Abstract
High-latitude coral reefs provide natural laboratories for investigating the mechanisms and limits of coral calcification. While the calcification processes of tropical corals have been studied intensively, little is known about how their temperate counterparts grow under much lower temperature and light conditions. Here, we report the results of a long-term (2-year) study of seasonal changes in calcification rates, photo-physiology and calcifying fluid (cf) chemistry (using boron isotope systematics and Raman spectroscopy) for the coral Turbinaria reniformis growing near its latitudinal limits (34.5° S) along the southern coast of Western Australia. In contrast with tropical corals, calcification rates were found to be threefold higher during winter (16 to 17° C) compared with summer (approx. 21° C), and negatively correlated with light, but lacking any correlation with temperature. These unexpected findings are attributed to a combination of higher chlorophyll a, and hence increased heterotrophy during winter compared with summer, together with the corals' ability to seasonally modulate pHcf, with carbonate ion concentration 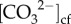 being the main controller of calcification rates. Conversely, calcium ion concentration [Ca2+]cf declined with increasing calcification rates, resulting in aragonite saturation states Ωcf that were stable yet elevated fourfold above seawater values. Our results show that corals growing near their latitudinal limits exert strong physiological control over their cf in order to maintain year-round calcification rates that are insensitive to the unfavourable temperature regimes typical of high-latitude reefs.
being the main controller of calcification rates. Conversely, calcium ion concentration [Ca2+]cf declined with increasing calcification rates, resulting in aragonite saturation states Ωcf that were stable yet elevated fourfold above seawater values. Our results show that corals growing near their latitudinal limits exert strong physiological control over their cf in order to maintain year-round calcification rates that are insensitive to the unfavourable temperature regimes typical of high-latitude reefs.
Keywords: coral calcification, pH upregulation, calcifying fluid, boron isotopes, high latitude, Western Australia
1. Introduction
Symbiotic corals are the foundation species of coral reef ecosystems, creating complex three-dimensional habitats that harbour over one-third of the oceans’ biodiversity [1]. Their distribution spans almost 70° of latitude, occupying a range of environments from tropical equatorial regions to cold temperate zones [2]. However, the future of these highly diverse and widespread ecosystems is threatened by the unprecedented impacts of both CO2-driven ocean acidification (OA) and warming [3]. Abrupt El Niño–Southern Oscillation (ENSO)-driven ocean-warming events cause widespread coral bleaching and mortality due to the loss of the algal symbiont [4,5], while declines in seawater pH and carbonate ion concentrations 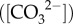 due to OA have often been shown to cause declines in coral calcification rates (e.g. [6,7]).
due to OA have often been shown to cause declines in coral calcification rates (e.g. [6,7]).
However, the effects of OA and rising seawater temperatures on symbiotic corals are likely to vary geographically. For example, high-latitude reefs (i.e. above 28° N and below 28° S) are already considered marginal, in part due to their low seawater aragonite saturation state (Ω), and will experience further declines in Ω due to OA, making them less suitable to support coral calcification [3]. By contrast, warming seawater temperatures may have a positive net effect on high-latitude coral calcification rates, particularly during winter when lower temperatures currently limit calcification rates [8–11]. However, during winter increased heterotrophic feeding at high latitude may help to offset the negative influences of lower temperatures and Ωsw by providing corals with the energy required for calcification processes [12,13]. Furthermore, while high-latitude warming would be expected to enhance coral calcification rates, and hence outweigh the negative effects of OA, increases in summertime temperatures beyond localized thermal optima may cause bleaching and declines to calcification [5,14]. Given the wide range of possible responses, and that atmospheric CO2 concentrations are projected to continue increasing even under stringent emissions reduction scenarios, it is therefore critical to better understand the coral calcification mechanisms and strategies available for corals at high latitude to endure ocean warming and acidification [15,16].
One such strategy to cope with and acquire resistance to OA is the corals' ability to modulate chemical conditions at the site of calcification (e.g. [15–20]). Reef-building corals create their calcium carbonate (CaCO3) skeletons within a seawater-supplied, semi-isolated extracellular calcifying fluid (cf), located between the sub-calicoblastic cells and the skeleton [21]. While this process is highly biologically modulated [21], the reaction kinetics are still nevertheless dependent on temperature and Ω in the cf [22]. Although aragonite is already supersaturated in ambient seawater (i.e. Ω > 3), corals possess mechanisms to upregulate both pHcf and dissolved inorganic carbon (DICcf) to increase Ωcf above seawater levels (i.e. approx. 10–25) [15,17,18], and thus promote rapid CaCO3 growth [22]. Additionally, the counter-regulation of pHcf and DICcf on seasonal time scales [18] may also act to dampen the effect of seasonally variable temperature on high-latitude calcification rates (see [23]). Thus, while laboratory experiments have demonstrated that declines in coral pHcf still typically occur under OA (e.g. [17,24]), it is clear that the corals’ strong ability to upregulate pHcf and DICcf is a critical mechanism for calcification, and provides corals with some resistance against the negative impacts of OA (e.g. [16,18]).
The ability to infer the calcium ion concentrations in the cf ([Ca2+]cf), pHcf, DICcf and Ωcf, however, has only recently become feasible due to the development of new geochemical approaches using boron isotope (δ11B) and elemental (B/Ca) systematics [18], together with Raman spectroscopy [25,26]. Thus, until recently, typically one [17,20,24,27] or at most two (e.g.[18,28]) aspects of the cf chemistry (i.e. pH and 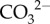 ) have been measured. Quantifying [Ca2+]cf remains, to date, a key knowledge gap given that [Ca2+]cf is a critical component of the coral calcification process. While it is often assumed that [Ca2+]cf is equal or similar to seawater, direct measurements of [Ca2+]cf are particularly limited. Recent work combining Raman spectroscopy with boron systematics suggests that active elevation of [Ca2+] in the cf (up to 25% higher than seawater) may be fundamental to the resistance of calcification to OA for some species [26]. Prior to that, the only other [Ca2+]cf measurements were made using micro-sensors, which showed that [Ca2+]cf was elevated above seawater by approximately 10% [29]. Yet, [Ca2+]cf concentrations may play an important role in controlling Ωcf in addition to
) have been measured. Quantifying [Ca2+]cf remains, to date, a key knowledge gap given that [Ca2+]cf is a critical component of the coral calcification process. While it is often assumed that [Ca2+]cf is equal or similar to seawater, direct measurements of [Ca2+]cf are particularly limited. Recent work combining Raman spectroscopy with boron systematics suggests that active elevation of [Ca2+] in the cf (up to 25% higher than seawater) may be fundamental to the resistance of calcification to OA for some species [26]. Prior to that, the only other [Ca2+]cf measurements were made using micro-sensors, which showed that [Ca2+]cf was elevated above seawater by approximately 10% [29]. Yet, [Ca2+]cf concentrations may play an important role in controlling Ωcf in addition to 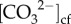 . Thus, all components of the cf carbonate chemistry need to be quantified to understand the mechanisms and responses of coral calcification to changing environmental conditions.
. Thus, all components of the cf carbonate chemistry need to be quantified to understand the mechanisms and responses of coral calcification to changing environmental conditions.
Here, we combine novel geochemical analyses (Raman spectroscopy and boron systematics) to quantify cf chemistry (i.e. pHcf, DICcf, 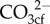 , Ca2+cf and Ωcf), together with calcification rates and photo-physiology as a proxy for coral health (photochemical efficiency; Fv/Fm), for the high-latitude coral species Turbinaria reniformis growing near the latitudinal limits for hermatypic coral growth in Bremer Bay, Western Australia (WA; 34.5° S; 17° C to 21° C). We show how corals seasonally regulate their cf chemistry to optimize calcification rates at high latitude. Our study is the first to constrain both aspects of Ωcf (i.e.
, Ca2+cf and Ωcf), together with calcification rates and photo-physiology as a proxy for coral health (photochemical efficiency; Fv/Fm), for the high-latitude coral species Turbinaria reniformis growing near the latitudinal limits for hermatypic coral growth in Bremer Bay, Western Australia (WA; 34.5° S; 17° C to 21° C). We show how corals seasonally regulate their cf chemistry to optimize calcification rates at high latitude. Our study is the first to constrain both aspects of Ωcf (i.e. 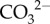 and Ca2+) as well as pHcf for corals growing in situ. Informed by this unique dataset, we present a conceptual model to elucidate the mechanisms of high-latitude coral calcification with respect to the full suite of carbonate system dynamics within the cf.
and Ca2+) as well as pHcf for corals growing in situ. Informed by this unique dataset, we present a conceptual model to elucidate the mechanisms of high-latitude coral calcification with respect to the full suite of carbonate system dynamics within the cf.
2. Material and methods
(a). Study sites and overview
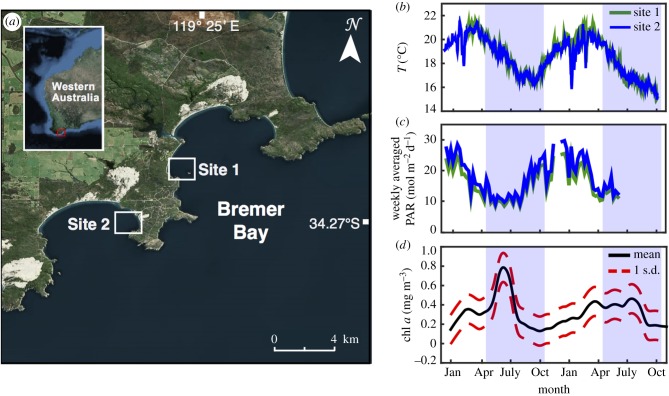
Bremer Bay is located approximately 500 km southeast of Perth in WA, bordering the Southern Ocean (34.4° S, 119.4° E; figure 1a). These waters support seven symbiotic species of coral, which probably migrated southwards via the Leeuwin Current, a pole-wards flowing current that transports warm water along the WA coastline [31]. Turbinaria reniformis is the dominant coral species at this location, with 100% coral cover in some areas, surrounded by macro-algae and seagrass. Seasonal mean monthly seawater temperatures typically range from just 17°C to 21°C, and light levels range from 13.5 to 21 mol m−2 d−1 (figure 1b,c).
Figure 1.
(a) Location of the study sites. (b) Daily averaged seawater temperature (°C), (c) weekly averaged photosynthetically active radiation (PAR) reaching the benthos and (d) monthly satellite-derived chlorophyll a for Bremer Bay (obtained from IMOS [30]). Shading denotes winter and no shading denotes summer. Seasons are defined based on changes in light and temperature.
We measured coral calcification rates, linear extension rates and photo-physiology (Fv/Fm), and analysed the chemical composition of the cf for T. reniformis at two sites in Bremer Bay: Back Beach (herein entitled Site 1; approx. 9 m water depth) and Little Boat Harbour (herein entitled Site 2; approx. 7 m water depth) (figure 1a). Measurements were taken every 3–4 months over an approximately 2-year period between December 2014 and October 2016 (see the electronic supplementary material for additional details).
(b). Environmental measurements and the 2016 El Niño
Photosynthetically active radiation (PAR), temperature, salinity, pH on the total scale (pHT), total alkalinity (TA) and nutrients (ammonium, nitrate + nitrite and phosphate) were measured throughout the study as per previously published methodology [32]. Monthly satellite-derived chlorophyll a for Bremer Bay was obtained from the Integrated Marine Observing System (IMOS) [30] (electronic supplementary material, figure S1). The 2015/16 global El Niño caused anomalously cold water conditions during the 2016 winter in southwest WA. Seasonal seawater temperatures in Bremer Bay during the 2016 El Niño winter were, on average, up to 1°C cooler than the 20-year long-term average [30], highlighting the chronically cold winter events (16°C) that high-latitude corals must cope with. Further details are provided in the electronic supplementary material.
(c). Photo-physiology
The maximal quantum yield of electron transport through photosystem II (Fv/Fm) was measured using a Diving-PAM (Walz, Germany). Measurements of Fv/Fm were performed after 1 h of dark acclimation. The fibre-optic probe on the PAM fluorometer was kept at a fixed distance (5 mm) using plastic tubing. The PAM settings used were: measuring intensity (3), gain (3), saturation intensity (12) and signal width (0.8).
(d). Coral calcification and linear extension rates
Calcification rates (mg CaCO3 cm−2 d−1) were measured on 35 individual coral colonies (n = 21 at Site 1 and n = 14 at Site 2) using samples taken from the natural population (one sample per parent colony, located approx. 3 to 5 m apart). The sample specimens were mounted on plastic tiles, and deployed in situ on aluminium frames (see [32]). Changes in weight were measured using the buoyant weight technique [33], and then normalized to surface area using a regression between surface area (measured using ImageJ software [14]) and dry weight (9 to 190 g; n = 23, r2 = 0.98; electronic supplementary material, figure S2). Linear extension rates were measured on naturally growing coral colonies at Site 2 only. We marked these corals with semi-permanent reference points by drilling three separate nails into two colonies. The extension rates were measured by taking the distance (± 1 mm) from the reference point to the growing edge at three-month intervals. Geochemical studies (see following) were undertaken on the outmost growing tips of specimens sampled every three months over the approximately 2-year period.
(e). Geochemical analyses
We used Raman spectroscopy to determine Ωcf, and boron systematics (δ11B, B/Ca) to determine coral pHcf, 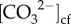 , and by inference [Ca2+]cf. Previous protocols were used for analysis of trace elements and boron isotopes (see [18,23]) and Raman spectroscopy [25]. Sampling distances of the coral skeletons were based on linear extension measurements (0.3 to 1.5 mm month1; electronic supplementary material, table S1). Additionally, we used the molar ratios of strontium to calcium (Sr/Ca) and lithium to magnesium (Li/Mg) temperature proxies to confirm the seasonal chronology of skeletal growth histories.
, and by inference [Ca2+]cf. Previous protocols were used for analysis of trace elements and boron isotopes (see [18,23]) and Raman spectroscopy [25]. Sampling distances of the coral skeletons were based on linear extension measurements (0.3 to 1.5 mm month1; electronic supplementary material, table S1). Additionally, we used the molar ratios of strontium to calcium (Sr/Ca) and lithium to magnesium (Li/Mg) temperature proxies to confirm the seasonal chronology of skeletal growth histories.
The pHcf was derived from the measured skeletal δ11B values according to the following equation [34]:
| 2.1 |
where pKB is the dissociation constant of boric acid in seawater [35] at the temperature and salinity of the seawater in Bremer Bay, δ11Bcarb and δ11BSW are the boron isotopic composition of the coral skeleton and average seawater (39.61‰), respectively, and αB is the isotopic fractionation factor (1.0272) [36].
We estimated 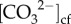 using molar ratios of boron to calcium (B/Ca) according to the following relationship [18,37]:
using molar ratios of boron to calcium (B/Ca) according to the following relationship [18,37]:
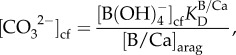 |
2.2 |
where 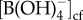 is the concentration of borate in the cf,
is the concentration of borate in the cf, 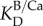 is the distribution coefficient for boron between aragonite and seawater [18], and [B/Ca]arag is the elemental ratio of boron to calcium measured in the coral skeleton. The concentration of DICcf was then calculated from the estimates of pHcf and
is the distribution coefficient for boron between aragonite and seawater [18], and [B/Ca]arag is the elemental ratio of boron to calcium measured in the coral skeleton. The concentration of DICcf was then calculated from the estimates of pHcf and 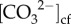 [18,28].
[18,28].
Raman spectroscopy was conducted to determine Ωcf using an abiogenic calibration to peak width [25]. Finally, the [Ca2+]cf was inferred from Ωcf (Raman) and 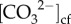 (δ11B and B/Ca) according to the following relationship:
(δ11B and B/Ca) according to the following relationship:
| 2.3 |
where K*sp is the solubility constant for aragonite as a function of temperature and salinity, Ωcf is the saturation state of the cf determined from Raman, and 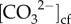 is the carbonate ion concentration of the cf estimated from δ11B and B/Ca (equation (2.2)). See the electronic supplementary material for additional details.
is the carbonate ion concentration of the cf estimated from δ11B and B/Ca (equation (2.2)). See the electronic supplementary material for additional details.
(f). Statistical analyses
A t-test was used to test for significant differences in calcification rates between the October 2015 time point and the October 2016 (unusually cold El Niño) time point. Repeated measures analysis of variance (rANOVA) was used to test for the effect of site on coral calcification rate, Fv/Fm, DICcf, pHcf, 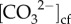 , [Ca2+]cf and Ωcf. Linear regression analysis was used to examine relationships between coral calcification rate, Fv/Fm, cf parameters and environmental data (see the electronic supplementary material for additional details).
, [Ca2+]cf and Ωcf. Linear regression analysis was used to examine relationships between coral calcification rate, Fv/Fm, cf parameters and environmental data (see the electronic supplementary material for additional details).
3. Results
(a). Environmental conditions
On average, monthly averaged seawater temperatures ranged from 16° to 21°C (figure 1b). Site 2 showed cold temperature ‘spikes’ during January and February 2016 that were not evident at Site 1 (figure 1b). However, for all months except January and February 2016, differences in mean temperatures generally did not differ by more than 0.30°C. The light attenuation coefficient (kd) measured for seawater was very low, signifying high water clarity (approx. 0.06 m−1 in summer and approx. 0.07 m−1 in winter; electronic supplementary material, table S3). On average, monthly averaged seasonal PAR reaching the benthos ranged from 9.8 to 22.3 mol m−2 d−1 at Site 1 and 10.8 to 26 mol m−2 d−1 at Site 2 (figure 1c). Diurnal measurements of ambient seawater pH (electronic supplementary material, figure S3) showed that the pH of near shore waters in Bremer Bay varied minimally (0.06 pH units) between seasons (8.05 in summer to 8.11 in winter; electronic supplementary material, table S3). Seawater Ωar was, on average, approximately 3.0 based on daytime water sampling (electronic supplementary material, table S3). Nutrient concentrations (total dissolved inorganic nitrogen and phosphate) were <1 µM (electronic supplementary material, table S3) and monthly satellite-derived chlorophyll a for Bremer Bay was, on average, higher in winter compared with summer for both years (i.e. 0.14 mg m−3 during 2014/15 summer to 0.79 mg m−3 during 2015 winter and 0.13 mg m−3 during 2015/16 summer to 0.46 mg m−3 during 2016 winter mg m−3; figure 1d).
(b). Photo-physiology
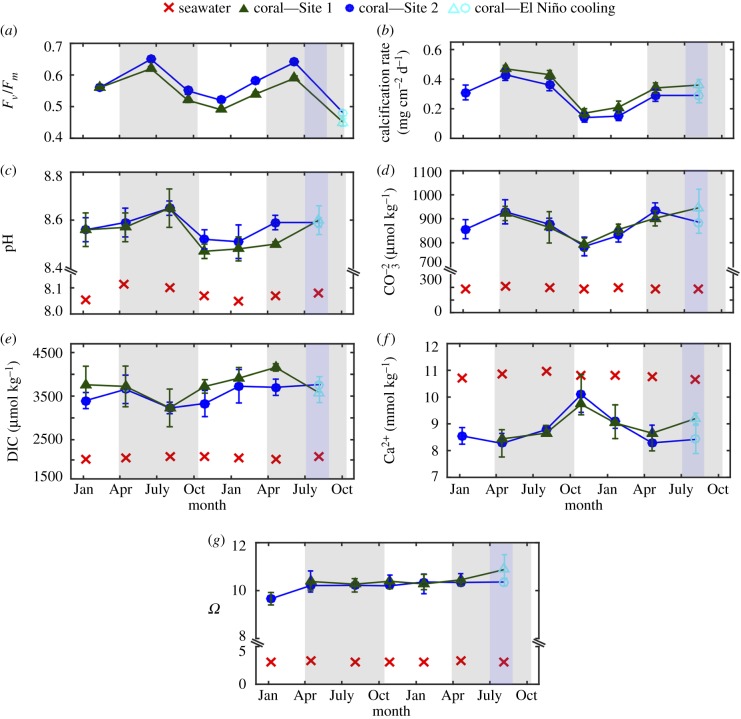
On average, Fv/Fm seasonally ranged from 0.45 to 0.65 (figure 2a). The lowest Fv/Fm (i.e. average of 0.45) occurred during the unusually cold El Niño winter period (figure 2a). Average seasonal Fv/Fm was significantly negatively correlated with light (r2 = 0.37, root mean squared error (RMSE) = 0.050 Fv/Fm, p = 0.027; electronic supplementary material, figure S4), and there was a significant nonlinear (polynomial) relationship between average seasonal Fv/Fm and temperature (r2 = 0.56, p = 0.012; electronic supplementary material, figure S4).
Figure 2.
Time series of (a) photochemical efficiency (Fv/Fm) (b) calcification rates, (c) pH, (d) DIC, (e) 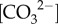 , (f) [Ca2+] and (g)
Ω for T. reniformis in Bremer Bay. Values represent mean ± 1 s.d for calcifying fluid (cf) parameters (n = 5 per site) and mean ± 1 s.e. for calcification rates and Fv/Fm (n = 14 at Site 1, n = 21 at site 2). No shading denotes summer, light shading denotes winter, and dark shading denotes El Niño winter cooling.
, (f) [Ca2+] and (g)
Ω for T. reniformis in Bremer Bay. Values represent mean ± 1 s.d for calcifying fluid (cf) parameters (n = 5 per site) and mean ± 1 s.e. for calcification rates and Fv/Fm (n = 14 at Site 1, n = 21 at site 2). No shading denotes summer, light shading denotes winter, and dark shading denotes El Niño winter cooling.
(c). Coral calcification rates and extension rates
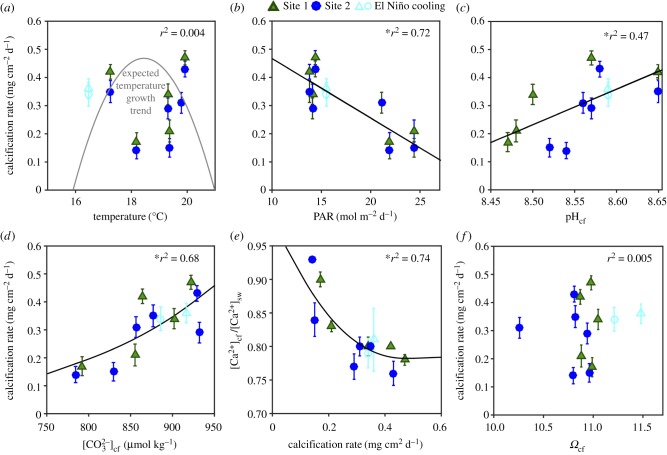
Mean rates of calcification over the entire study ranged from 0.16 to 0.45 mg cm−2 d−1, and were thus almost threefold higher during the winter months compared with the summer months (figure 2b). Calcification rates showed no significant relationship with temperature (r2 = 0.004, RMSE = 0.113 mg cm−2 d−2, p = 0.857; figure 3a), but were significantly negatively correlated with light (r2 = 0.71, RMSE = 0.061 mg cm−2 d−2, p < 0.001; figure 3b). The low Fv/Fm during the unusually cold El Niño winter period did not coincide with significantly lower calcification rates (i.e. compared with the October 2015 time point; t34 = 1.797, p = 0.081). Average linear extension rates ranged from 0.47 to 1.28 mm mo−1, with higher extension rates during winter compared with summer (electronic supplementary material, table S1).
Figure 3.
Sensitivity of coral calcification rate to (a) temperature (fitted with a conceptual nonlinear polynomial temperature growth curve), (b) photosynthetically active radiation (calcification rate = −0.021 PAR + 0.677), (c) pHcf (calcification rate = 1.27 pHcf – 10.57) and (d) 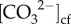 (best fitted by the exponential relationship calcification rate = 0.0007e0.0069x). (e) Relationship between [Ca2+]cf/[Ca2+]sw with calcification rates (best fitted by the polynomial relationship: [Ca2+]cf/[Ca2+]sw = 1.4313 calcification rate2 −1.2104G + 1.0377), and (f) sensitivity of calcification rate to Ωcf. Values represent the mean ± 1 s.e. (calcifying fluid: n = 5 per site; calcification: n = 14 at Site 1, n = 21 at site 2). Asterisks denote statistical significance.
(best fitted by the exponential relationship calcification rate = 0.0007e0.0069x). (e) Relationship between [Ca2+]cf/[Ca2+]sw with calcification rates (best fitted by the polynomial relationship: [Ca2+]cf/[Ca2+]sw = 1.4313 calcification rate2 −1.2104G + 1.0377), and (f) sensitivity of calcification rate to Ωcf. Values represent the mean ± 1 s.e. (calcifying fluid: n = 5 per site; calcification: n = 14 at Site 1, n = 21 at site 2). Asterisks denote statistical significance.
(d). Coral calcifying fluid chemical composition
Both Sr/Ca (Site 1: r2 = 0.62, Site 2: r2 = 0.71) and Li/Mg (Site 1: r2 = 0.67, Site 2: r2 = 0.62) were significantly correlated with ambient seawater temperature (p < 0.001; electronic supplementary material, figure S5a,b), supporting that our sampling scheme captured different seasons of growth. On average, the δ11B compositions varied seasonally by 2‰ (δ11B approx. 23 to 25‰; electronic supplementary material, figure S6a), corresponding to seasonal ranges in pHcf of 8.50 (summer) to 8.65 (winter) (figure 2c). Seasonal changes in pHcf and temperature were inversely correlated (r2 = 0.46, p = 0.007; electronic supplementary material, figure S7a), and changes in pHcf were significantly positively linearly correlated with calcification rates (r2 = 0.45, RMSE = 0.086 mg cm−2 d−2, p = 0.017; figure 3c). Skeletal ratios of boron to calcium (B/Ca) ranged from 0.57 to 0.73 mmol mol−1 (electronic supplementary material, figure S6b), which corresponded to mean 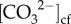 from approximately 780 to 930 µmol kg−1, with higher
from approximately 780 to 930 µmol kg−1, with higher 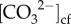 during winter compared with summer (figure 2d). Furthermore,
during winter compared with summer (figure 2d). Furthermore, 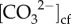 showed a significant positive relationship with calcification rates (r2 = 0.68; RMSE = 0.069 mg cm−2 d−2, p = 0.001; figure 3d). DICcf was substantially elevated relative to ambient seawater by a factor of 1.5 to 2 (figure 2e), with lower DICcf during the first winter period, and anomalously high DICcf during the second winter period (i.e. during the 2016 El Niño winter) (figure 2e). Thus, there was no significant linear correlation between DICcf/DICsw and temperature (r2 = 0.10, RMSE = 0.134, p = 0.301; electronic supplementary material, figure S7b), nor between DICcf/DICsw and calcification rates (r2 = 0.002, RMSE = 0.112 mg cm−2 d−2, p = 0.886). There was, however, a significant negative correlation between DICcf/DICsw and pHcf (r2 = 0.50, RMSE = 0.022, p = 0.009; electronic supplementary material, figure S7c).
showed a significant positive relationship with calcification rates (r2 = 0.68; RMSE = 0.069 mg cm−2 d−2, p = 0.001; figure 3d). DICcf was substantially elevated relative to ambient seawater by a factor of 1.5 to 2 (figure 2e), with lower DICcf during the first winter period, and anomalously high DICcf during the second winter period (i.e. during the 2016 El Niño winter) (figure 2e). Thus, there was no significant linear correlation between DICcf/DICsw and temperature (r2 = 0.10, RMSE = 0.134, p = 0.301; electronic supplementary material, figure S7b), nor between DICcf/DICsw and calcification rates (r2 = 0.002, RMSE = 0.112 mg cm−2 d−2, p = 0.886). There was, however, a significant negative correlation between DICcf/DICsw and pHcf (r2 = 0.50, RMSE = 0.022, p = 0.009; electronic supplementary material, figure S7c).
On average, [Ca2+]cf ranged from 8.3 to 9.7 mmol kg−1, and were thus 10 to 30% lower than seawater values (approx. 10.7 to 11 mmol kg−1; figure 2f). [Ca2+]cf/[Ca2+]sw was negatively correlated with calcification rates (r2 = 0.64; RMSE = 0.072 mg cm−2 d−2, p = 0.002; figure 3e), and showed a significant correlation with pHcf (r2 = 0.32, RMSE = 0.044, p = 0.037; electronic supplementary material, figure S7d). Lastly, mean Ωcf was relatively stable year-round ranging from just 10.3 to 11.2 (figure 2g); and thus, there was no significant linear relationship between Ωcf and calcification rates (r2 = 0.005, RMSE = 0.112 mg cm−2 d−2, p = 0.823; figure 3f).
4. Discussion
(a). Drivers of the seasonal patterns of coral calcification
We found that the high-latitude coral T. reniformis exhibited unusual seasonal patterns of calcification, with threefold higher calcification rates during winter compared with summer. This is in strong contrast to the well-established pattern of enhanced summer calcification in both tropical [38,39] and high-latitude corals [8–11], providing novel insights into the drivers and mechanisms supporting coral growth at its latitudinal limits. Our findings are unexpected given that seasonally higher temperatures should have promoted faster growth during summer compared to winter due to the strong temperature-dependence of aragonite precipitation rates [22] and light-enhanced calcification [40]. For example, in tropical corals, calcification rates typically increase with temperature until an optimum is reached, which is usually equal to, or slightly above, the annual average temperatures experienced by the coral (figure 3a) [38,39,41]. Moreover, along latitudinal temperature gradients, coral calcification rates generally decline with increasing latitude (decreasing temperatures) and are much lower than their tropical counterparts [42,43]. By contrast, our findings demonstrate that the positive relationship between temperature and calcification rate observed in tropical corals is not applicable to all high-latitude coral species.
Few other studies report area-normalized field-based calcification for T. reniformis. Our rates of calcification during the winter (34.5° S; 0.3 to 0.5 mg cm−2 d−1), however, were within the range of other tropical and sub-tropical corals, such as Pocillopora damicornis at both Rottnest Island (32° S; 0.3 to 0.9 mg cm−2 d−1) [32] and Coral Bay, Ningaloo (21.5° S; 0.4 to 0.9 mg cm−2 d−1) [14], despite the much cooler temperatures (i.e. 16 to 21°C at Bremer Bay versus 18 to 24°C at Rottnest and 22 to 28°C in Coral Bay, Ningaloo). This implies that corals in Bremer Bay have altered their thermal tolerance range, via either adaptation or acclimatization, to support rates of calcification similar to tropical corals but at cooler temperatures and lower light levels [44]. Thus, our findings based on corals in Bremer Bay suggest that warming seawater temperatures may not necessarily accelerate coral calcification at high latitude, or be required to sustain calcification during winter. Our results also provide additional support to a growing number of studies that have documented unusual seasonal or latitudinal variability in coral calcification rates, and/or instances where coral calcification rates were not maximized under the warmest conditions (i.e. summer and at low-latitude [8,14,32,45,46]).
However, the mechanisms underlying faster growth during winter and at high latitude are not yet fully understood, particularly for corals growing near their latitudinal limits [23], and our study is among the first to provide insights into the mechanisms enabling these patterns. One possible explanation for the lower growth rates during summer compared with winter is that the corals may have experienced heat stress during the warmer summer months resulting in suppressed summer growth rates [14,28]. However, this hypothesis is not supported by measurements of Fv/Fm, which showed no signs of chronic photo-inhibition during the summer months (figure 2a). Furthermore, no visible signs of bleaching (i.e. paling of corals due to loss of photosynthetic pigments and/or symbionts) were observed throughout the study (authors' observations). Nevertheless, Fv/Fm was negatively correlated with seasonal changes in light (r2 = 0.37; electronic supplementary material, figure S4), such that these corals showed a seasonal photo-acclamatory response, with higher photosynthetic efficiency during winter under low light levels; this is similar to results from previous work, albeit in tropical locations [47].
(b). Mechanisms of coral calcification at their latitudinal limits
A more plausible explanation for the unusually high calcification rates in winter compared with summer is that the corals were able to dictate seasonal rates of calcification by modulating their internal chemistry to counteract changes in the external conditions (i.e. temperature and light) [23]. For example, the absence of any temperature and light-dependent seasonality in the calcification rates can be largely explained by higher wintertime pHcf and 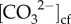 to support higher calcification rates during winter, despite the seasonally lower light, temperature and DICcf. Meanwhile, during summer, reduced pHcf and
to support higher calcification rates during winter, despite the seasonally lower light, temperature and DICcf. Meanwhile, during summer, reduced pHcf and 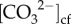 corresponded to lower rates of calcification (figures 1 and 4). Therefore, the unexpected negative relationship between seasonal light and calcification rates may have indirectly resulted from the concurrently higher upregulation of pHcf (and thus higher
corresponded to lower rates of calcification (figures 1 and 4). Therefore, the unexpected negative relationship between seasonal light and calcification rates may have indirectly resulted from the concurrently higher upregulation of pHcf (and thus higher 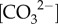 ) during winter (i.e. low light) compared to summer (i.e. high light).
) during winter (i.e. low light) compared to summer (i.e. high light).
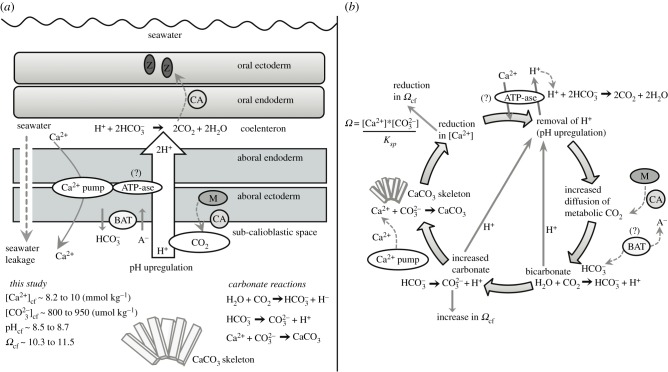
Figure 4.
Schematics of the (a) coral calcification mechanisms [18,21,48], and (b) calcification processes. Scleractinian corals create their calcium carbonate skeletons within an extracellular calcifying fluid located between the sub-calioblastic cells and the skeleton with fluid supplied from the seawater [48]. Coral pH upregulation occurs via the pumping of H+ out of the calcifying fluid, promoting the diffusion of metabolic CO2 from the mitochondria (M) into the calcifying fluid. CO2 is converted into bicarbonate using carbonic anhydrase (CA) producing additional H+, and active transport using bicarbonate transporters (BAT) also occurs [49]. Metabolic CO2 is also supplied to the symbionts (Z) [49,50]. pH upregulation shifts the equilibrium of DIC in favour of carbonate 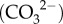 relative to bicarbonate
relative to bicarbonate 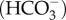 , producing additional H+. Calcification occurs and Ca2+ is depleted from the calcifying fluid, causing a decrease in Ωcf. Multiple mechanisms of calcium transport may operate (e.g. Ca-ATPase, Ca-channels, and Ca2+/Na+ exchange), combined with seawater renewal, to re-supply Ca2+ for calcification.
, producing additional H+. Calcification occurs and Ca2+ is depleted from the calcifying fluid, causing a decrease in Ωcf. Multiple mechanisms of calcium transport may operate (e.g. Ca-ATPase, Ca-channels, and Ca2+/Na+ exchange), combined with seawater renewal, to re-supply Ca2+ for calcification.
Furthermore, we found that pHcf was negatively correlated with DICcf, consistent with previous studies for tropical and sub-tropical corals (approx. 0.1 to 0.2 pH units) [18,23]. Given that the process of pH upregulation is thought to be relatively energetically inexpensive [15], healthy corals are able to systematically counter-regulate DICcf and pHcf to maintain elevated Ωcf [16,18,23], therefore potentially regulating their calcification rates [15,23]. These seasonal changes in DICcf [18,23] were pro-cyclical with temperature and light (except during the 2016 El Niño winter), and thus consistent with temperature- and/or light-driven seasonal changes in metabolic CO2 [21,50]. Furthermore, our results support a recent study showing that corals growing in a sub-tropical environment at Rottnest Island (located approx. 550 km northwest of Bremer Bay in WA) can maintain stable calcification rates year-round by modulating their cf carbonate chemistry, such that the effect of seasonally varying temperature and light on rates of bulk calcification was dampened (see [23]).
Interestingly, the counter-regulation of DICcf and pHcf appears to systematically shift biogeographically. Although we cannot rule out species specific differences, T. reniformis shows higher pHcf (approx. 8.5 to 8.65 at 16 to 21°C) than both the sub-tropical corals at Rottnest Island (approx. 8.4 to 8.6 at 18 to 24°C) and the tropical corals at Coral Bay (approx. 8.3 to 8.55 at 22 to 28°C) and Lizard Island (approx. 8.25 to 8.5 at 23 to 28.5°C) [18,23], consistent with a temperature-dependence of pHcf regulation [18,23]. This trend occurs in the absence of any differences in absolute pHsw values and independently of the magnitude of seasonal changes in pHsw (i.e. approx. 8.03 to 8.10 at all locations). Thus, this physiological control on pH upregulation appears to be a ubiquitous strategy among both high-latitude and tropical corals for counter-acting declines in metabolically supplied DICcf [18,23].
However, other factors probably contribute to the capacity of T. reniformis to tolerate sub-optimal conditions (i.e. low light and temperature) and to calcify at faster rates during winter compared to summer. One possible explanation is that only the species that can heavily rely on heterotrophic feeding or have high environmental tolerance are able to survive in temperate reefs at high latitude [12,51]. For example, in addition to the energy provided by their photosynthetic symbionts, corals can also meet their energy requirements by heterotrophic feeding on plankton, although this ability varies between species (e.g. see review [52]). Thus, another potential explanation for the unusual seasonality of calcification rates is a higher reliance on heterotrophy during winter when temperatures and light levels are low. The positive relationship between heterotrophic feeding and calcification rates is well documented, particularly for temperate corals [12,53], and seasonal variability in feeding has been shown previously, for example, in the symbiotic temperate coral Cladocora caespitosa in the Mediterranean Sea, which switches between autotrophy during summer and heterotrophy during winter [54]. Chlorophyll a levels were higher in winter compared with summer in Bremer Bay (figure 1d), and a positive correlation between calcification rates and chlorophyll a has been demonstrated previously [11,43]. Assuming the higher wintertime chlorophyll a corresponded to increased heterotrophy, this may have contributed to higher calcification rates during winter compared with summer by maintaining metabolic energy required for key growth processes, such as pHcf upregulation, tissue growth and composition, and organic matrix synthesis (e.g. see review [52]).
The higher rate of skeletal CaCO3 formation during winter resulted in a concurrent decline in [Ca2+]cf (figure 3e). During summer, the opposite occurred whereby a reduction in calcification rate resulted in higher [Ca2+]cf (albeit still 10% lower than the surrounding seawater), consistent with less Ca2+ being depleted during periods of lower calcification. The observed reduction in [Ca2+]cf relative to seawater values (10 to 30% depending on rates of calcification; figures 2b and 3e) is not surprising given that (i) [Ca2+]cf should decline as it is depleted from the cf during calcification (figure 4), and that (ii) [Ca2+]cf is most probably not limiting calcification in T. reniformis because it is far greater (approx. 8.3 to 9.8 mmol kg−1) than 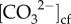 (approx. 0.7 to 0.95 mmol kg−1; figure 2). Thus, while elevating [Ca2+]cf above seawater concentrations is important for driving calcification in some coral species (such as P. damicornis and Galaxea fascicularis) [26,29], this does not appear to be the case for T. reniformis.
(approx. 0.7 to 0.95 mmol kg−1; figure 2). Thus, while elevating [Ca2+]cf above seawater concentrations is important for driving calcification in some coral species (such as P. damicornis and Galaxea fascicularis) [26,29], this does not appear to be the case for T. reniformis.
Multiple mechanisms may operate to transport calcium and protons between seawater and the cf. However, the active exchange of Ca2+ with H+ using the enzyme Ca-ATPase at the site of calcification has long been considered the primary mechanism for corals to upregulate both [Ca2+]cf and pHcf [17,48,55]. If Ca2+ upregulation were operational in these corals due to the enzyme Ca-ATPase exchange of Ca2+ with H+, we could expect simultaneously high [Ca2+]cf and pHcf caused by the pumping in of Ca2+ and the pumping out of H+ to elevate pHcf [29]. Yet the opposite is observed here, such that [Ca2+]cf was lowest when both pHcf and calcification rates were highest (figure 2). [Ca2+]cf may be initially elevated, but is then clearly outweighed by precipitation, given that [Ca2+]cf/[Ca2+]sw is less than 1. Furthermore, if seasonally higher rates of calcification were causing a systematic decline in pHcf due to an increase in protons when 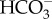 is converted to
is converted to 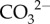 (i.e. instead of counter-balancing DICcf), then the lowest pHcf would have occurred when calcification rates were highest. Conversely, we show here that seasonally higher rates of calcification did not cause a systematic decline in pHcf, as would be expected if pHcf and [Ca2+]cf were controlled by the same processes (i.e. Ca-ATPase and CaCO3 precipitation). Thus, while the upregulation of [Ca2+]cf may still be occurring [26], the reduction in [Ca2+]cf when pH upregulation is highest nevertheless suggests that mechanisms other than the ATPase driven exchange of H+ for Ca2+ must be involved in the supply of Ca2+ and upregulation of pHcf (e.g. Ca-channels, Ca2+/Na+ exchange,
(i.e. instead of counter-balancing DICcf), then the lowest pHcf would have occurred when calcification rates were highest. Conversely, we show here that seasonally higher rates of calcification did not cause a systematic decline in pHcf, as would be expected if pHcf and [Ca2+]cf were controlled by the same processes (i.e. Ca-ATPase and CaCO3 precipitation). Thus, while the upregulation of [Ca2+]cf may still be occurring [26], the reduction in [Ca2+]cf when pH upregulation is highest nevertheless suggests that mechanisms other than the ATPase driven exchange of H+ for Ca2+ must be involved in the supply of Ca2+ and upregulation of pHcf (e.g. Ca-channels, Ca2+/Na+ exchange, 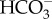 pumps; figure 4) [21,48].
pumps; figure 4) [21,48].
The seasonal dynamics of pHcf, 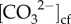 , [Ca2+]cf and calcification act to maintain relatively stable and elevated Ωcf (10.7 ± 0.5) year-round (figure 2). However, the wintertime Ωcf, for example, was far lower than it would be if constant levels of [Ca2+]cf were maintained (e.g. at seawater concentrations) or if [Ca2+]cf, were upregulated above seawater values as it was for OA-resistant P. damicornis corals in a laboratory study [26]. Thus, Ωcf is modulated by calcification through the depletion of [Ca2+]cf, such that decoupling between Ωcf and rates of calcification can occur. This would explain why the observed seasonally fluctuating growth rates do not mirror the relatively stable levels of Ωcf (figure 2). If this is true, it implies that bulk coral calcification rates may not depend strongly on the instantaneous rate of aragonite crystal growth (i.e. Ωcf), but rather on the supply of carbon to the site of calcification. Further, our results are consistent with recent work, albeit in tropical corals under both heat and acidification stress, which suggests an optimum threshold of Ωcf is required for calcification to occur [16,28], but Ωcf may not be the primary driver of calcification rates beyond this threshold. Thus, our results provide additional support to the hypothesis that maintaining a stable, yet elevated Ωcf, is likely to be a prerequisite for biomineralization.
, [Ca2+]cf and calcification act to maintain relatively stable and elevated Ωcf (10.7 ± 0.5) year-round (figure 2). However, the wintertime Ωcf, for example, was far lower than it would be if constant levels of [Ca2+]cf were maintained (e.g. at seawater concentrations) or if [Ca2+]cf, were upregulated above seawater values as it was for OA-resistant P. damicornis corals in a laboratory study [26]. Thus, Ωcf is modulated by calcification through the depletion of [Ca2+]cf, such that decoupling between Ωcf and rates of calcification can occur. This would explain why the observed seasonally fluctuating growth rates do not mirror the relatively stable levels of Ωcf (figure 2). If this is true, it implies that bulk coral calcification rates may not depend strongly on the instantaneous rate of aragonite crystal growth (i.e. Ωcf), but rather on the supply of carbon to the site of calcification. Further, our results are consistent with recent work, albeit in tropical corals under both heat and acidification stress, which suggests an optimum threshold of Ωcf is required for calcification to occur [16,28], but Ωcf may not be the primary driver of calcification rates beyond this threshold. Thus, our results provide additional support to the hypothesis that maintaining a stable, yet elevated Ωcf, is likely to be a prerequisite for biomineralization.
To explain how the modulation of the cf carbonate chemistry resulted in the observed calcification rates, we have integrated our findings with the literature [18,21,48] to form a conceptual model that includes our measurements of both aspects of Ωcf (i.e. 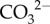 and Ca2+) for corals growing at high latitude (figure 4a,b). First, pH upregulation (removal of H+) raises the availability of
and Ca2+) for corals growing at high latitude (figure 4a,b). First, pH upregulation (removal of H+) raises the availability of 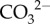 at the site of calcification and elevates Ωcf, thus promoting calcification (figure 4b) [15]. This is driven by (i) the diffusion of metabolic CO2 into the cf [21] and (ii) a shift in the equilibrium of DIC in favour of
at the site of calcification and elevates Ωcf, thus promoting calcification (figure 4b) [15]. This is driven by (i) the diffusion of metabolic CO2 into the cf [21] and (ii) a shift in the equilibrium of DIC in favour of 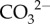 over bicarbonate
over bicarbonate 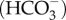 (figure 4b). CO2 is converted into bicarbonate using carbonic anhydrase (CA), producing additional H+ and
(figure 4b). CO2 is converted into bicarbonate using carbonic anhydrase (CA), producing additional H+ and 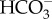 may be brought to the site of calcification using bicarbonate transporters (BAT) [49]. Elevating pH works in conjunction with the supply of DIC to increase the availability of
may be brought to the site of calcification using bicarbonate transporters (BAT) [49]. Elevating pH works in conjunction with the supply of DIC to increase the availability of 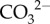 (figure 4b). Lastly, calcification occurs and Ca2+ is depleted from the cf, thereby causing a decrease in Ωcf (figure 4b). Thus, the same Ωcf can be achieved by either high
(figure 4b). Lastly, calcification occurs and Ca2+ is depleted from the cf, thereby causing a decrease in Ωcf (figure 4b). Thus, the same Ωcf can be achieved by either high 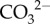 upregulation and high calcification (i.e. more [Ca2+]cf depleted), or low
upregulation and high calcification (i.e. more [Ca2+]cf depleted), or low 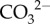 upregulation and low calcification (i.e. less [Ca2+]cf depleted; figure 4b).
upregulation and low calcification (i.e. less [Ca2+]cf depleted; figure 4b).
(c). Drivers and mechanisms of calcification during the 2016 El Niño winter cooling
The anomalous cold temperatures during the 2016 El Niño winter provide further insight into the extreme cold periods that are likely to threaten high-latitude corals more frequently than tropical corals [56]. Turbinaria reniformis showed significant cold stress (Fv/Fm: 0.47 ± 0.01) when temperatures dropped 1°C cooler (approx. 16°C) than the normal winter minimum (approx. 17°C). However, T. reniformis calcification rates did not appear to be affected by the El Niño-driven cold stress (figure 2), given that lower Fv/Fm did not correspond to significantly lower calcification rates (i.e. compared to the 2015 winter). The high calcification rates during this period of El Niño-driven cold stress were supported via unusually high upregulation of wintertime DICcf. This could potentially be explained by an increase in metabolic DIC supply via increased heterotrophy during sub-optimal or stressful conditions [57].
Our results show, however, that the higher levels of DICcf during the El Niño cold period were still counter-regulated systematically by lower pHcf during winter in order to maintain stable Ωcf and elevated 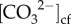 to support high rates of calcification (figure 3). Thus, Ωcf during the 2016 winter was lower than it would be if the mechanism of pHcf and DICcf counter-regulation were not operational. These results have important implications for high-latitude coral calcification during periods of ENSO-driven cold stress [56], as they demonstrate that corals are able to biologically modulate the carbonate chemistry of the cf, with a shift to higher DICcf, to maintain calcification rates.
to support high rates of calcification (figure 3). Thus, Ωcf during the 2016 winter was lower than it would be if the mechanism of pHcf and DICcf counter-regulation were not operational. These results have important implications for high-latitude coral calcification during periods of ENSO-driven cold stress [56], as they demonstrate that corals are able to biologically modulate the carbonate chemistry of the cf, with a shift to higher DICcf, to maintain calcification rates.
(d). High-latitude coral calcification mechanisms and the future of high-latitude corals
Under future climate change, rising temperatures at high latitude could potentially have a positive effect on calcification rates, particularly during winter, when growth rates are thought to be limited by lower temperatures [10]. We show here, however, that seasonally lower wintertime temperatures and light levels did not limit the calcification rates of T. reniformis corals at their latitudinal limits. Furthermore, these corals were able to maintain high rates of calcification despite an ENSO-driven cold stress event. The absence of any clear relationship between temperature and coral calcification rate can be explained by (i) higher chlorophyll a during winter compared with summer providing the coral nutrition for increased heterotrophy, and (ii) the corals' ability to modulate pHcf in response to seasonally variable DICcf, such that seasonal changes in bulk rates of calcification appear to depend strongly on 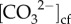 , rather than Ωcf or temperature. Thus, warmer seawater temperatures due to continued ocean warming may not necessarily promote faster rates of calcification at high latitude, and are not necessarily required to support high-latitude coral calcification during winter [32]. Moreover, marine heatwaves have recently caused mass bleaching of high-latitude reefs [5]. Thus, further work is required to establish how high-latitude coral calcification rates will respond to the combined effects of OA, warming and marine heatwaves.
, rather than Ωcf or temperature. Thus, warmer seawater temperatures due to continued ocean warming may not necessarily promote faster rates of calcification at high latitude, and are not necessarily required to support high-latitude coral calcification during winter [32]. Moreover, marine heatwaves have recently caused mass bleaching of high-latitude reefs [5]. Thus, further work is required to establish how high-latitude coral calcification rates will respond to the combined effects of OA, warming and marine heatwaves.
Supplementary Material
Supplementary Material
Acknowledgements
Thanks to A. Comeau, K. Rankenburg and J. Pablo D'Olivo for assistance in the coral isotope and mass spectrometry laboratories. We are grateful to J. Falter, Craig and Anne Lebens at Bremer Bay Dive and all volunteers (H. Clarke, C. Bowyer, A. Kuret, M. Cuttler, S. Bell, E. Lester, Y. Mulders, G. Ellwood and C. Krause) for assistance in the field. The authors acknowledge the facilities and the scientific and technical assistance of the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy, Characterisation & Analysis, The University of Western Australia, a facility funded by the University, State and Commonwealth Governments.
Ethics
The activities for this study were conducted under permission from the Government of Western Australia Department of Parks and Wildlife (DPaW) with research permits and licence to take fauna for scientific purposes (nos. SF010109 and SF010963) and the Government of Western Australia Department of Fisheries with research permits and exemption from the Fish Resources Management Act 1994 (nos. 2944 and 2410). All local regulations and permit requirements were followed. This study did not require clearance by the UWA Animal Ethics Committee.
Data accessibility
Data are available at the Zenodo Digital Repository (http://dx.doi.org/10.5281/zenodo.1220102).
Authors' contributions
C.L.R. designed the experiments, conducted all fieldwork and laboratory work, analysed the data, and wrote the manuscript. V.S contributed to experimental design, participated in fieldwork and guided data analysis. T.M.D. conducted laboratory work, and guided data analysis. M.T.M. contributed to experimental design and participated in fieldwork. All authors contributed to manuscript drafts.
Competing interests
There are no competing financial interests.
Funding
This research was supported by funding provided by an ARC Laureate Fellowship (LF120100049) awarded to Prof. M. McCulloch, the ARC Centre of Excellence for Coral Reef Studies (CE140100020), and an Australian Post Graduate Scholarship awarded to C. Ross.
References
- 1.Reaka-Kudla ML. 1997. The global biodiversity of coral reefs: a comparison with rain forests. In Biodiversity II: understanding and protecting our biological resources (eds Reaka- Kudla M, Wilson DE, Wilson EO), pp. 83–108. Washington, DC: Joseph Henry Press. [Google Scholar]
- 2.Veron J. 1995. Corals in space and time: the biogeography and evolution of the scleractinia, pp. 1–321. Ithaca, NY: Cornell University Press. [Google Scholar]
- 3.Hoegh-Guldberg O, et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. ( 10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- 4.Hughes TP, et al. 2017. Global warming and recurrent mass bleaching of corals. Nature 543, 373–377. ( 10.1038/nature21707) [DOI] [PubMed] [Google Scholar]
- 5.Le Nohaïc M, Ross CL, Cornwall CE, Comeau S, Lowe R, McCulloch MT, Schoepf V. 2017. Marine heatwave causes unprecedented regional mass bleaching of thermally resistant corals in northwestern Australia. Sci. Rep. 7, 14999 ( 10.1038/s41598-017-14794-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marubini F, Ferrier-Pages C, Cuif J-P. 2003. Suppression of skeletal growth in scleractinian corals by decreasing ambient carbonate-ion concentration: a cross-family comparison. Proc. R. Soc. Lond. B 270, 179–184. ( 10.1098/rspb.2002.2212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comeau S, Edmunds PJ, Spindel NB, Carpenter RC. 2014. Fast coral reef calcifiers are more sensitive to ocean acidification in short-term laboratory incubations. Limnol. Oceanogr. 59, 1081–1091. ( 10.4319/lo.2014.59.3.1081) [DOI] [Google Scholar]
- 8.Sawall Y, Al-Sofyani A, Hohn S, Banguera-Hinestroza E, Voolstra CR, Wahl M. 2015. Extensive phenotypic plasticity of a Red Sea coral over a strong latitudinal temperature gradient suggests limited acclimatization potential to warming. Sci. Rep. 5, 8940 ( 10.1038/srep08940) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuffner IB, Hickey TD, Morrison JM. 2013. Calcification rates of the massive coral Siderastrea siderea and crustose coralline algae along the Florida Keys (USA) outer-reef tract. Coral Reefs 32, 987–997. ( 10.1007/s00338-013-1047-8) [DOI] [Google Scholar]
- 10.Crossland C. 1984. Seasonal variations in the rates of calcification and productivity in the coral Acropora formosa on a high-latitude reef. Mar. Ecol. Prog. Ser. 15, 135–140. ( 10.3354/meps015135) [DOI] [Google Scholar]
- 11.Courtney TA, et al. 2017. Environmental controls on modern scleractinian coral and reef-scale calcification. Sci. Adv. 3, e1701356 ( 10.1126/sciadv.1701356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller MW. 1995. Growth of a temperate coral: effects of temperature, light, depth, and heterotrophy. Mar. Ecol. Prog. Ser. 122, 217–225. ( 10.3354/meps122217) [DOI] [Google Scholar]
- 13.Edmunds PJ. 2011. Zooplanktivory ameliorates the effects of ocean acidification on the reef coral Porites spp. Limnol. Oceanogr. 56, 2402–2410. ( 10.4319/lo.2011.56.6.2402) [DOI] [Google Scholar]
- 14.Foster T, Short J., Falter JL, Ross C, McCulloch MT. 2014. Reduced calcification in Western Australian corals during anomalously high summer water temperatures. J. Exp. Mar. Biol. Ecol. 461, 133–143. ( 10.1016/j.jembe.2014.07.014) [DOI] [Google Scholar]
- 15.McCulloch MT, Falter JL, Trotter J, Montagna P. 2012. Coral resilience to ocean acidification and global warming through pH up-regulation. Nat. Clim. Chang. 2, 1–5. ( 10.1038/nclimate1473) [DOI] [Google Scholar]
- 16.Schoepf V, Jury CP, Toonen R, McCulloch M. 2017. Coral calcification mechanisms facilitate adaptive response to ocean acidification. Proc. R. Soc. B 284, 20172117 ( 10.1098/rspb.2017.2117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venn A, Tambutté É, Holcomb M, Allemand D, Tambutté S. 2011. Live tissue imaging shows reef corals elevate pH under their calcifying tissue relative to seawater. PLoS ONE 6, e20013 ( 10.1371/journal.pone.0020013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCulloch MT, D'Olivo Cordero JP, Falter J, Holcomb M, Trotter JA. 2017. Coral calcification in a changing World: the interactive dynamics of pH and DIC up-regulation. Nat. Commun. 8, 15686 ( 10.1038/ncomms15686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wall M, Ragazzola F, Foster LC, Form A, Schmidt DN. 2015. Enhanced pH up-regulation enables the cold-water coral Lophelia pertusa to sustain growth in aragonite undersaturated conditions. Biogeosci. Discuss. 12, 6757–6781. ( 10.5194/bgd-12-6757-2015) [DOI] [Google Scholar]
- 20.Georgiou L, Falter JL, Trotter J, Kline DI, Holcomb M, Dove SG, Hoegh-Guldberg O, McCulloch MT. 2015. pH homeostasis during coral calcification in a free ocean CO2 enrichment (FOCE) experiment, Heron Island reef flat, Great Barrier Reef. Proc. Natl Acad. Sci. USA 112, 13 219–13 224. ( 10.1073/pnas.1505586112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen AL, McConnaughey TA. 2003. Geochemical perspectives on coral mineralization. Rev. Mineral. Geochem. 54, 151–187. ( 10.2113/0540151) [DOI] [Google Scholar]
- 22.Burton EA, Walter LM. 1987. Relative precipitation rates of aragonite and Mg calcite from seawater: temperature or carbonate ion control? Geology 15, 111 ( 10.1130/0091-7613(1987)15%3C111:RPROAA%3E2.0.CO;2) [DOI] [Google Scholar]
- 23.Ross CL, Falter JL, McCulloch MT. 2017. Active modulation of the calcifying fluid carbonate chemistry (δ11B, B/Ca) and seasonally invariant coral calcification at sub-tropical limits. Sci. Rep. 7, 1–11. ( 10.1038/s41598-017-14066-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holcomb M, Venn AA, Tambutté E, Tambutté S, Allemand D, Trotter J, McCulloch M. 2014. Coral calcifying fluid pH dictates response to ocean acidification. Sci. Rep. 4, 5207 ( 10.1038/srep05207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeCarlo TM, D'Olivo JP, Foster T, Holcomb M, Becker T, McCulloch MT. 2017. Coral calcifying fluid aragonite saturation states derived from Raman spectroscopy. Biogeosci. 14, 5253–5269. ( 10.5194/bg-14-5253-2017) [DOI] [Google Scholar]
- 26.DeCarlo TM, Comeau S, Cornwall CE, Mcculloch MT. 2018. Coral resistance to ocean acidification linked to increased calcium at the site of calcification. Proc. R. Soc. B 285, 20180564 ( 10.1098/rspb.2018.0564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wall M, Fietzke J, Schmidt GM, Fink A, Hofmann LC, De Beer D, Fabricius KE. 2016. Internal pH regulation facilitates in situ long-term acclimation of massive corals to end-of-century carbon dioxide conditions. Sci. Rep. 6, 30688 ( 10.1038/srep30688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D'Olivo JP, McCulloch MT. 2017. Response of coral calcification and calcifying fluid composition to thermally induced bleaching stress. Sci. Rep. 7, 2207 ( 10.1038/s41598-017-02306-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Horani FA, Al-Moghrabi SM, de Beer D. 2003. The mechanism of calcification and its relation to photosynthesis and respiration in the scleractinian coral Galaxea fascicularis. Mar. Biol. 142, 419–426. ( 10.1007/s00227-002-0981-8) [DOI] [Google Scholar]
- 30.IMOS. 2018. IMOS ocean colour (2015 and 2016). See http://oceancurrent.imos.org.au/oceancolour.php (accessed 11 April 2018).
- 31.Veron JEN, Marsh LM. 1988. Hermatypic corals of Western Australia: records and annotated species list. Rec. West Aust. Mus. 29, 1–136. [Google Scholar]
- 32.Ross CL, Falter JL, Schoepf V, McCulloch MT. 2015. Perennial growth of hermatypic corals at Rottnest Island, Western Australia (32°S). PeerJ 3, e781 ( 10.7717/peerj.781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bak R. 1973. Coral weight increment in situ. A new method to determine coral growth. Mar. Biol. 20, 45–49. ( 10.1007/BF00387673) [DOI] [Google Scholar]
- 34.Trotter J, et al. 2011. Quantifying the pH ‘vital effect’ in the temperate zooxanthellate coral Cladocora caespitosa: validation of the boron seawater pH proxy. Earth Planet. Sci. Lett. 303, 163–173. ( 10.1016/j.epsl.2011.01.030) [DOI] [Google Scholar]
- 35.Dickson AG. 1990. Thermodynamics of the dissociation of boric acid in synthetic seawater from 273.15 to 318.15 K. Deep Sea Res. Part A. Oceanogr. Res. Pap. 37, 755–766. ( 10.1016/0198-0149(90)90004-F) [DOI] [Google Scholar]
- 36.Klochko K, Kaufman AJ, Yao W, Byrne RH, Tossell JA. 2006. Experimental measurement of boron isotope fractionation in seawater. Earth Planet. Sci. Lett. 248, 261–270. ( 10.1016/j.epsl.2006.05.034) [DOI] [Google Scholar]
- 37.Holcomb M, DeCarlo TM, Gaetani GA, McCulloch MT. 2016. Factors affecting B/Ca ratios in synthetic aragonite. Chem. Geol. 437, 67–76. ( 10.1016/j.chemgeo.2016.05.007) [DOI] [Google Scholar]
- 38.Marshall AT, Clode P. 2004. Calcification rate and the effect of temperature in a zooxanthellate and an azooxanthellate scleractinian reef coral. Coral Reefs 23, 218–224. ( 10.1007/s00338-004-0369-y) [DOI] [Google Scholar]
- 39.Vajed Samiei J, Saleh A, Mehdinia A, Shirvani A, Kayal M. 2015. Photosynthetic response of Persian Gulf acroporid corals to summer versus winter temperature deviations. PeerJ 3, e1062 ( 10.7717/peerj.1062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gattuso J-P, Allemand D, Frankignoulle M. 1999. Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: a review on Interactions and control by carbonate chemistry. Am. Zool. 39, 160–183. ( 10.1093/icb/39.1.160) [DOI] [Google Scholar]
- 41.Jokiel PL, Coles SL. 1977. Effects of temperature on the mortality and growth of hawaiian reef corals*. Mar. Biol. 208, 201–208. ( 10.1007/BF00402312) [DOI] [Google Scholar]
- 42.Carricart-Ganivet JP. 2004. Sea surface temperature and the growth of the West Atlantic reef-building coral Montastraea annularis. J. Exp. Mar. Bio. Ecol. 302, 249–260. ( 10.1016/j.jembe.2003.10.015) [DOI] [Google Scholar]
- 43.Lough JM, Cantin NE, Benthuysen JA, Cooper TF. 2016. Environmental drivers of growth in massive Porites corals over 16 degrees of latitude along Australia's northwest shelf. Limnol. Oceanogr. 61, 684–700. ( 10.1002/lno.10244) [DOI] [Google Scholar]
- 44.Clausen CD, Roth AA. 1975. Effect of temperature and temperature adaption on calcification rate in the hermatypic coral Pocillopora damicornis. Mar. Biol. 33, 93–100. ( 10.1007/BF00390713) [DOI] [Google Scholar]
- 45.Roik A, Roder C, Röthig T, Voolstra CR. 2015. Spatial and seasonal reef calcification in corals and calcareous crusts in the central Red Sea. Coral Reefs 35, 681–693. ( 10.1007/s00338-015-1383-y) [DOI] [Google Scholar]
- 46.Goffredo S, Caroselli E, Mattioli G, Pignotti E, Dubinsky Z, Zaccanti F. 2009. Inferred level of calcification decreases along an increasing temperature gradient in a Mediterranean endemic coral. Limnol. Oceanogr. 54, 930–937. ( 10.4319/lo.2009.54.3.0930) [DOI] [Google Scholar]
- 47.Warner ME, Chilcoat GC, McFarland FK, Fitt WK. 2002. Seasonal fluctuations in the photosynthetic capacity of photosystem II in symbiotic dinoflagellates in the Caribbean reef-building coral Montastraea. Mar. Biol. 141, 31–38. ( 10.1007/s00227-002-0807-8) [DOI] [Google Scholar]
- 48.Allemand D, Ferrier-Pagès C, Furla P, Houlbrèque F, Puverel S, Reynaud S, Tambutté É, Tambutté S, Zoccola D. 2004. Biomineralisation in reef-building corals: from molecular mechanisms to environmental control. C.R. Palevol 3, 453–467. ( 10.1016/j.crpv.2004.07.011) [DOI] [Google Scholar]
- 49.Zoccola D, et al. 2015. Bicarbonate transporters in corals point towards a key step in the evolution of cnidarian calcification. Sci. Rep. 5, 9983 ( 10.1038/srep09983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furla P, Galgani I, Durand I, Allemand D. 2000. Sources and mechanisms of inorganic carbon transport for coral calcification and photosynthesis. J. Exp. Biol. 203, 3445–3457. [DOI] [PubMed] [Google Scholar]
- 51.Mizerek TL, Baird AH, Beaumont LJ, Madin JS. 2016. Environmental tolerance governs the presence of reef corals at latitudes beyond reef growth. Glob. Ecol. Biogeogr. 25, 979–987. ( 10.1111/geb.12459) [DOI] [Google Scholar]
- 52.Houlbrèque F, Ferrier-Pagès C. 2009. Heterotrophy in tropical scleractinian corals. Biol Rev. 84, 1–17. ( 10.1111/j.1469-185X.2008.00058.x) [DOI] [PubMed] [Google Scholar]
- 53.Rodolfo-Metalpa R, Peirano A, Houlbrèque F, Abbate M, Ferrier-Pagès C. 2008. Effects of temperature, light and heterotrophy on the growth rate and budding of the temperate coral Cladocora caespitosa. Coral Reefs 27, 17–25. ( 10.1007/s00338-007-0283-1) [DOI] [Google Scholar]
- 54.Ferrier-Pages C, Peirano A, Abbate M, Cocito S, Negri A, Rottier C, Riera P, Rodolfo-Metalpa R, Reynaud S. 2011. Summer autotrophy and winter heterotrophy in the temperate symbiotic coral Cladocora caespitosa. Limnol. Oceanogr. 56, 1429–1438. ( 10.4319/lo.2011.56.4.1429) [DOI] [Google Scholar]
- 55.McConnaughey TA. 1994. Ion transport and the generation of biomineral supersaturation. In 7th international symposium biomineralization (eds Allemand D, Cuif J-P), pp. 1–18. Monaco: Institut Oceanographique Monaco. [Google Scholar]
- 56.Saxby T, Dennison WC, Hoegh-Guldberg O. 2003. Photosynthetic responses of the coral Montipora digitata to cold temperature stress. Mar. Ecol. Prog. Ser. 248, 85–97. ( 10.3354/meps248085) [DOI] [Google Scholar]
- 57.Ezzat L, Towle E, Irisson J-O, Langdon C, Ferrier-Pagès C. 2015. The relationship between heterotrophic feeding and inorganic nutrient availability in the scleractinian coral T. reniformis under a short-term temperature increase. Limnol. Oceanogr. 61, 89–102. ( 10.1002/lno.10200) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available at the Zenodo Digital Repository (http://dx.doi.org/10.5281/zenodo.1220102).