Abstract
Herbivorous attack induces plant defenses. There is evidence that some pests suppress these defenses by interfering with signaling pathways. We here report that infestation by the white-backed planthopper, Sogatella furcifera, induces defense responses in rice and infection of the southern rice black-streaked dwarf virus in the planthoppers partially suppresses the planthopper-induced plant defenses. Salicylic acid (SA) levels generally showed a temporal increase pattern while jasmonic acid (JA) levels generally exhibited a decrease pattern in the planthopper-infested plants, irrespective of virus infection status in the insects. The increase in SA was less while the decrease in JA was more in the viruliferous insect-infested plants than in the nonviruliferous insect-infested plants at both 48 and 72 h post infestation. The phytohormone levels corresponded to the patterns of relative expression levels of SA-marker genes (ICS1 and NPR1) and JA-marker gene (AOS2) in the plant treatments. Planthoppers performed better on the uninfested plants than on the previously infested plants and were of not significant increase in performance on the plants previously attacked by viruliferous planthoppers in comparison with the plants previously attacked by nonviruliferous insects. Our results indicate that the virus plays a role in partially suppressing the plant defenses induced by the planthopper. These findings provide a new perspective on plant–virus-vector interactions.
Introduction
Most of the plant viruses rely on insects for spread1. Complex interplay has evolved in the triangle relationship among virus, plant and insect vector. The direct (by infection of the vector) or indirect (by infection of the host plant) interaction between plant virus and vector can be beneficial, neutral, or deleterious for the vector2–4.
The southern rice black-streaked dwarf virus (SRBSDV) is a Fijivirus transmitted in a persistent propagative manner5. In recent years, SRBSDV have devastated rice crops in south China and Vietnam and caused large economic losses6,7, and occurrence was also reported in Japan8. The white-backed planthopper (WBPH), Sogatella furcifera Horváh, is the only known vector of SRBSDV7. The latent periods of SRBSDV in WBPH varies from 6 to 14 d9. Viruliferous WBPH nymphs experience extended development than nonviruliferous nymphs and nonviruliferous WBPH adults live longer when they are fed on SRBSDV-infected plants, which are believed to favor virus spread4.
When attacked by herbivores or pathogens, plants usually mobilize an array of defensive responses to counteract the attack, which are primed by phytohormones such as salicylic acid (SA), jasmonic acid (JA) and ethylene (ET)10. Biotrophic pathogens, virus included, and most phloem-feeding insects may induce SA pathway, while necrotrophic pathogens including some viruses, some chewing herbivores, and some phloem-feeding insects may induce JA response11–14.
A number of functional genes have been identified for the biosynthesis of phytohormones in priming of the induced defense responses. For example, NPR1 genes are first reported in SA-mediated systemic acquired resistance in Arabidopsis15. ICS1 genes are required for pathogen-induced biosynthesis of salicylic acid16. AOS and LOX genes are involved in JA biosynthesis17. In rice plants damaged by the brown planthopper Nilaparvata lugens, SA level is increased and SA pathway genes are up-regulated, but there are no significant changes in JA level and expression of JA pathway genes in comparison with those in the undamaged plants18. In SRBSDV-infected rice plants, the expression of JA and SA biosynthesis genes changed dynamically and the JA gene OsAOS1 was down-regulated at 40 days post inoculation while the SA gene OsICS was up-regulated at 35 days post inoculation when the viral titers were the highest19. The induced defense responses triggered by a previous attack by the spider mite Tetranychus evansi and T. urticae are modulated by JA-related genes and show influence on the performance of subsequent T. evansi infestation20. However, in the SRBSDV-rice-WBPH system, it remains unknown whether virus infection of WBPH would affect plant defenses induced by a previous attack and what is the consequence for subsequent conspecific infestation.
Proteinase inhibitor (PI) is an inducible defense-related protein that is regulated by JA signal pathway21,22. PI is known to inhibit activities of digestive enzymes in insects’ midguts, thus reducing the growth and development of insects23. The spider mite T. evansi performed better on the previously conspecifics-infested plants due to these plants having lower PI activity20, PI activity in tomato leaves infested by tomato yellow leaf curl virus (TYLCV)-infected Bemisia tabaci biotype Q was lower than in leaves infested by nonviruliferous insects3. Whether previous infestation by viruliferous vector would influence on plant PI activity differentially and further affect conspecifics performance is unclear.
In this study, we measured JA and SA concentrations and PI activity, and quantified JA- and SA-related gene expression in healthy plants and plants previously exposed to heavy infestation of viruliferous or nonviruliferous WBPH. Further, we compared the performance of nonviruliferous WBPH on these plants. Our goals are to understand how induced plant defenses would affect conspecifics performance and to determine whether virus infection of the planthopper will affect the induced plant defense responses.
Results
Plant endogenous SA and JA concentrations
Endogenous SA and JA concentrations in the infested rice plants were measured dynamically (Fig. 1). SA concentrations in the WBPH-infested plants generally showed a temporal increase pattern post WBPH infestation (Fig. 1A). Between the plants infested by viruliferous and nonviruliferous WBPH, SA concentrations showed no significant differences at 6, 12 and 24 h post infestation (hpi) (t ≤ 0.659, P ≥ 0.534), but were 33.3% and 31.7% lower at 48 and 72 hpi in the viruliferous WBPH-infested plants than in the nonviruliferous WBPH-infested plants (t ≥ 3.088, P ≤ 0.021), respectively (Fig. 1A).
Figure 1.
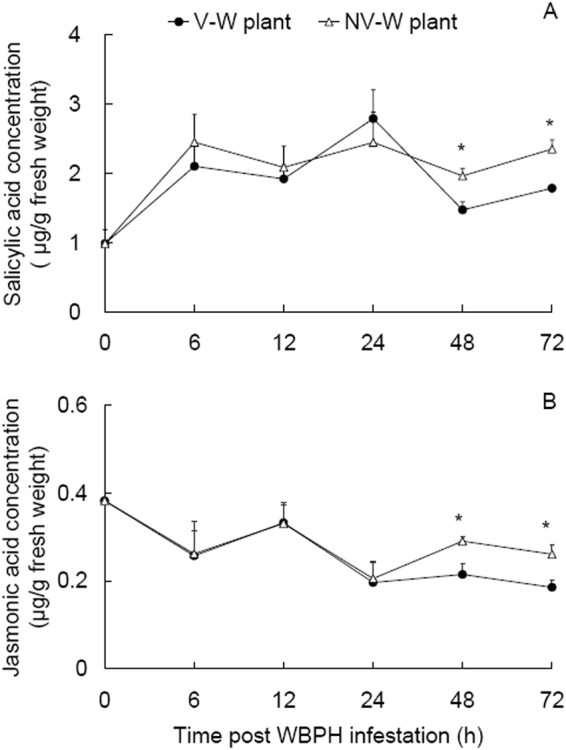
Dynamic concentrations of salicylic acid (A) and jasmonic acid (B) in viruliferous and nonviruliferous WBPH-infested plants (V-W and NV-W plant, respectively) and uninfested plants (0 h post WBPH infestation). Values are means ± SE. * indicates significant differences between the viruliferous and nonviruliferous WBPH-infested plants at a certain time point post WBPH infestation (independent sample t-test, P < 0.05).
Unlike SA, JA levels in the WBPH-infested plants generally exhibited a temporal decrease pattern post WBPH infestation (Fig. 1B). JA levels were not different between the viruliferous and nonviruliferous WBPH-infested plants at 6, 12 and 24 hpi (t ≤ 0.144, P ≥ 0.89), but were 35.3% and 40.7% lower at 48 and 72 hpi in the former than in the latter (t ≥ 3.131, P ≤ 0.033), respectively (Fig. 1B).
Relative expression levels of SA and JA genes
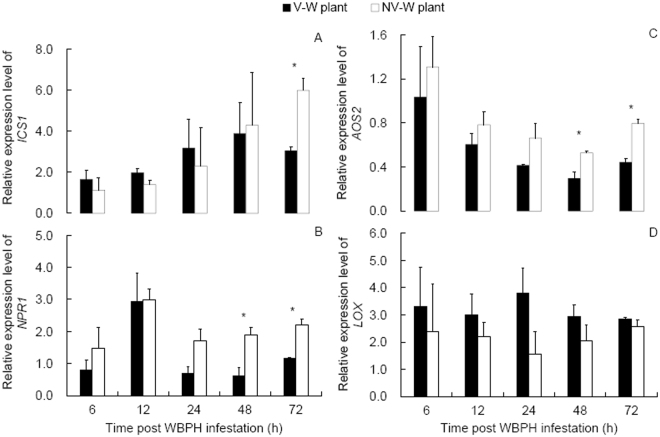
Expression levels of the SA-marker genes ICS1 and NPR1 and the JA-marker gene AOS2 all showed significant changes with the time post WBPH infestation of the plants (ICS1: F = 3.624, df = 5,30, P = 0.011; Fig. 2A; NPR1: F = 5.671, df = 5,30, P = 0.001; Fig. 2B; AOS2: F = 3.770, df = 5,30, P = 0.009; Fig. 2C), while the JA-marker gene LOX did not (F = 1.483, df = 5,30, P = 0.225; Fig. 2D). In the SA pathway, ICS1 was expressed at a lower level in the plants infested by viruliferous than nonviruliferous WBPH at 72 hpi (t = 4.867, P = 0.008; Fig. 2A), and NPR1 showed similar patterns at both 48 and 72 hpi (t ≥ 3.835, P ≤ 0.024; Fig. 2B). For AOS2, expression was at lower levels at both 48 and 72 hpi in the viruliferous than in the nonviruliferous WBPH-infested plans (t ≥ 3.657, P ≤ 0.022; Fig. 2C). The JA-marker gene LOX showed no significant differences in expression level between the viruliferous and nonviruliferous WBPH-infested plants at all the time points post WBPH infestation (t ≤ 1.802, P ≥ 0.146; Fig. 2D).
Figure 2.
Dynamic expression levels of salicylic acid-marker genes (A: ICS1, B: NPR1) and jasmonic acid-marker genes (C: AOS2, D: LOX) in the viruliferous and nonviruliferous WBPH-infested plants (V-W plant and NV-W plant, respectively) relative to those in the uninfested plants. Values are means ± SE. * indicates significant differences between the viruliferous and nonviruliferous WBPH-infested plants at a certain time point post WBPH infestation (Independent sample t-test, P < 0.05).
Proteinase inhibitor activity
Proteinase inhibitor activity was reduced in the infested plants (F = 9.956, df = 2,14, P = 0.003; Fig. 3). It was significantly lower in the viruliferous WBPH-infested plants than in the uninfested plants (Tukey HSD test, P = 0.002), but was not significantly different between the viruliferous and nonviruliferous WBPH-infested plants.
Figure 3.
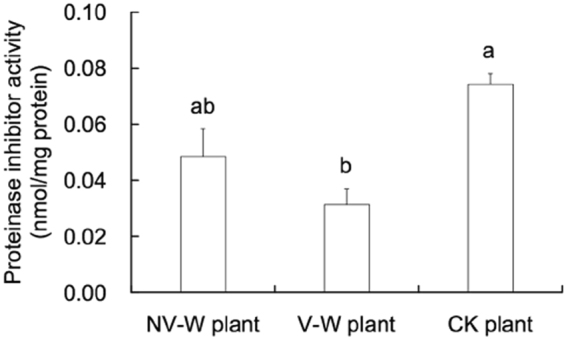
Proteinase inhibitor activity in the viruliferous and nonviruliferous WBPH-infested plants (NV-W plant and V-W plant, respectively) and uninfested plants (CK plant). Values are means ± SE. Different letters over the bars indicate significant differences (Tukey HSD test, P < 0.05).
WBPH performance
When the plants previously infested by WBPH or not were subsequently subjected to nonviruliferous WBPH females, significant difference was observed in the insects’ longevity (F = 9.023, df = 2,74, P < 0.001; Fig. 4A). The females lived shorter on the previously WBPH-infested plants than on the uninfested plants (Tukey honestly significant difference (HSD) test, P ≤ 0.008); on the infested plants, the females lived 14.2 d on the viruliferous WBPH-infested plants and 13.6 d on the non-viruliferous WBPH-infested plants; however, the difference was not significant. Fecundity of the females was not significantly different between the plant treatments (F = 1.44, df = 2,60, P = 0.245; Fig. 4B), although was reduced by 15.8% on the infested plants in comparison with the uninfested plants and by 5.8% on the non-viruliferous WBPH-infested plants compared to the viruliferous WBPH-infested plants.
Figure 4.
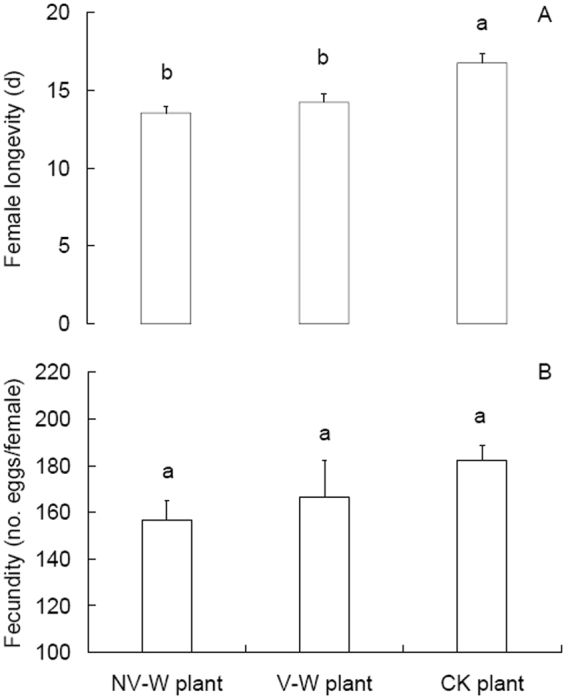
Longevity (A) and fecundity (B) of nonviruliferous WBPH females feeding on the rice plants previously infested or not. NV-W plant: plants previously infested by nonviruliferous females; V-W plant: plants previously infested by viruliferous females; CK plant: plants previously not infested. Values are means ± SE. Different letters over the bars indicate significant differences (Tukey HSD test, P < 0.05).
Discussion
We found that the WBPH on the plants previously infested by the conspecifics did not perform as well as those on the uninfested plants, as indicated by shorter longevity and a not significant reduction (by 15.8%) of fecundity. This corresponds to what is normally observed as the effect of induced plant defense, i.e., herbivore performance is lower on previously damaged plants than on undamaged plants24. However, this result contrasts to the findings by Sarmento et al.20, where the spider mite T. evansi performed much better on tomato leaves previously attacked by the conspecifics than on unattacked leaves while had reduced performance on leaves previously attacked by its congener T. urticae in comparison with uninfested plants. The recorded patterns of higher performance of T. evansi on the plants coincided with these plants having lower PI activity20. The low PI activity in leaves previously attacked by T. evansi is due to lack of up-regulation of the JA and SA defensive pathways20. In our current study, PI activity was lower in the viruliferous WBPH-infested plants or not significantly reduced in the nonviruliferous WBPH-infested plants than that in the undamaged plants (Fig. 3), showing that the insect performance was negatively connected with PI activity, as reported for the brown planthopper N. lugens25. Therefore, in contrast to the results for T. evansi20, our results show no positive connection between WBPH performance and PI activity in the previously damaged and undamaged plants.
Plant SA concentrations showed a general temporal increase pattern within 72 h of previous infestation by WBPH (Fig. 1A). Although SA levels and expression of SA-related genes may show circadian rhythm, as that reported for the expression of the serine/threonine protein kinase gene OsPBL126, Silverman et al.27 reported no significant changes in SA levels in healthy rice seedlings during a five week monitoring (7–35 days after sowing). Even with circadian rhythm, the SA levels and SA-related gene expression may show similar circadian changes in different treatments in the present study, as in the case of OsPBL126. Therefore, although not measured dynamically in the uninfested plants, it can be reasoned that SA levels in the uninfested plants may be low in comparison with those in the infested plants. The general temporal increase pattern of SA concentrations in the infested plants (Fig. 1A) is linked with the low WBPH performance on these plants (Fig. 4). In the brown planthopper N. lugens, the insect performance was positively correlated with planthopper-induced H2O2 and SA concentrations25. In another study, WBPH showed no performance difference on the rice mutants with impaired JA biosynthesis and the wild lines28. Our current results and previous reports indicate that the up-regulated SA signaling pathway may explain the relatively poor performance of WBPH on the previously infested plants in comparison with that on the uninfested plants.
Between the viruliferous and nonviruliferous WBPH-infested plants, we observed a not significant reduction in conspecifics performance on the latter in comparison with the former (Fig. 4), which corresponds to the higher SA concentrations in the latter than in the former at 48 and 72 hpi (Fig. 1). In the interaction between B. tabaci and TYLCV, B. tabaci biotype Q has a mutualistic relationship with TYLCV in that viruliferous biotype Q down-regulated while viruliferous biotype B up-regulated SA signaling, which is believed to be the reason for the wide spread of B. tabaci biotype Q and TYLCV in China3. Our results indicate that SRBSDV infection of WBPH functions in a way to down-regulate the induced SA-related plant defenses.
Plant defense responses to sucking insect pests are principally regulated by SA pathways and may act on subsequent infestation by conspecifics or congeners29,30. Attack by phloem-feeding insects usually induce SA accumulation25,31, as observed in our results (Fig. 1A). The temporally increased SA concentration in the infested plants coincides with the up-regulation of the SA-marker genes NPR1 and ICS1 in these plants (Fig. 2A,B). These results confirm previous reports that planthopper infestation induces the SA pathway25,28. Interestingly, the recorded lower SA concentration in the viruliferous than in the nonviruliferous WBPH-infested plants corresponds to the down-regulation of the SA-marker genes NPR1 and ICS1 in the former than in the latter (Figs 1A and 2A,B). In contrast to our results, the SA gene OsICS was up-regulated in SRBSDV-infected rice plants when the viral titers were the highest at 35 days after virus inoculation in comparison with that in uninfected plants19. However, this result19 has to be taken as the plant defense response to SRBSDV alone; while in the present study, the SA levels were measured within 3 days of infestation by the planthoppers and the plants may have responded to both virus infection and WBPH infestation. In a pathosystem consisting of a DNA virus TYLCV, B. tabaci and tomato plants, different patterns were reported. Infestation by viruliferous B. tabaci biotype B increased plant SA levels and up-regulated SA genes (NPR1 and PR1) in comparison with infestation by the nonviruliferous counterparts while infestation by viruliferous B. tabaci biotype Q showed no influence on SA levels and expression of SA genes (NPR1 and PR1)3. These results indicate that the responses of plant SA signal pathway to the infestation of viruliferous vectors may vary with the specific pathosystems in case.
We recorded temporally reduced JA concentrations in the infested plants (Fig. 1). Although not measured dynamically, as reasoned for SA levels, JA levels in the infested plants may be low in comparison with those in the uninfested plants, which coincides with the down-regulation of the JA-marker gene AOS2 in the infested plants (Fig. 2C), while the gene LOX is up-regulated in the infested plants. Previous reports showed similar JA marker gene expression patterns in rice plants after bacterial blight disease inoculation, i.e., up-regulated LOX and reduced AOS231 and in tomato plants infested by B. tabaci, i.e. up-regulated JA upstream gene LOX and reduced downstream gene PI II and JA concentrations32.
In conclusion, our results demonstrate that previous WBPH infestation increases the SA-mediated plant defenses, which accounts for the reduced performance of subsequent WBPH infestation on the infested vs uninfested rice plants. Compared to previous infestation by nonviruliferous WBPH, previous infestation by viruliferous insects up-regulates SA to a lesser extent, which contributes to the not significantly increased WBPH performance on the plants previously infested by viruliferous WBPH over the plants infested by nonviruliferous insects. The results show that infection with SRBSDV in WBPH plays a role in partially suppressing the plant defenses induced by the vector, which provides a new perspective on plant–virus-vector interactions and additional information for assessing SRBSDV transmission risks and field epidemiology.
Methods
Insects and plants
Potted seedlings of a SRBSDV-susceptible rice variety (Diantun 502) were cultured within 80-mesh insect-proof cages (50 by 50 by 50 cm) in a greenhouse (30 ± 5°, 15 L: 9D). WBPH colonies were maintained using caged rice seedlings in a climatic chamber (30 ± 1°, 15 L: 9D). SRBSDV-positive seedlings collected from paddy fields, as determined by reverse transcription polymerase chain reaction (RT-PCR), were used to establish a stock culture of infected rice plants in cages within another climatic chamber.
To obtain viruliferous WBPH adults for the experiments, nonviruliferous young nymphs (1st to 2nd instars) of WBPH were confined with SRBSDV-positive plants for 5 d and then transferred to caged virus-free plants for development. Newly emerged adult insects (<24 h) were used in assays.
SRBSDV detection by RT-PCR
Virus infection status was detected by one-step RT-PCR as described by Li et al.33. Briefly, total RNA of each sample was amplified using primers (forward: 5′-CGCGTCATCTCAAACTACAG-3′, reverse: 5′-TTTGTCAGCATCTAAAGCGC-3′)34. The amplified fragment of the expected size (682 bp) of SRBSDV-S10 fragment was confirmed by electrophoresis in agarose gels. An insect designated as viruliferous was confirmed by RT-PCR as SRBSDV-positive after the experiment with the insect was finished, and a plant designated as infected was confirmed as SRBSDV-positive using a portion of the plant material. In our laboratory colonies, about 80% of the insects and plants designated as SRBSDV-positive were confirmed to be really SRBSDV-positive.
Sampling for determination of defense-related phytohormone pathways and proteinase inhibitor activity
To determine the effects of WBPH infestation and SRBSDV infection of the WBPH on defense related phytohormone pathways and proteinase inhibitor activity, one 35–45 day old rice seedling was exposed to 20 viruliferous or nonviruliferous macropterous females in a plastic tube (3 cm × 8 cm) within a climatic chamber (27 ± 2 °C, 15 L: 9D, RH 75%). A sponge disc (3 cm in diameter and 2 cm thick) was used to secure the seedling at 6 cm above roots and another sponge disc was used to seal the tube opening, thus leaving a space of 4-cm height in the tube, where the insects were left to feed ad lib. Leaf sheaths of the 4-cm stem segments were sampled at 0 (not infested), 6, 12, 24, 48, or 72 h post infestation (hpi) by WBPH and frozen in liquid nitrogen. The leaf sheath samples thus collected were used to measure the concentrations of salicylic acid and jasmonic acid and to determine the relative expression levels of phytohormones-related genes. Additionally, the 72 hpi samples were used to determine proteinase inhibitor activity.
Quantification of phytohormone concentrations
Phytohormones were quantified by liquid chromatography-mass spectrometry to detect the influence of WBPH infestation of the plants and SRBSDV infection of the WBPH on phytohormone levels in rice plants. Total phytohormones were extracted and purified as described by Kojima et al.35. Briefly, radio-labeled internal standard containing 50 ng D6-SA (Sigma, cat no. 616796) and 50 ng H2-JA (OIChemim, cat no. 0145324) was added to the sample during the extraction36. The extracted sample was transferred by pipette to a brown glass vial and then analyzed using a triple quadruple liquid chromatography-mass spectrometry system (XEVO TQ-S-Quantum Access, Waters, USA). The reaction monitoring conditions and gradient parameters were listed in the supplemental file (Tables S1 and S2). The hormone concentration was normalized as ng per g of fresh weight of leaf sheath using the mass of fresh plant tissue measured before extraction. The quantification was repeated from four to eight samples and for each sample, technically repeated for three times.
Analysis of relative expression of phytohormones-related genes
To measure the impact of WBPH infestation of the plants and SRBSDV infection of the WBPH on induced phytohormone response in rice plants, we quantified transcript levels of SA- and JA-related genes in rice leaf sheath using quantitative real-time PCR (qPCR). ICS1 (isochorismate synthase 1) and NPR1 (homolog of Arabidopsis nonexpressor of pathogenesis-related genes 1) were selected as SA-marker genes, and LOX (lipoxygenase) and AOS2 (allene oxide synthase 2) were selected as JA-marker genes (Table 1). The extraction of total RNA from the leaf sheath samples and the synthesis of cDNA was described by Li et al.37. Every attempt was made to adhere to minimum information for quantitative real-time PCR experiments (MIQE) guidelines to ensure proper and accurate reporting of qPCR data38. The qPCR reaction was performed using the Bester SybrGreen qPCR mastermix (DBI Bioscience, Germany) with the 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Amplification reactions were performed in a 20 μL final volume containing 10 μL of Bester SybrGreen qPCR mastermix (DBI), 0.4 μL of forward primer (10 μM) and reverse primer (10 μM) pairs (Table 1), 0.04 μL of 50 × Rox, and 5 μL of cDNA (4 ng/μL) and 5.16 μL of sterilized H2O. Reaction conditions were as follows: 95 °C for 2 min followed by 40 cycles of 10 sec at 95 °C, 34 sec at 60 °C, and then followed by melt curves stages. Negative controls without template were included in each experiment. The qPCR reaction was performed for three samples and for each sample, technically repeated for three times. The comparative 2−ΔΔCT method was used to calculate the relative gene expression levels in different samples39. The average of CT values was used to calculate ΔΔCTs with the following equation:
Table 1.
Nucleotide sequence of primers used for qPCR analysis.
| Gene name | GenBank No. | Sequence (5′-3′) | Expected length (bp) | Reference |
|---|---|---|---|---|
| Target gene | ||||
| ICS1 | AK120689 | TATGGTGCTATCCGCTTCGAT | 120 | Qiu et al.31 |
| CGAGAACCGAGCTCTCTTCAA | ||||
| NPR1 | AY923983 | TTTCCGATGGAGGCAAGAG | 120 | Chern et al.43 |
| GCTGTCATCCGAGCTAAGTGTT | ||||
| LOX | D14000 | GCATCCCCAACAGCACATC | 110 | Qiu et al.31 |
| AATAAAGATTTGGGAGTGACATA | ||||
| AOS2 | AY062258 | CTCGTCGGAAGGCTGTTGCT | 120 | Qiu et al.31 |
| ACGATTGACGGCGGAGGTT | ||||
| Reference gene | ||||
| UBQ5 | AK061988 | AACCACTTCGACCGCCACT | 120 | Li et al.44 |
| GTTCGATTTCCTCCTCCTTCC | ||||
| OsActin | AB047313 | CAGCACATTCCAGCAGAT | 108 | Hao et al.45 |
| GGCTTAGCATTCTTGGGT | ||||
Determination of proteinase inhibitor activity
A leaf sheath sample was ground at 4 °C using a TissueLyser, and proteinase inhibitor was extracted as described by Sarmento et al.20. The proteinase inhibitor activity was represented by trypsin activity that was detected at 410 nm with a spectrophotometer as the difference between the absorbance monitored at 150 s and 60 s40. The trypsin activity was expressed as mg of trypsin inhibited per g of protein41. The measurement was performed for five samples and for each sample, technically repeated for three times.
Influence of previous WBPH infestation on subsequent conspecifics performance
To measure the performance of WBPH females on plants previously exposed to WBPH of different virus infection status, 35–45 day old potted rice plants were individually exposed to 100 newly emerged viruliferous or nonviruliferous WBPH for 72 h in an insect-proof cage in a completely randomized design. Control plants not exposed to WBPH were also placed in cages. After 72 h, the insects in the cages were removed and the plants were each transplanted into a glass tube (2.5 cm in diameter and 15 cm in length) with nutrient solution42. Then one nonviruliferous WBPH female and two males (1 day old) were confined with one plant either previously infested by nonviruliferous or viruliferous WBPH or left uninfested in the tubes in a completely randomized design. The glass tubes were observed daily and nymphs, if any, were removed after their number was recorded. Upon the death of females, leaf sheaths of the rice seedlings were dissected under a stereomicroscope and the number of unhatched WBPH eggs therein was recorded. Female longevity was calculated using dates of emergence and death. Fecundity was calculated as the sum of nymph numbers and number of the unhatched eggs. For each treatment, the bioassays for longevity and fecundity were repeated 15–34 times.
Statistical Analysis
One-way analysis of variance (ANOVA) was used to detect differences of WBPH performance, relative expression of the genes and PI activity between the plant treatments, i.e., uninfested, nonviruliferous and viruliferous WBPH-infested plants. Tukey HSD test was used to separate the means where there was a significant effects on WBPH performance and PI activity. Differences in JA/SA concentration and relative expression of the genes between nonviruliferous and viruliferous WBPH-infested plants at a specific time post WBPH infestation were compared using independent sample t-test (SPSS version 19.0, SPSS Inc., Chicago, IL).
Electronic supplementary material
Acknowledgements
We thank Xiaoqin Sun, Luyao Jia, Qi Cai, and Wen Su for technical assistance with the experiments. The research was funded by the National Natural Science Foundation of China (31371951) and Ministry of Science and Technology of China (2016YFD0300701). The granting agency had no role in the design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
M.H. conceived and together with P.L. and X.L. designed the experiments. P.L., H.L., F.L. and S.A. performed the experiments. M.H. contributed reagents/materials. P.L. and M.H. analyzed the results. All the authors joined writing and reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-27354-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaolan Liao, Email: 359437372@qq.com.
Maolin Hou, Email: mlhou@ippcaas.cn.
References
- 1.Hohn T. Plant virus transmission from the insect point of view. Proc. Natl. Acad. Sci. USA. 2007;104:17905–17906. doi: 10.1073/pnas.0709178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belliure B, et al. Herbivore arthropods benefit from vectoring plant viruses. Ecol. Lett. 2005;8:70–79. doi: 10.1111/j.1461-0248.2004.00699.x. [DOI] [Google Scholar]
- 3.Shi X, et al. Plant virus differentially alters the plant’s defense response to its closely related vectors. Plos One. 2013;8:e83520. doi: 10.1371/journal.pone.0083520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lei W, Liu D, Li P, Hou M. Interactive effects of southern rice black-streaked dwarf virus infection of host plant and vector on performance of the vector, Sogatella furcifera (Homoptera: Delphacidae) J. Econ. Entomol. 2014;107:1721–1727. doi: 10.1603/EC13569. [DOI] [PubMed] [Google Scholar]
- 5.Zhou G, Zhang S, Zou S, Xu Z, Zhou Z. Occurrence and damage analysis of a new rice dwarf disease caused by southern rice black-streaked dwarf virus. Plant Protect. 2010;36:144–146. [Google Scholar]
- 6.Hoang AT, et al. Identification, characterization, and distribution of southern rice black-streaked dwarf virus in Vietnam. Plant Dis. 2011;95:1063–1069. doi: 10.1094/PDIS-07-10-0535. [DOI] [PubMed] [Google Scholar]
- 7.Tu Z, Ling B, Xu D, Zhang M, Zhou G. Effects of southern rice black-streaked dwarf virus on the development and fecundity of its vector. Sogatella furcifera. Virol. J. 2013;10:145. doi: 10.1186/1743-422X-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsukura K, et al. Dynamics of southern rice black-streaked dwarf virus in rice and implication for virus acquisition. Phytopathology. 2013;103:509–512. doi: 10.1094/PHYTO-10-12-0261-R. [DOI] [PubMed] [Google Scholar]
- 9.Pu L, et al. Transmission characteristics of southern rice black-streaked dwarf virus by rice planthoppers. Crop Prot. 2012;41:71–75. doi: 10.1016/j.cropro.2012.04.026. [DOI] [Google Scholar]
- 10.Ellinger D, et al. Elevated early callose deposition results in complete penetration resistance to powdery mildew in Arabidopsis. Plant Physiol. 2013;161:1433–1444. doi: 10.1104/pp.112.211011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pieterse CMJ, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SCM. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012;28:489–521. doi: 10.1146/annurev-cellbio-092910-154055. [DOI] [PubMed] [Google Scholar]
- 12.Zhang T, et al. Begomovirus–whitefly mutualism is achieved through repression of plant defences by a virus pathogenicity factor. Mol. Ecol. 2012;21:1294–1304. doi: 10.1111/j.1365-294X.2012.05457.x. [DOI] [PubMed] [Google Scholar]
- 13.Luan J-B, et al. Suppression of terpenoid synthesis in plants by a virus promotes its mutualism with vectors. Ecol. Lett. 2013;16:390–398. doi: 10.1111/ele.12055. [DOI] [PubMed] [Google Scholar]
- 14.Bostock RM. Signal crosstalk and induced resistance: straddling the line between cost and benefit. Annu. Rev. Phytopathol. 2005;43:545–580. doi: 10.1146/annurev.phyto.41.052002.095505. [DOI] [PubMed] [Google Scholar]
- 15.Cao H, Bowling SA, Gordon AS, Dong X. Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell. 1994;6:1583–1592. doi: 10.1105/tpc.6.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wildermuth MC, Dewdney J, Wu G, Ausubel FM. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature. 2001;414:562–565. doi: 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- 17.Turner JG, Ellis C, Devoto A. The jasmonate signal pathway. Plant Cell. 2002;14:153–164. doi: 10.1105/tpc.000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, et al. β-glucosidase treatment and infestation by the rice brown planthopper Nilaparvata lugens, elicit similar signaling pathways in rice plants. Chin. Sci. Bull. 2008;53:53–57. doi: 10.1007/s11434-008-0048-4. [DOI] [Google Scholar]
- 19.Lu G, Zhang T, He Y, Zhou G. Virus altered rice attractiveness to planthoppers is mediated by volatiles and related to virus titre and expression of defence and volatile-biosynthesis genes. Sci. Rep. 2016;6:38581. doi: 10.1038/srep38581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarmento RA, et al. A herbivore that manipulates plant defence. Ecol. Lett. 2011;14:229–236. doi: 10.1111/j.1461-0248.2010.01575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farmer EE, Johnson RR, Ryan CA. Regulation of expression of proteinase inhibitor genes by methyl jasmonate and jasmonic acid. Plant Physiol. 1992;98:995–1002. doi: 10.1104/pp.98.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farmer EE, Ryan CA. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell. 1992;4:129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhattacharyya A, Mazumdar LS, Babu CR. Bioinsecticidal activity of Archidendron ellipticum trypsin inhibitor on growth and serine digestive enzymes during larval development of Spodoptera litura. Comp. Biochem. Phys. C. 2007;145:669–677. doi: 10.1016/j.cbpc.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Walling LL. The myriad plant responses to herbivores. J. Plant Growth Regul. 2000;19:195–216. doi: 10.1007/s003440000026. [DOI] [PubMed] [Google Scholar]
- 25.Zhou G, et al. Silencing OsHI-LOX makes rice more susceptible to chewing herbivores, but enhances resistance to a phloem feeder. Plant J. 2009;60:638–648. doi: 10.1111/j.1365-313X.2009.03988.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee K-J, Kim K. The rice serine/threonine protein kinase OsPBL1 (ORYZA SATIVA ARABIDOPSIS PBS1-LIKE 1) is potentially involved in resistance to rice stripe disease. Plant Growth Regul. 2015;77:67–75. doi: 10.1007/s10725-015-0036-z. [DOI] [Google Scholar]
- 27.Silverman P, et al. Salicylic acid biosynthesis, conjugation in rice and possible role. Plant Physiol. 1995;108:633–639. doi: 10.1104/pp.108.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang B, Zhou G, Xin Z, Ji R, Lou Y. (Z)−3-Hexenal, one of the green leaf volatiles, increases susceptibility of rice to the white-backed planthopper Sogatella furcifera. Plant Mol. Biol. Rep. 2015;33:377–387. doi: 10.1007/s11105-014-0756-7. [DOI] [Google Scholar]
- 29.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 30.Agrawal AA. Current trends in the evolutionary ecology of plant defence. Funct. Ecol. 2011;25:420–432. doi: 10.1111/j.1365-2435.2010.01796.x. [DOI] [Google Scholar]
- 31.Qiu D, et al. OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol. Plant-Microbe Interact. 2007;20:492–499. doi: 10.1094/MPMI-20-5-0492. [DOI] [PubMed] [Google Scholar]
- 32.Shi X, et al. Bemisia tabaci Q carrying tomato yellow leaf curl virus strongly suppresses host plant defenses. Sci. Rep. 2014;4:5230. doi: 10.1038/srep05230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li P, et al. Asymmetric spread of SRBSDV between rice and corn plants by the vector Sogatella furcifera (Hemiptera: Delphacidae) Plos One. 2016;11:e0165014. doi: 10.1371/journal.pone.0165014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, Zhou G, Zhang S. Detection of southern rice black-streaked dwarf virus using one-step dual RT-PCR. Acta Phytopathol. Sin. 2012;42:84–87. [Google Scholar]
- 35.Kojima M, et al. Highly sensitive and high throughput analysis of plant hormones using MS-Probe modification and Liquid Chromatography-Tandem Mass Spectrometry: an application for hormone profiling in Oryza sativa. Plant Cell Physiol. 2009;50:1201–1214. doi: 10.1093/pcp/pcp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan X, Welti R, Wang X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat. Protocols. 2010;5:986–992. doi: 10.1038/nprot.2010.37. [DOI] [PubMed] [Google Scholar]
- 37.Li Z, An X-K, Liu Y-D, Hou M-L. Transcriptomic and expression analysis of the salivary glands in white-backed planthoppers, Sogatella furcifera. Plos One. 2016;11:e0159393. doi: 10.1371/journal.pone.0159393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bustin SA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Kakade ML, Rackis JJ, Mcghee JE, Puski G. Determination of trypsin inhibitor activity of soy products: a collaborative analysis of an improved procedure. Cereal Chem. 1974;51:376–382. [Google Scholar]
- 41.Kant MR, Ament K, Sabelis MW, Haring MA, Schuurink RC. Differential timing of spider mite-induced direct and indirect defenses in tomato plants. Plant Physiol. 2004;135:483–495. doi: 10.1104/pp.103.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshida, S., Forno, D. A. & Cock, J. H. Laboratory manual for physiological studies of rice. International Rice Research Institute, Los Baños, Laguna, Philippines (1976).
- 43.Chern M, Fitzgerald HA, Canlas PE, Navarre DA, Ronald PC. Overexpression of a rice NPR1 homolog leads to constitutive activation of defense response and hypersensitivity to light. Mol. Plant-Microbe Interact. 2005;18:511–520. doi: 10.1094/MPMI-18-0511. [DOI] [PubMed] [Google Scholar]
- 44.Li R, Li J, Zhou G, Lou Y. Validation of rice candidate reference genes for herbivore-induced quantitative real-time PCRanalysis. Chin. Bull. Bot. 2013;48:184–191. doi: 10.3724/SP.J.1259.2013.00184. [DOI] [Google Scholar]
- 45.Hao P, et al. Herbivore-induced callose deposition on the sieve plates of rice: an important mechanism for host resistance. Plant Physiol. 2008;146:1810–1820. doi: 10.1104/pp.107.111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.