Abstract
Background
Heart failure affects 1–2% of the population and is associated with elevated morbidity and mortality. Cardiac arrhythmias are often a result of heart failure, but they can cause left-ventricular systolic dysfunction (LVSD) as an arrhythmia-induced cardiomyopathy (AIC). This causal relationship should be borne in mind by the physician treating a patient with systolic heart failure in association with cardiac arrhythmia.
Methods
This review is based on pertinent publications retrieved by a selective search in PubMed (1987–2017) and on the recommendations in current guidelines.
Results
The key criterion for the diagnosis of an AIC is the demonstration of a persistent arrhythmia (including pathological tachycardia) together with an LVSD whose origin cannot be explained on any other basis. Nearly any type of tachyarrhythmia or frequent ventricular extrasystoles can lead, if persistent, to a progressively severe LVSD. The underlying pathophysiologic mechanisms are incompletely understood; the increased ventricular rate, asynchronous cardiac contractions, and neurohumoral activation all seem to play a role. The most common precipitating factors are supraventricular tachycardias in children and atrial fibrillation in adults. Recent studies have shown that the causal significance of atrial fibrillation in otherwise unexplained LVSD is underappreciated. The treatment of AIC consists primarily of the treatment of the underlying arrhythmia, generally with drugs such as beta-blockers and amiodarone. Depending on the type of arrhythmia, catheter ablation for long-term treatment should also be considered where appropriate. The diagnosis of AIC is considered to be well established when the LVSD normalizes or improves within a few weeks or months of the start of targeted treatment of the arrhythmia.
Conclusion
An AIC is potentially reversible. The timely recognition of this condition and the appropriate treatment of the underlying arrhythmia can substantially improve patient outcomes.
Heart failure is one of the major causes of morbidity and mortality in western countries. Of the total population, approximately 1%–2% of adults and >10% of over 70-year-olds are affected (1– 3). Together with the ever-aging population, heart failure poses one of the greatest challenges to modern medicine and health economics.
The diagnostic algorithm of systolic heart failure places paramount importance on identifying the underlying cause of ventricular contractile dysfunction in order for causal treatment to be initiated and prognosis to be assessed (4).
Arrhythmia-induced cardiomyopathy (also referred to as tachymyopathy or tachycardia-induced cardiomyopathy) is a subform of (non-familial) dilated cardiomyopathy (5, 6). It is characterized by left ventricular systolic dysfunction (LVSD) which is caused by rapid and/or irregular ventricular rate. Arrhythmia-induced cardiomyopathy can be resolved by eliminating or effectively treating the causal arrhythmia (7). In the typical form, arrhythmia is the only underlying disorder and LVSD is fully reversible. However, in the case of pre-existing structural heart disease, arrhythmia-induced cardiomyopathy may aggravate LVSD, rendering it only partially reversible (8, 9).
Arrhythmia-induced cardiomyopathy appears to occur at any age. Although the clinical picture has been known for decades (10) and is well-characterized in animal models, prospective clinical or reliable epidemiological data are lacking and its prevalence is ultimately unknown.
What is striking in more recent treatment studies is that approximately one third of patients with atrial fibrillation and systolic heart failure exhibited primarily idiopathic LVSD and that arrhythmia-induced cardiomyopathy, or a relevant component of this disease, was detected in 58%–88% of cases (11, 12). Similarly, in a cohort study of 1269 consecutive patients with atrial flutter, arrhythmia-induced cardiomyopathy was diagnosed in 56% (103/184) of cases in the subgroup with LVSD after ablation (13). In contrast, the relevance of arrhythmia-induced cardiomyopathy is probably underestimated in clinical practice, given that arrhythmia is often considered to be solely a result of rather than a possible cause of cardiomyopathy.
Methods
PubMed literature for the period 1987–2017 was selectively searched on the basis of the authors‘ clinical and scientific experience. A detailed description can be found in the eMethods.
Pathophysiology
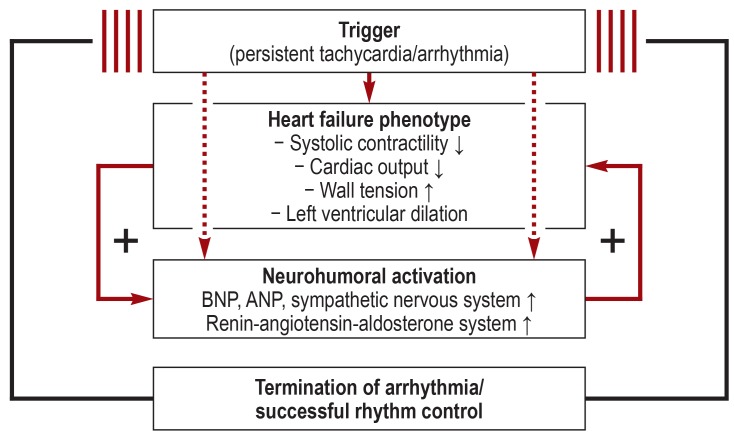
Persistent tachycardia induces systolic heart failure with a cell-typical phenotype of heart failure in animal models in a reproducible manner (figure 1) (14– 16). Here one observes reduced systolic contractility and resultant diminished cardiac output, increased wall dilatation, greater wall tension, and cardiac chamber enlargement within days to weeks (15, 17). In the further course (generally after several weeks depending on the model), the full heart failure phenotype with marked LVSD and cardiac chamber dilatation develops as a result of persistent tachycardia and excessive neurohumoral activation (8, 15, 17– 20). Once tachycardia ceases, the described changes normalize within days to weeks in animal models and within weeks to months in humans (figure 1) (21).
Figure 1.
Schematic representation of the pathophysiology of arrhythmia-induced cardiomyopathy. Persistent tachycardia (due to pacemaker stimulation) reproducibly induces systolic heart failure in various animal models within days to weeks. As part of this, one sees neurohumoral activation typical of heart failure with increased plasma levels of brain natriuretic peptide (BNP) and atrial natriuretic peptide (ANP), sympathetic activation, and excessive activity of the renin-angiotensin-aldosterone system. If tachycardia stimulation is stopped, these changes normalize within days to weeks in animal models and within weeks to months in humans (21).
In animal models, the degree of LVSD increases according to the rate and duration of pacing (22). Other factors also appear to influence the pathogenesis of arrhythmia-induced cardiomyopathy. For example, LVSD progresses more rapidly and is more pronounced upon tachy-pacing from the ventricles as compared to rapid atrial stimulation. (8, 23). The dyssynchronous electrical excitation of the heart resulting from ventricular stimulation (loss of physiological AV sequence and synchronous ventricular activation) thus appears to magnify the effect of tachycardia as a cofactor.
The functional and molecular effects of prolonged tachycardia on the myocardium and the development of heart failure have been investigated almost exclusively in animal models and, as such, can potentially only be extrapolated to humans to a limited extent.
General diagnostic tests and treatment
The key diagnostic criterion of arrhythmia-induced cardiomyopathy is the detection of persistent arrhythmia or pathological tachycardia in conjunction with LVSD that cannot be otherwise explained. A number of arrhythmias can cause arrhythmia-induced cardiomyopathy (box) (8). Although the duration and ventricular rate of the tachycardia are important factors, there are no threshold values for these parameters (8). Relevant arrhythmia cannot always be diagnosed by means of a one-off 12-channel ECG. ECG holter monitoring is able to identify, e.g., recurrent tachycardia, mean ventricular rate in atrial fibrillation, and the frequency of premature ventricular contraction.
BOX. Arrhythmias that can trigger arrhythmia-induced cardiomyopathy.
Supraventricular
Atrial fibrillation
Atrial flutter
-
Supraventricular tachycardia
-
Atrial tachycardia
Junctional ectopic tachycardia
AV node reentrant tachycardia
Dual (not circling) AV node tachycardia (1 : 2)
-
AV reentrant tachycardia
Permanent junctional reentrant tachycardia (PJRT)
-
Ventricular
Frequent premature ventricular contractions
Idiopathic ventricular tachycardia
Fascicular tachycardia
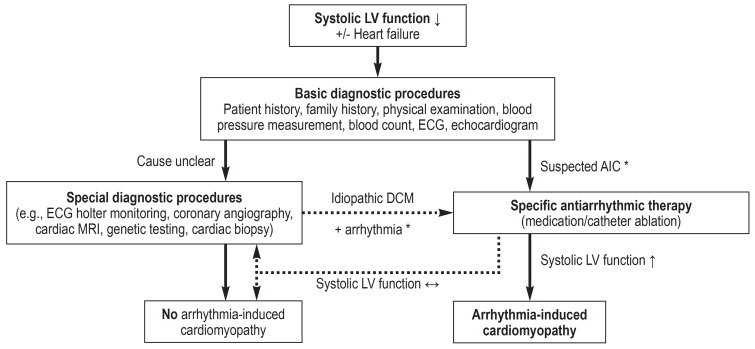
The causal link between arrhythmia and LVSD is often difficult to assess at first, since any form of LVSD can, in principle, lead to arrhythmia (the “chicken-and-egg” question). Figure 2 summarizes the possible diagnostic approach to suspected arrhythmia-induced cardiomyopathy. Once a basic diagnostic work-up has been performed, potential causes need to be excluded using targeted diagnostic methods. Clinical investigations have shown that the extent of initial left ventricular dilatation in patients with arrhythmia-induced cardiomyopathy is less pronounced than it is in patients with dilated cardiomyopathy and secondary tachycardia (24, 25). In a retrospective analysis, an end-diastolic left ventricular (LV) diameter = 61 mm predicted arrhythmia-induced cardiomyopathy with a sensitivity of 100% and a specificity of 71% (24).
Figure 2.
Flow diagram for the diagnosis of arrhythmia-induced cardiomyopathy (AIC).
DCM: dilated cardiomyopathy; LV: left ventricular
*see Box for triggering arrhythmias
Furthermore, a number of studies point to the potential of magnetic resonance imaging (MRI) in the diagnosis of arrhythmia-induced cardiomyopathy (8, 11, 26– 28). Thus, in cases of unclear cardiomyopathy, the absence of ventricular late-gadolinium enhancement (LGE) differentiates arrhythmia-induced cardiomyopathy from other heart diseases. In LGE, magnetic resonance images are acquired approximately 15 min following the administration of gadolinium contrast medium, and necrosis, areas of scarring, and myocardial fibrosis are visualized as contrast enhanced, hyperintense areas. MRI is considered superior in the differential diagnosis between cardiomyopathy and inflammatory heart disease compared with other methods (29) and, in the authors‘ view, can be recommended for the diagnosis of arrhythmia-induced cardiomyopathy.
Myocardial biopsies from patients with arrhythmia-induced cardiomyopathy and cardiomyopathy of other origin (dilated or inflammatory) recently underwent histopathological comparison (30). Arrhythmia-induced cardiomyopathy was characterized for instance by scant or absent myocardial fibrosis, increased expression of major histocompatibility complex class II molecules, and CD68+ macrophage infiltration. However, the potential role of the myocardial biopsy in the diagnosis of arrhythmia-induced cardiomyopathy requires additional prospective investigations.
At present, the diagnosis of arrhythmia-induced cardiomyopathy is considered conclusive if LVSD is completely (or partially in the case of pre-existing heart disease) reversible within only a few weeks or months of successful treatment of arrhythmia (8).
The treatment of arrhythmia-induced cardiomyopathy focuses on treating the arrhythmia—primarily its elimination—or alternatively, e.g., in the case of permanent ventricular fibrillation, by controlling ventricular rate (8, 31– 38). In LVSD, the spectrum of drugs suitable for the treatment of arrhythmia narrows to essentially beta-blockers, digitalis preparations, and amiodarone. Other antiarrhythmic agents may only be used on the basis of a careful benefit-risk assessment. Therefore, depending on the patient‘s age, comorbidities, and type of arrhythmia, catheter ablation is generally the treatment of choice in the long term.
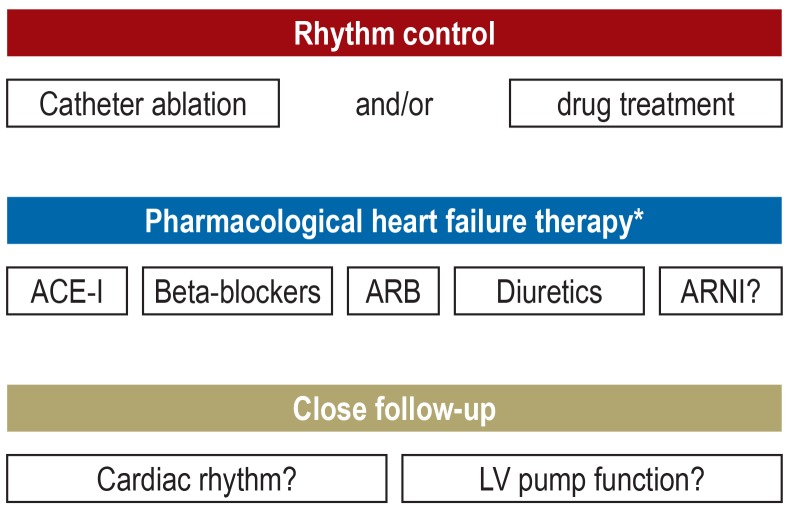
In addition to treating the causal arrhythmia, arrhythmia-induced cardiomyopathy is also treated with the medication generally recommended for systolic heart failure (figure 3) (1, 4). However, it should be noted that there are no specific study data evidencing the beneficial effect of this heart failure medication on the course or prognosis of arrhythmia-induced cardiomyopathy. This also applies to the administration of drugs following recovery of LV pump function. Even if one takes into consideration the available knowledge on the pathophysiology of arrhythmia-induced cardiomyopathy, heart failure medication still appears to be indicated, not least since the importance of this treatment in systolic heart failure in general (irrespective of etiology) is supported by a high level of evidence and enshrined in the guidelines (1, 4). Figure 3 summarizes the general treatment principles in arrhythmia-induced cardiomyopathy.
Figure 3.
Therapy and clinical treatment of arrhythmia-induced cardiomyopathy
* Medication depending on the stage of heart failure as well as the extent of left ventricular systolic dysfunction and in accordance with the guidelines (1, 4).
ACE-I, angiotensin-converting enzyme inhibitor
ARB, aldosterone receptor blocker
ARNI, angiotensin receptor neprilysin inhibitor
Since arrhythmia-induced cardiomyopathy is a reversible form of cardiomyopathy, primary prophylactic defibrillator placement is not indicated, even in initially severe LVSD (39). The temporary use of a wearable cardioverter defibrillator can be considered in patients with suspected arrhythmia-induced cardiomyopathy as a bridging treatment option (40).
Arrhythmia-specific diagnostic tests and treatment
Atrial fibrillation
Atrial fibrillation is the most common persistent arrhythmia in adults and the most common cause of arrhythmia-induced cardiomyopathy (24, e1). LVSD is found in 20%–30% of all patients with atrial fibrillation (e2), and 10%–50% of patients with heart failure have atrial fibrillation (e3). A structural heart disease is often present in the case of concomitant atrial fibrillation and LVSD. However, 25%–50% of those affected probably have at least a component of arrhythmia-induced cardiomyopathy (8, 11, e4). It should be emphasized that arrhythmia-induced cardiomyopathy also occurs in atrial fibrillation and normal ventricular rates (11).
It is not uncommon in clinical practice for atrial fibrillation and LVSD to be diagnosed simultaneously for the first time, making a causal link unclear. In such cases, the initial aim should be pharmacological rate control (beta-blockers ± digitalis) and drug therapy for heart failure (e2), since atrial fibrillation in the setting of heart failure may be present at the time of first contact. Optimal ventricular rates have not been definitively established as yet. At present, 60–100/min at rest and <110/min during light exercise are recommended (1). Particularly in the case of continued symptoms, inadequate rate control, and/or persistent LVSD, an attempt (if not contraindicated) at rhythm control using electrical cardioversion and, where appropriate, amiodarone are recommended. Depending on disease course and taking other diagnostic tests (figure 2) into consideration, it is possible to differentiate whether LVSD is caused by:
True arrhythmia-induced cardiomyopathy (due to atrial fibrillation)
Another heart disease (resulting in atrial fibrillation)
A mixed form (another heart disease and worsening of LVSD due to atrial fibrillation).
The majority of randomized studies on the treatment of atrial fibrillation in LVSD predominantly included patients with chronic systolic heart failure and structural heart disease. Compared with rate control only, rhythm control using electrical cardioversion and amiodarone resulted in improved left ventricular pump function and quality of life (e5), but not in reduced mortality (e6). In a meta-analysis (26 studies, 1838 LVSD patients, 61% structural heart disease, 39% idiopathic), the average left ventricular ejection fraction improved from 40% to 53% following left atrial ablation of atrial fibrillation (e7). Although this positive effect was seen in an earlier observational study also on structural heart disease, it was less pronounced compared with primary idiopathic LVSD (increase in left ventricular ejection fraction of 16% ± 14% and 24% ± 10%) (12).
Only a handful of prospective studies have investigated the effect of catheter ablation in atrial fibrillation specifically in patients with primary idiopathic LVSD (and hence most likely purely arrhythmia-induced cardiomyopathy). In an investigation of 16 patients, left ventricular pump function normalized (ejection fraction 40% ± 10% at baseline and 60% ± 6% after 6 months, p <0.001) in 15 subjects with stable sinus rhythm (27). A randomized study of 68 patients with primary idiopathic LVSD recently showed that even in the case of prior optimal pharmacological rate control, ablation of atrial fibrillation significantly improves left ventricular ejection fraction (32% ± 9% to 50% ± 11% versus 34% ± 8% to 38% ± 9% in continued rate control, p <0.0001) (11). If no ventricular LGE was detected on previous MRI, left ventricular ejection fraction normalizes in 75% of patients following ablation.
In summary, the preservation of sinus rhythm should be the goal in arrhythmia-induced cardiomyopathy secondary to atrial fibrillation. In terms of treatment, catheter ablation in the left atrium should be considered, taking the age, comorbidities, symptoms, and desires of the patient into account (e2). The risk of relevant procedure-related complications is around 5%, and more than one procedure is required to stabilize sinus rhythm in approximately 50% of cases (e2). In the case of treatment-refractory recurrent or permanent atrial fibrillation accompanied by a ventricular rate that cannot be adequately controlled pharmacologically, a combination of atrioventricular node ablation and (potentially bi-) ventricular stimulation (ablate and pace) is a safe and effective treatment option (e2, e8, e9).
Atrial flutter
An analysis of >1000 patients with atrial flutter found the incidence of arrhythmia-induced cardiomyopathy to be approximately 8% (13). Arrhythmia-induced cardiomyopathy patients had higher ventricular rates (109 ± 19 versus 84 ± 23/min) during atrial flutter compared with LVSD of other etiology (e10). Pharmacological control of ventricular rate is more challenging in atrial flutter than it is in atrial fibrillation, necessitating rhythm control more often. Electrical cardioversion is an effective method for the acute restoration of sinus rhythm. However, in view of the risk of recurrence, one should consider catheter ablation, which is associated with high success and low complication rates in typical atrial flutter (e11). Upon ablation of atrial flutter in patients with concomitant LVSD, an improvement in left ventricular function was seen in over 50% of the patients, and complete normalization in approximately 75% of these cases. (13, e10).
Supraventricular tachycardia
The arrhythmias that go to make up the group of supraventricular tachycardias (SVT) in the narrower sense are shown in the Box. SVT rarely trigger arrhythmia-induced cardiomyopathy in adulthood. According to a multicenter study on 81 children, on the other hand, persistent SVT is the underlying disorder in approximately 90% of pediatric cases of arrhythmia-induced cardiomyopathy (e12). This analysis found permanent focal atrial tachycardia in 59% and permanent junctional reentrant tachycardia in 23% of children with arrhythmia-induced cardiomyopathy. These forms of tachycardia tend to be rare in adulthood and often result in arrhythmia-induced cardiomyopathy in affected children (18%–28% of cases) (8), presumably since the persistent but relatively moderate increase in heart rate to 150–200/min frequently does not cause acute symptoms (e13).
Vagal maneuvers or the rapid administration of adenosine are recommended for the acute management of SVT (e11, e14). However, in the case of SVT-related arrhythmia-induced cardiomyopathy, these measures generally achieve a transient cessation of SVT at best. Where possible, catheter ablation should be aimed for in terms of curative treatment (e11, e14). Success rates vary between 80% and 95% depending on the mechanism of SVT (e11, e14). Ablation is only recommended in infants and young children if pharmacological treatment is not feasible or unsuccessful (e14).
Frequent premature ventricular contractions and ventricular tachycardias
Frequent premature ventricular contractions (PVC) can cause arrhythmia-induced cardiomyopathy in patients without structural heart disease (33) and worsen left ventricular function in the case of pre-existing LVSD (e15, e16). In a study on patients with relatively frequent idiopathic premature ventricular contractions (>1000/day), arrhythmia-induced cardiomyopathy occurred in 13 of the 239 patients (5.4%) during a follow-up period of 5.6 years (e17). The probability of arrhythmia-induced cardiomyopathy developing increases with the frequency of premature ventricular contractions (e17). This was quantified utilizing ECG holter monitoring as an absolute (premature ventricular contractions/day) or relative frequency (premature ventricular contraction burden as a percentage of all QRS complexes). The literature gives threshold values for premature ventricular contraction burden and the risk of arrhythmia-induced cardiomyopathy, ranging from a burden of >10% to >24% (38, 39). The likelihood of arrhythmia-induced cardiomyopathy also increases with the QRS duration of premature ventricular contractions, which correlates with the extent of ventricular asynchrony (e18). According to retrospective analyses, the risk of arrhythmia-induced cardiomyopathy increases at a premature ventricular contraction QRS duration >150 ms (e18– e20). In the case of frequent premature ventricular contractions and associated risk, left ventricular pump function should be regularly checked (every 6–12 months in the authors‘ opinion) in order to rule out arrhythmia-induced cardiomyopathy (e17, e21).
Ventricular tachycardia only rarely causes arrhythmia-induced cardiomyopathy, likely due to the fact that it generally causes acute symptoms, thereby leading to treatment.
With long-term success rates of between 66% and 90%, catheter ablation is the treatment of first choice for the elimination of premature ventricular contractions that trigger arrhythmia-induced cardiomyopathy (e22, e23). Alternatively, one can consider an attempt at pharmacological treatment, primarily with amiodarone, depending on age, comorbidities, and the presumed focus of premature ventricular contractions (e24).
Disease course and prognosis
Following effective treatment, left ventricular pump function normally recovers within a few weeks to months (8, 33, 36, e1, e25, e26). In the authors‘ opinion, initial outpatient follow-up should be frequent (e.g., every 1–3 months) and should include both ECG holter monitoring and an echocardiogram. Depending on the triggering arrhythmia, and despite primarily successful catheter ablation, arrhythmia recurrence can occur at an incidence of approximately 5% (SVT) to around 50% (atrial fibrillation). This can result in failure of left ventricular pump function to recover completely during follow-up (e27) or, following initial normalization, to rapidly worsen again (e1). The recurrence rate of arrhythmia-induced cardiomyopathy following initially successful treatment has not been definitively elucidated: a study on 12 patients (observation period of 53 ± 24 months) put it at approximately 25% (e25).
According to current knowledge, if left ventricular pump function recovers following arrhythmia treatment, the prognosis in terms of survival is good (13, e1, e25, e28). However, one study showed that, even years after arrhythmia-induced cardiomyopathy and normalization of LVSD on MRI, mild left ventricular dilatation and ultrastructural myocardial lesions may be seen (e29). This observation may also explain the apparently extremely rare yet nevertheless reported cases of sudden cardiac death observed in arrhythmia-induced cardiomyopathy patients in whom left ventricular pump function had normalized (e1). Therefore, the preliminary continuation of heart failure medication following the resolution of LVSD appears advisable, even if no evidence for this practice exists specifically for arrhythmia-induced cardiomyopathy (8).
Supplementary Material
eMETHODS
A selective literature search was performed in PubMed for the period 1987–2017 based on the authors‘ clinical and scientific experience. Search terms included, among others, “arrhythmia-induced cardiomyopathy,” “tachymyopathy,” “tachycardia-induced cardiomyopathy,” “heart failure,” “arrhythmia,” “ablation therapy,” “atrial fibrillation,” “atrial flutter,” “premature ventricular contraction.” Original articles, case reports, and review articles were included. It was borne in mind here that mainly retrospective analyses of selected collectives with limited case numbers and only scant prospective studies are available on the topic. There are no guidelines on arrhythmia-induced cardiomyopathy; however, where relevant, the guidelines and statements of the European Society of Cardiology (ESC) on the respective forms of arrhythmia and the topics heart failure and cardiomyopathy were taken into account
Acknowledgments
Translated from the original German by Christine Schaefer-Tsorpatzidis
Footnotes
Conflict of interest statement
Prof. Sossalla received reimbursement of travel and accommodation expenses from Berlin Chemie, Menarini, Novartis, Bayer, Böhringer Medtronic, and Servier. He received lecture fees from Novartis, Menarini, and Berlin-Chemie. He received funding from Novartis for a research project of his own initiation.
Prof. Vollmann was reimbursed for congress participation fees from Boston Scientific, Medtronic, and St. Jude Medical. He was reimbursed for travel and accommodation expenses by Biosense Webster, Boston Scientific, Medtronic, and St. Jude Medical. He received lecture fees from Medtronic, St. Jude Medical, Böhringer Ingelheim, and Novartis.
References
- 1.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 2.van Riet EE, Hoes AW, Limburg A, Landman MA, van der Hoeven H, Rutten FH. Prevalence of unrecognized heart failure in older persons with shortness of breath on exertion. Eur J Heart Fail. 2014;16:772–777. doi: 10.1002/ejhf.110. [DOI] [PubMed] [Google Scholar]
- 3.Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 4.Bundesärztekammer (BÄK), KBK, Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF) Nationale VersorgungsLeitlinie Chronische Herzinsuffizienz - Langfassung Version 1 2017. DOI: 106101/AZQ/000386 2017. [Google Scholar]
- 5.Santangeli P, Marzo F, Camporeale A, Bellocci F, Crea F, Pieroni M. What do tachycardiomyopathy belong to? Eur Heart J. 2008;29:1073–1074. doi: 10.1093/eurheartj/ehn092. [DOI] [PubMed] [Google Scholar]
- 6.Pinto YM, Elliott PM, Arbustini E, et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2016;37:1850–1858. doi: 10.1093/eurheartj/ehv727. [DOI] [PubMed] [Google Scholar]
- 7.Shinbane JS, Wood MA, Jensen DN, Ellenbogen KA, Fitzpatrick AP, Scheinman MM. Tachycardia-induced cardiomyopathy: a review of animal models and clinical studies. J Am Coll Cardiol. 1997;29:709–715. doi: 10.1016/s0735-1097(96)00592-x. [DOI] [PubMed] [Google Scholar]
- 8.Gopinathannair R, Etheridge SP, Marchlinski FE, Spinale FG, Lakkireddy D, Olshansky B. Arrhythmia-induced cardiomyopathies: mechanisms, recognition, and management. J Am Coll Cardiol. 2015;66:1714–1728. doi: 10.1016/j.jacc.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenelon G, Wijns W, Andries E, Brugada P. Tachycardiomyopathy: mechanisms and clinical implications. Pacing Clin Electrophysiol. 1996;19:95–106. doi: 10.1111/j.1540-8159.1996.tb04796.x. [DOI] [PubMed] [Google Scholar]
- 10.Brill IC. Auricular fibrillation with congestive failure and no other evidence of organic heart disease. Am Heart J. 1937:175–182. [Google Scholar]
- 11.Prabhu S, Taylor AJ, Costello BT, et al. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction (CAMERA-MRI) J Am Coll Cardiol. 2017;70:1949–1961. doi: 10.1016/j.jacc.2017.08.041. [DOI] [PubMed] [Google Scholar]
- 12.Hsu LF, Jais P, Sanders P, et al. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004;351:2373–2383. doi: 10.1056/NEJMoa041018. [DOI] [PubMed] [Google Scholar]
- 13.Brembilla-Perrot B, Ferreira JP, Manenti V, et al. Predictors and prognostic significance of tachycardiomyopathy: insights from a cohort of 1269 patients undergoing atrial flutter ablation. Eur J Heart Fail. 2016;18:394–401. doi: 10.1002/ejhf.482. [DOI] [PubMed] [Google Scholar]
- 14.Coleman HN, Taylor RR, Pool PE, et al. Congestive heart failure following chronic tachycardia. Am Heart J. 1971;81:790–798. doi: 10.1016/0002-8703(71)90083-4. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong PW, Stopps TP, Ford SE, de Bold AJ. Rapid ventricular pacing in the dog: pathophysiologic studies of heart failure. Circulation. 1986;74:1075–1084. doi: 10.1161/01.cir.74.5.1075. [DOI] [PubMed] [Google Scholar]
- 16.Schillinger W, Teucher N, Christians C, et al. High intracellular Na+ preserves myocardial function at low heart rates in isolated myocardium from failing hearts. Eur J Heart Fail. 2006;8:673–680. doi: 10.1016/j.ejheart.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Spinale FG, Hendrick DA, Crawford FA, Smith AC, Hamada Y, Carabello BA. Chronic supraventricular tachycardia causes ventricular dysfunction and subendocardial injury in swine. Am J Physiol. 1990;259:H218–H229. doi: 10.1152/ajpheart.1990.259.1.H218. [DOI] [PubMed] [Google Scholar]
- 18.Ellis ER, Josephson ME. Heart failure and tachycardia-induced cardiomyopathy. Curr Heart Fail Rep. 2013;10:296–306. doi: 10.1007/s11897-013-0150-z. [DOI] [PubMed] [Google Scholar]
- 19.Moe GW, Stopps TP, Angus C, Forster C, De Bold AJ, Armstrong PW. Alterations in serum sodium in relation to atrial natriuretic factor and other neuroendocrine variables in experimental pacing-induced heart failure. J Am Coll Cardiol. 1989;13:173–179. doi: 10.1016/0735-1097(89)90567-6. [DOI] [PubMed] [Google Scholar]
- 20.Riegger AJ, Liebau G. The renin-angiotensin-aldosterone system, antidiuretic hormone and sympathetic nerve activity in an experimental model of congestive heart failure in the dog. Clinical Science. 1982;62:465–469. doi: 10.1042/cs0620465. [DOI] [PubMed] [Google Scholar]
- 21.Moe GW, Stopps TP, Howard RJ, Armstrong PW. Early recovery from heart failure: insights into the pathogenesis of experimental chronic pacing-induced heart failure. J Lab Clin Med. 1988;112:426–432. [PubMed] [Google Scholar]
- 22.Ellis ER, Josephson ME. What about tachycardia-induced cardiomyopathy? Arrhythm Electrophysiol Rev. 2013;2:82–90. doi: 10.15420/aer.2013.2.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zupan I, Rakovec P, Budihna N, Brecelj A, Kozelj M. Tachycardia induced cardiomyopathy in dogs; relation between chronic supraventricular and chronic ventricular tachycardia. Int J Cardiol. 1996;56:75–81. doi: 10.1016/0167-5273(96)02728-3. [DOI] [PubMed] [Google Scholar]
- 24.Jeong YH, Choi KJ, Song JM, et al. Diagnostic approach and treatment strategy in tachycardia-induced cardiomyopathy. Clin Cardiol. 2008;31:172–178. doi: 10.1002/clc.20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujino T, Yamashita T, Suzuki S, et al. Characteristics of congestive heart failure accompanied by atrial fibrillation with special reference to tachycardia-induced cardiomyopathy. Circ J. 2007;71:936–940. doi: 10.1253/circj.71.936. [DOI] [PubMed] [Google Scholar]
- 26.Lishmanov A, Chockalingam P, Senthilkumar A, Chockalingam A. Tachycardia-induced cardiomyopathy: evaluation and therapeutic options. Congest Heart Fail. 2010;16:122–126. doi: 10.1111/j.1751-7133.2010.00147.x. [DOI] [PubMed] [Google Scholar]
- 27.Ling LH, Taylor AJ, Ellims AH, et al. Sinus rhythm restores ventricular function in patients with cardiomyopathy and no late gadolinium enhancement on cardiac magnetic resonance imaging who undergo catheter ablation for atrial fibrillation. Heart Rhythm. 2013;10:1334–1339. doi: 10.1016/j.hrthm.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 28.Addison D, Farhad H, Shah RV, et al. Effect of late gadolinium enhancement on the recovery of left ventricular systolic function after pulmonary vein isolation. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003570. pii: e003570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Achenbach S, Barkhausen J, Beer M, et al. Konsensusempfehlungen der DRG/DGK/DGPK zum Einsatz der Herzbildgebung mit Computertomographie und Magnetresonanztomographie. Der Kardiologe. 2012;6:105–125. [Google Scholar]
- 30.Mueller KAL, Heinzmann D, Klingel K, et al. Histopathological and immunological characteristics of tachycardia-induced cardiomyopathy. J Am Coll Cardiol. 2017;69:2160–2172. doi: 10.1016/j.jacc.2017.02.049. [DOI] [PubMed] [Google Scholar]
- 31.Medi C, Kalman JM, Haqqani H, et al. Tachycardia-mediated cardiomyopathy secondary to focal atrial tachycardia: long-term outcome after catheter ablation. J Am Coll Cardiol. 2009;53:1791–1797. doi: 10.1016/j.jacc.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Zhong L, Lee YH, Huang XM, et al. Relative efficacy of catheter ablation vs antiarrhythmic drugs in treating premature ventricular contractions: a single-center retrospective study. Heart Rhythm. 2014;11:187–193. doi: 10.1016/j.hrthm.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 33.Bogun F, Crawford T, Reich S, et al. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: comparison with a control group without intervention. Heart Rhythm. 2007;4:863–867. doi: 10.1016/j.hrthm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 34.van Gelder IC, Crijns HJ, Blanksma PK, et al. Time course of hemodynamic changes and improvement of exercise tolerance after cardioversion of chronic atrial fibrillation unassociated with cardiac valve disease. Am J Cardiol. 1993;72:560–566. doi: 10.1016/0002-9149(93)90352-d. [DOI] [PubMed] [Google Scholar]
- 35.Rabbani LE, Wang PJ, Couper GL, Friedman PL. Time course of improvement in ventricular function after ablation of incessant automatic atrial tachycardia. Am Heart J. 1991;121:816–819. doi: 10.1016/0002-8703(91)90193-l. [DOI] [PubMed] [Google Scholar]
- 36.Yokokawa M, Good E, Crawford T, et al. Recovery from left ventricular dysfunction after ablation of frequent premature ventricular complexes. Heart Rhythm. 2013;10:172–175. doi: 10.1016/j.hrthm.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 37.Hasdemir C, Kartal Y, Simsek E, Yavuzgil O, Aydin M, Can LH. Time course of recovery of left ventricular systolic dysfunction in patients with premature ventricular contraction-induced cardiomyopathy. Pacing Clin Electrophysiol. 2013;36:612–617. doi: 10.1111/pace.12087. [DOI] [PubMed] [Google Scholar]
- 38.Baman TS, Lange DC, Ilg KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010;7:865–869. doi: 10.1016/j.hrthm.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 39.Hasdemir C, Ulucan C, Yavuzgil O, et al. Tachycardia-induced cardiomyopathy in patients with idiopathic ventricular arrhythmias: the incidence, clinical and electrophysiologic characteristics, and the predictors. J Cardiovasc Electrophysiol. 2011;22:663–668. doi: 10.1111/j.1540-8167.2010.01986.x. [DOI] [PubMed] [Google Scholar]
- 40.Erath JW, Vamos M, Benz AP, Hohnloser SH. Usefulness of the WCD in patients with suspected tachymyopathy. Clin Res Cardiol. 2018;107:70–75. doi: 10.1007/s00392-017-1159-1. [DOI] [PubMed] [Google Scholar]
- E1.Nerheim P, Birger-Botkin S, Piracha L, Olshansky B. Heart failure and sudden death in patients with tachycardia-induced cardiomyopathy and recurrent tachycardia. Circulation. 2004;110:247–252. doi: 10.1161/01.CIR.0000135472.28234.CC. [DOI] [PubMed] [Google Scholar]
- E2.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- E3.Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91:2–8. doi: 10.1016/s0002-9149(02)03373-8. [DOI] [PubMed] [Google Scholar]
- E4.Edner M, Caidahl K, Bergfeldt L, Darpo B, Edvardsson N, Rosenqvist M. Prospective study of left ventricular function after radiofrequency ablation of atrioventricular junction in patients with atrial fibrillation. Br Heart J. 1995;74:261–267. doi: 10.1136/hrt.74.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E5.Shelton RJ, Clark AL, Goode K, et al. A randomised, controlled study of rate versus rhythm control in patients with chronic atrial fibrillation and heart failure: (CAFE-II Study) Heart. 2009;95:924–930. doi: 10.1136/hrt.2008.158931. [DOI] [PubMed] [Google Scholar]
- E6.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- E7.Anselmino M, Matta M, D‘Ascenzo F, et al. Catheter ablation of atrial fibrillation in patients with left ventricular systolic dysfunction: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2014;7:1011–1018. doi: 10.1161/CIRCEP.114.001938. [DOI] [PubMed] [Google Scholar]
- E8.Ozcan C, Jahangir A, Friedman PA, et al. Significant effects of atrioventricular node ablation and pacemaker implantation on left ventricular function and long-term survival in patients with atrial fibrillation and left ventricular dysfunction. Am J Cardiol. 2003;92:33–37. doi: 10.1016/s0002-9149(03)00460-0. [DOI] [PubMed] [Google Scholar]
- E9.Chatterjee NA, Upadhyay GA, Ellenbogen KA, McAlister FA, Choudhry NK, Singh JP. Atrioventricular nodal ablation in atrial fibrillation: a meta-analysis and systematic review. Circ Arrhythm Electrophysiol. 2012;5:68–76. doi: 10.1161/CIRCEP.111.967810. [DOI] [PubMed] [Google Scholar]
- E10.Pizzale S, Lemery R, Green MS, Gollob MH, Tang AS, Birnie DH. Frequency and predictors of tachycardia-induced cardiomyopathy in patients with persistent atrial flutter. Can J Cardiol. 2009;25:469–472. doi: 10.1016/s0828-282x(09)70119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E11.Katritsis DGC, Boriani G, Cosio FG, et al. European Heart Rhythm Association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLAECE) Europace. 2017;19:465–511. doi: 10.1093/europace/euw301. [DOI] [PubMed] [Google Scholar]
- E12.Moore JP, Patel PA, Shannon KM, et al. Predictors of myocardial recovery in pediatric tachycardia-induced cardiomyopathy. Heart Rhythm. 2014;11:1163–1169. doi: 10.1016/j.hrthm.2014.04.023. [DOI] [PubMed] [Google Scholar]
- E13.Paul T, Bertram H, Kriebel T, Windhagen-Mahnert B, Tebbenjohanns J, Hausdorf G. Supraventricular tachycardia in infants, children and adolescents: diagnosis, drug and interventional therapy. Z Kardiol. 2000;89:546–558. doi: 10.1007/s003920070227. [DOI] [PubMed] [Google Scholar]
- E14.Philip Saul J, Kanter RJ, Writing C, et al. PACES/HRS expert consensus statement on the use of catheter ablation in children and patients with congenital heart disease: developed in partnership with the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American Academy of Pediatrics (AAP), the American Heart Association (AHA), and the Association for European Pediatric and Congenital Cardiology (AEPC) Heart Rhythm. 2016;13:251–289. doi: 10.1016/j.hrthm.2016.02.009. [DOI] [PubMed] [Google Scholar]
- E15.Sarrazin JF, Labounty T, Kuhne M, et al. Impact of radiofrequency ablation of frequent post-infarction premature ventricular complexes on left ventricular ejection fraction. Heart Rhythm. 2009;6:1543–1549. doi: 10.1016/j.hrthm.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E16.El Kadri M, Yokokawa M, Labounty T, et al. Effect of ablation of frequent premature ventricular complexes on left ventricular function in patients with nonischemic cardiomyopathy. Heart Rhythm. 2015;12:706–713. doi: 10.1016/j.hrthm.2014.12.017. [DOI] [PubMed] [Google Scholar]
- E17.Niwano S, Wakisaka Y, Niwano H, et al. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart. 2009;95:1230–1237. doi: 10.1136/hrt.2008.159558. [DOI] [PubMed] [Google Scholar]
- E18.Yokokawa M, Kim HM, Good E, et al. Impact of QRS duration of frequent premature ventricular complexes on the development of cardiomyopathy. Heart Rhythm. 2012;9:1460–1464. doi: 10.1016/j.hrthm.2012.04.036. [DOI] [PubMed] [Google Scholar]
- E19.Carballeira Pol L, Deyell MW, Frankel DS, et al. Ventricular premature depolarization QRS duration as a new marker of risk for the development of ventricular premature depolarization-induced cardiomyopathy. Heart Rhythm. 2014;11:299–306. doi: 10.1016/j.hrthm.2013.10.055. [DOI] [PubMed] [Google Scholar]
- E20.Deneke T, Borggrefe M, Hindricks G, et al. Kommentar zu den ESC-Leitlinien 2015. Ventrikuläre Arrhythmien und Prävention des plötzlichen Herztodes. Kardiologe. 2017;11:27–43. [Google Scholar]
- E21.Laplante L, Benzaquen BS. A review of the potential pathogenicity and management of frequent premature ventricular contractions. Pacing Clin Electrophysiol. 2016;39:723–730. doi: 10.1111/pace.12870. [DOI] [PubMed] [Google Scholar]
- E22.Zang M, Zhang T, Mao J, Zhou S, He B. Beneficial effects of catheter ablation of frequent premature ventricular complexes on left ventricular function. Heart. 2014;100:787–793. doi: 10.1136/heartjnl-2013-305175. [DOI] [PubMed] [Google Scholar]
- E23.Priori SG, Blomstrom-Lundqvist C, Mazzanti A, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) Eur Heart J. 2015;36:2793–2867. doi: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]
- E24.Ling Z, Liu Z, Su L, et al. Radiofrequency ablation versus antiarrhythmic medication for treatment of ventricular premature beats from the right ventricular outflow tract: prospective randomized study. Circ Arrhythm Electrophysiol. 2014;7:237–243. doi: 10.1161/CIRCEP.113.000805. [DOI] [PubMed] [Google Scholar]
- E25.Watanabe H, Okamura K, Chinushi M, et al. Clinical characteristics, treatment, and outcome of tachycardia induced cardiomyopathy. Int Heart J. 2008;49:39–47. doi: 10.1536/ihj.49.39. [DOI] [PubMed] [Google Scholar]
- E26.Ilkhanoff L, Gerstenfeld EP, Zado ES, Marchlinski FE. Changes in ventricular dimensions and function during recovery of atrial tachycardia-induced cardiomyopathy treated with catheter ablation. J Cardiovasc Electrophysiol. 2007;18:1104–1106. doi: 10.1111/j.1540-8167.2007.00811.x. [DOI] [PubMed] [Google Scholar]
- E27.Nedios S, Sommer P, Dagres N, et al. Long-term follow-up after atrial fibrillation ablation in patients with impaired left ventricular systolic function: the importance of rhythm and rate control. Heart Rhythm. 2014;11:344–351. doi: 10.1016/j.hrthm.2013.12.031. [DOI] [PubMed] [Google Scholar]
- E28.Ju W, Yang B, Li M, et al. Tachycardiomyopathy complicated by focal atrial tachycardia: incidence, risk factors, and long-term outcome. J Cardiovasc Electrophysiol. 2014;25:953–957. doi: 10.1111/jce.12428. [DOI] [PubMed] [Google Scholar]
- E29.Ling LH, Kalman JM, Ellims AH, et al. Diffuse ventricular fibrosis is a late outcome of tachycardia-mediated cardiomyopathy after successful ablation. Circ Arrhythm Electrophysiol. 2013;6:697–704. doi: 10.1161/CIRCEP.113.000681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMETHODS
A selective literature search was performed in PubMed for the period 1987–2017 based on the authors‘ clinical and scientific experience. Search terms included, among others, “arrhythmia-induced cardiomyopathy,” “tachymyopathy,” “tachycardia-induced cardiomyopathy,” “heart failure,” “arrhythmia,” “ablation therapy,” “atrial fibrillation,” “atrial flutter,” “premature ventricular contraction.” Original articles, case reports, and review articles were included. It was borne in mind here that mainly retrospective analyses of selected collectives with limited case numbers and only scant prospective studies are available on the topic. There are no guidelines on arrhythmia-induced cardiomyopathy; however, where relevant, the guidelines and statements of the European Society of Cardiology (ESC) on the respective forms of arrhythmia and the topics heart failure and cardiomyopathy were taken into account