J.J., Vallely M.P., McCaughan B.C., Klebe S., van Zandwijk N., Lin R.C., Reid G. KCa1.1, a calcium-activated potassium channel subunit alpha 1, is targeted by miR-17-5p and modulates cell migration in malignant pleural mesothelioma. Mol. Cancer. 2016;15(1):44. doi: 10.1186/s12943-016-0529-z. [http://dx.doi. org/10.1186/s12943-016-0529-z]. [PMID: 27245839]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edalat L., Stegen B., Klumpp L., Haehl E., Schilbach K., Lukowski R., Kühnle M., Bernhardt G., Buschauer A., Zips D., Ruth P., Huber S.M.B.K.K.B.K.K. + channel blockade inhibits radiation-induced migration/brain infiltration of glioblastoma cells. Oncotarget. 2016;7(12):14259–14278. doi: 10.18632/oncotarget.7423. [http://dx.doi.org/10. 18632/oncotarget.7423]. [PMID: 26893360]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weaver A.K., Bomben V.C., Sontheimer H. Expression and function of calcium-activated potassium channels in human glioma cells. Glia. 2006;54(3):223–233. doi: 10.1002/glia.20364. [http://dx.doi.org/10.1002/glia. 20364]. [PMID: 16817201]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khaitan D., Sankpal U.T., Weksler B., Meister E.A., Romero I.A., Couraud P.O., Ningaraj N.S. Role of KCNMA1 gene in breast cancer invasion and metastasis to brain. BMC Cancer. 2009;9:258. doi: 10.1186/1471-2407-9-258. [http://dx.doi.org/10.1186/1471-2407-9-258]. [PMID: 19640305]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwab A., Schuricht B., Seeger P., Reinhardt J., Dartsch P.C. Migration of transformed renal epithelial cells is regulated by K+ channel modulation of actin cytoskeleton and cell volume. Pflugers Arch. 1999;438(3):330–337. doi: 10.1007/s004240050917. [http://dx.doi.org/10.1007/ s004240050917]. [PMID: 10398863]. [DOI] [PubMed] [Google Scholar]
- 35.Wen P.Y., Kesari S. Malignant gliomas in adults. N. Engl. J. Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [http://dx.doi.org/10.1056/NEJMra 0708126]. [PMID: 18669428]. [DOI] [PubMed] [Google Scholar]
- 36.Scherer H.J. Structural development in gliomas. Am. J. Cancer. 1938;34:333–351. [Google Scholar]
- 37.Burger P.C., Scheithauer B.W., Vogel F.S. Surgical Pathology of the Nervous System and Its Coverings. 4th ed. New York: Churchill Livingstone; 2002. p. 225. [Google Scholar]
- 38.Zagzag D., Esencay M., Mendez O., Yee H., Smirnova I., Huang Y., Chiriboga L., Lukyanov E., Liu M., Newcomb E.W. Hypoxia- and vascular endothelial growth factor-induced stromal cell-derived factor-1alpha/CXCR4 expression in glioblastomas: one plausible explanation of Scherer’s structures. Am. J. Pathol. 2008;173(2):545–560. doi: 10.2353/ajpath.2008.071197. [http://dx.doi.org/10.2353/ajpath.2008. 071197]. [PMID: 18599607]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richardson P.J. CXCR4 and Glioblastoma. Anticancer. Agents Med. Chem. 2016;16(1):59–74. doi: 10.2174/1871520615666150824153032. [http://dx.doi.org/10.2174/ 1871520615666150824153032]. [PMID: 26299663]. [DOI] [PubMed] [Google Scholar]
- 40.Sciaccaluga M., Fioretti B., Catacuzzeno L., Pagani F., Bertollini C., Rosito M., Catalano M., D’Alessandro G., Santoro A., Cantore G., Ragozzino D., Castigli E., Franciolini F., Limatola C. CXCL12-induced glioblastoma cell migration requires intermediate conductance Ca2+-activated K+ channel activity. Am. J. Physiol. Cell Physiol. 2010;299(1):C175–C184. doi: 10.1152/ajpcell.00344.2009. [http://dx.doi.org/10.1152/ ajpcell.00344.2009]. [PMID: 20392929]. [DOI] [PubMed] [Google Scholar]
- 41.D’Alessandro G., Catalano M., Sciaccaluga M., Chece G., Cipriani R., Rosito M., Grimaldi A., Lauro C., Cantore G., Santoro A., Fioretti B., Franciolini F., Wulff H., Limatola C. KCa3.1 channels are involved in the infiltrative behavior of glioblastoma in vivo. Cell Death Dis. 2013;4:e773. doi: 10.1038/cddis.2013.279. [http://dx. doi.org/10.1038/cddis.2013.279]. [PMID: 23949222]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guillamo J.S., Lisovoski F., Christov C., Le Guérinel C., Defer G.L., Peschanski M., Lefrançois T. Migration pathways of human glioblastoma cells xenografted into the immunosuppressed rat brain. J. Neurooncol. 2001;52(3):205–215. doi: 10.1023/a:1010620420241. [http://dx.doi.org/ 10.1023/A:1010620420241]. [PMID: 11519850]. [DOI] [PubMed] [Google Scholar]
- 43.Kanzawa T., Germano I.M., Komata T., Ito H., Kondo Y., Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11(4):448–457. doi: 10.1038/sj.cdd.4401359. [http://dx.doi.org/10.1038/sj.cdd.4401359]. [PMID: 14713959]. [DOI] [PubMed] [Google Scholar]
- 44.Hirose Y., Berger M.S., Pieper R.O. p53 effects both the duration of G2/M arrest and the fate of temozolomide-treated human glioblastoma cells. Cancer Res. 2001;61(5):1957–1963. [PMID: 11280752]. [PubMed] [Google Scholar]
- 45.Quast S.A., Berger A., Buttstädt N., Friebel K., Schönherr R., Eberle J. General Sensitization of melanoma cells for TRAIL-induced apoptosis by the potassium channel inhibitor TRAM-34 depends on release of SMAC. PLoS One. 2012;7(6):e39290. doi: 10.1371/journal.pone.0039290. [http://dx.doi.org/10.1371/journal.pone.0039290]. [PMID: 22723988]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D’Alessandro G., Grimaldi A., Chece G., Porzia A., Esposito V., Santoro A., Salvati M., Mainiero F., Ragozzino D., Di Angelantonio S., Wulff H., Catalano M., Limatola C. KCa3.1 channel inhibition sensitizes malignant gliomas to temozolomide treatment. Oncotarget. 2016;7(21):30781–30796. doi: 10.18632/oncotarget.8761. [http://dx.doi.org/ 10.18632/oncotarget.8761]. [PMID: 27096953]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stegen B., Butz L., Klumpp L., Zips D., Dittmann K., Ruth P., Huber S.M. Ca2+-Activated IK K+ Channel Blockade Radiosensitizes Glioblastoma Cells. Mol. Cancer Res. 2015;13(9):1283–1295. doi: 10.1158/1541-7786.MCR-15-0075. [http://dx.doi.org/10.1158/1541-7786.MCR-15-0075]. [PMID: 26041939]. [DOI] [PubMed] [Google Scholar]
- 48.Tyagi A.K., Singh R.P., Agarwal C., Chan D.C., Agarwal R. Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth Inhibition, G2-M arrest, and apoptosis. Clin. Cancer Res. 2002;8(11):3512–3519. [PMID: 12429642]. [PubMed] [Google Scholar]
- 49.Grimaldi A., D’Alessandro G., Golia M.T., Grössinger E.M., Di Angelantonio S., Ragozzino D., Santoro A., Esposito V., Wulff H., Catalano M., Limatola C. KCa3.1 inhibition switches the phenotype of glioma-infiltrating microglia/macrophages. Cell Death Dis. 2016;7:e2174. doi: 10.1038/cddis.2016.73. [http://dx.doi.org/10.1038/cddis.2016.73]. [PMID: 27054329]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bouhy D., Ghasemlou N., Lively S., Redensek A., Rathore K.I., Schlichter L.C., David S. Inhibition of the Ca2+-dependent K+ channel, KCNN4/KCa3.1, improves tissue protection and locomotor recovery after spinal cord injury. J. Neurosci. 2011;31(45):16298–16308. doi: 10.1523/JNEUROSCI.0047-11.2011. [http://dx.doi.org/10.1523/JNEUROSCI.0047-11. 2011]. [PMID: 22072681]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ataga K.I., Smith W.R., De Castro L.M., Swerdlow P., Saunthararajah Y., Castro O., Vichinsky E., Kutlar A., Orringer E.P., Rigdon G.C., Stocker J.W. Efficacy and safety of the Gardos channel blocker, senicapoc (ICA-17043), in patients with sickle cell anemia. Blood. 2008;111(8):3991–3997. doi: 10.1182/blood-2007-08-110098. [http://dx.doi.org/ 10.1182/blood-2007-08-110098]. [PMID: 18192510]. [DOI] [PubMed] [Google Scholar]
- 52.Yi M., Yu P., Lu Q., Geller H.M., Yu Z., Chen H. KCa3.1 constitutes a pharmacological target for astrogliosis associated with Alzheimer’s disease. Mol. Cell. Neurosci. 2016;76:21–32. doi: 10.1016/j.mcn.2016.08.008. [http://dx.doi.org/10.1016/j.mcn.2016.08.008]. [PMID: 27567685]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y.J., Raman G., Bodendiek S., O’Donnell M.E., Wulff H. The KCa3.1 blocker TRAM-34 reduces infarction and neurological deficit in a rat model of ischemia/reperfusion stroke. J. Cereb. Blood Flow Metab. 2011;31(12):2363–2374. doi: 10.1038/jcbfm.2011.101. [http://dx.doi.org/10. 1038/jcbfm.2011.101]. [PMID: 21750563]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Y.J., Wallace B.K., Yuen N., Jenkins D.P., Wulff H., O’Donnell M.E. Blood-brain barrier KCa3.1 channels: evidence for a role in brain Na uptake and edema in ischemic stroke. Stroke. 2015;46(1):237–244. doi: 10.1161/STROKEAHA.114.007445. [http://dx.doi.org/10.1161/STROKEAHA. 114.007445]. [PMID: 25477223]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaushal V., Koeberle P.D., Wang Y., Schlichter L.C. The Ca2+-activated K+ channel KCNN4/KCa3.1 contributes to microglia activation and nitric oxide-dependent neurodegeneration. J. Neurosci. 2007;27(1):234–244. doi: 10.1523/JNEUROSCI.3593-06.2007. [http://dx.doi.org/10.1523/JNEUROSCI. 3593-06.2007]. [PMID: 17202491]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mauler F., Hinz V., Horváth E., Schuhmacher J., Hofmann H.A., Wirtz S., Hahn M.G., Urbahns K. Selective intermediate-/small-conductance calcium-activated potassium channel (KCNN4) blockers are potent and effective therapeutics in experimental brain oedema and traumatic brain injury caused by acute subdural haematoma. Eur. J. Neurosci. 2004;20(7):1761–1768. doi: 10.1111/j.1460-9568.2004.03615.x. [http://dx.doi. org/10.1111/j.1460-9568.2004.03615.x]. [PMID: 15379997]. [DOI] [PubMed] [Google Scholar]
- 57.Reich E.P., Cui L., Yang L., Pugliese-Sivo C., Golovko A., Petro M., Vassileva G., Chu I., Nomeir A.A., Zhang L.K., Liang X., Kozlowski J.A., Narula S.K., Zavodny P.J., Chou C.C. Blocking ion channel KCNN4 alleviates the symptoms of experimental autoimmune encephalomyelitis in mice. Eur. J. Immunol. 2005;35(4):1027–1036. doi: 10.1002/eji.200425954. [http://dx.doi.org/10.1002/eji. 200425954]. [PMID: 15770697]. [DOI] [PubMed] [Google Scholar]
- 58.Urbahns K., Goldmann S., Krüger J., Horváth E., Schuhmacher J., Grosser R., Hinz V., Mauler F. IKCa-channel blockers. Part 2: discovery of cyclohexadienes. Bioorg. Med. Chem. Lett. 2005;15(2):401–404. doi: 10.1016/j.bmcl.2004.10.063. [http://dx.doi.org/10.1016/j.bmcl.2004.10.063]. [PMID: 15603962]. [DOI] [PubMed] [Google Scholar]
- 59.Urbahns K., Horváth E., Stasch J.P., Mauler F. 4-Phenyl-4H-pyrans as IK(Ca) channel blockers. Bioorg. Med. Chem. Lett. 2003;13(16):2637–2639. doi: 10.1016/s0960-894x(03)00560-2. [http://dx.doi.org/10.1016/S0960-894X (03)00560-2]. [PMID: 12873483]. [DOI] [PubMed] [Google Scholar]
- 60.Marcus H.J., Carpenter K.L., Price S.J., Hutchinson P.J. In vivo assessment of high-grade glioma biochemistry using microdialysis: a study of energy-related molecules, growth factors and cytokines. J. Neurooncol. 2010;97(1):11–23. doi: 10.1007/s11060-009-9990-5. [http://dx.doi.org/10.1007/ s11060-009-9990-5]. [PMID: 19714445]. [DOI] [PubMed] [Google Scholar]
- 61.Yao P.S., Kang D.Z., Lin R.Y., Ye B., Wang W., Ye Z.C. Glutamate/glutamine metabolism coupling between astrocytes and glioma cells: neuroprotection and inhibition of glioma growth. Biochem. Biophys. Res. Commun. 2014;450(1):295–299. doi: 10.1016/j.bbrc.2014.05.120. [http://dx. doi.org/10.1016/j.bbrc.2014.05.120]. [PMID: 24944014]. [DOI] [PubMed] [Google Scholar]
- 62.Savaskan N.E., Heckel A., Hahnen E., Engelhorn T., Doerfler A., Ganslandt O., Nimsky C., Buchfelder M., Eyüpoglu I.Y. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat. Med. 2008;14(6):629–632. doi: 10.1038/nm1772. [http://dx.doi.org/10.1038/nm1772]. [PMID: 18469825]. [DOI] [PubMed] [Google Scholar]
- 63.Buckingham S.C., Campbell S.L., Haas B.R., Montana V., Robel S., Ogunrinu T., Sontheimer H. Glutamate release by primary brain tumors induces epileptic activity. Nat. Med. 2011;17(10):1269–1274. doi: 10.1038/nm.2453. [http://dx.doi.org/10.1038/nm.2453]. [PMID: 21909104]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eder C., Klee R., Heinemann U. Pharmacological properties of Ca2+-activated K+ currents of ramified murine brain macrophages. Naunyn Schmiedebergs Arch. Pharmacol. 1997;356(2):233–239. doi: 10.1007/pl00005046. [http://dx.doi.org/10.1007/PL00005046]. [PMID: 9272730]. [DOI] [PubMed] [Google Scholar]
- 65.Khanna R., Myers M.P., Lainé M., Papazian D.M. Glycosylation increases potassium channel stability and surface expression in mammalian cells. J. Biol. Chem. 2001;276(36):34028–34034. doi: 10.1074/jbc.M105248200. [http://dx.doi.org/10.1074/jbc.M105248200]. [PMID: 11427541]. [DOI] [PubMed] [Google Scholar]
- 66.Schilling T., Repp H., Richter H., Koschinski A., Heinemann U., Dreyer F., Eder C. Lysophospholipids induce membrane hyperpolarization in microglia by activation of IKCa1 Ca(2+)-dependent K(+) channels. Neuroscience. 2002;109(4):827–835. doi: 10.1016/s0306-4522(01)00534-6. [http://dx.doi.org/10.1016/S0306-4522(01)00534-6]. [PMID: 11927165]. [DOI] [PubMed] [Google Scholar]
- 67.Schilling T., Stock C., Schwab A., Eder C. Functional importance of Ca2+-activated K+ channels for lysophosphatidic acid-induced microglial migration. Eur. J. Neurosci. 2004;19(6):1469–1474. doi: 10.1111/j.1460-9568.2004.03265.x. [http://dx.doi.org/10.1111/j.1460-9568.2004.03265.x]. [PMID: 15066143]. [DOI] [PubMed] [Google Scholar]
- 68.Lively S., Schlichter L.C. The microglial activation state regulates migration and roles of matrix-dissolving enzymes for invasion. J. Neuroinflammation. 2013;10:75. doi: 10.1186/1742-2094-10-75. [http://dx.doi.org/10.1186/1742-2094-10-75]. [PMID: 23786632]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferreira R., Wong R., Schlichter L.C. KCa3.1/IK1 channel regulation by cGMP-dependent protein kinase (PKG) via reactive oxygen species and CaMKII in microglia: An immune modulating feedback system? Front. Immunol. 2015;6:153. doi: 10.3389/fimmu.2015.00153. [http://dx.doi.org/ 10.3389/fimmu.2015.00153]. [PMID: 25904916]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scheiblich H., Roloff F., Singh V., Stangel M., Stern M., Bicker G. Nitric oxide/cyclic GMP signaling regulates motility of a microglial cell line and primary microglia in vitro. Brain Res. 2014;1564:9–21. doi: 10.1016/j.brainres.2014.03.048. [http://dx.doi.org/10.1016/j.brainres.2014.03. 048]. [PMID: 24713349]. [DOI] [PubMed] [Google Scholar]
- 71.Casella G., Garzetti L., Gatta A.T., Finardi A., Maiorino C., Ruffini F., Martino G., Muzio L., Furlan R. IL4 induces IL6-producing M2 macrophages associated to inhibition of neuroinflammation in vitro and in vivo. J. Neuroinflammation. 2016;13(1):139. doi: 10.1186/s12974-016-0596-5. [http://dx.doi.org/10.1186/s12974-016-0596-5]. [PMID: 27266518]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou X., Spittau B., Krieglstein K. TGFβ signalling plays an important role in IL4-induced alternative activation of microglia. J. Neuroinflammation. 2012;9:210. doi: 10.1186/1742-2094-9-210. [http://dx.doi.org/10.1186/1742-2094-9-210]. [PMID: 22947253]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [http://dx.doi. org/10.1172/JCI59643]. [PMID: 22378047]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ransohoff R.M. How neuroinflammation contributes to neurodegeneration. Science. 2016;353(6301):777–783. doi: 10.1126/science.aag2590. [http://dx.doi.org/ 10.1126/science.aag2590]. [PMID: 27540165]. [DOI] [PubMed] [Google Scholar]
- 75.Gutmann D.H. Microglia in the tumor microenvironment: taking their TOLL on glioma biology. Neuro-oncol. 2015;17(2):171–173. doi: 10.1093/neuonc/nou346. [http://dx.doi.org/10.1093/neuonc/nou346]. [PMID: 25523594]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simmons G.W., Pong W.W., Emnett R.J., White C.R., Gianino S.M., Rodriguez F.J., Gutmann D.H. Neurofibromatosis-1 heterozygosity increases microglia in a spatially and temporally restricted pattern relevant to mouse optic glioma formation and growth. J. Neuropathol. Exp. Neurol. 2011;70(1):51–62. doi: 10.1097/NEN.0b013e3182032d37. [http://dx.doi.org/10.1097/NEN.0b013e3182032d37]. [PMID: 21157378]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martinez F.O., Sica A., Mantovani A., Locati M. Macrophage activation and polarization. Front. Biosci. 2008;13:453–461. doi: 10.2741/2692. [http://dx.doi.org/10.2741/2692]. [PMID: 17981560]. [DOI] [PubMed] [Google Scholar]
- 78.Komohara Y., Ohnishi K., Kuratsu J., Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J. Pathol. 2008;216(1):15–24. doi: 10.1002/path.2370. [http://dx.doi.org/10.1002/path.2370]. [PMID: 18553315]. [DOI] [PubMed] [Google Scholar]
- 79.Li W., Graeber M.B. The molecular profile of microglia under the influence of glioma. Neuro-oncol. 2012;14(8):958–978. doi: 10.1093/neuonc/nos116. [http://dx.doi.org/10.1093/neuonc/nos116]. [PMID: 22573310]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang S.C., Hong J.H., Hsueh C., Chiang C.S. Tumor-secreted SDF-1 promotes glioma invasiveness and TAM tropism toward hypoxia in a murine astrocytoma model. Lab. Invest. 2012;92(1):151–162. doi: 10.1038/labinvest.2011.128. [http://dx.doi.org/10.1038/labinvest.2011.128]. [PMID: 21894147]. [DOI] [PubMed] [Google Scholar]
- 81.Platten M., Kretz A., Naumann U., Aulwurm S., Egashira K., Isenmann S., Weller M. Monocyte chemoattractant protein-1 increases microglial infiltration and aggressiveness of gliomas. Ann. Neurol. 2003;54(3):388–392. doi: 10.1002/ana.10679. [http://dx.doi.org/10.1002/ana.10679]. [PMID: 12953273]. [DOI] [PubMed] [Google Scholar]
- 82.Okada M., Saio M., Kito Y., Ohe N., Yano H., Yoshimura S., Iwama T., Takami T. Tumor-associated macrophage/microglia infiltration in human gliomas is correlated with MCP-3, but not MCP-1. Int. J. Oncol. 2009;34(6):1621–1627. doi: 10.3892/ijo_00000292. [PMID: 19424580]. [DOI] [PubMed] [Google Scholar]
- 83.Ku M.C., Wolf S.A., Respondek D., Matyash V., Pohlmann A., Waiczies S., Waiczies H., Niendorf T., Synowitz M., Glass R., Kettenmann H. GDNF mediates glioblastoma-induced microglia attraction but not astrogliosis. Acta Neuropathol. 2013;125(4):609–620. doi: 10.1007/s00401-013-1079-8. [http://dx.doi.org/10.1007/s00401-013-1079-8]. [PMID: 23344256]. [DOI] [PubMed] [Google Scholar]
- 84.Coniglio S.J., Eugenin E., Dobrenis K., Stanley E.R., West B.L., Symons M.H., Segall J.E. Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Mol. Med. 2012;18:519–527. doi: 10.2119/molmed.2011.00217. [http://dx.doi.org/10.2119/ molmed.2011.00217]. [PMID: 22294205]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sielska M., Przanowski P., Wylot B., Gabrusiewicz K., Maleszewska M., Kijewska M., Zawadzka M., Kucharska J., Vinnakota K., Kettenmann H., Kotulska K., Grajkowska W., Kaminska B. Distinct roles of CSF family cytokines in macrophage infiltration and activation in glioma progression and injury response. J. Pathol. 2013;230(3):310–321. doi: 10.1002/path.4192. [http://dx.doi.org/10. 1002/path.4192]. [PMID: 23520016]. [DOI] [PubMed] [Google Scholar]
- 86.Bettinger I., Thanos S., Paulus W. Microglia promote glioma migration. Acta Neuropathol. 2002;103(4):351–355. doi: 10.1007/s00401-001-0472-x. [http://dx. doi.org/10.1007/s00401-001-0472-x]. [PMID: 11904754]. [DOI] [PubMed] [Google Scholar]
- 87.Carvalho da Fonseca A.C., Wang H., Fan H., Chen X., Zhang I., Zhang L., Lima F.R., Badie B. Increased expression of stress inducible protein 1 in glioma-associated microglia/macrophages. J. Neuroimmunol. 2014;274(1-2):71–77. doi: 10.1016/j.jneuroim.2014.06.021. [http://dx.doi.org/10.1016/ j.jneuroim.2014.06.021]. [PMID: 25042352]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saederup N., Cardona A.E., Croft K., Mizutani M., Cotleur A.C., Tsou C.L., Ransohoff R.M., Charo I.F. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One. 2010;5(10):e13693. doi: 10.1371/journal.pone.0013693. [http://dx.doi.org/10.1371/journal.pone.0013693]. [PMID: 21060874]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wesolowska A., Kwiatkowska A., Slomnicki L., Dembinski M., Master A., Sliwa M., Franciszkiewicz K., Chouaib S., Kaminska B. Microglia-derived TGF-beta as an important regulator of glioblastoma invasion--an inhibition of TGF-beta-dependent effects by shRNA against human TGF-beta type II receptor. Oncogene. 2008;27(7):918–930. doi: 10.1038/sj.onc.1210683. [http://dx.doi.org/10.1038/sj.onc.1210683]. [PMID: 17684491]. [DOI] [PubMed] [Google Scholar]
- 90.Wick W., Platten M., Weller M. Glioma cell invasion: regulation of metalloproteinase activity by TGF-beta. J. Neurooncol. 2001;53(2):177–185. doi: 10.1023/a:1012209518843. [http://dx.doi.org/10.1023/A:1012209518843]. [PMID: 11716069]. [DOI] [PubMed] [Google Scholar]
- 91.Markovic D.S., Vinnakota K., Chirasani S., Synowitz M., Raguet H., Stock K., Sliwa M., Lehmann S., Kälin R., van Rooijen N., Holmbeck K., Heppner F.L., Kiwit J., Matyash V., Lehnardt S., Kaminska B., Glass R., Kettenmann H. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc. Natl. Acad. Sci. USA. 2009;106(30):12530–12535. doi: 10.1073/pnas.0804273106. [http://dx.doi.org/10.1073/pnas.0804273106]. [PMID: 19617536]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Markovic D.S., Glass R., Synowitz M., Rooijen Nv., Kettenmann H. Microglia stimulate the invasiveness of glioma cells by increasing the activity of metalloprotease-2. J. Neuropathol. Exp. Neurol. 2005;64(9):754–762. doi: 10.1097/01.jnen.0000178445.33972.a9. [http://dx.doi.org/10.1097/01.jnen. 0000178445.33972.a9]. [PMID: 16141784]. [DOI] [PubMed] [Google Scholar]
- 93.Markovic D.S., Vinnakota K., van Rooijen N., Kiwit J., Synowitz M., Glass R., Kettenmann H. Minocycline reduces glioma expansion and invasion by attenuating microglial MT1-MMP expression. Brain Behav. Immun. 2011;25(4):624–628. doi: 10.1016/j.bbi.2011.01.015. [http://dx.doi. org/10.1016/j.bbi.2011.01.015]. [PMID: 21324352]. [DOI] [PubMed] [Google Scholar]
- 94.Fulci G., Dmitrieva N., Gianni D., Fontana E.J., Pan X., Lu Y., Kaufman C.S., Kaur B., Lawler S.E., Lee R.J., Marsh C.B., Brat D.J., van Rooijen N., Stemmer-Rachamimov A.O., Hochberg F.H., Weissleder R., Martuza R.L., Chiocca E.A. Depletion of peripheral macrophages and brain microglia increases brain tumor titers of oncolytic viruses. Cancer Res. 2007;67(19):9398–9406. doi: 10.1158/0008-5472.CAN-07-1063. [http://dx.doi.org/10.1158/0008-5472.CAN-07-1063]. [PMID: 17909049]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gabrusiewicz K., Ellert-Miklaszewska A., Lipko M., Sielska M., Frankowska M., Kaminska B. Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS One. 2011;6(8):e23902. doi: 10.1371/journal.pone.0023902. [http://dx.doi. org/10.1371/journal.pone.0023902]. [PMID: 21901144]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lisi L., Stigliano E., Lauriola L., Navarra P., Dello Russo C. Proinflammatory-activated glioma cells induce a switch in microglial polarization and activation status, from a predominant M2b phenotype to a mixture of M1 and M2a/B polarized cells. ASN Neuro. 2014;6(3):171–183. doi: 10.1042/AN20130045. [http://dx.doi.org/10.1042/AN20130045]. [PMID: 24689533]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dello Russo C., Lisi L., Tentori L., Navarra P., Graziani G., Combs C.K. Exploiting microglial functions for the treatment of glioblastoma. Curr. Cancer Drug Targets. 2017;17(3):267–281. doi: 10.2174/1568009616666160813191240. [http://dx.doi.org/10.2174/1568009616666160813191240]. [PMID: 27528361]. [DOI] [PubMed] [Google Scholar]