Abstract
Background
The human gut microbiome comprise a huge number of microorganisms with co-evolutionary associations with humans. It has been repeatedly revealed that bidirectional communication exists between the brain and the gut and involves neural, hormonal, and immunological pathways. Evidences from neuroscience researches over the past few years suggest that microbiota is essential for the development and maturation of brain systems that are associated to stress responses.
Method
This review provides that the summarization of the communication among microbiota, gut and brain and the results of preclinical and clinical studies on gut microbiota used in treatments for neuropsychiatric disorders.
Result
Recent studies have reported that diverse forms of neuropsychiatric disorders (such as autism, depression, anxiety, and schizophrenia) are associated with or modulated by variations in the microbiome, by microbial substrates, and by exogenous prebiotics, antibiotics, and probiotics.
Conclusion
The microbiota–gut–brain axis might provide novel targets for prevention and treatment of neuropsychiatric disorders. However, further studies are required to substantiate the clinical use of probiotics, prebiotics and FMT.
Keywords: Microbiota-gut-brain axis, gut-brain axis, gut microbiota, enteric microbiota, probiotics, prebiotics
1. INTRODUCTION
There is a very complex group of microorganisms on the inner surface of the human gut. The number of microorganisms in the human body is 10 times greater than the number of human cells, and these microorganisms collectively contain over 100 times more genes than the number of human genes [1, 2]. In healthy individuals, intestinal microbial flora has physiological function, fermenting food ingredients that are not digestible in order to supply nutrients and energy to the host and to maintain the balance of the immune system [3]. Over the past few decades, researchers have found that there is a complex interaction between the host and its microorganisms, which not only affects energy utilization and digestion, but also brain function and behavior. In fact, these microorganisms and humans have evolved together for a long time and have formed a symbiotic relationship. The microbiome is now recognized by some as an organ of the human body. The intestinal microbiome is a significant part of the complete human microbiome and has a bi-directional relationship with various organ systems, particularly the central nervous system.
Neuropsychiatric diseases have various causes. Therefore, initial treatment success rates are often low due to a failure to treat the accurate biological targets [4, 5]. Groundbreaking studies showing reduced anxiety in germ-free mice in a stressful situation [6], the development of depression after a large-scale E. coli outbreak and reduced anxiety in rats after treatment with probiotics [7] suggest the role of the microbiome in neuropsychiatric illnesses. Emerging evidence of the interactions among the brain, gut, and microbiome might help to explain the mechanisms underlying these complex interactions.
In this review, the concept of the microbiota-gut-brain axis is discussed. We summarize the known studies to examine how each part of the axis interacts with the others. Finally, we present the results of preclinical and clinical studies on gut microbiota used in treatments for neuropsychiatric disorders and offer perspectives on the future uses of probiotics. This article is a non-systematic narrative review for providing descriptive information.
2. GUT MICROBIOTA
2.1. Structure
The term microbiota refers to microbial communities that include bacteria, archaea, eukaryotes, and viruses that are present in a host [8]. Among them, bacteria are predominant, particularly certain types of bacteria. A metagenomics analysis of the gut microbiome in 124 subjects (a cohort composed of healthy subjects, overweight subjects, and inflammatory bowel disease [IBD] patients) showed that 99% of the genes were bacterial, and 1,000-1,150 species were found in the entire cohort [9, 10]. Each individual harbored at least 160 bacterial species and more than three million microbial genes.
Recent metagenomic technologies have revealed that the human gut microbiome forms three distinct enterotypes, each including the Bacteroides, Prevotella, and Ruminococcus genera [11]. The composition of the gut microbiota depends on an individual's genetic predisposition, age, nutrition, physical activity, environmental factors, stress, infection, other diseases, and use of antibiotics.
2.2. Development
The gut microbiome varies according to age and is generally formed in three stages. Because the inside of the uterus is aseptic, an infant is sterile until birth. When passing through the birth canal, an infant encounters some bacteria, which then colonize the gastrointestinal tract, mouth, skin, and conjunctiva. The gut microbiota acquired during vaginal delivery include Bifidobacterium, Lactobacillus, and Prevotella as predominant genera [12]. Staphylococcus and Corynebacterium are the main genera acquired by newborns born via cesarean section [13]. The gut microbiome of a newborn is representative of the bacterial composition of the external environment and the maternal skin, and the birth method greatly affects the initial microbial settlement [14, 15]. Breastfed infants at this early stage have a lower diversity of gut microflora than infants fed formula, but the composition tends to be more stable. After initiating solid foods, the diversity of the gut microbiota increases [16, 17]. The proportion of anaerobic bacteria classified as Firmicutes begins to increase [17], and the microbiome becomes similar to that of an adult gut by three years of age [14].
2.3. Factors Affecting the Structure of the Gut Microbiome
Long-term dietary habits are associated with gut microbiome compositions. People with high protein and animal fat diets have enterotypes characterized by high levels of Bacteroides, while those who eat a high fiber diet have enterotypes characterized by high levels of Prevotella [18]. Prebiotics refer to indigestible food ingredients that selectively stimulate the growth and activities of beneficial microorganisms, such as Lactobacillus and Bifidobacterium [19].
Infection, antibiotics, and other factors can temporarily alter the stability of the gut microbiota composition, which might have detrimental effects on the host [20]. Even the short-term use of antibiotics can lead to long-term dysbiosis, inducing accelerated maturation and exacerbation of diseases [21]. The subject of stress-induced changes in the gut microbiome represents a challenging topic and will be discussed in more detail in this article.
3. THE MICROBIOTA-GUT-BRAIN (MGB) AXIS
Early studies of the interaction between the gastrointestinal tract and the brain were primarily related to digestive function and satiety [22]. The brain-gut axis is composed of the central nervous system (CNS, represented by the brain), the enteric nervous system, and the digestive system. It is involved in gut motility, secretion of hormones, and production of acid, bicarbonates, and mucus. Studies to date have revealed that gut microbiota have a symbiotic relationship with intestinal cells and play a role in basic physiological processes, including digestion, growth, and immune defense.
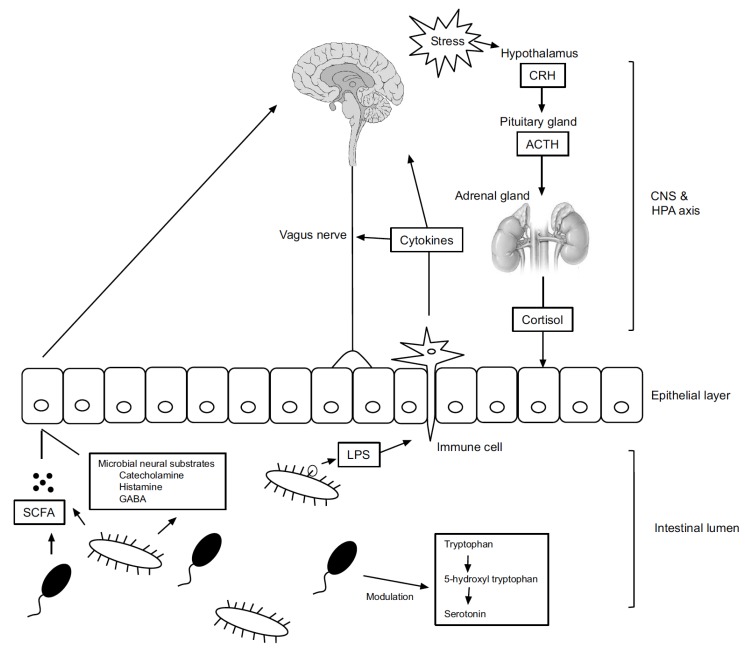
There is a high correlation between stress-related mental symptoms (such as anxiety) and irritable bowel syndrome (IBS). This correlation has provided a stimulus to study the importance of the gut-brain axis. More than 50% of IBS patients have comorbid depression or anxiety [23]. Recent studies on germ-free rodents, antibiotics, probiotics, gastrointestinal tract infections, and stool microbial transplantation provide evidence of the notion that intestinal microorganisms regulate the brain, behaviors, and stress responses by forming the microbiota-gut-brain (MGB) axis [24, 25]. Gut microbiota maintain a bidirectional interaction with major parts of the CNS via direct and indirect pathways. The MGB axis is part of a comprehensive physiological network including the endocrine system (hypothalamic-pituitary-adrenal axis), the immune system (cytokines, chemokines), the autonomic nervous system (including efferent and afferent neurons), and the enteric nervous system. Gut microbiota are thought to act on the hypothalamic-pituitary-adrenal (HPA) axis and the vagus nerve by producing bacterial metabolites via tryptophan metabolism.
3.1. Brain Development and Gut Microbiota
Gut microbiota seem to play a very important role in CNS development. There is evidence that the intestinal microbiota program the early activation of the HPA axis as a stress response in individuals. The elevated activation of stress hormones in GF mice also suggests that the gut microbiota play an important role in the development of the HPA axis [26].
3.2. Interactions between the Human CNS and Gut Microbiota
3.2.1. Evidence of the CNS Affecting Gut Microbiota
The gut microbiome composition is influenced by emotional and physiological stress (Fig. 1). In a mouse model, even two hours of social disruption altered the profile of the microorganism community, where the Lactobacillus population declined [27]. When separation anxiety was provoked in rhesus monkeys by separating them from their mothers at age 6-9 months, their fecal Lactobacillus concentrations decreased [28]. In another study, the Lactobacillus concentration in feces was lower in healthy students during a period of extreme stress than during a period of mild stress.
Fig. (1).
Interaction pathways of the microbiota–gut–brain axis. Gut microbiota and the brain interact in a variety of pathways. Activation of the HPA axis by stress leads to cortisol secretion. It can affect gut integrity, motility and mucus secretion, leading to the change the composition of the gut microbiota. LPS on the surface of gram negative bacteria can affect the brain by mediating immune cells and vagus nerves. Microbial metabolite such as SCFA and microbial neural substrate including catecholamine, histamine and GABA may affect the brain directly or indirectly. Intestinal microorganisms control the amount of serotonin by modulating the tryptophan metabolism. CRH, Corticotropin-releasing hormone; ACTH, Adrenocorticotropic hormone; CNS, Central nervous system; HPA, Hypothalamus-pituitary-adrenal; SCFA, Short-chain fatty acid; GABA, Gamma-aminobutyric acid; LPS, Lipopolysaccharide.
Stress alters the pattern of mucus secretion [29], which can have a grave impact on the proliferation of intestinal microorganisms facilitated by prebiotics and dietary fibers. Audio stress affects the gastrointestinal postprandial motility and induces a temporary reduction of gastric emptying in dogs [29]. Inducing stress in mice through maternal separation leads to changes in intestinal motility and gut microbiota composition [30].
Stress mediators cause local immune activity by altering intestinal permeability [31] and can change the germ composition through a series of processes [32]. Provoking chronic stress in adult mice reduced the relative abundance of Bacteroides species and increased Clostridium species in the cecum. Furthermore, the immune system was activated, and both interleukin-6 and C-C chemokine ligand 2 were elevated. Gastrointestinal and blood-brain permeability were improved when acute stress caused secretion of corticotropin-releasing hormone (CRH) in the CNS; this activated mast cells, which have a high affinity for CRH [33]. Chronic stress also disrupted the intestinal barrier by activating mast cells, in turn allowing antibodies, microbial metabolites, toxins, and lipopolysaccharides in the gut to enter the systemic circulation and CNS.
3.2.2. Evidence of Gut Microbiota Affecting the CNS
3.2.2.1. Immune Regulation
When gut microbiota cause an infection, the cells can gain access to the CNS and directly induce inflammatory reactions. Chronic low-grade inflammation causes cytokines to be released into the blood, further affecting the immune system. Intestinal microbiota contain molecules that can cause inflammation. For example, LPS and peptidoglycan are typical inflammation-inducing substances. LPS is recognized by the TLR-4 receptor, which is widely distributed in monocytes, macrophages, and microglia of the brain. Activation of TLR-4-mediated inflammatory responses by gut microbiota in IBS patients with depression has been reported [34, 35]. The indirect effects of intestinal microorganisms and probiotics on the innate immune system can cause alterations in the circulatory levels of proinflammatory and anti-inflammatory cytokines, which in turn have direct impacts on brain functions. When E. coli was introduced into germ-free mice, macrophage infiltration and activation in adipose tissue led to an increase in proinflammatory cytokine (IFN) expression [36] (Fig. 1).
3.2.2.2. Modulation of Afferent Nerves
Gut microbiota can activate the vagus nerve, and such activation plays an important role in mediating effects on the brain and behavior. The vagus nerve is an important part of the sensory pathway connecting the intestines to the brain. The evidence that the gut microbiota affect the vagus nerve is accumulating steadily.
Lipopolysaccharide (LPS) is a major component of the outer membrane of gram-negative bacteria. LPS activates cytokines such as IL-1β, which in turn induce illness through the vagus nerve. After vagotomy, cytokine induction is blocked [37, 38]. Goehler et al. found that c-Fos expression (an indicator of neural activity of vagal sensory ganglia and solitary neurons) in mice increased with time after C. jejuni administration [39]. In that study, neural activity after C. jejuni infection increased in a short time without a concomitant increase in proinflammatory cytokines, indicating that bacteria can directly affect behavior through the vagus nerve. Subsequent studies have also shown that early infection with Campylobacter affects vagally-mediated neural circuits, leading to anxiety-like behavior [40].
Bravo et al. reported that the vagus nerve plays an important role in the brain-gut bidirectional pathway [7]. In rats and mice receiving chronic administration of the probiotic Lactobacillus rhamnosus, anxiety and depressive behaviors were reduced, and this reduction was accompanied by reduction in gamma aminobutyric acid (GABA) receptor subunit mRNA expression and corticosterone levels. In contrast, no neurochemical or behavioral effects were observed after treatment with Lactobacillus rhamnosus in vagotomized rats and mice. Similarly, in mice with chemically induced colitis, the anxiolytic effect of Bifidobacterium longum NCC3001 disappeared after vagotomy [41]. In that study, vagal integrity was important for the anxiolytic effect, but it was not involved in immune modulation or production of hippocampal brain-derived neurotrophic factor (BDNF) in the gut.
3.2.2.3. Tryptophan Metabolism
Tryptophan is an amino acid that acts as an essential precursor for the production of serotonin and kynurenine. A reduction in tryptophan is known to be associated with clinical depression [42]. Gut microbiota appear to play an important role in the metabolism of tryptophan. Germ-free mice were reported to have elevated serum levels of tryptophan and reduced serotonin levels in the blood compared to normally colonized mice, suggesting that tryptophan hydroxylase expression in the intestines might be reduced in germ-free mice [43, 44].
In further animal studies, administration of the probiotic Bifidobacterium infantis increased inflammatory markers and tryptophan levels and decreased the kynurenine-to-tryptophan ratio [45].
3.2.2.4. Microbial Metabolites (Short-Chain Fatty Acids)
Gut microbiota produce short-chain fatty acids (SCFAs) including acetic acid, propionic acid, and butyric acid. These SCFAs have a role as inhibitors of histone deacetylases [46], and they bind to G protein-coupled receptors to induce intracellular signaling [47]. Therefore, SCFAs act as mediators between the internal microbiota and the brain and contribute to mechanisms by which intestinal bacteria affect brain physiology and behavior.
SCFAs can directly affect brain physiology and behavior. The microbial-derived SCFAs butyric acid and propionic acid increase the gene expression of tyrosine hydroxylase, an enzyme that limits the rate of synthesis of dopamine and noradrenaline, and dopamine-β-hydroxylase, an enzyme that converts dopamine to noradrenaline [48]. Long-term treatment of propionic acid in germ-free rats lowered in vivo levels of GABA, serotonin, and dopamine [49]. Microbial-derived SCFAs are therefore involved in a neural circuit that can affect physiology and behavior. Propionate generated by gut microbiota activated intestinal gluconeogenesis gene expression through a gut-brain circuit involving the fatty acid receptor FFAR3 [50].
SCFAs formed by gut microbiota appear to affect the CNS by acting on glial cells including microglial cells and astrocytes, but the specific effect depends on the type of SCFA and the target cell. It has been reported that an anti-inflammatory action of LPS-induced microglial cells in rats was induced by butyric acid [51]. Erny et al. reported that SCFA-treated germ-free mice showed restoration of microglial malformation and immaturity following activation of FFAR2 [52]. In rat experiments, propionic acid affected cytoskeletal integration and increased glial fibrillary acidic protein (GFAP) in cultured astrocytes [53]. When bacteria-derived propionic acid was injected into rats, cognitive and sensorimotor impairment were induced [54].
3.2.2.5. Microbial Neural Substrates
Bacteria have the ability to produce diverse neurotransmitters or similar substances. Some strains of gut bacteria can locally produce and release neurotransmitters, including GABA, serotonin, catecholamine, and histamine. These bacteria-derived neurotransmitters can deliver signals to the CNS through enterochromaffin cells and enteric nerve receptors.
GABA, a major inhibitory neurotransmitter in the CNS whose dysfunction is associated with depression, anxiety, autism, and schizophrenia, is efficiently produced by Lactobacillus brevis and Bifidobacterium dentium in human intestines [55]. Takanaga et al. suggested in their animal study that GABA produced by intestinal bacteria crosses the blood-brain barrier (BBB) and enters the CNS [56]. Lactobacillus rhamnosus has been found to reduce anxiety and depression-related behaviors in mice and to increase GABA concentration in the hippocampus [57, 58]. Considering that such effects manifest only when the vagus nerve is intact, it is possible that intestinal microorganisms indirectly regulate GABA signaling through the vagus nerve.
Dopamine and noradrenaline are neurotransmitters that act on the CNS and are produced by GI microorganisms. The fact that germ-free mice have markedly lower levels of noradrenaline and dopamine in the cecum than do SPF mice implies that gut microbiota can possibly supply catecholamine [59]. Some bacterial species have a gene for a transcript with a similar sequence to that of tyrosine hydroxylase, the rate-limiting enzyme of noradrenaline and dopamine synthesis [60]. Lactobacillus bacteria are known to synthesize dopamine during culture [61]. However, evidence that catecholamines produced by microorganisms influence the CNS is still lacking, as dopamine synthesized in the periphery cannot cross the BBB. Nevertheless, germ-free mice show lower tyrosine (the rate-limiting substrate of noradrenaline and dopamine synthesis) levels compared to those in ex-germ-free mice, which implies that gut microbiota elevate dopamine levels in the brains of germ-free mice [62]. Such a notion is supported by a study that compared ex-germ-free mice and germ-free mice and found that catecholamine levels were elevated in the brains of germ-free mice, but restoring the gut microbiota modulated catecholamine levels via dopamine and noradrenaline turnover in the brain [63].
Histamine, an immunomodulator and neurotransmitter, is involved in the regulatory processes of important functions such as waking, cognition, circadian rhythm, and neuroendocrine regulation [64]. Some gut microbiota can synthesize histamine. Lactobacillus reuteri expresses a gene for histidine decarboxylase and synthesizes histamine [65]. Adding histidine to Lactobacillus reuteri cultures not only leads to histidine decarboxylase expression, but also increases histamine production. Moreover, Lactobacillus reuteri inhibits the proinflammatory cytokine TNF-α in myeloid progenitor cells by producing histamine. Such an immunomodulatory role of histamine has also been observed in intestinal lymphoid organs, where it regulates Yersinia enterocolitica infection [2]. In addition, it has been revealed that an H2 receptor blockade reduces mucus secretion and intensifies impairment of the intestinal barrier, which in turn might contribute to translocation of bacteria in the intestinal lumen through the circulatory system [66].
4. PATHOPHYSIOLOGY OF THE MICROBIOTA-GUT-BRAIN AXIS IN NEUROPSYCHIATRIC ILLNESSES
4.1. Stress and Anxiety
Anxiety is an emotional state that develops via neural, endocrine, and immunologic mechanisms. Exposure to stress (including biological, environmental, or psychological stimuli) can provoke anxiety responses involving activation of the HPA axis or the immune response [67, 68]. The coexistence of anxiety and acute or mild intestinal dysfunction has been documented extensively, and the role of gut-brain signals, such as neurotransmitters and immunologic factors, has been emphasized [69-71].
Germ-free mice show increased motor activity and lower anxiety compared to SPF mice with normal microbiota. A reduction of anxiety behaviors in the GF condition is associated with elevated 5-HT and tryptophan metabolism and with long-lasting modulation of synaptic transmission by reducing PSD-95 and synaptophysin expression [67]. These findings imply that gut microbiota regulate the degree of the HPA axis response.
Dysbiosis caused by pathogenic bacteria in the intestine can induce and exacerbate anxiety via immunologic and metabolic pathways of the MGB axis. C. jejuni infection increased behaviors such as anxiety by activating c-Fos proteins, markers of neuronal activation, without elevating proinflammatory cytokine levels [72]. In the previously mentioned study by Goehler et al., anxiety increased during Campylobacter jejuni infection, with c-Fos protein induction [40]. Citrobacter rodentium infection led to an increase of anxiety, presumably mediated by the vagal sensory neurons, but without an increase of inflammatory factors [73]. Trichuris muris infection increased anxiety via immunologic and metabolic mechanisms [74].
In contrast, probiotics might ameliorate anxiety. Some species of Lactobacillus and Bifidobacterium have anxiety-relieving effects. Probiotic treatment involving specific strains of B. longum, B. infantis, L. helveticus, or L. rhamnosus alone or in combination normalized behavioral phenotypes in animal anxiety models via correction of immunologic factors and regulation of GABA receptors [7, 74-76].
4.2. Depression
Depression is a mood disorder associated with imbalance of the HPA axis, dysregulation of the immune system, and deficiency of tryptophan metabolism [77-79]. Although it is not known whether an imbalance of intestinal microorganisms is the cause or the effect of depression, several observational studies show a bidirectional interaction between depression and the gut microbiome.
Depression is associated with dysregulation of the HPA axis [80]. Profound changes in gut commensals were observed in mice that experienced maternal separation [81, 82]. De Palma et al. reported behavioral despair with changes in the HPA axis in maternal-separated rats [83]. A direct association between microbes and the HPA axis can be seen by the increased corticosterone and adrenocorticotropin responses to restraint stress in germ-free (GF) mice compared to specific pathogen-free (SPF) mice [84].
In the past few decades of observational studies, the contribution of low-grade inflammation to depression has been consistently demonstrated, but its source has not been clearly elucidated [85, 86]. The observation that a malfunctioning intestinal wall or a leaky gut causes inflammation due to microorganisms has been reported [87, 88]. Under normal conditions, immune cells are separated from intestinal gram-negative bacteria. However, when intestinal permeability changes and barrier integrity is disrupted, certain gram-negative bacteria such as Enterobacteriaceae are translocated across the barrier, and the inflammatory process is activated [89]. Such translocation can result in immunoglobulin (IgA and IgM)-mediated immune responses to the LPS of gram-negative bacteria, which can secondarily lead to depression [90]. Patients with depression had significantly higher TLR-4 expression compared to a control group, which might be related to bacterial translocation [91].
In another recent study, transplantation of fecal microbiota from depressed patients to microbiota-depleted rats altered tryptophan metabolism, leading to anhedonia and anxiety-like behavior in recipient animals. This was the first experimental study to demonstrate that the gut microbiota can play a causal role in the development of depression [92].
4.3. Cognitive Decline
Recent studies have shown a clear link between microbial changes and cognition. Intestinal dysbiosis has been shown to affect cognitive behavior, including learning and memory, in GF mice with intestinal pathogenic bacterial infections and probiotic administration.
GF mice exhibit defective memory and cognitive abilities, with or without exposure to stress [93]. In the same study, learning ability decreased in mice infected with pathogenic C. rodentium, and this cognitive decline was restored by probiotics. This study suggests that the HPA axis, which is not exposed to stress under GF conditions, comprises a neuroendocrine system vulnerable to external threats.
Hepatic encephalopathy (HE) in patients with liver cirrhosis is associated with alteration of the gut microbiota when intestinal barrier dysfunction is present. Dysbiosis with an increase in microbiota such as Porphyromonadaceae and Alcaligenaceae correlated with HE and decreased cognitive function [94]. Antibiotic-induced dysbiosis impaired cognitive performance, including novel object recognition, which is associated with BDNF, N-methyl-d-aspartate receptor subunit 2B, serotonin transporter, and neuropeptide Y [95].
Tests of cognitive function in animals treated with probiotics have repeatedly shown probiotics to be beneficial to memory function. Lactobacillus helveticus improved the stress response and cognitive dysfunction induced by chronic restraint stress in rats [96]. In the hyperammonemia state characteristic of the rat hepatic encephalopathy model, Lactobacillus helveticus led to improvement in both spatial memory and anxiety-like symptoms [97]. Bifidobacterium strains were also effective in improving spatial and non-spatial memory [98, 99].
Increased permeability of the intestine induced by microbial dysbiosis might directly or indirectly affect neurodegenerative disorders, including Alzheimer's disease (AD). In addition, bacteria in the gut can secrete large amounts of amyloid and lipopolysaccharide, which can contribute to the regulation of signaling pathways and the production of proinflammatory cytokines associated with AD. Also, an imbalance of the gut microbiota is closely related to other factors involved in the pathogenesis of AD, such as obesity and type 2 diabetes.
Although the gut microbiome is not known to affect age-related cognitive decline, it might play a role in the vulnerability associated with the aging process. Bacteroidetes and Firmicutes, both dominant phyla, appear increasingly prominent with age. There is potential for an increase of pathogenic bacteria at the expense of beneficial bacteria with age, which could manifest as an increase in the relative abundance of Proteobacteria and a decrease in Bifidobacterium species. This might cause chronic low-grade inflammation, although there is no conclusive evidence for this hypothesis. In mice fed a mixture of Lactobacillus plantarum and Lactobacillus curvatus, age-dependent memory deficits improved through inhibition of NF-κB activation [100].
4.4. Schizophrenia and Bipolar Disorder
Schizophrenia is a severe mental disease in which patients present with delusions, hallucinations, disorganized thoughts and speech, abnormal motor behavior (catatonia or stereotyped movements), and negative symptoms. Previous studies have shown that schizophrenia is associated with various gastrointestinal comorbidities, such as IBS, IBD and celiac disease [101-103]. These findings suggest that gut and microbiota may be involved in patients with schizophrenia.
Chronic inflammation is reportedly involved in the onset and progression of schizophrenia [104, 105]. Structural damage to the intestine can lead to chronic inflammation cause by gut microbiota. An autopsy study of patients with schizophrenia revealed various inflammatory bowel conditions that could lead to instability in the intestinal wall structure [106]. Severance et al. reported that the presence of soluble CD14, a surrogate marker of bacterial translocation in patients with schizophrenia, increased the risk of schizophrenia by 3-fold. However, it was not associated with LPS binding protein, suggesting that gut microbiota components other than LPS may stimulate immune responses [107]. Infection of the GI tract with Toxoplasma gondii changes the commensal bacteria in the intestinal lumen, resulting in dysbiosis of gut microbiota [108]. A cohort-based, case-control study suggested that infection by T. gondii was a risk factor for early-onset schizophrenia [109]. Bacteriophages have also been associated with schizophrenia, as they can alter the metabolism of bacteria and microbial community composition [110]. An increased load of the Lactobacillus bacteriophage has been detected in the oropharyngeal lumen of patients with schizophrenia.
Although inflammation can contribute to bipolar disorder, the role of gut microbiota is unclear [111]. However, several observational studies support the concept that bipolar disorder is associated with gut microbiota. As in schizophrenia, increases in IgM antibodies, suggesting an infection by T. gondii, have been observed in patients with manic episodes [112]. Antibodies to commensal microorganisms are increased in patients with bipolar disorder, which can induce gastrointestinal inflammation, resulting in translocation of gut microbiota [113]. A problem with the brain-gut axis was suspected in a patient without a previous psychiatric history who had a manic episode following total gastrectomy [114]. Treatment with charcoal, a non-psychotropic medication, improves the level of inflammation and limits manic symptoms.
4.5. Autism Spectrum Disorder
Autism spectrum disorder (ASD) describes a set of neurodevelopmental disorders characterized by defects in social interaction, communication, and repetitive stereotyped behaviors. The relationship of ASD to the gut microbiota began with a consideration of Clostridium and a hypothesis that the neurotoxic effects of Clostridium were involved in the onset of ASD [115]. Two human gut microbiome studies showed that the number of species belonging to the genus Clostridium was greater in fecal specimens of autistic children [116, 117]. This was supported by studies demonstrating the apparent benefits of oral vancomycin administration in ASD [118].
It has been hypothesized that dysbiosis of the gut microbiota is involved in the late onset of autism spectrum disorder accompanied by gastrointestinal symptoms, including distention, abdominal discomfort, and changes in bowel habits. A metagenomics analysis of gut bacteria in late-onset autism patients showed a marked decrease in Bacteroidetes (in contrast to an abundance of Bacteroidetes in the control group) and an increase in Sutterella species [119].
This difference in microbial composition was associated with a decrease in gray matter transcription of disaccharidases and hexose transporters, a condition under which carbohydrate malabsorption occurs [120]. Malabsorption of carbohydrates is thought to result from changes in the nutritional resources available to the intestinal bacteria, which in turn causes a change in the microbial composition and dysbiosis.
Subsequent molecular-based studies have found larger numbers of microorganisms that are altered in autistic children. Altered levels of Bifidobacterium, Lactobacillus, Sutterella, Prevotella, Ruminococcus, and Alcaligenaceae have been associated with autism [119, 121, 122].
4.6. Obesity
Food intake and obesity are complex processes involving both the peripheral and central nervous systems [123]. GF rats showed lower total body fat than naturally-fed rats and were resistant to diet-induced obesity [124]. Several human studies have found causal relationships between gut microbiota and obesity. Roux-en-Y gastric bypass surgery has altered fat metabolism by altering the gut microbiota, resulting in a much larger body mass index reduction than expected [125, 126].
Most studies investigating the potential role of gut microbiota in obesity have focused on peripheral control of food intake. The contribution of gut dysbiosis to the pathophysiology of obesity is based on an increased ability to harvest energy in the diet, an altered regulation of lipid and glucose metabolism in the periphery, systemic low-grade inflammation against LPS, and an ability to increase insulin resistance [127-129]. However, it is unclear whether gut microbiota can also directly affect the central regulation of food intake.
Obesity might be an adverse effect of psychotropic drugs (including atypical antipsychotics) acting on the central nervous system. Whether intestinal microbes mediate this effect is under investigation. These questions are based on recent results showing that gut microbiota composition changed after treatment with olanzapine in rats [130]. Morgan et al. showed that olanzapine induces subtle changes in the composition of the gut microbiota, beyond the effects of high-fat diet therapy [131].
5. TREATMENTS TARGETING THE GUT MICRO-BIOTA FOR NEUROPSYCHIATRIC ILLNESSES
5.1. Probiotics
5.1.1. Overview
Probiotics are defined as live microorganisms that provide health benefits to humans or animals when consumed [132]. Lactobacillus and Bifidobacterium species account for most probiotics. Animal studies on probiotic involvement in various neurological diseases have been performed, and many promising results have been reported. There are many studies on probiotics, but only studies reporting changes in neuropsychiatric symptoms in healthy subjects or in subjects with neuropsychiatric illness will be described in this review. Most of these are studies on cognition, autism spectrum disorder, or anxiety and depressive-like behavior (Table 1).
Table 1.
Clinical evidences of gut-microbiota targeting treatments.
|
Clinical
Concern |
Target Population | Treatment | Evaluation Tool |
Behavioral and
Psychological Outcomes |
Biological
Outcome |
Refs. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prebiotics | |||||||||||||
| Anxiety and cognition | Healthy volunteers | B-GOS | Emotional processing tasks Attentional dot-probe task Facial expression recognition task Emotional categorisation and memory |
Increased vigilance attention | Decreased waking cortisol level | Schmidt et al. | |||||||
| Probiotics | |||||||||||||
| Anxiety and Depression | 124 Healthy volunteers | Lactobacillus casei Shirota | POMS Wechsler Memory Scale Retrieval from long-term memory Verbal fluency Eating-associated behavior NART |
Improvement of mood of those who initially poor Better retrieval of long-term memory in placebo |
NA | Benton et al. | |||||||
| Anxiety and Depression | 55 Healthy volunteers | L. helveticus, B. longum | HSCL-90, HADS, PSS | Improvement of anxiety and depression |
Decreased urinary free cortisol | Messaoudi et al. | |||||||
| Anxiety and Depression | 70 healthy petrochemical workers |
L. acidophilus, L. casei, L. rhamnosus, L. bulgaricus, B. lactis, B. breve, B. longum, S. thermophilus |
GHQ, DASS | Improvement of anxiety and depression |
No changes in HPA axis activity | Mohammadi et al. | |||||||
| Cognitive reactivity to sad mood, anxiety and depression | 40 healthy volunteers |
B. bifidum, B. lactis, L. acidophilus, L. brevis, L. casei, L. salivarius, Lactococcus lactis |
LEIDS-r, BDI, BAI | Improvement of total score and rumination item of LEIDS-r, No changes in BDI and BAI scores | NA | Steenbergen et al. | |||||||
| IBS severity | 50 IBS patients |
L. casei | HADS | No change in HADS | IBS severity decreased in only subgroup with predominance of diarrhea | Dapoigny et al. | |||||||
| IBS severity | 74 IBS patients |
L. bulgaricus, S. thermophiles, L. paracasei, L. acidophilus |
HADS | No change in HADS | IBS severity was similarly improved between treatment and control groups | Simren et al. | |||||||
| Stress and anxiety | 140 Healthy medical students | L. casei Shirota | STAI | No change in self-reported anxiety | Decreased salivary cortisol level | Takada et al. | |||||||
| Anxiety and Depression | 35 Chronic fatigue syndrome patients | L. casei Shirota | BDI, BAI | Decreased anxiety symptoms |
Microbiota composition change | Rao et al. | |||||||
|
Clinical Concern |
Target Population | Treatment | Evaluation Tool |
Behavioral and Psychological Outcomes |
Biological Outcome |
Refs. | |||||||
| Probiotics | |||||||||||||
| Autism symptoms |
15 ASD children | L. plantarum | DBC | No change in DBC score | Change in stool Consistency, change of fecal microbiota |
Parracho et al. |
|||||||
| Schizophrenia | 65 Schizophrenia patients | L. rhamnosus, B. animalis | PANSS | No difference of PANSS | Bowel movement improvement | Dickerson et al. |
|||||||
| Fecal Microbiota Transplantation | |||||||||||||
| Autism symptoms |
Autism patients | Human fecal extract | CARS, ABC, SRS | ASD and GI symptoms improvement | Microbiota composition change | Kang et al. |
|||||||
POMS, Profile of mood states; NART, National Adult Reading Test; NA, not applicable; HADS, Hospital anxiety and depression scale; HSCL-90, Hopkins symptoms checklist; PSS, Perceived stress scale; GHQ, General Health Questionnaire; DASS, Depression Anxiety Stress Scales; LEIDS-r, Leiden Index of Depression Sensitivity; BDI, Beck depression inventory; BAI, Beck anxiety inventory; IBS, Irritable bowel syndrome; STAI, State-Trait Anxiety Inventory; PANSS, Positive and Negative Syndrome Scale; DBC, Development Behavior Checklist; CARS, Childhood Autism Rating Scale; ABC, Aberrant Behavior Checklist, SRS, The Social Responsiveness Scale.
Studies on probiotics with positive results have shown that low-grade inflammation is reduced, neurotrophic factors such as BDNF are modulated, gut permeability is restored, and the composition of the gut microbiome changes.
5.1.2. Clinical Studies
Studies administering probiotics to humans are relatively rare. Usually, questionnaires are used to examine the overall mental status of healthy populations. Many studies have been conducted on healthy participants, rather than on patients with a specific diagnosis, to assess behavioral and psychological symptoms (e.g., anxiety, depression, stress, and behavioral problems).
In these studies, concentrations of the probiotic strains administered ranged from 107 to 1010 CFU/ml, and their usage by recipients varied. The strains were administered alone or as mixed strains.
5.1.2.1. Studies on Healthy Populations
Benton et al. conducted the first study of probiotics, using L. casei Shirota-containing milk in depressed and healthy subjects [133]. The main findings were that probiotics improved mood in the bottom third of the depressed group and memory in all of the subjects. In a six-week clinical trial with petrochemical workers, groups receiving a probiotic capsule (containing L. casei, L. acidophilus, L. rhamnosus, L. bulgaricus, B. breve, B. longum, and Streptococcus thermophiles) or probiotic yogurt (containing B. lactis and L. acidophilus) showed alleviated anxiety and depression compared to groups receiving a placebo capsule or conventional yogurt [134].
According to two studies conducted by Messaoudi et al., a probiotic formulation of B. longum and L. helveticus also improved both anxiety and depression [135].
A study using L. casei Shirota showed a decrease in salivary cortisol levels in college students in response to stress, but there was no change in the average State-Trait Anxiety Inventory score [136].
5.1.2.2. Chronic Fatigue Syndrome
In a study of patients with chronic fatigue syndrome, L. casei Shirota was used, and anxiety levels decreased after treatment [137].
5.1.2.3. Schizophrenia
In a clinical study of 65 patients with asthma, PANSS scores did not change after four weeks of administration of L. rhamnosus GG (ATCC 53103) and B. animalis subsp. lactis Bb12, but bowel function was improved [138].
5.1.2.4. Autism Spectrum Disorder
Studies investigating the effects of probiotics in children with autism spectrum disorder are limited. There is one
longitudinal study of probiotics that considers certain autistic symptoms as the primary outcome. In this study, after two months of receiving probiotics capsules containing Lactobacillus acidophilus, the ability to concentrate and follow commands improved for the study subjects [139].
Recently, intriguing research has suggested that the administration of probiotics might have a preventive effect on ASD. In a study of Lactobacillus rhamnosus given at early postnatal times and followed for 13 years, the group receiving no probiotics was more affected by Asperger syndrome and attention-deficit hyperactivity disorder than the group receiving the probiotics, which was not prone to develop those disorders [140].
5.1.2.5. Cognitive Function
In human studies, intestinal microorganisms have been shown to have different effects on both cognitive function and intestinal disease, including IBD and IBS, diabetes, and hepatic encephalopathy.
Benton et al. reported that, in a placebo-controlled study with milk containing L. casei Shirota in healthy subjects, episodic memory and verbal fluency were not better in the probiotics group [141]. Sharma et al. reported improved psychomotor, attention, and visuo-constructive abilities after administration of probiotics (a mixture of Lactobacillus and Bifidobacterium) to subjects with minimal hepatic encephalopathy symptoms [142].
Clinical evidence of improvement of age-related cognitive decline or Alzheimer's disease with probiotics use is still lacking, despite the fact that probiotic supplements are commonly used by the elderly. In a recent, randomized, double-blind trial, a significant increase in MMSE scores was reported for patients with Alzheimer's disease after they received daily doses of probiotics for 12 weeks [143].
5.1.2.6. Obesity
Altering obesity through probiotics is an attractive concept. To date, studies have shown that probiotic effects vary greatly depending on the probiotic strain(s) administered. Diet-induced obese mice treated with L. curvatus and L. plantarum showed marked weight loss and down-regulation of inflammation-inducing genes (TNF-α, IL-6, IL-1β, and MCP) in adipose and liver tissue [144]. Administration of fermented milk containing Lactobacillus gasseri in two human clinical trials was also associated with a decrease in BMI, indicative of beneficial and sustained energy expenditure [145, 146].
5.2. Prebiotics and Diet
The term prebiotics refers to non-digestible fibers that are selectively metabolized in the small intestine and promote the growth of beneficial gut microbiota such as Lactobacillus and Bifidobacterium.
Major prebiotics include galacto-oligosaccharide (GOS) and fructo-oligosaccharide (FOS), inulins, and oligofructose. These prebiotics increase the level of Bifidobacterium in the intestinal tract, benefiting the microbial-gut-brain axis [147]. This bifidogenic effect of prebiotics seems to normalize the composition of Lactobacillus, Bacteroides, and Bifidobacterium [143, 148]. GOS and FOS further augment the production of SCFAs as a result of the normalized gut microbiota composition [149]. In both animal and human studies, prebiotics have been reported to improve inflammatory profiles and to alleviate psychological distress [150].
Clinical studies on the effects of prebiotics on behavior are very limited. In a recent study, healthy volunteers consumed Bimuno®-galactooligosaccharides (B-GOS), FOS, or placebo for three weeks. Researchers measured the waking cortisol response to assess stress levels and performed neuropsychological tests to evaluate attentional vigilance and emotional processes. The subjects who ingested B-GOS had significantly lower waking cortisol levels and greater attentional vigilance [151]. Another recent study on wheat dextrin in overweight adults demonstrated a progressive and significant increase in satiety and a decrease in hunger feelings [152].
5.3. Fecal Microbiota Transplantation
Fecal Microbiota Transplantation (FMT) refers to transplantation of healthy human feces to a suspected gut dysbiosis patient to regulate the intestinal microbiota. When the normal gut microbiota are destroyed by antibiotic treatment and C. difficile enteritis occurs, the recovery of normal bacterial flora by FMT can be outstanding [153]. As a result, interest in FMT has begun to increase.
FMT can also be an effective treatment for IBS. IBS showed a remission rate of 36-89% after FMT treatment [154]. Recently, Kang et al. published the first FMT trial in a neuropsychiatric area. In this eight-week, open-label clinical trial to evaluate the impact of FMT on GI and ASD symptoms, both were significantly reduced. Improvements in GI and ASD symptoms persisted eight weeks after treatment [155].
CONCLUSION
Many recent studies over the last decade have played an important role in recognizing the importance of gut microbiota in brain function. The results of major studies show that the bidirectional interaction between microorganisms and the brain affects various CNS activities (such as stress response, behavior, and mood) through immune and neuroendocrine system pathways. It is now clear that the gut microbiota directly or indirectly affect neuropsychiatric illness. Whether microbial dysbiosis is the cause or a complication of illness must be further investigated.
It might be premature to discuss the therapeutic potential of probiotics at this point. Several preclinical trials have shown that administering probiotics in animal subjects positively alleviates psychological symptoms. In a summary of studies to date, B. longum, B. breve, B. infantis, L. helveticus, L. rhamnosus, L. plantarum, and L. casei are presumed to be the most effective in ameliorating CNS functions related to mental disorders (anxiety, depression, mood disorders, and stress responses). Although a few promising clinical results of prebiotics and FMT have been reported, the number of clinical studies is still small.
Additional studies are required to substantiate the clinical use of probiotics, prebiotics and FMT. First, probiotic or prebiotic-induced endocrine, immune, neurochemical, and metabolic changes at the physiological level must be observed in humans. There have been too few controlled trials of probiotics, prebiotics and FMT based on precise neuropsychiatric diagnoses. Further investments into large-scale clinical trials by food and pharmaceutical companies are needed to shed light on the efficacy of probiotics and prebiotics for conditions such as depression, anxiety, and autism.
Acknowledgements
Declared none.
LIST OF ABBREVIATIONS
- IBD
Irritable Bowel Disease
- CNS
Central Nervous System
- IBS
Irritable Bowel Syndrome
- HPA
Hypothalamic-Pituitary-Adrenal
- GF
Germ Free
- SPF
Specific Pathogen Free
- MGB
Microbiota-Gut-Brain
- SCFA
Short Chain Fatty Acid
- GABA
Gamma Aminobutyric Acid
- ASD
Autism Spectrum Disorder
- FMT
Fecal Microbiota Transplantation
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Gill S.R., Pop M., Deboy R.T., Eckburg P.B., Turnbaugh P.J., Samuel B.S., Gordon J.I., Relman D.A., Fraser-Liggett C.M., Nelson K.E. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355–1359. doi: 10.1126/science.1124234. [http://dx.doi.org/ 10.1126/science.1124234]. [PMID: 16741115]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Handley S.A., Dube P.H., Miller V.L. Histamine signaling through the H(2) receptor in the Peyer’s patch is important for controlling Yersinia enterocolitica infection. Proc. Natl. Acad. Sci. USA. 2006;103(24):9268–9273. doi: 10.1073/pnas.0510414103. [http://dx.doi.org/10.1073/pnas. 0510414103]. [PMID: 16717182]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jandhyala S.M., Talukdar R., Subramanyam C., Vuyyuru H., Sasikala M., Nageshwar R.D. Role of the normal gut microbiota. World J. Gastroenterol. 2015;21(29):8787–8803. doi: 10.3748/wjg.v21.i29.8787. [http://dx.doi. org/10.3748/wjg.v21.i29.8787]. [PMID: 26269668]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szymanski S.R., Cannon T.D., Gallacher F., Erwin R.J., Gur R.E. Course of treatment response in first-episode and chronic schizophrenia. Am. J. Psychiatry. 1996;153(4):519–525. doi: 10.1176/ajp.153.4.519. [http://dx. doi.org/10.1176/ajp.153.4.519]. [PMID: 8599400]. [DOI] [PubMed] [Google Scholar]
- 5.Rush A.J., Trivedi M.H., Wisniewski S.R., Nierenberg A.A., Stewart J.W., Warden D., Niederehe G., Thase M.E., Lavori P.W., Lebowitz B.D., McGrath P.J., Rosenbaum J.F., Sackeim H.A., Kupfer D.J., Luther J., Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [http://dx.doi.org/10.1176/ajp.2006.163.11.1905]. [PMID: 17074942]. [DOI] [PubMed] [Google Scholar]
- 6.Neufeld K.M., Kang N., Bienenstock J., Foster J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011;23(3):255–264. doi: 10.1111/j.1365-2982.2010.01620.x. [http:// dx.doi.org/10.1111/j.1365-2982.2010.01620.x]. [DOI] [PubMed] [Google Scholar]
- 7.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [http://dx.doi.org/10.1073/ pnas.1102999108]. [PMID: 21876150]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ursell L.K., Metcalf J.L., Parfrey L.W., Knight R. Defining the human microbiome. Nutr. Rev. 2012;70(Suppl. 1):S38–S44. doi: 10.1111/j.1753-4887.2012.00493.x. [http:// dx.doi.org/10.1111/j.1753-4887.2012.00493.x]. [PMID: 22861806]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., Mende D.R., Li J., Xu J., Li S., Li D., Cao J., Wang B., Liang H., Zheng H., Xie Y., Tap J., Lepage P., Bertalan M., Batto J.M., Hansen T., Le Paslier D., Linneberg A., Nielsen H.B., Pelletier E., Renault P., Sicheritz-Ponten T., Turner K., Zhu H., Yu C., Li S., Jian M., Zhou Y., Li Y., Zhang X., Li S., Qin N., Yang H., Wang J., Brunak S., Doré J., Guarner F., Kristiansen K., Pedersen O., Parkhill J., Weissenbach J., Bork P., Ehrlich S.D., Wang J., Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [http://dx.doi.org/10.1038/nature08821]. [PMID: 20203603]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckburg P.B., Bik E.M., Bernstein C.N., Purdom E., Dethlefsen L., Sargent M., Gill S.R., Nelson K.E., Relman D.A. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. doi: 10.1126/science.1110591. [http://dx.doi.org/10.1126/science.1110591]. [PMID: 15831718]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arumugam M., Raes J., Pelletier E., Le Paslier D., Yamada T., Mende D.R., Fernandes G.R., Tap J., Bruls T., Batto J.M., Bertalan M., Borruel N., Casellas F., Fernandez L., Gautier L., Hansen T., Hattori M., Hayashi T., Kleerebezem M., Kurokawa K., Leclerc M., Levenez F., Manichanh C., Nielsen H.B., Nielsen T., Pons N., Poulain J., Qin J., Sicheritz-Ponten T., Tims S., Torrents D., Ugarte E., Zoetendal E.G., Wang J., Guarner F., Pedersen O., de Vos W.M., Brunak S., Doré J., Antolín M., Artiguenave F., Blottiere H.M., Almeida M., Brechot C., Cara C., Chervaux C., Cultrone A., Delorme C., Denariaz G., Dervyn R., Foerstner K.U., Friss C., van de Guchte M., Guedon E., Haimet F., Huber W., van Hylckama-Vlieg J., Jamet A., Juste C., Kaci G., Knol J., Lakhdari O., Layec S., Le Roux K., Maguin E., Mérieux A., Melo Minardi R., M’rini C., Muller J., Oozeer R., Parkhill J., Renault P., Rescigno M., Sanchez N., Sunagawa S., Torrejon A., Turner K., Vandemeulebrouck G., Varela E., Winogradsky Y., Zeller G., Weissenbach J., Ehrlich S.D., Bork P., Bork P. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. doi: 10.1038/nature09944. [http://dx.doi.org/10.1038/nature09944]. [PMID: 21508958]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlsson C.L., Molin G., Cilio C.M., Ahrné S. The pioneer gut microbiota in human neonates vaginally born at term-a pilot study. Pediatr. Res. 2011;70(3):282–286. doi: 10.1203/PDR.0b013e318225f765. [http://dx.doi.org/10.1203/ PDR.0b013e318225f765]. [PMID: 21629156]. [DOI] [PubMed] [Google Scholar]
- 13.Dominguez-Bello M.G., Costello E.K., Contreras M., Magris M., Hidalgo G., Fierer N., Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [http://dx.doi.org/10.1073/pnas.1002601107]. [PMID: 20566857]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biasucci G., Rubini M., Riboni S., Morelli L., Bessi E., Retetangos C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum. Dev. 2010;86(Suppl. 1):13–15. doi: 10.1016/j.earlhumdev.2010.01.004. [http:// dx.doi.org/10.1016/j.earlhumdev.2010.01.004]. [PMID: 20133091]. [DOI] [PubMed] [Google Scholar]
- 15.Huurre A., Kalliomäki M., Rautava S., Rinne M., Salminen S., Isolauri E. Mode of delivery - effects on gut microbiota and humoral immunity. Neonatology. 2008;93(4):236–240. doi: 10.1159/000111102. [http://dx.doi. org/10.1159/000111102]. [PMID: 18025796]. [DOI] [PubMed] [Google Scholar]
- 16.Roger L.C., McCartney A.L. Longitudinal investigation of the faecal microbiota of healthy full-term infants using fluorescence in situ hybridization and denaturing gradient gel electrophoresis. Microbiology. 2010;156(Pt 11):3317–3328. doi: 10.1099/mic.0.041913-0. [http://dx.doi.org/10. 1099/mic.0.041913-0]. [PMID: 20829292]. [DOI] [PubMed] [Google Scholar]
- 17.Favier C.F., Vaughan E.E., De Vos W.M., Akkermans A.D. Molecular monitoring of succession of bacterial communities in human neonates. Appl. Environ. Microbiol. 2002;68(1):219–226. doi: 10.1128/AEM.68.1.219-226.2002. [http://dx.doi.org/10.1128/AEM.68.1.219-226.2002]. [PMID: 11772630]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu G.D., Chen J., Hoffmann C., Bittinger K., Chen Y-Y., Keilbaugh S.A., Bewtra M., Knights D., Walters W.A., Knight R., Sinha R., Gilroy E., Gupta K., Baldassano R., Nessel L., Li H., Bushman F.D., Lewis J.D. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. doi: 10.1126/science.1208344. [http://dx.doi.org/10.1126/science.1208344]. [PMID: 21885731]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberfroid M., Gibson G.R., Hoyles L., McCartney A.L., Rastall R., Rowland I., Wolvers D., Watzl B., Szajewska H., Stahl B., Guarner F., Respondek F., Whelan K., Coxam V., Davicco M.J., Léotoing L., Wittrant Y., Delzenne N.M., Cani P.D., Neyrinck A.M., Meheust A. Prebiotic effects: metabolic and health benefits. Br. J. Nutr. 2010;104(S2) Suppl. 2:S1–S63. doi: 10.1017/S0007114510003363. [http://dx. doi.org/10.1017/S0007114510003363]. [PMID: 20920376]. [DOI] [PubMed] [Google Scholar]
- 20.Forsythe P., Sudo N., Dinan T., Taylor V.H., Bienenstock J. Mood and gut feelings. Brain Behav. Immun. 2010;24(1):9–16. doi: 10.1016/j.bbi.2009.05.058. [http://dx.doi.org/10.1016/j.bbi.2009.05.058]. [PMID: 19481599]. [DOI] [PubMed] [Google Scholar]
- 21.Lange K., Buerger M., Stallmach A., Bruns T. Effects of Antibiotics on Gut Microbiota. Dig. Dis. 2016;34(3):260–268. doi: 10.1159/000443360. [http://dx.doi.org/10.1159/000443360]. [PMID: 27028893]. [DOI] [PubMed] [Google Scholar]
- 22.Konturek S.J., Konturek J.W., Pawlik T., Brzozowski T. Brain-gut axis and its role in the control of food intake. J. Physiol. Pharmacol. 2004;55(1 Pt 2):137–154. [PMID: 15082874]. [PubMed] [Google Scholar]
- 23.Whitehead W.E., Palsson O., Jones K.R. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122(4):1140–1156. doi: 10.1053/gast.2002.32392. [http://dx.doi.org/10.1053/gast.2002.32392]. [PMID: 11910364]. [DOI] [PubMed] [Google Scholar]
- 24.Cryan J.F., Dinan T.G. Gut microbiota: Microbiota and neuroimmune signalling-Metchnikoff to microglia. Nat. Rev. Gastroenterol. Hepatol. 2015;12(9):494–496. doi: 10.1038/nrgastro.2015.127. [http://dx.doi.org/10.1038/nrgastro. 2015.127]. [PMID: 26215386]. [DOI] [PubMed] [Google Scholar]
- 25.Sherwin E., Sandhu K.V., Dinan T.G., Cryan J.F. May the force be with you: the light and dark sides of the microbiota–gut–brain axis in neuropsychiatry. CNS Drugs. 2016;30(11):1019–1041. doi: 10.1007/s40263-016-0370-3. [http://dx.doi.org/10.1007/s40263-016-0370-3]. [PMID: 27417321]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sudo N., Chida Y., Aiba Y., Sonoda J., Oyama N., Yu X.N., Kubo C., Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004;558(Pt 1):263–275. doi: 10.1113/jphysiol.2004.063388. [http://dx.doi.org/10.1113/ jphysiol.2004.063388]. [PMID: 15133062]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galley J.D., Nelson M.C., Yu Z., Dowd S.E., Walter J., Kumar P.S., Lyte M., Bailey M.T. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014;14:189. doi: 10.1186/1471-2180-14-189. [http://dx.doi.org/10.1186/ 1471-2180-14-189]. [PMID: 25028050]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey M.T., Coe C.L. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev. Psychobiol. 1999;35(2):146–155. [http://dx.doi.org/10.1002/(SICI)1098-2302(199909)35:2<146:AID-DEV7>3.0.CO;2-G]. [PMID: 10461128]. [PubMed] [Google Scholar]
- 29.Rubio C.A., Huang C.B. Quantification of the sulphomucin-producing cell population of the colonic mucosa during protracted stress in rats. In Vivo. 1992;6(1):81–84. [PMID: 1627747]. [PubMed] [Google Scholar]
- 30.Murakami T., Kamada K., Mizushima K., Higashimura Y., Katada K., Uchiyama K., Handa O., Takagi T., Naito Y., Itoh Y. Changes in intestinal motility and gut microbiota composition in a rat stress model. Digestion. 2017;95(1):55–60. doi: 10.1159/000452364. [http://dx.doi.org/ 10.1159/000452364]. [PMID: 28052282]. [DOI] [PubMed] [Google Scholar]
- 31.Spitz J., Hecht G., Taveras M., Aoys E., Alverdy J. The effect of dexamethasone administration on rat intestinal permeability: the role of bacterial adherence. Gastroenterology. 1994;106(1):35–41. doi: 10.1016/s0016-5085(94)94155-6. [http://dx.doi.org/10.1016/S0016-5085(94)94155-6]. [PMID: 8276206]. [DOI] [PubMed] [Google Scholar]
- 32.Ünsal H., Balkaya M., Ünsal C., Biyik H., Başbülbül G., Poyrazoğlu E. The short-term effects of different doses of dexamethasone on the numbers of some bacteria in the ileum. Dig. Dis. Sci. 2008;53(7):1842–1845. doi: 10.1007/s10620-007-0089-6. [http://dx.doi.org/10.1007/s10620-007-0089-6]. [PMID: 18049898]. [DOI] [PubMed] [Google Scholar]
- 33.Wallon C., Yang P.C., Keita A.V., Ericson A.C., McKay D.M., Sherman P.M., Perdue M.H., Söderholm J.D. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut. 2008;57(1):50–58. doi: 10.1136/gut.2006.117549. [http://dx.doi.org/10.1136/gut.2006.117549]. [PMID: 17525093]. [DOI] [PubMed] [Google Scholar]
- 34.Daulatzai M.A. Chronic functional bowel syndrome enhances gut-brain axis dysfunction, neuroinflammation, cognitive impairment, and vulnerability to dementia. Neurochem. Res. 2014;39(4):624–644. doi: 10.1007/s11064-014-1266-6. [http://dx.doi.org/10.1007/s11064-014-1266-6]. [PMID: 24590859]. [DOI] [PubMed] [Google Scholar]
- 35.Kelly J.R., Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G., Hyland N.P. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [http://dx.doi.org/10.3389/fncel.2015. 00392]. [PMID: 26528128]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caesar R., Reigstad C.S., Bäckhed H.K., Reinhardt C., Ketonen M., Lundén G.O., Cani P.D., Bäckhed F. Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut. 2012;61(12):1701–1707. doi: 10.1136/gutjnl-2011-301689. [http://dx.doi.org/10.1136/gutjnl-2011-301689]. [PMID: 22535377]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bluthé R.M., Walter V., Parnet P., Layé S., Lestage J., Verrier D., Poole S., Stenning B.E., Kelley K.W., Dantzer R. Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C. R. Acad. Sci. III. 1994;317(6):499–503. [PMID: 7987701]. [PubMed] [Google Scholar]
- 38.Layé S., Bluthé R.M., Kent S., Combe C., Médina C., Parnet P., Kelley K., Dantzer R. Subdiaphragmatic vagotomy blocks induction of IL-1 beta mRNA in mice brain in response to peripheral LPS. Am. J. Physiol. 1995;268(5 Pt 2):R1327–R1331. doi: 10.1152/ajpregu.1995.268.5.R1327. [PMID: 7771597]. [DOI] [PubMed] [Google Scholar]
- 39.Goehler L.E., Gaykema R.P., Opitz N., Reddaway R., Badr N., Lyte M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav. Immun. 2005;19(4):334–344. doi: 10.1016/j.bbi.2004.09.002. [http://dx.doi. org/10.1016/j.bbi.2004.09.002]. [PMID: 15944073]. [DOI] [PubMed] [Google Scholar]
- 40.Goehler L.E., Park S.M., Opitz N., Lyte M., Gaykema R.P. Campylobacter jejuni infection increases anxiety-like behavior in the holeboard: possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain Behav. Immun. 2008;22(3):354–366. doi: 10.1016/j.bbi.2007.08.009. [http://dx.doi.org/10.1016/j.bbi.2007.08.009]. [PMID: 17920243]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bercik P., Park A.J., Sinclair D., Khoshdel A., Lu J., Huang X., Deng Y., Blennerhassett P.A., Fahnestock M., Moine D., Berger B., Huizinga J.D., Kunze W., McLean P.G., Bergonzelli G.E., Collins S.M., Verdu E.F. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011;23(12):1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [http:// dx.doi.org/10.1111/j.1365-2982.2011.01796.x]. [PMID: 21988661]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogawa S., Fujii T., Koga N., Hori H., Teraishi T., Hattori K., Noda T., Higuchi T., Motohashi N., Kunugi H. Plasma L-tryptophan concentration in major depressive dis-order: new data and meta-analysis. J. Clin. Psychiatry. 2014;75(9):e906–e915. doi: 10.4088/JCP.13r08908. [DOI] [PubMed] [Google Scholar]
- 43.Yano J.M., Yu K., Donaldson G.P., Shastri G.G., Ann P., Ma L., Nagler C.R., Ismagilov R.F., Mazmanian S.K., Hsiao E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [http://dx.doi.org/10. 1016/j.cell.2015.02.047]. [PMID: 25860609]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wikoff W.R., Anfora A.T., Liu J., Schultz P.G., Lesley S.A., Peters E.C., Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA. 2009;106(10):3698–3703. doi: 10.1073/pnas.0812874106. [http://dx.doi.org/ 10.1073/pnas.0812874106]. [PMID: 19234110]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desbonnet L., Garrett L., Clarke G., Bienenstock J., Dinan T.G. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008;43(2):164–174. doi: 10.1016/j.jpsychires.2008.03.009. [http://dx.doi.org/10.1016/j.jpsychires.2008.03.009]. [PMID: 18456279]. [DOI] [PubMed] [Google Scholar]
- 46.Dokmanovic M., Clarke C., Marks P.A. Histone deacetylase inhibitors: overview and perspectives. Mol. Cancer Res. 2007;5(10):981–989. doi: 10.1158/1541-7786.MCR-07-0324. [http://dx.doi.org/10.1158/1541-7786.MCR-07-0324]. [PMID: 17951399]. [DOI] [PubMed] [Google Scholar]
- 47.Kimura I., Inoue D., Maeda T., Hara T., Ichimura A., Miyauchi S., Kobayashi M., Hirasawa A., Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA. 2011;108(19):8030–8035. doi: 10.1073/pnas.1016088108. [http://dx.doi.org/10.1073/pnas. 1016088108]. [PMID: 21518883]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nankova B.B., Agarwal R., MacFabe D.F., La Gamma E.F. Enteric bacterial metabolites propionic and butyric acid modulate gene expression, including CREB-dependent catecholaminergic neurotransmission, in PC12 cells--possible relevance to autism spectrum disorders. PLoS One. 2014;9(8):e103740. doi: 10.1371/journal.pone.0103740. [http://dx.doi. org/10.1371/journal.pone.0103740]. [PMID: 25170769]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El-Ansary A.K., Ben Bacha A., Kotb M. Etiology of autistic features: the persisting neurotoxic effects of propionic acid. J. Neuroinflammation. 2012;9:74. doi: 10.1186/1742-2094-9-74. [http://dx.doi.org/10.1186/1742-2094-9-74]. [PMID: 22531301]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Vadder F., Kovatcheva-Datchary P., Goncalves D., Vinera J., Zitoun C., Duchampt A., Bäckhed F., Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1-2):84–96. doi: 10.1016/j.cell.2013.12.016. [http://dx.doi.org/10. 1016/j.cell.2013.12.016]. [PMID: 24412651]. [DOI] [PubMed] [Google Scholar]
- 51.Huuskonen J., Suuronen T., Nuutinen T., Kyrylenko S., Salminen A. Regulation of microglial inflammatory response by sodium butyrate and short-chain fatty acids. Br. J. Pharmacol. 2004;141(5):874–880. doi: 10.1038/sj.bjp.0705682. [http://dx.doi.org/10.1038/sj.bjp.0705682]. [PMID: 14744800]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Erny D., Hrabě de Angelis A.L., Jaitin D., Wieghofer P., Staszewski O., David E., Keren-Shaul H., Mahlakoiv T., Jakobshagen K., Buch T., Schwierzeck V., Utermöhlen O., Chun E., Garrett W.S., McCoy K.D., Diefenbach A., Staeheli P., Stecher B., Amit I., Prinz M. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015;18(7):965–977. doi: 10.1038/nn.4030. [http://dx.doi.org/10.1038/nn.4030]. [PMID: 26030851]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Almeida L.M., Funchal C., Gottfried C., Wajner M., Pessoa-Pureur R. Propionic acid induces cytoskeletal alterations in cultured astrocytes from rat cerebral cortex. Metab. Brain Dis. 2006;21(1):51–62. doi: 10.1007/s11011-006-9002-9. [http://dx.doi.org/10.1007/s11011-006-9002-9]. [PMID: 16773470]. [DOI] [PubMed] [Google Scholar]
- 54.Shultz S.R., Macfabe D.F., Martin S., Jackson J., Taylor R., Boon F., Ossenkopp K.P., Cain D.P. Intracerebroventricular injections of the enteric bacterial metabolic product propionic acid impair cognition and sensorimotor ability in the Long-Evans rat: further development of a rodent model of autism. Behav. Brain Res. 2009;200(1):33–41. doi: 10.1016/j.bbr.2008.12.023. [http://dx.doi.org/10.1016/j.bbr.2008.12. 023]. [PMID: 19154758]. [DOI] [PubMed] [Google Scholar]
- 55.Barrett E., Ross R.P., O’Toole P.W., Fitzgerald G.F., Stanton C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012;113(2):411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [http:// dx.doi.org/10.1111/j.1365-2672.2012.05344.x]. [PMID: 22612585]. [DOI] [PubMed] [Google Scholar]
- 56.Takanaga H., Ohtsuki S. Hosoya Ki; Terasaki, T. GAT2/BGT-1 as a system responsible for the transport of γ-aminobutyric acid at the mouse blood-brain barrier. J. Cereb. Blood Flow Metab. 2001;21(10):1232–1239. doi: 10.1097/00004647-200110000-00012. [http://dx.doi.org/10.1097/00004647-200110000-00012]. [PMID: 11598501]. [DOI] [PubMed] [Google Scholar]
- 57.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [http://dx.doi.org/10.1073/ pnas.1102999108]. [PMID: 21876150]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Janik R., Thomason L.A.M., Stanisz A.M., Forsythe P., Bienenstock J., Stanisz G.J. Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. Neuroimage. 2016;125:988–995. doi: 10.1016/j.neuroimage.2015.11.018. [http:// dx.doi.org/10.1016/j.neuroimage.2015.11.018]. [PMID: 26577887]. [DOI] [PubMed] [Google Scholar]
- 59.Asano Y., Hiramoto T., Nishino R., Aiba Y., Kimura T., Yoshihara K., Koga Y., Sudo N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;303(11):G1288–G1295. doi: 10.1152/ajpgi.00341.2012. [http://dx.doi.org/10.1152/ajpgi.00341. 2012]. [PMID: 23064760]. [DOI] [PubMed] [Google Scholar]
- 60.Hernández-Romero D., Sanchez-Amat A., Solano F. A tyrosinase with an abnormally high tyrosine hydroxylase/dopa oxidase ratio. FEBS J. 2006;273(2):257–270. doi: 10.1111/j.1742-4658.2005.05038.x. [http://dx.doi.org/10.1111/j.1742-4658.2005.05038.x]. [PMID: 16403014]. [DOI] [PubMed] [Google Scholar]
- 61.Kuley E., Balıkcı E., Özoğul I., Gökdogan S., Ozoğul F. Stimulation of cadaverine production by foodborne pathogens in the presence of Lactobacillus, Lactococcus, and Streptococcus spp. J. Food Sci. 2012;77(12):M650–M658. doi: 10.1111/j.1750-3841.2012.02825.x. [http://dx.doi.org/10.1111/j. 1750-3841.2012.02825.x]. [PMID: 22853653]. [DOI] [PubMed] [Google Scholar]
- 62.Matsumoto M., Kibe R., Ooga T., Aiba Y., Sawaki E., Koga Y., Benno Y. Cerebral low-molecular metabolites influenced by intestinal microbiota: a pilot study. Front. Syst. Neurosci. 2013;7:9. doi: 10.3389/fnsys.2013.00009. [http://dx.doi.org/10.3389/fnsys.2013.00009]. [PMID: 23630473]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishino R., Mikami K., Takahashi H., Tomonaga S., Furuse M., Hiramoto T., Aiba Y., Koga Y., Sudo N. Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. Neurogastroenterol. Motil. 2013;25(6):521–528. doi: 10.1111/nmo.12110. [http://dx.doi.org/10.1111/nmo.12110]. [PMID: 23480302]. [DOI] [PubMed] [Google Scholar]
- 64.Fernández-Novoa L., Cacabelos R. Histamine function in brain disorders. Behav. Brain Res. 2001;124(2):213–233. doi: 10.1016/s0166-4328(01)00215-7. [http://dx. doi.org/10.1016/S0166-4328(01)00215-7]. [PMID: 11640975]. [DOI] [PubMed] [Google Scholar]
- 65.Thomas C.M., Hong T., van Pijkeren J.P., Hemarajata P., Trinh D.V., Hu W., Britton R.A., Kalkum M., Versalovic J. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One. 2012;7(2):e31951. doi: 10.1371/journal.pone.0031951. [http://dx.doi.org/10.1371/journal.pone.0031951]. [PMID: 22384111]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diebel L.N., Liberati D.M., Hall-Zimmerman L. H2 blockers decrease gut mucus production and lead to barrier dysfunction in vitro. Surgery. 2011;150(4):736–743. doi: 10.1016/j.surg.2011.07.067. [http://dx.doi.org/10.1016/ j.surg.2011.07.067]. [PMID: 22000186]. [DOI] [PubMed] [Google Scholar]
- 67.Clarke G., Grenham S., Scully P., Fitzgerald P., Moloney R.D., Shanahan F., Dinan T.G., Cryan J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry. 2013;18(6):666–673. doi: 10.1038/mp.2012.77. [http://dx.doi.org/10.1038/mp.2012.77]. [PMID: 22688187]. [DOI] [PubMed] [Google Scholar]
- 68.Leonard B.E. The HPA and immune axes in stress: the involvement of the serotonergic system. Eur. Psychiatry. 2005;20(Suppl. 3):S302–S306. doi: 10.1016/s0924-9338(05)80180-4. [http://dx.doi.org/10.1016/S0924-9338(05)80180-4]. [PMID: 16459240]. [DOI] [PubMed] [Google Scholar]
- 69.Dinan T.G., Cryan J.F. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37(9):1369–1378. doi: 10.1016/j.psyneuen.2012.03.007. [http://dx.doi.org/ 10.1016/j.psyneuen.2012.03.007]. [PMID: 22483040]. [DOI] [PubMed] [Google Scholar]
- 70.Fukudo S., Kanazawa M. Gene, environment, and brain-gut interactions in irritable bowel syndrome. J. Gastroenterol. Hepatol. 2011;26(s3) Suppl. 3:110–115. doi: 10.1111/j.1440-1746.2011.06631.x. [http://dx.doi.org/10.1111/j.1440-1746.2011.06631.x]. [PMID: 21443722]. [DOI] [PubMed] [Google Scholar]
- 71.O’Malley D., Quigley E.M., Dinan T.G., Cryan J.F. Do interactions between stress and immune responses lead to symptom exacerbations in irritable bowel syndrome? Brain Behav. Immun. 2011;25(7):1333–1341. doi: 10.1016/j.bbi.2011.04.009. [http://dx.doi.org/10.1016/j.bbi.2011.04.009]. [PMID: 21536124]. [DOI] [PubMed] [Google Scholar]
- 72.Gaykema R.P., Goehler L.E., Lyte M. Brain response to cecal infection with Campylobacter jejuni: analysis with Fos immunohistochemistry. Brain Behav. Immun. 2004;18(3):238–245. doi: 10.1016/j.bbi.2003.08.002. [http:// dx.doi.org/10.1016/j.bbi.2003.08.002]. [PMID: 15050651]. [DOI] [PubMed] [Google Scholar]
- 73.Lyte M., Li W., Opitz N., Gaykema R.P., Goehler L.E. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol. Behav. 2006;89(3):350–357. doi: 10.1016/j.physbeh.2006.06.019. [http://dx.doi. org/10.1016/j.physbeh.2006.06.019]. [PMID: 16887154]. [DOI] [PubMed] [Google Scholar]
- 74.Bercik P., Verdu E.F., Foster J.A., Macri J., Potter M., Huang X., Malinowski P., Jackson W., Blennerhassett P., Neufeld K.A., Lu J., Khan W.I., Corthesy-Theulaz I., Cherbut C., Bergonzelli G.E., Collins S.M. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139(6):2102–2112. doi: 10.1053/j.gastro.2010.06.063. [http://dx.doi.org/10.1053/j.gastro.2010.06.063]. [DOI] [PubMed] [Google Scholar]
- 75.McKernan D.P., Fitzgerald P., Dinan T.G., Cryan J.F. The probiotic Bifidobacterium infantis 35624 displays viscer-al antinociceptive effects in the rat. Neurogastroenterol. Motil. 2010;22(9):1029–1035. doi: 10.1111/j.1365-2982.2010.01520.x. [http://dx.doi.org/10.1111/j.1365-2982.2010.01520.x]. [DOI] [PubMed] [Google Scholar]
- 76.Ohland C.L., Kish L., Bell H., Thiesen A., Hotte N., Pankiv E., Madsen K.L. Effects of Lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. Psychoneuroendocrinology. 2013;38(9):1738–1747. doi: 10.1016/j.psyneuen.2013.02.008. [http://dx.doi.org/10.1016/j.psyneuen.2013.02. 008]. [PMID: 23566632]. [DOI] [PubMed] [Google Scholar]
- 77.Dantzer R., O’Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [http://dx.doi.org/10.1038/nrn2297]. [PMID: 18073775]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stetler C., Miller G.E. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom. Med. 2011;73(2):114–126. doi: 10.1097/PSY.0b013e31820ad12b. [http://dx.doi.org/ 10.1097/PSY.0b013e31820ad12b]. [PMID: 21257974]. [DOI] [PubMed] [Google Scholar]
- 79.Schwarcz R., Bruno J.P., Muchowski P.J., Wu H-Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci. 2012;13(7):465–477. doi: 10.1038/nrn3257. [http://dx.doi. org/10.1038/nrn3257]. [PMID: 22678511]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barden N. Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. J. Psychiatry Neurosci. 2004;29(3):185–193. [PMID: 15173895]. [PMC free article] [PubMed] [Google Scholar]
- 81.O’Mahony S.M., Marchesi J.R., Scully P., Codling C., Ceolho A.M., Quigley E.M., Cryan J.F., Dinan T.G. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry. 2009;65(3):263–267. doi: 10.1016/j.biopsych.2008.06.026. [http://dx.doi.org/10.1016/j.biopsych.2008. 06.026]. [PMID: 18723164]. [DOI] [PubMed] [Google Scholar]
- 82.O’Mahony S.M., Hyland N.P., Dinan T.G., Cryan J.F. Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology (Berl.) 2011;214(1):71–88. doi: 10.1007/s00213-010-2010-9. [http://dx.doi.org/10.1007/ s00213-010-2010-9]. [PMID: 20886335]. [DOI] [PubMed] [Google Scholar]
- 83.De Palma G., Blennerhassett P., Lu J., Deng Y., Park A.J., Green W., Denou E., Silva M.A., Santacruz A., Sanz Y., Surette M.G., Verdu E.F., Collins S.M., Bercik P. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat. Commun. 2015;6:7735. doi: 10.1038/ncomms8735. [http://dx.doi.org/ 10.1038/ncomms8735]. [PMID: 26218677]. [DOI] [PubMed] [Google Scholar]
- 84.Sudo N., Chida Y., Aiba Y., Sonoda J., Oyama N., Yu X.N., Kubo C., Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004;558(Pt 1):263–275. doi: 10.1113/jphysiol.2004.063388. [http://dx.doi.org/10.1113/ jphysiol.2004.063388]. [PMID: 15133062]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16(1):22–34. doi: 10.1038/nri.2015.5. [http://dx.doi.org/10.1038/nri.2015. 5]. [PMID: 26711676]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berk M., Williams L.J., Jacka F.N., O’Neil A., Pasco J.A., Moylan S., Allen N.B., Stuart A.L., Hayley A.C., Byrne M.L., Maes M. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11(1):200. doi: 10.1186/1741-7015-11-200. [http://dx.doi.org/10.1186/1741-7015-11-200]. [PMID: 24228900]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maes M., Kubera M., Leunis J-C. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuroendocrinol. Lett. 2008;29(1):117–124. [PMID: 18283240]. [PubMed] [Google Scholar]
- 88.Kelly J.R., Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G., Hyland N.P. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [http://dx.doi.org/10.3389/fncel. 2015.00392]. [PMID: 26528128]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wiest R., Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41(3):422–433. doi: 10.1002/hep.20632. [http://dx.doi.org/10.1002/ hep.20632]. [PMID: 15723320]. [DOI] [PubMed] [Google Scholar]
- 90.Maes M., Kubera M., Leunis J.C., Berk M. Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J. Affect. Disord. 2012;141(1):55–62. doi: 10.1016/j.jad.2012.02.023. [http://dx.doi.org/10.1016/j. jad.2012.02.023]. [PMID: 22410503]. [DOI] [PubMed] [Google Scholar]
- 91.Kéri S., Szabó C., Kelemen O. Expression of Toll-Like Receptors in peripheral blood mononuclear cells and response to cognitive-behavioral therapy in major depressive disorder. Brain Behav. Immun. 2014;40:235–243. doi: 10.1016/j.bbi.2014.03.020. [http://dx.doi.org/10.1016/j.bbi. 2014.03.020]. [PMID: 24726793]. [DOI] [PubMed] [Google Scholar]
- 92.Kelly J.R., Borre Y., O’ Brien C., Patterson E., El Aidy S., Deane J., Kennedy P.J., Beers S., Scott K., Moloney G., Hoban A.E., Scott L., Fitzgerald P., Ross P., Stanton C., Clarke G., Cryan J.F., Dinan T.G. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [http://dx.doi.org/10. 1016/j.jpsychires.2016.07.019]. [PMID: 27491067]. [DOI] [PubMed] [Google Scholar]
- 93.Gareau M.G., Wine E., Rodrigues D.M., Cho J.H., Whary M.T., Philpott D.J., Macqueen G., Sherman P.M. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307–317. doi: 10.1136/gut.2009.202515. [http://dx.doi.org/10.1136/gut.2009.202515]. [PMID: 20966022]. [DOI] [PubMed] [Google Scholar]
- 94.Bajaj J.S., Ridlon J.M., Hylemon P.B., Thacker L.R., Heuman D.M., Smith S., Sikaroodi M., Gillevet P.M. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302(1):G168–G175. doi: 10.1152/ajpgi.00190.2011. [http://dx. doi.org/10.1152/ajpgi.00190.2011]. [PMID: 21940902]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fröhlich E.E., Farzi A., Mayerhofer R., Reichmann F., Jačan A., Wagner B., Zinser E., Bordag N., Magnes C., Fröhlich E., Kashofer K., Gorkiewicz G., Holzer P. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav. Immun. 2016;56:140–155. doi: 10.1016/j.bbi.2016.02.020. [http:// dx.doi.org/10.1016/j.bbi.2016.02.020]. [PMID: 26923630]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liang S., Wang T., Hu X., Luo J., Li W., Wu X., Duan Y., Jin F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015;310:561–577. doi: 10.1016/j.neuroscience.2015.09.033. [http://dx.doi.org/ 10.1016/j.neuroscience.2015.09.033]. [PMID: 26408987]. [DOI] [PubMed] [Google Scholar]
- 97.Luo J., Wang T., Liang S., Hu X., Li W., Jin F. Ingestion of Lactobacillus strain reduces anxiety and improves cognitive function in the hyperammonemia rat. Sci. China Life Sci. 2014;57(3):327–335. doi: 10.1007/s11427-014-4615-4. [http://dx.doi.org/10.1007/s11427-014-4615-4]. [PMID: 24554471]. [DOI] [PubMed] [Google Scholar]
- 98.Ohland C.L., Kish L., Bell H., Thiesen A., Hotte N., Pankiv E., Madsen K.L. Effects of Lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. Psychoneuroendocrinology. 2013;38(9):1738–1747. doi: 10.1016/j.psyneuen.2013.02.008. [http://dx.doi.org/10.1016/j.psyneuen.2013.02. 008]. [PMID: 23566632]. [DOI] [PubMed] [Google Scholar]
- 99.Savignac H.M., Tramullas M., Kiely B., Dinan T.G., Cryan J.F. Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav. Brain Res. 2015;287:59–72. doi: 10.1016/j.bbr.2015.02.044. [http://dx.doi.org/10. 1016/j.bbr.2015.02.044]. [PMID: 25794930]. [DOI] [PubMed] [Google Scholar]
- 100.Jeong J.J., Kim K.A., Ahn Y.T., Sim J.H., Woo J.Y., Huh C.S., Kim D.H. Probiotic mixture KF attenuates age-dependent memory deficit and lipidemia in fischer 344 rats. J. Microbiol. Biotechnol. 2015;25(9):1532–1536. doi: 10.4014/jmb.1505.05002. [http://dx.doi.org/10.4014/jmb.1505.05002]. [PMID: 25975611]. [DOI] [PubMed] [Google Scholar]
- 101.Gupta S., Masand P.S., Kaplan D., Bhandary A., Hendricks S. The relationship between schizophrenia and irritable bowel syndrome (IBS). Schizophr. Res. 1997;23(3):265–268. doi: 10.1016/s0920-9964(96)00099-0. [http://dx. doi.org/10.1016/S0920-9964(96)00099-0]. [PMID: 9075306]. [DOI] [PubMed] [Google Scholar]
- 102.Filipovic B.R., Filipovic B.F. Psychiatric comorbidity in the treatment of patients with inflammatory bowel disease. World J. Gastroenterol. 2014;20(13):3552–3563. doi: 10.3748/wjg.v20.i13.3552. [http://dx.doi.org/10. 3748/wjg.v20.i13.3552]. [PMID: 24707138]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eaton W., Mortensen P.B., Agerbo E., Byrne M., Mors O., Ewald H. Coeliac disease and schizophrenia: population based case control study with linkage of Danish national registers. BMJ. 2004;328(7437):438–439. doi: 10.1136/bmj.328.7437.438. [http://dx.doi.org/10.1136/bmj.328. 7437.438]. [PMID: 14976100]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kirkpatrick B., Miller B.J. Inflammation and schizophrenia. Schizophr. Bull. 2013;39(6):1174–1179. doi: 10.1093/schbul/sbt141. [http://dx.doi.org/10. 1093/schbul/sbt141]. [PMID: 24072812]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miller B.J., Buckley P., Seabolt W., Mellor A., Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol. Psychiatry. 2011;70(7):663–671. doi: 10.1016/j.biopsych.2011.04.013. [http://dx.doi.org/10.1016/j.biopsych.2011.04.013]. [PMID: 21641581]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hemmings G. Schizophrenia. Nature. 1992;355(6358):291. doi: 10.1038/355291d0. [http://dx.doi.org/10.1038/355291d0]. [PMID: 1731240]. [DOI] [PubMed] [Google Scholar]
- 107.Severance E.G., Gressitt K.L., Stallings C.R., Origoni A.E., Khushalani S., Leweke F.M., Dickerson F.B., Yolken R.H. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr. Res. 2013;148(1-3):130–137. doi: 10.1016/j.schres.2013.05.018. [http://dx.doi.org/10.1016/j.schres.2013. 05.018]. [PMID: 23746484]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hand T.W., Dos Santos L.M., Bouladoux N., Molloy M.J., Pagán A.J., Pepper M., Maynard C.L., Elson C.O., III, Belkaid Y. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science. 2012;337(6101):1553–1556. doi: 10.1126/science.1220961. [http://dx.doi.org/10.1126/science.1220961]. [PMID: 22923434]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mortensen P.B., Nørgaard-Pedersen B., Waltoft B.L., Sørensen T.L., Hougaard D., Torrey E.F., Yolken R.H. Toxoplasma gondii as a risk factor for early-onset schizophrenia: analysis of filter paper blood samples obtained at birth. Biol. Psychiatry. 2007;61(5):688–693. doi: 10.1016/j.biopsych.2006.05.024. [http://dx.doi.org/10.1016/j.biopsych.2006.05.024]. [PMID: 16920078]. [DOI] [PubMed] [Google Scholar]
- 110.Mills S., Shanahan F., Stanton C., Hill C., Coffey A., Ross R.P. Movers and shakers: influence of bacteriophages in shaping the mammalian gut microbiota. Gut Microbes. 2013;4(1):4–16. doi: 10.4161/gmic.22371. [http://dx.doi.org/10.4161/gmic.22371]. [PMID: 23022738]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Goldstein B.I., Kemp D.E., Soczynska J.K., McIntyre R.S. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. J. Clin. Psychiatry. 2009;70(8):1078–1090. doi: 10.4088/JCP.08r04505. [http://dx. doi.org/10.4088/JCP.08r04505]. [PMID: 19497250]. [DOI] [PubMed] [Google Scholar]
- 112.Dickerson F., Stallings C., Origoni A., Vaughan C., Katsafanas E., Khushalani S., Yolken R. Antibodies to Toxoplasma gondii in individuals with mania. Bipolar Disord. 2014;16(2):129–136. doi: 10.1111/bdi.12123. [http://dx.doi.org/10.1111/bdi.12123]. [PMID: 24102676]. [DOI] [PubMed] [Google Scholar]
- 113.Severance E.G., Gressitt K.L., Yang S., Stallings C.R., Origoni A.E., Vaughan C., Khushalani S., Alaedini A., Dickerson F.B., Yolken R.H. Seroreactive marker for inflammatory bowel disease and associations with antibodies to dietary proteins in bipolar disorder. Bipolar Disord. 2014;16(3):230–240. doi: 10.1111/bdi.12159. [http://dx.doi.org/ 10.1111/bdi.12159]. [PMID: 24313887]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hamdani N., Boukouaci W., Hallouche M.R., Charron D., Krishnamoorthy R., Leboyer M., Tamouza R. Resolution of a manic episode treated with activated charcoal: Evidence for a brain-gut axis in bipolar disorder. Aust. N. Z. J. Psychiatry. 2015;49(12):1221–1223. doi: 10.1177/0004867415595873. [http://dx.doi.org/10.1177/0004867415595873]. [PMID: 26209321]. [DOI] [PubMed] [Google Scholar]
- 115.Bolte E.R. Autism and Clostridium tetani. Med. Hypotheses. 1998;51(2):133–144. doi: 10.1016/s0306-9877(98)90107-4. [http://dx.doi.org/10.1016/S0306-9877(98)90107-4]. [PMID: 9881820]. [DOI] [PubMed] [Google Scholar]
- 116.Finegold S.M., Molitoris D., Song Y., Liu C., Vaisanen M.L., Bolte E., McTeague M., Sandler R., Wexler H., Marlowe E.M., Collins M.D., Lawson P.A., Summanen P., Baysallar M., Tomzynski T.J., Read E., Johnson E., Rolfe R., Nasir P., Shah H., Haake D.A., Manning P., Kaul A. Gastrointestinal microflora studies in late-onset autism. Clin. Infect. Dis. 2002;35(Suppl. 1):S6–S16. doi: 10.1086/341914. [http://dx.doi.org/10.1086/341914]. [PMID: 12173102]. [DOI] [PubMed] [Google Scholar]
- 117.Parracho H.M., Bingham M.O., Gibson G.R., McCartney A.L. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 2005;54(Pt 10):987–991. doi: 10.1099/jmm.0.46101-0. [http://dx.doi.org/10.1099/jmm.0.46101-0]. [PMID: 16157555]. [DOI] [PubMed] [Google Scholar]
- 118.Sandler R.H., Finegold S.M., Bolte E.R., Buchanan C.P., Maxwell A.P., Väisänen M-L., Nelson M.N., Wexler H.M. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J. Child Neurol. 2000;15(7):429–435. doi: 10.1177/088307380001500701. [http://dx.doi.org/ 10.1177/088307380001500701]. [PMID: 10921511]. [DOI] [PubMed] [Google Scholar]
- 119.Williams B.L., Hornig M., Parekh T., Lipkin W.I. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. MBio. 2012;3(1):e00261–e11. doi: 10.1128/mBio.00261-11. [http://dx.doi.org/10.1128/mBio. 00261-11]. [PMID: 22233678]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Williams B.L., Hornig M., Buie T., Bauman M.L., Cho Paik M., Wick I., Bennett A., Jabado O., Hirschberg D.L., Lipkin W.I. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One. 2011;6(9):e24585. doi: 10.1371/journal.pone.0024585. [http://dx.doi.org/ 10.1371/journal.pone.0024585]. [PMID: 21949732]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Adams J.B., Johansen L.J., Powell L.D., Quig D., Rubin R.A. Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11(1):22. doi: 10.1186/1471-230X-11-22. [http://dx.doi.org/ 10.1186/1471-230X-11-22]. [PMID: 21410934]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kang D-W., Park J.G., Ilhan Z.E., Wallstrom G., Labaer J., Adams J.B., Krajmalnik-Brown R. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 2013;8(7):e68322. doi: 10.1371/journal.pone.0068322. [http://dx.doi.org/10.1371/ journal.pone.0068322]. [PMID: 23844187]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Morton G.J., Cummings D.E., Baskin D.G., Barsh G.S., Schwartz M.W. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–295. doi: 10.1038/nature05026. [http://dx.doi.org/ 10.1038/nature05026]. [PMID: 16988703]. [DOI] [PubMed] [Google Scholar]
- 124.Bäckhed F., Manchester J.K., Semenkovich C.F., Gordon J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA. 2007;104(3):979–984. doi: 10.1073/pnas.0605374104. [http://dx.doi.org/10.1073/pnas.0605374104]. [PMID: 17210919]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tremaroli V., Karlsson F., Werling M., Ståhlman M., Kovatcheva-Datchary P., Olbers T., Fändriks L., le Roux C.W., Nielsen J., Bäckhed F. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 2015;22(2):228–238. doi: 10.1016/j.cmet.2015.07.009. [http://dx.doi.org/10.1016/j.cmet.2015.07.009]. [PMID: 26244932]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang H., DiBaise J.K., Zuccolo A., Kudrna D., Braidotti M., Yu Y., Parameswaran P., Crowell M.D., Wing R., Rittmann B.E., Krajmalnik-Brown R. Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. USA. 2009;106(7):2365–2370. doi: 10.1073/pnas.0812600106. [http://dx.doi.org/10.1073/pnas.0812600106]. [PMID: 19164560]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Manco M., Putignani L., Bottazzo G.F. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr. Rev. 2010;31(6):817–844. doi: 10.1210/er.2009-0030. [http://dx.doi.org/10.1210/er.2009-0030]. [PMID: 20592272]. [DOI] [PubMed] [Google Scholar]
- 128.Cani P.D., Osto M., Geurts L., Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012;3(4):279–288. doi: 10.4161/gmic.19625. [http://dx.doi.org/10.4161/gmic.19625]. [PMID: 22572877]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cox L.M., Blaser M.J. Pathways in microbe-induced obesity. Cell Metab. 2013;17(6):883–894. doi: 10.1016/j.cmet.2013.05.004. [http://dx.doi.org/10.1016/j.cmet. 2013.05.004]. [PMID: 23747247]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Davey K.J., O’Mahony S.M., Schellekens H., O’Sullivan O., Bienenstock J., Cotter P.D., Dinan T.G., Cryan J.F. Gender-dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology (Berl.) 2012;221(1):155–169. doi: 10.1007/s00213-011-2555-2. [http://dx. doi.org/10.1007/s00213-011-2555-2]. [PMID: 22234378]. [DOI] [PubMed] [Google Scholar]
- 131.Morgan A.P., Crowley J.J., Nonneman R.J., Quackenbush C.R., Miller C.N., Ryan A.K., Bogue M.A., Paredes S.H., Yourstone S., Carroll I.M., Kawula T.H., Bower M.A., Sartor R.B., Sullivan P.F. The antipsychotic olanzapine interacts with the gut microbiome to cause weight gain in mouse. PLoS One. 2014;9(12):e115225. doi: 10.1371/journal.pone.0115225. [http://dx.doi.org/10.1371/journal.pone.0115225]. [PMID: 25506936]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fuller R. Probiotics in man and animals. J. Appl. Bacteriol. 1989;66(5):365–378. [http://dx.doi.org/10.1111/j.1365-2672.1989. tb05105.x]. [PMID: 2666378]. [PubMed] [Google Scholar]
- 133.Benton D., Williams C., Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 2007;61(3):355–361. doi: 10.1038/sj.ejcn.1602546. [http://dx.doi.org/10.1038/sj.ejcn. 1602546]. [PMID: 17151594]. [DOI] [PubMed] [Google Scholar]
- 134.Mohammadi A.A., Jazayeri S., Khosravi-Darani K., Solati Z., Mohammadpour N., Asemi Z., Adab Z., Djalali M., Tehrani-Doost M., Hosseini M., Eghtesadi S. The effects of probiotics on mental health and hypothalamic-pituitary-adrenal axis: A randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr. Neurosci. 2016;19(9):387–395. doi: 10.1179/1476830515Y.0000000023. [http://dx.doi.org/ 10.1179/1476830515Y.0000000023]. [PMID: 25879690]. [DOI] [PubMed] [Google Scholar]
- 135.Messaoudi M., Violle N., Bisson J-F., Desor D., Javelot H., Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2011;2(4):256–261. doi: 10.4161/gmic.2.4.16108. [http://dx.doi.org/10.4161/gmic.2.4.16108]. [PMID: 21983070]. [DOI] [PubMed] [Google Scholar]
- 136.Takada M., Nishida K., Kataoka-Kato A., Gondo Y., Ishikawa H., Suda K., Kawai M., Hoshi R., Watanabe O., Igarashi T., Kuwano Y., Miyazaki K., Rokutan K. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut-brain interaction in human and animal models. Neurogastroenterol. Motil. 2016;28(7):1027–1036. doi: 10.1111/nmo.12804. [http://dx.doi.org/10. 1111/nmo.12804]. [PMID: 26896291]. [DOI] [PubMed] [Google Scholar]
- 137.Rao A.V., Bested A.C., Beaulne T.M., Katzman M.A., Iorio C., Berardi J.M., Logan A.C. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009;1(1):6. doi: 10.1186/1757-4749-1-6. [http://dx.doi.org/10.1186/1757-4749-1-6]. [PMID: 19338686]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dickerson F.B., Stallings C., Origoni A., Katsafanas E., Savage C.L., Schweinfurth L.A., Goga J., Khushalani S., Yolken R.H. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. 2014. [DOI] [PMC free article] [PubMed]
- 139.Kałużna-Czaplińska J., Błaszczyk S. The level of arabinitol in autistic children after probiotic therapy. Nutrition. 2012;28(2):124–126. doi: 10.1016/j.nut.2011.08.002. [http://dx.doi.org/10.1016/j.nut.2011.08.002]. [PMID: 22079796]. [DOI] [PubMed] [Google Scholar]
- 140.Pärtty A., Kalliomäki M., Wacklin P., Salminen S., Isolauri E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: a randomized trial. Pediatr. Res. 2015;77(6):823–828. doi: 10.1038/pr.2015.51. [http://dx.doi.org/10.1038/ pr.2015.51]. [PMID: 25760553]. [DOI] [PubMed] [Google Scholar]
- 141.Benton D., Williams C., Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 2007;61(3):355–361. doi: 10.1038/sj.ejcn.1602546. [http://dx.doi.org/10.1038/sj.ejcn. 1602546]. [PMID: 17151594]. [DOI] [PubMed] [Google Scholar]
- 142.Sharma K., Pant S., Misra S., Dwivedi M., Misra A., Narang S., Tewari R., Bhadoria A.S. Effect of rifaximin, probiotics, and l-ornithine l-aspartate on minimal hepatic encephalopathy: a randomized controlled trial. Saudi J. Gastroenterol. 2014;20(4):225–232. doi: 10.4103/1319-3767.136975. [http://dx.doi.org/10.4103/1319-3767.136975]. [PMID: 25038208]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Akbari E., Asemi Z., Daneshvar Kakhaki R., Bahmani F., Kouchaki E., Tamtaji O.R., Hamidi G.A., Salami M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016;8:256. doi: 10.3389/fnagi.2016.00256. [http://dx. doi.org/10.3389/fnagi.2016.00256]. [PMID: 27891089]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Park D-Y., Ahn Y-T., Park S-H., Huh C-S., Yoo S-R., Yu R., Sung M-K., McGregor R.A., Choi M-S. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS One. 2013;8(3):e59470. doi: 10.1371/journal.pone.0059470. [http://dx. doi.org/10.1371/journal.pone.0059470]. [PMID: 23555678]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kadooka Y., Sato M., Imaizumi K., Ogawa A., Ikuyama K., Akai Y., Okano M., Kagoshima M., Tsuchida T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010;64(6):636–643. doi: 10.1038/ejcn.2010.19. [http://dx.doi.org/10. 1038/ejcn.2010.19]. [PMID: 20216555]. [DOI] [PubMed] [Google Scholar]
- 146.Kadooka Y., Sato M., Ogawa A., Miyoshi M., Uenishi H., Ogawa H., Ikuyama K., Kagoshima M., Tsuchida T. Effect of Lactobacillus gasseri SBT2055 in fermented milk on abdominal adiposity in adults in a randomised controlled trial. Br. J. Nutr. 2013;110(9):1696–1703. doi: 10.1017/S0007114513001037. [http://dx.doi.org/10.1017/ S0007114513001037]. [PMID: 23614897]. [DOI] [PubMed] [Google Scholar]
- 147.Depeint F., Tzortzis G., Vulevic J., I’anson K., Gibson G.R. Prebiotic evaluation of a novel galactooligosaccharide mixture produced by the enzymatic activity of Bifidobacterium bifidum NCIMB 41171, in healthy humans: a randomized, double-blind, crossover, placebo-controlled intervention study. Am. J. Clin. Nutr. 2008;87(3):785–791. doi: 10.1093/ajcn/87.3.785. [http://dx.doi.org/10.1093/ajcn/87.3.785]. [PMID: 18326619]. [DOI] [PubMed] [Google Scholar]
- 148.Vulevic J., Juric A., Walton G.E., Claus S.P., Tzortzis G., Toward R.E., Gibson G.R. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br. J. Nutr. 2015;114(4):586–595. doi: 10.1017/S0007114515001889. [http://dx.doi.org/10.1017/S0007114515001889]. [PMID: 26218845]. [DOI] [PubMed] [Google Scholar]
- 149.Nyangale E.P., Farmer S., Keller D., Chernoff D., Gibson G.R. Effect of prebiotics on the fecal microbiota of elderly volunteers after dietary supplementation of Bacillus coagulans GBI-30, 6086. Anaerobe. 2014;30:75–81. doi: 10.1016/j.anaerobe.2014.09.002. [http://dx.doi.org/10.1016/j.anaerobe. 2014.09.002]. [PMID: 25219857]. [DOI] [PubMed] [Google Scholar]
- 150.Savignac H.M., Couch Y., Stratford M., Bannerman D.M., Tzortzis G., Anthony D.C., Burnet P.W.J. Prebiotic administration normalizes lipopolysaccharide (LPS)-induced anxiety and cortical 5-HT2A receptor and IL1-β levels in male mice. Brain Behav. Immun. 2016;52:120–131. doi: 10.1016/j.bbi.2015.10.007. [http://dx.doi.org/10.1016/j.bbi. 2015.10.007]. [PMID: 26476141]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schmidt K., Cowen P.J., Harmer C.J., Tzortzis G., Errington S., Burnet P.W. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology (Berl.) 2015;232(10):1793–1801. doi: 10.1007/s00213-014-3810-0. [http://dx.doi.org/10. 1007/s00213-014-3810-0]. [PMID: 25449699]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Erejuwa O.O., Sulaiman S.A., Ab Wahab M.S. Modulation of gut microbiota in the management of metabolic disorders: the prospects and challenges. Int. J. Mol. Sci. 2014;15(3):4158–4188. doi: 10.3390/ijms15034158. [http:// dx.doi.org/10.3390/ijms15034158]. [PMID: 24608927]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bakken J.S., Borody T., Brandt L.J., Brill J.V., Demarco D.C., Franzos M.A., Kelly C., Khoruts A., Louie T., Martinelli L.P., Moore T.A., Russell G., Surawicz C. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin. Gastroenterol. Hepatol. 2011;9(12):1044–1049. doi: 10.1016/j.cgh.2011.08.014. [http://dx.doi.org/10.1016/ j.cgh.2011.08.014]. [PMID: 21871249]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Evrensel A., Ceylan M.E. Fecal microbiota transplantation and its usage in neuropsychiatric disorders. Clin. Psychopharmacol. Neurosci. 2016;14(3):231–237. doi: 10.9758/cpn.2016.14.3.231. [http://dx.doi.org/10.9758/cpn. 2016.14.3.231]. [PMID: 27489376]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kang D.W., Adams J.B., Gregory A.C., Borody T., Chittick L., Fasano A., Khoruts A., Geis E., Maldonado J., McDonough-Means S., Pollard E.L., Roux S., Sadowsky M.J., Lipson K.S., Sullivan M.B., Caporaso J.G., Krajmalnik-Brown R. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5(1):10. doi: 10.1186/s40168-016-0225-7. [http://dx.doi.org/10.1186/s40168-016-0225-7]. [PMID: 28122648]. [DOI] [PMC free article] [PubMed] [Google Scholar]