Abstract
Accumulating evidence has shown the importance of glial cells in the neurobiology of bipolar disorder. Activated microglia and inflammatory cytokines have been pointed out as potential biomarkers of bipolar disorder. Indeed, recent studies have shown that bipolar disorder involves microglial activation in the hippocampus and alterations in peripheral cytokines, suggesting a potential link between neuroinflammation and peripheral toxicity. These abnormalities may also be the biological underpinnings of outcomes related to neuroprogression, such as cognitive impairment and brain changes. Additionally, astrocytes may have a role in the progression of bipolar disorder, as these cells amplify inflammatory response and maintain glutamate homeostasis, preventing excitotoxicity. The present review aims to discuss neuron-glia interactions and their role in the pathophysiology and treatment of bipolar disorder.
Keywords: Bipolar disorder, mania, neuron, glia, microglia, astrocyte, oligodendrocyte, brain-blood barrier
1. Introduction
Bipolar disorder has a prevalence of about 2%, and subclinical variants affect another 2% of the population [1]. The World Health Organization mental health surveys have shown that bipolar disorder ranks second in the number of days out of role [2]. In addition, the incidence of death by suicide among patients with bipolar disorder is high, 20-30 times higher than for the general population [3]. Following a chronic course, bipolar disorder often results in enduring functional and cognitive impairment [4]. Also, the condition is associated with illnesses marked by immune activation, such as metabolic syndrome [5], obesity, type 2 diabetes mellitus and cardiovascular diseases [6]. Finally, bipolar disorder is known to be associated with an increased incidence of autoimmune and inflammatory disorders [7, 8].
Early studies on the pathophysiology of bipolar disorder followed the findings reported for major depressive disorder and pointed towards monoamine disturbances [9, 10]. Afterwards, the glutamatergic hypothesis emerged [11], and more recently, glial dysfunctions have been investigated [12]. For instance, a study on excitotoxicity and neuroinflammatory markers in postmortem frontal cortices showed alterations in astroglial and microglial markers in patients with bipolar disorder when compared with control subjects [13]. Furthermore, a positron emission tomography (PET) scan study found microglial activation and thus neuroinflammation in the hippocampus of euthymic patients with bipolar disorder [14].
Recently, a connection has been suggested between neuroinflammation and inflammatory peripheral markers in the pathophysiology of bipolar disorder [15]. A meta-analysis
reported significantly higher cytokine levels, such as soluble interleukins, tumor necrosis factor-alpha (TNF-α) and soluble cytokine receptors, in patients with bipolar disorder compared with healthy controls [16]. Later on, another meta-analysis showed changes in cytokine levels during acute mood episodes, during euthymia, and after treatment [17]. Finally, a recent study proposed a model of disruption of blood-brain barrier (BBB) integrity and increased permeability of signaling molecules, potentially triggering neuroinflammation [18]. Thus, the aim of the present narrative review is to discuss the role of glial cells and inflammatory markers in the pathophysiology of bipolar disorder and to review potential targets for future therapeutic approaches.
2. Bipolar disorder and glial cells
Neuropathological findings have shown some glial alterations in bipolar disorder [12, 19, 20-22]. A histological study of the subgenual portion of Brodmann area 24 showed a reduced number of glial cells in the brains of patients with bipolar disorder, especially in those with a family history of mood disorder [23]. Specifically, it has been suggested that patients with bipolar disorder have abnormal microglial function [12]. A study that used PET scan images after intravenous injection of the radiopharmaceutical [11C]-(R)-PK11195 was the first to report in vivo neuroinflammation in patients with bipolar disorder [14]. The study showed focal microglial activation in the right hippocampus and a non-significant similar trend in the left hippocampus of euthymic patients with bipolar I disorder [14].
As a follow-up to that study, the same group combined PET scan, magnetic resonance imaging and spectroscopy to investigate the hippocampus of patients with bipolar disorder [24]. The authors found decreased N-acetylaspartate (NAA) + N-acetyl-aspartyl-glutamate (NAAG) concentrations in the left hippocampus of patients with bipolar I disorder, suggesting decreased neuronal viability in this region [24]. In addition, a significant positive association between microglial activation and NAA+NAAG concentration in the left hippocampus was observed [24]. Although the authors argued this association was due to the activation of peripheral immune system by microglia, these results may be due to activation of astrocyte and indicate adaptive response. It is worth mentioning that the sample size was small and the author chose not to apply any correction for multiple testing in the analyses [24]. In relation to microglia, a postmortem study showed significantly higher microglial markers in the frontal cortex of patients with bipolar disorder when compared with control subjects [13]. Microglial activation was characterized by monoclonal human leukocyte antigen D-related (HLA-DR) antibodies and mRNA levels of CD11b in the brain tissue of patients with bipolar disorder [13].
Some studies involving patients with bipolar disorder have shown increased levels of microglial biomarkers in vivo [25]. Monocyte chemoattractant protein 1 (MCP-1), also called C-C motif chemokine ligand 2 (CCL-2), is a chemokine produced by microglial cells that have complex functions with both pro-inflammatory and neuromodulatory activity [26, 27]. YKL-40, also called chitinase-3-like protein 1 (CHI3L1), is a glycoprotein produced by reactive microglia and astrocytes, and it has been associated with systemic inflammatory and immune diseases, as well as with neurological disorders [28], especially the active relapsing-remitting and secondary progressive forms of multiple sclerosis [29, 30]. Soluble cluster of differentiation 14 (sCD14) is another marker secreted by microglial cells; it is involved in innate immune responses to infections and also mediates phagocytosis [25]. A study comparing euthymic patients with bipolar disorder and healthy controls showed increased cerebrospinal fluid (CSF) levels of MCP-1 and YKL-40 and increased serum levels of sCD14 and YKL-40 in the former [25]. Another study showed that CSF YKL-40 was associated with executive performance in euthymic bipolar disorder [31].
In addition to microglial dysfunction, some studies have reported abnormalities in astrocytes of patients with bipolar disorder [12]. S100-B is a calcium-binding protein produced by astrocytes. A neuropathological analysis has shown bilateral decrease in the numerical density of S100-B immunopositive astrocytes in hippocampal CA1 pyramidal layers of patients with bipolar depression [21]. Another brain analysis showed a decrease in S100-B levels in Brodmann area 9 and an increase in Brodmann area 40 in tissues from patients with bipolar disorder [22]. Interestingly, a meta-analysis (n=104 patients) showed higher levels of peripheral S100-B in patients with bipolar disorder [32].
Glutamate homeostasis is rooted in two astrocytic functions, namely: clearance of excess glutamate, preventing excitotoxicity; and glutamate restorage and transportation to neurons in the form of glutamine provided by glial cells [33]. Glial fibrillary acidic protein (GFAP) is a classical astrocyte protein marker [34, 35], and it is commonly altered in the brain of patients with bipolar disorder [13]. For instance, a study has reported significant increases in GFAP band intensity in the dorsolateral prefrontal cortex of patients with bipolar disorder [36]. Conversely, other studies failed to find positive results in other brain structures, such as the amygdala [37, 38] and other cortical areas [38, 39]. Furthermore, a study used proton magnetic resonance spectroscopy to evaluate glutamine/glutamate ratio and NAA levels in the anterior cingulate cortex (ACC) and parieto-occipital cortex (POC) in patients with bipolar disorder [40]. Authors found that the glutamine/glutamate ratio was significantly higher in the ACC and POC of patients in acute mania when compared with healthy controls [40]. It is worth mentioning that elevated glutamine/glutamate ratio is consistent with glutamatergic overactivity and with abnormal neuron-glia interactions. Moreover, a neuropathological study showed a significant decrease in the intensity and density of glutamate receptor ASCT-1 (neutral amino acid transporter 1), expressed in neurons and glial cells, in the brains of patients with bipolar disorder when compared to healthy controls [41].
At last, it is worth mentioning that a morphometric study showed reduction in the numerical density of oligodendroglial cells in layer VI and Broadmann area 9 in the brains of patients with bipolar disorder [19]. Another postmortem study showed a higher number of oligodendrocytes in the hippocampal CA1 area of patients with bipolar disorder [20]. Some neuropathological findings of glia in bipolar disorder are summarized in the Table 1.
Table 1.
Neuropathological findings of glia in bipolar disorder.
| Author/Year | N | Sex (m/f) | Age (y) | Suicide | Brain Region | Results |
|---|---|---|---|---|---|---|
| Malchow et al., 2015 | 08 | 04/04 | 56.38 (± 11.1) | 03 | Hippocampus | ↑ number of oligodendrocytes in CA1 when compared to HC. |
| Feresten et al., 2013 | 34 | 16/18 | 45.40 (± 10.7) | 15 | DLPFC | ↑ GFAP when compared to HC. |
| Gos et al., 2013 | 06 | 03/03 | 55.70 (± 13.3) | 00 | Hippocampus | ↓ numerical density of S100B-immunopositive astrocytes in the CA1 pyramidal compared HC. ↓density of S100B-immunopositive oligodendrocytes in the left alveus. |
| †Torres-Platas et al., 2011 | 10* | 07/03 | 48.6 (± 5.3) | 10 | ACC | Astrocytes with larger cell bodies, longer and more ramified processes when compared to HC. |
| Pantazopoulos et al., 2010 | 11 | 070/4 | 66.7 (± 17.3) | 00 | Deep amygdala nuclei Entorhinal cortex |
↑positive glial cells marker in the lateral nuclei of the amigdala. ↓ glial cell marker in layer III of the entorhinal cortex. |
| Rao et al., 2010 | 10 | 06/04 | 49.0 (± 7.2) | 05 | Frontal cortex | ↑ GFAP expression and ↑ level of CD11b mRNA. |
| Altshuler et al., 2010 | 10 | 06/04 | 44.5 (± 10.7) | 07 | Amygdala | ↓ density of GFAP-immunoreactive astrocytes in the amygdala of subjects with MDD compared to the bipolar disorder. |
| Dean et al., 2006 | 08 | 04/04 | 59.0 (± 3.6) | 00 | Frontal and parietal cortices | ↓S100β in BA 9 and ↑ S100β in BA 40 when compared to HC. |
| Uranova et al., 2004 | 15 | 09/06 | 42.3 (± 13.1) | 09 | Frontal cortex | ↓numerical density of oligodendroglial cells was found in layer VI when compared to HC. |
| Ongür et al., 1998 | 18 | 11/07 | 54.8 (± 12.6)/ 44.9 (± 3.2)** | 06 | Subgenual part of BA 24 | ↓ numbers of glia cells when compared to HC. |
| Damadzic et al., 2001 | 13 | 07/06 | 44.0 (± 12.0) | 07 | Entorhinal cortex | No significant difference in density of GFAP-positive astrocytes between the psychiatric diagnostic groups and the HC. |
ACC: anterior cingulate cortex; BA: Brodmann area; CA: Cornu Ammonis 1; CD11b: a marker of astrocyte and microglial activation; DLPFC: Dorsolateral prefrontal cortex GFAP: glial fibrillary acidic protein; HC: healthy controls; HLA-DR: monoclonal human leukocyte antigen-D realted antibody; ↑: increased/elevated; ↓: decreased; *: unipolar and bipolar depression subjects; **: two different brain banks used.
3. Neuroimaging findings related to brain white matter in bipolar disorder
Abnormalities in brain white matter are among the most consistent neuroimaging findings in bipolar disorder as demonstrated in meta-analyses of voxel-based morphometry [42] and diffusion tension imaging (DTI) studies [43, 44]. These alterations may be due to abnormalities in myelin and oligodendrocytes [45], and the glial cells responsible for generating and maintaining the myelin sheath in the brain [46]. Oligodendrocyte progenitor cells, which represent nearly 5% of the total of brain cells, are proliferative glial cells distributed in the CNS [46] and are the precursor of mature oligodendroglia. The oligodendrocyte generating the myelin sheaths during the neurodevelopment as well as in the remyelination process of damaged sheaths in neurodegenerative illness [47, 48]. The myelin membrane has a high metabolic stability [49], while oligodendrocyte has the capacity to renew its myelin sheath due to its high metabolic rate [50]. In addition, oligodendroglia is vulnerable to oxidative stress and neuroinflammation [51-54], both processes related to bipolar disorder.
A voxel-based morphometry meta-analytical study showed lower white matter concentrations in the left inferior longitudinal fasciculus, left superior corona radiata, and left posterior cingulum in subjects with bipolar disorder [42]. Furthermore, a meta-analysis of 10 studies of whole-brain DTI identified two significant clusters of decreased fractional anisotropy (FA) on the right side of the brain, close to the parahippocampal gyrus and to the anterior and subgenual cingulate cortex, two regions crossed by several white matter tracts [43]. Lastly, another meta-analysis of whole-brain DTI studies showed that all major classes of white matter (commissural tracts, association tracts, and projection tracts) are affected in bipolar disorder [44].
White matter microstructural integrity abnormalities in DTI have been studied both as possible trait and state-dependent changes [55, 56]. In this sense, a study investigated differences in white matter microstructure among patients with bipolar I disorder in different phases of illness [55]. The authors found an increasing gradient of white matter abnormalities from the euthymic to the manic and to the depressive phases. Euthymic subjects showed low degree and white matter alterations in the midline structures, while depressive subjects had high degree and widespread white matter alterations. Manic subjects showed more diffused white matter alterations in the midline and lateral structures [55]. Despite the result suggesting abnormalities in acute episodes, the study has a cross-sectional design and the true state-dependency needs to be confirmed in longitudinal studies.
In contrast, white matter microstructural integrity abnormalities in DTI also have been described as possible trait-based marker or endophenotype for bipolar disorder [56]. For instance, a study showed decreased FA in the fornix, left posterior thalamic radiation and left sagittal stratum in subjects with bipolar disorder and in unaffected siblings when compared to healthy controls without a first-degree relative with psychiatric disorder [56]. Another study also showed reductions in the FA in the superior longitudinal fasciculus of unaffected siblings of patients with bipolar disorder [57]. All these findings suggest that patients with bipolar disorder and their first-degree relatives show similar alterations in microstructural integrity of white matter tracts. Although these findings do not comply with all criteria for true endophenotypes [58], future longitudinal studies with additional populations may define this.
It is worth mentioning that, alterations in white matter have also been evaluated in youths at high risk of developing bipolar disorder. A neuroimaging study showed white matter volume reduction in the frontal, occipital and parietal lobes prior to the development of any psychiatric symptom in 115 offsprings of subjects with bipolar disorder type I [59]. A result pointed out that white matter volume reductions are correlated with familial risk to bipolar disorder. It is possible that vulnerabilities related to oligodendrocytes and myelin sheaths exist earlier in developmental brain processes of patients with bipolar disorder and may be disrupted by other pathophysiological mechanisms throughout the course of illness.
4. Neuron-glia interactions
4.1. Microglial Activation and Neuroimmunology
Microglial cells are resident macrophages of the central nervous system (CNS), and derive from yolk sac hematopoietic stem cells [60]. While other macrophages activate the peripheral immune system, microglial cells are responsible for CNS homeostasis and synaptic modulation. Their activation regulates cytokine production, neuronal plasticity, and also neurotransmission [61]. Microglial cells can adopt different phenotypes and functions. In the steady state, characterized by ramified processes, microglia are stimulated by factors such as adenosine triphosphate (ATP) and other nucleotides and reduced by factors such as CX3CL1 [41]. In the surveillance role, microglial cells produce substances such as insulin-like growth factor 1 (IGF-1), BDNF, transforming growth factor-beta (TGF-β), and nerve growth factor (NGF) [60, 62]. Through these secreted factors, microglia affects the development of other cell types of CNS and play a role in survival and proliferation of neural precursor cells, as well as oligodendrocyte precursors cells [63].
When stimulated by pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) via toll-like receptors (TLR) or ATP receptors [64], microglial cells polarize to the M1 phenotype in the presence of lipopolysaccharide (LPS) and interferon gamma (IFN-γ) [65]. In this activated role, microglial cells can produce pro-inflammatory cytokines and chemokines such as interleukin 1 beta (IL-1β), IL-6, TNF-α, MCP-1 or CCL2, as well as reactive oxygen and nitrogen species, characterizing a profile with killing activity [65, 66]. Interestingly, drug-free patients with bipolar disorder evaluated during an acute mood episode showed increased levels of DAMPs, such as circulating cell-free nuclear DNA and heat shock protein (HSP) 70 and 90, when compared to healthy subjects [67]. Thus, DAMP activation of TLR signaling pathways may be the link between an initial cytotoxic insult (drug abuse, high stress, affective episode) and the subsequent sterile systemic inflammatory response.
The suppression of anti-inflammatory activity and gene transcription of pro-inflammatory cytokines also occur during M1 polarization [68]. Conversely, cytokines such as IL-4 and IL-13 can induce microglial activation to the M2 phenotype, which down-regulates M1 functions via the anti-inflammatory cytokine IL-10 [65]. The M2 phenotype includes different subtypes with different functions [69]: M2a is associated with the production of anti-inflammatory cytokines, inhibits nuclear factor κB (NFκB) isoforms, and enhances the expression of phagocytic receptors [70]; M2b is considered to be a combination of subtypes M1 and M2a [69]; M2c is associated with phagocytosis and suppression of the innate immune system [71]. It is likely that M1/M2 polarization of microglial cells and macrophages plays an important role in controlling the balance between induction and resolution of neuroinflammation and immune response, shifting from a pro-inflammatory to an anti-inflammatory response and vice-versa [65, 66].
Microglia also plays a neuromodulatory role, especially in the context of the brain development, acting on pruning and remodeling synaptic plasticity, because it is involved in neural cell death [62, 63]. With the important role of autophagy, microglial cells are involved in the synaptic refinement process, which is demonstrated by a pre-clinical model of a neurodevelopmental disorder [72]. Also, an in vivo study showed that microglial cells phagocytize early neural precursor cells from the hippocampal neurogenic niche as part of an apoptotic process, suggesting a role of microglia in maintaining homeostasis of the baseline neurogenic cascade [73]. Another in vitro study showed that milk fat globule epidermal growth factor-8 mediates phagocytosis of viable neurons during an inflammatory process induced by LPS [74].
Lithium is a cornerstone in the treatment of bipolar disorder [75]. In addition to the evidence from clinical studies, pre-clinical investigations have demonstrated some of the molecular properties of this drug. In one study, lithium was administered to mice to evaluate cell death and proliferation in the hippocampus after irradiation; the findings showed that the drug could decrease neural progenitor cell apoptosis, reduce the number and size of microglia, and ameliorate learning impairments [76]. In fact, microglia plays a role in autophagy, and a possible mechanism of the therapeutic action of lithium may be due to its property of modulating the autophagy process [77-79]. Another therapeutic action of this drug is the inflammation. A cell culture study showed that lithium significantly inhibited LPS-induced microglial activation and pro-inflammatory cytokine production [34]. Also, an animal model of mania induced by d-amphetamine showed increased levels of IL-4, IL-6, IL-10, and TNF-α in the frontal cortex, striatum, and serum of rats [80] Cytokine levels were reversed by treatment with lithium, suggesting that the action of this drug on inflammation may contribute to its therapeutic efficacy [80].
4.2. Astrocytes and Glutamatergic Excitotoxicity
Astrocytes play roles in all essential functions of the CNS, such as BBB maintenance, immune defense, neurotransmission, and synapse formation [81]. Astrocytes can differentiate into two different morphological phenotypes: type 1 or protoplasmic astrocytes, located in the gray matter and promoting synapses and BBB functions; and type 2 or fibrous astrocytes, located in the white matter and contacting the nodes of Ranvier and blood vessels [82]. Astrocytes are one of the first responders to injury and infections of the CNS. When activated, they produce and secrete cytokines (e.g., IL-1, IL-6, IL-10) that affect themselves and other brain cells such as neurons, microglia, and oligodendrocytes, leading to excitotoxicity and inflammation [34, 35]. Interactions of astrocytes and microglial cells with neurons have been observed in the hippocampal region, where they act on glutamatergic excitatory synaptic transmission and provide a regulatory system to maintain microglial inflammatory pathways under control [62]. The release of cytokines during an inflammatory process might induce BBB permeability, and consequently allows the entrance of peripheral proinflammatory mediators in the brain [83].
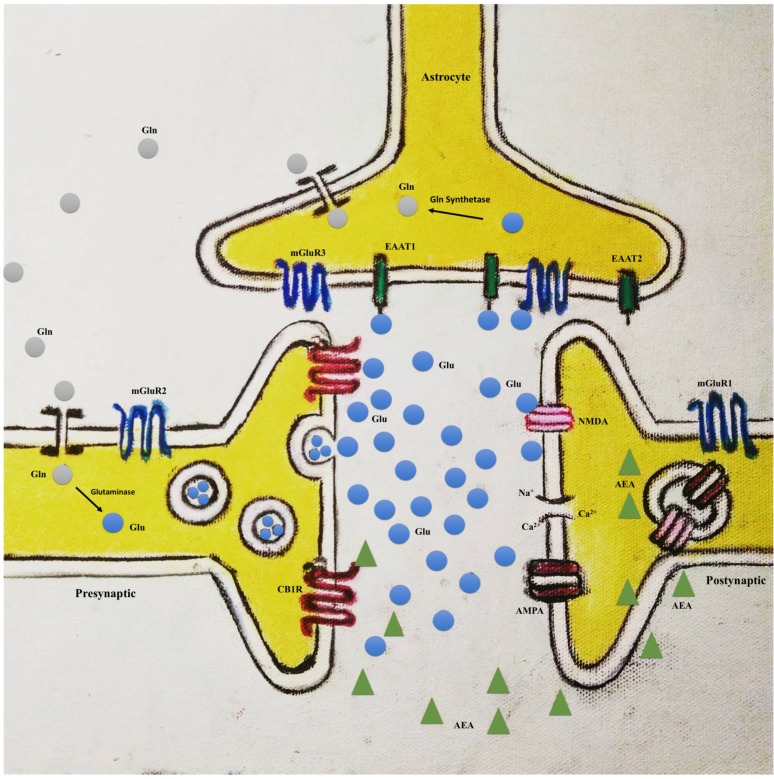
Glutamate is the main excitatory neurotransmitter of the CNS, and glial cells, especially astrocytes, are implicated in the regulation of its homeostasis [84]. Clearance of excess glutamate occurs in the synaptic cleft, avoiding excitotoxicity [84] (Fig. 1). Glutamate uptake is performed in humans by the excitatory amino acid transporter subtype 1 and 2 (EAAT1 and EAAT2, respectively) present in astrocytes [85]. Additionally, these cells play a role in transferring glutamate back to neurons, maintaining the neuronal pool of this neurotransmitter in a process called the glutamate-glutamine cycle. In this cycle, glutamate is converted by a specific astrocytic enzyme in the CNS, glutamine synthetase, in glutamine, which is transferred to neurons. Thereafter, glutamine is converted back to glutamate by the enzyme glutaminase [85, 86]. Moreover, astrocytes are the only brain cells that synthesize glutamate from glucose, because its expression of pyruvate carboxylase [85]. Interestingly, as discussed previously, a proton magnetic resonance spectroscopy study demonstrated a significantly higher glutamate/glutamine ratio in the ACC and POC of patients with bipolar disorder in manic episodes when compared to healthy control subjects [40].
Fig. (1).
Tripartite synapses model. AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; CB1R: cannabinoid receptor type 1; AEA: N-arachidonoylethanolamine (anandamide); EAAT1: excitatory amino acid transporter subtype 1; EAAT2: excitatory amino acid transporter subtype 2; Gln: glutamine; GlnT: glutamine transportes; Glu: glutamate; mGluR1: metabotropic glutamate receptor 1; mGluR2: metabotropic glutamate receptor 2; mGluR3: metabotropic glutamate receptor 3; NMDA: N-methyl-D-aspartate receptor. (The color version of the figure is available in the electronic copy of the article).
5. Brain-blood barrier disruption and peripheral inflammatory markers in bipolar disorder
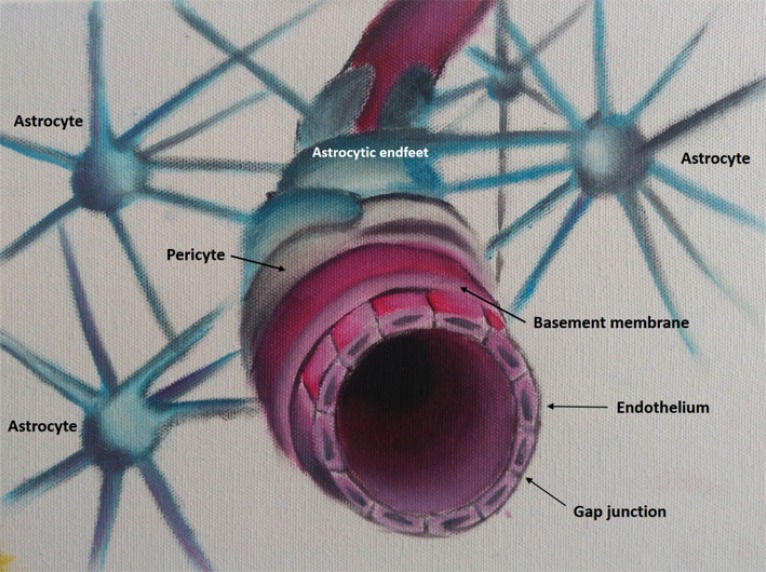
The BBB is a diffusion barrier comprised of glial cells, such as pericytes and astrocytes, and other types of cells, such as endothelial cells, perivascular macrophages, and the basal membrane [18] (Fig. 2). The BBB regulates the transport of macro and micromolecules and establishes and maintains a specific and stable fluid environment to meet the needs of the brain and protect the CNS against neurotoxins and other potentially damaging substances [18]. There is growing evidence of BBB disruption in neurological diseases with inflammatory components, such as Alzheimer’s disease and multiple sclerosis, and a model of BBB disruption in bipolar disorder has been proposed by Patel and Frey [18], where disruption in the BBB is associated with less protection and more influx of inflammatory molecules from the periphery to the brain.
Fig. (2).
Blood-brain barrier model. A blood-brain barrier model showing its components: astrocyte endfeet, pericytes, basement membrane, endothelium and the gap junctions. (The color version of the figure is available in the electronic copy of the article).
Despite the lack of other direct evidences demonstrating the disruption of BBB in bipolar disorder, indirect evidence points in direction to this hypothesis. This is the case of matrix metalloproteinase-9 (MMP-9), a proteinase that may lead to increased BBB permeability due to proteolysis of the basal lamina and extracellular matrix [18]. It has been shown that MMP-9 inhibition restores BBB integrity [87]. In addition, a study has demonstrated increased serum levels of MMP-9 in patients with bipolar disorder in an acute depressive episode, and also in remission after a depressive episode, when compared to patients in a manic episode and healthy subjects [88]. Another indirect evidence is the result of a cross-sectional study with DTI that showed some peripheral cytokines, such as TNF-α, IFN-γ, IL-8, IL-10, had significant associations with lower FA and higher radial diffusivity (RD) and mean diffusivity (MD) in cortico-limbic networks [89]. These findings suggest an inversely association of the peripheral inflammatory biomarkers and integrity of myelin sheaths.
Evidences from studies with lithium also demonstrate that differences in permeability of BBB may occur in bipolar disorder. Lithium passes unchanged through the BBB and its transport mechanisms from periphery to the brain may be involved in treatment resistance in bipolar disorder [90]. A study using 7Li magnetic resonance spectroscopy (7Li-MRS) in 10 patients with bipolar disorder showed that lithium concentrations in the brain increased markedly during manic episodes, while serum concentrations were unchanged [91]. Another study from the same group using a 7Li-MRS evaluated lithium concentrations in the brain of 14 patients with bipolar disorder showed that the reduction of manic symptoms after the initiation of lithium treatment was correlated with concentrations in the brain, but was not with blood levels [92]. A more recent study with 23 patients with bipolar disorder during depressive episode found a significant association between central and peripheral lithium levels only in remitters but not in non-remitters [90]. The authors concluded that non-remitters may not transport lithium properly to the brain, which may underlie treatment resistance to lithium in bipolar disorder [90].
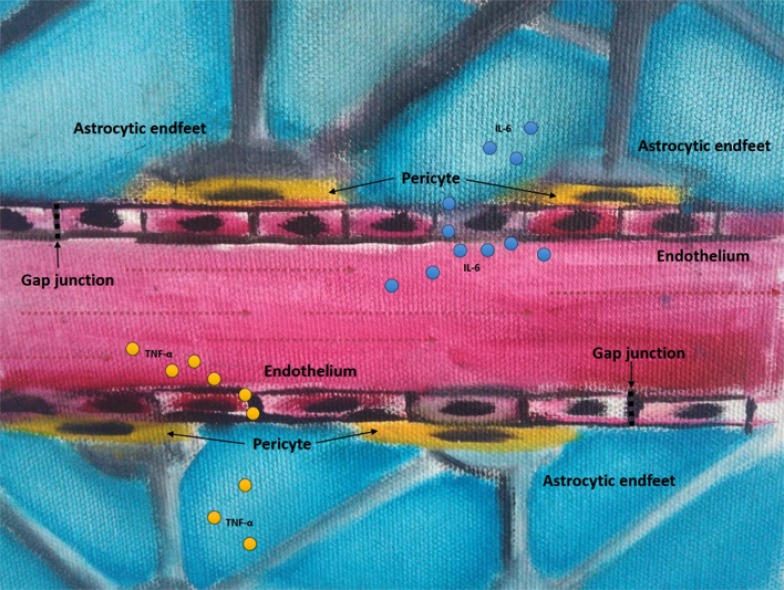
BBB disruption may explain the connection between changes in peripheral inflammatory markers and the abovementioned neuroinflammation in the pathophysiology of bipolar disorder [17] (Fig. 3). Several meta-analyses reported increased levels of inflammatory markers in patients with BD [16, 17, 93, 94] A meta-analysis showed that peripheral concentrations of cytokines such as IL-6, IL-10 and TNF-α were significantly elevated in patients with bipolar disorder [16, 17, 94] Mood episode-related differences were also shown for cytokines with increased levels of TNF-α and sTNF-α-R1 in patients in manic state compared to healthy controls [93]. However, there were no significant differences between patients with bipolar depression and healthy controls [17, 93]. These evidence support the implication of immune disruption in the pathophysiology of bipolar disorder and, in this sense, a study challenged retinoic acid-differentiated human neuroblastoma SH-SY5Y cells with serum from patients with bipolar disorder at early and late stages of illness [95]. The authors showed that cells treated with the serum of patients with bipolar disorder, particularly at late stages, showed decreased neurite density and decreased cell viability [95]. Despite the lack of evidence of altered cytokine penetration into brain in patients with bipolar disorder, the findings of differences in BBB permeability and others indirect evidence described above points into direction of this hypothesis, notwithstanding further studies are needed to clarify it.
Fig. (3).
Blood-brain barrier disruption. A model of blood-brain barrier disruption showing cytokines and the blood-brain barrier components: astrocyte endfeet, pericytes, basement membrane, endothelium, and the gap junctions. IL-6: interleukin-6; TNF-α: tumor necrosis factor-alpha. (The color version of the figure is available in the electronic copy of the article).
6. Glial cells: pathways of neuro-progression?
Bipolar disorder is an illness with a chronic course and different forms of progression, with subsets of patients presenting worse outcomes, marked by cognitive and functional impairment [96]. These clinical findings have been organized under the concept of neuroprogression, i.e., clinical and neurocognitive impairment secondary to the pathological rewiring of the brain in patients with bipolar disorder [15]. Indeed, several studies have reported reduced volumes of the left hippocampus [97], corpus callosum [98], and frontal cortices in patients with late-stage bipolar disorder [99] as a consequence of prior manic episodes. These findings are in line with the pioneering study that reported increased ventricular volumes in multiple-episode vs. first-episode patients with bipolar disorder [100].
Moreover, one study has examined whether structural neuroimaging scans coupled with a machine learning algorithm could distinguish between patients with bipolar disorder and healthy control subjects [101]. Authors found that the machine algorithm distinguished patients from healthy control subjects at a 70.3% accuracy (p < .005) using white matter density data, and at a 64.9% accuracy using gray matter density. Multiple brain regions, largely covering the fronto-limbic system, have been identified as the “most relevant” ones in distinguishing between the groups. Furthermore, patients identified by the algorithm with high certainty belonged to the late-stage bipolar disorder group, which included patients with >10 total lifetime manic episodes, including hospitalizations [101]. Finally, a meta-analysis of magnetic resonance imaging studies showed white matter volume reduction but no gray matter reduction among first-episode patients with bipolar disorder [102]. Recently, bipolar disorder has been associated with neurodevelopmental factors [103-106] and it is possible that the presence of these brain abnormalities may be explained by the involvement of astrocytes and oligodendrocytes in the developmental process of brain connectivity and circuitry. However, it is also possible that an initial pathology based on neuroplasticity impairments and involving exclusively the white matter may progress to a gray matter pathology associated with neuronal apoptosis and more substantial brain rewiring.
It has been hypothesized that impaired neuroplasticity in patients with bipolar disorder may be translated into shrinkage of the abovementioned brain structures by reducing neurites and intercellular connections in the neuronal network [15]. It is also suggested that the aforementioned biochemical changes in inflammatory pathways may play a causal role in this scenario, which has been referred to as systemic toxicity [107]. As discussed above, a previous study has revealed that the serum of patients with bipolar disorder, mainly those at late stages of the illness, may contain chemicals that could be toxic to neural cells, as proposed by the systemic toxicity hypothesis [95]. Abnormalities in inflammatory stress markers may play an important role in this process, given the emerging evidence of derangement in the immune system as a function of illness progression [108]. For example, there is evidence of increased serum IL-6 and TNF-α in late-stage patients with bipolar disorder [108], not to mention increased levels of CCL-11 and C-X-C motif ligand 10 (CXCL10) chemokines [109]. These are pro-inflammatory cytokines, which rely on the PI3K-Akt pathway for signaling [109].
It is worth mentioning that the interpretation of elevated levels of inflammatory markers in bipolar disorder is a matter of debate. For instance, it is not clear whether these signaling molecules can cross the BBB. As proposed by Patel and Frey [18] and discussed above, a connection between peripheral toxicity and CNS due to BBB disruption may lead to microglial activation. This was demonstrated in vivo with PET scan studies, which showed microglial activation in patients with bipolar I disorder [14, 24]. In the healthy brain, surveillant microglia and astrocytes communicate with neurons and other CNS cells to maintain their functions and brain homeostasis [62]. Conversely, when activated by brain injury, astrocytes lose control of the BBB, leading to its disruption and increasing permeability of peripheral proinflammatory markers into the brain [18]. This process contributes to maintaining chronic immune activation by transforming resting microglial cells to an activated form or M1 phenotype and leading to neuronal damage, in a process similar to that of neurodegenerative diseases [62].
7. Glial cells as a therapeutic target in bipolar disorder: towards new treatment strategies
Lithium treatment is indicated for all phases of bipolar disorder [110]. Meta-analyses studies have supported the role of lithium in acute manic episodes and also in maintenance treatments [111, 112]. In particular, two meta-analyses of neuroimaging studies have supported the neuroprotective effect of lithium in the hippocampus of patients with bipolar disorder [113, 114]. Other studies of white matter also suggest protective effects of lithium. A study with DTI found that euthymic bipolar I disorder patients using lithium had a higher FA and lower radial diffusivity when compared to non-lithium-using patients in the corpus callosum and left anterior corona radiate [115]. A study with older adults showed that among subjects with bipolar disorder, longer duration of lithium treatment was related to higher white matter integrity [116]. A clinical trial comparing protective effects of lithium and quetiapine in a prospective cohort of first-episode mania showed that, compared with baseline, lithium was more effective in slowing the progression of white matter volume reduction after 12 months [117].
Also, pre-clinical studies suggest some action of lithium in glial cells. A study of LPS-induced inflammation in rat primary glial cells found that lithium significantly reduced the secretion of TNF-α, IL1-β, prostaglandin E2, and nitric oxide [118]. Another study with microglial cells pretreated with lithium and stimulated with LPS showed that lithium significantly inhibited LPS-induced microglial activation and pro-inflammatory cytokine production [119]. Moreover, in a pre-clinical study, lithium inhibited astrocyte activation and pro-inflammatory cytokine production [34], reinforcing the neuroprotective role of this drug.
Valproic acid is another first-line drug in the treatment of bipolar disorder [110]. The anti-inflammatory properties of this drug have been demonstrated in an animal model of autoimmune encephalomyelitis [120]. Another study with mouse neurons in organotypic hippocampal slice cultures demonstrated a microglia-mediated neuroprotective function of valproic acid [121]. Primary rat neuronal, astroglial, and neuro-glial mixed culture systems have also been used to show that valproic acid may affect the synaptic excitatory-inhibitory balance through its effect on astrocytes [122]. Finally, a study with primary rat microglial cultures found that valproic acid can modulate microglial response to inflammatory insults mediated by LPS [123].
Clozapine, in turn, is an antipsychotic more commonly used in late-stage bipolar disorder [124]. A three-year follow-up study showed that clozapine was effective in reducing emergency service visits, hospitalizations, and total hospital days in patients with bipolar disorder [125]. A systematic review showed that clozapine was associated with improvement of mood and psychotic symptoms, suicidal and aggressive behaviors, and social functioning [126]. An in vitro study with astroglial and oligodendroglial cells found that treatment with clozapine reduced the release of S100B from both cells under basal conditions and serum and glucose deprivation [127]. In a schizophrenia animal model, the administration of clozapine reversed microglial activation as well as inducible nitric oxide synthase increase [128]. Finally, primary neuron-glia cell cultures showed a neuroprotection role of clozapine metabolites through microglial inactivation by inhibition of NADPH oxidase [129].
Conclusion
Given the evidence described above, it is possible that glial cells and the neuron-glia interaction play a role in the pathophysiology and neurodevelopmental process of bipolar disorder, even though the exact mechanisms implicated in the process are still not fully understood. It is also possible that changes in inflammatory markers and microglial function play an important role in neuroprogression in bipolar disorder. Both postmortem and in vivo studies have shown that microglial activation is involved in bipolar disorder neurobiology, which is in line with the presence of peripheral inflammatory markers described in meta-analyses and the BBB disruption hypothesis. Furthermore, neuroimaging studies have supported the presence of white matter microstructure abnormalities, pointing towards the involvement of oligodendrocytes. In addition, some drugs used in the treatment of bipolar disorder have effects on glial cells, and future studies may use these cells as targets for the development of new treatments. Further research is needed to improve our knowledge of neuron-glia interactions and their mechanisms to help improve detection and treatment of bipolar disorder.
Role of the Funding Source
Dr. Kapczinski and Dr. Kauer-Sant'Anna received funding resources from the institutions CAPES and CNPq belonging to the Brazilian Government. Dr. Quincozes-Satnos received funding resources from the institution CNPq.
Acknowledgements
We thank the Laboratory of Molecular Psychiatry team from Hospital de Clinicas de Porto Alegre.
Footnotes
Ref. [130].
Consent for Publication
Not applicable.
Conflict of Interest
Dr. Pinto, Dr. Passos, Dr. Librenza-Garcia, Dr. Schneider, Dr. Marcon, Dr. Quincozes-Santos and the authors Conte, Silva and Lima reported no biomedical financial interests or potential conflicts of interest. Dr. Kauer-Sant’Anna reports grants from CNPQ - UNIVERSAL, grants from SMRI, personal fees from ELI-LILLY, personal fees from NOVARTIS, personal fees from SHIRE, grants from FIPE-HCPA, grants and personal fees from CNPQ Produtividade em Pesquisa, outside the submitted work. Dr. Kapczinski is on speaker/advisor for Ache, Janssen and Daiichi Sankyo.
References
- 1.Merikangas K.R., Jin R., He J.P., Kessler R.C., Lee S., Sampson N.A., Viana M.C., Andrade L.H., Hu C., Karam E.G., Ladea M., Medina-Mora M.E., Ono Y., Posada-Villa J., Sagar R., Wells J.E., Zarkov Z. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch. Gen. Psychiatry. 2011;68(3):241–251. doi: 10.1001/archgenpsychiatry.2011.12. [http://dx.doi.org/10. 1001/archgenpsychiatry.2011.12]. [PMID: 21383262]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso J., Petukhova M., Vilagut G., Chatterji S., Heeringa S., Üstün T.B., Alhamzawi A.O., Viana M.C., Angermeyer M., Bromet E., Bruffaerts R., de Girolamo G., Florescu S., Gureje O., Haro J.M., Hinkov H., Hu C.Y., Karam E.G., Kovess V., Levinson D., Medina-Mora M.E., Nakamura Y., Ormel J., Posada-Villa J., Sagar R., Scott K.M., Tsang A., Williams D.R., Kessler R.C. Days out of role due to common physical and mental conditions: results from the WHO World Mental Health surveys. Mol. Psychiatry. 2011;16(12):1234–1246. doi: 10.1038/mp.2010.101. [http://dx.doi.org/10. 1038/mp.2010.101]. [PMID: 20938433]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pompili M., Gonda X., Serafini G., Innamorati M., Sher L., Amore M. Epidemiology of suicide in bipolar disorders : a systematic review of the literature. Bipolar Disord. 2013;15(5):457–490. doi: 10.1111/bdi.12087. [DOI] [PubMed] [Google Scholar]
- 4.Passos I.C., Mwangi B., Vieta E., Berk M., Kapczinski F. Areas of controversy in neuroprogression in bipolar disorder. Acta Psychiatr. Scand. 2016;134(2):91–103. doi: 10.1111/acps.12581. [http://dx.doi.org/10.1111/ acps.12581]. [PMID: 27097559]. [DOI] [PubMed] [Google Scholar]
- 5.Godin O., Etain B., Henry C., Bougerol T., Courtet P., Mayliss L., Passerieux C., Azorin J-M., Kahn J-P., Gard S., Costagliola D., Leboyer M. Metabolic syndrome in a French cohort of patients with bipolar disorder: results from the FACE-BD cohort. J. Clin. Psychiatry. 2014;75(10):1078–1085. doi: 10.4088/JCP.14m09038. [http://dx.doi.org/10.4088/ JCP.14m09038]. [PMID: 25373115]. [DOI] [PubMed] [Google Scholar]
- 6.Jerrell J.M., McIntyre R.S., Tripathi A. A cohort study of the prevalence and impact of comorbid medical conditions in pediatric bipolar disorder. J. Clin. Psychiatry. 2010;71(11):1518–1525. doi: 10.4088/JCP.09m05585ora. [http://dx.doi.org/10.4088/JCP.09m05585ora]. [PMID: 20584522]. [DOI] [PubMed] [Google Scholar]
- 7.Perugi G., Quaranta G., Belletti S., Casalini F., Mosti N., Toni C., Dell’Osso L. General medical conditions in 347 bipolar disorder patients: clinical correlates of metabolic and autoimmune-allergic diseases. J. Affect. Disord. 2015;170:95–103. doi: 10.1016/j.jad.2014.08.052. [http://dx. doi.org/10.1016/j.jad.2014.08.052]. [PMID: 25237732]. [DOI] [PubMed] [Google Scholar]
- 8.Farhi A., Cohen A.D., Shovman O., Comaneshter D., Amital H., Amital D. Bipolar disorder associated with rheumatoid arthritis: A case-control study. J. Affect. Disord. 2016;189:287–289. doi: 10.1016/j.jad.2015.09.058. [http://dx.doi.org/10.1016/j.jad.2015.09.058]. [PMID: 26454334]. [DOI] [PubMed] [Google Scholar]
- 9.Maletic V., Raison C. Integrated neurobiology of bipolar disorder. Front. Psychiatry. 2014;5:98. doi: 10.3389/fpsyt.2014.00098. [http://dx.doi.org/10.3389/fpsyt. 2014.00098]. [PMID: 25202283]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berk M., Dodd S., Kauer-Sant’anna M., Malhi G.S., Bourin M., Kapczinski F., Norman T. Dopamine dysregulation syndrome: implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr. Scand. Suppl. 2007;116(434):41–49. doi: 10.1111/j.1600-0447.2007.01058.x. [http://dx.doi.org/ 10.1111/j.1600-0447.2007.01058.x]. [PMID: 17688462]. [DOI] [PubMed] [Google Scholar]
- 11.Gigante A.D., Bond D.J., Lafer B., Lam R.W., Young L.T., Yatham L.N. Brain glutamate levels measured by magnetic resonance spectroscopy in patients with bipolar disorder: a meta-analysis. Bipolar Disord. 2012;14(5):478–487. doi: 10.1111/j.1399-5618.2012.01033.x. [http://dx.doi.org/ 10.1111/j.1399-5618.2012.01033.x]. [PMID: 22834460]. [DOI] [PubMed] [Google Scholar]
- 12.Réus G.Z., Fries G.R., Stertz L., Badawy M., Passos I.C., Barichello T., Kapczinski F., Quevedo J. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience. 2015;300:141–154. doi: 10.1016/j.neuroscience.2015.05.018. [http://dx.doi.org/10. 1016/j.neuroscience.2015.05.018]. [PMID: 25981208]. [DOI] [PubMed] [Google Scholar]
- 13.Rao J.S., Harry G.J., Rapoport S.I., Kim H.W. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol. Psychiatry. 2010;15(4):384–392. doi: 10.1038/mp.2009.47. [http://dx.doi.org/10.1038/mp.2009.47]. [PMID: 19488045]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haarman B.C., Riemersma-Van der Lek R.F., de Groot J.C., Ruhé H.G., Klein H.C., Zandstra T.E., Burger H., Schoevers R.A., de Vries E.F.J., Drexhage H.A., Nolen W.A., Doorduin J. Neuroinflammation in bipolar disorder - A [(11)C]-(R)-PK11195 positron emission tomography study. Brain Behav. Immun. 2014;40:219–225. doi: 10.1016/j.bbi.2014.03.016. [http://dx.doi.org/10.1016/j.bbi.2014.03.016]. [PMID: 24703991]. [DOI] [PubMed] [Google Scholar]
- 15.Berk M., Kapczinski F., Andreazza A.C., Dean O.M., Giorlando F., Maes M., Yücel M., Gama C.S., Dodd S., Dean B., Magalhães P.V., Amminger P., McGorry P., Malhi G.S. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci. Biobehav. Rev. 2011;35(3):804–817. doi: 10.1016/j.neubiorev.2010.10.001. [http://dx.doi.org/10.1016/j. neubiorev.2010.10.001]. [PMID: 20934453]. [DOI] [PubMed] [Google Scholar]
- 16.Munkholm K., Braüner J.V., Kessing L.V., Vinberg M. Cytokines in bipolar disorder vs. healthy control subjects: a systematic review and meta-analysis. J. Psychiatr. Res. 2013;47(9):1119–1133. doi: 10.1016/j.jpsychires.2013.05.018. [http://dx.doi.org/10.1016/j.jpsychires.2013.05.018]. [PMID: 23768870]. [DOI] [PubMed] [Google Scholar]
- 17.Goldsmith D.R., Rapaport M.H., Miller B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry. 2016;21(12):1696–1709. doi: 10.1038/mp.2016.3. [http://dx.doi.org/10.1038/ mp.2016.3]. [PMID: 26903267]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel J.P., Frey B.N. Disruption in the blood-brain barrier: The missing link between brain and body inflammation in bipolar disorder? Neural Plast. 2015;2015:708306. doi: 10.1155/2015/708306. [http://dx.doi.org/10. 1155/2015/708306]. [PMID: 26075104]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uranova N.A., Vostrikov V.M., Orlovskaya D.D., Rachmanova V.I. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr. Res. 2004;67(2-3):269–275. doi: 10.1016/S0920-9964(03)00181-6. [http:// dx.doi.org/10.1016/S0920-9964(03)00181-6]. [PMID: 14984887]. [DOI] [PubMed] [Google Scholar]
- 20.Malchow B., Strocka S., Frank F., Bernstein H-G., Steiner J., Schneider-Axmann T., Hasan A., Reich-Erkelenz D., Schmitz C., Bogerts B., Falkai P., Schmitt A. Stereological investigation of the posterior hippocampus in affective disorders. J. Neural Transm. (Vienna) 2015;122(7):1019–1033. doi: 10.1007/s00702-014-1316-x. [http://dx.doi.org/10. 1007/s00702-014-1316-x]. [PMID: 25307869]. [DOI] [PubMed] [Google Scholar]
- 21.Gos T., Schroeter M.L., Lessel W., Bernstein H.G., Dobrowolny H., Schiltz K., Bogerts B., Steiner J. S100B-immunopositive astrocytes and oligodendrocytes in the hippocampus are differentially afflicted in unipolar and bipolar depression: a postmortem study. J. Psychiatr. Res. 2013;47(11):1694–1699. doi: 10.1016/j.jpsychires.2013.07.005. [http:// dx.doi.org/10.1016/j.jpsychires.2013.07.005]. [PMID: 23896207]. [DOI] [PubMed] [Google Scholar]
- 22.Dean B., Gray L., Scarr E. Regionally specific changes in levels of cortical S100beta in bipolar 1 disorder but not schizophrenia. Aust. N. Z. J. Psychiatry. 2006;40(3):217–224. doi: 10.1080/j.1440-1614.2006.01777.x. [PMID: 16476148]. [DOI] [PubMed] [Google Scholar]
- 23.Ongür D., Drevets W.C., Price J.L. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc. Natl. Acad. Sci. USA. 1998;95(22):13290–13295. doi: 10.1073/pnas.95.22.13290. [http://dx.doi.org/10.1073/pnas. 95.22.13290]. [PMID: 9789081]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haarman B.C.M., Burger H., Doorduin J., Renken R.J.R.J., Sibeijn-Kuiper A.J.A.J., Marsman J-B.C.J.B.C., de Vries E.F.J.E.F.J., de Groot J.C.J.C., Drexhage H.A.H.A., Mendes R., Nolen W.A.W.A., Riemersma-Van der Lek R.F.R.F. Volume, metabolites and neuroinflammation of the hippocampus in bipolar disorder - A combined magnetic resonance imaging and positron emission tomography study. Brain Behav. Immun. 2015;60:1–5. [PMID: 26348581]. [Google Scholar]
- 25.Jakobsson J., Bjerke M., Sahebi S., Isgren A., Ekman C.J., Sellgren C., Olsson B., Zetterberg H., Blennow K., Pålsson E., Landén M. Monocyte and microglial activation in patients with mood-stabilized bipolar disorder. J. Psychiatry Neurosci. 2015;40(4):250–258. doi: 10.1503/jpn.140183. [http://dx.doi.org/10.1503/jpn.140183]. [PMID: 25768030]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y., Sonobe Y., Akahori T., Jin S., Kawanokuchi J., Noda M., Iwakura Y., Mizuno T., Suzumura A. IL-9 promotes Th17 cell migration into the central nervous system via CC chemokine ligand-20 produced by astrocytes. J. Immunol. 2011;186(7):4415–4421. doi: 10.4049/jimmunol.1003307. [http://dx.doi.org/10.4049/jimmunol.1003307]. [PMID: 21346235]. [DOI] [PubMed] [Google Scholar]
- 27.Goodkind M., Eickhoff S.B., Oathes D.J., Jiang Y., Chang A., Jones-Hagata L.B., Ortega B.N., Zaiko Y.V., Roach E.L., Korgaonkar M.S., Grieve S.M., Galatzer-Levy I., Fox P.T., Etkin A. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305–315. doi: 10.1001/jamapsychiatry.2014.2206. [http://dx.doi.org/ 10.1001/jamapsychiatry.2014.2206]. [PMID: 25651064]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonneh-Barkay D., Wang G., Starkey A., Hamilton R.L., Wiley C.A. In vivo CHI3L1 (YKL-40) expression in astrocytes in acute and chronic neurological diseases. J. Neuroinflammation. 2010;7:34. doi: 10.1186/1742-2094-7-34. [http://dx.doi.org/10.1186/1742-2094-7-34]. [PMID: 20540736]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burman J., Raininko R., Blennow K., Zetterberg H., Axelsson M., Malmeström C. YKL-40 is a CSF biomarker of intrathecal inflammation in secondary progressive multiple sclerosis. J. Neuroimmunol. 2016;292:52–57. doi: 10.1016/j.jneuroim.2016.01.013. [http://dx.doi.org/10.1016/j.jneuroim. 2016.01.013]. [PMID: 26943959]. [DOI] [PubMed] [Google Scholar]
- 30.Cantó E., Tintoré M., Villar L.M., Costa C., Nurtdinov R., Álvarez-Cermeño J.C., Arrambide G., Reverter F., Deisenhammer F., Hegen H., Khademi M., Olsson T., Tumani H., Rodríguez-Martín E., Piehl F., Bartos A., Zimova D., Kotoucova J., Kuhle J., Kappos L., García-Merino J.A., Sánchez A.J., Saiz A., Blanco Y., Hintzen R., Jafari N., Brassat D., Lauda F., Roesler R., Rejdak K., Papuc E., de Andrés C., Rauch S., Khalil M., Enzinger C., Galimberti D., Scarpini E., Teunissen C., Sánchez A., Rovira A., Montalban X., Comabella M. Chitinase 3-like 1: prognostic biomarker in clinically isolated syndromes. Brain. 2015;138(Pt 4):918–931. doi: 10.1093/brain/awv017. [http://dx.doi.org/10. 1093/brain/awv017]. [PMID: 25688078]. [DOI] [PubMed] [Google Scholar]
- 31.Rolstad S., Jakobsson J., Sellgren C., Isgren A., Ekman C.J., Bjerke M., Blennow K., Zetterberg H., Pålsson E., Landén M. CSF neuroinflammatory biomarkers in bipolar disorder are associated with cognitive impairment. Eur. Neuropsychopharmacol. 2015;25(8):1091–1098. doi: 10.1016/j.euroneuro.2015.04.023. [http://dx.doi.org/10.1016/j.euroneuro. 2015.04.023]. [PMID: 26024928]. [DOI] [PubMed] [Google Scholar]
- 32.da Rosa M.I., Simon C., Grande A.J., Barichello T., Oses J.P., Quevedo J. Serum S100B in manic bipolar disorder patients: Systematic review and meta-analysis. J. Affect. Disord. 2016;206:210–215. doi: 10.1016/j.jad.2016.07.030. [http://dx.doi.org/10.1016/j.jad.2016.07.030]. [PMID: 27475892]. [DOI] [PubMed] [Google Scholar]
- 33.Diaz-Ruiz A., Salgado-Ceballos H., Montes S., Maldonado V., Tristan L., Alcaraz-Zubeldia M., Ríos C. Acute alterations of glutamate, glutamine, GABA, and other amino acids after spinal cord contusion in rats. Neurochem. Res. 2007;32(1):57–63. doi: 10.1007/s11064-006-9225-5. [http://dx. doi.org/10.1007/s11064-006-9225-5]. [PMID: 17160506]. [DOI] [PubMed] [Google Scholar]
- 34.Li N., Zhang X., Dong H., Zhang S., Sun J., Qian Y. 2016.
- 35.Colombo E., Farina C. Astrocytes: Key regulators of neuroinflammation. Trends Immunol. 2016;37(9):608–620. doi: 10.1016/j.it.2016.06.006. [http://dx.doi. org/10.1016/j.it.2016.06.006]. [PMID: 27443914]. [DOI] [PubMed] [Google Scholar]
- 36.Feresten A.H., Barakauskas V., Ypsilanti A., Barr A.M., Beasley C.L. Increased expression of glial fibrillary acidic protein in prefrontal cortex in psychotic illness. Schizophr. Res. 2013;150(1):252–257. doi: 10.1016/j.schres.2013.07.024. [http://dx.doi.org/10.1016/j.schres.2013.07.024]. [PMID: 23911257]. [DOI] [PubMed] [Google Scholar]
- 37.Altshuler L.L., Abulseoud O.A., Foland-Ross L., Bartzokis G., Chang S., Mintz J., Hellemann G., Vinters H.V. Amygdala astrocyte reduction in subjects with major depressive disorder but not bipolar disorder. Bipolar Disord. 2010;12(5):541–549. doi: 10.1111/j.1399-5618.2010.00838.x. [http://dx. doi.org/10.1111/j.1399-5618.2010.00838.x]. [PMID: 20712756]. [DOI] [PubMed] [Google Scholar]
- 38.Pantazopoulos H., Woo T-U.W., Lim M.P., Lange N., Berretta S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch. Gen. Psychiatry. 2010;67(2):155–166. doi: 10.1001/archgenpsychiatry.2009.196. [http://dx.doi.org/10.1001/ archgenpsychiatry.2009.196]. [PMID: 20124115]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Damadzic R., Bigelow L.B., Krimer L.S., Goldenson D.A., Saunders R.C., Kleinman J.E., Herman M.M. A quantitative immunohistochemical study of astrocytes in the entorhinal cortex in schizophrenia, bipolar disorder and major depression: absence of significant astrocytosis. Brain Res. Bull. 2001;55(5):611–618. doi: 10.1016/s0361-9230(01)00529-9. [http:// dx.doi.org/10.1016/S0361-9230(01)00529-9]. [PMID: 11576757]. [DOI] [PubMed] [Google Scholar]
- 40.Ongür D., Jensen J.E., Prescot A.P., Stork C., Lundy M., Cohen B.M., Renshaw P.F. Abnormal glutamatergic neurotransmission and neuronal-glial interactions in acute mania. Biol. Psychiatry. 2008;64(8):718–726. doi: 10.1016/j.biopsych.2008.05.014. [http://dx.doi.org/10.1016/j.biopsych.2008. 05.014]. [PMID: 18602089]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weis S., Llenos I.C., Dulay J.R., Verma N., Sabunciyan S., Yolken R.H. Changes in region- and cell type-specific expression patterns of neutral amino acid transporter 1 (ASCT-1) in the anterior cingulate cortex and hippocampus in schizophrenia, bipolar disorder and major depression. J. Neural Transm. (Vienna) 2007;114(2):261–271. doi: 10.1007/s00702-006-0544-0. [http://dx.doi.org/10.1007/s00702-006-0544-0]. [PMID: 16897601]. [DOI] [PubMed] [Google Scholar]
- 42.Ganzola R., Duchesne S. Voxel-based morphometry meta-analysis of gray and white matter finds significant areas of differences in bipolar patients from healthy controls. Bipolar Disord. 2017;19(2):74–83. doi: 10.1111/bdi.12488. [http://dx.doi.org/10.1111/bdi.12488]. [PMID: 28444949]. [DOI] [PubMed] [Google Scholar]
- 43.Vederine F-E., Wessa M., Leboyer M., Houenou J. A meta-analysis of whole-brain diffusion tensor imaging studies in bipolar disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(8):1820–1826. doi: 10.1016/j.pnpbp.2011.05.009. [http://dx.doi.org/10.1016/j.pnpbp.2011.05.009]. [PMID: 21624424]. [DOI] [PubMed] [Google Scholar]
- 44.Nortje G., Stein D.J., Radua J., Mataix-Cols D., Horn N. Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. J. Affect. Disord. 2013;150(2):192–200. doi: 10.1016/j.jad.2013.05.034. [http://dx.doi.org/10.1016/j.jad.2013.05.034]. [PMID: 23810479]. [DOI] [PubMed] [Google Scholar]
- 45.Konradi C., Sillivan S.E., Clay H.B. Mitochondria, oligodendrocytes and inflammation in bipolar disorder: evidence from transcriptome studies points to intriguing parallels with multiple sclerosis. Neurobiol. Dis. 2012;45(1):37–47. doi: 10.1016/j.nbd.2011.01.025. [http://dx.doi.org/10. 1016/j.nbd.2011.01.025]. [PMID: 21310238]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosa P.M., Martins L.A.M., Souza D.O., Quincozes-Santos A. Glioprotective effect of resveratrol: An emerging therapeutic role for oligodendroglial cells. Mol. Neurobiol. 2017 doi: 10.1007/s12035-017-0510-x. [http://dx. doi.org/10.1007/s12035-017-0510-x]. [PMID: 28456938]. [DOI] [PubMed] [Google Scholar]
- 47.Young K.M., Psachoulia K., Tripathi R.B., Dunn S-J., Cossell L., Attwell D., Tohyama K., Richardson W.D. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77(5):873–885. doi: 10.1016/j.neuron.2013.01.006. [http://dx.doi.org/10.1016/j. neuron.2013.01.006]. [PMID: 23473318]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ziemka-Nalecz M., Janowska J., Strojek L., Jaworska J., Zalewska T., Frontczak-Baniewicz M., Sypecka J. Impact of neonatal hypoxia-ischaemia on oligodendrocyte survival, maturation and myelinating potential. J. Cell. Mol. Med. 2017 doi: 10.1111/jcmm.13309. [PMID: 28782169]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toyama B.H., Savas J.N., Park S.K., Harris M.S., Ingolia N.T., Yates J.R., III, Hetzer M.W. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell. 2013;154(5):971–982. doi: 10.1016/j.cell.2013.07.037. [http://dx.doi.org/10.1016/j.cell.2013.07.037]. [PMID: 23993091]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McTigue D.M., Tripathi R.B. The life, death, and replacement of oligodendrocytes in the adult CNS. J. Neurochem. 2008;107(1):1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [http://dx.doi.org/10.1111/j.1471-4159.2008.05570.x]. [PMID: 18643793]. [DOI] [PubMed] [Google Scholar]
- 51.Thorburne S.K., Juurlink B.H. Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J. Neurochem. 1996;67(3):1014–1022. doi: 10.1046/j.1471-4159.1996.67031014.x. [http://dx. doi.org/10.1046/j.1471-4159.1996.67031014.x]. [PMID: 8752107]. [DOI] [PubMed] [Google Scholar]
- 52.Ibarretxe G., Sánchez-Gómez M.V., Campos-Esparza M.R., Alberdi E., Matute C. Differential oxidative stress in oligodendrocytes and neurons after excitotoxic insults and protection by natural polyphenols. Glia. 2006;53(2):201–211. doi: 10.1002/glia.20267. [http://dx.doi.org/10. 1002/glia.20267]. [PMID: 16206167]. [DOI] [PubMed] [Google Scholar]
- 53.Shao Y., Peng H., Huang Q., Kong J., Xu H. Quetiapine mitigates the neuroinflammation and oligodendrocyte loss in the brain of C57BL/6 mouse following cuprizone exposure for one week. Eur. J. Pharmacol. 2015;765:249–257. doi: 10.1016/j.ejphar.2015.08.046. [http://dx.doi.org/ 10.1016/j.ejphar.2015.08.046]. [PMID: 26321148]. [DOI] [PubMed] [Google Scholar]
- 54.Olympiou M., Sargiannidou I., Markoullis K., Karaiskos C., Kagiava A., Kyriakoudi S., Abrams C.K., Kleopa K.A. Systemic inflammation disrupts oligodendrocyte gap junctions and induces ER stress in a model of CNS manifestations of X-linked Charcot-Marie-Tooth disease. Acta Neuropathol. Commun. 2016;4(1):95. doi: 10.1186/s40478-016-0369-5. [http://dx.doi.org/10.1186/s40478-016-0369-5]. [PMID: 27585976]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Magioncalda P., Martino M., Conio B., Piaggio N., Teodorescu R., Escelsior A., Marozzi V., Rocchi G., Roccatagliata L., Northoff G., Inglese M., Amore M. Patterns of microstructural white matter abnormalities and their impact on cognitive dysfunction in the various phases of type I bipolar disorder. J. Affect. Disord. 2016;193:39–50. doi: 10.1016/j.jad.2015.12.050. [http://dx.doi.org/10.1016/j.jad.2015.12.050]. [PMID: 26766032]. [DOI] [PubMed] [Google Scholar]
- 56.Sarıçiçek A., Zorlu N., Yalın N., Hıdıroğlu C., Çavuşoğlu B., Ceylan D., Ada E., Tunca Z., Özerdem A. Abnormal white matter integrity as a structural endophenotype for bipolar disorder. Psychol. Med. 2016;46(7):1547–1558. doi: 10.1017/S0033291716000180. [http://dx.doi.org/10.1017/ S0033291716000180]. [PMID: 26947335]. [DOI] [PubMed] [Google Scholar]
- 57.Sprooten E., Barrett J., McKay D.R., Knowles E.E., Mathias S.R., Winkler A.M., Brumbaugh M.S., Landau S., Cyr L., Kochunov P., Glahn D.C. A comprehensive tractography study of patients with bipolar disorder and their unaffected siblings. Hum. Brain Mapp. 2016;37(10):3474–3485. doi: 10.1002/hbm.23253. [http://dx.doi.org/10.1002/ hbm.23253]. [PMID: 27198848]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gottesman I.I., Gould T.D. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [http://dx.doi.org/10.1176/appi.ajp.160.4.636]. [PMID: 12668349]. [DOI] [PubMed] [Google Scholar]
- 59.Nery F.G., Norris M., Eliassen J.C., Weber W.A., Blom T.J., Welge J.A., Barzman D.A., Strawn J.R., Adler C.M., Strakowski S.M., DelBello M.P. White matter volumes in youth offspring of bipolar parents. J. Affect. Disord. 2017;209:246–253. doi: 10.1016/j.jad.2016.11.023. [http://dx.doi.org/10.1016/j.jad.2016.11.023]. [PMID: 27936454]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saijo K., Glass C.K. Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol. 2011;11(11):775–787. doi: 10.1038/nri3086. [http://dx.doi.org/10.1038/nri3086]. [PMID: 22025055]. [DOI] [PubMed] [Google Scholar]
- 61.Watkins C.C., Sawa A., Pomper M.G. Glia and immune cell signaling in bipolar disorder: insights from neuropharmacology and molecular imaging to clinical application. Transl. Psychiatry. 2014;4:e350. doi: 10.1038/tp.2013.119. [http://dx.doi.org/10.1038/tp.2013.119]. [PMID: 24448212]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gomez-Nicola D., Perry V.H. Microglial dynamics and role in the healthy and diseased brain: a paradigm of functional plasticity. Neuroscientist. 2015;21(2):169–184. doi: 10.1177/1073858414530512. [http://dx.doi.org/10.1177/ 1073858414530512]. [PMID: 24722525]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frost J.L., Schafer D.P. Microglia: Architects of the developing nervous system. Trends Cell Biol. 2016;26(8):587–597. doi: 10.1016/j.tcb.2016.02.006. [http:// dx.doi.org/10.1016/j.tcb.2016.02.006]. [PMID: 27004698]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [http://dx.doi.org/10.1038/nri2448]. [PMID: 19029990]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakagawa Y., Chiba K. Role of microglial m1/m2 polarization in relapse and remission of psychiatric disorders and diseases. Pharmaceuticals (Basel) 2014;7(12):1028–1048. doi: 10.3390/ph7121028. [http://dx.doi.org/ 10.3390/ph7121028]. [PMID: 25429645]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ascoli B. M., Géa L. P., Colombo R., Barbé-Tuana F. M., Kapczinski F., Rosa A. R. 2016. [DOI] [PubMed]
- 67.Stertz L., Fries G.R., Rosa A.R., Kauer-Sant’anna M., Ferrari P., Paz A.V.C., Green C., Cunha A.B.M., Dal-Pizzol F., Gottfried C., Kapczinski F. Damage-associated molecular patterns and immune activation in bipolar disorder. Acta Psychiatr. Scand. 2015;132(3):211–217. doi: 10.1111/acps.12417. [http://dx.doi.org/10.1111/acps.12417]. [PMID: 25891376]. [DOI] [PubMed] [Google Scholar]
- 68.Ferrante C.J., Leibovich S.J. Regulation of macrophage polarization and wound healing. Adv. Wound Care (New Rochelle) 2012;1(1):10–16. doi: 10.1089/wound.2011.0307. [http://dx.doi.org/10.1089/wound.2011.0307]. [PMID: 24527272]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brites D., Fernandes A. Neuroinflammation and depression: Microglia activation, extracellular microvesicles and microRNA dysregulation. Front. Cell. Neurosci. 2015;9:476. doi: 10.3389/fncel.2015.00476. [http://dx.doi. org/10.3389/fncel.2015.00476]. [PMID: 26733805]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duluc D., Delneste Y., Tan F., Moles M.P., Grimaud L., Lenoir J., Preisser L., Anegon I., Catala L., Ifrah N., Descamps P., Gamelin E., Gascan H., Hebbar M., Jeannin P. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood. 2007;110(13):4319–4330. doi: 10.1182/blood-2007-02-072587. [http://dx.doi.org/10.1182/blood-2007-02-072587]. [PMID: 17848619]. [DOI] [PubMed] [Google Scholar]
- 71.Brites D., Vaz A.R. Microglia centered pathogenesis in ALS: insights in cell interconnectivity. Front. Cell. Neurosci. 2014;8:117. doi: 10.3389/fncel.2014.00117. [http://dx.doi.org/10.3389/fncel.2014.00117]. [PMID: 24904276]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim H-J., Cho M-H., Shim W.H., Kim J.K., Jeon E-Y., Kim D-H., Yoon S-Y. Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Mol. Psychiatry. 2017;22:1576–1584. doi: 10.1038/mp.2016.103. [PMID: 27400854]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sierra A., Encinas J.M., Deudero J.J., Chancey J.H., Enikolopov G., Overstreet-Wadiche L.S., Tsirka S.E., Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7(4):483–495. doi: 10.1016/j.stem.2010.08.014. [http://dx.doi.org/10.1016/j.stem.2010.08.014]. [PMID: 20887954]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fricker M., Neher J.J., Zhao J-W., Théry C., Tolkovsky A.M., Brown G.C. MFG-E8 mediates primary phagocytosis of viable neurons during neuroinflammation. J. Neurosci. 2012;32(8):2657–2666. doi: 10.1523/JNEUROSCI.4837-11.2012. [http://dx.doi.org/10.1523/JNEUROSCI.4837-11.2012]. [PMID: 22357850]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nivoli A.M.A., Murru A., Vieta E. Lithium: still a cornerstone in the long-term treatment in bipolar disorder? Neuropsychobiology. 2010;62(1):27–35. doi: 10.1159/000314307. [http://dx.doi.org/10.1159/000314307]. [PMID: 20453532]. [DOI] [PubMed] [Google Scholar]
- 76.Huo K., Sun Y., Li H., Du X., Wang X., Karlsson N., Zhu C., Blomgren K. Lithium reduced neural progenitor apoptosis in the hippocampus and ameliorated functional deficits after irradiation to the immature mouse brain. Mol. Cell. Neurosci. 2012;51(1-2):32–42. doi: 10.1016/j.mcn.2012.07.002. [http://dx.doi.org/10.1016/j.mcn.2012.07.002]. [PMID: 22800605]. [DOI] [PubMed] [Google Scholar]
- 77.Liu P., Zhang Z., Wang Q., Guo R., Mei W. Lithium chloride facilitates autophagy following spinal cord injury via ERK-dependent pathway. Neurotox. Res. 2017;32(4):535–543. doi: 10.1007/s12640-017-9758-1. [http:// dx.doi.org/10.1007/s12640-017-9758-1]. [PMID: 28593525]. [DOI] [PubMed] [Google Scholar]
- 78.Li Q., Li H., Roughton K., Wang X., Kroemer G., Blomgren K., Zhu C. Lithium reduces apoptosis and autophagy after neonatal hypoxia-ischemia. Cell Death Dis. 2010;1:e56. doi: 10.1038/cddis.2010.33. [http://dx.doi. org/10.1038/cddis.2010.33]. [PMID: 21364661]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sarkar S., Floto R.A., Berger Z., Imarisio S., Cordenier A., Pasco M., Cook L.J., Rubinsztein D.C. Lithium induces autophagy by inhibiting inositol monophosphatase. J. Cell Biol. 2005;170(7):1101–1111. doi: 10.1083/jcb.200504035. [http://dx.doi.org/10.1083/jcb.200504035]. [PMID: 16186256]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valvassori S.S., Tonin P.T., Varela R.B., Carvalho A.F., Mariot E., Amboni R.T., Bianchini G., Andersen M.L., Quevedo J. Lithium modulates the production of peripheral and cerebral cytokines in an animal model of mania induced by dextroamphetamine. Bipolar Disord. 2015;17(5):507–517. doi: 10.1111/bdi.12299. [http://dx.doi.org/10.1111/ bdi.12299]. [PMID: 25929806]. [DOI] [PubMed] [Google Scholar]
- 81.Domingues H.S., Portugal C.C., Socodato R., Relvas J.B. Oligodendrocyte, astrocyte, and microglia crosstalk in myelin development, damage, and repair. Front. Cell Dev. Biol. 2016;4:1–16. doi: 10.3389/fcell.2016.00071. [PMID: 26858948]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barres B.A. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60(3):430–440. doi: 10.1016/j.neuron.2008.10.013. [http:// dx.doi.org/10.1016/j.neuron.2008.10.013]. [PMID: 18995817]. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y., Jin S., Sonobe Y., Cheng Y., Horiuchi H., Parajuli B., Kawanokuchi J., Mizuno T., Takeuchi H., Suzumura A. Interleukin-1β induces blood-brain barrier disruption by downregulating Sonic hedgehog in astrocytes. PLoS One. 2014;9(10):e110024. doi: 10.1371/journal.pone.0110024. [http://dx.doi.org/10.1371/journal.pone.0110024]. [PMID: 25313834]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Papa M., De Luca C., Petta F., Alberghina L., Cirillo G. Astrocyte-neuron interplay in maladaptive plasticity. Neurosci. Biobehav. Rev. 2014;42:35–54. doi: 10.1016/j.neubiorev.2014.01.010. [http://dx.doi.org/10.1016/j.neubiorev. 2014.01.010]. [PMID: 24509064]. [DOI] [PubMed] [Google Scholar]
- 85.Bélanger M., Allaman I., Magistretti P.J. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14(6):724–738. doi: 10.1016/j.cmet.2011.08.016. [http://dx.doi.org/10.1016/j.cmet.2011. 08.016]. [PMID: 22152301]. [DOI] [PubMed] [Google Scholar]
- 86.McKenna M.C. The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain. J. Neurosci. Res. 2007;85(15):3347–3358. doi: 10.1002/jnr.21444. [http://dx.doi.org/10.1002/jnr.21444]. [PMID: 17847118]. [DOI] [PubMed] [Google Scholar]
- 87.Gasche Y., Copin J., Sugawara T., Fujimura M., Chan P.H. Increased serum matrix metalloproteinase-9 (MMP-9) levels in young patients during bipolar depression. J. Affect. Disord. 2013;146:286–289. doi: 10.1016/j.jad.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 88.Rybakowski J.K., Remlinger-Molenda A., Czech-Kucharska A., Wojcicka M., Michalak M., Losy J. Increased serum matrix metalloproteinase-9 (MMP-9) levels in young patients during bipolar depression. J. Affect. Disord. 2013;146(2):286–289. doi: 10.1016/j.jad.2012.07.019. [http://dx.doi. org/10.1016/j.jad.2012.07.019]. [PMID: 22858217]. [DOI] [PubMed] [Google Scholar]
- 89.Benedetti F., Poletti S., Hoogenboezem T.A., Mazza E., Ambrée O., de Wit H., Wijkhuijs A.J.M., Locatelli C., Bollettini I., Colombo C., Arolt V., Drexhage H.A. Inflammatory cytokines influence measures of white matter integrity in Bipolar Disorder. J. Affect. Disord. 2016;202:1–9. doi: 10.1016/j.jad.2016.05.047. [http://dx.doi.org/10.1016/j.jad. 2016.05.047]. [PMID: 27253210]. [DOI] [PubMed] [Google Scholar]
- 90.Machado-Vieira R., Otaduy M.C., Zanetti M.V., De Sousa R.T., Dias V.V., Leite C.C., Forlenza O.V., Busatto G.F., Soares J.C., Gattaz W.F. A selective association between central and peripheral lithium levels in remitters in bipolar depression: A 3T-(7) li magnetic resonance spectroscopy study. Acta Psychiatr. Scand. 2016;133(3):214–220. doi: 10.1111/acps.12511. [http://dx.doi.org/10.1111/acps.12511]. [PMID: 26513535]. [DOI] [PubMed] [Google Scholar]
- 91.Kato T., Takahashi S., Inubushi T. Brain lithium concentration by 7Li- and 1H-magnetic resonance spectroscopy in bipolar disorder. Psychiatry Res. 1992;45(1):53–63. doi: 10.1016/0925-4927(92)90013-t. [http://dx.doi.org/10.1016/ 0925-4927(92)90013-T]. [PMID: 1410078]. [DOI] [PubMed] [Google Scholar]
- 92.Kato T., Inubushi T., Takahashi S. Relationship of lithium concentrations in the brain measured by lithium-7 magnetic resonance spectroscopy to treatment response in mania. J. Clin. Psychopharmacol. 1994;14(5):330–335. [PMID: 7806688]. [PubMed] [Google Scholar]
- 93.Munkholm K., Vinberg M., Vedel Kessing L. Cytokines in bipolar disorder: a systematic review and meta-analysis. J. Affect. Disord. 2013;144(1-2):16–27. doi: 10.1016/j.jad.2012.06.010. [http://dx.doi.org/10.1016/j.jad.2012. 06.010]. [PMID: 22749156]. [DOI] [PubMed] [Google Scholar]
- 94.Modabbernia A., Taslimi S., Brietzke E., Ashrafi M. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol. Psychiatry. 2013;74(1):15–25. doi: 10.1016/j.biopsych.2013.01.007. [http://dx.doi.org/10.1016/j. biopsych.2013.01.007]. [PMID: 23419545]. [DOI] [PubMed] [Google Scholar]
- 95.Wollenhaupt-Aguiar B., Pfaffenseller B., Chagas V.S., Castro M.A.A., Passos I.C., Kauer-Sant’Anna M., Kapczinski F., Klamt F. Reduced neurite density in neuronal cell cultures exposed to serum of patients with bipolar disorder. Int. J. Neuropsychopharmacol. 2016;•••:pyw051. doi: 10.1093/ijnp/pyw051. [http://dx.doi.org/10.1093/ijnp/pyw051]. [PMID: 27207915]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosa A.R., Magalhães P.V.S., Czepielewski L., Sulzbach M.V., Goi P.D., Vieta E., Gama C.S., Kapczinski F. Clinical staging in bipolar disorder: focus on cognition and functioning. J. Clin. Psychiatry. 2014;75(5):e450–e456. doi: 10.4088/JCP.13m08625. [http://dx.doi.org/10.4088/JCP. 13m08625]. [PMID: 24922497]. [DOI] [PubMed] [Google Scholar]
- 97.Cao B., Passos I.C., Mwangi B., Bauer I.E., Zunta-Soares G.B., Kapczinski F., Soares J.C. Hippocampal volume and verbal memory performance in late-stage bipolar disorder. J. Psychiatr. Res. 2016;73:102–107. doi: 10.1016/j.jpsychires.2015.12.012. [http://dx.doi.org/10.1016/j.jpsychires.2015. 12.012]. [PMID: 26714201]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lavagnino L., Cao B., Mwangi B., Wu M-J., Sanches M., Zunta-Soares G.B., Kapczinski F., Soares J. Changes in the corpus callosum in women with late-stage bipolar disorder. Acta Psychiatr. Scand. 2015;131(6):458–464. doi: 10.1111/acps.12397. [http://dx.doi.org/10.1111/ acps.12397]. [PMID: 25640667]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abé C., Ekman C-J., Sellgren C., Petrovic P., Ingvar M., Landén M. Manic episodes are related to changes in frontal cortex: a longitudinal neuroimaging study of bipolar disorder 1. Brain. 2015;138(Pt 11):3440–3448. doi: 10.1093/brain/awv266. [http://dx.doi.org/10.1093/brain/awv266]. [PMID: 26373602]. [DOI] [PubMed] [Google Scholar]
- 100.Strakowski S.M., DelBello M.P., Zimmerman M.E., Getz G.E., Mills N.P., Ret J., Shear P., Adler C.M. Ventricular and periventricular structural volumes in first- versus multiple-episode bipolar disorder. Am. J. Psychiatry. 2002;159(11):1841–1847. doi: 10.1176/appi.ajp.159.11.1841. [DOI] [PubMed] [Google Scholar]
- 101.Mwangi B., Wu M-J., Cao B., Passos I.C., Lavagnino L., Keser Z., Zunta-Soares G.B., Hasan K.M., Kapczinski F., Soares J.C. Individualized prediction and clinical staging of bipolar disorders using neuroanatomical biomarkers. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2016;1:186–194. doi: 10.1016/j.bpsc.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vita A. L, D. P.; Gray, S. E. Gray matter, white matter, brain, and intracranial volumes in first-episode bipolar disorder: a meta-analysis of magnetic resonance imaging studies. Bipolar Disord. 2009;11:807–814. doi: 10.1111/j.1399-5618.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 103.Bora E., Pantelis C. Meta-analysis of cognitive impairment in first-episode bipolar disorder: Comparison with first-episode schizophrenia and healthy controls. Schizophr. Bull. 2015;41(5):1095–1104. doi: 10.1093/schbul/sbu198. [http://dx.doi.org/10.1093/schbul/sbu198]. [PMID: 25616505]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bora E., Özerdem A. A meta-analysis of neurocognition in youth with familial high risk for bipolar disorder. Eur. Psychiatry. 2017;44:17–23. doi: 10.1016/j.eurpsy.2017.02.483. [http://dx.doi.org/10.1016/j.eurpsy.2017.02.483]. [PMID: 28531561]. [DOI] [PubMed] [Google Scholar]
- 105.Bora E., Özerdem A. Meta-analysis of longitudinal studies of cognition in bipolar disorder: comparison with healthy controls and schizophrenia. Psychol. Med. 2017;47(16):2753–2766. doi: 10.1017/S0033291717001490. [http://dx. doi.org/10.1017/S0033291717001490]. [PMID: 28585513]. [DOI] [PubMed] [Google Scholar]
- 106.Betts K.S., Williams G.M., Najman J.M., Alati R. Predicting spectrums of adult mania, psychosis and depression by prospectively ascertained childhood neurodevelopment. J. Psychiatr. Res. 2016;72:22–29. doi: 10.1016/j.jpsychires.2015.10.013. [http://dx.doi.org/10.1016/j.jpsychires.2015.10.013]. [PMID: 26519766]. [DOI] [PubMed] [Google Scholar]
- 107.Kapczinski F., Dal-Pizzol F., Teixeira A.L., Magalhaes P.V. Kauer-Sant’Anna, M.; Klamt, F.; Pasquali, M.A.; Quevedo, J.; Gama, C.S.; Post, R. A systemic toxicity index developed to assess peripheral changes in mood episodes. Mol. Psychiatry. 2010;15(8):784–786. doi: 10.1038/mp.2009.112. [http://dx.doi.org/10.1038/mp.2009.112]. [PMID: 20351717]. [DOI] [PubMed] [Google Scholar]
- 108.Kauer-Sant’Anna M., Kapczinski F., Andreazza A.C., Bond D.J., Lam R.W., Young L.T., Yatham L.N. Brain-derived neurotrophic factor and inflammatory markers in patients with early- vs. late-stage bipolar disorder. Int. J. Neuropsychopharmacol. 2009;12(4):447–458. doi: 10.1017/S1461145708009310. [http://dx.doi.org/10.1017/S1461145708009310]. [PMID: 18771602]. [DOI] [PubMed] [Google Scholar]
- 109.Panizzutti B., Gubert C., Schuh A.L., Ferrari P., Bristot G., Fries G.R., Massuda R., Walz J., Rocha N.P., Berk M., Teixeira A.L., Gama C.S. Increased serum levels of eotaxin/CCL11 in late-stage patients with bipolar disorder: An accelerated aging biomarker? J. Affect. Disord. 2015;182:64–69. doi: 10.1016/j.jad.2014.12.010. [http://dx.doi.org/10.1016/j.jad.2014.12.010]. [PMID: 25973785]. [DOI] [PubMed] [Google Scholar]
- 110.Yatham L.N., Kennedy S.H., Parikh S.V., Schaffer A., Beaulieu S., Alda M., O’Donovan C., Macqueen G., McIntyre R.S., Sharma V., Ravindran A., Young L.T., Milev R., Bond D.J., Frey B.N., Goldstein B.I., Lafer B., Birmaher B., Ha K., Nolen W.A., Berk M. Canadian network for mood and anxiety treatments (CANMAT) and international society for bipolar disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord. 2013;15(1):1–44. doi: 10.1111/bdi.12025. [http://dx.doi.org/10.1111/bdi.12025]. [PMID: 23237061]. [DOI] [PubMed] [Google Scholar]
- 111.Cipriani A., Barbui C., Salanti G., Rendell J., Brown R., Stockton S., Purgato M., Spineli L.M., Goodwin G.M., Geddes J.R. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378(9799):1306–1315. doi: 10.1016/S0140-6736(11)60873-8. [http://dx.doi.org/10.1016/S0140-6736(11) 60873-8]. [PMID: 21851976]. [DOI] [PubMed] [Google Scholar]
- 112.Miura T., Noma H., Furukawa T.A., Mitsuyasu H., Tanaka S., Stockton S., Salanti G., Motomura K., Shimano-Katsuki S., Leucht S., Cipriani A., Geddes J.R., Kanba S. Comparative efficacy and tolerability of pharmacological treatments in the maintenance treatment of bipolar disorder: a systematic review and network meta-analysis. Lancet Psychiatry. 2014;1(5):351–359. doi: 10.1016/S2215-0366(14)70314-1. [http://dx. doi.org/10.1016/S2215-0366(14)70314-1]. [PMID: 26360999]. [DOI] [PubMed] [Google Scholar]
- 113.Hajek T., Kopecek M., Höschl C., Alda M. Smaller hippocampal volumes in patients with bipolar disorder are masked by exposure to lithium: a meta-analysis. J. Psychiatry Neurosci. 2012;37(5):333–343. doi: 10.1503/jpn.110143. [http://dx.doi.org/10.1503/jpn.110143]. [PMID: 22498078]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Otten M., Meeter M. Hippocampal structure and function in individuals with bipolar disorder: a systematic review. J. Affect. Disord. 2015;174:113–125. doi: 10.1016/j.jad.2014.11.001. [http://dx.doi.org/10.1016/j.jad.2014.11.001]. [PMID: 25496759]. [DOI] [PubMed] [Google Scholar]
- 115.Haarman B.C.M., Riemersma-Van der Lek R.F., Burger H., de Groot J.C., Drexhage H.A., Nolen W.A., Cerliani L. Diffusion tensor imaging in euthymic bipolar disorder - A tract-based spatial statistics study. J. Affect. Disord. 2016;203:281–291. doi: 10.1016/j.jad.2016.05.040. [http://dx. doi.org/10.1016/j.jad.2016.05.040]. [PMID: 27317921]. [DOI] [PubMed] [Google Scholar]
- 116.Gildengers A.G., Butters M.A., Aizenstein H.J., Marron M.M., Emanuel J., Anderson S.J., Weissfeld L.A., Becker J.T., Lopez O.L., Mulsant B.H., Reynolds C.F., III Longer lithium exposure is associated with better white matter integrity in older adults with bipolar disorder. Bipolar Disord. 2015;17(3):248–256. doi: 10.1111/bdi.12260. [http://dx. doi.org/10.1111/bdi.12260]. [PMID: 25257942]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Berk M., Dandash O., Daglas R., Cotton S.M., Allott K., Fornito A., Suo C., Klauser P., Liberg B., Henry L., Macneil C., Hasty M., McGorry P., Pantelis C., Yücel M. Neuroprotection after a first episode of mania: a randomized controlled maintenance trial comparing the effects of lithium and quetiapine on grey and white matter volume. Transl. Psychiatry. 2017;7(1):e1011. doi: 10.1038/tp.2016.281. [http://dx.doi.org/10.1038/tp.2016.281]. [PMID: 28117843]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nahman S., Belmaker R.H., Azab A.N. Effects of lithium on lipopolysaccharide-induced inflammation in rat primary glia cells. Innate Immun. 2012;18(3):447–458. doi: 10.1177/1753425911421512. [http://dx.doi.org/10.1177/ 1753425911421512]. [PMID: 21994254]. [DOI] [PubMed] [Google Scholar]
- 119.Dong H., Zhang X., Dai X., Lu S., Gui B., Jin W., Zhang S., Zhang S., Qian Y. Lithium ameliorates lipopolysaccharide-induced microglial activation via inhibition of toll-like receptor 4 expression by activating the PI3K/Akt/FoxO1 pathway. J. Neuroinflammation. 2014;11:140. doi: 10.1186/s12974-014-0140-4. [http://dx.doi.org/10.1186/s12974-014-0140-4]. [PMID: 25115727]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang Z., Zhang Z.Y., Wu Y., Schluesener H.J. Valproic acid ameliorates inflammation in experimental autoimmune encephalomyelitis rats. Neuroscience. 2012;221:140–150. doi: 10.1016/j.neuroscience.2012.07.013. [http://dx.doi.org/ 10.1016/j.neuroscience.2012.07.013]. [PMID: 22800566]. [DOI] [PubMed] [Google Scholar]
- 121.Masuch A., Shieh C.H., van Rooijen N., van Calker D., Biber K. Mechanism of microglia neuroprotection: Involvement of P2X7, TNFα, and valproic acid. Glia. 2016;64(1):76–89. doi: 10.1002/glia.22904. [http://dx.doi. org/10.1002/glia.22904]. [PMID: 26295445]. [DOI] [PubMed] [Google Scholar]
- 122.Wang C.C., Chen P.S., Hsu C.W., Wu S.J., Lin C.T., Gean P.W. Valproic acid mediates the synaptic excitatory/inhibitory balance through astrocytes--a preliminary study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;37(1):111–120. doi: 10.1016/j.pnpbp.2012.01.017. [http://dx.doi. org/10.1016/j.pnpbp.2012.01.017]. [PMID: 22343008]. [DOI] [PubMed] [Google Scholar]
- 123.Singh V., Bhatia H.S., Kumar A., de Oliveira A.C.P., Fiebich B.L. Histone deacetylase inhibitors valproic acid and sodium butyrate enhance prostaglandins release in lipopolysaccharide-activated primary microglia. Neuroscience. 2014;265:147–157. doi: 10.1016/j.neuroscience.2014.01.037. [http://dx.doi.org/10.1016/j.neuroscience.2014.01.037]. [PMID: 24480366]. [DOI] [PubMed] [Google Scholar]
- 124.Goi P.D., Bücker J., Vianna-Sulzbach M., Rosa A.R., Grande I., Chendo I., Sodré L.A., Kauer-Sant’Anna M., Silveira L., Kunz M., Ceresér K.M., Gama C.S., Massuda R. Pharmacological treatment and staging in bipolar disorder: evidence from clinical practice. Rev. Bras. Psiquiatr. 2015;37(2):121–125. doi: 10.1590/1516-4446-2014-1554. [http://dx.doi. org/10.1590/1516-4446-2014-1554]. [PMID: 26018648]. [DOI] [PubMed] [Google Scholar]
- 125.Wu C-S., Wang S-C., Liu S-K. Clozapine use reduced psychiatric hospitalization and emergency room visits in patients with bipolar disorder independent of improved treatment regularity in a three-year follow-up period. Bipolar Disord. 2015;17(4):415–423. doi: 10.1111/bdi.12261. [http://dx.doi.org/10.1111/bdi.12261]. [PMID: 25295837]. [DOI] [PubMed] [Google Scholar]
- 126.Li X-B., Tang Y-L., Wang C-Y., de Leon J. Clozapine for treatment-resistant bipolar disorder: a systematic review. Bipolar Disord. 2015;17(3):235–247. doi: 10.1111/bdi.12272. [http://dx.doi.org/10.1111/bdi.12272]. [PMID: 25346322]. [DOI] [PubMed] [Google Scholar]
- 127.Steiner J., Schroeter M.L., Schiltz K., Bernstein H.G., Müller U.J., Richter-Landsberg C., Müller W.E., Walter M., Gos T., Bogerts B., Keilhoff G. Haloperidol and clozapine decrease S100B release from glial cells. Neuroscience. 2010;167(4):1025–1031. doi: 10.1016/j.neuroscience.2010.03.010. [http://dx.doi.org/10.1016/j.neuroscience.2010.03.010]. [PMID: 20226844]. [DOI] [PubMed] [Google Scholar]
- 128.Ribeiro B.M.M., do Carmo M.R.S., Freire R.S., Rocha N.F.M., Borella V.C.M., de Menezes A.T., Monte A.S., Gomes P.X.L., de Sousa F.C.F., Vale M.L., de Lucena D.F., Gama C.S., Macêdo D. Evidences for a progressive microglial activation and increase in iNOS expression in rats submitted to a neurodevelopmental model of schizophrenia: reversal by clozapine. Schizophr. Res. 2013;151(1-3):12–19. doi: 10.1016/j.schres.2013.10.040. [http://dx.doi.org/10.1016/j.schres. 2013.10.040]. [PMID: 24257517]. [DOI] [PubMed] [Google Scholar]
- 129.Jiang L., Wu X., Wang S., Chen S-H., Zhou H., Wilson B., Jin C-Y., Lu R-B., Xie K., Wang Q., Hong J-S. Clozapine metabolites protect dopaminergic neurons through inhibition of microglial NADPH oxidase. J. Neuroinflammation. 2016;13(1):110. doi: 10.1186/s12974-016-0573-z. [http://dx.doi.org/10.1186/s12974-016-0573-z]. [PMID: 27184631]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Torres-Platas S.G., Hercher C., Davoli M.A., Maussion G., Labonté B., Turecki G., Mechawar N. Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology. 2011;36(13):2650–2658. doi: 10.1038/npp.2011.154. [http://dx.doi.org/ 10.1038/npp.2011.154]. [PMID: 21814185]. [DOI] [PMC free article] [PubMed] [Google Scholar]