Abstract
Background
Studies investigating the impact of a variety of inflammatory stimuli on the brain and behavior have reported evidence that inflammation and release of inflammatory cytokines affect circuitry relevant to both reward and threat sensitivity to contribute to behavioral change. Of relevance to mood and anxiety-related disorders, biomarkers of inflammation such as inflammatory cytokines and acute-phase proteins are reliably elevated in a significant proportion of patients with major depressive disorder (MDD), bipolar disorder, anxiety disorders and post-traumatic stress disorder (PTSD).
Methods
This review summarized clinical and translational work demonstrating the impact of peripheral inflammation on brain regions and neurotransmitter systems relevant to both reward and threat sensitivity, with a focus on neuroimaging studies involving administration of inflammatory stimuli. Recent translation of these findings to further understand the role of inflammation in mood and anxiety-related disorders is also discussed.
Results
Inflammation was consistently found to affect basal ganglia and cortical reward and motor circuits to drive reduced motivation and motor activity, as well as anxiety-related brain regions including amygdala, insula and anterior cingulate cortex, which may result from cytokine effects on monoamines and glutamate. Similar relationships between inflammation and altered neurocircuitry have been observed in MDD patients with increased peripheral inflammatory markers, and such work is on the horizon for anxiety disorders and PTSD.
Conclusion
Neuroimaging effects of inflammation on reward and threat circuitry may be used as biomarkers of inflammation for future development of novel therapeutic strategies to better treat mood and anxiety-related disorders in patients with high inflammation.
Keywords: Neuroimaging, inflammation, cytokines, microglia, depression, anxiety, PTSD, motivation, motor slowing
1. Introduction
Findings from numerous laboratories have consistently indicated that innate immune activation and the release of inflammatory cytokines preferentially affect brain regions relevant to both reward and threat sensitivity. Indeed, cytokines have been shown to impact basal ganglia and cortical reward and motor circuitry, as well as to affect fear and anxiety-related structures including amygdala, insula and anterior cingulate cortex (ACC) [1-4] (Fig. 1). It has recently been appreciated that the effects of inflammation and cytokines on these brain regions produce potentially adaptive and beneficial behavioral responses [5, 6]. While decreased motivation and motor activity may subserve the conservation of energy and allocation of resources to fight infection and heal wounds, increased vigilance and threat sensitivity may guard against future attack or trauma. Thus, these inflammation-associated behavioral changes conferred an evolutionary advantage by promoting containment of pathogens and the initiation of wound healing. Our propensity to mount aggressive immune responses, not only to pathogens but also to a host of environmental insults including psychological stress, exist as the legacy of survival and reproductive fitness in unsafe and hostile environments of ancestral times [5, 6].
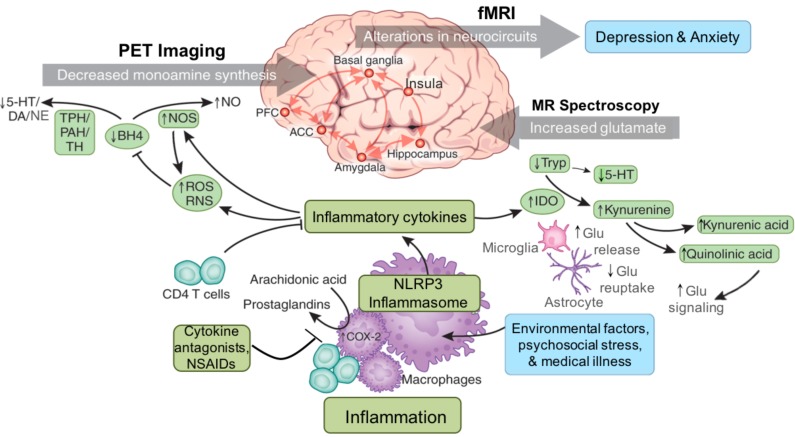
Fig. (1).
Inflammation is increased in patients with mood and anxiety-related disorders due to environmental challenges such as diet and lifestyle factors, medical illness and psychosocial stress. Immune activation involves intracellular signal transduction pathways and the inflammasome, which can respond to a variety of environmental stressors beyond pathogens including psychosocial stress, to produce release of inflammatory cytokines. Increased inflammatory cytokines are in turn associated with increased oxidative stress and generation of reactive oxygen and reactive nitrogen species (ROS and RNS). Increased ROS and RNS contribute to oxidation of tetrahydrobiopterin (BH4), a cofactor required for the enzymatic synthesis of monoamines via phenylalanine hydroxylase (PAH), tryptophan hydroxylase (TPH) and tyrosine hydroxylase (TH), thus disrupting the synthesis of serotonin (5-HT), dopamine (DA) and norepinephrine (NE) through effects on the availability of monoamine precursors such as tryptophan and tyrosine. Inflammation and the release of cytokines also stimulate enzyme pathways such as indoleamine 2,3 dioxygenase (IDO), which can then lead to the release of neurotoxic metabolites of kynurenine that affect glutamate (Glu) including quinolinic acid. In the brain, inflammation causes release of Glu from microglia and reduced uptake by astrocytes. These actions of inflammatory cytokines ultimately contribute to alterations in neurocircuits in the brain including those related to basal ganglia to prefrontal reward and motor circuits, as well as fear and anxiety-related amygdala, prefrontal and insular circuitry, which may contribute to symptoms of depression and anxiety. 5-HT - serotonin; ACC - anterior cingulate cortex; BH4 - tetrahydrobiopterin; DA - dopamine; Glu - glutamate; NLRP3 - NACHT domain- leucine-rich repeat- and pyrin domain-containing protein 3; I - imaging; IDO - indoleamine 2,3 dioxygenase; MR – magnetic resonance; NE - norepinephrine; NO-nitric oxide; NOS-nitric oxide synthase; NSAIDS-non-steroidal anti-inflammatory drugs; PFC - prefrontal cortex; PHA - phenylalanine hydroxylase; ROS - reactive oxygen species; TH - tyrosine hydroxylase; Trp – tryptophan. (The color version of the figure is available in the electronic copy of the article).
In modern times, this bias toward inflammatory responses to both physiologic and psychological challenge in part drives the high prevalence of persons exhibiting a maladaptive, low-grade chronic inflammatory state. Chronically increased inflammation in the body is associated with risk for medical illnesses such cardiovascular disease, metabolic disorders and cancer [7, 8], while its effects on the brain may cause behavioral symptoms relevant to mood and anxiety disorders, which are often comorbid [9]. It has been well established that a significant proportion of patients with mood and anxiety-related disorders exhibit evidence of elevated inflammatory markers including increases in cerebrospinal fluid (CSF) and circulating concentrations of inflammatory cytokines, chemokines and acute phase reactants, changes in gene expression and increased presence of inflammatory immune cell phenotypes [4, 10-12]. While mood disorders may have complex pathophysiology with heterogeneous etiologies, it is thought that increased inflammation may be involved in the disease process and contribute to discreet symptomologies in a subset of patients. Although the source of inflammation may vary across patients and disorders, it is thought to often be a combination primarily of lifestyle factors such as diet, exercise and body weight, sleep impairments, genetic inflammatory bias, medical illness and exposure to psychosocial stress and/or trauma (Fig. 1) [4, 11, 13]. Inflammatory signals elaborated in the body are thought to then impact the brain to drive behavioral symptoms relevant to mood and anxiety-related disorders (Fig. 1). Access of peripheral inflammatory signals to the brain may involve trafficking of peripheral immune cells to the brain as well as cytokine-induced activation of local inflammatory signaling pathways and microglia [14, 15].
Interest in the role of inflammation in mood and anxiety-related disorders has prompted research on the blockade of inflammation as a potential treatment strategy [16]. Thus, attempts have been made to image inflammation in the brains of psychiatric patients, in part to establish imaging biomarkers of target engagement in the brain to be used in treatment trials for anti-inflammatories [16]. Positron emission tomography (PET) imaging of activated microglia has been reported in a number of psychiatric populations; however, an association between inflammation in the CNS and circulating inflammatory markers and/or behavioral symptoms has been less clear [16]. Conversely, imaging of neural activation, functional and structural connectivity and change in neurotransmitter systems in humans and non-human primates has reliably provided information about the effects of inflammation on the brain that have been highly correlated with peripheral and CSF inflammatory markers as well as inflammation-related behavioral alterations [1-4] (Fig. 1). These findings, interpreted in conjunction with a wealth of information from basic science studies, have allowed us to understand how peripheral inflammation affects the brain, even in the absence of CNS immune cell activation detectable by PET neuroimaging. These translational neuroimaging and basic science studies have elucidated a number of neurotransmitter systems affected by inflammation that may serve as more proximal targets for existing therapeutics used in psychiatry and neurology that may improve symptoms of mood and anxiety-related disorders in patients with increased inflammation [2, 16, 17]. This review will highlight the wealth of clinical and translational work demonstrating the impact of peripheral inflammation on brain regions relevant to both reward and threat sensitivity, with a focus on neuroimaging studies involving administration of inflammatory stimuli and the translation of these findings to the investigation of similar relationships in patients with mood and anxiety-related disorders who exhibit high inflammation.
2. Increased inflammation in mood and anxiety-related disorders
2.1. Peripheral and Central Cytokines and Acute Phase Reactants
A growing body of evidence suggests that a significant proportion of patients with mood (e.g. major depressive disorder [MDD] and bipolar disorder [BD]) and anxiety-related disorders (e.g. generalized anxiety disorder [GAD], panic disorder and post-traumatic stress disorder [PTSD]) exhibit a chronic low-grade inflammation, as measured by increased peripheral and central inflammatory cytokines, inflammatory mediators and acute phase reactants (for review see [10-12, 18, 19]. Numerous studies have reported increased circulating inflammatory cytokines, e.g. interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF), their soluble receptors, and acute phase reactants such as C-reactive protein (CRP), in patients with mood and anxiety-related disorders [10, 20-22]. For example, recent studies have indicated that ‘high inflammation’ (plasma CRP concentrations >3mg/L, as defined by the American Heart Association) [23], is consistently found in 20-40% of patients with MDD, with higher concentrations observed in patients who are resistant to standard antidepressant therapies [24-28]. These findings have been corroborated by meta-analyses in MDD [29, 30], BD [19, 31, 32], and PTSD [33]. Increased inflammatory cytokine concentrations in the cerebrospinal fluid (CSF) of patients with MDD, BD and PTSD have also been observed [34-38]. In MDD, CSF cytokines have been shown to be associated with severity of depression, or with change in symptoms in response to successful treatment [34, 39, 40], and in BD they were higher in patients that have had more recent manic or hypomanic episodes [37].
2.2. Gene Expression and Genetic Predisposition
Several functional allelic variants and single-nucleotide polymorphisms (SNPs) of genes encoding immune and inflammatory molecules have been associated with MDD, including those encoding expression of inflammatory cytokines, major histocompatibility complex proteins, B and T cells, and inflammatory mediators like cyclo-oxygenase2 [5, 41-44]. In PTSD, results from a genome-wide association study (GWAS) indicated that polymorphisms for genes involved in inflammatory pathways were enriched in women with PTSD [45]. Furthermore, in patients from a high risk urban setting with a history of trauma, those who carried a CRP genotype (rs1130864) that is associated with elevated CRP concentrations had higher rates of PTSD and reported higher severity of symptoms of being overly alert and losing interest in activities [46]. As alluded to in the introduction, such findings have engendered speculation as to whether alleles that promote enhanced inflammatory cytokine secretion were evolutionarily advantageous and thus conserved [5]. Indeed, heightened inflammatory responses to environmental stimuli may have improved survival by conferring greater protection from bacterial and viral infection [5], and genetic priming to respond to stress and the environment with increased inflammatory and antiviral responses could contribute to the high prevalence of psychiatric disorders comorbid with medical illnesses that are associated with inflammation (e.g. cardiovascular disease, metabolic disorders, and autoimmune disorders) [47-52].
In addition to genetic polymorphisms, increased inflammatory gene expression in circulating immune cells has been found in patients with MDD, PTSD and other psychiatric disorders [53-55], and may predict treatment response in MDD. For instance, a targeted analysis of leukocyte mRNA expression of a subset of genes related to inflammation, glucocorticoid receptor signaling, and neuroplasticity revealed higher baseline mRNA levels of interleukin (IL)-1β, macrophage inhibitory factor (MIF), and TNF in patients with MDD who failed to respond to 8 weeks of treatment with escitalopram or nortriptyline [56]. Interestingly, increased expression of a number of inflammatory markers has been observed in the brains of patients with mood disorders [53, 57].
2.3. Sources of Innate Immune Activation and Inflammation
Factors that may activate the innate immune system and contribute to increased inflammation in psychiatric patients who are otherwise medically stable include psychosocial stress (and particularly early life stress or trauma), sleep disturbance, inflammatory diet and gastrointestinal permeability, obesity and other lifestyle factors such as smoking [13]. Persons with a history of childhood trauma exhibit elevated inflammatory biomarkers and higher rates of depression as adults [58, 59], and a “biological embedding” or imprinting of stress through inflammatory processes in childhood has been proposed [60, 61]. For instance, subjects with MDD and a history of early life stress responded to psychological stress (the Trier Social Stress Test) with exaggerated circulating IL-6 production and increased DNA binding of nuclear factor (NF)-kB in peripheral blood mononuclear cells compared to non-depressed controls [62]. Increased IL-6 production in adolescents with histories of childhood adversity has been shown to precede subsequent development of depression 6 months later [63]. Similarly, peripheral CRP prior to trauma has been shown to be a significant predictor of future development of PTSD after trauma, [64] and inflammatory cytokine production early after trauma was also predictive of the development of PTSD [65]. Together these findings suggest causal relationships between early life stress or trauma, increased inflammation, and development of MDD and/or PTSD.
Considerable evidence now exists supporting mechanisms by which stress activates the inflammatory response [66, 67]. On a cellular level, the immune system can detect danger signals in the absence of a pathogen through the release of danger associated molecular patterns (DAMPs) such as heat shock protein-72, uric acid, and adenosine triphosphate (ATP), through a process termed “sterile inflammation” [66, 68]. These DAMPS are released during psychological stress, and one key mechanism by which they may elicit an immune response is through the NLRP3 inflammasome (Fig. 1), a multi-protein complex that is involved in the processing of IL-1beta. DAMPs are known to stimulate the inflammasome in the presence of endotoxin to activate caspase-1, which cleaves the immature precursor of interleukin IL-1beta as well as IL-18 into their mature, releasable forms [67, 68]. This release of IL-1beta can then induce the production of other inflammatory cytokines that are produced during stress [67]. Therefore, DAMPS and the NLRP3 inflammasome may serve as a primary link by which stress is translated into damage signals that promote inflammatory activity in patients exposed to chronic psychological stress and trauma.
Sleep disturbance may be another variable that is related to inflammation [69-72]. Sleep deprivation results in increased circulating levels of IL-6, TNF, and CRP when compared to periods of undisturbed sleep [73-75]. Disturbed sleep also increase circulating IL-6, TNF and CRP [73-75], and sleep impairments in psychiatric illnesses such as MDD have been associated with increased inflammation [69-72]. In terms of lifestyle factors, inflammatory diets that promote gut permeability and changes in the microbiota, smoking, and increased body mass index (BMI) all contribute to increased inflammation and may interact with genetics and stress to contribute to behavioral symptoms and poor overall health outcomes in patients with psychiatric illness [13, 76]. For example, obesity from consumption of a high-fat diet in rodents induces changes in the gut microbiota and increases ileal inflammation and permeability [77]. Obesity and high BMI are associated with increased concentrations of IL-6 and other inflammatory markers in humans [78, 79] thought to be the result of macrophage accumulation in adipose tissue, and especially visceral adiposity, which can release cytokines into portal circulation [80-82]. Interestingly, adiposity has been suggested as a link between psychiatric illness, increased inflammatory markers and increased risk of coronary heart disease [83, 84].
2.4. Peripheral and Central Immune Cell Activation
Peripheral inflammatory cytokines may access the CNS to initiate local immune activation by several mechanisms, including 1.) passage through leaky regions in the blood-brain-barrier at circumventricular organs [85, 86], 2.) active uptake mechanisms of cytokines across the blood-brain-barrier [87-89], and 3.) local actions at peripheral vagal nerve afferents that relay cytokine signals to relevant brain regions, including the nucleus of the solitary tract and hypothalamus (the so called ‘neural route’) [90-93]. However, recent translational data indicates that during peripheral inflammation, activated monocytes/macrophages traffic to the brain in response to monocyte chemoattractant protein (MCP-1), a chemokine produced by activated microglial cells in response to cytokine signaling in from the periphery [14, 94]. These monocytes/macrophages traffic primarily to perivascular and meningeal spaces, and have been shown to contribute to behavioral changes in rodent models of stress-induced depressive and anxiety behaviors [15, 95, 96]. Interestingly, patterns of gene expression in the peripheral blood of patients with psychiatric disorders exhibit increased signatures consistent with pro-inflammatory “M1” activation of monocyte/macrophages [55, 97, 98]. Furthermore, recruitment of activated peripheral macrophages to perivascular spaces, as well as localized activation of microglia neighboring theses blood vessels and increased expression of MCP-1, has been observed in the dorsal ACC of post mortem tissue from suicide patients with mood disorders [99, 100]. These findings indicate that accumulation of peripheral immune cells in vascular compartments in association with restricted and/or regionally specific activation of microglia may be characteristic of patients with mood disorders who exhibit high inflammation.
3. In vivo imaging of CNS immune cell activation in psychiatric disorders
To better understand the consequence of increased inflammation in the periphery of patients with psychiatric illness, there has been growing interest in measuring microglial activation using neuroimaging strategies including positron emission tomography (PET). These strategies have aimed to understand the role of inflammation in the CNS in psychiatric disorders over time as well as the ability to determine whether anti-inflammatory therapies reduce inflammation in the brain. One primary strategy has been the development of radioligands that bind to macrophage as well as microglia in the brain when they become activated and increase surface expression of the translocator protein (TSPO) [101]. PET ligands that bind TSPO are used as potential markers of activated microglia, such as [11C]PK 11195, which show elevated non-displaceable binding potential (BPND) [102] in animal models of neuroinflammation [103, 104]. Unfortunately, the specific-activity of PK 11195 has proved too low to detect subtler inflammatory effects. However, structurally similar second-generation TSPO ligands such as [11C]PBR28 [105] and [18F]FEPPA [106] have been developed that have improved sensitivity and have been used to examine activated CNS macrophage and microglia in patients with mood disorders with mixed results [107-110]. [111].
In mild to moderate depression, [11C]PBR28 binding was not different between patients and controls in BPND, though the sample was small and the depression severity was relatively low [108]. In contrast the 18F analogue of [11C]PBR28, [18F]FEPPA, was used to examine microglia activity in a more severely depressed sample, and showed significant increases in BPND in the striatum, hippocampus, insula and prefrontal areas [107] that correlated with depression symptom severity [112]. However, in this study, no significant relationship was found between peripheral inflammatory markers and central TSPO binding. Similarly, although marked increases in TSPO binding were observed after administration of endotoxin to humans, no correlations between increased peripheral blood cytokine concentrations or increased symptoms of depression and increased TSPO binding in the brain were found [113]. This is in contrast to the measurement of TSPO binding in nonhuman primates administered endotoxin which demonstrated increased TSPO binding in the brain that correlated with increased peripheral blood concentrations of IL-1 beta, IL-6 and TNF depending on the time point examined post endotoxin administration [114]. Moreover, histological examination in non-human primates confirmed that TSPO binding following endotoxin was primarily increased in microglia as opposed astrocytes, which also express TSPO [114].
The inconsistencies between TSPO binding and circulating levels of inflammatory markers in humans, considering they are highly correlated in animal studies, raise questions about what TSPO binding is actually measuring in the brain of patients. Currently it is also unknown, yet highly plausible, that elaboration of immune signals such as cytokines, prostaglandins, nitric oxide and kynurenine pathway metabolites across the blood brain barrier may be sufficient to drive behavioral changes in the absence of appreciable CNS immune cell activation that is detectable by PET. Additional caveats of PET imaging to measure potential microglia activation are worth mentioning. Even at rest, microglia are now known to play a variety of ongoing sentinel-type functions [101]. Moreover, microglia exhibit graded activation responses [115], and increases in some activation markers–such as TSPO– may not indicate a pure inflammatory phenotype [116, 117], thus complicating interpretation of TSPO expression. Additionally, lack of correlation between TSPO BPND and peripheral cytokine concentrations may be an artifact of the volume transmission analysis model, as TSPO BPND is determined through the use of an arterial input function. It is possible that evidence of increased inflammation may also be associated with elevated TSPO expression in peripheral blood cells outside the CNS. In this light, the use of an arterial input function may obscure associations between peripheral and central inflammatory measures. Additionally, the assessment of TSPO binding is complicated by the presence of a TSPO polymorphism (rs6971) that is known to influence the TSPO binding, leading to high-affinity, mixed-affinity and low-affinity binders [111]. Low affinity binders are typically excluded from study analyses, albeit they represent only about 10% of the population in North America. In sum, while PET measures of CNS immune cell activation provide some evidence that macrophage and microglia are activated in mood disorders, further studies are needed to validate the efficiency of TSPO-ligands as markers of inflammation in the brain, in mood as well as anxiety-related disorders, and their relationships with peripheral measures of inflammation and behavioral symptom profiles.
4. Inflammation effects on reward and motor circuitry
Findings from numerous laboratories have consistently indicated that innate immune activation and the release of inflammatory cytokines preferentially affect reward circuitry and dopamine (DA) in the basal ganglia to contribute to reduced motivation and motor slowing [3, 118-124]. In humans, this evidence stems primarily from studies using PET and functional magnetic resonance imaging (fMRI) neuroimaging strategies to investigate the effects of cytokines in healthy volunteers acutely administered cytokine inducers (e.g. endotoxin or typhoid vaccination) [121, 123], and from patients chronically administered inflammatory cytokines (e.g. interferon [IFN]-α) as therapy for some cancers and infectious diseases [118, 119]. In terms of effects on behavior, depending on the dose, up to 50% of patients administered IFN-α as treatment for hepatitis C virus (HCV) or malignant melanoma meet symptom criteria for major depression, and up to 80% experience significant fatigue, lack of energy and motor slowing [125-132]. Additionally, reduced motivation and anhedonia are frequently reported in IFN-α-treated patients [118, 122, 126]. Indeed, targeted instruments that assess aspects of anhedonia, including the Snaith–Hamilton Pleasure Scale (SHAPS) and Reduced Motivation subscale of the Multidimensional Fatigue Inventory (MFI), have yielded comparable effect sizes (all r=0.47-0.49) as for increases in self-reported depression or fatigue scores after chronic IFN-α treatment [118, 122].
Preclinical neuroimaging and high performance liquid chromatography (HPLC) data in non-human primates and rodents suggest that the effects of inflammation on reward circuitry and motivation are mediated by cytokine-induced reductions in striatal DA [133-135]. Additionally, a wealth of in vitro and in vivo studies in laboratory animals have demonstrated a myriad of mechanisms by which inflammation may affect the glutamate system to increase both extracellular glutamate concentrations and glutamate receptor signaling to ultimately contribute to excitotoxicity (as discussed in section 6 below) [17]. Increased glutamate may contribute to inflammation-related functional changes in the striatum and medical prefrontal cortex, and/or drive some of the observed changes in the DA system either directly or through increasing excitetoxicity and oxidative stress, and is consistent with neuroimaging findings in patients administered IFN-alpha [136, 137]. Table 1 summarizes findings from studies investigating the effects of administration of cytokines and inflammatory stimuli on neural activation, structural changes, and DA and glutamate transmission in reward circuitry including regions of striatum and prefrontal cortex (PFC). Studies were identified by pubmed (NLM) query using search terms for inflammatory stimuli (typhoid OR endotoxin OR interferon) AND technique (neuroimaging OR PET OR spectroscopy) AND brain region (striatum OR basal ganglia OR substantia nigra OR prefrontal cortex). Studies were limited to those primary papers that involved administration of inflammatory stimuli to humans or nonhuman primates. Review papers were used to provide cross-references of papers not identified by the query. Translation of these findings to investigate associations between inflammation and changes in neural activity and neurotransmitters in patients with mood disorders is also discussed.
Table 1.
Summary of neuroimaging findings of changes in neural activity, glucose metabolism, qMT, or the dopamine or glutamate systems in reward-related circuitry following administration of peripheral inflammatory stimuli or cytokines.
| Neuroimaging Technique | Subjects | Inflammatory Stimulus | Region | Finding | Study |
|---|---|---|---|---|---|
|
PET [18F]FDOPA, uptake [18F]FDOPA, turnover [18F]FDG, glucose metabolism [18F]FDG, glucose metabolism [18F]FDG, glucose metabolism [18F]FDG, glucose metabolism [11C]raclopride, D2R binding [11C]raclopride, AMPH displacement [11C]raclopride, AMPH displacement |
HCV+ patients HCV+ patients HCV+ patients HCV+ patients MM patients MM patients Rhesus monkeys Rhesus monkeys Healthy controls |
4-6 weeks IFN-α 4-6 weeks IFN-α 12 weeks IFN-α 12 weeks IFN-α 4 weeks IFN-α 4 weeks IFN-α 4 weeks IFN-α 4 weeks IFN-α 1.5 hr endotoxin |
VS, DS VS, DS DS PFC VS, DS PFC VS, DS VS, DS DS |
↑ ↓ ↑ ↓ ↑ ↓ ↓ ↓ ↑ |
Capuron et al., 2012 Capuron et al., 2012 Juengling et al, 2000 Juengling et al, 2000 Capuron et al., 2010 Capuron et al., 2010 Felger et al., 2013 Felger et al., 2013 Petrulli et al., 2017 |
|
fMRI/MRI Activation to receipt of reward (gambling) Activation to reward anticipation (MIDT) Activation to social support figures Activation to positive social feedback Activation to emotional stimuli Activation to RPEs (probabilistic learning) Activation to PPEs (probabilistic learning) Activation to cognitive Stroop Activation to visual stimuli Activation to novel stimuli qMT - kf qMT - T2f |
HCV+ patients Healthy controls Healthy controls Healthy controls Healthy controls Healthy controls Healthy controls Healthy controls Healthy controls Healthy controls HCV+ patients HCV+ patients |
4-6 weeks IFN-α 2 hr endotoxin 2 hr endotoxin 2 hr endotoxin 2 hr endotoxin 3 hr vaccination 3 hr vaccination 3 hr vaccination 3 hr vaccination 3 hr vaccination 4 hr IFN-α 4 hr IFN-α |
VS VS VS VS, vmPFC OFC VS AI SN SN SN VS, DS VS, DS |
↓ ↓ ↑ ↑ ↑ ↓ ↑ ↑ ↓ ↓ ↑ ↓ |
Capuron et al., 2012 Eisenberger et al., 2010 Inagaki et al., 2015 Muscatell et al., 2016 Kullmann et al., 2013 Harrison et al., 2015a Harrison et al., 2015a Brydon et al., 2008 Brydon et al., 2008 Harrison et al., 2015b Dowell et al., 2016 Dowell et al., 2016 |
|
MRS Glutamate/Creatine |
HCV+ patients | 4 weeks IFN-α | DS, ACC | ↑ | Haroon et al., 2014 Haroon et al., 2015 |
↑ increased; ↓ deceased; ACC - anterior cingulate cortex; AI - anterior insula; AMPH - amphetamine; D2R - dopamine 2 receptor; DS - dorsal striatum; FDG - fludeoxyglucose; FDOPA - fluorodopa; fMRI - functional magnet resonance imaging; HCV - hepatitis C virus; IFN - interferon; kf - rate magnetization transfer from free (water) to molecular-bound protons; MIDT - monetary incentive delay task; MM - malignant melanoma; MRS - magnetic resonance spectroscopy; OFC - orbitofrontal cortex; PET - positron emission tomography; PFC - prefrontal cortex; PPE - punishment prediction error; qMT - quantitative magnetization transfer; RPE - reward prediction error; SN - substantia nigra; T2f - free water spin-spin relaxation time; vmPFC - ventromedial prefrontal cortex; VS - ventral striatum.
4.1. Translational Biochemical and Neuroimaging Studies
Initial evidence that inflammation can affect brain DA originates from neurochemical and behavioral studies in rodents administered acute or sub-chronic IFN-α that measured DA and/or DA metabolites in concert with depressive behaviors and changes in locomotor activity [134, 138-141]. Some studies reported increases [138, 140] while others have reported decreases [134, 139, 141] in brain DA and/or metabolites following acute or sub-chronic IFN-α administration. These discrepancies were likely due to differences in dosing, length of exposure, and most importantly, the fact that species-specific IFN-α was variably used and rodents do not respond to human IFN-α with activation of classic type I IFN receptor signaling [142-144]. Moreover, human IFN-α administered to rodents binds to opioid receptors, which may be responsible for some of the observed changes in brain monoamines [145-147]. Moreover, chronic (6 days to 4 weeks) peripheral administration of both human and species-specific IFN-a administered to rodents has demonstrated only limited ability to reliably induce depressive behaviors (for example see [142, 144, 148-154].
Rhesus monkeys exposed to chronic IFN-α exhibit immune, neuroendocrine and behavioral responses similar to that of cytokine-treated patients, including decreases in psychomotor activity and increases in depressive-like huddling behavior (in ~50% of animals) [3, 155]. Of note, depressive huddling behavior in non-human primates has been previously described following chronic administration of the monoamine-depleting agent reserpine, and DA receptor antagonists and partial agonists [156, 157]. Only animals that displayed depressive behavior following IFN-α administration were found to have significantly lower cerebrospinal fluid (CSF) concentrations of the DA metabolites homovanillic acid (HVA) and 3,4 dihydroxy-phenylacetic acid (DOPAC), which also correlated with decreased locomotor activity [3, 155]. Moreover, chronic IFN-α administration reduced effort-based but not freely available sucrose consumption by the monkeys [133]. Similar to the effects of IFN-α, peripheral administration of IL-1β to rodents has been shown to decrease effortful responding for sucrose reward over freely available chow, in the absence of a decrease in preference for freely available sucrose over chow [135]; an effect which was reversed by lisdexamfetamine [158]. Interestingly, peripheral administration of IL-1beta in mice at 24 hours has been shown to decrease locomotor (wheel running) activity, which was improved by methylphenidate but not modafinil [159]. Furthermore, similar to IL-1beta administration, an overall decrease in responding for food reward has been reported in mice following peripheral administration of lipopolysaccharide (LPS; i.e. endotoxin) with no decrease in reward sensitivity (preference for high value sucrose rewards) [160].
To further explore the effects of inflammatory cytokines on synaptic availability and release of striatal DA that may underlie inflammation effects on motivation and motor function, in vivo microdialysis was conducted on IFN-α-treated monkeys [133]. Results indicated that stimulated DA release was indeed decreased in the striatum after chronic administration of IFN-α (4 weeks), which correlated with reduced effort-based sucrose consumption [133]. These in vivo microdialysis findings of cytokine-induced decreases in striatal DA release were corroborated by translational neuroimaging studies in IFN-α-treated monkeys using [11C]raclopride PET that found decreased DA release in putamen and ventral striatum [133]. Furthermore, IFN-α-induced decreases in striatal DA release were reversed by the DA precursor levodopa (L-DOPA) administered via reverse in vivo microdialysis, indicating that cytokines may reduced DA synthesis and availability [161]. In addition to IFN-α administration, models of peripheral inflammation in rodents have also been shown to decrease DA availability. For example, single injections of septic doses of LPS (5 mg/kg) cause progressive neurodegeneration of the nigrostriatal DArgic system [162, 163]. It should be noted that acute systemic administration of low dose LPS (~100 μg/kg) has been reported to either decrease tissue DA content or increase extracellular DA metabolites in the nucleus accumbens (NAcc) [164, 165]. However, these studies assessed DA and “anhedonic” behavior (sucrose preference or responding for brain stimulation) at 2 to 4 hours post-LPS [164-166], a time point that may be confounded due to febrile effects of LPS and related sickness behaviors [167-169]. These early effects of LPS may also reflect, for instance, acute activation of neuroendocrine peptides and hormones (minutes to hours) which can have stimulatory effects on turnover or release of brain catecholamines [170-173], and which may occur ahead of the more chronic mechanisms by which inflammation is thought to contribute to decreased DA availability (see Section 6 below for detailed discussion). Together, these results from animal studies indicate that a variety of inflammatory stimuli have been consistently found to affect brain DA to lead to relevant behavioral symptoms, and have prompt further investigation into inflammation effects on DA and the basal ganglia in clinical populations.
4.2. Human Neuroimaging Studies
Neuroimaging studies across several laboratories suggest that disruption of the basal ganglia and changes in the DA or glutamate systems are major contributors to inflammation-induced behavioral change (Table 1). In the first study to examine IFN-α effects on the brain, in addition to decreased metabolism in PFC, increased glucose metabolism was found in the basal ganglia and particularly the DA-rich putamen [174], as assessed by PET neuroimaging with fluorine-18-labeled-fluorodeoxyglucose (FDG). More recently, FDG PET revealed increased basal ganglia glucose metabolism in patients receiving high dose IFN-α as therapy for malignant melanoma [119]. Increased glucose metabolism in the left putamen and left ventral striatum (NAcc) correlated significantly with reports of fatigue in these patients, as assessed by the 'energy' subscale of the Visual Analog Scale of Fatigue (VAS-F) [119]. This pattern of increased glucose metabolism in basal ganglia nuclei is similar to that seen in patients with Parkinson’s disease (PD) [175-177], where it is thought to reflect increased oscillatory burst activity in relevant basal ganglia nuclei secondary to loss of inhibitory nigral DA input [178, 179]. Interestingly, this pattern of increased metabolism in striatum is also similar to the effects of transient catecholamine depletion in patients with MDD reported by Hasler and colleagues, which correlated with anhedonic symptoms [180].
In fMRI studies conducted by Capuron and colleagues decreased neural activation in the basal ganglia, including ventral striatum, was observed in response to unexpected delivery of reward (winning in a gambling task [181]) in HCV+ patients undergoing IFN-α administration, which correlated with self-reported reduced motivation [118]. Acute administration of IFN-alpha has also been shown to induced an change in striatal microstructure, as measured by quantitative magnetization transfer (qMT) imaging, that predicted an increase in symptoms of fatigue [182]. Administration of the cytokine-inducers endotoxin and typhoid vaccination to healthy volunteers produces similar effects on the ventral striatum in response to rewarding stimuli, suggesting that findings from IFN-α generalize to other inflammatory stimuli [121, 123]. Indeed, Eisenberger and colleagues demonstrated that endotoxin administration led to reduced activation of ventral striatum to reward-predicting cues during a monetary incentive delay task (MIDT), which was associated with increases in self-reported depressed mood as measured by the Profile of Mood States (POMS) depression subscale [121]. Similar blunting of neural responses to reward anticipation have been observed following dietary depletion of precursors for DA synthesis [183]. Moreover, typhoid vaccination was found to cause a shift in reward versus punishment sensitivity in a probabilistic instrumental learning task combined with fMRI [123]. Compared to saline control, Harrison and colleagues determined that vaccination reduced behavioral attractiveness of rewards while making punishments more aversive, effects that were related to decreased neural activation of ventral striatum to RPEs and increased activation of anterior insula to punishment prediction errors [123]. Healthy female control subjects who have heightened stress-induced production of IL-6 have also been found to exhibit decreased ventral striatal RPE signaling during reinforcement learning [184], suggesting that persons with chronically elevated inflammatory responses exhibit decreased neural sensitivity to reward. Of relevance to potential effects of inflammation on DA, the magnitude of response to prediction error signaling is fundamentally modulated by DA-dependent striatal activity as determined by administration of drugs that enhance (L-DOPA) or inhibit (haloperidol) DAergic function [185]. Additionally, typhoid vaccination compared to saline has been shown to affect activity in the substantia nigra, including increased activation during a cognitive Stroop task and decreased activation in response to visual or novel stimuli, which correlated with both psychomotor slowing and increased peripheral blood concentrations of IL-6 [120, 186]. Finally, it should be mentioned that neural activation in reward circuitry (ventral striatum and ventromedial PFC [vmPFC]) has also been shown to encode endotoxin-induced increased sensitivity to social rewards, including positive social feedback and increased approach to familiar others, in healthy control subjects [187, 188]. Healthy subjects administered endotoxin were also found to have exaggerated increases in neural activation of the reward-related orbitofrontal cortex to emotional stimuli [189].
To further determine the role of DA in the effects of inflammation on neural activation and metabolism in reward circuitry, Capuron and colleagues conducted a PET study in HCV+, IFN-α-treated subjects using [18F]fluorodopa (FDOPA). Like the DA precursor L-DOPA, FDOPA is taken up by DAergic neurons and converted by DA decarboxylase to DA, whereupon it is stored in vesicles for release. Interestingly, both increased uptake and decreased turnover of FDOPA in the caudate, putamen and ventral striatum of IFN-α-treated patients was found [118]. Baseline and percent change in FDOPA uptake was in turn correlated with IFN-α-induced behavioral alterations including depression and fatigue, as measured by the Montgomery Asberg Depression Rating Scale (MADRS) and MFI, respectively [118]. Increased uptake and decreased turnover of FDOPA in the basal ganglia following IFN-α administration is in stark contrast to that observed in patients with PD where decreased uptake and increased FDOPA turnover is seen. Decreased uptake of FDOPA in PD is believed to be a function of loss of DAergic neurons and/or their projections throughout the basal ganglia [190-192], and intact or increased turnover suggests that the surviving neurons are capable of normal release [191, 193]. Increased FDOPA uptake during IFN-α treatment suggests intact terminals that exhibit a potential depletion of DA and increased synthetic capacity. These findings are consistent with that of the above described decreases in striatal DA release observed in IFN-α-treated non-human primates (rhesus monkeys), as measured by both [11C]raclopride PET with amphetamine displacement (in putamen and NAcc) and in vivo microdialysis - which was reversed by L-DOPA administration [133, 161]. As mentioned above, whereas chronic administration of inflammatory stimuli to laboratory animals may decrease DA availability, acute administration of inflammatory stimuli such as LPS (i.e. endotoxin) has been shown to stimulate efflux or turnover of monoamine neurotramsitters such as DA, serotonin (5-HT) and norepinephrine (NE) [138, 140, 194-196]. Accordingly, acute administration of endotoxin to healthy humans was found to increase DA release at 4 hours as measured by [11C]raclopride PET [197].
In regard to glutamate, there is an extensive basic science literature demonstrating that inflammation can increase extracellular glutamate concentrations and glutamate receptor signaling (for further discussion see [17] and section 6 below). In the clinical literature, patients treated with IFN-α exhibited significantly increased concentrations of glutamate in the basal ganglia, as measured by magnetic resonance spectroscopy (MRS)[137]. Increases in glutamate as measured by MRS were in turn associated with IFN-alpha-induced depressive symptoms, and also with increasing age [136, 137].
Despite the abundance of reports indicating changes in basal ganglia, prefrontal cortex, DA and glutamate function in subjects administered cytokines and inflammatory stimuli, little work has been done to investigate similar relationships in patients who exhibit high inflammation as a function of medical or neuropsychiatric illnesses. One study in patients with chronic fatigue syndrome, who have been frequently reported to exhibit elevated inflammatory markers including cytokines, observed decreased activation of basal ganglia structures, such as caudate and globus pallidus, in response to hedonic reward [198] using the gambling task mentioned above [118]. In medically stable patients with MDD, we observed a relationship between increased inflammation and decreased functional connectivity within reward-related corticostriatal neurocircuitry [24]. Indeed, increased inflammation (plasma concentrations of CRP as well as cytokines and their soluble receptors) was associated with decreased functional connectivity between the ventral striatum and vmPFC, and the dorsal striatum and the vmPFC and pre-supplementary motor area (pre-SMA), which correlated with self-reported symptoms of anhedonia and objective measures of psychomotor slowing, respectively [24]. Interestingly, dorsal striatum and pre-SMA/SMA are key components of corticostriatal circuitry involved in linking motivation to motor output [199, 200], and like the ventral striatum, vmPFC is part of classic reward circuitry that receives significant mesocorticolimbic DA innervation [201, 202]. Accordingly, inflammation-related decreases in corticostriatal connectivity within reward and motor circuitry in depression may involve cytokine-induced decreases in DA, and have potential for reversal with pharmacological strategies that increase DA availability or receptor signaling (see Sections 6 below for further discussion) [3]. Additionally, these changes in corticostriatal circuitry may be related to inflammation effects on glutamate. Like patients chronically administered INF-α, increased glutamate as measured by MRS has been observed in MDD patients with increased inflammation that correlated with anhedonia and psychomotor slowing [203].
Together these data from humans and laboratory animals indicate that inflammation-related decreases in DA availability and release and increased glutamate may have functional consequences on reward and motor circuitry that are associated with fundamental alterations in motivation and motor function, to contribute to symptoms of anhedonia and psychomotor retardation in mood and anxiety disorders. This work supports further consideration of the mechanisms of cytokine affects on DA and glutamate, which may lead to the development of novel therapeutic strategies for patients with increased inflammation (see section 6).
5. Inflammation effects on neural circuitry relevant to fear and anxiety
Of particular interest in considering the consequences of increased inflammation in anxiety-related disorders are the effects of inflammatory cytokines on brain regions involved in fear, anxiety and threat detection, such as the amygdala, insula, medial prefrontal cortex and anterior cingulate cortex (ACC), which may contribute to anxiety and PTSD symptoms. Administration of inflammatory cytokines or cytokine-inducers is well known to induce a myriad of behavioral symptoms in laboratory animals and humans including those relevant to anxiety disorders and PTSD [204]. In addition to the effects on reward and motor circuitry, PET and fMRI studies employing administration of cytokines or cytokine inducers (e.g. endotoxin or vaccination) acutely to healthy subjects, as well as chronic administration of IFN-α to patients with HCV, have observed effects on circuitry relevant to fear, anxiety and emotional regulation as presented in Table 2. Studies were identified by pubmed (NLM) query using search terms for inflammatory stimuli (typhoid OR endotoxin OR interferon) AND technique (neuroimaging OR PET OR spectroscopy) AND brain region (amygdala OR cingulate OR insula), and refined using similar procedures as described above, while excluding those that were most relevant to Table 1. Several studies have also examined alterations in neural activity associated with stress-induced peripheral inflammatory responses in healthy subjects, which are consistent with neuroimaging findings in patients with fear and anxiety-related disorders. Although no work to date has established relationships between brain function and inflammation in these disorders, based on recent interest in this area [10, 33, 205], such work is currently being conducted, particularly in PTSD.
Table 2.
Summary of neuroimaging findings of changes in neural activation and functional connectivity, and glucose and glutamate metabolism, in circuitry related to fear, anxiety and emotional regulation after administration of peripheral inflammatory stimuli or cytokines.
| Neuroimaging Technique | Subjects | Inflammatory Stimulus | Region | Finding | Study |
|---|---|---|---|---|---|
| fMRI Activation to Stroop (congruent and incongruent; incongruent > congruent) Activation to Stroop (incongruent > congruent) Activation to socially threatening images Activation to emotional face task Functional connectivity to emotional faces Activation to visuo-spatial attention errors Resting state functional connectivity Activation to social exclusion (ball toss) |
Healthy controls Healthy controls Healthy controls Healthy controls Healthy controls HCV+ patients Healthy men Healthy women |
3 hr vaccination 3 hr vaccination 2 hr endotoxin 3 hr vaccination 3 hr vaccination 12 weeks IFN-α 3.5 hr endotoxin 2 hr endotoxin |
Amygdala, AI, DMI ACC, DPI Amygdala sgACC sgACC to Amygdala, mPFC & VS dACC Amygdala, AI, CC ACC, AI |
↑ ↑ ↓ ↑ ↓ ↑ ↓ ↑ |
Harrison et al., 2009a Harrison et al., 2009a Inagaki et al., 2012 Harrison et al., 2009b Harrison et al., 2009b Capuron et al., 2005 Labrenz et al., 2016 Eisenberger et al., 2009 |
| PET [18F]FDG, glucose metabolism [18F]FDG, glucose metabolism |
Healthy controls Healthy controls |
1.5 hr endotoxin 1.5 hr endotoxin |
Insula ACC |
↑ ↓ |
Hannestaed et al., 2012 Hannestaed et al., 2012 |
| MRS Glutamine Glutamine/glutamate Glutamate/Creatine |
HCV+ patients HCV+ patients HCV+ patients |
4-6 weeks IFN-α 4-6 weeks IFN-α 12 weeks IFN-α |
sgACC sgACC dACC |
↑ ↑ ↑ |
Taylor et al., 2014 Taylor et al., 2014 Haroon et al., 2014 |
↑ increased; ↓ deceased; ACC - anterior cingulate cortex; AI - anterior insula; AMPH - amphetamine; d - dorsal; CC - cingulate cortex; DMI - dorsal middle/posterior insula; DPI - dorsal posterior insula; FDG - fludeoxyglucose; fMRI - functional magnet resonance imaging; HCV - hepatitis C virus; IFN - interferon; PET - positron emission tomography; sg - subgenual; vmPFC - ventromedial prefrontal cortex; VS - ventral striatum.
5.1. Amygdala
The amygdala is a principal brain region involved in fear and anxiety, and it is thought to play a crucial role in the neural circuitry of PTSD [206]. Neuroimaging findings in humans indicate that not only does inflammation increase amygdala activity, but also that increased amygdala responses to stress are associated with increased production of inflammatory cytokines [207-209]. Increased IL-6 and TNF after administration of endotoxin to healthy subjects has been shown to increase amygdala activity in response to socially threatening images, which was associated with enhanced feelings of social disconnection [208]. Administration of typhoid vaccination, which increases IL-6 and induces behavioral changes including cognitive disturbance and fatigue, also increased activation of the amygdala during presentation of congruent and incongruent stimuli [210]. In terms of stress sensitivity and neural pathways involved in the inflammatory response to stress, heightened neural activity in the amygdala in response to a psychosocial laboratory stressor was associated with greater stress-induced increases in IL-6 [209]. Thus, greater amygdala sensitivity to stress may lead to higher inflammatory cytokine production, which may in turn affect amygdala activity to create a feed-forward effect of inflammation on neural circuitry relevant to anxiety and PTSD symptoms.
5.2. Medial Prefrontal Cortex and Subgenual ACC
The medial prefrontal cortex, including the rostral ACC, subgenual ACC (BA 25) and medial frontal gyrus, is extensively connected to the amygdala in primates and thought to be involved in fear extinction and emotional regulation in PTSD [206, 211]. Several studies have reported a relationship between peripheral inflammatory cytokines and activity of medial prefrontal cortical regions, including the subgenual ACC, either in individuals undergoing stress or following administration of cytokine-inducers [207, 212]. Indeed, administration of typhoid vaccination to healthy controls induced mood changes that correlated with enhanced activity within subgenual ACC during an implicit emotional face perception task. Typhoid vaccination also reduced task-related functional connectivity of the subgenual ACC to the amygdala and other medial prefrontal cortical regions, as well as the nucleus accumbens and superior temporal sulcus, which correlated with peripheral blood IL-6 [207]. As mentioned above, heightened neural activity in the amygdala was associated with increased IL-6 responses to a psychosocial laboratory stressor, and functional connectivity analyses also indicated that individuals who showed a heightened inflammatory response to the stressor exhibited stronger coupling between the amygdala and the dorsomedial prefrontal cortex [209]. In a separate study of women undergoing the chronic emotional stress of bereavement, elevations in salivary concentrations of IL-1beta and soluble TNF receptor II have been shown to positively correlate with the degree of ventral prefrontal activation (including subgenual ACC and the orbitofrontal cortex) to a grief-elicitation task [212], suggesting a relationship between stress, inflammation, and medial prefrontal cortical activation that may be relevant for emotional processing in stress and trauma-related disorders.
5.3. Insula
Another region associated with amygdala activity and thought to contribute to emotional distress in anxiety disorders and PTSD is the insula [213, 214]. For instance, increased activation of the anterior insula and amygdala, and decreased connectivity among the anterior insula, amygdala, and ACC, have been observed in women with PTSD from intimate partner violence while matching to fearful or angry versus happy target faces [215]. Given the role of the insula in interoception, it is not surprising that this brain region has also been reported to be activated in response to peripheral inflammatory stimuli [210]. As mentioned above, in addition to activation of the amygdala, administration of typhoid vaccination increased activation of the insula during presentation of congruent and incongruent stimuli [210]. Among females but not males administered endotoxin, increases in IL-6 were associated with increases in neural activity in brain regions including the anterior insula in response to social exclusion during a virtual ball-tossing game [216]. Endotoxin administration has also been shown to increase cerebral glucose metabolism in the insula as measured by PET [217], and to decrease its functional connectivity with cingulate cortex [218]. Therefore, heightened sensitivity of the insula to inflammatory cytokines in the periphery, particularly in the presence of emotional stimuli, may contribute to altered neural circuitry involving the amygdala, medial prefrontal cortex and ACC to precipitate symptoms of fear, anxiety and emotional disturbance.
5.4. Dorsal anterior cingulate cortex (dACC)
A major CNS target of cytokines that has received considerable attention is the dorsal ACC (dACC). The dACC [Brodmann’s Area (BA) 24] has been shown to play an important role in error detection and conflict monitoring [219]. In addition, the dACC has been found to process social pain and therefore has been suggested to comprise a neural “alarm system”, which can both detect and respond to threatening environmental stimuli in the social domain [220]. The dACC’s downstream activation of the autonomic arousal system provides a further component of this alarm system which can thus both identify and respond to threat cognitively, emotionally and physically [221]. Increased activity of the dorsal ACC has been proposed as a mediator of hyperarousal symptoms in PTSD [222], as well as a potential familial risk factor for PTSD development [223], and veterans with PTSD and their twins have also been shown to exhibit increased resting metabolic activity in the dACC, as measured by PET [224]. Increased dACC activation has also been found in subjects with increased neuroticism, high trait anxiety and obsessive-compulsive disorder [225-227].
Similar to increased dACC activation in anxiety disorders and PTSD, early work in patients treated with IFN-α for hepatitis C virus using fMRI and a task of visuo-spatial attention demonstrated that chronic IFN-α administration was associated with increased dACC activation [228]. Activation of the dACC was highly correlated with the number of task-related errors made by IFN-alpha-treated patients, whereas no correlation was found between dACC activation and task-related errors in controls [228]. Administration of typhoid vaccination has also been shown to lead to increased dACC activation using fMRI and the Stroop task, which through its presentation of congruent and non-congruent stimuli has been shown to increase blood flow to the dACC [210, 220]. In participants administered endotoxin prior to a neuroimaging session in which they were socially excluded during a virtual ball-tossing game, increases in IL-6 were associated with increases in social pain-related neural activity in dACC in females but not males [225]. This increased interaction between emotional and neural sensitivity and inflammation is consistent with reports of increased inflammatory activity in women with PTSD [229, 230]. As mentioned above, another study administering typhoid vaccination to induce IL-6 and behavioral changes found increased activation of the amygdala, but also the dACC and insula, during presentation of congruent and incongruent stimuli [210]. In terms of mechanisms of cytokine effects on the dACC and as discussed in section 6 below, increased glutamine and glutamate in dACC as measured by MRS has been observed in patients administered IFN-a that correlated with IFN-a-induced behavioral symptoms [231, 232]. Taken together, these data indicate that cytokines can increase the reactivity of the dACC, possibly through effects on glutamate, and thereby increase sensitivity to the external environment. This heightened sensitivity of dACC in the presence of cytokines maybe further contribute to symptoms in anxiety disorders and PTSD stress and trauma-exposed patients with increased inflammation.
6. Mechanisms of inflammation affects on threat and reward circuitry and translational implications
The data summarized herein demonstrate that inflammation originating in the periphery targets brain structures relevant to mood and anxiety disorders, and may be related to cytokine effects on neurotransmitters such as monoamines and particularly DA as well as glutamate. These findings are based largely on clinical and translational neuroimaging results, which have opened opportunities to develop novel treatment strategies to block inflammation or target its effects on the brain to treat these symptoms in patients with mood and anxiety-related disorders that exhibit high inflammation. Current therapies are effective for many patients with mood and anxiety disorders. However, up to 30% fail to achieve remission and even responders often exhibit significant residual symptoms that are consistent with those that are caused by exposure to cytokines and inflammation, such as anhedonia, fatigue and psychomotor retardation [233-237]. Therefore, new conceptual frameworks are needed to treat these inflammation-associated symptoms [26, 56, 238], and are discussed below.
6.1. Therapies that Block Inflammation
A number of recent studies have begun to test the potential of anti-inflammatory compounds as possible antidepressant therapies. Most studies to date have focused on compounds such as cyclooxygenase (COX) inhibitors or minocycline, which have relatively mild anti-inflammatory effects and numerous “off target” effects that may confound data interpretation [16, 239]. A recent meta-analysis reported anti-depressant effects for anti-inflammatory agents in BD [240], however some of the selected trials used drugs that block oxidative stress, which may have many other sources in addition to inflammation. Recent meta-analyses in MDD also reported mild to moderate effect sizes showing that COX inhibitors may reduce depressive symptoms, yet heterogeneity across studies and mostly small sample sizes complicate results [241, 242]. It should be noted that the majority of studies included in the meta-analyses described above have not selected patients with increased inflammation, nor have they measured peripheral inflammatory markers to establish anti-inflammatory activity of the treatments. Interestingly, a recent study tested the efficacy of infliximab, a highly-selective TNF antagonist, in treatment-resistant MDD (TRD) as a function of peripheral inflammation. Treatment with infliximab was associated with robust decreases in plasma CRP concentrations, as well as a strong anti-depressant effect, but only in patients with elevated CRP at baseline [27]. Moreover, the greatest area of symptom improvement was related to motivation (interest in activities), followed by motor retardation and anxiety [27], which is consistent with the hypothesis that blockade of inflammation may exert anti-depressant properties through reversing inflammation effects on corticostriatal reward and motor as well as anxiety circuitry.
A number of holistic and alternative strategies, such as exercise, weight reduction or diet modification, dietary supplements, yoga, massage, tai chi and meditation, that have been shown to reduce depressive and anxiety symptoms are thought to either directly or indirectly reduce inflammation [243]. Weight loss and exercise have been the most studied of these interventions, both of which have been shown to have antidepressant and anti-anxiety effects [244, 245] and to reduce inflammation over time [246, 247]. Another therapy thought to decrease inflammation is supplementation with omega-3 fatty acids, which has been shown to have antidepressant efficacy, especially for eicosapentaenoic acid (EPA) [248], as verified by meta-analysis [249]. Indeed, treatment with EPA has been shown to prevent depression induced by IFN-α [250], and patients who are vulnerable to the development of this inflammation-induced depression have both higher risk alleles for the phospholipase A2 (PLA2) gene and lower levels of EPA in the blood [251]. In MDD, a recent study demonstrated that patients with high CRP and IL-1ra had a greater antidepressant response to EPA, whereas patients with increased CRP and IL-6 were less responsive to placebo [28]. Interestingly, omega-3 fatty acids have been shown to impact brain structure, function, and pathology in human neuroimaging studies, indicating that they may exert antidepressant efficacy in part through affecting pathways in the brain that are targeted by inflammation [252].
Although little work has been done to examine anti-inflammatory treatments in anxiety disorders, normalization of inflammatory cytokines in patients with remitted PTSD [253] or following response to selective serotonin reuptake inhibitor (SSRI) therapy [254], suggests a close and potentially causal association between inflammation and PTSD symptoms. Behavioral interventions that reduce stress, such as exercise, yoga or meditation, can affect the HPA and SNS axes as well as the immune system [255], and may also serve as potential treatments for PTSD and related comorbidities.
6.2. Monoamine Synthesis, Availability and Receptor Signaling
In addition to effects on DA as described above, inflammation also affects other monoamines such as 5-HT. For example, acute administration of inflammatory cytokines increased 5-HT turnover, as determined by increased 5 hydroxyindoleacetic acid (5-HIAA) or 5HIAA/5-HT ratios, in brain regions such as the cortex and nucleus accumbens [194, 195], and these changes in 5-HT turnover occurred in concert with the appearance of later, more persistent depressive-like behaviors [167, 168]. In patients treated chronically with IFN-a for hepatitis C virus (HCV), CSF concentrations of IL-6 negatively correlated with 5-HIAA concentrations, which in turn negatively correlated with IFN-a-induced depression severity [131]. Lower plasma concentrations of 5-HT and higher circulating TNF at baseline have also been associated with somatic symptoms of depression during IFN-a treatment [256].
One primary mechanism by which inflammation may decrease monoamine and particularly DA availability is by negatively impacting synthesis. Indeed, DA synthesis relies on the conversion of tyrosine to L-DOPA by tyrosine hydroxylase (TH), the rate-limiting enzyme for DA synthesis. A major source of tyrosine is phenylalanine, which is converted to tyrosine by phenylalanine hydroxylase (PAH). Likewise, 5-HT synthesis requires conversion of tryptophan to 5-HT by tryptophan hydroxylase (TPH). All of these enzymes, TH, PAH and TPH, require the enzyme cofactor tetrahydrobiopterin (BH4). Although inflammation and cytokines have been shown to induce GTP-cyclohydrolase I, the enzyme necessary for BH4 synthesis, inflammation may in turn decrease BH4 availability [257]. BH4 is also a cofactor for nitric oxide synthases (NOS). Inflammation-induced increases in inducible NOS (iNOS) activity can usurp available BH4, which results in NOS uncoupling and the generation of reactive oxygen species instead of NO [258, 259]. This increase in oxidative stress can then contribute to oxidative reduction of BH4 itself (which is highly redox-sensitive), leaving even less BH4 available for monoamine synthesis (Fig. 1) [258]. Indeed, intramuscular injection of rats with IFN-α has been shown to decrease CNS concentrations of BH4 through stimulation of NO, and inhibition of NOS was found to reverse IFN-α’s inhibitory effects on brain concentrations of both BH4 and DA [134]. Of note, IL-6 treatment has also been shown to reduce BH4 content in sympathetic neurons [260].
With relevance to DA availability, concentrations of phenylalanine, tyrosine, BH4 and BH2 can be measured in the peripheral blood and CSF, and the BH4/BH2 and phenylalanine/tyrosine ratios have been proposed as indicators of BH4 availability and PAH activity, and may serve as indirect biomarkers of DA synthetic capacity [257, 261-264]. For example, a number of patient populations with increased inflammation, including patients with trauma, sepsis, cancer, and HIV, have been found to exhibit increased peripheral blood concentrations of phenylalanine [257]. Evidence of reduced BH4 activity has also been observed in IFN-α-treated patients [265, 266]. For example, IFN-α administration was associated with increased peripheral blood phenylalanine/tyrosine ratio, which in turn correlated with decreased CSF DA and its major metabolite HVA [265], but not CSF 5-HT or its metabolites. Increased cerebrospinal fluid (CSF) IL-6 was also correlated with decreased BH4 in CSF of IFN-α-treated patients, and the phenylalanine/tyrosine ratio significantly correlated with IFN-α-induced depressive symptoms [265]. These findings are consistent with decreased DA metabolites in the CSF of both IFN-α-treated patients and monkeys [3, 155], and with the complete reversal of IFN-α-induced decreases in DA by L-DOPA administered via reverse in vivo microdialysis in monkeys [267]. Of note, during L-DOPA administration, no changes were found in the DOPAC/DA ratio, which increases when DA is not properly packaged in synaptic vesicles and is subsequently metabolized via monoamine oxidase [268].
Considering the strong evidence presented above indicating that inflammation can inhibit key components of monoamine and DA synthesis, pharmacologic strategies that increase DA may effectively treat inflammation-related symptoms of anhedonia, fatigue and psychomotor slowing. For instance, a number of compounds are available than can boost BH4 availability or activity, which may facility the capacity of PAH and TH to synthesize DA and TPH to synthesis 5-HT. These include administration of BH4 itself [269], which is currently approved in a synthetic form to treat phenylketonuria [270-272], and folic acid or S-adenosyl-methionine (SAMe), which have a role in the synthesis and/or regeneration of BH4 [3, 273, 274]. Although effects of BH4 administration on behavioral symptoms have not been reported outside of case reports [275, 276], folic acid (in the form of L-methylfolate) and SAMe have demonstrated efficacy as adjuvants in depression trials [277-281]. Administration of L-methylfolate (marketed as Deplin and Zervalx) to patients with MDD has been shown to augment the efficacy of standard antidepressant therapy in two studies [278, 279], however mixed results were reported in two parallel-sequential trials with only one trial finding efficacy over placebo [280]. Treatment with SAMe adjunctive to SSRIs led to significantly higher rates of remission and 50% or greater decreases in depressive symptoms compared with placebo [277]. Interestingly, post-hoc analysis of the two parallel-sequential adjuvant trials of L-methylfolate in patients with MDD [280] considered inflammatory markers. It was found in one study that BMI >30 as well as concentrations of CRP, TNF, IL-8, and leptin, alone or in combination with each other or with IL-6, predicted greater symptom improvement [281]. Therefore, strategies to augment BH4 activity exhibit potential for restoring monoamine availability and treating symptoms of depression in patients with increased inflammation.
In addition to compounds that may increase DA availability, adenosine (A2A) receptor antagonists, which are thought to facilitate activation of DA D2 receptors, are efficacious in reversing decreased effort-based sucrose consumption after DA depletion with tetrabenazine (a vesicular monoamine transporter inhibitor) and after peripheral administration of IL-1β in rats [135, 282-285]. The DA receptor agonist, pramipexole, has been shown to block endotoxin-induced degeneration of nigrostriatal DA cells [286], and has also demonstrated efficacy in unipolar MDD and BD patients with TRD [287-290].
6.3. Monoamine Reuptake
Attention has been paid to the effects of cytokines and inflammatory signaling pathways on monoamine reuptake pumps, and particularly the 5-HT transporter (5-HTT) [291-294]. Both in vitro and in vivo data have established that stimulation of p38 mitogen-activated protein kinase (MAPK), a major signaling pathway activated by IFN-α and other cytokines, can increase the expression and function of the 5-HTT, leading to increased 5-HT reuptake [292-294]. MAPK pathways have also been found to influence the DA transporter (DAT). For example, DAT-expressing cells transfected with a constitutively activate MAPK kinase (MEK) show increased DA reuptake (Vmax), whereas treatment of rat striatal synaptosomes with MEK inhibitors was associated with decreased DA reuptake in a concentration and time-dependent manner [291]. Furthermore, subjects with neuropsychiatric disturbances as a result of HIV infection and subsequent neuroinflammation are thought to have increased expression of DAT [295, 296]. Therefore, reduced DA turnover secondary to inflammatory cytokines may be mediated, in part, by increased DAT expression or function. However, no change in DAT binding, as measured by PET with 18F-labeled FECNT, was observed in monkeys exposed to chronic IFN-α [133]. However, increased plasma TNF significantly correlated with higher 5-HTT binding determined by [123I]-beta-CIT SPECT imaging in healthy adult women [297].
In terms of treatment implications, relationships exist between high levels of inflammation and resistance to standard antidepressant therapy with SSRIs, particularly in MDD [26, 56, 298, 299]. Non-responsiveness of inflammation-related symptoms to standard SSRI therapies has been exemplified in patients receiving IFN-α and those experiencing cancer-related behavioral symptoms that are associated with increased inflammation. Indeed, SSRIs have been found to alleviate cancer-related or IFN-α-induced anxiety and some depressive symptoms, but not those of fatigue or psychomotor retardation [126, 300, 301]. Moreover symptoms of anhedonia and motor slowing in psychiatric patients are difficult to treat even in SSRI responders [233-236], suggesting that other neurotransmitter systems, such as DA, may be involved in SSRI-resistant, inflammation-related symptoms [302].
Although classical stimulant medications that increase DA release and/or block DA reuptake increase motivation in rodent models [283, 303], they have demonstrated only limited efficacy in chronically treating fatigue and other DA-related symptoms in trials for patients with cancer and other medical illnesses that are associated with inflammation [304-313]. Since stimulants act to increase DA release and block DAT function to increase synaptic levels of available DA, these drugs may not provide long-term efficacy in the context of inflammation during medical illness. However, new evidence indicates that in MDD patients with high inflammation (as measured by CRP) exhibit a greater antidepressant response to SSRIs used in combination with the DAT blocker bupropion compared to SSRI monotherapy [314]. Interestingly, the use of the DAT blocker methylphenidate in patients with traumatic brain injury has also been found to improve cognitive symptoms as well as those of PTSD [315], which is consistent with findings that the combination of methylphenidate and desipramine improved behavioral symptoms in an animal model of PTSD [316].
6.4. Glutamate Neurotransmission
Another mechanism by which cytokines may influence reward, motor and threat-related circuitry is through effects on glutamate neurotransmission. For example, inflammation is known to stimulate production of indoleamine 2,3 dioxygenase (IDO) and kynurenine pathway metabolites produced in the periphery or CNS, which can then exert downstream effects on neurotransmitters in the brain such as glutamate (Fig. 1) [317, 318], and have been shown to play a role in TRD and suicide [319]. Immune-mediated activation of IDO catabolizes tryptophan, the primary amino-acid precursor of serotonin, to kynurenine. Kynurenine is further catabolized in the CNS into the neuroactive metabolites kynurenic acid (KA) (in astrocytes) and quinolinic acid (QUIN) (in microglia), both of which have been found to be increased in the plasma and CSF of IFN-α-treated patients [127, 320-322]. Of note, CSF QUIN significantly correlated with depressive symptoms during IFN-α administration, as measured by MADRS [321]. In addition to increasing oxidative stress [323, 324], the neurotoxic metabolite QUIN can also directly activate the n-methyl-d-aspartate (NMDA) receptor to induce the release of glutamate to lead excitotoxicity in the brain [320, 325, 326], thus further increasing inflammation and its potential effects on the monoamine systems as described above [327, 328]. In contrast to QUIN, KA reduces glutamate release, and has been shown to be an antagonist of NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors [329]. DA release is in part under glutamatergic control, and thereby KA may exert downstream effects on DA [320]. Indeed, intra-striatal administration of KA to rodents leads to marked reductions in extracellular dopamine concentrations, as determined by in vivo microdialysis [330].
Finally, cytokines and inflammation have been shown to increase glutamate by effects on microglia and astrocytes. A rich literature has shown that cytokines can decrease the astrocytic expression of glutamate transporters, and increase release of glutamate from astrocytes and activated microglia in vitro [317, 331-333]. Of note, glutamate released from glia may have preferential access to extrasynaptic NMDA receptors, which lead to decreased production of trophic factors including brain-derived neurotrophic factor [334, 335]. Given the sensitivity of DA neurons to oxidative stress and excitotoxicity, inflammation effects on glutamate may contribute to decreased striatal DA availability and eventual neurodegeneration [336, 337].
Inhibition of the IDO pathway or glutamate may be an important target in reversing the impact of inflammation in the brain. The IDO antagonist, 1-methyl tryptophan (1-MT) has been shown to abrogate the impact of LPS, as well as an attenuated form of Mycobacterium bovis, on depressive-like behavior [338, 339]. Given that the neurotoxic effects of QUIN may be mediated by excessive glutamate excitotoxicity [320, 325, 326], glutamate antagonists may be useful in preventing excitotoxic and oxidative effects and even protecting sensitive DA neurons. Indeed, metabotropic glutamate receptor antagonists that modulate glutamate transmission in the basal ganglia have been successful in reducing DA cell loss in an animal model of PD [340]. Antagonism of the NMDA receptor with memantine in monkeys infected with simian immunodeficiency virus (SIV) also reversed loss of DA in the striatum [341] that occurs during SIV and HIV infection secondary to immune cell activation in the basal ganglia [342, 343], in association with increased brain derived neurotrophic factor (BDNF). Interestingly, inflammation-induced release of glutamate from glia cells may preferentially activate extrasynaptic NMDA receptors, which has been shown to decrease BDNF [334]. Administration of the NMDA antagonist ketamine has potent antidepressant effects in depressed patients who are resistant to standard therapies and who often exhibit increased inflammation [26, 344, 345]. Interestingly, a recent study in treatment resistant depression found that patients who were most responsive to ketamine were those with the highest concentrations of serum IL-6 [346]. Therefore, blockade of kynurenine pathways or modulation of glutamate neurotransmission may confer protection against inflammation and/or IDO-mediated effects to improve behavioral symptoms in patients with mood or anxiety-related disorders with increased inflammation.
6.5. Limitations and Future Directions
The above described literature in Sections 1-5 examining the neurochemical and immunologic mechanisms of the effects of inflammation on the brain have provided much insight into the role of inflammation in depression and anxiety-related disorders and potential therapeutic targets, yet the process by which this information has been translated toward novel treatments for depression and anxiety has been slow. Although studies described in Section 6 have provided evidence that therapies which target inflammation or its effects on the brain may be efficacious in treating depressive and anxiety symptoms, important limitations in this literature exist. Considerations for future clinical trials warrant discussion in order to benefit this area and move the field forward [16]. Despite a number of studies showing that patients with high inflammation are more or less likely to respond to standard versus novel anti-depressant strategies [27, 56, 346, 347], very few trials to date using anti-inflammatory or novel antidepressant or anti-anxiety treatments that are thought to be beneficial against the effects of high inflammation have used inflammatory markers to predict or inform treatment response. Neuroimaging findings from the administration studies presented in Tables 1 and 2 have been seminal in shaping our understanding of how peripheral inflammation affects neurotransmitters and circuits to lead to behavioral symptoms. However, there is a dearth of studies using this knowledge to examine whether similar pathways are affected in psychiatric patients who have increased inflammation. Moreover, careful designed of studies and trials in the future will need to 1) measure inflammatory markers to be used for targeting of treatments to select patients or stratification for statistical analysis, 2) selection of therapies based on inflammatory markers and/or the presence of select symptom profiles, and 3) the used of a combination of inflammatory biomarker, behavioral and neuroimaging tools to assess treatment effects in longitudinal studies. In sum, while the work presented herein has been invaluable in determining mechanisms and potential clinical implications for the role of inflammation in depression and anxiety-related disorders, the translation of these findings into novel treatments will rely on future intelligently designed and hypothesis-driven clinical studies that incorporate neuroimaging methodologies.
Conclusion
This review has presented evidence that peripheral inflammation can affect brain regions relevant to reward and threat sensitivity to lead to symptoms of reduced motivation, motor slowing and anxiety. These findings, which stem largely from over a decade of clinical and translational neuroimaging studies examining the effects of the acute and chronic administration of inflammatory stimuli on the brain, are now being translated to understand the role of inflammation in behavioral symptoms in patients with mood and anxiety-related disorders. Biomarkers of inflammation such as inflammatory cytokines and acute-phase proteins are reliably elevated in a significant proportion of patients with MDD, BD, anxiety disorders and PTSD, and may be a causal factor driving behavioral symptoms. Although attempts have been made to image local activation of CNS immune cells in these patients, evidence of the functional and neurotransmitter effects of increased inflammation on the brain as measured by fMRI, PET and MRS has more consistently been associated with increases in peripheral inflammatory markers as well as behavior. More evidence is needed regarding the mechanisms of inflammation effects on the brain in psychiatric patients, and particularly those with anxiety disorders and PTSD. Nevertheless, the use of MRI and PET imaging to assess inflammation effects on neurotransmitters and neurocircuits relevant to CNS reward and anxiety pathways may serve as brain biomarkers of treatment response in future trials blocking inflammation or reversing its effects on the brain and behavior in patients with increased inflammation.
Funding sources
This work was supported by funds from the National Institute of Mental Health (R21MH106904), National Institute of Mental Health (R01MH 109637) and the Dana Foundation (CADF49143). In addition, the study was supported in part by PHS Grants UL1TR000454 from the Clinical and Translational Science Award program, by Winship Cancer Institute ACS IRG #126815-IRG-14-188-01-IRG from the American Cancer Society and by the NIH/NCI under award number P30CA138292.
Financial Disclosure
There are no conflicts of interest to declare. In the past 3 years, JCF has consulted for Proctor and Gamble and Pfizer.
Consent for Publication
Not applicable.
Acknowledgements
Declared none.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Miller A.H., Haroon E., Raison C.L., Felger J.C. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress. Anxiety. 2013;30(4):297–306. doi: 10.1002/da.22084. [http://dx.doi.org/10. 1002/da.22084]. [PMID: 23468190]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felger J.C., Treadway M.T. Inflammation effects on motivation and motor activity: Role of dopamine. Neuropsychopharmacology. 2017;42(1):216–241. doi: 10.1038/npp.2016.143. [http://dx.doi.org/10.1038/npp.2016.143]. [PMID: 27480574]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felger J.C., Miller A.H. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front. Neuroendocrinol. 2012;33(3):315–327. doi: 10.1016/j.yfrne.2012.09.003. [http://dx.doi.org/ 10.1016/j.yfrne.2012.09.003]. [PMID: 23000204]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felger J.C., Haroon E., Miller A.H. 2016. [Google Scholar]
- 5.Raison C.L., Miller A.H. The evolutionary significance of depression in pathogen host defense (PATHOS-D). Mol. Psychiatry. 2013;18(1):15–37. doi: 10.1038/mp.2012.2. [http://dx.doi.org/10.1038/mp.2012.2]. [PMID: 22290120]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raison C.L., Miller A.H. Pathogen-host defense in the evolution of depression: Insights into epidemiology, genetics, bioregional differences and female preponderance. Neuropsychopharmacology. 2017;42(1):5–27. doi: 10.1038/npp.2016.194. [http://dx.doi.org/10.1038/npp.2016.194]. [PMID: 27629366]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridker P.M. Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: implications for longevity. Nutr. Rev. 2007;65(12 Pt 2):S253–S259. doi: 10.1111/j.1753-4887.2007.tb00372.x. [http://dx.doi. org/10.1301/nr.2007.dec.S253-S259]. [PMID: 18240558]. [DOI] [PubMed] [Google Scholar]
- 8.Ridker P.M. Cardiology Patient Page. C-reactive protein: a simple test to help predict risk of heart attack and stroke. Circulation. 2003;108(12):e81–e85. doi: 10.1161/01.CIR.0000093381.57779.67. [http://dx.doi.org/10.1161/01.CIR.0000093381. 57779.67]. [PMID: 14504253]. [DOI] [PubMed] [Google Scholar]
- 9.Gorman J.M. Comorbid depression and anxiety spectrum disorders. Depress. Anxiety. 1996-1997;4(4):160–168. doi: 10.1002/(SICI)1520-6394(1996)4:4<160::AID-DA2>3.0.CO;2-J. [http://dx.doi. org/10.1002/(SICI)1520-6394(1996)4:4<160:AID-DA2>3.0.CO;2-J]. [PMID: 9166648]. [DOI] [PubMed] [Google Scholar]
- 10.Michopoulos V., Powers A., Gillespie C.F., Ressler K.J., Jovanovic T. Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology. 2017;42(1):254–270. doi: 10.1038/npp.2016.146. [http://dx.doi.org/10.1038/npp.2016.146]. [PMID: 27510423]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felger J.C., Lotrich F.E. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [http://dx.doi.org/10.1016/j.neuroscience. 2013.04.060]. [PMID: 23644052]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haroon E., Raison C.L., Miller A.H. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37(1):137–162. doi: 10.1038/npp.2011.205. [http://dx.doi.org/10.1038/npp.2011.205]. [PMID: 21918508]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berk M., Williams L.J., Jacka F.N., O’Neil A., Pasco J.A., Moylan S., Allen N.B., Stuart A.L., Hayley A.C., Byrne M.L., Maes M. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. doi: 10.1186/1741-7015-11-200. [http:// dx.doi.org/10.1186/1741-7015-11-200]. [PMID: 24228900]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Mello C., Le T., Swain M.G. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J. Neurosci. 2009;29(7):2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [http://dx.doi.org/10.1523/JNEUROSCI.3567-08.2009]. [PMID: 19228962]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wohleb E.S., Fenn A.M., Pacenta A.M., Powell N.D., Sheridan J.F., Godbout J.P. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. 2012;37(9):1491–1505. doi: 10.1016/j.psyneuen.2012.02.003. [http:// dx.doi.org/10.1016/j.psyneuen.2012.02.003]. [PMID: 22386198]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller A.H., Haroon E., Felger J.C. Therapeutic implications of brain-immune interactions: Treatment in translation. Neuropsychopharmacology. 2017;42(1):334–359. doi: 10.1038/npp.2016.167. [http://dx.doi.org/10.1038/ npp.2016.167]. [PMID: 27555382]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haroon E., Miller A.H., Sanacora G. Inflammation, glutamate, and glia: A trio of trouble in mood disorders. Neuropsychopharmacology. 2017;42(1):193–215. doi: 10.1038/npp.2016.199. [http://dx.doi.org/10.1038/npp.2016. 199]. [PMID: 27629368]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbosa I.G., Bauer M.E., Machado-Vieira R., Teixeira A.L. Cytokines in bipolar disorder: paving the way for neuroprogression. Neural Plast. 2014;2014:360481. doi: 10.1155/2014/360481. [http://dx.doi.org/10.1155/ 2014/360481]. [PMID: 25313338]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldsmith D.R., Rapaport M.H., Miller B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry. 2016;21(12):1696–1709. doi: 10.1038/mp.2016.3. [http://dx.doi.org/10.1038/ mp.2016.3]. [PMID: 26903267]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maes M. Major depression and activation of the inflammatory response system. Adv. Exp. Med. Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [http://dx. doi.org/10.1007/978-0-585-37970-8_2]. [PMID: 10442165]. [DOI] [PubMed] [Google Scholar]
- 21.Maes M., Lambrechts J., Bosmans E., Jacobs J., Suy E., Vandervorst C., de Jonckheere C., Minner B., Raus J. Evidence for a systemic immune activation during depression: results of leukocyte enumeration by flow cytometry in conjunction with monoclonal antibody staining. Psychol. Med. 1992;22(1):45–53. doi: 10.1017/s0033291700032712. [http://dx.doi. org/10.1017/S0033291700032712]. [PMID: 1574566]. [DOI] [PubMed] [Google Scholar]
- 22.Sluzewska A. Indicators of immune activation in depressed patients. Adv. Exp. Med. Biol. 1999;461:59–73. doi: 10.1007/978-0-585-37970-8_4. [http://dx.doi.org/10. 1007/978-0-585-37970-8_4]. [PMID: 10442167]. [DOI] [PubMed] [Google Scholar]
- 23.Ridker P.M. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [http://dx.doi.org/10.1161/01.CIR.0000053730. 47739.3C]. [PMID: 12551853]. [DOI] [PubMed] [Google Scholar]
- 24.Felger J.C. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry. 2015 doi: 10.1038/mp.2015.168. [PMID: 26552591]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haroon E., Fleischer C.C., Felger J.C., Chen X., Woolwine B.J., Patel T., Hu X.P., Miller A.H. Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol. Psychiatry. 2016;21(10):1351–1357. doi: 10.1038/mp.2015.206. [http://dx.doi.org/10.1038/mp.2015.206]. [PMID: 26754953]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raison C.L., Felger J.C., Miller A.H. Inflammation and treatment resistance in major depression: a perfect storm. Psychiatr. Times. 2013:12. [Google Scholar]
- 27.Raison C.L., Rutherford R.E., Woolwine B.J., Shuo C., Schettler P., Drake D.F., Haroon E., Miller A.H. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70(1):31–41. doi: 10.1001/2013.jamapsychiatry.4. [http://dx.doi. org/10.1001/2013.jamapsychiatry.4]. [PMID: 22945416]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rapaport M.H., Nierenberg A.A., Schettler P.J., Kinkead B., Cardoos A., Walker R., Mischoulon D. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol. Psychiatry. 2016;21(1):71–79. doi: 10.1038/mp.2015.22. [http://dx.doi.org/10.1038/mp.2015.22]. [PMID: 25802980]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howren M.B., Lamkin D.M., Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom. Med. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [http://dx.doi.org/10.1097/PSY. 0b013e3181907c1b]. [PMID: 19188531]. [DOI] [PubMed] [Google Scholar]
- 30.Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K., Lanctôt K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [http://dx.doi. org/10.1016/j.biopsych.2009.09.033]. [PMID: 20015486]. [DOI] [PubMed] [Google Scholar]
- 31.Potvin S., Stip E., Sepehry A.A., Gendron A., Bah R., Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol. Psychiatry. 2008;63(8):801–808. doi: 10.1016/j.biopsych.2007.09.024. [http:// dx.doi.org/10.1016/j.biopsych.2007.09.024]. [PMID: 18005941]. [DOI] [PubMed] [Google Scholar]
- 32.Miller B.J., Buckley P., Seabolt W., Mellor A., Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol. Psychiatry. 2011;70(7):663–671. doi: 10.1016/j.biopsych.2011.04.013. [http://dx.doi.org/10.1016/j.biopsych.2011.04.013]. [PMID: 21641581]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Passos I.C., Vasconcelos-Moreno M.P., Costa L.G., Kunz M., Brietzke E., Quevedo J., Salum G., Magalhães P.V., Kapczinski F., Kauer-Sant’Anna M. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2015;2(11):1002–1012. doi: 10.1016/S2215-0366(15)00309-0. [http://dx. doi.org/10.1016/S2215-0366(15)00309-0]. [PMID: 26544749]. [DOI] [PubMed] [Google Scholar]
- 34.Levine J., Barak Y., Chengappa K.N., Rapoport A., Rebey M., Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40(4):171–176. doi: 10.1159/000026615. [http://dx.doi. org/10.1159/000026615]. [PMID: 10559698]. [DOI] [PubMed] [Google Scholar]
- 35.Schwieler L., Larsson M.K., Skogh E., Kegel M.E., Orhan F., Abdelmoaty S., Finn A., Bhat M., Samuelsson M., Lundberg K., Dahl M.L., Sellgren C., Schuppe-Koistinen I., Svensson C., Erhardt S., Engberg G. Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia--significance for activation of the kynurenine pathway. J. Psychiatry Neurosci. 2015;40(2):126–133. doi: 10.1503/jpn.140126. [PMID: 25455350]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garver D.L., Tamas R.L., Holcomb J.A. Elevated interleukin-6 in the cerebrospinal fluid of a previously delineated schizophrenia subtype. Neuropsychopharmacology. 2003;28(8):1515–1520. doi: 10.1038/sj.npp.1300217. [http://dx.doi.org/10.1038/sj.npp.1300217]. [PMID: 12799618]. [DOI] [PubMed] [Google Scholar]
- 37.Söderlund J., Olsson S.K., Samuelsson M., Walther-Jallow L., Johansson C., Erhardt S., Landén M., Engberg G. Elevation of cerebrospinal fluid interleukin-1ß in bipolar disorder. J. Psychiatry Neurosci. 2011;36(2):114–118. doi: 10.1503/jpn.100080. [http://dx.doi.org/10.1503/jpn. 100080]. [PMID: 21138659]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Söderlund J., Schröder J., Nordin C., Samuelsson M., Walther-Jallow L., Karlsson H., Erhardt S., Engberg G. Activation of brain interleukin-1beta in schizophrenia. Mol. Psychiatry. 2009;14(12):1069–1071. doi: 10.1038/mp.2009.52. [http://dx.doi.org/10.1038/mp.2009.52]. [PMID: 19920835]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindqvist D., Janelidze S., Hagell P., Erhardt S., Samuelsson M., Minthon L., Hansson O., Björkqvist M., Träskman-Bendz L., Brundin L. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol. Psychiatry. 2009;66(3):287–292. doi: 10.1016/j.biopsych.2009.01.030. [http://dx.doi.org/10.1016/j.biopsych. 2009.01.030]. [PMID: 19268915]. [DOI] [PubMed] [Google Scholar]
- 40.Martinez J.M., Garakani A., Yehuda R., Gorman J.M. Proinflammatory and “resiliency” proteins in the CSF of patients with major depression. Depress. Anxiety. 2012;29(1):32–38. doi: 10.1002/da.20876. [http://dx. doi.org/10.1002/da.20876]. [PMID: 21898706]. [DOI] [PubMed] [Google Scholar]
- 41.Bosker F.J., Hartman C.A., Nolte I.M., Prins B.P., Terpstra P., Posthuma D., van Veen T., Willemsen G., DeRijk R.H., de Geus E.J., Hoogendijk W.J., Sullivan P.F., Penninx B.W., Boomsma D.I., Snieder H., Nolen W.A. Poor replication of candidate genes for major depressive disorder using genome-wide association data. Mol. Psychiatry. 2011;16(5):516–532. doi: 10.1038/mp.2010.38. [http://dx. doi.org/10.1038/mp.2010.38]. [PMID: 20351714]. [DOI] [PubMed] [Google Scholar]
- 42.Bufalino C. The role of immune genes in the association between depression and inflammation: A review of recent clinical studies. Brain Behav. Immun. 2013;31:31–47. doi: 10.1016/j.bbi.2012.04.009. [PMID: 22580182]. [DOI] [PubMed] [Google Scholar]
- 43.Bamne M., Wood J., Chowdari K., Watson A.M., Celik C., Mansour H., Klei L., Gur R.C., Bradford L.D., Calkins M.E., Santos A.B., Edwards N., Kwentus J., McEvoy J.P., Allen T.B., Savage R.M., Nasrallah H.A., Gur R.E., Perry R.T., Go R.C., Devlin B., Yolken R., Nimgaonkar V.L. Evaluation of HLA polymorphisms in relation to schizophrenia risk and infectious exposure. Schizophr. Bull. 2012;38(6):1149–1154. doi: 10.1093/schbul/sbs087. [http://dx.doi.org/ 10.1093/schbul/sbs087]. [PMID: 22966150]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ripke, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. [http://dx.doi. org/10.1038/nature13595]. [PMID: 25056061]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guffanti G., Galea S., Yan L., Roberts A.L., Solovieff N., Aiello A.E., Smoller J.W., De Vivo I., Ranu H., Uddin M., Wildman D.E., Purcell S., Koenen K.C. Genome-wide association study implicates a novel RNA gene, the lincRNA AC068718.1, as a risk factor for post-traumatic stress disorder in women. Psychoneuroendocrinology. 2013;38(12):3029–3038. doi: 10.1016/j.psyneuen.2013.08.014. [http://dx.doi. org/10.1016/j.psyneuen.2013.08.014]. [PMID: 24080187]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michopoulos V., Rothbaum A.O., Jovanovic T., Almli L.M., Bradley B., Rothbaum B.O., Gillespie C.F., Ressler K.J. Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am. J. Psychiatry. 2015;172(4):353–362. doi: 10.1176/appi.ajp.2014.14020263. [http://dx.doi.org/10.1176/appi.ajp.2014.14020263]. [PMID: 25827033]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yirmiya R., Weidenfeld J., Pollak Y., Morag M., Morag A., Avitsur R., Barak O., Reichenberg A., Cohen E., Shavit Y., Ovadia H. Cytokines, “depression due to a general medical condition,” and antidepressant drugs. Adv. Exp. Med. Biol. 1999;461:283–316. doi: 10.1007/978-0-585-37970-8_16. [http://dx.doi.org/10.1007/978-0-585-37970-8_16]. [PMID: 10442179]. [DOI] [PubMed] [Google Scholar]
- 48.Yirmiya R., Pollak Y., Morag M., Reichenberg A., Barak O., Avitsur R., Shavit Y., Ovadia H., Weidenfeld J., Morag A., Newman M.E., Pollmächer T. Illness, cytokines, and depression. Ann. N. Y. Acad. Sci. 2000;917:478–487. doi: 10.1111/j.1749-6632.2000.tb05412.x. [http://dx.doi.org/10. 1111/j.1749-6632.2000.tb05412.x]. [PMID: 11268375]. [DOI] [PubMed] [Google Scholar]
- 49.Raison C.L., Miller A.H. Depression in cancer: new developments regarding diagnosis and treatment. Biol. Psychiatry. 2003;54(3):283–294. doi: 10.1016/s0006-3223(03)00413-x. [http://dx.doi.org/10.1016/S0006-3223(03)00413-X]. [PMID: 12893104]. [DOI] [PubMed] [Google Scholar]
- 50.Evans D.L., Staab J.P., Petitto J.M., Morrison M.F., Szuba M.P., Ward H.E., Wingate B., Luber M.P., O’Reardon J.P. Depression in the medical setting: biopsychological interactions and treatment considerations. J. Clin. Psychiatry. 1999;60(Suppl. 4):40–55. [PMID: 10086482]. [PubMed] [Google Scholar]
- 51.Pollak Y., Yirmiya R. Cytokine-induced changes in mood and behaviour: implications for ‘depression due to a general medical condition’, immunotherapy and antidepressive treatment. Int. J. Neuropsychopharmacol. 2002;5(4):389–399. doi: 10.1017/S1461145702003152. [http://dx.doi. org/10.1017/S1461145702003152]. [PMID: 12466037]. [DOI] [PubMed] [Google Scholar]
- 52.Shelton R.C., Miller A.H. Eating ourselves to death (and despair): the contribution of adiposity and inflammation to depression. Prog. Neurobiol. 2010;91(4):275–299. doi: 10.1016/j.pneurobio.2010.04.004. [http://dx.doi.org/10.1016/j. pneurobio.2010.04.004]. [PMID: 20417247]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fillman S.G., Sinclair D., Fung S.J., Webster M.J., Shannon Weickert C. Markers of inflammation and stress distinguish subsets of individuals with schizophrenia and bipolar disorder. Transl. Psychiatry. 2014;4:e365. doi: 10.1038/tp.2014.8. [http://dx.doi.org/10.1038/tp.2014.8]. [PMID: 24569695]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chase K.A., Rosen C., Gin H., Bjorkquist O., Feiner B., Marvin R., Conrin S., Sharma R.P. Metabolic and inflammatory genes in schizophrenia. Psychiatry Res. 2015;225(1-2):208–211. doi: 10.1016/j.psychres.2014.11.007. [http://dx. doi.org/10.1016/j.psychres.2014.11.007]. [PMID: 25433960]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mostafavi S., Battle A., Zhu X., Potash J.B., Weissman M.M., Shi J., Beckman K., Haudenschild C., McCormick C., Mei R., Gameroff M.J., Gindes H., Adams P., Goes F.S., Mondimore F.M., MacKinnon D.F., Notes L., Schweizer B., Furman D., Montgomery S.B., Urban A.E., Koller D., Levinson D.F. Type I interferon signaling genes in recurrent major depression: increased expression detected by whole-blood RNA sequencing. Mol. Psychiatry. 2014;19(12):1267–1274. doi: 10.1038/mp.2013.161. [http://dx.doi.org/10.1038/ mp.2013.161]. [PMID: 24296977]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cattaneo A., Gennarelli M., Uher R., Breen G., Farmer A., Aitchison K.J., Craig I.W., Anacker C., Zunsztain P.A., McGuffin P., Pariante C.M. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline ‘predictors’ and longitudinal ‘targets’. Neuropsychopharmacology. 2013;38(3):377–385. doi: 10.1038/npp.2012.191. [http://dx.doi. org/10.1038/npp.2012.191]. [PMID: 22990943]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shelton R.C., Claiborne J., Sidoryk-Wegrzynowicz M., Reddy R., Aschner M., Lewis D.A., Mirnics K. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol. Psychiatry. 2011;16(7):751–762. doi: 10.1038/mp.2010.52. [http:// dx.doi.org/10.1038/mp.2010.52]. [PMID: 20479761]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Danese A., Moffitt T.E., Pariante C.M., Ambler A., Poulton R., Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch. Gen. Psychiatry. 2008;65(4):409–415. doi: 10.1001/archpsyc.65.4.409. [http://dx.doi.org/10.1001/archpsyc.65.4.409]. [PMID: 18391129]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Danese A., Pariante C.M., Caspi A., Taylor A., Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. Sci. USA. 2007;104(4):1319–1324. doi: 10.1073/pnas.0610362104. [http://dx.doi.org/10.1073/pnas.0610362104]. [PMID: 17229839]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Danese A., Caspi A., Williams B., Ambler A., Sugden K., Mika J., Werts H., Freeman J., Pariante C.M., Moffitt T.E., Arseneault L. Biological embedding of stress through inflammation processes in childhood. Mol. Psychiatry. 2011;16(3):244–246. doi: 10.1038/mp.2010.5. [http://dx.doi.org/10.1038/mp.2010.5]. [PMID: 20157309]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nusslock R., Miller G.E. Early-life adversity and physical and emotional health across the lifespan: A neuroimmune network hypothesis. Biol. Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.05.017. [PMID: 26166230]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pace T.W., Mletzko T.C., Alagbe O., Musselman D.L., Nemeroff C.B., Miller A.H., Heim C.M. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatry. 2006;163(9):1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [http://dx.doi.org/10.1176/ajp.2006.163.9.1630]. [PMID: 16946190]. [DOI] [PubMed] [Google Scholar]
- 63.Miller G.E., Cole S.W. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biol. Psychiatry. 2012;72(1):34–40. doi: 10.1016/j.biopsych.2012.02.034. [http://dx.doi.org/10.1016/j. biopsych.2012.02.034]. [PMID: 22494534]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eraly S.A., Nievergelt C.M., Maihofer A.X., Barkauskas D.A., Biswas N., Agorastos A., O’Connor D.T., Baker D.G. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry. 2014;71(4):423–431. doi: 10.1001/jamapsychiatry.2013.4374. [http:// dx.doi.org/10.1001/jamapsychiatry.2013.4374]. [PMID: 24576974]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pervanidou P., Kolaitis G., Charitaki S., Margeli A., Ferentinos S., Bakoula C., Lazaropoulou C., Papassotiriou I., Tsiantis J., Chrousos G.P. Elevated morning serum interleukin (IL)-6 or evening salivary cortisol concentrations predict posttraumatic stress disorder in children and adolescents six months after a motor vehicle accident. Psychoneuroendocrinology. 2007;32(8-10):991–999. doi: 10.1016/j.psyneuen.2007.07.001. [http://dx.doi.org/10.1016/j.psyneuen.2007.07.001]. [PMID: 17825995]. [DOI] [PubMed] [Google Scholar]
- 66.Fleshner M. Stress-evoked sterile inflammation, danger associated molecular patterns (DAMPs), microbial associated molecular patterns (MAMPs) and the inflammasome. Brain Behav. Immun. 2013;27(1):1–7. doi: 10.1016/j.bbi.2012.08.012. [http://dx.doi.org/10.1016/j.bbi.2012.08.012]. [PMID: 22964544]. [DOI] [PubMed] [Google Scholar]
- 67.Iwata M., Ota K.T., Duman R.S. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav. Immun. 2013;31:105–114. doi: 10.1016/j.bbi.2012.12.008. [http://dx.doi.org/10. 1016/j.bbi.2012.12.008]. [PMID: 23261775]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maslanik T. The inflammasome and danger associated molecular patterns (DAMPs) are implicated in cytokine and chemokine responses following stressor exposure. Brain Behav. Immun. 2012;28:54–62. doi: 10.1016/j.bbi.2012.10.014. [PMID: 23103443]. [DOI] [PubMed] [Google Scholar]
- 69.Bryant P.A., Trinder J., Curtis N. Sick and tired: Does sleep have a vital role in the immune system? Nat. Rev. Immunol. 2004;4(6):457–467. doi: 10.1038/nri1369. [http://dx.doi.org/10.1038/nri1369]. [PMID: 15173834]. [DOI] [PubMed] [Google Scholar]
- 70.Motivala S.J., Sarfatti A., Olmos L., Irwin M.R. Inflammatory markers and sleep disturbance in major depression. Psychosom. Med. 2005;67(2):187–194. doi: 10.1097/01.psy.0000149259.72488.09. [http://dx.doi.org/10.1097/01.psy. 0000149259.72488.09]. [PMID: 15784782]. [DOI] [PubMed] [Google Scholar]
- 71.Opp M.R., Born J., Irwin M.R. Irwin, Sleep and the immune system. In: Psychoneuroim-munology R.A., editor. Academic Press. New York, NY: 2007. pp. 570–618. [Google Scholar]
- 72.Suarez E.C. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain Behav. Immun. 2008;22(6):960–968. doi: 10.1016/j.bbi.2008.01.011. [http://dx.doi.org/10.1016/j.bbi.2008.01.011]. [PMID: 18328671]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meier-Ewert H.K., Ridker P.M., Rifai N., Regan M.M., Price N.J., Dinges D.F., Mullington J.M. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J. Am. Coll. Cardiol. 2004;43(4):678–683. doi: 10.1016/j.jacc.2003.07.050. [http://dx.doi.org/10. 1016/j.jacc.2003.07.050]. [PMID: 14975482]. [DOI] [PubMed] [Google Scholar]
- 74.Vgontzas A.N., Papanicolaou D.A., Bixler E.O., Lotsikas A., Zachman K., Kales A., Prolo P., Wong M.L., Licinio J., Gold P.W., Hermida R.C., Mastorakos G., Chrousos G.P. Circadian interleukin-6 secretion and quantity and depth of sleep. J. Clin. Endocrinol. Metab. 1999;84(8):2603–2607. doi: 10.1210/jcem.84.8.5894. [http://dx.doi.org/10. 1210/jcem.84.8.5894]. [PMID: 10443646]. [DOI] [PubMed] [Google Scholar]
- 75.Vgontzas A.N., Zoumakis E., Bixler E.O., Lin H.M., Follett H., Kales A., Chrousos G.P. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J. Clin. Endocrinol. Metab. 2004;89(5):2119–2126. doi: 10.1210/jc.2003-031562. [http://dx.doi.org/10. 1210/jc.2003-031562]. [PMID: 15126529]. [DOI] [PubMed] [Google Scholar]
- 76.Jamal O., Aneni E.C., Shaharyar S., Ali S.S., Parris D., McEvoy J.W., Veledar E., Blaha M.J., Blumenthal R.S., Agatston A.S., Conceição R.D., Feldman T., Carvalho J.A., Santos R.D., Nasir K. Cigarette smoking worsens systemic inflammation in persons with metabolic syndrome. Diabetol. Metab. Syndr. 2014;6:79–86. doi: 10.1186/1758-5996-6-79. [http://dx.doi.org/10.1186/1758-5996-6-79]. [PMID: 25960769]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de La Serre C.B., Ellis C.L., Lee J., Hartman A.L., Rutledge J.C., Raybould H.E. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299(2):G440–G448. doi: 10.1152/ajpgi.00098.2010. [http://dx.doi.org/10.1152/ajpgi.00098.2010]. [PMID: 20508158]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khaodhiar L., Ling P.R., Blackburn G.L., Bistrian B.R. Serum levels of interleukin-6 and C-reactive protein correlate with body mass index across the broad range of obesity. JPEN J. Parenter. Enteral Nutr. 2004;28(6):410–415. doi: 10.1177/0148607104028006410. [http://dx.doi.org/10.1177/ 0148607104028006410]. [PMID: 15568287]. [DOI] [PubMed] [Google Scholar]
- 79.Lim W., Hong S., Nelesen R., Dimsdale J.E. The association of obesity, cytokine levels, and depressive symptoms with diverse measures of fatigue in healthy subjects. Arch. Intern. Med. 2005;165(8):910–915. doi: 10.1001/archinte.165.8.910. [http://dx.doi.org/10.1001/archinte.165.8.910]. [PMID: 15851643]. [DOI] [PubMed] [Google Scholar]
- 80.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [http://dx.doi.org/10.1172/JCI200319246]. [PMID: 14679176]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suganami T., Ogawa Y. Adipose tissue macrophages: their role in adipose tissue remodeling. J. Leukoc. Biol. 2010;88(1):33–39. doi: 10.1189/jlb.0210072. [http://dx.doi.org/10.1189/jlb.0210072]. [PMID: 20360405]. [DOI] [PubMed] [Google Scholar]
- 82.Park H.S., Park J.Y., Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res. Clin. Pract. 2005;69(1):29–35. doi: 10.1016/j.diabres.2004.11.007. [http://dx.doi.org/ 10.1016/j.diabres.2004.11.007]. [PMID: 15955385]. [DOI] [PubMed] [Google Scholar]
- 83.Miller G.E., Freedland K.E., Carney R.M., Stetler C.A., Banks W.A. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain Behav. Immun. 2003;17(4):276–285. doi: 10.1016/s0889-1591(03)00057-6. [http://dx.doi.org/10.1016/S0889-1591(03)00057-6]. [PMID: 12831830]. [DOI] [PubMed] [Google Scholar]
- 84.Miller G.E., Stetler C.A., Carney R.M., Freedland K.E., Banks W.A. Clinical depression and inflammatory risk markers for coronary heart disease. Am. J. Cardiol. 2002;90(12):1279–1283. doi: 10.1016/s0002-9149(02)02863-1. [http:// dx.doi.org/10.1016/S0002-9149(02)02863-1]. [PMID: 12480034]. [DOI] [PubMed] [Google Scholar]
- 85.Katsuura G., Arimura A., Koves K., Gottschall P.E. Involvement of organum vasculosum of lamina terminalis and preoptic area in interleukin 1 beta-induced ACTH release. Am. J. Physiol. 1990;258(1 Pt 1):E163–E171. doi: 10.1152/ajpendo.1990.258.1.E163. [PMID: 1967907]. [DOI] [PubMed] [Google Scholar]
- 86.Pan W., Kastin A.J. Interactions of cytokines with the blood-brain barrier: implications for feeding. Curr. Pharm. Des. 2003;9(10):827–831. doi: 10.2174/1381612033455332. [http://dx.doi.org/10.2174/1381612033455332]. [PMID: 12678882]. [DOI] [PubMed] [Google Scholar]
- 87.Banks W.A., Erickson M.A. The blood-brain barrier and immune function and dysfunction. Neurobiol. Dis. 2010;37(1):26–32. doi: 10.1016/j.nbd.2009.07.031. [http://dx.doi.org/10.1016/j.nbd.2009.07.031]. [PMID: 19664708]. [DOI] [PubMed] [Google Scholar]
- 88.Banks W.A., Farr S.A., Morley J.E. Entry of blood-borne cytokines into the central nervous system: effects on cognitive processes. Neuroimmunomodulation. 2002-2003;10(6):319–327. doi: 10.1159/000071472. [http://dx. doi.org/10.1159/000071472]. [PMID: 12907838]. [DOI] [PubMed] [Google Scholar]
- 89.Banks W.A., Kastin A.J., Broadwell R.D. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2(4):241–248. doi: 10.1159/000097202. [http://dx.doi.org/10.1159/000097202]. [PMID: 8963753]. [DOI] [PubMed] [Google Scholar]
- 90.Bluthé R.M., Walter V., Parnet P., Layé S., Lestage J., Verrier D., Poole S., Stenning B.E., Kelley K.W., Dantzer R. Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C. R. Acad. Sci. III. 1994;317(6):499–503. [PMID: 7987701]. [PubMed] [Google Scholar]
- 91.Ericsson A., Kovács K.J., Sawchenko P.E. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J. Neurosci. 1994;14(2):897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [PMID: 8301368]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Watkins L.R., Goehler L.E., Relton J.K., Tartaglia N., Silbert L., Martin D., Maier S.F. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci. Lett. 1995;183(1-2):27–31. doi: 10.1016/0304-3940(94)11105-r. [http://dx.doi.org/10.1016/0304-3940(94)11105-R]. [PMID: 7746479]. [DOI] [PubMed] [Google Scholar]
- 93.Watkins L.R., Wiertelak E.P., Goehler L.E., Mooney-Heiberger K., Martinez J., Furness L., Smith K.P., Maier S.F. Neurocircuitry of illness-induced hyperalgesia. Brain Res. 1994;639(2):283–299. doi: 10.1016/0006-8993(94)91742-6. [http://dx.doi.org/10.1016/0006-8993(94)91742-6]. [PMID: 8205482]. [DOI] [PubMed] [Google Scholar]
- 94.D’Mello C., Ronaghan N., Zaheer R., Dicay M., Le T., MacNaughton W.K., Surrette M.G., Swain M.G. Probiotics improve inflammation-associated sickness behavior by altering communication between the peripheral immune system and the brain. J. Neurosci. 2015;35(30):10821–10830. doi: 10.1523/JNEUROSCI.0575-15.2015. [http://dx.doi.org/10.1523/ JNEUROSCI.0575-15.2015]. [PMID: 26224864]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wohleb E.S., McKim D.B., Shea D.T., Powell N.D., Tarr A.J., Sheridan J.F., Godbout J.P. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol. Psychiatry. 2014;75(12):970–981. doi: 10.1016/j.biopsych.2013.11.029. [http://dx. doi.org/10.1016/j.biopsych.2013.11.029]. [PMID: 24439304]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hodes G.E., Pfau M.L., Leboeuf M., Golden S.A., Christoffel D.J., Bregman D., Rebusi N., Heshmati M., Aleyasin H., Warren B.L., Lebonté B., Horn S., Lapidus K.A., Stelzhammer V., Wong E.H., Bahn S., Krishnan V., Bolaños-Guzman C.A., Murrough J.W., Merad M., Russo S.J. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl. Acad. Sci. USA. 2014;111(45):16136–16141. doi: 10.1073/pnas.1415191111. [http://dx.doi.org/10.1073/pnas.1415191111]. [PMID: 25331895]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brambilla P., Bellani M., Isola M., Bergami A., Marinelli V., Dusi N., Rambaldelli G., Tansella M., Finardi A.M., Martino G., Perlini C., Furlan R. Increased M1/decreased M2 signature and signs of Th1/Th2 shift in chronic patients with bipolar disorder, but not in those with schizophrenia. Transl. Psychiatry. 2014;4:e406. doi: 10.1038/tp.2014.46. [http://dx.doi.org/10.1038/tp.2014.46]. [PMID: 24984193]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Drago A., Crisafulli C., Calabrò M., Serretti A. Enrichment pathway analysis. the inflammatory genetic background in bipolar disorder. J. Affect. Disord. 2015;179:88–94. doi: 10.1016/j.jad.2015.03.032. [http://dx.doi.org/10. 1016/j.jad.2015.03.032]. [PMID: 25855618]. [DOI] [PubMed] [Google Scholar]
- 99.Steiner J., Walter M., Gos T., Guillemin G.J., Bernstein H.G., Sarnyai Z., Mawrin C., Brisch R., Bielau H., Meyer zu Schwabedissen L., Bogerts B., Myint A.M. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J. Neuroinflammation. 2011;8:94. doi: 10.1186/1742-2094-8-94. [http://dx.doi.org/10.1186/1742-2094-8-94]. [PMID: 21831269]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Torres-Platas S.G., Cruceanu C., Chen G.G., Turecki G., Mechawar N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav. Immun. 2014;42:50–59. doi: 10.1016/j.bbi.2014.05.007. [http://dx.doi.org/10.1016/j.bbi.2014.05.007]. [PMID: 24858659]. [DOI] [PubMed] [Google Scholar]
- 101.Tremblay M.E., Stevens B., Sierra A., Wake H., Bessis A., Nimmerjahn A. The role of microglia in the healthy brain. J. Neurosci. 2011;31(45):16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [http://dx.doi.org/10.1523/ JNEUROSCI.4158-11.2011]. [PMID: 22072657]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lockhart A., Davis B., Matthews J.C., Rahmoune H., Hong G., Gee A., Earnshaw D., Brown J. The peripheral benzodiazepine receptor ligand PK11195 binds with high affinity to the acute phase reactant α1-acid glycoprotein: implications for the use of the ligand as a CNS inflammatory marker. Nucl. Med. Biol. 2003;30(2):199–206. doi: 10.1016/s0969-8051(02)00410-9. [http://dx.doi.org/10.1016/S0969-8051(02)00410-9]. [PMID: 12623120]. [DOI] [PubMed] [Google Scholar]
- 103.Venneti S., Lopresti B.J., Wang G., Slagel S.L., Mason N.S., Mathis C.A., Fischer M.L., Larsen N.J., Mortimer A.D., Hastings T.G., Smith A.D., Zigmond M.J., Suhara T., Higuchi M., Wiley C.A. A comparison of the high-affinity peripheral benzodiazepine receptor ligands DAA1106 and (R)-PK11195 in rat models of neuroinflammation: implications for PET imaging of microglial activation. J. Neurochem. 2007;102(6):2118–2131. doi: 10.1111/j.1471-4159.2007.04690.x. [http://dx.doi. org/10.1111/j.1471-4159.2007.04690.x]. [PMID: 17555551]. [DOI] [PubMed] [Google Scholar]
- 104.Cagnin A., Kassiou M., Meikle S.R., Banati R.B. Positron emission tomography imaging of neuroinflammation. Neurotherapeutics. 2007;4(3):443–452. doi: 10.1016/j.nurt.2007.04.006. [http://dx.doi.org/10.1016/j.nurt.2007.04.006]. [PMID: 17599710]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Imaizumi M., Kim H.J., Zoghbi S.S., Briard E., Hong J., Musachio J.L., Ruetzler C., Chuang D.M., Pike V.W., Innis R.B., Fujita M. PET imaging with [11C]PBR28 can localize and quantify upregulated peripheral benzodiazepine receptors associated with cerebral ischemia in rat. Neurosci. Lett. 2007;411(3):200–205. doi: 10.1016/j.neulet.2006.09.093. [http://dx.doi.org/10.1016/j.neulet.2006.09.093]. [PMID: 17127001]. [DOI] [PubMed] [Google Scholar]
- 106.Wilson A.A., Garcia A., Parkes J., McCormick P., Stephenson K.A., Houle S., Vasdev N. Radiosynthesis and initial evaluation of [18F]-FEPPA for PET imaging of peripheral benzodiazepine receptors. Nucl. Med. Biol. 2008;35(3):305–314. doi: 10.1016/j.nucmedbio.2007.12.009. [http://dx.doi. org/10.1016/j.nucmedbio.2007.12.009]. [PMID: 18355686]. [DOI] [PubMed] [Google Scholar]
- 107.Setiawan E., Wilson A.A., Mizrahi R., Rusjan P.M., Miler L., Rajkowska G., Suridjan I., Kennedy J.L., Rekkas P.V., Houle S., Meyer J.H. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry. 2015;72(3):268–275. doi: 10.1001/jamapsychiatry.2014.2427. [http://dx.doi.org/10.1001/ jamapsychiatry.2014.2427]. [PMID: 25629589]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hannestad J., Gallezot J.D., Schafbauer T., Lim K., Kloczynski T., Morris E.D., Carson R.E., Ding Y.S., Cosgrove K.P. Endotoxin-induced systemic inflammation activates microglia: [11C]PBR28 positron emission tomography in nonhuman primates. Neuroimage. 2012;63(1):232–239. doi: 10.1016/j.neuroimage.2012.06.055. [http://dx.doi.org/10.1016/j.neuroimage. 2012.06.055]. [PMID: 22776451]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bloomfield P.S. Microglial activity in people at ultra high risk of psychosis and in schizophrenia: An [11C] PBR28 PET brain imaging study. Am. J. Psychiatry. 2015;173(1):44–52. doi: 10.1176/appi.ajp.2015.14101358. [PMID: 26472628]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kenk M., Selvanathan T., Rao N., Suridjan I., Rusjan P., Remington G., Meyer J.H., Wilson A.A., Houle S., Mizrahi R. Imaging neuroinflammation in gray and white matter in schizophrenia: an in-vivo PET study with [18F]-FEPPA. Schizophr. Bull. 2015;41(1):85–93. doi: 10.1093/schbul/sbu157. [http://dx.doi.org/10.1093/schbul/sbu157]. [PMID: 25385788]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vivash L., O’Brien T.J. Imaging microglial activation with TSPO PET: Lighting up neurologic diseases? J. Nucl. Med. 2016;57(2):165–168. doi: 10.2967/jnumed.114.141713. [http://dx.doi.org/10.2967/jnumed.114.141713]. [PMID: 26697963]. [DOI] [PubMed] [Google Scholar]
- 112.Setiawan E., Wilson A.A., Mizrahi R., Rusjan P.M., Miler L., Rajkowska G., Suridjan I., Kennedy J.L., Rekkas P.V., Houle S., Meyer J.H. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry. 2015;72(3):268–275. doi: 10.1001/jamapsychiatry.2014.2427. [http://dx.doi.org/10.1001/ jamapsychiatry.2014.2427]. [PMID: 25629589]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sandiego C.M., Gallezot J.D., Pittman B., Nabulsi N., Lim K., Lin S.F., Matuskey D., Lee J.Y., O’Connor K.C., Huang Y., Carson R.E., Hannestad J., Cosgrove K.P. Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc. Natl. Acad. Sci. USA. 2015;112(40):12468–12473. doi: 10.1073/pnas.1511003112. [http://dx.doi.org/10.1073/pnas.1511003112]. [PMID: 26385967]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hannestad J., Gallezot J.D., Schafbauer T., Lim K., Kloczynski T., Morris E.D., Carson R.E., Ding Y.S., Cosgrove K.P. Endotoxin-induced systemic inflammation activates microglia: [11C]PBR28 positron emission tomography in nonhuman primates. Neuroimage. 2012;63(1):232–239. doi: 10.1016/j.neuroimage.2012.06.055. [http://dx.doi.org/10.1016/j. neuroimage.2012.06.055]. [PMID: 22776451]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Raivich G., Bohatschek M., Kloss C.U., Werner A., Jones L.L., Kreutzberg G.W. Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res. Brain Res. Rev. 1999;30(1):77–105. doi: 10.1016/s0165-0173(99)00007-7. [http://dx.doi.org/10.1016/S0165-0173(99)00007-7]. [PMID: 10407127]. [DOI] [PubMed] [Google Scholar]
- 116.Saijo K., Glass C.K. Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol. 2011;11(11):775–787. doi: 10.1038/nri3086. [http://dx.doi.org/10.1038/nri3086]. [PMID: 22025055]. [DOI] [PubMed] [Google Scholar]
- 117.Marshall S.A., McClain J.A., Kelso M.L., Hopkins D.M., Pauly J.R., Nixon K. Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiol. Dis. 2013;54:239–251. doi: 10.1016/j.nbd.2012.12.016. [http://dx.doi.org/10.1016/j.nbd.2012.12.016]. [PMID: 23313316]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Capuron L., Pagnoni G., Drake D.F., Woolwine B.J., Spivey J.R., Crowe R.J., Votaw J.R., Goodman M.M., Miller A.H. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch. Gen. Psychiatry. 2012;69(10):1044–1053. doi: 10.1001/archgenpsychiatry.2011.2094. [http://dx.doi.org/10.1001/ archgenpsychiatry.2011.2094]. [PMID: 23026954]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Capuron L., Pagnoni G., Demetrashvili M.F., Lawson D.H., Fornwalt F.B., Woolwine B., Berns G.S., Nemeroff C.B., Miller A.H. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology. 2007;32(11):2384–2392. doi: 10.1038/sj.npp.1301362. [http://dx.doi.org/10.1038/sj.npp.1301362]. [PMID: 17327884]. [DOI] [PubMed] [Google Scholar]
- 120.Brydon L., Harrison N.A., Walker C., Steptoe A., Critchley H.D. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol. Psychiatry. 2008;63(11):1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [http://dx.doi.org/10.1016/j.biopsych. 2007.12.007]. [PMID: 18242584]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eisenberger N.I., Berkman E.T., Inagaki T.K., Rameson L.T., Mashal N.M., Irwin M.R. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol. Psychiatry. 2010;68(8):748–754. doi: 10.1016/j.biopsych.2010.06.010. [http://dx.doi.org/10.1016/j.biopsych. 2010.06.010]. [PMID: 20719303]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Majer M., Welberg L.A., Capuron L., Pagnoni G., Raison C.L., Miller A.H. IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav. Immun. 2008;22(6):870–880. doi: 10.1016/j.bbi.2007.12.009. [http://dx.doi.org/ 10.1016/j.bbi.2007.12.009]. [PMID: 18258414]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Harrison N.A. A neurocomputational account of how inflammation enhances sensitivity to punishments versus rewards. Biol. Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.07.018. [PMID: 26359113]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Goldsmith D.R., Haroon E., Woolwine B.J., Jung M.Y., Wommack E.C., Harvey P.D., Treadway M.T., Felger J.C., Miller A.H. Inflammatory markers are associated with decreased psychomotor speed in patients with major depressive disorder. Brain Behav. Immun. 2016;56:281–288. doi: 10.1016/j.bbi.2016.03.025. [http://dx.doi.org/10.1016/j.bbi. 2016.03.025]. [PMID: 27040122]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Donnelly S. Patient management strategies for interferon alfa-2b as adjuvant therapy of high-risk melanoma. Oncol. Nurs. Forum. 1998;25(5):921–927. [PMID: 9644709]. [PubMed] [Google Scholar]
- 126.Capuron L., Gumnick J.F., Musselman D.L., Lawson D.H., Reemsnyder A., Nemeroff C.B., Miller A.H. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26(5):643–652. doi: 10.1016/S0893-133X(01)00407-9. [http://dx.doi.org/10.1016/ S0893-133X(01)00407-9]. [PMID: 11927189]. [DOI] [PubMed] [Google Scholar]
- 127.Capuron L., Neurauter G., Musselman D.L., Lawson D.H., Nemeroff C.B., Fuchs D., Miller A.H. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol. Psychiatry. 2003;54(9):906–914. doi: 10.1016/s0006-3223(03)00173-2. [http:// dx.doi.org/10.1016/S0006-3223(03)00173-2]. [PMID: 14573318]. [DOI] [PubMed] [Google Scholar]
- 128.Musselman D.L., Lawson D.H., Gumnick J.F., Manatunga A.K., Penna S., Goodkin R.S., Greiner K., Nemeroff C.B., Miller A.H. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N. Engl. J. Med. 2001;344(13):961–966. doi: 10.1056/NEJM200103293441303. [http:// dx.doi.org/10.1056/NEJM200103293441303]. [PMID: 11274622]. [DOI] [PubMed] [Google Scholar]
- 129.Raison C.L., Borisov A.S., Broadwell S.D., Capuron L., Woolwine B.J., Jacobson I.M., Nemeroff C.B., Miller A.H. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J. Clin. Psychiatry. 2005;66(1):41–48. doi: 10.4088/jcp.v66n0106. [http://dx.doi.org/10.4088/JCP.v66n0106]. [PMID: 15669887]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Raison C.L., Rye D.B., Woolwine B.J., Vogt G.J., Bautista B.M., Spivey J.R., Miller A.H. Chronic interferon-alpha administration disrupts sleep continuity and depth in patients with hepatitis C: association with fatigue, motor slowing, and increased evening cortisol. Biol. Psychiatry. 2010;68(10):942–949. doi: 10.1016/j.biopsych.2010.04.019. [http://dx. doi.org/10.1016/j.biopsych.2010.04.019]. [PMID: 20537611]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Raison C.L., Borisov A.S., Majer M., Drake D.F., Pagnoni G., Woolwine B.J., Vogt G.J., Massung B., Miller A.H. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol. Psychiatry. 2009;65(4):296–303. doi: 10.1016/j.biopsych.2008.08.010. [http://dx.doi.org/10.1016/j.biopsych. 2008.08.010]. [PMID: 18801471]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Capuron L., Hauser P., Hinze-Selch D., Miller A.H., Neveu P.J. Treatment of cytokine-induced depression. Brain Behav. Immun. 2002;16(5):575–580. doi: 10.1016/s0889-1591(02)00007-7. [http://dx.doi.org/10.1016/S0889-1591(02) 00007-7]. [PMID: 12401471]. [DOI] [PubMed] [Google Scholar]
- 133.Felger J.C., Mun J., Kimmel H.L., Nye J.A., Drake D.F., Hernandez C.R., Freeman A.A., Rye D.B., Goodman M.M., Howell L.L., Miller A.H. Chronic interferon-α decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology. 2013;38(11):2179–2187. doi: 10.1038/npp.2013.115. [http://dx.doi.org/10.1038/ npp.2013.115]. [PMID: 23657438]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kitagami T., Yamada K., Miura H., Hashimoto R., Nabeshima T., Ohta T. Mechanism of systemically injected interferon-alpha impeding monoamine biosynthesis in rats: role of nitric oxide as a signal crossing the blood-brain barrier. Brain Res. 2003;978(1-2):104–114. doi: 10.1016/s0006-8993(03)02776-8. [http://dx.doi.org/10.1016/S0006-8993(03)02776-8]. [PMID: 12834904]. [DOI] [PubMed] [Google Scholar]
- 135.Nunes E.J., Randall P.A., Estrada A., Epling B., Hart E.E., Lee C.A., Baqi Y., Müller C.E., Correa M., Salamone J.D. Effort-related motivational effects of the pro-inflammatory cytokine interleukin 1-beta: studies with the concurrent fixed ratio 5/ chow feeding choice task. Psychopharmacology (Berl.) 2014;231(4):727–736. doi: 10.1007/s00213-013-3285-4. [http://dx.doi.org/10.1007/s00213-013-3285-4]. [PMID: 24136220]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Haroon E., Felger J.C., Woolwine B.J., Chen X., Parekh S., Spivey J.R., Hu X.P., Miller A.H. Age-related increases in basal ganglia glutamate are associated with TNF, reduced motivation and decreased psychomotor speed during IFN-alpha treatment: Preliminary findings. Brain Behav. Immun. 2015;46:17–22. doi: 10.1016/j.bbi.2014.12.004. [http://dx. doi.org/10.1016/j.bbi.2014.12.004]. [PMID: 25500218]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Haroon E., Woolwine B.J., Chen X., Pace T.W., Parekh S., Spivey J.R., Hu X.P., Miller A.H. IFN-alpha-induced cortical and subcortical glutamate changes assessed by magnetic resonance spectroscopy. Neuropsychopharmacology. 2014;39(7):1777–1785. doi: 10.1038/npp.2014.25. [http://dx.doi.org/10.1038/npp.2014.25]. [PMID: 24481242]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kumai T., Tateishi T., Tanaka M., Watanabe M., Shimizu H., Kobayashi S. Effect of interferon-alpha on tyrosine hydroxylase and catecholamine levels in the brain of rats. Life Sci. 2000;67(6):663–669. doi: 10.1016/s0024-3205(00)00660-3. [http://dx.doi.org/10.1016/S0024-3205(00)00660-3]. [PMID: 12659172]. [DOI] [PubMed] [Google Scholar]
- 139.Kamata M., Higuchi H., Yoshimoto M., Yoshida K., Shimizu T. Effect of single intracerebroventricular injection of alpha-interferon on monoamine concentrations in the rat brain. Eur. Neuropsychopharmacol. 2000;10(2):129–132. doi: 10.1016/s0924-977x(99)00067-x. [http://dx.doi.org/10.1016/ S0924-977X(99)00067-X]. [PMID: 10706995]. [DOI] [PubMed] [Google Scholar]
- 140.Sato T., Suzuki E., Yokoyama M., Semba J., Watanabe S., Miyaoka H. Chronic intraperitoneal injection of interferon-alpha reduces serotonin levels in various regions of rat brain, but does not change levels of serotonin transporter mRNA, nitrite or nitrate. Psychiatry Clin. Neurosci. 2006;60(4):499–506. doi: 10.1111/j.1440-1819.2006.01538.x. [http://dx.doi. org/10.1111/j.1440-1819.2006.01538.x]. [PMID: 16884454]. [DOI] [PubMed] [Google Scholar]
- 141.Shuto H., Kataoka Y., Horikawa T., Fujihara N., Oishi R. Repeated interferon-alpha administration inhibits dopaminergic neural activity in the mouse brain. Brain Res. 1997;747(2):348–351. doi: 10.1016/s0006-8993(96)01371-6. [http://dx.doi.org/10.1016/S0006-8993(96)01371-6]. [PMID: 9046014]. [DOI] [PubMed] [Google Scholar]
- 142.Loftis J.M., Hauser P., Macey T.A., Lowe J.D. Can rodents be used to model interferon-alpha-induced depressive symptoms? Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30(7):1364–1365. doi: 10.1016/j.pnpbp.2006.04.004. [http://dx.doi.org/10.1016/j.pnpbp.2006.04.004]. [PMID: 16682106]. [DOI] [PubMed] [Google Scholar]
- 143.Wang J., Campbell I.L., Zhang H. Systemic interferon-alpha regulates interferon-stimulated genes in the central nervous system. Mol. Psychiatry. 2008;13(3):293–301. doi: 10.1038/sj.mp.4002013. [http://dx.doi.org/10.1038/ sj.mp.4002013]. [PMID: 17486106]. [DOI] [PubMed] [Google Scholar]
- 144.Loftis J.M., Wall J.M., Pagel R.L., Hauser P. Administration of pegylated interferon-alpha-2a or -2b does not induce sickness behavior in Lewis rats. Psychoneuroendocrinology. 2006;31(10):1289–1294. doi: 10.1016/j.psyneuen.2006.07.006. [http://dx.doi.org/10.1016/j.psyneuen.2006.07.006]. [PMID: 17049181]. [DOI] [PubMed] [Google Scholar]
- 145.Blalock J.E., Smith E.M. Human leukocyte interferon (HuIFN-alpha): potent endorphin-like opioid activity. Biochem. Biophys. Res. Commun. 1981;101(2):472–478. doi: 10.1016/0006-291x(81)91284-5. [http://dx.doi.org/10.1016/ 0006-291X(81)91284-5]. [PMID: 6171272]. [DOI] [PubMed] [Google Scholar]
- 146.Ho B.T., Huo Y.Y., Lu J.G., Tansey L.W., Levin V.A. Opioid-dopaminergic mechanisms in the potentiation of d-amphetamine discrimination by interferon-alpha. Pharmacol. Biochem. Behav. 1992;42(1):57–60. doi: 10.1016/0091-3057(92)90446-m. [http://dx.doi.org/10.1016/0091-3057(92)90446-M]. [PMID: 1528947]. [DOI] [PubMed] [Google Scholar]
- 147.Wang J.Y., Zeng X.Y., Fan G.X., Yuan Y.K., Tang J.S. mu- but not delta- and kappa-opioid receptor mediates the nucleus submedius interferon-alpha-evoked antinociception in the rat. Neurosci. Lett. 2006;397(3):254–258. doi: 10.1016/j.neulet.2005.12.046. [http://dx.doi.org/10.1016/j.neulet. 2005.12.046]. [PMID: 16406668]. [DOI] [PubMed] [Google Scholar]
- 148.Kosel M., Bilkei-Gorzo A., Zawatzky R., Zimmer A., Schlaepfer T.E. Pegylated human interferon alpha 2a does not induce depression-associated changes in mice. Psychiatry Res. 2011;185(1-2):243–247. doi: 10.1016/j.psychres.2009.10.012. [http://dx.doi.org/10.1016/j.psychres.2009.10. 012]. [PMID: 20580843]. [DOI] [PubMed] [Google Scholar]
- 149.Orsal A.S., Blois S.M., Bermpohl D., Schaefer M., Coquery N. Administration of interferon-alpha in mice provokes peripheral and central modulation of immune cells, accompanied by behavioral effects. Neuropsychobiology. 2008;58(3-4):211–222. doi: 10.1159/000201718. [http://dx.doi. org/10.1159/000201718]. [PMID: 19212136]. [DOI] [PubMed] [Google Scholar]
- 150.Fahey B., Hickey B., Kelleher D., O’Dwyer A.M., O’Mara S.M. The widely-used anti-viral drug interferon-alpha induces depressive- and anxiogenic-like effects in healthy rats. Behav. Brain Res. 2007;182(1):80–87. doi: 10.1016/j.bbr.2007.05.005. [http://dx.doi.org/10.1016/j.bbr.2007. 05.005]. [PMID: 17588681]. [DOI] [PubMed] [Google Scholar]
- 151.Zheng L.S., Hitoshi S., Kaneko N., Takao K., Miyakawa T., Tanaka Y., Xia H., Kalinke U., Kudo K., Kanba S., Ikenaka K., Sawamoto K. Mechanisms for interferon-α-induced depression and neural stem cell dysfunction. Stem Cell Reports. 2014;3(1):73–84. doi: 10.1016/j.stemcr.2014.05.015. [http://dx.doi.org/10.1016/j.stemcr.2014.05.015]. [PMID: 25068123]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Guo J., Zhang W., Zhang L., Ding H., Zhang J., Song C., Zhang Y., Xia N., Li M., Liang Y., Hu X., Luan H., Wang H. Probable involvement of p11 with interferon alpha induced depression. Sci. Rep. 2016;6:17029. doi: 10.1038/srep17029. [http://dx.doi.org/10.1038/srep17029]. [PMID: 26821757]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Makino M., Kitano Y., Komiyama C., Hirohashi M., Kohno M., Moriyama M., Takasuna K. Human interferon-alpha induces immobility in the mouse forced swimming test: involvement of the opioid system. Brain Res. 2000;852(2):482–484. doi: 10.1016/s0006-8993(99)02235-0. [http://dx.doi. org/10.1016/S0006-8993(99)02235-0]. [PMID: 10678778]. [DOI] [PubMed] [Google Scholar]
- 154.Makino M., Kitano Y., Komiyama C., Takasuna K. Human interferon-alpha increases immobility in the forced swimming test in rats. Psychopharmacology (Berl.) 2000;148(1):106–110. doi: 10.1007/s002130050031. [http://dx.doi.org/10.1007/s002130050031]. [PMID: 10663424]. [DOI] [PubMed] [Google Scholar]
- 155.Felger J.C., Alagbe O., Hu F., Mook D., Freeman A.A., Sanchez M.M., Kalin N.H., Ratti E., Nemeroff C.B., Miller A.H. Effects of interferon-alpha on rhesus monkeys: a nonhuman primate model of cytokine-induced depression. Biol. Psychiatry. 2007;62(11):1324–1333. doi: 10.1016/j.biopsych.2007.05.026. [http://dx.doi.org/10.1016/j.biopsych. 2007.05.026]. [PMID: 17678633]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rosenzweig-Lipson S., Hesterberg P., Bergman J. Observational studies of dopamine D1 and D2 agonists in squirrel monkeys. Psychopharmacology (Berl.) 1994;116(1):9–18. doi: 10.1007/BF02244865. [http://dx.doi.org/ 10.1007/BF02244865]. [PMID: 7862937]. [DOI] [PubMed] [Google Scholar]
- 157.McKinney W.T., Jr, Eising R.G., Moran E.C., Suomi S.J., Harlow H.F. Effects of reserpine on the social behavior of rhesus monkeys. Dis. Nerv. Syst. 1971;32(11):735–741. [PMID: 5002259]. [PubMed] [Google Scholar]
- 158.Yohn S.E., Lopez-Cruz L., Hutson P.H., Correa M., Salamone J.D. Effects of lisdexamfetamine and s-citalopram, alone and in combination, on effort-related choice behavior in the rat. Psychopharmacology (Berl.) 2016;233(6):949–960. doi: 10.1007/s00213-015-4176-7. [http://dx.doi.org/ 10.1007/s00213-015-4176-7]. [PMID: 26694811]. [DOI] [PubMed] [Google Scholar]
- 159.Bonsall D.R., Kim H., Tocci C., Ndiaye A., Petronzio A., McKay-Corkum G., Molyneux P.C., Scammell T.E., Harrington M.E. Suppression of locomotor activity in female C57Bl/6J mice treated with interleukin-1β: Investigating a method for the study of fatigue in laboratory animals. PLoS One. 2015;10(10):e0140678. doi: 10.1371/journal.pone.0140678. [http://dx.doi.org/10.1371/journal.pone.0140678]. [PMID: 26469939]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vichaya E.G., Hunt S.C., Dantzer R. Lipopolysaccharide reduces incentive motivation while boosting preference for high reward in mice. Neuropsychopharmacology. 2014;39(12):2884–2890. doi: 10.1038/npp.2014.141. [http://dx.doi.org/10.1038/npp.2014.141]. [PMID: 24917202]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Felger J.C., Hernandez C.R., Miller A.H. Levodopa reverses cytokine-induced reductions in striatal dopamine release. Int. J. Neuropsychopharmacol. 2015;18(4):pyu084. doi: 10.1093/ijnp/pyu084. [http://dx.doi.org/ 10.1093/ijnp/pyu084]. [PMID: 25638816]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Qin L., Wu X., Block M.L., Liu Y., Breese G.R., Hong J.S., Knapp D.J., Crews F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55(5):453–462. doi: 10.1002/glia.20467. [http://dx.doi.org/10.1002/glia.20467]. [PMID: 17203472]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Reinert K.R., Umphlet C.D., Quattlebaum A., Boger H.A. Short-term effects of an endotoxin on substantia nigra dopamine neurons. Brain Res. 2014;1557:164–170. doi: 10.1016/j.brainres.2014.02.005. [http://dx.doi.org/10.1016/j. brainres.2014.02.005]. [PMID: 24513404]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yeh K.Y., Shou S.S., Lin Y.X., Chen C.C., Chiang C.Y., Yeh C.Y. Effect of Ginkgo biloba extract on lipopolysaccharide-induced anhedonic depressive-like behavior in male rats. Phytother. Res. 2015;29(2):260–266. doi: 10.1002/ptr.5247. [http://dx.doi.org/10.1002/ptr.5247]. [PMID: 25346240]. [DOI] [PubMed] [Google Scholar]
- 165.van Heesch F., Prins J., Konsman J.P., Korte-Bouws G.A., Westphal K.G., Rybka J., Olivier B., Kraneveld A.D., Korte S.M. Lipopolysaccharide increases degradation of central monoamines: an in vivo microdialysis study in the nucleus accumbens and medial prefrontal cortex of mice. Eur. J. Pharmacol. 2014;725:55–63. doi: 10.1016/j.ejphar.2014.01.014. [http://dx.doi.org/10.1016/j.ejphar.2014.01.014]. [PMID: 24444442]. [DOI] [PubMed] [Google Scholar]
- 166.van Heesch F., Prins J., Konsman J.P., Westphal K.G., Olivier B., Kraneveld A.D., Korte S.M. Lipopolysaccharide-induced anhedonia is abolished in male serotonin transporter knockout rats: an intracranial self-stimulation study. Brain Behav. Immun. 2013;29:98–103. doi: 10.1016/j.bbi.2012.12.013. [http://dx.doi.org/10.1016/j.bbi.2012.12.013]. [PMID: 23274951]. [DOI] [PubMed] [Google Scholar]
- 167.O’Connor J.C., Lawson M.A., André C., Moreau M., Lestage J., Castanon N., Kelley K.W., Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry. 2009;14(5):511–522. doi: 10.1038/sj.mp.4002148. [http://dx.doi.org/10.1038/sj.mp.4002148]. [PMID: 18195714]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Frenois F., Moreau M., O’Connor J., Lawson M., Micon C., Lestage J., Kelley K.W., Dantzer R., Castanon N. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32(5):516–531. doi: 10.1016/j.psyneuen.2007.03.005. [http://dx.doi.org/10.1016/ j.psyneuen.2007.03.005]. [PMID: 17482371]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dinarello C.A. Infection, fever, and exogenous and endogenous pyrogens: some concepts have changed. J. Endotoxin Res. 2004;10(4):201–222. doi: 10.1179/096805104225006129. [PMID: 15373964]. [DOI] [PubMed] [Google Scholar]
- 170.Barrot M., Marinelli M., Abrous D.N., Rougé-Pont F., Le Moal M., Piazza P.V. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur. J. Neurosci. 2000;12(3):973–979. doi: 10.1046/j.1460-9568.2000.00996.x. [http://dx.doi.org/10.1046/j.1460-9568.2000.00996.x]. [PMID: 10762327]. [DOI] [PubMed] [Google Scholar]
- 171.Lavicky J., Dunn A.J. Corticotropin-releasing factor stimulates catecholamine release in hypothalamus and prefrontal cortex in freely moving rats as assessed by microdialysis. J. Neurochem. 1993;60(2):602–612. doi: 10.1111/j.1471-4159.1993.tb03191.x. [http://dx.doi.org/10.1111/j.1471-4159.1993. tb03191.x]. [PMID: 7678287]. [DOI] [PubMed] [Google Scholar]
- 172.Mekaouche M., Siaud P., Givalois L., Barbanel G., Malaval F., Maurel D., Assenmacher I., Ixart G. Different responses of plasma ACTH and corticosterone and of plasma interleukin-1 beta to single and recurrent endotoxin challenges. J. Leukoc. Biol. 1996;59(3):341–346. doi: 10.1002/jlb.59.3.341. [http://dx.doi.org/10.1002/jlb.59.3.341]. [PMID: 8604011]. [DOI] [PubMed] [Google Scholar]
- 173.Matsuzaki I., Takamatsu Y., Moroji T. The effects of intracerebroventricularly injected corticotropin-releasing factor (CRF) on the central nervous system: behavioural and biochemical studies. Neuropeptides. 1989;13(3):147–155. doi: 10.1016/0143-4179(89)90085-1. [http://dx.doi.org/10. 1016/0143-4179(89)90085-1]. [PMID: 2785246]. [DOI] [PubMed] [Google Scholar]
- 174.Juengling F.D., Ebert D., Gut O., Engelbrecht M.A., Rasenack J., Nitzsche E.U., Bauer J., Lieb K. Prefrontal cortical hypometabolism during low-dose interferon alpha treatment. Psychopharmacology (Berl.) 2000;152(4):383–389. doi: 10.1007/s002130000549. [http://dx.doi.org/ 10.1007/s002130000549]. [PMID: 11140330]. [DOI] [PubMed] [Google Scholar]
- 175.Spetsieris P.G., Moeller J.R., Dhawan V., Ishikawa T., Eidelberg D. Visualizing the evolution of abnormal metabolic networks in the brain using PET. Comput. Med. Imaging Graph. 1995;19(3):295–306. doi: 10.1016/0895-6111(95)00011-e. [http://dx.doi.org/10.1016/0895-6111(95)00011-E]. [PMID: 7641174]. [DOI] [PubMed] [Google Scholar]
- 176.Eidelberg D., Moeller J.R., Dhawan V., Spetsieris P., Takikawa S., Ishikawa T., Chaly T., Robeson W., Margouleff D., Przedborski S. The metabolic topography of parkinsonism. J. Cereb. Blood Flow Metab. 1994;14(5):783–801. doi: 10.1038/jcbfm.1994.99. [http://dx.doi.org/ 10.1038/jcbfm.1994.99]. [PMID: 8063874]. [DOI] [PubMed] [Google Scholar]
- 177.Mentis M.J., McIntosh A.R., Perrine K., Dhawan V., Berlin B., Feigin A., Edwards C., Mattis P., Eidelberg D. Relationships among the metabolic patterns that correlate with mnemonic, visuospatial, and mood symptoms in Parkinson’s disease. Am. J. Psychiatry. 2002;159(5):746–754. doi: 10.1176/appi.ajp.159.5.746. [http://dx.doi.org/10.1176/appi.ajp. 159.5.746]. [PMID: 11986127]. [DOI] [PubMed] [Google Scholar]
- 178.Wichmann T., DeLong M.R. Oscillations in the basal ganglia. Nature. 1999;400(6745):621–622. doi: 10.1038/23148. [http://dx.doi.org/10.1038/ 23148]. [PMID: 10458157]. [DOI] [PubMed] [Google Scholar]
- 179.Wichmann T., DeLong M.R. Functional neuroanatomy of the basal ganglia in Parkinson’s disease. Adv. Neurol. 2003;91:9–18. [PMID: 12442660]. [PubMed] [Google Scholar]
- 180.Hasler G., Fromm S., Carlson P.J., Luckenbaugh D.A., Waldeck T., Geraci M., Roiser J.P., Neumeister A., Meyers N., Charney D.S., Drevets W.C. Neural response to catecholamine depletion in unmedicated subjects with major depressive disorder in remission and healthy subjects. Arch. Gen. Psychiatry. 2008;65(5):521–531. doi: 10.1001/archpsyc.65.5.521. [http://dx.doi.org/10.1001/archpsyc.65.5.521]. [PMID: 18458204]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Reuter J., Raedler T., Rose M., Hand I., Gläscher J., Büchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat. Neurosci. 2005;8(2):147–148. doi: 10.1038/nn1378. [http://dx.doi.org/10.1038/nn1378]. [PMID: 15643429]. [DOI] [PubMed] [Google Scholar]
- 182.Dowell N.G., Cooper E.A., Tibble J., Voon V., Critchley H.D., Cercignani M., Harrison N.A. Acute changes in striatal microstructure predict the development of interferon-alpha induced fatigue. Biol. Psychiatry. 2016;79(4):320–328. doi: 10.1016/j.biopsych.2015.05.015. [http://dx.doi.org/10. 1016/j.biopsych.2015.05.015]. [PMID: 26169252]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bjork J.M., Grant S.J., Chen G., Hommer D.W. Dietary tyrosine/phenylalanine depletion effects on behavioral and brain signatures of human motivational processing. Neuropsychopharmacology. 2014;39(3):595–604. doi: 10.1038/npp.2013.232. [http://dx.doi.org/10.1038/npp.2013. 232]. [PMID: 23995581]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Treadway M.T. Association between interleukin-6 and striatal prediction-error signals following acute stress in healthy females. Biol. Psychiatry. doi: 10.1016/j.biopsych.2017.02.1183. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pessiglione M., Seymour B., Flandin G., Dolan R.J., Frith C.D. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442(7106):1042–1045. doi: 10.1038/nature05051. [http://dx.doi.org/10.1038/nature05051]. [PMID: 16929307]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Harrison N.A., Cercignani M., Voon V., Critchley H.D. Effects of inflammation on hippocampus and substantia nigra responses to novelty in healthy human participants. Neuropsychopharmacology. 2015;40(4):831–838. doi: 10.1038/npp.2014.222. [http://dx.doi.org/10.1038/npp.2014.222]. [PMID: 25154706]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Inagaki T.K., Muscatell K.A., Irwin M.R., Moieni M., Dutcher J.M., Jevtic I., Breen E.C., Eisenberger N.I. The role of the ventral striatum in inflammatory-induced approach toward support figures. Brain Behav. Immun. 2015;44:247–252. doi: 10.1016/j.bbi.2014.10.006. [http://dx.doi. org/10.1016/j.bbi.2014.10.006]. [PMID: 25459101]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Muscatell K.A., Moieni M., Inagaki T.K., Dutcher J.M., Jevtic I., Breen E.C., Irwin M.R., Eisenberger N.I. Exposure to an inflammatory challenge enhances neural sensitivity to negative and positive social feedback. Brain Behav. Immun. 2016;57:21–29. doi: 10.1016/j.bbi.2016.03.022. [http://dx.doi.org/10.1016/j.bbi.2016.03.022]. [PMID: 27032568]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kullmann J.S., Grigoleit J.S., Lichte P., Kobbe P., Rosenberger C., Banner C., Wolf O.T., Engler H., Oberbeck R., Elsenbruch S., Bingel U., Forsting M., Gizewski E.R., Schedlowski M. Neural response to emotional stimuli during experimental human endotoxemia. Hum. Brain Mapp. 2013;34(9):2217–2227. doi: 10.1002/hbm.22063. [http://dx. doi.org/10.1002/hbm.22063]. [PMID: 22461242]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kaasinen V., Nurmi E., Brück A., Eskola O., Bergman J., Solin O., Rinne J.O. Increased frontal [(18)F]fluorodopa uptake in early Parkinson’s disease: sex differences in the prefrontal cortex. Brain. 2001;124(Pt 6):1125–1130. doi: 10.1093/brain/124.6.1125. [http://dx.doi.org/10.1093/brain/124.6. 1125]. [PMID: 11353728]. [DOI] [PubMed] [Google Scholar]
- 191.Kumakura Y., Cumming P. PET studies of cerebral levoaaaaaaaaadopa metabolism: a review of clinical findings and modeling approaches. Neuroscientist. 2009;15(6):635–650. doi: 10.1177/1073858409338217. [http://dx.doi.org/10.1177/ 1073858409338217]. [PMID: 19793723]. [DOI] [PubMed] [Google Scholar]
- 192.Leenders K.L., Palmer A.J., Quinn N., Clark J.C., Firnau G., Garnett E.S., Nahmias C., Jones T., Marsden C.D. Brain dopamine metabolism in patients with Parkinson’s disease measured with positron emission tomography. J. Neurol. Neurosurg. Psychiatry. 1986;49(8):853–860. doi: 10.1136/jnnp.49.8.853. [http://dx.doi.org/10.1136/jnnp.49. 8.853]. [PMID: 3091770]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kumakura Y., Gjedde A., Danielsen E.H., Christensen S., Cumming P. Dopamine storage capacity in caudate and putamen of patients with early Parkinson’s disease: correlation with asymmetry of motor symptoms. J. Cereb. Blood Flow Metab. 2006;26(3):358–370. doi: 10.1038/sj.jcbfm.9600202. [http://dx.doi.org/10.1038/sj.jcbfm.9600202]. [PMID: 16079784]. [DOI] [PubMed] [Google Scholar]
- 194.Song C., Merali Z., Anisman H. Variations of nucleus accumbens dopamine and serotonin following systemic interleukin-1, interleukin-2 or interleukin-6 treatment. Neuroscience. 1999;88(3):823–836. doi: 10.1016/s0306-4522(98)00271-1. [http://dx.doi.org/10.1016/S0306-4522(98)00271-1]. [PMID: 10363820]. [DOI] [PubMed] [Google Scholar]
- 195.De La Garza R.I.I., Asnis G.M. The non-steroidal anti-inflammatory drug diclofenac sodium attenuates IFN-alpha induced alterations to monoamine turnover in prefrontal cortex and hippocampus. Brain Res. 2003;977(1):70–79. doi: 10.1016/s0006-8993(03)02757-4. [http://dx.doi.org/10. 1016/S0006-8993(03)02757-4]. [PMID: 12788515]. [DOI] [PubMed] [Google Scholar]
- 196.Dunn A.J. Systemic interleukin-1 administration stimulates hypothalamic norepinephrine metabolism parallelling the increased plasma corticosterone. Life Sci. 1988;43(5):429–435. doi: 10.1016/0024-3205(88)90522-x. [http://dx. doi.org/10.1016/0024-3205(88)90522-X]. [PMID: 3260987]. [DOI] [PubMed] [Google Scholar]
- 197.Petrulli J.R., Kalish B., Nabulsi N.B., Huang Y., Hannestad J., Morris E.D. Systemic inflammation enhances stimulant-induced striatal dopamine elevation. Transl. Psychiatry. 2017;7(3):e1076. doi: 10.1038/tp.2017.18. [http://dx.doi.org/10.1038/tp.2017.18]. [PMID: 28350401]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Miller A.H., Jones J.F., Drake D.F., Tian H., Unger E.R., Pagnoni G. Decreased basal ganglia activation in subjects with chronic fatigue syndrome: association with symptoms of fatigue. PLoS One. 2014;9(5):e98156. doi: 10.1371/journal.pone.0098156. [http://dx.doi.org/10.1371/journal.pone. 0098156]. [PMID: 24858857]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Haber S.N., Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [http://dx.doi.org/10.1038/npp.2009.129]. [PMID: 19812543]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Samanez-Larkin G.R., Knutson B. Decision making in the ageing brain: changes in affective and motivational circuits. Nat. Rev. Neurosci. 2015;16(5):278–289. doi: 10.1038/nrn3917. [http://dx.doi.org/10.1038/nrn3917]. [PMID: 25873038]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Russo S.J., Nestler E.J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013;14(9):609–625. doi: 10.1038/nrn3381. [http://dx.doi.org/ 10.1038/nrn3381]. [PMID: 23942470]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Diekhof E.K., Kaps L., Falkai P., Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude - an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia. 2012;50(7):1252–1266. doi: 10.1016/j.neuropsychologia.2012.02.007. [http://dx.doi.org/10.1016/j.neuropsychologia.2012.02.007]. [PMID: 22366111]. [DOI] [PubMed] [Google Scholar]
- 203.Haroon E., Fleischer C.C., Felger J.C., Chen X., Woolwine B.J., Patel T., Hu X.P., Miller A.H. Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol. Psychiatry. 2016;21(10):1351–1357. doi: 10.1038/mp.2015.206. [http://dx.doi.org/10.1038/mp.2015.206]. [PMID: 26754953]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dantzer R., O’Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [http://dx.doi.org/10.1038/nrn2297]. [PMID: 18073775]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang Z., Young M.R. PTSD, a disorder with an immunological component. Front. Immunol. 2016;7:219. doi: 10.3389/fimmu.2016.00219. [http://dx.doi.org/ 10.3389/fimmu.2016.00219]. [PMID: 27375619]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Shin L.M., Rauch S.L., Pitman R.K. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann. N. Y. Acad. Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [http://dx.doi.org/10.1196/annals.1364.007]. [PMID: 16891563]. [DOI] [PubMed] [Google Scholar]
- 207.Harrison N.A., Brydon L., Walker C., Gray M.A., Steptoe A., Critchley H.D. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol. Psychiatry. 2009;66(5):407–414. doi: 10.1016/j.biopsych.2009.03.015. [http://dx.doi.org/10.1016/j. biopsych.2009.03.015]. [PMID: 19423079]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Inagaki T.K., Muscatell K.A., Irwin M.R., Cole S.W., Eisenberger N.I. Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage. 2012;59(4):3222–3226. doi: 10.1016/j.neuroimage.2011.10.090. [http://dx.doi.org/10.1016/j.neuroimage.2011.10.090]. [PMID: 22079507]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Muscatell K.A. Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav. Immun. 2014 doi: 10.1016/j.bbi.2014.06.201. [PMID: 25016200]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Harrison N.A., Brydon L., Walker C., Gray M.A., Steptoe A., Dolan R.J., Critchley H.D. Neural origins of human sickness in interoceptive responses to inflammation. Biol. Psychiatry. 2009;66(5):415–422. doi: 10.1016/j.biopsych.2009.03.007. [http://dx.doi.org/10.1016/j.biopsych.2009.03.007]. [PMID: 19409533]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Koenigs M., Grafman J. Posttraumatic stress disorder: the role of medial prefrontal cortex and amygdala. Neuroscientist. 2009;15(5):540–548. doi: 10.1177/1073858409333072. [http://dx.doi.org/10.1177/1073858409333072]. [PMID: 19359671]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.O’Connor M.F., Irwin M.R., Wellisch D.K. When grief heats up: pro-inflammatory cytokines predict regional brain activation. Neuroimage. 2009;47(3):891–896. doi: 10.1016/j.neuroimage.2009.05.049. [http://dx.doi.org/10.1016/j. neuroimage.2009.05.049]. [PMID: 19481155]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Simmons A., Strigo I.A., Matthews S.C., Paulus M.P., Stein M.B. Initial evidence of a failure to activate right anterior insula during affective set shifting in posttraumatic stress disorder. Psychosom. Med. 2009;71(4):373–377. doi: 10.1097/PSY.0b013e3181a56ed8. [http://dx.doi.org/10.1097/ PSY.0b013e3181a56ed8]. [PMID: 19398499]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Paulus M.P., Stein M.B. An insular view of anxiety. Biol. Psychiatry. 2006;60(4):383–387. doi: 10.1016/j.biopsych.2006.03.042. [http://dx.doi.org/10.1016/j.biopsych. 2006.03.042]. [PMID: 16780813]. [DOI] [PubMed] [Google Scholar]
- 215.Fonzo G.A., Simmons A.N., Thorp S.R., Norman S.B., Paulus M.P., Stein M.B. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol. Psychiatry. 2010;68(5):433–441. doi: 10.1016/j.biopsych.2010.04.028. [http:// dx.doi.org/10.1016/j.biopsych.2010.04.028]. [PMID: 20573339]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Eisenberger N.I., Inagaki T.K., Rameson L.T., Mashal N.M., Irwin M.R. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage. 2009;47(3):881–890. doi: 10.1016/j.neuroimage.2009.04.040. [http://dx.doi.org/10.1016/j.neuroimage.2009.04. 040]. [PMID: 19376240]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hannestad J., Subramanyam K., Dellagioia N., Planeta-Wilson B., Weinzimmer D., Pittman B., Carson R.E. Glucose metabolism in the insula and cingulate is affected by systemic inflammation in humans. J. Nucl. Med. 2012;53(4):601–607. doi: 10.2967/jnumed.111.097014. [http://dx. doi.org/10.2967/jnumed.111.097014]. [PMID: 22414635]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Labrenz F., Wrede K., Forsting M., Engler H., Schedlowski M., Elsenbruch S., Benson S. Alterations in functional connectivity of resting state networks during experimental endotoxemia - An exploratory study in healthy men. Brain Behav. Immun. 2016;54:17–26. doi: 10.1016/j.bbi.2015.11.010. [http://dx.doi.org/10.1016/j.bbi.2015.11.010]. [PMID: 26597151]. [DOI] [PubMed] [Google Scholar]
- 219.Carter C.S., Braver T.S., Barch D.M., Botvinick M.M., Noll D., Cohen J.D. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [http://dx.doi.org/10.1126/science.280.5364.747]. [PMID: 9563953]. [DOI] [PubMed] [Google Scholar]
- 220.Eisenberger N.I., Lieberman M.D. 2004. [Google Scholar]
- 221.Critchley H.D., Tang J., Glaser D., Butterworth B., Dolan R.J. Anterior cingulate activity during error and autonomic response. Neuroimage. 2005;27(4):885–895. doi: 10.1016/j.neuroimage.2005.05.047. [http://dx.doi.org/10.1016/j. neuroimage.2005.05.047]. [PMID: 15996878]. [DOI] [PubMed] [Google Scholar]
- 222.Hamner M.B., Lorberbaum J.P., George M.S. Potential role of the anterior cingulate cortex in PTSD: review and hypothesis. Depress. Anxiety. 1999;9(1):1–14. [http://dx.doi.org/10.1002/(SICI) 1520-6394(1999)9:1<1:AID-DA1>3.0.CO;2-4]. [PMID: 9989344]. [PubMed] [Google Scholar]
- 223.Shin L.M., Bush G., Milad M.R., Lasko N.B., Brohawn K.H., Hughes K.C., Macklin M.L., Gold A.L., Karpf R.D., Orr S.P., Rauch S.L., Pitman R.K. Exaggerated activation of dorsal anterior cingulate cortex during cognitive interference: a monozygotic twin study of posttraumatic stress disorder. Am. J. Psychiatry. 2011;168(9):979–985. doi: 10.1176/appi.ajp.2011.09121812. [http://dx.doi.org/10.1176/appi.ajp.2011.09121812]. [PMID: 21724666]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Shin L.M., Lasko N.B., Macklin M.L., Karpf R.D., Milad M.R., Orr S.P., Goetz J.M., Fischman A.J., Rauch S.L., Pitman R.K. Resting metabolic activity in the cingulate cortex and vulnerability to posttraumatic stress disorder. Arch. Gen. Psychiatry. 2009;66(10):1099–1107. doi: 10.1001/archgenpsychiatry.2009.138. [http://dx.doi.org/10.1001/archgenpsychiatry. 2009.138]. [PMID: 19805700]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Eisenberger N.I., Lieberman M.D., Satpute A.B. Personality from a controlled processing perspective: an fMRI study of neuroticism, extraversion, and self-consciousness. Cogn. Affect. Behav. Neurosci. 2005;5(2):169–181. doi: 10.3758/cabn.5.2.169. [http://dx.doi.org/10.3758/CABN.5.2.169]. [PMID: 16180623]. [DOI] [PubMed] [Google Scholar]
- 226.Ursu S., Stenger V.A., Shear M.K., Jones M.R., Carter C.S. Overactive action monitoring in obsessive-compulsive disorder: evidence from functional magnetic resonance imaging. Psychol. Sci. 2003;14(4):347–353. doi: 10.1111/1467-9280.24411. [http://dx.doi.org/10.1111/1467-9280. 24411]. [PMID: 12807408]. [DOI] [PubMed] [Google Scholar]
- 227.Paulus M.P., Feinstein J.S., Simmons A., Stein M.B. Anterior cingulate activation in high trait anxious subjects is related to altered error processing during decision making. Biol. Psychiatry. 2004;55(12):1179–1187. doi: 10.1016/j.biopsych.2004.02.023. [http://dx.doi.org/10.1016/j.biopsych. 2004.02.023]. [PMID: 15184037]. [DOI] [PubMed] [Google Scholar]
- 228.Capuron L., Pagnoni G., Demetrashvili M., Woolwine B.J., Nemeroff C.B., Berns G.S., Miller A.H. Anterior cingulate activation and error processing during interferon-alpha treatment. Biol. Psychiatry. 2005;58(3):190–196. doi: 10.1016/j.biopsych.2005.03.033. [http://dx.doi.org/10.1016/j. biopsych.2005.03.033]. [PMID: 16084839]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gill J.M., Szanton S.L., Page G.G. Biological underpinnings of health alterations in women with PTSD: a sex disparity. Biol. Res. Nurs. 2005;7(1):44–54. doi: 10.1177/1099800405276709. [http://dx.doi.org/10.1177/ 1099800405276709]. [PMID: 15920002]. [DOI] [PubMed] [Google Scholar]
- 230.Neylan T.C., Sun B., Rempel H., Ross J., Lenoci M., O’Donovan A., Pulliam L. Suppressed monocyte gene expression profile in men versus women with PTSD. Brain Behav. Immun. 2011;25(3):524–531. doi: 10.1016/j.bbi.2010.12.001. [http://dx.doi.org/10.1016/j.bbi.2010.12.001]. [PMID: 21145962]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Haroon E., Woolwine B.J., Chen X., Pace T.W., Parekh S., Spivey J.R., Hu X.P., Miller A.H. IFN-alpha-induced cortical and subcortical glutamate changes assessed by magnetic resonance spectroscopy. Neuropsychopharmacology. 2014;39(7):1777–1785. doi: 10.1038/npp.2014.25. [http://dx.doi.org/10.1038/npp.2014.25]. [PMID: 24481242]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Taylor M.J., Godlewska B., Near J., Christmas D., Potokar J., Collier J., Klenerman P., Barnes E., Cowen P.J. Effect of interferon-α on cortical glutamate in patients with hepatitis C: a proton magnetic resonance spectroscopy study. Psychol. Med. 2014;44(4):789–795. doi: 10.1017/S0033291713001062. [http://dx.doi.org/10.1017/S0033291713001062]. [PMID: 23659574]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Targum S.D., Fava M. Fatigue as a residual symptom of depression. Innov. Clin. Neurosci. 2011;8(10):40–43. [PMID: 22132370]. [PMC free article] [PubMed] [Google Scholar]
- 234.Rush A.J. STAR*D: what have we learned? Am. J. Psychiatry. 2007;164(2):201–204. doi: 10.1176/ajp.2007.164.2.201. [http://dx.doi.org/10.1176/ajp.2007.164.2. 201]. [PMID: 17267779]. [DOI] [PubMed] [Google Scholar]
- 235.Dunlop B.W., Nemeroff C.B. The role of dopamine in the pathophysiology of depression. Arch. Gen. Psychiatry. 2007;64(3):327–337. doi: 10.1001/archpsyc.64.3.327. [http://dx.doi.org/10.1001/archpsyc.64.3.327]. [PMID: 17339521]. [DOI] [PubMed] [Google Scholar]
- 236.Trivedi M.H., Hollander E., Nutt D., Blier P. Clinical evidence and potential neurobiological underpinnings of unresolved symptoms of depression. J. Clin. Psychiatry. 2008;69(2):246–258. doi: 10.4088/jcp.v69n0211. [http://dx.doi.org/10.4088/JCP.v69n0211]. [PMID: 18363453]. [DOI] [PubMed] [Google Scholar]
- 237.Nierenberg A.A. Residual symptoms in depression: prevalence and impact. J. Clin. Psychiatry. 2015;76(11):e1480. doi: 10.4088/JCP.13097TX1C. [http://dx.doi. org/10.4088/JCP.13097TX1C]. [PMID: 26646047]. [DOI] [PubMed] [Google Scholar]
- 238.Capuron L., Miller A.H. Cytokines and psychopathology: lessons from interferon-alpha. Biol. Psychiatry. 2004;56(11):819–824. doi: 10.1016/j.biopsych.2004.02.009. [http:// dx.doi.org/10.1016/j.biopsych.2004.02.009]. [PMID: 15576057]. [DOI] [PubMed] [Google Scholar]
- 239.Miller A.H., Raison C.L. Are anti-inflammatory therapies viable treatments for psychiatric disorders?: Where the rubber meets the road. JAMA Psychiatry. 2015;72(6):527–528. doi: 10.1001/jamapsychiatry.2015.22. [http://dx.doi.org/ 10.1001/jamapsychiatry.2015.22]. [PMID: 25853989]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rosenblat J.D., Kakar R., Berk M., Kessing L.V., Vinberg M., Baune B.T., Mansur R.B., Brietzke E., Goldstein B.I., McIntyre R.S. Anti-inflammatory agents in the treatment of bipolar depression: a systematic review and meta-analysis. Bipolar Disord. 2016;18(2):89–101. doi: 10.1111/bdi.12373. [http://dx.doi.org/10.1111/bdi.12373]. [PMID: 26990051]. [DOI] [PubMed] [Google Scholar]
- 241.Sommer I.E. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J. Clin. Psychol. 2011;73(4):478–419. doi: 10.4088/JCP.10r06823. [DOI] [PubMed] [Google Scholar]
- 242.Köhler O., Benros M.E., Nordentoft M., Farkouh M.E., Iyengar R.L., Mors O., Krogh J. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12):1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [http://dx.doi.org/10.1001/ jamapsychiatry.2014.1611]. [PMID: 25322082]. [DOI] [PubMed] [Google Scholar]
- 243.Bower J.E., Irwin M.R. Mind-body therapies and control of inflammatory biology: A descriptive review. Brain Behav. Immun. 2016;51:1–11. doi: 10.1016/j.bbi.2015.06.012. [http://dx.doi.org/10.1016/j.bbi.2015.06.012]. [PMID: 26116436]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Schuch F.B., Vancampfort D., Richards J., Rosenbaum S., Ward P.B., Stubbs B. Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. J. Psychiatr. Res. 2016;77:42–51. doi: 10.1016/j.jpsychires.2016.02.023. [http://dx.doi.org/10.1016/j.jpsychires.2016.02. 023]. [PMID: 26978184]. [DOI] [PubMed] [Google Scholar]
- 245.Fabricatore A.N., Wadden T.A., Higginbotham A.J., Faulconbridge L.F., Nguyen A.M., Heymsfield S.B., Faith M.S. Intentional weight loss and changes in symptoms of depression: a systematic review and meta-analysis. Int. J. Obes. 2011;35(11):1363–1376. doi: 10.1038/ijo.2011.2. [http://dx.doi.org/10.1038/ijo.2011.2]. [PMID: 21343903]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Forsythe L.K., Wallace J.M., Livingstone M.B. Obesity and inflammation: the effects of weight loss. Nutr. Res. Rev. 2008;21(2):117–133. doi: 10.1017/S0954422408138732. [http://dx.doi.org/10.1017/S0954422408138732]. [PMID: 19087366]. [DOI] [PubMed] [Google Scholar]
- 247.Woods J.A., Vieira V.J., Keylock K.T. Exercise, inflammation, and innate immunity. Immunol. Allergy Clin. North Am. 2009;29(2):381–393. doi: 10.1016/j.iac.2009.02.011. [http://dx.doi.org/10.1016/j.iac.2009.02.011]. [PMID: 19389588]. [DOI] [PubMed] [Google Scholar]
- 248.Rapaport M.H. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol. Psychiatry. 2015 doi: 10.1038/mp.2015.22. [PMID: 25802980]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Mocking R.J., Harmsen I., Assies J., Koeter M.W., Ruhé H.G., Schene A.H. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl. Psychiatry. 2016;6:e756. doi: 10.1038/tp.2016.29. [http://dx.doi.org/10.1038/ tp.2016.29]. [PMID: 26978738]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Su K.P., Lai H.C., Yang H.T., Su W.P., Peng C.Y., Chang J.P., Chang H.C., Pariante C.M. Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: results from a randomized, controlled trial. Biol. Psychiatry. 2014;76(7):559–566. doi: 10.1016/j.biopsych.2014.01.008. [http:// dx.doi.org/10.1016/j.biopsych.2014.01.008]. [PMID: 24602409]. [DOI] [PubMed] [Google Scholar]
- 251.Su K.P., Huang S.Y., Peng C.Y., Lai H.C., Huang C.L., Chen Y.C., Aitchison K.J., Pariante C.M. Phospholipase A2 and cyclooxygenase 2 genes influence the risk of interferon-alpha-induced depression by regulating polyunsaturated fatty acids levels. Biol. Psychiatry. 2010;67(6):550–557. doi: 10.1016/j.biopsych.2009.11.005. [http://dx.doi.org/10. 1016/j.biopsych.2009.11.005]. [PMID: 20034614]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.McNamara R.K., Asch R.H., Lindquist D.M., Krikorian R. 2017.
- 253.Gill J.M., Saligan L., Lee H., Rotolo S., Szanton S. Women in recovery from PTSD have similar inflammation and quality of life as non-traumatized controls. J. Psychosom. Res. 2013;74(4):301–306. doi: 10.1016/j.jpsychores.2012.10.013. [http://dx.doi.org/10.1016/j.jpsychores.2012.10.013]. [PMID: 23497831]. [DOI] [PubMed] [Google Scholar]
- 254.Tucker P., Ruwe W.D., Masters B., Parker D.E., Hossain A., Trautman R.P., Wyatt D.B. Neuroimmune and cortisol changes in selective serotonin reuptake inhibitor and placebo treatment of chronic posttraumatic stress disorder. Biol. Psychiatry. 2004;56(2):121–128. doi: 10.1016/j.biopsych.2004.03.009. [http://dx.doi.org/10.1016/j.biopsych.2004.03.009]. [PMID: 15231444]. [DOI] [PubMed] [Google Scholar]
- 255.Pace T.W., Negi L.T., Adame D.D., Cole S.P., Sivilli T.I., Brown T.D., Issa M.J., Raison C.L. Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology. 2009;34(1):87–98. doi: 10.1016/j.psyneuen.2008.08.011. [http://dx.doi.org/10.1016/j.psyneuen.2008.08.011]. [PMID: 18835662]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Loftis J.M., Patterson A.L., Wilhelm C.J., McNett H., Morasco B.J., Huckans M., Morgan T., Saperstein S., Asghar A., Hauser P. Vulnerability to somatic symptoms of depression during interferon-alpha therapy for hepatitis C: a 16-week prospective study. J. Psychosom. Res. 2013;74(1):57–63. doi: 10.1016/j.jpsychores.2012.10.012. [http://dx.doi.org/10.1016/j. jpsychores.2012.10.012]. [PMID: 23272989]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Neurauter G., Schröcksnadel K., Scholl-Bürgi S., Sperner-Unterweger B., Schubert C., Ledochowski M., Fuchs D. Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr. Drug Metab. 2008;9(7):622–627. doi: 10.2174/138920008785821738. [http://dx.doi.org/ 10.2174/138920008785821738]. [PMID: 18781914]. [DOI] [PubMed] [Google Scholar]
- 258.Cunnington C., Channon K.M. Tetrahydrobiopterin: pleiotropic roles in cardiovascular pathophysiology. Heart. 2010;96(23):1872–1877. doi: 10.1136/hrt.2009.180430. [http://dx.doi.org/10.1136/hrt.2009.180430]. [PMID: 20837663]. [DOI] [PubMed] [Google Scholar]
- 259.Xia Y., Tsai A.L., Berka V., Zweier J.L. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J. Biol. Chem. 1998;273(40):25804–25808. doi: 10.1074/jbc.273.40.25804. [http://dx.doi.org/10.1074/ jbc.273.40.25804]. [PMID: 9748253]. [DOI] [PubMed] [Google Scholar]
- 260.Li W., Knowlton D., Woodward W.R., Habecker B.A. Regulation of noradrenergic function by inflammatory cytokines and depolarization. J. Neurochem. 2003;86(3):774–783. doi: 10.1046/j.1471-4159.2003.01890.x. [http://dx.doi. org/10.1046/j.1471-4159.2003.01890.x]. [PMID: 12859689]. [DOI] [PubMed] [Google Scholar]
- 261.Capuron L., Schroecksnadel S., Féart C., Aubert A., Higueret D., Barberger-Gateau P., Layé S., Fuchs D. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol. Psychiatry. 2011;70(2):175–182. doi: 10.1016/j.biopsych.2010.12.006. [http://dx.doi.org/10. 1016/j.biopsych.2010.12.006]. [PMID: 21277567]. [DOI] [PubMed] [Google Scholar]
- 262.Hashimoto R., Nagatsu T., Ohta T., Mizutani M., Omura I. Changes in the concentrations of tetrahydrobiopterin, the cofactor of tyrosine hydroxylase, in blood under physical stress and in depression. Ann. N. Y. Acad. Sci. 2004;1018:378–386. doi: 10.1196/annals.1296.047. [http:// dx.doi.org/10.1196/annals.1296.047]. [PMID: 15240393]. [DOI] [PubMed] [Google Scholar]
- 263.Candito M., Nagatsu T., Chambon P., Chatel M. High-performance liquid chromatographic measurement of cerebrospinal fluid tetrahydrobiopterin, neopterin, homovanillic acid and 5-hydroxindoleacetic acid in neurological diseases. J. Chromatogr. B Biomed. Appl. 1994;657(1):61–66. doi: 10.1016/0378-4347(94)80070-7. [http://dx.doi.org/10.1016/ 0378-4347(94)80070-7]. [PMID: 7524948]. [DOI] [PubMed] [Google Scholar]
- 264.Yokoyama K., Tajima M., Yoshida H., Nakayama M., Tokutome G., Sakagami H., Hosoya T. Plasma pteridine concentrations in patients with chronic renal failure. Nephrol. Dial. Transplant. 2002;17(6):1032–1036. doi: 10.1093/ndt/17.6.1032. [http://dx.doi.org/10.1093/ndt/17.6. 1032]. [PMID: 12032193]. [DOI] [PubMed] [Google Scholar]
- 265.Felger J.C., Li L., Marvar P.J., Woolwine B.J., Harrison D.G., Raison C.L., Miller A.H. Tyrosine metabolism during interferon-alpha administration: association with fatigue and CSF dopamine concentrations. Brain Behav. Immun. 2013;31:153–160. doi: 10.1016/j.bbi.2012.10.010. [http:// dx.doi.org/10.1016/j.bbi.2012.10.010]. [PMID: 23072726]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zoller H., Schloegl A., Schroecksnadel S., Vogel W., Fuchs D. Interferon-alpha therapy in patients with hepatitis C virus infection increases plasma phenylalanine and the phenylalanine to tyrosine ratio. J. Interferon Cytokine Res. 2012;32(5):216–220. doi: 10.1089/jir.2011.0093. [http://dx. doi.org/10.1089/jir.2011.0093]. [PMID: 22191466]. [DOI] [PubMed] [Google Scholar]
- 267.Felger J.C., Hernandez C.R., Miller A.H. Levodopa reverses cytokine-induced reductions in striatal dopamine release. Int. J. Neuropsychopharmacol. 2015;18(4):pyu084. doi: 10.1093/ijnp/pyu084. [http://dx.doi.org/ 10.1093/ijnp/pyu084]. [PMID: 25638816]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Caudle W.M., Richardson J.R., Wang M.Z., Taylor T.N., Guillot T.S., McCormack A.L., Colebrooke R.E., Di Monte D.A., Emson P.C., Miller G.W. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J. Neurosci. 2007;27(30):8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [http://dx.doi.org/10.1523/JNEUROSCI. 0319-07.2007]. [PMID: 17652604]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Douglas T.D., Jinnah H.A., Bernhard D., Singh R.H. The effects of sapropterin on urinary monoamine metabolites in phenylketonuria. Mol. Genet. Metab. 2013;109(3):243–250. doi: 10.1016/j.ymgme.2013.04.017. [http://dx.doi.org/ 10.1016/j.ymgme.2013.04.017]. [PMID: 23712020]. [DOI] [PubMed] [Google Scholar]
- 270.Burton B.K., Bausell H., Katz R., Laduca H., Sullivan C. Sapropterin therapy increases stability of blood phenylalanine levels in patients with BH4-responsive phenylketonuria (PKU). Mol. Genet. Metab. 2010;101(2-3):110–114. doi: 10.1016/j.ymgme.2010.06.015. [http://dx.doi.org/10.1016/j. ymgme.2010.06.015]. [PMID: 20638313]. [DOI] [PubMed] [Google Scholar]
- 271.Trefz F.K., Burton B.K., Longo N., Casanova M.M., Gruskin D.J., Dorenbaum A., Kakkis E.D., Crombez E.A., Grange D.K., Harmatz P., Lipson M.H., Milanowski A., Randolph L.M., Vockley J., Whitley C.B., Wolff J.A., Bebchuk J., Christ-Schmidt H., Hennermann J.B. Efficacy of sapropterin dihydrochloride in increasing phenylalanine tolerance in children with phenylketonuria: a phase III, randomized, double-blind, placebo-controlled study. J. Pediatr. 2009;154(5):700–707. doi: 10.1016/j.jpeds.2008.11.040. [http://dx. doi.org/10.1016/j.jpeds.2008.11.040]. [PMID: 19261295]. [DOI] [PubMed] [Google Scholar]
- 272.Utz J.R., Lorentz C.P., Markowitz D., Rudser K.D., Diethelm-Okita B., Erickson D., Whitley C.B. START, a double blind, placebo-controlled pharmacogenetic test of responsiveness to sapropterin dihydrochloride in phenylketonuria patients. Mol. Genet. Metab. 2012;105(2):193–197. doi: 10.1016/j.ymgme.2011.10.014. [http://dx.doi.org/10.1016/j.ymgme.2011. 10.014]. [PMID: 22112818]. [DOI] [PubMed] [Google Scholar]
- 273.Stahl S.M. Novel therapeutics for depression: L-methylfolate as a trimonoamine modulator and antidepressant-augmenting agent. CNS Spectr. 2007;12(10):739–744. doi: 10.1017/s1092852900015418. [http://dx.doi.org/10.1017/ S1092852900015418]. [PMID: 17934378]. [DOI] [PubMed] [Google Scholar]
- 274.Shintaku H. Disorders of tetrahydrobiopterin metabolism and their treatment. Curr. Drug Metab. 2002;3(2):123–131. doi: 10.2174/1389200024605145. [http://dx.doi. org/10.2174/1389200024605145]. [PMID: 12003346]. [DOI] [PubMed] [Google Scholar]
- 275.Pan L. 2011.
- 276.Sato H., Uematsu M., Endo W., Nakayama T., Kobayashi T., Hino-Fukuyo N., Sakamoto O., Shintaku H., Kure S. Early replacement therapy in a first Japanese case with autosomal recessive guanosine triphosphate cyclohydrolase I deficiency with a novel point mutation. Brain Dev. 2014;36(3):268–271. doi: 10.1016/j.braindev.2013.04.003. [http://dx. doi.org/10.1016/j.braindev.2013.04.003]. [PMID: 23660475]. [DOI] [PubMed] [Google Scholar]
- 277.Papakostas G.I., Mischoulon D., Shyu I., Alpert J.E., Fava M. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double-blind, randomized clinical trial. Am. J. Psychiatry. 2010;167(8):942–948. doi: 10.1176/appi.ajp.2009.09081198. [http://dx.doi.org/10.1176/appi.ajp. 2009.09081198]. [PMID: 20595412]. [DOI] [PubMed] [Google Scholar]
- 278.Godfrey P.S., Toone B.K., Carney M.W., Flynn T.G., Bottiglieri T., Laundy M., Chanarin I., Reynolds E.H. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392–395. doi: 10.1016/0140-6736(90)91942-4. [http://dx.doi.org/10.1016/0140-6736(90) 91942-4]. [PMID: 1974941]. [DOI] [PubMed] [Google Scholar]
- 279.Ginsberg L.D., Oubre A.Y., Daoud Y.A. L-methylfolate Plus SSRI or SNRI from treatment initiation compared to SSRI or SNRI monotherapy in a major depressive episode. Innov. Clin. Neurosci. 2011;8(1):19–28. [PMID: 21311704]. [PMC free article] [PubMed] [Google Scholar]
- 280.Papakostas G.I., Shelton R.C., Zajecka J.M., Etemad B., Rickels K., Clain A., Baer L., Dalton E.D., Sacco G.R., Schoenfeld D., Pencina M., Meisner A., Bottiglieri T., Nelson E., Mischoulon D., Alpert J.E., Barbee J.G., Zisook S., Fava M. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am. J. Psychiatry. 2012;169(12):1267–1274. doi: 10.1176/appi.ajp.2012.11071114. [http://dx.doi.org/10.1176/ appi.ajp.2012.11071114]. [PMID: 23212058]. [DOI] [PubMed] [Google Scholar]
- 281.Shelton R.C., Pencina M.J., Barrentine L.W., Ruiz J.A., Fava M., Zajecka J.M., Papakostas G.I. Association of obesity and inflammatory marker levels on treatment outcome: results from a double-blind, randomized study of adjunctive L-methylfolate calcium in patients with MDD who are inadequate responders to SSRIs. J. Clin. Psychiatry. 2015;76(12):1635–1641. doi: 10.4088/JCP.14m09587. [http://dx.doi. org/10.4088/JCP.14m09587]. [PMID: 26613389]. [DOI] [PubMed] [Google Scholar]
- 282.Collins L.E., Galtieri D.J., Collins P., Jones S.K., Port R.G., Paul N.E., Hockemeyer J., Müller C.E., Salamone J.D. Interactions between adenosine and dopamine receptor antagonists with different selectivity profiles: Effects on locomotor activity. Behav. Brain Res. 2010;211(2):148–155. doi: 10.1016/j.bbr.2010.03.003. [http://dx.doi.org/10.1016/j. bbr.2010.03.003]. [PMID: 20211657]. [DOI] [PubMed] [Google Scholar]
- 283.Yohn S.E., Thompson C., Randall P.A., Lee C.A., Müller C.E., Baqi Y., Correa M., Salamone J.D. The VMAT-2 inhibitor tetrabenazine alters effort-related decision making as measured by the T-maze barrier choice task: reversal with the adenosine A2A antagonist MSX-3 and the catecholamine uptake blocker bupropion. Psychopharmacology (Berl.) 2015;232(7):1313–1323. doi: 10.1007/s00213-014-3766-0. [http://dx. doi.org/10.1007/s00213-014-3766-0]. [PMID: 25323625]. [DOI] [PubMed] [Google Scholar]
- 284.Chen J.F., Moratalla R., Impagnatiello F., Grandy D.K., Cuellar B., Rubinstein M., Beilstein M.A., Hackett E., Fink J.S., Low M.J., Ongini E., Schwarzschild M.A. The role of the D(2) dopamine receptor (D(2)R) in A(2A) adenosine receptor (A(2A)R)-mediated behavioral and cellular responses as revealed by A(2A) and D(2) receptor knockout mice. Proc. Natl. Acad. Sci. USA. 2001;98(4):1970–1975. doi: 10.1073/pnas.98.4.1970. [http://dx.doi.org/10.1073/pnas.98.4.1970]. [PMID: 11172060]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Xie X., Mhaskar Y., Arbogast L.A., Trammell R.A., Hughes L.F., Toth L.A. Adenosine receptor antagonists and behavioral activation in NF-kappaB p50 subunit knockout mice. Life Sci. 2009;85(5-6):226–234. doi: 10.1016/j.lfs.2009.05.016. [http://dx.doi.org/10.1016/j.lfs.2009.05.016]. [PMID: 19508875]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Iravani M.M., Sadeghian M., Leung C.C., Tel B.C., Rose S., Schapira A.H., Jenner P. Continuous subcutaneous infusion of pramipexole protects against lipopolysaccharide-induced dopaminergic cell death without affecting the inflammatory response. Exp. Neurol. 2008;212(2):522–531. doi: 10.1016/j.expneurol.2008.04.037. [http://dx.doi.org/10.1016/j. expneurol.2008.04.037]. [PMID: 18571649]. [DOI] [PubMed] [Google Scholar]
- 287.Cassano P., Lattanzi L., Soldani F., Navari S., Battistini G., Gemignani A., Cassano G.B. Pramipexole in treatment-resistant depression: an extended follow-up. Depress. Anxiety. 2004;20(3):131–138. doi: 10.1002/da.20038. [http://dx.doi.org/10.1002/da.20038]. [PMID: 15549689]. [DOI] [PubMed] [Google Scholar]
- 288.Cusin C., Iovieno N., Iosifescu D.V., Nierenberg A.A., Fava M., Rush A.J., Perlis R.H. A randomized, double-blind, placebo-controlled trial of pramipexole augmentation in treatment-resistant major depressive disorder. J. Clin. Psychiatry. 2013;74(7):e636–e641. doi: 10.4088/JCP.12m08093. [http://dx.doi.org/10.4088/JCP.12m08093]. [PMID: 23945458]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Franco-Chaves J.A., Mateus C.F., Luckenbaugh D.A., Martinez P.E., Mallinger A.G., Zarate C.A., Jr Combining a dopamine agonist and selective serotonin reuptake inhibitor for the treatment of depression: a double-blind, randomized pilot study. J. Affect. Disord. 2013;149(1-3):319–325. doi: 10.1016/j.jad.2013.02.003. [http://dx.doi.org/10.1016/j.jad.2013. 02.003]. [PMID: 23517885]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Fawcett J., Rush A.J., Vukelich J., Diaz S.H., Dunklee L., Romo P., Yarns B.C., Escalona R. Clinical experience with high-dosage pramipexole in patients with treatment-resistant depressive episodes in unipolar and bipolar depression. Am. J. Psychiatry. 2016;173(2):107–111. doi: 10.1176/appi.ajp.2015.15060788. [http://dx.doi.org/10.1176/appi.ajp.2015. 15060788]. [PMID: 26844792]. [DOI] [PubMed] [Google Scholar]
- 291.Morón J.A., Zakharova I., Ferrer J.V., Merrill G.A., Hope B., Lafer E.M., Lin Z.C., Wang J.B., Javitch J.A., Galli A., Shippenberg T.S. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J. Neurosci. 2003;23(24):8480–8488. doi: 10.1523/JNEUROSCI.23-24-08480.2003. [PMID: 13679416]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Zhu C.B., Blakely R.D., Hewlett W.A. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31(10):2121–2131. doi: 10.1038/sj.npp.1301029. [PMID: 16452991]. [DOI] [PubMed] [Google Scholar]
- 293.Zhu C.B., Carneiro A.M., Dostmann W.R., Hewlett W.A., Blakely R.D. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J. Biol. Chem. 2005;280(16):15649–15658. doi: 10.1074/jbc.M410858200. [http://dx.doi.org/10.1074/jbc.M410858200]. [PMID: 15728187]. [DOI] [PubMed] [Google Scholar]
- 294.Zhu C.B., Lindler K.M., Owens A.W., Daws L.C., Blakely R.D., Hewlett W.A. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology. 2010;35(13):2510–2520. doi: 10.1038/npp.2010.116. [http://dx.doi.org/10.1038/npp. 2010.116]. [PMID: 20827273]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Gelman B.B., Spencer J.A., Holzer C.E., III, Soukup V.M. Abnormal striatal dopaminergic synapses in National NeuroAIDS Tissue Consortium subjects with HIV encephalitis. J. Neuroimmune Pharmacol. 2006;1(4):410–420. doi: 10.1007/s11481-006-9030-6. [http://dx.doi.org/10.1007/s11481-006-9030-6]. [PMID: 18040813]. [DOI] [PubMed] [Google Scholar]
- 296.Ferris M.J., Mactutus C.F., Booze R.M. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in NeuroAIDS. Neurosci. Biobehav. Rev. 2008;32(5):883–909. doi: 10.1016/j.neubiorev.2008.01.004. [http:// dx.doi.org/10.1016/j.neubiorev.2008.01.004]. [PMID: 18430470]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Krishnadas R., Nicol A., Sassarini J., Puri N., Burden A.D., Leman J., Combet E., Pimlott S., Hadley D., McInnes I.B., Cavanagh J. Circulating tumour necrosis factor is highly correlated with brainstem serotonin transporter availability in humans. Brain Behav. Immun. 2016;51:29–38. doi: 10.1016/j.bbi.2015.08.005. [http://dx.doi.org/10.1016/j.bbi. 2015.08.005]. [PMID: 26255693]. [DOI] [PubMed] [Google Scholar]
- 298.Lanquillon S., Krieg J.C., Bening-Abu-Shach U., Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22(4):370–379. doi: 10.1016/S0893-133X(99)00134-7. [http:// dx.doi.org/10.1016/S0893-133X(99)00134-7]. [PMID: 10700656]. [DOI] [PubMed] [Google Scholar]
- 299.Sluzewska A., Sobieska M., Rybakowski J.K. Changes in acute-phase proteins during lithium potentiation of antidepressants in refractory depression. Neuropsychobiology. 1997;35(3):123–127. doi: 10.1159/000119332. [http://dx.doi.org/10.1159/000119332]. [PMID: 9170116]. [DOI] [PubMed] [Google Scholar]
- 300.Raison C.L., Demetrashvili M., Capuron L., Miller A.H. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19(2):105–123. doi: 10.2165/00023210-200519020-00002. [http://dx.doi.org/ 10.2165/00023210-200519020-00002]. [PMID: 15697325]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Morrow G.R., Hickok J.T., Roscoe J.A., Raubertas R.F., Andrews P.L., Flynn P.J., Hynes H.E., Banerjee T.K., Kirshner J.J., King D.K. Differential effects of paroxetine on fatigue and depression: a randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. J. Clin. Oncol. 2003;21(24):4635–4641. doi: 10.1200/JCO.2003.04.070. [http://dx.doi.org/10. 1200/JCO.2003.04.070]. [PMID: 14673053]. [DOI] [PubMed] [Google Scholar]
- 302.Nutt D., Demyttenaere K., Janka Z., Aarre T., Bourin M., Canonico P.L., Carrasco J.L., Stahl S. The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. J. Psychopharmacol. (Oxford) 2007;21(5):461–471. doi: 10.1177/0269881106069938. [http:// dx.doi.org/10.1177/0269881106069938]. [PMID: 17050654]. [DOI] [PubMed] [Google Scholar]
- 303.Randall P.A., Lee C.A., Podurgiel S.J., Hart E., Yohn S.E., Jones M., Rowland M., López-Cruz L., Correa M., Salamone J.D. Bupropion increases selection of high effort activity in rats tested on a progressive ratio/chow feeding choice procedure: implications for treatment of effort-related motivational symptoms. Int. J. Neuropsychopharmacol. 2014;18(2):pyu017. doi: 10.1093/ijnp/pyu017. [http://dx.doi.org/ 10.1093/ijnp/pyu017]. [PMID: 25575584]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Mar Fan H.G., Clemons M., Xu W., Chemerynsky I., Breunis H., Braganza S., Tannock I.F. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support. Care Cancer. 2008;16(6):577–583. doi: 10.1007/s00520-007-0341-9. [http://dx.doi.org/10.1007/s00520-007-0341-9]. [PMID: 17972110]. [DOI] [PubMed] [Google Scholar]
- 305.Moraska A.R., Sood A., Dakhil S.R., Sloan J.A., Barton D., Atherton P.J., Suh J.J., Griffin P.C., Johnson D.B., Ali A., Silberstein P.T., Duane S.F., Loprinzi C.L. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J. Clin. Oncol. 2010;28(23):3673–3679. doi: 10.1200/JCO.2010.28.1444. [http://dx.doi.org/10.1200/JCO.2010.28.1444]. [PMID: 20625123]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Butler J.M., Jr, Case L.D., Atkins J., Frizzell B., Sanders G., Griffin P., Lesser G., McMullen K., McQuellon R., Naughton M., Rapp S., Stieber V., Shaw E.G. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of d-threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2007;69(5):1496–1501. doi: 10.1016/j.ijrobp.2007.05.076. [http://dx.doi.org/10.1016/j.ijrobp.2007.05.076]. [PMID: 17869448]. [DOI] [PubMed] [Google Scholar]
- 307.Sugawara Y., Akechi T., Shima Y., Okuyama T., Akizuki N., Nakano T., Uchitomi Y. Efficacy of methylphenidate for fatigue in advanced cancer patients: a preliminary study. Palliat. Med. 2002;16(3):261–263. doi: 10.1191/0269216302pm547xx. [http://dx.doi.org/10.1191/0269216302pm547xx]. [PMID: 12047006]. [DOI] [PubMed] [Google Scholar]
- 308.Pucci E. Amantadine for fatigue in multiple sclerosis. Cochrane Datab. System. Rev. (Online) 2007;1:CD002818. doi: 10.1002/14651858.CD002818.pub2. [http://dx.doi. org/10.1002/14651858.CD002818.pub2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Stankoff B., Waubant E., Confavreux C., Edan G., Debouverie M., Rumbach L., Moreau T., Pelletier J., Lubetzki C., Clanet M. Modafinil for fatigue in MS: a randomized placebo-controlled double-blind study. Neurology. 2005;64(7):1139–1143. doi: 10.1212/01.WNL.0000158272.27070.6A. [http://dx.doi. org/10.1212/01.WNL.0000158272.27070.6A]. [PMID: 15824337]. [DOI] [PubMed] [Google Scholar]
- 310.Bruera E., Yennurajalingam S., Palmer J.L., Perez-Cruz P.E., Frisbee-Hume S., Allo J.A., Williams J.L., Cohen M.Z. Methylphenidate and/or a nursing telephone intervention for fatigue in patients with advanced cancer: a randomized, placebo-controlled, phase II trial. J. Clin. Oncol. 2013;31(19):2421–2427. doi: 10.1200/JCO.2012.45.3696. [http://dx. doi.org/10.1200/JCO.2012.45.3696]. [PMID: 23690414]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Ruddy K.J., Barton D., Loprinzi C.L. Laying to rest psychostimulants for cancer-related fatigue? J. Clin. Oncol. 2014;32(18):1865–1867. doi: 10.1200/JCO.2014.55.8353. [http://dx.doi.org/10.1200/JCO.2014.55.8353]. [PMID: 24799492]. [DOI] [PubMed] [Google Scholar]
- 312.Escalante C.P., Meyers C., Reuben J.M., Wang X., Qiao W., Manzullo E., Alvarez R.H., Morrow P.K., Gonzalez-Angulo A.M., Wang X.S., Mendoza T., Liu W., Holmes H., Hwang J., Pisters K., Overman M., Cleeland C. A randomized, double-blind, 2-period, placebo-controlled crossover trial of a sustained-release methylphenidate in the treatment of fatigue in cancer patients. Cancer J. 2014;20(1):8–14. doi: 10.1097/PPO.0000000000000018. [http://dx.doi.org/10.1097/ PPO.0000000000000018]. [PMID: 24445757]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Gong S., Sheng P., Jin H., He H., Qi E., Chen W., Dong Y., Hou L. Effect of methylphenidate in patients with cancer-related fatigue: a systematic review and meta-analysis. PLoS One. 2014;9(1):e84391. doi: 10.1371/journal.pone.0084391. [http://dx.doi.org/10.1371/journal.pone.0084391]. [PMID: 24416225]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Jha M.K., Minhajuddin A., Gadad B.S., Greer T., Grannemann B., Soyombo A., Mayes T.L., Rush A.J., Trivedi M.H. Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology. 2017;78:105–113. doi: 10.1016/j.psyneuen.2017.01.023. [http://dx.doi.org/10.1016/ j.psyneuen.2017.01.023]. [PMID: 28187400]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.McAllister T.W., Zafonte R., Jain S., Flashman L.A., George M.S., Grant G.A., He F., Lohr J.B., Andaluz N., Summerall L., Paulus M.P., Raman R., Stein M.B. Randomized placebo-controlled trial of methylphenidate or galantamine for persistent emotional and cognitive symptoms associated with PTSD and/or traumatic brain injury. Neuropsychopharmacology. 2016;41(5):1191–1198. doi: 10.1038/npp.2015.282. [http://dx.doi.org/10.1038/npp.2015.282]. [PMID: 26361060]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Aga-Mizrachi S., Cymerblit-Sabba A., Gurman O., Balan A., Shwam G., Deshe R., Miller L., Gorodetsky N., Heinrich N., Tzezana O., Zubedat S., Grinstein D., Avital A. Methylphenidate and desipramine combined treatment improves PTSD symptomatology in a rat model. Transl. Psychiatry. 2014;4:e447. doi: 10.1038/tp.2014.82. [http://dx.doi.org/10.1038/tp.2014.82]. [PMID: 25247592]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Dantzer R., Walker A.K. Is there a role for glutamate-mediated excitotoxicity in inflammation-induced depression? J. Neural Transm. (Vienna) 2014;121(8):925–932. doi: 10.1007/s00702-014-1187-1. [http://dx.doi.org/10. 1007/s00702-014-1187-1]. [PMID: 24633997]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Dantzer R., O’Connor J.C., Lawson M.A., Kelley K.W. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36(3):426–436. doi: 10.1016/j.psyneuen.2010.09.012. [http://dx.doi.org/ 10.1016/j.psyneuen.2010.09.012]. [PMID: 21041030]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Serafini G., Adavastro G., Canepa G., Capobianco L., Conigliaro C., Pittaluga F., Murri M.B., Valchera A., De Berardis D., Pompili M., Lindqvist D., Brundin L., Amore M. Abnormalities in kynurenine pathway metabolism in treatment-resistant depression and suicidality: A systematic review. CNS Neurol. Disord. Drug Targets. 2017;16(4):440–453. doi: 10.2174/1871527316666170413110605. [http://dx. doi.org/10.2174/1871527316666170413110605]. [PMID: 28412922]. [DOI] [PubMed] [Google Scholar]
- 320.Schwarcz R., Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J. Pharmacol. Exp. Ther. 2002;303(1):1–10. doi: 10.1124/jpet.102.034439. [http://dx.doi.org/10.1124/ jpet.102.034439]. [PMID: 12235226]. [DOI] [PubMed] [Google Scholar]
- 321.Raison C.L., Dantzer R., Kelley K.W., Lawson M.A., Woolwine B.J., Vogt G., Spivey J.R., Saito K., Miller A.H. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol. Psychiatry. 2010;15(4):393–403. doi: 10.1038/mp.2009.116. [http://dx. doi.org/10.1038/mp.2009.116]. [PMID: 19918244]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Bonaccorso S., Marino V., Puzella A., Pasquini M., Biondi M., Artini M., Almerighi C., Verkerk R., Meltzer H., Maes M. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J. Clin. Psychopharmacol. 2002;22(1):86–90. doi: 10.1097/00004714-200202000-00014. [http://dx.doi.org/10.1097/ 00004714-200202000-00014]. [PMID: 11799348]. [DOI] [PubMed] [Google Scholar]
- 323.Santamaría A., Flores-Escartín A., Martínez J.C., Osorio L., Galván-Arzate S., Pedraza-Chaverrí J., Maldonado P.D., Medina-Campos O.N., Jiménez-Capdeville M.E., Manjarrez J., Ríos C. Copper blocks quinolinic acid neurotoxicity in rats: contribution of antioxidant systems. Free Radic. Biol. Med. 2003;35(4):418–427. doi: 10.1016/s0891-5849(03)00317-4. [http://dx.doi.org/10.1016/S0891-5849(03)00317-4]. [PMID: 12899943]. [DOI] [PubMed] [Google Scholar]
- 324.Behan W.M., McDonald M., Darlington L.G., Stone T.W. Oxidative stress as a mechanism for quinolinic acid-induced hippocampal damage: protection by melatonin and deprenyl. Br. J. Pharmacol. 1999;128(8):1754–1760. doi: 10.1038/sj.bjp.0702940. [http://dx.doi.org/10.1038/ sj.bjp.0702940]. [PMID: 10588931]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Tavares R.G., Tasca C.I., Santos C.E., Alves L.B., Porciúncula L.O., Emanuelli T., Souza D.O. Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes. Neurochem. Int. 2002;40(7):621–627. doi: 10.1016/s0197-0186(01)00133-4. [http://dx.doi.org/10. 1016/S0197-0186(01)00133-4]. [PMID: 11900857]. [DOI] [PubMed] [Google Scholar]
- 326.Tavares R.G., Schmidt A.P., Abud J., Tasca C.I., Souza D.O. In vivo quinolinic acid increases synaptosomal glutamate release in rats: reversal by guanosine. Neurochem. Res. 2005;30(4):439–444. doi: 10.1007/s11064-005-2678-0. [http://dx.doi.org/10.1007/s11064-005-2678-0]. [PMID: 16076013]. [DOI] [PubMed] [Google Scholar]
- 327.Guillemin G.J., Croitoru-Lamoury J., Dormont D., Armati P.J., Brew B.J. Quinolinic acid upregulates chemokine production and chemokine receptor expression in astrocytes. Glia. 2003;41(4):371–381. doi: 10.1002/glia.10175. [http://dx.doi.org/10.1002/glia.10175]. [PMID: 12555204]. [DOI] [PubMed] [Google Scholar]
- 328.Guillemin G.J. Quinolinic acid: neurotoxicity. FEBS J. 2012;279(8):1355. doi: 10.1111/j.1742-4658.2012.08493.x. [http://dx.doi.org/10.1111/j.1742-4658.2012.08493.x]. [PMID: 22251552]. [DOI] [PubMed] [Google Scholar]
- 329.Stone T.W. Development and therapeutic potential of kynurenic acid and kynurenine derivatives for neuroprotection. Trends Pharmacol. Sci. 2000;21(4):149–154. doi: 10.1016/s0165-6147(00)01451-6. [http://dx.doi.org/10.1016/ S0165-6147(00)01451-6]. [PMID: 10740291]. [DOI] [PubMed] [Google Scholar]
- 330.Wu H.Q., Rassoulpour A., Schwarcz R. Kynurenic acid leads, dopamine follows: a new case of volume transmission in the brain? J. Neural Transm. (Vienna) 2007;114(1):33–41. doi: 10.1007/s00702-006-0562-y. [http://dx.doi.org/ 10.1007/s00702-006-0562-y]. [PMID: 16932989]. [DOI] [PubMed] [Google Scholar]
- 331.Tilleux S., Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J. Neurosci. Res. 2007;85(10):2059–2070. doi: 10.1002/jnr.21325. [http://dx.doi.org/10.1002/jnr.21325]. [PMID: 17497670]. [DOI] [PubMed] [Google Scholar]
- 332.Ida T., Hara M., Nakamura Y., Kozaki S., Tsunoda S., Ihara H. Cytokine-induced enhancement of calcium-dependent glutamate release from astrocytes mediated by nitric oxide. Neurosci. Lett. 2008;432(3):232–236. doi: 10.1016/j.neulet.2007.12.047. [http://dx.doi.org/10.1016/j.neulet.2007.12. 047]. [PMID: 18255223]. [DOI] [PubMed] [Google Scholar]
- 333.Takaki J., Fujimori K., Miura M., Suzuki T., Sekino Y., Sato K. L-glutamate released from activated microglia downregulates astrocytic L-glutamate transporter expression in neuroinflammation: the ‘collusion’ hypothesis for increased extracellular L-glutamate concentration in neuroinflammation. J. Neuroinflammation. 2012;9:275. doi: 10.1186/1742-2094-9-275. [http://dx.doi.org/10.1186/1742-2094-9-275]. [PMID: 23259598]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Hardingham G.E., Fukunaga Y., Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002;5(5):405–414. doi: 10.1038/nn835. [http://dx.doi.org/10.1038/nn835]. [PMID: 11953750]. [DOI] [PubMed] [Google Scholar]
- 335.Haydon P.G., Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006;86(3):1009–1031. doi: 10.1152/physrev.00049.2005. [http://dx.doi.org/10.1152/physrev.00049.2005]. [PMID: 16816144]. [DOI] [PubMed] [Google Scholar]
- 336.Schwarcz R., Köhler C. Differential vulnerability of central neurons of the rat to quinolinic acid. Neurosci. Lett. 1983;38(1):85–90. doi: 10.1016/0304-3940(83)90115-5. [http://dx.doi.org/10.1016/0304-3940(83)90115-5]. [PMID: 6225037]. [DOI] [PubMed] [Google Scholar]
- 337.Miranda A.F., Boegman R.J., Beninger R.J., Jhamandas K. Protection against quinolinic acid-mediated excitotoxicity in nigrostriatal dopaminergic neurons by endogenous kynurenic acid. Neuroscience. 1997;78(4):967–975. doi: 10.1016/s0306-4522(96)00655-0. [http://dx.doi.org/10.1016/ S0306-4522(96)00655-0]. [PMID: 9174065]. [DOI] [PubMed] [Google Scholar]
- 338.O’Connor J.C., Lawson M.A., André C., Briley E.M., Szegedi S.S., Lestage J., Castanon N., Herkenham M., Dantzer R., Kelley K.W. Induction of IDO by bacille Calmette-Guérin is responsible for development of murine depressive-like behavior. J. Immunol. 2009;182(5):3202–3212. doi: 10.4049/jimmunol.0802722. [http://dx.doi.org/10.4049/jimmunol. 0802722]. [PMID: 19234218]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.O’Connor J.C. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry. 2008 doi: 10.1038/sj.mp.4002148. [PMID: 18195714]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Masilamoni G.J., Bogenpohl J.W., Alagille D., Delevich K., Tamagnan G., Votaw J.R., Wichmann T., Smith Y. Metabotropic glutamate receptor 5 antagonist protects dopaminergic and noradrenergic neurons from degeneration in MPTP-treated monkeys. Brain. 2011;134(Pt 7):2057–2073. doi: 10.1093/brain/awr137. [http://dx.doi.org/ 10.1093/brain/awr137]. [PMID: 21705423]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Meisner F., Scheller C., Kneitz S., Sopper S., Neuen-Jacob E., Riederer P., ter Meulen V., Koutsilieri E. Memantine upregulates BDNF and prevents dopamine deficits in SIV-infected macaques: a novel pharmacological action of memantine. Neuropsychopharmacology. 2008;33(9):2228–2236. doi: 10.1038/sj.npp.1301615. [http://dx.doi.org/10.1038/sj.npp. 1301615]. [PMID: 17971830]. [DOI] [PubMed] [Google Scholar]
- 342.Scheller C., Sopper S., Jenuwein M., Neuen-Jacob E., Tatschner T., Grünblatt E., ter Meulen V., Riederer P., Koutsilieri E. Early impairment in dopaminergic neurotransmission in brains of SIV-infected rhesus monkeys due to microglia activation. J. Neurochem. 2005;95(2):377–387. doi: 10.1111/j.1471-4159.2005.03373.x. [http://dx.doi.org/10.1111/j.1471-4159.2005.03373.x]. [PMID: 16190867]. [DOI] [PubMed] [Google Scholar]
- 343.Kumar A.M., Ownby R.L., Waldrop-Valverde D., Fernandez B., Kumar M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. J. Neurovirol. 2011;17(1):26–40. doi: 10.1007/s13365-010-0003-4. [http://dx.doi.org/10.1007/s13365-010-0003-4]. [PMID: 21165787]. [DOI] [PubMed] [Google Scholar]
- 344.aan het Rot, M. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol. Psychiatry. 2010;67(2):139–12. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 345.Price R.B., Nock M.K., Charney D.S., Mathew S.J. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol. Psychiatry. 2009;66(5):522–526. doi: 10.1016/j.biopsych.2009.04.029. [http://dx.doi.org/10.1016/j.biopsych.2009.04.029]. [PMID: 19545857]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Yang J.J., Wang N., Yang C., Shi J.Y., Yu H.Y., Hashimoto K. Serum interleukin-6 is a predictive biomarker for ketamine’s antidepressant effect in treatment-resistant patients with major depression. Biol. Psychiatry. 2015;77(3):e19–e20. doi: 10.1016/j.biopsych.2014.06.021. [http://dx.doi.org/10. 1016/j.biopsych.2014.06.021]. [PMID: 25104172]. [DOI] [PubMed] [Google Scholar]
- 347.Uher R., Tansey K.E., Dew T., Maier W., Mors O., Hauser J., Dernovsek M.Z., Henigsberg N., Souery D., Farmer A., McGuffin P. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am. J. Psychiatry. 2014;171(12):1278–1286. doi: 10.1176/appi.ajp.2014.14010094. [http://dx.doi. org/10.1176/appi.ajp.2014.14010094]. [PMID: 25017001]. [DOI] [PubMed] [Google Scholar]