Abstract
Repeated use of the same areas may benefit animals as they exploit familiar sites, leading to consistent home ranges over time that can span generations. Changing risk landscapes may reduce benefits associated with home range fidelity, however, and philopatric animals may alter movement in response to new pressures. Despite the importance of range changes to ecological and evolutionary processes, little tracking data have been collected over the long-term nor has range change been recorded in response to human pressures across generations. Here, we investigate the relationships between ecological, demographic and human variables and elephant ranging behaviour across generations using 16 years of tracking data from nine distinct female social groups in a population of elephants in northern Kenya that was heavily affected by ivory poaching during the latter half of the study. Nearly all groups—including those that did not experience loss of mature adults—exhibited a shift north over time, apparently in response to increased poaching in the southern extent of the study area. However, loss of mature adults appeared to be the primary indicator of range shifts and expansions, as generational turnover was a significant predictor of range size increases and range centroid shifts. Range expansions and northward shifts were associated with higher primary productivity and lower poached carcass densities, while westward shifts exhibited a trend to areas with higher values of primary productivity and higher poached carcass densities relative to former ranges. Together these results suggest a trade-off between resource access, mobility and safety. We discuss the relevance of these results to elephant conservation efforts and directions meriting further exploration in this disrupted society of a keystone species.
Keywords: home range, ivory poaching, landscape of fear, matriarch, radio-tracking
1. Introduction
Spatial philopatry, animals staying in or habitually returning to an area, is a widespread phenomenon found across diverse species [1–3]. The greater familiarity of particular areas or routes is thought to enhance the ability of animals to effectively exploit sites [4,5]. Some species show consistency over multiple generations, both in migration routes and in seasonal use of the same sites [6]. For example, southern right whales exhibit maternally inherited site fidelity to summer feeding grounds off South Georgia [7], and the expansive pronghorn antelope migration in the Yellowstone ecosystem passes annually through the same narrow corridor [8]. In long-lived species with overlapping generations, the role of social learning is thought to be critical for the maintenance of such consistent long-term movement patterns [7,9,10].
Human pressures present challenges to ranging animals [11–13]. As wildlife are exposed to landscape change or intensified risk on the landscape, the advantages of site familiarity may be lost or outweighed by the risks associated with those sites [14,15]. For animals that exhibit plasticity in their ranging, this may lead to range shifts or expansions as animals seek out new areas or to range constrictions as animals seek to avoid areas [16,17]. Changes in movement carry broad implications for life-history evolution, community dynamics and species management, but how risk from humans influences spatial behaviour over the long-term is not well understood [18]. Some work has investigated ranging behaviour in relation to changing levels of risk for specific individuals over time [1,18,19], but rarely have longer term investigations been made.
African savannah elephants (Loxodonta africana africana) are keystone generalist herbivores that range widely for seasonally variable resources [20,21] and have exceptional spatial memory [22]. Female elephants form stable core social groups with overlapping generations that facilitate prolonged learning [23–25]. This close knit social structure is thought to have evolved in part to enable elephants to successfully exploit large and complex landscapes. Transmission of knowledge across generations is hypothesized to be a key aspect of this process [26]. Range use might, therefore, be expected to be consistent between generations within families [27], yet substantial shifts in range use over decades have been documented in elephant populations [28]. Comparison of movement across generations in elephants offers the opportunity to gain insight into long-term patterns in a widely ranging, social species.
Over the last decade poaching for ivory has increased across African elephant range leading to population declines [29–32]. Whether elephants are changing their use of space in response to increased risk remains undetermined, but is critical to understand in a species dependent on complex movement strategies [33]. Given the importance of movement to survival and access to resources like water and fresh forage for elephants [26,27], the influence of poaching on movement behaviour may have consequences for population processes. Additionally, changes in elephant movement over time carry ecosystem level implications, as elephant herbivory and seed dispersal are linked to ecological functioning [34,35].
The Samburu elephant population of northern Kenya has been studied continuously since 1997 [36], a period that included high variability in anthropogenic threats to elephants. Disruption in the form of a severe drought and a persistent period of ivory poaching between 2009 and 2013 resulted in high mortality, especially among adult elephants [29,37]. However, this mortality was not uniform across the population, and it is hypothesized that poaching pressure across this system is variable [38] and drives different behaviours that affect survival. The elephants in disrupted families have been studied in an ongoing project documenting behavioural responses to social disruption caused by illegal killing [39–41]. Here, we compare the ranging behaviour of female elephants from groups that have been disrupted with those of their older maternal relatives prior to the disruption to gain greater insight into range fidelity and range change. Specifically, we asked (1) whether ranges changed over time, (2) how younger generation ranges related to those of their older relatives and (3) what ecological, human or demographic variables might account for differences. We discuss the implications of our findings for elephant response to human predation and for elephant conservation in large landscapes.
2. Material and methods
(a). Study site
This study is part of a long-term individual-based elephant monitoring project centred in Samburu and Buffalo Springs National Reserves in northern Kenya within the Laikipia–Samburu ecosystem (0.3–2.0° N, 36.2–38.3° E) [36] (figure 1). The animals using the unfenced reserves are a part of the second largest elephant population in Kenya [42]. Laikipia–Samburu is made up of a patchwork of land use types including community conservancies, human settlements, agriculture and protected areas [38], and has been monitored intensively for poaching since 2002 as a part of the Convention on International Trade in Endangered Species' (CITES) Monitoring Illegal Killing of Elephants (MIKE) programme [43].
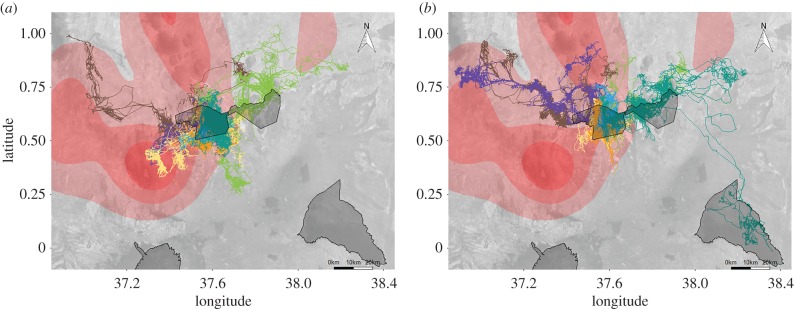
Figure 1.
Movement tracks plotted from earlier (a) and later (b) generations from seven groups collared between 2001 and 2017 (data from groups where only one generation was tracked are not shown). Lines represent 20 (a) and 18 (b) tracked years on a Google Maps base map. Outlines demarcate national reserves and parks, with the two central outlines demarcating the Samburu and Buffalo Springs (left) and Shaba (right) National Reserves complex. Underlying red contours represent poached carcass density during the period when the later generation was tracked (2013–2017). Carcass data were not available for the ecosystem encompassing Meru National Park in the southeast corner.
(b). Social and demographic data
The elephants that use the reserves are monitored by field teams that survey the parks most days along set routes [44]. Records of individuals are maintained through a photo-identification system, and the ages and family histories of most animals are known [37,45]. Analyses of association data recorded during daily surveys were used to define social groups within the hierarchical society [44]. The most cohesive and closely bonded social level in elephant society is the core group, often but not always a family unit of close maternal relatives and their offspring [23,39,44–46]. Members of inter-generational pairs of tracked elephants in this study belonged to the same core social group, such that each pair represented a distinct core group over different time periods.
(c). Movement data
GPS collars recording hourly positions were fitted on immobilized elephants according to protocol of the government of Kenya, administered by a Kenya Wildlife Service veterinary team jointly with the Save the Elephants field team. After collection, movement datasets were cleaned for errors by removing coordinates that could only be reached with speeds exceeding 10 km h−1 (deemed biologically unrealistic), duplicate and incomplete records. For analyses, data were organized into annual datasets with start dates that maximized the number of sampled days in each dataset. Annual datasets had high fix success, averaging 91.5% (ranging from 76.6 to 98.0%) of expected hourly coverage (table 1). The female with the lowest fix success (Amayeta) died 2 months prior to a complete year of tracking. Inter-generational pairs of collared elephants in this study represented disrupted families and included five mother–daughter pairs and two grandmother–granddaughter pairs (table 1). For comparison, we included movement records from two families that did not experience generational turnover that were continuously tracked over the same period, such that nine family lineages were represented in this study.
Table 1.
Individuals tracked over the course of the study with birth years in parentheses. The Royals dataset represents three alternately collared relatives that were in the same core social unit throughout the study. See main text for more detail on group characterizations. Distinct core groups correspond to consistent colours across figures.
| radio-collared female | no. years analysed | period tracked | range of combined adult years in group | percent coordinate fix success | relationship (core group) |
|---|---|---|---|---|---|
| Rosemary (1966) | 4 | 2002–2006 | 84–93 | 89.3 | mother–daughter (Spice Girls) |
| Nutmeg (1995) | 3 | 2014–2017 | 76–84 | 96.4 | |
| Maua (1972) | 4 | 2002–2013 | 46–64 | 97.2 | mother–daughter (Flowers) |
| Orchid (2004) | 2 | 2015–2017 | 60–63 | 97.1 | |
| Goya (1960) | 2 | 2001–2003 | 99–103 | 93.9 | mother–daughter (Artists) |
| Flaubert (1989) | 3 | 2013–2016 | 128–138 | 92.3 | |
| Aztec (1975) | 1 | 2003–2004 | 120 | 98.0 | grandmother–granddaughter (American Indians) |
| Amayeta (2000) | 1 | 2013–2014 | 52 | 76.6 | |
| Amina (1956) | 2 | 2002–2004 | 123–127 | 80.6 | grandmother–granddaughter (Swahili Ladies) |
| Habiba (2001) | 3 | 2013–2016 | 12–26 | 92.4 | |
| Maya (1976) | 3 | 2002–2005 | 50–93 | 87.4 | mother–daughter (First Ladies) |
| Salma (1999) | 3 | 2014–2017 | 34–38 | 97.8 | |
| Neptune (1966) | 4 | 2001–2008 | 121–181 | 86.5 | mother–daughter (Planets) |
| Luna (2000) | 3 | 2013–2016 | 54–60 | 95.6 | |
| Jerusalem (1968) | 7 | 2003–2014 | 121–194 | 91.2 | (Biblical Towns) |
| Cleopatra, Anastasia, Anabelle (1965, 1973, 1986) | 12 | 2001–2017 | 122–182 | 91.6 | (Royals) |
(d). Analyses
Continuous time stochastic process models that account for inherent autocorrelation were fitted to annual tracking datasets for each dataset in the study (n = 17; Cleopatra and Anastasia are closely associated sisters that were alternately collared and were, therefore, considered a single dataset) to estimate annual autocorrelated kernel density estimation (AKDE) home ranges [47,48]. We estimated both 95% (general) and 50% (core) AKDE home ranges [33]. AKDE home range estimation is robust to inconsistencies in sampling schedules [47] and, therefore, suited to this tracking dataset that spanned several years with variable fix successes. Because tracking datasets varied across individual elephants, we conducted home range analyses on each individual year. Analyses were done using the package ctmm in R v. 3.4.2 [47,49].
To understand whether and how annual home ranges within families change over time, we constructed two sets of normally distributed hierarchical models predicting the response variables latitudinal centroid of home range, longitudinal centroid of home range and home range size. The first set of models predicted these response variables as a function of time to ascertain if range changes were occurring over time, with individual elephant as a random effect and the day on which tracking began as the single predictor variable. The second set of models used the difference in centroids or the difference in home range size between pairs of annual home ranges within a core group over different years (later minus earlier). This set included covariates corresponding to differences in ecological, human, demographic and control variables characterizing the different annual ranges of the focal groups. Specifically, we calculated the difference in mean normalized difference vegetation index values between the former and later annual ranges using data from the MODIS satellite at 250 m spatial resolution and 16-day temporal resolution (https://lpdaac.usgs.gov; product MOD13Q1) averaged over both range areas during the later period (NDVI); the difference in poaching carcass density between the two ranges during the later period calculated as the number of illegally killed carcasses [43] divided by the home range size (poaching); the difference in the combined age of core group adults between the two periods calculated as the sum of the ages of adults in a core group where elephants were considered adults at breeding age (age adults); the difference in the number of coordinates collected between the two datasets (fixes); and whether each comparison of annual home ranges was inter-generational, where the covariate inter-generational was assigned as 0 when the comparison of annual ranges was within one generation (i.e. same individual or among females in the Royals group) and 1 when the comparison was across different generations before and after the older tracked individual died (i.e. mother–daughter or grandmother–granddaughter pairs). As with response variables, all covariates that compared conditions between ranges during the later time period were defined as the value for the later range minus the value for the former range, such that positive differences indicated comparatively higher values in the later range. This structure allowed insight into how later conditions changed between the two ranges, not how conditions changed over time. We excluded Orchid's most recent range from this comparative analysis because she ventured into a different ecosystem for which we did not have poaching data (figure 1). We included core group identity as a random effect, and standardized continuous predictor variables prior to running models for ease of interpretation. No covariates were correlated above r = |0.5|.
3. Results
The average size of annual ranges among all females studied was 1690.5 km2 (s.d. = 2660.9 km2) for 95% home ranges and 396.2 km2 (s.d. = 688.2 km2) for 50% home ranges. Among groups that spanned more than one generation, ranges of older generations tended to be smaller at 1326.2 km2 (s.d. = 1691.2 km2) for 95% home ranges and 285.4 km2 (s.d. = 360.8 km2) for 50% home ranges when compared to their younger relatives, which averaged 3267.2 km2 (s.d. = 3984.3 km2) for 95% home ranges and 811.7 km2 (s.d. = 1061.3 km2) for 50% home ranges. Ranges in the early part of the study (collared prior to 2009 when poaching intensified) versus the latter part of the study (collared in 2009 or later) averaged 1148.7 km2 (s.d. = 1448.2 km2) for 95% ranges and 248.8 km2 (s.d. = 310.2 km2) for 50% ranges when compared to 2213.7 km2 (s.d. = 3400.2 km2) for 95% ranges and 538.4 km2 (s.d. = 901.0 km2) for 50% ranges, respectively.
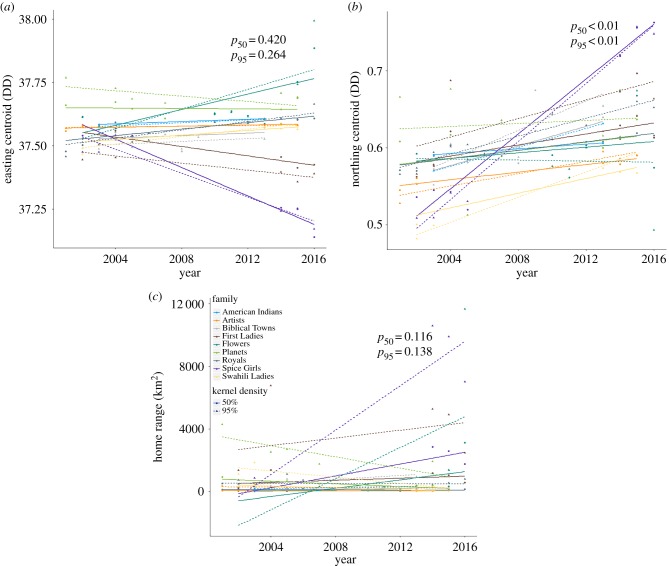
There was a clear latitudinal shift north in the home ranges of the elephants collared over time. Shifts in longitudinal centroids exhibited inconsistency, with some families maintaining similar easting centroids and others shifting west or east with time (figures 1 and 2; table 2). Changes in home range size over time were found in the behaviour of about half of the core groups (figure 2), with the spectrum of responses (increasing, decreasing or staying the same) resulting in no significant changes detected in the overall model (table 2).
Figure 2.
Easting (a) and northing (b) range centroids and home range size (c) across the years of the study for 95% and 50% home ranges. Ranges shifted north over the study across the sample and increased in size for about half of the studied groups. Longitudinal shifts were mixed among families. Distinct colours correspond to different core groups and are consistent across panels and with figure 1.
Table 2.
Coefficient estimates (s.e.) and p-values for home range characteristics as a function of tracking year demonstrate a shift north in the home range centroid. Home range for this analysis was log-transformed. p-Values less than 0.1 are italicized.
| response variable | centroid easting (95%) | centroid easting (50%) | centroid northing (95%) | centroid northing (50%) | home range size (95%) | home range size (50%) |
|---|---|---|---|---|---|---|
| date coefficient estimate (s.e.) | 0.015 (0.014) p = 0.264 |
0.009 (0.011) p = 0.42 |
0.030 (0.009) p < 0.01 |
0.019 (0.006) p < 0.01 |
0.257 (0.170) p = 0.138 |
0.298 (0.187) p = 0.116 |
We found evidence for range shifts between generations within core groups, with 50% and 95% home ranges exhibiting similar changes (table 3). We analysed ecological and human properties that were associated with these shifts by examining differences between conditions in current and former ranges during the latter period. When controlling for the number of fixes between years, shifts north and west were associated with accessing areas with higher primary productivity. Later ranges were associated with higher poaching carcass density for westward shifts in the 50% range model and lower poaching carcass density for northward shifts in the 95% range model, though the latter result was not significant at the α = 0.05 level. Home range centroid shifts were significantly related to whether comparisons were inter-generational, with shifts north and west occurring between relative to within generations and younger families tending eastward.
Table 3.
Coefficient estimates (s.e.) and p-values for models of home range changes, with those with p-values less than 0.1 italicized. Covariates represent the value in the later range minus the value in the earlier range for pairwise comparisons, such that positive values indicate an increase in the covariate in the later range was associated with an increase in the response variable.
| covariate | easting centroid (95%) | easting centroid (50%) | northing centroid (95%) | northing centroid (50%) | home range size (95%) | home range size (50%) |
|---|---|---|---|---|---|---|
| age of adults | 0.008 (0.007) p = 0.234 |
−0.013 (0.007) p = 0.052 |
−0.005 (0.005) p = 0.336 |
0.001 (0.004) p = 0.844 |
−418.30 (161.65) p < 0.01 |
83.24 (50.43) p = 0.101 |
| poaching carcass density | 0.000 (0.005) p = 0.957 |
−0.012 (0.005) p < 0.05 |
−0.007 (0.004) p = 0.069 |
0.001 (0.003) p = 0.783 |
−206.47 (110.93) p = 0.069 |
47.57 (37.70) p = 0.209 |
| number of fixes |
0.014 (0.005) p < 0.01 |
0.005 (0.006) p = 0.368 |
−0.011 (0.004) p < 0.01 |
−0.004 (0.003) p = 0.204 |
−343.34 (123.48) p < 0.01 |
−19.30 (40.52) p = 0.634 |
| inter-generational |
−0.070 (0.015) p < 0.001 |
−0.100 (0.016) p < 0.001 |
0.091 (0.012) p < 0.001 |
0.086 (0.009) p < 0.001 |
1812.75 (347.01) p < 0.001 |
753.09 (116.37) p < 0.001 |
| primary productivity |
−0.044 (0.006) p < 0.001 |
−0.018 (0.006) p < 0.01 |
0.010 (0.005) p < 0.05 |
−0.004 (0.003) p = 0.182 |
1837.06 (138.85) p < 0.001 |
215.38 (41.66) p < 0.001 |
Increases in 95% home range size were associated with inter-generational comparisons, indicating that younger generations increased their ranges relative to older generations to a greater extent than found in same generation comparisons. Increases in home range area were negatively related to poaching carcass density, indicating lower poaching pressure was found the greater the differences in range size between generations. In addition, increased range size was characteristic of core groups with younger adults and positively related to NDVI, suggesting expanded ranges were associated with higher primary productivity. For 50% ranges, only the inter-generational and NDVI covariates were significant for the range size analysis.
4. Discussion
The areas animals use can have far-reaching implications for their survival and reproduction [50]. To date little work has explored how animals change ranges across generations, but such information can provide unique insight into what drives movement and the development of philopatric behaviours. We examined tracking data collected from multiple generations of known elephant core groups over a period spanning variable ecological conditions and both low and high rates of ivory poaching, allowing investigation of range fidelity and drivers of range change in a well-studied elephant population. Female elephants exhibit matriarchal social structure [23,24], and older adults are linked to resource access [27], ecological and social knowledge [25,51] and calf survival [26,52,53]. Ivory poaching tends to target older animals for their larger tusks, reducing population age structure [54,55]. Since matriarchs are targeted, this causes orphaning and alters family unit structure. The ability of orphans and families that have lost their mature adults to survive within their dynamic landscapes is a concern for population recovery [26]. Our analysis of space use patterns in elephant core groups with different demographic histories tracked over a 16-year period demonstrated substantive change in range use, with generational turnover associated with range expansions and shifts. However, families demonstrated highly individualistic space use patterns over time. For example, the sprawling movements of the later generations of the Spice Girls and Flowers were markedly different from the more recursive movements of their mothers. By contrast, the Swahili Ladies and Artists maintained the recursive movements of their matriarchs but contracted their ranges to centre around the protected areas relative to those of their older relatives. Such differences among families underscore the complexity inherent in the ranging behaviour of this species and the potential role of multiple factors at play.
(a). Environmental drivers of range shifts
The general trend of northward shifts in home range centroids demonstrated across nearly all collared families (figure 2) was caused by less use of the areas to the south and southwest of Samburu and Buffalo Springs National Reserves as well as more use of the areas north of these reserves (figure 1). The less used southern areas were subject to heavy poaching during the latter half of the study and were the known location of adult killings from some of the tracked families. Notably, greater use of northern areas occurred regardless of the disruption experienced by individual families, with control families that did not experience poaching exhibiting this pattern as well (electronic supplementary material). The observed shifts in range appear to reflect a general response to illegal killing and avoidance of higher risk areas; model outputs showed a general avoidance of poaching associated with northern shifts, though the coefficient was not significant at the α = 0.05 level. The areas directly north of the reserves had lower poaching pressure, largely because they are older community conservancies that have benefited from ecotourism and the security it brings [38]. Northward shifts were also associated with higher relative primary productivity, suggesting multiple benefits to these range shifts as elephants are known to select habitat with high greenness at broad spatial scales [21,56]. Like northward shifts, westward shifts were associated with higher primary productivity. Unlike northward shifts, westward shifts were also associated with higher poaching, suggesting some families may be trading off productivity for safety in their ranging strategies. The drivers of the divergence in ranging strategies between those families shifting east toward relative safety and those shifting west are unclear from the present study, but such variability in ranging strategies deserves further study.
The influence of the risk landscape on animal foraging behaviour has been characterized in numerous systems [57], including this study system where shifts in elephant circadian patterns were found in human dominated areas [58–60]. It is worth noting that poaching is a cryptic activity [61], and our metric of poached carcasses serves as a coarse proxy for more nuanced human activity that elephants are likely responding to on the landscape. Despite the absence of finer resolution data on poaching risk, our analyses did suggest a trend away from areas with successful poaching attempts for those families that shifted north and east.
While larger home ranges can be associated with energetically expensive large-scale movements, the movement requirements of such large ranges may be offset by access to greater forage quantity and possibly quality, as indicated by the positive correlation with primary productivity (table 3). Thus, range size expansions and westward shifts are potentially both associated with trade-offs in this ecosystem, signifying the influence of human pressures and ecological resources on ranging behaviour. While home range provides a broad metric of space use, future work detailing differences in habitat selection across this sample will provide deeper insight to the mechanisms structuring changes in range.
(b). Social and demographic drivers of range change
Before poaching intensified in this population, range size was found to be inversely correlated to social dominance, where less dominant, younger families had larger ranges [27]. Our results are consistent with the idea that following disruption from age-selective poaching, younger families may be more inclined to expand their range and move into new areas. Our analysis, however, suggests a range of strategies that do not always follow this pattern. For example, the most disrupted family in our sample which lost all its adult females in the period of increased poaching—the Swahili Ladies—contracted their range to encompass little more than the protected areas (electronic supplementary material). However, orphans have less interaction with adults, limiting their access to ecological knowledge [41]. Therefore, constricted ranges around preferred areas do not necessarily indicate priority of access to resources.
The strategy by younger families to expand their range contrasts with age-related range expansions recorded in the Tarangire ecosystem in Tanzania, which occurred during droughts in families led by older matriarchs [26]. There are several possible drivers as to these contrasting results, particularly the different ecology of the two study systems (Tarangire receives two to three times the annual rainfall of Samburu). However, we suspect the recorded range shifts are at least partly influenced by orphans changing their primary social partners. Social instability stemming from age-selective mortality results in elephants restructuring their relationships [39,41]. Access to other animals with different spatial knowledge that may inform movement decisions [62] may be critical in some cases for surviving orphans during periods of change.
As elephant families alter their ranges in different ways, the question of how movement influences survival and reproduction is increasingly important to interpret the repercussions of such behavioural changes. Further work that integrates demographic data with information on movement and social strategies will provide a more comprehensive understanding of the implications of altered social landscapes for this threatened keystone species. Long-term, multi-generational movement studies provide novel understanding of how populations shift spatial behaviours in response to landscape dynamics and can be used to discern critical mechanisms shaping spatial behaviour over time. Such information is valuable for applied conservation by illuminating drivers of spatial behaviour, preferred landscape features and areas to target for protection as important refuge habitats. This unique inter-generational dataset offers insight into range changes over time, but the variable responses of the nine families included in our analysis underscore the complexity of response to dynamic landscapes in this species and the need for greater sampling to better illuminate this process. Our inter-generational study demonstrates the importance of continuous monitoring and the complexity of wildlife response to increasingly anthropogenically altered landscapes.
Supplementary Material
Acknowledgements
We thank the Kenyan Office of the President, the Kenya Wildlife Service and the county governments in Samburu and Isiolo counties for permission to conduct research in the national reserves. C. Thouless and three reviewers provided constructive feedback on the manuscript. G. Bastille-Rousseau provided technical advice.
Ethics
Protocols adhered to standards of the Kenya Wildlife Service, Samburu and Isiolo governments and Colorado State University (IACUC 12-3414A).
Data accessibility
The data used in this study have not been archived because of the protected status of African elephants.
Authors' contributions
All authors designed the study and collected the data. S.Z.G. and G.W. conducted the analyses and wrote the manuscript. S.Z.G. made the figures. I.D.-H. provided revisions to the manuscript.
Competing interests
We declare we have no competing interests.
Funding
We are grateful to private donors of Save the Elephants and WorldWomenWork for funding this study.
References
- 1.Northrup JM, Anderson CR, Wittemyer G. 2016. Environmental dynamics and anthropogenic development alter philopatry and space-use in a North American cervid. Divers. Distrib. 22, 547–557. ( 10.1111/ddi.12417) [DOI] [Google Scholar]
- 2.Arnaud CM, Dobson FS, Murie JO. 2012. Philopatry and within-colony movements in Columbian ground squirrels. Mol. Ecol. 21, 493–504. ( 10.1111/j.1365-294X.2011.05219.x) [DOI] [PubMed] [Google Scholar]
- 3.Meise K, Krüger O, Piedrahita P, Trillmich F. 2013. Site fidelity of male Galápagos sea lions: a lifetime perspective. Behav. Ecol. Sociobiol. 67, 1001–1011. ( 10.1007/s00265-013-1526-5) [DOI] [Google Scholar]
- 4.Piper WH. 2011. Making habitat selection more ‘familiar’: a review. Behav. Ecol. Sociobiol. 65, 1329–1351. ( 10.1007/s00265-011-1195-1) [DOI] [Google Scholar]
- 5.Spencer WD. 2012. Home ranges and the value of spatial information. J. Mammal. 93, 929–947. ( 10.1644/12-MAMM-S-061.1) [DOI] [Google Scholar]
- 6.Berger J, Cain SL, Berger KM. 2006. Connecting the dots: an invariant migration corridor links the Holocene to the present. Biol. Lett. 2, 528–531. ( 10.1098/rsbl.2006.0508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valenzuela LO, Sironi M, Rowntree VJ, Seger J. 2009. Isotopic and genetic evidence for culturally inherited site fidelity to feeding grounds in southern right whales (Eubalaena australis). Mol. Ecol. 18, 782–791. ( 10.1111/j.1365-294X.2008.04069.x) [DOI] [PubMed] [Google Scholar]
- 8.Berger J. 2004. The last mile: how to sustain long-distance migration in mammals. Conserv. Biol. 18, 320–331. ( 10.1111/j.1523-1739.2004.00548.x) [DOI] [Google Scholar]
- 9.Mueller T, O'Hara RB, Converse SJ, Urbanek RP, Fagan WF. 2013. Social learning of migratory performance. Science 341, 999–1002. ( 10.1126/science.1237139) [DOI] [PubMed] [Google Scholar]
- 10.Fagan WF, Cantrell RS, Cosner C, Mueller T, Noble AE. 2012. Leadership, social learning, and the maintenance (or collapse) of migratory populations. Theor. Ecol. 5, 253–264. ( 10.1007/s12080-011-0124-2) [DOI] [Google Scholar]
- 11.Seidler RG, Long RA, Berger J, Bergen S, Beckmann JP. 2015. Identifying impediments to long-distance mammal migrations. Conserv. Biol. 29, 99–109. ( 10.1111/cobi.12376) [DOI] [PubMed] [Google Scholar]
- 12.Winkler DW, et al. 2014. Cues, strategies, and outcomes: how migrating vertebrates track environmental change. Mov. Ecol. 2, 10 ( 10.1186/2051-3933-2-10) [DOI] [Google Scholar]
- 13.Løvschal M, Bøcher K, Pilgaard J, Amoke I, Odingo A, Thuo A, Svenning J-C. 2017. Fencing bodes a rapid collapse of the unique Greater Mara ecosystem. Sci. Rep. 7, 41450 ( 10.1038/srep41450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown J, Laundré J, Gurung M. 1999. The ecology of fear: optimal foraging, game theory, and trophic interactions. J. Mammal. 80, 385–399. ( 10.2307/1383287) [DOI] [Google Scholar]
- 15.Lafontaine A, Drapeau P, Fortin D, St-Laurent MH. 2017. Many places called home: the adaptive value of seasonal adjustments in range fidelity. J. Anim. Ecol. 86, 624–633. ( 10.1111/1365-2656.12645) [DOI] [PubMed] [Google Scholar]
- 16.Forrester TD, Casady DS, Wittmer HU. 2015. Home sweet home: fitness consequences of site familiarity in female black-tailed deer. Behav. Ecol. Sociobiol. 69, 603–612. ( 10.1007/s00265-014-1871-z) [DOI] [Google Scholar]
- 17.Padie S, Morellet N, Hewison AJM, Martin J-L, Bonnot N, Cargnelutti B, Chamaille-Jammes S. 2015. Roe deer at risk: teasing apart habitat selection and landscape constraints in risk exposure at multiple scales. Oikos 124, 1536–1546. ( 10.1111/oik.02115) [DOI] [Google Scholar]
- 18.Marantz SA, Long JA, Webb SL, Gee KL, Little AR, Demarais S. 2016. Impacts of human hunting on spatial behavior of white-tailed deer (Odocoileus virginianus). Can. J. Zool. 94, 853–861. ( 10.1139/cjz-2016-0125) [DOI] [Google Scholar]
- 19.Tolon V, Dray S, Loison A, Zeileis A, Fischer C, Baubet E. 2009. Responding to spatial and temporal variations in predation risk: space use of a game species in a changing landscape of fear. Can. J. Zool. 87, 1129–1137. ( 10.1139/Z09-101) [DOI] [Google Scholar]
- 20.Loarie SR, Aarde RJ, Pimm SL. 2009. Fences and artificial water affect African savannah elephant movement patterns. Biol. Conserv. 142, 3086–3098. ( 10.1016/j.biocon.2009.08.008) [DOI] [Google Scholar]
- 21.Wall J, Wittemyer G, Klinkenberg B, LeMay V, Douglas-Hamilton I. 2013. Characterizing properties and drivers of long distance movements by elephants (Loxodonta africana) in the Gourma, Mali. Biol. Conserv. 157, 60–68. ( 10.1016/j.biocon.2012.07.019) [DOI] [Google Scholar]
- 22.Polansky L, Kilian W, Wittemyer G. 2015. Elucidating the significance of spatial memory on movement decisions by African savannah elephants using state-space models. Proc. R. Soc. B 282, 20143042 ( 10.1098/rspb.2014.3042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Douglas-Hamilton I. 1972. On the ecology and behaviour of the African elephant. PhD thesis, University of Oxford, Oxford, UK. [Google Scholar]
- 24.Moss CJ. 1988. Elephant memories: thirteen years in the life of an elephant family. Chicago, IL: University of Chicago Press. [Google Scholar]
- 25.McComb K, Moss C, Durant SM, Baker L, Sayialel S. 2001. Matriarchs as repositories of social knowledge in African elephants. Science 292, 491–494. ( 10.1126/science.1057895) [DOI] [PubMed] [Google Scholar]
- 26.Foley C, Pettorelli N, Foley L. 2008. Severe drought and calf survival in elephants. Biol. Lett. 4, 541–544. ( 10.1098/rsbl.2008.0370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wittemyer G, Getz WM, Vollrath F, Douglas-Hamilton I. 2007. Social dominance, seasonal movements, and spatial segregation in African elephants: a contribution to conservation behavior. Behav. Ecol. Sociobiol. 61, 1919–1931. ( 10.1007/s00265-007-0432-0) [DOI] [Google Scholar]
- 28.Thouless CR. 1995. Long distance movements of elephants in northern Kenya. Afr. J. Ecol. 33, 321–334. ( 10.1111/j.1365-2028.1995.tb01042.x) [DOI] [Google Scholar]
- 29.Wittemyer G, Northrup JM, Blanc J, Douglas-Hamilton I, Omondi P, Burnham KP. 2014. Illegal killing for ivory drives global decline in African elephants. Proc. Natl Acad. Sci. USA 111, 13 117–13 121. ( 10.1073/pnas.1403984111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maisels F, et al. 2013. Devastating decline of forest elephants in central Africa. PLoS ONE 8, e59469 ( 10.1371/journal.pone.0059469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouché P, Douglas-Hamilton I, Wittemyer G, Nianogo AJ, Doucet J-L, Lejeune P, Vermeulen C. 2011. Will elephants soon disappear from West African savannahs? PLoS ONE 6, e20619 ( 10.1371/journal.pone.0020619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chase MJ, et al. 2016. Continent-wide survey reveals massive decline in African savannah elephants. PeerJ 4, e2354 ( 10.7717/peerj.2354) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blake S, Deem SL, Strindberg S, Maisels F, Momont L, Isia IB, Douglas-Hamilton I, Karesh WB, Kock MD. 2008. Roadless wilderness area determines forest elephant movements in the Congo Basin. PLoS ONE 3, e3546 ( 10.1371/journal.pone.0003546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Western D. 1989. The ecological role of elephants in Africa. Pachyderm 12, 42–45. [Google Scholar]
- 35.Campos-Arceiz A, Blake S. 2011. Megagardeners of the forest – the role of elephants in seed dispersal. Acta Oecol. 37, 542–553. ( 10.1016/j.actao.2011.01.014) [DOI] [Google Scholar]
- 36.Wittemyer G. 2001. The elephant population of Samburu and buffalo springs national reserves, Kenya. Afr. J. Ecol. 39, 357–365. ( 10.1046/j.1365-2028.2001.00324.x) [DOI] [Google Scholar]
- 37.Wittemyer G, Daballen D, Douglas-Hamilton I. 2013. Comparative demography of an at-risk African elephant population. PLoS ONE 8, e53726 ( 10.1371/journal.pone.0053726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ihwagi FW, et al. 2015. Using poaching pevels and elephant distribution to assess the conservation efficacy of private, communal and government land in northern Kenya. PLoS ONE 10, e0139079 ( 10.1371/journal.pone.0139079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldenberg SZ, Douglas-Hamilton I, Wittemyer G. 2016. Vertical transmission of social roles drives resilience to poaching in elephant networks. Curr. Biol. 26, 75–79. ( 10.1016/j.cub.2015.11.005) [DOI] [PubMed] [Google Scholar]
- 40.Goldenberg SZ. 2016. Ivory poaching, sociality, and the role of behavior in conservation. Fort Collins, CO: Colorado State University. [Google Scholar]
- 41.Goldenberg SZ, Wittemyer G. 2017. Orphaned female elephant social bonds reflect lack of access to mature adults. Sci. Rep. 7, 14408 ( 10.1038/s41598-017-14712-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Omondi P, Bitok E, Kahindi O, Mayienda R. 2002. Total aerial count of elephants in Samburu/Laikipia. Kenya Wildl. Serv. Rep.
- 43.Kahindi O, Wittemyer G, King J, Ihwagi F, Omondi P, Douglas-Hamilton I. 2010. Employing participatory surveys to monitor the illegal killing of elephants across diverse land uses in Laikipia–Samburu, Kenya. Afr. J. Ecol. 48, 972–983. ( 10.1111/j.1365-2028.2009.01200.x) [DOI] [Google Scholar]
- 44.Wittemyer G, Douglas-Hamilton I, Getz W. 2005. The socioecology of elephants: analysis of the processes creating multitiered social structures. Anim. Behav. 69, 1357–1371. ( 10.1016/j.anbehav.2004.08.018) [DOI] [Google Scholar]
- 45.Wittemyer G, Okello JB. A., Rasmussen HB, Arctander P, Nyakaana S, Douglas-Hamilton I, Siegismund HR. 2009. Where sociality and relatedness diverge: the genetic basis for hierarchical social organization in African elephants. Proc. R. Soc. B 276, 3513–3521. ( 10.1098/rspb.2009.0941) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Archie EA, Moss CJ, Alberts SC. 2006. The ties that bind: genetic relatedness predicts the fission and fusion of social groups in wild African elephants. Proc. R. Soc. B 273, 513–522. ( 10.1098/rspb.2005.3361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calabrese JM, Fleming CH, Gurarie E. 2016. ctmm: An R package for analyzing animal relocation data as a continuous-time stochastic process. Methods Ecol. Evol. 7, 1124–1132. ( 10.1111/2041-210X.12559) [DOI] [Google Scholar]
- 48.Fleming CH, Fagan WF, Mueller T, Olson KA, Leimgruber P, Calabrese JM. 2015. Rigorous home range estimation with movement data: a new autocorrelated kernel density estimator. Ecology 96, 1182–1188. ( 10.1890/14-2010.1) [DOI] [PubMed] [Google Scholar]
- 49.Core Team R. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 50.Bose S, Forrester TD, Brazeal JL, Sacks BN, Casady DS, Wittmer HU. 2017. Implications of fidelity and philopatry for the population structure of female black-tailed deer. Behav. Ecol. 28, 983–990. ( 10.1093/beheco/arx047) [DOI] [Google Scholar]
- 51.McComb K, Shannon G, Durant SM, Sayialel K, Slotow R, Poole J, Moss C. 2011. Leadership in elephants: the adaptive value of age. Proc. R. Soc. B 278, 3270–3276. ( 10.1098/rspb.2011.0168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee PC, Fishlock V, Webber CE, Moss CJ. 2016. The reproductive advantages of a long life: longevity and senescence in wild female African elephants. Behav. Ecol. Sociobiol. 70, 337–345. ( 10.1007/s00265-015-2051-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lahdenperä M, Mar KU, Lummaa V. 2016. Nearby grandmother enhances calf survival and reproduction in Asian elephants. Sci. Rep. 6, 27213 ( 10.1038/srep27213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiyo PI, Obanda V, Korir DK. 2015. Illegal tusk harvest and the decline of tusk size in the African elephant. Ecol. Evol. 5, 5216–5229. ( 10.1002/ece3.1769) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wittemyer G, Daballen D, Douglas-Hamilton I. 2011. Rising ivory prices threaten elephants. Nature 476, 282 ( 10.1038/476282c) [DOI] [PubMed] [Google Scholar]
- 56.Marshal JP, Rajah A, Parrini F, Henley M, Henley SR, Erasmus BFN. 2010. Scale-dependent selection of greenness by African elephants in the Kruger–private reserve transboundary region, South Africa. Eur. J. Wildl. Res. 57, 537–548. ( 10.1007/s10344-010-0462-1) [DOI] [Google Scholar]
- 57.Frid A, Dill L. 2002. Human-caused disturbance stimuli as a form of predation risk. Conserv. Ecol. 6, 11 ( 10.5751/ES-00404-060111) [DOI] [Google Scholar]
- 58.Wittemyer G, Polansky L, Douglas-Hamilton I, Getz WM. 2008. Disentangling the effects of forage, social rank, and risk on movement autocorrelation of elephants using Fourier and wavelet analyses. Proc. Natl Acad. Sci. USA 105, 19 108–19 113. ( 10.1073/pnas.0801744105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wittemyer G, Keating LM, Vollrath F, Douglas-Hamilton I. 2017. Graph theory illustrates spatial and temporal features that structure elephant rest locations and reflect risk perception. Ecography 40, 598–605. ( 10.1111/oik.03181) [DOI] [Google Scholar]
- 60.Ihwagi FW, Thouless C, Wang T, Skidmore AK, Omondi P, Douglas-hamilton I. 2018. Night–day speed ratio of elephants as indicator of poaching levels. Ecol. Indic. 84, 38–44. ( 10.1016/j.ecolind.2017.08.039) [DOI] [Google Scholar]
- 61.Liberg O, Chapron G, Wabakken P, Pedersen HC, Hobbs NT, Sand H. 2012. Shoot, shovel and shut up: cryptic poaching slows restoration of a large carnivore in Europe. Proc. R. Soc. B 279, 910–915. ( 10.1098/rspb.2011.1275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shrader AM, Owen-Smith N. 2002. The role of companionship in the dispersal of white rhinoceroses (Ceratotherium simum). Behav. Ecol. Sociobiol. 52, 255–261. ( 10.1007/s00265-002-0506-y) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study have not been archived because of the protected status of African elephants.