Abstract
The catalytic system generated in-situ from the tetranuclear Ru–H complex with a catechol ligand (1/L1) was found to be effective for the direct deaminative coupling of two primary amines to form secondary amines. The catalyst 1/L1 was highly chemoselective for promoting the coupling of two different primary amines to afford unsymmetric secondary amines. The analogous coupling of aniline with primary amines formed aryl-substituted secondary amines. The treatment of aniline-d7 with 4-methoxybenzylamine led to the coupling product with significant deuterium incorporation on CH2 (18% D). The most pronounced carbon isotope effect was observed on the α-carbon of the product isolated from the coupling reaction of 4-methoxybenzylamine (C(1) = 1.015(2)). Hammett plot was constructed from measuring the rates of the coupling reaction of 4-methoxyaniline with a series of para-substituted benzylamines 4-X-C6H4CH2NH2 (X = OMe, Me, H, F, CF3). (ρ = −0.79 ± 0.1). A plausible mechanistic scheme has been proposed for the coupling reaction on the basis of these results. The catalytic coupling method provides an operationally simple and chemoselective synthesis of secondary amine products without using any reactive reagents or forming wasteful byproducts.
Keywords: secondary amine, deamination, ruthenium catalyst
Graphical Abstract

Introduction
Designing catalytic C–N bond cleavage methods has attracted considerable research efforts in the field of homogeneous catalysis since such methods are of fundamental importance in a variety of industrial chemical syntheses as well as in biochemical processes of nitrogen compounds.1 Even though catalytic C–N bond cleavage methods have been recognized as an essential protocol for harnessing nitrogen compounds from both petroleum and biomass feedstocks, these catalytic methods have long been hampered by a relatively strong C–N bond strength and catalyst poisoning by the nitrogen substrates.2 In a pioneering report, Fujiwara and co-workers first demonstrated the direct coupling of olefins with arylamines, in which arene sp2 C–N bond cleavage was promoted by a stoichiometric amount of Pd(II) salts.3 More recently, Kakiuchi and co-workers devised a ruthenium-catalyzed C–C coupling reaction of arylamines with organoboranes, by exploiting a directing group assisted sp2 C–N bond activation strategy.4 A few remarkably efficient amide-to-ester conversion methods via amide C–N bond cleavage have been achieved by using Ni catalysts.5 A number of Pd-catalyzed C–N cleavage methods have also been developed, including intramolecular Heck-type coupling reactions6 and allylamine coupling reactions.7 Selective catalytic C–C coupling methods via sp3 C–N bond activation of allyl- and benzylic amines have been achieved.8 Other notable examples of catalytic C–N bond cleavage reactions include: oxidative C–H coupling reaction of amides with alkynes,9 and Milstein group’s report on the Ru-catalyzed hydrogenolysis of amides.10 Catalytic C–N cleavage methods have also been successfully utilized for constructing nitrogen heterocycles.11
Chemoselective synthesis of amines has long been a pertinent issue in organic synthesis. Much research efforts has been devoted to the development of new catalytic synthesis of amines, and transition metal catalyzed dehydrogenative coupling strategies have emerged as highly effective ways to activate amines and alcohols.12 In particular, late transition metal catalysts have been successfully employed to promote amine-to-alcohol coupling reactions via hydrogen borrowing strategy to synthesize unsymmetric secondary amines.13 Traditionally, the reductive amination methods have also been commonly used for the synthesis of secondary amines.14 Since these methods often require stoichiometric reducing agents and/or additives, which result in the formation of copious amounts of salt byproducts, catalytic deaminative coupling methods have been recognized as an alternative strategy for the synthesis of secondary amines. Beller and co-workers reported a highly selective dealkylation of amines by using Shvo catalyst.15 Zhang group also devised the synthesis of unsymmetric secondary amines from the coupling of aniline with primary amines by using soluble Co catalysts.16 From both economic and environmental perspectives, the development of chemoselective catalytic coupling methods via C–N bond cleavage of amines and related nitrogen compounds still remains an essential goal in the fields of homogeneous catalysis and organic synthesis.
We previously disclosed the synthesis of unsymmetrical ethers from the dehydrative coupling reactions of alcohols and aldehydes that are catalyzed by a well-defined cationic ruthenium-hydride complex.17 Since these coupling reactions are driven by the formation of water, we reasoned that the analogous deaminative coupling reactions of amines could be achieved, where the formation of ammonia would serve as the driving force for such coupling reactions. Herein, we report the synthesis of symmetric and unsymmetric secondary amines from the catalytic deaminative coupling of primary amines. The catalytic method exhibits high chemoselectivity as well as a broad substrate scope in forming secondary amines, while generating ammonia as the only byproduct.
Results and Discussion
We previously devised a catalytic system comprised of the cationic Ru–H complex and a phenol ligand, which was found to exhibit high catalytic activity for the hydrogenolysis of carbonyl compounds to yield the corresponding aliphatic products.18 By adopting the similar ligand controlled catalysis strategy, we initially screened soluble Ru catalysts with phenol and related oxygen and nitrogen ligands to promote the C–N bond activation reactions. We have chosen the coupling reaction of benzylamine with cyclohexylamine as a test case to screen both Ru catalysts and the ligands (eq 1).
 |
(1) |
Among the initially screened Ru catalysts, both the tetranuclear Ru-H complex [(PCy3)(CO)RuH]4(O)(OH)2 (1) and the cationic [(C6H6)(PCy3)(CO)RuH]+BF4− (2) with 1,2-catechol ligand exhibited the most promising activity for the coupling reaction, as analyzed by both GC and NMR spectroscopic methods (Table 1). Among the screened oxygen and nitrogen ligands, 4-(1,1-dimethylethyl)-1,2-benzenediol (L1) was found to give the highest activity and selectivity for these Ru catalysts in giving the unsymmetric amine product 3a over the symmetric one 4a (entries 9 and 13). After further ligand screening and optimization studies, we have chosen the standard condition for the 1.0 mmol scale coupling reaction as: 1 (0.75 mol %, 3 Ru mol %)/L1 (10 mol %) in chlorobenzene (2 mL) at 130 °C (Tables S1 and S2, Supporting Information (SI)). A 3:1 ratio of catechol ligand to Ru catalyst was found to be the optimum for the catalytic activity, as a lower ratio (2:1) typically gave a lower product yield. Non-protic polar solvents such as chlorobenzene and dioxane afforded the highest product yields and selectivity, and the for the sake of consistency, we have chosen chlorobenzene as the solvent for all coupling reactions. The formation of the byproduct ammonia was detected in the crude mixture as analyzed by both NMR and GC-MS methods.
Table 1.
Catalyst and Ligand Screening Studya
| entry | catalyst | ligandb | yield (3a:4a)c |
|---|---|---|---|
| 1 | 1 | none | 27:7 |
| 2 | 1 | phenol | 52:16 |
| 3 | 1 | 1,2-catechol | 68:20 |
| 4 | 1 | aniline | 19:6 |
| 5 | 1 | 2-NH2PhCOMe | 28:16 |
| 6 | 1 | PhCONH2 | trace |
| 7 | 1 | 1,1′-BINOL | trace |
| 8 | 1 | 1,2-C6H4(NH2)2 | 27:12 |
| 9 | 1 | L1 | 74:22 |
| 10 | 1 | L2 | 67:6 |
| 11 | 1 | L3 | 62:13 |
| 12 | 1/HBF4·OEt2 | L1 | 65:1 |
| 13 | 2 | L1 | 70:16 |
| 14 | 2/HBF4·OEt2 | L1 | 60:5 |
| 15 | [Ru(COD)Cl2]x | L1 | 14:7 |
| 16 | RuCl3·3H2O | L1 | 0 |
| 17 | (PPh3)3(CO)RuH2 | L1 | 0 |
| 18 | [(p-cymene)RuCl2]2 | L1 | <1 |
| 19 | RuHCl(CO)(PCy3)2 | L1 | 70:15 |
| 20 | [(PCy3)2(CO)(CH3CN)2RuH]BF4 | L1 | 0 |
Reaction conditions: benzylamine (0.5 mmol), cyclohexylamine (0.7 mmol), catalyst (3 mol %), ligand (10 mol %), chlorobenzene (2 mL), 130 °C, 16 h.
See the Supporting Information for extensive list and structure of ligands.
The product yield was determined by 1H NMR using C6Me6 as the internal standard.
We explored the substrate scope of the deaminative coupling reaction by using the optimized catalyst system 1/L1 under the standard condition (Table 2). The coupling of benzylic amines with a variety of aliphatic and benzylic primary amines selectively formed the unsymmetric secondary amine products 3a–3m. An excess amount (1.4 equiv) of the second amine (usually more electron rich amine) was found to improve the product selectivity for unsymmetric amines 3. Typically, <20 % of the symmetric amine products was formed in the crude mixture in most cases, and analytically pure unsymmetric amine products were readily isolated after silica gel column chromatographic separation. The major byproducts, symmetric amine products 4 were detected by TLC and GC/MS, but were not isolated in these cases. The coupling of phenethyl amines with both benzylic and aliphatic amines also gave the selective formation of 3n–3v. Indole-, Furanyl- and phenol-substituted amines with cyclohexylamine selectively yielded the unsymmetric secondary amine products 3bb–3dd. For these cases, the coupling of benzylic and other aryl-substituted amines with electron-rich amines tend to favor the formation of unsymmetrical amines over the symmetrical ones. In contrast, the coupling of two different aliphatic amines with sterically non-demanding group yielded in a mixture of symmetric and unsymmetric amines. The coupling reaction with secondary and tertiary amines was found to be very sluggish and unselective, resulting in a complex mixture of products. The catalytic coupling method is operationally simple and exhibits high chemoselectivity toward the formation of unsymmetric secondary amines without resorting to employing any reactive reagents.
Table 2.
Synthesis of Unsymmetric Secondary Amines from the Deaminative Coupling of Primary Aminesa
Reaction conditions: R-NH2 (1.0 mmol), R′-NH2 (1.4 mmol), 1 (0.75 mol %), L1 (10 mol %), chlorobenzene (2 mL), 130 °C, 16 h. Ar = C6H4-4-OMe.
We next explored the substrate scope for the formation of symmetric secondary amines by using the catalyst system 1/L1 (Table 3). Benzylic primary amines reacted smoothly to afford the secondary amine products 4a–4k without the formation of tertiary amines or other side products. The coupling of both phenethyl and indanyl amines formed the corresponding secondary amine products 4m–4r. While cyclohexyl and thiophene-substituted amines predictively yielded the corresponding secondary amine products 4s and 4t, respectively, a mixture of secondary and tertiary amines was formed for sterically non-demanding aliphatic amines 4u–4x. Generally, the coupling of chiral primary amines led to a 1:1 diastereomeric mixture of products as illustrated by the formation of 4p–4q, but interestingly, a diastereoselective formation of the product 4l was obtained in case of (R)-4-methoxy-α-methylbenzenemethaneamine (d.r. = 7:1). The catalytic method delivers a operationally simple synthesis of symmetric secondary amines from readily available primary amines without using any reactive reagents via a deaminative coupling method.14,16
Table 3.
Synthesis of Symmetric Secondary Aminesa
Reaction conditions: amine (1.0 mmol), 1 (0.75 mol %), L1 (10 mol %), chlorobenzene (2 mL), 130 °C, 16 h.
40–50% of tertiary amines formed.
To further illustrate the synthetic versatility of catalytic coupling method, we next explored the coupling reaction of aniline derivatives with primary amine substrates of biological relevance (Table 4). The coupling of para-substituted anilines with benzylic amines led to the selective formation of unsymmetric amine products 5a–5e without any significant amount of the symmetric amine products. Similarly, the reaction of para-substituted anilines with 3,4,5-trimethoxybenzylamine afforded the coupling products 5f–5h in high yields. Single crystals of 5f were obtained by slow evaporation in CH2Cl2/hexanes, and its structure was determined by X-ray crystallography.
Table 4.
Synthesis of Unsymmetric Secondary Amines from the Deaminative Coupling of Anilines with Aminesa
Reaction conditions: aniline (0.5 mmol), R-NH2 (0.7 mmol), 1 (0.75 mol %), L1 (10 mol %), chlorobenzene (2 mL), 140 °C, 20 h.
The treatment of 3-amino-9-ethylcarbazole with n-hexylamine predictively yielded the product 5q. The coupling of (R)-(+)-aminoglutathimide with 3,4,5-trimethoxybenzylamine led to the optically active coupling product (R)-5r, without any detectable racemization. The coupling of L-glutamine with 3-phenylpropylamine and 4-methoxybenzylamine led to the cyclized amine products 5s and 5t in 75% and 70% yields, respectively. The formation of cyclized products 5s and 5t can be rationalized by initial dehydrative cyclization of glutamine followed by the deaminative coupling with the primary amine substrates. The optimized standard conditions were used for all of these coupling reactions as described in Tables 2–4, with slight modifications on the reaction time and temperature.
We monitored the amine coupling reaction by NMR spectroscopy to probe the overall reaction profile. In a J-Young NMR tube equipped with Teflon stopcock, 4-methoxybenzylamine (34 mg, 0.25 mmol) and the catalyst system 1 (3 mg, 0.75 mol %)/L1 (4 mg, 10 mol %) were dissolved in toluene-d8 (0.5 mL). The tube was immersed in an oil bath at 130 °C, and the reaction progress was monitored by 1H NMR in 20 min intervals.
As shown in Figure 1, the secondary amine product 4c was formed steadily at the expense of the benzylamine substrate. Initially, the formation of a minor product was also observed (~10%), which gradually disappeared within 200 min of the reaction time. The structure of minor product was subsequently determined to be ArCH=NCH2Ar (Ar = 4-methoxyphenyl) by both NMR and GC/MS, as obtained from a separate preparatory scale experiment.
Figure 1.
Reaction Profile for the Coupling of 4-Methoxybenzylamine. 4-Methoxybenzylamine (●), 4c (■) and ArCH=NCH2Ar (▲).
 |
(2) |
Next, we examined the deuterium labeling pattern on the product from the reaction of aniline-d7 with 4-methoxybenzylamine (eq 2). The reaction tube consisted of aniline-d7 (50 mg, 0.5 mmol) with 4-methoxybenzylamine (69 mg, 0.5 mmol) in the presence of 1 (7 mg, 0.75 mol %)/L1 (8 mg, 10 mol %) in chlorobenzene (1 mL) was heated in an oil bath at 140 °C for 20 h. The product 5a-d was isolated by silica gel column chromatography, and its deuterium content was analyzed by 1H and 2H NMR (Figure S1, SI). A significant amount of deuterium was incorporated to the CH2 position of 5a-d (18% D), without any deuterium exchange on the arene C–H positions. In a control experiment, the treatment of the isolated product 5a with aniline-d7 in the presence of 1 (0.75 mol %)/L1 (10 mol %) did not lead to any significant deuterium exchange into the benzyl position of 5a under similar reaction conditions after 20 h. The significant amount of the deuterium incorporation suggests that the imine-to-amine hydrogenation-dehydrogenation process might have occurred during the product formation of 5a.
 |
(3) |
To discern the rate-limiting step of the catalytic reaction, we employed Singleton’s high precision NMR technique to measure the carbon isotope effect for the coupling reaction (eq 3).19 The reaction tube of 4-methoxybenzylamine (2 mmol) and 1 (0.75 mol %)/L1 (10 mol %) in chlorobenzene (4 mL) was heated at 130 °C for 16 h for high conversion and for 2–3 h for low conversion cases. The product 4c was isolated by a column chromatography on silica gel, and was analyzed by 13C NMR. The most pronounced carbon isotope effect was observed on the α-carbon of the product 4c when the 13C ratio of the product at three high conversions (86–89%) was compared with the sample obtained at low conversions (12–15%) [(average of 13C at 87% conversion)/(average of 13C at 13% conversion) at C(1) = 1.015(2)] (Table S2, SI).
The significant carbon isotope effect on the α-CH2 carbon is consistent with the C–N bond cleavage turnover-limiting step. In support of this notion, Singleton and co-workers showed that the observation of most pronounced carbon isotope effect has been a definitive tool for establishing the rate-limiting step for both C–C and C–O bond forming reactions.19c While C–N bond cleavage has been found to be the turnover limiting step for a number of chemical and biochemical coupling reactions,20 very few carbon kinetic isotope effect measurements have been reported for the catalytic coupling reactions of nitrogen compounds. Eubanks and co-workers measured pronounced carbon isotope effects in Hofmann elimination reaction of para-substituted (2-phenylethyl)-trimethylammonium bromides, which indicated an E2 mechanism involving C–N bond cleavage step.21 In a urease-catalyzed hydrolysis of hydroxyurea, Cleland and co-workers observed the significant carbon isotope effect on the carbonyl carbon but not on the nitrogen atom, which argues for the formation of a common intermediate prior to the C–N bond cleavage step.22
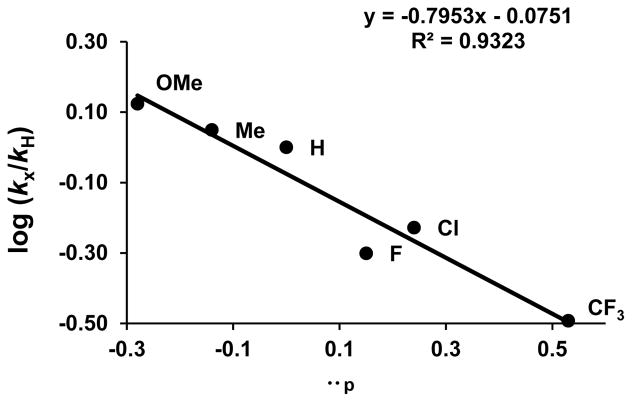
The Hammett plot was constructed from measuring the rate of the coupling reaction of a series of para-substituted benzylamines 4-X-C6H4CH2NH2 (X = OMe, Me, H, Cl, F, CF3) in the presence of 1 (0.75 mol %)/L1 (10 mol %) in toluene-d8 (Figure 2). The rate of each substrate was obtained by measuring the appearance of the product peaks, which were normalized against an internal standard (C6Me6) as analyzed by 1H NMR. The kobs for each catalytic run was determined from a first order plot of ln[(benzylamine)t/(benzylamine)0] vs time. The Hammett plot of log(kX/kH) vs σp showed a linearly correlated pattern with ρ = −0.79 ± 0.1. A relatively high negative slope suggests a significant cationic character build-up on the amine substrate during the coupling reaction.
Figure 2.
Hammett Plot from the Coupling of 4-Methoxyaniline with 4-X-C6H4CH2NH2 (X = OMe, Me, H, Cl, F, CF3).
We present a plausible mechanistic hypothesis for the deaminative coupling reaction on the basis of these results (Scheme 1). In light of the observation of imine product ArCH=NCH2Ar, we propose the formation of a Ru-imine species 6 as a catalytically active species, which would be initially generated from the dehydrogenation of amine substrate.23 In support of this notion, some oxidative C–N bond cleavage reactions are known to proceed via the formation of an imine intermediate.24 The coordination of imine substrate to the Ru center would increase the electrophilic nature of the imine carbon, and the nucleophilic addition of the second amine substrate would proceed to form the Ru-1,1-diamine species 7. Many structurally similar transition metal-urea complexes have been synthesized and their bonding and reactivity patterns have been well established.25 The observation of carbon isotope effect provides an experimental support for the rate-limiting C–N cleavage step in forming the Ru-aminoalkyl species 8. The dehydrogenation of second amine substrate in conjunction with the hydrogen transfer would form the coupling product 4 with the regeneration of imine species 6. Both the detection of imine product and the selective deuterium incorporation on the α-CH2 of 5a suggest that the dehydrogenation and hydrogen transfer steps are likely facile and reversible under the reaction conditions.
Scheme 1.
Proposed Mechanism of the Deaminative Coupling of Primary Amines
Conclusion
In conclusion, we successfully devised a highly chemoselective synthesis of secondary amines from the deaminative coupling of primary amines. The catechol ligand promoted ruthenium catalytic system was found to exhibit a uniquely high activity and selectivity in forming both symmetric and unsymmetric secondary amines. The catalytic method has a number of salient features that it is operationally simple, exhibits a broad substrate scope, tolerates common organic functional groups, and forms ammonia as the sole byproduct without employing any reactive reagents. The kinetic and spectroscopic studies thus far indicate that the coupling reaction proceeds via the formation of imine species with the turnover limiting C–N bond cleavage step. At this time, we have not been able to ascertain the exact role of catechol ligand on promoting the Ru catalyst, and we are currently pursuing the detection and/or the isolation of catalytically relevant Ru-catechol species.26 We are also devoting our efforts to extend synthetic utility of the catalytic method for the synthesis of nitrogen heterocycles of biological importance.
Experimental Section
General Information
All operations were carried out in a nitrogen-filled glove box or by using standard high vacuum and Schlenk techniques unless otherwise noted. All solvents used were freshly distilled over appropriate drying reagents. Chlorobenzene was distilled from purple solutions of sodium and benzophenone, and hexanes was dried over calcium hydride prior to use. All organic substrates were received from commercial sources and were used without further purification. The 1H, 2H, 13C, and 31P NMR spectra were recorded on a Varian 400 MHz FT-NMR spectrometer, and the data are reported as: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad, app = apparent; coupling constant(s) in Hz; integration. Mass spectra were recorded from Agilent 6850 GC-MS spectrometer by using a HP-5 (5% phenylmethylpolysiloxane) column (30 m, 0.32 mm, 0.25 μm). High resolution mass spectra were obtained at the Mass Spectrometry/ICP Lab, Department of Chemistry and Biochemistry, University of Wisconsin-Milwaukee, Milwaukee, WI. Elemental analyses were performed at the Midwest Microlab, Indianapolis, IN.
General Procedure for the Catalytic Synthesis of Secondary Amines
In a glove box, complex 1 (13 mg, 0.75 mol %) and 4-(1,1-dimethylethyl)-1,2-benzenediol (L1), (16 mg, 10 mol %) were dissolved in chlorobenzene (1 mL) in a 25 mL Schlenk tube equipped with a Teflon screw cap stopcock and a magnetic stirring bar. The resulting mixture was stirred for 5 to 10 minutes until the solution turned to a reddish green color. In an alternative procedure, the complex 2 (17 mg, 3 mol %) and L1 (16 mg, 10 mol %) were dissolved in anhydrous 1,4-dioxane (1 mL). Amine substrate (1.0 mmol and 1.4 mmol) in chlorobenzene (1 mL) was added to the reaction tube. After the tube was sealed, it was brought out of the glove box, and was stirred in an oil bath maintained at 130–140 °C for 16–20 h. The reaction tube was taken out of the oil bath, and was cooled to room temperature. After the tube was open to air, the solution was filtered through a short silica gel column by eluting with CH2Cl2 (10 mL), and the filtrate was analyzed by GC-MS. Analytically pure product was isolated by a simple column chromatography on silica gel (280–400 mesh, hexanes/EtOAc or hexanes/EtOAc/methanol).
Synthesis of Benzylcyclohexylamine (3a)
In a glove box, complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) were dissolved in chlorobenzene (1 mL) in a 25 mL Schlenk tube equipped with a Teflon screw cap stopcock and a magnetic stirring bar. The resulting mixture was stirred for 5–10 min until the solution was turned reddish green color. Benzylamine (1.0 mmol) and cyclohexylamine (1.4 mmol) were dissolved in chlorobenzene (1 mL), and the solution was added to the reaction tube. The tube was brought out of the glove box, and was stirred in an oil bath maintained at 130 °C for 16 h. The reaction tube was taken out of the oil bath, and was cooled to room temperature. The resulting solution was filtered through a short silica gel column by eluting with CH2Cl2 (10 mL), and the filtrate was analyzed by GC-MS. Analytically pure product 3a was isolated by a simple column chromatography on silica gel (280–400 mesh, n-hexane/EtOAc).
Synthesis of Dibenzylamine (4a)
In a glove box, complex 1 (13 mg, 0.75 mol %) and L1 (16 mg, 10 mol %) were dissolved in chlorobenzene (1 mL) in a 25 mL Schlenk tube equipped with a Teflon screw cap stopcock and a magnetic stirring bar. The resulting mixture was stirred for 5 to 10 min until the solution was turned reddish green color. Benzylamine (107 mg, 1.0 mmol) in chlorobenzene (1 mL) was added to the reaction mixture. After the tube was sealed, it was brought out of the glove box, and was stirred in an oil bath maintained at 130 °C for 16 h. The reaction tube was taken out of the oil bath, and was cooled to room temperature. The resulting solution was filtered through a short silica gel column by eluting with CH2Cl2 (10 mL), and the filtrate was analyzed by GC-MS. Analytically pure product 4a was isolated by a simple column chromatography on silica gel (280–400 mesh, n-hexane/EtOAc).
Catalyst and Ligand Screening Study
In a glove box, a Ru catalyst (3 mol % Ru atom) and a ligand (10 mol %) were dissolved in a solvent (1 mL) in a 25 mL Schlenk tube equipped with a Teflon screw cap stopcock and a magnetic stirring bar. After stirring for 5 to 10 min, benzylamine (54 mg, 0.5 mmol) and cyclohexylamine (69 mg, 0.7 mmol) were dissolved in chlorobenzene (1 mL), and the solution was added to the reaction tube. The tube was brought out of the glove box, and was stirred in an oil bath at 130 °C for 16 h. The product yield was determined by 1H NMR by using hexamethylbenzene as an internal standard. The results are summarized in Table S1 and S2.
N-Cyclohexylbenzenemethanamine (3a)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), benzylamine (107 mg, 1.0 mmol) and cyclohexylamine (139 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3a was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 138 mg, 73%). TLC; Rf = 0.3 (10% EtOAc in hexanes). Data for 3a: 1H NMR (400 MHz, CDCl3) δ 7.35–7.29 (m, 4H), 7.28–7.20 (m, 1H), 3.81 (s, 2H), 2.49 (tt, J = 10.3, 3.7 Hz, 1H), 1.96–1.88 (m, 2H), 1.78–1.70 (m, 2H), 1.65–1.58 (m, 1H), 1.32–1.07 (m, 5H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 140.8, 128.3, 128.0, 126.7, 56.1, 51.0, 33.5, 26.1, 25.0 ppm; GC-MS for C13H19N, m/z = 189 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.27
N-Cyclohexyl-4-methoxybenzenemethanamine (3b)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 4-methoxybenzylamine (137 mg, 1.0 mmol) and cyclohexylamine (139 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3b was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 171 mg, 78%). TLC; Rf = 0.3 (20% EtOAc in hexanes). Data for 3b: 1HNMR (400 MHz, CDCl3) δ 7.25–7.21 (m, 2H), 6.88–6.83 (m, 2H), 3.79 (s, 3H), 3.74 (s, 2H), 2.47 (tt, J = 10.4, 3.7 Hz, 1H), 1.96–1.86 (m, 2H), 1.77–1.68 (m, 2H), 1.65– 1.56 (m, 1H), 1.31 (br s, 1H), 1.30–1.05 (m, 5H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 158.4, 133.0, 129.2, 113.7, 56.0, 55.2, 50.4, 33.5, 26.1, 25.0 ppm; GC-MS for C14H21NO, m/z = 219 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.28
N-Cyclohexyl-4-fluorobenzenemethanamine (3c)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 4-fluorobenzylamine (125 mg, 1.0 mmol) and cyclohexylamine (139 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3c was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 147 mg, 71%). TLC; Rf = 0.4 (20% EtOAc in hexanes). Data for 3c: 1H NMR (400 MHz, CDCl3) δ 7.31–7.23 (m, 2H), 7.02–6.95 (m, 2H), 3.76 (s, 2H), 2.45 (tt, J = 10.3, 3.8 Hz, 1H), 1.94–1.85 (m, 2H), 1.77–1.68 (m, 2H), 1.64–1.56 (m, 1H), 1.37 (br s, 1H), 1.32–1.04 (m, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 161.7 (d, JCF = 244.2 Hz), 136.6 (d, JCF = 3.1 Hz), 129.5 (d, JCF = 7.9 Hz), 115.1 (d, JCF = 21.2 Hz), 56.1, 50.2, 33.5, 26.1, 24.9 ppm; GC-MS for C13H18FN, m/z = 207 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.29
N-Cyclohexyl-4-(trifluoromethyl)benzenemethanamine (3d)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 4-trifluromethylbenzylamine (175 mg, 1.0 mmol) and cyclohexylamine (139 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3d was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 152 mg, 59%). TLC; Rf = 0.4 (20% EtOAc in hexanes). Data for 3d: 1H NMR (400 MHz, CDCl3) δ 7.58–7.53 (m, 2H), 7.45–7.41 (m, 2H), 3.86 (s, 2H), 2.46 (tt, J = 10.3, 3.7 Hz, 1H), 1.95–1.86 (m, 2H), 1.77–1.69 (m, 2H), 1.64–1.56 (m, 1H), 1.30–1.05 (m, 5H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 145.1, 129.0 (q, JCF = 32.2 Hz), 128.2, 125.2 (q, JCF = 3.8 Hz), 124.3 (q, JCF = 271.9 Hz), 56.2, 50.4, 33.5, 26.0, 24.9 ppm; GC-MS for C14H18F3N, m/z = 257 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.30
N-(4-Chlorobenzyl)-2-(4-methoxyphenyl)ethanamine (3e)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 4-chlorobenzylamine (141 mg, 1.0 mmol) and 4-methoxybenzeneethanamine (211 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3e was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 158 mg, 57%). TLC; Rf = 0.4 (20% EtOAc in hexanes). Data for 3e: 1HNMR (400 MHz, CDCl3) δ 7.30–7.25 (m, 2H), 7.24–7.19 (m, 2H), 7.15–7.09 (m, 2H), 6.87–6.82 (m, 2H), 3.79 (s, 3H), 3.76 (s, 2H), 2.87–2.82 (m, 2H), 2.80–2.74 (m, 2H), 1.73 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 157.9, 138.6, 132.5, 131.7, 129.5, 129.3, 128.4, 113.8, 55.1, 53.0, 50.5, 35.2 ppm; GC-MS for C16H18ClNO, m/z = 275 (M+). HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C16H18ClNOH 276.1150; Found 276.1122.
N-(Biphenyl-4-ylmethyl)hexan-1-amine (3f)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 4-pheylbenzylamine (183 mg, 1.0 mmol) and 1-hexamine (141 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3f was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 176 mg, 66%). TLC; Rf = 0.3 (20% EtOAc in hexanes). Data for 3f: 1HNMR (400 MHz, CDCl3) δ 7.61–7.54 (m, 4H), 7.46–7.37 (m, 4H), 7.36–7.31 (m, 1H), 3.83 (s, 2H), 2.66 (t, J = 7.3 Hz, 2H), 1.58–1.50 (m, 2H), 1.36–1.26 (m, 6H), 0.89 (t, J = 7.0 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 141.0, 139.8, 139.6, 128.7, 128.5, 128.4, 127.1, 127.0, 53.7, 49.6, 31.8, 30.1, 27.0, 22.6, 14.1 ppm; GC-MS for C19H25N, m/z = 267 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C19H25NH 268.2060; Found 268.2061.
Benzo[d][1,3]dioxol-5-yl-N-(biphenyl-4-ylmethyl)methanamine (3g)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 4-pheylbenzylamine (183 mg, 1.0 mmol) and 1,3-benzodioxol-5-ylmethylamine (211 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3g was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 178 mg, 56%). TLC; Rf = 0.3 (20% EtOAc in hexanes). Data for 3g: 1HNMR (400 MHz, CDCl3) δ 7.62–7.55 (m, 4H), 7.47–7.39 (m, 4H), 7.37–7.32 (m, 1H), 6.90–6.88 (m, 1H), 6.82–6.76 (m, 2H), 5.95 (s, 2H), 3.84 (s, 2H), 3.75 (s, 2H), 1.67 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 147.7, 146.5, 140.9, 139.9, 139.3, 134.2, 128.7, 128.6, 127.1, 127.1, 127.0, 121.2, 108.7, 108.0, 100.9, 52.9, 52.6 ppm; GC-MS for C21H19NO2, m/z = 317 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C21H19NO2H 318.1489, Found 318.1486.
N-(4-(Trifluoromethyl)benzyl)hexan-1-amine (3h)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 4-trifluromethylbenzylamine (175 mg, 1.0 mmol) and 1-hexamine (141 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3h was isolated by a column chromatography on silica gel (n-hexanes/EtOAc = 100:1 to 10:1; 142 mg, 55%). TLC; Rf = 0.4 (20% EtOAc in hexanes). Data for 3h: 1H NMR (400 MHz, CDCl3) δ 7.57 (d, J = 8.2 Hz, 2H), 7.44 (d, J = 8.2 Hz, 2H), 3.84 (s, 2H), 2.61 (t, J = 7.2 Hz, 2H), 1.58 (br s, 1H), 1.55–1.46 (m, 2H), 1.37–1.22 (m, 6H), 0.91–0.84 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 144.6, 129.1 (q, JCF = 32.3 Hz), 128.3, 125.3 (q, JCF = 3.8 Hz), 124.2 (q, JCF = 272.0 Hz), 53.5, 49.5, 31.7, 30.0, 27.0, 22.6, 14.0; GC-MS for C14H20F3N, m/z = 259 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C14H20F3NH 260.1621; Found 260.1627.
Ethyl 3-(benzylamino)propanoate (3i)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), β-alanine ethyl ester (117 mg, 1.0 mmol) and benzylamine (150 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3i was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 149 mg, 72%). TLC; Rf = 0.3 (20% EtOAc in hexanes). Data for 3i: 1HNMR (400 MHz, CDCl3) δ 7.33–7.19 (m, 5H), 4.11 (q, J = 7.2 Hz, 2H), 3.78 (s, 2H), 2.87 (t, J = 6.5 Hz, 2H), 2.50 (t, J = 6.5 Hz, 2H), 1.82 (brs, 1H), 1.23 (t, J = 7.2 Hz, 3H), ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 172.7, 140.0, 128.3, 128.0, 126.8, 60.3, 53.7, 44.4, 34.7, 14.1 ppm; GC-MS for C12H17NO2, m/z = 207 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.31
4-Fluoro-N-hexylbenzenemethanamine (3j)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 4-flurobenzylamine (125 mg, 1.0 mmol) and 1-hexamine (141 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3j was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 132 mg, 63%). TLC; Rf = 0.3 (20% EtOAc in hexanes). Data for 3j: 1HNMR (400 MHz, CDCl3) δ 7.30–7.24 (m, 2H), 7.02–6.95 (m, 2H), 3.74 (s, 2H), 2.59 (t, J = 7.3, 2H), 1.53–1.44 (m, 2H), 1.35–1.21 (m, 6H), 0.90–0.84 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 161.8 (d, JCF = 244.6 Hz), 136.2 (d, JCF = 3.1 Hz), 129.6 (d, JCF = 7.9 Hz), 115.0 (d, JCF = 21.2 Hz), 53.3, 49.4, 31.7, 30.0, 27.0, 22.6, 14.0 ppm; GC-MS for C13H20FN, m/z = 209 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C13H20FNH 210.1653; Found 260.1654.
N-(4-fluorobenzyl)-N-(4-methoxybenzyl)amine (3k)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 4-flurobenzylamine (125 mg, 1.0 mmol) and 4-methoxybenzylamine (192 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3k was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 162 mg, 66%). TLC; Rf = 0.3 (20% EtOAc in hexanes). Data for 3k: 1H NMR (400 MHz, CDCl3) δ 7.35–7.29 (m, 2H), 7.27 (d, J = 8.3 Hz, 2H), 7.06–6.99 (m, 2H), 6.90 (d, J = 8.3 Hz, 2H), 3.81 (s, 3H), 3.76 (s, 2H), 3.74 (s, 2H), 1.78 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 161.8 (d, JCF = 244.6 Hz), 158.5, 135.9 (d, JCF = 3.1 Hz), 132.1, 129.6 (d, JCF = 7.9 Hz), 129.2, 115.0 (d, JCF = 21.2 Hz), 113.7, 55.1, 52.4, 52.1; GC-MS for C15H16FNO, m/z = 245 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.32
N-[(4-fluorophenyl)methyl]-4-methoxy-benzeneethanamine (3l)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 4-flurobenzylamine (125 mg, 1.0 mmol) and 4-methoxybenzeneethanamine (211 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3l was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 142 mg, 55%). TLC; Rf = 0.3 (20% EtOAc in hexanes). Data for 3l: 1H NMR (400 MHz, CDCl3) δ 7.27–7.21 (m, 2H), 7.15–7.10 (m, 2H), 7.03–6.96 (m, 2H), 6.87–6.82 (m, 2H), 3.79 (s, 3H), 3.76 (s, 2H), 2.88–2.83 (m, 2H), 2.80–2.74 (m, 2H), 1.69 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 161.8 (d, JCF = 244.6 Hz), 157.9, 135.8 (d, JCF = 3.1 Hz), 131.8, 129.5, 129.5 (d, JCF = 7.9 Hz), 115.0 (d, JCF = 21.2 Hz), 113.8, 55.1, 53.0, 50.6, 35.2 ppm; GC-MS for C16H18FNO, m/z = 259 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C16H18FNOH 260.1445; Found 260.1439.
N-[(4-Fluorophenyl)methyl]-β-methylbenzeneethanamine (3m)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 4-flurobenzylamine (125 mg, 1.0 mmol) and β-methylphenethylamine (189 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3m was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 141 mg, 58%). TLC; Rf = 0.4 (20% EtOAc in hexanes). Data for 3m: 1H NMR (400 MHz, CDCl3) δ 7.37–7.30 (m, 2H), 7.26–7.19 (m, 5H), 7.03–6.96 (m, 2H), 3.74 (ABq, J = 13.5 Hz, 2H), 2.99 (qt, J = 7.1, 7.0 Hz, 1H), 2.84–2.77 (m, 2H), 1.55 (br s, 1H), 1.28 (d, J = 7.0 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 161.7 (d, JCF = 244.2 Hz), 145.1, 135.8 (d, JCF = 3.1 Hz), 129.4 (d, JCF = 7.9 Hz), 128.5, 127.1, 126.3, 115.0 (d, JCF = 21.2 Hz), 56.1, 52.9, 39.9, 20.0 ppm; GC-MS for C16H18FN, m/z = 243 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C16H18FNH 244.1496; Found 244.1500.
4-Methoxy-N-(phenylmethyl)benzeneethanamine (3n)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 4-methoxybenzeneethanamine (151 mg, 1.0 mmol) and benzylamine (150 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3n was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 169 mg, 70%). TLC; Rf = 0.3 (10% EtOAc in hexanes). Data for 3n: 1H NMR (400 MHz, CDCl3) δ 7.37–7.23 (m, 5H), 7.15 (d, J = 8.3 Hz, 2H), 6.86 (d, J = 8.3 Hz, 2H), 3.82 (s, 2H), 3.80 (s, 3H), 2.89 (t, J = 6.9 Hz, 2H), 2.80 (t, J = 6.9 Hz, 2H), 1.66 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 157.9, 140.1, 131.9, 129.5, 128.3, 128.0, 126.8, 113.8, 55.1, 53.8, 50.6, 35.3 ppm; GC-MS for C16H19NO, m/z = 241 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.33
N-(4-Methoxyphenethyl)hexan-1-amine (3o)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 4-methoxybenzeneethanamine (151 mg, 1.0 mmol) and 1-hexamine (141 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3o was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 119 mg, 51%). TLC; Rf = 0.3 (10% EtOAc in hexanes). Data for 3o: 1H NMR (400 MHz, CDCl3) δ 7.12 (d, J = 8.7 Hz, 2H), 6.83 (d, J = 8.7 Hz, 2H), 3.78 (s, 3H), 2.86–2.81 (m, 2H), 2.78–2.72 (m, 2H), 2.62–2.57 (m, 2H), 1.68 (br s, 1H), 1.50–1.41 (m, 2H), 1.33–1.23 (m, 6H), 0.89–0.84 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 157.9, 132.0, 129.6, 113.8, 55.2, 51.4, 49.9, 35.3, 31.7, 29.9, 27.0, 22.6, 14.0 ppm; GC-MS for C15H25NO, m/z = 235 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C15H25NOH 236.2009; Found 236.2005.
N-Cyclohexyl-4-methoxybenzeneethanamine (3p)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 4-methoxybenzeneethanamine (151 mg, 1.0 mmol) and cyclohexylamine (139 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3p was isolated by a column chromatography on silica gel (n-hexanes/EtOAc = 100:1 to 10:1; 187 mg, 80%). TLC; Rf = 0.3 (20% EtOAc in hexanes). Data for 3p: 1H NMR (400 MHz, CDCl3) δ 7.10 (d, J = 8.7 Hz, 2H), 6.81 (d, J = 8.7 Hz, 2H), 3.75 (s, 3H), 2.86–2.81 (m, 2H), 2.74–2.68 (m, 2H), 2.39 (tt, J = 10.6, 3.8 Hz, 1H), 1.88–1.78 (m, 2H), 1.74–1.64 (m, 2H), 1.62–1.54 (m, 1H), 1.48 (br s, 1H), 1.29–0.95 (m, 5H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 157.8, 132.0, 129.4, 113.7, 56.6, 55.0, 48.3, 35.5, 33.4, 26.0, 24.9 ppm; GC-MS for C15H23NO, m/z = 233 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C15H23NOH 234.1852; Found 234.1854.
N-(4-Methoxyphenethyl)-2,3-dihydro-1H-inden-2-amine (3q)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 4-methoxybenzeneethanamine (151 mg, 1.0 mmol) and 2-aminoindane (186 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3q was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 144 mg, 54%). TLC; Rf = 0.3 (20% EtOAc in hexanes). Data for 3q: 1H NMR (400 MHz, CDCl3) δ 7.22–7.12 (m, 6H), 6.89–6.83 (m, 2H), 3.80 (s, 3H), 3.65 (quintet, J = 7.0 Hz, 1H), 3.17 (dd, J = 7.2 Hz, 2H), 2.96–2.90 (m, 2H), 2.82–2.70 (m, 4H), 1.67 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 158.0, 141.6, 131.9, 129.5, 126.3, 124.6, 113.9, 59.5, 55.2, 49.7, 39.9, 35.5 ppm; GC-MS for C18H21NO, m/z = 267 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C18H21NOH 268.1696; Found 268.1699.
N-(4-Methoxyphenethyl)-1-(4-methoxyphenyl)ethanamine (3r)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 4-methoxybenzeneethanamine (151 mg, 1.0 mmol) and (R)-(+)-1-(4-methoxyphenyl)ethylamine (211 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3r was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 186 mg, 65%). TLC; Rf = 0.3 (20% EtOAc in hexanes). Data for 3r: 1H NMR (400 MHz, CDCl3) δ 7.21–7.15 (m, 2H), 7.10–7.05 (m, 2H), 6.87–6.79 (m, 4H), 3.79 (s, 3H), 3.78 (s, 3H), 3.73 (q, J = 6.6 Hz, 1H), 2.79–2.60 (m, 4H), 1.86 (br s, 1H), 1.32 (d, J = 6.6, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 158.3, 157.8, 137.4, 131.9, 129.4, 127.4, 113.7, 113.6, 57.4, 55.0, 48.9, 35.2, 24.1 ppm (one carbon signal obscured or overlapping); GC-MS for C18H23NO2, m/z = 285 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C18H23NO2H 286.1802; Found 286.1798.
N-(4-Methoxyphenethyl)-4-phenylbutan-2-amine (3s)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 4-methoxybenzeneethanamine (151 mg, 1.0 mmol) and (±)1-methyl-3-phenyl-1-propanamine (209 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3s was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 208 mg, 73%. TLC; Rf = 0.3 (20% EtOAc in hexanes). Data for 3s: 1H NMR (400 MHz, CDCl3) δ 7.30–7.25 (m, 2H), 7.21–7.11 (m, 5H), 6.88–6.83 (m, 2H), 3.79 (s, 3H), 2.94–2.84 (m, 1H), 2.82–2.70 (m, 3H), 2.70–2.52 (m, 3H), 1.82–1.72 (m, 1H), 1.66–1.56 (m, 1H), 1.54 (br s, 1H), 1.10 (d, J = 6.3 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 157.9, 142.2, 132.0, 129.6, 128.3, 128.2, 125.6, 113.8, 55.2, 52.4, 48.5, 38.5, 35.5, 32.2, 20.2 ppm; GC-MS for C19H25NO, m/z = 283 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C19H25NOH 284.2009; Found 284.2010.
4-Methoxy-N-[(4-methoxyphenyl)methyl]benzeneethanamine (3t)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 4-methoxybenzeneethanamine (151 mg, 1.0 mmol) and 4-methoxybenzylamine (192 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3t was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 149 mg, 55%). TLC; Rf = 0.3 (20% EtOAc in hexanes). Data for 3t: 1H NMR (400 MHz, CDCl3) δ 7.21 (d, J = 8.3 Hz, 2H), 7.13 (d, J = 8.3 Hz, 2H), 6.90–6.82 (m, 4H), 3.80 (s, 3H), 3.79 (s, 3H), 3.75 (s, 2H), 2.87 (t, J = 7.1 Hz, 2H), 2.78 (t, J = 7.1 Hz, 2H), 1.71 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 158.5, 157.9, 132.3, 131.9, 129.5, 129.2, 113.7, 113.6, 55.1, 55.1, 53.2, 50.6, 35.2 ppm; GC-MS for C17H21NO2, m/z = 271 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.33
N-(3-Fluorophenethyl)-2,3-dihydro-1H-inden-2-amine (3u)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 2-(3-fluorophenyl)ethylamine (139 mg, 1.0 mmol) and 2-aminoindane (186 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3u was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 193 mg, 76%). TLC; Rf = 0.4 (10% EtOAc in hexanes). Data for 3u: 1H NMR (400 MHz, CDCl3) δ 7.3–7.24 (m, 1H), 7.24–7.12 (m, 4H), 7.06–7.00 (m, 1H), 6.99–6.89 (m, 2H), 3.67 (quintet, J = 6.9 Hz, 1H), 3.18 (dd, J = 15.5, 7.2 Hz, 2H), 3.01–2.92 (m, 2H), 2.89–2.81 (m, 2H), 2.75 (dd, J = 15.5, 6.6 Hz, 2H), 1.63 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 162.8 (d, JCF = 245.5 Hz), 142.5 (d, JCF = 7.2 Hz), 141.5, 129.8 (d, JCF = 8.3 Hz), 126.4, 124.6, 124.3 (d, JCF = 2.7 Hz), 115.4 (d, JCF = 20.8 Hz), 113.0 (d, JCF = 21.0 Hz), 59.4, 49.2, 39.9, 36.2 (d, JCF = 1.6 Hz) ppm; GC-MS for C17H18FN, m/z = 255 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C17H18FNH 256.1496; Found 256.1501.
N-(3-Fluorophenethyl)-1-(4-methoxyphenyl)ethanamine (3v)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 2-(3-fluorophenyl)ethylamine (139 mg, 1.0 mmol) and (R)-(+)-1-(4-methoxyphenyl)ethylamine (211 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3v was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 157 mg, 58%). TLC; Rf = 0.3 (20% EtOAc in hexanes). Data for 3v: 1H NMR (400 MHz, CDCl3) δ 7.27–7.16 (m, 3H), 6.98–6.80 (m, 5H), 3.80 (s, 3H), 3.73 (q, J = 6.6 Hz, 1H), 2.83–2.63 (m, 4H), 1.60 (br s, 1H), 1.32 (d, J = 6.6 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 162.8 (d, JCF = 245.4 Hz), 158.5, 142.6 (d, JCF = 7.2 Hz), 137.3, 129.7 (d, JCF = 8.3 Hz), 127.5, 124.3 (d, JCF = 2.7 Hz), 115.4 (d, JCF = 20.9 Hz), 113.7, 112.9 (d, JCF = 21.0 Hz), 57.5, 55.2, 48.5, 36.1 (d, JCF = 1.7 Hz), 24.2 ppm; GC-MS for C17H20FNO, m/z = 273 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C17H20FNOH 274.1602; Found 274.1604.
N-Cyclohexyl-1,3-benzodioxole-5-methanamine (3w)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 1-(1,3-benzodioxol-5-yl)methanamine (151 mg, 1.0 mmol) and cyclohexylamine (139 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3w was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 138 mg, 59%). TLC; Rf = 0.3 (20% EtOAc in hexanes). Data for 3w: 1H NMR (400 MHz, CDCl3) δ 6.83–6.81 (m, 1H), 6.75–6.73 (m, 2H), 5.92 (s, 2H), 3.70 (s, 2H), 2.45 (tt, J = 10.4, 3.8 Hz, 1H), 1.95–1.84 (m, 2H), 1.77–1.67 (m, 2H), 1.64–1.54 (m, 1H), 1.30–1.04 (m, 5H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 147.6, 146.3, 134.8, 121.1, 108.6, 108.0, 100.8, 55.9, 50.7, 33.4, 26.1, 24.9 ppm; GC-MS for C14H19NO2, m/z = 233 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.34
N-(Benzo[d][1,3]dioxol-5-ylmethyl)-1-(4-methoxyphenyl)ethanamine (3x)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 1-(1,3-benzodioxol-5-yl)methanamine (151 mg, 1.0 mmol) and (R)-(+)-1-(4-methoxyphenyl)ethylamine (211 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3x was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 171 mg, 60%). TLC; Rf = 0.3 (20% EtOAc in hexanes). Data for 3x: 1H NMR (400 MHz, CDCl3) δ 7.30–7.24 (m, 2H), 6.93–6.86 (m, 2H), 6.82–6.78 (m, 1H), 6.77–6.67 (m, 2H), 5.93 (s, 2H), 3.82 (s, 3H), 3.76 (q, J = 6.6 Hz, 1H), 3.52 (ABq, J = 13.2 Hz, 2H), 1.70 (br s, 1H), 1.34 (d, J = 6.6 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 158.5, 147.6, 146.3, 137.4, 134.5, 127.7, 121.1, 113.8, 108.7, 108.0, 100.8, 56.5, 55.2, 51.3, 24.4 ppm; GC-MS for C17H19NO3, m/z = 285 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C17H19NO3H 286.1438; Found 286.1427.
N-(Benzo[d][1,3]dioxol-5-ylmethyl)-2-(4-methoxyphenyl)propan-1-amine (3y)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 1-(1,3-benzodioxol-5-yl)methanamine (151 mg, 1.0 mmol) and (±)-1-(4-methoxyphenyl)ethylamine (211 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3y was isolated by a column chromatography on silica gel (n-hexanes/EtOAc = 100:1 to 10:1; 152 mg, 56%). TLC; Rf = 0.3 (20% EtOAc in hexanes). Data for 3y: 1H NMR (400 MHz, CDCl3) δ 7.35–7.28 (m, 2H), 7.24–7.18 (m, 3H), 6.75 (d, J = 1.6 Hz, 2H), 6.73 (d, J = 7.9 Hz, 1H), 6.68 (dd, J = 7.9, 1.6 Hz, 1H), 5.93 (s, 2H), 3.66 (ABq, J = 13.2 Hz, 2H), 2.96 (sextet, J = 7.1 Hz, 1H), 2.77 (d, J = 7.2 Hz, 2H), 1.66 (br s, 1H), 1.26 (d, J = 7.1 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 147.6, 146.4, 145.2, 134.1, 128.5, 127.2, 126.4, 121.1, 108.6, 108.0, 100.8, 56.0, 53.5, 39.9, 20.1 ppm; GC-MS for C17H19NO2, m/z = 269 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C17H19NO2H 270.1489; Found 270.1463.
N-Hexyl-1,2,3,4-tetrahydro-1-naphthalenamine (3z)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 1,2,3,4-tetrahydro-1-naphthalenamine (147 mg, 1.0 mmol) 1-hexamine (141 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3z was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 106 mg, 46%). TLC; Rf = 0.3 (10% EtOAc in hexanes). Data for 3z: 1H NMR (400 MHz, CDCl3) 7.37–7.30 (m, 1H), 7.21–7.05 (m, 3H), 3.77 (t, J = 4.8 Hz, 1H), 2.87–2.61 (m, 1H), 2.03–1.91 (m, 1H), 1.91–1.81 (m, 2H), 1.78–1.68 (m, 1H), 1.56–1.46 (m, 2H), 1.41–1.19 (m, 7H), 0.94–0.85 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 139.3, 137.3, 129.0, 128.7, 126.5, 125.6, 55.4, 47.3, 31.8, 30.4, 29.3, 28.2, 27.1, 22.6, 18.9, 14.1 ppm; GC-MS for C16H25N, m/z = 231 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C16H25NH 232.2060; Found 232.2053.
N-[2-(4-Methoxyphenyl)ethyl]-1,2,3,4-tetrahydro-1-naphthalenamine (3aa)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 1,2,3,4-tetrahydro-1-naphthalenamine (147 mg, 1.0 mmol) and 4-methoxybenzeneethanamine (211 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3aa was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 177 mg, 63%). TLC; Rf = 0.3 (10% EtOAc in hexanes). Data for 3aa: 1H NMR (400 MHz, CDCl3) δ 7.28–7.23 (m, 1H), 7.21–7.13 (m, 4H), 7.12–7.07 (m, 1H), 6.90–6.85 (m, 2H), 3.82 (s, 3H), 3.81 (t, J = 4.7 Hz, 1H), 3.05–2.89 (m, 2H), 2.87–2.69 (m, 4H), 2.01–1.86 (m, 3H), 1.80–1.70 (m, 1H), 1.63 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 157.9, 139.0, 137.3, 132.1, 129.6, 129.0, 128.5, 126.5, 125.6, 113.7, 55.3, 55.2, 48.6, 35.7, 29.3, 28.2, 19.0 ppm; GC-MS for C19H23NO, m/z = 281 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C19H23NOH 282.1852; Found 282.1843.
N-Cyclohexyl-5-methoxytryptamine (3bb)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 5-methoxytryptamine (190 mg, 1.0 mmol) and cyclohexylamine (139 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3bb was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 218 mg, 80%). TLC; Rf = 0.2 (30% EtOAc in hexanes). Data for 3bb: 1H NMR (400 MHz, CDCl3) δ 8.21 (br s, 1H), 7.26–7.20 (m, 1H), 7.08–7.05 (m, 1H), 7.02–6.99 (m, 1H), 6.85 (dd, J = 8.8, 2.5 Hz, 1H), 3.86 (s, 3H), 3.07–2.90 (m, 4H), 2.45 (tt, J = 10.5, 3.7 Hz, 1H), 1.91–1.78 (m, 3H), 1.75–1.65 (m, 2H), 1.64–1.55 (m, 1H), 1.29–0.99 (m, 5H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 153.8, 131.5, 127.8, 122.8, 113.6, 112.1, 111.8, 100.6, 56.8, 55.9, 46.8, 33.5, 26.1, 25.9, 25.0 ppm; GC-MS for C17H24N2O, m/z = 272 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C17H24FN2OH 273.1961; Found 273.1960.
N-Cyclohexyl-2-furanmethanamine (3cc)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), 2-(2-aminoethyl)furan (97 mg, 1.0 mmol) and cyclohexylamine (139 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3cc was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 124 mg, 69%). TLC; Rf = 0.3 (10% EtOAc in hexanes). Data for 3cc: 1H NMR (400 MHz, CDCl3) δ 7.32 (dd, J = 1.9, 0.9 Hz, 1H), 6.28 (dd, J = 3.2, 1.9 Hz, 1H), 6.14–6.12 (m, 1H), 3.78 (s, 2H), 2.42 (tt, J = 10.4, 3.8, Hz, 1H), 1.90–1.81 (m, 2H), 1.74–1.66 (m, 2H), 1.63–1.46 (m, 2H), 1.28–1.02 (m, 5H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 154.2, 141.5, 110.0, 106.4, 55.7, 43.2, 33.2, 26.0, 24.9 ppm; GC-MS for C11H17NO, m/z = 179 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.35
4-[2-Cyclohexylaminoethyl]phenol (3dd)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %), tyramine (136 mg, 1.0 mmol) and cyclohexylamine (139 mg, 1.4 mmol) was stirred at 130 °C for 16 h. The product 3dd was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 152 mg, 69%). TLC; Rf = 0.3 (20% EtOAc in hexanes). Data for 3dd: 1H NMR (400 MHz, CDCl3) δ 7.02 (d, J = 8.3 Hz, 2H), 6.72 (d, J = 8.3 Hz, 2H), 4.71 (br s, 1H), 2.93 (t, J = 6.9 Hz, 2H), 2.75 (t, J = 6.9 Hz, 2H), 2.47 (tt, J = 10.6, 3.6 Hz, 1H), 1.92–1.85 (m, 2H), 1.75–1.66 (m, 2H), 1.64–1.55 (m, 1H), 1.28–1.08 (m, 5H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 155.5, 130.0, 129.7, 115.9, 56.9, 47.6, 34.7, 32.9, 25.9, 25.0 ppm; GC-MS for C14H21NO, m/z = 219 (M+); Anal. Calcd for C14H21NO: C, 76.67; H, 9.65. Found: C, 76.78; H, 9.35; HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C14H21NOH 220.1696; Found 220.1698.
Dibenzylamine (4a)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and benzylamine (107 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4a was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 88 mg, 89%). TLC; Rf = 0.3 (10% EtOAc in hexanes). Data for 4a: 1H NMR (400 MHz, CDCl3) 7.42–7.24 (m, 10H), 3.84 (s, 4H), 2.08 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 140.0, 128.4, 128.1, 126.9, 53.0 ppm; GC-MS for C14H15N, m/z = 197 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.36
Bis(4-methylbenzyl)amine (4b)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and 4-methylbenzylamine (121 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4b was isolated by a column chromatography on silica gel (n-hexane/ethyl acetate = 100:1 to 10:1; 106 mg, 94%). TLC; Rf = 0.4 (20% EtOAc in hexanes). Data for 4b: 1H NMR (400 MHz, CDCl3) δ 7.27 (d, J = 7.9 Hz, 4H), 7.18 (d, J = 7.9 Hz, 4H), 3.80 (s, 4H), 2.38 (s, 6H), 1.70 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 137.2, 136.4, 129.0, 128.1, 52.7, 21.1 ppm; GC-MS for C16H19N, m/z = 225 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.36,37
Bis(4-methoxylbenzyl)amine (4c)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol L1 (16 mg, 10 mol %) and 4-methoxylbenzylamine (137 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4c was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 111 mg, 86%). TLC; Rf = 0.3 (10% EtOAc in hexanes). Data for 4c 1H NMR (400 MHz, CDCl3) δ 7.29–7.24 (m, 4H), 6.91–6.86 (m, 4H), 3.81 (s, 6H), 3.74 (s, 4H), 1.69 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 158.5, 132.4, 129.2, 113.6, 55.1, 52.3 ppm; GC-MS for C16H19NO2, m/z = 257 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.36,37
N,N-Bis(4-chlorobenzyl)amine (4d)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and 4-chlorobenzylamine (141 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4d was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 122 mg, 92%). TLC; Rf = 0.5 (10% EtOAc in hexanes). Data for 4d 1H NMR (400 MHz, CDCl3) δ 7.33–7.28 (m, 8H), 3.76 (s, 4H), 1.64 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 138.5, 132.6, 129.4, 128.5, 52.2 ppm; GC-MS for C14H13Cl2N, m/z = 265 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.37,38
N-([1,1′-Biphenyl]-4-ylmethyl)-[1,1′-biphenyl]-4-methanamine (4e)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and 4-phenylbenzylamine (183 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4e was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 148 mg, 85%). TLC; Rf = 0.5 (20% EtOAc in hexanes). Data for 4e 1H NMR (400 MHz, CDCl3) δ 7.59 (t, J = 8.4 Hz, 8H), 7.49–7.42 (m, 8H), 7.34 (t, J = 7.6 Hz, 2H), 3.90 (s, 4H), 1.65 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 140.9, 139.9, 139.4, 128.7, 128.6, 127.1, 127.0, 52.8 ppm (one carbon signal obscured or overlapping); GC-MS for C26H23N, m/z = 349 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.39
4-Fluoro-N-[(4-fluorophenyl)methyl]benzenemethanamine (4f)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and 4-phenylbenzylamine (125 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4f was isolated by a column chromatography on silica gel (n-hexanes/EtOAc = 100:1 to 10:1; 106 mg, 91%). TLC; Rf = 0.4 (20% EtOAc in hexanes). Data for 4f 1H NMR (400 MHz, CDCl3) δ 7.36–7.28 (m, 4H), 7.07–6.99 (m, 4H), 3.78 (s, 4H), 1.67 (br s, 1H); 13C{1H} NMR (100 MHz, CDCl3) δ 161.8 (d, JCF = 244.5 Hz), 135.8 (d, JCF = 3.1 Hz), 129.6 (d, JCF = 8.0 Hz), 115.1 (d, JCF = 21.3 Hz), 52.3 ppm; GC-MS for C14H13F2N, m/z = 233 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.38
4-(Trifluoromethyl)-N-[[4-(trifluoromethyl)phenyl]methyl]benzenemethanamine (4g)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and 4-(trifluoromethyl)benzenemethanamine (175 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4g was isolated by a column chromatography on silica gel (n-hexanes/EtOAc = 100:1 to 10:1; 140 mg, 84%). TLC; Rf = 0.5 (20% EtOAc in hexanes). Data for 4g 1H NMR (400 MHz, CDCl3) δ 7.64–7.57 (m, 4H), 7.51–7.46 (m, 4H), 3.88 (s, 4H), 1.75 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 144.1, 129.4 (q, JCF = 32.4 Hz), 128.3, 125.3 (q, JCF = 3.8 Hz), 124.2 (q, JCF = 271.8 Hz), 52.6 ppm; GC-MS for C16H13F6N, m/z = 333 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.38,40
Bis(3-methoxylbenzyl)amine (4h)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and 3-methoxylbenzylamine (137 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4h was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 108 mg, 84%). TLC; Rf = 0.3 (10% EtOAc in hexanes). Data for 4h 1H NMR (400 MHz, CDCl3) δ 7.29–7.22 (m, 2H), 6.97–6.91 (m, 4H), 6.84–6.78 (m, 2H), 3.82 (s, 6H), 3.80 (s, 4H), 1.71 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.6, 141.9, 129.3, 120.4, 113.5, 112.4, 55.1, 53.0 ppm; GC-MS for C16H19NO2, m/z = 257 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.36
N,N-Bis(3-chlorobenzyl)amine (4i)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and 3-chlorobenzylamine (141 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4i was isolated by a column chromatography on silica gel (n-hexanes/EtOAc = 100:1 to 10:1; 118 mg, 89%). TLC; Rf = 0.3 (10% EtOAc in hexanes). Data for 4i 1H NMR (400 MHz, CDCl3) δ 7.37–7.34 (m, 2H), 7.29–7.19 (m, 6H), 3.77 (s, 4H), 1.65 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 142.1, 134.2, 129.6, 128.1, 127.2, 126.2, 52.5 ppm; GC-MS for C14H13Cl2N, m/z = 265 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.41
Bis(3,4-methylenedioxybenzyl)amine (4j)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and 1-(1,3-benzodioxol-5-yl)methanamine (151 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4j was isolated by a column chromatography on silica gel (n-hexanes/EtOAc = 100:1 to 10:1; 110 mg, 77%). TLC; Rf = 0.4 (40% EtOAc in hexanes). Data for 4j 1H NMR (400 MHz, CDCl3) δ 6.85 (s, 2H), 6.77–6.75 (m, 4H), 5.94 (s, 4H), 3.69 (s, 4H), 1.59 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 147.7, 146.5, 134.3, 121.2, 108.7, 108.0, 100.9, 52.7 ppm; GC-MS for C16H15NO4, m/z = 285 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C16H15NO4H 286.1074; Found 286.1070.
Bis(3,4,5-trimethoxylbenzyl)amine (4k)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and 3,4,5-trimethoxylbenzylamine (197 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4k was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 115 mg, 61%). TLC; Rf = 0.3 (50% EtOAc in hexanes). Data for 4k 1H NMR (400 MHz, CDCl3) 6.56 (s, 4H), 3.84 (s, 12H), 3.81 (s, 6H), 3.74 (s, 4H), 1.80 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 153.1, 136.6, 135.8, 104.7, 60.7, 55.9, 53.3 ppm; GC-MS for C20H27NO6, m/z = 377 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C20H27NO6H 378.1911; Found 378.1892.
4-Methoxy-N-[(1R)-1-(4-methoxyphenyl)ethyl]-α-methyl-(αR)-benzenemethanamine (4l)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and (R)-(+)-1-(4-methoxyphenyl)ethylamine (151 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4l was isolated by a column chromatography on silica gel (n-hexanes/EtOAc = 100:1 to 10:1; 107 mg, 75%), 7:1 mixture of diastereomers, = −211.1 (c = 0.12 in CH2Cl2); TLC; Rf = 0.3 (20% EtOAc in hexanes). Data for 4l 1H NMR (400 MHz, CDCl3) δ 7.13 (d, J = 8.6 Hz, 4H), 6.89–6.84 (m, 4H), 3.82 (s, 6H), 3.45 (q, J = 6.7 Hz, 2H), 1.54 (br s, 1H), 1.25 (d, J = 6.7 Hz, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 158.4, 137.7, 127.6, 113.7, 55.2, 54.2, 24.9 ppm; GC-MS for C18H23NO2, m/z = 285 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.42
4-Methoxy-N-[2-(4-methoxyphenyl)ethyl]benzeneethanamine (4m)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and 4-methoxybenzeneethanamine (151 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4m was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 127 mg, 89%). TLC; Rf = 0.3 (10% EtOAc in hexanes). Data for 4m 1H NMR (400 MHz, CDCl3) δ 7.10–7.05 (m, 4H), 6.84–6.79 (m, 4H), 3.79 (s, 6H), 2.88–2.82 (m, 4H), 2.76–2.70 (m, 4H), 1.56 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 157.9, 131.9, 129.5, 113.8, 55.2, 51.2, 35.3 ppm; GC-MS for C18H23NO2, m/z = 285 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C18H23NO2H 286.1802; Found 286.1800.
3-Methoxy-N-[2-(3-methoxyphenyl)ethyl]benzeneethanamine (4n)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and 3-methoxybenzeneethanamine (139 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4n was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1). Isolated yield: 128 mg, 90%. TLC; Rf = 0.3 (10% EtOAc in hexanes). Data for 4n 1H NMR (400 MHz, CDCl3) δ 7.23–7.16 (m, 2H), 6.80–6.71 (m, 6H), 3.79 (s, 6H), 2.91 (t, J = 7.2 Hz, 4H), 2.79 (t, J = 7.2 Hz, 4H), 1.91 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 159.6, 141.4, 129.4, 121.0, 114.3, 111.5, 55.1, 50.9, 36.2 ppm; GC-MS for C18H23NO2, m/z = 285 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C18H23NO2H 286.1802; Found 286.1798.
3-Fluoro-N-[2-(3-fluorophenyl)ethyl]benzeneethanamine (4o)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and 3-fluorobenzeneethanamine (139 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4o was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 114 mg, 87%). TLC; Rf = 0.4 (10% EtOAc in hexanes). Data for 4o 1H NMR (400 MHz, CDCl3) δ 7.26–7.18 (m, 2H), 6.98–6.83 (m, 6H), 2.93–2.86 (m, 4H), 2.82–2.76 (m, 4H), 1.68 (br s, 1H); 13C{1H} NMR (100 MHz, CDCl3) δ 162.9 (d, JCF = 245.7 Hz), 142.3 (d, JCF = 7.1 Hz), 129.9 (d, JCF = 8.3 Hz), 124.3 (d, JCF = 2.8 Hz), 115.4 (d, JCF = 20.9 Hz), 113.1 (d, JCF = 21.0 Hz), 50.6, 35.9 (d, JCF = 1.4 Hz) ppm; GC-MS for C16H17F2N, m/z = 261 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C16H17F2NH 262.1402; Found 262.1389.
β-Methyl-N-(2-phenylpropyl)benzeneethanamine (4p)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and (R)-(+)-β-methylphenethylamine (135 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4p was isolated by a column chromatography on silica gel (n-hexane/ethyl acetate = 100:1 to 10:1; 118 mg, 93%), 1.15:1 mixture of diastereomers. TLC; Rf = 0.5 (30% EtOAc in hexanes). Data for 4p 1H NMR (400 MHz, CDCl3) δ 7.26–7.21 (m, 4H), 7.20–7.14 (m, 2H), 7.13–7.07 (m, 4H), 2.95–2.84 (m, 2H), 2.84–2.66 (m, 4H), 1.49 (br s, 1H), 1.21–1.17 (2d, J = 6.9 Hz, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 145.1, 128.5, 127.0, 126.2, 56.7, 39.5, 19.8 ppm; GC-MS for C18H23N, m/z = 253 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.43
α-Methyl-N-(1-methyl-3-phenylpropyl)benzenepropanamine (4q)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and α-methylbenzenepropanamine (149 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4p was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 126 mg, 90%), 1:1 mixture of diastereomers. TLC; Rf = 0.5 (40% EtOAc in hexanes). Data for 4q 1H NMR (400 MHz, CDCl3) δ 7.58–6.97 (m, 10H), 2.85–2.74 (m, 2H), 2.73–2.58 (m, 4H), 1.84–1.70 (m, 2H), 1.69–1.57 (m, 2H), 1.15–1.04 (m, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 142.4 (isomers 1 and 2), 128.3 (isomers 1 and 2), 128.2 (isomers 1 and 2), 125.6 (isomers 1 and 2), 49.4 (isomer 1), 49.2 (isomer 2), 39.3 (isomer 1), 38.8 (isomer 2), 32.4 (isomer 1), 32.2 (isomer 2), 21.1 (isomer 1), 20.7 (isomer 2) ppm; GC-MS for C20H27N, m/z = 281 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.44
Di-[2-aminoindane] (4r)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and 2-indanamine (133 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4r was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 91 mg, 73%). TLC; Rf = 0.5 (20% EtOAc in hexanes). Data for 4r 1H NMR (400 MHz, CDCl3) δ 7.30–7.24 (m, 4H), 7.24–7.18 (m, 4H), 3.83 (quintet, J = 7.2 Hz, 2H), 3.26 (dd, J = 15.4, 7.2 Hz, 4H), 2.85 (dd, J = 15.4, 7.2 Hz, 4H), 1.77 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 141.5, 126.3, 124.5, 58.1, 40.2 ppm; GC-MS for C18H19N, m/z = 249 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C18H19NH 250.1590; Found 250.1598.
Bis(cyclohexyl)amine (4s)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and cyclohexylamine (99 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4s was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 86 mg, 95%). TLC; Rf = 0.3 (20% EtOAc in hexanes). Data for 4s 1H NMR (400 MHz, CDCl3) δ 2.47 (tt, J = 10.6, 3.7 Hz, 2H), 1.82–1.74 (m, 4H), 1.68–1.59 (m, 4H), 1.57–1.49 (m, 2H), 1.24–0.88 (m, 10H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 52.8, 34.1, 26.0, 25.1 ppm; GC-MS for C12H23N, m/z = 181 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.45,46
N-(2-Thienylethyl)-2-thiopheneethanamine (4t)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and 2-thiopheneethanamine (127 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4t was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 106 mg, 89%). TLC; Rf = 0.4 (30% EtOAc in hexanes). Data for 4t 1H NMR (400 MHz, CDCl3) δ 7.22–7.04 (m, 2H), 6.96–6.87 (m, 2H), 6.85–6.75 (m, 2H), 3.06–2.99 (m, 4H), 2.98–2.90 (m, 4H), 2.45 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 142.2, 126.8, 125.0, 123.5, 50.7, 30.2 ppm; GC-MS for C12H15NS2, m/z = 237 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C12H15NS2H 238.0719; Found 238.0716.
Di-n-hexylamine (4u)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and 1-hexamine (101 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4u was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 40 mg, 43%). TLC; Rf = 0.3 (30% EtOAc in hexanes). Data for 4u: 1H NMR (400 MHz, CDCl3) δ 2.59 (t, J = 7.4, 4H), 2.24 (br s, 1H), 1.54–1.44 (m, 4H), 1.35–1.19 (m, 12H), 0.90–0.82 (m, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 50.0, 31.7, 29.8, 27.1, 22.6, 14.0 ppm; GC-MS for C12H27N, m/z = 185 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.46,47
N,N-Dibutylamine (4v)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and n-butylamine (73 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4v was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 30 mg, 46%). TLC; Rf = 0.3 (30% EtOAc in hexanes). Data for 4v: 1H NMR (400 MHz, CDCl3) δ 2.43 (t, J = 7.2 Hz, 4H), 1.37–1.25 (m, 4H), 1.24–1.11 (m, 4H), 0.86 (br s, 1H), 0.75 (t, J = 7.3 Hz, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 49.6, 32.1, 20.2, 13.7 ppm; GC-MS for C8H19N, m/z = 129 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.47
N-(2-Furanylmethyl)-2-furanmethanamine (4w)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and Furfurylamine (97 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4w was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 70 mg, 79%). TLC; Rf = 0.3 (30% EtOAc in hexanes). Data for 4w: 1H NMR (400 MHz, CDCl3) δ 7.40–7.31 (m, 2H), 6.34–6.27 (m, 2H), 6.22–6.13 (m, 2H), 3.78 (s, 4H), 1.89 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 153.3, 141.9, 110.1, 107.2, 44.9 ppm; GC-MS for C10H11NO2, m/z = 177 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.48
N-(3-Phenylpropyl)benzenepropanamine (4x)
A chlorobenzene (2.0 mL) solution of complex 1 (13 mg, 0.75 mol %), L1 (16 mg, 10 mol %) and 3-phenyl-1-propanamine (135 mg, 1.0 mmol) was stirred at 130 °C for 16 h. The product 4x was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 100:1 to 10:1; 53 mg, 42%). TLC; Rf = 0.3 (30% EtOAc in hexanes). Data for 4x: 1H NMR (400 MHz, CDCl3) δ 7.34–7.14 (m, 10H), 2.65 (t, J = 7.4 Hz, 8H), 1.89 (brs, 1H), 1.84 (quintet, J = 7.4 Hz, 4H); 13C{1H} NMR (100 MHz, CDCl3) δ 142.0, 128.3, 128.3, 125.8, 49.4, 33.6, 31.5 ppm; GC-MS for C18H23N, m/z = 253 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.41
4-Methoxy-N-phenylbenzenemethanamine (5a)
A chlorobenzene (2.0 mL) solution of complex 1 (7 mg, 0.75 mol %), L1 (8 mg, 10 mol %), aniline (47 mg, 0.5 mmol) and 4-methoxybenzylamine (96 mg, 0.7 mmol) was stirred at 140 °C for 20 h. The product 5a was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 150:1 to 40:1; 76 mg, 71%). TLC; Rf = 0.4 (30% EtOAc in hexanes). Data for 5a: 1H NMR (400 MHz, CDCl3) δ 7.29 (d, J = 8.6 Hz, 2H), 7.22–7.16 (m, 2H), 6.88 (d, J = 8.6 Hz, 2H), 6.77–6.72 (m, 1H), 6.70–6.65 (m, 2H), 4.26 (s, 2H), 3.80 (s, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 158.9, 147.1, 130.6, 129.3, 129.1, 118.4, 114.0, 113.6, 55.3, 48.4 ppm; GC-MS for C14H15NO, m/z = 213 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.49,50
N-(4-Methoxyphenyl)phenylmethanamine (5b)
A chlorobenzene (2.0 mL) solution of complex 1 (7 mg, 0.75 mol %), L1 (8 mg, 10 mol %), 4-methoxyaniline (62 mg, 0.5 mmol) and benzylamine (75 mg, 0.7 mmol) was stirred at 140 °C for 20 h. The product 5b was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 150:1 to 40:1; 61 mg, 57%). TLC; Rf = 0.3 (20% EtOAc in hexanes). Data for 5b: 1HNMR (400 MHz, CDCl3) δ 7.46–7.29 (m, 5H), 6.87–6.81 (m, 2H), 6.69–6.63 (m, 2H), 4.33 (s, 2H), 3.79 (s, 3H), ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 152.1, 142.2, 139.5, 128.5, 127.5, 127.1, 114.8, 114.1, 55.7, 49.1 ppm; GC-MS for C14H15NO, m/z = 213 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.51,52
N-(4-Methoxyphenyl)[1,1′-biphenyl]-4-methanamine (5c)
A chlorobenzene (2.0 mL) solution of complex 1 (7 mg, 0.75 mol %), L1 (8 mg, 10 mol %), 4-methoxyaniline (62 mg, 0.5 mmol) and 4-phenylbenzylamine (128 mg, 0.7 mmol) was stirred at 140 °C for 20 h. The product 5c was isolated by a column chromatography on silica gel (n-hexane/ethyl acetate = 150:1 to 40:1; 101 mg, 70%). TLC; Rf = 0.4 (30% EtOAc in hexanes). Data for 5c: 1H NMR (400 MHz, CDCl3) δ 7.61–7.55 (m, 4H), 7.47–7.42 (m, 4H), 7.35 (tt, J = 7.3, 1.3, Hz, 1H), 6.82–6.77 (m, 2H), 6.69–6.65 (m, 2H), 4.34 (s, 2H), 3.75 (s, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 152.5, 141.7, 140.8, 140.2, 138.3, 128.7, 128.1, 127.3, 127.2, 127.0, 114.9, 114.6, 55.8, 49.2 ppm; GC-MS for C20H19NO m/z = 289 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.53
4-Fluoro-N-(4-methoxyphenyl)benzenemethanamine (5d)
A chlorobenzene (2.0 mL) solution of complex 1 (7 mg, 0.75 mol %), L1 (8 mg, 10 mol %), 4-methoxyaniline (62 mg, 0.5 mmol) and 4-fluorobenzylamine (88 mg, 0.7 mmol) was stirred at 140 °C for 20 h. The product 5d was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 150:1 to 40:1; 47 mg, 41%). TLC; Rf = 0.3 (20% EtOAc in hexanes). Data for 5d: 1H NMR (400 MHz, CDCl3) δ 7.38–7.30 (m, 2H), 7.07–6.99 (m, 2H), 6.83–6.76 (m, 2H), 6.66–6.59 (m, 2H), 4.26 (s, 2H), 4.09 (br s, 1H), 3.75 (s, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 162.0 (d, JCF = 245.1 Hz), 152.4, 141.7, 135.0 (d, JCF = 3.1 Hz), 129.1 (d, JCF = 8.0 Hz), 115.3 (d, JCF = 21.4 Hz), 114.8, 114.4, 55.7, 48.6 ppm; GC-MS for C14H14FNO m/z = 231 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.54,55
N-(4-Chlorophenyl)-4-methoxybenzenemethanamine (5e)
A chlorobenzene (2.0 mL) solution of complex 1 (7 mg, 0.75 mol %), L1 (8 mg, 10 mol %), 4-chloroaniline (64 mg, 0.5 mmol) and 4-methoxybenzylamine (96 mg, 0.7 mmol) was stirred at 140 °C for 20 h. The product 5e was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 150:1 to 40:1; 73 mg, 59%). TLC; Rf = 0.5 (20% EtOAc in hexanes). Data for 5e: 1H NMR (400 MHz, CDCl3) 7.30–7.25 (m, 2H), 7.14–7.09 (m, 2H), 6.92–6.87 (m, 2H), 6.59–6.53 (m, 2H), 4.23 (s, 2H), 4.15 (br s, 1H), 3.81 (s, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 158.9, 146.5, 130.7, 129.0, 128.7, 122.1, 114.0, 114.0, 55.3, 47.9 ppm; GC-MS for C14H14ClNO, m/z = 247 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.56
3,4,5-Trimethoxy-N-(4-methoxyphenyl)benzenemethanamine (5f)
A chlorobenzene (2.0 mL) solution of complex 1 (7 mg, 0.75 mol %), L1 (8 mg, 10 mol %), 4-methoxyaniline (62 mg, 0.5 mmol) and 3,4,5-trimethoxybenzylamine (138 mg, 0.7 mmol) was stirred at 140 °C for 20 h. The product 5f was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 150:1 to 40:1; 138 mg, 91%). TLC; Rf = 0.5 (50% EtOAc in hexanes). Data for 5f: 1H NMR (400 MHz, CDCl3) δ 6.81–6.76 (m, 2H), 6.65–6.60 (m, 4H), 4.21 (s, 2H), 3.84 (s, 9H), 3.74 (s, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 153.3, 152.3, 142.1, 136.9, 135.3, 114.8, 114.3, 104.3, 60.8, 56.0, 55.7, 49.7 ppm; GC-MS for C17H21NO4, m/z = 303 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.57,58
3,4,5-Trimethoxy-N-phenylbenzenemethanamine (5g)
A chlorobenzene (2.0 mL) solution of complex 1 (7 mg, 0.75 mol %), L1 (8 mg, 10 mol %), aniline (47 mg, 0.5 mmol) and 3,4,5-trimethoxybenzylamine (138 mg, 0.7 mmol) was stirred at 140 °C for 20 h. The product 5g was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 150:1 to 40:1; 97 mg, 71%). TLC; Rf = 0.6 (50% EtOAc in hexanes). Data for 5g: 1H NMR (400 MHz, CDCl3) δ 7.23–7.17 (m, 2H), 6.75 (tt, J = 7.3, 1.1 Hz, 1H), 6.69–6.65 (m, 2H), 6.63 (s, 2H), 4.27 (s, 2H), 4.13 (br s, 1H), 3.86 (s, 3H), 3.85 (s, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 153.3, 148.0, 136.8, 135.1, 129.1, 117.6, 112.8, 104.2, 60.7, 55.9, 48.7 ppm; GC-MS for C16H19NO3, m/z = 273 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.59,60
3,4,5-Trimethoxy-N-(4-chlorophenyl)benzenemethanamine (5h)
A chlorobenzene (2.0 mL) solution of complex 1 (7 mg, 0.75 mol %), L1 (8 mg, 10 mol %), 4-chloroaniline (64 mg, 0.5 mmol) and 3,4,5-trimethoxybenzylamine (138 mg, 0.7 mmol) was stirred at 140 °C for 20 h. The product 5h was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 150:1 to 40:1; 111 mg, 72%). TLC; Rf = 0.6 (50% EtOAc in hexanes). Data for 5h: 1H NMR (400 MHz, CDCl3) δ 7.12 (d, J = 9.0 Hz, 2H), 6.58 (s, 2H), 6.57 (d, J = 9.0 Hz, 2H), 4.23 (s, 2H), 3.84 (s, 3H), 3.84 (s, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 153.4, 146.4, 137.0, 134.5, 129.0, 122.3, 114.0, 104.1, 60.8, 56.0, 48.8 ppm; GC-MS for C16H18ClNO3, m/z = 307 (M+). Anal. Calcd for C16H18ClNO3: C, 62.44; H, 5.90. Found: C, 62.88; H, 6.05.
N-Hexyl-4-methoxybenzenamine (5i)
A chlorobenzene (2.0 mL) solution of complex 1 (7 mg, 0.75 mol %), L1 (8 mg, 10 mol %), 4-methoxyaniline (62 mg, 0.5 mmol) and 1-hexamine (71 mg, 0.7 mmol) was stirred at 140 °C for 20 h. The product 5i was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 150:1 to 40:1; 65 mg, 63%). TLC; Rf = 0.4 (20% EtOAc in hexanes). Data for 5i: 1H NMR (400 MHz, CDCl3) δ 6.82–6.77 (m, 2H), 6.63–6.58 (m, 2H), 3.75 (s, 3H), 3.39 (br s, 1H), 3.06 (t, J = 7.2 Hz, 2H), 1.61 (quintet, J = 7.4 Hz, 2H), 1.44–1.26 (m, 6H), 0.91 (t, J = 6.9 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 152.1, 142.5, 114.8, 114.2, 55.8, 45.2, 31.6, 29.5, 26.8, 22.6, 14.0 ppm; GC-MS for C13H21NO, m/z = 207 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.58
N-(4-Methoxyphenyl)benzenepropanamine (5j)
A chlorobenzene (2.0 mL) solution of complex 1 (7 mg, 0.75 mol %), L1 (8 mg, 10 mol %), 4-methoxyaniline (62 mg, 0.5 mmol) and 3-phenyl-1-propanamine (95 mg, 0.7 mmol) was stirred at 140 °C for 20 h. The product 5j was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 150:1 to 40:1; 54 mg, 45%). TLC; Rf = 0.9 (50% EtOAc in hexanes). Data for 5j: 1H NMR (400 MHz, CDCl3) δ 7.34–7.29 (m, 2H), 7.25–7.18 (m, 3H), 6.80 (d, J = 8.9 Hz, 2H), 6.60 (d, J = 8.9 Hz, 2H), 3.76 (s, 3H), 3.13 (t, J = 7.1 Hz, 2H), 2.75 (t, J = 7.6 Hz, 2H), 1.96 (quintet, J = 7.4 Hz, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 152.2, 142.2, 141.7, 128.4, 128.4, 125.9, 114.8, 114.3, 55.8, 44.6, 33.4, 31.0 ppm; GC-MS for C16H19NO, m/z = 241 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.61,62
N-(4-Methoxyphenyl)-2,3-dihydro-1H-inden-2-amine (5k)
A chlorobenzene (2.0 mL) solution of complex 1 (7 mg, 0.75 mol %), L1 (8 mg, 10 mol %), 3,4,5-trimethoxyaniline (62 mg, 0.5 mmol) and 2-aminoindan (93 mg, 0.7 mmol) was stirred at 140 °C for 20 h. The product 5k was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 150:1 to 40:1; 88 mg, 74%). TLC; Rf = 0.5 (30% EtOAc in hexanes). Data for 5k: 1H NMR (400 MHz, CDCl3) δ 7.29–7.17 (m, 4H), 6.86–6.80 (m, 2H), 6.67–6.62 (m, 2H), 4.33 (tt, J = 6.8, 4.4 Hz,1H), 3.79 (s, 3H), 3.37 (dd, J = 16.0, 6.8 Hz, 2H), 2.90 (dd, J = 16.0, 4.4 Hz, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 152.2, 141.4, 141.4, 126.5, 124.9, 114.9, 114.9, 55.7, 54.9, 40.1 ppm; GC-MS for C16H17NO, m/z = 239 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C16H17NOH 240.1383; Found 240.1377.
4-(2-(3,4,5-Trimethoxyphenylamino)ethyl)phenol (5l)
A chlorobenzene (2.0 mL) solution of complex 1 (7 mg, 0.75 mol %), L1 (8 mg, 10 mol %), 3,4,5-trimethoxyaniline (91 mg, 0.5 mmol) and 2-(4-hydroxyphenyl)ethanamine (96 mg, 0.7 mmol) was stirred at 140 °C for 20 h. The product 5l was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 150:1 to 40:1; 77 mg, 51%). TLC; Rf = 0.4 (50% EtOAc in hexanes). Data for 5l: 1H NMR (400 MHz, CDCl3) δ 7.09–7.05 (m, 2H), 6.81–6.77 (m, 2H), 5.90 (s, 2H), 3.81 (s, 6H), 3.77 (s, 3H), 3.33 (t, J = 7.1 Hz, 2H), 2.86 (t, J = 7.1 Hz, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 154.4, 153.9, 144.1, 130.7, 130.5, 129.8, 115.5, 91.1, 61.1, 55.9, 46.2, 34.4 ppm; GC-MS for C17H21NO4, m/z = 303 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C17H21NO4H 304.1543; Found 304.1516.
N-(3,4,5-Trimethoxybenzyl)-3,4-dihydro-2H-benzo[b][1,4]dioxepin-7-amine (5m)
A chlorobenzene (2.0 mL) solution of complex 1 (7 mg, 0.75 mol %), L1 (8 mg, 10 mol %), 3,4-dihydro-2H-1,5-benzodioxepin-7-amine (83 mg, 0.5 mmol) and 3,4,5-trimethoxybenzylamine (138 mg, 0.7 mmol) was stirred at 140 °C for 20 h. The product 5m was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 150:1 to 40:1; 129 mg, 75%). TLC; Rf = 0.4 (50% EtOAc in hexanes). Data for 5m: 1H NMR (400 MHz, CDCl3) δ 6.84 (d, J = 8.6 Hz, 1H), 6.59 (s, 2H), 6.38–6.34 (m, 1H), 6.31–6.26 (m, 1H), 4.19 (s, 2H), 4.15 (t, J = 5.4 Hz, 2H), 4.09 (t, J = 5.5 Hz, 2H), 3.84 (s, 6H), 3.83 (s, 3H), 2.14 (quintet, J = 5.5 Hz, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 153.3, 152.1, 143.9, 143.6, 137.0, 134.4, 122.2, 108.7, 106.5, 104.5, 70.9, 70.8, 60.8, 56.1, 49.8, 32.4 ppm; GC-MS for C19H23NO5, m/z = 345 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C19H23FNO5H 346.1649; Found 346.1618.
N-(3,5-Dimethoxybenzyl)-4-methoxybenzenamine (5n)
A chlorobenzene (2.0 mL) solution of complex 1 (7 mg, 0.75 mol %), L1 (8 mg, 10 mol %), 4-methoxyaniline (62 mg, 0.5 mmol) and 3,5-dimethoxybenzylamine (117 mg, 0.7 mmol) was stirred at 140 °C for 20 h. The product 5n was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 150:1 to 40:1; 115 mg, 84%). TLC; Rf = 0.7 (50% EtOAc in hexanes). Data for 5n: δ 1H NMR (400 MHz, CDCl3) 6.82–6.77 (m, 2H), 6.64–6.59 (m, 2H), 6.57 (d, J = 2.3 Hz, 2H), 6.40 (t, J = 2.3 Hz, 1H), 4.23 (s, 2H), 3.79 (s, 6H), 3.76 (s, 3H), ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 160.9, 152.0, 142.3, 142.2, 114.7, 114.0, 105.2, 98.9, 55.6, 55.2, 49.2 ppm; GC-MS for C16H19NO3, m/z = 273 (M+). 1H and 13C NMR spectral data were in good agreement with the literature values.63
4-Chloro-N-(3,5-dimethoxybenzyl)benzenamine (5o)
A chlorobenzene (2.0 mL) solution of complex 1 (7 mg, 0.75 mol %), L1 (8 mg, 10 mol %), 4-chloroaniline (64 mg, 0.5 mmol) and 3,5-dimethoxybenzylamine (117 mg, 0.7 mmol) was stirred at 140 °C for 20 h. The product 5o was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 150:1 to 40:1; 66 mg, 48%). TLC; Rf = 0.6 (30% EtOAc in hexanes). Data for 5o: 1H NMR (400 MHz, CDCl3) δ 7.13–7.08 (m, 2H), 6.58–6.53 (m, 2H), 6.52–6.49 (m, 2H), 6.38 (t, J = 2.2 Hz, 1H), 4.24 (s, 2H), 3.78 (s, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 161.1, 146.3, 141.3, 129.0, 122.3, 114.1, 105.2, 99.1, 55.3, 48.6 ppm; GC-MS for C15H16ClNO2, m/z = 277 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C15H16NO2ClH 278.0942; Found 278.0928.
N-(3,5-Dimethoxybenzyl)-4-(trifluoromethyl)benzenamine (5p)
A chlorobenzene (2.0 mL) solution of complex 1 (7 mg, 0.75 mol %), L1 (8 mg, 10 mol %), 4-trifluoromethylaniline (81 mg, 0.5 mmol) and 3,5-dimethoxybenzylamine (117 mg, 0.7 mmol) was stirred at 140 °C for 20 h. The product 5p was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 150:1 to 40:1; 58 mg, 37%). TLC; Rf = 0.4 (30% EtOAc in hexanes). Data for 5p: 1H NMR (400 MHz, CDCl3) δ 7.39 (d, J = 8.6 Hz, 2H), 6.63 (d, J = 8.6 Hz, 2H), 6.51 (d, J = 2.3 Hz, 2H) 6.39 (t, J = 2.3 Hz, 1H), 4.55 (br s, 1H), 4.30 (s, 2H), 3.78 (s, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 161.1, 150.3, 140.9, 126.6 (q, JCF = 3.8 Hz), 124.9 (q, JCF = 270.6 Hz), 119.2 (q, JCF = 32.6 Hz), 112.1 105.2, 99.1, 55.3, 48.0 ppm; GC-MS for C16H16F3NO2, m/z = 311 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C16H6F3NO2H 312.1206; Found 312.1207.
9-Ethyl-N-hexyl-9H-carbazol-3-amine (5q)
A chlorobenzene (2.0 mL) solution of complex 1 (7 mg, 0.75 mol %), L1 (8 mg, 10 mol %), 3-amino-9-ethylcarbazole (105 mg, 0.5 mmol) and 1-hexamine (71 mg, 0.7 mmol) was stirred at 140 °C for 20 h. The product 5q was isolated by a column chromatography on silica gel (n-hexane/EtOAc = 150:1 to 40:1; 103 mg, 70%). TLC; Rf = 0.4 (50% EtOAc in hexanes). Data for 5q: 1H NMR (400 MHz, CDCl3) δ 8.09–8.04 (m, 1H), 7.48–7.42 (m, 1H), 7.40–7.34 (m, 2H), 7.26 (d, J = 7.3 Hz, 1H), 7.19 (dd, J = 7.3, 1.2 Hz, 1H), 6.91 (dd, J = 8.6, 2.3 Hz, 1H), 4.32 (q, J = 7.2 Hz, 2H), 3.40 (br s, 1H), 3.25 (t, J = 7.2 Hz, 2H), 1.72 (quintet, J = 7.4 Hz, 2H), 1.55–1.45 (m, 2H), 1.42 (t, J = 7.2 Hz, 3H), 1.41–1.33 (m, 4H), 1.12–0.92 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 141.9, 140.2, 133.9, 125.2, 123.6, 122.6, 120.3, 117.8, 114.6, 109.0, 108.3, 103.3, 45.7, 37.4, 31.7, 29.7, 27.0, 22.6, 14.0, 13.8 ppm; GC-MS for C20H26N2, m/z = 294 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C20H26N2H 295.2169; Found 295.2167.
(R)-3-Ethyl-3-(4-(3,4,5-trimethoxybenzylamino)phenyl)piperidine-2,6-dione (5r)
A chlorobenzene (2.0 mL) solution of complex 1 (7 mg, 0.75 mol %), L1 (8 mg, 10 mol %), (R)-(+)-aminoglutethimide (116 mg, 0.5 mmol) and 3,4,5-trimethoxybenzylamine (138 mg, 0.7 mmol) was stirred at 140 °C for 20 h. The product 5r was isolated by a column chromatography on silica gel (n-hexane/ethyl acetate = 100:1 to 1:1; 82 mg, 40%), = +148.2 (c = 0.2 in CH2Cl2); TLC; Rf = 0.2 (50% EtOAc in hexanes). Data for 5r: δ 7.98 (br s, 1H), 7.07 (d, J = 8.7 Hz, 2H), 6.64 (d, J = 8.7 Hz, 2H), 6.59 (s, 2H), 4.24 (s, 2H), 3.83 (s, 9H), 2.62–2.52 (m, 1H), 2.44 (dd, J = 13.2, 4.9 Hz, 1H), 2.31 (ddd, J = 14.2, 4.8, 2.7 Hz, 1H), 2.16 (dd, J = 13.8, 4.8 Hz, 1H), 1.99 (sext, J = 7.4 Hz, 1H), 1.87 (sextet, J = 7.4 Hz, 1H), 0.85 (t, J = 7.4 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 175.5, 172.5, 153.4, 147.0, 137.1, 134.5, 127.1, 113.4, 113.4, 104.4, 60.8, 56.1, 50.2, 48.9, 32.9, 29.3, 26.9, 9.0 ppm; GC-MS for C23H28N2O5, m/z = 412 (M+); HRMS (IT-TOF/ESI) Calcd for C23H28N2O5-H ([M-H]−): 411.1925, Found: 411.1908.
3-(3-Phenylpropylamino)piperidine-2,6-dione (5s)
A chlorobenzene (2.0 mL) solution of complex 1 (7 mg, 0.75 mol %), L1 (8 mg, 10 mol %), L-glutamine (73 mg, 0.5 mmol) and 3-phenyl-1-propanamine (95 mg, 0.7 mmol) was stirred at 140 °C for 20 h. The product 5s was isolated by a column chromatography on silica gel (n-hexane/ethyl acetate = 50:1 to 1:1; 92 mg, 75%). TLC; Rf = 0.2 (50% EtOAc in hexanes). Data for 5s: δ 7.30–7.23 (m, 2H), 7.22–7.12 (m, 3H), 6.73 (br s, 1H), 4.08 (dd, J = 9.0, 4.8 Hz, 1H), 3.28 (dd, J = 8.9, 4.8 Hz, 1H), 2.63 (t, J = 7.5 Hz, 2H), 2.50–2.37 (m, 1H), 2.37–2.19 (m, 2H), 2.16–2.05 (m, 1H), 1.84 (quintet, J = 7.4 Hz, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 179.5, 172.1, 141.4, 128.4, 128.3, 126.0, 57.1, 39.3, 33.2, 30.8, 29.4, 25.7 ppm; GC-MS for C14H18N2O2, m/z = 246 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C14H18N2O2H 247.1441; Found 247.1408.
3-(4-Methoxybenzylamino)piperidine-2,6-dione (5t)
A chlorobenzene (2.0 mL) solution of complex 1 (7 mg, 0.75 mol %), L1 (8 mg, 10 mol %), L-glutamine (73 mg, 0.5 mmol) and 4-methoxybenzylamine (96 mg, 0.7 mmol) was stirred at 140 °C for 20 h. The product 5t was isolated by a column chromatography on silica gel (n-hexane/ethyl acetate = 50:1 to 1:1; 87 mg, 70%). TLC; Rf = 0.2 (50% EtOAc in hexanes). Data for 5t δ 7.18 (d, J = 8.5 Hz, 2H), 6.92 (br s, 1H), 6.83 (d, J = 8.5 Hz, 2H), 4.40–4.29 (m, 2H), 4.13 (dd, J = 9.0, 4.7 Hz, 1H), 3.77 (s, 3H), 2.53–2.41 (m, 1H), 2.34–2.20 (m, 2H), 2.20–2.09 (m, 1H), 1.87 (br s, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 179.5, 172.2, 158.8, 130.1, 128.9, 113.8, 57.0, 55.1, 42.7, 29.2, 25.5 ppm; GC-MS for C13H16N2O3, m/z = 248 (M+); HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C13H16N2O3H 249.1334; Found 249.1202.
Supplementary Material
Acknowledgments
Financial support from the National Science of Foundation (CHE-1358439, CHE-1664652) and National Institute of Health General Medical Sciences (R15 GM109273) is gratefully acknowledged.
Footnotes
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures, spectroscopic data and NMR spectra (PDF)
References
- 1.Recent reviews on the catalytic oxidative C–N activation reactions: Ouyang K, Hao W, Zhang WX, Xi Z. Transition-Metal-Catalyzed Cleavage of C–N Single Bonds. Chem Rev. 2015;115:12045–12090. doi: 10.1021/acs.chemrev.5b00386.Wang Q, Su Y, Li L, Huang H. Transition-metal catalysed C–N bond activation. Chem Soc Rev. 2016;45:1257–1272. doi: 10.1039/c5cs00534e.
- 2.Selected examples of catalytic C-N bond cleavage reactions: Gandelman M, Milstein D. Homogeneously catalyzed, chelate assisted hydrogenolysis of an amine C–N bond. Chem Commun. 2000:1603–1604.Hosokawa T, Kamiike T, Murahashi S-I, Shimada M, Sugafuji T. The Heck reaction in the presence of molecular oxygen. Tetrahedron Lett. 2002;43:9323–9325.Caddick S, Cloke FGN, Hitchcock PB, de Lewis KAK. Unusual Reactivity of a Nickel N-Heterocyclic Carbene Complex: tert-Butyl Group Cleavage and Silicone Grease Activation. Angew Chem, Int Ed. 2004;43:5824–5827. doi: 10.1002/anie.200460955.Takano K, Inagaki A, Akita M. Chelation-assisted Facile C–N Bond Oxidative Addition of Spironaphthoxazines by Ru(η4-cycloocta-1,5-diene)(η6-naphthalene) Chem Lett. 2006;35:434–435.Burling S, Mahon MF, Powell RE, Whittlesey MK, Williams JMJ. Ruthenium Induced C-N Bond Activation of an N-Heterocyclic Carbene: Isolation of C- and N-Bound Tautomers. J Am Chem Soc. 2006;128:13702–13703. doi: 10.1021/ja065582k.Ueno S, Chatani N, Kakiuchi F. Ruthenium-Catalyzed Carbon-Carbon Bond Formation via the Cleavage of an Unreactive Aryl Carbon-Nitrogen Bond in Aniline Derivatives with Organoboronates. J Am Chem Soc. 2007;129:6098–6099. doi: 10.1021/ja0713431.Zou B, Jiang H-F, Wang Z-Y. Palladium-Catalyzed C–N Bond Activation: The Synthesis of β-Amino Acid Derivatives from Triethylamine and Acrylates. Eur J Org Chem. 2007:4600–4604.Kruger K, Tillack A, Beller M. Recent Innovative Strategies for the Synthesis of Amines: From C-N Bond Formation to C-N Bond Activation. Chem Sus Chem. 2009;2:715–717. doi: 10.1002/cssc.200900121.Kuninobu Y, Nishi M, Takai K. Iron-catalyzed synthesis of glycine derivatives via carbon–nitrogen bond cleavage using diazoacetate. Chem Commun. 2010:8860–8862. doi: 10.1039/c0cc03781h.Shen H, Wang Y, Xie Z. Ti-amide Catalyzed Synthesis of Cyclic Guanidines from Di-/Triamines and Carbodiimides. Org Lett. 2011;13:4562–4565. doi: 10.1021/ol201752e.Jin Y-H, Fang F, Zhang X, Liu Q-Z, Wang H-B, Tian S-K. Four-Component Reaction of N-Sulfonylimines, (Cyanomethylene)triphenylphosphorane, Nitromethane, and Formaldehyde for the Synthesis of 3-Substituted 2-Methylene-4-nitrobutanenitriles. J Org Chem. 2011;76:4163–4167. doi: 10.1021/jo200483n.Fukumoto K, Oya T, Itazaki M, Nakazawa H. N-CN Bond Cleavage of Cyanamides by a Transition-Metal Complex. J Am Chem Soc. 2009;131:38–39. doi: 10.1021/ja807896b.Anbarasan P, Neumann H, Beller M. A General Rhodium-Catalyzed Cyanation of Aryl and Alkenyl Boronic Acids. Angew Chem, Int Ed. 2011;50:519–522. doi: 10.1002/anie.201006044.
- 3.Akiyama F, Miyazaki H, Kaneda K, Teranishi S, Fujiwara Y, Abe M, Taniguchi H. Arylation and Alkylation of Olefins by Arylamines or Hydrazines via Carbon-Nitrogen Bond Cleavage in the Presence of Palladium(II) Salts. J Org Chem. 1980;45:2359–2361. [Google Scholar]
- 4.Koreeda T, Kochi T, Kakiuchi F. Cleavage of C-N Bonds in Aniline Derivatives on a Ruthenium Center and Its Relevance to Catalytic C-C Bond Formation. J Am Chem Soc. 2009;131:7238–7239. doi: 10.1021/ja902829p. [DOI] [PubMed] [Google Scholar]
- 5.(a) Hie L, Fine NNF, Shah TK, Baker EL, Hong X, Yang YF, Liu P, Houk KN, Garg NK. Conversion of amides to esters by the nickel-catalysed activation of amide C–N bonds. Nature. 2015;524:79–83. doi: 10.1038/nature14615. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Meng G, Shi S, Szostak M. Cross-Coupling of Amides by N–C Bond Activation. Synlett. 2016;27:2530–2540. [Google Scholar]; (c) Liu C, Szostak M. Twisted Amides: From Obscurity to Broadly Useful Transition-Metal-Catalyzed Reactions by N−C Amide Bond Activation. Chem Eur J. 2017;23:7157–7173. doi: 10.1002/chem.201605012. [DOI] [PubMed] [Google Scholar]
- 6.(a) Weston MH, Parvez M, Back TG. J Org Chem. 2010;75:5402–5405. doi: 10.1021/jo101030b. [DOI] [PubMed] [Google Scholar]; (b) Wang M, Zhang X, Zhuang Y-X, Xu Y-H, Loh T-P. Pd-Catalyzed Intramolecular C−N Bond Cleavage, 1,4-Migration, sp3 C−H Activation, and Heck Reaction: Four Controllable Diverse Pathways Depending on the Judicious Choice of the Base and Ligand. J Am Chem Soc. 2015;137:1341–1347. doi: 10.1021/ja512212x. [DOI] [PubMed] [Google Scholar]; (c) Meng G, Szostak M. General Olefin Synthesis by the Palladium-Catalyzed Heck Reaction of Amides: Sterically Controlled Chemoselective Activation. Angew Chem, Int Ed. 2015;54:14518–14522. doi: 10.1002/anie.201507776. [DOI] [PubMed] [Google Scholar]; (d) Shi S, Meng G, Szostak M. Synthesis of Biaryls through Nickel-Catalyzed Suzuki–Miyaura Coupling of Amides by Carbon–Nitrogen Bond Cleavage. Angew Chem, Int Ed. 2016;55:6959–6963. doi: 10.1002/anie.201601914. [DOI] [PubMed] [Google Scholar]
- 7.(a) Mukherjee S, List B. Chiral Counteranions in Asymmetric Transition-Metal Catalysis: Highly Enantioselective Pd/Brønsted Acid-Catalyzed Direct γ-Allylation of Aldehydes. J Am Chem Soc. 2007;129:11336–11337. doi: 10.1021/ja074678r. [DOI] [PubMed] [Google Scholar]; (b) Wu X-S, Chen Y, Li M-B, Zhou M-G, Tian S-K. Direct Substitution of Primary Allylic Amines with Sulfinate Salts. J Am Chem Soc. 2012;134:14694–14697. doi: 10.1021/ja306407x. [DOI] [PubMed] [Google Scholar]; (c) Li MB, Wang Y, Tian S-K. Regioselective and Stereospecific Cross-Coupling of Primary Allylic Amines with Boronic Acids and Boronates through Palladium-Catalyzed C-N Bond Cleavage. Angew Chem, Int Ed. 2012;51:2968–2971. doi: 10.1002/anie.201109171. [DOI] [PubMed] [Google Scholar]; (d) Ma X-T, Wang Y, Dai R-H, Liu C-R, Tian S-K. Catalytic Allylation of Stabilized Phosphonium Ylides with Primary Allylic Amines. J Org Chem. 2013;78:11071–11075. doi: 10.1021/jo401736k. [DOI] [PubMed] [Google Scholar]
- 8.Liu CR, Li MB, Cheng DJ, Yang CF, Tian SK. Catalyst-Free Alkylation of Sulfinic Acids with Sulfonamides via sp3 C-N Bond Cleavage at Room Temperature. Org Lett. 2009;11:2543–2545. doi: 10.1021/ol900788r. [DOI] [PubMed] [Google Scholar]
- 9.Li BJ, Wang HY, Zhu QL, Shi ZJ. Rhodium/Copper-Catalyzed Annulation of Benzimides with Internal Alkynes: Indenone Synthesis through Sequential C-H and C-N Cleavage. Angew Chem, Int Ed. 2012;51:3948–3952. doi: 10.1002/anie.201200271. [DOI] [PubMed] [Google Scholar]
- 10.Balaraman E, Gnanaprakasam G, Shimon LJW, Milstein D. Direct Hydrogenation of Amides to Alcohols and Amines under Mild Conditions. J Am Chem Soc. 2010;132:16756–16758. doi: 10.1021/ja1080019. [DOI] [PubMed] [Google Scholar]
- 11.(a) Lei Y, Wrobleski AD, Golden JE, Powell DR, Aube J. Facile C-N Cleavage in a Series of Bridged Lactams. J Am Chem Soc. 2005;127:4552–4553. doi: 10.1021/ja050214m. [DOI] [PubMed] [Google Scholar]; (b) Kajita Y, Matsubara S, Kurahashi T. Nickel-Catalyzed Decarbonylative Addition of Phthalimides to Alkynes. J Am Chem Soc. 2008;130:6058–6059. doi: 10.1021/ja7114426. [DOI] [PubMed] [Google Scholar]; (c) Li C, Zhang W-T, Wang X-S. CuI-Catalyzed C−N Bond Formation and Cleavage for the Synthesis of Benzimidazo[1,2-α]quinazoline Derivatives. J Org Chem. 2014;79:5847–5851. doi: 10.1021/jo5007398. [DOI] [PubMed] [Google Scholar]; (d) Yang X-F, Hu X-H, Loh T-P. Expedient Synthesis of Pyrroloquinolinones by Rh-Catalyzed Annulation of N-Carbamoyl Indolines with Alkynes through a Directed C−H Functionalization/C−N Cleavage Sequence. Org Lett. 2015;17:1481–1484. doi: 10.1021/acs.orglett.5b00355. [DOI] [PubMed] [Google Scholar]; (e) Shi S, Szostak M. Nickel-Catalyzed Diaryl Ketone Synthesis by N−C Cleavage: Direct Negishi Cross-Coupling of Primary Amides by Site-Selective N, N-DiBoc Activation. Org Lett. 2016;18:5872–5875. doi: 10.1021/acs.orglett.6b02952. [DOI] [PubMed] [Google Scholar]; (f) Hu F, Lalancette R, Szostak M. Structural Characterization of N-Alkylated Twisted Amides: Consequences for Amide Bond Resonance and N−C Cleavage. Angew Chem, Int Ed. 2016;55:5062–5066. doi: 10.1002/anie.201600919. [DOI] [PubMed] [Google Scholar]
- 12.Recent reviews: Hamid MHSA, Slatford PA, Williams JMJ. Borrowing Hydrogen in the Activation of Alcohols. Adv Synth Catal. 2007;349:1555–1575.Dobereiner GE, Crabtree RH. Dehydrogenation as a Substrate-Activating Strategy in Homogeneous Transition-Metal Catalysis. Chem Rev. 2010;110:681–703. doi: 10.1021/cr900202j.Guillena G, Ramon DJ, Yus M. Hydrogen Autotransfer in the N-Alkylation of Amines and Related Compounds using Alcohols and Amines as Electrophiles. Chem Rev. 2010;110:1611–1641. doi: 10.1021/cr9002159.Yang Q, Wanga Q, Yu Z. Substitution of alcohols by N-nucleophiles via transition metal-catalyzed dehydrogenation. Chem Soc Rev. 2015;44:2305–2329. doi: 10.1039/c4cs00496e.
- 13.(a) Yan T, Feringa BL, Barta K. Iron catalysed direct alkylation of amines with alcohols. Nat Commun. 2014;5:5602–5609. doi: 10.1038/ncomms6602. [DOI] [PubMed] [Google Scholar]; (b) Rosler S, Ertl M, Irrgang T, Kempe R. Cobalt Catalyzed Alkylation of Aromatic Amines by Alcohols. Angew Chem, Int Ed. 2015;54:15046–15050. doi: 10.1002/anie.201507955. [DOI] [PubMed] [Google Scholar]; (c) Elangovan S, Neumann J, Sortais J-B, Junge K, Darcel C, Beller M. Efficient and selective N-alkylation of amines with alcohols catalysed by manganese pincer complexes. Nat Commun. 2016;7:12641–12649. doi: 10.1038/ncomms12641. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Mukherjee A, Nerush A, Leitus G, Shimon LJW, David YB, Jalapa NAE, Milstein D. Manganese-Catalyzed Environmentally Benign Dehydrogenative Coupling of Alcohols and Amines to Form Aldimines and H2: A Catalytic and Mechanistic Study. J Am Chem Soc. 2016;138:4298–4301. doi: 10.1021/jacs.5b13519. [DOI] [PubMed] [Google Scholar]; (e) Vellakkaran M, Singh K, Banerjee D. An Efficient and Selective Nickel-Catalyzed Direct N-Alkylation of Anilines with Alcohols. ACS Catal. 2017;7:8152–8158. [Google Scholar]
- 14.Recent reviews: Baxter EW, Reitz AB. Reductive Aminations of Carbonyl Compounds with Borohydride and Borane Reducing Agents. Org React. 2002;59:1–715.Gomez S, Peters JA, Maschmeyer T. The Reductive Amination of Aldehydes and Ketones and the Hydrogenation of Nitriles: Mechanistic Aspects and Selectivity Control. Adv Synth Catal. 2002;344:1037–1057.Tripathi RP, Verma SS, Pandey J, Tiwari VK. Recent Development on Catalytic Reductive Amination and Applications. Curr Org Chem. 2008;12:1093–1115.
- 15.Hollmann D, Bahn S, Tillack A, Beller M. N-Dealkylation of aliphatic amines and selective synthesis of monoalkylated aryl amines. Chem Commun. 2008:3199–3201. doi: 10.1039/b803114b. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X, Liu D, Guo H, Liu Y, Zhang W. C-N Bond Cleavage of Allylic Amines via Hydrogen Bond Activation with Alcohol Solvents in Pd-Catalyzed Allylic Alkylation of Carbonyl Compounds. J Am Chem Soc. 2011;133:19354–19357. doi: 10.1021/ja209373k. [DOI] [PubMed] [Google Scholar]
- 17.(a) Kim J, Lee DH, Kalutharage N, Yi CS. Selective Catalytic Synthesis of Unsymmetrical Ethers from the Dehydrative Etherification of Two Different Alcohols. ACS Catal. 2014;4:3881–3885. [Google Scholar]; (b) Kalutharage N, Yi CS. Chemoselective Formation of Unsymmetrically Substituted Ethers from Catalytic Reductive Coupling of Aldehydes and Ketones with Alcohols in Aqueous Solution. Org Lett. 2015;17:1778–1781. doi: 10.1021/acs.orglett.5b00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalutharage N, Yi CS. Scope and Mechanistic Analysis for Chemoselective Hydrogenolysis of Carbonyl Compounds Catalyzed by a Cationic Ruthenium Hydride Complex with a Tunable Phenol Ligand. J Am Chem Soc. 2015;137:11105–11114. doi: 10.1021/jacs.5b06097. [DOI] [PubMed] [Google Scholar]
- 19.(a) Singleton DA, Thomas AA. High-Precision Simultaneous Determination of Multiple Small Kinetic Isotope Effects at Natural Abundance. J Am Chem Soc. 1995;117:9357–9358. [Google Scholar]; (b) Frantz DE, Singleton DA, Snyder JP. 13C Kinetic Isotope Effects for the Addition of Lithium Dibutylcuprate to Cyclohexenone. Reductive Elimination Is Rate-Determining. J Am Chem Soc. 1997;119:3383–3384. [Google Scholar]; (c) Nowlan DT, Gregg TM, Davies HML, Singleton DA. Isotope Effects and the Nature of Selectivity in Rhodium-Catalyzed Cyclopropanations. J Am Chem Soc. 2003;125:15902–15911. doi: 10.1021/ja036025q. [DOI] [PubMed] [Google Scholar]
- 20.(a) Brown RS, Ulan JG. Hydrolysis of Imidazole-Containing Amide Acetals. J Am Chem Soc. 1983;105:2382–2388. [Google Scholar]; (b) Nuiry II, Hermes JD, Weiss PM, Chen CY, Cook PF. Kinetic Mechanism and Location of Rate-Determining Steps for Aspartase from Hafnia alvei. Biochemistry. 1984;23:5168–5175. doi: 10.1021/bi00317a013. [DOI] [PubMed] [Google Scholar]; (c) Manoharan TS, Brown RS. 1-(1-Ethoxyethyl): An Effective Protecting Group for Imidazole Nitrogen. J Org Chem. 1988;53:1107–1110. [Google Scholar]; (d) Reyes MB, Carpenter BK. Mechanism of Thermal Deazetization of 2,3-Diazabicyclo[2.2.1]hept-2-ene and Its Reaction Dynamics in Supercritical Fluids. J Am Chem Soc. 2000;122:10163–10176. [Google Scholar]; (e) Fast W, Wang Z, Benkovic SJ. Familial Mutations and Zinc Stoichiometry Determine the Rate-Limiting Step of Nitrocefin Hydrolysis by Metallo-α-lactamase from Bacteroides fragilis. Biochemistry. 2001;40:1640–1650. doi: 10.1021/bi001860v. [DOI] [PubMed] [Google Scholar]
- 21.Eubanks JRI, Sims LB, Fry A. Carbon Isotope Effect Studies of the Mechanism of the Hofmann Elimination Reaction of Para-Substituted (2-Phenylethyl-1-14C)- and (2-Phenylethyl-2-14C)trimethylammonium Bromides. J Am Chem Soc. 1991;113:8821–8829. [Google Scholar]
- 22.Marlier JF, Robins LI, Tucker KA, Rawlings J, Anderson MA, Cleland WW. A Kinetic and Isotope Effect Investigation of the Urease-Catalyzed Hydrolysis of Hydroxyurea. Biochemistry. 2010;49:8213–8219. doi: 10.1021/bi100890v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.In an effort to demonstrate the reversibility of the imine formation, we performed the reaction of PhN=CHPh (1.0 mmol) with cyclohexylamine (1.4 mmol) in the presence of 1/L1 at 130 °C. The reaction produced a complex mixture of aniline and the crossover products, PhN=CC5H10, (PhCH2)2NH, PhCH2NHCy and PhCH2N=CC5H10.
- 24.(a) Wu JC, Song RJ, Wang ZQ, Huang XC, Xie YX, Li JH. Copper-Catalyzed C-H Oxidation/Cross-Coupling of α-Amino Carbonyl Compounds. Angew Chem, Int Ed. 2012;51:3453–3457. doi: 10.1002/anie.201109027. [DOI] [PubMed] [Google Scholar]; (b) Tian J-S, Loh T-P. Copper-Catalyzed Rearrangement of Tertiary Amines through Oxidation of Aliphatic C-H Bonds in Air or Oxygen: Direct Synthesis of α-Amino Acetals. Angew Chem, Int Ed. 2010;49:8417–8420. doi: 10.1002/anie.201003646. [DOI] [PubMed] [Google Scholar]; (c) Guo S, Qian B, Xie Y, Xia C, Huang H. Copper-Catalyzed Oxidative Amination of Benzoxazoles via C-H and C-N Bond Activation: A New Strategy for Using Tertiary Amines as Nitrogen Group Sources. Org Lett. 2011;13:522–525. doi: 10.1021/ol1030298. [DOI] [PubMed] [Google Scholar]; (d) Wan J-P, Zhou Y, Cao S. Domino Reactions Involving the Branched C–N and C-C Cleavage of Enaminones Toward Pyridines Synthesis. J Org Chem. 2014;79:9872–9877. doi: 10.1021/jo5018266. [DOI] [PubMed] [Google Scholar]
- 25.Selected reviews: Theophanides T, Harvey PD. Structural and spectroscopic properties of metal-urea complexes. Coord Chem Rev. 1987;76:237–264.Borovik AS. Bioinspired Hydrogen Bond Motifs in Ligand Design: The Role of Noncovalent Interactions in Metal Ion Mediated Activation of Dioxygen. Acc Chem Res. 2005;38:54–61. doi: 10.1021/ar030160q.Shao J-Y, Gong Z-L, Zhong Y-W. Bridged cyclometalated diruthenium complexes for fundamental electron transfer studies and multi-stage redox switching. Dalton Trans. 2018;47:23–29. doi: 10.1039/c7dt04168c.
- 26.In a NMR tube, the heating of 1 (0.01 mmol) with L1 (0.04 mmol) in toluene-d8 at 80 °C for 6 h led to the formation of a new Ru complex, which exhibited two signals in the 31P{1H} NMR (δ 56.1 and 56.6 ppm) but did not show any Ru-H peaks in the 1H NMR.
- 27.Jadhav AR, Bandal HA, Kim H. Synthesis of Substituted Amines: Catalytic Reductive Amination of Carbonyl Compounds using Lewis Acid Zn–Co-Double Metal Cyanide/Polymethylhydrosiloxane. Chem Eng J. 2016;295:376–383. [Google Scholar]
- 28.Bregeot NF, Raushel J, Sandrock DL, Dreher SD, Molander GA. Rapid and Efficient Access to Secondary Arylmethylamines. Chem Eur J. 2012;18:9564–9570. doi: 10.1002/chem.201200831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehmke V, Winkler E, Banner DW, Haap W, Schweizer WB, Rottmann M, Kaiser M, Freymond C, Schirmeister T, Diederich F. Optimization of Triazine Nitriles as Rhodesain Inhibitors: Structure–Activity Relationships, Bioisosteric Imidazopyridine Nitriles, and X-ray Crystal Structure Analysis with Human Cathepsin. Chem Med Chem. 2013;8:967–975. doi: 10.1002/cmdc.201300112. [DOI] [PubMed] [Google Scholar]
- 30.Huang PQ, Geng H. Simple, Versatile, and Chemoselective Reduction of Secondary Amides and Lactams to Amines with the Tf2O–NaBH4 or Cp2ZrHCl–NaBH4 System. Org Chem Front. 2015;2:150–158. [Google Scholar]
- 31.(a) Majumdar B, Mandani S, Bhattacharya T, Sarma D, Sarma TK. Probing Carbocatalytic Activity of Carbon Nanodots for the Synthesis of Biologically Active Dihydro/Spiro/Glyco Quinazolinones and Aza-Michael Adducts. J Org Chem. 2017;82:2097–2106. doi: 10.1021/acs.joc.6b02914. [DOI] [PubMed] [Google Scholar]; (b) Kalita P, Pegu CD, Dutta P, Baruah PK. Room Temperature Solvent Free Aza-Michael Reactions over Nano-cage Mesoporous Materials. J Mol Cat A. 2014;394:145–150. [Google Scholar]
- 32.Smith CJ, Smith CD, Nikbin N, Ley SV, Baxendale IR. Flow Synthesis of Organic Azides and the Multistep Synthesis of Imines and Amines using a New Monolithic Triphenylphosphine Reagent. Org Biomol Chem. 2011;9:1927–1937. doi: 10.1039/c0ob00813c. [DOI] [PubMed] [Google Scholar]
- 33.Martínez PH, Hultzsch KC, Gil A, Branchadell V. Base-Catalyzed Anti-Markovnikov Hydroamination of Vinylarenes – Scope, Limitations and Computational Studies. Eur J Org Chem. 2007;20:3311–3325. [Google Scholar]
- 34.Ehmke V, Heindl C, Rottmann M, Freymond C, Schweizer WB, Brun R, Stich A, Schirmeister T, Diederich F. Potent and Selective Inhibition of Cysteine Proteases from Plasmodium falciparum and Trypanosoma brucei. Chem Med Chem. 2011;6:273–278. doi: 10.1002/cmdc.201000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murali R, Rao HSP, Scheeren HW. Intra-Molecular Diels–Alder Reactions of Citraconamic Acids from Furfurylamines and Citraconic Anhydride: Effects of Substitution in the Furan Ring on Regioselectivity. Tetrahedron. 2001;57:3165–3174. [Google Scholar]
- 36.Su C, Tandiana R, Balapanuru J, Tang W, Pareek K, Nai CT, Hayashi T, Loh KP. Tandem Catalysis of Amines using Porous Graphene Oxide. J Am Chem Soc. 2015;137:685–690. doi: 10.1021/ja512470t. [DOI] [PubMed] [Google Scholar]
- 37.He W, Wang L, Sun C, Wu K, He S, Chen J, Wu P, Yu Z. Pt–Sn/γ-Al2O3-Catalyzed Highly Efficient Direct Synthesis of Secondary and Tertiary Amines and Imines. Chem Eur J. 2011;17:13308–13317. doi: 10.1002/chem.201101725. [DOI] [PubMed] [Google Scholar]
- 38.Shao Z, Fu S, Wei M, Zhou S, Liu Q. Mild and Selective Cobalt-Catalyzed Chemodivergent Transfer Hydrogenation of Nitriles. Angew Chem, Int Ed. 2016;55:14653–14657. doi: 10.1002/anie.201608345. [DOI] [PubMed] [Google Scholar]
- 39.Fang Y-Q, Jacobsen EN. Cooperative, Highly Enantioselective Phosphinothiourea Catalysis of Imine–Allene [3+2] Cycloadditions. J Am Chem Soc. 2008;130:5660–5661. doi: 10.1021/ja801344w. [DOI] [PubMed] [Google Scholar]
- 40.Yuan H, Yoo WJ, Miyamura H, Kobayashi S. Discovery of a Metalloenzyme-like Cooperative Catalytic System of Metal Nanoclusters and Catechol Derivatives for the Aerobic Oxidation of Amines. J Am Chem Soc. 2012;134:13970–13973. doi: 10.1021/ja306934b. [DOI] [PubMed] [Google Scholar]
- 41.Zhao P-P, Zhou X-F, Dai J-J, Xu H-J. Catalyst-Free Reductive Amination of Aromatic Aldehydes with Ammonium Formate and Hantzsch Ester. Org Biomol Chem. 2014;12:9092–9096. doi: 10.1039/c4ob01590h. [DOI] [PubMed] [Google Scholar]
- 42.Polet D, Alexakis A. Kinetic Study of Various Phosphoramidite Ligands in the Iridium-Catalyzed Allylic Substitution. Org Lett. 2005;7:1621–1624. doi: 10.1021/ol050350w. [DOI] [PubMed] [Google Scholar]
- 43.Miriyala B, Bhattacharyya S, Williamson JS. Chemoselective Reductive Alkylation of Ammonia with Carbonyl Compounds: Synthesis of Primary and Symmetrical Secondary Amines. Tetrahedron. 2004;60:1463–1471. [Google Scholar]
- 44.Kawahara R, Fujita K, Yamaguchi R. Multialkylation of Aqueous Ammonia with Alcohols Catalyzed by Water-Soluble Cp*Ir–Ammine Complexes. J Am Chem Soc. 2010;132:15108–15111. doi: 10.1021/ja107274w. [DOI] [PubMed] [Google Scholar]
- 45.Kumar P, Cherian SK, Jain R, Show K. Chemoselective Deprotection of N-allylic Amines using DDQ. Tetrahedron Lett. 2014;55:7172–7176. [Google Scholar]
- 46.Yamaguchi R, Mingwen Z, Kawagoe S, Asai C, Fujita K. A New Atom-Economical and Selective Synthesis of Secondary and Tertiary Alkylamines by Means of Cp*Iridium Complex Catalyzed Multiple N-Alkylation of Ammonium Salts with Alcohols without Solvent. Synthesis. 2009;7:1220–1223. [Google Scholar]
- 47.Petersen LLR-L, Jensen P, Madsen R. Iridium-Catalyzed Condensation of Primary Amines to Form Secondary Amines. Synthesis. 2009;24:4110–4112. [Google Scholar]
- 48.Yin Z, Zeng H, Wu J, Zheng S, Zhang G. Cobalt-Catalyzed Synthesis of Aromatic, Aliphatic, and Cyclic Secondary Amines via a “Hydrogen-Borrowing” Strategy. ACS Catal. 2016;6:6546–6550. [Google Scholar]
- 49.Fasano V, Radcliffe JE, Ingleson MJ. B(C6F5)3-Catalyzed Reductive Amination using Hydrosilanes. ACS Catal. 2016;6:1793–1798. [Google Scholar]
- 50.Ji P, Manna K, Lin Z, Urban A, Greene FX, Lan G, Lin W. Single-Site Cobalt Catalysts at New Zr8(μ2-O)8(μ2-OH)4 Metal-Organic Framework Nodes for Highly Active Hydrogenation of Alkenes, Imines, Carbonyls, and Heterocycles. J Am Chem Soc. 2016;138:12234–12242. doi: 10.1021/jacs.6b06759. [DOI] [PubMed] [Google Scholar]
- 51.Too PC, Chan GH, Tnay YL, Hirao H, Chiba S. Hydride Reduction by a Sodium Hydride–Iodide Composite. Angew Chem. 2016;128:3783–3787. doi: 10.1002/anie.201600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corre Y, Iali W, Hamdaoui M, Trivelli X, Djukic J-P, -Niedercorn FA, Michon C. Efficient Hydrosilylation of Imines using Catalysts Based on Iridium(III) Metallacycles. Catal Sci Tech. 2015;5:1452–1458. [Google Scholar]
- 53.Champagne PA, Pomarole J, Therien M-E, Benhassine Y, Beaulieu S, Legault CY, Paquin J-F. Enabling Nucleophilic Substitution Reactions of Activated Alkyl Fluorides through Hydrogen Bonding. Org Lett. 2013;15:2210–2213. doi: 10.1021/ol400765a. [DOI] [PubMed] [Google Scholar]
- 54.Yagafarov NZ, Usanov DL, Moskovets AP, Kagramanov ND, Maleev VI, Chusov D. Reductive Transformations of Carbonyl Compounds Catalyzed by Rhodium Supported on a Carbon Matrix by using Carbon Monoxide as a Deoxygenative Agent. Chem Cat Chem. 2015;7:2590–2593. [Google Scholar]
- 55.Afanasyev OI, Tsygankov AA, Usanov DL, Perekalin DS, Shvydkiy NV, Maleev VI, Kudinov AR, Chusov D. Cyclobutadiene Metal Complexes: A New Class of Highly Selective Catalysts. An Application to Direct Reductive Amination. ACS Catal. 2016;6:2043–2046. [Google Scholar]
- 56.Sousa SCA, Fernandes AC. Efficient and Highly Chemoselective Direct Reductive Amination of Aldehydes using the System Silane/Oxorhenium Complexes. Adv Synth Catal. 2010;352:2218–2226. [Google Scholar]
- 57.Cushman M, Nagarathnam D, Gopal D, Chakraborti AK, Lin CM, Hamel E. Synthesis and Evaluation of Stilbene and Dihydrostilbene Derivatives as Potential Anticancer Agents that Inhibit Tubulin Polymerization. J Med Chem. 1991;34:2579–2588. doi: 10.1021/jm00112a036. [DOI] [PubMed] [Google Scholar]
- 58.Bhunia M, Hota PK, Vijaykumar G, Adhikari G, Mandal SK. A Highly Efficient Base-Metal Catalyst: Chemoselective Reduction of Imines to Amines Using An Abnormal-NHC–Fe(0) Complex. Organometallics. 2016;35:2930–2937. [Google Scholar]
- 59.Laha JK, Tummalapalli KSS, Jethava KP. Implications of Dynamic Imine Chemistry for the Sustainable Synthesis of Nitrogen Heterocycles via Transimination Followed by Intramolecular Cyclisation. Org Biomol Chem. 2016;14:2473–2479. doi: 10.1039/c5ob02670a. [DOI] [PubMed] [Google Scholar]
- 60.Yang M, Liu F. An Ullmann Coupling of Aryl Iodides and Amines Using an Air-Stable Diazaphospholane Ligand. J Org Chem. 2007;72:8969–8971. doi: 10.1021/jo0712291. [DOI] [PubMed] [Google Scholar]
- 61.Akisanya J, Danks TN, Garman RN. Reaction of (1-Azabuta-1, 3-diene)tricarbonyliron(0) Complexes with Sodium Borohydride under Microwave Conditions. J Organomet Chem. 2000;603:240–243. [Google Scholar]
- 62.Bollenbach M, Wagner P, Aquino PGV, Bourguignon J-J, Bihel F, Salomé C, Schmitt M. D-Glucose: An Efficient Reducing Agent for a Copper(II)-Mediated Arylation of Primary Amines in Water. Chem Sus Chem. 2016;9:3244–3249. doi: 10.1002/cssc.201600801. [DOI] [PubMed] [Google Scholar]
- 63.Aziz J, Brion J-D, Hamze A, Alami M. Copper Acetoacetonate [Cu(acac)2]/BINAP-Promoted Csp3-N Bond Formation via Reductive Coupling of N-Tosylhydrazones with Anilines. Adv Synth Catal. 2013;355:2417–2429. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.