Abstract
In addition to its role in gastric conditions, Helicobacter pylori has been found to contribute to the development of several non-gastric issues in recent years. Eradication therapy is the only effective management strategy to minimize the H. pylori-related gastric cancer and extra-gastric complications. For an effective “test and treat” strategy, diagnosis and therapy are both important. Because the infection is usually asymptomatic, patient selection is a critical issue for timely diagnosis and many clinical and demographic factors should be considered. Clarithromycin and metronidazole resistance rates also need to be considered while eradication therapy is offered. In this report, we discuss the issues which must be taken into account for the correct and timely diagnosis and for the antibiotic therapy-based management of H. pylori infection.
Keywords: Helicobacter pylori, virulence factors, eradication therapy, antibiotics resistance
Introduction
Helicobacter pylori is the causative agent of chronic gastric infections, and it has been estimated that at least half of the world’s population is infected. A recent meta-analysis on the global prevalence of H. pylori infection has shown an overall prevalence of 44.3%, and estimated prevalences are as high as 89.7% in Nigeria and as low as 10.0% in Indonesia and 8.9% in Yemen 1. Socio-economic status, together with the level of urbanization and sanitation conditions, likely reflects the differences of H. pylori prevalence from country to country 2. The exact route of this bacterium’s transmission is unclear; however, evidence supports person-to-person transmission via oral–oral or fecal–oral route between family members 3, 4. After it has transited to the gastric lumen, H. pylori localizes to specific locations such as the antrum and corpus, where it is well adapted to survive in acidic conditions and establish persistent infection 5. Once infection is established, several gastro-duodenal complications such as gastritis, gastric ulcer, duodenal ulcer, dyspeptic symptoms, gastric cancer, and gastric mucosa-associated lymphoid tissue (MALT) B-cell lymphoma may develop 6. Gastric cancer persists as a major public health issue and ranks as the third most common cause of cancer-related mortality; in 2012, it led to the deaths of about 723,100 individuals 7, 8. In addition to its association with gastro-duodenal complications, H. pylori in recent years has been reported to cause several extra-gastric complications.
Epidemiological studies have suggested an association between H. pylori infection and certain other extra-gastric complications such as ischemic heart disease, neurodegenerative diseases, and hematological disorders (iron deficiency anemia, immune-thrombocytopenic purpura, and vitamin B 12 deficiency) 6, 9, 10. Bellos et al. recently found that H. pylori infection in pregnant women increases the risk of developing preeclampsia, which is a potent contributor to maternal and fetal morbidity and mortality 11. Another complication, hyperemesis gravidarum, can be found in up to 2.0% of women with early pregnancy and its onset has been associated with H. pylori infection 12. Cen et al., in a meta-analysis comprising 18 studies involving 1,544 participants, found an overall threefold increased risk for gall bladder disease, such as cholecystitis and cholelithiasis, in association with H. pylori infection. In Asian populations, the risk is higher than in non-Asian populations 13. Serological evidence for H. pylori infection was found to be associated with the development of hepatic diseases such as non-alcoholic fatty liver disease 14. With regard to the conclusive evidence linking H. pylori infection with hematological disorders (iron deficiency anemia, immune-thrombocytopenic purpura, and vitamin B 12 deficiency), the Maastricht V/Florence consensus recommended H. pylori eradication therapy for these complications in addition to the gastric complications 15.
Eradication therapy significantly decreases the risk of developing gastric cancer if given before the onset of pre-cancerous lesions (atrophy, intestinal metaplasia, and dysplasia) 16 and has proven to be the only effective strategy for reducing the development of gastric cancer. When a population-based “test and treat” strategy in a geographic region is being considered, which tests are preferred for the diagnosis of H. pylori infection, which subjects should be offered the diagnosis, and which treatment should be prescribed remain critical issues. The main aim of this review is to summarize the information regarding the strategic approaches and indications for the diagnosis of H. pylori as well as appropriate antibiotic therapy-based management.
Virulence factors implicated in gastro-duodenal diseases
Although a declining trend of H. pylori infection has been reported in many countries, the incidence of gastric cancer remains a major public health issue for cancer-related deaths worldwide 7. Despite the role of host factors and environmental conditions of the stomach, bacterial virulence factors play an important role in H. pylori-related pathogenicity. The virulence factors such as cytotoxin-associated gene A (CagA) and vacuolating cytotoxin A (VacA) are the most studied and closely associated with gastric epithelial cell apoptosis and the development of severe gastric complications 17, 18. CagA is an oncogenic protein that possesses an EPIYA (Glu–Pro–Ile–Tyr–Ala) motif; after CagA’s internalization in the host epithelium by the type 4 secretory system (T4SS), which forms a needle-like structure 19, the tyrosine of the EPIYA motif undergoes phosphorylation. CagA can possess four different types of EPIYA motifs—EPIYA-A, -B, -C, and -D—depending on the geographic region. H. pylori strains from Western countries usually possess CagA with EPIYA-A, -B, and -C (one to three EPIYA-C), whereas those from most of the East Asian countries possess EPIYA-A, -B, and -D. EPIYA-A and -B are carried by almost all CagA, and the third EPIYA motif (C or D) is a geographic, genotypic, and virulence characteristic 20. The presence and characteristics of the third EPIYA motif (EPIYA-C or -D) determine the virulent characteristics of CagA. In a recent meta-analysis, CagA with a single EPIYA-D motif was significantly associated with the development of gastric cancer while CagA with multiple EPIYA-C motifs was found to be a significant risk factor for peptic ulcer disease (PUD) in Asian countries; however, in the US and Europe, CagA with multiple EPIYA-C motifs was associated with the development of gastric cancer 21. The VacA is an exotoxin which affects multiple cellular pathways and induces host cell vacuolation and cell death (reviewed in 22).
Blood group antigen-binding adhesin (BabA) is a major outer membrane protein and another major virulence factor that is involved in the attachment of bacteria to the host epithelium, which leads to double-strand DNA breaks and translocation of CagA to the host cells 23, 24. The specific location of bab-paralogous genes in three loci ( babA/babB/-) was found to be associated with the development of pre-cancerous lesion (atrophy) and peptic ulcer 25. The role and characteristics of many other proteins have been implicated in the development of H. pylori-related pathogenicity. The outer inflammatory protein A (OipA), duodenal ulcer-promoting gene A (DupA), sialic acid-binding adhesin (SabA), and protein which is induced by contact with epithelium (IceA) are implicated in the triggering of gastric epithelial cell apoptosis and the development of severe gastric complications such as peptic ulcer and gastric cancer 26– 30.
In addition to its acid-neutralization function, urease, a potent virulence factor, was recently reported to induce angiogenesis, the formation of new blood vessels from pre-existing vasculature, which is important for tumor growth and metastatic dissemination and plays a key role in the progression of gastric cancer 31, 32. In a study using in vitro endothelial cell tube formation assay and in vivo chorioallantoic membrane (CAM), the addition of H. pylori urease was found to induce the formation of tube-like structures by human umbilical vascular endothelial cells and CAM, respectively 33. Another gene, hp0169, the only gene annotated as collagenase in H. pylori that encodes the protein HpPrtC, which belongs to the protease family, was found to affect pathogenicity through cell viability, proliferation, and apoptosis 34. The H. pylori strains harboring these virulence factors are considered more pathogenic than the strains lacking these factors. Therefore, evaluation of these virulence factors provides insight for risk stratification and clinical outcome.
Diagnostic approaches for H. pylori infections
Currently, the diagnosis of H. pylori infection is carried out by invasive (for example, endoscopy and endoscopic biopsy for histopathology, culture, and rapid urease test) and non-invasive (for example, urea breath tests, stool antigen test, and serological tests) methods 35. However, the diagnostic preferences are based on the prevalence of H. pylori infection and age-related gastric cancer incidence in each area. For example, the non-invasive methods are preferred mostly in areas where the gastric cancer incidence is low, whereas endoscopy is recommended in those patients who have a high likelihood of developing gastric cancer, such as those over 60 years of age (or even in younger patients in some European countries), and who have a family history of gastric cancer or are in geographic regions with a high incidence of gastric cancer. The guidelines of the Japanese Society for Helicobacter Research put forth its recommendations suggesting that the diagnosis of H. pylori infection is performed by using at least one of several invasive and non-invasive methods; however, increased accuracy is obtained by using multiple diagnostic tests 36. Despite their high accuracy, the endoscopy-based diagnostic methods are not recommended for screening purposes and this is because of their invasiveness, high cost, and unavailability 37. The Maastricht V/Florence consensus report recommended using non-invasive methods such as locally validated serological tests over endoscopic procedures for the diagnosis of H. pylori infection in patients with dyspeptic symptoms 15. Moreover, the American College of Gastroenterology (ACG) and Canadian Association of Gastroenterology, considering the adverse effects that may occur because of endoscopy, suggested the use of upper gastrointestinal endoscopy in patients who present with dyspeptic symptoms and are over 60 years of age or if the patient belongs to a high-risk family or a region with gastric cancer 38. However, in some European countries, endoscopy is recommended in patients over 45 years of age who have predisposing factors such as a high chance of developing gastric cancer 39. In this context, the non-invasive methods are considered the preferred and recommended methods for the mass screening of H. pylori infection despite the possible drawbacks they may have. For example, the urea breath test is currently recommended as the best approach for the screening of H. pylori infection because of its non-invasiveness and high sensitivity 15; on the other hand, it is relatively expensive and requires mass spectrometric analysis (which may not be available at resource-limited centers) 40, and false-positive and -negative results may occur (albeit rarely). For example, Neisseria flavescens and Pseudomonas fluorescens, the urease-producing bacteria that were found to colonize the stomach of patients with gastritis, are potential pathogens that can give a false-positive result using the urea breath test 41, 42. The stool antigen test is the preferred method for the detection of H. pylori infection in children 43; however, low sensitivity and specificity have been reported in patients with low bacterial density and in those with peptic ulcer bleeding 44. Therefore, the preference of appropriate diagnostic tests depends on many factors such as the patient’s choice and the test’s accuracy and availability as well as its cost-effectiveness.
Indications for “test and treat” strategy
Almost all H. pylori-infected individuals have chronic active gastritis on biopsy, and the clinical outcome of the infection is quite unpredictable, ranging from asymptomatic to a severe complication such as peptic ulcer and gastric cancer; however, these are mostly preventable by eradication therapy 45. Several studies have reported that eradication therapy for H. pylori in healthy and asymptomatic patients reduces the risk of developing gastric cancer; however, in patients with pre-neoplastic lesions, such as intestinal metaplasia and dysplasia, reversal of this pathological progression was hardly achieved by eradication therapy 16, 46, 47. However, reports have found significant improvement in prognosis and reversal of atrophy and even intestinal metaplasia after successful therapy, though to a lesser degree in the case of intestinal metaplasia 48– 50. Moreover, a recent clinical trial conducted in South Korea reported that eradication therapy is able to significantly prevent the development of gastric cancer after endoscopic removal of early gastric cancer lesions 51. Treatment also reduces the risk of infection transmission from individual to individual, and therefore the financial burden that is associated with H. pylori infections may be avoided. The Kyoto global consensus report involving members of the Japanese Society of Gastroenterology, the European Helicobacter Study Group, the Asian Pacific Association of Gastroenterology, the Healthy Stomach Initiative, and the working group members of gastroenterology for International Classification of Diseases-11th revision (ICD-11) recommended screening for H. pylori gastritis after the age of 12 years and proposed that all positive cases be treated with eradication therapy even if they have no related symptoms or conditions 52. With regard to the Kyoto global consensus report, the Maastricht V/Florence consensus recommended the “test and treat” strategy for patients with dyspeptic symptoms. This report also made an important recommendation that patients with hematological disorders (iron deficiency anemia, immune-thrombocytopenic purpura, and vitamin B 12 deficiency) be administered eradication therapy because there is considerable evidence linking these complications with H. pylori infection 15. However, because of the low incidence of H. pylori-associated gastric cancer in the US, the ACG recommended testing for H. pylori infection in patients with predisposing factors such as PUD, a history of PUD, low-grade gastric MALT lymphoma, or a history of endoscopic resection of early gastric cancer 53, whereas the Bangkok consensus report for the Association of Southeast Asian Nations (ASEAN) countries (Indonesia, Thailand, the Philippines, Malaysia, Singapore, Vietnam, Myanmar, Cambodia, Laos, and Brunei) emphasized that H. pylori infection is more common in dyspeptic patients than in asymptomatic ones and recommended testing for H. pylori infection in patients with chronic dyspeptic symptoms 54. Thus, the diagnosis of H. pylori infection in a particular geographic region should take into account the prevalence of infection, the incidence of severe complications such as gastric cancer in that geographic region, predisposing factors, and the age of the patient (for example, screening using non-invasive tests in younger patients and endoscopy-based methods in patients in the upper extremity of life, usually over 60 years, or over 45 years in some European countries). Irrespective of the diagnostic methods used, all patients with diagnosed H. pylori infection should be offered eradication therapy, which is based on the antibiotic resistance rate of that geographic region.
Current first-line therapeutic strategies
The therapeutic strategy that is offered as the initial course (first-line) to patients with diagnosed H. pylori infection provides the greatest chance for eradication overall. Therefore, the first-line eradication therapy plays a key role in the cure of H. pylori infections. Additionally, careful selection of the pertinent first-line therapy is mandatory and this should be based on the local resistance rates of the antibiotic constituents. Clarithromycin (a macrolide) has been an important constituent of H. pylori eradication therapy, but proton pump inhibitor (PPI)-clarithromycin-based triple therapy with PPI, clarithromycin, and amoxicillin (or metronidazole where its resistance rate is low) is now recommended as the first-line eradication therapy only when clarithromycin resistance is below 15%. However, if clarithromycin resistance exceeds 15%, bismuth quadruple therapy (bismuth, PPI, tetracycline, and metronidazole) or non-bismuth quadruple therapy (PPI, amoxicillin, clarithromycin, and metronidazole; also known as concomitant therapy) may be offered for 10–14 days as an alternative to first-line triple therapy 15, 53. In most of the ASEAN countries, metronidazole resistance is high, and an increasing rate of clarithromycin resistance in recent years confers difficulty in achieving the goal of clarithromycin- and metronidazole-based therapy. A meta-analysis on primary antibiotic resistance conducted in the Asia-Pacific region in 2017 reported an increasing pattern of clarithromycin resistance rate in recent years, whereas metronidazole resistance rates were as high as 75% in Vietnam, 84% in Bangladesh, and 88% in Nepal 55. However, in most areas, amoxicillin resistance is rare (below 5%), and in some parts clarithromycin resistance is also lower than 15% 55; therefore, PPI-clarithromycin-based triple therapy for 14 days is effective 54. Another recent meta-analysis based on randomized controlled trials regarding eradication efficacy found an 84.3% cure rate by sequential therapy with PPI, amoxicillin, clarithromycin, and metronidazole in 2013 and this was superior to 7- or 10-day triple therapy but not to 14-day triple therapy and bismuth- or non-bismuth-based therapy 56. The ACG also included sequential therapy—consisting of PPI and amoxicillin for 5–7 days followed by PPI, clarithromycin, and metronidazole for a further 5–7 days—as an option for first-line triple therapy 53. The clarithromycin and metronidazole resistance rate in a particular geographic region determines the preferred constituents of eradication therapy. For example, in a geographic region where clarithromycin resistance exceeds 15%, it may be replaced with levofloxacin (a fluoroquinolone), and a levofloxacin-based triple therapy consisting of PPI, levofloxacin, and amoxicillin for 10–14 days or sequential therapy consisting of PPI and amoxicillin for 5–7 days followed by PPI, levofloxacin, and metronidazole for a further 5–7 days may be prescribed as an option for first-line therapy 53. However, the efficacy of sequential therapy may vary depending on geographic region and antibiotic resistance rate. In a meta-analysis conducted in China, the authors found that 10-day concomitant therapy was more efficacious than 10-day sequential therapy for infection with metronidazole-resistant strains or together with clarithromycin-resistant strains 57. The meta-analysis conducted in the Asia-Pacific region in 2017 also reported that in these countries with clarithromycin resistance higher than 15–20%, clarithromycin-based triple therapy as well as sequential and concomitant therapy showed less than 80% eradication efficacies 55. In countries with a high incidence of H. pylori-associated gastric cancer and clarithromycin resistance exceeding 15–20%, it is better to use alternative approaches to clarithromycin-based eradication therapy. Finally, after the completion of first-line antibiotic treatment, the eradication therapy’s efficacy should be assessed using the urea breath test 15. In agreement with the development of multi-drug resistance in other bacterial species, antibiotic resistance in H. pylori is an increasing trend because of the overuse and misuse of antibiotics for the treatment of other infections, especially in developing countries 58. Currently, the novel polymerase chain reaction-based approach is sensitive for the detection of H. pylori DNA in stool samples together with detecting mutations causing clarithromycin resistance 59. This non-invasive method could be able to significantly decrease endoscopy-based biopsy sampling for antibiotic resistance determination.
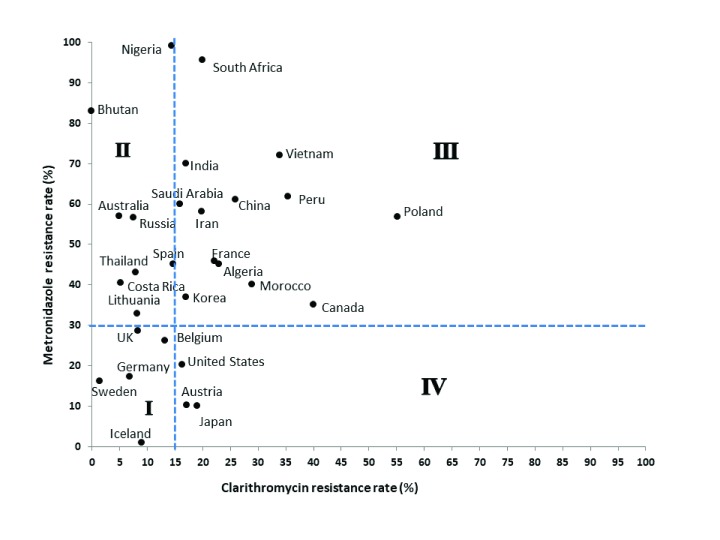
Geographic distribution of clarithromycin and metronidazole resistance
Although the antibiotic resistance rate differs from country to country and even a regional variation may be found within a country, an overall increasing pattern of resistance with time is an emerging problem in many countries 60. In 2017, based on the threat that may be imposed, H. pylori was listed in the World Health Organization’s “priority list of antibiotic resistance bacteria” and was ranked as top of the most common causes of community-acquired infections if the strain is clarithromycin-resistant 61. In general, the clarithromycin and metronidazole resistance rates predict the success rate of standard therapy, as these antibiotics are primary constituents of standard therapy and also resistance to these two antibiotics is frequently seen; therefore, to prescribe the therapy, one must have sound knowledge of regional resistance rates to these antibiotics. In European regions such as Sweden 62, Belgium 63, Iceland 64, Germany 65, and the UK 66, generally lower resistance rates to both clarithromycin and metronidazole (lower than 15% and 30%, respectively) have been reported ( Figure 1, area I). In countries such as Costa Rica 67, Spain 68, Nigeria 69, and Lithuania 70 and in some Asia-Pacific regions such as Thailand, Bhutan, Russia, and Australia 55, clarithromycin resistance is lower than 15%; however, metronidazole resistance rates of higher than 30% have been reported ( Figure 1, area II). According to a meta-analysis conducted in Asia-Pacific regions, no clarithromycin resistance was found in Bhutan, although more than 80% of the H. pylori strains were metronidazole-resistant 55. In Nigeria, metronidazole resistance was reported to be up to 99% 69. On the other hand, in South Africa 71, Peru 72, Algeria 73, Canada 74, and Morocco 75 and in other European countries such as Poland 76 and France 77, together with other Asia-Pacific regions (for example, India, Iran, Saudi Arabia, South Korea, China, and Vietnam) 55, higher resistance rates than the threshold levels for both clarithromycin and metronidazole have been reported ( Figure 1, area III). In most regions, the frequent use of antibiotics is the main contributor to drug resistance and the declining efficacy of eradication therapies. However, hetero-resistance (both resistant and susceptible strains together in one patient’s stomach) has also been reported to contribute to the reduced efficacy of eradication therapy 65. The resistance rate of metronidazole usually remains high in developing countries because it is most widely used for the treatment of parasitic infestations, whereas in the developed world its resistance tends to be low. In the US 78, Austria 79, and Japan 55, overall clarithromycin resistance was more than 15%; however, metronidazole resistance was lower than 30% ( Figure 1, area IV).
Figure 1. Geographic distribution of clarithromycin and metronidazole resistance.
The dotted lines show the threshold levels for clarithromycin and metronidazole resistance rates (15% and 30%, respectively). Both clarithromycin and metronidazole resistance rates are low in countries belonging to area I. Clarithromycin resistance is low but metronidazole resistance is high in countries of area II, whereas in the countries belonging to area III both clarithromycin and metronidazole resistance rates are high. In countries of area IV, the clarithromycin resistance is high but metronidazole resistance is low.
Last but not least
Regarding the current therapeutic management of H. pylori infections, we, the authors, are deeply concerned with two main points. First, we are well aware that the misuse and overuse of antibiotics pose a great threat to reaching the goal of eradication therapy efficacy and also can create a problem for the future by increasing the rate of antibiotic resistance, as “what does not kill you makes you stronger” and similarly “weaker antibiotics make stronger bacteria”. Thus, the selection of the most appropriate therapeutic strategy based on regional resistance rate is of the utmost importance. Second, H. pylori is transmitted from person to person and usually between family members, so there is the possibility of re-infection in cured patients living with other asymptomatic family members (carriers). Therefore, in the authors’ opinion, the “mass eradication” strategy may offer better efficacy of eradication therapy in regions with a high incidence of H. pylori-related gastric cancer. In the case of one member being offered eradication therapy owing to some clinical symptoms, the other members (>12 years) of the family should be screened as well and eradication therapy should be offered together to all who are positive for H. pylori infection. In this way, the possibility of re-infection from asymptomatic family members is avoided.
Key points and conclusions
As H. pylori-associated gastric complications are a challenging threat to public health, their effective management is of the utmost importance. Diagnosis and therapy are the major arms of management. Non-invasive methods should be the preferred option for diagnosis unless the patient has some predisposing factors necessitating endoscopy. A population-based approach to H. pylori eradication should be based on the prevalence of H. pylori infection and incidence of gastric cancer in that geographic locality. Moreover, first-line eradication therapy is the most efficacious; therefore, the choice of therapy should be based on the local resistance rate to clarithromycin and metronidazole primarily. Finally, after the completion of therapy, the eradication of H. pylori should be assessed.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Peter Malfertheiner, Department of Gastroenterology, Hepatology and Infectious Diseases, Otto-von-Guericke University Magdeburg, Magdeburg, Germany
Francis Mégraud, Department of Bacteriology, INSERM U1053, Université de Bordeaux, Bordeaux, France
Steven Moss, Department of Medicine, Warrren Alpert Medical School of Brown University, Providence, RI, USA
Funding Statement
This work was supported by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan (16H05191) (YY) and by the Japan Society for the Promotion of Science (Core-to-Core Program) (YY) and by National Institutes of Health grant DK62813 (YY). SA is a PhD student supported by the Japanese Government (MEXT) Scholarship Program for 2015.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 3 approved]
References
- 1. Zamani M, Ebrahimtabar F, Zamani V, et al. : Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47(7):868–76. 10.1111/apt.14561 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Hooi JKY, Lai WY, Ng WK, et al. : Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153(2):420–9. 10.1053/j.gastro.2017.04.022 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Mamishi S, Eshaghi H, Mahmoudi S, et al. : Intrafamilial transmission of Helicobacter pylori: genotyping of faecal samples. Br J Biomed Sci. 2016;73(1):38–43. 10.1080/09674845.2016.1150666 [DOI] [PubMed] [Google Scholar]
- 4. Bui D, Brown HE, Harris RB, et al. : Serologic Evidence for Fecal-Oral Transmission of Helicobacter pylori. Am J Trop Med Hyg. 2016;94(1):82–8. 10.4269/ajtmh.15-0297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ansari S, Yamaoka Y: Survival of Helicobacter pylori in gastric acidic territory. Helicobacter. 2017;22(4):e12386. 10.1111/hel.12386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang J, Chen Y, Shi J, et al. : Population attributable burden of Helicobacter pylori-related gastric cancer, coronary heart disease, and ischemic stroke in China. Eur J Clin Microbiol Infect Dis. 2017;36(2):199–212. 10.1007/s10096-016-2810-x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Ferlay J, Soerjomataram I, Dikshit R, et al. : Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 8. Torre LA, Bray F, Siegel RL, et al. : Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 9. Franceschi F, Gasbarrini A, Polyzos SA, et al. : Extragastric Diseases and Helicobacter pylori. Helicobacter. 2015;20 Suppl 1:40–6. 10.1111/hel.12256 [DOI] [PubMed] [Google Scholar]
- 10. Goni E, Franceschi F: Helicobacter pylori and extragastric diseases. Helicobacter. 2016;21 Suppl 1:45–8. 10.1111/hel.12340 [DOI] [PubMed] [Google Scholar]
- 11. Bellos I, Daskalakis G, Pergialiotis V: Helicobacter pylori infection increases the risk of developing preeclampsia: A meta-analysis of observational studies. Int J Clin Pract. 2018;72(2):e13064. 10.1111/ijcp.13064 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Ng QX, Venkatanarayanan N, De Deyn MLZQ, et al. : A meta-analysis of the association between Helicobacter pylori (H. pylori) infection and hyperemesis gravidarum. Helicobacter. 2018;23(1):e12455. 10.1111/hel.12455 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Cen L, Pan J, Zhou B, et al. : Helicobacter Pylori infection of the gallbladder and the risk of chronic cholecystitis and cholelithiasis: A systematic review and meta-analysis. Helicobacter. 2018;23(1):e12457. 10.1111/hel.12457 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Polyzos SA, Kountouras J, Papatheodorou A, et al. : Helicobacter pylori infection in patients with nonalcoholic fatty liver disease. Metabolism. 2013;62(1):121–6. 10.1016/j.metabol.2012.06.007 [DOI] [PubMed] [Google Scholar]
- 15. Malfertheiner P, Megraud F, O'Morain CA, et al. : Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66(1):6–30. 10.1136/gutjnl-2016-312288 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Rokkas T, Rokka A, Portincasa P: A systematic review and meta-analysis of the role of Helicobacter pylori eradication in preventing gastric cancer. Ann Gastroenterol. 2017;30(4):414–23. 10.20524/aog.2017.0144 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Wandler AM, Guillemin K: Transgenic expression of the Helicobacter pylori virulence factor CagA promotes apoptosis or tumorigenesis through JNK activation in Drosophila. PLoS Pathog. 2012;8(10):e1002939. 10.1371/journal.ppat.1002939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Akazawa Y, Isomoto H, Matsushima K, et al. : Endoplasmic reticulum stress contributes to Helicobacter pylori VacA-induced apoptosis. PLoS One. 2013;8(12):e82322. 10.1371/journal.pone.0082322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zanotti G, Cendron L: Structural and functional aspects of the Helicobacter pylori secretome. World J Gastroenterol. 2014;20(6):1402–23. 10.3748/wjg.v20.i6.1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tohidpour A: CagA-mediated pathogenesis of Helicobacter pylori. Microb Pathog. 2016;93:44–55. 10.1016/j.micpath.2016.01.005 [DOI] [PubMed] [Google Scholar]
- 21. Li Q, Liu J, Gong Y, et al. : Association of CagA EPIYA-D or EPIYA-C phosphorylation sites with peptic ulcer and gastric cancer risks: A meta-analysis. Medicine (Baltimore). 2017;96(17):e6620. 10.1097/MD.0000000000006620 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Thi Huyen Trang T, Thanh Binh T, Yamaoka Y: Relationship between vacA Types and Development of Gastroduodenal Diseases. Toxins (Basel). 2016;8(6): pii: E182. 10.3390/toxins8060182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Toller IM, Neelsen KJ, Steger M, et al. : Carcinogenic bacterial pathogen Helicobacter pylori triggers DNA double-strand breaks and a DNA damage response in its host cells. Proc Natl Acad Sci U S A. 2011;108(36):14944–9. 10.1073/pnas.1100959108 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Ishijima N, Suzuki M, Ashida H, et al. : BabA-mediated adherence is a potentiator of the Helicobacter pylori type IV secretion system activity. J Biol Chem. 2011;286(28):25256–64. 10.1074/jbc.M111.233601 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Ansari S, Kabamba ET, Shrestha PK, et al. : Helicobacter pylori bab characterization in clinical isolates from Bhutan, Myanmar, Nepal and Bangladesh. PLoS One. 2017;12(11):e0187225. 10.1371/journal.pone.0187225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Teymournejad O, Mobarez AM, Hassan ZM, et al. : Binding of the Helicobacter pylori OipA causes apoptosis of host cells via modulation of Bax/Bcl-2 levels. Sci Rep. 2017;7(1): 8036. 10.1038/s41598-017-08176-7 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Takahashi A, Shiota S, Matsunari O, et al. : Intact long-type dupA as a marker for gastroduodenal diseases in Okinawan subpopulation, Japan. Helicobacter. 2013;18(1):66–72. 10.1111/j.1523-5378.2012.00994.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu J, Leung WK, Go MY, et al. : Relationship between Helicobacter pylori babA2 status with gastric epithelial cell turnover and premalignant gastric lesions. Gut. 2002;51(4):480–4. 10.1136/gut.51.4.480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamaoka Y: Increasing evidence of the role of Helicobacter pylori SabA in the pathogenesis of gastroduodenal disease. J Infect Dev Ctries. 2008;2(3):174–81. 10.3855/jidc.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma YJ, Duan GC, Zhang RG, et al. : Mutation of iceA in Helicobacter pylori compromised IL-8 induction from human gastric epithelial cells. J Basic Microbiol. 2010;50 Suppl 1:S83–8. 10.1002/jobm.200900410 [DOI] [PubMed] [Google Scholar]
- 31. De Palma M, Biziato D, Petrova TV: Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17(8):457–74. 10.1038/nrc.2017.51 [DOI] [PubMed] [Google Scholar]
- 32. Macedo F, Ladeira K, Longatto-Filho A, et al. : Gastric Cancer and Angiogenesis: Is VEGF a Useful Biomarker to Assess Progression and Remission? J Gastric Cancer. 2017;17(1):1–10. 10.5230/jgc.2017.17.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Olivera-Severo D, Uberti AF, Marques MS, et al. : A New Role for Helicobacter pylori Urease: Contributions to Angiogenesis. Front Microbiol. 2017;8:1883. 10.3389/fmicb.2017.01883 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Zhao H, Ji X, Chen X, et al. : Functional study of gene hp0169 in Helicobacter pylori pathogenesis. Microb Pathog. 2017;104:225–31. 10.1016/j.micpath.2017.01.039 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Filomena A, Guenther A, Planatscher H, et al. : Performance of a Multiplex Serological Helicobacter pylori Assay on a Novel Microfluidic Assay Platform. Proteomes. 2017;5(4): pii: E24. 10.3390/proteomes5040024 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Asaka M, Kato M, Takahashi S, et al. : Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter. 2010;15(1):1–20. 10.1111/j.1523-5378.2009.00738.x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Siddique I, Al-Mekhaizeem K, Alateeqi N, et al. : Diagnosis of Helicobacter pylori: improving the sensitivity of CLOtest by increasing the number of gastric antral biopsies. J Clin Gastroenterol. 2008;42(4):356–60. 10.1097/MCG.0b013e31802b650d [DOI] [PubMed] [Google Scholar]
- 38. Moayyedi PM, Lacy BE, Andrews CN, et al. : ACG and CAG Clinical Guideline: Management of Dyspepsia. Am J Gastroenterol. 2017;112(7):988–1013. 10.1038/ajg.2017.154 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Smith S, Boyle B, Brennan D, et al. : The Irish Helicobacter pylori Working Group consensus for the diagnosis and treatment of H. pylori infection in adult patients in Ireland. Eur J Gastroenterol Hepatol. 2017;29(5):552–9. 10.1097/MEG.0000000000000822 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Kato M, Saito M, Fukuda S, et al. : 13C-Urea breath test, using a new compact nondispersive isotope-selective infrared spectrophotometer: comparison with mass spectrometry. J Gastroenterol. 2004;39(7):629–34. 10.1007/s00535-003-1357-7 [DOI] [PubMed] [Google Scholar]
- 41. Zeng B, Sun L, Chen Y, et al. : Neisseria flavescens: A Urease-Expressing Potential Pathogen Isolated from Gastritis Patients. Curr Microbiol. 2018;75(2):186–93. 10.1007/s00284-017-1364-1 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Patel SK, Pratap CB, Verma AK, et al. : Pseudomonas fluorescens-like bacteria from the stomach: a microbiological and molecular study. World J Gastroenterol. 2013;19(7):1056–67. 10.3748/wjg.v19.i7.1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Prell C, Osterrieder S, Lottspeich C, et al. : Improved performance of a rapid office-based stool test for detection of Helicobacter pylori in children before and after therapy. J Clin Microbiol. 2009;47(12):3980–4. 10.1128/JCM.01204-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lin HJ, Lo WC, Perng CL, et al. : Helicobacter pylori stool antigen test in patients with bleeding peptic ulcers. Helicobacter. 2004;9(6):663–8. 10.1111/j.1083-4389.2004.00276.x [DOI] [PubMed] [Google Scholar]
- 45. Rugge M, Genta RM, Di Mario F, et al. : Gastric Cancer as Preventable Disease. Clin Gastroenterol Hepatol. 2017;15(12):1833–43. 10.1016/j.cgh.2017.05.023 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Ford AC, Forman D, Hunt RH, et al. : Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174. 10.1136/bmj.g3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee YC, Chiang TH, Chou CK, et al. : Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology. 2016;150(5):1113–1124.e5. 10.1053/j.gastro.2016.01.028 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Kodama M, Murakami K, Okimoto T, et al. : Ten-year prospective follow-up of histological changes at five points on the gastric mucosa as recommended by the updated Sydney system after Helicobacter pylori eradication. J Gastroenterol. 2012;47(4):394–403. 10.1007/s00535-011-0504-9 [DOI] [PubMed] [Google Scholar]
- 49. Mera RM, Bravo LE, Camargo MC, et al. : Dynamics of Helicobacter pylori infection as a determinant of progression of gastric precancerous lesions: 16-year follow-up of an eradication trial. Gut. 2017;67(7): pii: gutjnl-2016-311685. 10.1136/gutjnl-2016-311685 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Hwang YJ, Kim N, Lee HS, et al. : Reversibility of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication - a prospective study for up to 10 years. Aliment Pharmacol Ther. 2018;47(3):380–90. 10.1111/apt.14424 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Choi IJ, Kook MC, Kim YI, et al. : Helicobacter pylori Therapy for the Prevention of Metachronous Gastric Cancer. N Engl J Med. 2018;378(12):1085–95. 10.1056/NEJMoa1708423 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Sugano K, Tack J, Kuipers EJ, et al. : Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353–67. 10.1136/gutjnl-2015-309252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chey WD, Leontiadis GI, Howden CW, et al. : ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112(2):212–39. 10.1038/ajg.2016.563 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Mahachai V, Vilaichone RK, Pittayanon R, et al. : Helicobacter pylori management in ASEAN: The Bangkok consensus report. J Gastroenterol Hepatol. 2018;33(1):37–56. 10.1111/jgh.13911 [DOI] [PubMed] [Google Scholar]
- 55. Kuo YT, Liou JM, El-Omar EM, et al. : Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2(10):707–15. 10.1016/S2468-1253(17)30219-4 [DOI] [PubMed] [Google Scholar]
- 56. Gatta L, Vakil N, Vaira D, et al. : Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ. 2013;347:f4587. 10.1136/bmj.f4587 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Wang Y, Zhao R, Wang B, et al. : Sequential versus concomitant therapy for treatment of Helicobacter pylori infection: an updated systematic review and meta-analysis. Eur J Clin Pharmacol. 2018;74(1):1–13. 10.1007/s00228-017-2347-7 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Mégraud F: Current recommendations for Helicobacter pylori therapies in a world of evolving resistance. Gut Microbes. 2013;4(6):541–8. 10.4161/gmic.25930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Beckman E, Saracino I, Fiorini G, et al. : A Novel Stool PCR Test for Helicobacter pylori May Predict Clarithromycin Resistance and Eradication of Infection at a High Rate. J Clin Microbiol. 2017;55(8):2400–5. 10.1128/JCM.00506-17 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Thung I, Aramin H, Vavinskaya V, et al. : Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43(4):514–33. 10.1111/apt.13497 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Tacconelli E, Carrara E, Savoldi A, et al. : Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–27. 10.1016/S1473-3099(17)30753-3 [DOI] [PubMed] [Google Scholar]
- 62. Storskrubb T, Aro P, Ronkainen J, et al. : Antimicrobial susceptibility of Helicobacter pylori strains in a random adult Swedish population. Helicobacter. 2006;11(4):224–30. 10.1111/j.1523-5378.2006.00414.x [DOI] [PubMed] [Google Scholar]
- 63. Vekens K, Vandebosch S, De Bel A, et al. : Primary antimicrobial resistance of Helicobacter pylori in Belgium. Acta Clin Belg. 2013;68(3):183–7. 10.2143/ACB.3233 [DOI] [PubMed] [Google Scholar]
- 64. Gunnarsdottir AI, Gudjonsson H, Hardardottir H, et al. : Antibiotic susceptibility of Helicobacter pylori in Iceland. Infect Dis (Lond). 2017;49(9):647–54. 10.1080/23744235.2017.1317359 [DOI] [PubMed] [Google Scholar]
- 65. Selgrad M, Tammer I, Langner C, et al. : Different antibiotic susceptibility between antrum and corpus of the stomach, a possible reason for treatment failure of Helicobacter pylori infection. World J Gastroenterol. 2014;20(43):16245–51. 10.3748/wjg.v20.i43.16245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chisholm SA, Teare EL, Davies K, et al. : Surveillance of primary antibiotic resistance of Helicobacter pylori at centres in England and Wales over a six-year period (2000-2005). Euro Surveill. 2007;12(7):E3–4. 10.2807/esm.12.07.00721-en [DOI] [PubMed] [Google Scholar]
- 67. Lang L, García F: Comparison of E-test and disk diffusion assay to evaluate resistance of Helicobacter pylori isolates to amoxicillin, clarithromycin, metronidazole and tetracycline in Costa Rica. Int J Antimicrob Agents. 2004;24(6):572–7. 10.1016/j.ijantimicag.2004.07.009 [DOI] [PubMed] [Google Scholar]
- 68. Cuadrado-Lavín A, Salcines-Caviedes JR, Carrascosa MF, et al. : Antimicrobial susceptibility of Helicobacter pylori to six antibiotics currently used in Spain. J Antimicrob Chemother. 2012;67(1):170–3. 10.1093/jac/dkr410 [DOI] [PubMed] [Google Scholar]
- 69. Harrison U, Fowora MA, Seriki AT, et al. : Helicobacter pylori strains from a Nigerian cohort show divergent antibiotic resistance rates and a uniform pathogenicity profile. PLoS One. 2017;12(5):e0176454. 10.1371/journal.pone.0176454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dargiene G, Kupcinskas J, Jonaitis L, et al. : Primary antibiotic resistance of Helicobacter pylori strains among adults and children in a tertiary referral centre in Lithuania. APMIS. 2018;126(1):21–8. 10.1111/apm.12752 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Tanih NF, Ndip LM, Ndip RN: Characterisation of the genes encoding resistance to metronidazole ( rdxA and frxA) and clarithromycin (the 23S-rRNA genes) in South African isolates of Helicobacter pylori. Ann Trop Med Parasitol. 2011;105(3):251–9. 10.1179/136485911X12899838683485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Boehnke KF, Valdivieso M, Bussalleu A, et al. : Antibiotic resistance among Helicobacter pylori clinical isolates in Lima, Peru. Infect Drug Resist. 2017;10:85–90. 10.2147/IDR.S123798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Raaf N, Amhis W, Saoula H, et al. : Prevalence, antibiotic resistance, and MLST typing of Helicobacter pylori in Algiers, Algeria. Helicobacter. 2017;22(6):e12446. 10.1111/hel.12446 [DOI] [PubMed] [Google Scholar]
- 74. Eng NF, Ybazeta G, Chapman K, et al. : Antimicrobial susceptibility of Canadian isolates of Helicobacter pylori in Northeastern Ontario. Can J Infect Dis Med Microbiol. 2015;26(3):137–44. 10.1155/2015/853287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bouihat N, Burucoa C, Benkirane A, et al. : Helicobacter pylori Primary Antibiotic Resistance in 2015 in Morocco: A Phenotypic and Genotypic Prospective and Multicenter Study. Microb Drug Resist. 2017;23(6):727–32. 10.1089/mdr.2016.0264 [DOI] [PubMed] [Google Scholar]
- 76. Ferenc S, Gnus J, Kościelna M, et al. : High antibiotic resistance of Helicobacter pylori and its effect on tailored and empiric eradication of the organism in Lower Silesia, Poland. Helicobacter. 2017;22(2):e12365. 10.1111/hel.12365 [DOI] [PubMed] [Google Scholar]
- 77. Ducournau A, Bénéjat L, Sifré E, et al. : Helicobacter pylori resistance to antibiotics in 2014 in France detected by phenotypic and genotypic methods. Clin Microbiol Infect. 2016;22(8):715–8. 10.1016/j.cmi.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 78. Shiota S, Reddy R, Alsarraj A, et al. : Antibiotic Resistance of Helicobacter pylori Among Male United States Veterans. Clin Gastroenterol Hepatol. 2015;13(9):1616–24. 10.1016/j.cgh.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zollner-Schwetz I, Leitner E, Plieschnegger W, et al. : Primary resistance of Helicobacter pylori is still low in Southern Austria. Int J Med Microbiol. 2016;306(4):206–11. 10.1016/j.ijmm.2016.04.003 [DOI] [PubMed] [Google Scholar]