Abstract
In this research paper the production of biodiesel from palm kernel oil (PKO) using CaO obtained from waste turkey bones (WTB) and analytical grade calcium oxide was investigated. Treated WTB was reduced to fine particulate size of <150 µm and then calcinated at 800 °C for 3 h to increase its catalytic activity by its conversion from Calcium phosphate hydroxide (Ca10P6O26H2) to CaO. X-ray diffraction (XRD) and X-ray fluorescent (XRF) analysis of the analytical grade CaO, uncalcined and calcined WTB were carried out to establish their elemental chemical composition. The transesterification reaction between the triglyceride of palm kernel oil (PKO) and methanol was carried out at a constant agitation speed of 600 rpm and temperature of 65 °C, with varied methanol to oil molar ratio (8–14), catalyst concentration (1–7 wt/wt%) and the reaction time (1–3 h). Minitab 17 software (using response surface method) was employed for the design of experiment and statistical analysis required in the transesterification process of biodiesel production. The qualities of the biodiesel produced were assessed and the results obtained showed conformity of the biodiesel produced to the ASTM standard for biodiesel.
Keywords: Biodiesel, Calcined waste turkey bone, Catalyst, Palm kernel oil
Specifications Table
| Subject area | Materials Science Engineering |
| More specific subject area | Renewable Energy |
| Type of data | Table, image |
| How data was acquired | XRF and XRD spectroscopy principles were employed in the elemental chemical analysis of CaO (analytical grade), uncalcined and calcined turkey bone used as catalyst. Biodiesel production through transesterification process (using Minitab 17, Box Benkhen design) was employed in generating data on the effects of the process parameters (main and interaction effects) on biodiesel yields. Analytical tests to determine the properties of the biodiesel obtained were carried out. |
| Data format | Raw, Analyzed |
| Experimental factors | Methanol/oil mole ratio (8–14), catalyst concentration (1–7 wt/wt%) and reaction time (1–3 hours) were the process parameters that were considered during biodiesel production (transesterification process). |
| Experimental features | X-ray Diffraction (XRD) analysis was carried out to determine the elemental components of the different catalyst used. The analysis involved Mac science X-ray diffraction system (MXP3A-HF) with CuKα X-ray source (λ=0.15 nm and k=1.5406 Å) operated at 30 mA and 40 kV. The diffractograms were recorded in the 2 h ranges of 5–70 with a 2 h step size of 0.03. This was done to determine the diffraction pattern of the finely grounded calcined and uncalcined waste turkey bones (WTB) catalyst. X-ray fluorescence (XRF) (Thermo Scientific ARL OP-TIM’X 166) gave the composition of both the calcined and uncalcined catalyst (WTB). That is, the elemental composition (in percent) of both the calcined and uncalcined WTB were determined. |
| Data source location | Department of Chemical Engineering, Covenant University, Ota, Nigeria and Metallurgical and Chemical Engineering Department, Amadu Bello University, Zaria, Kaduna State, Nigeria. |
| Data accessibility | Data are available within this article |
Value of the data
-
•
The data on biodiesel production was modelled to established the correlation and relationship between the process variables (methanol/oil mole ratio, catalyst concentration and reaction time) and the yields of biodiesel.
-
•
The given data will show authors in the field of material science and chemical engineering that the calcination of WTB (waste turkey bone) will aid conversion of Calcium phosphate hydroxide (Ca10P6O26H2) to CaO catalyst for biodiesel production.
-
•
The data will aid in the establishment of the optimum conditions for the production of PKO biodiesel yield.
-
•
The models obtained from the transesterification of PKO can be used to predict the yield, operating conditions using any other vegetable oils.
-
•
The data reveals that calcined WTB catalyst is a potential source of CaO heterogenous catalyst that can be used in the place of the conventional CaO catalyst during the transesterification of vegetable oil.
1. Data
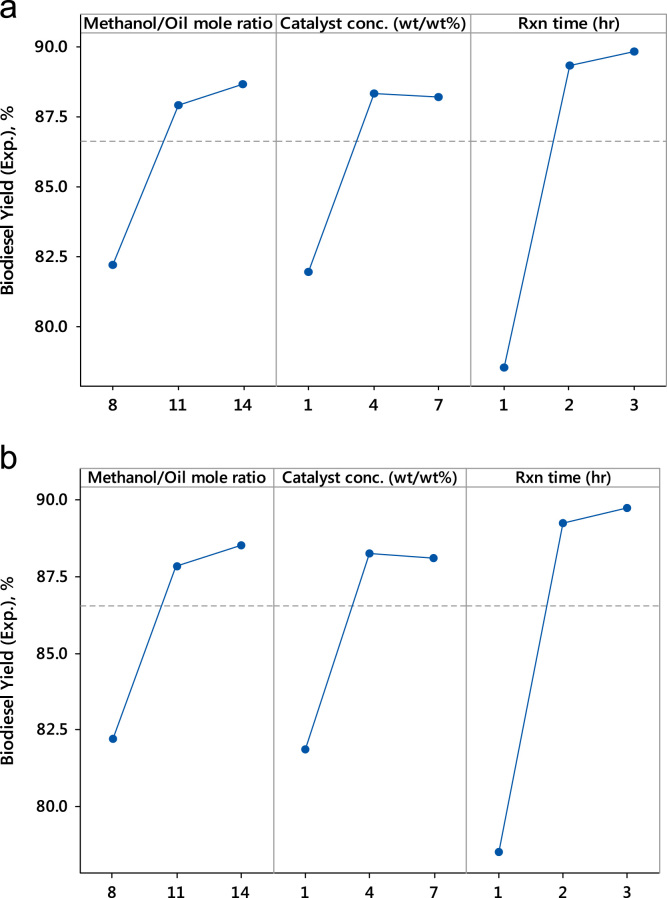
Table 1 shows the data obtained from XRF analysis of CaO, uncalcined waste turkey bones and calcined waste turkey bones (used as catalyst). Fig. 1 shows the data obtained from XRD analysis of both the uncalcined WTB and calcined WTB. Table 2 shows the design of experiment, experimental biodiesel yield and calculated biodiesel yield data obtained from the transesterification process of PKO, using the conventional CaO and calcined WTB catalysts. Fig. 2 shows the main effects of the process variables (methanol/oil mole ratio, catalyst concentration and reaction time) on the biodiesel yields obtained. The interactive effects of the process variables (methanol/oil mole ratio, catalyst concentration and reaction time) on the biodiesel yields (using CaO catalyst) are shown is Fig. 3, while the interactive effects of the process variables (using calcined WTB catalyst) are shown in Fig. 4. Properties of the PKO biodiesel obtained are tabulated in Table 3. The suitable model equations obtained for the yields of biodiesel are shown in Eqs. (1), (2). Table 4 shows the analysis of variance (ANOVA) for CaO catalysed process, while Table 5 shows the analysis of variance (ANOVA) for WTB catalysed process.
Table 1.
XRF analysis of CaO, uncalcined WTB and calcined WTB catalysts.
| Compounds |
Composition of catalyst (%) |
||
|---|---|---|---|
| Calcium Oxide | Uncalcined WTB | Calcined WTB at 800 °C | |
| CaO | 99.230 | 62.335 | 65.599 |
| P2O5 | _ | 30.084 | 32.119 |
| SiO2 | _ | 2.882 | 1.766 |
| MgO | 0.110 | 0.640 | 0.296 |
| Al2O3 | _ | 0.647 | 0.465 |
| SO3 | _ | 0.454 | 0.120 |
| Cl | – | 0.085 | 0.100 |
| K2O | _ | 0.103 | 0.128 |
| TiO2 | 0.010 | 0.000 | 0.063 |
| Cr2O3 | – | 0.002 | 0.000 |
| Mn2O3 | – | 0.004 | 0.005 |
| Fe2O3 | 0.110 | 0.158 | 0.184 |
| ZnO | 0.210 | 0.047 | 0.043 |
| SrO | 0.120 | 0.025 | 0.025 |
| Na2O | 0.020 | 0.214 | 0.356 |
Fig. 1.
XRD analysis of uncalcined WTB (in blue) and calcined WTB (in green) catalyst.
Table 2.
Design of experiment and biodiesel yields obtained.
| Exptal. Run | Methanol/Oil mole ratio | Catalyst conc. (wt/wt%) | Rxn Time (hour) | Experimental % Biodiesel Yield using CaO | Calculated % Biodiesel Yield using CaO | Experimental % Biodiesel Yield using WTB | Calculated % Biodiesel Yield using WTB |
|---|---|---|---|---|---|---|---|
| 1 | 8 | 1 | 2 | 78.65 | 79.57 | 73.00 | 72.94 |
| 2 | 14 | 1 | 2 | 87.56 | 86.68 | 84.00 | 83.54 |
| 3 | 8 | 7 | 2 | 85.90 | 86.60 | 85.30 | 85.74 |
| 4 | 14 | 7 | 2 | 93.23 | 92.13 | 87.20 | 87.23 |
| 5 | 8 | 4 | 1 | 82.30 | 80.22 | 78.00 | 77.67 |
| 6 | 14 | 4 | 1 | 78.00 | 77.54 | 74.20 | 74.27 |
| 7 | 8 | 4 | 3 | 82.20 | 82.48 | 77.90 | 77.81 |
| 8 | 14 | 4 | 3 | 95.90 | 97.80 | 93.00 | 93.31 |
| 9 | 11 | 1 | 1 | 75.56 | 76.54 | 72.89 | 73.26 |
| 10 | 11 | 7 | 1 | 78.50 | 79.70 | 82.30 | 82.17 |
| 11 | 11 | 1 | 3 | 86.10 | 84.731 | 83.40 | 83.51 |
| 12 | 11 | 7 | 3 | 95.20 | 94.04 | 91.50 | 91.11 |
| 13 | 11 | 4 | 2 | 94.30 | 93.30 | 90.90 | 90.62 |
| 14 | 11 | 4 | 2 | 93.46 | 93.30 | 91.00 | 90.63 |
| 15 | 11 | 4 | 2 | 92.40 | 93.30 | 90.00 | 90.62 |
Fig. 2.
Main effects of the process variables: (a) CaO catalyst, (b) calcined WTB catalyst.
Fig. 3.

Interactive effects of the process variables on yield, using CaO catalyst.
Fig. 4.

Interactive effects of the process variables on yield, using WTB catalyst.
Table 3.
Properties of the biodiesel obtained.
| Property | Unit | Method | PKO Biodiesel using CaO | PKO Biodiesel using WTB catalyst | ASTM Standard Values |
|---|---|---|---|---|---|
| Density (at 20 °C) | g/ml | ASTM D4052 | 0.882 | 0.876 | 0.860–0.890 |
| Viscosity (at 40°) | mm2/s | ASTM D445 | 3.74 | 3.81 | 1.9–6.0 |
| Cloud point | °C | ASTM D240 | 6 | 6 | (−3)–12 |
| Pour Point | °C | ASTM D92 | 2 | 1 | (−15)–13 |
| Flash point | °C | ASTM D93 | 128 | 130 | 93 minimum |
| Acid Value | mgKOH/g | ASTM D664 | 0.32 | 0.35 | 0.8 max |
Table 4.
Analysis of Variance (ANOVA) for the CaO catalysed process.
| Source | Df | Adj SS | Adj MS | F- Value | P-Value | Remarks |
|---|---|---|---|---|---|---|
| Model | 9 | 689.660 | 76.629 | 20.28 | 0.002 | Highly Significant |
| X1: Methanol-Oil mole ratio | 1 | 82.176 | 82.176 | 1.74 | 0.006 | |
| X2: Catalyst Concentration | 1 | 77.875 | 77.875 | 20.60 | 0.006 | |
| X3: Reaction time | 1 | 253.575 | 253.575 | 67.09 | 0.000 | |
| X1X1 | 1 | 36.540 | 36.540 | 9.67 | 0.027 | |
| X2X2 | 1 | 56.328 | 56.328 | 14.90 | 0.012 | |
| X3X3 | 1 | 117.486 | 117.486 | 31.09 | 0.003 | |
| X1X2 | 1 | 0.624 | 0.624 | 8.04 | 0.701 | |
| X1X3 | 1 | 81.000 | 81.000 | 0.17 | 0.006 | |
| X2X3 | 1 | 9.486 | 9.486 | 21.43 | 0.174 | Insignificant |
| Lack-of-Fit | 3 | 17.084 | 0.5.695 | 6.28 | 0.140 | Insignificant |
| Pure Error | 2 | 1.813 | 0.907 | – | – |
R-sq=0.9733, R-sq(adj)= 0.9253,
Table 5.
Analysis of Variance (ANOVA) for the calcined WTB catalysed process.
| Source | Df | Adj SS | Adj MS | F- Value | P-Value | Remarks |
|---|---|---|---|---|---|---|
| Model | 9 | 695.212 | 77.246 | 247.71 | 0.000 | Highly Significant |
| X1: Methanol/Oil mole ratio | 1 | 73.205 | 73.205 | 234.75 | 0.000 | |
| X2: Catalyst Concentration | 1 | 136.208 | 136.208 | 436.79 | 0.000 | |
| X3: Reaction time | 1 | 184.416 | 184.416 | 591.38 | 0.000 | |
| X1X1 | 1 | 92.415 | 92.415 | 296.36 | 0.000 | |
| X2X2 | 1 | 39.13 | 39.130 | 125.48 | 0.000 | |
| X3X3 | 1 | 87.046 | 87.046 | 279.14 | 0.000 | |
| X1X2 | 1 | 20.702 | 20.702 | 66.39 | 0.000 | |
| X1X3 | 1 | 89.302 | 89.302 | 286.37 | 0.000 | |
| X2X3 | 1 | 0.429 | 0.429 | 1.38 | 0.294 | Insignificant |
| Lack-of-Fit | 3 | 0.953 | 0.318 | 1.05 | 0.523 | Insignificant |
| Pure Error | 2 | 0.607 | 0.303 | – | – |
R-sq=0.9978, R-sq(adj)=0.9937,
2. Experimental design, materials and methods
Response surface experimental design (Box-Behnken method, Minitab 17 software) was used in the determination of the effects of the process variables (methanol/oil mole ratio, catalyst concentration and reaction time) on biodiesel yield. Materials and reagents used include waste turkey bones (WTB), palm kernel oil (PKO), Calcium Oxide (99% purity, Sigma-Aldrich UK), H2SO4 (98% purity, Sigma-Aldrich UK), methanol (99.8% purity, J.T Baker USA), Potassium hydroxide (96.5%, BDH AnalAR Inc.), sodium Hydroxide pellets (97.5%, BDH AnalAR Inc.), Isopropyl Alcohol (98% purity, J.T Baker USA) and Benzene (96% purity, J.T Baker USA). Equipment used include XRD and XRF spectrometers for the identification and quantification of the elemental composition of CaO, uncalcined and calcined WTB catalysts.
Waste turkey bones were boiled in hot water for 3 hours (the hot water was replaced every 30 min to remove impurities), dried and then crushed. Treated WTB was reduced to fine particulate size of <150 µm and then calcinated at 800 °C for 3 h to increase its catalytic activity by its conversion from Calcium phosphate hydroxide (Ca10P6O26H2) to CaO. X-ray diffraction (XRD) and X-ray fluorescent (XRF) analysis of the analytical grade CaO, uncalcined and calcined WTB were carried out to establish their elemental chemical composition. X-ray fluorescence (XRF) (Thermo Scientific ARL OP-TIM’X 166) gave the composition of both the calcined and uncalcined WTB catalystS (Table 1).
X-ray Diffraction (XRD) analysis involved Mac science X-ray diffraction system (MXP3A-HF) with CuKα X-ray source (λ=0.15 nm and k=1.5406Å) operated at 30 mA and 40 kV. The diffractograms were recorded in the 2 h ranges of 5–70 with a 2 h step size of 0.03. This was done to determine the diffraction pattern of the finely grounded calcined and uncalcined waste turkey bones (WTB) catalyst. For the uncalcined WTB, major peaks were observed at 2θ=32.0° and 34.5°, other peaks were noticed at 2θ=29.0°, 34.0°, 40.0°, and 46.5° (Fig. 1). These peak values were the characteristics of CaO and Ca10P6O26H2. While the peak for the calcined WTB catalyst was measured at 2θ=32.0° and 33.0°, the main peak values characteristics of calcium oxide [1].
The procedures followed in the experimental production of PKO biodiesel were clearly stated in our previous work [2], [3]. The transesterification reaction between the triglyceride of palm kernel oil (PKO) and methanol was carried out at a constant agitation speed of 600 rpm and temperature of 65 °C, with varied methanol to oil molar ratio (8–14), catalyst concentration (1–7 wt/wt%) and the reaction time (1–3 h) (Table 2, Table 3 and Fig. 2, Fig. 3, Fig. 4).
Models that established relationship between the response (biodiesel yields) and the process parameters were formulated, using Minitab 17 software (Eqs. (1), (2)). Fitness and suitability of the predicted models were statistically analyzed using ANOVA test. The results gave high level of accurate prediction, with R2 values between 0.9253 and 0.9978 (Table 4, Table 5).
| (1) |
| (2) |
where X1=Methanol/Oil mole ratio, X2=Catalyst concentration, X3=Reaction time
Acknowledgement
The financial commitment of the Covenant University Centre for Research Innovation and Discovery (CUCRID) Ota, Nigeria towards the publication of this research is greatly appreciated.
Footnotes
Supplementary data associated with this article can be found in the online version at 10.1016/j.dib.2018.05.103.
Transparency document. Supplementary material
Supplementary material.
.
References
- 1.Y.C. Sharma, B. Singh, J. Korstad, Application of an efficient nonconventional heterogeneous catalyst for biodiesel synthesis from pongamia pinnata oil, Energy & Fuels, DOI: 10.1021/ef901514a. [DOI]
- 2.Ayoola A.A., Hymore F.K., Omonhinmin C.A. Optimization of biodiesel production from selected waste oils using response surface methodology. Biotechnology. 2017;16(1):1–9. [Google Scholar]
- 3.Ayoola A.A., Hymore F.K., Obande M.A., Udeh I.N. Optimization of experimental conditions for biodiesel production. Int. J. Eng. Technol. 2012;12(6):130–133. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.