Abstract
Background
Schizophrenia is a complex illness in which genetic, environmental, and epigenetic components have been implicated. However, recently, psychiatric disorders appear to be related to a chronic inflammatory state, at the level of specific cerebral areas which have been found as well impaired and responsible for schizophrenia symptomatology. Hence, a role of inflammatory mediators and cytokines has been as well defined. Accordingly, the role of an acute inflammatory phase protein, the C-reactive protein (CRP) has been recently investigated.
Objective
The objective of the present study is to evaluate how PCR may represent a biomarker in schizophrenia, i.e. correlated with illness phases and/or clinical manifestation and/or psychopathological severity.
Methods
A systematic review was here carried out by searching the following keywords ((C-reactive protein AND ((schizophrenia) OR (psychotic disorder))) for the topics ‘PCR’ and ‘Schizophrenia’, by using MESH terms.
Results
An immune dysfunction and inflammation have been described amongst schizophrenic patients. Findings reported elevated CRP levels in schizophrenia, mainly correlated with the severity of illness and during the recrudescent phase. CRP levels are higher when catatonic features, negative symptomatology and aggressiveness are associated. CRP levels appeared not to be related to suicidal behaviour and ideation.
Conclusion
CRP and its blood levels have been reported higher amongst schizophrenic patients, by suggesting a role of inflammation in the pathogenesis of schizophrenia. Further studies are needed to better understand if CRP may be considered a biomarker in schizophrenia.
Keywords: C-reactive protein, schizophrenia, psychosis, inflammation, chronic illness, systematic review
1. INTRODUCTION
Schizophrenia is a complex psychiatric disease reported in around 1% of the general population [1], and associated with an average lifetime prevalence ranging from 0.7%-0.8% [2] and a low life expectancy, defined as the potential years of life lost due to schizophrenic illness [3, 4]. A recent literature review and meta-analysis observed a low life expectancy (of around 13-15 years), associated with a greater prevalence amongst males compared to females [5]. This has been extensively hypothesized depending on several factors, i.e. concomitant somatic diseases (e.g., cardiovascular diseases, diabetes, metabolic syndrome, etc.) [6-8]; long-term and chronic use of the second-generation antipsychotics may potentially lead to metabolic and cardiovascular side-effects [9-11]; increasing prevalence in tobacco, alcohol and illicit substances abuse use, amongst schizophrenic patients [12-14], a greater sedentary life style [15] and unhealthy dietary habits [16]. Overall, schizophrenic patients appear to suffer from a reduced health-seeking behaviour [17] and a higher suicide risk compared to general population (OR=22), especially within one year of their first hospital admission [18]. Furthermore, it has been as well supposed a possible major genetic disposition which may facilitate the development of metabolic and cardiovascular accidents [19, 20].
In general, the risk to develop a schizophrenic and/or a psychotic disorder may be determined by the complexity of genetic, environmental and social factors [21], linked to each other by epigenetic mechanisms regulating gene expression levels and consequently molecular pathways [22, 23]. It is well known that monozygotic twins show schizophrenia concordance rates of about 40%-50% with a heritability rate of 80% [25]. However, the genetic component is not fully able to explain the aetiology of schizophrenia [26, 27]; hence, recently genetic variants, epigenetic marks – such as cross-talk between DNA methylation and histone modification processes –, gene expression regulation and environmental factors are currently under study to better clarify the etiopatho-genesis of schizophrenia [23]. Genome-wide linkage studies (GWLSs), derivation of genome-wide association studies (GWASs), are focused on screening variants and high-throughput sequences [23]. Furthermore, it has been demonstrated that schizophrenic genetic risk is modulated by genes indexed by single nucleotide polymorphisms (SNPs) located in the minor allele frequency (MAF) spectrum [23]. Moreover, proliferate chromosomal duplications or deletions, better known as copy number variants (CNVs), seem to be part of schizophrenia pathogenic expression [23]. Environmental factors as well as the specific patterns of disturbing brain growth during susceptible periods (i.e., early life distresses, urban environment, drug use and/or abuse, etc.) may contribute to the onset of schizophrenia [28].
Furthermore, recently, the role of an inflammatory status as determinant in the etiopathophysiology of schizophrenia has also been proposed [29, 30]. Both psychiatric and neurodegenerative illnesses have been demonstrated to be related to a chronic inflammation of some specific brain areas, which are characterized by an infiltration of peripheral immune cells, which may worsen brain impairment, leading to symptomatic clinical presentation [31]. Generally, the Central Nervous System (CNS) is characterized by a mild inflammatory state associated with an immune activation/reaction. During the relapse phases of a neuropsychiatric disorder, the CNS is characterized by the absence of immune tolerance and, consequently, a worsening of the symptomatology; whilst, during remission phase, it presents an activation of immune tolerance which may as well ameliorates the symptomatology [31, 32]. Accordingly, evidence suggests that an increasing level of a stressing hormone may activate the inflammatory arm of the immune system and, hence, trigger the expression of genes responsible to elicit a chronic and low-grade inflammation state [33]. Within this context, the possible relationship between a psychotic breakdown evolving in schizophrenia and the C-reactive protein (CRP) plasma levels has been investigated [34, 35]. The CRP is an acute phase protein secreted by hepatocytes [36] during an inflammation, in response to the pro-inflammatory cytokines, other endogenous signals of innate immunity or a tissue damage [37]. CRP is easily measured through a blood sample in its high-sensitivity form (hs-CRP) [38, 39]. CRP is considered a biomarker of aging and chronic illness and/or chronic low grade inflammation [40]. In fact, in subjects affected by cardiovascular diseases, myocardial infarction, stroke [41-43], hypertension [44, 45], type-2 diabetes [46], chronic kidney injury [47, 48], cancer or Alzheimer's disease and Parkinson's disease [49-51], increased CPR levels have been reported in an age-dependent manner [52]. Comparing ageing adults with or without diseases/disability, CPR levels are reported higher in those with some physical illnesses [52]. Moreover, the survival, poor physical and cognitive performances appear to be dependent on PCR levels [52]. A meta-analysis evaluating eight studies reported that CRP levels were higher amongst patients affected with schizophrenia compared to comparison subjects [53]. CRP has been as well reported to be correlated with more severe psychopathology [54], especially negative symptoms [55, 56] and a worsening in cognitive functioning [57, 58]. Indeed, understanding the pathways involved in the pathogenesis of elevated serum CRP may provide crucial insights into the pathophysiology of schizophrenia, also in a microvascular perspective [59]. An assessment of CRP levels may have utility in improving our understanding of the trajectory towards schizophrenia, being considered a sort of biomarker of the schizophrenia status [5, 33, 40]. Furthermore, clearly understanding how the inflammation could play a significant role in schizophrenia, may significantly help in developing new treatment strategies and pharmaceutical targets.
Hence, the present systematic review aims: a) to evaluate the correlation (if any) between the CRP and its blood levels with the onset, exacerbation and maintenance of schizophrenia; b) to evaluate the association (if any) between CRP levels and cognitive pattern amongst schizophrenics; c) to evaluate the association (if any) between CRP levels and severity of schizophrenia psychopathology (including the influence on aggressiveness, impulsiveness, etc.). The final goal is to determine if serum CRP levels may be considered biomarkers of schizophrenia phases and severity of illness. In addition, better understanding of the role of CRP in schizophrenia may in turn allow to develop better treatment strategies for schizophrenic patients.
2. Material and Methods
2.1. Search Sources and Strategies
A systematic review was here conducted according to the methods recommended by the Cochrane Collaboration [60] and the process and results were documented in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [61]. Literature searches were performed by using PubMed/Medline databases. We combined the search strategy of free text terms and exploded MESH headings for the topics of PCR and Schizophrenia as following: ((C-reactive protein[Title/Abstract] AND ((schizophrenia [Title/Abstract]) OR (psychotic disorder[Title/Abstract]))). Only articles published during the last 20 years in English were here selected. Studies published through May 1, 2017 were included. In addition, secondary searches were performed using the reference listing of all eligible as well as relevant articles and in consultation with experts in the field and or manual search.
2.2. Study Selection
We considered studies evaluating the relationship between CRP and schizophrenia or psychotic disorders. We examined all titles and abstracts, and obtained full texts of potentially relevant papers. Working independently and in duplicate, two reviewers (FS and FV) read the papers and determined whether they met inclusion criteria. Duplicate publications were properly excluded. All English-language articles identified by the data sources, reporting original data related to CRP in schizophrenia or psychotic disorders, were evaluated in the present review. All experimental and observational study designs were included apart from case reports. Randomized, controlled clinical trials involving humans were prioritized in the present systematic review. Narrative and systematic reviews, letters to the editor and book chapters were excluded. To be included in the present review, studies were required to meet the following criteria: a) empirical and peer-reviewed study; b) at least an abstract with estimates and/or full results published in English; c) the role of CRP in schizophrenia and/or psychotic disorders; and d) human studies.
2.3. Data Extraction and Management
FV and FS, independently extracted the data on participant characteristics, intervention details and outcomes measures. Disagreements were resolved by discussion and consensus with a third member of the team (LO). Data were collected using an ad-hoc developed data extraction spreadsheet.
2.4. Characteristics of Included Studies
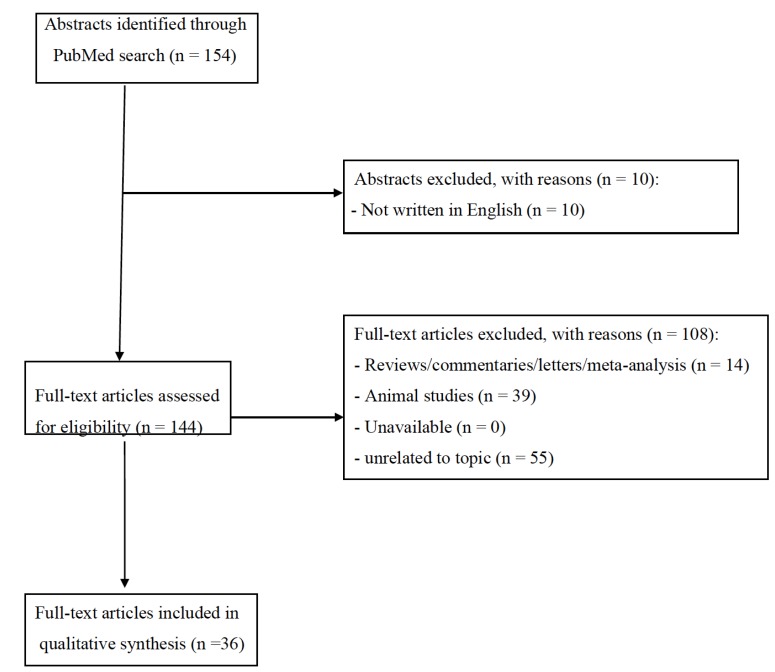
The set of keywords initially generated 154 results (Fig. 1). A total of 10 papers were excluded because they were not written in English; 14 papers were excluded because of reviews, letters to editors, or metanalysis; whilst 39 papers were not here included being based on non-human studies. Of the remaining 91 studies, further 55 studies were excluded because they did not meet the inclusion criteria. Finally, a total of 36 papers were included and accounted for in our analysis. Table 1 clearly explains the main characteristics (study design, sample size, main outcomes and findings) of all the studies here retrieved.
Fig. (1).
Selection of retrieved studies.
Table 1.
Summary of included studies evaluating the association between CRP and schizophrenia.
| Study | Study Design | Primary and Secondary Outcomes | Characteristics of Participants | Assessments | Main Findings | Correlation CRP and Schizophrenia? | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [62] | Cross-sectional study | Association between CRP levels and severity of clinical psychopathology | 26 inpatients schizophrenia or schizoaffective disorder (DSM-IV-TR criteria) 18-65 yy stable on antipsychotic medications |
•Serum CRP levels (cut-off value =5 mg/L) •PANSS total score |
Subjects with CRP levels above the normal range (n=5) scored significantly higher than those with CRP levels in the normal range (n=21) at the PANSS total score, negative symptom subscale score and general psychopathology subscale score. | •Positive correlation with severity of psychopathology | |||||||||||||||||||||||||||||
| [57] | Cross-sectional study | Association between CRP levels and severity of clinical psychopathology and cognitive impairment | 413 outpatients schizophrenia or schizoaffective disorder (DSM-IV criteria) 13-65 yy ongoing antipsychotic, anticholinergic, non-lithium mood stabilizer, antidepressant medications. |
•Serum CRP levels •(cut-off value =5 mg/L) •PANSS total score •RBANS total score •PDS |
Individuals with CRP ≥5.0 mg/μl had significantly lower RBANS cognitive scores than those with CRP <5.0 mg/μl (F=8.07, pb.005). CRP groups did not differ in the severity of positive, negative, or general PANSS symptoms. |
•Positive correlation with severity of cognitive impairment •No correlation with severity of psychopathology |
|||||||||||||||||||||||||||||
| [63] | Case-control study | Association between hsCRP levels and clinical psychopathology | 165 HC vs 207 in/outpatients schizophrenia (DSM-IV criteria) Arabic population 15-76 yy stable on antipsychotic medications |
•Serum CRP levels •(cut-off value =5 mg/L) •PANSS total score •SANS (Andreasen's Scale for Assessment of Negative Symptoms) total score •SCAN (WHO Schedule for Clinical Assessment in Neuropsychiatry – schizophrenia section) •CGI (Clinical Global Impression) •AIMS (Abnormal involuntary movement scale for tardive dyskinesia) |
Amongst schizophrenics, hsCRP levels were: (a) marginally higher in women with later age of disease onset; (b) highest with remission and with catatonic features; (c) lower with family history of psychosis. |
•Positive correlation with some psychopathological features | |||||||||||||||||||||||||||||
| [64] | Case-control study | Differences between CRP levels amongst bipolar disorder vs schizophrenia disorder Association between CRP levels and the presence of metabolic syndrome amongst these groups |
60 BD vs 63 inpatients schizophrenia (DSM-IV criteria) stable on antipsychotic medications |
•Serum CRP levels •(cut-off value =5 mg/L) •NCEP ATP III criteria for metabolic syndrome |
The prevalence of the metabolic syndrome was 31% in bipolar group compared to the 37% in schizophrenia group. Amongst bipolar disorder patients significantly higher CRP levels compared to schizophrenia group. |
•No significant correlation with psychopathology | |||||||||||||||||||||||||||||
| Study | Study Design | Primary and Secondary Outcomes | Characteristics of Participants | Assessments | Main Findings | Correlation CRP and Schizophrenia? | |||||||||||||||||||||||||||||
| [65] | Case-control study | Association between the CRP rs1417938, rs1800947, rs1205 variants with susceptibility to Schizophrenia |
105 HC vs 103 schizophrenia (DSM-IV criteria) Armenian population |
•Serum CRP levels (cut-off value =5 mg/L) •Genomic DNA extraction and gentotyping analysis |
•No significant differences found when the proportions of CRP variants were compared between the patients and HC (p>0.05). •Nevertheless, the rs1417938 AA homozygotes and rs1205*T carriers tended to be overrepresented in the patients by comparison with the HC (p=0.1 in both cases). |
•No significant correlation with psychopathology | |||||||||||||||||||||||||||||
| [55] | A cross-sectional case–control study | Association between hsCRP levels and clinical features of schizophrenia | 200 antipsychotic-free outpatients male schizophrenia (DSM-IV criteria) vs 200 HC Egyptian population |
•Serum CRP levels (cut-off value =5 mg/L) •PANSS total score |
•CRP levels for patients was significantly higher than that for controls (P=0.000). •PANSS scores and patients' data, which significantly correlated with serum hs-CRP levels •PANSS negative symptom score was second only to the waist circumference, with which they explained 54.7 % of the variation in serum hs-CRP. |
•Positive correlation with severity of psychopathology •Positive correlation with negative symptomatology |
|||||||||||||||||||||||||||||
| [66] | Case-control study | Association between hsCRP levels and schizophrenia, bipolar disorder and non-psychiatric subjects | 295 schizophrenia vs 192 bipolar disorder (DSM-IV criteria) vs 228 non-psychiatric subjects | •Serum CRP levels (cut-off value =5 mg/L) •PANSS total score •SCID |
•The individuals with schizophrenia had significantly increased odds of having elevated levels of CRP relative to both the 75th and 90th percentile •CRP levels of the controls adjusting for the same covariates (OR 1.79, 95% CI 1.14, 2.82; p=.012; OR 2.76, 95% CI 1.58, 4.83, p=b.001). |
•Positive correlation with psychopathology •Positive correlation with negative symptomatology |
|||||||||||||||||||||||||||||
| [67] | Case-control study | Association between hsCRP and three antipsychotic groups of schizophrenia subjects (clozapine, olanzapine and risperidone) | 36 HC vs 36 schizophrenia inpatients (DSM-IV criteria) Taiwanese population Stable on antipsychotic mediations (clozapine, olanzapine or risperidone) |
•Serum CRP levels (cut-off value =5 mg/L) •PANSS total score |
•An increase in the hsCRP levels in the schizophrenic group was observed in comparison with the HC (P = 0.013) | •Positive correlation with psychopathology •Positive correlation with negative symptomatology and illness duration |
|||||||||||||||||||||||||||||
| [68] | A longitudinal birth cohort study | Association between early-life atopic disorder, inflammatory markers at age 9 and the risk of onset of psychotic episodes at age 13 years | 6785 13-year adolescents with atopic disorders (asthma and eczema) | •Serum CRP levels (cut-off value =N/A) •Serum IL-6 levels |
•Risk of psychotic episodes at age 13 years was increased for both groups (asthma and eczema) •Atopy was associated with increased serum IL-6 and CRP levels •Inflammatory markers were not associated with later psychotic episodes |
•Positive association between atopy and CRP levels •No association between CRP levels and risk of psychotic episodes |
|||||||||||||||||||||||||||||
| Study | Study Design | Primary and Secondary Outcomes | Characteristics of Participants | Assessments | Main Findings | Correlation CRP and Schizophrenia? | |||||||||||||||||||||||||||||
| [69] | Prospective and cross-sectional study |
Association between hsCRP and risk of late and very-late onset schizophrenia | 78810 individuals from 2 large independent general population studies outpatients and inpatients |
•Serum CRP levels (cut-off value =5 mg/L) |
Age- and gender-adjusted hazard ratios vs individuals in the first quartile of CRP were 1.7 (95% CI: 0.3–8.9) for second quartile, 2.1 (0.4–10) for third quartile, and 11 (2.8–40) for fourth quartile individuals. The corresponding hazard ratio for fourth quartile individuals after multifactorial adjustment was 5.9 (1.4–24). Individuals with vs without schizophrenia had 63% increased plasma levels of CRP (P = 1 ×10−4). When CRP was on a continuous scale, a doubling in CRP yielded an age- and gender-adjusted observational OR of 1.5 (1.3–1.8) and a corresponding causal OR of 1.4 (0.5–4.3) (observed vs causal: P = .89). |
•Positive correlation with psychopathology •Positive correlation with the risk of late or very-late onset schizophrenia |
|||||||||||||||||||||||||||||
| [70] | Retrospective, cross-sectional, naturalistic study |
Association between CRP levels and schizophrenia, major depression and bipolar disorder | 485 schizophrenia vs 319 major depression vs 260 bipolar disorder (DSM-IV criteria) inpatients | •Serum CRP levels (cut-off value =3 mg/L) | •Mean concentration of CRP levels in study groups was 5.30 mg/l amongst schizophrenics •There was no difference for CRP levels between patients with schizophrenia, unipolar depression, bipolar depression and bipolar mania (P=0.58). •No significant differences in the risk of having high level of CRP between the clinical groups •The rate of patients being above high level was higher in women. •Patients with CRP level in the low range were significantly younger (48.2 _ 20.1 vs. 51.1 _ 20.6 years, t _ 2.22, P _ 0.001) •Patients with CRP levels in the high range were significantly older (53.1 _ 20.4 vs. 47.9 _ 20.2 years, t _ _ 3.83, P _ 0.001) |
•No correlation with psychopathology | |||||||||||||||||||||||||||||
| [71] | Case-control study | Association between CRP levels and different phases of schizophrenia | 79 with recent onset psychosis (inpatients); 249 with chronic schizophrenia (outpatients); 260 HC 18-65 yy |
•Serum CRP levels (cut-off value =5 mg/L) •PANSS total score •RBANS total score •FORM-A |
•The correlations were generally weak to moderate, ranging from r = 0.023 (antibodies to gliadin and CRP) to r = 0.27 (antibodies to gliadin and casein). •Generally, CRP was the most weakly correlated with the other measures, while the correlation between the level of casein antibodies and other measures was the strongest. •The chronic schizophrenia group differed from the control group for antibodies to gliadin (coefficient = .333, P = .007); CRP (coefficient = .469, P=<.001); ASCA (coefficient = .382, P=.005); pentraxin 3 (coefficient = .118, P = .023); and the composite inflammation score (coefficient = .453, P = <.001). The level of these markers was higher in the chronic schizophrenia group than in the control group. |
•Positive correlation with psychopathology •Positive correlation with chronic phase of schizophrenia |
|||||||||||||||||||||||||||||
| Study | Study Design | Primary and Secondary Outcomes | Characteristics of Participants | Assessments | Main Findings | Correlation CRP and Schizophrenia? | |||||||||||||||||||||||||||||
| [72] | Cross-sectional study | Association between quality of life and CRP levels amongst schizophrenia subjects | 256 schizophrenia (DSM-IV criteria) stable on antipsychotic medications |
•Serum CRP levels (cut-off value =3 mg/L) •SQoL score and dimensions •BMI •PANSS total score •FTND •AUDIT |
•Compared to patients with normal CRP levels, those with high CRP levels were significantly older (p= 0.027), with a lower education level (p= 0.021), a longer duration of disorder (p= 0.018), a higher BMI (p= 0.000) and a higher Fagerström score (p= 0.041). Concerning QoL, patients with high CRP levels reported lower QoL scores for the SQoL 18 index. the PhW and the SL dimensions (p= 0.016, p= 0.005 and p= 0.003, respectively). •An educational level ≤12 years, a higher BMI and a higher Fagerström score remained significantly associated with high CRP level. An investigation of the various dimensions of the SQoL 18 revealed that PsW, PhW and SL were the most salient features of QoL associated with CRP. A trend was observed for the SE dimension |
•Positive correlation with psychopathology •Positive correlation with quality of life |
|||||||||||||||||||||||||||||
| [40] | Post-hoc analysis on baseline data from a longitudinal study | Association between hsCRP levels and psychopathology amongst schizophrenia subjects | 65 outpatients schizophrenia or 23 schizoaffective disorder (DSM-IV criteria) vs 71 HC | •Serum CRP levels (cut-off value =N/A) •SAPS and SANS •D-KEFS •CIRS |
•hs-CRP levels were significantly higher in individuals with schizophrenia than in comparison subjects. •Higher hs-CRP levels in schizophrenia group were associated with female gender, more severe negative symptoms, greater medical comorbidity, and worse metabolic risk factors including BMI, fasting glucose, and haemoglobin A1c levels. Hs-CRP was not related to age, race, education, smoking status, antipsychotic dosage, or cognitive impairment. |
•Positive correlation with psychopathology •Positive correlation with severity of schizophrenia •No correlation with cognitive pattern |
|||||||||||||||||||||||||||||
| [73] | Observational study | Association between CRP levels in childhood and the risk for the onset of depression and/or psychosis in adulthood | 4585 individuals at age 9 were assessed for CRP levels and then evaluated at age 18 with clinical assessments | •Serum CRP levels (cut-off value =N/A) •CIS-R (Clinical Interview Schedule-Revised) •MFQ (Mood and Feelings Questionnaire) Participants were assessed at age 9 and the at age 18 years |
•Risks of psychotic episodes and psychotic disorder at age 18 years were increased with higher IL-6 levels but not with CRP levels, at baseline (adjusted OR, 1.81; 95% CI, 1.01-3.28; and adjusted OR, 2.40; 95% CI, 0.88-6.22, respectively). •Higher IL-6 levels (not CRP levels) in childhood were associated with subsequent risks of depression and Pes (Psychotic episodes) in a dose-dependent manner. |
•No correlation with psychopathology | |||||||||||||||||||||||||||||
| Study | Study Design | Primary and Secondary Outcomes | Characteristics of Participants | Assessments | Main Findings | Correlation CRP and Schizophrenia? | |||||||||||||||||||||||||||||
| [74] | Cross-sectional study | Association between vitamin D, CRP levels and risk of schizophrenia | 93 outpatients schizophrenia or schizoaffective disorder (DSM-IV criteria) vs 93 family-matched HC 18-65 yy |
•Serum CRP levels (cut-off value =N/A) •25(OH)D concentrations |
•Mean levels of CRP and 25(OH)D were 43.3% higher and 26.7% lower for patients compared to controls, respectively. •25(OH)D were inversely associated with CRP in the patients, but not in the controls. •The proportions of patients significantly increased with increasing quartiles of CRP, while significantly decreased with increasing quartiles of 25(OH)D. •Among individuals with high CRP, participants with high 25(OH)D have significantly lower proportion (adjusted OR = 0.217, 95% CI 0.063, 0.751) of schizophrenia compared to those with low 25(OH)D. •High levels of vitamin D may be linked to reduced risk of schizophrenia with elevated CRP |
•Inverse correlation between CRP and vitamin D levels | |||||||||||||||||||||||||||||
| [75] | Cross-sectional study | Association between aggressive behavior and inflammatory markers in schizophrenia | 213 schizophrenia (DSM-IV-TR) inpatients 19-89 yy |
•Serum CRP levels (cut-off value =N/A) •PANSS total score and subscales |
•CRP levels significantly correlated with other laboratory markers indicating increased inflammation including leukocyte count and neutrophil to lymphocyte ratio (r = 0.387, P< 0.0001 and r = 0.356, P < 0.0001) respectively. •Inpatients with elevated CRP levels displayed increased aggressive behaviour compared to patients with normal CRP levels (<1 mg/dL). This was manifested by higher rates of restraint during hospitalization (x 2= 5.22, P=0.031) and increased PANSS-EC score (U=5410.5, P=0.012). •Elevated CRP levels were not associated with suicidal behaviour. •Multivariate analysis revealed that higher PANSS-EC score was associated with elevated CRP after controlling for the covariates age, sex, BMI and smoking. |
•Positive correlation with psychopathology •Positive correlation with aggressive behavior •Negative correlation with suicidal behavior |
|||||||||||||||||||||||||||||
| [76] | Case-control study | Association between hsCRP levels and lipid profile amongst schizophrenia subjects | 40 male outpatients schizophrenia (DSM-IV criteria) Indian population 18-45 yy |
•Serum CRP levels (cut-off value =N/A) •PANSS total score •Lipid profile |
•Copper, ceruloplasmin, total cholesterol, LDL-Cholesterol and hs-CRP were significantly increased and HDL Cholesterol was significantly reduced in schizophrenia cases when compared with controls •In a subtest, when drug naïve cases (n = 22) were compared with controls, we found that cases showed significantly increased serum copper (21.80±6.27 vs 18.19±5.78, p = 0.026), ceruloplasmin (518.6±137.18 vs 214.9±58.8, p=0.001) and significantly reduced HDL-Cholesterol(0.87±0.14 vs ±1.01 ±0.22, p = 0.009) reflecting the main results. •Total cholesterol significantly correlated with both negative symptom score and general psycho pathology score, while triacylglycerol correlated only with general psycho pathology score in schizophrenia cases. Serum copper and hs-CRP levels did not show any significant correlation with the PANSS scores. In cases, serum copper correlated significantly with hs-CRP (r = 0.338, p = 0.003). |
•No correlation with psychopathology | |||||||||||||||||||||||||||||
| Study | Study Design | Primary and Secondary Outcomes | Characteristics of Participants | Assessments | Main Findings | Correlation CRP and Schizophrenia? | |||||||||||||||||||||||||||||
| [77] | Genetic cross-sectional study | Association between activated immune/inflammation response-related genes and schizophrenia via CRP levels | 19 schizophrenia (DSM-IV criteria) vs 19 HC | •Serum CRP levels (cut-off value =N/A) •RNA extraction from post-mortem at the level of the posterior lateral ventricle from subjects with schizophrenia and HC |
•CRP levels can be increased by infection and/or inflammation •Peripheral stimulus (e.g., viral and/or bacterial) may activate the co-expression module related to immune/inflammation responses in schizophrenics |
•Positive correlation with schizophrenia | |||||||||||||||||||||||||||||
| [78] | Case-control study and meta-analysis of case-control studies | Association between CRP levels and risk of schizophrenia onset | 418 inpatients schizophrenia (DSM-IV criteria) vs 1365 HC Japanese population |
•Serum CRP levels (cut-off value =N/A) •SNP selection and genotyping |
•The presence of higher serum CRP levels in patients with schizophrenia compared to controls in the Japanese population by conducting an ANCOVA with separate genotypes of the 2 SNPs (rs2794520 and rs1183910) identified in the meta-analyses of genome-wide association studies of CRP although the difference between 2 groups in the stratum of rs2794520 CC genotype did not reach statistical significance after the Bonferroni adjustment. | •Positive correlation with psychopathology | |||||||||||||||||||||||||||||
| [79] | Case-control study | Association between oxidative stress and immune dysregulation, CRP levels and schizophrenia and depression | 22 drug-naïve first episode patients with schizophrenia vs 18 drug-naïve first episode patients with major depression vs 43 HC |
• Serum CRP levels (cut-off value =N/A) •EEG •PANSS total score •HAM-D (21-items) •creatinine, glucose, lipids, liver enzymes and a urine drug screen |
•At baseline, 8-iso-PGF2α levels were higher in patients with schizophrenia (p = 0.004) and major depression (p = 0.037), with a trend toward higher SOD concentrations in schizophrenia (p = 0.053). •After treatment, schizophrenia patients showed a further increase in 8-iso-PGF2α (p = 0.016). These results were not related to age, sex, disease severity, medication or adipose tissue mass. •8-iso-PGF2α was associated with smoking, endocrine stress axis activation, CRP levels and low plasma concentrations of brain-derived neurotrophic factor. •Serum hsCRP levels tended to be higher in depressed patients at baseline compared to controls (p = 0.062) and increased in both patient groups following treatment to reach significance only in schizophrenia patients (p= 0.012). |
•Positive correlation with psychopathology | |||||||||||||||||||||||||||||
| [80] | Neuroimaging case-control study | Association with neuroimaging, inflammatory markers and schizophrenia | 28 early-course schizophrenia (DSM-IV criteria) vs 21 HC | •Serum CRP levels (cut-off value =N/A) •MRI data BPRS, SAPS, SANS, Go-No-Go test, WCST, WLMT, 3T Siemens Tim Trio system, peripheral blood sample |
•CRP levels did not correlate with psychopathology or neurocognitive measures •CRP levels did not correlate with positive symptomatology and WCST performance scale |
•No correlation with psychopathology •No correlation with cognitive pattern •No correlation with severity of illness |
|||||||||||||||||||||||||||||
| Study | Study Design | Primary and Secondary Outcomes | Characteristics of Participants | Assessments | Main Findings | Correlation CRP and Schizophrenia? | |||||||||||||||||||||||||||||
| [81] | Case-control study | Association between CRP levels and clinical manifestation, psychopathology and demographic characteristics in clinically acutely agitated schizophrenia subjects | 32 newly admitted schizophrenia subjects (DSM-IV criteria) vs 42 HC 20-65 yy Chinese population |
•Serum CRP levels (cut-off value =N/A) •PANSS total score |
•Serum hsCRP levels are significantly higher in acutely agitated patients with schizophrenia than in comparison subjects (P<0.001, Z=-5.828). •After medical treatment for 2 weeks, serum hsCRP levels were statistically decreased (P<0.001, Z=-3.935). •The reduction of serum hsCRP levels after treatment is consistent with the decrease of PANSS-EC scores of the patients. |
•Positive association with psychopathology •Positive correlation with antipsychotic medication |
|||||||||||||||||||||||||||||
| [82] | Cross-sectional study | Association between physical functional capacity and inflammatory markers in schizophrenia | 40 schizophrenia (DSM-IV criteria) outpatients Brazilian population stable on antipsychotic medications 16-60 yy |
•Serum CRP levels (cut-off value =5 mg/L) •Borg scale •6MWT |
•CRP levels were higher in clinical sample compared to general population •Impaired 6MWD and dyspnea on the Borg scale were correlated to CRP (r= −0.369, p = 0.019 and r = −0.376, p = 0.017 and r = 0.354, p = 0.025 and r = 0.535, p < 0.001, respectively). |
•Positive correlation with psychopathology | |||||||||||||||||||||||||||||
| [83] | Case-control study | Association between exposure to infectious agents, inflammatory markers and schizophrenia vs bipolar disorder vs HC | 28 schizophrenia or schizoaffective disorder (DSM-IV criteria) vs 32 Bipolar disorder (type I or II) outpatients (DSM-IV criteria) vs 60 HC | •Serum CRP levels (cut-off value =N/A) •Serum pentraxin-3 and sCD14 levels •Measurement of the antibody levels of infectious agents (T. Gondii, CMV, HSV-1 and HSV-2) •BACS •PANSS total score |
•No significant differences in the six antibody levels between schizophrenics vs HC •Schizophrenia patients showed a significant increase only in sCD14 (p=0.038) compared with controls. •No significant relationship between the PANSS/BACS scores and the antibody levels or inflammation markers, amongst schizophrenics |
•No correlation with psychopathology •No correlation with cognitive pattern |
|||||||||||||||||||||||||||||
| [84] | Case-control study | Association between inflammatory markers and BDNF genetic variants and schizophrenic phenotype | 44 unrelated outpatients schizophrenia (DSM-IV criteria) vs 50 HC Egyptian population |
•Serum CRP levels (cut-off value =N/A) •PANSS total score |
•Significantly higher CRP levels amongst schizophrenics vs HC | •Positive correlation with psychopathology | |||||||||||||||||||||||||||||
| [85] | Case-control study | Association between classic risk markers of cardiovascular disease and inflammation amongst schizophrenics | 105 schizophrenia inpatients (DSM-IV criteria) vs 148 HC Estonian population Stable antipsychotic medication |
•Serum CRP levels (cut-off value =N/A) •Serum levels of the following elements: TG, LDL-c, HDL-c, HbA1c, CRP, TNF-a, IFN-g, IL-1a, IL-1b, IL-2, IL-4, IL-6, IL-8, IL-10, MCP-1, VEGF and EGF •PANSS total score |
•IL-4 was significantly (P < 0.002) lower among patients. Conversely, the levels of other markers (IL-2, IL-6, IL-8, IFN-g and MCP-1, IL-10, VEGF, EGF) levels were significantly (P < 0.000001 and P < 0.05, respectively) elevated in patients compared to controls. •Higher but not significant CRP levels amongst schizophrenics compared to HC |
•No correlation with psychopathology | |||||||||||||||||||||||||||||
| Study | Study Design | Primary and Secondary Outcomes | Characteristics of Participants | Assessments | Main Findings | Correlation CRP and Schizophrenia? | |||||||||||||||||||||||||||||
| [86] | Population-based cross-cohort study | Association between CRP levels and white blood cells across individuals with schizophrenia, bipolar disorder and depression | 2472 schizophrenia (DSM-IV criteria) outpatients Danish population |
•Serum CRP levels (cut-off value=N/A) •White blood cells count |
•At first diagnosis with severe mental disorder, the median CRP level was 3 mg/L across the mental disorders •Overall CRP levels were higher among inpatients (p=.006), females (p<.001), individuals who had redeemed prescription(s) for antipsychotic drugs (p<.001) and those with somatic comorbidity (p<.001) •CRP levels were higher in the first period of the study and lowest in most recent years (p=.04) •Individuals with elevated CRP levels at baseline had increased rates of all-cause mortality •CRP levels were not associated with subsequent admission at a psychiatric hospital |
•Positive correlation with psychopathology •Positive correlation with mortality |
|||||||||||||||||||||||||||||
| [87] | Prospective birth cohort study | Association between CRP levels assessed at age 15/16 and subsequent hospitalization for schizophrenia and related psychosis until age 27 | 6362 schizophrenia or schizoaffective disorder (DSM-IV criteria) Finnish population 15-16 yy |
•Serum CRP levels (cut-off value =3 mg/L) | •Higher CRP levels at age 15/16 years were associated with female sex, increased BMI, lower maternal education, and increased smoking and alcohol use. •Serum CRP levels at age 15/16 years were associated with risk of schizophrenia by age 27 years. •Serum CRP levels were negatively correlated with age of onset (rs=-0.40; P=0.07), suggesting that higher CRP levels at age 15/16 years were associated with earlier illness onset. •There was no evidence of a sex difference in the association between CRP and schizophrenia. |
•Positive correlation with psychopathology •Positive correlation with a earlier onset of schizophrenia |
|||||||||||||||||||||||||||||
| [88] | Cross-sectional study | Association between brain damage, associated biomarkers, including inflammatory markers (e.g., PCR levels) and schizophrenia and mood disorder | 96 inpatients with schizophrenia (n=70) or mood disorder (n=26) (DSM-IV criteria) Japanese population |
•Serum CRP levels (cut-off value =N/A) |
•In both group of patients, the concentration of CRP (0.07 mg/dl for SZ and 0.14 mg/dl for mood disorders) was higher than that in control subjects (0.03 mg/dl). | •Positive correlation with psychopathology | |||||||||||||||||||||||||||||
| [89] | Case-control study | Association between CRP levels and clinical manifestation, psychopathology of schizophrenia. | 41 schizophrenia inpatients (DSM-IV criteria) vs 40 HC 18-65 yy Drug-free before enrollment |
•Serum CRP levels (cut-off value =N/A) •PANSS total score •MOAS |
•A significantly negative correlations between hsCRP levels and the thought disorder score of PANSS (r = -0.355, p = 0.023), and also between the plasma IL-10 levels and the total and general scores of PANSS (r =-0.325, p =0.038; r =-0.397, p =0.010). •A significantly positive correlation was found between hsCRP/IL-10 and the general score of PANSS (r = 0.393, p = 0.011). •No significant correlation between hsCRP, logIL-10, hsCRP/IL-10 and other variables about the PANSS scores. •Positive correlations were observed between hsCRP and total and verbal aggression score of MOAS (r =0.654, p =0.006; r =0.678, p = 0.015), and also between hsCRP/IL-10 and the total MOAS score (r =0.636, p =0.008). |
•Positive correlation with psychopathology •Positive correlation with aggressiveness |
|||||||||||||||||||||||||||||
| Study | Study Design | Primary and Secondary Outcomes | Characteristics of Participants | Assessments | Main Findings | Correlation CRP and Schizophrenia? | |||||||||||||||||||||||||||||
| [90] | Prospective cohort study | Association between temporal change of cognitive functioning and predictors of cognitive performance with CRP levels in schizophrenia | 132 schizophrenia or schizoaffective disorder (DSM-IV criteria) | •Serum CRP levels (cut-off value =N/A) •RBANS •Selected genetic polymorphisms |
•Immediate Memory and Attention showed modest but statistically significant improvements (gains of 0.89 ± 0.33 and 0.76 ± 0.29 points per year, respectively) •Visuospatial/Constructional performance showed a modest but statistically significant decline (of 0.80±0.25 points per year). •A greater psychiatric symptom severity was associated with worse cognitive performance for most cognitive measures. |
•No correlation with cognitive pattern | |||||||||||||||||||||||||||||
| [91] | Case-control study | Association between a set of proinflammatory markers and general cognitive functioning amongst psychotic subjects | 121 Schizophrenia spectrum disorder vs 111 bipolar spectrum disorder (DSM-IV criteria) vs 241 HC | •Serum CRP levels (cut-off value =N/A) •WAIS •PANSS total score •GAF •YMRS •CDSS •Other serum factors: soluble tumor necrosis factor receptor 1 (sTNF-R1) interleukin 1 receptor antagonist (IL-1Ra), osteoprotegerin, von Willebrand factor, C-reactive protein, interleukin-6 and CD40 ligand |
•Significant negative associations with general cognitive function were found for sTNF-R1 (p=2 × 10−5), IL-1Ra (p=0.002) and sCD40 ligand (p=0.003). •A significant negative association between inflammatory markers and general cognitive abilities after adjusting for possible confounders. |
•No association with psychopathology •No association with cognitive pattern |
|||||||||||||||||||||||||||||
| [92] | Cross-sectional study | Association between CRP levels and sensory gating deficit in schizophrenia | 55 schizophrenia (DSM-IV criteria) stable on antipsychotic medication |
•Serum CRP levels (cut-off value =5 mg/L) •sensory gating assessment with a conditioning-testing P50 procedure •PANSS total score |
•Elevated CRP levels were associated with higher rate of sensory gating deficit (60% vs. 12.5%, p b 0.001) |
•Positive correlation with cognitive pattern •No association with psychopathology •No association with severity of illness |
|||||||||||||||||||||||||||||
| Study | Study Design | Primary and Secondary Outcomes | Characteristics of Participants | Assessments | Main Findings | Correlation CRP and Schizophrenia? | |||||||||||||||||||||||||||||
| [93] | Prospective cohort study | Association between CRP levels and cognitive impairment | 369 schizophrenia or schizoaffective disorder (DSM-IV criteria) outpatients stable on antipsychotic medications |
•Serum CRP levels (cut-off value =3 mg/L) •PANSS total score •YMRS •National Adult Reading Test, Wechsler Adult Intelligence Scale, 3rd Ed., FSIQ, VIQ, PIQ, Letter Number Sequencing, Trail Making Test, California Verbal Learning Test, Doors Test, CPT-IP |
•Abnormal CRP levels, found in 104 patients (28.2%), were associated with impaired General Intellectual Ability and Abstract Reasoning (aOR = 0.56, 95% CI 0.35–0.90, P = .014), independently of age, sex, education level, psychotic symptomatology, treatments, and addiction comorbidities. •Abnormal CRP levels were associated with the decline of all components of working memory (respectively effect size [ES]= 0.25, P= .033; ES= 0.27, P= .04; ES= 0.33, P= .006; and ES= 0.38, P = .004) and a wide range of other impaired cognitive functions, including memory (ES= 0.26, P= .026), learning abilities (ES= 0.28, P= .035), semantic memory (ES= 0.26, P= .026), mental flexibility (ES= 0.26, P= .044), visual attention (ES= 0.23, P = .004) and speed of processing (ES= 0.23, P = .043). |
•Positive correlation with cognitive pattern | |||||||||||||||||||||||||||||
| [94] | RCT | Association between CRP levels and cognitive performance in schizophrenia | 124 schizophrenia (DSM-IV criteria) inpatients and 62 patients were retested at discharge or after 6 weeks at the latest 18-65 yy |
•Serum CRP levels (cut-off value =3 mg/L) •PANSS total score •CDSS •CGI-S •GAF-F •RBANS |
•There was an inverse relationship between overall cognitive performance and CRP level at admittance •The association increased in sub-analyses including only patients with schizophrenia. In cognitive subdomain analyses statistically significant inverse associations were found between the CRP level and Delayed memory and Attention, respectively. •No associations were found between CRP level and other measures of psychopathology including psychosis symptoms, depression, or functioning. •At follow-up the association between CRP level and cognition was no longer present. •There was a significant increase in cognitive performance between baseline and follow-up. •There was a stronger increase in overall cognition scores in patients with higher baseline CRP levels. |
•Positive correlation with cognitive pattern | |||||||||||||||||||||||||||||
HC: healthy controls; IL-10: Interleukin-10; PANSS: Positive and Negative Syndrome Scale; PANSS-EC: PANSS excitement component; CRP: C-reactive protein; hsCRP: high sensitive C-reactive protein; EEG: electroencephalography; HAMD-21: Hamilton Depression Scale (21 items); sCD14: soluble CD14; BACS: Brief Assessment of Cognition in Schizophrenia; MDD: major depression disorder; standard deviation; 6MWT: 6-min walk test; SNP: single-nucleotide polymorphisms; FSIQ: Full Scale IQ; VIQ: Verbal IQ; PIQ: Performance IQ; CPT-IP: The Continuous Performance Test—Identical Pairs; CGI-S: Clinical Global Impression—Severity of Illness scale; GAF-F: Global Assessment of Functioning—Split Version, Functions scale; SAPS: Scale for Assessment of Positive Symptoms; SANS: Scales for Assessment Of Negative Symptoms; D-KEFS: Delis-Kaplan Executive Function System; CIRS: Total Score from the Cumulative Illness Rating Scale; ASCA: Saccharomyces cerevisiae; SCID: Structured Clinical Interview for DSM-IV; BMI: body mass index; FTND 24: Fagerström Test for Nicotine Dependence; AUDIT: Alcohol Use Disorders Identification Test; SQoL: Quality of Life Screening; GAF: General Assessment of Functioning,; YMRS: Young Mania Rating Scale; CDSS Calgary Depression Scale for Schizophrenia; CPT-IP: Continuous Performance Test; WCST: Wisconsin Card Sorting Test; RBANS: Repeatable Battery for the Assessment of Neuropsychological Status classified as deficit or non-deficit; PDS: Proxy for the Deficit Syndrome derived from PANSS scores.
3. Results
Table 1 summarizes all the characteristics of studies here retrieved. Findings have been here dealt with according to the following subcategories: a) Association between CRP levels and schizophrenia and related clinical manifestations and severity of illness; c) Association between CRP levels and cognitive pattern in schizophrenia.
3.1. Studies on Correlation between CRP and Schizophrenia
A cross-sectional study evaluated the association between CRP levels and the clinical manifestation amongst 26 inpatients affected with schizophrenia or schizoaffective disorder, stable on antipsychotic medications [62]. No
differences were found between the normal (e.g.<5 mg/L)/elevated (e.g. >5mg/L) CRP levels of groups regarding general clinical characteristics (e.g., race, age, gender, education level, age of illness onset, history of substance use, smoking status, current antipsychotic medication) (p>.05). Amongst schizophrenic patients with elevated CRP levels, a statistically significant higher PANSS total score, negative symptom subscale score and general psychopathology subscale score have been found compared to normal CRP group (p>.05) [62].
A study investigated the association between the CRP levels and the severity of psychopathology and cognitive impairment amongst 413 schizophrenia patients [57]. No statistically significant association between CRP levels and the PANSS (Positive and Negative Syndrome Scale) total score (p=.386), positive scale score (p=.592), negative scale score (p=.258) or general scale score (p=.627) has been reported. However, the mean RBANS (Repeatable Battery for the Assessment of Neuropsychological Status) cognitive score in the CRP elevated group (i.e., CRP>5 mg/L) was lower than that of the CRP normal level group (i.e., CRP<5 mg/L). No significant association has been reported between CRP levels and the use of a psychotropic medication (p>.05) [57].
A case-control study investigating the association between high-sensitive CRP (hsCRP) levels with clinical phenotype of schizophrenia in a Kuwait Arab population, recruited 165 healthy controls vs 207 schizophrenia patients [63]. A statistically significant association has been found between hsCRP levels and schizophrenia patients clinically categorized as being in remission at the time of assessment (p<.01); whilst subjects with mild-to-moderate and severe illness had lower values. In addition, significantly lower hsCRP levels have been found amongst schizophrenia patients with a family history of psychosis to those without (p<.05). Higher and statistically significant hsCRP levels amongst patients with prominent catatonic features (p=.04) were compared to other clinical manifestation. No significant association between hsCRP and use of antipsychotic medication, current age of patient, gender, disease outcome, presence or absence of negative symptoms or age of onset of disease has been reported [63].
A study recruited 60 bipolar disorder and 63 schizophrenia subjects in order to investigate if there are any differences in CRP levels amongst the groups as well as an association between increased CRP levels and the presence of a metabolic syndrome [64]. No statistically significant differences in mean CRP values have been observed between bipolar and schizophrenia group. However, a statistically significant higher proportion of bipolar patients (around 33%) reported CRP levels higher than 5 mg/L in comparison to the schizophrenia group (22%)(p=.041). In both groups, a significant association between high CRP and metabolic syndrome has been found (p=.031). CRP higher than 5 mg/L represented a predictor for metabolic syndrome both in schizophrenia (OR=2.153) and bipolar disorder group (OR=2.627) [64].
A genetic pilot-study aimed at investigating the association of the CRP rs1417938, rs1800947, rs1205 variants with susceptibility to schizophrenia amongst 208 unrelated Armenians (103 patients and 105 healthy controls). However, no significant differences have been found when the proportions of CRP variants were compared between patients and controls (p>.05) [65].
A study examined the association of serum hsCRP with schizophrenia psychopathology amongst a sample of antipsychotic-free male Egyptian patients with schizophrenia, in order to test if there is any association with psychopathology, severity of psychopathology and hsCRP levels in schizophrenia vs healthy controls [55]. In drug-free male schizophrenic patients, serum CRP levels were higher than in healthy controls. Moreover, the severity of psychopathology seemed to be associated with higher hsCRP levels, particularly negative symptoms [55].
A large sample of individuals affected with schizophrenia (n= 295) was compared with bipolar disorder (n=192) and non-psychiatric controls (n=228) in order to evaluate the association with hsCRP [66]. CRP levels were significantly increased as compared to controls (p=.001). The CRP levels in bipolar disorder individuals were not significantly higher compared to controls (p=.120). Furthermore, schizophrenia individuals showed a significantly increased odd of having elevated levels of CRP (OR=1.79-2.76; p<.001). The odds for elevated CRP in the bipolar disorder group were not significantly elevated. Within the schizophrenia group, the CRP levels were associated with age (p=.001), female gender (p=.05), and BMI (p<.001), but not with PANSS total score, history of drug abuse, cigarette smoking, history of diabetes, education, maternal education, or current receipt of an atypical antipsychotic agent or specific antipsychotic medications including olanzapine, risperidone and clozapine [66].
A case-control Taiwanese study recruiting 36 schizophrenic patients undergoing clozapine, olanzapine or risperidone vs 36 sex-matched healthy controls was conducted, in order to examine the differences in hsCRP levels amongst these groups [67]. A significant increase in the hsCRP levels in the schizophrenic group has been observed compared to healthy controls (p=.013). A significant positive correlation with hsCRP level was found in illness duration (p=.047) and PANSS negative scale score (p=.038) in schizophrenia group. The only significant correlation with hsCRP level was PANSS negative scale score in the clozapine subgroup (p=.043) [67].
A population-based longitudinal birth cohort study evaluated the association between early-life atopic disorders, serum inflammatory markers (IL-6 and CRP) at age 9 years, and the risk of psychotic episodes at age 13 years [68]. Findings did not find an association between serum IL-6 and CRP levels at age 9 years and the risk of psychotic experiences at age 13 years [68].
A study evaluated 78,810 individuals from two large independent general population studies, the Copenhagen General Population Study and the Copenhagen City Heart Study, which followed up subjects for up to 20 years, to evaluate if there is an association between plasma CRP levels between individuals with and without schizophrenia and schizophrenia and schizophrenia-like psychosis combined [69]. A further analysis involved testing their genotypes for 4 single nucleotide polymorphisms (SNPs) in the CRP gene to perform a Mendelian randomization study. Elevated plasma CRP levels were associated with a 6- to 11-fold increased risk of late or very-late-onset schizophrenia [69].
A retrospective, cross-sectional, naturalistic study compared CRP levels between patients affected with schizophrenia, unipolar depression, bipolar depression and bipolar mania [70]. The authors did not find any differences between the groups (i.e. CRP level did not differ between subjects with schizophrenia, unipolar depression and bipolar disorder) [70].
A study recruiting 588 individuals (79 with a recent onset of psychosis, 249 with chronic schizophrenia, not of recent onset, and 260 controls without a history of psychiatric disorder) aimed at evaluating the role of a set of inflammatory markers, including CRP, amongst schizophrenic patients at different phases of illness [71]. Amongst subjects in chronic schizophrenia group and recent onset group, CRP levels were significantly higher than in control group (respectively, p<.001 and p=.003) [71].
A cross-sectional study aimed at investigating the relationship between quality of life and chronic inflammation by using CRP levels in a sample of 256 schizophrenic patients [72]. Amongst schizophrenia subjects recruited, those with high CRP levels (around 39.06% of the sample) were significantly older (p=.027), with a lower education level (p=.021), a longer duration of illness (p=.018), a higher BMI (p=.0) and lower quality of life scores for the SQoL (Schizophrenia Quality of Life) 18 index, the PhW (Physical Well-being) and the SL (Sentimental Life) dimensions (respectively, p=.016, p=.005 and p=.003). A multivariate analysis reported that PsW (Psychological Well-being), PhW and SL were the most salient dimensions of SQoL associated with CRP [72].
A post-hoc analysis based on baseline data coming from a longitudinal study of accelerated aging in schizophrenia, evaluated the relationships of hsCRP levels with demographic characteristics, current psychopathology, cognitive function, physical comorbidity, and metabolic markers amongst schizophrenic patients, compared to healthy subjects without history of a major psychiatric illness [40]. Schizophrenic subjects showed significantly higher hsCRP compared to healthy subjects. Moreover, in the schizophrenia sample, elevated hsCRP levels were correlated with greater medical comorbidity and severity of negative symptoms. Authors did not find a significant association of hsCRP levels with other psychopathology or with executive/cognitive impairment [40].
A population-based longitudinal cohort study recruited approximately 4500 participants at the age of 9 years aimed to test the hypothesis that higher serum hsCRP levels in childhood would increase future risks for the onset of depression and/or psychosis [73]. There was no evidence of an association between psychiatric outcomes and CRP levels [73].
A cross-sectional study enrolled outpatients affected with schizophrenia and healthy controls, by collecting simultaneously, at the time of the study visit, blood samples in order to measure CRP and 25-hydroxyvitamin [25(OH)D], to evaluate the relationship between vitamin D levels and risk of schizophrenia [74]. Patients with schizophrenia had significantly higher CRP and lower 25(OH)D compared to controls. Elevated levels of CRP or decreasing levels of 25(OH)D were independently associated with increased risk of schizophrenia. Findings suggested that high levels of vitamin D may be linked to reduced risk of schizophrenia with elevated CRP [74].
A retrospective cohort study identified and recruited 213 schizophrenic inpatients without affective symptoms according to their CRP levels at admission, by classifying them in two groups (elevated CRP>1 mg/L; normal CRP<1 mg/L) in order to investigate the association, if any, between aggressive behaviour and inflammatory markers [75]. CRP levels were significantly correlated with other inflammatory markers, i.e. leukocyte count and neutrophil to lymphocyte ratio (p<.0001). Higher CRP levels were significantly associated with aggressive behaviour compared to patients with normal CRP levels. Elevated CRP levels have not been associated with suicidal ideation and/or behaviour [75].
A case-control study, carried out in India, evaluated serum levels of copper, CRP and lipid profile and their association with psychopathology scores in drug-free schizophrenia patients [76]. Forty male (22 drug naïve and 18 drug free) schizophrenic patients and forty controls were enrolled. Copper, highly sensitive-CRP (hsCRP), total cholesterol and LDL-cholesterol were significantly increased and HDL-Cholesterol was significantly reduced in schizophrenia, compared to controls. However, serum copper and hsCRP levels did not show any significant correlation with the PANSS scores [76].
A genetic sequencing study analysed post-mortem cerebrospinal fluid of 20 schizophrenic patients and 26 healthy controls, in order to sequence mRNA, markers of immune response and inflammation [77]. CRP levels were positively correlated with the immune/inflammation-related co-expression module and several immune modulator proteins in the serum of schizophrenic individuals [77].
A case-control study and a meta-analysis of case-control studies between schizophrenia and serum CRP levels have been carried out to evaluate the causal association, if any, between CRP levels and the schizophrenia risk [78]. Four hundred and eighteen patients with schizophrenia and 1365 healthy controls were recruited in Japan. Findings reported that serum CRP levels were significantly higher in schizophrenic patients compared to controls. Moreover, a causal association between elevated CRP levels and increased schizophrenia risk has been demonstrated [78].
A set of oxidative stress biomarkers [e.g., urinary 8-iso-prostaglan- din F2α (8-iso-PGF2α), 8-OH-2-deoyxguanosine (8-OH- 2-dG), and blood levels of malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione S-transferase (GST), CRP] were assessed in drug-naïve first episode schizophrenic patients (n=22), major depression (n=18) and healthy controls (n=43) [79]. Serum hsCRP levels tended to be higher in depressed patients at baseline compared to controls (p=.062) and increased in both patient groups following treatment to reach significance only in schizophrenic patients (p=.012). Moreover, a positive correlation has been found between 8-iso-PGF2alfa with serum hsCRP, by underlining the potential link between oxidative stress and a proinflammatory state in psychiatric patients [79].
A neuroimaging study enrolled 28 early-course schizophrenia subjects and 21 healthy controls, by acquiring whole-brain multi-voxel, in vivo 31P MRS data in 3-dimensions from 12 brain regions, interleukin-6 and CRP serum levels, to determine membrane phospholipid (MPL) metabolites, cognitive, clinical and immunological correlates in schizophrenia [80]. Findings reported an increased level of MPL catabolite in the thalamus of schizophrenic patients, no differences in IL-6 and CRP levels between cases and controls. Furthermore, CRP levels did not correlate with psychopathology or neurocognitive measures; positive symptom severity and WCST performance did not correlate with CRP levels [80].
Thirty-two newly admitted acutely agitated schizophrenic patients were randomly recruited and compared to 42 healthy controls in a case-control study aiming at assessing hsCRP levels [81]. The authors reported significantly higher serum hsCRP levels in acutely agitated schizophrenic patients vs healthy controls (p<.001). Moreover, serum hsCRP significantly decreases after acute agitation is controlled by administration of anti-psychiatric medications for 2 weeks [81].
A cross-sectional study recruiting 40 schizophrenic patients stable with antipsychotic medication, evaluated their physical functional capacity (assess by the 6-min walk-test [6MWT]) and inflammatory markers, including CRP levels and Von Willebrand factor [82]. 6MWT and dyspnea in Borg Scale of Perceived Exertion scores (BSPE) were positively associated with CRP (p=.019 and p=.017, respectively). Elevated CRP levels were associated with measures of functional impairment amongst schizophrenics [82].
A study measured the antibody levels of some infectious agents and a pool of inflammatory markers (e.g., CRP, pentraxin-3 and soluble CD14) on a sample of outpatients affected with schizophrenics (n=28), bipolar disorder (n=32) vs healthy controls [83]. Amongst the three inflammatory markers here assessed, schizophrenic patients showed a significant increase only in sCD14. No differences were reported in CRP levels. Whilst bipolar disorder patients showed a significant increase in both CRP (p<.001) and sCD14 (p<.001) compared to controls [83].
Forty-four unrelated schizophrenic Egyptian SZ patients and 50 healthy controls, who were admitted to the outpatient clinics of the Neuropsychiatry Department in Cairo, were enrolled in order to investigate whether oxidative stress, inflammatory and immune activation markers may be implicated in schizophrenia pathogenesis, including CRP serum levels [84]. Schizophrenic patients showed significantly higher CRP levels compared to controls. Moreover, CRP appeared to better discriminate, compared to other immunological markers, between a schizophrenic patient and a healthy control [84].
A study recruited 105 schizophrenia patients with a stable antipsychotic medication regimen and a stable clinical status and 148 healthy controls, in order to assess some inflammatory, including CRP levels, and growth factors in their blood serum samples [85]. Findings reported higher (but not significant) CRP levels, together with triglycerides as well as high density (HDL) and low-density (LDL) lipoprotein cholesterol levels amongst schizophrenia patients compared to healthy control (p=.40) [85].
A large population-based cross-cohort Danish study aimed at evaluating the relationship between CRP levels and white blood cells across individuals affected with schizophrenia, bipolar depression and depression, and, also investigating the subsequent psychiatric admission and mortality [86]. Baseline median CRP differed significantly between mental disorders (p=.01) being highest in individuals with bipolar disorder (particularly during the manic phase) and schizophrenia. Elevated CRP levels appeared to be associated with increased all-cause mortality. No association has been reported between CRP levels and subsequent psychiatric admission [86].
A prospective bird cohort study assessed CRP in adolescence and subsequent risk of schizophrenia and related psychoses in the Northern Finland Birth Cohort 1986 [87]. The study reported that adolescents with higher hsCRP levels (<3 mg/L) compared to those with lower CRP (<1 mg/L) were more likely to develop schizophrenia (OR=4.25). Moreover, the authors suggested that probably higher CRP level may be associated with an earlier onset of schizophrenia (p=.07) [87].
A cross-sectional study evaluating the correlation between brain damage, associated biomarkers and medication, recruited 96 inpatients (70 schizophrenic and 26 affected with mood disorders) in Japan [88]. CRP levels were higher in plasma of both schizophrenic (0.07 mg/dl) and mood disorder (0.14 mg/dl) patients, compared to healthy controls (0.03 mg/dl) [88].
Forty-one schizophrenic patients who were hospitalized in Shanghai Mental Health Center, were enrolled in a study investigating the correlation between high-sensitivity CRP (hsCRP), interleukin-10 (IL-10) and clinical characteristics, particularly aggression, and their role as potential plasma biomarkers of schizophrenia [89]. Statistically significant higher levels of hsCRP (p<.001) and higher ratio of hsCRP and IL-10 were observed in the plasma of patients affected with schizophrenia, compared to healthy controls. Furthermore, a positive correlation has been observed between hsCRP and the total score and verbal aggression score of the modified Overt Aggression Scale (MOAS) (p<.01) and between hsCRP/IL-10 and the total score of MOAS (p<.01) [89].
3.2. Studies on Correlation between CRP Levels and Cognitive Pattern in Schizophrenia
The study abovementioned by Dickerson et al. [57] already reported an inverse correlation between the mean RBANS cognitive score and CRP levels [57]. Furthermore, the abovementioned neuroimaging study by Prasad et al. [80] did not report any correlation between CRP levels and WCST and cognitive pattern amongst schizophrenics enrolled.
A prospective longitudinal cohort study evaluated the temporal change of cognitive functioning and the predictors of cognitive performance in relation with CRP levels, in a sample of schizophrenia or schizoaffective disorder patients [90]. No significant associations have been found with CRP levels [90].
A case-control study aimed at determining how a set of proinflammatory markers, including CRP, may be related to general cognitive abilities, recruited 226 patients (121 affected with a schizophrenia spectrum disorder, e.g., 93 with schizophrenia, 8 with a schizophreniform disorder and 20 with a schizoaffective disorder; and 111 with a bipolar disorder spectrum disorder, e.g., 65 bipolar type I disorder, 40 bipolar type II disorder and 6 not otherwise specified patients) vs 241 healthy controls [91]. Findings did not report any association between hsCRP and symptom severity either with general cognitive abilities. The authors stated that a possible explanation for the lack of association may be due to a low detection limit of hsCRP which may have reduced probability to find a significant association. Moreover, the study supports that systemic inflammation markers are negatively associated with general cognitive abilities and show that some inflammatory markers are associated with cognitive function in schizophrenia and bipolar disorder subjects, even though there is not any association with hsCRP [91].
A study investigated the association between CRP and sensory gating deficit (e.g., abnormal P50 suppression, assessed with an auditory event-related potential method) amongst a sample of 55 outpatients affected with schizophrenia [92]. An elevated CRP (i.e., > 5 mg/L) was associated with a sensory gating deficit and a lower executive function performance assessed with the Stroop Interference test. Furthermore, no association has been found between CRP levels and severity of psychiatric symptoms [92].
A cohort study consecutively recruited 369 community-dwelling stable patients affected with schizophrenia or schizoaffective disorder, in order to investigate cognitive impairment associated with abnormal CRP levels, using a comprehensive neuropsychological battery [93]. Abnormal CRP levels (i.e, > 3 mg/L) were associated with impaired General Intellectual Ability and Abstract Reasoning (p=.014), independently of psychotic symptomatology, treatments and addiction comorbidities. Moreover, a decline in all components of working memory, memory, learning abilities, semantic memory, mental flexibility, attention and speed of processing has been as well demonstrated to be associated with abnormal CRP levels amongst schizophrenic patients [93].
A study consecutively recruited 124 schizophrenic patients who were evaluated for their serum CRP level measurements and cognitive assessments at admittance to hospital; whilst 62 patients were retested at discharge or after 6 weeks at the latest, through the measurement of CRP levels and alternative forms of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) [94]. Findings reported an inverse relationship between overall cognitive performance and CRP levels at admittance; no associations have been found between CRP levels and other measures of psychopathology including psychotic symptoms, depression or functioning. Whilst at the follow up, the association between CRP levels and cognition was no longer confirmed. The authors stated that inflammatory state may be a state-dependent marker of cognitive dysfunction in acute psychosis [94].
4. Discussion
Overall, an immune dysfunction and inflammation have been described amongst patients affected with schizophrenia [31-32, 95-97]. Furthermore, many immune-related diseases/problems, such as autoimmune thyroid diseases, cardiovascular disease, obesity, diabetes, hypertension, smoking, metabolic syndrome and infections have been found comorbidly with schizophrenia [98-99]. Patients affected with schizophrenia show significant inflammatory marker alterations [100-102]. Findings reported that some antipsychotic medications altered levels of cytokines and other inflammatory markers in schizophrenic patients [103]. Therefore, the interest in immune/inflammatory changes and their associated oxidative consequences as a potential determinant/key in the pathophysiology of schizophrenia has been recently restored [104, 105]. The latest evidence supports a model which describes that the onset of oxidative stress stimuli and a consequent immune dysfunction may alter cellular homeostasis which, in turn, may determine an aberrant growth of interneurons and, consequently, a psychotic symptomatology [106]. Parvalbumin interneurons (PVIs), particularly through the hypofunction of N-methyl-D-aspartate receptors (NMDARs), are involved in the schizophrenic development [107]. They are fast-spiking GABAergic neurons, involved during the synchronization of firing of pyramidal neurons in cortex and during the generation of gamma oscillations linked to higher-order cognitive processes (e.g., the working memory) which have been found interrupted amongst schizophrenics [108]. Moreover, NMDAR disruption could be prior to the well-known hyperactivity of dopamine (D2) receptors, at the level of mesolimbic and mesocortical projections [109]. Whilst the connection between the PVI disruption and the mesolimbic dopaminergic dysfunction has not been well established yet, the current scientific knowledge demonstrated that PVI disruption, through the loss of pyramidal cell inhibition in hippocampal subiculum, may cause dopamine dysfunction [110]. In addition, correct PVIs functioning depends on the antioxidant and inflammatory/anti-inflammatory activity [111]. Reactive oxygen species (ROS) are produced in the mitochondrial cells, during aerobic metabolism, and they are involved in the aging and in the onset of several diseases as ROS can chemically alter cellular components (e.g., lipids and proteins) [111]. In general, endogenous antioxidants, such as glutathione (GSH), nullify ROS preventing cellular damage [112]. Otherwise, when ROS are not controlled by antioxidants, they can damage macromolecules (DNA, proteins, fats) and alter normal cell signalling pathways [113], hence, by determining structural and functional alterations [114]. Therefore, oxidative and inflammatory pathways are strictly interconnected to each other, i.e. oxidative stresses induce inflammation through Nuclear Factor-κB (NF-κB) via activation, and consequently, determine the production of further free radicals [115, 116].
In general, the immunological response, and the consequent cytokines release, respond to different stimuli and the “sensitization” or “kindling” occurs when these are repetitive, by generating a sort of memory function of the immune system [117, 118]. A recent systematic review [119], involving population-based studies, described how a prenatal maternal infection [73, 120] may be a predisposing factor responsible in raising inflammatory markers during pregnancy, which in turn may increase the risk to develop a schizophrenia or a psychotic disorder in adolescence [121]. Prenatal immune activation, linked or not to infections, has been highlighted as an important risk factor determining the schizophrenia occurrence [122]. In animal models, infective (bacterial or viral) stimulation of maternal immune system during pregnancy develops typical schizophrenia/psychotic symptoms in the offsprings [122, 123]. The same phenomenon has been observed in humans as well, as the maternal infection during pregnancy acts on the foetus brain development [124-126]. In fact, increased maternal levels of cytokine Interleukin-8 (IL-8) during pregnancy hace been associated to an increased risk for schizophrenia [125] and a decreased brain volume amongst the new-borns (lower volumes of the right posterior cingulum and left entorhinal cortex and higher volumes of the ventricles) [127]. The attention was focused on maternal respiratory infections [128, 129], genital infections and reproductive tract infections [129, 130].
As the abovementioned evidence suggested an inflammation trajectory in the pathophysiology of schizophrenia, current research tried to identify specific inflammatory markers for schizophrenia, able to be considered pathognomonic biomarkers amongst schizophrenics. Identifying a specific inflammatory pattern amongst schizophrenic patients may allow the development of new pharmacological targets for schizophrenia. To date, various interesting findings have been described, such as higher serum levels of tumor necrosis factor alpha (TNF-alpha), interleukin-6 (IL-6), interleukin-2 (IL-2) and CRP associated with the ‘schizophrenic state’ [131-134]. Amongst these, CRP, a nonspecific plasma protein marker, produced by the liver as part of the acute-phase response in both acute and chronic inflammatory conditions, seems to play a significant role in the immune response in schizophrenia [135]. CRP is direct, sensitive, quantitative measure of the overall acute phase response and easy to be detected through blood sample. In fact, currently, highly sensitive assays are available for the measurement of relatively small serum concentrations of CRP (hsCRP), which may be useful for laboratory assessment amongst schizophrenia subjects. Furthermore, CRP levels begin to rise within 4-6 hours after tissue injury/infection and continue to increase exponentially, doubling every 8-9 hours and peaking at several hundred folds within 24-48 hours.
The present systematic review reported that most studies here retrieved demonstrated that elevated serum CRP levels are correlated with schizophrenia and are associated with a more severe schizophrenic symptomatology [40, 55, 62-63, 66-69, 71-72, 77-79, 81, 84, 86-88, 95, 97, 134]; whilst other studies did not confirm these findings [57, 70-71, 74, 76, 77, 80, 83, 85, 91-92]. Specifically, hsCRP levels are increased in stabilized schizophrenic patients and during the remission and chronic phase of illness [63, 71, 81], are increased in acutely agitated schizophrenics and decreased after acute agitation is controlled by administration of antipsychotics [81]. Moreover, increased CRP levels have been reported amongst schizophrenics with catatonic features associated to the illness [63], with a comorbid metabolic syndrome [40, 64], in acutely agitated schizophrenics with an increased cardiovascular risk [81], negative symptomatology [55, 67], with a low quality of life [72], a greater physical functional impairment [82], and a higher mortality [86], amongst schizophrenia subjects. Furthermore, an association has been as well reported between elevated CRP levels and more aggressive schizophrenics [77, 89], but not with suicidal behaviour [77].
Overall, CRP levels seemed to increase the general risk of late or very-late-onset schizophrenia [69] or to be associated with an earlier onset of schizophrenia [87]. However, a recent large-scale cross-consortium Mendelian randomization study used two genetic risk scores (GRSs), i.e. the first one consisting of four single nucleotide polymorphisms (SNPs) in the CRP gene; whilst the second one constituted 18 SNPs which were significantly associated with CRP levels in the largest genome-wide association study (GWAS), in order to determine if CRP could be a protective or risk factor to schizophrenia [136]. The authors reported indeed that genetically elevated CRP levels showed a significant potentially protective causal relationship with the risk of schizophrenia, by disconfirming previous hypotheses [136].
Cognitive symptomatology has been considered to own a higher impact on the outcomes of schizophrenics. Regarding the cognitive pattern amongst schizophrenics, a positive correlation has been reported between CRP levels and cognitive functioning in individuals with schizophrenia [57, 92-94], particularly an elevated CRP level was associated with a sensory gating deficit and a lower executive function performance assessed with the Stroop Interference test [92]. Moreover, increased CRP levels were associated with impaired General Intellectual Ability and Abstract Reasoning, independently of psychotic symptomatology, treatments and addiction comorbidities [93]. Working memory, memory, learning abilities, semantic memory, mental flexibility, attention and speed of processing worsened with increasing CRP levels [93]. Whilst other studies did not demonstrate any correlation with cognitive functioning [40, 80, 83, 90, 91].
However, there are several major limitations in the present review that should be acknowledged. First, most studies own methodological dis-homogeneities, such as inclusion/exclusion criteria (not all studies included only schizophrenic patients), different sample size (some of them included a small sample size with different clinical features) [40, 62, 64, 67, 71, 76, 77, 79, 80-84, 89, 92], various recruitment settings (i.e., inpatients, outpatients, community-based sample, etc.) and study design (Table 1). Furthermore, from a clinical point of view, studies here retrieved showed different lengths/courses of schizophrenia, different phases of illness (i.e., acutely agitated patients, acutely not-agitated patients, chronic and stable patients, early-onset patients, late-onset patients, etc.), different clinical subtypes of schizophrenia, however the antipsychotic medication has not been always reported (i.e., patients with stable antipsychotic medication, patients with not stable antipsychotic medication, free-drug patients, not specified antipsychotic medication, etc.) [64, 66, 68, 70, 73, 79, 91]. Regarding the sample, a dis-homogeneous sample has been reported of schizophrenic patients recruited in terms of different socio-demographic characteristics of sample (i.e., sex, gender, race, etc.); some studies specifically recruited some ethnic groups, such as Arabics [63], Armenians [65], Egyptians [55, 84], Taiwanese [67], Indians [76], Japanese [78, 88], Chinese [81], Brazilian [82], Estonian [85], Danish [86], Finnish [87], which may influence the findings here retrieved. Therefore, the above-mentioned limitations greatly limit the generalizability of findings here reported and have limited the possibility to carry out a clinically significant meta-analysis.
Moreover, one should argue that the duration of schizophrenia illness as well as the different phases and course of schizophrenia may influence the findings of studies here considered. In fact, some studies recruited acute patients [81, 94] whilst others assessed chronic and medication-stable patients [71]. Findings support the hypothesis of inflammatory and immune involvement of acute agitation in patients with schizophrenia, by suggesting that hsCRP may be a potential indicator in the prompt diagnosis of acute agitation amongst schizophrenics [81]. However, the role of CRP in the pathogenesis of acute agitation and acute recrudescence in schizophrenia still needs further studies.
Furthermore, other potential bias limiting the interpretation of findings here presented, may be represented by the influence that specific antipsychotic medications may have on the inflammatory process and immune function amongst schizophrenic patients [97]. Some studies specifically recruited participants stable on antipsychotic medications [62, 63, 64, 67, 72, 82, 85, 92, 93], while others, without any medication [55, 79, 89]. More studies specifically focused on the influence of an antipsychotic as well as an antidepressant medication on the inflammatory markers of schizophrenia are needed in order to evaluate how a psychopharmacological treatment may be more efficacious in attenuating schizophrenic symptomatology based on their specific effect on the inflammation associated to the illness [98, 99, 137, 138]. Furthermore, as recently discussed, schizophrenia research should as well consider the relationship between other psychopathological dimensions, e.g., the alexithymia, and the acute phase proteins and cytokines, amongst the schizophrenia patients, in order to evaluate how inflammatory markers may specifically influence other clinical dimensions of schizophrenia [139].
Alexithymia and its relationships with acute phase proteins and cytokine release: an updated review.
conclusion
In conclusion, even though it is still unclear if inflammation may be a by-product of the pathophysiology of schizophrenia or may directly contribute to the clinical manifestations of schizophrenia, findings here retrieved support the hypothesis of a potential role of CRP and its blood levels as biomarker in schizophrenia. Further longitudinal studies (based on an ethnically homogeneous and geographically defined region) taking into account: a) the different illness phases (i.e., early-stage, chronic state, acute recrudescence, remission phase, etc.); b) the different clinical subtypes and manifestations (including aggressiveness, violent behaviour, suicide ideation and behaviour, cognitive impairment, and so on); and, c) the role of antipsychotic medications in influencing the inflammatory state in schizophrenics, are needed in order to better understand if the inflammation pathways may be useful targets for new interventional strategies in schizophrenia as well as if some inflammatory markers (such as CRP) may be considered an useful biomarker.
Consent for Publication
Not applicable.
Acknowledgements
Declared none.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.MacDonald A.W., Schulz S.C. What we know: findings that every theory of schizophrenia should explain. Schizophr. Bull. 2009;35(3):493–508. doi: 10.1093/schbul/sbp017. [http://dx.doi.org/10.1093/schbul/sbp017]. [PMID: 19329559]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saha S., Chant D., Welham J., McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2(5):e141. doi: 10.1371/journal.pmed.0020141. [http://dx.doi.org/10.1371/journal.pmed.0020141]. [PMID: 15916472]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickerson F., Stallings C., Origoni A., Schroeder J., Khushalani S., Yolken R. Mortality in schizophrenia: clinical and serological predictors. Schizophr. Bull. 2014;40(4):796–803. doi: 10.1093/schbul/sbt113. [http://dx. doi.org/10.1093/schbul/sbt113]. [PMID: 23943410]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirkpatrick B., Messias E., Harvey P.D., Fernandez-Egea E., Bowie C.R. Is schizophrenia a syndrome of accelerated aging? Schizophr. Bull. 2008;34(6):1024–1032. doi: 10.1093/schbul/sbm140. [http://dx.doi.org/10. 1093/schbul/sbm140]. [PMID: 18156637]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hjorthøj C., Stürup A.E., McGrath J.J., Nordentoft M. Years of potential life lost and life expectancy in schizophrenia: A systematic review and meta-analysis. Lancet Psychiatry. 2017;4(4):295–301. doi: 10.1016/S2215-0366(17)30078-0. [http://dx.doi.org/10.1016/S2215-0366(17)30078-0]. [PMID: 28237639]. [DOI] [PubMed] [Google Scholar]
- 6.Laursen T.M., Munk-Olsen T., Vestergaard M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr. Opin. Psychiatry. 2012;25(2):83–88. doi: 10.1097/YCO.0b013e32835035ca. [http://dx.doi.org/10.1097/ YCO.0b013e32835035ca]. [DOI] [PubMed] [Google Scholar]
- 7.Stubbs B., Vancampfort D., De Hert M., Mitchell A.J. The prevalence and predictors of type two diabetes mellitus in people with schizophrenia: a systematic review and compara-tive meta-analysis. Acta Psychiatr. Scand. 2015;132(2):144–157. doi: 10.1111/acps.12439. [http://dx. doi.org/10.1111/acps.12439]. [DOI] [PubMed] [Google Scholar]
- 8.Suvisaari J., Keinänen J., Eskelinen S., Mantere O. Diabetes and Schizophrenia. Curr. Diab. Rep. 2016;16(2):16. doi: 10.1007/s11892-015-0704-4. [http://dx.doi. org/10.1007/s11892-015-0704-4]. [PMID: 26803652]. [DOI] [PubMed] [Google Scholar]
- 9.Rummel-Kluge C., Komossa K., Schwarz S., Hunger H., Schmid F., Lobos C.A., Kissling W., Davis J.M., Leucht S. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr. Res. 2010;123(2-3):225–233. doi: 10.1016/j.schres.2010.07.012. [http://dx.doi.org/10.1016/j.schres.2010.07.012]. [PMID: 20692814]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young S.L., Taylor M., Lawrie S.M. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J. Psychopharmacol. (Oxford) 2015;29(4):353–362. doi: 10.1177/0269881114562090. [http://dx.doi.org/10.1177/0269881114562090]. [PMID: 25516373]. [DOI] [PubMed] [Google Scholar]
- 11.Leung J.G., Hasassri M.E., Barreto J.N., Nelson S., Morgan R.J. III characterization of admission types in medically hospitalized patients prescribed clozapine. Psychosomatics. 2017;58(2):164–172. doi: 10.1016/j.psym.2016.11.013. [http://dx.doi.org/10.1016/j.psym.2016.11.013]. [PMID: 28153339]. [DOI] [PubMed] [Google Scholar]
- 12.Poirier M.F. Sensitivity to psychotropic drugs in schizophrenia. 2002. [PubMed]
- 13.Hjorthøj C., Østergaard M.L., Benros M.E., Toftdahl N.G., Erlangsen A., Andersen J.T., Nordentoft M. Association between alcohol and substance use disorders and all-cause and cause-specific mortality in schizophrenia, bipolar disorder, and unipolar depression: a nationwide, prospective, register-based study. Lancet Psychiatry. 2015;2(9):801–808. doi: 10.1016/S2215-0366(15)00207-2. [http://dx.doi.org/10.1016/S2215-0366(15)00207-2]. [PMID: 26277044]. [DOI] [PubMed] [Google Scholar]
- 14.Toftdahl N.G., Nordentoft M., Hjorthøj C. Prevalence of substance use disorders in psychiatric patients: a nationwide Danish population-based study. Soc. Psychiatry Psychiatr. Epidemiol. 2016;51(1):129–140. doi: 10.1007/s00127-015-1104-4. [http://dx.doi.org/10.1007/s00127-015-1104-4]. [PMID: 26260950]. [DOI] [PubMed] [Google Scholar]
- 15.Stubbs B., Williams J., Gaughran F., Craig T. How sedentary are people with psychosis? A systematic re-view and meta-analysis. Schizophr. Res. 2016;171(1-3):103–109. doi: 10.1016/j.schres.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 16.Dipasquale S., Pariante C.M., Dazzan P., Aguglia E., McGuire P., Mondelli V. The dietary pattern of patients with schizophrenia: a systematic review. J. Psychiatr. Res. 2013;47(2):197–207. doi: 10.1016/j.jpsychires.2012.10.005. [http:// dx.doi.org/10.1016/j.jpsychires.2012.10.005]. [PMID: 23153955]. [DOI] [PubMed] [Google Scholar]
- 17.Fleury M.J., Grenier G., Bamvita J.M., Caron J. Professional service utilisation among patients with severe mental disorders. BMC Health Serv. Res. 2010;10:141. doi: 10.1186/1472-6963-10-141. [http://dx.doi.org/10.1186/ 1472-6963-10-141]. [PMID: 20507597]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nordentoft M., Madsen T., Fedyszyn I. Suicidal behavior and mortality in first-episode psychosis. J. Nerv. Ment. Dis. 2015;203(5):387–392. doi: 10.1097/NMD.0000000000000296. [http://dx.doi.org/10.1097/NMD.0000000000000296]. [PMID: 25919385]. [DOI] [PubMed] [Google Scholar]
- 19.Andreassen O.A., Djurovic S., Thompson W.K., Schork A.J., Kendler K.S., O’Donovan M.C., Rujescu D., Werge T., van de Bunt M., Morris A.P., McCarthy M.I., Roddey J.C., McEvoy L.K., Desikan R.S., Dale A.M. Improved detection of common variants associated with schizophrenia by leveraging pleiotropy with cardiovascular-disease risk factors. Am. J. Hum. Genet. 2013;92(2):197–209. doi: 10.1016/j.ajhg.2013.01.001. [http://dx.doi.org/10.1016/j.ajhg.2013.01.001]. [PMID: 23375658]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malan-Müller S., Kilian S., van den Heuvel L.L., Bardien S., Asmal L., Warnich L., Emsley R.A., Hemmings S.M., Seedat S. A systematic review of genetic variants associated with metabolic syndrome in patients with schizophrenia. Schizophr. Res. 2016;170(1):1–17. doi: 10.1016/j.schres.2015.11.011. [http://dx.doi.org/10.1016/j.schres.2015.11.011]. [PMID: 26621002]. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan P.F., Kendler K.S., Neale M.C. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch. Gen. Psychiatry. 2003;60(12):1187–1192. doi: 10.1001/archpsyc.60.12.1187. [http://dx.doi.org/10. 1001/archpsyc.60.12.1187]. [PMID: 14662550]. [DOI] [PubMed] [Google Scholar]
- 22.Javidfar B., Park R., Kassim B.S., Bicks L.K., Akbarian S. The epigenomics of schizophrenia, in the mouse. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2017;174(6):631–640. doi: 10.1002/ajmg.b.32566. [http://dx.doi.org/ 10.1002/ajmg.b.32566]. [PMID: 28699694]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van de Leemput J., Hess J.L., Glatt S.J., Tsuang M.T. Genetics of schizophrenia: Historical insights and prevailing evidence. Adv. Genet. 2016;96:99–141. doi: 10.1016/bs.adgen.2016.08.001. [http://dx.doi.org/10.1016/bs.adgen.2016. 08.001]. [PMID: 27968732]. [DOI] [PubMed] [Google Scholar]
- 24.Gejman P.V., Sanders A.R., Duan J. The role of genetics in the etiology of schizophrenia. Psychiatr. Clin. North Am. 2010;33(1):35–66. doi: 10.1016/j.psc.2009.12.003. [http://dx.doi.org/10.1016/j.psc.2009.12.003]. [PMID: 20159339]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castellani C.A., Melka M.G., Gui J.L., O’Reilly R.L., Singh S.M. Integration of DNA sequence and DNA methylation changes in monozygotic twin pairs discordant for schizophrenia. Schizophr. Res. 2015;169(1-3):433–440. doi: 10.1016/j.schres.2015.09.021. [http://dx.doi.org/10.1016/j.schres. 2015.09.021]. [PMID: 26441003]. [DOI] [PubMed] [Google Scholar]
- 26.Akbarian S., Huang H.S. Epigenetic regulation in human brain-focus on histone lysine methylation. Biol. Psychiatry. 2009;65(3):198–203. doi: 10.1016/j.biopsych.2008.08.015. [http://dx.doi.org/10.1016/j.biopsych.2008.08.015]. [PMID: 18814864]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alelú-Paz R., González-Corpas A., Ashour N., Escanilla A., Monje A., Guerrero M.C., Algora Weber M., Ropero S. DNA methylation pattern of gene promoters of major neurotransmitter systems in older patients with schizophrenia with severe and mild cognitive impairment. Int. J. Geriatr. Psychiatry. 2015;30(6):558–565. doi: 10.1002/gps.4182. [http://dx.doi.org/10.1002/gps.4182]. [PMID: 25044034]. [DOI] [PubMed] [Google Scholar]
- 28.van Os J., Kenis G., Rutten B.P. The environment and schizophrenia. Nature. 2010;468(7321):203–212. doi: 10.1038/nature09563. [http://dx.doi.org/10. 1038/nature09563]. [PMID: 21068828]. [DOI] [PubMed] [Google Scholar]
- 29.Miller B.J., Buckley P., Seabolt W., Mellor A., Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol. Psychiatry. 2011;70(7):663–671. doi: 10.1016/j.biopsych.2011.04.013. [http://dx.doi.org/10.1016/j.biopsych.2011.04.013]. [PMID: 21641581]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedrini M., Massuda R., Fries G.R., de Bittencourt Pasquali M.A., Schnorr C.E., Moreira J.C.F., Teixeira A.L., Lobato M.I., Walz J.C., Belmonte-de-Abreu P.S. Kauer-Sant’Anna, M.; Kapczinski, F.; Gama, C.S. Similarities in serum oxidative stress markers and inflammatory cytokines in patients with overt schizophrenia at early and late stages of chronicity. J. Psychiatr. Res. 2012;46(6):819–824. doi: 10.1016/j.jpsychires.2012.03.019. [http://dx.doi.org/10.1016/j.jpsychires.2012. 03.019]. [PMID: 22520512]. [DOI] [PubMed] [Google Scholar]
- 31.Na K.S., Jung H.Y., Kim Y.K. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;48:277–286. doi: 10.1016/j.pnpbp.2012.10.022. [http://dx.doi.org/10.1016/j.pnpbp.2012.10.022]. [PMID: 23123365]. [DOI] [PubMed] [Google Scholar]
- 32.Bechter K. Updating the mild encephalitis hypothesis of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;42:71–91. doi: 10.1016/j.pnpbp.2012.06.019. [http://dx.doi.org/10.1016/j.pnpbp.2012.06.019]. [PMID: 22765923]. [DOI] [PubMed] [Google Scholar]
- 33.Singh B., Chaudhuri T.K. Role of C-reactive protein in schizophrenia: an overview. Psychiatry Res. 2014;216(2):277–285. doi: 10.1016/j.psychres.2014.02.004. [http:// dx.doi.org/10.1016/j.psychres.2014.02.004]. [PMID: 24565000]. [DOI] [PubMed] [Google Scholar]
- 34.Miller B.J., Culpepper N., Rapaport M.H. C-reactive protein levels in schizophrenia: a review and meta-analysis. Clin. Schizophr. Relat. Psychoses. 2014;7(4):223–230. doi: 10.3371/CSRP.MICU.020813. [http://dx.doi. org/10.3371/CSRP.MICU.020813]. [PMID: 23428789]. [DOI] [PubMed] [Google Scholar]
- 35.Fernandes B.S., Steiner J., Bernstein H.G., Dodd S., Pasco J.A., Dean O.M., Nardin P., Gonçalves C.A., Berk M. C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol. Psychiatry. 2016;21(4):554–564. doi: 10.1038/mp.2015.87. [http://dx.doi.org/10.1038/mp.2015.87]. [PMID: 26169974]. [DOI] [PubMed] [Google Scholar]
- 36.Pepys M.B., Hirschfield G.M. C-reactive protein: A critical update. J. Clin. Invest. 2003;111(12):1805–1812. doi: 10.1172/JCI18921. [http://dx.doi.org/ 10.1172/JCI200318921]. [PMID: 12813013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yo C.H., Lee S.H., Chang S.S., Lee M.C., Lee C.C. Value of high-sensitivity C-reactive protein assays in predicting atrial fibrillation recurrence: A systematic review and meta-analysis. BMJ Open. 2014;4(2):e004418. doi: 10.1136/bmjopen-2013-004418. [http://dx.doi.org/10.1136/bmjopen-2013-004418]. [PMID: 24556243]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Windgassen E.B., Funtowicz L., Lunsford T.N., Harris L.A., Mulvagh S.L. C-reactive protein and high-sensitivity C-reactive protein: an update for clinicians. Postgrad. Med. 2011;123(1):114–119. doi: 10.3810/pgm.2011.01.2252. [http://dx.doi.org/10.3810/pgm.2011.01.2252]. [PMID: 21293091]. [DOI] [PubMed] [Google Scholar]
- 39.Misiak B., Stańczykiewicz B., Kotowicz K., Rybakowski J.K., Samochowiec J., Frydecka D. Cytokines and C-reactive protein alterations with respect to cognitive impairment in schizophrenia and bipolar disorder: A systematic review. Schizophr. Res. 2017 doi: 10.1016/j.schres.2017.04.015. [http://dx.doi.org/10.1016/j.schres.2017.04.015]. [PMID: S0920-9964(17)30202-5]. [DOI] [PubMed] [Google Scholar]
- 40.Joseph J., Depp C., Martin A.S., Daly R.E., Glorioso D.K., Palmer B.W., Jeste D.V. Associations of high sensitivity C-reactive protein levels in schizophrenia and comparison groups. Schizophr. Res. 2015;168(1-2):456–460. doi: 10.1016/j.schres.2015.08.019. [http://dx.doi.org/10.1016/ j.schres.2015.08.019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tracy R.P., Lemaitre R.N., Psaty B.M., Ives D.G., Evans R.W., Cushman M., Meilahn E.N., Kuller L.H. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly. Results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler. Thromb. Vasc. Biol. 1997;17(6):1121–1127. doi: 10.1161/01.atv.17.6.1121. [http://dx.doi.org/10.1161/01.ATV.17.6.1121]. [PMID: 9194763]. [DOI] [PubMed] [Google Scholar]
- 42.Cesari M., Penninx B.W., Newman A.B., Kritchevsky S.B., Nicklas B.J., Sutton-Tyrrell K., Rubin S.M., Ding J., Simonsick E.M., Harris T.B., Pahor M. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108(19):2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [http://dx.doi.org/10.1161/01.CIR. 0000097109.90783.FC]. [PMID: 14568895]. [DOI] [PubMed] [Google Scholar]
- 43.Makita S., Nakamura M., Hiramori K. The association of C-reactive protein levels with carotid intima-media complex thickness and plaque formation in the general population. Stroke. 2005;36(10):2138–2142. doi: 10.1161/01.STR.0000181740.74005.ee. [http://dx.doi.org/10.1161/01.STR.0000181740.74005.ee]. [PMID: 16151032]. [DOI] [PubMed] [Google Scholar]
- 44.Labonté M.E., Dewailly E., Chateau-Degat M.L., Couture P., Lamarche B. Population-based study of high plasma C-reactive protein concentrations among the Inuit of Nunavik. Int. J. Circumpolar Health. 2012;•••:71. doi: 10.3402/ijch.v71i0.19066. [http://dx.doi.org/10.3402/ijch.v71i0.19066]. [PMID: 23087913]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hosford-Donovan A., Nilsson A., Wåhlin-Larsson B., Kadi F. Observational and mechanistic links between C-reactive protein and blood pressure in elderly women. Maturitas. 2016;89:52–57. doi: 10.1016/j.maturitas.2016.04.016. [http://dx.doi.org/10.1016/j.maturitas.2016.04.016]. [PMID: 27180160]. [DOI] [PubMed] [Google Scholar]
- 46.de Rekeneire N., Peila R., Ding J., Colbert L.H., Visser M., Shorr R.I., Kritchevsky S.B., Kuller L.H., Strotmeyer E.S., Schwartz A.V., Vellas B., Harris T.B. Diabetes, hyperglycemia, and inflammation in older individuals: the health, aging and body composition study. Diabetes Care. 2006;29(8):1902–1908. doi: 10.2337/dc05-2327. [http://dx.doi.org/10.2337/dc05-2327]. [PMID: 16873800]. [DOI] [PubMed] [Google Scholar]
- 47.Panickar K.S., Jewell D.E. The beneficial role of anti-inflammatory dietary ingredients in attenuating markers of chronic low-grade inflammation in aging. Horm. Mol. Biol. Clin. Investig. 2015;23(2):59–70. doi: 10.1515/hmbci-2015-0017. [http://dx.doi.org/10.1515/hmbci-2015-0017]. [PMID: 26124060]. [DOI] [PubMed] [Google Scholar]
- 48.Costello-White R., Ryff C.D., Coe C.L. Aging and low-grade inflammation reduce renal function in middle-aged and older adults in Japan and the USA. Age (Dordr.) 2015;37(4):9808. doi: 10.1007/s11357-015-9808-7. [http://dx. doi.org/10.1007/s11357-015-9808-7]. [PMID: 26187318]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song I.U., Cho H.J., Kim J.S., Park I.S., Lee K.S. Serum hs-CRP levels are increased in de Novo Parkinson’s disease independently from age of onset. Eur. Neurol. 2014;72(5-6):285–289. doi: 10.1159/000363570. [http:// dx.doi.org/10.1159/000363570]. [DOI] [PubMed] [Google Scholar]
- 50.Song I.U., Chung S.W., Kim Y.D., Maeng L.S. Relationship between the hs-CRP as non-specific biomarker and Alzheimer’s disease according to aging process. Int. J. Med. Sci. 2015;12(8):613–617. doi: 10.7150/ijms.12742. [http://dx.doi.org/10.7150/ijms.12742]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tegeler C., O’Sullivan J.L., Bucholtz N., Goldeck D., Pawelec G., Steinhagen-Thiessen E., Demuth I. The inflammatory markers CRP, IL-6, and IL-10 are associated with cognitive function--data from the Berlin Aging Study II. Neurobiol. Aging. 2016;38:112–117. doi: 10.1016/j.neurobiolaging.2015.10.039. [http://dx.doi.org/10.1016/j.neurobiolaging.2015.10.039]. [PMID: 26827649]. [DOI] [PubMed] [Google Scholar]
- 52.Puzianowska-Kuźnicka M., Owczarz M., Wieczorowska-Tobis K., Nadrowski P., Chudek J., Slusarczyk P., Skalska A., Jonas M., Franek E., Mossakowska M. 2016. [DOI] [PMC free article] [PubMed]
- 53.Miller B.J., Culpepper N., Rapaport M.H. 2014.
- 54.Fan X., Goff D.C., Henderson D.C. Inflammation and schizophrenia. Expert Rev. Neurother. 2007;7(7):789–796. doi: 10.1586/14737175.7.7.789. [http://dx. doi.org/10.1586/14737175.7.7.789]. [PMID: 17610386]. [DOI] [PubMed] [Google Scholar]
- 55.Fawzi M.H., Fawzi M.M., Fawzi M.M., Said N.S. C-reactive protein serum level in drug-free male Egyptian patients with schizophrenia. Psychiatry Res. 2011;190(1):91–97. doi: 10.1016/j.psychres.2011.05.010. [http://dx.doi. org/10.1016/j.psychres.2011.05.010]. [PMID: 21621854]. [DOI] [PubMed] [Google Scholar]
- 56.Garcia-Rizo C., Fernandez-Egea E., Oliveira C., Justicia A., Bernardo M., Kirkpatrick B. Inflammatory markers in antipsychotic-naive patients with nonaffective psychosis and deficit vs. nondeficit features. Psychol. Res. 2012;198(2):212–215. doi: 10.1016/j.psychres.2011.08.014. [http://dx.doi.org/10.1016/j.psychres.2011.08.014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dickerson F., Stallings C., Origoni A., Boronow J., Yolken R. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr. Res. 2007;93(1-3):261–265. doi: 10.1016/j.schres.2007.03.022. [http://dx. doi.org/10.1016/j.schres.2007.03.022]. [PMID: 17490859]. [DOI] [PubMed] [Google Scholar]
- 58.Dickerson F., Stallings C., Origoni A., Vaughan C., Khushalani S., Yolken R. Additive effects of elevated C-reactive protein and exposure to Herpes Simplex Virus type 1 on cognitive impairment in individuals with schizophrenia. Schizophr. Res. 2012;93(1-3):261–265. doi: 10.1016/j.schres.2011.10.003. [http://dx.doi.org/10.1016/j.schres.2011.10.003]. [DOI] [PubMed] [Google Scholar]
- 59.Dorajoo R., Li R., Ikram M.K., Liu J., Froguel P., Lee J., Sim X., Ong R.T., Tay W.T., Peng C., Young T.L., Blakemore A.I., Cheng C.Y., Aung T., Mitchell P., Wang J.J., Klaver C.C., Boerwinkle E., Klein R., Siscovick D.S., Jensen R.A., Gudnason V., Smith A.V., Teo Y.Y., Wong T.Y., Tai E.S., Heng C.K., Friedlander Y. Are C-reactive protein associated genetic variants associated with serum levels and retinal markers of microvascular pathology in Asian populations from Singapore? PLoS One. 2013;8(7):e67650. doi: 10.1371/journal.pone.0067650. [http://dx.doi.org/10.1371/journal.pone. 0067650]. [PMID: 23844046]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Higgins J.P.T., Green S. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Book Series. Wiley-Blackwell; 2008. [http://dx.doi.org/10.1002/9780470712184] [Google Scholar]
- 61.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interven-tions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [http://dx.doi.org/10.1136/bmj.b2700]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fan X., Pristach C., Liu E.Y., Freudenreich O., Henderson D.C., Goff D.C. Elevated serum levels of C-reactive protein are associated with more severe psychopathology in a subgroup of patients with schizophrenia. Psychiatry Res. 2007;149(1-3):267–271. doi: 10.1016/j.psychres.2006.07.011. [http://dx.doi.org/10.1016/j.psychres.2006.07.011]. [PMID: 17112596]. [DOI] [PubMed] [Google Scholar]
- 63.Akanji A.O., Ohaeri J.U., Al-Shammri S., Fatania H.R. Association of blood levels of C-reactive protein with clinical phenotypes in Arab schizophrenic patients. Psychiatry Res. 2009;169(1):56–61. doi: 10.1016/j.psychres.2008.06.010. [http://dx.doi.org/10.1016/j.psychres.2008.06.010]. [PMID: 19619902]. [DOI] [PubMed] [Google Scholar]
- 64.Vuksan-Cusa B., Sagud M., Jakovljević M. C-reactive protein and metabolic syndrome in patients with bipolar disorder compared to patients with schizophrenia. Psychiatr. Danub. 2010;22(2):275–277. [PMID: 20562761]. [PubMed] [Google Scholar]
- 65.Zakharyan R., Chavushyan A., Khoyetsyan A., Stahelova A., Arakelyan A., Boyajyan A., Mrazek F., Petrek M. Genetic variants of the inflammatory C-reactive protein and schizophrenia in Armenian population: a pilot study. Int. J. Immunogenet. 2010;37(5):407–410. doi: 10.1111/j.1744-313X.2010.00942.x. [http://dx.doi.org/10.1111/j.1744-313X.2010.00942. x]. [PMID: 21182750]. [DOI] [PubMed] [Google Scholar]
- 66.Dickerson F., Stallings C., Origoni A., Vaughan C., Khushalani S., Yolken R. Elevated C-reactive protein and cognitive deficits in individuals with bipolar disorder. J. Affect. Disord. 2013;150(2):456–459. doi: 10.1016/j.jad.2013.04.039. [http://dx.doi.org/10.1016/j.jad.2013.04.039]. [PMID: 23684514]. [DOI] [PubMed] [Google Scholar]
- 67.Lin C.C., Chang C.M., Liu C.Y., Huang T.L. Increased high-sensitivity C-reactive protein levels in Taiwanese schizophrenic patients. Asia-Pac. Psychiatry. 2013;5(2):E58–E63. doi: 10.1111/appy.12078. [http://dx.doi. org/10.1111/appy.12078]. [PMID: 23857813]. [DOI] [PubMed] [Google Scholar]
- 68.Khandaker G.M., Zammit S., Lewis G., Jones P.B. A population-based study of atopic disorders and inflammatory markers in childhood before psychotic experiences in adolescence. Schizophr. Res. 2014;152(1):139–145. doi: 10.1016/j.schres.2013.09.021. [http://dx.doi.org/10.1016/j.schres. 2013.09.021]. [PMID: 24268471]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wium-Andersen M.K., Ørsted D.D., Nordestgaard B.G. Elevated C-reactive protein associated with late- and very-late-onset schizophrenia in the general population: a prospective study. Schizophr. Bull. 2014;40(5):1117–1127. doi: 10.1093/schbul/sbt120. [http://dx.doi.org/10.1093/schbul/ sbt120]. [PMID: 23996346]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wysokiński A., Margulska A., Strzelecki D., Kłoszewska I. Levels of C-reactive protein (CRP) in patients with schizophrenia, unipolar depression and bipolar disorder. Nord. J. Psychiatry. 2015;69(5):346–353. doi: 10.3109/08039488.2014.984755. [http://dx.doi.org/10.3109/08039488.2014. 984755]. [PMID: 25495587]. [DOI] [PubMed] [Google Scholar]
- 71.Dickerson F., Stallings C., Origoni A., Schroeder J., Katsafanas E., Schweinfurth L., Savage C., Khushalani S., Yolken R. Inflammatory Markers in Recent Onset Psychosis and Chronic Schizophrenia. Schizophr. Bull. 2016;42(1):134–141. doi: 10.1093/schbul/sbv108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Faugere M., Micoulaud-Franchi J.A., Alessandrini M., Richieri R., Faget-Agius C., Auquier P., Lançon C., Boyer L. Quality of life is associated with chronic inflammation in schizophrenia: a cross-sectional study. Sci. Rep. 2015;5:10793. doi: 10.1038/srep10793. [http://dx.doi. org/10.1038/srep10793]. [PMID: 26041435]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khandaker G.M., Pearson R.M., Zammit S., Lewis G., Jones P.B. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry. 2014;71(10):1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [http://dx.doi.org/10.1001/jamapsychiatry. 2014.1332]. [PMID: 25133871]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu D.M., Liu Y., Zhang A.G., Chu Z.X., Wu Q., Li H., Ge J.F., Dong Y., Zhu P. High levels of vitamin D in relation to reduced risk of schizophrenia with elevated C-reactive protein. Psychiatry Res. 2015;30228(3):565–570. doi: 10.1016/j.psychres.2015.05.051. [DOI] [PubMed] [Google Scholar]
- 75.Barzilay R., Lobel T., Krivoy A., Shlosberg D., Weizman A., Katz N. Elevated C-reactive protein levels in schizophrenia inpatients is associated with aggressive behavior. Eur. Psychiatry. 2016;31:8–12. doi: 10.1016/j.eurpsy.2015.09.461. [http://dx.doi.org/10.1016/j.eurpsy.2015.09.461]. [PMID: 26657596]. [DOI] [PubMed] [Google Scholar]
- 76.Devanarayanan S., Nandeesha H., Kattimani S., Sarkar S., Jose J. Elevated copper, hs C-reactive protein and dyslipidemia in drug free schizophrenia: Relation with psychopathology score. Asian J. Psychiatr. 2016;24:99–102. doi: 10.1016/j.ajp.2016.08.025. [http://dx.doi.org/10.1016/j.ajp.2016. 08.025]. [PMID: 27931919]. [DOI] [PubMed] [Google Scholar]
- 77.Kim S., Hwang Y., Lee D., Webster M.J. Transcriptome sequencing of the choroid plexus in schizophrenia. Transl. Psychiatry. 2016;6(11):e964. doi: 10.1038/tp.2016.229. [http://dx.doi.org/10.1038/tp.2016.229]. [PMID: 27898074]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Inoshita M., Numata S., Tajima A., Kinoshita M., Umehara H., Nakataki M., Ikeda M., Maruyama S., Yamamori H., Kanazawa T., Shimodera S., Hashimoto R., Imoto I., Yoneda H., Iwata N., Ohmori T. A significant causal association between C-reactive protein levels and schizophrenia. Sci. Rep. 2016;6:26105. doi: 10.1038/srep26105. [http://dx.doi.org/10.1038/srep26105]. [PMID: 27193331]. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Jordan W., Dobrowolny H., Bahn S., Bernstein H.G., Brigadski T., Frodl T., Isermann B., Lessmann V., Pilz J., Rodenbeck A., Schiltz K., Schwedhelm E., Tumani H., Wiltfang J., Guest P.C., Steiner J. Oxidative stress in drug-naïve first episode patients with schizophrenia and major depression: effects of disease acuity and potential confounders. Eur. Arch. Psychiatry Clin. Neurosci. 2016 doi: 10.1007/s00406-016-0749-7. Epub ahead of print [PMID: 27913877]. [DOI] [PubMed] [Google Scholar]
- 80.Prasad K.M., Upton C.H., Nimgaonkar V.L., Keshavan M.S. Differential susceptibility of white matter tracts to inflammatory mediators in schizophrenia: an integrated DTI study. Schizophr. Res. 2015;161(1):119–125. doi: 10.1016/j.schres.2014.09.043. [http://dx.doi.org/10.1016/j.schres. 2014.09.043]. [PMID: 25449712]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pan S., Tan Y., Yao S., Zhao X., Xiong J. Serum high-sensitivity C-reactive protein: A delicate sentinel elevated in drug-free acutely agitated patients with schizophrenia. Psychiatry Res. 2016;246:89–94. doi: 10.1016/j.psychres.2016.09.033. [http://dx.doi.org/10.1016/j.psychres.2016.09. 033]. [PMID: 27669496]. [DOI] [PubMed] [Google Scholar]
- 82.Szortyka M.F., Cristiano V.B., Ceresér K.M., Francesconi L.P., Lobato M.I., Gama C., Belmonte-de-Abreu P. Physical functional capacity and C-reactive protein in schizophrenia. Front. Psychiatry. 2016;7:131. doi: 10.3389/fpsyt.2016.00131. [http://dx.doi.org/10.3389/fpsyt.2016.00131]. [PMID: 27547191]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tanaka T., Matsuda T., Hayes L.N., Yang S., Rodriguez K., Severance E.G., Yolken R.H., Sawa A., Eaton W.W. Infection and inflammation in schizophrenia and bipolar dis-order. 2016. [DOI] [PubMed]
- 84.Ali F.T., Abd El-Azeem E.M., Hamed M.A., Ali M.A., Abd Al-Kader N.M., Hassan E.A. 2017. [DOI] [PubMed]
- 85.Balõtšev R., Koido K., Vasar V., Janno S., Kriisa K., Mahlapuu R., Ljubajev U., Parksepp M., Veiksaar P., Volke V., Lang A., Haring L., Zilmer M., Vasar E. Inflammatory, cardio-metabolic and diabetic profiling of chronic schizophrenia. Eur. Psychiatry. 2017;39:1–10. doi: 10.1016/j.eurpsy.2016.05.010. [http://dx.doi.org/10.1016/j.eurpsy. 2016.05.010]. [PMID: 27810612]. [DOI] [PubMed] [Google Scholar]
- 86.Horsdal H.T., Köhler-Forsberg O., Benros M.E., Gasse C. C-reactive protein and white blood cell levels in schizophrenia, bipolar disorders and depression - associations with mortality and psychiatric outcomes: a population-based study. Eur. Psychiatry. 2017;44:164–172. doi: 10.1016/j.eurpsy.2017.04.012. [http://dx.doi.org/10.1016/j.eurpsy.2017.04.012]. [PMID: 28645055]. [DOI] [PubMed] [Google Scholar]
- 87.Metcalf S.A., Jones P.B., Nordstrom T., Timonen M., Mäki P., Miettunen J., Jääskeläinen E., Järvelin M.R., Stochl J., Murray G.K., Veijola J., Khandaker G.M. Serum C-reactive protein in adolescence and risk of schizophrenia in adulthood: A prospective birth cohort study. Brain Behav. Immun. 2017;59:253–259. doi: 10.1016/j.bbi.2016.09.008. [http://dx.doi.org/10.1016/j.bbi.2016.09.008]. [PMID: 27622678]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoshida M., Kanzaki T., Mizoi M., Nakamura M., Uemura T., Mimori S., Uju Y., Sekine K., Ishii Y., Yoshimi T., Yasui R., Yasukawa A., Sato M., Okamoto S., Hisaoka T., Miura M., Kusanishi S., Murakami K., Nakano C., Mizuta Y., Mishima S., Hayakawa T., Tsukada K., Kashiwagi K., Igarashi K. Correlation between brain damage, associated biomarkers, and medication in psychiatric inpatients: A cross-sectional study. Clin. Chim. Acta. 2017;464:50–56. doi: 10.1016/j.cca.2016.11.002. [http://dx.doi.org/10.1016/j.cca.2016.11.002]. [PMID: 27816667]. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Q., Hong W., Li H., Peng F., Wang F., Li N., Xiang H., Zhang Z., Su Y., Huang Y., Zhang S., Zhao G., Zhou R., Mao L., Lin Z., Cai W., Fang Y., Xie B., Zhao M. Increased ratio of high sensitivity C-reactive protein to interleukin-10 as a potential peripheral biomarker of schizophrenia and aggression. Int. J. Psychophysiol. 2017;114:9–15. doi: 10.1016/j.ijpsycho.2017.02.001. [http://dx.doi.org/10.1016/j.ijpsycho. 2017.02.001]. [PMID: 28174109]. [DOI] [PubMed] [Google Scholar]
- 90.Dickerson F., Stallings C., Origoni A., Vaughan C., Khushalani S., Yolken R. Elevated C-reactive protein and cognitive deficits in individuals with bipolar disorder. J. Affect. Disord. 2013;150(2):456–459. doi: 10.1016/j.jad.2013.04.039. [http://dx.doi.org/10.1016/j.jad.2013.04.039]. [PMID: 23684514]. [DOI] [PubMed] [Google Scholar]
- 91.Hope S., Hoseth E., Dieset I., Mørch R.H., Aas M., Aukrust P., Djurovic S., Melle I., Ueland T., Agartz I., Ueland T., Westlye L.T., Andreassen O.A. Inflammatory markers are associated with general cognitive abilities in schizophrenia and bipolar disorder patients and healthy controls. Schizophr. Res. 2015;165(2-3):188–194. doi: 10.1016/j.schres.2015.04.004. [http://dx.doi.org/10.1016/j.schres.2015.04.004]. [PMID: 25956633]. [DOI] [PubMed] [Google Scholar]
- 92.Micoulaud-Franchi J.A., Faugere M., Boyer L., Fond G., Richieri R., Faget C., Cermolacce M., Philip P., Vion-Dury J., Lancon C. Elevated C-reactive protein is associated with sensory gating deficit in schizophrenia. Schizophr. Res. 2015;165(1):94–96. doi: 10.1016/j.schres.2015.03.018. [http:// dx.doi.org/10.1016/j.schres.2015.03.018]. [PMID: 25864954]. [DOI] [PubMed] [Google Scholar]
- 93.Bulzacka E., Boyer L., Schürhoff F., Godin O., Berna F., Brunel L., Andrianarisoa M., Aouizerate B., Capdevielle D., Chéreau-Boudet I., Chesnoy-Servanin G., Danion J.M., Dubertret C., Dubreucq J., Faget C., Gabayet F., Le Gloahec T., Llorca P.M., Mallet J., Misdrahi D., Rey R., Richieri R., Passerieux C., Roux P., Yazbek H., Leboyer M., Fond G. Chronic peripheral inflammation is associated with cognitive impairment in schizophrenia: Results from the multicentric FACE-SZ dataset. Schizophr. Bull. 2016;42(5):1290–1302. doi: 10.1093/schbul/sbw029. [http://dx.doi.org/10. 1093/schbul/sbw029]. [PMID: 27143795]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Johnsen E., Fathian F., Kroken R.A., Steen V.M., Jørgensen H.A., Gjestad R., Løberg E.M. The serum level of C-reactive protein (CRP) is associated with cognitive performance in acute phase psychosis. BMC Psychiatry. 2016;16:60. doi: 10.1186/s12888-016-0769-x. [http://dx.doi.org/10. 1186/s12888-016-0769-x]. [PMID: 26973142]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rapaport M.H., Lohr J.B. Serum-soluble interleukin-2 receptors in neuroleptic-naive schizophrenic subjects and in medicated schizophrenic subjects with and without tardive dyskinesia. Acta Psychiatr. Scand. 1994;90(5):311–315. doi: 10.1111/j.1600-0447.1994.tb01599.x. [http://dx.doi.org/10.1111/ j.1600-0447.1994.tb01599.x]. [PMID: 7872033]. [DOI] [PubMed] [Google Scholar]
- 96.Müller N., Riedel M., Gruber R., Ackenheil M., Schwarz M.J. The immune system and schizophrenia. An integrative view. Ann. N. Y. Acad. Sci. 2000;917:456–467. doi: 10.1111/j.1749-6632.2000.tb05410.x. [http://dx.doi.org/10.1111/j. 1749-6632.2000.tb05410.x]. [PMID: 11268373]. [DOI] [PubMed] [Google Scholar]
- 97.Sirota P., Meiman M., Herschko R., Bessler H. Effect of neuroleptic administration on serum levels of soluble IL-2 receptor-alpha and IL-1 receptor antagonist in schizophrenic patients. Psychiatry Res. 2005;134(2):151–159. doi: 10.1016/j.psychres.2004.04.012. [http://dx.doi.org/10.1016/j. psychres.2004.04.012]. [PMID: 15840416]. [DOI] [PubMed] [Google Scholar]
- 98.Barnett A.H., Mackin P., Chaudhry I., Farooqi A., Gadsby R., Heald A., Hill J., Millar H., Peveler R., Rees A., Singh V., Taylor D., Vora J., Jones P.B. Minimising metabolic and cardiovascular risk in schizophrenia: diabetes, obesity and dyslipidaemia. J. Psychopharmacol. (Oxford) 2007;21(4):357–373. doi: 10.1177/0269881107075509. [http://dx. doi.org/10.1177/0269881107075509]. [PMID: 17656425]. [DOI] [PubMed] [Google Scholar]
- 99.Blomström Å., Karlsson H., Svensson A., Frisell T., Lee B.K., Dal H., Magnusson C., Dalman C. Hospital admission with infection during childhood and risk for psychotic illness--a population-based cohort study. Schizophr. Bull. 2014;40(6):1518–1525. doi: 10.1093/schbul/sbt195. [http://dx.doi.org/10.1093/schbul/sbt195]. [PMID: 24366719]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miller B.J., Buckley P., Seabolt W., Mellor A., Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol. Psychiatry. 2011;70(7):663–671. doi: 10.1016/j.biopsych.2011.04.013. [http://dx.doi.org/10.1016/j.biopsych.2011.04.013]. [PMID: 21641581]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Asevedo E., Rizzo L.B., Gadelha A., Mansur R.B., Ota V.K., Berberian A.A., Scarpato B.S., Teixeira A.L., Bressan R.A., Brietzke E. Peripheral interleukin-2 level is associated with negative symptoms and cognitive performance in schizophrenia. Physiol. Behav. 2014;129:194–198. doi: 10.1016/j.physbeh.2014.02.032. [http://dx.doi.org/10.1016/j. physbeh.2014.02.032]. [PMID: 24576679]. [DOI] [PubMed] [Google Scholar]
- 102.Dickerson F., Stallings C., Origoni A., Vaughan C., Khushalani S., Yang S., Yolken R. C-reactive protein is elevated in schizophrenia. Schizophr. Res. 2013;143(1):198–202. doi: 10.1016/j.schres.2012.10.041. [http://dx. doi.org/10.1016/j.schres.2012.10.041]. [PMID: 23218564]. [DOI] [PubMed] [Google Scholar]
- 103.Sugino H., Futamura T., Mitsumoto Y., Maeda K., Marunaka Y. Atypical antipsychotics suppress production of proinflammatory cytokines and up-regulate interleukin-10 in lipopolysaccharide-treated mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33(2):303–307. doi: 10.1016/j.pnpbp.2008.12.006. [http://dx.doi.org/10.1016/j.pnpbp.2008. 12.006]. [PMID: 19138716]. [DOI] [PubMed] [Google Scholar]
- 104.Kunz M., Ceresér K.M., Goi P.D., Fries G.R., Teixeira A.L., Fernandes B.S., Belmonte-de-Abreu P.S. Kauer-Sant’Anna, M.; Kapczinski, F.; Gama, C.S. Serum levels of IL-6, IL-10 and TNF-α in patients with bipolar disorder and schizophrenia: differences in pro- and anti-inflammatory balance. Rev. Bras. Psiquiatr. 2011;33(3):268–274. doi: 10.1590/s1516-44462011000300010. [PMID: 21971780]. [DOI] [PubMed] [Google Scholar]
- 105.Emiliani F.E., Sedlak T.W., Sawa A. Oxidative stress and schizophrenia: recent breakthroughs from an old story. Curr. Opin. Psychiatry. 2014;27(3):185–190. doi: 10.1097/YCO.0000000000000054. [http://dx.doi.org/10.1097/ YCO.0000000000000054]. [PMID: 24613987]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barron H., Hafizi S., Andreazza A.C., Mizrahi R. Neuroinflammation and oxidative stress in psychosis and psychosis risk. Int. J. Mol. Sci. 2017;18(3):E651. doi: 10.3390/ijms18030651. [http://dx.doi.org/10.3390/ijms 18030651]. [PMID: 28304340]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Steullet P., Cabungcal J.H., Coyle J., Didriksen M., Gill K., Grace A.A., Hensch T.K., LaMantia A.S., Lindemann L., Maynard T.M., Meyer U., Morishita H., O’Donnell P., Puhl M., Cuenod M., Do K.Q. Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Mol. Psychiatry. 2017;22(7):936–943. doi: 10.1038/mp.2017.47. [http://dx. doi.org/10.1038/mp.2017.47]. [PMID: 28322275]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hoftman G.D., Datta D., Lewis D.A. Layer 3 excitatory and inhibitory circuitry in the prefrontal cortex: Developmental trajectories and alterations in schizophrenia. Biol. Psychiatry. 2017;81(10):862–873. doi: 10.1016/j.biopsych.2016.05.022. [http://dx.doi.org/10.1016/j.biopsych.2016.05. 022]. [PMID: 27455897]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Howes O.D., Montgomery A.J., Asselin M.C., Murray R.M., Valli I., Tabraham P., Bramon-Bosch E., Valmaggia L., Johns L., Broome M., McGuire P.K., Grasby P.M. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch. Gen. Psychiatry. 2009;66(1):13–20. doi: 10.1001/archgenpsychiatry.2008.514. [http://dx.doi.org/10.1001/ archgenpsychiatry.2008.514]. [DOI] [PubMed] [Google Scholar]
- 110.Barron H., Hafizi S., Andreazza A.C., Mizrahi R. Neuroinflammation and Oxidative Stress in Psychosis and Psychosis Risk. Int. J. Mol. Sci. 2017;18(3):E651. doi: 10.3390/ijms18030651. [http://dx.doi.org/10.3390/ijms 18030651]. [PMID: 28304340]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Turrens J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552(Pt 2):335–344. doi: 10.1113/jphysiol.2003.049478. [http://dx.doi.org/10.1113/ jphysiol.2003.049478]. [PMID: 14561818]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Flatow J., Buckley P., Miller B.J. Meta-analysis of oxidative stress in schizophrenia. Biol. Psychiatry. 2013;74(6):400–409. doi: 10.1016/j.biopsych.2013.03.018. [http://dx.doi.org/10.1016/j.biopsych.2013.03.018]. [PMID: 23683390]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Machado A.K., Pan A.Y., da Silva T.M., Duong A., Andreazza A.C. Upstream pathways controlling mitochondrial function in major psychosis: A focus on bipolar disorder. Can. J. Psychiatry. 2016;61(8):446–456. doi: 10.1177/0706743716648297. [http://dx.doi.org/10.1177/0706743716648297]. [PMID: 27310240]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Prabakaran S., Swatton J.E., Ryan M.M., Huffaker S.J., Huang J.T., Griffin J.L., Wayland M., Freeman T., Dudbridge F., Lilley K.S., Karp N.A., Hester S., Tkachev D., Mimmack M.L., Yolken R.H., Webster M.J., Torrey E.F., Bahn S. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. 2004. [DOI] [PubMed]
- 115.Hayden M.S., Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18(18):2195–2224. doi: 10.1101/gad.1228704. [http://dx.doi.org/10.1101/gad.1228704]. [PMID: 15371334]. [DOI] [PubMed] [Google Scholar]
- 116.Bitanihirwe B.K., Woo T.U. Oxidative stress in schizophrenia: an integrated approach. Neurosci. Biobehav. Rev. 2011;35(3):878–893. doi: 10.1016/j.neubiorev.2010.10.008. [http://dx.doi.org/10.1016/j.neubiorev.2010.10.008]. [PMID: 20974172]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Furukawa H., del Rey A., Monge-Arditi G., Besedovsky H.O. Interleukin-1, but not stress, stimulates glucocorticoid output during early postnatal life in mice. Ann. N. Y. Acad. Sci. 1998;840:117–122. doi: 10.1111/j.1749-6632.1998.tb09555.x. [http://dx.doi.org/10.1111/j.1749-6632.1998.tb09555.x]. [PMID: 9629243]. [DOI] [PubMed] [Google Scholar]
- 118.Sparkman N.L., Johnson R.W. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15(4-6):323–330. doi: 10.1159/000156474. [http://dx.doi. org/10.1159/000156474]. [PMID: 19047808]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Khandaker G.M., Zimbron J., Lewis G., Jones P.B. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol. Med. 2013;43(2):239–257. doi: 10.1017/S0033291712000736. [http://dx.doi.org/10.1017/S0033291712000736]. [PMID: 22717193]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brown A.S., Derkits E.J. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am. J. Psychiatry. 2010;167(3):261–280. doi: 10.1176/appi.ajp.2009.09030361. [http://dx.doi.org/10.1176/appi.ajp. 2009.09030361]. [PMID: 20123911]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Canetta S., Sourander A., Surcel H.M., Hinkka-Yli-Salomäki S., Leiviskä J., Kellendonk C., McKeague I.W., Brown A.S. Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Am. J. Psychiatry. 2014;171(9):960–968. doi: 10.1176/appi.ajp.2014.13121579. [http://dx.doi.org/10.1176/appi.ajp.2014.13121579]. [PMID: 24969261]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meyer U., Schwarz M.J., Müller N. Inflammatory processes in schizophrenia: a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol. Ther. 2011;132(1):96–110. doi: 10.1016/j.pharmthera.2011.06.003. [http://dx.doi.org/10.1016/j.pharmthera. 2011.06.003]. [PMID: 21704074]. [DOI] [PubMed] [Google Scholar]
- 123.Meyer U., Feldon J. Neural basis of psychosis-related behaviour in the infection model of schizophrenia. Behav. Brain Res. 2009;204(2):322–334. doi: 10.1016/j.bbr.2008.12.022. [http://dx.doi.org/10.1016/j.bbr.2008.12.022]. [PMID: 19154759]. [DOI] [PubMed] [Google Scholar]
- 124.Pearce B.D. Schizophrenia and viral infection during neurodevelopment: a focus on mechanisms. Mol. Psychiatry. 2001;6(6):634–646. doi: 10.1038/sj.mp.4000956. [http://dx.doi.org/10.1038/sj.mp.4000956]. [PMID: 11673791]. [DOI] [PubMed] [Google Scholar]
- 125.Brown A.S., Begg M.D., Gravenstein S., Schaefer C.A., Wyatt R.J., Bresnahan M., Babulas V.P., Susser E.S. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch. Gen. Psychiatry. 2004;61(8):774–780. doi: 10.1001/archpsyc.61.8.774. [http://dx.doi.org/10.1001/ archpsyc.61.8.774]. [PMID: 15289276]. [DOI] [PubMed] [Google Scholar]
- 126.Buka S.L., Cannon T.D., Torrey E.F., Yolken R.H. Maternal exposure to herpes simplex virus and risk of psychosis among adult offspring. Biol. Psychiatry. 2008;63(8):809–815. doi: 10.1016/j.biopsych.2007.09.022. [http://dx.doi. org/10.1016/j.biopsych.2007.09.022]. [PMID: 17981263]. [DOI] [PubMed] [Google Scholar]
- 127.Ellman L.M., Deicken R.F., Vinogradov S., Kremen W.S., Poole J.H., Kern D.M., Tsai W.Y., Schaefer C.A., Brown A.S. Structural brain alterations in schizophrenia following fetal exposure to the inflammatory cytokine interleukin-8. Schizophr. Res. 2010;121(1-3):46–54. doi: 10.1016/j.schres.2010.05.014. [http://dx.doi.org/10.1016/j.schres.2010. 05.014]. [PMID: 20553865]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brown A.S., Schaefer C.A., Wyatt R.J., Goetz R., Begg M.D., Gorman J.M., Susser E.S. Maternal exposure to respiratory infections and adult schizophrenia spectrum disorders: a prospective birth cohort study. Schizophr. Bull. 2000;26(2):287–295. doi: 10.1093/oxfordjournals.schbul.a033453. [http:// dx.doi.org/10.1093/oxfordjournals.schbul.a033453]. [PMID: 10885631]. [DOI] [PubMed] [Google Scholar]
- 129.Sørensen H.J., Mortensen E.L., Reinisch J.M., Mednick S.A. Association between prenatal exposure to bacterial infection and risk of schizophrenia. Schizophr. Bull. 2009;35(3):631–637. doi: 10.1093/schbul/sbn121. [http://dx.doi.org/10.1093/schbul/sbn121]. [PMID: 18832344]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Babulas V., Factor-Litvak P., Goetz R., Schaefer C.A., Brown A.S. Prenatal exposure to maternal genital and reproductive infections and adult schizophrenia. Am. J. Psychiatry. 2006;163(5):927–929. doi: 10.1176/ajp.2006.163.5.927. [http://dx.doi.org/10.1176/ajp.2006.163.5.927]. [PMID: 16648337]. [DOI] [PubMed] [Google Scholar]
- 131.Licinio J., Seibyl J.P., Altemus M., Charney D.S., Krystal J.H. Elevated CSF levels of interleukin-2 in neuroleptic-free schizophrenic patients. Am. J. Psychiatry. 1993;150(9):1408–1410. doi: 10.1176/ajp.150.9.1408. [http://dx.doi.org/10.1176/ajp.150.9.1408]. [PMID: 8102512]. [DOI] [PubMed] [Google Scholar]
- 132.Schattner A., Cori Y., Hahn T., Sirota P. No evidence for autoimmunity in schizophrenia. J. Autoimmun. 1996;9(5):661–666. doi: 10.1006/jaut.1996.0086. [http://dx.doi.org/10.1006/jaut.1996.0086]. [PMID: 8933282]. [DOI] [PubMed] [Google Scholar]
- 133.Rapaport M.H., McAllister C.G., Pickar D., Tamarkin L., Kirch D.G., Paul S.M. CSF IL-1 and IL-2 in medicated schizophrenic patients and normal volunteers. Schizophr. Res. 1997;25(2):123–129. doi: 10.1016/S0920-9964(97)00008-X. [http://dx.doi.org/10.1016/S0920-9964(97)00008-X]. [PMID: 9187011]. [DOI] [PubMed] [Google Scholar]
- 134.Lin A., Kenis G., Bignotti S., Tura G.J., De Jong R., Bosmans E., Pioli R., Altamura C., Scharpé S., Maes M. The inflammatory response system in treatment-resistant schizophrenia: increased serum interleukin-6. Schizophr. Res. 1998;32(1):9–15. doi: 10.1016/s0920-9964(98)00034-6. [http://dx.doi.org/10.1016/S0920-9964(98)00034-6]. [PMID: 9690329]. [DOI] [PubMed] [Google Scholar]
- 135.Roberts W.L., Sedrick R., Moulton L., Spencer A., Rifai N. Evaluation of four automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications. Clin. Chem. 2000;46(4):461–468. [PMID: 10759469]. [PubMed] [Google Scholar]
- 136.Prins B.P., Abbasi A., Wong A., Vaez A., Nolte I., Franceschini N., Stuart P.E., Guterriez Achury J., Mistry V., Bradfield J.P., Valdes A.M., Bras J., Shatunov A., Lu C., Han B., Raychaudhuri S., Bevan S., Mayes M.D., Tsoi L.C., Evangelou E., Nair R.P., Grant S.F., Polychronakos C., Radstake T.R., van Heel D.A., Dunstan M.L., Wood N.W., Al-Chalabi A., Dehghan A., Hakonarson H., Markus H.S., Elder J.T., Knight J., Arking D.E., Spector T.D., Koeleman B.P., van Duijn C.M., Martin J., Morris A.P., Weersma R.K., Wijmenga C., Munroe P.B., Perry J.R., Pouget J.G., Jamshidi Y., Snieder H., Alizadeh B.Z. Investigating the causal relationship of C-reactive protein with 32 complex somatic and psychiatric outcomes: A large-scale cross-consortium mendelian randomization study. PLoS Med. 2016;13(6):e1001976. doi: 10.1371/journal.pmed.1001976. [http://dx.doi.org/10.1371/journal.pmed.1001976]. [PMID: 27327646]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Adzic M., Brkic Z., Mitic M., Francija E., Jovicic M.J., Radulovic J., Maric N.P. Therapeutic strategies for treatment of inflammation-related depression. Curr. Neuropharmacol. 2017 doi: 10.2174/1570159X15666170828163048. [http://dx.doi.org/10.2174/1570159X15666170828163048]. [PMID: 28847294]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Orsolini L., Tomasetti C., Valchera A., Iasevoli F., Buonaguro E.F., Fornaro M., Fiengo A.L.C., Martinotti G., Vellante F., Matarazzo I., Vecchiotti R., Perna G., Nicola M.D., Carano A., Bartolomeis A., Giannantonio M.D., Berardis D. Current and future perspectives on the major depressive disorder: Focus on the new multimodal antidepressant vortioxetine. CNS Neurol. Disord. Drug Targets. 2017;16(1):65–92. doi: 10.2174/1871527315666161025140111. [http://dx.doi.org/10.2174/ 1871527315666161025140111]. [PMID: 27781949]. [DOI] [PubMed] [Google Scholar]
- 139.De Berardis D., Conti C., Iasevoli F., Valchera A., Fornaro M., Cavuto M., Brucchi M., Perna G., Pompili M., Modabbernia A., Lucidi G., Mazza M., Martinotti G., Di Giannantonio M. Alexithymia and its relationships with acute phase proteins and cytokine release: an updated review. J. Biol. Regul. Homeost. Agents. 2014;28(4):795–799. [PMID: 25620189]. [PubMed] [Google Scholar]