Abstract
Background
Expert opinion recommends performing exercise testing with initiation of Class Ic antiarrhythmic medication.
Objective
To evaluate the rate and reason for discontinuation of Ic agent within the first year of follow up, with particular attention to rate of proarrhythmia and the value of routine treadmill testing.
Methods
This is a single center retrospective cohort study including consecutive patients with atrial arrhythmias who were initiated on a Class Ic agent from 2011 to 2016. Data was collated from chart review and pharmacy database.
Results
The study population included 300 patients (55% male, mean age 61; mean ejection fraction, 56%) started on flecainide (n = 153; 51%) and propafenone (n = 147; 49%). Drug initiation was completed while hospitalized on telemetry and the staff electrophysiologists directed dosing. There was one proarrhythmic event during initiation (0.3%). The primary reason for not being discharged on Ic agent was due to detection of proarrhythmia (n = 15) or ischemia (n = 1) with treadmill testing (5.3%). Exercise testing was the single significant variable to affect the decision to discontinue Ic drug, p < 0.0001 (95% CI: 1.89–6.08%). During follow up, the primary reason for discontinuation of Ic agent was lack of efficacy, 32%.
Conclusions
With proper screening, initiation of Class Ic agent is associated with very low rate of proarrhythmia. Treadmill testing is of incremental value and should be completed in all patients after loading Class Ic antiarrhythmic.
Keywords: Atrial fibrillation, Antiarrhythmic medication, Flecainide, Propafenone, Exercise stress testing, Proarrhythmia
1. Introduction
Propafenone and flecainide are Class Ic antiarrhythmic medications used for rhythm control in the management of paroxysmal and persistent atrial fibrillation (AF). These drugs are selective sodium channel blockers with use-dependent properties and risk of proarrhythmia at elevated heart rates. Although not recommended in patients with structural heart disease, Class Ic agents are often used in patients with normal hearts and no coronary artery disease. In these patients, the risk of proarrhythmia is low. The 2014 American College of Cardiology/American Heart Association/European Society of Cardiology guidelines, based on committee consensus, recommend exercise testing when starting a Ic agent to detect for increase in QRS duration that occurs only at higher heart rates [1]. QRS widening ≥25% of baseline is often used at the threshold value for proarrhythmia based on previous findings of Bordier et al. [2]. Currently, the data regarding the use of exercise testing in patients with normal ejection fractions is limited; furthermore, only a few centers routinely complete exercise treadmill testing to screen for this proarrhythmia. One small study evaluating the utility of routine exercise testing found that, contrary to guidelines, it is not warranted in patients with structurally normal hearts [3]. The purpose of this study is to evaluate the outcomes of initiation and chronic therapy with Class Ic agent and the incremental value of routinely performing exercise treadmill testing after drug loading.
2. Methods
2.1. Patient population
A retrospective chart review was performed of patients >18 years who were initiated on a Class Ic antiarrhythmic drug for treatment of atrial arrhythmias at the Ohio State University Wexner Medical Center from January 2011 to May 2016 (65 months). Patients who met this inclusion criterion were identified through query of the Ohio State University Pharmacy Antiarrhythmic Drug Clinic database and the electronic medical records through the Ohio State University Information Warehouse. Exclusion criteria included prisoners, pregnant patients, and patients who were lost to follow up. Patients who were prescribed Class Ic in an intermittent manner (ie, “pill in the pocket” - medication use only with onset of the arrhythmia) were also excluded. This study was approved by the Ohio State University institutional review board.
2.2. Class Ic inpatient initiation and exercise treadmill testing
All patients were admitted to a telemetry unit for 4–5 doses of drug initiation. Dosing of flecainide and propafenone as well as alterations in drug dosing were at the discretion of the attending electrophysiologist. A 12-lead ECG was obtained prior to drug initiation and then 2 h post each drug dosing.
Serial assessment of QRS duration during drug loading and completion of an exercise treadmill test were the primary methods to screen for proarrhythmia. Excessive QRS prolongation was defined as QRS widening equal to or greater than 25% of baseline based on previous findings by Bordier et al. [2]. QRS duration was measured for each patient at the following times: at baseline prior to starting the medication, 2 h after each drug dosing during inpatient initiation, at peak exertion during treadmill testing, and then at each follow up clinic evaluation. QRS duration was measured by computer analysis and verified by two clinicians, separately. The broadest QRS duration was collated.
An exercise treadmill testing was completed within 4 days after completion of loading antiarrhythmic therapy so to screen for proarrhythmic effects of rate-dependent sodium channel blockade. The exercise treadmill testing protocol utilized were: Bruce in 170 (75%), modified Bruce in 35 (15%), other protocol in 23 (10%). For patients who were not able to exercise on a treadmill, hallway ambulation was performed until achieving maximum exertion. The goal of exercise testing was to achieve peak exertion level, rather than a specific target heart rate.
2.3. Follow up and study endpoints
Patients were followed for 1 year or until the Ic drug was discontinued. Clinical care and management of the Class Ic agent was at the discretion of the attending electrophysiologist. The primary study endpoint was reason for discontinuation of Class Ic agent, with particular attention to understand the role of routine exercise treadmill testing, assessment for proarrhythmia and the incremental value of inpatient initiation. A proarrhythmic event was defined as either QRS widening of ≥25% above baseline QRS [1], occurrence of first documented episode of nonsustained ventricular tachycardia, unexplained syncope or new onset cavo-tricuspid isthmus-dependent atrial flutter.
2.4. Statistical analysis
Continuous variables are presented as a mean ± standard deviation. Chi square test was used to assess if an abnormal treadmill test resulted in discontinuation of Class Ic medical therapy. Logistic modeling was used to define an odds ratio. Multivariate logistic modeling including age, gender, body mass index, ejection fraction, baseline QRS duration and results of treadmill testing was used to assess for variables that impacted drug discontinuation during initiation.
3. Results
3.1. Patient characteristics
During the 65 month period, 300 patients were initiated on flecainide (n = 154) or propafenone (n = 146) for atrial tachyarrhythmias. Patient demographics and baseline characteristics are listed in Table 1. All patients underwent evaluation with a transthoracic echocardiogram. The mean ejection fraction was 56.0 ± 5.4%. An AV nodal blocking agent was used in 230 patients (76%: beta blocker in 44%, calcium channel blocker in 16%; both in 16%). Prior to drug initiation, 105 (35%) of patients were in low risk category for coronary artery disease (based on clinical risk factor analysis) and the remaining 195 (65%) patients completed an evaluation for ischemia.
Table 1.
Demographic and clinical characteristics of the study population, n = 300.
| Variables | |
|---|---|
| Patient Demographics | |
| Age (years) | 61 ± 11 |
| Body mass index (kg/m2) | 32.1 ± 8.4 |
| Men | 164 (55%) |
| Race | |
| White | 272 (90%) |
| Black | 23 (8%) |
| Other | 5 (2%) |
| Clinical Characteristics | |
| Flecainide | 153 (51%) |
| Propafenone | 147 (49%) |
| Concomitant AV nodal blockers | |
| Beta blockers | 133 (44%) |
| Calcium channel blockers | 49 (16%) |
| Both | 48 (16%) |
| CKD | 11 (4%) |
| COPD | 36 (12%) |
| Liver disease | 2 (1%) |
| Average EF (%) | 56.0 ± 5.4 |
| Prior ischemic evaluation | 195 (65%) |
Abbreviations: CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease EF = ejection fraction.
3.2. Drug initiation & treadmill testing (Table 2, Table 3)
Table 2.
Clinical outcomes.
| Variables | Number of Patients |
|---|---|
| Outcomes of Inpatient Initiation, n = 300 | |
| Discharged on Drug | (87%) |
| Dose unchanged | 237 |
| Dose was increased | 15 |
| Dose was decreased: | 10 |
| ECG changes prior to exercise: | 5 |
| Change in QRS width ≤ 15% | 1 |
| Asymptomatic bradycardia | 4 |
| Other | 5 |
| Drug Discontinued Before Discharge | 38 (13%) |
| Results of treadmill test | 16 |
| AF recurrence | 9 |
| Intolerance | 7 |
| Resting ECG changes: | 4 |
| Change in QRS width ≤ 15% | 1 |
| NSVT | 1 |
| Asymptomatic bradycardia | 1 |
| Increase in QTc | 1 |
| New diagnosis of CAD | 2 |
| Change in Drug Dosing During Follow Up, n = 262 | |
| Drug Continued | 144 (55%) |
| Dose unchanged | 130 |
| Dose increase | 6 |
| Dose decrease: | 8 |
| Intolerance | 4 |
| Change in QRS width ≤ 15% | 2 |
| Chronotropic incompetence | 2 |
| Drug Discontinued | 118 (45%) |
| AF recurrence | 83 |
| Intolerance | 13 |
| Low AF burden | 8 |
| ECG changes: | 8 |
| Change in QRS width ≤ 15% | 7 |
| Asymptomatic bradycardia | 1 |
| New diagnosis of CAD | 4 |
| New atrial flutter | 1 |
| Unexplained syncope | 1 |
Abbreviations: AF = atrial fibrillation; CAD = coronary artery disease; NSVT = nonsustained ventricular tachycardia.
Table 3.
Characteristics of patients with exercise induced changes.
| Patient | Ejection Fraction | Ic Drug | Daily Total Dose (mg) | Peak HR on Treadmill (bpm) | ECG changes during exercise |
|---|---|---|---|---|---|
| 1 | 60 | flecainide | 200 | 162 | NSVT |
| 2 | 53 | flecainide | 200 | 158 | WCT |
| 3 | 55 | flecainide | 200 | 193 | QRS prolongation |
| 4 | 60 | flecainide | 200 | 142 | QRS prolongation |
| 5 | 65 | flecainide | 150 | 157 | QRS prolongation |
| 6 | 60 | flecainide | 200 | 131 | QRS prolongation |
| 7 | 55 | flecainide | 200 | 88 | NSVT |
| 8 | 55 | flecainide | 300 | 92 | NSVT |
| 9 | 60 | flecainide | 200 | 146 | ischemic changes |
| 10 | 60 | propafenone | 450 | 129 | NSVT |
| 11 | 45 | propafenone | 450 | 136 | QRS prolongation |
| 12 | 45 | propafenone | 450 | 166 | QRS prolongation |
| 13 | 60 | propafenone | 675 | 157 | QRS prolongation |
| 14 | 60 | propafenone | 450 | 122 | QRS prolongation |
| 15 | 65 | propafenone | 675 | 158 | QRS prolongation |
QRS prolongation defined as ≥ 25% increase of QRS duration compared to baseline ECG recording. Abbreviations: HR = heart rate; NSVT = non-sustained ventricular tachycardia; WCT = wide complex tachycardia.
All patients were admitted to the hospital during initial drug loading. Average total daily dose for propafenone was 568 ± 137 mg and 175 ± 99 mg for flecainide, and 87% were discharged on a Class Ic drug. The Ic drug dose was adjusted in 25 patients (with an increase in 15 and a decrease in 10) (Table 2). The single adverse/proarrhythmic event that occurred during drug loading was detection of asymptomatic nonsustained ventricular tachycardia in one patient in whom the left ventricular ejection fraction was found to be 45%. No other ECG changes associated with drug initiation were associated with symptoms or proarrhythmia.
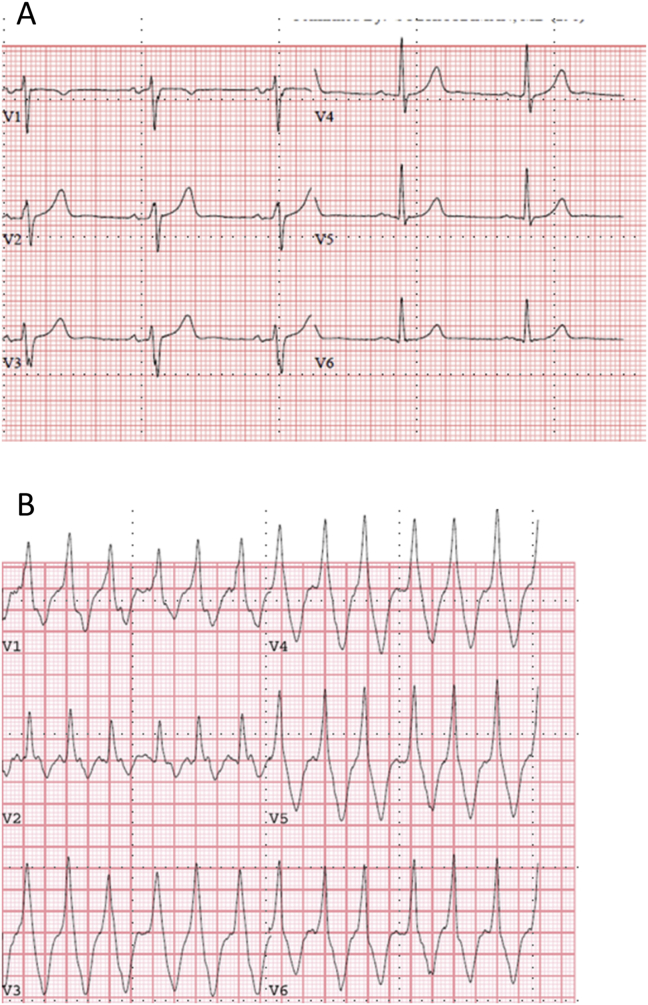
Of the 13% in whom Class Ic was not prescribed at discharge, the primary reason was the results of treadmill testing. Treadmill test was completed in 228 (76%) patients soon after drug loading. Proarrhythmia (QRS widening or nonsustained ventricular tachycardia) was noted during treadmill testing in 15 patients and ischemia in 1 patient (16/228; 7%) (Table 3). Fig. 1 shows an example of wide complex tachycardia during exercise. Tachycardia in this patient lasted 13.4 s. Exercise testing was not performed in 72 (24%) patients due to AF recurrence or drug discontinuation during drug loading (n = 10), inability to exercise (n = 40), or patient refusal (n = 22). By multivariate logistic regression analysis of the eight variables in the model (age, gender, body mass index, ejection fraction, renal function, hepatic function and baseline QRS duration and exercise testing), the only variable that significantly affecting the decision to discontinue Ic drug was results of exercise testing, p < 0.0001 (95% CI: 2.4–9.9%).
Fig. 1.
Examples of exercise testing ECG changes. Baseline ECGs of patient #2 (1A) with subsequent exercise induced wide complex tachycardia during exercise treadmill testing (1B).
3.3. Post discharge outcomes (Table 2)
During follow up through the electrophysiology clinic and through the specialty Antiarrhythmic Drug Clinic, there were no occurrences of sustained ventricular tachycardia or 1:1 conduction of atrial tachyarrhythmias identified. The Class Ic drug was discontinued within one year in 118/262 patients (45%). The reasons for drug discontinuation are detailed in Table 2. The primary reason for discontinuation is lack of efficacy, 83/262 patients (32%). The drug was also stopped for concern of proarrhythmia based on asymptomatic ECG changes in 8 patients; and, for symptomatic arrhythmia in 2 patients (new atrial flutter and unexplained syncope).
4. Discussion
There are 3 main conclusions of this retrospective study. First, the rate of important clinical events noted during inpatient monitoring was quite low. There was a single patient with an ejection fraction of 45% who had a potentially important ventricular arrhythmia (asymptomatic nonsustained ventricular tachycardia) during drug loading. Therefore, the clinical event rate for inpatient telemetry was 0.3% (1/300). Second, the results of routine treadmill testing noted important proarrhythmia (n = 15) and ischemia (n = 1) in 7% of patients. These findings would not be discerned without formal evaluation of Class Ic effect during exercise. The results of the treadmill test was the only variable that significantly affected the decision to discontinue Ic therapy. Third, during follow up, in addition to the common reason for discontinuation of antiarrhythmic drug due to lack of efficacy, routine periodic assessment of clinical and ECG outcomes lead to drug discontinuation in 35 patients. In total, therefore, of the 300 patients started on Class Ic medication, within 1 year, the drug was continued in 144 patients (48%). The findings of this study suggest that inpatient initiation of Class Ic drug is unnecessary in cohort of patients who have been screened for presence of structural heart disease or coronary artery disease. However, soon after loading, all patients should undergo a maximal exertion treadmill test to assess for proarrhythmic effects of use-dependent sodium channel blockade.
The risk of proarrhythmia with Class Ic agents is well recognized to be related to structural heart disease and ischemia [4], [5], [6], [7], [8]. However, a less well recognized proarrhythmia risk is related to use-dependent effects manifest only at higher ventricular rates [9], [10], [11]. Based upon this risk, current guidelines recommend monitoring QRS duration for patients initiating a Class Ic antiarrhythmic agent and exercise testing to detect QRS widening at elevated heart rates [1]. In a retrospective study, Vallurupati et al. [3] evaluated the utility of treadmill testing in 56 patients without structural heart disease and noted only one proarrhythmic event (2%), QRS widening ≥ 25%. The authors concluded that treadmill testing was not of value. A possible explanation for the higher rate of QRS widening in the current study is that Vallurupati et al. [3], completed treadmill testing only in patients with a total daily dose of at least 200 mg flecainide and 300 mg of propafenone; and, the QRS width was measured only in a single lead. The current study completed treadmill testing for all patients regardless of dosing and the widest QRS response during treadmill testing was selected from the 12L-ECG.
AV nodal blocking agents were used in 76% of patients. The remaining patients were found to have adequate AV node conduction slowing at baseline and did not require further pharmacologic therapy. There were no sustained ventricular arrhythmias or 1:1 conduction of rapid atrial arrhythmias during follow up despite the lack of AV nodal blocking agents in these 24% of patients.
4.1. Limitations
Drug dosing and discontinuation decisions were determined by the attending electrophysiologist and not by a study protocol. Nonetheless, this approach allows for an expert clinical decision based upon numerous clinical variables that could not be controlled with a specific protocol. Also, the exact rate of clinical proarrhythmia is not defined by this study since the physicians used the prior published criteria of QRS widening ≥25% [2] to be an indicator of increased risk for proarrhythmia. Most publications regarding proarrhythmia with class Ic agents included patients with impaired left ventricular function, so no other criteria were available. Additionally, this study was limited by its retrospective design, albeit the data was prospectively collated. Lastly, the clinical proarrhythmic event rate was quite low, thus subgroup analysis to assess for features associated with proarrhythmia is not feasible.
4.2. Clinical conclusions
The findings of this study support the following algorithm for safe initiation of a Class Ic agent. The first step is to screen patients for risk factors associated with poor outcomes, such as structural heart disease and coronary artery disease. Second, since the incidence of important side effects/proarrhythmia is low and with minimal risk when patients are at rest, a Class Ic agent may be prescribed as an outpatient with instructions provided to the patient not to perform activities associated with increased heart rate. Third, shortly after drug loading (≈5 doses), each patient should undergo a treadmill test to assess for important increase in QRS duration or other proarrhythmia related to use-dependent sodium channel blockade.
Financial support
None.
Disclosures & conflict of interest
None.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Fuster V., Ryden L.E., Cannom D.S. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123(10):e269–367. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 2.Bordier P., Garrigue S., Bernard V. Flecainide-induced increase in QRS duration and proarrhythmia during exercise. Clin Drug Investig. 1997;13(6):326–337. doi: 10.2165/00044011-199713060-00005. [DOI] [PubMed] [Google Scholar]
- 3.Vallurupalli S., Pothineni N.V., Deshmukh A., Paydak H. Utility of routine exercise testing to detect rate-related QRS widening in patients without structural heart disease on class Ic antiarrhythmic agents (flecainide and propafenone) Am J Cardiol. 2015;116(5):730–732. doi: 10.1016/j.amjcard.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 4.McNamara R.L., Tamariz L.J., Segal J.B., Bass E.B. Management of atrial fibrillation: review of the evidence for the role of pharmacologic therapy, electrical cardioversion, and echocardiography. Ann Intern Med. 2003;139(12):1018–1033. doi: 10.7326/0003-4819-139-12-200312160-00012. [DOI] [PubMed] [Google Scholar]
- 5.Echt D.S., Liebson P.R., Mitchell L.B. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324(12):781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 6.Anastasiou-Nana M.I., Anderson J.L., Stewart J.R. Occurrence of exercise-induced and spontaneous wide complex tachycardia during therapy with flecainide for complex ventricular arrhythmias: a probable proarrhythmic effect. Am Heart J. 1987;113(5):1071–1077. doi: 10.1016/0002-8703(87)90914-8. [DOI] [PubMed] [Google Scholar]
- 7.Herre J.M., Titus C., Oeff M. Inefficacy and proarrhythmic effects of flecainide and encainide for sustained ventricular tachycardia and ventricular fibrillation. Ann Intern Med. 1990;113(9):671–676. doi: 10.7326/0003-4819-113-9-671. [DOI] [PubMed] [Google Scholar]
- 8.Velebit V., Podrid P., Lown B., Cohen B.H., Graboys T.B. Aggravation and provocation of ventricular arrhythmias by antiarrhythmic drugs. Circulation. 1982;65(5):886–894. doi: 10.1161/01.cir.65.5.886. [DOI] [PubMed] [Google Scholar]
- 9.Ranger S., Talajic M., Lemery R., Roy D., Villemaire C., Nattel S. Kinetics of use-dependent ventricular conduction slowing by antiarrhythmic drugs in humans. Circulation. 1991;83(6):1987–1994. doi: 10.1161/01.cir.83.6.1987. [DOI] [PubMed] [Google Scholar]
- 10.Ranger S., Talajic M., Lemery R., Roy D., Nattel S. Amplification of flecainide-induced ventricular conduction slowing by exercise. A potentially significant clinical consequence of use-dependent sodium channel blockade. Circulation. 1989;79(5):1000–1006. doi: 10.1161/01.cir.79.5.1000. [DOI] [PubMed] [Google Scholar]
- 11.Kidwell G.A., Greenspon A.J., Greenberg R.M., Volosin K.J. Use-dependent prolongation of ventricular tachycardia cycle length by type I antiarrhythmic drugs in humans. Circulation. 1993;87(1):118–125. doi: 10.1161/01.cir.87.1.118. [DOI] [PubMed] [Google Scholar]