SUMMARY
Plasmodium infection begins with the bite of an anopheline mosquito, when sporozoites along with saliva are injected into a vertebrate host. The role of the host responses to mosquito saliva components in malaria remains unclear. We observed that antisera against Anopheles gambiae salivary glands partially protected mice from mosquito-borne Plasmodium infection. Specifically, antibodies to A. gambiae TRIO (AgTRIO), a mosquito salivary gland antigen, contributed to the protection. Mice administered AgTRIO antiserum showed lower Plasmodium liver burden and decreased parasitemia when exposed to infected mosquitoes. Active immunization with AgTRIO was also partially protective against Plasmodium berghei infection. A combination of AgTRIO antiserum and antibodies against Plasmodium circumsporozoite protein, a vaccine candidate, further decreased P. berghei infection. In humanized mice, AgTRIO antiserum afforded some protection against mosquito-transmitted Plasmodium falciparum. AgTRIO antiserum reduced the movement of sporozoites in the murine dermis. AgTRIO may serve as an arthropod-based target against Plasmodium to combat malaria.
Graphical abstract
In Brief Plasmodium infection begins with the bite of an anopheline mosquito, when sporozoites along with saliva are injected into a vertebrate host. Dragovic et al. demonstrate that antiserum against mosquito salivary glands decreases Plasmodium infection levels in mice, and antibodies against AgTRIO, a mosquito salivary protein, contribute to this effect.
INTRODUCTION
Despite recent efforts, malaria remains one of the most lethal infectious diseases worldwide, with approximately half a million deaths each year (Varghese et al., 2016). A highly effective malaria vaccine has not yet been developed. The most established vaccine formulation (RTS,S) is a recombinant protein containing regions of the Plasmodium circumsporozoite protein (CSP) (Chaudhury et al., 2016; Kaslow and Biernaux, 2015). The vaccine targets the pre-erythrocytic stage of the parasite and confers a degree of protection (less than 40% against clinical disease and severe malaria) that wanes over time (Chaudhury et al., 2016; Kaslow and Biernaux, 2015). This makes an understanding of additional factors that contribute to immunity against Plasmodium crucial.
Malaria begins when an infected female Anopheles mosquito, while probing for a blood meal, injects saliva together with Plasmodium sporozoites into the skin of the vertebrate host (Vanderberg and Frevert, 2004; Yamauchi et al., 2007). The saliva contains biologically active molecules, which modulate host responses, including coagulation, platelet aggregation, thrombin activation, and vasodilation (Fontaine et al., 2011; Hayashi et al., 2012; Ribeiro et al., 1994; Ronca et al., 2012). Indeed, the saliva of arthropods can enhance the infectivity of diverse pathogens (Edwards et al., 1998; Limesand et al., 2000; Schneider and Higgs, 2008; Schneider et al., 2006), including Aedes and Culex mosquito saliva and arboviruses (Le Coupanec et al., 2013; Reagan et al., 2012; Styer et al., 2011), sand fly saliva and Leishmania (Kamhawi, 2000; Norsworthy et al., 2004; Ockenfels et al., 2014; Theodos and Titus, 1993), tick saliva and Borrelia (Wikel et al., 1997), or tsetse fly saliva and trypanosomiasis (Caljon et al., 2006). Moreover, immunization with a phlebotomine salivary protein, PdSP15, prevents cutaneous leishmaniasis transmitted by sand flies (Oliveira et al., 2015; Valenzuela et al., 2001), and another sand fly salivary protein, LJM19, protects mammals from fatal infection associated with visceral leishmaniasis (Gomes et al., 2008).
The influence of the immune response to mosquito saliva on Plasmodium infection has been controversial. Early studies showed very modest protection against infection by Plasmodium berghei sporozoites when mice were immunized with mosquito salivary gland homogenates and challenged with sporozoites intraperitoneally (Alger and Harant, 1976; Alger et al., 1972). It was later suggested that non-specific immune responses contributed to the effect (Kebaier et al., 2010). It was subsequently demonstrated that chickens subjected to uninfected mosquito bites showed decreased infection by Plasmodium gallinaceum sporozoites as measured by parasitemia (Rocha et al., 2004). In addition, partial immunity against Plasmodium yoelii infection was reported in mice repeatedly pre-exposed to uninfected bites from Anopheles stephensi mosquitoes (Donovan et al., 2007). It was proposed that exposure to mosquito saliva resulted in a T-helper 1 response that contributed to a lower parasite burden (Donovan et al., 2007). Conversely, more recent studies using both the P. berghei and P. yoelii model systems showed that pre-exposure of mice to saliva via uninfected mosquito bites had no detectable effect on the number of sporozoites delivered by mosquitoes or their infectivity in the animals (Kebaier et al., 2010). Moreover, a subsequent report demonstrated that mice injected with sporozoites while mosquitoes were concomitantly feeding on the animals progressed more rapidly to cerebral malaria (Schneider et al., 2011). These results suggested that saliva can contribute to the disease evolution; however, the underlying interactions between salivary proteins and immune responses were not addressed (Schneider et al., 2011). Overall, these data are conflicting, and differences in methodology may partially explain the divergent results. In our opinion, the preponderance of the evidence suggests that the importance of host responses to mosquito saliva or saliva protein components in protection against malaria remains unclear. We therefore undertook studies to systematically examine the effect of antiserum generated against Anopheles gambiae salivary gland extracts in protection against Plasmodium infection in mice.
RESULTS
Antiserum against A. gambiae Salivary Glands Influences Mosquito-Borne P. berghei Infection of Mice
As natural exposure to mosquito bites does not seem to provide immunity against malaria (Remoue et al., 2006; Stone et al., 2012; Waitayakul et al., 2006; Ya-Umphan et al., 2017), we determined whether hyperimmune antiserum prepared against A. gambiae salivary gland extract (SGE) altered mosquito-borne P. berghei infection. Mice were administered SGE antiserum and then P. berghei-infected A. gambiae mosquitoes took a blood meal on the animals. At 40 hr after challenge with the infected mosquitoes, livers were removed to determine the parasite burden. Mice administered SGE antiserum had lower levels of Plasmodium in the liver than the control group (Figure 1A). On days 5 and 6 after infection, mice administered SGE antiserum also had lower levels of parasitemia (Figures 1B and 1C). Overall, antibodies raised against components of SGE had a partially protective effect against Plasmodium infection in mice.
Figure 1. A. gambiae SGE Antiserum Influences P. berghei Infection.
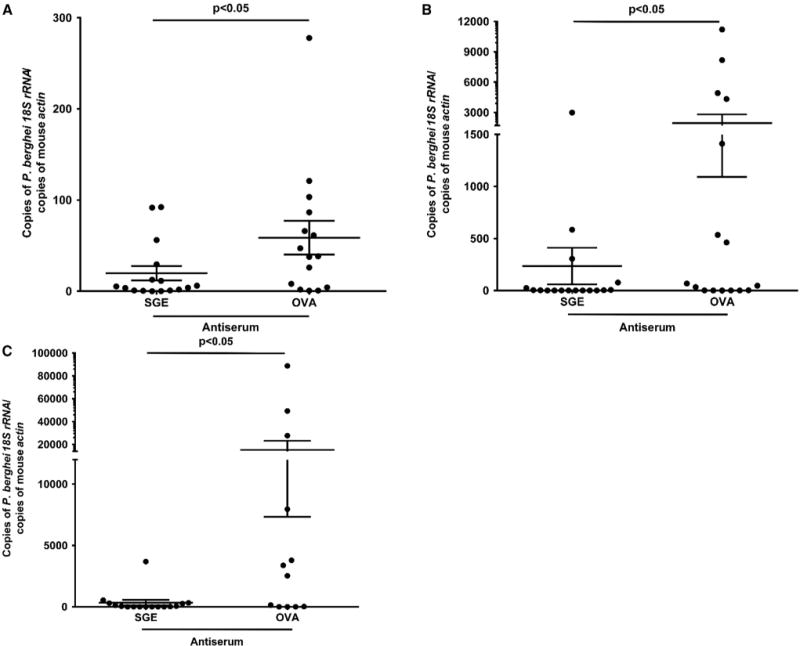
(A) Liver burden in mice administered SGE antiserum and challenged with P. berghei-infected mosquitoes. Mice administered ovalbumin (OVA) antiserum served as controls. The liver burden was determined 40 hr after the mosquitoes fed.
(B and C) Parasitemia levels on days 5 and 6 in mice that received SGE antiserum and were then engorged upon by infected mosquitoes. Mice immunized with OVA antiserum served as controls. Results depict two combined independent experiments. Each data point represents one mouse. Error bars represent mean ± SEM. Mean values were considered significantly different using the non-parametric Mann-Whitney test when p < 0.05.
See also Table S1.
A. gambiae TRIO Is Secreted into Mosquito Saliva and Recognized by SGE Antiserum
To identify antigens recognized by SGE antiserum that contributed to the diminished levels of Plasmodium infection, immunoglobulin G (IgG) purified from SGE antiserum was used to probe an A. gambiae salivary gland cDNA yeast surface display library. The screen identified genes encoding at least nine A. gambiae proteins with putative signal sequences, suggesting that they are secreted into saliva, as well as 12 proteins lacking a signal sequence (Table S1). We focused on AGAP001374 because it belongs to the Anopheles-specific SG1 gene family (Arca et al., 2005) and likely encoded for a secreted protein. The AGAP001374 protein was previously named TRIO because of a minimal degree of homology with Drosophila TRIO (Francischetti et al., 2002), an intracellular protein with Rho GTPase activity (Schmidt and Debant, 2014). We now call it A. gambiae TRIO (AgTRIO) to reflect that it is from A. gambiae, although functional domains required for Rho GTPase activity are lacking. AgTRIO was previously reported to be expressed in the salivary glands of female anopheline mosquitoes (Arca et al., 2005; Marie et al., 2014). We also found that AgTRIO was expressed only in salivary glands and not in other organs (Figure 2A). Consistent with this, we detected AgTRIO protein in the salivary glands (Figure 2B). The presence of P. berghei sporozoites in the salivary glands augmented the expression of AgTRIO (Figure 2C) (Marie et al., 2014; Zocevic et al., 2013) and the production of AgTRIO protein (Figure 2D). In addition, AgTRIO was secreted into saliva, as confirmed by a western blot with AgTRIO antiserum (Figure 2E).
Figure 2. Expression of AgTRIO in Mosquitoes.
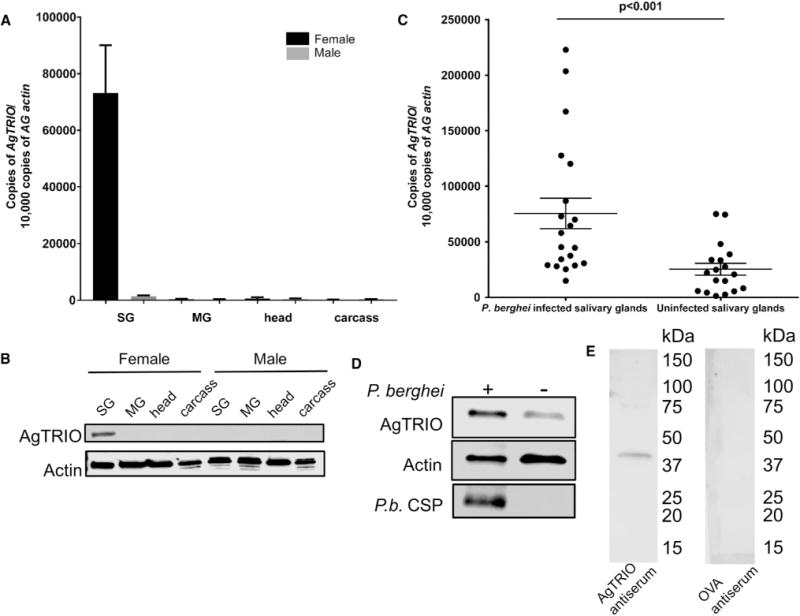
(A) qRT-PCR analysis of tissues from A. gambiae mosquitoes for AgTRIO gene expression. A. gambiae actin (AG actin) was used as the control. SG, salivary gland; MG, midgut.
(B) Mosquito organs were analyzed for AgTRIO protein using AgTRIO antiserum. Actin antibody was used to detect actin levels and normalized as a loading control.
(C) Female P. berghei-infected and uninfected A. gambiae salivary glands were analyzed for AgTRIO expression using qRT-PCR. Each data point represents one salivary gland. Error bars represent mean ± SEM. Mean values were considered significantly different using the non-parametric Mann-Whitney test when p < 0.05.
(D) Female P. berghei-infected salivary glands have higher AgTRIO protein levels compared with uninfected samples, as determined using AgTRIO antiserum. Actin levels serve as the loading control, and were detected using an actin-specific antibody. P. berghei CSP (P.b. CSP) is also shown to demonstrate the presence of sporozoites.
(E) Saliva was collected from uninfected A. gambiae mosquitoes, as described in the STAR Methods. Membranes were blotted with AgTRIO antiserum or OVA antiserum (control).
See also Figure S3 and Table S2.
Antibodies against AgTRIO Interfere with Mosquito-Borne P. berghei or Plasmodium falciparum Infection of Mice
Previous studies demonstrated that the depletion of AgTRIO in salivary glands does not alter mosquito probing time and blood-feeding behavior (Calvo et al., 2010). Since AgTRIO is a component of A. gambiae saliva and secreted into the mammalian host in conjunction with Plasmodium sporozoites, we examined whether antibodies against AgTRIO could influence the infectivity of sporozoites during the early stages of murine infection. We first assessed whether targeting AgTRIO could affect sporozoite infection of the liver. Naive mice received AgTRIO antiserum and were challenged with P. berghei-infected A. gambiae mosquitoes. The administration of AgTRIO antiserum led to a decrease in the parasite burden in the liver (Figure 3A). These results suggest that Plasmodium sporozoites are directly or indirectly affected by AgTRIO antibodies and are unable to establish a high level of hepatic infection as rapidly as control animals. Additional studies also showed a significant decrease in blood stage parasite levels in mice as measured by qRT-PCR and flow cytometric analysis (Figures 3D–3I). We purified the IgG fraction of the AgTRIO antiserum and performed passive transfer studies to extend our results and to delineate a dose-dependent effect of AgTRIO IgG on Plasmodium infection (Figure S1). These results suggest that antibodies against AgTRIO reduced the degree of murine infection with P. berghei in a dose-dependent manner.
Figure 3. Infection in Mice Administered AgTRIO Antiserum and Fed upon by P. berghei- or P. falciparum-Infected Mosquitoes.
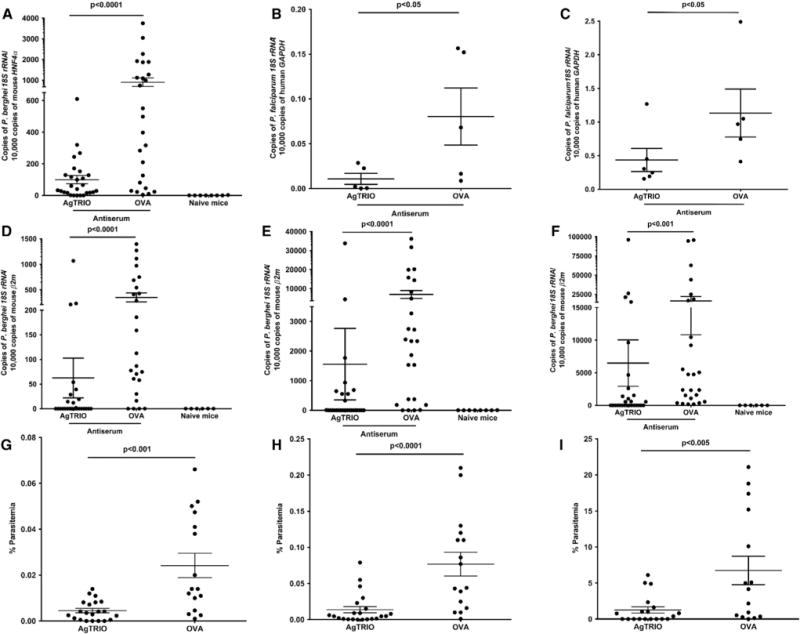
Mice were given AgTRIO antiserum or OVA antiserum (control).
(A) Each mouse was exposed to three P. berghei-infected mosquitoes and, 40 hr later, livers were excised and homogenized to isolate total RNA. qRT-PCR analysis was performed to detect levels of P. berghei RNA using 18S rRNA, and hepatocyte nuclear factor 4 alpha (HNF-4α) as the control gene. qRT-PCR was performed on naive mice to ensure that none of the samples were contaminated with Plasmodium DNA.
(B and C) Human hepatocyte engrafted mice (FNRG) were given either AgTRIO or OVA antiserum. Each mouse was exposed to 12 P. falciparum-infected A. gambiae mosquitoes. Seven days later, livers were removed to isolate RNA. qRT-PCR was performed using P. falciparum 18S rRNA and human GAPDH as the control gene. Results represent two independent experiments (C). Human liver chimeric FNRG mice were administered AgTRIO or OVA antiserum. Humanized mice were then exposed to six P. falciparum-infected A. stephensi mosquitoes. Livers were collected 7 days later to isolate RNA. qRT-PCR was performed using P. falciparum 18S rRNA and human GAPDH as the control gene.
(D–I) Each mouse was exposed to three P. berghei-infected mosquitoes, and blood was collected on days 4 (D and G), 5 (E and H), and 8 (F and I).
(D–F) Infected blood was used for RNA isolation and qRT-PCR analysis for P. berghei 18S rRNA with murine β2-microglobulin (mouse β2m) serving as a control. Results represent three combined independent experiments. As an additional control in the PCR studies (A and D–F), naive mice were not given antisera, nor were they exposed to infected mosquitoes.
(G–I) Mouse blood was isolated to perform flow cytometry and determine percentage parasitemia for each mouse. Results represent two combined independent experiments. Each data point represents one mouse. Error bars represent mean ± SEM. Mean values were considered significantly different using the non-parametric Mann-Whitney test when p < 0.05.
See also Figures S1 and S5 and Table S2.
We next examined whether the effect of AgTRIO antiserum extended to Plasmodium falciparum, a major cause of human disease. We utilized human liver chimeric mice, which support liver stage infection with P. falciparum (VanBuskirk et al., 2009; Vaughan et al., 2012). Specifically, FNRG mice (FAH−/−, NOD Rag1−/−, and IL2RgNULL) were transplanted with human adult hepatocytes (de Jong et al., 2014). Human liver chimeric FNRG mice were then injected with AgTRIO or OVA (control) antiserum. Humanized mice were exposed to P. falciparum-infected A. gambiae or A. stephensi mosquitoes. Seven days later, livers were isolated to determine the pathogen burden. Mice given AgTRIO antiserum had reduced infection levels compared with the control groups (Figures 3B and 3C). Therefore, AgTRIO antibodies can alter mosquito-borne P. falciparum infection in human liver chimeric mice.
AgTRIO and CSP Antibodies Synergize against Plasmodium in Mice
Antibodies against Plasmodium CSP can protect mammalian hosts against malaria (Mishra et al., 2012; Persson et al., 2002). This antigen is the main component of RTS,S, which is currently the most advanced malaria vaccine candidate (Kaslow and Biernaux, 2015). However, the CSP antibody titer generated by the RTS,S vaccine is often low and declines with time, and protective efficacy is not optimal (Long and Zavala, 2016; Penny et al., 2015). We therefore tested whether antibodies against AgTRIO could augment the protective capacity of a CSP monoclonal antibody (mAb). As expected, P. berghei CSP mAb 3D11 decreased the parasite burden in a dose-dependent manner in mosquito-borne P. berghei transmission to mice (Figure 4A). Protection was diminished at a 3D11 mAb dose of 50 μg per mouse (Figure 4A), so we chose a slightly lower dose of 30 μg per mouse for our combination studies. When AgTRIO and 3D11 antibodies were both administered to mice, Plasmodium parasitemia was decreased from day 4 to 8 (Figures 4B–4G), indicating that AgTRIO antibodies may enhance the efficacy of CSP antibodies against malaria.
Figure 4. AgTRIO and CSP Antibodies Work in Conjunction against Plasmodium Infection In Vivo.
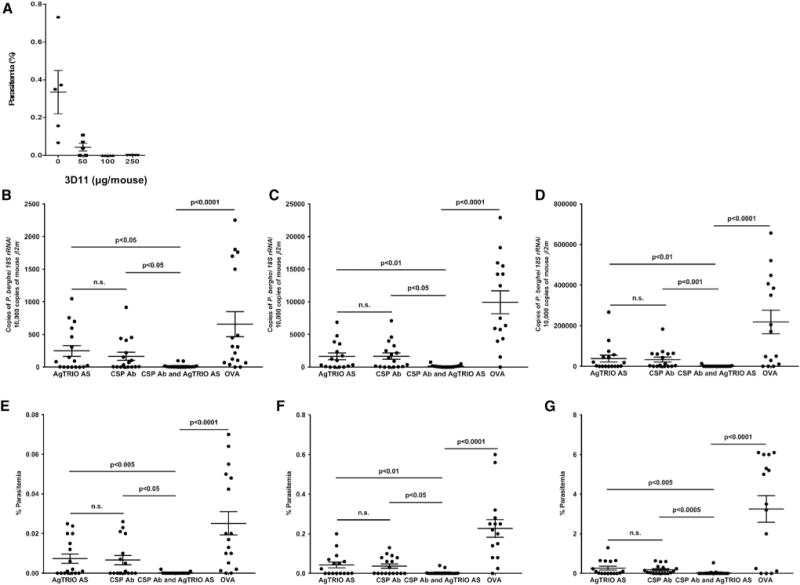
(A) A CSP mAb exhibits dose-dependent protection against P. berghei infection. Mice were injected with various amounts of the P. berghei-specific CSP mAb 3D11 (0, 50, 100, and 250 μg/animal) to determine the dose at which protection wanes. Twenty hours later, the mice were exposed to P. berghei-infected mosquitoes. Parasitemia was measured 5 days after infection. This experiment was performed two times.
(B–G) Mice were administered AgTRIO antiserum alone, a suboptimal dose of CSP mAb 3D11, a combination of AgTRIO and CSP mAb 3D11 (30 μg), or OVA antiserum (control). Mice were fed upon by three P. berghei-infected mosquitoes, and blood was collected on days 4 (B and E), 5 (C and F), and 8 (D and G). (B–D) qRT-PCR analysis was performed by examining P. berghei 18S rRNA and murine β2-microglobulin (mouse β2m) as the control gene. For the PCR studies, as an additional control, naive mice were not administered antibodies or exposed to P. berghei-infected mosquitoes.
(E–G) On days 4, 5, and 8, mouse blood was analyzed by flow cytometry and quantified as a percentage of total red blood cells. Results represent two combined independent experiments. Each data point represents one mouse. Error bars represent mean ± SEM. Mean values were considered significantly different using the non-parametric Mann-Whitney test when p < 0.05. n.s., not significant.
See also Table S2.
Antisera against Additional SG1 Family Members, SG1L3 and SG1b, or an Abundant Protein Recognized in the Yeast Display Assay (D7r1), Are Not Protective against Plasmodium
We then determined whether the effects observed with AgTRIO extended to other members of the SG1 family or another protein identified in our A. gambiae salivary gland yeast display screen. We chose two additional SG1 family members, SG1L3 and SG1b, which have been demonstrated to be expressed in female salivary glands (Arca et al., 2005). We also included D7r1 because it is one of the most abundant salivary components in A. gambiae (Baker et al., 2011) and was identified in our screen (Table S1). The D7 protein family is a distinct branch of the odorant-binding protein superfamily and D7r1 is one of the short forms (Calvo et al., 2006). We generated SG1L3, SG1b, and D7r1 antisera in a similar fashion to the AgTRIO antiserum. AgTRIO, SG1L3, SG1b, and D7r1 all carry signal sequences and display little similarity (Figure S2). Immunoblots demonstrated that each protein was present in mosquito salivary glands (Figures S3A–S3D) and saliva (Figures S3E–S3G and 2E). Each antiserum bound to its respective proteins in ELISA (optical density [OD] >0.8 at a dilution of 1:10,000). Mice were passively immunized with each antiserum and the animals were then exposed to P. berghei-infected mosquitoes. SG1L3, SG1b, or D7r1 antisera did not have an effect on mosquito-transmitted P. berghei infection of mice when liver burden levels (Figures S4A and S4B) or blood stage infection (Figures S4C–S4E) was measured.
AgTRIO Antibodies Are Not Generally Elicited by Natural Mosquito Bites; However, High Titers in Mice Are Detected Following Active Immunization
Since AgTRIO antibodies altered mosquito-borne Plasmodium infection in mice, we determined whether AgTRIO IgG responses developed in mice bitten by A. gambiae mosquitoes. The IgG responses against AgTRIO in mice exposed to 40 mosquito bites, at least six times over a period of several months, were measured in ELISA. AgTRIO IgG was not detected in these animals (Figure 5A). In contrast, mice bitten by A. gambiae mosquitoes elicited substantial IgG responses to A. gambiae SGE and recombinant D7r1 protein (Figure 5A) compared with control mice. The human response demonstrated similarities to the murine response. Individuals from Senegal, where malaria is endemic, had highly significant IgG responses to A. gambiae SGE compared with persons from Marseille, where malaria is not present (Figure 5B). In contrast, persons from Senegal and Marseille had low IgG responses to AgTRIO, which were not significantly different. In addition, a previous study with these groups of sera has shown that individuals from Senegal had IgG responses to selected A. gambiae components, while persons from Marseille did not (Ali et al., 2012), further suggesting that AgTRIO is not highly antigenic during a natural mosquito bite.
Figure 5. IgG Response of Mice Bitten by A. gambiae, and Humans to AgTRIO, A. gambiae SGE, and D7r1.
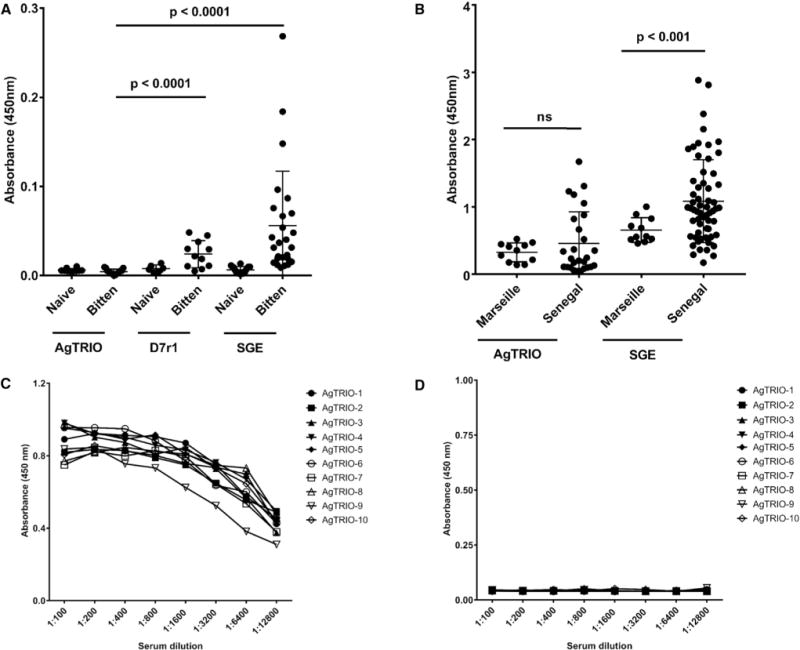
(A) Mice were fed upon by 40 mosquitoes (bitten mice), at least 6 times, over a period of several months. Control mice (naive mice) were never exposed to mosquitoes.
(B) Sera from individuals from a malaria-endemic region (Senegal) and a non-endemic region (Marseille) were examined for IgG to AgTRIO, D7r1, and A. gambiae SGE. Mean values were considered significantly different using the non-parametric Mann-Whitney test when p < 0.05. The cutoff value for seropositivity in mice and humans for AgTRIO (the mean OD ± 3 SDs) was defined at 0.01 and 0.75, respectively, based on the IgG reactivity of sera from naive, unexposed mice and naive, unexposed individuals from Marseille. These sera have previously been examined for responses to other A. gambiae salivary proteins (Ali et al., 2012). ns, not significant.
(C and D) A group of 10 C57Bl/6 female mice was injected with AgTRIO or OVA in Freund’s adjuvant. Two weeks after final boost, mice were bled and the sera examined for specific antibodies in ELISA. Sera from AgTRIO-immunized mice recognized AgTRIO (C), but not OVA (D).
Since natural mosquito bite did not elicit antibody responses both in mice and humans, we next examined whether AgTRIO immunization of mice could lead to productive antibody response. Mice were administered 10 μg AgTRIO with complete Freund’s adjuvant, followed by two boosts in incomplete Freund’s adjuvant. Following the final boost, AgTRIO antibody titers were determined in ELISA against cognate and irrelevant protein (OVA). Results suggest high antibody reactivity against AgTRIO (Figure 5C), while responses to an irrelevant protein were minimal (Figure 5D). Absorbance at 450 nm suggests high serum reactivity against AgTRIO (1:100 dilution; 1.0 OD), but not against a control protein, OVA (1:100 dilution; 0.06 OD).
Active Immunization with AgTRIO Reduces Plasmodium Infection in Mice
We then examined whether active immunization of mice with AgTRIO influenced Plasmodium infection. Animals were exposed to P. berghei-infected A. gambiae mosquitoes. Forty hours later, livers were excised and processed to isolate RNA. A reduced Plasmodium burden in the livers of mice that received AgTRIO compared with OVA was observed (Figure 6A). Mice immunized with AgTRIO also displayed reduced parasitemia at days 4, 5, and 8 compared with control animals (Figures 6B–6G). Therefore, active immunization of mice with AgTRIO influenced mosquito-transmitted P. berghei infection.
Figure 6. Mice Immunized with AgTRIO Protein Display Reduced Parasitemia.
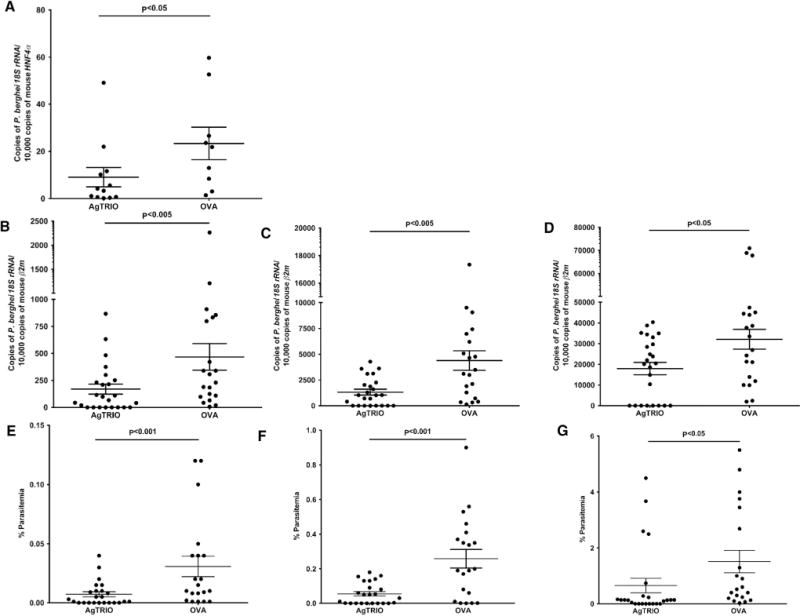
Mice were actively immunized with AgTRIO or OVA (control). Mice were fed upon by three P. berghei-infected mosquitoes.
(A–G) Forty hours following the mosquito challenge, livers were processed to isolate RNA. qRT-PCR determined levels of P. berghei 18S rRNA, and murine hepatocyte nuclear factor 4 alpha (HNF-4α) served as a control. In a subsequent experiment, blood was collected on days 4 (B and E), 5 (C and F), and 8 (D and G) to determine the levels of parasitemia.
(B–D) Blood was used to isolate RNA and perform qRT-PCR to detect P. berghei 18S rRNA, and murine β2-microglobulin (mouse β2m) as a control.
(E–G) Blood was used for flow cytometry to determine percentage parasitemia. Results represent two combined independent experiments. Each data pointrepresents one mouse. Error bars represent mean ± SEM. Mean values were considered significantly different using the non-parametric Mann-Whitney test when p < 0.05. See also Table S2.
AgTRIO Antiserum Decreases Sporozoite Motility and Dispersal in the Murine Skin In Vivo
Once deposited in the skin, sporozoites migrate from the extracellular matrix to a blood vessel in order to cause a systemic infection. Using two-photon microscopy, we examined whether AgTRIO antiserum influenced the velocity and dispersal of sporozoites in the dermis, following the deposition of sporozoites in the murine skin, using several different parameters. Sporozoites in the skin were visualized following direct injection, rather than a mosquito bite, because the number can be controlled and the location more precisely determined. Needle injection with a mixture of sporozoites and salivary gland material may cause local inflammation and damage that allows antibodies to access that extravascular space. AgTRIO antiserum significantly diminished the speed at which sporozoite moved in dermis (Figure 7A) as compared with the control (OVA antiserum) group. Sporozoite track straightness did not differ between the two groups (Figure 7B). Sporozoites in the AgTRIO antiserum-treated group had a lower mean squared displacement over time, indicating a reduced sporozoite deviation from the previous position over time (Figure 7C). Overall, AgTRIO antiserum reduced sporozoite velocity and movement in the murine skin.
Figure 7. AgTRIO Antiserum Reduces Sporozoite Movement and Dispersal in Murine Skin In Vivo.
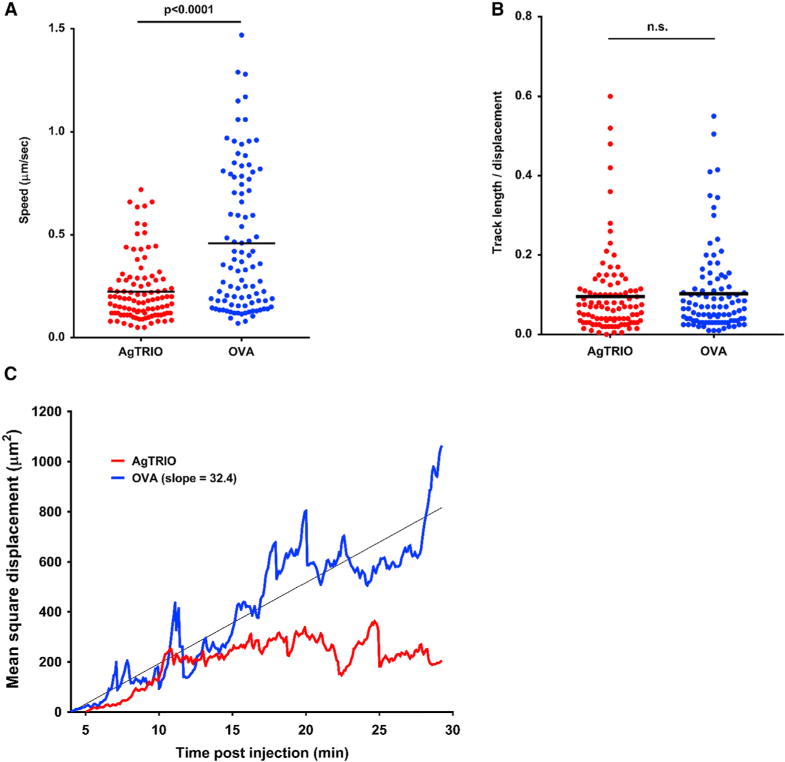
(A) Average speed of sporozoite tracks. Each sample includes 168 and 170 individual sporozoite tracks measured from two mice passively immunized with AgTRIO antiserum and two mice passively immunized with OVA antiserum, respectively. Sporozoite tracks were measures from 4 to 30 min post-intradermal injection.
(B) Sporozoite track straightness was calculated by dividing sporozoite track lengths by total distance displaced.
(C) Mean square displacement of all sporozoite tracks present at any point from 4 to 30 min post-injection. Linear regression of the OVA group fit a linear curve, indicating a random walk. Occasional spikes of faster-than-linear displacement are suggestive of directed motility of individual sporozoites. Sporozoite motility in the AgTRIO group decreased below the linear displacement curve after 10 min, suggesting these sporozoites exhibited a more confined behavior.
See also Figure S6.
DISCUSSION
An examination of how the immune response to mosquito saliva alters Plasmodium infection is increasingly important, particularly in the absence of a highly effective malaria vaccine (Birkett, 2016). We now demonstrate a role for antibodies against A. gambiae SGE in partial protection against malaria and characterize an antigen that contributes to this process. Antisera against A. gambiae SGE diminished murine infection with Plasmodium, including the early hepatic stage of infection and parasitemia. In contrast, the natural immune response to mosquito bites does not afford protection against malaria, and experimental models of mosquito exposure do not demonstrate substantial protection against Plasmodium (Kebaier et al., 2010; Pollock et al., 2011). This may be attributed to several factors, including the quantity of saliva secreted into the host (Abdeladhim et al., 2011), the short duration of a mosquito bite (Kebaier et al., 2010), and/or the relative immunogenicity of specific proteins in the natural milieu of saliva compared with artificial hyper-immunization. In contrast, the A. gambiae SGE antisera, prepared in rabbits using Freund’s adjuvant, elicited robust and diverse responses to numerous proteins in saliva.
Many studies have characterized mosquito salivary gland proteins using different techniques, including genomics, transcriptomic approaches, and proteomic approaches (Ali et al., 2012; Almeras et al., 2010; Arca et al., 2005, 2007; Calvo et al., 2004, 2009, 2010; Kalume et al., 2005; Valenzuela et al., 2003). Using a yeast surface display library from RNA isolated from female A. gambiae mosquitoes, and probing it with A. gambiae SGE antiserum, allows for the selection of antigens independent of the limits of detection due to protein quantity in saliva. We identified a panel of secreted salivary gland proteins, some of which have previously been identified and characterized as markers of exposure to mosquito bites. As AgTRIO was a member of the SG1 family of Anopheles-specific proteins, and D7r1 is a highly recognized and characterized protein, several SG1 family members and D7r1 were selected for further analysis, and only AgTRIO elicited a humoral response that diminished Plasmodium infection. Unlike D7r1, which is found in blood-sucking dipterans, AgTRIO is absent from Aedes and Culex mosquitoes, suggesting a specific role in Anopheles salivary gland biology. AgTRIO does not have conserved domains, including domains associated with the Rho GTPase activity of Drosophila TRIO (Francischetti et al., 2002), making it difficult to predict its function.
AgTRIO IgG and a CSP antibody (3D11) both diminished Plasmodium infection in a dose-dependent fashion (Figures 4 and S1). Immunity against Plasmodium depends on the quantity and quality of sporozoites that are inoculated via a mosquito bite, as well as the immediate levels of protective antibodies. Indeed, Anopheles mosquitoes in endemic areas normally transmit a low number of sporozoites, often less than 100 (Rosenberg et al., 1990; Voza et al., 2012). Such mosquitoes greatly differ from laboratory strains, which can achieve high numbers of parasites in the salivary glands. Laboratory mosquitoes also have optimal conditions in terms of food, temperature, and humidity. Therefore, it is possible that protection afforded in a laboratory setting may differ from that observed in nature. In addition, sporozoites are generally deposited in the extracellular matrix during mosquito probing, and only a small subpopulation migrates to a blood vessel to initiate infection (Amino et al., 2006; Voza et al., 2012). It is most likely that an arthropod-based target, such as AgTRIO, may enhance the efficacy of a traditional, pathogen-based vaccine approach, especially when the antibodies to a pathogen-specific antigen have declined to low levels.
Sporozoites deposited in the skin need to reach a local blood vessel in order to travel to the liver. We demonstrated that AgTRIO antiserum diminished sporozoite velocity and dispersal in the murine dermis. As only a few sporozoites in the skin ultimately reach a blood vessel, any impact on this process can greatly alter the initial pathogen burden during systemic infection. The primary site of action of any antiserum directed at a mosquito saliva protein would likely be the early stage of Plasmodium infection and influenced by the ability of the delivered sporozoites and saliva components to alter local vascular permeability and allow the antibodies to access the extravascular space. Moreover, both direct and indirect interactions between saliva components, the sporozoites, and the host environment, are possible in the skin. Indeed, analysis of cell populations in the skin of mice administered AgTRIO antiserum and then fed upon by Plasmodium-infected mosquitoes indicates that the AgTRIO antiserum increases the number of macrophages and reduces the number of neutrophils and dendritic cells at the bite site (Figure S6). As macrophages help to clear sporozoites (Krettli and Miller, 2001; Verhave et al., 1980), and neutrophils can increase vascular permeability that may enable sporozoites to easily find a blood vessel (Hopp and Sinnis, 2015), changes in these cell populations in response to AgTRIO could contribute to sporozoite infectivity.
Most of our studies were performed using P. berghei. We have, however, performed passive immunization studies using P. falciparum and humanized FNRG mice, which suggest that our data extend to a human pathogen (de Jong et al., 2014). Our studies used P. falciparum-infected A. gambiae or A. stephensi mosquitoes, also suggesting that there are conserved epitopes between AgTRIO and AsTRIO. Indeed, AgTRIO and AsTRIO share at least 50% homology, and AgTRIO antiserum recognizes both proteins in A. gambiae and A. stephensi salivary glands, respectively (Figure S5).
Under natural conditions, some salivary components of mosquitoes induce an antibody response in humans (Fontaine et al., 2011; Penneys et al., 1988; Poinsignon et al., 2008; Remoue et al., 2006; Waitayakul et al., 2006). Since AgTRIO elicits an antibody response after immunization with an adjuvant, we set out to measure the IgG responses to AgTRIO in mice and individuals that had been exposed to A. gambiae. We observed low IgG responses to AgTRIO in both the mice and human groups compared with A. gambiae SGE, indicating that AgTRIO does not elicit a strong IgG response following mosquito bites. This is in contrast with D7r1, which is one of the most abundant proteins in saliva and SGE, for which the responses were much higher. Interestingly, although D7r1 is antigenic, it was not immunogenic in our studies. The sera used for our human IgG response study had previously identified two Anopheles salivary protein candidates that are highly antigenic and potential biomarkers for exposure to mosquito bites (Ali et al., 2012). Several other studies have also identified such salivary protein candidates as biomarkers for exposure to mosquito bites and estimates of malaria transmission (Proietti et al., 2013; Rizzo et al., 2011, 2014; Stone et al., 2012; Ya-Umphan et al., 2017). The low IgG response to AgTRIO suggests a natural lack of antigenicity following mosquito exposure.
The identification of salivary protein targets that influence sporozoites after mosquito bite is a concept that may be incorporated into new approaches to combat malaria. Affecting sporozoites at the point of entry may lead to a decrease in initial parasite burden during the development of systemic infection. Targeting mosquito proteins may also enhance the efficacy of current CSP-based vaccines that are directed at Plasmodium. Furthermore, this tactic may prove useful for other vector-borne diseases and helps to expand the paradigm that arthropod proteins should be considered when new prevention strategies are being developed.
STAR★METHODS
Detailed methods are provided in the online version of this paper and include the following:
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Human Albumin Antibody | Bethyl | (Bethyl Cat# A80-129, RRID: AB_67016) |
| Mouse anti-human albumin | Abcam | ab10241, RRID: AB_296978 |
| Alexa Fluor 488 Goat anti-Rabbit IgG | Thermo | Cat #:A-11008, RRID: AB_143165 |
| Xpress-epitope antibody | Thermo | Cat #:R910-25, RRID: AB_2556552 |
| V5-HRP monoclonal antibody | Thermo | Cat #:R961-25, RRID: AB_2556565 |
| His-tag HRP antibody | Abcam | Cat# ab3553, RRID: AB_303900 |
| Bacterial and Virus Strains | ||
| One Shot Top 10 chemically competent cells | Invitrogen | C404003 |
| One Shot BL21(DE3) Chemically Competent E. coli | Invitrogen | C600003 |
| Biological Samples | ||
| Mouse antiserum | Lab | N/A |
| Human antiserum | Lab | N/A |
| Rabbit antiserum | Cocalico Biologicals, PA | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Nitisone | Sigma Aldrich | Cat# SML0269 |
| TRIzol | Thermo | 15596018 |
| TMZ | Sigma Aldrich | T0565 |
| Pilocarpine hydrochloride | Sigma Aldrich | P6503 |
| rTRIO protein | pET21b | N/A |
| rSG1L3 | pET21b | N/A |
| rSG1b | pET21b | N/A |
| rD7r1 | pET21b | N/A |
| Critical Commercial Assays | ||
| RNeasy Mini Kit (250) | Qiagen | Cat No./ID: 74106 |
| Complete and Incomplete Freund’s adjuvant | Thermo Scientific | 77140; 77145 |
| Experimental Models: Cell Lines | ||
| 3D11 murine hybridoma | ATTC | PTA4624 |
| Drosophila S2 cells | Thermo scientific | R69007 |
| Experimental Models: Organisms/Strains | ||
| A. gambiae 4arr strain of mosquito | ATCC | MRA-121 |
| Plasmodium berghei (ANKA GFPcon 259cl2) | ATCC | MRA-865 |
| Plasmodium berghei NK65 RedStar, | ATCC | MRA-905 |
| S. cerevisiae EBY100 | Invitrogen | C83900 |
| Swiss Webster mice | Charles River Lab | N/A |
| C57Bl/6 mice | Charles River Lab | Cat# CRL:27, RRID: IMSR_CRL:27 |
| Oligonucleotides | ||
| See Table S2 | N/A | N/A |
| Recombinant DNA | ||
| pMT-V5-His-AgTRIO | Invitrogen | Carlsbad, CA |
| pETt21b-TRIO | Novagen | Madison, WI |
| Software and Algorithms | ||
| Graphpad Prism | La Jolla, CA | Version 7 |
| FlowJo software | Ashland, OR | Version 10 |
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Erol Fikrig (erol.fikrig@yale.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Ethics Statement
The Senegal National Ethics Committee (Senegal) and the Marseille-2 Ethical Committee (France) approved the ethical collection of all human sera samples. The samples did not have any identifiable markers, and had previously been used for publication (Ali et al., 2012). Animals were housed and handled under the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal experimental protocol was approved by the Yale University Institutional Animal Care & Use Committee (Protocol Permit Number: 2017-07941). All infection experiments were performed in a biosafety level 2 animal facility, according to the regulations of Yale University.
Animals
A. gambiae (4arr strain, MRA-121, http://beiresources.org/, ATCC, Manassas, Virginia) mosquitoes were raised at 27°C, 80% humidity, under a 12/12-hour light/dark cycle and maintained with 10% sucrose under standard laboratory conditions. 5-6 week old female C57BL/6 and Swiss Webster mice were purchased from Charles River Laboratories (Wilmington, MA). Mice were randomly chosen for experimental groups. All mice were kept in the same mouse room under the same conditions, before and after infection with the parasite. For each experiment at least 8-10 mice were used.
P. berghei Infection
P. berghei (ANKA GFPcon 259cl2, MRA-865, or NK65 RedStar, MRA-905, http://beiresources.org/, ATCC Manassas, Virginia) were maintained by serial passage in 4 - 6 weeks old female Swiss Webster mice (Charles River) from frozen stocks. Murine parasitemia was monitored by light microscopy using air-dried blood smears that were methanol fixed and stained with 10% Giemsa. 3 - 5 days old mosquitoes were deprived of sucrose for 18 - 20 hours and then fed on anesthetized mice (5 mice/cage/200 mosquitoes). The fed mosquitoes were then maintained on 10% sucrose soaked in cotton pads. The cotton pads were changed every day. 3 - 4 days after blood feeding, the mosquitoes were allowed to lay eggs on the wet filter paper to propagate the mosquito colony. 17 - 18 days after infection, salivary glands on live, intact mosquitoes were observed under a fluorescent microscope to confirm P. berghei infection, and mosquitoes negative for infection were discarded.
Generation of Human Liver Chimeric Mice
FNRG mice were generated and transplanted as previously described (de Jong et al., 2014). Female mice greater than 6 weeks of age were transplanted with ca. 1 × 106 cryopreserved adult human hepatocytes. Primary hepatocytes were obtained from Bioreclamation (Westbury, NY). FNRG mice were cycled on NTBC (Yecuris, Tualatin, OR) supplemented in their water to block the accumulation of toxic metabolites. All surgical experiments were performed in accordance with protocols reviewed and approved by the IACUC of Princeton University (protocol number 1930).
Assessment of Engraftment by Human Albumin ELISA
Levels of human albumin in mouse serum were quantified by ELISA as described previously (de Jong et al., 2014; Winer et al., 2017); 96-well flat-bottomed plates (Nunc, Thermo Fisher Scientific, Witham MA) were coated with goat anti-human albumin antibody (1:500, Bethel) in coating buffer (1.59g Na2CO3, 2.93g NaHCO3, 1L dH2O, pH = 9.6) for 1 hour at 37°C. The plates were washed four times with wash buffer (0.05% Tween 20 (Sigma Aldrich, St. Luis MO) in 1x PBS) then incubated with superblock buffer (Fisher Scientific, Hampton NH) for 1 hour at 37°C. Plates were washed twice. Human serum albumin (Sigma Aldrich, St. Luis MO) was diluted to 1 μg/ml in sample diluent (10% Superblock, 90% wash buffer), then serial diluted 1:2 in 135 μl sample diluent to establish an albumin standard. Mouse serum (5 μl) was used for a 1:10 serial dilution in 135 μl sample diluent. The coated plates were incubated for 1 hour at 37°C, then washed three times. Mouse anti-human albumin (50 μl, 1:2000 in sample diluent, Abcam, Cambridge, UK) was added and plates were incubated for 2 hours at 37°C. Plates were washed four times and 50 μl of goat anti-mouse-HRP (1:10,000 in sample diluent, LifeTechnologies, Carlsbad, CA) was added and incubated for 1 hour at 37°C. Plates were washed six times. TMB (100 μl) substrate (Sigma Aldrich, St. Luis, MO) was added and the reaction was stopped with 12.5μl of 2N H2SO4. Absorbance was read at 450l on the BertholdTech TriStar (Bad Wildbad, Germany).
P. falciparum Infection
The transmission of P. falciparum was studied using a humanized mouse model (de Jong et al., 2014; Morosan et al., 2006; Sacci et al., 2006; VanBuskirk et al., 2009; Vaughan et al., 2012; Winer et al., 2017). Our goal was to determine whether AgTRIO antibodies affect mosquito-borne P. falciparum infection. Approximately 200 female A. stephensi (Liston strain, LIS) were fed through an artificial membrane on a blood culture containing P. falciparum NF54 gametocytes at Johns Hopkins University as previously described (Kumar et al., 2010). P. falciparum-infected wing-clipped A. stephensi were generated at Johns Hopkins University and shipped to Yale University for transmission studies. Upon arrival, the mosquitoes were anesthetized on ice and randomly separated into small paper cups (6 mosquitoes/cup) covered by a mesh net. In experiments with A. gambiae from Johns Hopkins University, we used 12 wing-clipped mosquitoes per cup, due to lower infection rates. The human liver chimeric FNRG mice were passively infused with 200 μL of OVA or AgTRIO rabbit antiserum 18 - 20 hours prior to the infectious mosquito challenge. Immediately before the transmission experiment, the mice were anesthetized (ketamine 100mg/kg and xylazine 10 mg/kg) and placed into a small plastic reservoir tray with the abdomen exposed. The small paper cup was inverted onto the mouse abdomen and the mosquitoes were allowed to feed for 1 hr. To determine the presence of P. falciparum infection, the livers were harvested 7 days following the infectious mosquito challenge and analyzed by RT-qPCR (Table S2). All mosquitoes were individually frozen at −80°C for DNA analysis and verification of P. falciparum infection using RT-qPCR.
METHOD DETAILS
Procedural Considerations
Female mice, 5-6 weeks of age, were randomly selected for all experimental groups. All mice appeared of good health. Blood collection was performed in a BL2 mouse room. Parasitemia experiments were performed 4, 5 and 8 days post infectious mosquitoes bite. RNA extraction was performed in the laboratory.
Mosquito Saliva Collection
The method of saliva collection from uninfected blood-fed female A. gambiae was modified from Remoue et al. (2006). We collected mosquito saliva to perform immunoblots to determine the levels of AgTRIO, and other saliva proteins. Briefly, 10 - 14 day old uninfected A. gambiae females were anesthetized on ice, followed by the removal of the legs and wings. The mosquitoes had been fed on mice 5 days before saliva collection. The proboscis of the mosquito was placed in a low-retention plastic pipette tip containing 5 μl of PBS that had been fixed on a glass slide by adhesive tape. Salivation was induced by topical application of 1 μl of 50 mg/ml pilocarpine (Sigma-Aldrich) in ethanol to the thorax. After 20 minutes of salivation at room temperature, the liquid in the tip (saliva in PBS) was collected and pooled from 30 - 50 mosquitoes. The saliva was stored at −80°C or immediately used for immunoblot analysis.
Recombinant Protein Expression and Antibodies Production
AgTRIO, D7r1, SG1L3 and SG1b coding sequences were amplified from A. gambiae female mosquito salivary gland cDNA. N-terminally His6-tagged AgTRIO without the signal peptide was cloned in a pET21b vector and transformed into E. coli BL21/DE3 cells (Table S2). The expression was induced by 1mM IPTG at 37°C and next overnight at 18°C. Recombinant protein was purified using a Ni-NTA column (Qiagen). Recombinant D7r1, SG1L3 and SG1b were expressed and purified using the same experimental approach (Table S2).
To generate rabbit polyclonal antisera, recombinant AgTRIO, SG1L3, SG1b, and D7r1 from E. coli were emulsified in complete Freund’s adjuvant and separately injected subcutaneously into rabbits (400 μg/animal/injection). Animals were boosted twice at 2-week intervals with the same dose of antigen in incomplete Freund’s adjuvant. Sera were collected 2 weeks after the last boost (Cocalico Biologicals, PA). Polyclonal rabbit IgG was purified using 1 mL NAb Protein A Plus spin columns according to manufacturer’s protocol (Thermo Fisher Scientific, MA).
The A. gambiae salivary gland extract rabbit antiserum was prepared using an identical regimen. Salivary glands were dissected from 300 uninfected adult female A. gambiae mosquitoes, and placed into sterile phosphate-buffered saline (PBS). The material was divided into 3 samples, and used to generate antisera (Cocalico Biologicals, PA).
Active immunization studies were performed with 2 groups of mice receiving either AgTRIO or OVA. Initial immunization was performed with 10 μg AgTRIO, or OVA per mouse emulsified in complete Freund’s adjuvant. Animals received 2 boosts (10-20 μg) at 2 week intervals in incomplete Freund’s adjuvant. Two weeks after the final boost, sera were collected to determine antibody titers.
Intravital Imaging
Swiss Webster mice passively immunized with TRIO or OVA antiserum were lightly anesthetized by intraperitoneal injection of ketamine/xylazine solution. Hair from the dorsal ears was removed using a depilatory cream applied for 2 minutes, then washed gently with PBS. The ear of an individual mouse was gently immobilized over a 14 ml falcon tube covered with double stick tape. Intradermal injection of P. berghei (stain NK65) expressing RedStar fluorescent protein, was performed under a stereoscope on a 37° heated plate. One hundred nanoliters containing 2000 sporozoites/μl were injected intradermally into the dorsal ear using glass micropipettes with a 80 μm diameter beveled opening made as described elsewhere (Balaban et al., 2018) and a Nanoject II Auto-Nanoliter Injector (Drummond). The total number of sporozoites at the injection site ranged between 50-200.
Intravital microscopy was performed within the Yale In Vivo Imaging Core Facility using an upright, laser scanning, two-photon microscope operated with a Titanium-Sapphire Laser (Chameleon Vision II, Coherent) tuned to 880 nm and an Olympus 20X water immersion objective. The mouse was placed on the 37° heated stage and anesthesia was maintained with isofluorane-oxygen gas mixture delivered via a nose cone. The injection site images were acquired every 5 seconds within a 500 μm × 500 μm × 30 μm (x 3 y 3 z) field using 10 μm z-steps.
Sporozoite Tracking and Image Analysis
Image hyperstacks were compiled using ImageJ software. Imaris software was used to semi-automatically track sporozoite movement over time. Imaris was also used to calculate track speed, track straightness, and mean square displacement. All movies and tracks were reviewed and edited frame by frame to ensure accurate sporozoite tracking.
Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA was performed according to standard procedures. Microtiter 96-well plates (Nunc Maxisorp) were coated overnight at 4°C with 1 μg/ml (100 μl/well) of recombinant AgTRIO, SG1L3, SG1b, D7r1, OVA, or salivary gland extract in 0.1M bicarbonate coating buffer (pH 9.6). Three washes were done with 200 μL of PBS plus 0.05% Tween-20 (Sigma) between each incubation. Plates were blocked for 1 hour at room temperature with 100 μL of blocking buffer consisting of PBS 0.05% Tween and 10% fetal bovine serum (FBS). Serum with serial dilutions in blocking buffer was added (100 μl/well) and incubated for 1 h. 100 μL horseradish peroxidase (HRP)-conjugated secondary IgG diluted in the blocking buffer was incubated for 1 h. Enzyme activity was detected by incubation with 100 μl of tetramethylbenzidine substrate (KPL, USA) for 10 min at room temperature in the dark. The reaction was stopped using 100 μl of 1 M H2SO4. The optical density (OD) at 450 nm was determined with a microplate reader (PowerWave XS, BioTek).
Quantification of Gene Expression and Plasmodium Load
Total RNA was extracted from targeted tissues using either the RNeasy Mini Kit (QIAGEN, CA) or TRIzol Reagent (Thermo Fisher Scientific, MA) following the manufacturer’s protocols. cDNA was synthesized using the iScript RT-qPCR kit (Bio-Rad, CA). Quantitative PCR was performed by using iTaq SYBR Green Supermix (Bio-Rad, CA) on a CFX96 real time system (Bio-Rad). PCR involved an initial denaturation at 95°C for 5 min, 45 cycles of 10 sec at 94°C, 10 sec at 58°C, and 10 sec at 72°C. Fluorescence readings were taken at 72°C after each cycle. At the end of each reaction, a melting curve (70 - 95°C) was checked to confirm the identity of the PCR product. Relative expression of AgTRIO was calculated by normalization to A. gambiae actin mRNA. The Plasmodium load in mice was determined by PCR using primers to amplify P. berghei 18S rRNA and normalized to M. musculus beta-2 microglobulin (Table S2). Parasitemia levels were also quantified by flow cytometric analysis based on the fact that parasites carry GFP. Flow cytometric analysis allows distinction between infected mice and healthy controls, and was confirmed by thin blood smears. S1000 flow cytometer (Stratedigm, CA) was used for data acquisition. Flow cytometry data were analyzed with FlowJo software (Ashland, OR).
Yeast Surface Display Library Screening
Three μg of total RNA purified from the salivary glands of female A. gambiae mosquitoes was used in cDNA synthesis using a modified SMART cDNA synthesis kit according to protocols by Bio S & T (Quebec, Canada). Double stranded cDNAs were obtained by primer extension and then purified for normalization. The cDNAs were directionally cloned into the EcoRI and XhoI sites of the modified yeast expression vector pYD1 (modified and prepared by Bio S & T, Quebec, Canada) to generate a salivary gland expression library where salivary proteins were expressed as Aga2 fusion proteins on the yeast surface. Digestion of the plasmids purified from 14 random clones of the pYD1-salivary gland library, showed an average insert size of 1.7 kb, and 100% of the clones contained inserts. The total number of primary clones were 1 million. The quality of the cDNA library was assessed by the random PCR amplification of 6 salivary gland genes - SG1L3, AgTRIO, Apyrase, gSG6, Lysozyme, and D7r1 - from the library (Table S2). Plasmid DNA was isolated from the library using the QIAGEN Plasmid Midi Kit (QIAGEN, CA, USA). 1 μg of the plasmid DNA was transformed into Saccharomyces cerevisiae EBY100 cells (Invitrogen, CA) as described in the literature (Chao et al., 2006).
Growth of transformed yeast cells was carried out in SDCAA medium (2% dextrose, 0.67% yeast nitrogen base, 0.5% bacto amino acids, 30 mM NaHPO4, 62 mM NaH2PO4) overnight at 30°C with shaking at 250 rpm. To induce surface protein expression, approximately 13109 transformed yeast cells, representing a thousand fold greater than the original clones, were grown overnight at 30°C in SGCAA medium (2% galactose, 0.67% yeast nitrogen base, 0.5% bacto amino acids, 30 mM NaHPO4, 62 mM NaH2PO4) with shaking at 250 rpm as previously described (Chao et al., 2006). Selection was done 4 times using AutoMACS (Miltenyi Biotec, Auburn, CA). The transformed yeast cells were washed 3 times with cold MACS buffer (0.5% BSA, 2 mM EDTA) and pelleted at 2,500 × g for 5 minutes. Next, cells were resuspended in 5 ml cold MACS buffer and incubated with 30 μg/ml of purified A. gambiae salivary gland extract rabbit IgG with gentle rotation for 30 minutes at 4°C. Subsequently, cells were washed twice and resuspended in 25 ml MACS buffer. 1 ml of goat anti-rabbit microbeads (Miltenyi Biotec, Auburn, CA) was then added and incubated for 30 minutes at 4°C with gentle inversion. The cells were magnetically sorted on the AutoMACS and eluted with 18 ml SDCAA media. The sorted cells were grown in SDCAA medium with Pen/Strep overnight at 30°C, induced in SGCAA medium overnight at 30°C and magnetically sorted using four rounds of AutoMACS sorting as described above. IgG purified from OVA-immunized rabbit serum was included as control. Plasmid DNA was isolated from the last sort using the Zymoprep II Yeast Plasmid Miniprep kit (Zymo research, CA), transformed into E. coli DH5α (Invitrogen, CA) and plated on LB plates containing 100 μg/ml ampicillin. Plasmid DNA was then isolated from bacterial colonies using the Plasmid Miniprep kit (QIAGEN, CA). After each sort, about 1 × 106 cells were incubated with 5 μg of the A. gambiae salivary gland extract rabbit IgG, washed and then incubated with Alexa Fluor 488 Goat anti-Rabbit IgG (Thermo Fisher Scientific, MA) to assess binding of the salivary gland proteins to the antibody. To assess surface expression, cells were stained with an Xpress-epitope antibody (Thermo Fisher Scientific, MA). The cells were then examined on a Stratedigm STD-13+L flow cytometer (Stratedigm, CA), and data analyzed using the FlowJo software (Ashland, OR).
P. berghei Challenge Experiments
P. berghei infection of mice by mosquito feeding was performed as described above. Infected mosquitoes were first screened under fluorescence dissecting microscope for the presence of GFP-positive sporozoites in their salivary glands at 17 days after an infective blood meal. Mosquitoes were fed on naïve mice with 3 mosquitoes per animal. 4, 5 and 8 days following infectious bites, parasitemia was quantified by RT-qPCR and flow cytometric analysis (Table S2).
For passive rabbit antiserum transfer experiments, mice were injected intraperitoneally with 100-120 μL per animal 18-20 hours before challenge, to allow maximum diffusion of antibodies into skin tissues. On the same day, infected mosquitoes were randomly aliquoted into individual paper cups with mesh covers. The infectious mosquito challenge was performed on the following day with 3 infectious bites per mouse. The liver burdens were quantified at 40 hours post infection. RNA from murine tissues was extracted in TRIzol Reagent and RT-qPCR was performed. Parasitemia of the blood was monitored starting at 4 days post infection by flow cytometric and RT-qPCR (Table S2).
Analysis of Immune Cells in Mice Following Mosquito Bite
5-week-old C57Bl/6 mice were passively immunized with either AgTRIO or OVA antiserum 24 hours prior to allowing 4 P. berghei-infected A. gambiae mosquitoes to feed on the right ear for approximately five minutes. After two hours, mice were sacrificed and both the bitten and unbitten (naive) ear were cut off at the base and split into dorsal and ventral halves. Ears were incubated for 1.5 hours in 1 mg/mL of Dispase I (Sigma) in RPMI media with 10% FBS and pen/strep, and then cut into small pieces using a scalpel. Small pieces were then incubated for 1.5 hours in 0.2 mg/mL of Collagenase (Gibco) in RPMI media with 10% FBS and pen/strep. Digested ears were then individually passed through 70μM filter to obtain single-cell suspension.
Cells were washed once with PBS with 2% FBS (FACS buffer) and then fixed with 2% PFA for ten minutes at room temperature before washing twice more with FACS buffer. Cells were then probed with antibodies against CD45 (PerCP - BD Pharmingen; Clone 30-F11), MHCII (APC-Cy7 – Biolegend; Clone M4/114.15.2), CD11b (Pacific Blue – Biolegend; Clone M1/70), CD11c (Pe-Cy7 – BD Pharmingen; Clone HL3), and Ly6G (FITC – Tonbo; Clone RB6-8C5) for 1 hour at room temperature, washed twice with FACS buffer, permeabilized and probed with CD207 (Langerin; AF647 - BD Pharmingen; Clone 81E2) for 30 minutes at room temperature. Samples were run on a BD LSRII flow cytometer and analyzed using FlowJo software.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical Analysis
All data analysis, graphing, and statistics were performed in Prism 7.0 software (GraphPad Software, CA). Results represent combined independent experiments. Each data point represents one mouse. Error bars represent mean ± SEM. Mean values were considered significantly different using the non-parametric Mann-Whitney test when p < 0.05. n.s. Not significant. Results of statistical analysis are included in the figures or figure legends.
Supplementary Material
Figure S1. Purified AgTRIO IgG reduces parasitemia in mice in a dose-dependent manner, Related to Figure 3 and Table S2. (A-F) Mice were administered a high dose of AgTRIO IgG (500 μg, AgTRIO IgG), a low dose of AgTRIO IgG (80 μg, Low TRIO IgG), or OVA IgG (500 μg). Mice were then exposed to bites from 3 Plasmodium berghei-infected mosquitoes. Blood was collected on day 4 (A and D), 5 (B and E), and 8 (C and F). Blood was used for RNA isolation and subsequent RT-qPCR analysis using P. berghei 18S rRNA with murine β2-microglobulin (mouse β2m) serving as a control (A-C) For the PCR studies, as an additional control, naïve mice were not administered IgG or exposed to P. berghei-infected mosquitoes (A-C). Flow cytometry analysis (D-F) The presence of P. berghei was determined based on the GFP signal in the blood sample. Results represent 3 combined independent experiments. Each data point represents one mouse. Error bars represent mean ± SEM. Mean values were considered significantly different using the non-parametric Mann-Whitney test when p < 0.05. Not significant (ns).
Figure S2. Amino acid sequence analysis of SG1 family members, as well as the D7-family member, D7r1, Related to the STAR Methods section, and Figures S3 and S4. 1. AgTRIO, 2. SG1L3, 3. SG1b, 4. D7r1. The signal peptide sequences are marked in red, and were determined using the SignalP 4.1 server.
Figure S3. AgTRIO, SG1L3, SG1b and D7r1 antisera recognize salivary proteins, Related to Figure 3, and Figures S2 and S4, and Table S2. (A-D). Rabbit antisera to AgTRIO (A), SG1L3 (B), SG1b (C), or D7r1 (D) recognize their respective proteins in salivary glands (SG). There is no reactivity with midgut proteins (MG). (E-G) Rabbit antisera to SG1L3 (E), SG1b (F) and D7r1 (G) identify their associated proteins in mosquito saliva.
Figure S4. SG1L3, SG1b and D7r1 antisera are not protective against mosquito-borne Plasmodium challenge, Related to Figure 3 and Figures S2 and S3, and Table S2. (A-B) Mice were given SG1L3 (A), or SG1b (B) antisera and then challenged with Plasmodium berghei-infected mosquitoes. Livers were collected at 40 hours and RNA isolated. RT-qPCR was performed to determine levels of P. berghei RNA using 18S rRNA and mouse actin (control). Each result depicts 3 independent experiments. (C-E) SG1L3 (C), SG1b (D) and D7r1 (E) antisera were administered to mice prior to exposure to P. berghei-infected mosquito. (C-D) On day 5, blood was collected from mice to isolate RNA and perform RT-qPCR. P. berghei levels were determined using 18S rRNA, while mouse actin served as control. Results represent 2 independent parasitemia experiments. (E) Flow cytometry analysis. The presence of P. berghei was determined based on the GFP signal in the blood sample on say 5. Each data point represents one mouse. Error bars represent mean ± SEM. Mean values were considered significantly different using the non-parametric Mann-Whitney test when p < 0.05. Not significant (ns).
Figure S5. Antibodies raised against Anopheles gambiae TRIO interact with Anopheles stephensi TRIO, Related to Figure 3. (A-B) Salivary glands and midguts were isolated from female, clean blood-fed A. gambiae and A. stephensi mosquitoes. Rabbit serum to AgTRIO was used to probe against AgTRIO and AsTRIO in salivary glands (A). Midgut served as negative control (B). Actin antibody was used to detect actin. Actin levels were normalized for the loading control.
Figure S6. AgTRIO antiserum affects immune cell populations in the skin of mice fed upon by Plasmodium berghei-infected Anopheles gambiae, Related to Figure 7. C57Bl/6 mice were passively immunized with AgTRIO or OVA antisera and 4 infected A. gambiae mosquitoes were allowed to feed on the ears of each mouse. Both bitten and unbitten (naïve) ears were harvested, enzymatically digested to single cell suspension and the percentage of CD45+ Ly6G− (B, C and D) or CD45+ (A) cells of each population of macrophages (B), dendritic cells (C), Langerhans cells (D) and neutrophils (A) were analyzed using flow cytometry. Data shown are pooled from 3 independent experiments, with an n=16 for each group. Significance was calculated using a one-way ANOVA with post-hoc Tukey test for multiple comparisons. Each dot represents one mouse. Results represent 3 combined independent experiments. n.s. not significant.
Highlights.
Antisera to A. gambiae salivary glands alter mosquito-borne Plasmodium infection in mice
Antiserum against AgTRIO, a mosquito protein, reduces murine Plasmodium infection
AgTRIO antiserum and an antibody to the vaccine candidate CSP synergize to protect
Sporozoite movement in the mouse dermis is diminished by AgTRIO antiserum
Acknowledgments
We thank Kathy Deponte, Jesse Hwang, Jenny Yang, and Julie Sellau for assistance and the Johns Hopkins Malaria Research Institute Parasitology and Insectary core facilities. This work was supported in part by grants from Princeton University and an Investigator in Pathogenesis Award by the Burroughs Wellcome Fund to A.P., and NIH/NIAID grant AI131574 to G.D. E.F. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Supplemental Information includes six figures and two tables and can be found with this article online at https://doi.org/10.1016/j.chom.2018.03.008.
AUTHOR CONTRIBUTIONS
Conceptualization E.F., S.M.D., and T.A.A.; Methodology, E.F., S.M.D., and T.A.A.; Investigation, S.M.D., T.A.A., M.F., J.Y., T.R.S., X.Z., S.C., F.G., A.K.H., Y.-M.C., Y.L., G.H., A.T., and G.M.; Resources, L.A., A.P., and G.D.; Writing – Original Draft, E.F., S.M.D., and T.A.A.; Writing – Review & Editing, E.F., A.P., G.D., L.A., S.M.D., and T.A.A.; Funding Acquisition, E.F., A.P., and G.D.; Supervision, E.F., G.D., A.P., S.M.D., and T.A.A.
DECLARATION OF INTERESTS
The authors declare no competing interests.
References
- Abdeladhim M, Ben Ahmed M, Marzouki S, Belhadj Hmida N, Boussoffara T, Belhaj Hamida N, Ben Salah A, Louzir H. Human cellular immune response to the saliva of Phlebotomus papatasi is mediated by IL-10-producing CD8+ T cells and Th1-polarized CD4+ lymphocytes. PLoS Negl Trop Dis. 2011;5:e1345. doi: 10.1371/journal.pntd.0001345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger NE, Harant J. Plasmodium berghei: sporozoite challenge, protection, and hypersensitivity in mice. Exp Parasitol. 1976;40:273–280. doi: 10.1016/0014-4894(76)90091-6. [DOI] [PubMed] [Google Scholar]
- Alger NE, Harant JA, Willis LC, Jorgensen GM. Sporozoite and normal salivary gland induced immunity in malaria. Nature. 1972;238:341. doi: 10.1038/238341a0. [DOI] [PubMed] [Google Scholar]
- Ali ZM, Bakli M, Fontaine A, Bakkali N, Vu Hai V, Audebert S, Boublik Y, Pages F, Remoue F, Rogier C, et al. Assessment of Anopheles salivary antigens as individual exposure biomarkers to species-specific malaria vector bites. Malar J. 2012;11:439. doi: 10.1186/1475-2875-11-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeras L, Fontaine A, Belghazi M, Bourdon S, Boucomont-Chapeaublanc E, Orlandi-Pradines E, Baragatti M, Corre-Catelin N, Reiter P, Pradines B, et al. Salivary gland protein repertoire from Aedes aegypti mosquitoes. Vector Borne Zoonotic Dis. 2010;10:391–402. doi: 10.1089/vbz.2009.0042. [DOI] [PubMed] [Google Scholar]
- Amino R, Thiberge S, Martin B, Celli S, Shorte S, Frischknecht F, Menard R. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat Med. 2006;12:220–224. doi: 10.1038/nm1350. [DOI] [PubMed] [Google Scholar]
- Arca B, Lombardo F, Francischetti IM, Pham VM, Mestres-Simon M, Andersen JF, Ribeiro JM. An insight into the sialome of the adult female mosquito Aedes albopictus. Insect Biochem Mol Biol. 2007;37:107–127. doi: 10.1016/j.ibmb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Arca B, Lombardo F, Valenzuela JG, Francischetti IM, Marinotti O, Coluzzi M, Ribeiro JM. An updated catalogue of salivary gland transcripts in the adult female mosquito, Anopheles gambiae. J Exp Biol. 2005;208:3971–3986. doi: 10.1242/jeb.01849. [DOI] [PubMed] [Google Scholar]
- Baker DA, Nolan T, Fischer B, Pinder A, Crisanti A, Russell S. A comprehensive gene expression atlas of sex- and tissue-specificity in the malaria vector, Anopheles gambiae. BMC Genomics. 2011;12:296. doi: 10.1186/1471-2164-12-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban AE, Neuman K, Sinnis P, Balaban RS. Robust fluorescent labelling of micropipettes for use in fluorescence microscopy: application to the observation of a mosquito borne parasite infection. J Microsc. 2018;269:78–84. doi: 10.1111/jmi.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkett AJ. Status of vaccine research and development of vaccines for malaria. Vaccine. 2016;34:2915–2920. doi: 10.1016/j.vaccine.2015.12.074. [DOI] [PubMed] [Google Scholar]
- Caljon G, Van Den Abbeele J, Sternberg JM, Coosemans M, De Baetselier P, Magez S. Tsetse fly saliva biases the immune response to Th2 and induces anti-vector antibodies that are a useful tool for exposure assessment. Int J Parasitol. 2006;36:1025–1035. doi: 10.1016/j.ijpara.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Calvo E, Andersen J, Francischetti IM, de LCM, de Bianchi AG, James AA, Ribeiro JM, Marinotti O. The transcriptome of adult female Anopheles darlingi salivary glands. Insect Mol Biol. 2004;13:73–88. doi: 10.1111/j.1365-2583.2004.00463.x. [DOI] [PubMed] [Google Scholar]
- Calvo E, Pham VM, Lombardo F, Arca B, Ribeiro JM. The sialotranscriptome of adult male Anopheles gambiae mosquitoes. Insect Biochem Mol Biol. 2006;36:570–575. doi: 10.1016/j.ibmb.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Calvo E, Pham VM, Marinotti O, Andersen JF, Ribeiro JM. The salivary gland transcriptome of the neotropical malaria vector Anopheles darlingi reveals accelerated evolution of genes relevant to hematophagy. BMC Genomics. 2009;10:57. doi: 10.1186/1471-2164-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo E, Sanchez-Vargas I, Kotsyfakis M, Favreau AJ, Barbian KD, Pham VM, Olson KE, Ribeiro JM. The salivary gland transcriptome of the eastern tree hole mosquito, Ochlerotatus triseriatus. J Med Entomol. 2010;47:376–386. doi: 10.1603/me09226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao G, Lau WL, Hackel BJ, Sazinsky SL, Lippow SM, Wittrup KD. Isolating and engineering human antibodies using yeast surface display. Nat Protoc. 2006;1:755–768. doi: 10.1038/nprot.2006.94. [DOI] [PubMed] [Google Scholar]
- Chaudhury S, Ockenhouse CF, Regules JA, Dutta S, Wallqvist A, Jongert E, Waters NC, Lemiale F, Bergmann-Leitner E. The biological function of antibodies induced by the RTS, S/AS01 malaria vaccine candidate is determined by their fine specificity. Malar J. 2016;15:301. doi: 10.1186/s12936-016-1348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong YP, Dorner M, Mommersteeg MC, Xiao JW, Balazs AB, Robbins JB, Winer BY, Gerges S, Vega K, Labitt RN, et al. Broadly neutralizing antibodies abrogate established hepatitis C virus infection. Sci Transl Med. 2014;6:254ra129. doi: 10.1126/scitranslmed.3009512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan MJ, Messmore AS, Scrafford DA, Sacks DL, Kamhawi S, McDowell MA. Uninfected mosquito bites confer protection against infection with malaria parasites. Infect Immun. 2007;75:2523–2530. doi: 10.1128/IAI.01928-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JF, Higgs S, Beaty BJ. Mosquito feeding-induced enhancement of Cache Valley Virus (Bunyaviridae) infection in mice. J Med Entomol. 1998;35:261–265. doi: 10.1093/jmedent/35.3.261. [DOI] [PubMed] [Google Scholar]
- Fontaine A, Diouf I, Bakkali N, Misse D, Pages F, Fusai T, Rogier C, Almeras L. Implication of haematophagous arthropod salivary proteins in host-vector interactions. Parasit Vectors. 2011;4:187. doi: 10.1186/1756-3305-4-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francischetti IM, Valenzuela JG, Pham VM, Garfield MK, Ribeiro JM. Toward a catalog for the transcripts and proteins (sialome) from the salivary gland of the malaria vector Anopheles gambiae. J Exp Biol. 2002;205:2429–2451. doi: 10.1242/jeb.205.16.2429. [DOI] [PubMed] [Google Scholar]
- Gomes R, Teixeira C, Teixeira MJ, Oliveira F, Menezes MJ, Silva C, de Oliveira CI, Miranda JC, Elnaiem DE, Kamhawi S, et al. Immunity to a salivary protein of a sand fly vector protects against the fatal outcome of visceral leishmaniasis in a hamster model. Proc Natl Acad Sci USA. 2008;105:7845–7850. doi: 10.1073/pnas.0712153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Kyushiki H, Nagano K, Sudo T, Matsuoka H, Yoshida S. Anopheline anti-platelet protein from a malaria vector mosquito has anti-thrombotic effects in vivo without compromising hemostasis. Thromb Res. 2012;129:169–175. doi: 10.1016/j.thromres.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Hopp CS, Sinnis P. The innate and adaptive response to mosquito saliva and Plasmodium sporozoites in the skin. Ann N Y Acad Sci. 2015;1342:37–43. doi: 10.1111/nyas.12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalume DE, Okulate M, Zhong J, Reddy R, Suresh S, Deshpande N, Kumar N, Pandey A. A proteomic analysis of salivary glands of female Anopheles gambiae mosquito. Proteomics. 2005;5:3765–3777. doi: 10.1002/pmic.200401210. [DOI] [PubMed] [Google Scholar]
- Kamhawi S. The biological and immunomodulatory properties of sand fly saliva and its role in the establishment of Leishmania infections. Microbes Infect. 2000;2:1765–1773. doi: 10.1016/s1286-4579(00)01331-9. [DOI] [PubMed] [Google Scholar]
- Kaslow DC, Biernaux S. RTS, S: toward a first landmark on the Malaria Vaccine Technology Roadmap. Vaccine. 2015;33:7425–7432. doi: 10.1016/j.vaccine.2015.09.061. [DOI] [PubMed] [Google Scholar]
- Kebaier C, Voza T, Vanderberg J. Neither mosquito saliva nor immunity to saliva has a detectable effect on the infectivity of Plasmodium sporozoites injected into mice. Infect Immun. 2010;78:545–551. doi: 10.1128/IAI.00807-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krettli AU, Miller LH. Malaria: a sporozoite runs through it. Curr Biol. 2001;11:R409–R412. doi: 10.1016/s0960-9822(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Kumar S, Molina-Cruz A, Gupta L, Rodrigues J, Barillas-Mury C. A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science. 2010;327:1644–1648. doi: 10.1126/science.1184008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Coupanec A, Babin D, Fiette L, Jouvion G, Ave P, Misse D, Bouloy M, Choumet V. Aedes mosquito saliva modulates Rift Valley fever virus pathogenicity. PLoS Negl Trop Dis. 2013;7:e2237. doi: 10.1371/journal.pntd.0002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limesand KH, Higgs S, Pearson LD, Beaty BJ. Potentiation of vesicular stomatitis New Jersey virus infection in mice by mosquito saliva. Parasite Immunol. 2000;22:461–467. doi: 10.1046/j.1365-3024.2000.00326.x. [DOI] [PubMed] [Google Scholar]
- Long CA, Zavala F. Malaria vaccines and human immune responses. Curr Opin Microbiol. 2016;32:96–102. doi: 10.1016/j.mib.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie A, Holzmuller P, Tchioffo MT, Rossignol M, Demettre E, Seveno M, Corbel V, Awono-Ambene P, Morlais I, Remoue F, et al. Anopheles gambiae salivary protein expression modulated by wild Plasmodium falciparum infection: highlighting of new antigenic peptides as candidates of An. gambiae bites. Parasit Vectors. 2014;7:599. doi: 10.1186/s13071-014-0599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Nussenzweig RS, Nussenzweig V. Antibodies to Plasmodium circumsporozoite protein (CSP) inhibit sporozoite’s cell traversal activity. J Immunol Methods. 2012;377:47–52. doi: 10.1016/j.jim.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morosan S, Hez-Deroubaix S, Lunel F, Renia L, Giannini C, Van Rooijen N, Battaglia S, Blanc C, Eling W, Sauerwein R, et al. Liver-stage development of Plasmodium falciparum, in a humanized mouse model. J Infect Dis. 2006;193:996–1004. doi: 10.1086/500840. [DOI] [PubMed] [Google Scholar]
- Norsworthy NB, Sun J, Elnaiem D, Lanzaro G, Soong L. Sand fly saliva enhances Leishmania amazonensis infection by modulating interleukin-10 production. Infect Immun. 2004;72:1240–1247. doi: 10.1128/IAI.72.3.1240-1247.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockenfels B, Michael E, McDowell MA. Meta-analysis of the effects of insect vector saliva on host immune responses and infection of vector-transmitted pathogens: a focus on leishmaniasis. PLoS Negl Trop Dis. 2014;8:e3197. doi: 10.1371/journal.pntd.0003197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira F, Rowton E, Aslan H, Gomes R, Castrovinci PA, Alvarenga PH, Abdeladhim M, Teixeira C, Meneses C, Kleeman LT, et al. A sand fly salivary protein vaccine shows efficacy against vector-transmitted cutaneous leishmaniasis in nonhuman primates. Sci Transl Med. 2015;7:290ra290. doi: 10.1126/scitranslmed.aaa3043. [DOI] [PubMed] [Google Scholar]
- Penneys NS, Nayar JK, Bernstein H, Knight JW. Circulating antibody detection in human serum to mosquito salivary gland proteins by the avidin-biotin-peroxidase technique. J Am Acad Dermatol. 1988;18:87–92. doi: 10.1016/s0190-9622(88)70013-4. [DOI] [PubMed] [Google Scholar]
- Penny MA, Pemberton-Ross P, Smith TA. The time-course of protection of the RTS, S vaccine against malaria infections and clinical disease. Malar J. 2015;14:437. doi: 10.1186/s12936-015-0969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson C, Oliveira GA, Sultan AA, Bhanot P, Nussenzweig V, Nardin E. Cutting edge: a new tool to evaluate human preerythrocytic malaria vaccines: rodent parasites bearing a hybrid Plasmodium falciparum circumsporozoite protein. J Immunol. 2002;169:6681–6685. doi: 10.4049/jimmunol.169.12.6681. [DOI] [PubMed] [Google Scholar]
- Poinsignon A, Remoue F, Rossignol M, Cornelie S, Courtin D, Grebaut P, Garcia A, Simondon F. Human IgG antibody response to Glossina saliva: an epidemiologic marker of exposure to Glossina bites. Am J Trop Med Hyg. 2008;78:750–753. [PubMed] [Google Scholar]
- Pollock T, Leitao R, Galan-Rodriguez C, Wong KA, Rodriguez A. Daily Plasmodium yoelii infective mosquito bites do not generate protection or suppress previous immunity against the liver stage. Malar J. 2011;10:97. [Google Scholar]
- Proietti C, Verra F, Bretscher MT, Stone W, Kanoi BN, Balikagala B, Egwang TG, Corran P, Ronca R, Arca B, et al. Influence of infection on malaria-specific antibody dynamics in a cohort exposed to intense malaria transmission in northern Uganda. Parasite Immunol. 2013;35:164–173. doi: 10.1111/pim.12031. [DOI] [PubMed] [Google Scholar]
- Reagan KL, Machain-Williams C, Wang T, Blair CD. Immunization of mice with recombinant mosquito salivary protein D7 enhances mortality from subsequent West Nile virus infection via mosquito bite. PLoS Negl Trop Dis. 2012;6:e1935. doi: 10.1371/journal.pntd.0001935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remoue F, Cisse B, Ba F, Sokhna C, Herve JP, Boulanger D, Simondon F. Evaluation of the antibody response to Anopheles salivary antigens as a potential marker of risk of malaria. Trans R Soc Trop Med Hyg. 2006;100:363–370. doi: 10.1016/j.trstmh.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Nussenzveig RH, Tortorella G. Salivary vasodilators of Aedes triseriatus and Anopheles gambiae (Diptera: Culicidae) J Med Entomol. 1994;31:747–753. doi: 10.1093/jmedent/31.5.747. [DOI] [PubMed] [Google Scholar]
- Rizzo C, Lombardo F, Ronca R, Mangano V, Sirima SB, Nebie I, Fiorentino G, Modiano D, Arca B. Differential antibody response to the Anopheles gambiae gSG6 and cE5 salivary proteins in individuals naturally exposed to bites of malaria vectors. Parasit Vectors. 2014;7:549. doi: 10.1186/s13071-014-0549-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo C, Ronca R, Fiorentino G, Mangano VD, Sirima SB, Nebie I, Petrarca V, Modiano D, Arca B. Wide cross-reactivity between Anopheles gambiae and Anopheles funestus SG6 salivary proteins supports exploitation of gSG6 as a marker of human exposure to major malaria vectors in tropical Africa. Malar J. 2011;10:206. doi: 10.1186/1475-2875-10-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha AC, Braga EM, Araujo MS, Franklin BS, Pimenta PF. Effect of the Aedes fluviatilis saliva on the development of Plasmodium gallinaceum infection in Gallus (gallus) domesticus. Mem Inst Oswaldo Cruz. 2004;99:709–715. doi: 10.1590/s0074-02762004000700008. [DOI] [PubMed] [Google Scholar]
- Ronca R, Kotsyfakis M, Lombardo F, Rizzo C, Curra C, Ponzi M, Fiorentino G, Ribeiro JM, Arca B. The Anopheles gambiae cE5, a tight- and fast-binding thrombin inhibitor with post-transcriptionally regulated salivary-restricted expression. Insect Biochem Mol Biol. 2012;42:610–620. doi: 10.1016/j.ibmb.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R, Wirtz RA, Schneider I, Burge R. An estimation of the number of malaria sporozoites ejected by a feeding mosquito. Trans R Soc Trop Med Hyg. 1990;84:209–212. doi: 10.1016/0035-9203(90)90258-g. [DOI] [PubMed] [Google Scholar]
- Sacci JB, Jr, Alam U, Douglas D, Lewis J, Tyrrell DL, Azad AF, Kneteman NM. Plasmodium falciparum infection and exoerythrocytic development in mice with chimeric human livers. Int J Parasitol. 2006;36:353–360. doi: 10.1016/j.ijpara.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Debant A. Function and regulation of the Rho guanine nucleotide exchange factor Trio. Small GTPases. 2014;5:e29769. doi: 10.4161/sgtp.29769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BS, Higgs S. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans R Soc Trop Med Hyg. 2008;102:400–408. doi: 10.1016/j.trstmh.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BS, Mathieu C, Peronet R, Mecheri S. Anopheles stephensi saliva enhances progression of cerebral malaria in a murine model. Vector Borne Zoonotic Dis. 2011;11:423–432. doi: 10.1089/vbz.2010.0120. [DOI] [PubMed] [Google Scholar]
- Schneider BS, Soong L, Girard YA, Campbell G, Mason P, Higgs S. Potentiation of West Nile encephalitis by mosquito feeding. Viral Immunol. 2006;19:74–82. doi: 10.1089/vim.2006.19.74. [DOI] [PubMed] [Google Scholar]
- Stone W, Bousema T, Jones S, Gesase S, Hashim R, Gosling R, Carneiro I, Chandramohan D, Theander T, Ronca R, et al. IgG responses to Anopheles gambiae salivary antigen gSG6 detect variation in exposure to malaria vectors and disease risk. PLoS One. 2012;7:e40170. doi: 10.1371/journal.pone.0040170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styer LM, Lim PY, Louie KL, Albright RG, Kramer LD, Bernard KA. Mosquito saliva causes enhancement of West Nile virus infection in mice. J Virol. 2011;85:1517–1527. doi: 10.1128/JVI.01112-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodos CM, Titus RG. Salivary gland material from the sand fly Lutzomyia longipalpis has an inhibitory effect on macrophage function in vitro. Parasite Immunol. 1993;15:481–487. doi: 10.1111/j.1365-3024.1993.tb00634.x. [DOI] [PubMed] [Google Scholar]
- Valenzuela JG, Belkaid Y, Garfield MK, Mendez S, Kamhawi S, Rowton ED, Sacks DL, Ribeiro JM. Toward a defined anti-Leishmania vaccine targeting vector antigens: characterization of a protective salivary protein. J Exp Med. 2001;194:331–342. doi: 10.1084/jem.194.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela JG, Francischetti IM, Pham VM, Garfield MK, Ribeiro JM. Exploring the salivary gland transcriptome and proteome of the Anopheles stephensi mosquito. Insect Biochem Mol Biol. 2003;33:717–732. doi: 10.1016/s0965-1748(03)00067-5. [DOI] [PubMed] [Google Scholar]
- VanBuskirk KM, O’Neill MT, De La Vega P, Maier AG, Krzych U, Williams J, Dowler MG, Sacci JB, Jr, Kangwanrangsan N, Tsuboi T, et al. Preerythrocytic, live-attenuated Plasmodium falciparum vaccine candidates by design. Proc Natl Acad Sci USA. 2009;106:13004–13009. doi: 10.1073/pnas.0906387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderberg JP, Frevert U. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int J Parasitol. 2004;34:991–996. doi: 10.1016/j.ijpara.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Varghese C, Oyere O, Cowan M, Davis S, Norrving B. World Health Organization. Stroke. 2016;47:e210. doi: 10.1161/STROKEAHA.116.014233. [DOI] [PubMed] [Google Scholar]
- Vaughan AM, Mikolajczak SA, Wilson EM, Grompe M, Kaushansky A, Camargo N, Bial J, Ploss A, Kappe SH. Complete Plasmodium falciparum liver-stage development in liver-chimeric mice. J Clin Invest. 2012;122:3618–3628. doi: 10.1172/JCI62684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhave JP, Meuwissen JH, Golenser J. The dual role of macrophages in the sporozoite-induced malaria infection. A hypothesis Int J Nucl Med Biol. 1980;7:149–156. doi: 10.1016/0047-0740(80)90033-9. [DOI] [PubMed] [Google Scholar]
- Voza T, Miller JL, Kappe SH, Sinnis P. Extrahepatic exoerythrocytic forms of rodent malaria parasites at the site of inoculation: clearance after immunization, susceptibility to primaquine, and contribution to blood-stage infection. Infect Immun. 2012;80:2158–2164. doi: 10.1128/IAI.00246-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitayakul A, Somsri S, Sattabongkot J, Looareesuwan S, Cui L, Udomsangpetch R. Natural human humoral response to salivary gland proteins of Anopheles mosquitoes in Thailand. Acta Trop. 2006;98:66–73. doi: 10.1016/j.actatropica.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Wikel SK, Ramachandra RN, Bergman DK, Burkot TR, Piesman J. Infestation with pathogen-free nymphs of the tick Ixodes scapularis induces host resistance to transmission of Borrelia burgdorferi by ticks. Infect Immun. 1997;65:335–338. doi: 10.1128/iai.65.1.335-338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer BY, Huang T, Low BE, Avery C, Pais MA, Hrebikova G, Siu E, Chiriboga L, Wiles MV, Ploss A. Recapitulation of treatment response patterns in a novel humanized mouse model for chronic hepatitis B virus infection. Virology. 2017;502:63–72. doi: 10.1016/j.virol.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ya-Umphan P, Cerqueira D, Parker DM, Cottrell G, Poinsignon A, Remoue F, Brengues C, Chareonviriyaphap T, Nosten F, Corbel V. Use of an Anopheles salivary biomarker to assess malaria transmission risk along the Thailand-Myanmar border. J Infect Dis. 2017;215:396–404. doi: 10.1093/infdis/jiw543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi LM, Coppi A, Snounou G, Sinnis P. Plasmodium sporozoites trickle out of the injection site. Cell Microbiol. 2007;9:1215–1222. doi: 10.1111/j.1462-5822.2006.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zocevic A, Carmi-Leroy A, Sautereau J, d’Alayer J, Lenormand P, Rousselle JC, Namane A, Choumet V. New markers in Anopheles gambiae salivary glands after Plasmodium berghei infection. Vector Borne Zoonotic Dis. 2013;13:119–127. doi: 10.1089/vbz.2012.0964. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Purified AgTRIO IgG reduces parasitemia in mice in a dose-dependent manner, Related to Figure 3 and Table S2. (A-F) Mice were administered a high dose of AgTRIO IgG (500 μg, AgTRIO IgG), a low dose of AgTRIO IgG (80 μg, Low TRIO IgG), or OVA IgG (500 μg). Mice were then exposed to bites from 3 Plasmodium berghei-infected mosquitoes. Blood was collected on day 4 (A and D), 5 (B and E), and 8 (C and F). Blood was used for RNA isolation and subsequent RT-qPCR analysis using P. berghei 18S rRNA with murine β2-microglobulin (mouse β2m) serving as a control (A-C) For the PCR studies, as an additional control, naïve mice were not administered IgG or exposed to P. berghei-infected mosquitoes (A-C). Flow cytometry analysis (D-F) The presence of P. berghei was determined based on the GFP signal in the blood sample. Results represent 3 combined independent experiments. Each data point represents one mouse. Error bars represent mean ± SEM. Mean values were considered significantly different using the non-parametric Mann-Whitney test when p < 0.05. Not significant (ns).
Figure S2. Amino acid sequence analysis of SG1 family members, as well as the D7-family member, D7r1, Related to the STAR Methods section, and Figures S3 and S4. 1. AgTRIO, 2. SG1L3, 3. SG1b, 4. D7r1. The signal peptide sequences are marked in red, and were determined using the SignalP 4.1 server.
Figure S3. AgTRIO, SG1L3, SG1b and D7r1 antisera recognize salivary proteins, Related to Figure 3, and Figures S2 and S4, and Table S2. (A-D). Rabbit antisera to AgTRIO (A), SG1L3 (B), SG1b (C), or D7r1 (D) recognize their respective proteins in salivary glands (SG). There is no reactivity with midgut proteins (MG). (E-G) Rabbit antisera to SG1L3 (E), SG1b (F) and D7r1 (G) identify their associated proteins in mosquito saliva.
Figure S4. SG1L3, SG1b and D7r1 antisera are not protective against mosquito-borne Plasmodium challenge, Related to Figure 3 and Figures S2 and S3, and Table S2. (A-B) Mice were given SG1L3 (A), or SG1b (B) antisera and then challenged with Plasmodium berghei-infected mosquitoes. Livers were collected at 40 hours and RNA isolated. RT-qPCR was performed to determine levels of P. berghei RNA using 18S rRNA and mouse actin (control). Each result depicts 3 independent experiments. (C-E) SG1L3 (C), SG1b (D) and D7r1 (E) antisera were administered to mice prior to exposure to P. berghei-infected mosquito. (C-D) On day 5, blood was collected from mice to isolate RNA and perform RT-qPCR. P. berghei levels were determined using 18S rRNA, while mouse actin served as control. Results represent 2 independent parasitemia experiments. (E) Flow cytometry analysis. The presence of P. berghei was determined based on the GFP signal in the blood sample on say 5. Each data point represents one mouse. Error bars represent mean ± SEM. Mean values were considered significantly different using the non-parametric Mann-Whitney test when p < 0.05. Not significant (ns).
Figure S5. Antibodies raised against Anopheles gambiae TRIO interact with Anopheles stephensi TRIO, Related to Figure 3. (A-B) Salivary glands and midguts were isolated from female, clean blood-fed A. gambiae and A. stephensi mosquitoes. Rabbit serum to AgTRIO was used to probe against AgTRIO and AsTRIO in salivary glands (A). Midgut served as negative control (B). Actin antibody was used to detect actin. Actin levels were normalized for the loading control.
Figure S6. AgTRIO antiserum affects immune cell populations in the skin of mice fed upon by Plasmodium berghei-infected Anopheles gambiae, Related to Figure 7. C57Bl/6 mice were passively immunized with AgTRIO or OVA antisera and 4 infected A. gambiae mosquitoes were allowed to feed on the ears of each mouse. Both bitten and unbitten (naïve) ears were harvested, enzymatically digested to single cell suspension and the percentage of CD45+ Ly6G− (B, C and D) or CD45+ (A) cells of each population of macrophages (B), dendritic cells (C), Langerhans cells (D) and neutrophils (A) were analyzed using flow cytometry. Data shown are pooled from 3 independent experiments, with an n=16 for each group. Significance was calculated using a one-way ANOVA with post-hoc Tukey test for multiple comparisons. Each dot represents one mouse. Results represent 3 combined independent experiments. n.s. not significant.


