ABSTRACT
Introduction: The aim of our study is to determine the clinical, biochemical, and imaging factors that affect the duration of hospital stay in patients admitted with normotensive acute pulmonary embolism.
Methods: This was a single-center retrospective study conducted in a community hospital in New York metropolitan area for patients admitted from October 2015 to October 2017.
Results: A total of 79 patients were included, the mean age was 55.76 (SD = 17.33), 29 cases were males (37%) and 50 cases were females (63%). Among all patients, 17 cases had short length of stay (LOS) (≤2 days) and 62 cases had long LOS (>2 days). There were statistically significant differences in age (p = .041), presence of lung disease (p = .036), number of comorbidities (p = .043), and pulmonary embolism severity index (PESI) scores (original and simplified; p = .002 and .001, respectively). Logistic regression analysis showed that PESI score significantly predicted long LOS (OR 1.067, 95% CI [1.001, 1.137], p = .048). Similarly, sPESI significantly predicted long LOS (OR 0.223, 95% CI [0.050, 0.999], p = .050). Both regression models were adjusted for age, lung disease, and number of comorbidities.
Conclusion: Both original and simplified PESI scores were statistically significant predictors of duration of hospital stay. Patients with multiple comorbidities or with chronic lung disease were also likely to have prolonged hospital stay. None of the cardiac biomarkers affected the duration of hospital stay, neither did the presence of right ventricular dysfunction nor treatment modality.
KEYWORDS: Pulmonary embolism, severity index, prognosis, risk stratification, hospital stay
1. Introduction
Acute pulmonary embolism (PE) is a leading cause of cardiovascular mortality worldwide; it is the third most frequent acute cardiovascular disease after acute coronary syndrome and stroke [1], with an incidence of 112 cases per 100,000 [2]. The aggregate cost estimation for newly diagnosed venous thromboembolism (VTE) in the USA is 7–10 billion dollars annually [3]. The mortality rate for untreated acute PE is as high as 30%, which drops to 8% if timely diagnosed and appropriately managed [4].
Patients with acute PE are commonly admitted to US hospitals for risk stratification and initiation of anticoagulation therapy [5]. Several risk stratification tools have been developed and implemented to help predict mortality in acute PE. Among the common prognostic scores are pulmonary embolism severity index (PESI) (original and simplified), Bova score, and Geneva prognostic score (GPS) [6–9]. Current recommendations suggest that low-risk PE (LRPE) patients are suitable for treatment out of the hospital or they can be treated as in-patients but to be discharged early [10]. The aim of this study is to evaluate these ‘mortality scores’ as predictors of early hospital discharge for patients who are being admitted for acute PE.
2. Patients and methods
2.1. Study design
We retrospectively reviewed electronic medical records of patients who presented to the emergency department of a single community hospital in Metropolitan Area in New York state and admitted with a diagnosis of acute PE (International Classification of Diseases, 10th Revision, ICD-10 code: I26) in the period from October 2015 to October 2017. Only patients who were normotensive on admission were included in this study. Patients who developed PE during hospitalization were excluded. The study protocol was approved by Institutional Review Board (IRB) prior to data collection. Informed consent was waived due to the retrospective nature of the study.
2.2. Patient selection
Patients were included in the study if they (1) were ≥18 years of age, (2) had at least one admission with acute PE confirmed by pulmonary CT angiography (CTA) or high-probability ventilation/perfusion scan (V/Q scan), (3) had a systolic blood pressure on presentation ≥90 mmHg, (4) had troponin I (TnI) and/or B-natriuretic peptide (BNP) levels obtained within 24 h of admission, and (4) had an echocardiogram obtained within 48 h of admission.
Length of stay (LOS) was calculated for each eligible patient. Patients were later stratified, based on their LOS, into short LOS (≤2 days) and long LOS (>2 days) cohorts.
2.3. Methods and measurements
Based on detailed chart review, the patients’ demographics, medical comorbidities, risk factors, clinical presentation, vital signs, and initial workup were collected.
Demographic parameters: age, gender, race and body mass index (BMI).
Medical comorbidities: diabetes, hypertension, hyperlipidemia, smoking history, heart disease, lung disease, previous stroke, and renal impairment.
Risk factors: immobilization (≥3 days), recent surgery (within the last 4 weeks), recent travel (≥4 h), recent estrogen therapy (within the last 3 months), concomitant documented deep-venous thrombosis (DVT), previous history of VTE, and history of cancer.
Clinical features on presentation: chest pain, dyspnea, palpitations, syncope, cough, hemoptysis, wheezing, leg pain or swelling, altered mentation, and hemodynamic instability.
Initial workup: complete blood count, renal functions, D-dimer, lactic acid and electrocardiogram (EKG), TnI, and BNP obtained within 24 h of admission and echocardiogram obtained within 48 h of admission.
Echocardiograms were evaluated for right ventricular (RV) dysfunction and were reported by a board-certified cardiologist.
A cutoff values for TnI of 0.10 ng/mL and for BNP of 100 pg/mL were predefined. Based on the available data, Bova score, GPS, PESI, and sPESI were determined for each eligible patient (Table 1).
Table 1.
Clinical prognostic scores.
| 1.1 Bova score | |
| Variables | Points |
| SBP ≤100 mmHg | +2 |
| Elevated cardiac troponin | +2 |
| RV dysfunction | +2 |
| Heart rate ≥110 beats/min | +1 |
| The total score is calculated as the sum of the points assigned to each variable and is used to classify patients as Stage I, II, or III | |
| Stages | Points |
| Stage I | 0–2 |
| Stage II | 3–4 |
| Stage III | >4 |
| Low/intermediate risk is defined by a score ≤4, those with a score >4 are considered high risk | |
| 1.2 Geneva prognostic score (GPS) | |
| Variables | Points |
| Cancer | +2 |
| Heart failure | +1 |
| Previous DVT | +1 |
| SBP <100 mmHg | +2 |
| SaO2 < 90% | +1 |
| Concomitant proximal DVT | +1 |
| The total score is calculated as the sum of the points assigned to each variable. Low risk is defined by a score of ≤2. | |
| 1.3 Pulmonary embolism severity index (PESI) | |
| Variables | Points |
| Age | Age in years |
| Male gender | +10 |
| Cancer | +30 |
| Heart failure | +10 |
| Chronic lung disease | +10 |
| Heart rate ≥110 beats/min | +20 |
| SBP <100 mmHg | +30 |
| Respiratory rate >30 breath/min | +20 |
| Temperature <36°C/96.8°F | +20 |
| Altered mental status | +60 |
| SaO2 <90% | +20 |
| The total score is calculated as the sum of the age in years and the points assigned to each variable and used to classify patients as class I, II, III, IV, or V. | |
| Classes | Points |
| Class I | ≤65 |
| Class II | 66–85 |
| Class III | 86–105 |
| Class IV | 106–125 |
| Class V | >125 |
| Low risk is defined by a score of ≤85 or a risk class ≤II. | |
| 1.4 Simplified Pulmonary Embolism Severity Index (sPESI) | |
| Variables | Points |
| Age >80 years | +1 |
| Cancer | +1 |
| Chronic cardiopulmonary disease | +1 |
| Heart rate ≥110 beats/min | +1 |
| SBP <100 mmHg | +1 |
| SaO2 < 90% | +1 |
| The total score is calculated as the sum of the points assigned to each variable. Patients with a score of 0 were determined to be low risk, while those with a score of 1 or more were considered high risk. | |
SBP, systolic blood pressure; RV dysfunction, right ventricular dysfunction; DVT, deep-venous thrombosis; SaO2, oxygen saturation.
The primary outcome of this study was the LOS in days. This was subdivided into two cohorts: LOS of 2 days or less and LOS of more than 2 days. The secondary outcomes were the association between clinical and biochemical variables and LOS.
2.4. Statistical analyses
Statistical analyses were performed using statistical package for the social sciences (SPSS), version 24.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were provided for all variables and presented as mean ± standard deviation for continuous variables and as number and percentage for categorical variables. Statistical tests of significance (Student’s t test or Mann–Whitney U test for continuous variables and Chi-square test or Fischer’s exact test for categorical variables) were conducted to assess the differences between the cohorts (short LOS versus long LOS). A two-tailed p value ≤.05 was regarded as statistically significant. Binary logistic regression analysis was used to identify predictors of LOS among the study population. Variables with a p value ≤.20 were included in multivariate logistic regression analysis. A multivariate model was made using the Enter-method. To interpret the results of multivariate analyses, p values ≤.05 were considered significant.
3. Results
3.1. Patient characteristics
After applying inclusion and exclusion criteria, a total of 79 patients were included in the study. The demographic and clinical characteristics of the patients are shown in Table 2. Seventeen patients (22%) had short LOS (≤2 days) and 62 (78%) had long LOS (>2 days).
Table 2.
Demographic and clinical characteristics of patients at baseline.
| Characteristics | All patients (N = 79) | Length of stay |
p Value | |
|---|---|---|---|---|
| Short (≤2 days) (N = 17) |
Long (>2 days) (N = 62) |
|||
| Age (years) | 55.76 ± 17.330 | 48.18 ± 14.838 | 57.84 ± 17.489 | 0.041* |
| Age group | 0.028* | |||
| <65 years | 52 (65.8) | 15 (88.2) | 37 (59.7) | |
| ≥65 years | 27 (34.2) | 2 (11.8) | 25 (40.3) | |
| Gender | 0.481 | |||
| Male | 29 (36.7) | 5 (29.4) | 24 (38.7) | |
| Female | 50 (63.3) | 12 (70.6) | 38 (61.3) | |
| Comorbidities | ||||
| Diabetes | 18 (22.8) | 2 (11.8) | 16 (25.8) | 0.332 |
| Hypertension | 45 (57.0) | 9 (52.9) | 36 (58.1) | 0.705 |
| Hyperlipidemia | 15 (19.0) | 1 (5.9) | 14 (22.6) | 0.170 |
| Smoking | 19 (24.1) | 4 (23.5) | 15 (24.2) | 1.000 |
| Heart disease | 17 (21.5) | 2 (11.8) | 15 (24.2) | 0.338 |
| Lung disease | 26 (32.9) | 2 (11.8) | 24 (38.7) | 0.036* |
| Stroke | 12 (15.2) | 3 (17.6) | 9 (14.5) | 0.714 |
| Renal impairment | 13 (16.5) | 1 (5.9) | 12 (19.4) | 0.278 |
| Number of comorbidities | 2.09 ± 1.562 | 1.41 ± 1.372 | 2.27 ± 1.570 | 0.043* |
| Risk factors | ||||
| Immobilization | 13 (16.5) | 2 (11.8) | 11 (17.7) | 0.723 |
| Recent surgery | 4 (5.1) | 0 | 4 (6.5) | 0.572 |
| Recent travel | 4 (5.1) | 3 (17.6) | 1 (1.6) | 0.030* |
| Recent estrogen therapy | 6 (7.6) | 2 (11.8) | 4 (6.5) | 0.604 |
| Concomitant DVT | 11 (13.9) | 4 (23.5) | 7 (11.3) | 0.238 |
| Previous VTE (DVT/PE) | 13 (16.5) | 1 (5.9) | 12 (19.4) | 0.278 |
| Active cancer | 6 (7.6) | 0 | 6 (9.7) | 0.331 |
| Symptoms | ||||
| Chest pain | 31 (39.2) | 6 (35.3) | 25 (40.3) | 0.707 |
| Dyspnea | 52 (65.8) | 12 (70.6) | 40 (64.5) | 0.640 |
| Palpitations | 13 (16.5) | 2 (11.8) | 11 (17.7) | 0.723 |
| Syncope | 6 (7.6) | 1 (5.9) | 5 (8.1) | 1.000 |
| Cough | 10 (12.7) | 3 (17.6) | 7 (11.3) | 0.441 |
| Hemoptysis | 6 (7.6) | 2 (11.8) | 4 (6.5) | 0.604 |
| Wheezing | 3 (3.8) | 1 (5.9) | 2 (3.2) | 0.522 |
| Leg pain/swelling | 22 (27.8) | 5 (29.4) | 17 (27.4) | 1.000 |
| Altered mentation | 1 (1.3) | 0 | 1 (1.6) | 1.000 |
| Initial vital signs | ||||
| Heart rate (beats/min) | 97.75 ± 20.465 | 89.71 ± 15.846 | 99.95 ± 21.136 | 0.067 |
| Systolic blood pressure (mmHg) | 136.33 ± 23.580 | 137.18 ± 17.802 | 136.10 ± 25.051 | 0.868 |
| Oxygen saturation (%) | 96.44 ± 3.957 | 97.18 ± 2.128 | 96.24 ± 4.318 | 0.392 |
| Initial EKG | ||||
| Normal | 39 (49.4) | 10 (58.8) | 29 (46.8) | 0.379 |
| Sinus bradycardia | 3 (3.8) | 1 (5.9) | 2 (3.2) | 0.522 |
| Sinus tachycardia | 14 (17.7) | 2 (11.8) | 12 (19.4) | 0.722 |
| Atrial arrhythmia | 8 (10.1) | 3 (17.6) | 5 (8.1) | 0.359 |
| New RBBB | 2 (2.5) | 1 (5.9) | 1 (1.6) | 0.386 |
| New inferior Q waves | 4 (5.1) | 0 | 4 (6.5) | 0.572 |
| New anterior ST-T changes | 4 (5.1) | 2 (11.8) | 2 (3.2) | 0.201 |
| S1Q3T3 | 2 (2.5) | 0 | 2 (3.2) | 1.000 |
| Troponin I (cutoff 0.10ng/mL) | ||||
| Negative | 53 (67.1) | 14 (82.4) | 39 (62.9) | 0.131 |
| Positive | 26 (32.9) | 3 (17.6) | 23 (37.1) | |
| B-natriuretic peptide (cutoff 100 pg/mL) | ||||
| Negative | 26 (39.4) | 7 (53.8) | 19 (35.8) | 0.234 |
| Positive | 40 (60.6) | 6 (46.2) | 34 (64.2) | |
| RV dysfunction | 22 (27.8) | 4 (23.5) | 18 (29.0) | 0.767 |
| Bova score (points) | 1.59 ± 1.971 | 0.88 ± 1.409 | 1.79 ± 2.066 | 0.094 |
| Bova stage | 0.164 | |||
| Stage I | 56 (70.9) | 15 (88.2) | 41 (66.1) | |
| Stage II | 16 (20.3) | 2 (11.8) | 14 (22.6) | |
| Stage III | 7 (8.9) | 0 | 7 (11.3) | |
| PESI score (points) | 77.28 ± 33.170 | 55.24 ± 16.758 | 83.32 ± 34.069 | 0.002* |
| PESI score | 0.002* | |||
| ≤85 | 55 (69.6) | 17 (100) | 38 (61.3) | |
| >85 | 24 (30.4) | 0 | 24 (38.7) | |
| Simplified PESI | 0.001* | |||
| Low risk | 33 (41.8) | 13 (76.5) | 20 (32.3) | |
| High risk | 46 (58.2) | 4 (23.5) | 42 (67.7) | |
| Geneva prognostic score (points) | 0.73 ± 1.118 | 0.41 ± 0.712 | 0.82 ± 1.195 | 0.232 |
| Geneva prognostic score | 0.331 | |||
| ≤2 | 73 (92.4) | 17 (100) | 56 (90.3) | |
| >2 | 6 (7.6) | 0 | 6 (9.7) | |
Data are presented as mean ± SD or count (percentage).
BMI, body mass index; DVT, deep-venous thrombosis; VTE, venous thromboembolism; PE, pulmonary embolism; RBBB, right bundle branch block; CTA, computed tomography angiography; V/Q scan, ventilation/perfusion scan; RDW, red cell distribution width; BUN, blood urea nitrogen; GFR, glomerular filtration rate; RV dysfunction, right ventricular dysfunction; LMWH, low-molecular-weight heparin; NOAC, novel oral anticoagulants; IVC, inferior vena cava; PESI, pulmonary embolism severity index.
*p value <.05 is considered statistically significant.
In our study population, 29 patients (37%) were males and 50 (63%) were females. Elderly patients (defined as age ≥65 years) comprised 34% of the study population compared to younger patients with age <65 years (66%). Majority of our patients (94%) were African-Americans. Age groups showed statistically significant difference in LOS; χ2 (1, N = 79) = 4.84, p = .028, with patients in short LOS being younger (M = 48.18, SD = 14.84) compared to long LOS (M = 57.84, SD = 17.49). However, gender did not show any statistically significant difference in LOS between the two groups; χ2 (1, N = 79) = 0.50, p = .481. In 86% of the patients, PE was confirmed by pulmonary CTA, while V/Q scan was utilized when renal impairment precluded the use of contrast in 14% of cases.
Hypertension was the most common comorbidity present on more than half of the patients (57%) followed by lung disease (33%), smoking (24%), and diabetes (23%), in descending order of prevalence. Patients with long LOS were more likely to have more comorbidities than patients with short LOS, but no statistically significant differences were found between the two cohorts for each comorbidity evaluated with the exception of lung disease, which was more common in long LOS group (39%) compared to short LOS group (12%), χ2 (1, N = 79) = 4.39, p = .036. In general, there was a statistically significant difference in the total number of comorbidities between patients with LOS ≤2 days and >2 days (M = 1.41, SD = 1.37 and M = 2.27, SD = 1.57, respectively), t (77) = 2.06, p = .043.
The most common risk factors were recent immobilization (≥3 days), history of previous VTE, and concomitant proximal DVT diagnosed at the time of admission representing 17%, 17%, and 14%, respectively. Recent surgery and associated cancer were only present in long LOS group. On the other hand, there was a statistically significant difference in the history of recent travel as a risk factor between the two groups and was associated with short LOS, with most patients with history of travel (18%) being discharged within 2 days of admission (p = .030).
Dyspnea was the most frequent presenting complaint (66%) followed by chest pain (39%), leg swelling and/or pain (28%), and palpitations (17%). Very few patients presented with altered mentation (1%), but they had long LOS. There were no statistically significant differences in vital signs between the two groups: pulse rate, systolic blood pressure, or oxygen saturation (t(77) = 1.86, p = .067; t(77) = 0.17, p = .868; and t(77) = 0.86, p = .392, respectively).
Half of our patients (49%) had a normal EKG on presentation. EKG abnormalities when present were mostly sinus tachycardia (18%). Few patients had atrial arrhythmias (10%), new inferior Q waves or anterior ST-T changes (5%), sinus bradycardia (4%), S1Q3T3 pattern (3%), and new RBBB (3%). Both biochemical marker values, TnI and BNP, were more likely to be positive in long LOS than in short LOS, but no statistically significant difference was found (χ2(1, N = 79) = 2.29, p = .131 and χ2(1, N = 66) = 1.42, p = .234, respectively). Patients with RV dysfunction accounted for 28% and more than three-quarters (82%) were in long LOS group (p = .767).
Most patients were treated with low-molecular-weight heparin (LMWH) and/or warfarin (39% and 47%, respectively). Around 35% received novel oral anticoagulants (NOACs) and only three patients received inferior vena cava filters for secondary prevention of PE. Only one patient had serious bleeding as a complication of anticoagulation during admission. Most patients were safely discharged (96%). In-hospital mortality was 4%, occurring only in the long LOS group. Among all patients, 41% required ICU stay.
Among the clinical prognostic scores, both PESI (original and simplified) scores showed statistically significant difference between short and long LOS (χ2(1, N = 79) = 9.45, p = .002 and χ2(1, N = 79) = 10.72, p = .001, respectively). On the other hand, both Bova and GPS scores showed no statistically significant difference in mean between the two groups (U = 395.50, Z = −1.67, p = .094; U = 438.50, Z = −1.20, p = .0232; respectively).
3.2. Predictors of length of stay
Binary logistic regression models revealed a PESI score that significantly predicted long LOS (OR 1.067, 95% CI [1.001, 1.137], p = .048), even when adjusted for age, presence of lung disease, and the number of comorbidities. Similarly, sPESI also significantly predicted long LOS (OR 0.223, 95% CI [0.050, 0.999], p = .050), adjusted for age group, the presence of lung disease, and the number of comorbidities. Bova score was not a statistically significant predictor of LOS. Additionally, lung disease was not a statistically significant predictor of prolonged hospital stay in both regression models tested.
3.3. Optimal cutoff of prediction
The calculated receiver operating characteristic (ROC) analysis for PESI score predicting prolonged hospital showed an area under the curve (AUC) of .743 with a p value of .002 for differentiation. A PESI score cutoff value of 50.00 had a sensitivity and a specificity of 84% and 41%, respectively (Figure 1).
Figure 1.
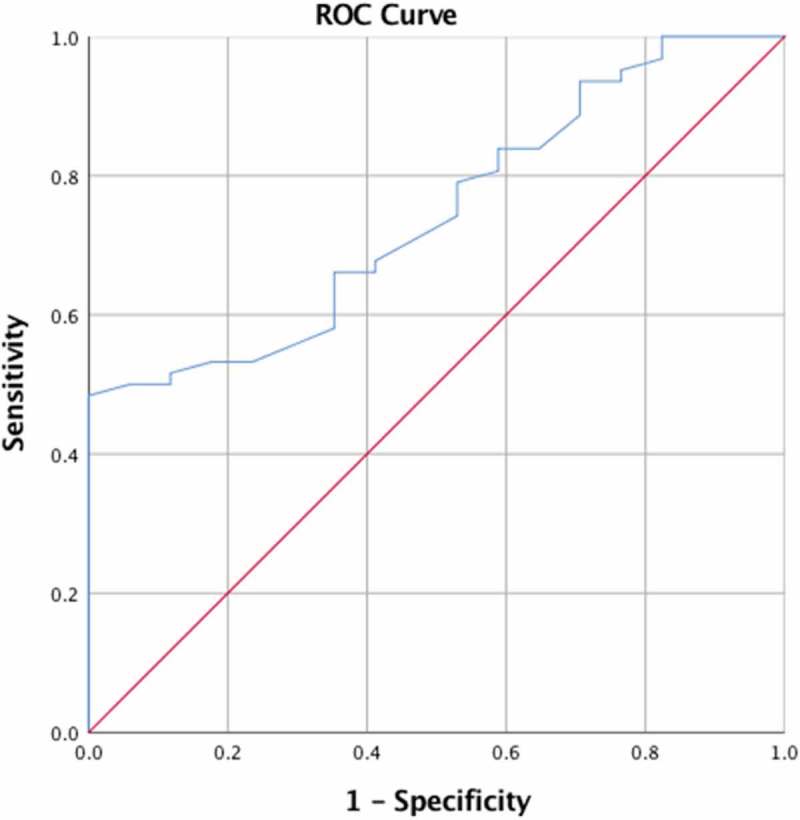
ROC curve with AUC to test the effectiveness of PESI score as a predictor of prolonged LOS.
4. Discussion
Acute PE is one of the leading causes of morbidity and mortality [1]. During the acute phase, it is of supreme importance to stratify the severity of the disease because it may help guide decision-making in terms of appropriate setting of treatment. In a study conducted by Wang et al. [11], LRPE patients with a short LOS had better clinical outcomes than those with long LOS which was complicated with a higher rate of hospital-acquired morbidities. Our investigation about the association between the parameters and LOS is unique as it allowed us to determine the factors which lead to prolonged LOS.
The mortality of acute PE can be predicted with myocardial injury, RV dysfunction, and other biochemical parameters. Meta-analysis of five prospective studies of hemodynamically stable PE demonstrated that 44% of patients had RV dysfunction and a higher mortality rate with a sensitivity of 70%, a positive predictive value of 58%, and a negative predictive value of 60% for prediction of mortality [12]. In another study, the prognostic value of echocardiogram in hemodynamically stable PE patients was moderate [13]. In our study, 29% of patients had RV dysfunction, of which about (82%) belonged to long LOS group. However, these results were not statistically significant (p = .767). Yet, this finding was limited by its operator dependence, a relatively small sample size, and lack of reference echocardiograms for comparison prior to diagnosis of RV dysfunction.
Higher values of cardiac biomarkers (TnI and BNP) were noted in patients who belonged to long LOS when compared with short LOS group, but no statistically significant difference was found between the two groups, as well. Elevated cardiac biomarkers have been linked with an increased risk of mortality during the acute phase of PE [14]. Furthermore, Becatinni et al. published meta-analysis which supported the fact that troponin I and/or T elevations were associated with higher medical conditions and mortality even in the subgroup of low-risk and hemodynamically stable patients [15]. Nevertheless, large-scale investigations are clearly necessary to define the precise correlation of cardiac biomarkers and its association with duration of in-hospital stay for patients with an acute PE.
PESI, the most extensively validated prognostic model for acute PE, stratifies patients into five classes (Class I–V) based on 11 clinical parameters with different prognostic weights. Similar to the above parameters, PESI score was initially devised to predict mortality and to identify low-risk patients who may be candidates for outpatient treatment or abbreviated hospital stay [16]. sPESI, with similar prognostic accuracy, reduces complexity by including 6 of the 11 original PESI variables [7], and we aimed to analyze the usefulness of PESI and sPESI scores to predict LOS in normotensive patients with acute PE. Our investigation showed that a PESI score of <85 points and a sPESI score of 0 (low-risk class) identified patients at low risk and accurately predicted short LOS. Other factors that may result in prolonged LOS are the presence of lung disease and the total number of comorbidities. The calculated effectiveness in predicting prolonged LOS showed AUC of 0.743 (p = .002) with a sensitivity and specificity of 84% and 41% when the diagnostic cutoff was further lowered to score of 50, respectively. Based on these findings, physicians should be more comfortable with outpatient treatment especially when home services are adequate which is further supported by recently updated guidelines [12].
In summary, the results of this study showed that PESI score is a major predictor in LRPE patients to define short LOS. The risk stratification is of utmost importance as it can safely categorize patients eligible for short-term hospital stay which in turn reduces disease’s clinical and financial burden. Even though cardiac biomarkers and RV dysfunction are predictors of mortality, their role in defining LOS is yet to be established.
5. Conclusion
PE severity stratification is essential in determining LOS. Several clinical, laboratory, and imaging parameters have been used to assess PE severity. Physicians are expected to take into consideration all these variables in their management plan. PE predictors of LOS might help to assess PE severity and may effectively help to reduce LOS, health care utilization, and cost. However, more studies with a larger sample size are needed to come up with a more accurate tool for assessment of PE severity, which can assist physicians to take decision prospectively about hospital stay for optimal treatment of PE patients.
6. Limitations
This study is subject to the limitations inherent in its retrospective design. We conducted a review of 149 charts over the period of 2 years, and only 79 patients were eligible to be included in this study as per our selection criteria. As a result, the number of patients is relatively small, which potentially affects the statistical power of the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Dentali F, Ageno W, Pomero F, et al. Time trends and case fatality rate of in-hospital treated pulmonary embolism during 11 years of observation in Northwestern Italy. Thromb Haemost. 2016January;115(2):399–405. PubMed PMID: 26422774. [DOI] [PubMed] [Google Scholar]
- [2].Wiener RS, Schwartz LM, Woloshin S.. Time trends in pulmonary embolism in the USA: evidence of overdiagnosis. Arch Intern Med. 2011May9;171(9):831–837. PubMed PMID: 21555660; PubMed Central PMCID: PMCPMC3140219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Grosse SD, Nelson RE, Nyarko KA, et al. The economic burden of incident venous thromboembolism in the USA: a review of estimated attributable healthcare costs. Thromb Res. 2016January;137:3–10. PubMed PMID: 26654719; PubMed Central PMCID: PMCPMC4706477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Belohlavek J, Dytrych V, Linhart A. Pulmonary embolism, part I: epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp Clin Cardiol. 2013Spring;18(2):129–138. PubMed PMID: 23940438; PubMed Central PMCID: PMCPMC3718593. [PMC free article] [PubMed] [Google Scholar]
- [5].Kearon C. Diagnosis of pulmonary embolism. Cmaj. 2003January21;168(2):183–194. PubMed PMID: 12538548; PubMed Central PMCID: PMCPMC140429. [PMC free article] [PubMed] [Google Scholar]
- [6].Donze J, Le Gal G, Fine MJ, et al. Prospective validation of the pulmonary embolism severity index. A clinical prognostic model for pulmonary embolism. Thromb Haemost. 2008November;100(5):943–948. PubMed PMID: 18989542. [DOI] [PubMed] [Google Scholar]
- [7].Jimenez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010August9;170(15):1383–1389. PubMed PMID: 20696966. [DOI] [PubMed] [Google Scholar]
- [8].Bova C, Sanchez O, Prandoni P, et al. Identification of intermediate-risk patients with acute symptomatic pulmonary embolism. Eur Respir J. 2014September;44(3):694–703. PubMed PMID: 24696111. [DOI] [PubMed] [Google Scholar]
- [9].Subramaniam RM, Mandrekar J, Blair D, et al. The Geneva prognostic score and mortality in patients diagnosed with pulmonary embolism by CT pulmonary angiogram. J Med Imaging Radiat Oncol. 2009August;53(4):361–365. PubMed PMID: 19695042. [DOI] [PubMed] [Google Scholar]
- [10].Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016February;149(2):315–352. PubMed PMID: 26867832. [DOI] [PubMed] [Google Scholar]
- [11].Wang L, Baser O, Wells P, et al. Benefit of early discharge among patients with low-risk pulmonary embolism. PLoS One. 2017;12(10):e0185022 PubMed PMID: 29016692; PubMed Central PMCID: PMCPMC5634547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012February;141(2 Suppl):e419S–e496S. PubMed PMID: 22315268; PubMed Central PMCID: PMCPMC3278049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J. 2008June;29(12):1569–1577. PubMed PMID: 18495689. [DOI] [PubMed] [Google Scholar]
- [14].Klok FA, Mos IC, Huisman MV. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med. 2008August15;178(4):425–430. PubMed PMID: 18556626. [DOI] [PubMed] [Google Scholar]
- [15].Bajaj A, Saleeb M, Rathor P, et al. Prognostic value of troponins in acute nonmassive pulmonary embolism: A meta-analysis. Heart Lung. 2015Jul-Aug;44(4):327–334. PubMed PMID: 25976228. [DOI] [PubMed] [Google Scholar]
- [16].Wicki J, Perrier A, Perneger TV, et al. Predicting adverse outcome in patients with acute pulmonary embolism: a risk score. Thromb Haemost. 2000October;84(4):548–552. PubMed PMID: 11057848. [PubMed] [Google Scholar]


