Abstract
Gadoxetic acid (Gd-EOB-DTPA) is a paramagnetic MRI contrast agent with raising popularity and has been used for evaluation of imaging-based liver function in recent years. In order to verify whether liver function as determined by real-time breath analysis using the intravenous administration of 13C-methacetin can be estimated quantitatively from Gd-EOB-DTPA-enhanced MRI using signal intensity (SI) values. 110 patients underwent Gd-EOB-DTPA-enhanced 3-T MRI and, for the evaluation of liver function, a 13C-methacetin breath test (13C-MBT). SI values from before (SIpre) and 20 min after (SIpost) contrast media injection were acquired by T1-weighted volume-interpolated breath-hold examination (VIBE) sequences with fat suppression. The relative enhancement (RE) between the plain and contrast-enhanced SI values was calculated and evaluated in a correlation analysis of 13C-MBT values to SIpost and RE to obtain a SI-based estimation of 13C-MBT values. The simple regression model showed a log-linear correlation of 13C-MBT values with SIpost and RE (p < 0.001). Stratified by 3 different categories of 13C-MBT readouts, there was a constant significant decrease in both SIpost (p ≤ 0.002) and RE (p ≤ 0.033) with increasing liver disease progression as assessed by the 13C-MBT. Liver function as determined using real-time 13C-methacetin breath analysis can be estimated quantitatively from Gd-EOB-DTPA-enhanced MRI using SI-based indices.
Introduction
To monitor patients with hepatic dysfunction, various liver function tests are used routinely in clinical practice. These tests may evaluate the increasing severity of illness, differ between stages of disease and offer a prediction of therapy outcome1–3. The routine part of clinical investigations is based on analysis, where the levels of non-volatile compounds, such as proteins and ions, are measured and checked for abnormalities. As a complement to blood parameters, volatile compounds carry information concerning the biochemical status of the individual, which might be examined via breath tests4–10.
Different orally or intravenously administered 13C-labeled substrates can reflect the function of specific hepatocyte compartments in real time as they are processed by liver function-dependent metabolic pathways. Therefore, cytosolic, mitochondrial and microsomal processes can be investigated non-invasively to obtain information about site-specific physiological and pathological metabolism5. A novel approach, which is already used in clinical routine, is the 13C-methacetin breath test (13C-MBT) established by Stockmann et al.11. The principle underlying this real-time test is the ability to metabolize 13C-labeled methacetin by the hepatocyte endoplasmic reticulum-located cytochrome P450 1A2 (CYP1A2) into paracetamol and 13C-labeled formaldehyde, which will be eliminated as 13CO25,11–13. After intravenous (i.v.) injection, 13CO2 will be exhaled, and a 13CO2:12CO2 ratio can be determined by a suitable device for breath analysis. Based on the values obtained by the 13C-MBT, real-time information of patients’ liver function can be directly obtained.
The hepatocyte-specific magnetic resonance imaging (MRI) contrast agent gadoxetic acid (Gd-EOB-DTPA) has been established and used for diagnostic purposes to detect and characterize focal liver lesions. Several studies have reported the use of Gd-EOB-DTPA-based MRI to evaluate liver function, usually expressed via the Child-Pugh score. However, no studies have yet compared the direct assessment of hepatic metabolic capacity and the indirect estimation of liver function based on MRI.
The purpose of this retrospective analysis was to evaluate the diagnostic performance of the Gd-EOB-DTPA-enhanced signal intensity (SI)-based quantification of liver function compared with an established liver function test, the 13C-MBT.
Results
The baseline characteristics of the 110 patients (83 men and 27 women; mean age, 60.84 ± 9.52 years; range, 39–82 years) who were included in this study and underwent a 13C-MBT and T1-weighted VIBE MRI are summarized in Table 1. Representative T1-weighted MRI scans and the corresponding SI-based indices, SIpre, SIpost and RE, are shown in Figs 1 and 2.
Table 1.
Characteristics for all patients and each 13C-MBT readout category.
| parameters | n = 110 | 13C-MBT categories | ||
|---|---|---|---|---|
| 1 >315.0 [µg/kg/h] n = 33 |
2 140.0–315.0 [µg/kg/h] n = 46 |
3 <140.0 [µg/kg/h] n = 31 |
||
| male | 83 (75.5%) | 14 (42.4%) | 43 (93.5%) | 26 (83.9%) |
| female | 27 (24.5%) | 19 (57.6%) | 3 (6.5%) | 5 (16.1%) |
| age [years] | 60.84 ± 9.52 | 59.88 ± 11.02 | 62.33 ± 9.40 | 59.65 ± 7.81 |
| height [cm] | 172.92 ± 7.62 | 168.97 ± 8.62 | 175.37 ± 7.27 | 173.48 ± 5.05 |
| weight [kg] | 85.84 ± 16.03 | 77.74 ± 19.80 | 89.96 ± 12.83 | 88.35 ± 12.80 |
| SIpre | 192.98 ± 38.40 | 204.80 ± 41.08 | 185.30 ± 35.71 | 191.80 ± 37.42 |
| SIpost | 347.36 ± 100.38 | 414.03 ± 87.85 | 345.59 ± 97.14 | 279.03 ± 67.09 |
| RE | 0.80 ± 0.37 | 1.04 ± 0.27 | 0.86 ± 0.35 | 0.46 ± 0.20 |
| 13C-MBT [µg/kg/h] | 234.66 ± 124.08 | 391.21 ± 53.28 | 217.78 ± 49.39 | 93.03 ± 33.59 |
Values indicate the mean ± standard deviation. RE: relative enhancement as a function of SI-based indices. SI: mean signal intensity of the liver. 13C-MBT: 13C-labeled methacetin metabolism liver function breath test.
Figure 1.
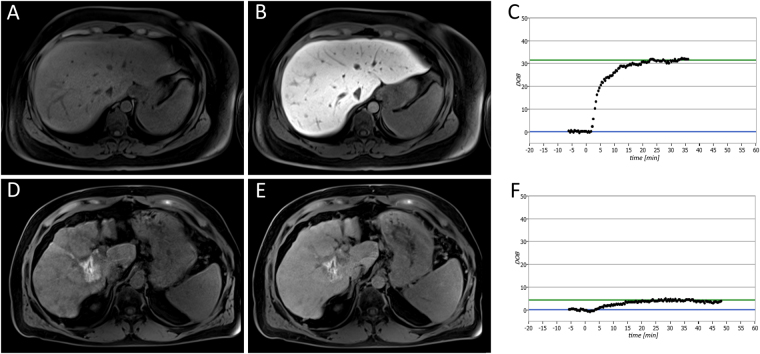
Liver function analysis in patients with normal liver function (A,B,C) and severely impaired liver function (D,E,F). The patients underwent plain (A,D) and Gd-EOB-DTPA-enhanced HBP (B,E) T1-weighted VIBE sequences with fat suppression, as well as a 13C-MBT, over a maximum time span of 60 min (C,F). In the case of the displayed patients, a 13C-MBT readout value of 409 µg/kg/h (C) was considered normal liver function (SIpre, 182.67; SIpost, 441.67; RE, 1.42), while a 13C-MBT readout value of 57 µg/kg/h (F) was considered impaired liver function (SIpre, 258.50; SIpost, 332.83; RE, 0.29). The lesion observed in the liver with impaired function (D,E) was caused by former radiofrequency ablations treatments. The delta-over-baseline (DOB) was assessed inline automatically and describes the increase in the RPDB-corrected 13CO2:12CO2 ratio to the basal value (blue line). The evaluated 13C-MBT value was calculated as the product of the DOBmax, RPDB, CO2 production and molar mass of 13C-methacetin per body weight13. The DOBmax (green line) was defined after an increase in DOB was no longer observable. At the time point 0, the 13C-methacetin was applied via bolus injection.
Figure 2.
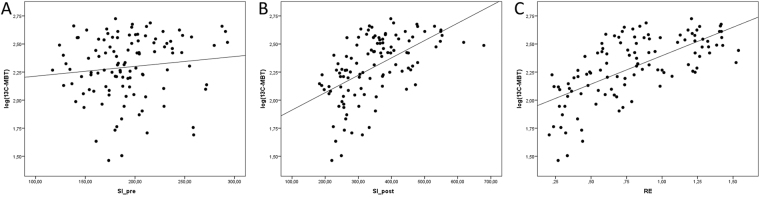
Correlation analysis of SI-based liver function indices to logarithmic values of 13C-MBT readout in scatterplots. The SI values obtained without contrast enhancement (SI_pre) show no predictive power for the logarithmic values of 13C-MBT (r = 0.213, p = 0.120; A), while the contrast-enhanced SI values (SI_post) show a significant correlation (r = 0.554, p < 0.001; B). A strong linear prediction of logarithmic 13C-MBT values can be observed for the relative enhancement (RE) values (r = 0.665, p < 0.001; C).
SI in T1-weighted MRI scans compared with 13C-MBT readout
In a simple linear regression model, RE values were strong linear predictors of the logarithmic values of uncategorized 13C-MBT readout values (r = 0.665, p < 0.001), while the SI values obtained from the HBP showed less predictive power (r = 0.554, p < 0.001). In contrast, SI values obtained without contrast enhancement showed no significant correlation (p = 0.120) (Table 2). Scatterplots of SIpre, SIpost and RE values plotted against the logarithmic values of 13C-MBT are shown in Fig. 2.
Table 2.
Simple linear regression models of SI-based indices with logarithmic values of 13C-MBT.
| B (95% CI) | p-value | r | ||
|---|---|---|---|---|
| Log(13C-MBT) | RE | 0.507 (0.398–0.615) | <0.001 | 0.665 |
| SIpre | 0.001 (−0.001–0.002) | 0.213 | 0.120 | |
| SIpost | 0.002 (0.001–0.002) | <0.001 | 0.554 |
RE: relative enhancement as a function of SI-based indices. SI: mean signal intensity of the liver. 13C-MBT: 13C-labeled methacetin metabolism liver function breath test. r: correlation coefficient. B: linear regression coefficient. CI: confidence interval.
SI-based results compared with 13C-MBT readout categories
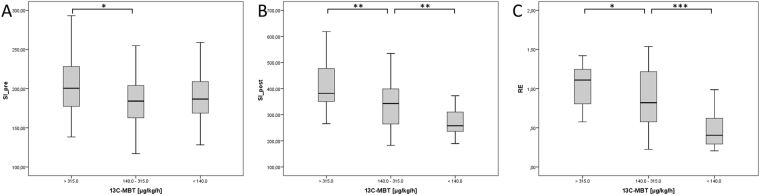
Patients with normal liver function (Category 1) had a mean 13C-MBT readout of 391.21 ± 53.28 µg/kg/h, whereas patients with intermediate liver function (category 2) had a mean 13C-MBT readout value of 217.78 ± 49.39 µg/kg/h, and patients with severely impaired liver function had a mean 13C-MBT readout value of 93.03 ± 33.59 µg/kg/h (Table 1). All pairwise comparisons between Categories 1, 2 and 3 showed significant differences (p ≤ 0.05) in the evaluated SI-based indices, except for SIpre values between Categories 1 and 3 and Categories 2 and 3 (Fig. 3A). The SI values obtained from the HBP (SIpost; p < 0.005; Fig. 3B) and the RE values (p < 0.05; Fig. 3C) differed significantly among the three 13C-MBT based categories.
Figure 3.
Boxplot analysis of SI-based indices separated by 13C-MBT readout categories. Native SI values (SI_pre; A) show no significant difference between the different 13C-MBT readout categories, except for Categories 1 and 2. SI values obtained after contrast enhancement (SI_post; B) and corrected by native SI values (RE; C) show significant differences among the 13C-MBT readout categories. *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
One of the most common types of liver cancer is HCC, with a global cancer mortality rate of 9.1%14. As HCC is mostly based on preexisting liver cirrhosis, the early detection and assessment of liver cirrhosis is of high clinical relevance. Patients benefit from rapid and accurate examinations of the liver condition. Thus, the 13C-MBT has become more common, as it can provide liver failure predictions, liver transplant control, and liver disease severity estimations13,15–17. The test is based on the analysis of volatile components exhaled during the 13C-methacetin metabolism by CYP1A2 in the endoplasmic reticulum12. Different studies have demonstrated the predictive power of enzymatic hepatocyte functionality for liver resection13,15,17–21. Even in cases of non-cirrhotic, early-stage and primary biliary cirrhosis, the 13C-MBT can reliably indicate decreased liver function10. Nevertheless, this tool has some diagnostic restrictions, as it is unable to determine and distinguish between areas of reduced hepatocyte function and healthy areas. Therefore, 13C-MBT values describe the function of the whole liver. However, diagnostic imaging techniques might provide these details, which are crucial for both liver resection and liver transplantation. To reduce the number of different examinations, researchers aim to establish liver tests capable of not only reliably reflecting liver function but also revealing hepatic lesions in a single examination.
To this end, Gd-EOB-DTPA-enhanced MRI has been established as a promising approach for assessing liver function in the past few years. Gd-EOB-DTPA consists of a gadolinium ion covalently bound to a lipophilic ethoxybenzyl group and is absorbed by intact hepatocytes via triggered ATP-dependent organic anion-transporting polypeptide (OATP1 B1/B3) channels. Subsequently, Gd-EOB-DTPA will be excreted by multidrug-resistance protein 2 (MRP2) into the biliary system22–24. The strength of Gd-EOB-DTPA is its hepatocyte-specific character, which improves the contrast-to-noise ratio, as it will not be taken up by cells other than hepatocytes (e.g., cells in metastatic lesions)25–27. At 20 min after contrast agent application, a significant increase in the SI of the liver parenchyma is recorded, and the extent of this phenomenon is dependent on the integrity of the liver parenchyma. The highest SI is exhibited by healthy liver tissue, while cirrhotic liver parenchyma shows only a slight increase in SI28,29. A loss of functioning hepatocytes, bridging of portal spaces or nodular regeneration of the liver parenchyma is associated with liver cirrhosis and hindered hepatocyte contrast agent uptake, causing decreased SI-based values30.
The estimation of SI-based indices provides insights into regional hepatocyte-specific function, efficiency, functionality and condition31. Nevertheless, the analysis is restricted by the relative character of the obtained SI values32. However, by the mathematical computation of SIpost with SIpre, a liver function index with increased reliability can be achieved. By calculating the RE, we correct the enhanced SI values to gain a SI ratio independent from artificial signal enhancement with increased explanatory power29,33–38.
To the best of our knowledge, this is the first study comparing SI-based MRI values reflecting hepatocyte OATP1 B1/B3 and MRP2 pathway activity with the 13C-MBT.
We could demonstrate that the SI-based MRI values assessed in the HBP reflect liver function in a suitable manner, as estimated by 13C-MBT readout values. Similar to a previous study39, we observed that patients with normal liver function expressed the highest SI-based values (Category 1: SIpre, 204.80 ± 41.08; SIpost, 414.03 ± 87.85; RE, 1.04 ± 0.27), while patients with decreased liver function showed decreased SI-based values (Category 2: SIpre, 185.30 ± 35.71; SIpost, 345.59 ± 97.14, RE, 0.86 ± 0.35; Category 3: SIpre, 191.80 ± 37.42; SIpost, 279.03 ± 67.09; RE, 0.46 ± 0.20). In a correlation analysis of SI-based indices to 13C-MBT values, we were able to show that SI-based RE values significantly support the 13C-MBT findings (RE, r = 0.665, p < 0.001) and therefore reflect liver functionality. Similar to Utsunomiya et al.31, in this study, we tested the correlation of the mean SI obtained after contrast agent application (SIpost) to the results of a liver function test. In their case, the tested SI values showed a higher prediction of indocyanine green dye (ICG) retention at 15 min (r = −0.67, p < 0.01) than we obtained when testing against 13C-MBT values (r = 0.554, p < 0.001). However, this difference seems plausible because the ICG clearance test is, similar to the GD-EOB-DTPA pathway, dependent from OATP transporter activity. Therefore, we expected a slightly less pronounced correlation between the 13C-MBT and SI-based values, as the 13C-MBT relies on an enzymatic metabolism, whereas contrast-enhanced MRI relies on OATP channel-triggered contrast agent uptake. In general, the ICG clearance test has certain limitations, as constant hemodynamic conditions (stable liver perfusion rate and hepatic blood flow) are required for liver function analysis40–42 and, in cases of cholestasis and hyperbilirubinemia, carrier competition of bilirubin and ICG at the OATP1 transporter might occur43,44. It is also known that various drugs (e.g., rifampicin) exert inhibitory effects on the OATP pathway and might influence hepatocyte ICG uptake45,46. Similar to the findings of Tamada et al.36, we could show that the RE of the liver parenchyma serves as a reliable tool for liver function classification, as the RE significantly differs among different stages of liver function (p ≤ 0.02). Additionally, it has been shown that the hepatic enhancement during Gd-EOB-DTPA-enhanced MRI is strongly affected by the degree of liver cirrhosis, as expressed by the ICG test, the Child-Pugh score or the MELD score34,47. These studies have shown that Gd-EOB-DTPA-enhanced MRI has potential as a reliable tool for liver function estimation in addition to its already established implementation for hepatic lesion detection.
Our study has several limitations. First, ROI placement may cause some variations due to the possible nonhomogeneous distribution of parenchymal changes. However, using the average of six repeated ROI measurements across an area of the liver parenchyma should provide reliable values. Second, this study was retrospective in nature, with only a limited patient population. Third, the lack of histopathology is another potential limitation.
In conclusion, SI-based indices, such as the RE and contrast-enhanced SI values, can be used to determine liver function as assessed by 13C-MBT.
Materials and Methods
Patients
Local institutional review board approval of the University Hospital Regensburg was obtained for 13C-MBT and Gd-EOB-DTPA-enhanced MRI at 3 T. Only data from written informed consent patients were included for this analysis, also the study was performed in accordance with the relevant guidelines and regulations.
The retrospective analysis includes 110 patients (83 men and 27 women; median age, 61 years) who underwent both a 13C-MBT and Gd-EOB-DTPA-enhanced MRI at 3 T. The patients underwent Gd-EOB-DTPA-enhanced T1-weighted volume-interpolated breath-hold examination (VIBE) MRI sequences with fat suppression. The included patients did not have known reactivity to liver-specific MRI contrast media, 13C-methacetin intolerance or renal-specific contraindications to either MRI and Gd-EOB-DTPA administration.
The patients underwent MRI and a liver function test for the following reasons:
active hepatocellular carcinoma monitoring in the case of no (n = 1) or known liver cirrhosis (n = 30)
follow-up in the case of known secondary malignancy (cholangiocarcinoma, n = 3; duodenal carcinoma, n = 1; rectal cancer, n = 4; sigma carcinoma, n = 2; uveal melanoma, n = 1) or benign hepatic lesion (focal nodular hyperplasia (FNH), n = 1; hemangioma, n = 3)
preinterventional assessment in the case of known hepatocellular carcinoma (HCC) (n = 6) or known secondary liver malignancy / focal hepatic lesion (cholangiocarcinoma, n = 2; hemangioma, n = 1; mamma carcinoma, n = 1; rectal cancer, n = 4; sigma carcinoma, n = 1)
postinterventional assessment in the case of known HCC (n = 19) or known secondary liver malignancy (cholangiocarcinoma, n = 3; colon carcinoma, n = 1; mamma carcinoma, n = 1; rectal cancer, n = 1; sigma carcinoma, n = 2; thymoma, n = 1)
in the case of suspected liver disease or focal hepatic lesions with known cirrhosis (n = 12), overlap syndrome (n = 1), Budd-Chiari syndrome (n = 1), cholangiocarcinoma (n = 2), carcinoid of the ileum (n = 1), rectal cancer (n = 3), or sigma carcinoma (n = 1)
The main reasons for liver cirrhosis were alcoholic steatohepatitis (n = 30), hepatitis B infection (n = 12) and hepatitis C infection (n = 13). Only 2 patients suffered from non-alcoholic steatohepatitis (NASH). Detailed insights in underlying disease can be seen in Table 3.
Table 3.
Underlying diseases for MRI examination and 13C-MBT for each medical case.
| patients (n = 110) | ||
|---|---|---|
| HCC | with cirrhosis | 62 |
| without cirrhosis | 1 | |
| liver disease | liver cirrhosis | 5 |
| autoimmune disease | overlap syndrome (AIH and PBC) | 1 |
| benign liver lesion | focal nodular hyperplasia (FNH) | 1 |
| hemangioma | 4 | |
| hepatic vein thrombosis | Budd-Chiari syndrome | 1 |
| secondary liver malignancies | carcinoma of the ileum or duodenum | 2 |
| cholangiocarcinoma | 10 | |
| colon carcinoma | 1 | |
| mamma carcinoma | 2 | |
| rectal cancer | 12 | |
| sigma carcinoma | 6 | |
| thymoma | 1 | |
| uveal melanoma | 1 |
13C-MBT
The 13C-MBT was performed 24 h before or after the MRI scan, according to published recommendations11,13. The patients fasted for at least 3 hours before the 13C-MBT. Ten minutes before the i.v. injection of 13C-methacetin, a breath 13CO2:12CO2 ratio control was recorded to calculate the delta-over-baseline (DOB). Then, 2 mg/kg body weight 13C-methacetin was injected via i.v. bolus and flushed with 20 mL of 0.9% sodium chloride. The volatile analysis, performed by modified nondispersive isotope-selective infrared spectroscopy (FANci2-db16, Fischer Analysen Instrumente, Leipzig, Germany), was started immediately after the injection to track the hepatocyte-specific enzymatic 13CO2 production.
For the statistical analysis, the patients were grouped according to their 13C-MBT readout into 3 categories: patients with normal liver function (Category 1): 13C-MBT > 315 [µg/kg/h]; patients with intermediate liver function (Category 2): 13C-MBT 315–140 [µg/kg/h]; and patients with severely impaired liver function (Category 3): 13C-MBT < 140 [µg/kg/h]13,16.
MRI
All imaging was performed using a clinical whole-body 3-T system (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany). T1-weighted VIBE sequences with fat suppression (repetition time (TR), 3.09 ms; echo time (TE), 1.17 ms, 2.49 ms; flip angle, 10°; parallel imaging factor, 2; slices, 64; reconstructed voxel size, 1.25 × 1.25 × 3.0 mm3; measured voxel size, 1.71 × 1.25 × 4.5 mm3; acquisition time, 14 sec) were acquired during breath-holding before and 20 min after Gd-EOB-DTPA (Primovist®, Bayer Healthcare, Berlin) administration. Every sequence covered the entire liver before Gd-EOB-DTPA administration and in the hepatobiliary phase (HBP) after 20 min.
The patients received a Gd-EOB-DTPA dose (0.025 mmol/kg body weight) adapted to their body weight administered via bolus injection at a flow rate of 1 mL/s, followed by 20 mL of 0.9% sodium chloride.
Image analysis
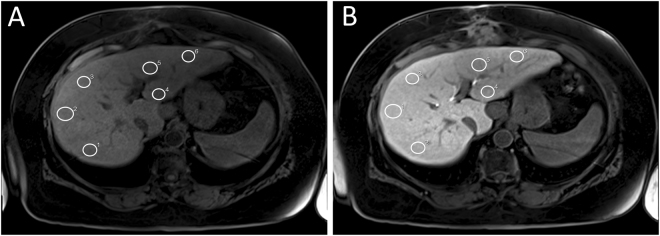
Operator-defined region-of-interest (ROI) measurements were used to obtain the mean SI values from the T1-weighted VIBE images (before and after Gd-EOB-DTPA injection). ROIs were manually placed at identical locations in every sequence, avoiding liver lesions, major branches of the portal and hepatic veins, and imaging artifacts. In total, 6 ROIs (3 each in the right and left lobes) were defined in the VIBE images (Fig. 4). Each ROI was a circle that was made as large as possible (liver parenchyma: 1.1 cm2–4.6 cm2) and manually adjusted between sequences if necessary.
Figure 4.
Representative example of ROI placement in unenhanced (A) and Gd-EOB-DTPA-enhanced VIBE (B) scans of a patient with normal liver function. White circles mark ROIs manually placed at identical places in the right and left lobe of the liver.
The relative enhancement (RE) of the liver was calculated according to following formula:
| 1 |
Statistical analysis
The different 13C-MBT readout categories were compared as non-parametric independent samples by the Mann-Whitney-U test. The predictive power of SI-based indices was determined by simple linear regression models, and the optimal curve fit was assessed visually. In all tests, the statistical significance level was set to 0.05 (two-sided). All analyses were performed using SPSS software (version 24; IBM, Chicago, IL, USA).
Data availability
All data that support the findings of this study are provided in the manuscript. Raw data used in this work are available on reasonable request.
Author Contributions
M.H. did the literature search, interpreted data and drafted the manuscript. U.P. participated in the literature search, statistics and manuscript drafting. S.P. collected the data and helped in interpretation of data. L.B., C.F. and P.W. helped in data acquisition, literature search and interpretation of data. M.S. and M.H. revised the manuscript critically for important intellectual content and made substantial contributions to data analysis. C.S. participated in its design, coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Morris-Stiff G, Gomez D, Prasad R. Quantitative assessment of hepatic function and its relevance to the liver surgeon. J Gastrointest Surg. 2009;13:374–385. doi: 10.1007/s11605-008-0564-1. [DOI] [PubMed] [Google Scholar]
- 2.Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Dig Surg. 2012;29:6–17. doi: 10.1159/000335713. [DOI] [PubMed] [Google Scholar]
- 3.Schreckenbach T, Liese J, Bechstein WO, Moench C. Posthepatectomy liver failure. Dig Surg. 2012;29:79–85. doi: 10.1159/000335741. [DOI] [PubMed] [Google Scholar]
- 4.Candelli M, et al. 13C-methionine breath tests for mitochondrial liver function assessment. Eur Rev Med Pharmacol Sci. 2008;12:245–249. [PubMed] [Google Scholar]
- 5.Afolabi P, Wright M, Wootton SA, Jackson AA. Clinical utility of 13C-liver-function breath tests for assessment of hepatic function. Dig Dis Sci. 2013;58:33–41. doi: 10.1007/s10620-012-2340-z. [DOI] [PubMed] [Google Scholar]
- 6.Armuzzi A, et al. Breath testing for human liver function assessment. Aliment Pharmacol Ther. 2002;16:1977–1996. doi: 10.1046/j.1365-2036.2002.01374.x. [DOI] [PubMed] [Google Scholar]
- 7.Candelli M, et al. 13C-methacetin breath test for monitoring hepatic function in cirrhotic patients before and after liver transplantation. Aliment Pharmacol Ther. 2004;19:243. doi: 10.1046/j.1365-2036.2003.01824.x. [DOI] [PubMed] [Google Scholar]
- 8.Candelli M, et al. 13C-breath tests in the study of mitochondrial liver function. Eur Rev Med Pharmacol Sci. 2004;8:23–31. [PubMed] [Google Scholar]
- 9.Grattagliano I, Lauterburg BH, Palasciano G, Portincasa P. 13C-breath tests for clinical investigation of liver mitochondrial function. Eur J Clin Invest. 2010;40:843–850. doi: 10.1111/j.1365-2362.2010.02331.x. [DOI] [PubMed] [Google Scholar]
- 10.Holtmeier J, et al. 13C-methacetin and 13C-galactose breath tests can assess restricted liver function even in early stages of primary biliary cirrhosis. Scand J Gastroenterol. 2006;41:1336–1341. doi: 10.1080/00365520600670125. [DOI] [PubMed] [Google Scholar]
- 11.Stockmann M, et al. Prediction of postoperative outcome after hepatectomy with a new bedside test for maximal liver function capacity. Ann Surg. 2009;250:119–125. doi: 10.1097/SLA.0b013e3181ad85b5. [DOI] [PubMed] [Google Scholar]
- 12.Palmer CNA, Coates PJ, Davies SE, Shephard EA, Phillips IR. Localization of cytochrome P-450 gene expression in normal and diseased human liver by in situ hybridization of wax-embedded archival material. Hepatology. 1992;16:682–687. doi: 10.1002/hep.1840160311. [DOI] [PubMed] [Google Scholar]
- 13.Stockmann M, et al. The LiMAx test: a new liver function test for predicting postoperative outcome in liver surgery. HPB (Oxford) 2010;12:139–146. doi: 10.1111/j.1477-2574.2009.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferlay J, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 15.Lock JF, et al. Early diagnosis of primary nonfunction and indication for reoperation after liver transplantation. Liver Transpl. 2010;16:172–180. doi: 10.1002/lt.21973. [DOI] [PubMed] [Google Scholar]
- 16.Stockmann M, et al. How to define initial poor graft function after liver transplantation? - a new functional definition by the LiMAx test. Transpl Int. 2010;23:1023–1032. doi: 10.1111/j.1432-2277.2010.01089.x. [DOI] [PubMed] [Google Scholar]
- 17.Stockmann M, et al. 1073 Accurate Diagnosis and grading of cirrhosis using the new LiMAx test. J Hepatol. 2012;56:S422. doi: 10.1016/S0168-8278(12)61085-X. [DOI] [Google Scholar]
- 18.Jara M, et al. Prognostic value of enzymatic liver function for the estimation of short-term survival of liver transplant candidates: a prospective study with the LiMAx test. Transpl Int. 2015;28:52–58. doi: 10.1111/tri.12441. [DOI] [PubMed] [Google Scholar]
- 19.Malinowski M, et al. Enzymatic liver function capacity correlates with disease severity of patients with liver cirrhosis: a study with the LiMAx test. Dig Dis Sci. 2014;59:2983–2991. doi: 10.1007/s10620-014-3250-z. [DOI] [PubMed] [Google Scholar]
- 20.Kaffarnik MF, et al. Early diagnosis of sepsis-related hepatic dysfunction and its prognostic impact on survival: a prospective study with the LiMAx test. Crit Care. 2013;17:R259. doi: 10.1186/cc13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lock JF, et al. Predicting the prognosis in acute liver failure: results from a retrospective pilot study using the LiMAx test. Ann Hepatol. 2013;12:556–562. [PubMed] [Google Scholar]
- 22.Ringe KI. Diagnosis and differential diagnosis of focal liver lesions using the hepatocyte-specific contrast agent Gd-EOB-DTPA. Radiologie up2date. 2016;16:15–32. doi: 10.1055/s-0042-102037. [DOI] [Google Scholar]
- 23.Leonhardt M, et al. Hepatic uptake of the magnetic resonance imaging contrast agent Gd-EOB-DTPA: role of human organic anion transporters. Drug Metab Dispos. 2010;38:1024–1028. doi: 10.1124/dmd.110.032862. [DOI] [PubMed] [Google Scholar]
- 24.Nassif A, et al. Visualization of hepatic uptake transporter function in healthy subjects by using gadoxetic acid-enhanced MR imaging. Radiology. 2012;264:741–750. doi: 10.1148/radiol.12112061. [DOI] [PubMed] [Google Scholar]
- 25.Reimer P, et al. Phase II clinical evaluation of Gd-EOB-DTPA: dose, safety aspects, and pulse sequence. Radiology. 1996;199:177–183. doi: 10.1148/radiology.199.1.8633143. [DOI] [PubMed] [Google Scholar]
- 26.Clemént O, et al. Comparison of Gd-EOB-DTPA and Gd-DTPA for contrast-enhanced MR imaging of liver tumors. J Magn Reson Imaging. 1993;3:71–77. doi: 10.1002/jmri.1880030113. [DOI] [PubMed] [Google Scholar]
- 27.Stern W, et al. Dynamic MR imaging of liver metastases with Gd-EOB-DTPA. Acta Radiol. 2000;41:255–262. doi: 10.1080/028418500127345208. [DOI] [PubMed] [Google Scholar]
- 28.Hamm B, et al. Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology. 1995;195:785–792. doi: 10.1148/radiology.195.3.7754011. [DOI] [PubMed] [Google Scholar]
- 29.Nishie A, et al. MR prediction of liver fibrosis using a liver-specific contrast agent: superparamagnetic iron oxide versus Gd-EOB-DTPA. J Magn Reson Imaging. 2012;36:664–671. doi: 10.1002/jmri.23691. [DOI] [PubMed] [Google Scholar]
- 30.Popper H. Pathologic aspects of cirrhosis. A review. The American Journal of Pathology. 1977;87:228–264. [PMC free article] [PubMed] [Google Scholar]
- 31.Utsunomiya T, et al. Possible utility of MRI using Gd-EOB-DTPA for estimating liver functional reserve. J Gastroenterol. 2012;47:470–476. doi: 10.1007/s00535-011-0513-8. [DOI] [PubMed] [Google Scholar]
- 32.Haimerl M, et al. Gd-EOB-DTPA-enhanced MRI for evaluation of liver function: Comparison between signal-intensity-based indices and T1 relaxometry. Sci Rep. 2017;7:43347. doi: 10.1038/srep43347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoneyama T, et al. Efficacy of liver parenchymal enhancement and liver volume to standard liver volume ratio on Gd-EOB-DTPA-enhanced MRI for estimation of liver function. Eur Radiol. 2014;24:857–865. doi: 10.1007/s00330-013-3086-5. [DOI] [PubMed] [Google Scholar]
- 34.Verloh N, et al. Assessing liver function by liver enhancement during the hepatobiliary phase with Gd-EOB-DTPA-enhanced MRI at 3 Tesla. Eur Radiol. 2014;24:1013–1019. doi: 10.1007/s00330-014-3108-y. [DOI] [PubMed] [Google Scholar]
- 35.Motosugi U, et al. Staging liver fibrosis by using liver-enhancement ratio of gadoxetic acid-enhanced MR imaging: comparison with aspartate aminotransferase-to-platelet ratio index. Magn Reson Imaging. 2011;29:1047–1052. doi: 10.1016/j.mri.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Tamada T, et al. Gd-EOB-DTPA-enhanced MR imaging: evaluation of hepatic enhancement effects in normal and cirrhotic livers. Eur J Radiol. 2011;80:e311–316. doi: 10.1016/j.ejrad.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe H, et al. Staging hepatic fibrosis: comparison of gadoxetate disodium-enhanced and diffusion-weighted MR imaging–preliminary observations. Radiology. 2011;259:142–150. doi: 10.1148/radiol.10100621. [DOI] [PubMed] [Google Scholar]
- 38.Motosugi U, et al. Liver parenchymal enhancement of hepatocyte-phase images in Gd-EOB-DTPA-enhanced MR imaging: which biological markers of the liver function affect the enhancement? J Magn Reson Imaging. 2009;30:1042–1046. doi: 10.1002/jmri.21956. [DOI] [PubMed] [Google Scholar]
- 39.Verloh N, et al. Liver fibrosis and Gd-EOB-DTPA-enhanced MRI: A histopathologic correlation. Sci Rep. 2015;5:15408. doi: 10.1038/srep15408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janssen MWW, et al. Indocyanine green R15 ratio depends directly on liver perfusion flow rate. J Hepatobiliary Pancreat Sci. 2010;17:180–185. doi: 10.1007/s00534-009-0160-0. [DOI] [PubMed] [Google Scholar]
- 41.Sakka SG. Indocyanine green plasma disappearance rate during relief of increased abdominal pressure. Intensive Care Med. 2006;32:2090–2091. doi: 10.1007/s00134-006-0411-3. [DOI] [PubMed] [Google Scholar]
- 42.Spiegel T, et al. Perioperative monitoring of indocyanine green clearance and plasma disappearance rate in patients undergoing liver transplantation. Der Anaesthesist. 2002;51:359–366. doi: 10.1007/s00101-002-0290-0. [DOI] [PubMed] [Google Scholar]
- 43.De Gasperi A, Mazza E, Prosperi M. Indocyanine green kinetics to assess liver function: Ready for a clinical dynamic assessment in major liver surgery? World J Hepatol. 2016;8:355–367. doi: 10.4254/wjh.v8.i7.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stockmann M, Malinowski M, Lock JF, Seehofer D, Neuhaus P. Factors influencing the indocyanine green (ICG) test: Additional impact of acute cholestasis. Hepatogastroenterology. 2009;56:734–738. [PubMed] [Google Scholar]
- 45.Vavricka SR, van Montfoort J, Ha HR, Meier PJ, Fattinger K. Interactions of rifamycin SV and rifampicin with organic anion uptake systems of human liver. Hepatology. 2002;36:164–172. doi: 10.1053/jhep.2002.34133. [DOI] [PubMed] [Google Scholar]
- 46.Cusin F, et al. Hepatocyte Concentrations of Indocyanine Green Reflect Transfer Rates Across Membrane Transporters. Basic and clinical pharmacology and toxicology. 2017;120:171–178. doi: 10.1111/bcpt.12671. [DOI] [PubMed] [Google Scholar]
- 47.Verloh N, et al. Impact of liver cirrhosis on liver enhancement at Gd-EOB-DTPA enhanced MRI at 3 Tesla. Eur J Radiol. 2013;82:1710–1715. doi: 10.1016/j.ejrad.2013.05.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that support the findings of this study are provided in the manuscript. Raw data used in this work are available on reasonable request.