Abstract
Study of extracellular vesicles (EVs), particularly exosomes, holds significant promise; however, it is technically challenging to define these small and molecularly diverse nanovesicles. With intrinsic molecular payload and biodegradability, molecular engineering of exosomes opens new avenues for mediating cellular responses and developing novel nano-delivery systems in precision therapeutics. Microfluidic lab-on-chip technology is playing pivotal roles in this emerging field. In this review, we have examined scientific advancements of microfluidic technology for engineering exosomes and assessed future applications and perspectives in developing precision therapeutics; this can serve the community via identification of potential new research areas or technologies that are urgently needed in precision therapeutics.
Graphical Abstract
This review examines scientific advancements of microfluidic technology for engineering exosomes and assesses future applications and perspectives in developing precision therapeutics, which can serve the community by identifying potential new research areas or technologies that are urgently needed in precision therapeutics.

Introduction
Since the 2013 Nobel Prize in Medicine for the discovery of vesicles, substantial scientific interests have been devoted to a sub-group of vesicles called exosomes. Exosome-based precision medicine for cancer diagnosis and prognosis has gained significant attention and holds great promise1–2. However, studying exosomes is extremely challenging due to enormous technical difficulties in defining and analyzing these small and molecularly diverse nanovesicles3–5.
In this review, we have discussed exosomes through the term extracellular vesicle to provide a clear elucidation. Extracellular vesicle (EV) is a loose term, which typically describes three types of vesicles: exosomes, microvesicles, and apoptotic bodies.6 The major differences between these three vesicles are their cellular origins and molecular pathways. As illustrated in Fig. 1, the formation of exosomes begins with the creation of endosomes as intracellular vesicles.7 Inward invagination occurs at the endosomal membrane, which creates small membrane-bound intraluminal vesicles (ILVs). At this point, the endosome is referred to as a multivesicular body (MVB). The MVBs then follow one of the two paths to exit: the lysosomal pathway or the secretory pathway.8–9 In the lysosomal pathway, the MVB releases its contents into a lysosome for degradation. In the secretory pathway, the MVB fuses with the plasma membrane and secretes its contents into the extracellular space that are then referred to as exosomes, and their size ranges between 30 and 150 nm.
Fig. 1.

Schematic of exosome biogenesis and comparison to microvesicles.
By contrast, as shown in Fig. 1, microvesicles are formed directly through outward budding of the plasma membrane and can range from around 50 nm to 1 μm in diameter. Compared to exosomes and microvesicles, apoptotic bodies fragmented from apoptotic cells are currently of little interest for therapeutic applications and have not been discussed in this perspective review.
Both exosomes and microvesicles contain a lipid bilayer and protein content derived from their parent cells.10 However, due to their different biogenesis, the lipid bilayer of exosomes contains lipid types from both the plasma membrane and the Golgi apparatus, whereas the microvesicles contain the lipid types from the plasma membrane only6. The protein content of EVs reflects the presence of proteins in the parent cell at the time of formation of EVs9. As a result, both exosomes and microvesicles contain biomolecules, such as, but not limited to, cytosolic proteins (tubulin, actin, and actin-binding proteins), signal transduction proteins (protein kinases, 14-3-3, and heterotrimeric G proteins), nucleic acids, metabolic enzymes (peroxidases, pyruvate, lipid kinases, and enolase 1), tetraspanins, and heat shock proteins (HSP70, HSP90), specific to parent cellular function and status.11–13 However, during the formation of ILVs, specific proteins and nucleic acids are sorted into the cytosolic interior and membrane of exosomes; this makes their surface and contents slightly different from those of microvesicles.14 Although many studies have attempted to identify biomarkers specific only to exosomes, these studies collectively struggle to find these specific markers likely due to the difficulties in completely isolating exosomes from microvesicles and the heterogeneity of exosome subtypes found from the parent cells. Certain markers, such as tetraspanins CD63, CD81, CD9, and CD45, FLOT1, Alix, HSP70, TFRC, and TSG101, have been used to detect the presence of exosomes. 15–17 Unfortunately, there are no good solutions to precisely differentiate neither exosomes from microvesicles nor subtypes of exosomes secreted in variable cellular status. Current exosomes are pooled from a large population of cells, and the understanding of exosome biology completely stems from these ensemble-average measurements of exosome properties. There is still a long path for the clear elucidation of biogenesis, consistent classification of exosome subpopulations, and a good understanding of their molecular packaging.
However, exosomes, sometimes so-called EVs, have been observed to play a vital role in communication, delivery, and mediation of diseases without the need of cell-cell contact.6, 9, 18–24 Upon their release from the parent cell, EVs can either bind to local cells or the extracellular matrix, or enter the body fluids such as blood or cerebrospinal fluid.9, 22 This movement allows EVs to deliver important contents and signals to cells both locally and distantly. In fact, upon injection of marked EVs into the bloodstream, they have been found to be delivered to tissues around the body within minutes;19 this makes them one of the fastest delivery vehicles. Moreover, markers on the EV surface act as targeting subunits, allowing them to bind to targeted cell types for mediating the exchange of genetic information and signal transductions.9, 24 Typically, three mechanisms have been proposed for interpreting the cellular uptake of exosomes: 1) endocytotic mechanisms, in which the exosome is engulfed into the cell; 2) fusion with the cell membrane directly for the release of contents into the cytoplasm; and 3) receptor-ligand-type interactions for signal internalization.6–7, 22–23;
Due to large quantity of EVs in many body fluids and enclosure of a group of proteins and RNA representatives to their parent cells, EVs are very promising for precision medicine in diagnosing diseases and potentially replacing invasive procedures such as biopsies.20, 22, 25–26 The expansive potential of EVs as a therapeutic delivery vehicle is even more impressive. It has been found that altered surface molecules on exosomes can avoid circulation clearance, such as blocking the scavenger receptor class A family (SR-A) to decrease liver clearance,24 as compared to natural exosomes without alteration, undergoing rapid clearance through the kidneys, liver, spleen, and lungs.6, 9,24 Additionally, the small size and slight negative charge of exosomes allow them to avoid clearance through the reticuloendothelial system (RES) and, in turn, decrease renal clearance10, 23 and result in a longer circulation time at the therapeutic sites. Future research on the molecular engineering of exosomes can lead to even more tailored clearance routes.27 Many studies have shown that engineering surface molecules on exosomes allows specific tissue targeting,6, 19, 28–29 which opens a new avenue for speeding up precision therapeutics. Cancer immunotherapy can benefit the most from these engineered molecular targeting mechanisms as they increase the accuracy of drug delivery and decrease the systemic toxic effects of therapy. EVs have a natural tendency to accumulate in solid tumors due to their high penetration and enhanced retention in the dense tissue microenvironment. The abnormally formed blood vessels and surrounding compromised lymphatic system around tumor tissues also delay the efficient drainage of EVs, leading to the accumulation of EVs.23–24 Another advantage of using EVs as a delivery vehicle is their ability to load both hydrophobic and hydrophilic contents either in the interior or in the lipid bilayer.22–23 The bilayer membrane effectively protects the cargo and prevents it from enzymatic degradation during circulation. 6, 23 Multiple signaling molecules and co-stimulating factors can be loaded at the same time for delivery to specific cell types.22, 30 EVs have also demonstrated the ability to easily cross tissue boundaries and spread into deep tissues10 such as the blood-brain barrier6, 23, 31–32 and the blood-tumor barrier22, which have traditionally been a challenge in delivery. EVs are highly biocompatible, biodegradable, and stable, exhibit low immunogenicity,6, 23–24, 29, 33–34 and have been shown to aid regeneration19, 35–36 and induce stem cell differentiation35, 37 and specific immune responses.33, 38 All these traits, as summarized in Fig. 2, make EVs a promising therapeutic delivery device. This review focuses on the state-of-the-art approaches for engineering EVs or exosomes as well as the important and innovative roles that microfluidic lab-on-chip technology can play in unlocking the power of EVs and exosomes.
Fig. 2.
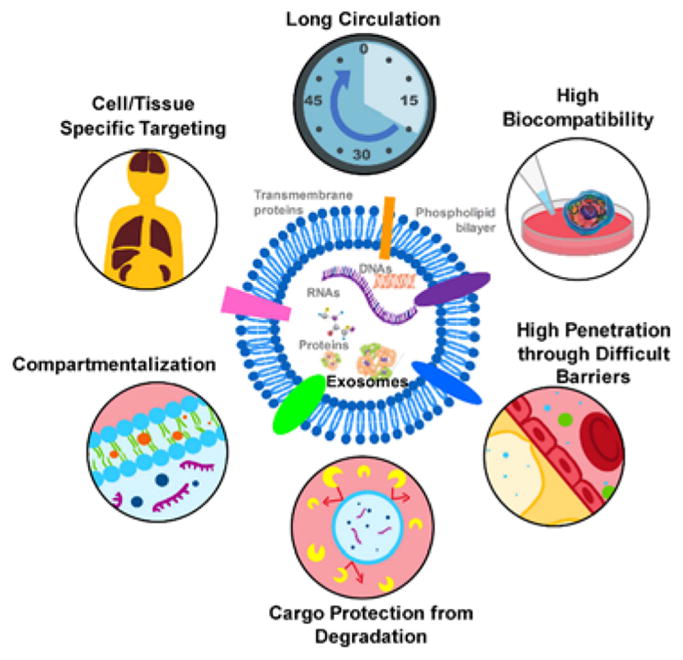
Schematic of exosome structures and delivery advancements.
Advances in the engineering of exosomes
Bioengineered exosomes as emerging immunotherapeutics have gained significant attention in the development of a new generation of cancer vaccines38–43, which have shown fascinating results in pre-clinical studies and early-phase clinical trials39–40, 44–45 with increased stability, solubility, and bioavailability.46 A recent phase-II trial that evaluated IFN-DC-derived exosomes loaded with MHC I/II-restricted cancer antigens as maintenance immunotherapy for non-small cell lung cancer patients47 showed the capability of these exosomes for promoting T cell and natural killer (NK) cell-based immune responses in patients.41 Several phase-II clinical studies have recently been initiated as well for treating malignant ascites and pleural effusion using tumor cell-derived vesicles loaded with chemotherapeutic drugs (ClinicalTrials.gov). Exosome-encapsulated drugs have been proven to be valuable in addressing multiple clinical issues such as therapeutic resistance and toxicity to central nervous system.48–49 Exosomes are also very promising for gene therapies because they naturally perform horizontal transfer of genetic information to specific recipient cells in a pathophysiological contest and preserve the functionality of genetic cargos.50–52 Different surface markers on exosomes, which are varied based on the types of parent cells, can influence functional therapy.
Generally, there are two broad strategies for exosome engineering: 1) manipulation of parent cells either through genetic or metabolic engineering and 2) functionalization of purified exosomes using surface molecular engineering and membrane permeabilization.53 We have summarized the latest engineering strategies for modifying and reconstructing exosomes employed in drug delivery and cancer immunotherapy.
Surface engineering
There are multiple strategies developed recently for molecular surface engineering of exosomes, as illustrated in Fig. 3 with their advantages and drawbacks. Surface display technology via donor cell manipulation has been applied to modify exosome surface structures in targeted drug delivery.54–59 Genetic modification of parent cells is a popular method to display potent proteins or peptides on the surface of exosome membranes.59 In a study reported by Tian et al., tumor-specific targeting was achieved by transfecting immature dendritic cells (DCs) for expressing Lamp2b exosomal membrane proteins fused with the breast cancer cell-specific iRGD peptide. Over 60% exosomes secreted from the engineered DCs displayed Lamp2b on their surface. In addition, by growing parent cells in a modified medium containing 40 μg/mL biotin-functionalized DSPE,60 almost 100% exosomes can inherit biotinylated membrane from the parent cells. Manipulation of donor cells leads to the expression of markers on the exosome surface with high efficiency. However, the transfection efficiency is not consistent, which is highly dependent on RNA species. For instance, in a study reported by Kooijmans et al., only 15–25% exosomes displayed anti-epidermal growth factor receptor (EGFR) nanobodies on their membrane via transfection of Neuro2A cells.61
Fig. 3.

Schematic of strategies for surface engineering of exosomes.
Receptor-ligand binding can also be used for modifying the exosome surface. Qi et al. reported a technique by anchoring superparamagnetic nanoparticles onto reticulocyte-derived exosome surfaces through transferrin-transferrin receptor interactions62 that yielded a superparamagnetic drug delivery system for targeting diseased cells via responding to an external magnetic field. Alternatively, anchoring of binding groups on exosome surfaces can be managed via transgene expression in parent cells. In a recent study, murine melanoma B16BL6 cells were transfected with a plasmid vector encoding streptavidin and lactadherin to obtain SAV-LA-modified exosomes; this enabled the introduction of radioactive labeling of exosomes subsequently via streptavidin-biotin binding.63 Maguire et al. introduced a method employing specific binding between transgenic biotin-acceptor peptides on the surface of exosome with biotinylated magnetic nanoparticles.64 The receptor-ligand binding approach offers an effective way for exosome surface reconstruction. More importantly, this approach is highly specific for activating or eliminating signaling pathways associated with exosomal surface membrane proteins and receptors.
Covalent bonding has also been investigated for exosome surface engineering. Covalent bonds typically have bond energies in the range of 200–900 kJ mol−1, which are much stronger than noncovalent interactions (cf. 2–13 kJ mol−1). Unlike cells, exosomes are nonliving entities. Thus, the bioconjugation and click chemistry reactions can be introduced without the concern of impairing the biological activity.65 Smyth et al. applied alkyne-based cross-linking reactions and successfully attached azide-Fluor 545 fluorescent molecules to the surface of mouse 4T1 breast cancer cell-derived exosomes.66 To date, conjugation chemistry has reported neither the effect of these reactions on the size of exosomes nor any changes in adherence or internalization with recipient cells. However, chemically engineered exosomes are expected to affect bio-distribution. Efforts have been made to improve the tracking of exosomes in vivo for studying their bio-distribution.67
Membrane permeabilization-mediated cargo loading
Due to the lipid membrane bilayer of EVs and exosomes, hydrophobic drugs can be passively loaded via hydrophobic binding during incubation; one successful example is the case of anti-inflammatory and antioxidant treatment using exosomes carrying curcumin, which is difficult to deliver in aqueous solutions traditionally.68 Sun et al. developed curcumin-loaded mouse lymphoma EL-4 exosomes via direct incubation allowing membrane hydrophobic binding.46 Haney et al. loaded a tetramer protein (250 kDa) into monocyte-derived exosomes by incubation at room temperature for 18 h.69 However, the nonspecific hydrophobic binding between cargos and exosomes makes the passive loading suffer from long incubation times and low loading capacity.
For the delivery of hydrophilic compounds, such as RNA, the hydrophilicity actually prevents passive loading through the hydrophobic lipid bilayer membrane. Therefore, membrane permeabilization strategies, including electroporation, sonication, direct transfection, and saponin permeabilization, adapted from the liposome field have been developed for exosome cargo loading, as shown in Fig. 4.70 These methods are termed as active loading, and all require the disruption of the exosome membrane, but they differ in terms of loading scalability and product quality.
Fig. 4.
Illustration of strategies for membrane permeabilization mediated cargo loading of exosomes.
By creating small transient pores in the lipid bilayer membrane via the electrical field-induced cross membrane potential, electroporation has been widely employed for cell transfection since the 1980s.71–72 Adapted in 2012, electroporation was successfully applied to load siRNA into exosomes by Alvarez-Erviti and colleagues.73, 74,75 Subsequent systemic administration of engineered exosomes in mice showed the inhibition of beta-site APP-cleaving enzyme and protein expression in the brain. Walhgren et al. electroporated siRNA to plasma exosomes76 for delivery to human monocytes and lymphocytes. Electroporation for exosome cargo loading minimizes perturbation of sensitive exosome components (e.g., ligands and receptors) without introducing additional chemicals. To date, no Joule heating-induced thermal damage has been observed to membrane components; this is believed to be due to the application of instant electrical pulses in the millisecond range.74
Other commonly applied active cargo-loading methods for exosomes are sonication, freeze-thaw cycles, and incubation with membrane permeabilizers.77–78 Haney et al. investigated several loading methods, including simple incubation at room temperature, saponin-mediated permeabilization, sonication, freeze-thaw cycles, and extrusion, to load exosomes with the antioxidant enzyme catalase.69 It was observed that reformed exosomes upon sonication, electroporation, as well as saponin-mediated permeabilization resulted in high loading efficiency, sustained release, and catalase preservation against degradation. However, sonication and extrusion-derived vesicles showed significant size increase via nanoparticle tracking analysis. Unfortunately, the influence of these different methods on the delivery capability of reconstructed exosomes has not been much investigated and is worth exploring in the future.
Exosome mimetics are a type of vesicles produced by extruding donor cells through membrane filters with a 100–400 nm pore size.70 The vesicles are fabricated artificially by breaking the cells and then reforming the contents into exosome mimetics. This extrusion method can produce exosomes as high as 100-fold in quantity when compared with the exosomes naturally released by cells.79–81 By subjecting mammalian cells with doxorubicin to extrusion through a serial filtering device (e.g., pore sizes 10, 5, and 1 μm), Jang et al. generated high quantities of mimetic exosomes carrying sheltered drug. The sheltered doxorubicin can travel to the tumor tissue and reduce tumor growth.79 Compared to the case of free drug, a 20-fold lower amount of drug was needed via exosome mimetic delivery for reducing the tumor growth to the same extent. However, Fuhrmann et al. have observed that harsh mechanical forces used in extrusion may cause the alteration of zeta potential on the surface of the exosomal membrane. Cytotoxicity was observed in the cell uptake experiment using extruded exosomes carrying porphyrin from the MDA-MB 231 breast cancer cells.95 Although these observations have been speculated for attributing to the intensive extrusion process, further characterization of the exosome mimetics is necessary.
Currently, the successful applications of exosomes in therapeutics are entirely dependent on the capacity of cargo loading. Another effective approach to load therapeutic nucleic acid cargos into exosomes is the modification of parent cells, i.e. through genetic engineering or medication with cytotoxic drugs. Variable therapeutic cargos, including small molecule compounds, proteins, and nucleic acid drugs, have been studied for loading into exosomes via different methods and techniques, as summarized in Table 1. As research continues to progress, more side effects have been observed for several types of engineered exosomes. For instance, harsh electroporation conditions may trigger the aggregation of exosomes and change their morphological characteristics.96–98 The harsh extrusion conditions were reported to alter the zeta potential of the exosomes, which could cause cytotoxicity.95 Consequently, surface-engineering of exosomes is widely accepted as a better alternative to liposomes and exosome mimetics.58–59, 99 Presently, there are still many challenges and pitfalls in this research field. As shown in Table 1, the cargo loading efficiency is still quite low (~ 30%) among all the approaches. In addition, exosomes released from cells are usually in a limited quantity with dynamic molecular contents. The technologies for quality control and mass production of exosomes are desperately needed to achieve fast, high-throughput, and highly efficient cargo loading. These concerns are currently being addressed by researchers using microfluidic platforms.
Table 1.
Summary of the variable applications of engineered exosomes in precision therapeutics.
| Surface Engineering | Membrane Permeabilization | ||||||
|---|---|---|---|---|---|---|---|
| Cell Manipulation | Affinity Binding | Covalent bonding | Incubation | Electroporation | Sonication | Cell Extrusion (Vesicle Mimetics) | |
| Exosome source | Cell culture: imDC,29 HepG260, DCs from 57BL/6 mice82, Neuro2A61, MCA10183 | Cell culture: Hela84, HepG260 Blood62 | Cell culture: 4T1 cell line66, B16F10 cells (AHA-integrated exosomes)85 | Cell culture: EL-446, U-87 MG86, Raw 264.748, 87, U8788 Bovine milk89–90 | Cell culture: imDC29, CRL 647591, DC from C57BL/6 mice82, HEK293T92, Human plasma76 | Cell culture: Raw 64.748, 87 | Cell suspension: Raw 264.787, ES-D393, U93779 Grapefruit94 |
| Cargo type | iRGD peptide29, biotin60, Lamp2b82, GPI linked nanobody61, Chicken egg ovalbumin83 | Lipofectamine LTX84, Transferrin-conjugated superparamagnetic nanoparticle clusters62, Avidin60 | Azide-Fluor 54566, DBCO-Cy385 | Curcumin46, 89, rhodamine86, paclitaxel48, 86, doxorubicin86, catalase87, withaferin89, anthocyanidin89, paclitaxel89, docetaxel89, paclitaxel90, hydrophobic siRNA88 | Doxorubicin29, Superparamagnetic iron oxide nanoparticles91, siRNA76, 82, dsDNA92 | catalase87, paclitaxel48 | Catalase87, Polystyrene beads93, Doxorubicin79, Inflammatory related receptor enriched plasma membrane94 |
| Efficiency (%) or binding capacity | 60%29, 100%60, Not reported82–83, 15–25%61 | Not reported62, 84, 91%60 | ~1.5 alkyne groups in 150 kDa exosomal protein66, 790 nM in 1 mg/mL exosome85 | 2.9 g in 1 g exosomes46, 0.008–0.1 g in 1 g exosomes86, 4.9%87, 10–40%89, 18.5% with saponion90, 30%88, 1.4%48 | 20%29, 0.5 μg iron particles per μg exosome protein91, 25%82, 27%76, 2%92 | 26.1%87, 28%48 | 22.2%87, 60%93, 0.052–0.332 g in 1 g exosomes79, 83%94 |
Microfluidic engineering of exosomes
Molecular engineering offers alluring prospect for making exosomes versatile therapeutics beyond their native functions. The approaches for modifying exosomes are often adapted from well-established cell manipulation technologies such as electroporation, sonication, incubation, etc. Exosomes are significantly smaller than cells; this results in a higher degree of membrane curvature with less surface area and more difficult conditions for transfection. Microfluidic systems overcome many drawbacks of benchtop systems because they are intrinsically customizable, automatable, scalable, and capable of highly efficient mass transport. In fact, microfluidic lab-on-chip technology has been proven as a highly effective method for triggering the fast growth of exosome research.25, 100–104 Although the development is still at an early stage, microfluidic technology has proven its superior performance in isolation, molecular analysis, and detection105–109. As the advances of microfluidic technology, the high throughput analysis of EVs and exosomes has been achieved up to 100 μL/min, and multiple on-chip detection systems have been developed with detection limits as low as ~50 exosomes per μL.25, 102. In addition to the significant contribution of microfluidic isolation and molecular analysis of EVs and exosomes, microfluidic engineering of exosomes has also emerged in recent years. Due to the relatively short history of EVs and exosomes being discovered, characterized, and utilized in therapeutics, there are only a few studies employing microfluidic technology for engineering exosomes, and the full potential and capability have not yet been well explored. In this section, we have reviewed the advances of microfluidic technology for engineering EVs and exosomes, which is the area that we anticipate will grow exponentially in the next five years. The future perspectives and pitfalls in precision therapeutics have been discussed as well.
Microfluidic extrusion for engineering of exosome mimetics
Park’s group has reported the microfluidic extrusion method to generate exosome mimetics. In the first report in 2014, Jo et al. developed constriction microchannels with small dimensions for mechanically breaking down the cells into exosome mimetics.110 The microfluidic device is shown in Fig. 5a with a length of 100–400 μm and a width of 3–7 μm. When cells were squeezed into a microchannel by a syringe pump, the membranes were elongated due to the resistant shear force on the surface of the microchannel. As a result, the elongated lipid bilayer was broken and re-assembled as small nanoparticles thermodynamically. These exosome mimetics were fabricated endogenously using living embryonic stem cells containing mRNA, intracellular proteins, and plasma membrane proteins at the stage of re-assembling. The size of the formed mimetic exosomes was controllable and found to be dependent on the microchannel geometry (e.g., cross-section area and length) in a range of 60–120 nm. The successful cellular uptake of exosome mimetics encapsulated with cytosols staining was demonstrated. The delivery ability of the engineered exosome mimetics was found to be similar to that of the naturally secreted exosomes by comparing the exogenous genes in the recipient cells. With conventional soft-lithography and polydimethylsiloxane (PDMS) device molding, this microfluidic approach is facile and scalable, with a high flow throughput of 6.5 μL/min. However, the measured total amount of RNAs and proteins indicates that exosome mimetics are generated from about one-fifth of donor cells. This small production may be partially due to cell clogging at the entrance of microchannels and adherence to the inside surface of the microchannel. However, it holds promise for large-scale exosome mimetic production and modification.
Fig. 5.
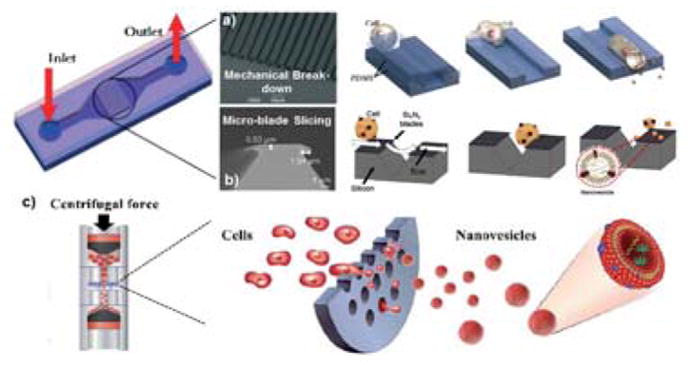
The microfluidic extrusion method for fabricating exosome mimetics from donor cells. (a) Mechanical elongation and cellular breaking down into exosome mimetics. Adapted from Ref. 110 with permission from the Royal Society of Chemistry. (b) Micro-blade slicing of cells. Adapted from Ref. 111, with permission from Elsevier copyright 2015. (c) Micro-sized pore squeezing of cells into exosome mimetics. Figures were adapted from Ref. 113 with permission from the Royal Society of Chemistry.
An improved microfluidic device was reported by Park’s group subsequently, which produced exosome mimetics by slicing living cell membrane with micro-fabricated silicon nitride blades (500 nm-thick), as shown in Fig. 5b.111 The device fabrication used conventional bulk silicon fabrication processes and soft lithography. The fascinating part of this device is the patterning of cantilever-blades by growing a 100-nm-thick silicon oxide layer and subsequently depositing a 500-nm-thick SixNy. The pattern of SixNy is designable using AZ photoresist and lithography, followed by inductively-coupled plasma reaction-ion-etching (RIE). Dry etching removes the SixNy layer and the silicon oxide layer for exposing the silicon substrate. Living cells entered into the flow are subjected to slicing by touching them with the sharp edge of the silicon blade. The sliced cell fragments can re-assemble into exosome mimetics due to minimization of the free energy of lipid bilayers. The high throughput production of exosome mimetics was achieved at ~1.50 × 1010 vesicles with a particle size of ~100–300 nm per million cells. The number of produced vesicles is ~100 times higher than the number of exosomes secreted from the same number of cells.112 Under an encapsulating test, ~30% of fluorescent beads were enveloped during cell fragment re-assembly. Compared to those obtained from previous cell disruption methods enabled by mechanical shear forces,110, 113 both the protein content and the number of vesicles obtained using microfluidic cell slicing were significantly higher.
In a subsequent study, Jo et al. introduced a micro-filter device consisting of a polycarbonate filter with micro-sized pores by utilizing the cell extrusion principle in micro-scale. Using a common centrifuge, the large-scale generation of cell-derived exosome mimetics can be achieved in high automation and efficiency.113 As illustrated in Fig. 5c, exosome mimetics were directly produced by fragmenting the cells during centrifugation (1 × 108 cells at 2000 rpm) due to the shear force and elongation of cells while passing through hydrophilic and micro-sized pores. The quantity of exosome mimetics produced by this centrifuge micro-device could be 250-fold higher than that of naturally secreted exosomes. Most importantly, the intracellular molecular contents were 2-fold higher as compared to those of the naturally secreted exosomes.
Microfluidic surface engineering of living-cell-derived exosomes
Compared to the microfluidic extrusion approaches for random packaging and re-assembly, surface engineering is more promising for producing less impaired exosomes secreted from living cells. Although production throughput needs to be enhanced, microfluidic surface engineering of exosomes is an excellent approach for studying exosome packaging mechanisms and biogenesis and understanding delivery signaling pathways. It has been shown that parent cells possess a sorting mechanism for guiding a selective subset of microRNAs to be loaded into exosomes, and a few proteins (e.g., Y-box protein 1114, RNA-Binding Protein SYNCRIP115) may be involved in this sorting process116. This evidence suggests that specific molecular sorting into exosomes may be a mechanism for long-distance export and signal transduction14, 117. By contrast, the delivery ability of exosome mimetics may require further validation for comparison with that of the naturally secreted exosomes with sorting mechanism.
Compared to benchtop surface engineering approaches, microfluidic technology offers tremendous advancements. Due to its micro-scale nature, mass transfer is much more efficient, and it is able to achieve thousand-fold enhancements for fluid mixing, specific molecular binding, and transport.118 The functional integration also allows multiple processing steps to be automated in one device for high throughput and scale up. Our research group recently introduced 3D printing technology for building microfluidic devices that adds third-dimensional control over 3D microstructures for enhancing the mixing capability of microfluidics119. We devised a 3D-molded PDMS microfluidic chip integrated with on-chip cell culture and streamlined surface engineering of culture-derived exosomes. Fig. 6a shows the PDMS chip with integrated functionalities, including cell culture, exosome capture, and surface engineering.120 Current microfluidic technology has been well developed for exosome isolation and molecular analysis25. However, the processed exosomes are either in small quantities or bound to solid surfaces/particles and unable to stay intact for downstream therapeutic preparations. To overcome these challenges, we introduced a photo-cleavable linker functionalized on magnetic nanoparticles for selectively capturing MHC-1 positive exosomes secreted from the on-chip culture (Fig. 6b). The immunogenic exosomes derived from dendritic cells carry an intrinsic payload of MHC class I and II molecules and other co-stimulatory molecules for mediating immune responses. Our microfluidic cell culture system allows surface engineering of the cultured immunogenic exosomes (MHC I+) with tumor antigenic peptides. Moreover, the functional exosomes can be photo-released at downstream for immunity stimulation. We have tested the cellular uptake of engineered exosomes by antigen-presenting cells, and the engineered exosomes show much-improved internalization capability as compared to non-engineered exosomes, as shown in Fig. 6c (engineered exosomes) and d (native exosomes), which are promising for developing novel cancer vaccines and delivery in immunotherapy.
Fig. 6.

Microfluidic surface engineering of living-cell-derived exosomes. (a) A 3D-molded PDMS microfluidic chip integrated with on-chip cell culture and streamlined surface engineering of culture-derived exosomes. (b) Schematic of the surface engineering process for exosome capture, surface binding, and photo-release. (c) Antigen-presenting cell uptake of gp-100 tumor peptide surface-modified exosomes as compared to the cellular uptake of native exosomes without surface engineering (d). The cellular nucleus was stained with DAPI in blue, and exosomes were stained with PKH67 in green.
Applications and future perspectives
Microfluidic technology has shown unique capability for speeding up exosome research towards precision medicine. However, the enormous potential has not been fully exploited yet. We envision that substantial research in investigating exosomes can be facilitated using microfluidic technology. For instance, microfluidic platforms have been introduced in cell electroporation for the past two decades,121 which will have great adaptability for electroporating extracellular vesicles and exosomes. Although transfection of exosomes via the microfluidic electroporation approach has not been reported to date, several unmatched capabilities offered by microfluidic electroporation are obvious, including 1) precisely controlled electric field and electric pulse in spatial and temporary for high-efficiency electroporation; 2) microscale dimension allows low potential difference, which greatly reduces side effects often observed in the benchtop approach; 3) the pH variation and Joule heating caused by electric fields can be minimized in microscale; and 4) the high functional integration, high throughput, and scale up are amenable and straightforward.
Precise liquid handling and mixing via the microfluidic approach is another promising feature for engineering nanovesicles and exosomes. Laminar flow is a typical flow profile in microfluidic devices dominated by molecular diffusion, which can be precisely controlled for achieving highly efficient mass transport between streams with different species.122 Efficient mixing within microfluidic channels has been proven to improve the reaction rate123 and can be integrated with various sample processing methods and molecular analysis.122, 124–127 Our research group has previously developed an ExoSearch chip with a serpentine microfluidic mixer to enhance immunomagnetic bead-based exosome isolation and detection.105 This highly efficient mass transport as well as a high surface-to-volume ratio of the microstructures perfectly meet the needs of surface engineering for either delivering hydrophobic therapeutics to exosome membrane or facilitating affinity binding reactions.
Exosomes are increasingly being recognized as contributing factors in many diseases, and their potential as therapeutics holds substantial promise for developing exciting strategies in drug delivery and cancer immunotherapy.128 Thus, bioengineering of exosomes is becoming significantly important. Traditional benchtop methods for exosome modifications will continue to play a significant role in the future. However, as microfluidic technology develops, we believe that microfluidic approaches will eventually replace benchtop methods for engineering exosomes in speeding up the precision therapeutics. With the advances of on-chip cell culture technologies,129–131 a fully integrated microfluidic system, including cell culture and exosome isolation and engineering, as well as exosome-mediated therapeutic delivery, will be an essential research direction for understanding exosome biogenesis fundamentally and seeking novel therapeutic strategies.
Exosomes are secreted from all living cells and can be harvested from a variety of sources including cow milk, plant, bacterium, and various human body fluids, as shown in Fig. 7. Thus, the heterogeneous subtypes and complicated molecular contents presented in exosomes pose a daunting challenge for precision engineering and processing of exosomes. In fact, microfluidic technology can play a unique role in solving this challenge by integration with sophisticated sample preparation functionalities such as sorting, filtration, subtyping, and molecular probing. The high specificity and high sensitivity offered by the microfluidic approach would also contribute to high-resolution manipulation of exosomes for clinical applications. Presently, the biggest challenge for microfluidic engineering of exosomes still lies in the processing volume that is required for meeting the large-scale therapeutic demands. Although there has been an attempt at large-scale production of exosome mimetics via microfluidic cell extrusion, the molecular contents of the engineered exosomes still need to be well characterized for meeting the desired bioactivity. Thus, interfacing high throughput cell culture and combining continuous flow processing are necessary. Considering that substantial quantities of exosomes are needed to achieve a therapeutic effect,132 highly scalable approaches for the mass production of therapeutic exosomes will be emerging for future precision therapeutics but have not been explored yet. In addition, there is also a continuous need to deal with the manufacturing, storage, and administration of therapeutic exosomes, which have not been well standardized. However, the potential therapeutic values of EVs and exosomes have been increasingly promising. As a versatile tool, microfluidic technology is expected to fully unlock the potential of these diverse nanovesicles in the near future for speeding up precision medicine.
Fig. 7.
Schematic of the “power” of microfluidic technology in exosome research for speeding up precision medicine.
Acknowledgments
Our work was funded by USDA-NIFA 2017-67021-26600, NIH NIGMS P20 GM103418, and NIH NIGMS P20 GM103638.
Biographies

Dr. Qingfu Zhu received his M.S. in Medicinal Chemistry from Wuhan University, China, in 2011, and his Ph.D. degree in Pharmacy from University of Jena, Germany, in 2015. Then, he became a Postdoctoral fellow at the Wichita State University for 1 year. Currently, he is a Postdoctoral Research Associate at the University of Kansas working with Dr. Mei He. His research interests focus on the development of microfluidic devices for programming and monitoring biomimetic immunity via exosome communications.

Mikala Heon earned her Bachelor of Science in Biomedical Engineering from the University of Houston, USA, in 2017. She is currently a graduate student in Bioengineering at the University of Kansas, where she has joined Dr. Mei He’s research group. Her research is in magnetic bead capturing of exosomes for use in isolation techniques.

Zheng Zhao graduated with Bachelor of Science in Biological System Engineering from Kansas State University, USA, in 2015. Then he joined Dr. Mei He’s research group as a graduate research student. Currently, he is a Ph.D. student at the University of Kansas. His research is focusing on 2D and 3D microfluidic devise platforms with exosome related early cancer diagnosis and immunotherapy.

Dr. Mei He currently is an assistant professor at the University of Kansas and she directs a research group focusing on the 3D microfluidic technology and 3D bioprinting for studying extracellular vesicles associated immunity communications. Dr. He received her Ph. D. degree in Chemistry from professor Jed Harrison’s research group at the University of Alberta in 2008. She conducted the postdoctoral research at the University of California, Berkeley with professor Amy Herr between 2008 and 2012.
Footnotes
Author contributions
In the authorship, Dr. Qingfu Zhu and Mikala Heon contributed to the original draft and preparation of review manuscript, Zheng Zhao contributed to the data collection, and Dr. Mei He contributed to the conceptualization, review and editing, and supervision.
Conflicts of interest
There are no conflicts to declare.
References
- 1.Hodson R. Precision medicine. Nature. 2016;537(7619):S49. doi: 10.1038/537S49a. [DOI] [PubMed] [Google Scholar]
- 2.Mora EM, Alvarez-Cubela S, Oltra E. Biobanking of Exosomes in the Era of Precision Medicine: Are We There Yet? Int J Mol Sci. 2015;17(1) doi: 10.3390/ijms17010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu M, Ouyang Y, Wang Z, Zhang R, Huang PH, Chen C, Li H, Li P, Quinn D, Dao M, Suresh S, Sadovsky Y, Huang TJ. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci U S A. 2017;114(40):10584–10589. doi: 10.1073/pnas.1709210114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamashita T, Takahashi Y, Nishikawa M, Takakura Y. Effect of exosome isolation methods on physicochemical properties of exosomes and clearance of exosomes from the blood circulation. Eur J Pharm Biopharm. 2016;98:1–8. doi: 10.1016/j.ejpb.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Zeringer E, Barta T, Li M, Vlassov AV. Strategies for isolation of exosomes. Cold Spring Harb Protoc. 2015;2015(4):319–23. doi: 10.1101/pdb.top074476. [DOI] [PubMed] [Google Scholar]
- 6.Barile L, Vassalli G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacology & Therapeutics. 2017;174(Supplement C):63–78. doi: 10.1016/j.pharmthera.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 7.Tkach M, Théry C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016;164(6):1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 8.Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–25. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nature Reviews Immunology. 2014;14:195. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacologica Sinica. 2017;38:754. doi: 10.1038/aps.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–79. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 12.Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113(1):1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 14.Stoorvogel W. Resolving sorting mechanisms into exosomes. Cell Res. 2015;25(5):531–2. doi: 10.1038/cr.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soo CY, Song Y, Zheng Y, Campbell EC, Riches AC, Gunn-Moore F, Powis SJ. Nanoparticle tracking analysis monitors microvesicle and exosome secretion from immune cells. Immunology. 2012;136(2):192–197. doi: 10.1111/j.1365-2567.2012.03569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalra H, Adda CG, Liem M, Ang C-S, Mechler A, Simpson RJ, Hulett MD, Mathivanan S. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. PROTEOMICS. 2013;13(22):3354–3364. doi: 10.1002/pmic.201300282. [DOI] [PubMed] [Google Scholar]
- 17.Dragovic RA, Collett GP, Hole P, Ferguson DJP, Redman CW, Sargent IL, Tannetta DS. Isolation of syncytiotrophoblast microvesicles and exosomes and their characterisation by multicolour flow cytometry and fluorescence Nanoparticle Tracking Analysis. Methods. 2015;87:64–74. doi: 10.1016/j.ymeth.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurwitz SN, Conlon MM, Rider MA, Brownstein NC, Meckes DG. Nanoparticle analysis sheds budding insights into genetic drivers of extracellular vesicle biogenesis. Journal of Extracellular Vesicles. 2016;5(1):31295. doi: 10.3402/jev.v5.31295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teixeira JH, Silva AM, Almeida MI, Barbosa MA, Santos SG. Circulating extracellular vesicles: Their role in tissue repair and regeneration. Transfusion and Apheresis Science. 2016;55(1):53–61. doi: 10.1016/j.transci.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Fuhrmann G, Herrmann IK, Stevens MM. Cell-derived vesicles for drug therapy and diagnostics: Opportunities and challenges. Nano Today. 2015;10(3):397–409. doi: 10.1016/j.nantod.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gehrmann U, Näslund TI, Hiltbrunner S, Larssen P, Gabrielsson S. Harnessing the exosome-induced immune response for cancer immunotherapy. Seminars in Cancer Biology. 2014;28(Supplement C):58–67. doi: 10.1016/j.semcancer.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Tang X-J, Sun X-Y, Huang K-M, Zhang L, Yang Z-S, Zou D-D, Wang B, Warnock GL, Dai L-J, Luo J. Therapeutic potential of CAR-T cell-derived exosomes: a cell-free modality for targeted cancer therapy. Oncotarget. 2015;6(42):44179–44190. doi: 10.18632/oncotarget.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren J, He W, Zheng L, Duan H. From structures to functions: insights into exosomes as promising drug delivery vehicles. Biomaterials Science. 2016;4(6):910–921. doi: 10.1039/c5bm00583c. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Zheng Y, Zhao M. Exosome-Based Cancer Therapy: Implication for Targeting Cancer Stem Cells. Frontiers in Pharmacology. 2017;7(533) doi: 10.3389/fphar.2016.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He M, Zeng Y. Microfluidic Exosome Analysis toward Liquid Biopsy for Cancer. J Lab Autom. 2016;21(4):599–608. doi: 10.1177/2211068216651035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Contreras-Naranjo JC, Wu HJ, Ugaz VM. Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine. Lab Chip. 2017;17(21):3558–3577. doi: 10.1039/c7lc00592j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parodi A, Molinaro R, Sushnitha M, Evangelopoulos M, Martinez JO, Arrighetti N, Corbo C, Tasciotti E. Bio-inspired engineering of cell- and virus-like nanoparticles for drug delivery. Biomaterials. 2017;147:155–168. doi: 10.1016/j.biomaterials.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Xitong D, Xiaorong Z. Targeted therapeutic delivery using engineered exosomes and its applications in cardiovascular diseases. Gene. 2016;575(2 Part 2):377–384. doi: 10.1016/j.gene.2015.08.067. [DOI] [PubMed] [Google Scholar]
- 29.Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 30.Tian X, Zhu M, Nie G. How can nanotechnology help membrane vesicle-based cancer immunotherapy development? Human Vaccines & Immunotherapeutics. 2013;9(1):222–225. doi: 10.4161/hv.22130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sterzenbach U, Putz U, Low L-H, Silke J, Tan S-S, Howitt J. Engineered Exosomes as Vehicles for Biologically Active Proteins. Molecular Therapy. 2017;25(6):1269–1278. doi: 10.1016/j.ymthe.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilligan KE, Dwyer RM. Engineering Exosomes for Cancer Therapy. International Journal of Molecular Sciences. 2017;18(6):1–12. doi: 10.3390/ijms18061122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan A, De La Peña H, Seifalian AM. The application of exosomes as a nanoscale cancer vaccine. International Journal of Nanomedicine. 2010;5:889–900. doi: 10.2147/IJN.S13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen H, Zeng Y, Liu W, Zhao S, Wu J, Du Y. Multifaceted applications of nanomaterials in cell engineering and therapy. Biotechnology Advances. 2013;31(5):638–653. doi: 10.1016/j.biotechadv.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Huang C-C, Narayanan R, Alapati S, Ravindran S. Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterials. 2016;111(Supplement C):103–115. doi: 10.1016/j.biomaterials.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su S-A, Xie Y, Fu Z, Wang Y, Wang J-A, Xiang M. Emerging role of exosome-mediated intercellular communication in vascular remodeling. Oncotarget. 2017;8(15):25700–25712. doi: 10.18632/oncotarget.14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin Y, Lee JS, Min S, Park H-J, Kang TJ, Cho S-W. Bioengineered Extracellular Membranous Nanovesicles for Efficient Small-Interfering RNA Delivery: Versatile Platforms for Stem Cell Engineering and In Vivo Delivery. Advanced Functional Materials. 2016;26(32):5804–5817. [Google Scholar]
- 38.Morishita M, Takahashi Y, Matsumoto A, Nishikawa M, Takakura Y. Exosome-based tumor antigens–adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials. 2016;111(Supplement C):55–65. doi: 10.1016/j.biomaterials.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 39.Syn NL, Wang L, Chow EK, Lim CT, Goh BC. Exosomes in Cancer Nanomedicine and Immunotherapy: Prospects and Challenges. Trends Biotechnol. 2017;35(7):665–676. doi: 10.1016/j.tibtech.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Bell BM, Kirk ID, Hiltbrunner S, Gabrielsson S, Bultema JJ. Designer exosomes as next-generation cancer immunotherapy. Nanomedicine. 2016;12(1):163–9. doi: 10.1016/j.nano.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Pitt JM, André F, Amigorena S, Soria J-C, Eggermont A, Kroemer G, Zitvogel L. Dendritic cell–derived exosomes for cancer therapy. The Journal of Clinical Investigation. 2016;126(4):1224–1232. doi: 10.1172/JCI81137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian H, Li W. Dendritic cell-derived exosomes for cancer immunotherapy: hope and challenges. Ann Transl Med. 2017;5(10):221. doi: 10.21037/atm.2017.02.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Viaud S, Théry C, Ploix S, Tursz T, Lapierre V, Lantz O, Zitvogel L, Chaput N. Dendritic Cell-Derived Exosomes for Cancer Immunotherapy: What's Next? Cancer Research. 2010;70(4):1281. doi: 10.1158/0008-5472.CAN-09-3276. [DOI] [PubMed] [Google Scholar]
- 44.Tran TH, Mattheolabakis G, Aldawsari H, Amiji M. Exosomes as nanocarriers for immunotherapy of cancer and inflammatory diseases. Clin Immunol. 2015;160(1):46–58. doi: 10.1016/j.clim.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 45.Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol. 2015;40:72–81. doi: 10.1016/j.semcdb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 46.Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang H-G. A Novel Nanoparticle Drug Delivery System: The Anti-inflammatory Activity of Curcumin Is Enhanced When Encapsulated in Exosomes. Molecular Therapy. 2010;18(9):1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, Le Chevalier T, Livartoski A, Barlesi F, Laplanche A, Ploix S, Vimond N, Peguillet I, Théry C, Lacroix L, Zoernig I, Dhodapkar K, Dhodapkar M, Viaud S, Soria J-C, Reiners KS, Pogge von Strandmann E, Vély F, Rusakiewicz S, Eggermont A, Pitt JM, Zitvogel L, Chaput N. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. 2016;5(4):e1071008. doi: 10.1080/2162402X.2015.1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O, Hingtgen SD, Kabanov AV, Batrakova EV. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine: Nanotechnology, Biology and Medicine. 12(3):655–664. doi: 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heusermann W, Hean J, Trojer D, Steib E, von Bueren S, Graff-Meyer A, Genoud C, Martin K, Pizzato N, Voshol J, Morrissey DV, Andaloussi SEL, Wood MJ, Meisner-Kober NC. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. The Journal of Cell Biology. 2016;213(2):173. doi: 10.1083/jcb.201506084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Melo Sonia A, Sugimoto H, O’Connell Joyce T, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman Lev T, Melo Carlos A, Lucci A, Ivan C, Calin George A, Kalluri R. Cancer Exosomes Perform Cell-Independent MicroRNA Biogenesis and Promote Tumorigenesis. Cancer Cell. 26(5):707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mizrak A, Bolukbasi MF, Ozdener GB, Brenner GJ, Madlener S, Erkan EP, Strobel T, Breakefield XO, Saydam O. Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol Ther. 2013;21(1):101–8. doi: 10.1038/mt.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stewart MP, Sharei A, Ding X, Sahay G, Langer R, Jensen KF. In vitro and ex vivo strategies for intracellular delivery. Nature. 2016;538:183. doi: 10.1038/nature19764. [DOI] [PubMed] [Google Scholar]
- 53.Armstrong JPK, Holme MN, Stevens MM. Re-Engineering Extracellular Vesicles as Smart Nanoscale Therapeutics. ACS Nano. 2017;11(1):69–83. doi: 10.1021/acsnano.6b07607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Andaloussi S, Lee Y, Lakhal-Littleton S, Li J, Seow Y, Gardiner C, Alvarez-Erviti L, Sargent IL, Wood MJA. Exosome-mediated delivery of siRNA in vitro and in vivo. Nature Protocols. 2012;7:2112. doi: 10.1038/nprot.2012.131. [DOI] [PubMed] [Google Scholar]
- 55.Kuate S, Cinatl J, Doerr HW, Überla K. Exosomal vaccines containing the S protein of the SARS coronavirus induce high levels of neutralizing antibodies. Virology. 2007;362(1):26–37. doi: 10.1016/j.virol.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yim N, Ryu SW, Choi K, Lee KR, Lee S, Choi H, Kim J, Shaker MR, Sun W, Park JH, Kim D, Do Heo W, Choi C. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein–protein interaction module. Nature Communications. 2016;7:12277. doi: 10.1038/ncomms12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stickney Z, Losacco J, McDevitt S, Zhang Z, Lu B. Development of exosome surface display technology in living human cells. Biochemical and Biophysical Research Communications. 2016;472(1):53–59. doi: 10.1016/j.bbrc.2016.02.058. [DOI] [PubMed] [Google Scholar]
- 58.Hood JL. Post isolation modification of exosomes for nanomedicine applications. Nanomedicine. 2016;11(13):1745–1756. doi: 10.2217/nnm-2016-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kutralam-Muniasamy G, Perez-Guevara F. Recombinant surface engineering to enhance and expand the potential of biologically produced nanoparticles: A review. Process Biochemistry. 2017;59(Part A):4–17. [Google Scholar]
- 60.Wang J, Li W, Zhang L, Ban L, Chen P, Du W, Feng X, Liu BF. Chemically Edited Exosomes with Dual Ligand Purified by Microfluidic Device for Active Targeted Drug Delivery to Tumor Cells. ACS Appl Mater Interfaces. 2017;9(33):27441–27452. doi: 10.1021/acsami.7b06464. [DOI] [PubMed] [Google Scholar]
- 61.Kooijmans SAA, Aleza CG, Roffler SR, van Solinge WW, Vader P, Schiffelers RM. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. Journal of Extracellular Vesicles. 2016;5(1):31053. doi: 10.3402/jev.v5.31053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qi H, Liu C, Long L, Ren Y, Zhang S, Chang X, Qian X, Jia H, Zhao J, Sun J, Hou X, Yuan X, Kang C. Blood Exosomes Endowed with Magnetic and Targeting Properties for Cancer Therapy. ACS Nano. 2016;10(3):3323–3333. doi: 10.1021/acsnano.5b06939. [DOI] [PubMed] [Google Scholar]
- 63.Morishita M, Takahashi Y, Nishikawa M, Ariizumi R, Takakura Y. Enhanced Class I Tumor Antigen Presentation via Cytosolic Delivery of Exosomal Cargos by Tumor-Cell-Derived Exosomes Displaying a pH-Sensitive Fusogenic Peptide. Molecular Pharmaceutics. 2017;14(11):4079–4086. doi: 10.1021/acs.molpharmaceut.7b00760. [DOI] [PubMed] [Google Scholar]
- 64.Maguire CA, Balaj L, Sivaraman S, Crommentuijn MHW, Ericsson M, Mincheva-Nilsson L, Baranov V, Gianni D, Tannous BA, Sena-Esteves M, Breakefield XO, Skog J. Microvesicle-associated AAV Vector as a Novel Gene Delivery System. Molecular Therapy. 2012;20(5):960–971. doi: 10.1038/mt.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oude Blenke E, Klaasse G, Merten H, Plückthun A, Mastrobattista E, Martin NI. Liposome functionalization with copper-free “click chemistry”. Journal of Controlled Release. 2015;202(Supplement C):14–20. doi: 10.1016/j.jconrel.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 66.Smyth T, Petrova K, Payton NM, Persaud I, Redzic JS, Graner MW, Smith-Jones P, Anchordoquy TJ. Surface Functionalization of Exosomes Using Click Chemistry. Bioconjugate Chemistry. 2014;25(10):1777–1784. doi: 10.1021/bc500291r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, Takakura Y. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. 2013;165(2):77–84. doi: 10.1016/j.jbiotec.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 68.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15(1):195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. Journal of Controlled Release. 2015;207(Supplement C):18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sutaria DS, Badawi M, Phelps MA, Schmittgen TD. Achieving the Promise of Therapeutic Extracellular Vesicles: The Devil is in Details of Therapeutic Loading. Pharm Res. 2017;34(5):1053–1066. doi: 10.1007/s11095-017-2123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol. 2011;23(3):421–9. doi: 10.1016/j.coi.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Impellizeri JA, Ciliberto G, Aurisicchio L. Electro-gene-transfer as a new tool for cancer immunotherapy in animals. Vet Comp Oncol. 2014;12(4):310–8. doi: 10.1111/vco.12006. [DOI] [PubMed] [Google Scholar]
- 73.Wahlgren J, Statello L, Skogberg G, Telemo E, Valadi H. Delivery of Small Interfering RNAs to Cells via Exosomes. In: Shum K, Rossi J, editors. SiRNA Delivery Methods: Methods and Protocols. Springer; New York: New York, NY: 2016. pp. 105–125. [DOI] [PubMed] [Google Scholar]
- 74.Weaver JC. Electroporation: A general phenomenon for manipulating cells and tissues. Journal of Cellular Biochemistry. 1993;51(4):426–435. doi: 10.1002/jcb.2400510407. [DOI] [PubMed] [Google Scholar]
- 75.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnology. 2011;29:341. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 76.Wahlgren J, Karlson TDL, Brisslert M, Vaziri Sani F, Telemo E, Sunnerhagen P, Valadi H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Research. 2012;40(17):e130–e130. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38(6):754–763. doi: 10.1038/aps.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106(Pt A):148–156. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 79.Jang SC, Kim OY, Yoon CM, Choi D-S, Roh T-Y, Park J, Nilsson J, Lötvall J, Kim Y-K, Gho YS. Bioinspired Exosome-Mimetic Nanovesicles for Targeted Delivery of Chemotherapeutics to Malignant Tumors. ACS Nano. 2013;7(9):7698–7710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- 80.Jang SC, Gho YS. Could bioengineered exosome-mimetic nanovesicles be an efficient strategy for the delivery of chemotherapeutics? Nanomedicine. 2014;9(2):177–180. doi: 10.2217/nnm.13.206. [DOI] [PubMed] [Google Scholar]
- 81.Lunavat TR, Jang SC, Nilsson L, Park HT, Repiska G, Lässer C, Nilsson JA, Gho YS, Lötvall J. RNAi delivery by exosome-mimetic nanovesicles – Implications for targeting c-Myc in cancer. Biomaterials. 2016;102(Supplement C):231–238. doi: 10.1016/j.biomaterials.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 82.Alvarez-Erviti L, Seow YQ, Yin HF, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341-U179. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 83.Zeelenberg IS, Ostrowski M, Krumeich S, Bobrie A, Jancic C, Boissonnas A, Delcayre A, Le Pecq JB, Combadiere B, Amigorena S, Thery C. Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses. Cancer Res. 2008;68(4):1228–35. doi: 10.1158/0008-5472.CAN-07-3163. [DOI] [PubMed] [Google Scholar]
- 84.Nakase I, Futaki S. Combined treatment with a pH-sensitive fusogenic peptide and cationic lipids achieves enhanced cytosolic delivery of exosomes. Sci Rep. 2015;5:10112. doi: 10.1038/srep10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang M, Altinoglu S, Takeda YS, Xu Q. Integrating Protein Engineering and Bioorthogonal Click Conjugation for Extracellular Vesicle Modulation and Intracellular Delivery. PLOS ONE. 2015;10(11):e0141860. doi: 10.1371/journal.pone.0141860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Bai S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res. 2015;32(6):2003–14. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Didiot M-C, Hall LM, Coles AH, Haraszti RA, Godinho BMDC, Chase K, Sapp E, Ly S, Alterman JF, Hassler MR, Echeverria D, Raj L, Morrissey DV, DiFiglia M, Aronin N, Khvorova A. Exosome-mediated Delivery of Hydrophobically Modified siRNA for Huntingtin mRNA Silencing. Molecular Therapy. 2016;24(10):1836–1847. doi: 10.1038/mt.2016.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371(1):48–61. doi: 10.1016/j.canlet.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Agrawal AK, Aqil F, Jeyabalan J, Spencer WA, Beck J, Gachuki BW, Alhakeem SS, Oben K, Munagala R, Bondada S, Gupta RC. Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine: Nanotechnology, Biology and Medicine. 2017;13(5):1627–1636. doi: 10.1016/j.nano.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 91.Hu L, Wickline SA, Hood JL. Magnetic resonance imaging of melanoma exosomes in lymph nodes. Magn Reson Med. :2014. doi: 10.1002/mrm.25376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lamichhane TN, Raiker RS, Jay SM. Exogenous DNA Loading into Extracellular Vesicles via Electroporation is Size-Dependent and Enables Limited Gene Delivery. Mol Pharm. 2015;12(10):3650–7. doi: 10.1021/acs.molpharmaceut.5b00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yoon J, Jo W, Jeong D, Kim J, Jeong H, Park J. Generation of nanovesicles with sliced cellular membrane fragments for exogenous material delivery. Biomaterials. 2015;59:12–20. doi: 10.1016/j.biomaterials.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 94.Wang Q, Ren Y, Mu J, Egilmez NK, Zhuang X, Deng Z, Zhang L, Yan J, Miller D, Zhang H-G. Grapefruit-Derived Nanovectors Use an Activated Leukocyte Trafficking Pathway to Deliver Therapeutic Agents to Inflammatory Tumor Sites. Cancer Research. 2015;75(12):2520. doi: 10.1158/0008-5472.CAN-14-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. Journal of Controlled Release. 2015;205(Supplement C):35–44. doi: 10.1016/j.jconrel.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 96.Hood JL, Scott MJ, Wickline SA. Maximizing exosome colloidal stability following electroporation. Analytical Biochemistry. 2014;448(Supplement C):41–49. doi: 10.1016/j.ab.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Johnsen KB, Gudbergsson JM, Skov MN, Christiansen G, Gurevich L, Moos T, Duroux M. Evaluation of electroporation-induced adverse effects on adipose-derived stem cell exosomes. Cytotechnology. 2016;68(5):2125–38. doi: 10.1007/s10616-016-9952-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kooijmans SAA, Stremersch S, Braeckmans K, de Smedt SC, Hendrix A, Wood MJA, Schiffelers RM, Raemdonck K, Vader P. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. Journal of Controlled Release. 2013;172(1):229–238. doi: 10.1016/j.jconrel.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 99.van der Meel R, Fens MHAM, Vader P, van Solinge WW, Eniola-Adefeso O, Schiffelers RM. Extracellular vesicles as drug delivery systems: Lessons from the liposome field. Journal of Controlled Release. 2014;195(Supplement C):72–85. doi: 10.1016/j.jconrel.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 100.Liga A, Vliegenthart ADB, Oosthuyzen W, Dear JW, Kersaudy-Kerhoas M. Exosome isolation: a microfluidic road-map. Lab on a Chip. 2015;15(11):2388–2394. doi: 10.1039/c5lc00240k. [DOI] [PubMed] [Google Scholar]
- 101.Yang F, Liao X, Tian Y, Li G. Exosome separation using microfluidic systems: size-based, immunoaffinity-based and dynamic methodologies. Biotechnology Journal. 2017;12(4):1600699. doi: 10.1002/biot.201600699. n/a. [DOI] [PubMed] [Google Scholar]
- 102.Contreras-Naranjo JC, Wu H-J, Ugaz VM. Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine. Lab on a Chip. 2017;17(21):3558–3577. doi: 10.1039/c7lc00592j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Salafi T, Zeming KK, Zhang Y. Advancements in microfluidics for nanoparticle separation. Lab on a Chip. 2017;17(1):11–33. doi: 10.1039/c6lc01045h. [DOI] [PubMed] [Google Scholar]
- 104.Gholizadeh S, Shehata Draz M, Zarghooni M, Sanati-Nezhad A, Ghavami S, Shafiee H, Akbari M. Microfluidic approaches for isolation, detection, and characterization of extracellular vesicles: Current status and future directions. Biosens Bioelectron. 2017;91:588–605. doi: 10.1016/j.bios.2016.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao Z, Yang Y, Zeng Y, He M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip. 2016;16(3):489–96. doi: 10.1039/c5lc01117e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang P, He M, Zeng Y. Ultrasensitive microfluidic analysis of circulating exosomes using a nanostructured graphene oxide/polydopamine coating. Lab Chip. 2016;16(16):3033–42. doi: 10.1039/c6lc00279j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shao H, Chung J, Balaj L, Charest A, Bigner DD, Carter BS, Hochberg FH, Breakefield XO, Weissleder R, Lee H. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat Med. 2012;18(12):1835–40. doi: 10.1038/nm.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Im H, Shao H, Park YI, Peterson VM, Castro CM, Weissleder R, Lee H. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat Biotechnol. 2014;32(5):490–5. doi: 10.1038/nbt.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Im H, Lee K, Weissleder R, Lee H, Castro CM. Novel nanosensing technologies for exosome detection and profiling. Lab Chip. 2017;17(17):2892–2898. doi: 10.1039/c7lc00247e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jo W, Jeong D, Kim J, Cho S, Jang SC, Han C, Kang JY, Gho YS, Park J. Microfluidic fabrication of cell-derived nanovesicles as endogenous RNA carriers. Lab on a Chip. 2014;14(7):1261–1269. doi: 10.1039/c3lc50993a. [DOI] [PubMed] [Google Scholar]
- 111.Yoon J, Jo W, Jeong D, Kim J, Jeong H, Park J. Generation of nanovesicles with sliced cellular membrane fragments for exogenous material delivery. Biomaterials. 2015;59(Supplement C):12–20. doi: 10.1016/j.biomaterials.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 112.Théry C, Amigorena S, Raposo G, Clayton A. Current Protocols in Cell Biology. John Wiley & Sons, Inc; 2001. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. [DOI] [PubMed] [Google Scholar]
- 113.Jo W, Kim J, Yoon J, Jeong D, Cho S, Jeong H, Yoon YJ, Kim SC, Gho YS, Park J. Large-scale generation of cell-derived nanovesicles. Nanoscale. 2014;6(20):12056–12064. doi: 10.1039/c4nr02391a. [DOI] [PubMed] [Google Scholar]
- 114.Shurtleff MJ, Temoche-Diaz MM, Karfilis KV, Ri S, Schekman R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Elife. 2016;5 doi: 10.7554/eLife.19276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Santangelo L, Giurato G, Cicchini C, Montaldo C, Mancone C, Tarallo R, Battistelli C, Alonzi T, Weisz A, Tripodi M. The RNA-Binding Protein SYNCRIP Is a Component of the Hepatocyte Exosomal Machinery Controlling MicroRNA Sorting. Cell Rep. 2016;17(3):799–808. doi: 10.1016/j.celrep.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 116.Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13(1):17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Villarroya-Beltri C, Baixauli F, Gutierrez-Vazquez C, Sanchez-Madrid F, Mittelbrunn M. Sorting it out: regulation of exosome loading. Semin Cancer Biol. 2014;28:3–13. doi: 10.1016/j.semcancer.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kuo JS, Chiu DT. Controlling mass transport in microfluidic devices. Annu Rev Anal Chem (Palo Alto Calif) 2011;4:275–96. doi: 10.1146/annurev-anchem-061010-113926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Plevniak K, Campbell M, Myers T, Hodges A, He M. 3D printed auto-mixing chip enables rapid smartphone diagnosis of anemia. Biomicrofluidics. 2016;10(5):054113. doi: 10.1063/1.4964499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhao Z, He JSM. On-chip harvesting and photo-release of immunogenic extracellular vesicles for cancer immunotherapy. Proceedings of the 21st International Conference on Miniaturized Systems for Chemistry and Life Sciences; 2017; pp. 898–899. [Google Scholar]
- 121.Wang SN, Lee LJ. Micro-/nanofluidics based cell electroporation. Biomicrofluidics. 2013;7(1) doi: 10.1063/1.4774071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee C-Y, Chang C-L, Wang Y-N, Fu L-M. Microfluidic Mixing: A Review. International Journal of Molecular Sciences. 2011;12(5):3263. doi: 10.3390/ijms12053263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Seong GH, Crooks RM. Efficient Mixing and Reactions within Microfluidic Channels Using Microbead-Supported Catalysts. Journal of the American Chemical Society. 2002;124(45):13360–13361. doi: 10.1021/ja020932y. [DOI] [PubMed] [Google Scholar]
- 124.Ward K, Fan ZH. Mixing in microfluidic devices and enhancement methods. J Micromech Microeng. 2015;25(9) doi: 10.1088/0960-1317/25/9/094001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Suh YK, Kang S. A Review on Mixing in Microfluidics. Micromachines. 2010;1(3):82. [Google Scholar]
- 126.Johnson TJ, Ross D, Locascio LE. Rapid Microfluidic Mixing. Analytical Chemistry. 2002;74(1):45–51. doi: 10.1021/ac010895d. [DOI] [PubMed] [Google Scholar]
- 127.Kevin W, Fan ZH. Mixing in microfluidic devices and enhancement methods. Journal of Micromechanics and Microengineering. 2015;25(9):094001. doi: 10.1088/0960-1317/25/9/094001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Perkel JM. Membrane messengers: Extracellular vesicles. Science. 2016;352(6291):1349–1351. [Google Scholar]
- 129.Halldorsson S, Lucumi E, Gómez-Sjöberg R, Fleming RMT. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosensors and Bioelectronics. 2015;63(Supplement C):218–231. doi: 10.1016/j.bios.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 130.Yang Y, Leong KW. Microfluidic Cell Culture Systems. William Andrew Publishing; Oxford: 2013. Chapter 1 - Microfluidic Cell Culture Platforms with Embedded Nanoscale Features; pp. 3–26. [Google Scholar]
- 131.Xiao Y, Zhang B, Hsieh A, Thavandiran N, Martin C, Radisic M. Microfluidic Cell Culture Systems. William Andrew Publishing; Oxford: 2013. Chapter 12 - Microfluidic Cell Culture Techniques; pp. 303–321. [Google Scholar]
- 132.Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW. Analysis of nanoparticle delivery to tumours. Nature Reviews Materials. 2016;1:16014. [Google Scholar]




