Abstract
Abstract: Background
Recently, neuroinflammation and the immune-kynurenine pathway have received increased attention in the psychoimmunology field of major depressive disorder (MDD), while studies related to anxiety disorders have been very limited.
Objective
This study reviewed possible mechanisms by which stress or inflammation modulate anxiety through tryptophan metabolism and the kynurenine pathway.
Methods
Relevant literature was identified through a search of MEDLINE via PubMed.
Results
Accumulating evidence has indicated the modulatory effects of the immune-kynurenine pathway on anxiety. The tryptophan catabolites (TRYCATs) in the kynurenine pathway imbalanced by stress or inflammation induce serotonin and melatonin deficiency, making anxiety reactions more sensitive. In addition, TRYCATs cause or sustain anxiety by acting as endogenous anxiogens or anxiolytics, an NMDA agonist or antagonist, or a free radical generator.
Conclusion
We hope that our understanding of the psychoimmunological mechanisms of anxiety will be expanded and anxiety-related studies will receive greater attention.
Keywords: Anxiety, kynurenine, tryptophan catabolites, serotonin, psychoimmunology, neuroinflammation
1. INTRODUCTION
Anxiety disorders and major depressive disorder (MDD) are complex diseases resulting from the interaction of various psychological, environmental, and biological factors. Over the past several decades, active research has been conducted into the biological factors of these diseases: neuroanatomical abnormalities, neurochemical factors, such as norepinephrine and serotonin, and neuroendocrine abnormalities of the hypothalamus-pituitary-adrenal axis [1]. However, none of these factors can fully explain anxiety and depression.
The most actively studied psychiatric disorder in the field of psychoimmunology is MDD. Recently, among the biological mechanisms of MDD, neuroinflammation and the immune-kynurenine pathway are drawing attention, and are known to play key roles in inflammation-induced depression [2, 3]. In contrast, few studies have been conducted on anxiety disorders. Of those few studies, most are laboratory research or animal experiments, and even fewer studies have been conducted since 2000. This is likely due to the lack of consistent reproducible findings or insufficient explanations for the biological mechanisms of anxiety. Although both anxiety and depression are phenomenologically observed states, anxiety disorder can appear in a more diverse form of illness than MDD. This is supported by the fact that, unlike MDD, which is a single disease, anxiety disorders are sub-classified into various diseases, such as panic disorder, phobia and generalized anxiety disorder. Furthermore, the anxiety state is more difficult to define than a depressive episode, and the pathologic state period varies widely. For this reason, psychoimmunological studies on anxiety may have been more difficult than those on depression.
However, we need to rethink this field in relation to anxiety disorders, for a variety of reasons. First, depression and anxiety often coexist and have many similarities in terms of therapies. According to the hypothesis that anxiety and depression are distributed in one dimension, the middle point of this dimension is where anxiety and depression coexist, which is where the biological mechanism is shared [4]. Therefore, comparable studies on anxiety are needed in parallel, in that the biological mechanisms of MDD and anxiety can explain each other’s pathophysiology. Second, anxiety disorders are brain disorders in which various heterogeneous pathogenic mechanisms are expressed [5], and the pathogenic factors of anxiety disorders associated with inflammation and the kynurenine pathway can enable a more comprehensive understanding of anxiety.
This study reviewed possible mechanisms by which stress or inflammation modulates anxiety through tryptophan metabolism and the kynurenine pathway. Because there are relatively few past studies, we sought to investigate all the work that has been conducted to date, regardless of the subjects of the experiment (laboratory/animals/humans) and the dates of study. Through this review, we hope that our understanding about the psychoimmunological mechanisms of anxiety will be expanded and anxiety-related studies will receive renewed attention.
2. METHODS
The source of literature was the electronic database MEDLINE via PubMed (1950-2017). The initial search strategy included the use of a combination of the following thesaurus terms: ‘anxiety disorder,’ ‘anxiety,’ ‘kynurenine,’ ‘neuroinflammation,’ ‘tryptophan,’ and ‘serotonin.’ However, as there are limited studies in this field, we expanded our search by adding the terms ‘indoleamine 2,3-dioxygenase (IDO),’ ‘kynurenic acid,’ ‘quinolinic acid (QUIN),’ ‘melatonin,’ ‘benzodiazepine,’ ‘N-methyl-D-aspartate (NMDA),’ ‘neurogenesis’ and ‘oxidative stress.’ The inclusion criteria included: (i) studies examining immune-kynurenine mechanisms underlying an anxious state or anxiety, regardless of the subjects of experiment (laboratory/animals/humans); (ii) review articles on immune-kynurenine and anxiety; (iii) articles written in English. The exclusion criteria included: (i) letters to editors and editorials without data; and (ii) studies outside the time window (1950-2017), as these were not available electronically. Based on the inclusion and exclusion criteria, we reviewed the titles of all citations and retrieved relevant abstracts for more detailed evaluation. When there was uncertainty, we studied the full article. We also hand-searched the reference list of relevant studies and reviews to aid identification of further studies. We identified 142 references and 38 were included in this review.
3. RESULTS
3.1. Tryptophan Metabolism without Stress or Immune Challenges
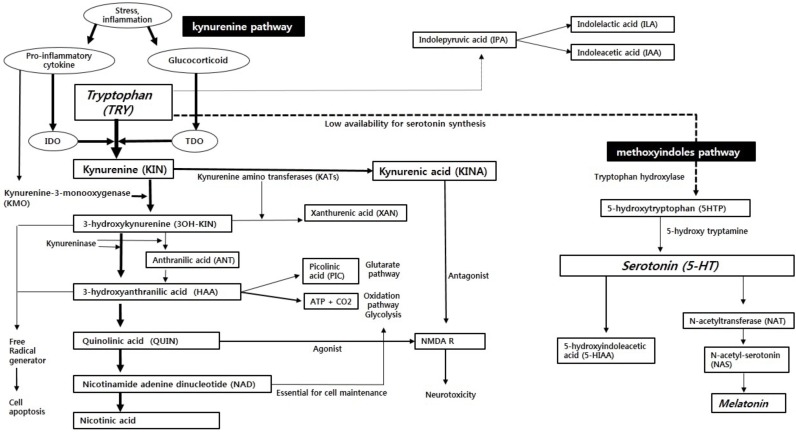
Tryptophan (TRY) metabolism has two large pathways: the methoxyindoles pathway and the kynurenine pathway (Fig. 1). Among the available TRYs in the body, approximately 1~5% are synthesized as serotonin (5-HT) through the methoxyindoles pathway [6]. This serotonin synthesis occurs primarily in the enterochromaffin cells of the gut, and the remaining 10-20% occurs in the brain through the blood brain barrier (BBB) [6]. Because less than 5% of TRYs are metabolized through the methoxyindoles pathway, the availability of TRYs, which are the substrates of serotonin, is an important rate-limiting factor for serotonin synthesis [6]. Meanwhile, because serotonin is the substrate of melatonin, serotonin deficiency not only affects the serotonin receptor function, but also reduces the synthesis of melatonin [7].
Fig. (1).
The tryptophan breakdown metabolic pathway in immune challenges. IDO: indoleamine 2,3-dioxygenase; TDO: tryptophan 2,3-dioxygenase; NMDA R: N-methyl-D-aspartate receptor.
Approximately 95~99% of TRYs are metabolized through the kynurenine pathway and form tryptophan catabolites (TRYCATs) [6]. These TRYCATs are important metabolites that may contribute to the pathophysiology of anxiety [8]. The greater part of the flow in the kynurenine pathway is the formation of kynurenines (KYNs) from TRYs in the liver by tryptophan 2,3-dioxygenase (TDO), which is the rate-limiting enzyme [6, 9]. Meanwhile, indoleamine 2,3-dioxygenase (IDO), which is another rate-limiting enzyme, also forms KYNs [10]. Although TDOs and IDOs both metabolize TRYs to KYNs, TDOs and IDOs exist in different parts of the body and their activation methods are slightly different. TDOs are primarily distributed in the liver, and IDOs are primarily distributed in extrahepatic tissues, such as the brain, blood, spleen, kidney, and lung [10]. IDOs generally exist in astrocytes, microglia, microvascular endothelial cells, and macrophages, and are usually activated by pro-inflammatory cytokines [10]. TDOs are generally activated by TRY itself, but they can be also activated by glucocorticoids [11]. Quantitatively, most TRYs are metabolized by TDOs in the liver, but in the brain, the role of IDOs is critical and IDO is an important enzyme associated with the psychoimmunological mechanism of anxiety [12].
KYNs are metabolized through two distinct routes: the KYN - nicotinamide adenine dinucleotide (NAD) pathway and the KYN - kynurenic acid (KYNA) pathway. These two routes compete with one another for KYN.
In the KYN-NAD pathway, KYN is catabolized to 3-hydroxykynurenine (3OH-KIN) by the kynurenine-3-monooxygenase (KMO) enzyme, and 3OH-KIN is catabolized to hydroxyanthranilic acid (HAA) by kynureniase. The subsequent catabolism progresses in two routes: the complete oxidation pathway, which forms adenosine triphosphate (ATP) in the liver, and the degrading pathway in the order of quinolinic acid (QUIN) - nicotinamide adenine dinucleotide (NAD) - nicotinic acid (NIC). HAA also forms a small amount of picolinic acid (PIC) besides ATP. Under general conditions, the major part of catabolism is the formation of ATP; the formation of NAD is relatively minor [13]. In summary, the KYN-NAD pathway plays an important role, not only in the brain's glycogen storage, but also in supplying NAD, which is an essential substance for the functioning of the central nervous system [13].
The KYN-kynurenic acid (KYNA) pathway is a process by which KYN is catabolized to KYNA by KYN-amino-transferases (KATs). KYNA has a functional antagonistic effect on KIN, 3OH-KIN, and QUIN [8].
In addition to the methoxyindoles pathway and the kynurenine pathway, TRYs are also catabolized to indolepyruvic acid (IPA), which is a minor pathway of TRY metabolism. Then, IPA is catabolized to indolelactic acid (ILA) and indoleacetic acid (IAA).
3.2. Tryptophan Metabolism in Stress or Immune Challenges
3.2.1. Imbalance between Kynurenine and Methoxyindoles Pathways
The increased pro-inflammatory cytokines induced by stress or inflammation activate IDO enzymes, which exist primarily in extrahepatic tissues, such as those in the brain [14]. Thus, activated IDOs shift TRY metabolism from the liver to the extrahepatic regions. Meanwhile, the TDO enzymes can also be activated by cortisol secretion that has been strengthened by inflammation [11]. Eventually, inflammatory states activate pro-inflammatory cytokines and cortisols, which activate IDOs and TDOs, respectively, and then TRY metabolism is shifted from the methoxyindoles pathway to the kynurenine pathway (Fig. 1). As a result, the amount of KYN formation increases. Because KYNs can pass through the BBB, additional peripheral KYNs are supplied to the brain, and the kynurenine pathway is highly activated in astrocytes and microglia in the brain [15].
3.2.2. Imbalance between 3OH-KIN and KYNA
Pro-inflammatory cytokines intensify the activity of KMO enzymes, as well as IDOs [16]. Therefore, during the inflammatory state, KYN metabolism is shifted from KYNA to 3OH-KIN (Fig. 1). Such an imbalance between 3OH-KIN and KYNA is more pronounced in activated monocytes [17]. The increase of 3OH-KINs leads to an increase in the creation of QUIN, which is the next metabolite. Eventually, inflammation causes an imbalance between KINA, which has anxiolytic and neuroprotective effects, and 3OH-KIN and QUIN, which have anxiogenic and neurodegenerative effects [8, 18].
3.3. Neuroactivities of Tryptophan Catabolites (TRYCATs)
The neurotropic activity of TRYCATs has been found through laboratory experiments and animal studies. KYN, 3OH-KIN, and QUIN (and PIC in some experiments), which are initial metabolites in the kynurenine pathway, act as excitants and convulsants [19-23]. In contrast, most final metabolites, such as KYNA, xanthurenic acid (XAN), NAD, and IPA have anti-excitatory neuroactivities, such as inhibitory, tranquillizing, and anticonvulsant effects (anthranilic acid [ANT] seems to have a minimal effect) [19, 24, 25]. It has been well-demonstrated that [KYN and XAN], [QUIN and KINA], [KYN and NAD], and [KYN and IPA] have a functionally antagonistic relationship with one another in tests of seizures and in animal models for anxiety-measurements of locomotor activity [8, 23]. Such antagonistic relationships are one of the mechanisms of self-regulation in the kynurenine pathway [15, 23]. Meanwhile, excitatory TRYCATs are known to have antagonism for other metabolites of tryptophan, namely 5-HT, 5-hydroxytryptophan (5-HTP), and melatonins, which are in the methoxyindoles pathway (from outside the kynurenine pathway). In rats or mice, the pretreatment of excitatory TRYCATs reduces the central effects of 5-HT, 5-HTP, and melatonin, such as head-twitches and sedation [23, 26].
These neurotropic TRYCATs also interact with the adrenergic, dopaminergic, serotonergic, cholinergic, and glutaminergic systems. The pressor effect in rats and the hyperthermic effect in mice from noradrenaline, which is induced by amphetamines (subcutaneously [SC]), are potentiated by KYN, 3OH-KIN, ANT, and NIC [8, 25]. PIC has the opposite effect [8, 25]. Amphetamine-induced (SC) locomotor activation in mice and rats is potentiated by QUIN and ANT [23, 25]. Seizures in mice, which are induced by the central administration (intracerebroventricularly [ICV]) of KYN, 3OH-KIN, and QUIN, are prevented by centrally injected (ICV) serotonin and dopamine, and diminished by melatonin, noradrenaline, and adrenaline [19]. The oxotremorine hypothermia in mice, which is mediated by acetylcholine secretion, is potentiated by KYN, PIC, and NIC [23]. Two arms of the TRYCATs are associated with glutamate receptor. The increased formation of NMDA agonist, QUIN, leads to a hyperglutamatergic status associated with psychiatric illness such as depression. KYNA, an NMDA antagonist, counteracts the excitotoxicity of QUIN to protect it.
Excitatory TRYCATs have a functional antagonism with γ-aminobutyric acid (GABA), glycine, and taurine, which are inhibitory amino acids. Seizures induced by QUIN and KYN are prevented by these amino acids (ICV) [22]. It would be due to GABA inhibition of TRYCATs effects on glutamate. The behavioral inhibitory effects of these amino acids are diminished by pretreatment with KYN, 3OH-KIN, and QUIN [22].
In summary, anti-excitatory TRYCATs, such as KINA, XAN, NAD, and IPA have anticonvulsant and neuroprotective neuroactivities, and play an adaptogenic role in stress, as they also exert anxiolytic effects, which will be explained below. In contrast, excitatory TRYCATs, such as KYN, 3OH-KIN, and QUIN have antagonism for anti-excitatory TRYCATs and even have proconvulsive, neurotoxic neuroactivities and anxiogenic effects (Fig. 2). Under general conditions, such internal and external antagonisms of the kynurenine pathway make the neuroactivities of excitatory TRYCATs stable and flexible [8]. If the balance of antagonisms collapses under continuous stress, and the concentration of excitatory TRYCATs rises in a long-lasting manner, this can lead to pathologic consequences, such as anxiety, depression, and dementia [27].
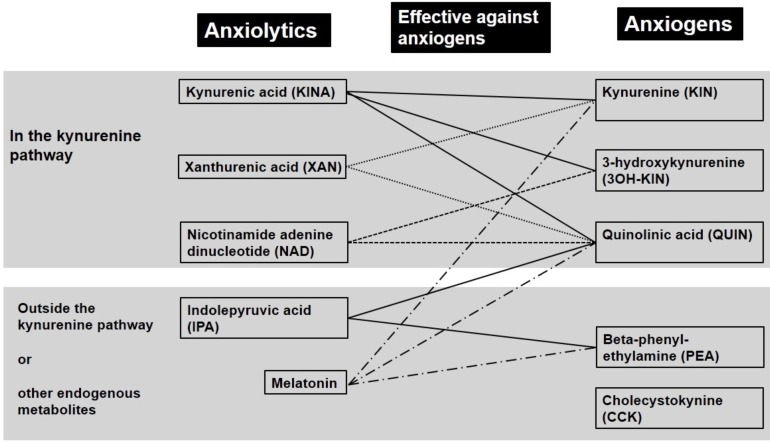
Fig. (2).
Putative endogenous anxiogens, anxiolytics, and functional antagonistic relationship in animal models of anxiety.
3.4. Anxiety and Tryptophan Catabolites (TRYCATs)
In general, all standard anxiogens (pentylenetetrazole, caffeine and yohimbine) can cause seizures in mice. In mice, the ratio between the convulsant and the anxiogenic doses of standard anxiogens is known to be approximately 10:1 [28]. This ratio applies to excitatory TRYCATs as well [19]. Therefore, excitatory TRYCATs, which have convulsive effects, are likely to act as anxiogens in the body, whereas anti-excitatory TRYCATs are likely to act as anxiolytics, because they antagonize excitatory TRYCATs.
KYN, 3OH-KIN and QUIN, which are excitatory TRYCATs, have anxiogenic activity in animal models of anxiety, such as in the social interaction test (diminution of number and duration of contacts), the elevated plus-maze and the conflict situation in a dark-light chamber (diminution of transitions) [23, 28, 29]. Meanwhile, KYNA, XAN and IPA, which are anti-excitatory TRYCATs, have the pharmacological profile of anxiolytics [29-31]. KYNA, XAN, and IPA antagonize both anxiogenic TRYCATs and standard anxiogens, such as caffeine, pentylenetetrazole and yohimbine [8]. As shown through animal models of anxiety, the endogenous anxiogens, except TRYCATs, are beta-phenyl-ethylamine (PEA) and cholecystokinin (CCK) [32]. Some anxiolytic TRYCATs have antagonism against PEA and CCK [32] (Fig. 2).
These effects of TRYCATs on anxiety have been revealed through laboratory and animal experiments, but little has been revealed in experiments on human subjects. Among putative endogenous anxiogens, a major emphasis has been on KYN. The relationship between KIN and anxiety has been revealed in some studies with healthy volunteers and psychiatric patients [33].
In 15 healthy volunteers for whom anxiety was provoked by administrating an anxiogenic dose (25mg/kg) of caffeine, the plasma KIN concentration markedly increased at the peak of anxiety and returned to normal when the caffeine-induced anxiety disappeared. The correlation between plasma KYN concentration and anxiety scale (Spielberger-Khanin scale and Hamilton scale) scores was significant at baseline. This correlation disappeared at the peak of anxiety. This result suggests that pharmacologically-induced anxiety was correlated with changes in plasma KYN concentrations [33-35].
In another study with 30 psychiatric patients with affective state, the Dexamethasone Suppression Test (DST), Diazepam Test (DT), Hamilton anxiety scale (HAM-A), and Hamilton depression scale (HAMD) were used to differentiate between an endogenous anxiety group and an endogenous depression group. Patients with endogenous anxiety showed an increased plasma KIN concentration, whereas patients with endogenous depression showed a decreased plasma KIN concentration. Consequently, the patients in anxiety group were effectively treated with anxiolytics, and those in depression group with antidepressants. In both groups, the plasma KIN concentration returned to normal after treatment. The plasma KYN concentration was significantly correlated with the severity of anxiety. The present study proposes that together with DST and DT, KIN concentration can be a biological marker that distinguishes between endogenous anxiety disorders and endogenous depression [34].
In a study with healthy, nonpregnant women, pregnant women at the end of term, and women in the early puerperium, the plasma KIN concentration and the KIN/TRY ratio increased greatly in women in early puerperium relative to nonpregnant women. Furthermore, the KIN and KIN/TRY ratio increased more markedly in women in early puerperium who showed higher anxiety and depression scores. In early puerperium, which is a period of high immune activation, a large amount of KINs are created due to IDO activation, and these increased anxiogenic KINs may have contributed to depressive and anxiety symptoms [35].
3.5. TRYCATs and Benzodiazepine
Other evidence that supports the correlation of TRYCATs with anxiety includes the structure-activity relationship between KYN, diazepam, and some putative endogenous ligands of the benzodiazepine receptors. KIN antagonizes the anti-caffeine effect of diazepam [21, 36]. Among the various convulsants, KIN has the most resistant convulsant effect against the protective action of diazepam [37]. The chemical structural formula of KIN is similar to that of benzophenones, which are the metabolites of diazepam, and shares four structural fragments with diazepam. The putative endogenous and non-endogenous ligands of benzodiazepine receptors share 1-3 of these structural fragments. The benzodiazepine receptor is estimated to be a phylogenetically transformed kynurenine receptor. The biggest difference in the chemical structural formulas between KIN and diazepam is that KIN does not have diazo-moiety, and this moiety plays a key role in binding to benzodiazepine receptor. Such similarity in the chemical structural formulas can be also found in QUIN, which is a catabolite of KYN [21, 37].
3.6. Serotonin Deficiency and Anxiety
As discussed above, stress and inflammatory states trigger serotonin deficiency, due to the shunt of TRY from serotonin synthesis to KYN formation. What effects does this induced serotonin deficiency have on anxiety? This is the basic question about whether serotonin itself increases or decreases anxiety. At present, serotonergic agents, such as the 5HT1A receptor partial agonist (Buspirone) and selective serotonin reuptake inhibitors (SSRIs), are the first-line treatment for all anxiety disorders. Therefore, serotonin can be seen as an anxiolytic, because chronic SSRI treatment decreases anxiety by boosting serotonin levels. Some studies have shown that the serotonin-boosting effect of SSRIs resulted in anxiolysis [38-40]. Various serotonergic agents, such as m-chlorophenylpiperazine (mCPP), a mixed serotonin receptor agonist, can elevate anxiety [41]. Furthermore, monoamine oxidase (MAO)-A, which degrades serotonin and lowers its availability, is increased in panic disorder patients relative to healthy controls [42]. These results are aligned with reports that serotonin has an anxiolytic effect.
Tryptophan depletion in the body causes an acute reduction in serotonin levels [40]. In healthy people, tryptophan depletion followed by administration of panicogenic agents, such as carbon dioxide or yohimbine, significantly increased anxiety [43, 44]. The same results were observed in patients with panic disorder. When panicogens were administered after pretreatment with tryptophan depletion in patients with panic disorder, anxiety ratings and panic attack rates increased [45, 46]. However, tryptophan depletion alone, in the absence of panicogens, did not increase general anxiety in both healthy controls and panic disorder patients [45]. Tryptophan depletion appears to sensitize anxiety-related mechanisms, and the increase in anxiety by tryptophan depletion seems to become apparent after provocation. Therefore, serotonin has the function of protecting against anxiety and panic attacks, but it is unlikely that tonically elevated serotonin levels underlie SSRI’s anxiolytic effect. The reason for this is that the mechanism of the action of serotonin on anxiety is not only associated with the simple amount of serotonin, but also with the type of 5-HT receptor, as well as with genetic associations, such as the serotonin transporter gene polymorphism [47].
In conclusion, serotonin itself is thought to have an anxiolytic effect and the quantitative deficiency of serotonin due to stress and inflammation appears to increase the sensitivity to anxiety.
3.7. Melatonin Deficiency and Anxiety
Melatonin has psychotropic effects in rodents, such as sedative, analgesic, anticonvulsant, hypnotic, and anxiolytic effects [48]. In models based on exploratory behavior, melatonin has been found to be associated with stress and anxiety-related behaviors and has an anxiolytic-like effect [49]. Interestingly, melatonin interacts with GABA neurotransmission. Melatonin intensifies the binding of GABA and muscimol (a GABAA receptor agonist) in rat brain tissues in vitro [50, 51]. In vivo, melatonin administration increases GABA levels in several rat brain regions [52, 53]. In mice, flumazenil, which is a benzodiazepine receptor antagonist, blunts the anxiolytic-like activity of melatonin [54]. Furthermore, the combined treatment of ineffective doses of melatonin and diazepam has been reported to show an anxiolytic-like effect [55]. In recent human studies, agomelatine, a melatonergic receptor agonist, was effective in the treatment of generalized anxiety disorder [56, 57]. The agomelatine also demonstrated efficacy in the treatment of panic disorder [58, 59]. The deficiency of serotonin itself can increase the sensitivity to anxiety and lead to a decrease in the synthesis of melatonin, thus making the increase of anxiety more sensitive.
3.8. NMDA, TRYCATs, and Anxiety
In the KYN-NAD pathway, QUIN and picolinic acid, which are N-methyl-D-aspartate (NMDA) agonists, are synthesized. Meanwhile, KYNA is the only known endogenous antagonist to NMDA receptors (Fig. 1). The NMDA receptors not only are involved in learning and memory, but also affect emotionality, such as fear, anxiety, and depression [60]. The general pharmacological profile of an NMDA antagonist is similar to the spectrum of activity of benzodiazepines and barbiturates [61]. Both benzodiazepines and barbiturates have amnestic, anticonvulsant and anxiolytic potency. CPP (3-(2-carboxy piperazine-4yl)-propyl-1-phosphonic-acid), AP5 (2-amino-5-phosphonoheptanoate), and AP7 (2-amino-7-phosphonoheptanoate), which are competitive NMDA antagonists, have been found to have anxiolytic effects in an animal model of anxiety (elevated plus maze and increased social interaction) [62-64]. In addition, non-competitive NMDA antagonists, such as MK-801 (dizocilpine) and PCP (phencyclidine), also revealed anxiolytic effects in an animal model of anxiety [62-64]. Much preclinical evidence suggests the possibility that NMDA antagonists can be used as treatment for anxiety disorders [60]. Surprisingly, ketamine, which is another widely studied non-competitive NMDA antagonist, has been found to have an anxiety-reducing effect in an animal experiment, but has been also shown to decrease anxiety in healthy human volunteers at doses as low as 0.1mg/kg i.v. [65]. In addition, there is a recent study indicating that ketamine may be effective in the treatment of treatment resistant anxiety disorders [66].
Therefore, the increase of QUIN (an NMDA agonist) and the decrease of KINA (an NMDA antagonist), which are induced by stress and inflammation, leads to an increase of endogenous anxiogenic metabolites, and it is likely to be a pathway for inducing or continuing anxiety.
3.9. Hippocampal Neurogenesis, TRYCATs, and Anxiety
The hippocampus is not only an important structure for cognitive function, but also a key structure of the so-called emotional brain. The hippocampus modulates affective states, and is particularly associated with the modulation of anxiety states [67]. The hippocampus is a major target for antidepressants with respect to regulating anxiety. Several categories of antidepressant are known to increase hippocampal neurogenesis, and it has been found that such a mechanism is required for the anxiolytic effect of antidepressants [68, 69]. Furthermore, the hippocampus is a key locus for the anxiolytic effects that allow NMDA receptor antagonists to modulate anxiety [60]. The fact that anxiety-related behaviors increase strikingly in transgenic animals specifically impaired in hippocampal neurogenesis suggests that anxiety is directly correlated with hippocampal neurogenesis [70]. Another method of intuitive observation for the relationship between hippocampal neurogenesis and anxiety is to observe the reduction in the hippocampal volume by imaging patients with anxiety disorders. A significant hippocampal volume reduction in structural MRI studies was observed in patients with posttraumatic stress disorder (PTSD) and social anxiety disorder compared with healthy controls, and this volume reduction was significantly correlated with a risk factor for vulnerability to these anxiety disorders [71-74].
TRYCATs can directly contribute to neuroprotective or neurodegenerative changes in the brain through NMDA receptors, especially in the hippocampus [3, 60, 68, 70]. The accumulation of QUINs, which are NMDA agonists, causes a hyperglutamatergic state, which is associated with anxiety and depression [75]. Hippocampal atrophy, which is frequently observed in anxiety disorders and depression, is related to the activation of such QUINs [76]. Meanwhile, KYNA, which is an NMDA antagonist, protects QUINs by offsetting their excitotoxicity [76].
In the inflammatory state, the balance between 3OH-KIN and KYNA is shifted from KYNA to 3OH-KIN [17]. However, KYNA also increases relative to the normal state, due to the general increase in KIN. Such increased KYNA complements the negative effects of QUIN and maintains the homeostasis of the NMDA receptor. However, if the inflammatory state becomes chronic or stronger, the balance of KIN metabolites collapses, causing a disturbance in neurotransmission and neurodegenerative changes in the brain. The neuroprotective and neurotoxic effects due to the imbalance of TRYCATs have been proven in human brain studies, as well [77, 78]. Such imbalances of TRYCATs are believed to partially contribute to hippocampal neurodegenerative changes, which are frequently reported in anxiety disorders.
3.10. Oxidative Stress, TRYCATs and Anxiety
The KYN-NAD pathway synthesizes 3OH-KIN and HAA, which are free radical generators (Fig. 1). 3OH-KIN, which is activated in the inflammatory state, causes neuronal apoptosis in the brain by producing more reactive oxygen species (ROS) [9]. When many ROS are generated, it changes the membrane viscosity, as well as apoptosis, and eventually damages monoamine surface receptors, such as serotonin receptors, enzymes, and membrane proteins, such as ion channels [79]. Oxidative stress can alter neurotransmission, neuronal function, and overall brain activity, and thereby contributes to the inducement of anxiety [80].
Many studies have been conducted to evaluate correlations between obsessive-compulsive disorder, panic disorder and oxidative stress [81, 82]. However, oxidative stress does not appear to have a specific link only with anxiety disorders. As it is also associated with many neurodegenerative diseases and psychiatric illnesses, such as schizophrenia and MDD, oxidative stress appears to cause an intrinsic oxidative vulnerability of the brain and triggers various psychiatric diseases [83]. The presence of TRYCATs, such as 3OH-KIN and HAA, which can activate ROS, may explain the triangular relationship between stress/inflammation, oxidative stress and anxiety [80]. While some data demonstrate that there is a link between oxidative stress and anxiety, a cause-effect relationship has yet to be completely established.
CONCLUSION
Anxiety studies on neuroinflammation and the immune-kynurenine pathway have been limited to laboratory and animal experiments, and few studies on anxiety have been conducted after the period of the 1970s to 1990s. TRYCATs can act as endogenous anxiogens or anxiolytics by themselves, and have functionally antagonistic relationships with one another in the kynurenine pathway. Furthermore, outside the kynurenine pathway, TRYCATs have potentiation or antagonism relationships with various neurotransmission systems. Serotonin and melatonin deficiency, which are caused by stress or inflammation, seem to increase the sensitivity to anxiety. Some TRYCATs act as NMDA agonists or antagonists, and this path can cause or sustain anxiety disorders. Furthermore, TRYCATs can influence anxiety by directly contributing to neuroprotective-neurodegenerative changes in the brain, especially in the hippocampus, through the NMDA receptor. Some TRYCATs that have a free radical generator function, cause oxidative stress and contribute to anxiety inducement by changing neurotransmission and neuronal function. In the future, more human studies should be conducted based on this evidence, which should also be used in the development of new therapies for the treatment of anxiety disorders.
Author Contributions
Sang Won Jeon and Yong-Ku Kim designed the study and wrote the manuscript together and have approved the final manuscript.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HC15C1405).
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Ionescu D.F., Niciu M.J., Mathews D.C., Richards E.M., Zarate C.A., Jr Neurobiology of anxious depression: a review. Depress. Anxiety. 2013;30(4):374–385. doi: 10.1002/da.22095. [http://dx.doi.org/10.1002/da.22095]. [PMID: 23495126]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wichers M.C., Maes M. The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-alpha-induced depression. J. Psychiatry Neurosci. 2004;29(1):11–17. [PMID: 14719046]. [PMC free article] [PubMed] [Google Scholar]
- 3.Savitz J., Drevets W.C., Smith C.M., Victor T.A., Wurfel B.E., Bellgowan P.S., Bodurka J., Teague T.K., Dantzer R. Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology. 2015;40(2):463–471. doi: 10.1038/npp.2014.194. [http://dx.doi.org/10.1038/npp.2014.194]. [PMID: 25074636]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krueger R.F., Piasecki T.M. Toward a dimensional and psychometrically-informed approach to conceptualizing psychopathology. Behav. Res. Ther. 2002;40(5):485–499. doi: 10.1016/s0005-7967(02)00016-5. [http://dx.doi.org/10. 1016/S0005-7967(02)00016-5]. [PMID: 12038642]. [DOI] [PubMed] [Google Scholar]
- 5.Garakani A., Mathew S.J., Charney D.S. Neurobiology of anxiety disorders and implications for treatment. Mt. Sinai J. Med. 2006;73(7):941–949. [PMID: 17195879]. [PubMed] [Google Scholar]
- 6.Gál E.M., Sherman A.D. L-kynurenine: its synthesis and possible regulatory function in brain. Neurochem. Res. 1980;5(3):223–239. doi: 10.1007/BF00964611. [http://dx.doi.org/10.1007/BF00964611]. [PMID: 6154900]. [DOI] [PubMed] [Google Scholar]
- 7.Zimmermann R.C., McDougle C.J., Schumacher M., Olcese J., Mason J.W., Heninger G.R., Price L.H. Effects of acute tryptophan depletion on nocturnal melatonin secretion in humans. J. Clin. Endocrinol. Metab. 1993;76(5):1160–1164. doi: 10.1210/jcem.76.5.8496306. [PMID: 8496306]. [DOI] [PubMed] [Google Scholar]
- 8.Lapin I.P. Neurokynurenines (NEKY) as common neurochemical links of stress and anxiety. Adv. Exp. Med. Biol. 2003;527:121–125. doi: 10.1007/978-1-4615-0135-0_14. [http://dx.doi.org/10.1007/978-1-4615-0135-0_14]. [PMID: 15206724]. [DOI] [PubMed] [Google Scholar]
- 9.Maes M., Leonard B.E., Myint A.M., Kubera M., Verkerk R. The new ‘5-HT’ hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(3):702–721. doi: 10.1016/j.pnpbp.2010.12.017. [http://dx.doi.org/10.1016/j.pnpbp.2010. 12.017]. [PMID: 21185346]. [DOI] [PubMed] [Google Scholar]
- 10.Hayaishi O. Properties and function of indoleamine 2,3-dioxygenase. J. Biochem. 1976;79(4):13P–21P. doi: 10.1093/oxfordjournals.jbchem.a131115. [http://dx.doi.org/ 10.1093/oxfordjournals.jbchem.a131115]. [PMID: 931956]. [DOI] [PubMed] [Google Scholar]
- 11.Salter M., Pogson C.I. The role of tryptophan 2,3-dioxygenase in the hormonal control of tryptophan metabolism in isolated rat liver cells. Effects of glucocorticoids and experimental diabetes. Biochem. J. 1985;229(2):499–504. doi: 10.1042/bj2290499. [http://dx.doi.org/10.1042/ bj2290499]. [PMID: 3899109]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salazar A., Gonzalez-Rivera B.L., Redus L., Parrott J.M., O’Connor J.C. Indoleamine 2,3-dioxygenase mediates anhedonia and anxiety-like behaviors caused by peripheral lipopolysaccharide immune challenge. Horm. Behav. 2012;62(3):202–209. doi: 10.1016/j.yhbeh.2012.03.010. [http://dx. doi.org/10.1016/j.yhbeh.2012.03.010]. [PMID: 22504306]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leklem J.E. Quantitative aspects of tryptophan metabolism in humans and other species: a review. Am. J. Clin. Nutr. 1971;24(6):659–672. doi: 10.1093/ajcn/24.6.659. [http://dx.doi.org/10.1093/ajcn/24.6.659]. [PMID: 4253043]. [DOI] [PubMed] [Google Scholar]
- 14.Carlin J.M., Borden E.C., Sondel P.M., Byrne G.I. Biologic-response-modifier-induced indoleamine 2,3-dioxygenase activity in human peripheral blood mononuclear cell cultures. J. Immunol. 1987;139(7):2414–2418. [PMID: 2443564]. [PubMed] [Google Scholar]
- 15.Grant R.S., Naif H., Espinosa M., Kapoor V. IDO induction in IFN-gamma activated astroglia: a role in improving cell viability during oxidative stress. Redox Rep. 2000;5(2-3):101–104. doi: 10.1179/135100000101535357. [http:// dx.doi.org/10.1179/135100000101535357]. [PMID: 10939283]. [DOI] [PubMed] [Google Scholar]
- 16.Mellor A.L., Munn D.H. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol. Today. 1999;20(10):469–473. doi: 10.1016/s0167-5699(99)01520-0. [http://dx.doi.org/10.1016/S0167-5699(99)01520-0]. [PMID: 10500295]. [DOI] [PubMed] [Google Scholar]
- 17.Chiarugi A., Calvani M., Meli E., Traggiai E., Moroni F. Synthesis and release of neurotoxic kynurenine metabolites by human monocyte-derived macrophages. J. Neuroimmunol. 2001;120(1-2):190–198. doi: 10.1016/s0165-5728(01)00418-0. [http://dx.doi.org/10.1016/S0165-5728(01)00418-0]. [PMID: 11694334]. [DOI] [PubMed] [Google Scholar]
- 18.Myint A.M., Kim Y.K. Network beyond IDO in psychiatric disorders: revisiting neurodegeneration hypothesis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;48:304–313. doi: 10.1016/j.pnpbp.2013.08.008. [http://dx. doi.org/10.1016/j.pnpbp.2013.08.008]. [PMID: 24184687]. [DOI] [PubMed] [Google Scholar]
- 19.Lapin I.P. Kynurenines and seizures. Epilepsia. 1981;22(3):257–265. doi: 10.1111/j.1528-1157.1981.tb04108.x. [http://dx.doi.org/10.1111/j.1528-1157.1981.tb04108.x]. [PMID: 6263604]. [DOI] [PubMed] [Google Scholar]
- 20.Melnikova N.V. Neurokynurenines--seizures or/and anxiety in children with epilepsy? Adv. Exp. Med. Biol. 2003;527:191–195. doi: 10.1007/978-1-4615-0135-0_22. [http://dx.doi.org/10.1007/978-1-4615-0135-0_22]. [PMID: 15206732]. [DOI] [PubMed] [Google Scholar]
- 21.Lapin I.P. Structure-activity relationships in kynurenine, diazepam and some putative endogenous ligands of the benzodiazepine receptors. Neurosci. Biobehav. Rev. 1983;7(2):107–118. doi: 10.1016/0149-7634(83)90013-1. [http://dx. doi.org/10.1016/0149-7634(83)90013-1]. [PMID: 6308529]. [DOI] [PubMed] [Google Scholar]
- 22.Lapin I.P., Prakhie I.B., Kiseleva I.P. Excitatory effects of kynurenine and its metabolites, amino acids and convulsants administered into brain ventricles: differences between rats and mice. J. Neural Transm. (Vienna) 1982;54(3-4):229–238. doi: 10.1007/BF01254932. [http://dx.doi. org/10.1007/BF01254932]. [PMID: 7130975]. [DOI] [PubMed] [Google Scholar]
- 23.Lapin I. Experimental studies on kynurenines as neuroactive tryptophan metabolites: past, present and future. Trends Pharmacol. Sci. 1980;1(2):410–412. [http://dx.doi.org/10.1016/0165-6147(80) 90066-8]. [Google Scholar]
- 24.Lapin I.P. Endogenous antagonists of quinolinic acid and kynurenine as links of the defensive mechanism in epilepsy. Acta Neurol. (Napoli) 1985;7(3-4):203–206. [PMID: 2932891]. [PubMed] [Google Scholar]
- 25.Lapin I. Behavioral and convulsant effects of kynurenines. Quinolinic Acid and the Kynurenines; 1989. pp. 193–211. [Google Scholar]
- 26.Lapin I.P. Interaction of kynurenine and its metabolites with tryptamine, serotonin and its precursors and oxotremorine. Psychopharmacology (Berl.) 1972;26(3):237–247. doi: 10.1007/BF00422699. [http://dx.doi.org/ 10.1007/BF00422699]. [PMID: 4672450]. [DOI] [PubMed] [Google Scholar]
- 27.Stone T.W. Kynurenines in the CNS: from endogenous obscurity to therapeutic importance. Prog. Neurobiol. 2001;64(2):185–218. doi: 10.1016/s0301-0082(00)00032-0. [http:// dx.doi.org/10.1016/S0301-0082(00)00032-0]. [PMID: 11240212]. [DOI] [PubMed] [Google Scholar]
- 28.Lapin I.P., Mutovkina L.G., Ryzov I.V., Mirzaev S. Anxiogenic activity of quinolinic acid and kynurenine in the social interaction test in mice. J. Psychopharmacol. (Oxford) 1996;10(3):246–249. doi: 10.1177/026988119601000312. [http:// dx.doi.org/10.1177/026988119601000312]. [PMID: 22302953]. [DOI] [PubMed] [Google Scholar]
- 29.Lapin I.P. Antagonism of kynurenic acid to anxiogens in mice. Life Sci. 1998;63(15):PL231–PL236. doi: 10.1016/s0024-3205(98)00404-4. [http://dx.doi.org/10.1016/ S0024-3205(98)00404-4]. [PMID: 9768878]. [DOI] [PubMed] [Google Scholar]
- 30.Robinson K.S., Stewart A.M., Cachat J., Landsman S., Gebhardt M., Kalueff A.V. Psychopharmacological effects of acute exposure to kynurenic acid (KYNA) in zebrafish. Pharmacol. Biochem. Behav. 2013;108:54–60. doi: 10.1016/j.pbb.2013.04.002. [http://dx.doi.org/10.1016/j. pbb.2013.04.002]. [PMID: 23583441]. [DOI] [PubMed] [Google Scholar]
- 31.Laugeray A., Launay J.M., Callebert J., Surget A., Belzung C., Barone P.R. Evidence for a key role of the peripheral kynurenine pathway in the modulation of anxiety- and depression-like behaviours in mice: focus on individual differences. Pharmacol. Biochem. Behav. 2011;98(1):161–168. doi: 10.1016/j.pbb.2010.12.008. [http://dx.doi.org/10.1016/j. pbb.2010.12.008]. [PMID: 21167857]. [DOI] [PubMed] [Google Scholar]
- 32.Lapin I.P. Beta-phenylethylamine (PEA): an endogenous anxiogen? Three series of experimental data. Biol. Psychiatry. 1990;28(11):997–1003. doi: 10.1016/0006-3223(90)90065-a. [http://dx.doi.org/10.1016/0006-3223(90)90065-A]. [PMID: 1980422]. [DOI] [PubMed] [Google Scholar]
- 33.Orlikov A., Ryzov I. Caffeine-induced anxiety and increase of kynurenine concentration in plasma of healthy subjects: a pilot study. Biol. Psychiatry. 1991;29(4):391–396. doi: 10.1016/0006-3223(91)90225-b. [http://dx.doi.org/10. 1016/0006-3223(91)90225-B]. [PMID: 2036480]. [DOI] [PubMed] [Google Scholar]
- 34.Orlikov A.B., Prakhye I.B., Ryzov I.V. Kynurenine in blood plasma and DST in patients with endogenous anxiety and endogenous depression. Biol. Psychiatry. 1994;36(2):97–102. doi: 10.1016/0006-3223(94)91189-4. [http:// dx.doi.org/10.1016/0006-3223(94)91189-4]. [PMID: 7948450]. [DOI] [PubMed] [Google Scholar]
- 35.Maes M., Verkerk R., Bonaccorso S., Ombelet W., Bosmans E., Scharpé S. Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation. Life Sci. 2002;71(16):1837–1848. doi: 10.1016/s0024-3205(02)01853-2. [http://dx.doi.org/10.1016/ S0024-3205(02)01853-2]. [PMID: 12175700]. [DOI] [PubMed] [Google Scholar]
- 36.Song L.C., Zhou T.C. Putative endogenous ligands for the benzodiazepine receptor. Sheng Li Ke Xue Jin Zhan. 1989;20(1):70–72. [PMID: 2549623]. [PubMed] [Google Scholar]
- 37.Zarkovsky A.M. The inhibitory effect of endogenous convulsants quinolinic acid and kynurenine on the pentobarbital stimulation of [3H]flunitrazepam binding. Pharmacol. Biochem. Behav. 1986;24(5):1215–1217. doi: 10.1016/0091-3057(86)90173-5. [http://dx.doi.org/10.1016/0091-3057(86)90173-5]. [PMID: 3014563]. [DOI] [PubMed] [Google Scholar]
- 38.Fuller R.W. Uptake inhibitors increase extracellular serotonin concentration measured by brain microdialysis. Life Sci. 1994;55(3):163–167. doi: 10.1016/0024-3205(94)00876-0. [http://dx.doi.org/10.1016/0024-3205(94)00876-0]. [PMID: 8007758]. [DOI] [PubMed] [Google Scholar]
- 39.Kreiss D.S., Lucki I. Effects of acute and repeated administration of antidepressant drugs on extracellular levels of 5-hydroxytryptamine measured in vivo. J. Pharmacol. Exp. Ther. 1995;274(2):866–876. [PMID: 7636750]. [PubMed] [Google Scholar]
- 40.Bell C., Abrams J., Nutt D. Tryptophan depletion and its implications for psychiatry. Br. J. Psychiatry. 2001;178:399–405. doi: 10.1192/bjp.178.5.399. [http://dx.doi.org/10.1192/bjp.178.5.399]. [PMID: 11331552]. [DOI] [PubMed] [Google Scholar]
- 41.Baumann M.H., Mash D.C., Staley J.K. The serotonin agonist m-chlorophenylpiperazine (mCPP) binds to serotonin transporter sites in human brain. Neuroreport. 1995;6(16):2150–2152. doi: 10.1097/00001756-199511000-00013. [http://dx. doi.org/10.1097/00001756-199511000-00013]. [PMID: 8595191]. [DOI] [PubMed] [Google Scholar]
- 42.Deckert J., Catalano M., Syagailo Y.V., Bosi M., Okladnova O., Di Bella D., Nöthen M.M., Maffei P., Franke P., Fritze J., Maier W., Propping P., Beckmann H., Bellodi L., Lesch K.P. Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum. Mol. Genet. 1999;8(4):621–624. doi: 10.1093/hmg/8.4.621. [http://dx.doi.org/10.1093/hmg/8.4.621]. [PMID: 10072430]. [DOI] [PubMed] [Google Scholar]
- 43.Klaassen T., Klumperbeek J., Deutz N.E., van Praag H.M., Griez E. Effects of tryptophan depletion on anxiety and on panic provoked by carbon dioxide challenge. Psychiatry Res. 1998;77(3):167–174. doi: 10.1016/s0165-1781(98)00004-3. [http://dx.doi.org/10.1016/S0165-1781(98)00004-3]. [PMID: 9707299]. [DOI] [PubMed] [Google Scholar]
- 44.Goddard A.W., Charney D.S., Germine M., Woods S.W., Heninger G.R., Krystal J.H., Goodman W.K., Price L.H. Effects of tryptophan depletion on responses to yohimbine in healthy human subjects. Biol. Psychiatry. 1995;38(2):74–85. doi: 10.1016/0006-3223(94)00223-P. [http://dx.doi.org/10. 1016/0006-3223(94)00223-P]. [PMID: 7578653]. [DOI] [PubMed] [Google Scholar]
- 45.Miller H.E., Deakin J.F., Anderson I.M. Effect of acute tryptophan depletion on CO2-induced anxiety in patients with panic disorder and normal volunteers. Br. J. Psychiatry. 2000;176:182–188. doi: 10.1192/bjp.176.2.182. [http://dx.doi.org/10.1192/bjp.176.2.182]. [PMID: 10755058]. [DOI] [PubMed] [Google Scholar]
- 46.Schruers K., Klaassen T., Pols H., Overbeek T., Deutz N.E., Griez E. Effects of tryptophan depletion on carbon dioxide provoked panic in panic disorder patients. Psychiatry Res. 2000;93(3):179–187. doi: 10.1016/s0165-1781(00)00117-7. [http://dx.doi.org/10.1016/S0165-1781(00)00117-7]. [PMID: 10760376]. [DOI] [PubMed] [Google Scholar]
- 47.Gordon J.A., Hen R. The serotonergic system and anxiety. Neuromolecular Med. 2004;5(1):27–40. doi: 10.1385/NMM:5:1:027. [http://dx.doi.org/10.1385/ NMM:5:1:027]. [PMID: 15001810]. [DOI] [PubMed] [Google Scholar]
- 48.Sugden D. Psychopharmacological effects of melatonin in mouse and rat. J. Pharmacol. Exp. Ther. 1983;227(3):587–591. [PMID: 6655558]. [PubMed] [Google Scholar]
- 49.Papp M., Litwa E., Gruca P., Mocaër E. Anxiolytic-like activity of agomelatine and melatonin in three animal models of anxiety. Behav. Pharmacol. 2006;17(1):9–18. doi: 10.1097/01.fbp.0000181601.72535.9d. [PMID: 16377959]. [DOI] [PubMed] [Google Scholar]
- 50.Niles L.P., Pickering D.S., Arciszewski M.A. Effects of chronic melatonin administration on GABA and diazepam binding in rat brain. J. Neural Transm. (Vienna) 1987;70(1-2):117–124. doi: 10.1007/BF01252513. [http://dx.doi.org/10.1007/BF01252513]. [PMID: 3668517]. [DOI] [PubMed] [Google Scholar]
- 51.Coloma F.M., Niles L.P. Melatonin enhancement of [3H]-gamma-aminobutyric acid and [3H]muscimol binding in rat brain. Biochem. Pharmacol. 1988;37(7):1271–1274. doi: 10.1016/0006-2952(88)90781-2. [http://dx.doi.org/10. 1016/0006-2952(88)90781-2]. [PMID: 2833276]. [DOI] [PubMed] [Google Scholar]
- 52.Loiseau F., Le Bihan C., Hamon M., Thiébot M.H. Effects of melatonin and agomelatine in anxiety-related procedures in rats: interaction with diazepam. Eur. Neuropsychopharmacol. 2006;16(6):417–428. doi: 10.1016/j.euroneuro.2005.11.007. [http://dx.doi.org/10.1016/j.euroneuro.2005.11.007]. [PMID: 16376525]. [DOI] [PubMed] [Google Scholar]
- 53.Xu F., Li J.C., Ma K.C., Wang M. Effects of melatonin on hypothalamic gamma-aminobutyric acid, aspartic acid, glutamic acid, beta-endorphin and serotonin levels in male mice. Biol. Signals. 1995;4(4):225–231. doi: 10.1159/000109446. [http://dx.doi.org/10.1159/000109446]. [PMID: 8720689]. [DOI] [PubMed] [Google Scholar]
- 54.Kopp C., Vogel E., Rettori M.C., Delagrange P., Guardiola-Lemaître B., Misslin R. Effects of melatonin on neophobic responses in different strains of mice. Pharmacol. Biochem. Behav. 1999;63(4):521–526. doi: 10.1016/s0091-3057(99)00023-4. [http://dx.doi.org/10.1016/S0091-3057(99) 00023-4]. [PMID: 10462179]. [DOI] [PubMed] [Google Scholar]
- 55.Guardiola-Lemaître B., Lenègre A., Porsolt R.D. Combined effects of diazepam and melatonin in two tests for anxiolytic activity in the mouse. Pharmacol. Biochem. Behav. 1992;41(2):405–408. doi: 10.1016/0091-3057(92)90118-y. [http://dx.doi.org/10.1016/0091-3057(92)90118-Y]. [PMID: 1349438]. [DOI] [PubMed] [Google Scholar]
- 56.Stein D.J., Ahokas A.A., de Bodinat C. Efficacy of agomelatine in generalized anxiety disorder: a randomized, double-blind, placebo-controlled study. J. Clin. Psychopharmacol. 2008;28(5):561–566. doi: 10.1097/JCP.0b013e318184ff5b. [http://dx.doi.org/10.1097/JCP.0b013e318184ff5b]. [PMID: 18794654]. [DOI] [PubMed] [Google Scholar]
- 57.Stein D.J., Ahokas A., Jarema M., Avedisova A.S., Vavrusova L., Chaban O., Gruget C., Olivier V., Picarel-Blanchot F., de Bodinat C. Efficacy and safety of agomelatine (10 or 25 mg/day) in non-depressed out-patients with generalized anxiety disorder: A 12-week, double-blind, placebo-controlled study. Eur. Neuropsychopharmacol. 2017;27(5):526–537. doi: 10.1016/j.euroneuro.2017.02.007. [http://dx.doi.org/10.1016/ j.euroneuro.2017.02.007]. [PMID: 28298261]. [DOI] [PubMed] [Google Scholar]
- 58.Huijbregts K.M., Batelaan N.M., Schonenberg J., Veen G., van Balkom A.J. Agomelatine as a novel treatment option in panic disorder, results from an 8-week open-label trial. J. Clin. Psychopharmacol. 2015;35(3):336–338. doi: 10.1097/JCP.0000000000000313. [http://dx.doi.org/10.1097/JCP. 0000000000000313]. [PMID: 25856784]. [DOI] [PubMed] [Google Scholar]
- 59.Levitan M.N., Papelbaum M., Soares G., Simões P., Zugliani M., Freire R.C., Mochcovitch M., Nardi A.E. Agolmelatine in Panic Disorder: A 6 week follow-up case series. J. Clin. Psychopharmacol. 2016;36(4):395–396. [http://dx.doi.org/10.1097/JCP. 0000000000000524]. [PMID: 27285660]. [Google Scholar]
- 60.Barkus C., McHugh S.B., Sprengel R., Seeburg P.H., Rawlins J.N., Bannerman D.M. Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion. Eur. J. Pharmacol. 2010;626(1):49–56. doi: 10.1016/j.ejphar.2009.10.014. [http://dx.doi.org/10.1016/j. ejphar.2009.10.014]. [PMID: 19836379]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willetts J., Balster R.L., Leander J.D. The behavioral pharmacology of NMDA receptor antagonists. Trends Pharmacol. Sci. 1990;11(10):423–428. doi: 10.1016/0165-6147(90)90150-7. [http://dx.doi.org/10.1016/0165-6147(90)90150-7]. [PMID: 2147794]. [DOI] [PubMed] [Google Scholar]
- 62.Corbett R., Dunn R.W. Effects of 5,7 dichlorokynurenic acid on conflict, social interaction and plus maze behaviors. Neuropharmacology. 1993;32(5):461–466. doi: 10.1016/0028-3908(93)90170-8. [http://dx.doi.org/10.1016/0028-3908(93)90170-8]. [PMID: 8100622]. [DOI] [PubMed] [Google Scholar]
- 63.Dunn R.W., Corbett R., Fielding S. Effects of 5-HT1A receptor agonists and NMDA receptor antagonists in the social interaction test and the elevated plus maze. Eur. J. Pharmacol. 1989;169(1):1–10. doi: 10.1016/0014-2999(89)90811-x. [http://dx.doi.org/10.1016/0014-2999(89)90811-X]. [PMID: 2574684]. [DOI] [PubMed] [Google Scholar]
- 64.Plaznik A., Palejko W., Nazar M., Jessa M. Effects of antagonists at the NMDA receptor complex in two models of anxiety. Eur. Neuropsychopharmacol. 1994;4(4):503–512. doi: 10.1016/0924-977x(94)90299-2. [http://dx. doi.org/10.1016/0924-977X(94)90299-2]. [PMID: 7894261]. [DOI] [PubMed] [Google Scholar]
- 65.Krystal J.H., Karper L.P., Seibyl J.P., Freeman G.K., Delaney R., Bremner J.D., Heninger G.R., Bowers M.B., Jr, Charney D.S. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry. 1994;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [http://dx.doi.org/10.1001/archpsyc.1994.03950030035004]. [PMID: 8122957]. [DOI] [PubMed] [Google Scholar]
- 66.Glue P., Medlicott N.J., Harland S., Neehoff S., Anderson-Fahey B., Le Nedelec M., Gray A., McNaughton N. Ketamine’s dose-related effects on anxiety symptoms in patients with treatment refractory anxiety disorders. J. Psychopharmacol. (Oxford) 2017;31(10):1302–1305. doi: 10.1177/0269881117705089. Epub ahead of print [http://dx.doi.org/ 10.1177/0269881117705089]. [PMID: 28441895]. [DOI] [PubMed] [Google Scholar]
- 67.Bannerman D.M., Sprengel R., Sanderson D.J., McHugh S.B., Rawlins J.N., Monyer H., Seeburg P.H. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat. Rev. Neurosci. 2014;15(3):181–192. doi: 10.1038/nrn3677. [http://dx.doi.org/10.1038/nrn3677]. [PMID: 24552786]. [DOI] [PubMed] [Google Scholar]
- 68.Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S., Weisstaub N., Lee J., Duman R., Arancio O., Belzung C., Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [http://dx.doi.org/10.1126/science.1083328]. [PMID: 12907793]. [DOI] [PubMed] [Google Scholar]
- 69.David D.J., Samuels B.A., Rainer Q., Wang J.W., Marsteller D., Mendez I., Drew M., Craig D.A., Guiard B.P., Guilloux J.P., Artymyshyn R.P., Gardier A.M., Gerald C., Antonijevic I.A., Leonardo E.D., Hen R. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62(4):479–493. doi: 10.1016/j.neuron.2009.04.017. [http://dx.doi.org/10.1016/j.neuron. 2009.04.017]. [PMID: 19477151]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Revest J.M., Dupret D., Koehl M., Funk-Reiter C., Grosjean N., Piazza P.V., Abrous D.N. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol. Psychiatry. 2009;14(10):959–967. doi: 10.1038/mp.2009.15. [http://dx.doi.org/10.1038/mp.2009.15]. [PMID: 19255582]. [DOI] [PubMed] [Google Scholar]
- 71.Kitayama N., Vaccarino V., Kutner M., Weiss P., Bremner J.D. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J. Affect. Disord. 2005;88(1):79–86. doi: 10.1016/j.jad.2005.05.014. [http://dx.doi.org/10.1016/j.jad.2005. 05.014]. [PMID: 16033700]. [DOI] [PubMed] [Google Scholar]
- 72.Irle E., Ruhleder M., Lange C., Seidler-Brandler U., Salzer S., Dechent P., Weniger G., Leibing E., Leichsenring F. Reduced amygdalar and hippocampal size in adults with generalized social phobia. J. Psychiatry Neurosci. 2010;35(2):126–131. doi: 10.1503/jpn.090041. [http://dx. doi.org/10.1503/jpn.090041]. [PMID: 20184810]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gilbertson M.W., Shenton M.E., Ciszewski A., Kasai K., Lasko N.B., Orr S.P., Pitman R.K. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat. Neurosci. 2002;5(11):1242–1247. doi: 10.1038/nn958. [http://dx.doi.org/10.1038/nn958]. [PMID: 12379862]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dannlowski U., Stuhrmann A., Beutelmann V., Zwanzger P., Lenzen T., Grotegerd D., Domschke K., Hohoff C., Ohrmann P., Bauer J., Lindner C., Postert C., Konrad C., Arolt V., Heindel W., Suslow T., Kugel H. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol. Psychiatry. 2012;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. [http://dx.doi.org/10.1016/j.biopsych.2011.10.021]. [PMID: 22112927]. [DOI] [PubMed] [Google Scholar]
- 75.Müller N., Schwarz M.J. A psychoneuroimmunological perspective to Emil Kraepelins dichotomy: schizophrenia and major depression as inflammatory CNS disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2008;258(Suppl. 2):97–106. doi: 10.1007/s00406-008-2012-3. [http://dx.doi.org/10. 1007/s00406-008-2012-3]. [PMID: 18516521]. [DOI] [PubMed] [Google Scholar]
- 76.Wichers M.C., Koek G.H., Robaeys G., Verkerk R., Scharpé S., Maes M. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol. Psychiatry. 2005;10(6):538–544. doi: 10.1038/sj.mp.4001600. [http://dx.doi.org/10. 1038/sj.mp.4001600]. [PMID: 15494706]. [DOI] [PubMed] [Google Scholar]
- 77.Kim Y.K., Na K.S., Myint A.M., Leonard B.E. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;64:277–284. doi: 10.1016/j.pnpbp.2015.06.008. [http://dx. doi.org/10.1016/j.pnpbp.2015.06.008]. [PMID: 26111720]. [DOI] [PubMed] [Google Scholar]
- 78.Kheirbek M.A., Klemenhagen K.C., Sahay A., Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat. Neurosci. 2012;15(12):1613–1620. doi: 10.1038/nn.3262. [http://dx.doi.org/10.1038/nn.3262]. [PMID: 23187693]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [http://dx.doi.org/10.1016/j.biocel.2006.07.001]. [PMID: 16978905]. [DOI] [PubMed] [Google Scholar]
- 80.Bouayed J., Rammal H., Soulimani R. Oxidative stress and anxiety: relationship and cellular pathways. Oxid. Med. Cell. Longev. 2009;2(2):63–67. doi: 10.4161/oxim.2.2.7944. [http://dx.doi.org/10.4161/oxim.2.2.7944]. [PMID: 20357926]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuloglu M., Atmaca M., Tezcan E., Ustundag B., Bulut S. Antioxidant enzyme and malondialdehyde levels in patients with panic disorder. Neuropsychobiology. 2002;46(4):186–189. doi: 10.1159/000067810. [http:// dx.doi.org/10.1159/000067810]. [PMID: 12566935]. [DOI] [PubMed] [Google Scholar]
- 82.Kuloglu M., Atmaca M., Tezcan E., Gecici O., Tunckol H., Ustundag B. Antioxidant enzyme activities and malondialdehyde levels in patients with obsessive-compulsive disorder. Neuropsychobiology. 2002;46(1):27–32. doi: 10.1159/000063573. [http://dx.doi.org/10.1159/000063573]. [PMID: 12207144]. [DOI] [PubMed] [Google Scholar]
- 83.Smaga I., Niedzielska E., Gawlik M., Moniczewski A., Krzek J., Przegaliński E., Pera J., Filip M. Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacol. Rep. 2015;67(3):569–580. doi: 10.1016/j.pharep.2014.12.015. [http://dx.doi.org/10.1016/j.pharep. 2014.12.015]. [PMID: 25933971]. [DOI] [PubMed] [Google Scholar]