ABSTRACT
Posterior reversible encephalopathy syndrome (PRES) is an increasingly recognizable neuro-clinical syndrome. Clinical and neurological manifestations of PRES include hypertension, headache, encephalopathy, seizures, and symmetrical white matter changes on brain MRI. Most common precipitants of PRES are acute medical illness, hypertensive crisis, eclampsia, immunosuppressive therapy, and chemotherapy. Bevacizumab is a monoclonal antibody that halts angiogenesis by inhibiting vascular endothelial growth factor. It has gained widespread popularity in oncology world especially for metastatic and recurrent cancers due to its inherent ability to stop angiogenesis; a vital step for tumor growth. Bevacizumab has also been implicated as the cause of PRES due to dysregulation of the blood-brain barrier. We are reporting a case of PRES induced by Bevacizumab in a patient of colorectal cancer.
KEYWORDS: PRES, RPLS, BRES, VEGF, Bevacizumab, FOLFOX, angiogenesis
1. Introduction
Hinchey et al. first described posterior reversible encephalopathy syndrome (PRES) or reversible posterior leukoencephalopathy syndrome (RPLS) in 1996 [1]. PRES has been an increasingly recognizable neuro-clinical syndrome due to widespread and early use of neuroimaging in modern medicine. Pathophysiology of PRES is related to cytotoxic-mediated endothelial dysfunction and failure of blood-brain barrier or resulting hypertension-induced autoregulatory failure, although other mechanisms have also been proposed. The presence of both neurological findings and clinical manifestations are required for diagnosis. A majority of patients with PRES are hypertensive at presentation; most common symptoms are headaches, visual disturbance, and seizures; whereas, the most common radiological finding is the presence of symmetrical white matter edema in the parietal-occipital area [2]. PRES has been increasingly reported as a side effect of anti-vascular endothelial growth factor (VEGF) agents and tyrosine kinase inhibitors, due to increased accessibility and frequent use of medications in these classes of drugs. Patients presenting with possible PRES should prompt the physicians to identify and discontinue any such medications that might have precipitated it. We are hereby reporting a case of bevacizumab-induced PRES, and a brief review of literature of its side-effect profile.
2. Case presentation
48-year-old woman with recently diagnosed stage IV sigmoid adenocarcinoma with liver metastasis was started on FOLFOX (folinic acid, 5-fluorouracil, and oxaliplatin) therapy. She had no known history of hypertension or migraines. Bevacizumab was held initially due to concerns of rectal bleeding; however, it was added back into her third cycle of chemotherapy. She responded well to her treatment, and a repeat CT abdomen/pelvis after 3 months of starting chemotherapy showed interval decrease in the size of the tumor. After the addition of bevacizumab in the third cycle of FOLFOX, she started having high blood pressure (BP) readings with a range of 160–180 mmHg systolic BP. About a week after the sixth cycle of FOLFOX, which included the fourth dose of bevacizumab, she started complaining of a headache. Her headache was mainly frontal in location, throbbing in nature, continuous and radiating behind her eyes. She explained this headache as the worst headaches of her life, prompting a visit to the emergency department (ED). A non-contrast CT scan of her head was performed and found to be unremarkable. She was managed with supportive care with acetaminophen and metoclopramide with partial relief of headache, and discharged home. Two days later, while driving, she had a tonic-clonic seizure and was brought to the ED.
On examination, vital signs included BP ranging from 170s to 190s systolic and 80s to 95s diastolic, temperature: 100 F, pulse: 110/min, oxygen saturation: 99% on ambient air, and respiratory rate of 18/min. Physical examination in the ED was pertinent for confusion and agitation, rest of the physical examination was unremarkable. An initial laboratory workup including complete blood count, blood chemistry, coagulation studies, urinalysis, toxicology screen, lumbar puncture was negative. Radiographic imaging including non-contrast CT head was also negative. She was started on an antiepileptic regimen, levetiracetam for seizure prophylaxis. Electroencephalography (EEG) was unremarkable. Brain MRI (Figure 1) showed hyperintense T2 signal intensity in both occipital lobes involving the gray-white matter consistent with PRES syndrome.
Figure 1.
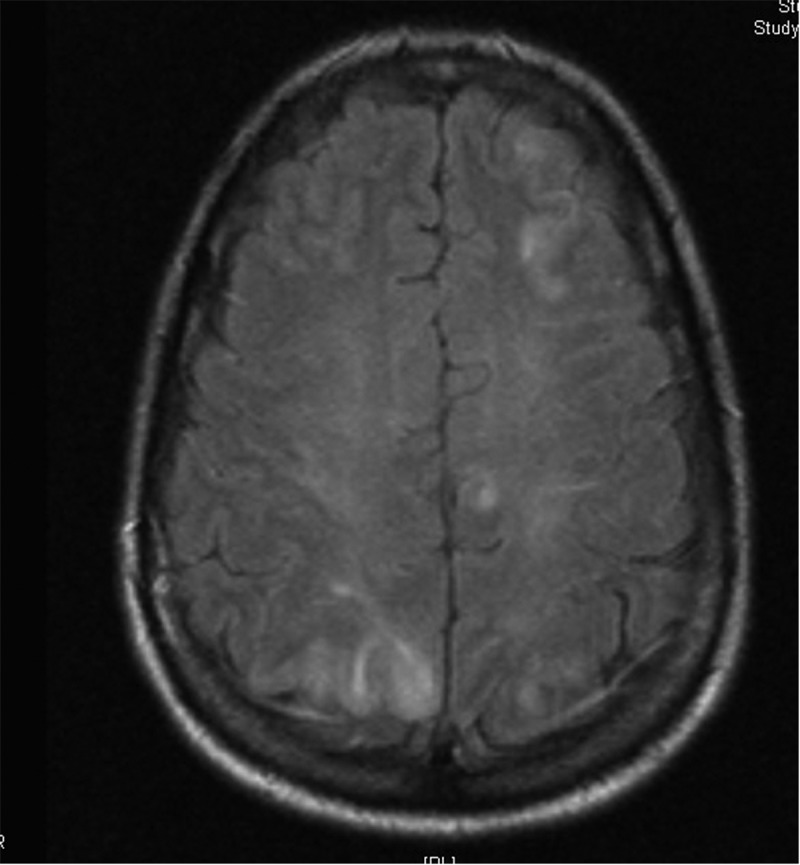
Brain MRI showing abnormal signal intensity in the gray-white matter in both occipital lobes and parietal lobes posteriorly and in the left frontal lobe.
She was initially observed in the neuro-critical care unit. Intravenous hydralazine was given for persistently elevated BP, which was later switched to oral amlodipine once she started taking oral diet resulting in good BP control. Her symptoms resolved by the third day of hospitalization and she was discharged on amlodipine for hypertension and levetiracetam as seizure prophylaxis. A repeat MRI one week after presentation showed resolving gray-white matter changes (Figure 2). Later on, FOLFOX was continued while bevacizumab was held. She did well on chemotherapy with good control of her BP and didn’t report any recurrent headaches. Repeat MRI after one month of the initial presentation showed complete resolution and seizure prophylaxis was discontinued.
Figure 2.
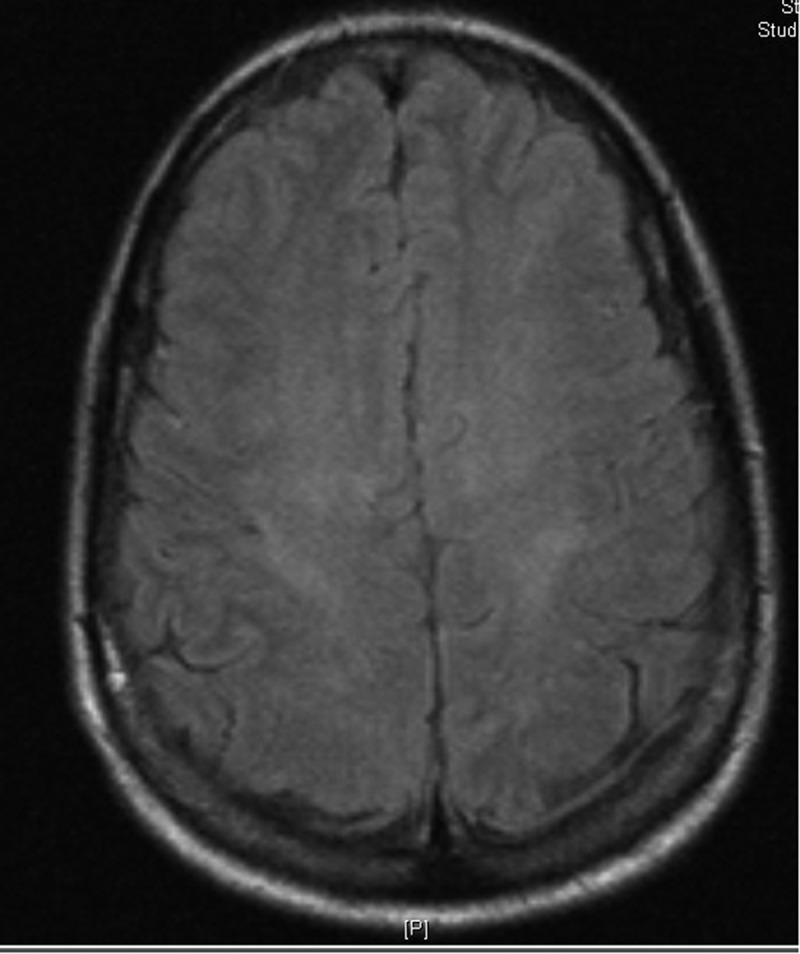
Brain MRI showing resolving signal changes of frontal and parietal-occipital regions representing resolving PRES.
3. Discussion
VEGF plays a major role as an anti-angiogenic factor that prevents malignant cells from surviving, dividing, and ultimately metastasizing. VEGF inhibitors were developed to halt angiogenesis and stop the exponential growth of malignant cells. Bevacizumab is a humanized monoclonal antibody that impairs the interaction of VEGF and VEGF receptor by binding to VEGF-A (a subtype of VEGF) [3]. It was first approved by the Federal Drug Agency (FDA) in 2004 for metastatic colorectal cancer. It was later approved for lung, breast, and ovarian cancer, among others, which has led to its increased use in oncologic practice [4].
Although well tolerated, the most common adverse effects reported are hypertension, proteinuria, arterial thrombosis, delayed wound healing, bleeding, and gastrointestinal perforation. Hypertension is the most common side effect, and the proposed mechanism is a decrease in the release of nitric oxide from endothelial cells as a result of VEGF inhibition [5]. The incidence of hypertension varies from 16–38% [6]; however, the incidence of grade 3 or 4 hypertension is 16–18% and is dose-dependent [7]. The single most important risk factor for the development of hypertension is the age with one study finding the incidence of hypertension in patients aged 75 years or older to be 29% as compared to 10–11% in patients aged 65–74 [8]. Hypertension usually occurs within the first year of starting therapy but can occur later as well. Patients should be carefully followed for new onset or worsening hypertension, National Cancer Institute recommends checking BP every week during the first cycle and then every 2–3 weeks for the rest of the treatment. Continue to monitor BP 3–6 months after discontinuation of therapy [9]. BP goals and choice of antihypertensive therapy should be dictated by guidelines of Joint National Committee on hypertension [10]. Grade I hypertension defined as asymptomatic or transient (<24 h) increase of BP to >150/100 mmHg can be managed with careful monitoring, grade II and III recurrent or persistent (>24 h) of BP to above 150/100 mmHg necessitate initiation and addition of antihypertensive therapy; for grade IV hypertension (hypertensive crises, e.g. PRES), bevacizumab should be discontinued.
Proteinuria is the second most common side effect of bevacizumab with an incidence ranging from 23–38%. However, the incidence of grade III/IV proteinuria is very low, 3% or less and progression to the florid nephrotic syndrome is rare. Although no direct association has been found between the development of proteinuria and PRES, a review done by Tlemsani et al. [11] suggested that patients who develop new-onset proteinuria while on treatment with bevacizumab are at high risk of PRES. Patients should be screened for proteinuria with a urine dipstick every month and if 2+(100 mg/dL), it should be confirmed by 24-h urine collection or urine protein to creatinine (UPC) ratio for further evaluation. Bevacizumab can be continued if UPC <2 mg/g or 24-h urine protein is ≤2 g. Bevacizumab should be suspended if UPC> 2 mg/g and restarted only if proteinuria has resolved, failure to resolve within 3 months should result in permanent discontinuation. Development of nephrotic syndrome is another reason for permanent discontinuation [12]. No therapy has been shown to improve or at least prevent the worsening of proteinuria, the role of ACE inhibitors has been controversial in this regard, with some studies suggesting it might worsen it [13].
Two years after its formal approval, bevacizumab was noticed to be related to PRES for the first time [14]. The mechanism by which bevacizumab causes PRES is not fully understood, although it is hypothesized that it disrupts the blood-brain barrier through endothelial dysregulation resulting in hyperperfusion and vasogenic edema leading to failure of autoregulation. Bevacizumab-induced hypertension might also play some role, although cases of bevacizumab-induced PRES have been reported in normotensive patients [15,16]. The incidence of PRES with bevacizumab is <0.5% according to the package insert. Typically, PRES occurs within the half-life of bevacizumab which is 20 days, as in our patient symptoms started about 12 days after the last dose, but it can occur from 16 h to 1 year. Seizures are the most common presenting complaint, and tonic-clonic in nature. Encephalopathy is an integral part of presentation present in about 50–80% of patients [17]. Headache is present in about 50% of patients and mostly gradual in onset and dull in character. Visual changes, focal neurological signs, and status epilepticus can be present [1]. Diagnosis is challenging and can be wrongly attributed to stroke, infection, or metastasis. A high index of suspicion should be maintained in someone with new-onset seizures, encephalopathy in the background of elevated BP and ongoing/previous chemotherapy with drugs known to cause PRES, such as bevacizumab. Lumbar puncture is done routinely to rule out infection, but has no role in identifying PRES. MRI remains the diagnostic modality of choice and helps to rule out other differentials as described earlier. Removal of the inciting agent and BP control is the most critical thing in the management.
No specific drug is recommended for the control of BP in the acute phase, and continuous intravenous infusion of any antihypertensive that is easily titrated might be needed [18]. Severe fluctuation in BP should be avoided, and the goal is to reduce the SBP not more than 25% or diastolic BP to 100–105 mmHg in the first 2–6 hours as there is a risk of ischemia with a more aggressive decrease in BP. Seizures can be controlled with benzodiazepines. However, any antiepileptic can be used for the acute management of seizures with no role of antiepileptic drugs long term. Antiepileptics should be tapered quickly in patients without a seizure recurrence and after resolution of MRI abnormalities. Seizures associated with PRES have a good prognosis without any long-term sequelae [17].
The overall prognosis of PRES after bevacizumab is favorable, and there have been calls to change its name to BRES (benign reversible encephalopathy syndrome) [19]. Successful re-challenge of bevacizumab has also been reported but should be done under close supervision, and appropriate BP control should be ensured [20]. The median time to recovery of the neurological symptoms is 7.5 days [21].
In conclusion, bevacizumab use in on the rise in the oncology practice, and potentially severe complications should be considered when implementing this agent. Physicians should be reminded of its potentially devastating side effect of PRES and the need to maintain a high index of suspicion in patients presenting with neurological symptoms who have been or are currently treated with Bevacizumab.
Funding Statement
No funding source involved
Declaration of interest
Authors disclose no potential conflicts of interest.
References
- [1].Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494–500. [DOI] [PubMed] [Google Scholar]
- [2].McKinney AM, Short J, Truwit CL, et al. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. Am J Roentgenology. 2007;189(4):904–912. [DOI] [PubMed] [Google Scholar]
- [3].Ignoffo RJ.Overview of bevacizumab: a new cancer therapeutic strategy targeting vascular endothelial growth factor. Am J Health Syst Pharm. 2004;61(21 Suppl 5):21. [DOI] [PubMed] [Google Scholar]
- [4].FDA approval for bevacizumab National cancer institute web site. https://www.cancer.gov/about-cancer/treatment/drugs/fda-bevacizumab.
- [5].Kiefer FN, Neysari S, Humar R, et al. Hypertension and angiogenesis. Curr Pharm Des. 2003;9(21):1733–1744. [DOI] [PubMed] [Google Scholar]
- [6].Zhu X, Wu S, Dahut WL, et al. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49(2):186–193. [DOI] [PubMed] [Google Scholar]
- [7].Saif MW, Mehra R. Incidence and management of bevacizumab-related toxicities in colorectal cancer. Expert Opin Drug Saf. 2006;5(4):553–566. [DOI] [PubMed] [Google Scholar]
- [8].Raman AK, Lombardo JC, Chandrasekhar R, et al. Bevacizumab (BV) related adverse events among various age groups of elderly patients with advanced colorectal cancer. JCO. 2007;25(18_suppl):14546. [Google Scholar]
- [9].Mourad J, Des Guetz G, Debbabi H, et al. Blood pressure rise following angiogenesis inhibition by bevacizumab. A crucial role for microcirculation. Ann Oncol. 2008;19(5):927–934. [DOI] [PubMed] [Google Scholar]
- [10].James PA, Suzanne Oparil MD, Barry MD, et al. 2014 evidence-based guideline for the management of high blood pressure in adults. JAMA. 2014;311(5):507–520. [DOI] [PubMed] [Google Scholar]
- [11].Tlemsani C, Mir O, Boudou-Rouquette P, et al. Posterior reversible encephalopathy syndrome induced by anti-VEGF agents. Target Oncol. 2011;6(4):253–258. [DOI] [PubMed] [Google Scholar]
- [12].Gordon MS, Cunningham D. Managing patients treated with bevacizumab combination therapy. OCL. 2005;69(Suppl. 3):25–33. [DOI] [PubMed] [Google Scholar]
- [13].Siddiqui AJ, Mansson-Broberg A, Gustafsson T, et al. Antagonism of the renin-angiotensin system can counteract cardiac angiogenic vascular endothelial growth factor gene therapy and myocardial angiogenesis in the normal heart. Am J Hypertens. 2005;18(10):1347–1352. [DOI] [PubMed] [Google Scholar]
- [14].Glusker P, Recht L, Lane B, et al. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354(9):980–982. [DOI] [PubMed] [Google Scholar]
- [15].Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29(6):1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Eryılmaz MK, Mutlu H, Salim DK, et al. Fatal posterior revesible leukoencephalopathy syndrome associated coma induced by bevacizumab in metastatic colorectal cancer and review of literature. J Oncol Pharm Pract. 2016;22(6):806–810. [DOI] [PubMed] [Google Scholar]
- [17].Kastrup O, Gerwig M, Frings M, et al. Posterior reversible encephalopathy syndrome (PRES): electroencephalographic findings and seizure patterns. J Neurol. 2012;259(7):1383–1389. [DOI] [PubMed] [Google Scholar]
- [18].Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14(9):914–925. [DOI] [PubMed] [Google Scholar]
- [19].Sawaya R, Radwan W, Hammoud S. Benign reversible encephalopathy syndrome after bevacizumab therapy for metastatic ovarian cancer. Med Oncol. 2014;31(2):831. [DOI] [PubMed] [Google Scholar]
- [20].Lou E, Turner S, Sumrall A, et al. Bevacizumab-induced reversible posterior leukoencephalopathy syndrome and successful retreatment in a patient with glioblastoma. J Clin Oncol. 2011;29(28):739. [DOI] [PubMed] [Google Scholar]
- [21].Singer S, Grommes C, Reiner AS, et al. Posterior reversible encephalopathy syndrome in patients with cancer. Oncologist. 2015;20(7):806–811. [DOI] [PMC free article] [PubMed] [Google Scholar]


